Topical sildenafil cream 3.6% improved outcomes among women with female sexual arousal disorder, particularly an exploratory subset of women with female sexual arousal disorder with or without concomitant decreased desire.
Abstract
OBJECTIVE:
To assess the efficacy of topical sildenafil cream, 3.6% among healthy premenopausal women with female sexual arousal disorder.
METHODS:
We conducted a phase 2b, exploratory, randomized, placebo-controlled, double-blind study of sildenafil cream. Coprimary efficacy endpoints were the change from baseline to week 12 in the Arousal Sensation domain of the SFQ28 (Sexual Function Questionnaire) and question 14 of the FSDS-DAO (Female Sexual Distress Scale—Desire, Arousal, Orgasm).
RESULTS:
Two hundred women with female sexual arousal disorder were randomized to sildenafil cream (n=101) or placebo cream (n=99). A total of 174 participants completed the study (sildenafil 90, placebo 84). Among the intention-to-treat (ITT) population, which included women with only female sexual arousal disorder and those with female sexual arousal disorder with concomitant sexual dysfunction diagnoses or genital pain, although the sildenafil cream group demonstrated greater improvement in the SFQ28 Arousal Sensation domain scores, there were no statistically significant differences between sildenafil and placebo cream users in the coprimary and secondary efficacy endpoints. An exploratory post hoc subset of the ITT population with an enrollment diagnosis of female sexual arousal disorder with or without concomitant decreased desire randomized to sildenafil cream reported significant increases in their SFQ28 Arousal Sensation domain score (least squares mean 2.03 [SE 0.62]) compared with placebo cream (least squares mean 0.08 [SE 0.71], P=.04). This subset achieved a larger mean improvement in the SFQ28 Desire and Orgasm domain scores. This subset population also had significantly reduced sexual distress and interpersonal difficulties with sildenafil cream use as measured by FSDS-DAO questions 3, 5, and 10 (all P≤.04).
CONCLUSION:
Topical sildenafil cream improved outcomes among women with female sexual arousal disorder, most significantly in those who did not have concomitant orgasmic dysfunction. In particular, in an exploratory analysis of a subset of women with female sexual arousal disorder with or without concomitant decreased desire, topical sildenafil cream increased sexual arousal sensation, desire, and orgasm and reduced sexual distress.
CLINICAL TRIAL REGISTRATION:
Female sexual arousal disorder is a persistent or recurrent inability to attain, or to maintain until completion of the sexual activity, an adequate lubrication-swelling response of sexual excitement that causes marked distress or interpersonal difficulty.1,2 Despite the high prevalence of female sexual arousal disorder (about 20% of U.S. women3–5), to date there are no U.S. Food and Drug Administration (FDA)–approved pharmacologic treatments for female sexual arousal disorder.
Female sexual arousal disorder is clinically analogous to male erectile dysfunction, characterized by impaired blood flow to genital tissues. Oral sildenafil citrate (Viagra) was approved in 1998 for the treatment of erectile dysfunction. Published placebo-controlled trials of oral sildenafil administered in premenopausal and postmenopausal women afflicted with broad-spectrum female sexual dysfunction (eg, hypoactive sexual desire disorder, female sexual arousal disorder, female orgasmic disorder, dyspareunia) demonstrated marginal efficacy compared with placebo6–9 with high incidences of intolerable side effects.
Topical sildenafil cream 3.6% (sildenafil cream) has been developed for the treatment of female sexual arousal disorder. We hypothesized that by delivering sildenafil citrate topically with a fast-absorbing delivery technology specifically targeting genital anatomy central to the vascular arousal response,10 there would be less systemic exposure, fewer systemic side effects, and a more immediate biological efficacy response.
METHODS
Before the start of the study, we conducted a qualitative content validity study among women with female sexual arousal disorder to ensure that the efficacy endpoints, which were patient-reported outcome measures, were fit for purpose and valid for this specific population.11
This clinical trial was conducted at 49 sites in the United States, approved by the Advarra IRB (Pro00049161), and registered at ClinicalTrials.gov (NCT04948151). Healthy premenopausal women aged 18 years or older and their sexual partners were screened for the study. Volunteers and their sexual partners provided written informed consent before the performance of any study-related procedures. Women at risk of pregnancy used effective contraception during the study (eg, hormonal contraception). Male condoms could be used for sexually transmitted infection prevention. The coprimary efficacy endpoints were the change from baseline to week 12 in the Arousal Sensation domain of the SFQ38 (Sexual Function Questionnaire) and question 14 of the FSDS-DAO (Female Sexual Distress Scale—Desire, Arousal, Orgasm). A one-on-one clinical interview was conducted with each potential participant, using interviewers who were not employees of the study sponsor and who were recognized experts in the field, to establish the diagnosis of female sexual arousal disorder and to verify that female sexual arousal disorder was the woman's main sexual dysfunction concern if other concomitant sexual dysfunction diagnoses or symptoms existed. The clinical interview documented those concomitant diagnoses or symptoms for exploratory post hoc analyses based on sexual dysfunction diagnoses or symptoms at enrollment.
The first screening occurred in July 2021, and the last participant visit was in April 2023. Appendix 1, available online at http://links.lww.com/AOG/D723, outlines the schedule of efficacy evaluations. In brief, volunteers underwent informed consent and screening safety procedures at visit 1. A major barrier to recruitment was the requirement for sexual partners to be consented and involved in adverse event reporting because many volunteers did not want to disclose their female sexual arousal disorder symptoms to their sexual partners. Therefore, amendment 3 of the protocol allowed women to enroll in the trial if they did not have a sexual partner or if their sexual partner did not want to participate in the informed consent and safety monitoring process. In this latter case, investigational product was used only for unpartnered sexual events.
As shown in Appendix 1 (http://links.lww.com/AOG/D723), the study included a 28-day no-drug run-in period starting at visit 2, followed by a 28-day single-blind placebo run-in period beginning at visit 3. Participants were screen failed during these 2 months if they were not compliant with recording sexual events, adverse events, or concomitant medications in the daily electronic diary or if they had a predetermined placebo response during the single-blind run-in period. Eligible participants were randomized at visit 4 in a 1:1 manner to sildenafil cream or placebo cream.
The double-blind dosing period lasted 12 weeks, and responses at visit 4 and electronic diary data from the single-blind placebo run-in period were baseline scores. Participants were seen monthly during the double-blind dosing period at weeks 4 (visit 5), 8 (visit 6), and 12 (visit 7) and answered the 1-month recall SFQ28 and FSDS-DAO surveys. Participants recorded data in the electronic diary within 24 hours of a sexual event between monthly appointments.
Participants were randomized with a concealed, interactive response technology system, after the single-blind run-in period, to either sildenafil cream or placebo cream in a 1:1 ratio. The allocation was blinded, and there were no allocation errors.
The placebo cream contained the same ingredients as the sildenafil cream but without the active ingredient, sildenafil citrate. Each month, the investigational product was dispensed in a 30-g tube, along with nine 2-g dosing cards. Participants were instructed to apply no more than one 2-g application of product per calendar day and no more than 9 applications per month. Participants applied the investigational product about 10–20 minutes before the sexual event. One gram was applied externally to the anterior labial commissure, prepuce of the clitoris, glans, frenulum, vestibule, and labia minora. The other gram was applied to the anterior distal vagina to an approximate depth of 0–3 cm or about halfway between the distal and proximal interphalangeal joints, targeting the distal, lower one-third of the vagina. Participants were instructed to wash off the cream and have their partner wash off the cream, if relevant, after the sexual experience.
The Arousal Sensation domain of the SFQ28 questionnaire is made up of questions 6 through 9 (Appendix 2, available online at http://links.lww.com/AOG/D723). These four questions were answered using a Likert scale ranging from 1–5, with 1 being no sensation and 5 being a very strong sensation. The range of scores for the Arousal Sensation domain is 4–20, with higher scores indicating higher sexual function.
The FSDS-DAO survey includes 15 questions, each of which began with the phrase, “How often in the past 30 days did you feel,” with responses of 0–4, with 0 indicating never feeling this way and 4 indicating always feeling this way (Appendix 2, http://links.lww.com/AOG/D723). Question 14 asks, “How often in the past 30 days did you feel concerned by difficulties with sexual arousal?” The range of scores for question 14 of the FSDS-DAO is 0–4, with lower scores indicating decreased levels of sexual distress.
For the secondary efficacy endpoint, the electronic diary prompted women every 24 hours to report whether there had been a sexual event. If there was a sexual event, the electronic diary prompted the participant to answer several questions, including, “Did you consider sexual activity satisfactory for you?” The secondary endpoint was the change from baseline at week 12 in the average number and proportion of satisfactory sexual events per product cohort.
Prespecified exploratory endpoints are detailed in Appendix 2, http://links.lww.com/AOG/D723. This study was designed as an exploratory, preliminary efficacy study. Assuming an estimated dropout rate of 25%, about 400–590 patients were planned to be randomized to the double-blind dosing period to ensure that a total of 300–440 patients successfully completed the double-blind dosing period (150–220 patients for each arm). Under the assumption of an SD of 4.7 for change from baseline to end of study in the SFQ28 Arousal Sensation domain and based on a previous study of oral sildenafil use in women with female sexual arousal disorder12 and a SD of 1.2 for change from baseline to end of study in question 14 of the FSDS-DAO, 300 patients would have provided more than 90% marginal power for the primary objective tests on the coprimary endpoints, with a target difference of +2 points in the change from baseline at week 12 in the SFQ28 Arousal Sensation domain and −0.5 in the change from baseline at week 12 in question 14 of the FSDS-DAO. Several studies further support the initial sample size calculations include.13–17
However, as a result of slower-than-anticipated enrollment (because of the requirement to obtain consent from and to enroll the patient's sexual partner and the effect of the coronavirus disease 2019 [COVID-19] pandemic) and considering the exploratory nature of the study, the sponsor decided that a smaller sample size, although resulting in underpowering, was acceptable and halted recruitment in August 2022. The protocol was revised to randomize 169–175 patients to ensure that a minimum of 150 patients completed the study, even though that sample size would not be sufficient to achieve the 300 patients estimated to provide more than 90% marginal power for the primary objective tests on the coprimary endpoints, per the original sample size estimates.
Age, baseline hormonal contraceptive use, race, and ethnicity were collected for preplanned subset analyses on the effect of these factors on efficacy endpoints (results reported elsewhere). The analysis was conducted with a mixed model for repeated measures, which handles missing data through maximum likelihood under the assumption of missing at random. For the nonprimary endpoints, we did not adjust for multiple hypothesis testing; therefore, all post hoc subset analyses are considered exploratory.
The individual clinical interviews (conducted at enrollment) for the 174 participants in the intention-to-treat (ITT) population who completed all study visits were reviewed retrospectively. All participants had a main diagnosis of female sexual arousal disorder and were categorized according to the presence or absence of concomitant sexual dysfunction diagnoses or symptoms, including decreased desire, orgasmic dysfunction, and genital pain. The efficacy endpoints were then evaluated in an exploratory post hoc analysis by enrollment diagnoses groups and product assignment.
All statistical analyses used SAS 9.4 or higher. The primary analysis of the SFQ28 (Arousal Sensation) and the FSDS-DAO (question 14) included a two-sided test with a significant level of 0.05.
RESULTS
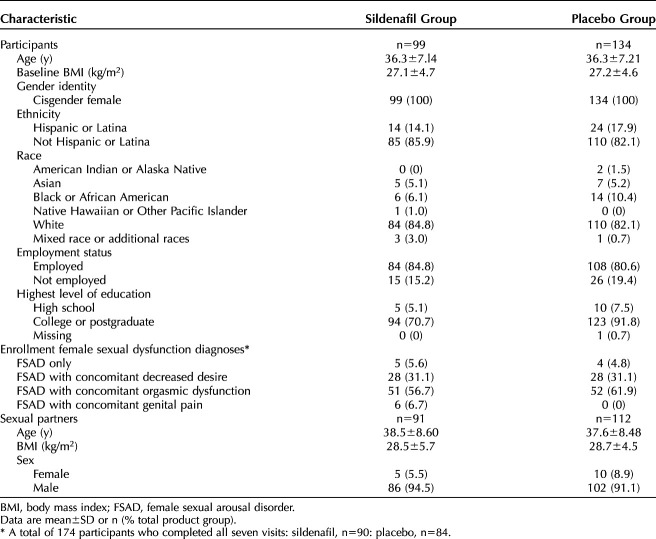
As shown in Figure 1, volunteers (n=833) and their sexual partners (n=605) underwent informed consent. Of the consented patients, 277 eligible participants entered the no-drug run-in period and 252 entered the single-blind placebo run-in period. A total of 200 participants were randomized to sildenafil cream (n=101) or placebo cream (n=99); 99 sildenafil cream–assigned women and 94 placebo cream–assigned women who received at least one dose of the investigational product made up the ITT population. A total of 174 women (sildenafil 90 and placebo 84) completed all study visits. Table 1 displays the baseline characteristics of participants and their sexual partners, if applicable, included in the safety analysis population.
Fig. 1. Disposition of participants.
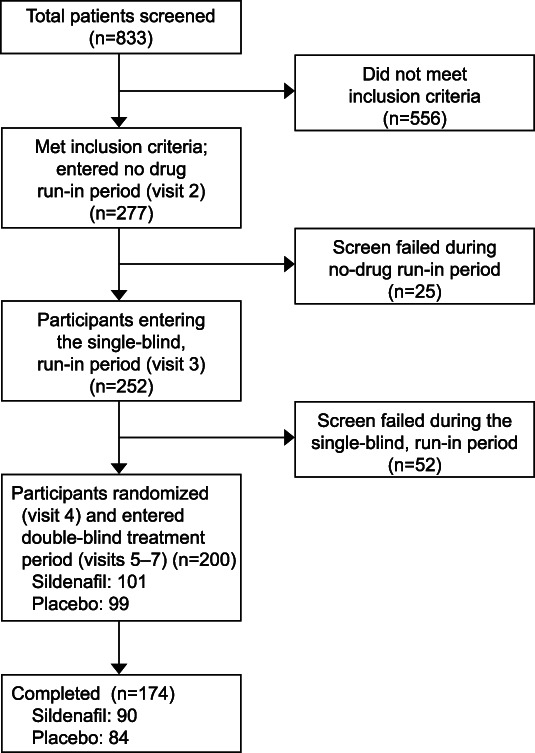
Johnson. Sildenafil Cream for Female Sexual Arousal Disorder. Obstet Gynecol 2024.
Table 1.
Demographic and Baseline Characteristics of Participants and Sexual Partners (Safety Population)
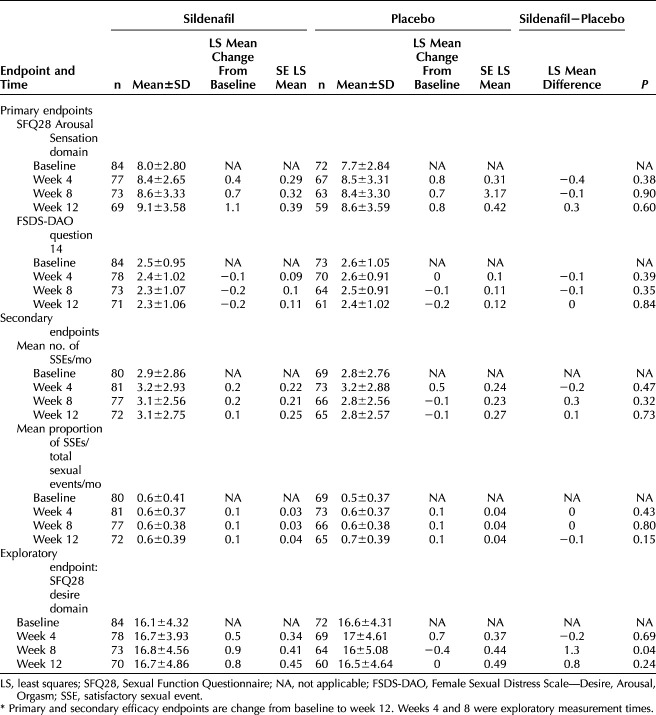
Table 2 displays the efficacy results for the ITT population. Although the sildenafil cream group demonstrated greater improvement than the placebo cream group in the SFQ28 Arousal Sensation domain (P=.60), we did not find any statistically significant improvement in either coprimary efficacy endpoint during the double-blind dosing period (all P>.05) in the ITT population. There were no statistically significant differences in the mean number of satisfactory sexual events between sildenafil cream and placebo cream users at any point during the double-blind dosing period (all P>.32). The mean proportion of satisfactory sexual events was also similar between the two dosing cohorts (all P>.15). The only statistically significant change noted for the ITT population was a statistically significant increase in the SFQ28 Desire domain score at week 8 for sildenafil cream users (P=.04, Table 2), which was a predefined exploratory endpoint.
Table 2.
Efficacy Endpoints for the Intention-to-Treat Population*
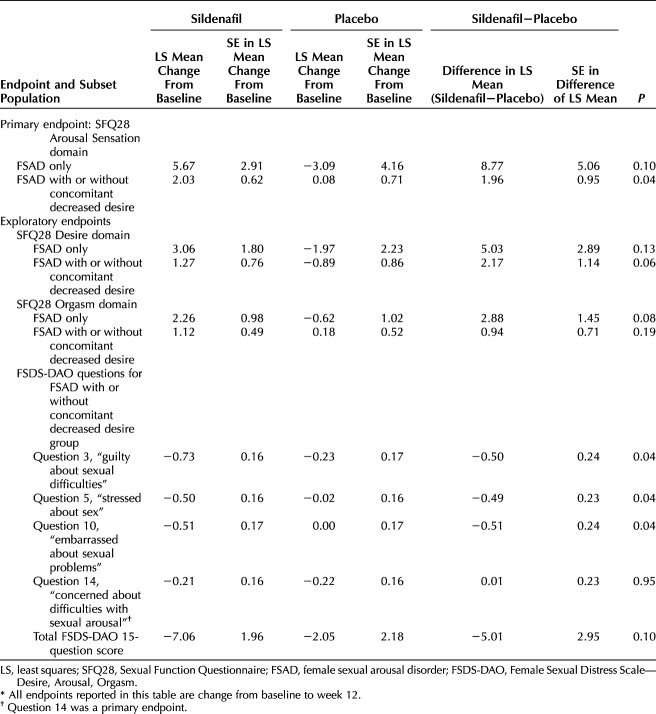
When the efficacy endpoints were compared in an exploratory post hoc analysis based on enrollment sexual dysfunction diagnoses, women with female sexual arousal disorder with concomitant orgasmic dysfunction did not experience as large a treatment benefit at week 12 from sildenafil cream use (data not shown, all P>.22) as those with other sexual dysfunction diagnoses. Specifically, women with female sexual arousal disorder as their only sexual dysfunction, although uncommon, experienced the largest improvements in sexual function at week 12 with sildenafil cream (Table 3), which were not statistically significant, likely because of the small sample size. When participants with female sexual arousal disorder with concomitant orgasmic dysfunction and female sexual arousal disorder with concomitant genital pain were removed from the analysis, a subset of participants (sildenafil 33 and placebo 32) remained. This subset of participants, who had female sexual arousal disorder only or female sexual arousal disorder with concomitant decreased desire, experienced significant improvements with sildenafil cream use at week 12 in the coprimary endpoint of SFQ28 Arousal Sensation domain (P=.04, Table 3) and had large improvements in the SFQ28 Desire domain (P=.06, Table 3) and the SFQ28 Orgasm domain (P=.19, Table 3) at week 12 compared with placebo cream users.
Table 3.
Efficacy Endpoints for Enrollment Female Sexual Dysfunction Diagnoses Subset Population*
Although the subset population of women randomized to sildenafil cream with either female sexual arousal disorder only or female sexual arousal disorder with concomitant decreased desire did not demonstrate statistically significant decreased sexual distress as measured by the coprimary endpoint of FSDS-DAO question 14 (P=.95, Table 3), which assesses concern about difficulties with sexual arousal, we found that several other FSDS-DAO questions, which asked about generalized feelings related to sexual distress and interpersonal difficulties, showed significant improvement with sildenafil cream compared with placebo cream (questions 3, 5 and 10, all P≤.04, Table 3) in this exploratory subset. The total FSDS-DAO score decreased by about 7 points for sildenafil cream users in the subset population (a clinically meaningful decrease in sexual distress11) compared with a 2-point decrease for placebo cream users (P=.10).
DISCUSSION
Topical sildenafil cream used over 12 weeks among healthy premenopausal women with female sexual arousal disorder did not show statistically significant improvement over placebo in the coprimary (SFQ28 Arousal Sensation domain, FSDS-DAO question 14) or secondary (mean number and mean proportion of satisfactory sexual events) endpoints among the ITT population, all of whom had female sexual arousal disorder but had a wide variety of concomitant sexual dysfunction diagnoses or symptoms, during this exploratory phase 2b study.
This study required the participants' main sexual dysfunction to be related to arousal because the mechanism of action of topical sildenafil citrate is to augment genital tissue blood flow to treat female sexual arousal disorder.18–28 Not surprisingly, we saw that female sexual arousal disorder was often accompanied by concomitant sexual dysfunction diagnoses or symptoms.29,30 When the efficacy endpoints were compared in an exploratory post hoc analysis among a subset of women with female sexual arousal disorder only or female sexual arousal disorder with concomitant decreased desire, we found either trends or significant improvements in sexual functioning with sildenafil cream compared with placebo cream across multiple aspects of sexual function. We also found significant reductions in several measures of sexual distress and interpersonal difficulties among this subset population. Although we recognize that exploratory post hoc subset analyses must be interpreted with caution and can introduce type I errors, we believe that the trends observed in this subset population are promising, are clinically meaningful, and warrant further study because an important objective of this exploratory phase 2 study was to identify which women with female sexual arousal disorder are most likely to benefit from the mechanism of action of sildenafil citrate to increase genital blood flow.18–28
Participants with female sexual arousal disorder with concomitant orgasmic dysfunction did not derive as much benefit from sildenafil cream use. Although it was important to assess this subset population in this exploratory study, it was expected that women with female sexual arousal disorder with concomitant orgasmic dysfunction may not benefit as greatly from sildenafil cream because orgasmic dysfunction is often associated with neurologic problems or other comorbid medical or psychological conditions that would not be treated by the mechanism of action of sildenafil citrate (ie, increased blood flow to the genital tissue)18–28 and can be challenging to distinguish from female sexual arousal disorder temporally in terms of onset.
A limitation of this exploratory study was that it was underpowered to demonstrate statistically significant changes in the primary and secondary efficacy endpoints among the ITT population, all of whom had female sexual arousal disorder but were heterogeneous in their concomitant sexual dysfunction diagnoses or symptoms. Because this study was the first efficacy study of topical sildenafil cream among healthy premenopausal women with a main complaint of female sexual arousal disorder and because there are no FDA-approved treatments for female sexual arousal disorder, the main benefits of this study were to characterize the sexual response affected by arousal dysfunction, to evaluate the patient population based on concomitant diagnoses and medications, to identify endpoints to take forward in clinical development, and to provide data for psychometric validation of the study endpoints, including identifying the magnitude of within-patient change corresponding to a meaningful within-patient improvement.
We based our sample size calculations on previous studies of FDA-approved products for hypoactive sexual desire disorder with or without concomitant decreased sexual arousal16,17,31–34 because there are no approved products for female sexual arousal disorder. We reviewed the published data for female sexual dysfunction treatments and expected a two-point increase in the SFQ28 domains and a 0.5-point decrease in question 14 of the FSDS-DAO. Although we did not achieve these changes in the entire ITT population, we consistently achieved or exceeded these improvements among the subset population of women with female sexual arousal disorder only or female sexual arousal disorder with concomitant decreased desire.
As seen in registration trials for hypoactive sexual desire disorder treatments,16,17,31–34 another limitation of our study was the relatively homogeneous population of college-educated White women. We acknowledge that the requirement to enroll sexual partners likely limited enrollment of Hispanic and non-Hispanic Black women and hypothesize that removing this requirement from future studies will result in a more diverse patient population.
Topical sildenafil cream improved outcomes among women with female sexual arousal disorder, most significantly in those who did not have concomitant orgasmic dysfunction. In particular, in an exploratory analysis of a subset of women with female sexual arousal disorder with or without concomitant decreased desire, topical sildenafil increased sexual arousal sensation, desire, and orgasm and reduced sexual distress.
Authors' Data Sharing Statement
Will individual participant data be available (including data dictionaries)? No.
What data in particular will be shared? Not available.
What other documents will be available? Not available.
When will data be available (start and end dates)? Not applicable.
By what access criteria will data be shared (including with whom, for what types of analyses, and by what mechanism)? Not applicable.
Footnotes
This study was funded by Daré Bioscience.
Financial Disclosure Clint Dart reports money was paid to his institution from Daré Bioscience. Isabella Johnson, Andrea Ries Thurman, Jessica Hatheway, David R. Friend, and Andrew Goldstein are employees of Daré Bioscience. Katherine A. Cornell is an employee of Strategic Science & Technologies, LLC. She indicated that this article discusses off-label use, in that sildenafil cream for FSAD is investigated. Clint Dart provided independent data verification and is an employee of Premier Research. C. Paige Brainard was the principal investigator of this study and was financially compensated by Del Sol Research Management for that service. Dr. Brainard's practice received funding from Daré Bioscience for study specific activities. Dr. Goldstein also reported receiving payments from Nuvig, Ipsen, and AbbVie.
This is a phase 2b study of topical sildenafil cream 3.6%, which is being developed clinically for the treatment of female sexual arousal disorder. The study was conducted under a U.S. Food and Drug Administration–approved investigational new drug application.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/D724.
REFERENCES
- 1.Parish SJ, Cottler-Casanova S, Clayton AH, McCabe MP, Coleman E, Reed GM. The evolution of the female sexual disorder/dysfunction definitions, nomenclature, and classifications: a review of DSM, ICSM, ISSWSH, and ICD. Sex Med Rev 2021;9:36–56. doi: 10.1016/j.sxmr.2020.05.001 [DOI] [PubMed] [Google Scholar]
- 2.Graham CA. The DSM diagnostic criteria for female sexual arousal disorder. Arch Sex Behav 2010;39:240–55. doi: 10.1007/s10508-009-9535-1 [DOI] [PubMed] [Google Scholar]
- 3.Laumann EO, Michael RT, Gagnon JH. A political history of the national sex survey of adults. Fam Plann Perspect 1994;26:34–8. doi: 10.2307/2136095 [DOI] [PubMed] [Google Scholar]
- 4.Witherow-Parkanyi M. Female sexual interest/arousal disorder: history of diagnostic considerations and their implications for clinical practice. Psychiatr Hung 2022;37:133–49. [PubMed] [Google Scholar]
- 5.Jayne C, Gago BA. Diagnosis and treatment of female sexual arousal disorder. Clin Obstet Gynecol 2009;52:675–81. doi: 10.1097/GRF.0b013e3181bf4982 [DOI] [PubMed] [Google Scholar]
- 6.Shields KM, Hrometz SL. Use of sildenafil for female sexual dysfunction. Ann Pharmacother 2006;40:931–4. doi: 10.1345/aph.1G471 [DOI] [PubMed] [Google Scholar]
- 7.Schoen C, Bachmann G. Sildenafil citrate for female sexual arousal disorder: a future possibility? Nat Rev Urol 2009;6:216–22. doi: 10.1038/nrurol.2009.25 [DOI] [PubMed] [Google Scholar]
- 8.Brown DA, Kyle JA, Ferrill MJ. Assessing the clinical efficacy of sildenafil for the treatment of female sexual dysfunction. Ann Pharmacother 2009;43:1275–85. doi: 10.1345/aph.1L691 [DOI] [PubMed] [Google Scholar]
- 9.Chivers ML, Rosen RC. Phosphodiesterase type 5 inhibitors and female sexual response: faulty protocols or paradigms? J Sex Med 2010;7:858–72. doi: 10.1111/j.1743-6109.2009.01599.x [DOI] [PubMed] [Google Scholar]
- 10.Goldstein I. Female sexual arousal disorder: new insights. Int J Impot Res 2000;12(suppl 4):S152–7. doi: 10.1038/sj.ijir.3900596 [DOI] [PubMed] [Google Scholar]
- 11.Symonds T, Kingsberg SA, Simon JA, Kroll R, Althof SE, Parish SJ, et al. Symptoms and associated impact in pre- and postmenopausal women with sexual arousal disorder: a concept elicitation study. J Sex Med 2023;20:277–86. doi: 10.1093/jsxmed/qdac043 [DOI] [PubMed] [Google Scholar]
- 12.Nurnberg HG, Hensley PL, Heiman JR, Croft HA, Debattista C, Paine S. Sildenafil treatment of women with antidepressant-associated sexual dysfunction: a randomized controlled trial. JAMA 2008;300:395–404. doi: 10.1001/jama.300.4.395 [DOI] [PubMed] [Google Scholar]
- 13.Berman JR, Berman LA, Toler SM, Gill J, Haughie S; Sildenafil Study Group. Safety and efficacy of sildenafil citrate for the treatment of female sexual arousal disorder: a double-blind, placebo controlled study. J Urol 2003;170:2333–8. doi: 10.1097/01.ju.0000090966.74607.34 [DOI] [PubMed] [Google Scholar]
- 14.Clayton AH, Althof SE, Kingsberg S, DeRogatis LR, Kroll R, Goldstein I, et al. Bremelanotide for female sexual dysfunctions in premenopausal women: a randomized, placebo-controlled dose-finding trial. Womens Health (Lond) 2016;12:325–37. doi: 10.2217/whe-2016-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labrie F, Archer D, Bouchard C, Fortier M, Cusan L, Gomez JL, et al. Effect of intravaginal dehydroepiandrosterone (Prasterone) on libido and sexual dysfunction in postmenopausal women. Menopause 2009;16:923–31. doi: 10.1097/gme.0b013e31819e85c6 [DOI] [PubMed] [Google Scholar]
- 16.Derogatis LR, Komer L, Katz M, Moreau M, Kimura T, Garcia M, Jr, et al. Treatment of hypoactive sexual desire disorder in premenopausal women: efficacy of flibanserin in the VIOLET Study. J Sex Med 2012;9:1074–85. doi: 10.1111/j.1743-6109.2011.02626.x [DOI] [PubMed] [Google Scholar]
- 17.Thorp J, Simon J, Dattani D, Taylor L, Kimura T, Garcia M, Jr, et al. Treatment of hypoactive sexual desire disorder in premenopausal women: efficacy of flibanserin in the DAISY study. J Sex Med 2012;9:793–804. doi: 10.1111/j.1743-6109.2011.02595.x [DOI] [PubMed] [Google Scholar]
- 18.D'Amati G, di Gioia CR, Bologna M, Giordano D, Giorgi M, Dolci S, et al. Type 5 phosphodiesterase expression in the human vagina. Urology 2002;60:191–5. doi: 10.1016/s0090-4295(02)01663-1 [DOI] [PubMed] [Google Scholar]
- 19.Francis SH, Corbin JD. Molecular mechanisms and pharmacokinetics of phosphodiesterase-5 antagonists. Curr Urol Rep 2003;4:457–65. doi: 10.1007/s11934-003-0027-x [DOI] [PubMed] [Google Scholar]
- 20.Mayer M, Stief CG, Truss MC, Uckert S. Phosphodiesterase inhibitors in female sexual dysfunction. World J Urol 2005;23:393–7. doi: 10.1007/s00345-005-0015-5 [DOI] [PubMed] [Google Scholar]
- 21.Oelke M, Hedlund P, Albrecht K, Ellinghaus P, Stief CG, Jonas U, et al. Expression of cAMP and cGMP-phosphodiesterase isoenzymes 3, 4, and 5 in the human clitoris: immunohistochemical and molecular biology study. Urology 2006;67:1111–6. doi: 10.1016/j.urology.2005.11.055 [DOI] [PubMed] [Google Scholar]
- 22.Park K, Moreland RB, Goldstein I, Atala A, Traish A. Sildenafil inhibits phosphodiesterase type 5 in human clitoral corpus cavernosum smooth muscle. Biochem Biophys Res Commun 1998;249:612–7. doi: 10.1006/bbrc.1998.9206 [DOI] [PubMed] [Google Scholar]
- 23.Traish AM, Kim NN, Munarriz R, Moreland R, Goldstein I. Biochemical and physiological mechanisms of female genital sexual arousal. Arch Sex Behav 2002;31:393–400. doi: 10.1023/a:1019831906508 [DOI] [PubMed] [Google Scholar]
- 24.Uckert S, Ehlers V, Nuser V, Oelke M, Kauffels W, Scheller F, et al. In vitro functional responses of isolated human vaginal tissue to selective phosphodiesterase inhibitors. World J Urol 2005;23:398–404. doi: 10.1007/s00345-005-0014-6 [DOI] [PubMed] [Google Scholar]
- 25.Uckert S, Ellinghaus P, Albrecht K, Jonas U, Oelke M. Expression of messenger ribonucleic acid encoding for phosphodiesterase isoenzymes in human female genital tissues. J Sex Med 2007;4:1604–9. doi: 10.1111/j.1743-6109.2007.00595.x [DOI] [PubMed] [Google Scholar]
- 26.Uckert S, Oelke M, Albrecht K, Breitmeier D, Kuczyk MA, Hedlund P. Expression and distribution of key enzymes of the cyclic GMP signaling in the human clitoris: relation to phosphodiesterase type 5 (PDE5). Int J Impot Res 2011;23:206–12. doi: 10.1038/ijir.2011.29 [DOI] [PubMed] [Google Scholar]
- 27.Uckert S, Oelke M, Albrecht K, Stief C, Jonas U, Hedlund P. Immunohistochemical description of cyclic nucleotide phosphodiesterase (PDE) isoenzymes in the human labia minora. J Sex Med 2007;4:602–8. doi: 10.1111/j.1743-6109.2007.00490.x [DOI] [PubMed] [Google Scholar]
- 28.Vemulapalli S, Kurowski S. Sildenafil relaxes rabbit clitoral corpus cavernosum. Life Sci 2000;67:23–9. doi: 10.1016/s0024-3205(00)00596-8 [DOI] [PubMed] [Google Scholar]
- 29.Bockaj A, Rosen NO, Muise A. Sexual motivation in couples coping with female sexual interest/arousal disorder: a comparison with control couples. J Sex Marital Ther 2019;45:796–808. doi: 10.1080/0092623X.2019.1623356 [DOI] [PubMed] [Google Scholar]
- 30.Lim-Watson MZ, Hays RD, Kingsberg S, Kallich JD, Murimi-Worstell IB. A systematic literature review of health-related quality of life measures for women with hypoactive sexual desire disorder and female sexual interest/arousal disorder. Sex Med Rev 2022;10:23–41. doi: 10.1016/j.sxmr.2021.07.003 [DOI] [PubMed] [Google Scholar]
- 31.Katz M, DeRogatis LR, Ackerman R, Hedges P, Lesko L, Garcia M, Jr, et al. Efficacy of flibanserin in women with hypoactive sexual desire disorder: results from the BEGONIA trial. J Sex Med 2013;10:1807–15. doi: 10.1111/jsm.12189 [DOI] [PubMed] [Google Scholar]
- 32.Simon JA, Thorp J, Millheiser L. Flibanserin for premenopausal hypoactive sexual desire disorder: pooled analysis of clinical trials. J Womens Health (Larchmt) 2019;28:769–77. doi: 10.1089/jwh.2018.7516 [DOI] [PubMed] [Google Scholar]
- 33.Simon JA, Kingsberg SA, Portman D, Williams LA, Krop J, Jordan R, et al. Long-term safety and efficacy of bremelanotide for hypoactive sexual desire disorder. Obstet Gynecol 2019;134:909–17. doi: 10.1097/AOG.0000000000003514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kingsberg SA, Clayton AH, Portman D, Williams LA, Krop J, Jordan R, et al. Bremelanotide for the treatment of hypoactive sexual desire disorder: two randomized phase 3 trials. Obstet Gynecol 2019;134:899–908. doi: 10.1097/AOG.0000000000003500 [DOI] [PMC free article] [PubMed] [Google Scholar]