Abstract
Introduction
Acute Leriche syndrome is a rare but potentially life-threatening condition. Pain, pallor, and coldness of the lower extremities serve as clues for suspecting Leriche syndrome. However, the absence of these findings may pose a diagnostic challenge.
Case Presentation
An 83-year-old man presented at our emergency department with a complaint of sudden-onset paraparesis. Initially, spinal cord infarction was suspected due to clinical course and neurological findings, but thoracolumbar MRI showed normal findings. On admission, symptoms associated with aortoiliac occlusion were not present, except for muscle atrophy in the thigh. CT angiography revealed aortoiliac occlusion, leading to a diagnosis of Leriche syndrome.
Conclusion
Leriche syndrome should be considered as a potential differential diagnosis in patients with acute paraparesis. Muscle atrophy of the lower limbs disproportionate to the clinical course may be the clue for suspecting acute Leriche syndrome with symptoms related to atherosclerotic occlusion which are inconspicuous.
Keywords: Leriche syndrome, Spinal cord infarction, Muscle atrophy, Contrast-enhanced computed tomography
Introduction
Leriche syndrome is an aortoiliac occlusive disease caused by chronic atherosclerotic occlusion. It is characterized by the clinical triad of intermittent claudication in the lower extremities, erectile dysfunction, and decreased or absent femoral pulses [1]. Typically, arterial stenosis progresses gradually, and Leriche syndrome manifests as chronic ischemic symptoms [2, 3]. However, its acute form has been reported and is known to be associated with high mortality [4–7]. Therefore, an accurate diagnosis and prompt initiation of an appropriate therapeutic intervention are crucial.
Here, we present a case of a patient with the acute form of Leriche syndrome. The patient did not experience any preceding symptoms related to peripheral ischemia in the lower extremities. The sudden onset of paraparesis mimicked spinal cord infarction.
Case Report
An 83-year-old man was taken to the emergency room by ambulance with complaints of sudden-onset paraparesis while walking outdoors. He denied having prior intermittent claudication. He had a history of hyperlipidemia, which was treated with statin drugs. He had no history of diabetes and was a non-smoker. His blood pressure was 194/77 mm Hg, and his heart rate was 60/min with a sinus rhythm. Neurological examinations revealed weakness of both lower extremities and paresthesia below the proximal part of the thigh on both sides; however, a definitive sensory level was not observed. The lower extremities showed diffuse weakness predominantly on the left (manual muscle testing of the proximal and distal muscles of the lower extremities was 4/5 and 4−/5 on the right and left sides, respectively, according to the Medical Research Council Scale). The left thigh was atrophic compared to the right (Fig. 1). Patellar and Achilles tendon reflexes were absent bilaterally. Babinski’s sign was negative bilaterally, and bladder bowel disturbance (BBD) was not observed. Pain, pallor, and coldness of the lower extremities were not observed at this time.
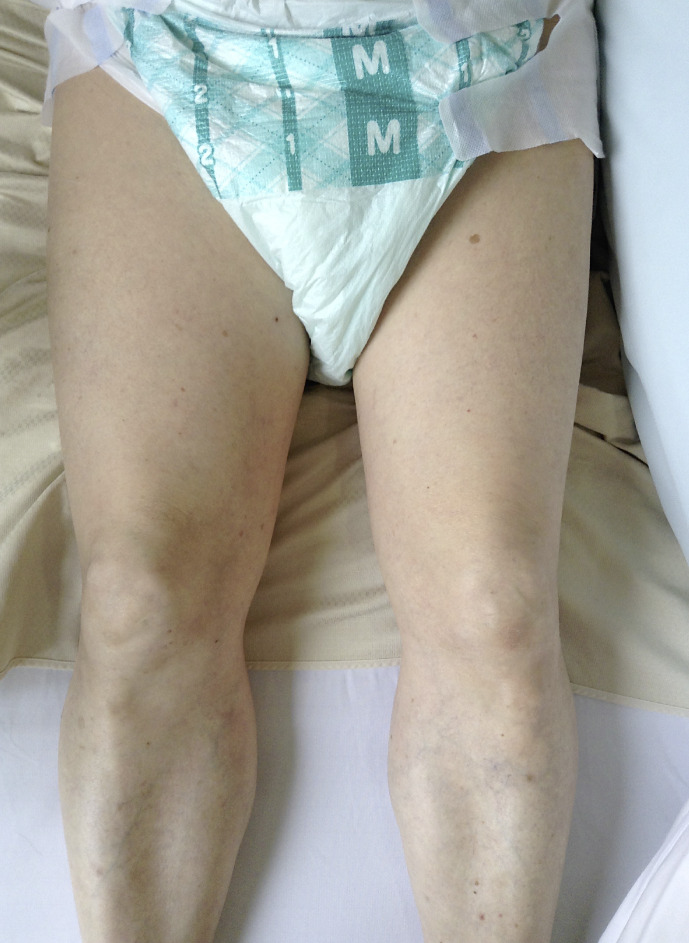
Fig. 1.
Photograph of lower extremities at admission. Atrophy of the left thigh is observed when compared to the appearance of the right thigh. The circumferences of the mid-thigh were 45.0 and 51.3 cm on the left and right sides, respectively.
Blood tests performed during admission revealed elevated D-dimer levels (2.9 μg/mL; normal reference range: 0–0.9 μg/mL), whereas the other laboratory findings were normal including the creatine kinase level. Electrocardiogram and chest X-ray showed no abnormalities. The clinical course of the acute-onset paraparesis raised the diagnosis of spinal cord infarction. However, thoracolumbar MRI did not detect abnormal findings. Contrast-enhanced computed tomography was performed to rule out aortic dissection and revealed no contrast filling between the abdominal aorta distal to the renal artery branching and the proximal portions of the bilateral external iliac arteries (Fig. 2). Contrast filling of the bilateral internal iliac arteries was preserved. The ankle-brachial indices were decreased (left, 0.32; right, 0.26; cutoff: 0.9) [8]. Additionally, the absence of pulses in the dorsal pedis and femoral arteries was observed bilaterally by detailed re-examination. Based on these findings, he was diagnosed with Leriche syndrome. We started treatment with intravenous heparin and oral cilostazol and limaprost alfadex. Blood tests performed on the day after admission revealed elevated creatine kinase levels (2,845 U/L; normal reference range: 59–248 U/L), which decreased to a normal range after adequate infusion of extracellular fluid.
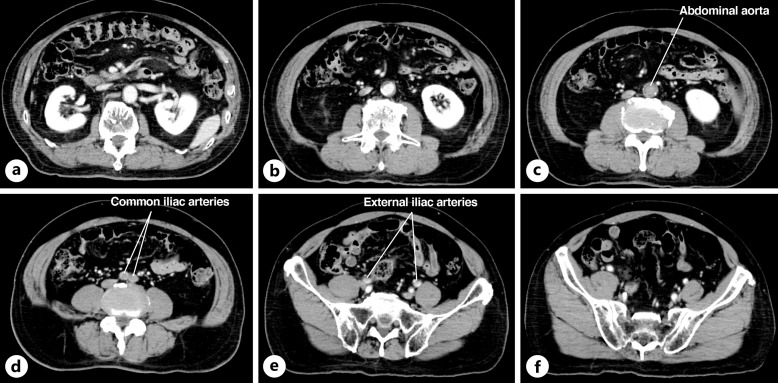
Fig. 2.
a–f Contrast-enhanced computed tomography (CT) findings on admission. Contrast-enhanced CT showed a lack of perfusion between the distal to the renal artery bifurcation to the proximal portions of the bilateral external iliac arteries.
Nerve conduction studies performed on day 10 after admission showed a markedly reduced amplitude of compound muscle action potentials in the tibial nerve and sensory action potentials in both sural nerves (Fig. 3a). Somatosensory evoked potentials with the left tibial nerve stimulation did not elicit any potentials of peripheral nerve origin, but elicited only the P38 potential, the first cortical component derived from the primary somatosensory cortex with prolonged latency (Fig. 3b). The tibial nerve somatosensory evoked potentials were performed according to the established standard procedures at our laboratory, and the results were judged based on the published normal values [9].
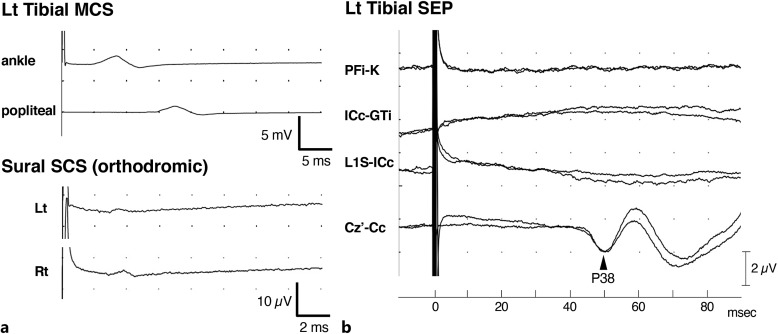
Fig. 3.
Findings of the nerve conduction studies and tibial nerve somatosensory evoked potentials (SEPs). a Motor and sensory nerve conduction studies showed a reduced amplitude of compound muscle action potentials in the tibial nerve and sensory action potentials in the bilateral sural nerves. b The left tibial nerve SEPs showed a P38 potential with prolonged latency (Z-score value was 4.91). However, the amplitudes of the N8, P15, and N21 potentials, which originated from the peripheral nerve, were almost lost. Each parameter was evaluated using our institutional reference values. Cc, contralateral C3 or C4; Cz′, 2 cm posterior to Cz; GTi, ipsilateral greater trochanter; ICc, contralateral iliac crest; K, adjacent knee bone; L1S, spinous process of the first lumbar vertebra; PFi, ipsilateral popliteal fossa.
Follow-up thoracolumbar MRI showed no abnormalities in the spinal cord. 3D-computed tomography angiography from the abdominal aorta to the femoral artery was performed on day 16 after admission and revealed severe stenosis of the abdominal aorta distal to the renal artery bifurcation (Fig. 4). Pain in both lower extremities gradually appeared during nocturnal rest. We considered this as a warning sign of ischemia [10], and on day 28 of admission, he underwent aorto-bilateral external iliac artery bypass. After the surgery, he was transferred to a rehabilitation hospital, and ankle-brachial index values 6 months after surgery had improved to 1.12 and 1.03 on the right and left sides, respectively. The CARE Checklist has been completed by the authors for this case report, attached as online supplementary material (for all online suppl. material, see https://doi.org/10.1159/000539456).
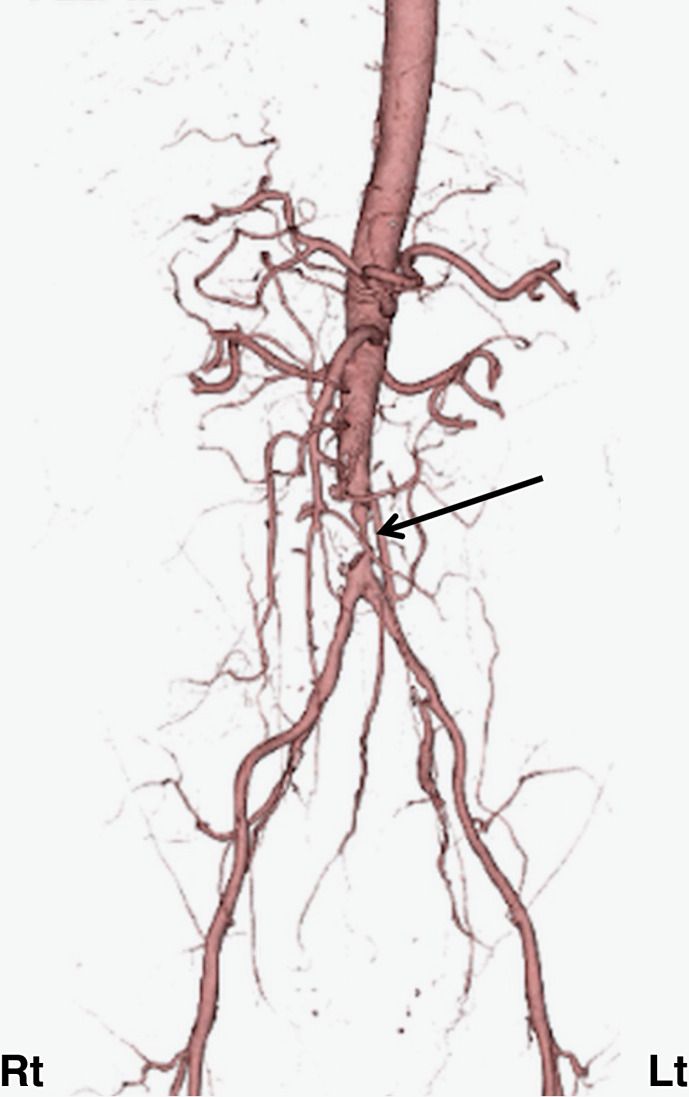
Fig. 4.
Findings of 3D-computed tomography (CT) angiography. Severe stenosis of the abdominal aorta distal to the renal artery bifurcation was observed (black arrow).
Discussion
Typically, patients with Leriche syndrome present symptoms associated with chronic atherosclerotic occlusion, including pain, pallor, coldness in the lower extremities, impotence in males, and intermittent claudication [1]. However, it was challenging to diagnose our patient who presented with sudden-onset paraparesis without these chronic ischemic symptoms. We observed thigh muscle atrophy, indicating chronic subclinical ischemic condition. We hypothesized that acute ischemia had been superimposed on the pathophysiology of conventional Leriche syndrome.
Although reports on acute Leriche syndrome are rare, it is often controversial whether the neurological symptoms are caused by spinal cord infarction due to ischemia through the Adamkiewicz artery (AKA) or by peripheral ischemia in the lower extremities [6, 7, 11]. Anatomically, the AKA is known to branch at a vertebral level between T8 and L1 in over 90% of cases [12]. Given that arterial occlusion in Leriche syndrome typically takes place distal to the bifurcation of the renal artery (at the vertebral L1–2 level) [13], it seems unlikely that Leriche syndrome causes spinal cord infarction through AKA occlusion. Furthermore, in the current case, electrodiagnostic tests also indicated the presence of ischemic peripheral neuropathy.
In cases of acute paraparesis, physicians might exhibit a bias toward contemplating neurogenic disorders, particularly spinal cord infarction, thereby overlooking the possibility of aortoiliac occlusive disease. However, early and accurate diagnosis of acute form Leriche syndrome is crucial because delays in initiating treatment can contribute to high mortality rates. The present case provides important lessons in this context. First, the normal MRI findings of the spinal cord served as a hint to consider a lesion outside the spinal cord. Second, the observed thigh muscle atrophy indicated chronic local ischemia/malnutrition. Third, BBD may help the differentiation as many patients with spinal cord infarction manifest BBD and need bladder catheterization [14]. Conversely, BBD has seldom been reported in cases of acute Leriche syndrome [4–7].
Leriche syndrome with acute-onset clinical presentation, as in the present case, is not a typical case. However, Leriche syndrome should be listed as one of the differential diagnoses for cases of acute-onset paraparesis, even when symptoms associated with chronic atherosclerotic occlusion are absent. For an accurate diagnosis of acute Leriche syndrome, it may also be helpful to examine muscle atrophy in the lower extremities disproportionate to the rapid clinical course.
Statement of Ethics
This study was conducted in line with the principles of the Declaration of Helsinki. Written informed consent was obtained from the patient for publication of the details of his medical case and any accompanying images. Ethical approval was not required for this study in accordance with local guidelines.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was supported by Japan Society for the Promotion of Science, Grants-in-Aid for Young Scientists (JP22K15738).
Author Contributions
Takamichi Kanbayashi contributed to the conception, design, data collection and interpretation, and drafting and revising the manuscript. Sonoko Tanaka and Kiyoshi Matsukura contributed to the data interpretation. Masahiro Sonoo contributed to the data interpretation and supervision of the study. Shunsuke Kobayashi contributed to the data interpretation and critically revised and updated the manuscript. All of the authors read and approved the final version.
Funding Statement
This study was supported by Japan Society for the Promotion of Science, Grants-in-Aid for Young Scientists (JP22K15738).
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material files. Further inquiries can be directed to the corresponding author.
Supplementary Material.
References
- 1. Leriche R, Morel A. The syndrome of thrombotic obliteration of the aortic bifurcation. Ann Surg. 1948;127(2):193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mavioglu I, Veli Dogan O, Ozeren M, Dolgun A, Yucel E. Surgical management of chronic total occlusion of abdominal aorta. J Cardiovasc Surg. 2003;44(1):87–93. [PubMed] [Google Scholar]
- 3. Bhatia MS, Gautam P, Saha R. Leriche syndrome presenting as depression with erectile dysfunction. J Clin Diagn Res. 2016;10(3):VD01–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gouëffic Y, Piffeteau T, Patra P. Acute leriche syndrome due to paradoxical embolism. Eur J Vasc Endovasc Surg. 2007;33:220–2. [DOI] [PubMed] [Google Scholar]
- 5. Mosa E, Manouvelou S, Tolia M, Tsoukalas N, Ardavanis A, Stasinopoulou M, et al. Acute leriche syndrome in pancreatic adenocarcinoma: a case report. Curr Med Imaging. 2020;16(5):622–4. [DOI] [PubMed] [Google Scholar]
- 6. Zankl AR, Blessing E, Volz HC, Krumsdorf U, Katus HA, Andrassy M. Neurological symptoms in acute Leriche’s syndrome. Clin Res Cardiol. 2010;99(7):459–62. [DOI] [PubMed] [Google Scholar]
- 7. Akhaddar A, Eljebbouri B, Saouab R, Boucetta M. Acute paraplegia revealing Leriche syndrome. Intern Med. 2012;51(8):981–2. [DOI] [PubMed] [Google Scholar]
- 8. Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;124(18):2020–45, [DOI] [PubMed] [Google Scholar]
- 9. Miura T, Sonoo M, Shimizu T. Establishment of standard values for the latency, interval and amplitude parameters of tibial nerve somatosensory evoked potentials (SEPs). Clin Neurophysiol. 2003;114(7):1367–78. [DOI] [PubMed] [Google Scholar]
- 10. Second European consensus document on chronic critical leg ischemia. Circulation. 1991;84: IV1–26. [PubMed] [Google Scholar]
- 11. Mahendrakar SM, Sandhu HS, Khan AH, Loya YS. Leriche syndrome: acute onset painful paraplegia of vascular origin with catastrophic consequences. J Clin Diagn Res. 2017;11(5):OD22–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koshino T, Murakami G, Morishita K, Mawatari T, Abe T. Does the Adamkiewicz artery originate from the larger segmental arteries? J Thorac Cardiovasc Surg. 1999;117(5):898–905. [DOI] [PubMed] [Google Scholar]
- 13. Aubert J, Koumare K. Variations of origin of the renal artery: a review covering 403 aortographies. Eur Urol. 1975;1(4):182–8. [PubMed] [Google Scholar]
- 14. Robertson CE, Brown RD Jr, Wijdicks EF, Rabinstein AA. Recovery after spinal cord infarcts: long-term outcome in 115 patients. Neurology. 2012;78(2):114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material files. Further inquiries can be directed to the corresponding author.