Abstract
Introduction
We report a case of pseudoxanthoma elasticum (PXE) with an atypical phenotype likely related to a hypomorphic variant in ABCC6.
Case Presentation
A 66-year-old Caucasian female with a history of a maculopathy interpreted as either age-related macular degeneration or a pattern dystrophy underwent a detailed ophthalmic evaluation. Visual acuities were 20/25, OD, and 20/20, OS. Spectral domain optical coherence and fluorescein angiography demonstrated outer retinal disruptions and breaks in retinal pigment epithelium (RPE)/Bruch’s membrane bilaterally, consistent with angioid streaks. A large area of hypo- and hyperautofluorescence extending from the central retina into the peripapillary retina was documented with short-wavelength excitation autofluorescence. The area of hypoautofluorescence, which was much larger on near-infrared excitation, spared the temporal retina. Two-color dark-adapted perimetries documented severe rod sensitivity losses and less severe cone sensitivity abnormalities co-localizing with the RPE abnormalities. No obvious skin findings were observed, and initial dermatologic biopsy was negative. Gene screening identified a pathogenic ABCC6 gene variant c.1552C>T and a previously reported variant of uncertain significance c.1171A>G. A second dermatologic biopsy demonstrated positive findings consistent with PXE.
Conclusion
Although this patient had minimal skin findings, this patient had characteristic structural and functional abnormalities of a pattern dystrophy with angioid streaks and histologic evidence of PXE, suggesting compound heterozygous variants involving the hypomorphic ABCC6 c.1171A>G variant. These findings support the pathogenic role of both variants.
Keywords: ABCC6, Angioid streaks, Pattern dystrophy, Pseudoxanthoma elasticum, Case report
Introduction
Pseudoxanthoma elasticum (PXE; OMIM #264800) is a genetically heterogeneous condition with systemic manifestations including significant cardiovascular risks [1, 2]. The prevalence ranges from 1 to 4 per 100,000 with a slight female predilection [3]. PXE exhibits a spectrum of disease severity ranging from multisystemic manifestations to isolated organ involvement. In classic descriptions of PXE, multi-organ pathology begins in childhood or adolescence and involves widespread ectopic calcification. Most notably, aberrant mineralization is found in peripheral vascular beds, inelastic yellow flexural papules described as “plucked chicken skin,” and breaks in Bruch’s membrane named angioid streaks (AS). In this report, we present a detailed phenotypic description of an older patient with a hypomorphic variant in ABCC6 that may be more easily diagnosed with genetic testing.
Case Report
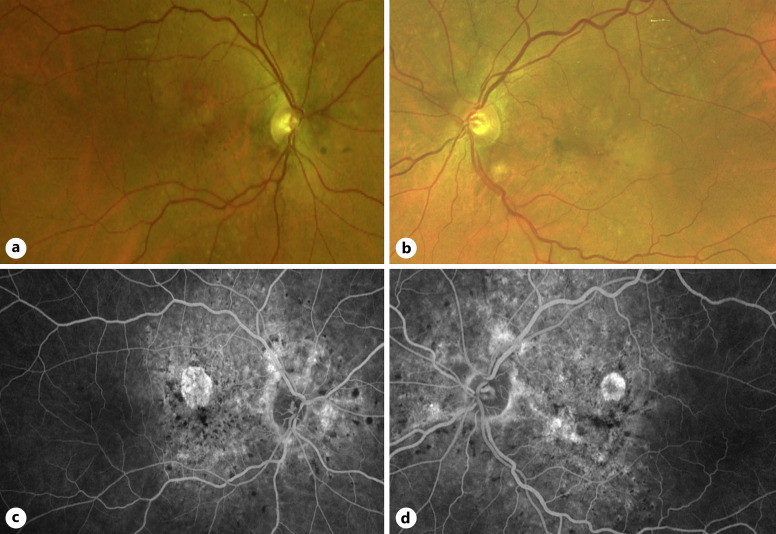
A 66-year-old Caucasian female with a history of hyperlipidemia, coronary calcification, and essential hypertension was referred for evaluation of age-related macular degeneration (AMD). She had a family history of premature coronary artery disease and no history of inherited retinal degeneration, thalassemia, sickle cell, or bone/connective tissue disease. Macular pigmentary changes had been noted incidentally during a routine eye exam around age 62. On initial evaluation with us at age 66, best corrected visual acuity was 20/25 in each eye. Intraocular pressures and anterior segment examinations were normal. Fundus examination demonstrated bilateral irregular macular pigmentary changes, localized areas of retinal pigment epithelium (RPE) atrophy, and drusen-like lesions extending from the peripapillary retina, along the vascular arcades, and into the temporal pericentral retina (Fig. 1a, b). Fluorescein angiography revealed hyperfluorescent radial lines emanating from the disc, a large non-homogenous hyperfluorescent area caused by window defects that co-localized with RPE changes, and scattered hypofluorescent foci (Fig. 1c, d).
Fig. 1.
Fundus photos and fluorescein angiography. Color fundus photos of the right (a) and left (b) eyes demonstrating macular pigmentary changes. Fluorescein angiography of the right (c) and left (d) eyes illuminating AS, posterior hypofluorescent spots, and macular atrophy.
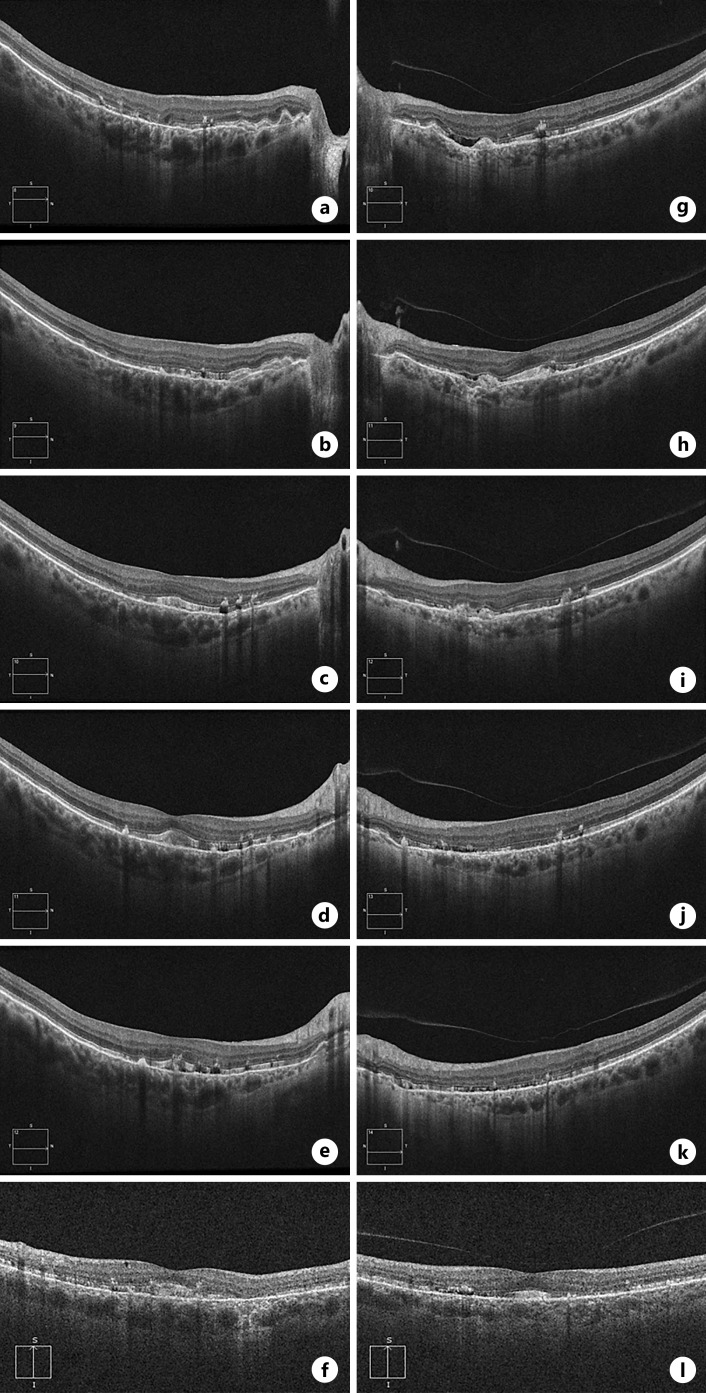
Spectral domain optical coherence tomography (SD-OCT) and fundus autofluorescence (FAF) imaging using short-wavelength (SW) and near-infrared excitation lights was performed using a Spectralis HRA system (Heidelberg Engineering GmbH, Heidelberg, Germany). SD-OCT demonstrated hyperreflective subretinal deposits, most obvious near the foveal center, and irregularities of the RPE nasally (Fig. 2a, b). There were segments with abrupt thinning of the photoreceptor outer nuclear layer and interruptions of the inner segment ellipsoid and outer segment interdigitation bands, often crossed by vertical hyperreflective tracks (Fig. 2a, b). In peripapillary retina, the hyperreflective deposits were mostly sub-RPE, often associated with interruptions and posterior displacement of the RPE/BrM, consistent with AS. There also was suggestion of mild foveal hypoplasia, but caution is needed before making this conclusion.
Fig. 2.
Optical coherence tomography. Several horizontal scans are shown for the right (a–e) and left (g–k) eyes as well as a vertical foveal scan for the right (f) and left (l) eye. Diffuse hyperreflective subretinal deposits with outer retinal irregularity is seen. Subretinal fluid was present without evidence of neovascularization (g) [4].
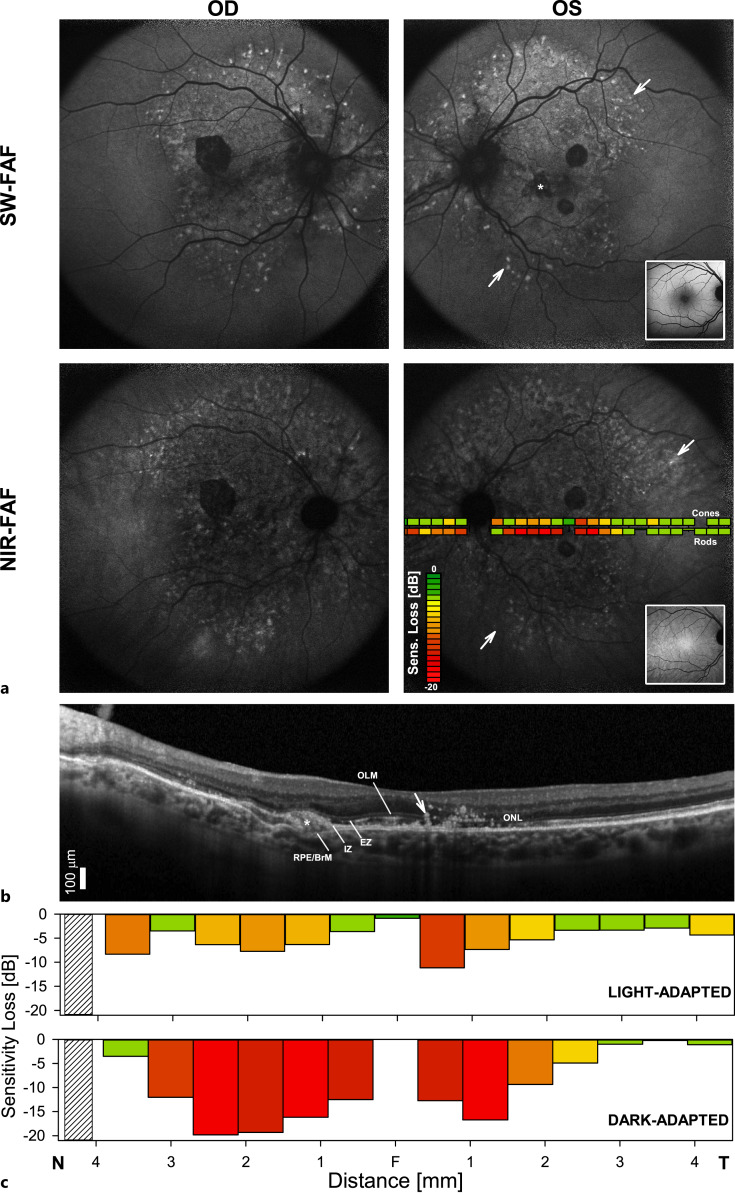
SW-FAF demonstrated a mostly diffuse pattern of hyperautofluorescence interrupted by well-circumscribed hypoautofluorescent lesions in areas of RPE atrophy and a peripheral leading edge of punctate hyperautofluorescent lesions (Fig. 3a). Near-infrared-FAF showed a large, mostly hypoautofluorescent area extending from the optic nerve into the temporal and nasal near midperiphery, beyond the boundaries of the SW-FAF abnormalities but similarly delimited in the periphery by a leading edge of punctate hyperautofluorescent lesions. Measures of photoreceptor specific sensitivities by two-color dark-adapted perimetry disclosed major rod sensitivity losses along this area of hypoautofluorescence, confirming earlier observations in patients with PXE (Fig. 3a) [5–7]. Cone sensitivity losses were much less severe. The sensitivity losses co-localized with outer retinal abnormalities seen in the SD-OCT cross-section (Fig. 3b, c). The greatest abnormalities corresponded to segments of outer nuclear layer thinning in the parafoveal and pericentral nasal retina (Fig. 3b, c). Note the abrupt normalization of the sensitivities for both photoreceptor mechanisms in the temporal well-laminated retina, as well as the relative preservation of light-adapted sensitivities near the foveal center (Fig. 3c).
Fig. 3.
Visual function and retinal structure in the patient. a Fundus autofluorescence (FAF) imaging with short-wavelength (SW-FAF) and near-infrared (NIR-FAF) excitation lights in each eye of the patient. Insets on the left eye panels are representative normal images for each FAF modality. Overlaid on the NIR-FAF image of the left eye is perimetric sensitivity losses measured in the light-adapted (cone) and dark-adapted (rod) states plotted following a pseudocolor scheme. A diffuse pattern of hyperautofluorescence interrupted by well-circumscribed hypoautofluorescent lesions in areas of RPE atrophy is seen (asterisk). There is a peripheral leading edge of punctate hyperautofluorescent lesions (arrows). b 9 mm SD-OCT cross-section through the patient’s left fovea. The temporal parafovea retina shows multiple round hyperreflective foci tracking from the RPE toward the inner retina, likely representative of RPE migration. Outer retinal sublaminae are labeled according to conventional nomenclature: outer nuclear layer (ONL); outer limiting membrane (OLM); inner segment ellipsoid region (EZ or ISe); contact cylinder between the apical RPE microvilli and the photoreceptor outer segments tips, or interdigitation zone (IZ); basal RPE and Bruch’s membrane (RPE/BrM). A subretinal deposit replaces a focal EZ and IZ signal laying superficial to the RPR/BrM (arrow). c Light-adapted achromatic and dark-adapted chromatic (500 nm) sensitivity losses (calculated from lower limit of normal = mean − 2 standard deviations) along the horizontal meridian in the patient. Photoreceptor mediation in the dark-adapted state was confirmed rod-mediated for all locations with the use of spectral sensitivity (500 nm–650 nm) differences. Hatched bars denote the location of the blind spot. N, nasal. T, temporal.
On physical exam, the patient did not demonstrate any neck or flexural skin changes. Dermatology was consulted, and examination did not reveal any abnormal lesions except for a solitary right thigh papule. Biopsy of this lesion showed no malignant features or background calcification. As the structural and functional retinal phenotype was consistent with PXE, genetic testing (Blueprint Genetics, CLIA-certified, next-generation sequencing assay and Sanger sequencing, Seattle, WA, USA) was subsequently performed, and two compound heterozygous variants in adenosine triphosphate-binding cassette subfamily C member 6 (ABCC6) were identified: a known deletion in exon 12 [c.1552C>T, p.(Arg518*), pathogenic, previously associated with PXE in compound heterozygous state, autosomal recessive], and a previously reported variant [c.1171A>G, p.(Arg391Gly), uncertain significance, PXE, autosomal recessive] [1, 8, 9]. This R391G [c.1171A>G, p.(Arg391Gly)] variant, which is enriched in populations with PXE, involves an intracellular loop at the cytoplasmic surface of the protein, thought to interact with other residues [8, 10]. In silico analysis also supported a deleterious effect.
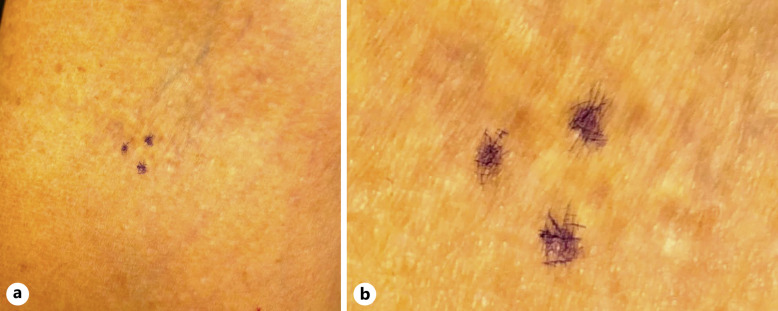
The retinal phenotype and the molecular findings further supported the possibility of PXE prompting a referral back to dermatology for a flexural skin biopsy, upon which a subtle macule (Fig. 4) was positive for fragmented elastic fibers, consistent with a final diagnosis of PXE. The patient was under the care of a cardiologist around this time, and echography demonstrated moderate coronary calcification in the left anterior descending and right coronary arteries. Exercise single photon emission computed tomography stress testing revealed abnormal myocardial perfusion suggestive of a prior small myocardial infarction in the left anterior descending distribution.
Fig. 4.
External photos of flexural skin biopsy site. a Subtle areas of speckled dermal hyper- and hypopigmentation outlined by surgical marking (purple) at the biopsy location. b Magnification of focal skin lesions outlined by surgical marking (purple) without classic pseudoxanthomatous lesions.
The CARE Checklist has been completed by the authors for this case report, attached as online supplementary material (for all online suppl. material, see https://doi.org/10.1159/000538045).
Discussion
The diagnostic criteria of PXE have evolved within the past few decades from solely clinical criteria to include positive ABCC6 variants [2, 11, 12]. Most recently, some authors suggest that the presence of biallelic genetic variants is sufficient as a standalone criterion for diagnosis of this condition [2]. Diagnostic challenges persist in cases with variants of uncertain significance including our patient’s missense variant c.1171A>G (p.Arg391Gly). This variant was first reported in 2004 and holds conflicting interpretations of pathogenicity. While there is evidence of benign homozygosity in the Genome Aggregation Database (https://gnomad.broadinstitute.org; variant ID: 16-16295863-T-C, accessed August 7, 2022) [13, 14], this variant has also been described as pathogenic in several patients, supported by targeted next-generation sequencing and comparative molecular modeling [8, 14]. Our patient’s findings strengthen the argument for pathogenicity, at least when this variant is combined with another pathogenic allele (compound heterozygosity) [8, 14], as our patient demonstrated positive ocular findings, skin biopsy, and genetic testing consistent with PXE. Although it is theoretically possible that the pathogenic variant (c.1552C>T, p.Arg518*) may be sufficient to cause the phenotype as single heterozygosity, this variant has only been reported in the compound heterozygous state (National Center of Biotechnology Information, ClinVar; https://www.ncbi.nlm.nih.gov/clinvar/variation/VCV000030339.17, accessed February 23, 2023) [9, 15]. The evidence thus far, however, supports the need of two variants in compound heterozygosity [1, 15]. In our case, this was the combination of a variant (p.Arg518*) that causes loss of function of the gene product and a variably penetrant hypomorphic allele (p.Arg391Gly) [8, 16, 17].
The phenotype of this form of PXE is generally an atypically late-onset presentation ranging from the fifth to ninth decade of life [8, 14]. Commonly, reticular pseudodrusen and macular pigmentary changes are seen which can be easily confused with drusen in AMD [8, 18]. A pattern dystrophy phenotype, including that associated with PXE, most commonly presents as fundus pulverulentus which can mimic pigment migration in AMD [19]. While neovascularization can develop at AS, PXE can also have subretinal fluid at the macula, mimicking exudative macular neovascularization (MNV) associated with other conditions. Interestingly, one study reported subretinal fluid in 14 out of 42 eyes in 21 patients with PXE [4]. Of these fourteen eyes, seven eyes had MNV, six were treated with anti-vascular endothelial growth factor injections, and no eyes responded to treatment [4]. This nonresponsive subretinal fluid is believed to be secondary to retinal pigment epithelial dysfunction and loss in PXE, with MNV non-requisite [4]. Nonetheless, anti-vascular endothelial growth factor has been shown to be effective in treating neovascularization related to AS [20]. Lastly, additional confounding challenges in the vast spectrum of PXE findings include peau d’orange, coquille d’ouef atrophy, comet lesions, comet rain, optic nerve drusen, retinal crystalline bodies, outer retinal tubulations, and vitelliform lesions [4, 8, 19, 21, 22].
PXE associated with a hypomorphic variant also demonstrates subtle AS such that fulfilling clinical criteria may not be straightforward [8]. Our patient had AS that were not clearly evident on exam alone (Fig. 1a, b). Fluorescein and/or indocyanine green angiography can be essential in uncovering AS as seen in this case (Fig. 1c, d) and others [2, 8]. FAF may also be useful in measuring the degree of atrophy beyond what is visible on conventional fundus imaging [4, 23, 24]. Our patient demonstrated atrophic areas which appears to be a consistent finding in this variant. Additionally, FAF demonstrated areas of symmetric hyperautofluorescent macular deposits (Fig. 3a) [8, 4].
Another challenge in diagnosing PXE associated with a hypomorphic variant is that patients can appear to demonstrate mild to no dermatologic findings [8, 14]. Our patient lacked classic yellow papules and plaques in flexural and neck skin, and initially had a negative biopsy from a thigh papule. There is a small number of other reports of this variant, and patients had age-related skin changes or no characteristic skin lesions such that biopsy was not performed [8, 14]. These cases benefit from genetic testing as initial negative skin biopsies may deter clinicians from identifying PXE. This is not limited to the R391G [c.1171A>G, p.(Arg391Gly)] variant as PXE cases with other variants (homozygous R1141X; heterozygous c.2542delG, deletions of exon 2 and exon 4; homozygous c.2542delG) have been similarly reported with positive ocular findings, negative neck/flexural biopsies, yet positive genetic variants [25, 26]. Blind skin and scar biopsies have been shown to be useful in establishing a histopathologic diagnosis, but may prove unreliable with this form of PXE [11, 27]. Importantly, this is the first reported case known to the authors of initial negative skin biopsy testing with this hypomorphic variant.
Finally, it is noteworthy that any patient diagnosed with PXE should undergo cardiac evaluation due to associated cardiovascular risks [2]. Common involvement includes calcification of the internal elastic laminae of arteries, endocardium, and atrioventricular valves, leading to claudication, restrictive cardiomyopathy, and myocardial infarction [2]. The phenotype of this hypomorphic variant includes hypertension most commonly [8].
Conclusions
For this patient referred for an AMD evaluation, we present a detailed phenotypic description of a Caucasian female with a known pathogenic ABCC6 gene variant c.1552C>T and a hypomorphic variant c.1171A>G. First, our case suggests that this hypomorphic variant in ABCC6 is pathogenic as well, although a key limitation of this report is that additional affected or unaffected family members were not available for segregation analyses to confirm pathogenicity and phase of the variants. Second, this case is a reminder that clinicians should consider alternative diagnoses when AMD referrals present at a younger age or with atypical drusen-like outer retinal changes. Finally, this carefully phenotyped case highlights the value of genetic testing when there is clinical suspicion of PXE based on ophthalmologic exam despite initial negative dermatologic evaluation, as skin findings may be mild or have negative biopsy results.
Statement of Ethics
Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images. This retrospective review of patient data did not require ethical approval in accordance with local/national guidelines. Ethical approval is not required for this study in accordance with local or national guidelines.
Conflict of Interest Statement
The authors have no financial disclosures.
Funding Sources
No funding or grant support was received.
Author Contributions
All authors attest that they meet the current ICMJE criteria for authorship. J.C.T.: drafting of the manuscript and critical review of the manuscript. T.S.A. and B.J.K.: drafting of the manuscript, acquisition of data, and critical review of the manuscript. P.J.T.: critical review of the manuscript.
Funding Statement
No funding or grant support was received.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material files. Further inquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Pfendner EG, Vanakker OM, Terry SF, Vourthis S, McAndrew PE, McClain MR, et al. Mutation detection in the ABCC6 gene and genotype-phenotype analysis in a large international case series affected by pseudoxanthoma elasticum. J Med Genet. 2007;44(10):621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Uitto J, Jiang Q, Váradi A, Bercovitch LG, Terry SF. Pseudoxanthoma elasticum: diagnostic features, classification, and treatment options. Expert Opin Orphan Drugs. 2014;2(6):567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Germain DP. Pseudoxanthoma elasticum. Orphanet J Rare Dis. 2017;12(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zweifel SA, Imamura Y, Freund KB, Spaide RF. Multimodal fundus imaging of pseudoxanthoma elasticum. Retina. 2011;31(3):482–91. [DOI] [PubMed] [Google Scholar]
- 5. Hess K, Gliem M, Birtel J, Müller P, Hendig D, Andrews C, et al. Impaired dark adaptation associated with a diseased bruch membrane in pseudoxanthoma elasticum. Retina. 2020;40(10):1988–95. [DOI] [PubMed] [Google Scholar]
- 6. Hess K, Gliem M, Charbel Issa P, Birtel J, Müller PL, von der Emde L, et al. Mesopic and scotopic light sensitivity and its microstructural correlates in pseudoxanthoma elasticum. JAMA Ophthalmol. 2020;138(12):1272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aleman TS, Han G, Serrano LW, Fuerst NM, Charlson ES, Pearson DJ, et al. Natural history of the central structural abnormalities in choroideremia: a prospective cross-sectional study. Ophthalmology. 2017;124(3):359–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Charbel Issa P, Tysoe C, Caswell R. Late-onset pseudoxanthoma elasticum associated with a hypomorphic ABCC6 variant. Am J Ophthalmol. 2020;218:255–60. [DOI] [PubMed] [Google Scholar]
- 9. Meloni I, Rubegni P, De Aloe G, Bruttini M, Pianigiani E, Cusano R, et al. Pseudoxanthoma elasticum: point mutations in the ABCC6 gene and a large deletion including also ABCC1 and MYH11. Hum Mutat. 2001;18(1):85. [DOI] [PubMed] [Google Scholar]
- 10. Szeri F, Niaziorimi F, Donnelly S, Orndorff J, van de Wetering K. Generation of fully functional fluorescent fusion proteins to gain insights into ABCC6 biology. FEBS Lett. 2021;595(6):799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lebwohl M, Phelps RG, Yannuzzi L, Chang S, Schwartz I, Fuchs W. Diagnosis of pseudoxanthoma elasticum by scar biopsy in patients without characteristic skin lesions. N Engl J Med. 1987;317(6):347–50. [DOI] [PubMed] [Google Scholar]
- 12. Plomp AS, Toonstra J, Bergen AAB, van Dijk MR, de Jong PTVM. Proposal for updating the pseudoxanthoma elasticum classification system and a review of the clinical findings. Am J Med Genet. 2010;152A(4):1049–58. [DOI] [PubMed] [Google Scholar]
- 13. Chassaing N, Martin L, Mazereeuw J, Barrié L, Nizard S, Bonafé JL, et al. Novel ABCC6 mutations in pseudoxanthoma elasticum. J Invest Dermatol. 2004;122(3):608–13. [DOI] [PubMed] [Google Scholar]
- 14. Ringpfeil F, McGuigan K, Fuchsel L, Kozic H, Larralde M, Lebwohl M, et al. Pseudoxanthoma elasticum is a recessive disease characterized by compound heterozygosity. J Invest Dermatol. 2006;126(4):782–6. [DOI] [PubMed] [Google Scholar]
- 15. Nitschke Y, Baujat G, Botschen U, Wittkampf T, du Moulin M, Stella J, et al. Generalized arterial calcification of infancy and pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6. Am J Hum Genet. 2012;90(1):25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Legrand A, Cornez L, Samkari W, Mazzella JM, Venisse A, Boccio V, et al. Mutation spectrum in the ABCC6 gene and genotype-phenotype correlations in a French cohort with pseudoxanthoma elasticum. Genet Med. 2017;19(8):909–17. [DOI] [PubMed] [Google Scholar]
- 17. Szeri F, Miko A, Navasiolava N, Kaposi A, Verschuere S, Molnar B, et al. The pathogenic c.1171A>G (p.Arg391Gly) and c.2359G>A (p.Val787Ile) ABCC6 variants display incomplete penetrance causing pseudoxanthoma elasticum in a subset of individuals. Hum Mutat. 2022;43(12):1872–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lebwohl M, Halperin J, Phelps RG. Brief report: occult pseudoxanthoma elasticum in patients with premature cardiovascular disease. N Engl J Med. 1993;329(17):1237–9. [DOI] [PubMed] [Google Scholar]
- 19. Agarwal A, Patel P, Adkins T, Gass JDM. Spectrum of pattern dystrophy in pseudoxanthoma elasticum. Arch Ophthalmol. 2005;123(7):923–8. [DOI] [PubMed] [Google Scholar]
- 20. Parodi MB, Cicinelli MV, Marchese A, Giuffrè C, Viola F, Staurenghi G, et al. Intravitreal aflibercept for management of choroidal neovascularization secondary to angioid streaks: the Italian EYLEA-STRIE study. Eur J Ophthalmol. 2021;31(3):1146–53. [DOI] [PubMed] [Google Scholar]
- 21. Murro V, Mucciolo DP, Giorgio D, Sodi A, Boraldi F, Quaglino D, et al. Pattern dystrophy-like changes and coquille d’oeuf atrophy in elderly patients affected by pseudoxanthoma elasticum. Graefes Arch Clin Exp Ophthalmol. 2020;258(9):1881–92. [DOI] [PubMed] [Google Scholar]
- 22. Murro V, Mucciolo DP, Sodi A, Boraldi F, Quaglino D, Virgili G, et al. Peripapillary comet lesions and comet rain in PXE-related retinopathy. Graefes Arch Clin Exp Ophthalmol. 2018;256(9):1605–14. [DOI] [PubMed] [Google Scholar]
- 23. Finger RP, Charbel Issa P, Ladewig M, Götting C, Holz FG, Scholl HPN. Fundus autofluorescence in pseudoxanthoma elasticum. Retina. 2009;29(10):1496–505. [DOI] [PubMed] [Google Scholar]
- 24. Sawa M, Ober MD, Freund KB, Spaide RF. Fundus autofluorescence in patients with pseudoxanthoma elasticum. Ophthalmology. 2006;113(5):814–20.e2. [DOI] [PubMed] [Google Scholar]
- 25. Aerts C, De Keyser C, Leys A. Visual loss and presumed pseudoxanthoma elasticum confirmed with genetic analysis but not with skin examination and biopsies. GMS Ophthalmol Cases. 2011;1:Doc03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fukumoto T, Iwanaga A, Fukunaga A, Wataya-Kaneda M, Koike Y, Nishigori C, et al. First-genetic analysis of atypical phenotype of pseudoxanthoma elasticum with ocular manifestations in the absence of characteristic skin lesions. J Eur Acad Dermatol Venereol. 2018;32(4):e147–9. [DOI] [PubMed] [Google Scholar]
- 27. Stembridge N, Rytina E, Holden S, Burrows NP. Pseudoxanthoma elasticum presenting without typical skin changes. Clin Exp Dermatol. 2020;45(4):518–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material files. Further inquiries can be directed to the corresponding author.