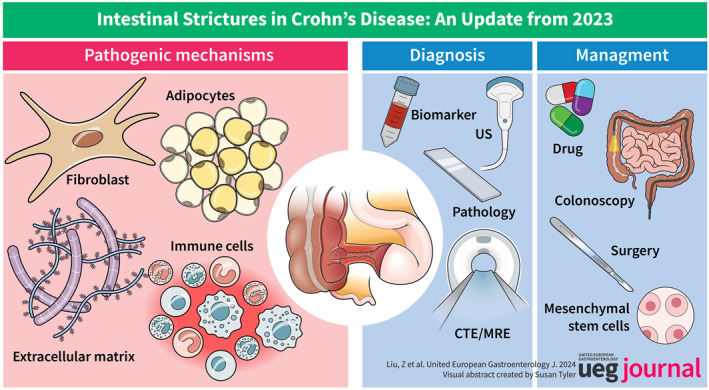
Abstract
Crohn's disease (CD) is a chronic inflammatory disease that leads to intestinal stricture in nearly 35% of cases within 10 years of initial diagnosis. The unknown pathogenesis, lack of universally accepted criteria, and absence of an effective management approach remain unconquered challenges in structuring CD. The pathogenesis of stricturing CD involves intricate interactions between factors such as immune cell dysbiosis, fibroblast activation, and microecology imbalance. New techniques such as single‐cell sequencing provide a fresh perspective. Non‐invasive diagnostic tools such as serum biomarkers and novel cross‐sectional imaging techniques offer a precise understanding of intestinal fibrostenosis. Here, we provide a timely and comprehensive review of the worthy advancements in intestinal strictures in 2023, aiming to dispense cutting‐edge information regarding fibrosis and to build a cornerstone for researchers and clinicians to make greater progress in the field of intestinal strictures.
Keywords: biomarkers, Crohn disease, diagnosis, dysbiosis, fibroblast, imaging, immune system, intestinal fibrosis, pathogenesis, stenosing
INTRODUCTION
Crohn's disease (CD) is a chronic inflammatory disease with high prevalence. 1 Complications requiring surgery may occur in up to 70% of patients within 10 years of diagnosis, with half of them attributed to the development of strictures. 2 The stricture may progress to form a vicious circle, as associated complications such as abscess or fistula formation can be treated with surgery, which may lead to re‐stricturing. 3 , 4 Intestinal stricture is an unsolved health‐threatening problem. Although the therapeutic management of CD has improved with the advent of novel therapeutic agents, an effective approach to deal with CD‐related stricture has not yet been discovered. Intestinal stricture is often manifested as intestinal fibrosis at the microscopic level. The mechanisms behind fibrosis pathogenesis are still not fully understood. There is currently a dearth of efficacious drugs for intestinal fibrosis. 5 , 6 Here, we summarize a curated set of articles from the year 2023 that display some refreshing breakthroughs concerning the underlying mechanisms and diagnostic methods, alongside reports on improved and new‐advent management strategies for intestinal stricture, with the hope of setting a stage for researchers and clinicians to have a more inclusive look into intestinal stricture.
SEARCH STRATEGY
A comprehensive literature review was conducted using the PubMed database, Web of Science, and Embase for relevant literature published in 2023. The search terms were as follows (all fields): (“stenotic” OR “stenosis” OR “fibrosis” OR “fibrotic” OR “fibrostenotic” OR “fibrostenosis” OR “stricture” OR “strictures” OR “stricturing” OR “strictured” OR “constriction” OR “constrictions”) AND (“Crohn's disease” OR “inflammatory bowel disease” OR “CD” OR “IBD”) AND (Filters: from 1 January 2023 to 31 December 2023). References to the identified articles were also examined for additional studies meeting these criteria.
MECHANISM
Genetic markers associated with intestinal fibrosis
Genetic and epigenetic factors are involved in the induction and progression of intestinal stricture in inflammatory bowel disease (IBD). 7 Nucleotide‐binding oligomerization domain 2 (NOD2) is one of the most studied genes. Patients with NOD2 mutations show increased expression of genes in activated fibroblasts and macrophages, especially those related to glycoprotein 130 (gp130). 8 A more recent study hypothesized that NOD2 might be a solitary genetic factor contributing to the development of disease and is specifically associated with the distinct ileal‐stricturing phenotype, therefore providing opportunities for personalized diagnosis, disease prediction, and targeted treatment. 9 In addition, a study showed that the genomic data of NOD2 alone can accurately predict the occurrence of stricturing disease even without taking any clinical features into consideration at the time of diagnosis (Figure 1b). 10 Through bioinformatics approaches, the genes COL1A1, CXCL10, MMP2, and FGF2 were identified as being associated with CD. Among them, further investigation revealed an upregulation of MMP2 and COL1A1 in animal models of CD fibrosis, suggesting the potential implication of these genes in intestinal fibrosis. 11
FIGURE 1.
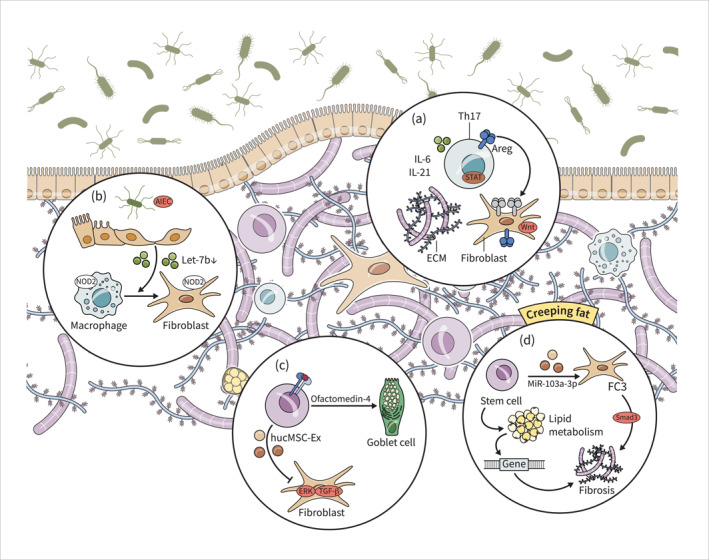
Fibroblasts play a key role in excessive ECM deposition and stricture formation in IBD. Th17 cells and Stat3 signalling pathway drive fibrosis in IBD via activation of fibroblasts (a). AIEC suppresses let‐7b secretion from intestinal epithelial cells, leading to the transformation of intestinal macrophages into fibroblasts and intestinal fibrosis. NOD2 mutations contribute to the activation of fibroblasts and macrophages, potentially leading to development of strictures (b). HucMSCs and ISCs exhibit therapeutic effects in IBD‐related fibrosis by inhibiting proliferation, migration, and activation of fibroblasts and promoting tissue regeneration (c). Dysregulated lipid metabolism genes contribute to fibrogenesis in adipose tissue, and the FC3 subset of fibroblasts is associated with intestinal fibrosis. Additionally, stem cells promote fibrogenesis through modulation of lipid metabolism and fibroblast function (d). AIEC, adherent‐invasive Escherichia coli; HucMSCs, Human umbilical cord mesenchymal stem cells; IBD, inflammatory bowel disease; ISCs, intestinal stem cells.
Cellular players in fibrosis
Intestinal stricture is a macroscopic pathological condition, and its microscopic manifestations typically include intestinal fibrosis. Fibroblasts and myofibroblasts are the two primary cells responsible for intestinal fibrosis. In a previous study, four subsets of fibroblasts in intestine fibrosis were classified based on unbiased single‐cell profiling, each expressing different transcriptional regulators and functional pathways. Fibroblasts and myofibroblasts are relevant to fibrosis as they produce various extracellular matrix (ECM) proteins. 12 A study collected samples of normal ileal and colon tissues and their counterparts with CD stricture or inflammation. Using single‐cell sequencing and timing analysis, CHMP1A, TBX3, and RNF168 have been identified as regulatory factors associated with collagen expression in myofibroblasts, showing a strong correlation with the development of intestinal fibrosis. 13 Cell‐cell interaction modelling unveiled the pivotal signaling role of CXCL14+ fibroblasts and MMP/WNT5A + fibroblasts in CD strictures. The expression of CDH11 (a cell‐cell adhesion molecule specific to fibroblasts) was widely observed and upregulated, indicating its significant involvement in stricture formation. 14 Wnt‐β‐catenin signaling in myofibroblasts may be connected to stricture formation. There is an elevation in the number of cells expressing β‐catenin in the fibrotic strictured intestine. Activation of the Wnt‐β‐catenin signaling pathway results in upregulation of Collagen‐I expression in CCD‐18Co myofibroblasts. Besides directly activating β‐catenin, Wnt ligands oligomerization of Frizzled (Fzd) also plays a role. Upregulation of the Wnt receptor Fzd8 by TGF‐β directly contributes to the increase in Collagen‐I expression independent of the β‐catenin pathway. 15 Fzd5 exhibits specific expression and is necessary for epithelial proliferation in the intestine (Figure 1a). 16 The cross‐way between Wnt and Fzd has great potential to be further explored.
Research investigating the mechanisms and preclinical studies in various organs have unveiled the indispensable involvement of immune cells in both the initiation and regression of fibrosis. 17 A previous study has demonstrated that IBD patients commonly exhibit an increase in PDE4B‐ and TNF‐expressing macrophages, as well as a decrease in CD39‐expressing intraepithelial T cells in the colonic mucosae. But the role of macrophages and T cells in the pathogenesis of fibrosis still needs digging. 18 Macrophages, in particular, are classified into two main subsets: M1 and M2 macrophages. M1 macrophages typically promote tissue inflammation, whereas M2 macrophages release cytokines that facilitate tissue repair and mitigate inflammation. Nevertheless, excessive accumulation of M2 macrophages can lead to an overactive tissue repair response, culminating in fibrosis and stricture. 19 Th1 and Th17 cells (particularly gut microbiota antigen‐specific T cells) are linked to the development of CD. Th17 cells cause more severe fibrosis despite inducing similar levels of intestinal inflammation to Th1 cells. 20 Many genes were differently expressed between Th1 and Th17 cells, of which Amphiregulin (Areg, a member of the epidermal growth factor (EGF) family that is overexpressed in Th17 cells) is of great significance as it promotes the proliferation and motility of human intestinal myofibroblast through activation of mammalian target of rapamycin (mTOR), thus triggering fibrosis. Mechanically, interleukin‐6 (IL‐6) and interleukin‐21 (IL‐21) promote Areg expression through activation of Stat3 in Th17 cells. Thus, AREG may be a potential therapeutic target for CD fibrosis. 20
Intestinal stem cells maintain homeostasis and contribute to disease pathogenesis. Mesenchymal stem cells (MSCs) can regulate both local and systemic innate and adaptive immune responses by releasing a range of mediators, such as immunosuppressive molecules, growth factors, exosomes, chemokines, complement components, and metabolites, when facing the exposure of an inflammatory environment. 21 They have strong self‐renewal and multi‐lineage differentiation potential and have significant therapeutic effects. Human intestinal organoids were developed in a planar system with open lumens; Increasing matrix hardness levels were seen in patients with IBD, which decreased stem cell homeostasis, resulting in fewer epithelial cells. 2 Stiffness also reduced the number of LGR5+, ISCs, and KI‐67+ proliferating cells. 22 Exosomes derived from human umbilical cord mesenchymal stem cells (hucMSC‐Ex) mitigate IBD‐related fibrosis by suppressing the extracellular regulated protein kinase (ERK) pathway and TGF‐β‐induced proliferation, migration, and activation of human intestinal fibroblasts (Figure 1c). 23 Additionally, Olfactomedin‐4‐expressing cells promote ISC differentiation into goblet cells. Blocking molecular pathways mitigates fibrosis. 22
Molecular pathways in fibrosis
Fibrosis is characterized by excessive extracellular matrix (ECM) deposition. Matrix metalloproteinases (MMPs) participate in this process. Abnormalities in TGF‐beta signaling and MMP production were detected in the mucosal tissue that overlies strictures in Crohn's disease. 24 In the fibrotic terminal CD ileum, MMP‐1, ‐14, and TIMP‐1 expression are significantly increased, suggesting their potential involvement in fibrosis development. Similarly, MMP‐1 and ‐3 are upregulated in the muscularis and submucosal tissue of the fibrotic intestine. In CD patients with stricturing disease, the balance between collagen types I and III leans towards degradation, whereas type V collagen leans towards formation. 25 Besides, Elevated expression of MMP‐13 is observed in CD patients with strictures, regulated by IL36R signaling. Targeting MMPs via the IL36R pathway may represent a potential therapeutic strategy for combating fibrosis in CD. 26
Several other pathways also directly contribute to fibrosis and ECM accumulation. For instance, the absence of P2X7 in mice resulted in an augmented accumulation of collagen and elevated expression of various profibrotic markers, such as Col4 and Col5a1 in fibroblasts, which increased the risk of stricture. 27 Dysregulation of the NOD‐like receptor thermal protein domain associated protein 3 (NLRP3) signaling pathway contributes to excessive ECM production by intestinal fibroblasts. Inhibition of NLRP3 decreases the proliferation of intestinal fibroblasts and collagen production, suggesting that targeting NLRP3 can be a potential therapeutic strategy to prevent the development of intestinal fibrosis in IBD. 28 Sirtuin 4 (SIRT4) and Sirtuin 5 (SIRT5) are two other mitochondrial cytokines that interact in fibrosis. SIRI4 hinders SIRT5's stabilizing interaction with glutaminase 1, therefore impeding ECM deposition by inhibiting glutaminolysis. The decrease in SIRT4 is negatively associated with disease severity. 29
Furthermore, according to classical cognition, chronic inflammation and fibrosis in the intestine are closely linked. Cytokines, including interleukins, contribute to the formation of fibrosis. Nox4, miRNAs, the AGE/RAGE pathway, senescence pathway, and the Nrf2/ARE signaling pathway are also implicated in the mechanisms underlying fibrosis (Table 1). 25 , 26 , 30 , 31 , 32 , 33 , 34 , 35
TABLE 1.
Mechanisms and molecular pathways associated with intestinal fibrosis in 2023.
| Molecular | In vitro/in vivo models | Mechanism | Reference |
|---|---|---|---|
| ECM proteins | Colon biopsies, Patients' serum | MMP‐1, ‐3, ‐14, and TIMP‐1 expression increased in fibrosis CD; Collagen types I and III leans towards degradation; Collagen type V collagen leans towards formation. | Biel C, et al 25 |
| Interleukin‐36 | Colon biopsies, Mice‐derived fibroblasts, MMP13 deficient mice | IL36R signaling pathway regulates the expression of MMP13. | Koop K, et al 26 |
| NOX4 | Colitis NOX4−/− mice | NOX4 inhibits TGF‐β‐mediated inflammation injury and fibrosis. | Lee Y, et al 30 |
| Smad7 | Smad7−/− mice | Smad7 inhibits the increase of collagen content and protein expression of α‐smooth muscle actin infibrosis. | Schuler C, et al 31 |
| COX‐2 PEG2 | TNBS‐induced mice | The expression of COX‐2 and PGE2 were increased in the inflammatory site and pre inflammatory dilated site of themice model of intestinal stenosis. | Johnson JC, et al 32 |
| MicroRNAs | Both in vivo and in vitro studies | MiR‐155 inhibited high mobility group box transcription factor 1 (HBP1), resulting in the activation of fibrosis‐related pathway. | Aggeletopoulou I, et al 33 |
| AGE/RAGE | Both in vivo and in vitro studies | AGE/RAGE increased in patients and DSS‐treated mice compared to controls. | Pompili S, et al 34 |
| Nrf2/ARE | Both in vivo and in vitro studies | Nrf2 agonists prevented transformation of intestinal fibroblasts into myofibroblasts by inhibiting the TGF‐/Smad axis. | Li B, et al 35 |
Abbreviations: AGE/RAGE, advanced glycation end products/receptor of AGEs; ARE, antioxidant response element; CD, Crohn’s disease; COX‐2, cyclooxygenase‐2; DSS, dextran sulfate sodium; ECM, extracellular matrix; EMT, epithelial‐mesenchymal transition; MMP, matrix metalloproteinases; NOX, nicotinamide adenine dinucleotide phosphate oxidases; Nrf2, Nuclear factor erythroid 2‐related factor; PEG2, prostaglandin E2; TGF‐β, transforming growth factor‐β.
Bacterial modulation of intestinal fibrosis
Emerging evidence suggests that the gut microbiome plays a significant role in fibrosis. While fibrosis can occur in various tissues and organs, the involvement of the gut microbiota in the fibrotic process appears to be exclusive to the intestine. Bacterial translocation into the lamina propria activates immune and non‐immune cells through pattern recognition receptors (PRRs) such as Toll‐like receptors (TLRs) and nucleotide‐binding and oligomerization domain (NOD)‐like receptors (NLRs), resulting in the production of pro‐fibrotic factors. 12 Previous research has indicated that adherent‐invasive Escherichia coli (AIEC) and its flagellin can bind to TLR5, leading to the activation of IL‐33/ST2 signaling, thus promoting intestinal fibrosis. 36 A recent study also uncovered the mechanisms by which AIEC interacts with intestinal cells, contributing to the development of fibrosis in CD. AIEC suppress the secretion of extracellular vesicle let‐7b from intestinal epithelial cells, either directly promoting the transformation of intestinal macrophages into fibroblasts or indirectly causing abnormal expression of TGF‐βR1; both outcomes ultimately result in the development of intestinal fibrosis (Figure 1a). 37
Microbiota may affect the disease course through the adipose tissue. In a previous study, it was found that bacterial translocation from the gut to mesenteric adipose tissue (MAT) occurs to some extent in healthy tissue. However, in conditions of chronic intestinal inflammation like in CD, the gut microbiota is constantly disrupted. 38 Crohn's disease mesenteric adipose tissue (CD‐MAT) exhibits a significant enrichment of Enterobacteriaceae members compared to non‐CD controls, among which viable Klebsiella variicola are exclusively isolated in CD‐MAT. This specific strain of K. variicola triggers a pro‐inflammatory response in vitro and worsens colitis in animal models by disrupting the intestinal barrier via inhibition of the zonula occludens (ZO‐1) expression. 39 Derived from the gut microbiota, indole‐3‐propionic acid (IPA) interacts with pregnane X receptor (PXR) to regulate intestinal inflammation and fibrosis. Deletion of PXR leads to exacerbated fibrosis, increased neutrophil infiltration, and enhanced innate cytokine production, while administration of IPA reduces inflammation and fibrosis. 40 A significant reduction in the diversity of mucosal‐associated microbiota was observed in stricturing CD compared to the non‐stricture areas. Specifically, the abundance of Lactobacillus, Oscillospira, Subdoligranulum, Hydrogenophaga, Clostridium, and Allobaculum decreased in stenotic segments. Among them, the decrease of Oscillospira was the most significant, and this particular bacterial genus is negatively associated with fibrosis and surgery recurrence. 41
Creeping fat in intestinal fibrosis
Crohn's disease can be characterized by the presence of creeping fat (CrF), which refers to mesenteric fat that wraps around the impaired bowel wall. 42 Previous studies have suggested that adipose tissue, particularly CrF, secretes adipokines and cytokines that contribute to intestinal damage and fibrogenesis. The excessive secretion of fatty acids serves as a potent stimulus for human intestinal myofibroblasts (HIMCs) proliferation, contributing to muscular layer thickening and fibrotic constriction. 43 Transcriptomic analysis shows that genes in CrF exhibiting an increasing trend are associated with immune cell responses, specifically B‐cell and T‐cell activation. Conversely, genes showing a decreasing trend in CrF are involved in cell trafficking and migration, suggesting a heightened state for inflammatory responses. 44 Single‐cell sequencing technology analysis discovered a novel subpopulation of fibroblasts known as FC3 that predominantly accumulate in CrF. FC3 can induce inflammatory responses and modulate Smad phosphorylation, which is associated with intestinal fibrosis regulation. 45
Dysfunction of lipid metabolism is also an important factor to be taken into consideration. During the progression of intestinal fibrosis, a close correlation was observed between 15 hub genes associated with lipid metabolism and significant alterations in 14 lipid metabolites. 46 Single‐cell RNA‐seq (scRNA‐seq) analysis identified a subset of mesenchymal stem cells that promote adipogenic differentiation, leading to the formation of CrF. 47 Besides, adipose‐derived stem cells (ASCs) separate from fibrosis and excrete exosomal miR‐103a‐3p, thereby activating fibroblasts via the transforming growth factor beta receptor 3 (TGFBR3) targeting pathway and facilitating Smad2/3 phosphorylation (Figure 1d). Targeting this pathway could be a promising approach to mitigate intestinal fibrosis. 48
DIAGNOSIS
Precise assessment of intestinal fibrosis in individuals with CD continues to pose a challenge.
Pathological diagnosis
Pathological diagnosis remains the gold standard. Histopathological evaluation of strictures involves assessing various microscopic features. Fibrosis of the submucosa and increased thickness of the bowel wall layers are considered appropriate for diagnosing small bowel strictures. However, scoring systems for inflammation and fibrosis have a large degree of heterogeneity and theres is still no uncertain appropriateness. It is worth noting that increased muscularis propria thickness is a characteristic of CD strictures, with muscular hypertrophy and fibrosis being considered as appropriate causes of these changes. 49 (Table 2).
TABLE 2.
Imaging technology contributions to intestinal fibrosis assessment in 2023.
| Techniques/markers | Superiority | Utility | Reference | |
|---|---|---|---|---|
| Magnetic resonance imaging (MRI) | MTR and TA | MTR and TA were linked to histopathological fibrosis. | Evaluate the efficacy of antifibrotic therapy. | De Kock I, et al 50 |
| 68Ga‐FAPI PET/MR enterography | FAPI uptake is positive correlated with histopathological confirmed fibrosis in the bowel wall. | Differentiate fibrotic from mixed strictures. | Scharitzer M, et al 51 | |
| Radiomics | Radiomic features were associated with fibrosis but not inflammation in CD strictures | Evaluate fibrosis and guide anti‐fibrotic therapies. | Sleiman J, et al 52 | |
| Computed tomography (CT) | [68Ga] Ga‐FAPI‐04 PET/CT | Measuring FAP uptake allows for quantitative and precise localization of fibrosis. | High sensitivity, better performance than CTE. | Chen L, et al 53 |
| CT‐based VAT features | The developed VAT‐radiomics model blended 1130 radiomics features VAT and offers notable advantages for identifying high‐risk patients. | Predict disease progression (strictures, penetrations, or surgery) better than a SAT‐radiomics model. | Li X, et al 54 | |
| ES, RS and upstream dilatation in RS | Strictures with upstream dilation and those that meet both RS and ES are associated with a higher risk of CAO. | Radiology is crucial in identifying strictures. Upstream dilation significantly impacts RS outcomes. | Shi L, et al 55 | |
| Intestinal ultrasound (IUS) | Ultrasound elastography | Ultrasound elastography demonstrated moderate to good overall accuracy in diagnosing intestinal fibrosis. | Emphasize the importance of ultrasound elastography. | Xu C, et al 56 |
| Point shear wave elastography showed higher accuracy in this regard. | ||||
| Ileocecal valve | Children with CD may display imaging features of the ileocecal valve, such as loss of mural stratification and severe fibrofatty proliferation. | Associated with both active inflammation and chronic fibrosis, and future surgical resection. | Manzotti C, et al 57 | |
| IUS | Younger patients with ileocolonic disease had higher stenosis‐detection rates by IUS. | A detection rate of 70.0% sensitivity, 98.2% specificity, and 88.4% accuracy. | Takeuchi K, et al 58 | |
| Endoscopy | The number, severity, length of strictures, and the presence of prestenotic dilatation and surrounding fistulas or abscesses | Endoscopy allows direct visualization of the narrowing bowel and can be used as a therapeutic tool. | Critical when evaluating stricture. | Shen B, et al 59 |
| Pennazio M, et al 60 | ||||
| The patency capsule procedure | Those CD patients with failed patency capsule procedure tended to suffer worse outcomes such as intestinal surgery and endoscopic dilation than those without. | Rule out small bowel stenosis | Ukashi O, et al 61 | |
| Consensus on escalation and de‐escalation treatment decisions | It is recommended to complete colonoscopy, even if imaging tests, such as MRE, are negative, especially when patients have obstructive symptoms. | Determine the necessity for intervention. | Nakase H, et al 62 |
Abbreviations: CAO, clinical adverse outcomes; CTE, computed tomography enterography; ES, endoscopic stricture; FAP, fibroblast activation protein; FAPI, fibroblast activation protein inhibitor; MCFI, mesenteric creeping fat index; MRE, magnetic resonance enterography; MTR, magnetization transfer MRI; RM, radiomics model; RS, radiological stricture; SAT, subcutaneous adipose tissue; TA, texture analysis; VAT, visceral adipose tissue.
Intestinal ultrasound
Ultrasound is a valuable tool for assessing fibrosis. The relationship between ultrasound parameters and histopathological findings in patients with CD was investigated. Previous studies demonstrated that ultrasonography (US)‐based real‐time elastography (RTE) has good efficacy in assessing fibrosis. 63 In 2023, the presence of hyperechogenic spiculates was found to be associated with fibrosis, while marked vascular signals were indicative of inflammation. 64 Additionally, ultrasound measurements of wall thickness showed good reliability compared to histological measurements. Another study focused on ultrasound shear wave elastography, which demonstrated correlations between ultrasound stiffness measurements and mucosal inflammation and smooth muscle hypertrophy, providing further insights into the pathophysiology of stricturing ileal Crohn's disease. 65 Comparing findings from double balloon enteroscopy (DBE) and IUS in 86 CD patients, IUS demonstrated a detection rate of 70.0% sensitivity, 98.2% specificity, and 88.4% accuracy for diagnosing small intestinal stenosis when meeting two or more parameters. Younger patients with ileocolonic disease had higher stenosis‐detection rates by IUS. 58 A systematic review compared ultrasound (US) and histopathology for evaluating intestinal fibrosis. Ultrasound elastography showed moderate to good accuracy in diagnosing fibrosis, with point shear wave elastography being more accurate. Children diagnosed with ileal CD requiring surgical resection may display more severe imaging features of the ileocecal valve, such as loss of mural stratification and severe fibrofatty proliferation. These features are associated with both active inflammation and chronic fibrosis, and may not be observed in children who are receiving medical treatment. 56
Cross‐sectional imaging techniques
Currently, the most commonly used diagnostic methods are magnetic resonance imaging (MRI) and computed tomography (CT) since they are non‐invasive and quickly evaluate active transmural inflammation and detect intestinal strictures (Table 2). 50 , 51 , 53 , 54 , 55 , 57 , 59 , 60 , 62 Comparing CT and MRE, a previous study suggested that the latter one is free of ionising radiation and therefore has become the most widely applied method for stricture differentiation. 66 Novel MRI techniques, such as magnetization transfer MRI (MTR) and texture analysis (TA), show promise in assessing intestinal fibrosis. Both MTR and texture entropy are correlated with histopathological fibrosis, with entropy demonstrating better results in tracking fibrosis in the presence of inflammation. 50 68Ga‐FAPI PET/MR enterography utilizing fibroblast activation protein (FAP) inhibition shows potential diagnostic value in detecting fibrosis. 57 Additionally, the application of AI technology has shown significant advancements in the diagnosis of intestinal fibrosis. 65 A study evaluating the efficacy of [68Ga] Ga‐FAPI‐04 PET/CT showed high sensitivity in detecting endoscopic lesions and correlated well with clinical and biomarker assessments, indicating the value of AI technology in facilitating a more comprehensive and precise evaluation of fibrosis characterization. 58 Through exploring radiomics in MR enterography (MRE), another study found that radiomic features were significantly associated with severe fibrosis but not inflammation in Crohn's disease strictures. Radiomic analysis could be a useful tool for evaluating fibrosis and guiding anti‐fibrotic therapies. 52
Endoscopy
Endoscopy plays an important role in the management of intestinal disease and allows direct visualization of the narrowing bowel. It is recommended to complete colonoscopy, even if imaging tests, such as MRE, are negative, especially when patients have obstructive symptoms, and to perform endoscopic balloon dilatation if necessary. 62 However, before performing video capsule endoscopy, it is recommended to use the patency capsule procedure for ruling out small bowel stenosis. 61 Those CD patients with failed patency capsule procedure tended to suffer worse outcomes such as intestinal surgery and endoscopic dilation than those without. When performing endoscopy, it is essential to clearly characterize the number, severity and length of strictures, as well as the presence of prestenotic dilatation and surrounding fistulas or abscesses. Combining clinical presentation and endoscopic examination allows for the assessment of the risk of future interventions in patients with strictures (Table 2). 59 , 60
Biomarkers
Serological markers are highly valued for diagnosing and predicting fibrosis owing to their non‐invasive nature, accessibility, cost‐effectiveness, and efficiency. A previous system review showed that no biomarkers could pass the validation to diagnose intestinal fibrosis. 67 A predictive model was developed to forecast disease progression over 5 years following diagnosis utilizing data from a 67 paediatric cohort in France. The best predictive model with an area under the curve (AUC) of 0.80 consisted of 8 biomarkers, that is, the location at diagnosis, 6 single nucleotide polymorphisms (SNPs), and pANCA. 68 In another cohort, 1129 proteomic markers and antimicrobial antibodies were analysed, identifying 22 biomarkers related to complicated CD, among which six were related to fibrosis. 69 Microbiota and serum lipidomic biomarkers also show promise in diagnosing fibrosis. 41 , 70 , 71 However, their specificity and sensitivity require validation, and larger cohorts are needed for more comprehensive data (Table 3). 72
TABLE 3.
Serological markers for early detection of intestinal stenosis.
| Markers | Potency | Correlation to fibrosis | Reference |
|---|---|---|---|
| pANCA | Prediction | Positive | Sarter H, et al 68 |
| MMP‐1, PAPP‐A, IGFBP‐2 | Diagnosis | Positive | Choung RS, et al 69 |
| KIT, FAP, NTRK2 | Negative | ||
| Bacterial flagellin antigens | Diagnosis | Positive | Bourgonje AR, et al 70 |
| Oscillospira | Diagnosis | Negative | Zhan S, et al 41 |
| PEO‐, SM, CE, LCDCA, ST | Diagnosis | Positive/Negative | Ferru‐Clément R, et al 71 |
Abbreviations: CE, cholesterol ester; FAP, fibroblast activation protein; IGFBP‐2, insulin‐like growth factor binding protein 2; LCDCA, long‐chain dicarboxylic acid; MMP‐1, matrix metalloproteinase‐1; NTRK2, neurotrophic tyrosine kinase receptor; pANCA, perinuclear anti‐neutrophil cytoplasmic antibody; PAPP‐A, pregnancy‐associated plasma protein A; PEO, phosphatidylethanolamine ether; SM, sphingomyelin; ST, sitosterol sulfate.
MANAGEMENT
Endoscopy and surgery are primarily used to alleviate stricture rather than directly targeting fibrosis for treatment. As a result, there is a growing need for the development of new drugs and innovative techniques that specifically address fibrosis. In 2023, multiple studies were conducted to assess the effectiveness of existing management strategies and investigate potential new anti‐fibrotic treatments (Table 4). 23 , 73 , 74 , 75 , 76 , 77 , 78 , 80 , 81 , 82 , 83
TABLE 4.
Cutting‐edge breakthroughs in the management of strictures in 2023.
| Treatment | Application scenario/pharmacodynamics mechanism | Reference | |
|---|---|---|---|
| Endoscopic Management | ES | Non‐fistulizing patients with anastomotic strictures; stenosis accompanied by ulcers | Jia Y, et al 73 |
| EBD | Patients with IPAA | Darlington K, et al 74 | |
| BAE‐based ES | Deep small bowel strictures | Ning SB, et al 75 | |
| Surgery | Surgery | Strictures persist despite endoscopic interventions or when there is significant obstruction | Senior K. 76 |
| Laparoscopic ileocecal resection | Limited involvement (affected segment ≤40 cm) and predominantly inflammatory terminal ileitis. | Popivanov G, et al 77 | |
| Ileocolonic resection | Anastomotic configuration, temporary diversion at the time of ileocolonic resection do not increase risk of anastomotic stricturing | Bachour SP, et al 78 | |
| Surgery | Surgery yields a lower re‐interventions rate compared with endotherapy | Pal P, et al 79 | |
| Drug | D‐ceo2 | Suppressing the expression of fibrosis‐related genes, specifically α‐SMA and Collagen 1/3; Reversing the activation of fibroblasts induced by TGF‐β1 | Cao Y, et al 80 |
| Fraxinellone | Inhibiting TGF‐β‐induced fibrosis responses by disrupting the TGF‐β/Smad2/3 signaling pathway and interfering with the interaction between HSP47 and collagen. | Wang J, et al 81 | |
| sEVs | Anti‐inflammatory oxylipins in sEVs prevent inflammation and fibrosis. | Gómez‐Ferrer M, et al 82 | |
| MSCs | Allogenic bone marrow‐derived MSCs | When injected with MSCs, some patients showed resolution of the stricture at week 12, and some achieved complete resolution at week 48. | Didamoony MA, et al 83 |
| hucMSC‐Ex | HucMSC‐Ex reduced inflammation‐induced fibrosis in mouse models, and inhibited fibroblast proliferation and migration through inhibiting ERK phosphorylation. | Wang Y, et al 23 | |
Abbreviations: BAE‐based ES, Balloon‐assisted enteroscopy‐based endoscopic stricturotomy; CD, Crohn’s disease; D‐CeO2, dextran‐coated cerium oxide; EBD, endoscopic balloon dilation; ES, endoscopic strictureplasty; FCSEMS, self‐expandable metal stent; HSP, heat shock protein; hucMSC‐Ex, human umbilical cord mesenchymal stem cell‐derived exosomes; IPAA, ileoanal and rectal strictures following ileal pouch‐anal anastomosis; MSCs, Mesenchymal stem cells; sEVs, human milk‐derived small extracellular vesicles; TGF‐β, transform growth factor‐β; WT1, Wilms tumor 1; α‐SMA, α‐smooth muscle actin.
Endoscopic management
Endoscopic intervention is the primary approach for treating intestinal strictures due to its convenience and minimal invasiveness compared to surgery. Common techniques include endoscopic strictureplasty (ES) and endoscopic balloon dilation (EBD). ES is effective for non‐fistulizing Crohn's disease patients with anastomotic strictures, while strictureplasty may be safer in cases with accompanying ulcers. 73 Both mechanical dilation and EBD have shown similar safety profiles and effectiveness for ileoanal and rectal strictures following ileal pouch‐anal anastomosis (IPAA), with no significant difference in complications. 79 Balloon‐assisted enteroscopy‐based endoscopic stricturotomy (BAE‐based ES) has also been found to be effective and safe for deep small bowel strictures. 75 With ongoing advancements in endoscopic techniques, there is great potential for further improvements in safety, effectiveness, and patient outcomes. As research continues to explore innovative methods, the future of endoscopic interventions holds promise for enhanced precision and expanded applications, ultimately benefiting patients with intestinal strictures.
Surgery
Surgery plays a crucial role in managing IBD. Approximately 70% of individuals with Crohn's disease will undergo surgical intervention at some point according to previous epidemiology study. 84 In situations where strictures persist despite endoscopic interventions or when there is significant obstruction, surgical management provides better outcomes. 76 Resection surgery yields a lower re‐intervention rate for CD strictures compared with endotherapy. In most cases, re‐operation can also be avoided with comparable symptom free survival at 1 year. If, resection is needed, minimizing the interval between diagnosis and surgery is important to reduce postoperative complications. 79 The LIR!C trial provided evidence that early surgery, defined as resection for luminal involvement without prior surgeries, is not only a viable alternative to biologics but also reduces costs significantly. 77
Drug therapy
A previous STRIDENT study has proved that intensifying treat‐to‐target drug therapy led to fewer treatment failures, decreased inflammation associated with strictures, and greater enhancement in stricture morphology. 85 It is noted that this study focused solely on evaluating drugs for stricture treatment, but not fibrosis The anti‐fibrotic effects of some emerging drugs are in focus. CeO2 has the ability to suppress the expression of fibrosis‐related genes as well as serve as a contrast agent in CT. 74 Another drug, fraxinellone, has shown significant promise in preventing fibrosis by disrupting the TGF‐β/Smad2/3 signaling pathway. 75 What's more, docosahexaenoic acid, an omega‐3 fatty acid, which derived from human milk was found to be anti‐ fibrotic. 82
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) hold promise as a therapeutic approach for alleviating intestinal fibrosis associated with IBD. 23 Extracellular vesicles derived from MSCs may be the reason for the therapeutic effects. 83 Furthermore, human umbilical cord MSC‐derived exosomes reduce inflammation‐induced fibrosis and inhibit the proliferation and migration of fibroblasts. 23
FUTURE OUTLOOK
Tremendous progress has been made in understanding, diagnosing, and treating CD‐related intestinal strictures in 2023. Advanced technologies such as single‐cell sequencing and intestinal organoids have deepened our understanding of the roles of various players in intestinal fibrosis, including subpopulations of fibroblasts, signaling molecules like Areg, and pathways like Wnt. However, these studies are insufficient to fully depict the complexity of fibrosis. Creeping fat and gut microbiota play indispensable roles in fibrosis. Improvements in MRI and CT methods, together with various serum markers hold promise for more accurate, time‐ and cost‐effective assessments. However, current research faces some limitations. First, there is a lack of high‐fidelity animal models and simulated fibrotic microenvironments to fully elucidate the entirety of intestinal fibrosis. Second, the precise mechanisms underlying the formation of intestinal fibrosis remain incompletely elucidated. It may arise as a consequence of chronic inflammation or it could be independent of inflammation and associated with other pathways or interactions. Thirdly, it is still difficult to accurately evaluate intestinal fibrosis from clinical presentations and routine laboratory tests alone. The widely used Montreal classification system fails to accurately predict disease progression and even shows instances of disease regression. 86 Lastly, even today, there is no generally accepted therapeutic approach for intestinal fibrosis. In the future, advancements in more sophisticated technologies such as SNP analysis and spatial multi‐omics will contribute to deeper understanding of the mechanisms underlying fibrosis. 87 With the help of AI, novel advancements in the field of intestinal fibrosis diagnosis are on the horizon. These advancements will contribute to the certification of new approaches for diagnosing and predicting intestinal fibrosis, as well as facilitate clinical research on anti‐fibrotic drugs. Moreover, the ongoing research on new anti‐fibrotic drugs offers hope for improved treatment options in managing fibrosis. life. The advancements outlined in this review regarding intestinal strictures will be consistently upgraded and improved upon, confirming that a new era in the field of intestinal fibrosis is around the corner.
CONFLICT OF INTEREST STATEMENT
All authors disclosed no relevant relationships.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (81970483, 82170537 and 82222010).
Liu Z, Huang Z, Wang Y, Xiong S, Lin S, He J, et al. Intestinal strictures in Crohn's disease: an update from 2023. United European Gastroenterol J. 2024;12(6):802–13. 10.1002/ueg2.12568
[Correction added on 04 April 2024, after first online publication: The author contribution statement has been revised.]
Zishan Liu and Zhuoyan Huang contributed equally to this work. Yao Zhang, Longyuan Zhou, and Ren Mao contributed equally to this work.
Contributor Information
Yao Zhang, Email: zyrjxh97@sjtu.edu.cn.
Longyuan Zhou, Email: zhouly76@mail.sysu.edu.cn.
Ren Mao, Email: maor5@mail.sysu.edu.cn.
DATA AVAILABILITY STATEMENT
Data availability is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Lewis JD, Parlett LE, Jonsson Funk ML, Brensinger C, Pate V, Wu Q, et al. Incidence, prevalence, and racial and ethnic distribution of inflammatory bowel disease in the United States. Gastroenterology. 2023;165(5):1197–1205. 10.1053/j.gastro.2023.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ. Crohn’s disease complicated by strictures: a systematic review. Gut. 2013;62(7):1072–1084. 10.1136/gutjnl-2012-304353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tavares de Sousa H, Magro F. How to evaluate fibrosis in IBD? Diagnostics. 2023;13(13):2188. 10.3390/diagnostics13132188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chandan S, Dhindsa BS, Khan SR, Deliwala S, Kassab LL, Mohan BP, et al. Endoscopic stenting in Crohn’s disease‐related strictures: a systematic review and meta‐analysis of outcomes. Inflamm Bowel Dis. 2023;29(7):1145–1152. 10.1093/ibd/izac153 [DOI] [PubMed] [Google Scholar]
- 5. Welz L, Aden K. Fibrosis and inflammation in inflammatory bowel disease‐more than 2 sides of the same coin? Gastroenterology. 2023;164(1):19–21. 10.1053/j.gastro.2022.10.024 [DOI] [PubMed] [Google Scholar]
- 6. D’Alessio S, Ungaro F, Noviello D, Lovisa S, Peyrin‐Biroulet L, Danese S. Revisiting fibrosis in inflammatory bowel disease: the gut thickens. Nat Rev Gastro Hepat. 2022;19(3):169–184. 10.1038/s41575-021-00543-0 [DOI] [PubMed] [Google Scholar]
- 7. Jarmakiewicz‐Czaja S, Sokal A, Ferenc K, Motyka E, Helma K, Filip R. The role of genetic and epigenetic regulation in intestinal fibrosis in inflammatory bowel disease: a descending process or a programmed consequence? Genes. 2023;14(6):1167. 10.3390/genes14061167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nayar S, Morrison JK, Giri M, Gettler K, Chuang L, Walker LA, et al. A myeloid‐stromal niche and gp130 rescue in NOD2‐driven Crohn’s disease. Nature. 2021;593(7858):275–281. 10.1038/s41586-021-03484-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ashton JJ, Seaby EG, Beattie RM, Ennis S. NOD2 in Crohn’s disease‐unfinished business. J Crohn’s and Colitis. 2023;17(3):450–458. 10.1093/ecco-jcc/jjac124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ashton JJ, Cheng G, Stafford IS, Kellermann M, Seaby EG, Cummings JRF, et al. Prediction of Crohn’s disease stricturing phenotype using a NOD2‐derived genomic biomarker. Inflamm Bowel Dis. 2023;29(4):511–521. 10.1093/ibd/izac205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang R, Wang W, Chen Z, Chai J, Qi Q, Zheng H, et al. Identifying immune cell infiltration and effective diagnostic biomarkers in Crohn’s disease by bioinformatics analysis. Front Immunol. 2023;14:1162473. 10.3389/fimmu.2023.1162473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoo JH, Holubar S, Rieder F. Fibrostenotic strictures in Crohn’s disease. Intestinal Res. 2020;18(4):379–401. 10.5217/ir.2019.09148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kong L, Pokatayev V, Lefkovith A, Carter GT, Creasey EA, Krishna C, et al. The landscape of immune dysregulation in Crohn’s disease revealed through single‐cell transcriptomic profiling in the ileum and colon. Immunity. 2023;56(2):444–458. 10.1016/j.immuni.2023.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mukherjee PK, Nguyen QT, Li J, Zhao S, Christensen SM, West GA, et al. Stricturing Crohn’s disease single‐cell RNA sequencing reveals fibroblast heterogeneity and intercellular interactions. Gastroenterology. 2023;165(5):1180–1196. Published online July 26. 10.1053/j.gastro.2023.07.014 [DOI] [PubMed] [Google Scholar]
- 15. Lewis A, Sánchez S, Berti G, Pan‐Castillo B, Nijhuis A, Mehta S, et al. Small‐molecule Wnt inhibitors are a potential novel therapy for intestinal fibrosis in Crohns disease. Clin Sci. 2022;136(19):1405–1423. 10.1042/CS20210889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nabhan AN, Webster JD, Adams JJ, Blazer L, Everrett C, Eidenschenk C, et al. Targeted alveolar regeneration with Frizzled‐specific agonists. Cell. 2023;186(14):2995–3012. 10.1016/j.cell.2023.05.022 [DOI] [PubMed] [Google Scholar]
- 17. Bhattacharya M, Ramachandran P. Immunology of human fibrosis. Nat Immunol. 2023;24(9):1423–1433. 10.1038/s41590-023-01551-9 [DOI] [PubMed] [Google Scholar]
- 18. Huang B, Chen Z, Geng L, Wang J, Liang H, Cao Y, et al. Mucosal profiling of pediatric‐onset colitis and IBD reveals common pathogenics and therapeutic pathways. Cell. 2019;179(5):1160–1176. 10.1016/j.cell.2019.10.027 [DOI] [PubMed] [Google Scholar]
- 19. Mao R, Liu Z, Rieder F. The effects of mesenteric inflammation on intestinal fibrosis. In: Coffey JC, editor. The Mesentery and inflammation Vol 90 progress in inflammation research. Springer International Publishing; 2023. p. 149–163. 10.1007/978-3-031-17774-3_9 [DOI] [Google Scholar]
- 20. Zhao X, Yang W, Yu T, Yu Y, Cui X, Zhou Z, et al. Th17 cell‐derived Amphiregulin promotes colitis‐associated intestinal fibrosis through activation of mTOR and MEK in intestinal myofibroblasts. Gastroenterology. 2023;164(1):89–102. 10.1053/j.gastro.2022.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14(8):493–507. 10.1038/s41581-018-0023-5 [DOI] [PubMed] [Google Scholar]
- 22. He S, Lei P, Kang W, Cheung P, Xu T, Mana M, et al. Stiffness restricts the stemness of the intestinal stem cells and skews their differentiation toward goblet cells. Gastroenterology. 2023;164(7):1137–1151. 10.1053/j.gastro.2023.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang Y, Zhang Y, Lu B, Xi J, Ocansey DKW, Mao F, et al. hucMSC‐ex alleviates IBD‐associated intestinal fibrosis by inhibiting ERK phosphorylation in intestinal fibroblasts. Stem Cell Int. 2023;2023:2828981–2829014. 10.1155/2023/2828981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Di Sabatino A, Jackson CL, Pickard KM, Buckley M, Rovedatti L, Leakey NAB, et al. Transforming growth factor beta signalling and matrix metalloproteinases in the mucosa overlying Crohn’s disease strictures. Gut. 2009;58(6):777–789. 10.1136/gut.2008.149096 [DOI] [PubMed] [Google Scholar]
- 25. Biel C, Faber KN, Bank RA, Olinga P. Matrix metalloproteinases in intestinal fibrosis. J Crohn’s and Colitis. 2023;18(3):jjad178. Published online October 25. 10.1093/ecco-jcc/jjad178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koop K, Enderle K, Hillmann M, Ruspeckhofer L, Vieth M, Sturm G, et al. Interleukin 36 receptor‐inducible matrix metalloproteinase 13 mediates intestinal fibrosis. Front Immunol. 2023;14:1163198. 10.3389/fimmu.2023.1163198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lis‐López L, Bauset C, Seco‐Cervera M, Macias‐Ceja D, Navarro F, Álvarez Á, et al. P2X7 receptor regulates collagen expression in human intestinal fibroblasts: relevance in intestinal fibrosis. Int J Mol Sci. 2023;24(16):12936. 10.3390/ijms241612936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weber S, Sitte S, Voegele AL, Sologub L, Wilfer A, Rath T, et al. NLRP3 inhibition leads to impaired mucosal fibroblast function in patients with inflammatory bowel diseases. J Crohn’s and Colitis. 2023;18(3):jjad164. Published online September 25. 10.1093/ecco-jcc/jjad164 [DOI] [PubMed] [Google Scholar]
- 29. Xue X, Zeng X, Wu X, Mu K, Dai Y, Wei Z. SIRT4 protects against intestinal fibrosis by facilitating GLS1 degradation. Matrix Biol: J Int Soc Matrix Biol. 2023;122:33–45. 10.1016/j.matbio.2023.08.001 [DOI] [PubMed] [Google Scholar]
- 30. Lee Y, Kim SH, Jeong H, Kim KH, Jeon D, Cho Y, et al. Role of Nox4 in mitigating inflammation and fibrosis in dextran sulfate sodium‐induced colitis. Cell Mol Gastroenterol Hepatol. 2023;16(3):411–429. 10.1016/j.jcmgh.2023.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schuler C, Foti F, Perren L, Mamie C, Weder B, Stokmaier M, et al. Deletion of Smad7 ameliorates intestinal inflammation and contributes to fibrosis. Inflamm Bowel Dis. 2023;29(4):647–660. 10.1093/ibd/izac221 [DOI] [PubMed] [Google Scholar]
- 32. Johnson JC, Geesala R, Zhang K, Lin YM, M’Koma AE, Shi XZ. Smooth muscle dysfunction in the pre‐inflammation site in stenotic Crohn’s‐like colitis: implication of mechanical stress in bowel dysfunction in gut inflammation. Front Physiol. 2023;14:1215900. 10.3389/fphys.2023.1215900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aggeletopoulou I, Mouzaki A, Thomopoulos K, Triantos C. miRNA molecules‐late breaking treatment for inflammatory bowel diseases? Int J Mol Sci. 2023;24(3):2233. 10.3390/ijms24032233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pompili S, Vetuschi A, Latella G, Smakaj A, Sferra R, Cappariello A. PPAR‐gamma orchestrates EMT, AGE, and cellular senescence pathways in colonic epithelium and restrains the progression of IBDs. Int J Mol Sci. 2023;24(10):8952. 10.3390/ijms24108952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li B, Wang Y, Jiang X, Du H, Shi Y, Xiu M, et al. Natural products targeting Nrf2/ARE signaling pathway in the treatment of inflammatory bowel disease. Biomed and Pharmacother. 2023;164:114950. 10.1016/j.biopha.2023.114950 [DOI] [PubMed] [Google Scholar]
- 36. Imai J, Kitamoto S, Sugihara K, Nagao‐Kitamoto H, Hayashi A, Morhardt TL, et al. Flagellin‐mediated activation of IL‐33‐ST2 signaling by a pathobiont promotes intestinal fibrosis. Mucosal Immunol. 2019;12(3):632–643. 10.1038/s41385-019-0138-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu Y, Qian W, Huang L, Wen W, Li Y, Guo F, et al. Crohn’s disease‐associated AIEC inhibiting intestinal epithelial cell‐derived exosomal let‐7b expression regulates macrophage polarization to exacerbate intestinal fibrosis. Gut Microb. 2023;15(1):2193115. 10.1080/19490976.2023.2193115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Translocation of viable gut microbiota to mesenteric adipose drives formation of creeping fat in humans ‐ PubMed. Accessed February 26, 2024. https://pubmed.ncbi.nlm.nih.gov/32991841/ [DOI] [PMC free article] [PubMed]
- 39. Gong J, Yu J, Yin S, Ke J, Wu J, Liu C, et al. Mesenteric adipose tissue‐derived Klebsiella variicola disrupts intestinal barrier and promotes colitis by type VI secretion system. Adv Sci. 2023;10(12):e2205272. 10.1002/advs.202205272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Flannigan KL, Nieves KM, Szczepanski HE, Serra A, Lee JW, Alston LA, et al. The pregnane X receptor and indole‐3‐propionic acid shape the intestinal mesenchyme to restrain inflammation and fibrosis. Cell Mol Gastroenterol Hepatol. 2023;15(3):765–795. 10.1016/j.jcmgh.2022.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhan S, Liu C, Meng J, Mao R, Tu T, Lin J, et al. Mucosa‐associated Oscillospira sp. is related to intestinal stricture and post‐operative disease course in Crohn’s disease. Microorganisms. 2023;11(3):794. 10.3390/microorganisms11030794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dickson I. Creeping fat in Crohn’s disease explained. Nat Rev Gastroenterol Hepatol. 2020;17(12):713. 10.1038/s41575-020-00379-0 [DOI] [PubMed] [Google Scholar]
- 43. Mao R, Doyon G, Kurada S, Gordon I, Zhao S, Dejanovic D, et al. Creeping‐fat derived free fatty acids induce hyperplasia of intestinal muscularis propria muscle cells – A novel link between fat and intestinal stricture formation in Crohn’s disease. Gastroenterology. 2018;154(6):S–131. 10.1016/S0016-5085(18)30866-7 [DOI] [Google Scholar]
- 44. Kim K, Park S, Lee Y, Baek J, Kim Y, Hwang SW, et al. Transcriptomic profiling and cellular composition of creeping fat in Crohn’s disease. J Crohns Colitis. 2024;18(2):jjad141. 10.1093/ecco-jcc/jjad141 [DOI] [PubMed] [Google Scholar]
- 45. Shu W, Wang Y, Li C, Zhang L, Zhuoma D, Yang P, et al. Single‐cell expression atlas reveals cell heterogeneity in the creeping fat of Crohn’s disease. Inflamm Bowel Dis. 2023;29(6):850–865. 10.1093/ibd/izac266 [DOI] [PubMed] [Google Scholar]
- 46. Wu J, Tian Z, Zhuang X, Chen Y, Fan T, Li J, et al. Dynamic alterations in metabolomics and transcriptomics associated with intestinal fibrosis in a 2, 4, 6‐trinitrobenzene sulfonic acid‐induced murine model. J Transl Med. 2023;21(1):554. 10.1186/s12967-023-04392-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu F, Wu F, Zhou Q, Liu X, Fei J, Zhang D, et al. A CCL2+DPP4+ subset of mesenchymal stem cells expedites aberrant formation of creeping fat in humans. Nat Commun. 2023;14(1):5830. 10.1038/s41467-023-41418-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Qian W, Xu Y, Wen W, Huang L, Guo Z, Zhu W, et al. Exosomal miR‐103a‐3p from Crohn’s creeping fat‐derived adipose‐derived stem cells contributes to intestinal fibrosis by targeting TGFBR3 and activating fibroblasts. J Crohn’s and Colitis. 2023;17(8):1291–1308. 10.1093/ecco-jcc/jjad042 [DOI] [PubMed] [Google Scholar]
- 49. Gordon IO, Bettenworth D, Bokemeyer A, Srivastava A, Rosty C, de Hertogh G, et al. International consensus to standardise histopathological scoring for small bowel strictures in Crohn’s disease. Gut. 2022;71(3):479–486. 10.1136/gutjnl-2021-324374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. De Kock I, Bos S, Delrue L, et al. MRI texture analysis of T2‐weighted images is preferred over magnetization transfer imaging for readily longitudinal quantification of gut fibrosis. Eur Radiol. 2023;33(9):5943–5952. 10.1007/s00330-023-09624-x [DOI] [PubMed] [Google Scholar]
- 51. Scharitzer M, Macher‐Beer A, Mang T, et al. Evaluation of intestinal fibrosis with 68Ga‐FAPI PET/MR enterography in Crohn disease. Radiology. 2023;307(3):e222389. 10.1148/radiol.222389 [DOI] [PubMed] [Google Scholar]
- 52. Sleiman J, Chirra P, Gandhi N, et al. DOP12 Validation of radiomics features on MR enterography characterizing inflammation and fibrosis in stricturing Crohn’s disease. J Crohn’s Colitis. 2023;17(Suppl 1):i73. 10.1093/ecco-jcc/jjac190.0052 [DOI] [PubMed] [Google Scholar]
- 53. Chen L, Zhong X, Li L, et al. [68Ga]Ga‐FAPI‐04 PET/CT on assessing Crohn’s disease intestinal lesions. Eur J Nucl Med Mol Imag. 2023;50(5):1360–1370. 10.1007/s00259-023-06107-5 [DOI] [PubMed] [Google Scholar]
- 54. Li X, Zhang N, Hu C, et al. CT‐based radiomics signature of visceral adipose tissue for prediction of disease progression in patients with Crohn’s disease: a multicentre cohort study. EClinicalMedicine. 2023;56:101805. 10.1016/j.eclinm.2022.101805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shi L, Wang Y, Shen X, et al. Clinical outcome is distinct between radiological stricture and endoscopic stricture in ileal Crohn’s disease. Eur Radiol. 2023. 10.1007/s00330-023-09743-5 [DOI] [PubMed] [Google Scholar]
- 56. Xu C, Jiang W, Wang L, Mao X, Ye Z, Zhang H. Intestinal ultrasound for differentiating fibrotic or inflammatory stenosis in Crohn’s disease: a systematic review and meta‐analysis. J Crohn’s and Colitis. 2022;16(9):1493–1504. 10.1093/ecco-jcc/jjac052 [DOI] [PubMed] [Google Scholar]
- 57. Manzotti C, Colombo F, Zurleni T, Danelli P, Maconi G. Prognostic role of intestinal ultrasound in Crohn’s disease. World J Gastroenterol. 2023;29(23):3595–3605. 10.3748/wjg.v29.i23.3595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Takeuchi K, Inokuchi T, Takahara M, et al. Usefulness of intestinal ultrasound to detect small intestinal stenosis in patients with Crohn’s disease. J Ultrasound Med. 2023;42(2):373–383. 10.1002/jum.16038 [DOI] [PubMed] [Google Scholar]
- 59. Shen B, Kochhar G, Navaneethan U, et al. Practical guidelines on endoscopic treatment for Crohn’s disease strictures: a consensus statement from the Global Interventional Inflammatory Bowel Disease Group. Lancet Gastroenterol and Hepatol. 2020;5(4):393–405. 10.1016/S2468-1253(19)30366-8 [DOI] [PubMed] [Google Scholar]
- 60. Pennazio M, Rondonotti E, Despott EJ, et al. Small‐bowel capsule endoscopy and device‐assisted enteroscopy for diagnosis and treatment of small‐bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Guideline ‐ update 2022. Endoscopy. 2023;55(1):58–95. 10.1055/a-1973-3796 [DOI] [PubMed] [Google Scholar]
- 61. Ukashi O, Kopylov U, Ungar B, et al. Patency capsule: a novel independent predictor for long‐term outcomes among patients with Quiescent Crohn’s disease. Am J Gastroenterol. 2023;118(6):1019–1027. 10.14309/ajg.0000000000002118 [DOI] [PubMed] [Google Scholar]
- 62. Nakase H, Esaki M, Hirai F, et al. Treatment escalation and de‐escalation decisions in Crohn’s disease: Delphi consensus recommendations from Japan, 2021. J Gastroenterol. 2023;58(4):313–345. 10.1007/s00535-023-01958-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Baumgart DC, Müller HP, Grittner U, et al. US‐Based real‐time elastography for the detection of fibrotic gut tissue in patients with stricturing Crohn disease. Radiology. 2015;275(3):889–899. 10.1148/radiol.14141929 [DOI] [PubMed] [Google Scholar]
- 64. Allocca M, Dal Buono A, D’Alessio S, et al. Relationships between intestinal ultrasound parameters and histopathologic findings in a prospective cohort of patients with Crohn’s disease undergoing surgery. J Ultrasound Med. 2023;42(8):1717–1728. 10.1002/jum.16191 [DOI] [PubMed] [Google Scholar]
- 65. Abu‐Ata N, Dillman JR, Rubin JM, et al. Ultrasound shear wave elastography in pediatric stricturing small bowel Crohn disease: correlation with histology and second harmonic imaging microscopy. Pediatr Radiol. 2023;53(1):34–45. 10.1007/s00247-022-05446-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Assessment of Crohn’s disease‐associated small bowel strictures and fibrosis on cross‐sectional imaging: a systematic review ‐ PubMed. Accessed February 26, 2024. https://pubmed.ncbi.nlm.nih.gov/30944110/ [DOI] [PMC free article] [PubMed]
- 67. Steiner CA, Berinstein JA, Louissaint J, Higgins PD, Spence JR, Shannon C, et al. Biomarkers for the prediction and diagnosis of fibrostenosing Crohn’s disease: a systematic review. Clin Gastroenterol Hepatol: Official Clin Pract J Am Gastroenterological Assoc. 2022;20(4):817–846. 10.1016/j.cgh.2021.05.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sarter H, Savoye G, Marot G, Ley D, Turck D, Hugot JP, et al. A novel 8‐predictors signature to predict complicated disease course in pediatric‐onset Crohn’s disease: a population‐based study. Inflamm Bowel Dis. 2023;29(11):izad090. Published online June 2. 10.1093/ibd/izad090 [DOI] [PubMed] [Google Scholar]
- 69. Choung RS, Petralia F, Torres J, Ungaro RC, Porter C, Sato T, et al. Preclinical serological signatures are associated with complicated Crohn’s disease phenotype at diagnosis. Clin Gastroenterol Hepatol. 2023;21(11):2928–2937. Published online February 12. 10.1016/j.cgh.2023.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bourgonje AR, Andreu‐Sánchez S, Vogl T, Hu S, Vich Vila A, Gacesa R, et al. Phage‐display immunoprecipitation sequencing of the antibody epitope repertoire in inflammatory bowel disease reveals distinct antibody signatures. Immunity. 2023;56(6):1393–1409. 10.1016/j.immuni.2023.04.017 [DOI] [PubMed] [Google Scholar]
- 71. Ferru‐Clément R, Boucher G, Forest A, Bouchard B, Bitton A, Lesage S, et al. Serum lipidomic screen identifies key metabolites, pathways, and disease classifiers in Crohn’s disease. Inflamm Bowel Dis. 2023;29(7):1024–1037. 10.1093/ibd/izac281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pehrsson M, Alexdóttir MS, Karsdal MA, Thakker P, Mortensen JH. Novel fibro‐inflammatory biomarkers associated with disease activity in patients with Crohn’s disease. Expert Rev Gastroenterol Hepatol. 2023;17(6):1–13. Published online May 24. 10.1080/17474124.2023.2212158 [DOI] [PubMed] [Google Scholar]
- 73. Jia Y, Ma X, Tao Y, et al. Endoscopic treatment of a postoperative anastomotic stricture in a patient with refractory Crohn’s disease. Endoscopy. 2023;55(S 01):E135–E136. 10.1055/a-1941-8409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Darlington K, Wang A, Herfarth HH, Barnes EL. The safety of dilation of ileoanal strictures with mechanical or balloon dilation is similar among patients after ileal pouch‐anal anastomosis. Inflamm Bowel Dis. 2023:izad051. Published online April 12. 10.1093/ibd/izad051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ning SB, Yang H, Li B, et al. Balloon‐assisted enteroscopy‐based endoscopic stricturotomy for deep small bowel strictures from Crohn’s disease: first cohort study of a novel approach. Dig Liver Dis: Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2023;55(10):1397–1402. Published online June 12. 10.1016/j.dld.2023.05.033 [DOI] [PubMed] [Google Scholar]
- 76. Senior K. Surgery in Crohn’s disease: patients need MDT‐led holistic care. Lancet Gastroenterol and Hepatol. 2023;8(10):874. 10.1016/S2468-1253(23)00274-1 [DOI] [Google Scholar]
- 77. Popivanov G, Kjossev K, Stoyanova D, Konaktchieva M, Mutafchiyski V. Early surgery for Crohn’s disease ‐ an appeal for a reassessment of biologics. Dig Liver Dis: Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2023;118(12):2212–2219. Published online September 16. 10.1016/j.dld.2023.08.060 [DOI] [PubMed] [Google Scholar]
- 78. Bachour SP, Khan MZ, Shah RS, et al. Anastomotic configuration and temporary diverting ileostomy do not increase risk of anastomotic stricture in postoperative Crohn’s disease. Am J Gastroenterol. 2023;118(12):2212–2219. Published online August 4. 10.14309/ajg.0000000000002393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pal P, Gala J, Rebala P, et al. Re‐intervention rates and symptom‐free survival at 1 year after endoscopic versus surgical management of strictures in Crohn’s disease: a propensity matched analysis of a prospective inflammatory bowel disease cohort. J Gastroenterol Hepatol. 2023. Published online October 28. 10.1111/jgh.16384 [DOI] [PubMed] [Google Scholar]
- 80. Cao Y, Cheng K, Yang M, et al. Orally administration of cerium oxide nanozyme for computed tomography imaging and anti‐inflammatory/anti‐fibrotic therapy of inflammatory bowel disease. J Nanobiotechnol. 2023;21(1):21. 10.1186/s12951-023-01770-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wang J, Bai M, Zhang C, et al. Natural compound fraxinellone ameliorates intestinal fibrosis in mice via direct intervention of HSP47‐collagen interaction in the epithelium. Acta Pharmacol Sin. 2023. 44(12):2469–2478. Published online August 14. 10.1038/s41401-023-01143-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gómez‐Ferrer M, Amaro‐Prellezo E, Albiach‐Delgado A, Ten‐Domenech I, Kuligowski J, Sepúlveda P. Identification of omega‐3 oxylipins in human milk‐derived extracellular vesicles with pro‐resolutive actions in gastrointestinal inflammation. Front Immunol. 2023;14:1293737. 10.3389/fimmu.2023.1293737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Didamoony MA, Soubh AA, Atwa AM, Ahmed LA. Innovative preconditioning strategies for improving the therapeutic efficacy of extracellular vesicles derived from mesenchymal stem cells in gastrointestinal diseases. Inflammopharmacology. 2023;31(6):2973–2993. 10.1007/s10787-023-01350-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Surgical management of IBD‐‐from an open to a laparoscopic approach ‐ PubMed. Accessed February 27, 2024. https://pubmed.ncbi.nlm.nih.gov/23419288/ [DOI] [PubMed]
- 85. Schulberg JD, Wright EK, Holt BA, Hamilton AL, Sutherland TR, Ross AL, et al. Intensive drug therapy versus standard drug therapy for symptomatic intestinal Crohn’s disease strictures (STRIDENT): an open‐label, single‐centre, randomised controlled trial. Lancet Gastroenterol and Hepatol. 2022;7(4):318–331. 10.1016/S2468-1253(21)00393-9 [DOI] [PubMed] [Google Scholar]
- 86. Bokemeyer B, Plachta‐Danielzik S, di Giuseppe R, Helwig U, Teich N, Schmidt C, et al. Evaluation of a downstaging, bidirectional version of the Montreal classification of Crohn’s disease: analysis of 5‐year follow‐up data from the prospective BioCrohn study. Aliment Pharm Ther. 2023;58(1):35–47. 10.1111/apt.17512 [DOI] [PubMed] [Google Scholar]
- 87. Zou C, Zan X, Jia Z, Zheng L, Gu Y, Liu F, et al. Crosstalk between alternative splicing and inflammatory bowel disease: basic mechanisms, biotechnological progresses and future perspectives. Clin Transl Med. 2023;13(11):e1479. 10.1002/ctm2.1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availability is not applicable to this article as no new data were created or analyzed in this study.


