Abstract
Food allergy oral immunotherapy (OIT) has demonstrated efficacy in promoting clinically relevant immunomodulation that leads to desensitization (reduced reactivity while on OIT) in the majority of treated individuals; however, sustained unresponsiveness after OIT cessation for a specified interval has only been observed in a subset. The potential therapeutic benefits of OIT must be balanced with the risk for adverse events. These adverse events may range from self-limited or easily treated oropharyngeal, respiratory, or gastrointestinal symptoms to persistent abdominal symptoms that lead to cessation of therapy and to anaphylaxis. To date, the majority of studies have evaluated single-allergen OIT approaches; however, multi-allergen OIT has demonstrated favorable safety and efficacy outcomes, and is the subject of ongoing investigation. Recent U.S. Food and Drug Administration approval of the first licensed OIT product for peanut allergy challenges the long-standing paradigm of dietary food avoidance as the sole option for individuals with food allergy. Yet, the limitations of this “first-generation” treatment support the need for continued research and development of next-generation therapies to improve efficacy, minimize risk, and allow for broad applicability to both individuals with single-food allergy and those with multifood allergies. Optimizing future therapies will require developing novel approaches that maximize both efficacy and safety and/or tolerability outcomes, potentially through the combination with biologic therapies or adjuvants. Shared decision-making among patients, physicians, and parents and/or caregivers is critical to select optimal candidates for treatment with OIT by balancing the potential therapeutic benefit and possible risk reduction with a realistic consideration of OIT treatment burden and the risk of treatment-related adverse events.
Advances in the development of active therapies for immunoglobulin E (IgE) mediated food allergy such as oral immunotherapy (OIT)1 and epicutaneous immunotherapy2 have resulted from productive clinical and translational research over the past 2 decades. OIT for food allergy is a multiphase treatment strategy in which a food allergen(s) is administered in combination with a vehicle food and is consumed in incrementally increasing doses over a specified time interval, with the goal of promoting immunomodulation, which leads to clinically relevant outcomes, such as protection against accidental ingestion or ad libitum ingestion of the allergen.3–8
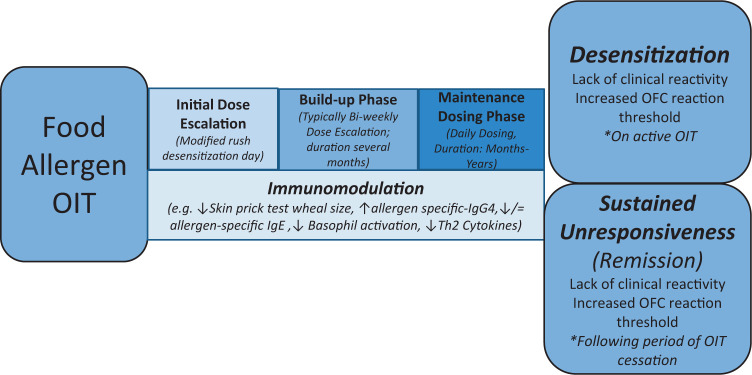
OIT protocols typically involve multiple treatment phases, including initial dose escalation (IDE), home OIT dosing, with observed (every two weeks) up-dosing, and a daily home dosing maintenance phase (Fig. 1). Clinical outcomes during OIT include desensitization, defined as a lack of clinical reactivity to a prespecified amount of food allergen while undergoing active therapy, and sustained unresponsiveness (SU), the absence of clinical reactivity to the food allergen after treatment has been discontinued for a specific time interval (typically 1–3 months). Because SU has been inconsistently defined in OIT studies, the exact duration of therapy necessary to achieve SU is uncertain. Use of the term “tolerance” has been eschewed because clinical reactivity can return after periods of allergen avoidance; as such, some investigators have proposed that sustained effects of immunotherapy may be more accurately represented by using the term “remission” (Fig. 1).
Figure 1.
Overview of food allergen oral immunotherapy (OIT), including phases of treatment and clinical outcome definitions.
Although collective evidence supports the efficacy of single-allergen OIT in achieving clinical desensitization, SU has only been demonstrated in a subset of individuals treated with OIT.3,6,9 Further, published meta-analyses observed heterogeneity in the published OIT clinical trial design and concluded that additional data are needed for full efficacy assessments, that safety concerns persist, and that the impact on quality of life (QoL) is variable.10,11 Nonetheless, decades of OIT research efforts have recently culminated in the U.S. Food and Drug Administration (FDA) approval, on January 31, 2020, of Palforzia (formerly AR101; Aimmune Therapeutics, Brisbane, CA) the first treatment option for food allergy for individuals ages 4–17 years with peanut allergy. Additional investigation is critical to refine existing approaches and develop novel treatment strategies, while addressing knowledge gaps, including the optimal dose and duration for different allergens, long-term outcomes, predictors of response, cost-effectiveness, risk reduction and psychosocial impact to maximize efficacy, minimize risk, and develop individualized approaches for future clinical application. Here, we provided a concise review of the available evidence with regard the efficacy, safety, and therapeutic application of food allergen OIT.
EFFICACY OF OIT
OIT has been evaluated for treatment of a variety of food allergies, with the majority of published trials focused on single-allergen peanut, egg, and milk OIT. Systematic reviews and meta-analyses have evaluated the collective evidence with regard to OIT, which concluded that the evidence for OIT-induced desensitization is strong; however, further evidence with regard to long-term safety and efficacy end points, dosing in adults, cost-effectiveness, risk reduction, and the impact on QoL is needed.10–12
Peanut
After the promising results of early peanut OIT studies,8,13,14 the first randomized, double-blind, placebo controlled multicenter trial of peanut OIT evaluated the effectiveness of peanut OIT (4000 mg maintenance dose) in 28 pediatric subjects.7 Investigators observed desensitization to 5000 mg of peanut protein (~16–20 peanuts) in participants on active OIT (versus 280 mg of placebo; p < 0.001) after 1 year of treatment. In another randomized controlled trial of peanut OIT (maintenance dose of 800 mg), the investigators observed desensitization in 62% of the active peanut OIT group.4 The efficacy and feasibility of peanut OIT in young children (ages 9–36 months) was evaluated, and, after 3 years of treatment, 85% of the participants who received low-dose peanut OIT (300 mg maintenance, approximately one peanut) were desensitized to 5 g of peanut versus 76% of the participants who received high-dose OIT (3000 mg).15 Interestingly, 78% of the total population achieved SU after discontinuation of OIT for 4 weeks. The outcomes observed in early OIT trials ultimately led to the development of industry-sponsored randomized double-blind, placebo controlled clinical trials by using a standardized peanut OIT product (AR101).16,17
In a multicenter phase II trial that used a 300-mg AR101 maintenance dose enrolled 55 subjects (ages 4–26 years) with clinical reactivity to ≤143 mg of peanut during a screening double-blind, placebo controlled food challenge (DBPCFC).16 After 34 weeks of therapy, 79% of the participants in the OIT-treatment arm tolerated a cumulative 443 mg of peanut during the exit DBPCFC compared with only 19% in the placebo arm (p < 0.001). In the pivotal phase III Peanut Allergy oral Immunotherapy Study of AR101 for Desensitization (PALISADE) trial, the investigators assessed the effectiveness of 300 mg daily maintenance dosing for 6 months to induce the primary end point of desensitization to ≥600 mg of a single peanut dose in the exit DBPCFC.17 All 496 subjects ages 4 to 17 years demonstrated clinical reactivity to a single dose of ≤100 mg during the entry food challenge. Sixty-seven percent of the AR101-treatment group met the primary end point versus 4% of the placebo group (p < 0.001). Notably, 55 participants ages ≥18 years were also enrolled, but this age group did not meet the desensitization end point.
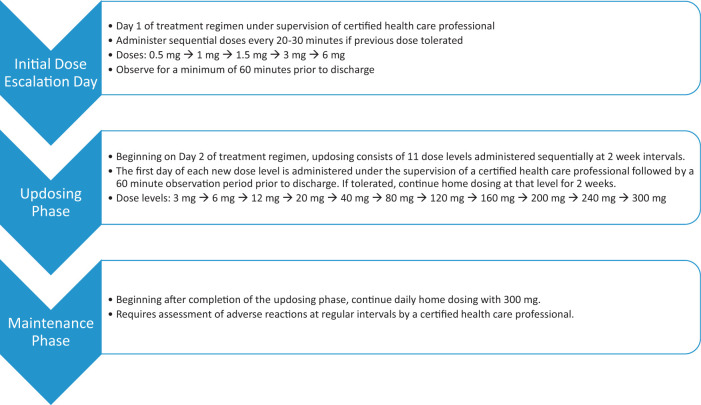
After completion of the phase III PALISADE trial,17 Aimmune Therapeutics submitted a Biologics License Application for AR101 to the FDA in December 2018, having previously received FDA Fast Track Designation in September 2014 and Breakthrough Therapy Designation in June 2015. The FDA granted approval of AR101 on January 31, 2020, under the proprietary name of Palforzia, for the treatment of peanut allergy in children ages 4–17 years. Aimed at mitigating allergic reactions secondary to accidental exposures to peanut, Palforzia is to be used daily in combination with a peanut-avoidance diet. The dosing protocol includes an IDE phase up to 6 mg on day 1 of treatment, an up-dosing phase that includes daily home dosing with observed increases in the dose level every 2 weeks up to 300 mg, and a maintenance phase of daily home dosing with 300 mg (Fig. 2). The IDE and administration of the firstdoseof each up-dosing level must be performed under direct supervision of a qualified health-care professional, followed by a minimum 60-minute monitoring period.
Figure 2.
Flow chart representation of three phase-dosing schema for Palforzia oral immunotherapy (OIT) treatment of peanut allergy adapted from the package insert. See package insert for full prescribing details.26
Milk
In the first double-blind, placebo controlled multicenter trial of milk OIT, 19 children ages 6–17 years with persistent cow's milk allergy were randomized (2:1) to receive 500 mg of OIT or placebo.18 After maintenance therapy for 3–4 months, the median dose that resulted in clinical reactivity in the active OIT treatment group increased from a baseline of 40 mg to 5140 mg, while remaining stable at 40 mg in the placebo group (p <0.001).18 In an open-label long-term follow-up study of this same cohort, researchers demonstrated the feasibility of gradual home dose escalation with the participants tolerating between 1000 and 16,000 mg, with one-third tolerating the maximum dose of 16,000 mg (~2 cups of milk).19 A more-recent systemic review and meta-analysis of randomized controlled trials of milk OIT supports the efficacy of milk OIT in inducing desensitization reported in these early studies.20 In addition, there is an analysis of data that dietary use of extensively heated or baked milk may accelerate unbaked milk tolerance.21 Evaluation of optimal dosing regimens in subgroups of individuals with baked-milk versus individuals with non–baked-milk allergy are needed to inform future milk OIT studies.
Egg
Egg OIT has also shown success in desensitizing a significant portion of children with egg allergy. Fiftyfive children ages 5–11 years were enrolled in the first double-blind, placebo controlled multicenter egg OIT trial.5 Desensitization was achieved in 55% and 75% of individuals who received active therapy after 10 months and 22 months of therapy, respectively. Of the children desensitized to 10 g of egg protein (one egg [~6–8 g protein]) after 22 months of OIT, only 28% demonstrated SU 4–6 weeks after OIT discontinuation. Subsequent investigation suggests that rates of SU are increased with a longer duration of egg OIT treatment.22 Similar to baked milk, extensively heated or baked egg shows promise as a form of OIT in inducing desensitization to lightly cooked egg.23
MULTI-ALLERGEN OIT
Investigators have evaluated the safety and efficacy of multi-allergen OIT to address the increased disease burden in individuals with multifood allergies. In a single-center phase I trial, multi-allergen OIT showed comparable safety to single-allergen peanut OIT and was effective in promoting a 10-fold increase in reaction threshold in the majority of the treated participants.24 A second phase I study demonstrated successful multifood desensitization after omalizumab pretreatment, with subsequent rush multi-allergen OIT.25 Clinical trials are ongoing to evaluate multi-allergen OIT in combination with omalizumab (www.clinicaltrials.gov, NCT03881696). The use of biologic therapies for food allergy is reviewed by Chen et al.26
OIT Safety and Tolerability
The benefits of OIT for inducing desensitization must be carefully balanced with consideration of potential untoward adverse events (AE), such as gastrointestinal symptoms and anaphylaxis (Table 1). When evaluating AE by using pooled data from three peanut OIT trials (n = 104 participants), investigators observed that 80% of the participants who received peanut OIT experienced AEs: 72% occurred during buildup and 47% during maintenance.27 Twenty percent of the participants on peanut OIT dropped out of the study due to AEs, half due to gastrointestinal symptoms. Asthma and allergic rhinitis predicted systemic symptoms, and a higher skin-prick test wheal size was predictive of gastrointestinal AEs.27
Table 1.
Potential adverse effects of oral immunotherapy
| Oropharyngeal | Oral itching |
|---|---|
| Respiratory | Cough, wheezing, rhinitis |
| Gastrointestinal | Abdominal pain, cramping, vomiting, eosinophilic esophagitis |
| Skin | Rash, hives, itching |
| Anaphylaxis | Multisystem |
In the PALISADE trial, 4.3% of the participants who received AR101 experienced severe symptoms compared with 0.8% in the placebo group; epinephrine use occurred in 14% of the participants treated with AR101 versus 6.5% of participants on placebo, with two-thirds of epinephrine doses administered after home dosing.17 Factors that may predispose to the development of AEs during OIT treatment include concurrent illness, exercise, menses, use of nonsteroidal medications, and poorly controlled asthma. Eosinophilic esophagitis has been identified in patients treated in OIT, with a prevalence rate of 2.7% (95% confidence interval, 1.7–4%) in a 2014 meta-analysis.28
In the 2019 Peanut Allergen Immunotherapy, Clarifying the Evidence systematic review and meta-analysis, high-certainty evidence demonstrated that available peanut OIT regimens increased allergic and anaphylactic reactions over peanut avoidance not receiving OIT; OIT was associated with a threefold increased risk of anaphylaxis and a twofold increase in epinephrine use compared with allergen avoidance and no difference in QoL.11 This study raises important questions with regard to safety and overall benefitof OIT-induced desensitization versus avoidance, highlighting the need for development of safer treatment approaches and rigorous evaluation of outcomes important to patients and/or caregivers in the design of future randomized controlled trials. Indeed, shared decision-making before OIT initiation should include frank discussions of not only efficacy, safety, and tolerability but also the treatment burden on patients and families.
Specific considerations include the need for multiple clinic visits, particularly during OIT dose escalation, alteration of daily activities to limit physical activity around daily dosing, and the risk of unexpected dosing reactions associated with illness or menses. Further, unknowns with regard to long-term treatment outcomes and the possibility of reversion to an allergic state with cessation of therapy as well as the lack of reliable predictors of individual treatment response and surrogate biomarkers of tolerability should be carefully considered.
Palforzia carries a black box warning for anaphylaxis and is contraindicated in those with uncontrolled asthma and eosinophilic gastrointestinal disorders.29 In addition, the FDA has mandated the implementation of a risk evaluation and mitigation strategy to ensure that the benefits of the medication outweigh the risks for each individual. Thus, an in-depth risk-benefit ratio discussion is a necessity before initiation of OIT to establish a well-informed, personalized decision about initiation of therapy.
IMMUNOMODULATION BY OIT
The pathophysiology of IgE-mediated food allergy is reviewed by Shreffler.30 Although the immune mechanisms associated with clinical outcomes during OIT are the subject of ongoing investigation, the typical parameters associated with beneficial outcomes in OIT trials to date include increased allergen-specific IgG4, decreased skin-prick test and basophil reactivity, reduced T-helper type 2 cytokine responses, and induction of regulatory T cells (Fig. 1).3,5,14 Investigators have observed that OIT-induced immunologic changes may be temporary, even while receiving maintenance treatment, which highlights individual variability in immunomodulation and the clinical response.
CONCLUSION
Advancements in OIT approaches have ushered in a paradigm-shifting era in food allergy, including the recent FDA approval of OIT for peanut allergy. However, the potential therapeutic benefit of OIT must be balanced with consideration of potential risks associated with its use and include shared decision-making among patients, caregivers, and providers to determine the best approach for each individual. Additional research is important to better understand long-term outcomes, predictors of response, cost-effectiveness, psychosocial impact, real-world risk reduction, and patient-prioritized outcomes to refine and optimize therapeutic application of OIT.
CLINICAL PEARLS
OIT induces desensitization in the majority of individuals with food allergy but not SU, which requires continued dosing to maintain protection against severe allergic reactions in the event of accidental exposure.
Mild-to-moderate AEs are common with OIT treatment and often self-limited or manageable with antihistamines alone.
Serious AEs, including anaphylaxis and eosinophilic esophagitis, are possible during any phase of OIT treatment, which necessitates follow-up at regular intervals while undergoing therapy.
Palforzia is the only FDA licensed OIT treatment and is approved in children ages 4–17 years with peanut allergy and without poorly controlled asthma or eosinophilic gastrointestinal disease.
Before deciding to initiate (or not) OIT, it is important to ensure that all stakeholders (physicians, patients, caregivers) understand the potential benefits and burdens of treatment to set appropriate therapeutic expectations in a shared decision-making process.
Footnotes
Dr. Scurlock reports grant support to her institution from NIH/NIAID, Immune Tolerance Network, Aimmune Therapeutics, DBV Technologies, Astellas, Regeneron, Genentech, and Food Allergy Research and Education (FARE). She reports clinical medical advisory board membership with DBV Technologies
The authors have no conflicts of interest to declare pertaining to this article
Funded by Food Allergy Research & Education (FARE)
REFERENCES
- 1.Sood AK, Scurlock AM.. Food allergy oral immunotherapy. J Food Allergy. 2020; 2:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chow T, Parrish C, Bird JA.. Food allergy epicutaneous immunotherapy. J Food Allergy. 2020; 2:81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vickery BP, Scurlock AM, Kulis Met al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol. 2014; 133:468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anagnostou K, Islam S, King Yet al. Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised controlled trial. Lancet. 2014; 383:1297–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burks AW, Jones SM, Wood RAet al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012; 367:233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keet CA, Seopaul S, Knorr Set al. Long-term follow-up of oral immunotherapy for cow's milk allergy. J Allergy Clin Immunol. 2013; 132:737–739.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varshney P, Jones SM, Scurlock AMet al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011; 127:654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blumchen K, Ulbricht H, Staden Uet al. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol. 2010; 126:83-91.e1. [DOI] [PubMed] [Google Scholar]
- 9.Chinthrajah RS, Purington N, Andorf Set al. Sustained outcomes in oral immunotherapy for peanut allergy (POISED study): a large, randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2019; 394:1437–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brożek JL, Terracciano L, Hsu Jet al. Oral immunotherapy for IgE-mediated cow's milk allergy: a systematic review and meta-analysis. Clin Exp Allergy. 2012; 42:363–374. [DOI] [PubMed] [Google Scholar]
- 11.Chu DK, Wood RA, French Set al. Oral immunotherapy for peanut allergy (PACE): a systematic review and meta-analysis of efficacy and safety. Lancet. 2019; 393:2222–2232. [DOI] [PubMed] [Google Scholar]
- 12.Nurmatov U, Dhami S, Arasi Set al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017; 72:1133–1147. [DOI] [PubMed] [Google Scholar]
- 13.Clark AT, Islam S, King Yet al. Successful oral tolerance induction in severe peanut allergy. Allergy. 2009; 64:1218–1220. [DOI] [PubMed] [Google Scholar]
- 14.Jones SM, Pons L, Roberts JLet al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009; 124:292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vickery BP, Berglund JP, Burk CMet al. Early oral immunotherapy in peanut-allergic preschool children is safe and highly effective. J Allergy Clin Immunol. 2017; 139:173–18.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bird JA, Spergel JM, Jones SMet al. Efficacy and safety of AR101 in oral immunotherapy for peanut allergy: results of ARC001, a randomized, double-blind, placebo-controlled phase 2 clinical trial. J Allergy Clin Immunol Pract. 2018; 6:476–485.e3. [DOI] [PubMed] [Google Scholar]
- 17.PALISADE Group of Clinical Investigators, Vickery BP, Vereda Aet al. AR101 oral immunotherapy for peanut allergy. N Engl J Med. 2018; 379:1991–2001. [DOI] [PubMed] [Google Scholar]
- 18.Skripak JM, Nash SD, Rowley Het al. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow's milk allergy. J Allergy Clin Immunol. 2008; 122:1154–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narisety SD, Skripak JM, Steele Pet al. Open-label maintenance after milk oral immunotherapy for IgE-mediated cow's milk allergy. J Allergy Clin Immunol. 2009; 124:610–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martorell Calatayud C, Muriel Garcia A, Martorell Aragonés Aet al. Safety and efficacy profile and immunological changes associated with oral immunotherapy for IgE-mediated cow's milk allergy in children: systematic review and meta-analysis. J Investig Allergol Clin Immunol. 2014; 24:298–307. [PubMed] [Google Scholar]
- 21.Esmaeilzadeh H, Alyasin S, Haghighat Met al. The effect of baked milk on accelerating unheated cow's milk tolerance: a control randomized clinical trial. Pediatr Allergy Immunol. 2018; 29:747–753. [DOI] [PubMed] [Google Scholar]
- 22.Jones SM, Burks AW, Keet C, Vickery BP, Scurlock AM, Wood RAet al. Long-term treatment with egg oral immunotherapy enhances sustained unresponsiveness that persists after cessation of therapy. J Allergy Clin Immunol. 2016; 137:1117–1127.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bird JA, Clark A, Dougherty Iet al. Baked egg oral immunotherapy desensitizes baked egg allergic children to lightly cooked egg. J Allergy Clin Immunol Pract. 2019; 7:667–669.e4. [DOI] [PubMed] [Google Scholar]
- 24.Bégin P, Winterroth LC, Dominguez Tet al. Safety and feasibility of oral immunotherapy to multiple allergens for food allergy. Allergy Asthma Clin Immunol. 2014; 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bégin P, Dominguez T, Wilson SPet al. Phase 1 results of safety and tolerability in a rush oral immunotherapy protocol to multiple foods using omalizumab. Allergy Asthma Clin Immunol. 2014; 10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen M, Zhang W, Lee Let al. Biologic therapy for food allergy. J Food Allergy. 2020; 2:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Virkud YV, Burks AW, Steele PHet al. Novel baseline predictors of adverse events during oral immunotherapy in children with peanut allergy. J Allergy Clin Immunol. 2017; 139:882–888.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lucendo AJ, Arias A, Tenias JM.. Relation between eosinophilic esophagitis and oral immunotherapy for food allergy: a systematic review and meta-analysis. Ann Allergy Asthma Immunol. 2014; 113:624–629. [DOI] [PubMed] [Google Scholar]
- 29.Aimmune Therapeutics, Inc. PALFORZIA [Peanut (Arachis hypogaea) Allergen Powder-dnfp] [package insert]. U.S. Food and Drug Administration website. https://www.fda.gov/media/134838/download. Revised January 2020. Accessed February 2020. [Google Scholar]
- 30.Shreffler W. Pathophysiology of IgE-mediated food allergy. J Food Allergy. 2020; 2:7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]