Abstract
Introduction
Although terlipressin is known to cause bradycardia, this adverse effect is usually described in association with hypertension and is considered a benign compensatory response mediated by arterial baroreceptors. Cardiac monitoring for patients receiving terlipressin is not routinely recommended.
Case Presentation
A 77-year-old female patient with no history of coronary artery disease and no other coexisting risk factors for cardiac arrhythmias or conduction disturbances was admitted to intensive care unit with severe cholangitis, complicated by variceal bleeding. She developed severe sinus bradycardia following the use of terlipressin, which was associated with significant hypotension that required the infusion of norepinephrine. The bradycardia occurred again when terlipressin therapy was reattempted.
Conclusion
Vasopressin is known to sensitize baroreceptor reflexes by a central mechanism though its actions on V1a receptors in the area postrema, and we speculate that vasopressin analogues such as terlipressin may act in the same manner. That this effect is not widely described in terlipressin safety literature may be due to the overall younger age range of the trial population. This raises the possibility that cardiac monitoring may be warranted for elderly patients receiving terlipressin.
Keywords: Terlipressin, Vasopressin, Bradycardia, Baroreflex, Baroreceptor reflex, Adverse drug event, Drug-related side effects and adverse reactions, Case report, Pharmacological intervention
Introduction
Vasoconstrictive drugs have a significant role in managing two major complications of hepatic disease: portal hypertensive bleeding such as bleeding oesophageal varices (BOV) and hepatorenal syndrome (HRS). One such drug is terlipressin, a vasopressin analogue which is a non-selective agonist of V1 and V2 receptors [1, 2]. The prodrug terlipressin undergoes enzymatic cleavage in the endothelium to release the active drug, lysine vasopressin, over 4–6 h. The usual therapeutic dose is 1 mg–2 mg of terlipressin acetate (equivalent to 0.85 mg–1.7 mg of terlipressin base) q4-6h [1, 2]. Its pharmacological effects are mediated through the V1a receptors which produce vasoconstriction by their effect on arterial smooth muscle of the splanchnic, portal, and systemic vasculature. This has the potential to increase mean arterial pressure (MAP), while the heart rate decreases – an effect that is attributed to the normal activity of the baroreceptor reflex [2–4].
Besides bradycardia and hypertension, other side effects identified in the literature include abdominal pain, diarrhoea, hyponatraemia, cardiac ischaemia, arrhythmia, stroke, seizures, and severe respiratory failure [1, 2, 5]. Most unwanted effects occurring in clinical practice are deemed reversible and mild to moderate in severity [4, 6]. Mortality and withdrawal of terlipressin therapy due to adverse effects have previously been reported in less than 1% of patients with BOV and/or HRS [6].
Terlipressin use in HRS (with therapeutic response as a predictor) has been positively associated with survival at 15 days and at 180 days after drug initiation, across multiple studies [7]. Compared to no vasoactive agent, terlipressin use in patients with active variceal bleeding significantly decreased in-hospital mortality, with no effect on mortality within 42 days [4]. Hence, terlipressin has been used internationally for decades in the treatment of HRS and acute BOV, with emerging indications including acute liver failure, septic shock, refractory ascites, and perioperatively during liver transplant and hepatobiliary surgeries [1]. Its safety profile is perceived by many clinicians as relatively benign, with many patients receiving therapy on general hospital wards without close haemodynamic monitoring. We present the first case of a patient who received terlipressin therapy for BOV and subsequently developed life-threatening bradycardia, without hypertension, requiring terlipressin cessation and chronotropic support. The CARE Checklist has been completed by the authors for this case report, attached as online supplementary material (for all online suppl. material, see https://doi.org/10.1159/000539439).
Case Presentation
A 77-year-old female presented to the Emergency Department (ED) of Westmead Hospital (Australia) with constant pain in the right upper quadrant and epigastrium, hypotension, tachypnoea, and fever to approximately 38°C. She had a background of primary sclerosing cholangitis with liver cirrhosis, essential tremor, and right neck of femur fracture requiring hip replacement in 2018. She did not smoke nor drink alcohol and was usually independent with daily activities. She did not have any history of ischaemic heart disease. Her medications included esomeprazole 20 mg daily, domperidone 10 mg BD, prochlorperazine 5 mg daily PRN, propranolol 10 mg daily or PRN (for tremor), spironolactone 25 mg mane, and ursodeoxycholic acid 1 g nocte. The patient had recently finished treatment for Klebsiella pneumoniae bacteraemia attributed to cholangitis, with a 7-day course of intravenous (IV) ceftriaxone followed by 7 days of oral amoxicillin/clavulanic acid (guided by drug sensitivity testing).
On examination, she was saturating 97% on room air, appeared clinically dehydrated, and had a systolic blood pressure 100 mm Hg and a heart rate of 100 beats per min. Her Glasgow Coma Score was 14–15 and she appeared jaundiced. Investigations revealed a lactate of 5.5 mmol/L, pO2 83 mm Hg, pCO2 26 mm Hg, bicarbonate 16 mmol/L, standard base excess −7.5 mmol/L, and serum creatinine 129 µmol/L (from baseline of 67 µmol/L). Total bilirubin had increased to 98 µmol/L from 26 µmol/L several weeks previously, international normalised ratio was 1.4 with no history of anticoagulant medication, white cell count was 1.0 × 109/L, C-reactive protein was 48 mg/L and procalcitonin 7.45 μg/L. An abdominal computed tomography scan with contrast demonstrated enhancement of the common bile duct wall, supporting a diagnosis of moderate acute cholangitis.
The patient did not appreciably respond to 3 L of fluid resuscitation in ED, metaraminol infusion, gentamicin 6.5 mg/kg of actual body weight, and initiation of IV piperacillin 4 g/tazobactam 0.5 g QID. As the systolic blood pressured deteriorated to 70–80 mm Hg and lactate minimally improved, the patient was transitioned to a norepinephrine infusion and subsequently transferred to the intensive care unit (ICU). On admission to the ICU, norepinephrine was continued with the additions of vasopressin and albumin to target a MAP of 65 mm Hg.
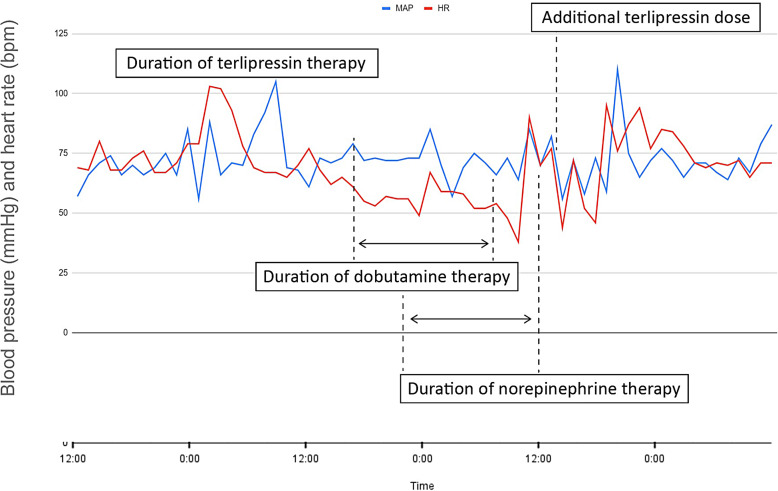
During the initial 2 days of the patient’s stay in the ICU, there was little clinical improvement and antibiotic therapy had been escalated empirically to IV meropenem 1 g TDS following the finding of Gram negative rods in the blood culture. This organism was subsequently identified as K. pneumoniae with similar sensitivity spectrum to the previous positive blood cultures, and the antibiotics were deescalated. The patient’s condition gradually improved, and vasopressor support was discontinued within 5 days. She had remained in the ICU awaiting an endoscopic retrograde cholangiopancreatography to investigate the CT finding of increased intrahepatic biliary tree dilatation, when her stay was further complicated by melaena and haematemesis associated with hypotension and tachycardia. The haemoglobin was observed to drop from 96 g/L to 74 g/L, and further to 66 g/L despite the transfusion of 500 mL (2 units) of packed red blood cells. An emergency gastroscopy was performed in the ICU, which revealed large oesophageal varices, and three variceal bands were placed with incomplete control of the bleeding. To reduce the risk of post-gastroscopy rebleeding the patient was commenced on an infusion of pantoprazole (8 mg/h) and IV terlipressin (1 mg six times a day, or 76 μg/kg/day in six divided doses). Shortly after the first dose of terlipressin, the patient’s heart rate started to decline. The nadir heart rate (38 bpm) was observed after 24 h of terlipressin therapy, with a corresponding MAP of 50 mm Hg, at which stage an infusion of norepinephrine was commenced to maintain a MAP of 65 mm Hg. At this stage, chronotrope therapy was commenced (initially boluses of IV glycopyrronium bromide 200 μg, followed by an infusion of dobutamine which was later escalated to isoproterenol). At no stage was the patient hypertensive. Figure 1 illustrates the patient’s heart rate and MAP over time with the duration of terlipressin therapy. Clinical findings documented by the attending medical staff reported that the patient was euvolemic on physical examination.
Fig. 1.
Patient vital signs and cardiovascular support during terlipressin therapy.
Terlipressin therapy was considered as a potential differential for the bradycardia, and terlipressin was discontinued. The patient’s axillary temperature ranged from 35.4°C to 36.7°C. Alternative explanations were sought, including endocrine dysfunction and myocardial infarction. These were excluded with ECG (sinus bradycardia with no ischaemic changes), two serial high sensitivity troponin measurements (8 and 9 ng/L, respectively), thyroid stimulating hormone (TSH, 1.77 mIU/L), free T4 (12.4 pmol/L), and random serum cortisol (359 nmol/L). Electrolytes during this period were within normal laboratory range (sodium 144 mmol/L, potassium 4.1 mmol/L, magnesium 0.85 mmol/L, corrected calcium 2.30 mmol/L). Transthoracic echocardiography did not reveal any significant abnormalities except for mildly increased left ventricular wall thickness and left atrial dilatation. The left ventricular ejection fraction was 64%, which needs to be considered in the context of concurrent dobutamine infusion.
When the haemodynamic disturbance had resolved and vasopressor support had been discontinued (approximately 10 h after the last dose of terlipressin), an attempt to continue terlipressin therapy had again resulted in clinically significant bradycardia with a nadir of 38 bpm. This bradycardia had persisted despite the terlipressin dose being halved (0.43 mg q6h for another four doses), and terlipressin therapy was discontinued.
On agreement between the gastroenterology and ICU clinical teams, terlipressin therapy was not re-commenced for the rest of the patient’s admission. An ultrasound of the abdomen revealed a patent portal vein of normal calibre (13 mm) with normal directional flow. She was discharged from the ICU and remained a hospital inpatient with stable vital signs and without any further episodes of melaena or haematemesis. Carvedilol (3.125 mg BD) was commenced in the ward setting without continuous cardiac monitoring, and the patient tolerated this well, with stable vital signs and no further episodes of bradycardia.
Discussion
Terlipressin-induced bradycardia is a well-documented side effect of terlipressin therapy [1]. The need to discontinue terlipressin therapy due to bradycardia is rare. Bradycardia down to rates of 50 [8] and 40 [9] are reported as a minor side effect that did not require the cessation of terlipressin therapy in either of the referenced studies. In more severe cases, one alternative may be to trial continuous infusion at a lower dose which confers therapeutic response and, compared to bolus dosing, has been associated with significantly fewer adverse effects including incidence of severe arrhythmias such as bradycardia [10].
Studies of the haemodynamic effects of terlipressin have demonstrated a small but statistically significant effect on heart rate [11]. In most patients, the change in heart rate (less than 10%) usually has little clinical significance and the use of terlipressin is thought to be safe even with concomitant use of non-selective β-blockers [12], with more recent works recommending that “in patients without pre-existing cardiac disease, terlipressin seems safe and extensive cardiac monitoring is not necessary” [3]. A part of this impression is likely related to the increase in peripheral vascular resistance and blood pressure. Though cardiac output and cardiac index are found to be decreased with terlipressin therapy, MAP is usually increased, leading to the classical interpretation of terlipressin-associated bradycardia – i.e., that it can be attributed to a reflex baroreceptor response [3]. However, in this case report we demonstrate bradycardia which was reproducibly associated with the use of terlipressin and which was not associated with an increase in blood pressure.
A possible explanation for this phenomenon is an increased central sensitivity to afferent baroreceptor stimuli. Animal studies have suggested that vasopressin sensitises the baroreceptors by acting on V1a receptors in the area postrema [13–15]. Microinjection of vasopressin into the area postrema of male Sprague Dawley rats reliably increased the baroreceptor responses to small increments of blood pressure produced by phenylephrine [15], and injections of vasopressin into the fourth ventricle of dogs produced marked bradycardia [16]. Systemic administration of vasopressin produced increased sympathetic inhibition by baroreceptors in normal animals but not in those whose area postrema was lesioned [17]. Mutant mice lacking in the V1a vasopressin receptors were found to have a consistently lower resting blood pressure and markedly impaired baroreceptor reflex responses to changes in blood pressure, suggesting an important role for vasopressin in the regulation of baroreflex sensitivity [14]. Vasopressin analogues, especially those with high affinity for V1 receptors, may have a similar effect.
Though terlipressin-associated bradycardia is dismissed as a trivial side effect in studies of terlipressin therapy, many patients enrolled into such trials have alcoholic liver disease and are of an age group younger than the patient presented in this case study. To illustrate, a systematic review of trials by Zhou et al. [4] included 3,344 patients with a median age of 55 [18]. Age-related changes in baroreflex sensitivity may result in an increased variability in blood pressure, with impaired central afferent-efferent coupling implicated as one possible mechanism [19]. However, the change is usually in the direction of decreased baroreflex sensitivity [20]. Further research on the influence of age on the effects of vasopressin and its analogues on baroreflex function is warranted.
This is the first case report of terlipressin being associated with significant life-threatening bradycardia in the context of a normal or decreased arterial blood pressure. This observation supports the idea that terlipressin, like vasopressin, can increase the sensitivity of the baroreceptor reflex, and may produce bradycardia without concurrent hypertension, or with a modest elevation of blood pressure [21]. Further studies of this mechanism in humans may yield further insights into the pathophysiology of terlipressin-associated bradycardia. We caution against the use of terlipressin without continuous cardiac monitoring in elderly patients, even with previously normal cardiac function.
Statement of Ethics
A written statement of informed patient consent is available on demand, and the case report was approved for publication by Westmead Hospital Human Research Ethics Committee (Approval No. 2301-06 CR, January 23, 2023). Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This research was performed as part of the employment of the authors; the employer is Western Sydney Area Local Health District.
Author Contributions
A. Yartsev: writing – original draft, conceptualization, and resources. J. Nguyen: writing – review and editing, data curation, and validation.
Funding Statement
This research was performed as part of the employment of the authors; the employer is Western Sydney Area Local Health District.
Data Availability Statement
The data that support the findings of this study are not publicly available due to privacy reasons but are available from the corresponding author upon reasonable request.
Supplementary Material.
References
- 1. Kulkarni AV, Arab JP, Premkumar M, Benítez C, Tirumalige Ravikumar S, Kumar P, et al. Terlipressin has stood the test of time: clinical overview in 2020 and future perspectives. Liver Int. 2020;40(12):2888–905. [DOI] [PubMed] [Google Scholar]
- 2. Glypressin Solution . MIMS Australia. 2020. [cited 04 January 2023]. Available from: https://www.mimsonline.com.au.acs.hcn.com.au/Search/FullPI.aspx?ModuleName=Product%20Info&searchKeyword=glypressin&PreviousPage=∼/Search/QuickSearch.aspx&SearchType=&ID=94940001_2 [Google Scholar]
- 3. Krag A, Bendtsen F, Mortensen C, Henriksen JH, Møller S. Effects of a single terlipressin administration on cardiac function and perfusion in cirrhosis. Eur J Gastroenterol Hepatol. 2010;22(9):1085–92. [DOI] [PubMed] [Google Scholar]
- 4. Zhou X, Tripathi D, Song T, Shao L, Han B, Zhu J, et al. Terlipressin for the treatment of acute variceal bleeding: a systematic review and meta-analysis of randomized controlled trials. Medicine. 2018;97(48):e13437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wong F, Pappas SC, Curry MP, Reddy KR, Rubin RA, Porayko MK, et al. Terlipressin plus albumin for the treatment of type 1 hepatorenal syndrome. N Engl J Med. 2021;384(9):818–28. [DOI] [PubMed] [Google Scholar]
- 6. Krag A, Borup T, Møller S, Bendtsen F. Efficacy and safety of terlipressin in cirrhotic patients with variceal bleeding or hepatorenal syndrome. Adv Ther. 2008;25(11):1105–40. [DOI] [PubMed] [Google Scholar]
- 7. Papaluca T, Gow P. Terlipressin: current and emerging indications in chronic liver disease. J Gastroenterol Hepatol. 2018;33(3):591–8. [DOI] [PubMed] [Google Scholar]
- 8. Pedretti G, Elia G, Calzetti C, Magnani G, Fiaccadori F. Octreotide versus terlypressin in acute variceal hemorrhage in liver cirrhosis Emergency control and prevention of early rebleeding. Clin Investig. 1994;72(9):653–9. [DOI] [PubMed] [Google Scholar]
- 9. Levacher S, Letoumelin P, Pateron D, Blaise M, Lapandry C, Pourriat JL. Early administration of terlipressin plus glyceryl trinitrate to control active upper gastrointestinal bleeding in cirrhotic patients. Lancet. 1995;346(8979):865–8. [DOI] [PubMed] [Google Scholar]
- 10. Cavallin M, Piano S, Romano A, Fasolato S, Frigo AC, Benetti G, et al. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: a randomized controlled study. Hepatology. 2016;63(3):983–92. [DOI] [PubMed] [Google Scholar]
- 11. Møller S, Hansen EF, Becker U, Brinch K, Henriksen JH, Bendtsen F. Central and systemic haemodynamic effects of terlipressin in portal hypertensive patients. Liver. 2000;20(1):51–9. [DOI] [PubMed] [Google Scholar]
- 12. Vachiery F, Moreau R, Gadano A, Yang S, Sogni P, Hadengue A, et al. Hemodynamic and metabolic effects of terlipressin in patients with cirrhosis receiving a nonselective beta-blocker. Dig Dis Sci. 1996;41(9):1722–6. [DOI] [PubMed] [Google Scholar]
- 13. Barrett LK, Singer M, Clapp LH. Vasopressin: mechanisms of action on the vasculature in health and in septic shock. Crit Care Med. 2007;35(1):33–40. [DOI] [PubMed] [Google Scholar]
- 14. Koshimizu TA, Nasa Y, Tanoue A, Oikawa R, Kawahara Y, Kiyono Y, et al. V1a vasopressin receptors maintain normal blood pressure by regulating circulating blood volume and baroreflex sensitivity. Proc Natl Acad Sci USA. 2006;103(20):7807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang X, Abdel-Rahman ARA, Wooles WR. Vasopressin receptors in the area postrema differentially modulate baroreceptor responses in rats. Eur J Pharmacol. 1992;222(1):81–91. [DOI] [PubMed] [Google Scholar]
- 16. Varma S, Jaju BP, Bhargava KP. Mechanism of vasopressin-induced bradycardia in dogs. Circ Res. 1969;24(6):787–92. [DOI] [PubMed] [Google Scholar]
- 17. Peuler JD, Edwards GL, Schmid PG, Johnson AK. Area postrema and differential reflex effects of vasopressin and phenylephrine in rats. Am J Physiol. 1990;258(4 Pt 2):H1255–9. [DOI] [PubMed] [Google Scholar]
- 18. Zhou X, Tripathi D, Song T, Shao L, Han B, Zhu J, et al. Supplementary material [PDF]. Wolters Kluwer Health, Inc.; 2018. [10 January 2023]. Supplementary Table 2. Patient characteristics]. Available from: https://cdn-links.lww.com/permalink/md/c/md_2018_11_09_qi_md-d-18-03719_sdc1.pdf [Google Scholar]
- 19. Monahan KD. Effect of aging on baroreflex function in humans. Am J Physiol Regul Integr Comp Physiol. 2007;293(1):R3–12. [DOI] [PubMed] [Google Scholar]
- 20. James MA, Robinson TG, Panerai RB, Potter JF. Arterial baroreceptor-cardiac reflex sensitivity in the elderly. Hypertension. 1996;28(6):953–60. [DOI] [PubMed] [Google Scholar]
- 21. Kim JW, Kim G, Kim TW, Han W, Kim MS, Jeong CY, et al. Hemodynamic changes following accidental infiltration of a high dose of vasopressin. J Int Med Res. 2020;48(9):0300060520959494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not publicly available due to privacy reasons but are available from the corresponding author upon reasonable request.