Abstract
Background
Splanchnic vein thrombosis is a complication of acute pancreatitis (AP) and is likely often underdiagnosed.
Objectives
We aimed to understand the time course and risk factors of splanchnic vein thrombosis in the early phase of AP.
Methods
A systematic search was conducted using the PRISMA guidelines (PROSPERO registration CRD42022367578). Inclusion criteria were appropriate imaging techniques in adult AP patients, studies that reported splanchnic vein thrombosis data from the early phase, and reliable information on the timing of imaging in relation to the onset of pancreatitis symptoms or hospital admission. The proportion of patients with thrombosis with 95% confidence intervals (CI) was calculated using random‐effects meta‐analyses, and multiple subgroup analyses were performed.
Results
Data from 1951 patients from 14 studies were analyzed. The proportion of patients with splanchnic vein thrombosis within 12 days after symptom onset was 0.13 (CI 0.07–0.23). The occurrence was lowest at 0.06 (CI 0.03–0.1) between 0 and 3 days after symptom onset, and increased fourfold to 0.23 (CI 0.16–0.31) between 3 and 11 days. On hospital admission, the proportion of patients affected was 0.12 (CI 0.02–0.49); it was 0.17 (CI 0.03–0.58) 1–5 days after admission. The prevalence in mild, moderate, and severe AP was 0.15 (CI 0.05–0.36), 0.26 (CI 0.15–0.43), and 0.27 (CI 0.17–0.4), respectively. Alcoholic etiology (0.31, CI 0.13–0.58) and pancreatic necrosis (0.55, CI 0.29–0.78, necrosis above 30%) correlated with increased SVT prevalence.
Conclusion
The risk of developing splanchnic vein thrombosis is significant in the early stages of AP and may affect up to a quarter of patients. Alcoholic etiology, pancreatic necrosis, and severity may increase the prevalence of splanchnic vein thrombosis.
Keywords: portal vein thrombosis, portosplenomesenteric venous thrombosis, splenic vein thrombosis, superior mesenteric vein thrombosis
Key summary.
Established knowledge on this subject:
Splanchnic vein thrombosis is a local complication of acute pancreatitis (AP) and is associated with worse patient outcomes.
There are important risk factors for thrombosis development, which include pancreatic necrosis, severity, and alcoholic etiology of pancreatitis.
Significant and/or new findings of this study:
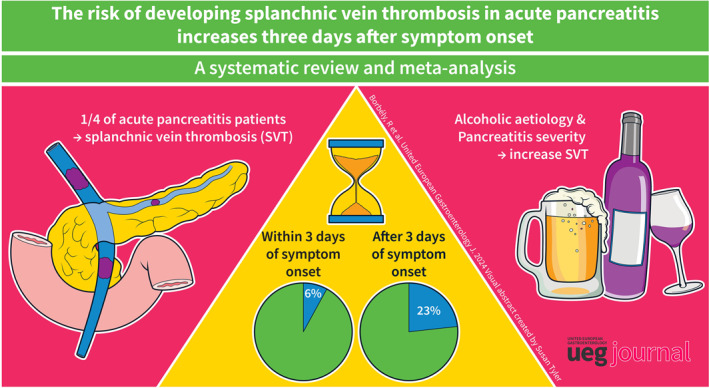
Splanchnic vein thrombosis affects up to one in four patients in the early phase of AP.
Thrombosis development takes 3 days after the onset of pancreatitis symptoms.
INTRODUCTION
Acute pancreatitis (AP) is a major gastrointestinal condition that often requires hospital admission; in its severe form, the mortality rate can reach 30%. 1 , 2 Disease development is confounded by potential local complications, of which splanchnic vein thrombosis (SVT) is a major one. SVT is associated with worse patient outcomes and may lead to further complications such as portal hypertension, gastrointestinal bleeding, and mesenteric ischemia. 3 , 4
SVT impacts the veins of the gastrointestinal system, specifically the splenic vein (SV), portal vein (PV), and superior mesenteric vein (SMV). The development of SVT is attributed to the proximity of inflammatory processes in the pancreatic region, which can affect the coagulation system and the compression due to the mass effect. 5 , 6 , 7 , 8 , 9
The early phase of AP is defined as the first week after symptom onset; the underlying pathogenesis of this phase may continue in the second week. 10 , 11 The diagnosis of SVT is based on imaging; however, in uncomplicated cases, current imaging protocols recommend the use of advanced imaging techniques such CT or MRI 48–72 h after symptom onset only in specific scenarios, such as diagnostic uncertainty or rapid deterioration of the clinical state. 12 In addition, the diagnostic value of ultrasound (US) is limited in these initial days. 13 These factors reduce the reporting of SVT in the initial days of AP. Our objective was to investigate the timeline of SVT development in the early phase of AP, despite challenges in detection and resulting scarcity of data. 14
Our hypotheses were threefold: first, that SVT is prevalent in the early phase; second, that its development has a time course; and third, that identifiable risk factors contribute to its occurrence. Our goal was to fill these research gaps, 14 , 15 , 16 as our investigation may serve as a cornerstone for the development of anticoagulation guidelines for AP patients with SVT, which are currently lacking 14 , 16 , 17 , 18 , 19 ; only nonspecific guidelines are available for the acutely ill. 20
MATERIALS AND METHODS
Protocol
Our systematic review and meta‐analysis followed both the recommendations of the PRISMA 2020 guidelines 21 (Supplementary Table S1) and the Cochrane Handbook. 22 Our study protocol was prospectively registered in PROSPERO, 23 CRD42022367578. We adhered to the protocol and made no amendments.
Eligibility criteria
Using the CoCoPop (Condition, Context, Population) framework, 24 we included cohort studies that provided data on SVT prevalence in adult patients diagnosed with AP by early‐phase imaging. Studies that reported data from the first week following symptom onset or hospital admission were included. Studies in all languages and publication years were considered; no additional filters were considered.
We excluded studies investigating pediatric patients and those involving participants with an active or recent history of malignancy or surgery. Conference abstracts, case reports, and case series were excluded.
Information sources, search strategy
A systematic search was conducted on 27.10.2022 in four databases: MEDLINE (via PubMed), Embase, Cochrane (CENTRAL), and Scopus. The search key can be found in Supplementary Table S2.
Selection process
We managed records using EndNote 25 ; after removing duplicates, we conducted the selection on Rayyan. 26 Two independent review authors (RB and BG) performed the selection, with an independent senior author (NF) responsible for resolving selection disagreements. We first performed the title and abstract selection and then screened the studies by full text. Cohen's kappa was calculated for each step to demonstrate the level of inter‐rater agreement.
Forward and backward citation chasing was employed with the citation chaser tool. 27
Early phase of AP
As there is no universal definition of the early phase of AP, with articles describing it as the first one to 2 weeks after symptom onset, 10 , 11 we considered studies that reported an SVT diagnostic interval that overlapped the first week of either symptom onset or hospital admission.
Pooled prevalence of SVT
To calculate the pooled prevalence of SVT, we included data from studies reporting timelines from both symptom onset and hospital admission. Symptom onset invariably precedes hospital admission, and empirical evidence suggests that approximately three‐quarters of patients are admitted within the first two to three days following symptom onset. 28 , 29 , 30 However, a notable proportion, around one‐quarter of patients, exhibits a delayed admission pattern, extending to a week 28 or more. 31 According to these data, we estimated that the majority of patients had symptom onset maximum a week before admission, and data reported from 0–12 days after symptom onset and from 0 to 5 days after admission were considered to cover the same disease period and pooled together. This adjusted pooling was performed exclusively for this particular analysis.
Study risk of bias assessment and level of evidence
The Joanna Briggs Institute Critical Appraisal Checklist for Prevalence Studies 32 was used to evaluate the risk of bias in the identified studies, and the assessment outcomes were visually represented. The evaluation was conducted independently by two researchers (RB and BG); any disagreements were resolved by consensus and consultation with the senior review author (NF). A study was considered to have an acceptable risk of bias if it had two or fewer “No" answers in the checklist. Studies with a higher risk of bias were excluded. We assessed the level of evidence for each outcome using the GRADEpro tool, 33 following the guidelines of the GRADE Handbook. 34
Statistical analysis
The proportion with a 95% confidence interval (CI) was used for the effect size measure. To calculate prevalence, the total number of patients and those with the event of interest were extracted from each study. We used a random‐effects model to pool effect sizes. “Classical 2‐level” meta‐analyses were performed to pool different studies. If more results were available in separate categories in the same article (subgroups), a “3‐level” model was used. 35
All statistical analyses were conducted with R, 36 using the meta 37 package for basic meta‐analysis calculations and plots, metafor 38 for 3‐level models, and dmetar 39 package for additional influential analysis calculations and plots.
For more details on data collection, calculations, data synthesis, publication bias assessment, and influential analyses, see Supporting Information S1.
RESULTS
Search and selection process
A total of 12,496 studies were identified by searching the relevant databases. Twelve studies were retrieved through a systematic search 3 , 5 , 6 , 7 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 and three studies via citation chasing, 48 , 49 , 50 resulting in 15 eligible studies included in the review. One article was excluded from the meta‐analysis as it had a substantial risk of bias. 7 Details of the selection process with Cohen's kappa values calculated are summarized in a PRISMA flow diagram, shown in Figure 1.
FIGURE 1.
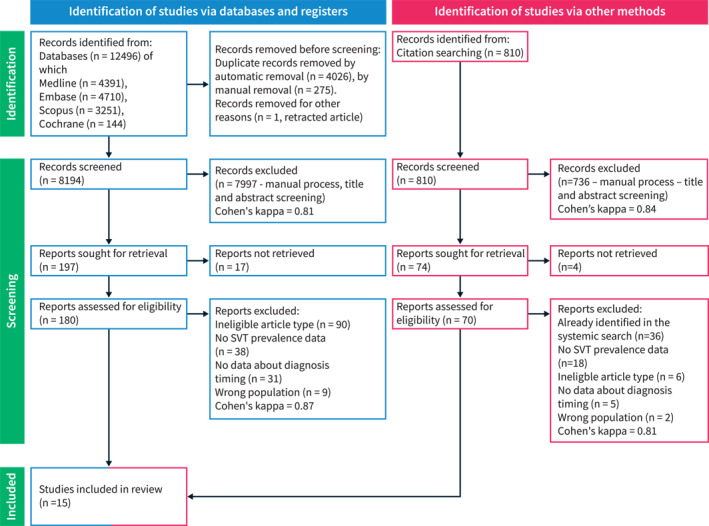
PRISMA 2020 flow diagram of the study screening process. SVT, splanchnic vein thrombosis.
Basic characteristics of studies included
The baseline characteristics of the studies included in this analysis are presented in Table 1. Six out of 14 studies were identified as prospective, 41 , 43 , 45 , 46 , 48 , 49 while eight were characterized as retrospective cohorts. 3 , 5 , 6 , 40 , 42 , 44 , 47 , 50 One prospective study was included only for the systematic review. 7
TABLE 1.
Basic characteristics of the studies included in the systematic review and meta‐analysis.
| Author | Year | Country | Study design | Number of patients | Mean age (years) | Gender distribution (%) | AP etiology distribution (%) | AP severity distribution (%) | Imaging relative to AP symptom onset (days) | Imaging modality | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Alcoholic | Biliary | Other | Mild | Moderate | Severe | ||||||||
| Ahmed et al. | 2018 | India | Prospective | 96 | 31.9 | 24 | 76 | 46 | 32 | 22 | 5 | 36 | 58 | 5–7 | CT, US |
| Alberti et al. | 2020 | Spain | Retrospective | 149 | 18 a | 38 | 62 | 17 | 50 | 32 | 37 | 45 | 18 | 2–3 | CT |
| Banday et al. | 2015 | India | Prospective | 50 | 42.3 | 34 | 66 | ‐ | ‐ | ‐ | 18 | 38 | 44 | 0–8 c | CT |
| Ding et al. | 2018 | China | Retrospective | 140 | 54.6 | 52 | 48 | 6 | 64 | 31 | 0 | 0 | 100 d | 3–10 | CT |
| Dorffel et al. | 2000 | Germany | Prospective | 189 | ‐ | 26 | 74 | 54 | 32 | 14 | ‐ | ‐ | ‐ | 3–56 c | CT, US |
| Fei et al. | 2017 | China | Retrospective | 72 | 20–70 a | 46 | 54 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 1–10 c | US |
| Gonzelez et al. e | 2011 | United Kingdom | Prospective | 28 | ‐ | ‐ | ‐ | ‐ | 0 | 0 | 100 | 0–8 c | CT | ||
| Nawacki et al. | 2021 | Poland | Retrospective | 111 | 18–79 a | 23 | 77 | 58 | 29 | 14 | 10 | 59 | 32 | 2–10 c | CT |
| Raghuwanshi et al. | 2016 | India | Prospective | 50 | ‐ | 30 | 70 | 37 | 38 | 25 | 42 | 24 | 34 | 0–8 c | CT |
| Sahu et al. | 2017 | India | Prospective | 60 | 36.6 | 40 | 60 | 50 | 25 | 25 | 43 | 20 | 37 | 5–11 | CT |
| Stimac et al. | 2007 | Croatia | Prospective | 101 | 62 | 51 | 49 | 12 | 63 | 25 | ‐ | ‐ | 34 d | 3–12 c | CT, MRI |
| Taydas et al. | 2018 | Turkey | Retrospective | 189 | 21‐93 a | 4 | 61 | 35 | ‐ | ‐ | ‐ | 0–3 | CT | ||
| Thejasvin et al. | 2022 | United Kingdom | Retrospective | 401 | 57 | 40 | 60 | 35 | 38 | 27 | 16 | 53 | 31 | 0–8 c | CT |
| Toque et al. | 2015 | France | Retrospective | 318 | 57 b | 39 | 61 | 25 | 49 | 26 | ‐ | ‐ | ‐ | 1–61 | CT |
| Tsushima et al. | 1999 | Japan | Retrospective | 25 | 53.4 | 28 | 72 | 48 | 24 | 28 | ‐ | ‐ | ‐ | 0–3 | CT |
Note: Percentages in the table are rounded to the nearest whole number, while mean age is rounded to the first decimal.
Abbreviations: AP, acute pancreatitis; AP severity, according to 2012 Revised Atlanta Criteria; US, ultrasound;.
Range.
Median.
Estimated.
AP severity according to Atlanta Criteria.
Study included only in the systematic review.
The risk of developing SVT increases over time in AP and can affect up to one in four patients
The proportion of patients who developed SVT in the early phase of AP (within 11 days after symptom onset or 5 days after admission) is 0.13 (CI 0.07–0.23), details are shown in Figure 2. Subgroup analysis revealed that the occurrence was lowest 0–3 days after symptom onset at 0.06 (CI 0.03–0.10); it increased to 0.23 (CI 0.16–0.31) between 3 and 11 days. On the day of hospital admission, the prevalence was 0.12 (CI 0.02–0.49) and increased to 0.17 (CI 0.03–0.58) 1–5 days after admission; see Figure 3a,b.
FIGURE 2.
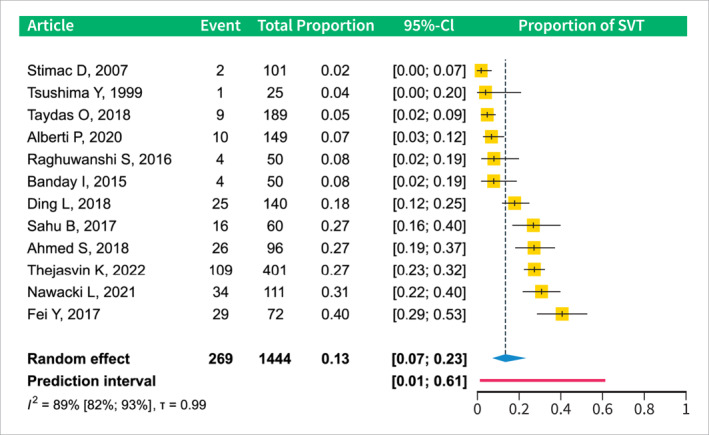
Forest plot of the analysis of the pooled prevalence of SVT in the early phase of AP (within 12 days of symptom onset). AP, acute pancreatitis; CI, confidence interval; SVT, splanchnic vein thrombosis.
FIGURE 3.
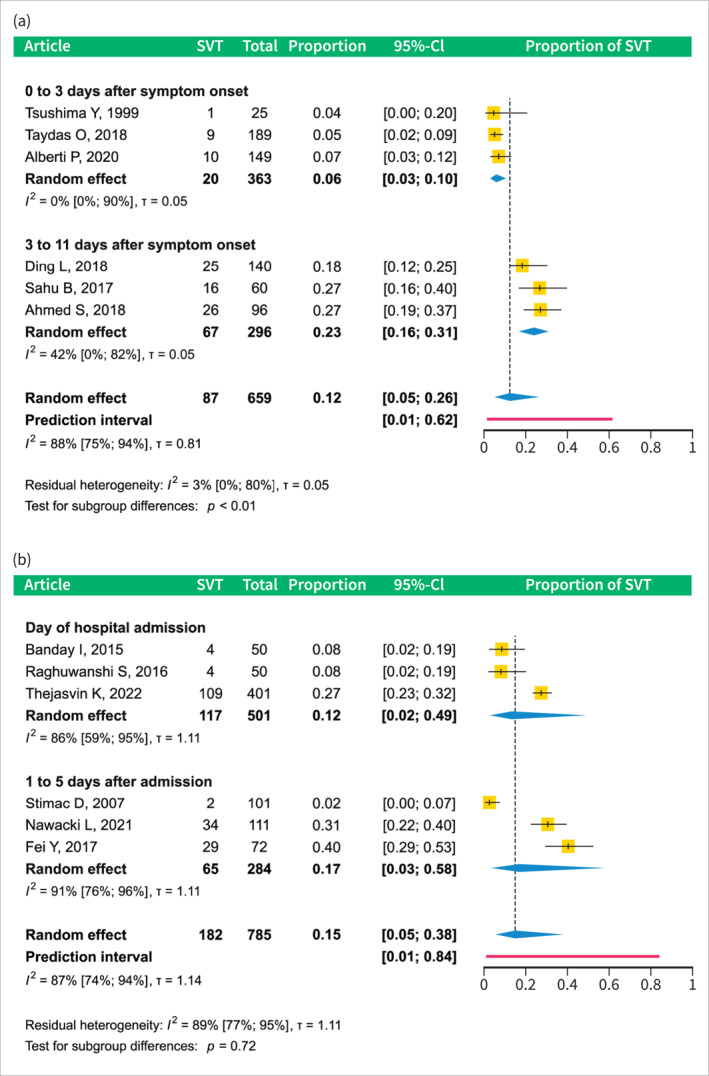
Subgroup analyses by SVT diagnosis timing in studies reporting SVT prevalence in the early phase of AP. (a) subgroup analysis of studies reporting SVT data relative to symptom onset. (b) subgroup analysis of studies reporting SVT data relative to the hospital admission. AP, acute pancreatitis; CI, confidence interval; SVT, splanchnic vein thrombosis.
Alcoholic etiology, pancreatic necrosis, and severity are associated with SVT development
Subgroup analysis was conducted to examine disease factors influencing the incidence of SVT, with prevalence calculated for each factor. Subgroup analysis by disease severity showed SVT occurrence as follows: mild patients had a prevalence of 0.15 (CI 0.05–0.36), moderate patients 0.26 (CI 0.15–0.43), and severe patients 0.27 (CI 0.17–0.4). These data suggest that the risk of SVT increases with increasing disease severity, see Supplementary Figure S1.
The etiology of AP also showed a correlation with SVT occurrence. The alcoholic AP group was the most affected, with a prevalence of 0.31 (CI 0.13–0.58), whereas the biliary group had a lower prevalence of 0.12 (CI 0.04–0.3). The difference between these subgroups was statistically significant; see Figure 4.
FIGURE 4.
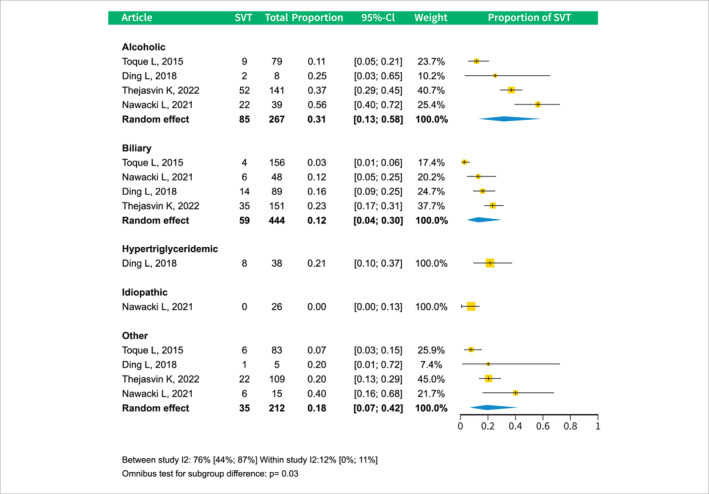
Forest plot of subgroup analysis of SVT prevalence by different AP etiologies. AP, acute pancreatitis; CI, confidence interval; SVT, splanchnic vein thrombosis.
The relationship between pancreatic necrosis and SVT occurrence was evaluated in two separate analyses, as the studies reported different necrosis categories. Both analyses demonstrated a significant and dose‐dependent association between the extent of necrosis and the incidence of SVT. In the first analysis, the prevalence was as follows: absent necrosis 0.11 (CI 0.05–0.25), <30% necrosis 0.25 (CI 0.11–0.47), and >30% necrosis 0.5 (CI 0.29–0.72). The second analysis yielded the following proportions: absence of necrosis 0.09 (CI 0.06–0.15), <50% necrosis 0.29 (CI 0.22–0.37), and >50% necrosis 0.46 (CI 0.36–0.56); see Figures 5a,b.
FIGURE 5.
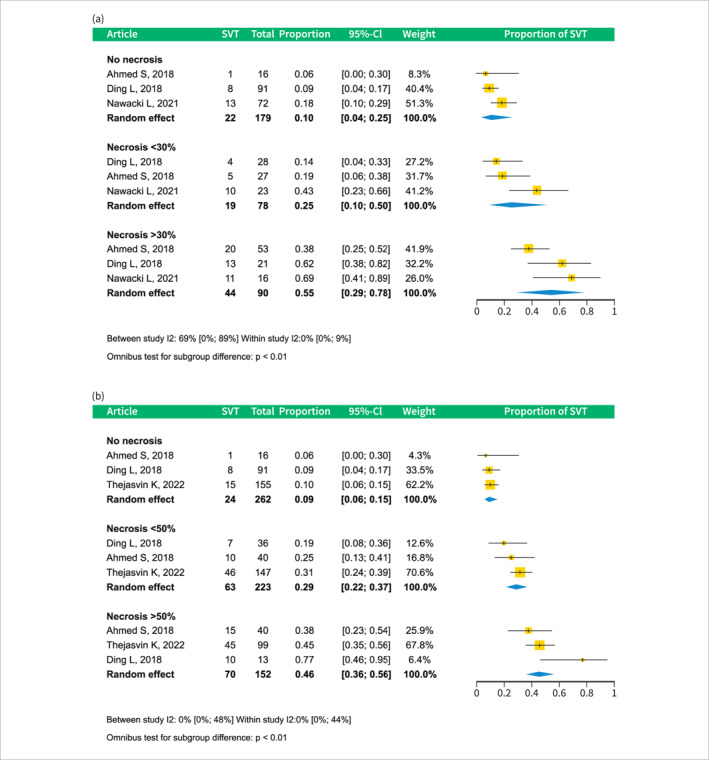
Forest plots of subgroup analyses of SVT prevalence by different amounts of pancreatic necrosis. A: subgroup analysis of studies reporting SVT prevalence by necrosis categories – necrosis absent, necrosis less than 30%, and more than 30%. (a): subgroup analysis of studies reporting SVT prevalence by necrosis categories – necrosis absent, necrosis less than 50%, and more than 50%. CI, confidence interval; SVT, splanchnic vein thrombosis.
Thrombosis rate in splenic (SV), portal (PV), and superior mesenteric veins (SMV)
The proportion of thrombosed veins in patients with SVT was as follows: SV 0.58 (CI 0.44–0.71), PV 0.43 (CI 0.3–0.56), and SMV 0.23 (CI 0.14–0.36); the sum was greater than one because some patients had multiple thrombosed vessels. The proportion of patients with a single vessel and specific combinations is shown in Supplementary Figures S2 and S3.
Gender
We found that the proportion of females affected was 0.09 and 0.16 for males; statistical significance was not reached; see Supplementary Figure S4.
Mortality, length of hospital stay
Because of methodological differences between the studies, we were unable to conduct a meta‐analysis for hospital stay and mortality outcomes. The data collected can be found in Supplementary Tables S3 and S4.
Risk of bias assessment, level of evidence
Of the 15 studies identified in the search and selection process, 14 were deemed suitable for the meta‐analysis, 3 , 5 , 6 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 and one was considered high risk 7 leading to exclusion. In general, the most significant source of bias was due to inadequate study population size, as most studies did not report sufficient patient numbers to accurately determine the prevalence of a condition estimated to have a prevalence rate of 15%. 32 , 51 Figure 6. The level of evidence ranged from very low to moderate; see Supplementary Table S5.
FIGURE 6.
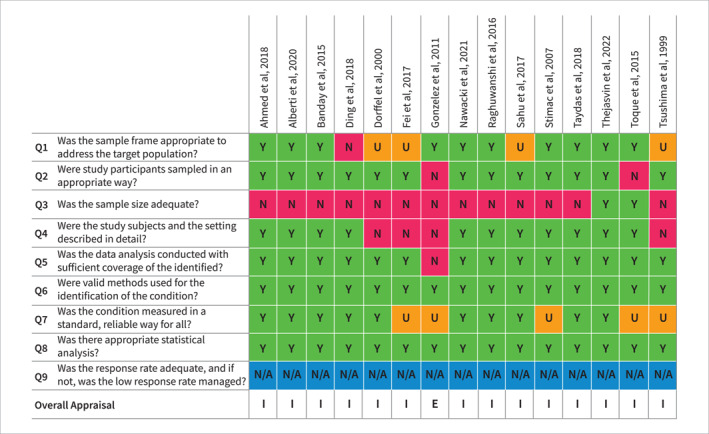
Risk of bias assessment according to the Joanna Briggs Institute Critical Appraisal Checklist for Studies Reporting Prevalence Data. E, exclude; I, include; N, no; N/A, not applicable; U, unclear; Y, yes.
Publication bias
In the overall SVT prevalence analysis, we found no significant publication bias based on visual inspection of the funnel plot and the fact that the Egger's test p‐value exceeded 0.1. Visual inspection of funnel plots and Begg's test for the prevalence subgroup analyses for severity, etiology, necrosis, gender, and affected veins found no evidence for publication bias. Supplementary Figures S5–S14.
Heterogeneity
We found high heterogeneity in most analyses; we report them in Figures with Forest plots.
DISCUSSION
Our investigation focused on the time dependence and risk factors for the development of SVT. We found that patients in the 0–3 days within the symptom onset group were the least affected, and the proportion of SVT rose significantly thereafter, suggesting that its development takes several days. The onset of AP was defined as the onset of abdominal pain. 10 However, patients often do not seek immediate medical help, resulting in a delay in hospital admission. 28 This delay contributes to our findings that the percentage of patients with SVT is higher upon admission compared to that in the 0–3 days within the symptom onset group and does not significantly increase 1–5 days after admission.
Our findings show that 13.2% of patients in the early phase are affected by SVT. One meta‐analysis found that the prevalence of SVT in patients with AP ‐ irrespective of imaging timing ‐ was 16.6%, with differences among the studies by geographical area, for example, European studies having the highest prevalence. 52 In a more recent meta‐analysis focusing on the treatment of SVT, 17% of AP patients were affected by it, and in a subgroup of patients with a first episode of AP, it was 15%. 9 Our meta‐analysis showed minimal overlap with these previous meta‐analyses in terms of studies included: one study was also a part of the analysis published in 2015, 41 two were in the 2022 analysis, 42 , 46 and a fourth study presented in both was included only in our systematic review. 7 Our pooled prevalence is comparable to previous results; the minor difference could be attributed to our selection of early‐phase AP cases, where the prevalence is lower due to the time required for SVT to develop. We synthesized data from studies where all patients underwent imaging, as asymptomatic patients in some studies did not receive imaging. 53
We found that pancreatic necrosis is associated with the development of SVT. The interaction is likely bidirectional in nature: firstly, the inflammatory response and cytokine storm associated with pancreatic necrosis create an environment conducive to SVT development; conversely, the presence of SVT may exacerbate the progression of pancreatitis by impairing circulatory function. Pancreatic necrosis causes inflammation ‐ both locally and systemically ‐ and mass effect; SVT development is fueled mainly by these local effects of necrosis, in particular venous endothelial damage by pancreatic enzymes, leading to the exposure of the tissue factor, impaired vasomotor function and compression from surrounding necrotic tissue, resulting in reduced capillary perfusion and stasis. 5 , 6 , 7 , 8 , 53 , 54 , 55 The release of pro‐inflammatory mediators from necrotic tissue also contributes to the systemic inflammatory response, which has been shown to tip the balance of the hemostasis toward a pro‐thrombotic state. 56 Confirming these findings, Roch et al. reported that 50% of necrotizing AP patients presented with SVT, 16% had deep vein thrombosis in the extremities, and 6% had pulmonary embolism. 57 In addition, this inflammation alters the pharmacokinetics and pharmacodynamics of anticoagulants in patients receiving prophylactic anticoagulation therapy, leading to inadequate prophylaxis and, eventually, thrombosis. 8
Furthermore, the impact of necrotizing AP extends beyond inflammation, the known depletion of Beta‐cells and insulin production over the course of necrotizing AP 58 , 59 can also lead to disease deterioration, 60 thus forming a vicious cycle. The presence of SVT might trigger this cycle by damaging circulation 61 and, consequently, the functionality of Beta‐cells.
Our data suggest that alcohol‐induced AP patients may have a higher prevalence of SVT, which could be explained by the higher proportion of severe cases and more frequent necrosis in such patients, 62 as well as the effect of alcohol on the coagulation system. In addition, cirrhosis in the chronic alcoholic population predisposes them to chronic and recurrent SVT. 63 , 64 Our results show that approximately one‐third of patients with alcohol‐induced AP presented with this complication compared with 12% of biliary patients. While there is an inverse relationship between mild‐to‐moderate alcohol consumption and thrombosis development, heavy alcohol consumption has been associated with an increased incidence of thrombotic events 65 , 66 , 67 , 68 and corresponding hemostatic factor changes. 69 Alcohol consumption required to induce an AP episode falls into the category of heavy alcohol consumption. 70 There is some confounding between pancreatic necrosis and alcoholic etiology, as they are known to co‐occur more frequently. 62
The splenic vein is the most affected
We observed that the veins of the splanchnic system were not equally involved in thrombosis. SV was the most frequently affected vein, followed by PV, and the SMV was the least frequently thrombosed vein alone. As local mechanisms play a significant role in SVT development, the proximity of the SV makes it the most exposed, while PV and SMV are more distant. Other studies confirm these findings. 9 , 17
Strengths and limitations
The strengths of our study include that this is the first meta‐analysis to explore this topic, we adhered to the guidelines of our methodology, which was prospectively published in PROSPERO, and we conducted detailed statistical subgroup analyses using data from 1951 patients.
A significant limitation is the substantial heterogeneity of the studies included; we conducted several prespecified subgroup analyses and were able to account for some of this heterogeneity.
The lack of individual patient data is another limitation; aggregate data from published studies limited our ability to control for potential confounders and explore effect modifiers beyond subgroup analyses. Specifically, information concerning preexisting conditions known to be pro‐thrombotic and potentially linked to increased SVT prevalence, such as cirrhosis 63 , 64 or diabetes, 71 , 72 , 73 was not reliably reported in the articles.
Our analyses consisted of pooling univariate data. Nevertheless, some articles in our review conducted supporting multivariate analyses, revealing that alcoholic AP etiology 6 , 42 and pancreatic necrosis 6 independently elevate the risk of SVT development.
Implications for practice and research
This study can be considered translational medicine, with implications for research and practical applications. 74 , 75
The existing guidelines for AP both in the initial days and later than 48–72 h after symptom onset recommend the use of both CT and MRI only in cases of diagnostic uncertainty, rapid deterioration of clinical status, or in critically ill patients. 12 , 76 These are not appropriate for SVT detection, as most hospitalized AP patients do not undergo imaging, and thrombosis may not be detected. 53 Routine CT or MRI imaging in hospitalized AP patients after 48–72 h should be considered to diagnose SVT without delay.
The most pivotal research direction lies in the prevention of SVT development. While certain studies have reported improved outcomes associated with early anticoagulation in AP, 19 , 77 , 78 the underlying mechanism remains to be fully elucidated. Our findings highlight the need for anticoagulation therapy as a routine element of AP therapy. However, additional studies are needed to establish the optimal agent and dosage required to achieve adequate anticoagulation without unnecessarily increasing bleeding complications.
CONCLUSION
The risk of developing Splanchnic Vein Thrombosis (SVT) is significant in AP, affecting up to a quarter of patients. The risk of occurrence increases with time in the early stages of AP. Alcoholic etiology, pancreatic necrosis, and most probably, severity are associated with an increased risk of SVT development in AP. Our findings highlight the need for anticoagulation therapy and advanced imaging (CT, MRI) to become a routine component of AP therapy.
AUTHOR CONTRIBUTION
Ruben Zsolt Borbély: Conceptualization, project administration, methodology, investigation, visualization, writing—original draft. Eszter Ágnes Szalai: Methodology, visualization, supervision, writing—review and editing. Bálint Gellért: Investigation, conceptualization, formal analysis, writing—review and editing. Bryan Mangalath Philip: Conceptualization, investigation, visualization, writing—review and editing. Dalma Dobszai: Conceptualization, writing—review and editing. Brigitta Teutsch: Methodology, supervision, writing—review and editing. Ádám Zolcsák: Formal analysis, data curation, writing—review and editing. Dániel Sándor Veres: Methodology, formal analysis, data curation, writing—review and editing. Bálint Erőss: Methodology, conceptualization, writing—review and editing, supervision. Péter Jenő Hegyi: Methodology, conceptualization, supervision, writing ‐ review and editing. Péter Hegyi: Methodology, conceptualization; supervision; writing—original draft, funding acquisition. Nándor Faluhelyi: Methodology, conceptualization; supervision; writing—original draft, funding acquisition. All authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript.
CONFLICT OF INTEREST STATEMENT
None to declare.
ETHICS APPROVAL
No ethical approval was required for this systematic review with meta‐analysis, as all data were already published in peer‐reviewed journals. No patients were directly involved in the design, conduct, or interpretation of our study.
Supporting information
Supporting Information S1
ACKNOWLEDGMENTS
The research was supported by the Hungarian Ministry of Innovation and Technology, National Research, Development and Innovation Fund (TKP2021‐EGA‐23 to PH), Translational Neuroscience National Laboratory Program (RRF‐2.3.1‐21‐2022‐00011 to PH), a project grant (K131996 to PH) and the Translational Medicine Foundation. Funding for Brigitta Teutsch was provided by the ÚNKP‐22‐3 New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund (to BT ‐ ÚNKP‐22‐3‐I‐PTE‐1693). The funders had no effect on the concept, data collection, analysis, or writing of the manuscript.
Borbély RZ, Szalai EÁ, Philip BM, Dobszai D, Teutsch B, Zolcsák Á, et al. The risk of developing splanchnic vein thrombosis in acute pancreatitis increases 3 days after symptom onset: a systematic review and meta‐analysis. United European Gastroenterol J. 2024;12(6):678–90. 10.1002/ueg2.12550
Péter Hegyi and Nándor Faluhelyi contributed equally.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the supplementary material of this article.
REFERENCES
- 1. Oppenlander KE, Chadwick C, Carman K. Acute pancreatitis: rapid evidence review. Am Fam Physician. 2022;106(1):44–50. [PubMed] [Google Scholar]
- 2. Mederos MA, Reber HA, Girgis MD. Acute pancreatitis: a review. JAMA. 2021;325(4):382–390. 10.1001/jama.2020.20317 [DOI] [PubMed] [Google Scholar]
- 3. Nawacki L, Matykiewicz J, Stochmal E, Gluszek S. Splanchnic vein thrombosis in acute pancreatitis and its consequences. Clin Appl Thromb Hemost. 2021;27:10760296211010260. 10.1177/10760296211010260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou J, Ke L, Tong Z, Li G, Li W, Li N, et al. Risk factors and outcome of splanchnic venous thrombosis in patients with necrotizing acute pancreatitis. Thromb Res. 2015;135(1):68–72. 10.1016/j.thromres.2014.10.021 [DOI] [PubMed] [Google Scholar]
- 5. Ding L, Deng F, Yu C, He WH, Xia L, Zhou M, et al. Portosplenomesenteric vein thrombosis in patients with early‐stage severe acute pancreatitis. World J Gastroenterol. 2018;24(35):4054–4060. 10.3748/wjg.v24.i35.4054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thejasvin K, Chan SJ, Varghese C, Lim WB, Cheemungtoo GM, Akter N, et al. A selective anticoagulation policy for splanchnic vein thrombosis in acute pancreatitis is associated with favourable outcomes: experience from a UK tertiary referral centre. HPB Oxf. 2022;24(11):1937–1943. 10.1016/j.hpb.2022.06.003 [DOI] [PubMed] [Google Scholar]
- 7. Gonzelez HJ, Sahay SJ, Samadi B, Davidson BR, Rahman SH. Splanchnic vein thrombosis in severe acute pancreatitis: a 2‐year, single‐institution experience. HPB Oxf. 2011;13(12):860–864. 10.1111/j.1477-2574.2011.00392.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maatman TK, McGuire SP, Lewellen KA, McGreevy KA, Ceppa EP, House MG, et al. Prospective analysis of the mechanisms underlying ineffective deep vein thrombosis prophylaxis in necrotizing pancreatitis. J Am Coll Surg. 2021;232(1):91–100. 10.1016/j.jamcollsurg.2020.08.774 [DOI] [PubMed] [Google Scholar]
- 9. Anis FS, Adiamah A, Lobo DN, Sanyal S. Incidence and treatment of splanchnic vein thrombosis in patients with acute pancreatitis: a systematic review and meta‐analysis. J Gastroenterol Hepatol. 2022;37(3):446–454. 10.1111/jgh.15711 [DOI] [PubMed] [Google Scholar]
- 10. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis‐‐2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. 10.1136/gutjnl-2012-302779 [DOI] [PubMed] [Google Scholar]
- 11. Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology. 2012;262(3):751–764. 10.1148/radiol.11110947 [DOI] [PubMed] [Google Scholar]
- 12. Expert Panel on Gastrointestinal I, Porter KK, Zaheer A, Kamel IR, Horowitz JM, Arif‐Tiwari H, et al. ACR appropriateness criteria(R) acute pancreatitis. J Am Coll Radiol. 2019;16(11S):S316–S330. 10.1016/j.jacr.2019.05.017 [DOI] [PubMed] [Google Scholar]
- 13. Turkvatan A, Erden A, Turkoglu MA, Secil M, Yener O. Imaging of acute pancreatitis and its complications. Part 1: acute pancreatitis. Diagn Interv Imaging. 2015;96(2):151–160. 10.1016/j.diii.2013.12.017 [DOI] [PubMed] [Google Scholar]
- 14. Sissingh NJ, Groen JV, Timmerhuis HC, Besselink MG, Boekestijn B, Bollen TL, et al. Therapeutic anticoagulation for splanchnic vein thrombosis in acute pancreatitis: a national survey and case‐vignette study. World J Gastroenterol. 2023;29(21):3328–3340. 10.3748/wjg.v29.i21.3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Besselink MG. Splanchnic vein thrombosis complicating severe acute pancreatitis. HPB Oxf. 2011;13(12):831–832. 10.1111/j.1477-2574.2011.00411.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Valeriani E, Riva N, Di Nisio M, Ageno W. Splanchnic vein thrombosis: current perspectives. Vasc Health Risk Manag. 2019;15:449–461. 10.2147/vhrm.s197732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sissingh NJ, Groen JV, Koole D, Klok FA, Boekestijn B, Bollen TL, et al. Therapeutic anticoagulation for splanchnic vein thrombosis in acute pancreatitis: a systematic review and meta‐analysis. Pancreatology. 2022;22(2):235–243. 10.1016/j.pan.2021.12.008 [DOI] [PubMed] [Google Scholar]
- 18. Norton W, Lazaraviciute G, Ramsay G, Kreis I, Ahmed I, Bekheit M. Current practice of anticoagulant in the treatment of splanchnic vein thrombosis secondary to acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2020;19(2):116–121. 10.1016/j.hbpd.2019.12.007 [DOI] [PubMed] [Google Scholar]
- 19. Zhou J, Zhang H, Mao W, Ke L, Li G, Ye B, et al. Efficacy and safety of early systemic anticoagulation for preventing splanchnic thrombosis in acute necrotizing pancreatitis. Pancreas. 2020;49(9):1220–1224. 10.1097/mpa.0000000000001661 [DOI] [PubMed] [Google Scholar]
- 20. Schünemann HJ, Cushman M, Burnett AE, Kahn SR, Beyer‐Westendorf J, Spencer FA, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198–3225. 10.1182/bloodadvances.2018022954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cochrane handbook for systematic reviews of interventions: Cochrane; 2022. www.training.cochrane.org/handbook
- 23. PROSPERO . International prospective register of systematic reviews [Internet]. https://www.crd.york.ac.uk/prospero/ [DOI] [PMC free article] [PubMed]
- 24. Munn Z, Stern C, Aromataris E, Lockwood C, Jordan Z. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med Res Methodol. 2018;18(1):5. 10.1186/s12874-017-0468-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. The EndNote Team . EndNote. EndNote. (20 ed.). Philadelphia: Clarivate; 2013. [Google Scholar]
- 26. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan‐a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haddaway NR, Grainger MJ, Gray CT. citationchaser: an R package for forward and backward citations chasing in academic searching; 2021. [DOI] [PubMed]
- 28. Parniczky A, Kui B, Szentesi A, Balazs A, Szucs A, Mosztbacher D, et al. Prospective, multicentre, nationwide clinical data from 600 cases of acute pancreatitis. PLoS One. 2016;11(10):e0165309. 10.1371/journal.pone.0165309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Phillip V, Schuster T, Hagemes F, Lorenz S, Matheis U, Preinfalk S, et al. Time period from onset of pain to hospital admission and patients' awareness in acute pancreatitis. Pancreas. 2013;42(4):647–654. 10.1097/mpa.0b013e3182714565 [DOI] [PubMed] [Google Scholar]
- 30. Földi M, Gede N, Kiss S, Vincze Á, Bajor J, Szabó I, et al. The characteristics and prognostic role of acute abdominal on‐admission pain in acute pancreatitis: a prospective cohort analysis of 1432 cases. Eur J Pain. 2022;26(3):610–623. 10.1002/ejp.1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mallick B, Dhaka N, Sharma V, Malik S, Sinha SK, Dutta U, et al. Impact of timing of presentation of acute pancreatitis to a tertiary care centre on the outcome. Pancreatology. 2019;19(1):143–148. 10.1016/j.pan.2018.10.005 [DOI] [PubMed] [Google Scholar]
- 32. Munn ZMS, Lisy K, Riitano D, Tufanaru C. Chapter 5: systematic reviews of prevalence and incidence. In: Aromataris EMZ, editor. JBI manual for evidence synthesis. JBI; 2020. [Google Scholar]
- 33. GRADEpro GDT. GRADEpro guideline development tool. [Software]; 2022. McMaster Univ Evid Prime. https://gdt.gradepro.org/app/ [Google Scholar]
- 34. Schünemann HBJ, Guyatt G, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group; 2013. Updated October 2013. [Google Scholar]
- 35. Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing meta‐analysis with R2021.
- 36. 2023 RCT. R. v4.3.0 ed. Vienna: R Foundation for Statistical Computing; 2023. [Google Scholar]
- 37. Schwarzer G. Meta: general package for meta‐analysis. 6.5.0; 2023.
- 38. Viechtbauer W. Metafor: meta‐analysis package for r. 4.2.0. 2023.
- 39. Cuijpers P, Furukawa T, Daniel Ebert D. Dmetar: companion r package for the guide doing meta‐analysis in r. 0.0.9000. 2023.
- 40. Tsushima Y, Tamura T, Tomioka K, Okada C, Kusano S, Endo K. Transient splenomegaly in acute pancreatitis. Br J Radiol. 1999;72(859):637–643. 10.1259/bjr.72.859.10624319 [DOI] [PubMed] [Google Scholar]
- 41. Dorffel T, Wruck T, Ruckert RI, Romaniuk P, Dorffel Q, Wermke W. Vascular complications in acute pancreatitis assessed by color duplex ultrasonography. Pancreas. 2000;21(2):126–133. 10.1097/00006676-200008000-00004 [DOI] [PubMed] [Google Scholar]
- 42. Toque L, Hamy A, Hamel JF, Cesbron E, Hulo P, Robert S, et al. Predictive factors of splanchnic vein thrombosis in acute pancreatitis: a 6‐year single‐center experience. J Dig Dis. 2015;16(12):734–740. 10.1111/1751-2980.12298 [DOI] [PubMed] [Google Scholar]
- 43. Raghuwanshi S, Gupta R, Vyas MM, Sharma R. CT evaluation of acute pancreatitis and its prognostic correlation with CT severity index. J Clin Diagn Res. 2016;10(6):TC06–TC11. 10.7860/jcdr/2016/19849.7934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fei Y, Hu J, Li WQ, Wang W, Zong GQ. Artificial neural networks predict the incidence of portosplenomesenteric venous thrombosis in patients with acute pancreatitis. J Thromb Haemost. 2017;15(3):439–445. 10.1111/jth.13588 [DOI] [PubMed] [Google Scholar]
- 45. Sahu B, Abbey P, Anand R, Kumar A, Tomer S, Malik E. Severity assessment of acute pancreatitis using CT severity index and modified CT severity index: correlation with clinical outcomes and severity grading as per the Revised Atlanta Classification. Indian J Radiol Imaging. 2017;27(2):152–160. 10.4103/ijri.ijri_300_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ahmed SU, Rana SS, Ahluwalia J, Varma N, Sharma R, Gupta R, et al. Role of thrombophilia in splanchnic venous thrombosis in acute pancreatitis. Ann Gastroenterol. 2018;31(3):371–378. 10.20524/aog.2018.0242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Taydas O, Unal E, Karaosmanoglu AD, Onur MR, Akpinar E. Accuracy of early CT findings for predicting disease course in patients with acute pancreatitis. Jpn J Radiol. 2018;36(2):151–158. 10.1007/s11604-017-0709-9 [DOI] [PubMed] [Google Scholar]
- 48. Stimac D, Miletic D, Radic M, Krznaric I, Mazur‐Grbac M, Perkovic D, et al. The role of nonenhanced magnetic resonance imaging in the early assessment of acute pancreatitis. Am J Gastroenterol. 2007;102(5):997–1004. 10.1111/j.1572-0241.2007.01164.x [DOI] [PubMed] [Google Scholar]
- 49. Banday IA, Gattoo I, Khan AM, Javeed J, Gupta G, Latief M. Modified computed tomography severity index for evaluation of acute pancreatitis and its correlation with clinical outcome: a tertiary care hospital based observational study. J Clin Diagn Res. 2015;9(8):TC01–TC05. 10.7860/jcdr/2015/14824.6368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Alberti P, Pando E, Mata R, Vidal L, Roson N, Mast R, et al. Evaluation of the modified computed tomography severity index (MCTSI) and computed tomography severity index (CTSI) in predicting severity and clinical outcomes in acute pancreatitis. J Dig Dis. 2021;22(1):41–48. 10.1111/1751-2980.12961 [DOI] [PubMed] [Google Scholar]
- 51. Naing L, Winn T, Nordin R. Pratical issues in calculating the sample size for prevalence studies, 1. Archives of Orofacial Sciences; 2006. [Google Scholar]
- 52. Xu W, Qi X, Chen J, Su C, Guo X. Prevalence of splanchnic vein thrombosis in pancreatitis: a systematic review and meta‐analysis of observational studies. Gastroenterol Res Pract. 2015;2015:245460. 10.1155/2015/245460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Harris S, Nadkarni NA, Naina HV, Vege SS. Splanchnic vein thrombosis in acute pancreatitis: a single‐center experience. Pancreas. 2013;42(8):1251–1254. 10.1097/mpa.0b013e3182968ff5 [DOI] [PubMed] [Google Scholar]
- 54. Pagliari D, Cianci R, Brizi MG, Mancarella FA, Musso M, Cintoni M, et al. Anticoagulant therapy in the treatment of splanchnic vein thrombosis associated to acute pancreatitis: a 3‐year single‐centre experience. Intern Emerg Med. 2020;15(6):1021–1029. 10.1007/s11739-019-02271-5 [DOI] [PubMed] [Google Scholar]
- 55. Rebours V, Boudaoud L, Vullierme MP, Vidaud D, Condat B, Hentic O, et al. Extrahepatic portal venous system thrombosis in recurrent acute and chronic alcoholic pancreatitis is caused by local inflammation and not thrombophilia. Am J Gastroenterol. 2012;107(10):1579–1585. 10.1038/ajg.2012.231 [DOI] [PubMed] [Google Scholar]
- 56. Stark K, Massberg S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol. 2021;18(9):666–682. 10.1038/s41569-021-00552-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Roch AM, Maatman TK, Carr RA, Colgate CL, Ceppa EP, House MG, et al. Venous thromboembolism in necrotizing pancreatitis: an underappreciated risk. J Gastrointest Surg. 2019;23(12):2430–2438. 10.1007/s11605-019-04124-0 [DOI] [PubMed] [Google Scholar]
- 58. Tu J, Yang Y, Zhang J, Yang Q, Lu G, Li B, et al. Effect of the disease severity on the risk of developing new‐onset diabetes after acute pancreatitis. Medicine (Baltim). 2018;97(22):e10713. 10.1097/md.0000000000010713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dekeryte I, Zviniene K, Bieliuniene E, Dambrauskas Z, Ignatavicius P. Volume, but not the location of necrosis, is associated with worse outcomes in acute pancreatitis: a prospective study. Medicina. 2022;58(5):645. 10.3390/medicina58050645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bruce JIE, Sánchez‐Alvarez R, Sans MD, Sugden SA, Qi N, James AD, et al. Insulin protects acinar cells during pancreatitis by preserving glycolytic ATP supply to calcium pumps. Nat Commun. 2021;12(1):4386. 10.1038/s41467-021-24506-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cuthbertson CM, Christophi C. Disturbances of the microcirculation in acute pancreatitis. Br J Surg. 2006;93(5):518–530. 10.1002/bjs.5316 [DOI] [PubMed] [Google Scholar]
- 62. Balint ER, Fur G, Kiss L, Nemeth DI, Soos A, Hegyi P, et al. Assessment of the course of acute pancreatitis in the light of aetiology: a systematic review and meta‐analysis. Sci Rep. 2020;10(1):17936. 10.1038/s41598-020-74943-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Senzolo M, Riva N, Dentali F, Rodriguez‐Castro K, Sartori MT, Bang SM, et al. Long‐term outcome of splanchnic vein thrombosis in cirrhosis. Clin Transl Gastroenterol. 2018;9(8):176. 10.1038/s41424-018-0043-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lesmana CR, Inggriani S, Cahyadinata L, Lesmana LA. Deep vein thrombosis in patients with advanced liver cirrhosis: a rare condition? Hepatol Int. 2010;4(1):433–438. 10.1007/s12072-010-9166-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Reynolds K, Lewis B, Nolen JD, Kinney GL, Sathya B, He J. Alcohol consumption and risk of stroke: a meta‐analysis. JAMA. 2003;289(5):579–588. 10.1001/jama.289.5.579 [DOI] [PubMed] [Google Scholar]
- 66. Patra J, Taylor B, Irving H, Roerecke M, Baliunas D, Mohapatra S, et al. Alcohol consumption and the risk of morbidity and mortality for different stroke types‐‐a systematic review and meta‐analysis. BMC Publ Health. 2010;10(1):258. 10.1186/1471-2458-10-258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zoller B, Ji J, Sundquist J, Sundquist K. Alcohol use disorders are associated with venous thromboembolism. J Thromb Thrombolysis. 2015;40(2):167–173. 10.1007/s11239-015-1168-8 [DOI] [PubMed] [Google Scholar]
- 68. Hansen‐Krone IJ, Braekkan SK, Enga KF, Wilsgaard T, Hansen JB. Alcohol consumption, types of alcoholic beverages and risk of venous thromboembolism ‐ the Tromso Study. Thromb Haemost. 2011;106(2):272–278. 10.1160/th-11-01-0043 [DOI] [PubMed] [Google Scholar]
- 69. Mukamal KJ, Jadhav PP, D'Agostino RB, Massaro JM, Mittleman MA, Lipinska I, et al. Alcohol consumption and hemostatic factors: analysis of the Framingham Offspring cohort. Circulation. 2001;104(12):1367–1373. 10.1161/hc3701.096067 [DOI] [PubMed] [Google Scholar]
- 70. Samokhvalov AV, Rehm J, Roerecke M. Alcohol consumption as a risk factor for acute and chronic pancreatitis: a systematic review and a series of meta‐analyses. EBioMedicine. 2015;2(12):1996–2002. 10.1016/j.ebiom.2015.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vazzana N, Ranalli P, Cuccurullo C, Davì G. Diabetes mellitus and thrombosis. Thromb Res. 2012;129(3):371–377. 10.1016/j.thromres.2011.11.052 [DOI] [PubMed] [Google Scholar]
- 72. Sobczak AIS, Stewart AJ. Coagulatory defects in type‐1 and type‐2 diabetes. Int J Mol Sci. 2019;20(24):6345. 10.3390/ijms20246345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hinton W, Nemeth B, de Lusignan S, Field B, Feher MD, Munro N, et al. Effect of type 1 diabetes and type 2 diabetes on the risk of venous thromboembolism. Diabet Med. 2021;38(5):e14452. 10.1111/dme.14452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hegyi P, Eross B, Izbeki F, Parniczky A, Szentesi A. Accelerating the translational medicine cycle: the Academia Europaea pilot. Nat Med. 2021;27(8):1317–1319. 10.1038/s41591-021-01458-8 [DOI] [PubMed] [Google Scholar]
- 75. Hegyi P, Petersen OH, Holgate S, Erőss B, Garami A, Szakács Z, et al. Academia Europaea position paper on translational medicine: the cycle model for translating scientific results into community benefits. J Clin Med. 2020;9(5):1532. 10.3390/jcm9051532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Working Group IAPAPAAPG . IAP/APA evidence‐based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4 (Suppl 2)):e1–e15. [DOI] [PubMed] [Google Scholar]
- 77. Patil B, Meena LN, Sharma DC, Agarwal G, Dadhich Y, Gupta G. Impact of low‐molecular‐weight heparin in the treatment of moderately severe and severe acute pancreatitis; a randomized, single blind, phase 3 control trial. Int J Surg. 2022;101:106621. 10.1016/j.ijsu.2022.106621 [DOI] [PubMed] [Google Scholar]
- 78. Tozlu M, Kayar Y, Ince AT, Baysal B, Senturk H. Low molecular weight heparin treatment of acute moderate and severe pancreatitis: a randomized, controlled,open‐label study. Turk J Gastroenterol. 2019;30(1):81–87. 10.5152/tjg.2018.18583 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.


