Abstract
Background:
Recent evidence challenges the conventional belief that hyaluronic acid (HA) fillers have a short lifespan of 3–12 months. This study, using extensive patient data and long-term imaging post-injection, suggests a need to reconsider refilling protocols and underscores the critical role of precise clinical photography for accurate comparisons.
Methods:
The study enrolled 33 patients who received HA fillers in the mid-face, excluding those with recent injections, permanent fillers, or specific medical histories. Magnetic resonance imaging (MRI) was conducted on 24 asymptomatic and nine edema-concerned patients over 2.5 years. Two blinded radiologists assessed filler presence and longevity based on requested MRI observations.
Results:
MRI scans confirmed HA presence in all 33 patients, with no complete dissipation observed over a 2-year period post injection. Among them, 21 had not received injections for 2–5 years, 12 for over 5 years, and some for up to 8–15 years. Varying volumes of HA were noted: mild in nine patients, moderate in 13, and severe in 11. The study reported HA longevity of up to 15 years across different products, with a 95% confidence interval of 84.47% ± 4.43%, demonstrating the persistence of cross-linked HA fillers in the mid-face.
Conclusions:
HA fillers remained detectable for at least 2 years in all 33 patients, with one patient showing filler longevity of up to 15 years. These findings suggest significant implications for filler management practices. Further research with larger cohorts and ongoing imaging follow-up is warranted to fully understand HA filler longevity and optimize clinical protocols.
Takeaways
Question: What is the longevity of hyaluronic acid (HA) facial fillers, traditionally considered temporary, based on magnetic resonance imaging findings?
Findings: A study of 33 patients using magnetic resonance imaging revealed evidence of HA filler in the mid-face lasting from 2 to 15 years. All patients showed HA presence, even after 2 years without injections. Different MRI depths of filler were observed, with 84.47% confidence in readings. Various HA products were used, with no single brand showing exceptional longevity.
Meaning: The study challenges traditional notions of HA filler longevity, suggesting it can persist for many years. This necessitates reevaluation of filler management practices.
INTRODUCTION
There are approximately 12 publications regarding magnetic resonance imaging (MRI) of dermal fillers.1–10 Longevity has been identified in two articles using MRI.1,2 Hyaluronic acid (HA) fillers have traditionally regarded as medium-term fillers, designed to last between 3 and 12 months.7 New information has evolved demonstrating longevity of HA of many years, contrary to traditional information.1,2,11 Only three MRI publications are known to the authors, which are specific to HA.1–3 This study delivers the largest database of patients with the greatest length of time from injection of the mid-face to radiological imaging. One study involving high-frequency ultrasound had a total of 22 patients.12 The longevity of cross-linked HA dermal fillers influences the techniques and amounts used. Refilling and “top-ups” commonly performed in the injectables space will require reconsideration, and updated protocols are needed. There needs to be encouragement for accurate controlled clinical photographs for comparison.
METHODS
A total of 33 patients have been included in the trial, with the inclusion criteria of HA filler injections in the mid-face and no history mid-face injections for greater than or equal to 2 years. In total, 32 women and one man were included. Any patient with a history of permanent filler was completely excluded from the study. Other exclusion criteria included any of the following within 6 weeks of the MRI; hyaluronidase dissolve, facial surgical interventions, tumor excisions, infection, or inflammation of the skin. Twenty-four asymptomatic patients were recruited using referral networks for an MRI to identify residual filler in the face with the inclusion criteria above. A questionnaire was used with detailed information and history of filler, including the exclusion criteria below. Nine patients were retrospectively included in the trial with the same criteria; however, they had concerns of periorbital edema or malar edema, requesting MRI investigation. “Blending” with other products such as lignocaine is rare in Australia, and there were no clinical notes or patient recall of its use. One principal investigator included 33 patients over 2½ years, from February 2020 to September 2022, all of which were performed on the on the 3T Pioneer scanner (GE Health Care). All were performed by three MRI technicians. The trial protocol used Axial T2 FS 2mm Flex and Coronal T2 FAT Sat 3mm of the mid-face. This is a simple “water sequence” protocol done in two planes. The diagnostic protocol was Sagittal T2 Fat Sat 3mm, Sagittal T1 3mm, Axial T2 FS 2mm Flex, Axial T1 2mm, Axial DWI b1000 4mm, Coronal T2 FAT Sat Face 3mm. This protocol has a number of further anatomical axial sequences for any additional information, such as superficial musculoaponeurotic system involvement or muscle involvement for additional diagnostic purposes. Of the 33 patients, 12 underwent the trial protocol, and the remaining 21 underwent the diagnostic protocol for additional diagnostic purposes.
Table 1.
Summary of Results for All Patients
| Patient Number | Age | Sex | Observer Avg Max Depth of Filler (mm) | Max Thickness T2 Signal | Years post Treatment | Protocol |
|---|---|---|---|---|---|---|
| 1 | 52 | F | 7.5 | Moderate | >5 | Standard full |
| 2 | 56 | F | 7 | Moderate | 2–5 | Standard full |
| 3 | 61 | F | 10 | Severe | 2–5 | Standard full |
| 4 | 52 | F | 9 | Moderate | 2–5 | Standard full |
| 5 | 46 | F | 11 | Severe | >5 | Standard full |
| 6 | 64 | F | 11 | Severe | 2–5 | Standard full |
| 7 | 53 | F | 3 | Mild | >5 | Standard full |
| 8 | 37 | F | 6 | Moderate | >5 | Standard full |
| 9 | 44 | F | 8.5 | Moderate | >5 | Trial (T2Fs ax/cor) |
| 10 | 62 | F | 2.5 | Mild | 2–5 | Standard full |
| 11 | 52 | F | 9.5 | Severe | 2–5 | Trial (T2Fs ax/cor) |
| 12 | 30 | F | 4.5 | Mild | 2–5 | Trial (T2Fs ax/cor) |
| 13 | 33 | F | 5.5 | Mod | 2–5 | Trial (T2Fs ax/cor) |
| 14 | 32 | F | 3.5 | Mild | 2–5 | Trial (T2Fs ax/cor) |
| 15 | 32 | F | 5 | Mild | 2–5 | Trial (T2Fs ax/cor) |
| 16 | 43 | F | 9.5 | Mod | 2–5 | Trial (T2Fs ax/cor) |
| 17 | 50 | M | 4.5 | Mild | 2–5 | Standard Full |
| 18 | 63 | F | 15 | Severe | >5 | Standard full |
| 19 | 46 | F | 8 | Moderate | 2–5 | Standard full |
| 20 | 36 | F | 10 | Moderate | 2–5 | Standard full |
| 21 | 50 | F | 19 | Severe | 2–5 | Standard full |
| 22 | 41 | F | 7 | Moderate | 2–5 | Standard full |
| 23 | 40 | F | 12 | Severe | 2–5 | Standard full |
| 24 | 36 | F | 9.5 | Severe | 2–5 | Standard full |
| 25 | 48 | F | 10.7 | Severe | 2–5 | Trial (T2Fs ax/cor) |
| 26 | 60 | F | 10 | Moderate | >5 | Standard full |
| 27 | 56 | F | 7 | Moderate | 2–5 | Trial (T2Fs ax/cor) |
| 28 | 42 | F | 11.3 | Severe | >5 | Standard full |
| 29 | 32 | F | 8.5 | Moderate | >5 | Trial (T2Fs ax/cor) |
| 30 | 60 | F | 5.5 | Mild | 2–5 | Trial (T2Fs ax/cor) |
| 31 | 40 | F | 10 | Severe | >5 | Trial (T2Fs ax/cor) |
| 32 | 69 | F | 7.5 | Moderate | 2–5 | Standard full |
| 33 | 52 | F | 7.5 | Moderate | >5 | Standard full |
Table 2.
Confidence Interval and Product Information [Internal Observer Agreement (IOA) with a 95% Confidence Interval of 84.47% ± 4.43.2]
| Patient | Study Type | Product | Total Filler Volume (mL) | Years post Treatment | IOA |
|---|---|---|---|---|---|
| 1 | Retrospective | Juvaderm Ultra | Unknown | 8 | 87.50 |
| 2 | Retrospective | Perlane | Unknown | Unknown | 100.00 |
| 3 | Retrospective | Juvaderm Ultra | Unknown | 4 | 81.82 |
| 4 | Retrospective | RHA3 | 3 | 3 | 100.00 |
| 5 | Retrospective | Perlane, Restylane, Juvaderm Ultra, Sub q | 7 | 8 | 100.00 |
| 6 | Retrospective | Volux, Volift | 6 | 3 | 100.00 |
| 7 | Retrospective | Resylane, Sub Q | Unknown | 15 | 50.00 |
| 8 | Retrospective | Hyaluronic acid | Unknown | 7 | 71.43 |
| 9 | Retrospective | Hyaluronic acid | Unknown | 7 | 88.89 |
| 10 | Prospective | Juvaderm Ultra | 8 | 5 | 66.67 |
| 11 | Prospective | Hyaluronic acid | 2 | 5 | 90.00 |
| 12 | Prospective | Hyaluronic acid | Unknown | 2 | 80.00 |
| 13 | Prospective | Hyaluronic acid | 2 | 2 | 83.33 |
| 14 | Prospective | Hyaluronic acid | 1 | 2 | 75.00 |
| 15 | Prospective | Restylane | 1 | 3 | 66.67 |
| 16 | Prospective | Hyaluronic acid | 1 | 2 | 90.00 |
| 17 | Prospective | Lyft, Reylane Defyne, Juvaderm Ultra, | 6 | 2 | 80.00 |
| 18 | Prospective | Voluma, Juvaderm Ultra | Unknown | 8 | 100.00 |
| 19 | Prospective | Volyme, Voluma, Volbella, Sub Q | 7 | 4 | 100.00 |
| 20 | Prospective | Juvaderm Ultra, Restylane | Unknown | 4 | 81.82 |
| 21 | Prospective | Hyaluronic acid | Unknown | 3 | 90.00 |
| 22 | Prospective | Hyaluronic acid | Unknown | 4 | 100.00 |
| 23 | Prospective | Radensity II, Juvaderm Ultra, Restylane | 5 | 4 | 71.43 |
| 24 | Prospective | Voluma, Restylane | 5 | 4 | 72.73 |
| 25 | Prospective | Hyaluronic acid | 10 | 2 | 78.33 |
| 26 | Prospective | Hyaluronic acid | Unknown | 6 | 81.82 |
| 27 | Prospective | Hyaluronic acid | Unknown | 3 | 75.00 |
| 28 | Prospective | Restylane | Unknown | 10 | 79.37 |
| 29 | Prospective | Voluma | 1 | 5 | 88.89 |
| 30 | Prospective | Hyaluronic acid | Unknown | 5 | 83.33 |
| 31 | Prospective | Hyaluronic acid | Unknown | 5 | 100.00 |
| 32 | Prospective | Juvaderm Ultra, Restylane | 20 | 2 | 87.50 |
| 33 | Retrospective | Juvaderm Ultra | Unknown | 8 | 87.50 |
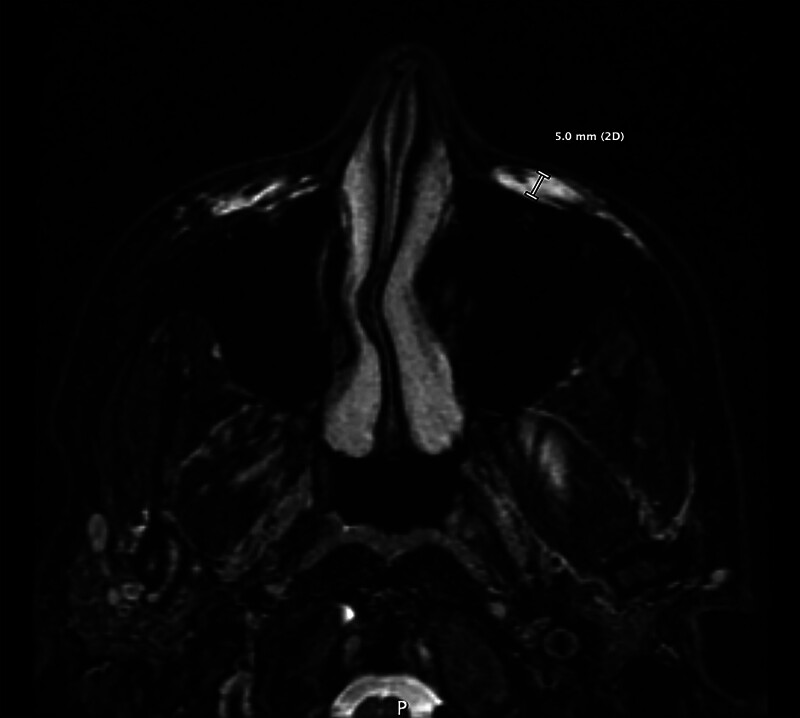
The total volume of HA filler in the mid-face was recorded from the patient’s history, and information on when and the range of products used was recorded. Some clinical notes were accessed with permission of the patients to clarify patient histories. MRIs were read by the principal investigator, a dual-qualified radiologist/aesthetic physician and a secondary investigator, a qualified radiologist. The filler appearance observed followed fluid signal on the water sequences (T2 fat saturation), and the typical described appearance of HA is seen on MRI in multiple publications.1–5 The degree of longevity was quantitatively measured by the greatest depth of continuous HA in any mid-face fat compartment using the axial T2 fat saturation images (Figs. 1–4).
Fig. 1.
MRI on the 3 Tesla (GE MRI Pioneer), axial T2 fat saturation demonstrating the technique of greatest depth of measurement. Mild amounts of HA were denoted as volumes of 1–5 mm in depth; moderate, 5–10 mm; and severe, more than 10 mm.
Fig. 4.
MRI scanning of the 64-year-old woman who had a total of 6 mL of a combination of Juvederm Volift and Volux (Allergan; Irvine, Calif.) to the mid-face between 2012 and 2017. MRI performed in Jan 2020. It demonstrates extensive filler signal in the infraorbital fat compartment (short arrow) and postseptal location (long arrow). The other filler remaining in the face is seen as bright signal (“white”).
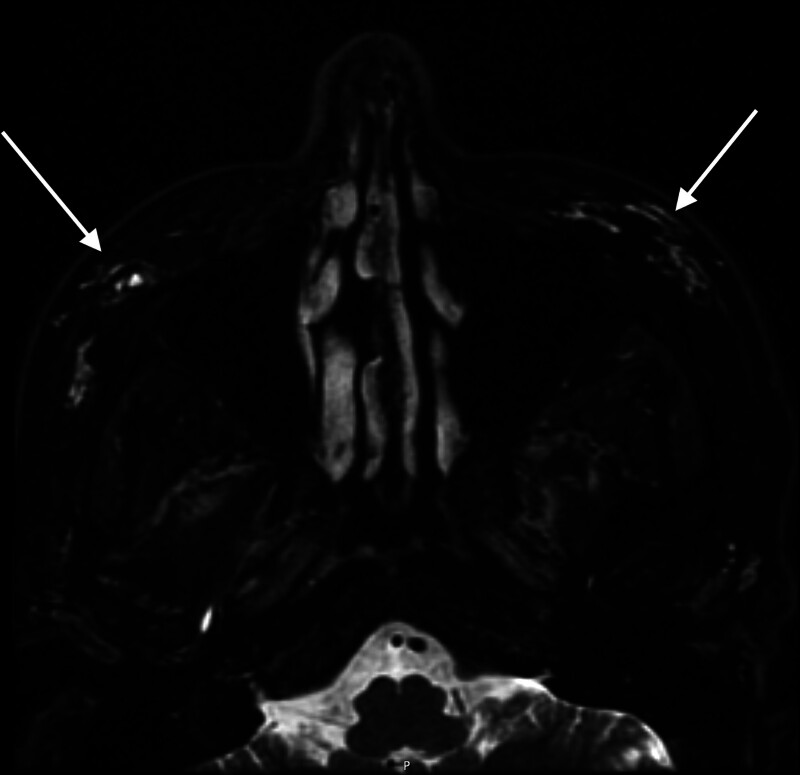
Fig. 2.
MRI on the 3 Tesla (GE MRI Pioneer) axial T2 fat saturation of the 53-year-old woman, who had unknown volume of Restylane lidocaine (Restylane, Galoderma, Switzerland) and SUB Q (Revolax, Fox pharma, UK) 15 years or more prior. This demonstrated scattered bight T2 (white) signal of malar residual HA filler. (Arrows)
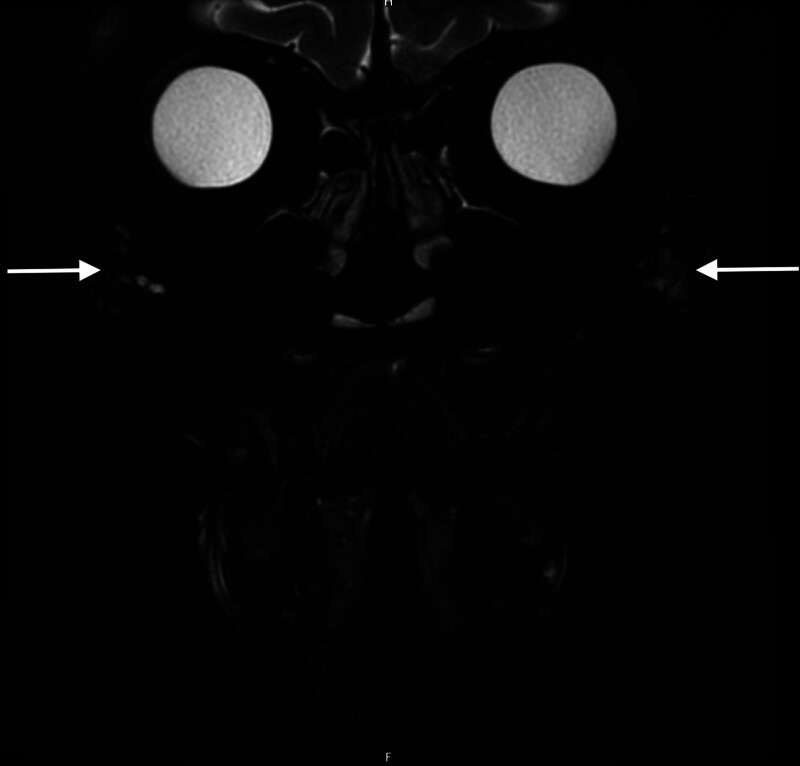
Fig. 3.
MRI on the 3 Tesla (GE MRI Pioneer) axial T2 fat saturation of the same 53-year-old in Figure 2, from over 15 years prior with HA filler in the lateral malar regions (arrows).
RESULTS
All 33 patients demonstrated evidence of HA on MRI. No patients in the trial showed complete resolution of HA injected over 2 years prior in the mid-face. Twenty-one patients denied mid-face injections for 2 to 5 years, and 12 denied injection for greater than 5 years. Four of these patients included going without injections for over 8 years, two patients for over 10–12 years, and one patient with no injections for over 15 years. Nine patients demonstrated mild volumes of HA, 13 patients demonstrated moderate volumes, and 11 patients demonstrated severe volumes in the mid-face on MRI.
The double-blinded confidence interval of 95% between readings was 84.47% ± 4.43%. Historical total volumes (which included both sides of the mid-face) in the mid-face ranged from 1 mL to 8 mL and for 17 of 33 patients the product was unknown.
The known products included Juvederm Ultra, Volbella, Voluma (Allergan; Irvine, Calif.), Perlane, Restylane, Defyne, Lyft (Galderma, Lausanne, Switzerland), Evolence (Ortho Neutogena,; Flemington, N.J.), Sub Q (Revolax, Fox pharma, UK), RHA3, Redensity II (Teoxane; Geneva, Switzerland). The unknown brand of HA made up the remainder of the patients, as confirmed by all the patient histories. All patients denied any injection of permanent dermal fillers.
This article is the largest cohort of patients to be studied using MRI, demonstrating a universal theme of longevity of over 2 years and up to 15 years. Many products have been represented in this trial, all with cross-linked HA technology.13–18 Measuring a volume on MRI would include the larger measurements of natural facial fat and other anatomical structures, which was thought to be a less-accurate technique. The exclusion criteria will successfully remove the confounders of inflammation, infection, or lymphedema, and successfully measure hydrophilic HA signal. Limitations included a large variation in techniques, locations, and volumes poorly recalled or recorded by the patient. The patients with unknown brands all confirmed a history of HA, and none admitted to any permanent or other types of fillers. Only cross-linked injectable HA was available in Australia at the time of the study, and all patients were injected by medical professionals in the country. The use of permanent fillers is very uncommon in Australia. Locations of the HA on reviewed MRIs included the deep medial cheek, medial SOOF, lateral SOOF, buccal, pyriform, infraorbital, middle cheek, and temporo-lateral cheek fat compartments.15 No specific products stood out regarding longevity. The vast majority of known HA products were in the Juvaderm and the Galderma range, most likely related to the time and dominance in the Australian market.
CONCLUSIONS
This article implies HA filler longevity in 33 of 33 patients, who denied filler injection in the mid-face for at least 2 years, with a range from 2 years to up to 15 years in one patient. This has far-reaching implications for ongoing management and maintenance of the previously considered temporary HA facial fillers. Further studies with larger cohorts on HA longevity are required. Post-injection imaging and ongoing imaging follow-up would be ideal.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
ACKNOLWEDGMENTS
The authors thank Rebecca Whelan and Lucy O’Neal (MRI technologists) at Lake Imaging Specialist and Research Center for their involvement in MRI scanning and obtaining patient data.
Footnotes
Published online 15 July 2024.
Disclosure statements are at the end of this article, following the correspondence information.
REFERENCES
- 1.Master M. Hyaluronic acid filler longevity and localization—MRI evidence. Plast Reconstr Surg. 2021;147:50e–53e. [DOI] [PubMed] [Google Scholar]
- 2.Master M, Roberts S. Long-term MRI follow-up of hyaluronic acid dermal filler. Plast Reconstr Surg Glob Open. 2022;10:e4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Master M. Novel treatment of inadvertent injection of post-septal hyaluronic acid filler. Plast Reconstr Surg. 2021;148:855e–856e. [DOI] [PubMed] [Google Scholar]
- 4.Jeong KH, Gwak MJ, Moon SK, et al. Efficacy and durability of hyaluronic acid fillers for malar enhancement: a prospective, randomized, split-face clinical controlled trial. J Cosmet Laser Ther. 2018;20:184–188. [DOI] [PubMed] [Google Scholar]
- 5.Kadouch JA, Tutein Nolthenius CJ, Kadouch DJ, et al. Complications after facial injections with permanent fillers: important limitations and considerations of MRI evaluation. Aesthet Surg J. 2014;34:913–923. [DOI] [PubMed] [Google Scholar]
- 6.Di Girolamo M, Mattei M, Signore A, et al. MRI in the evaluation of facial dermal fillers in normal and complicated cases. Eur Radiol. 2015;25:1431–1442. [DOI] [PubMed] [Google Scholar]
- 7.Tal S, Maresky HS, Bryan T, et al. MRI in detecting facial cosmetic injectable fillers. Head Face Med. 2016;12:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa ALF, Caliento R, da Rocha GBL, et al. Magnetic resonance imaging appearance of foreign-body granulomatous reactions to dermal cosmetic fillers. Imaging Sci Dent. 2017;47:281–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girolamo MD, Mattei M, Signore A, et al. MRI in the evaluation of facial dermal fillers in normal and complicated cases. Eur Radiol. 2015;25:1431–1442. [DOI] [PubMed] [Google Scholar]
- 10.Becker M, Balagué N, Montet X, et al. ; LIPO and Metabolism Group. Hyaluronic acid filler in HIV-associated facial lipoatrophy: evaluation of tissue distribution and morphology with MRI. Dermatology. 2015;230:367–374. [DOI] [PubMed] [Google Scholar]
- 11.Qiao J, Jia Q-N, Jin H-Z, et al. Long-term follow-up of longevity and diffusion pattern of hyaluronic acid in nasolabial fold correction through high-frequency ultrasound. Plast Reconstr Surg. 2019;144:189e–196e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Pasquale A, Russa G, Pulvirenti M, et al. Hyaluronic acid filler injections for tear-trough deformity: injection technique and high frequency ultrasound follow-up evaluation. Aesthet Plast Surg. 2013;37:587–591. [DOI] [PubMed] [Google Scholar]
- 13.American Society of Plastic Surgeons. Plastic surgery statistics. 2017. Available at https://www.plasticsurgery.org/documents/News/Statistics/2017/plastic-surgery-statistics-report-2017.pdf [Google Scholar]
- 14.Beasley KL, Weiss MA, Weiss RA. Hyaluronic acid fillers: a comprehensive review. Facial Plast Surg. 2009;25:86–94. [DOI] [PubMed] [Google Scholar]
- 15.Fundarò S. Anatomy and aging of cheek fat compartments. Med Dent Res. 2018;2. [Google Scholar]
- 16.Lemperle G, Duffy DM. Treatment options for dermal filler complications. Aesthet Surg J. 2006;26:356–364. [DOI] [PubMed] [Google Scholar]
- 17.Soparkar C, Patrinely J, Tschen J. Erasing restylane. Ophthalmic Plast Reconstruct Surg. 2004;20:317–318. [DOI] [PubMed] [Google Scholar]
- 18.Skippen B, Baldelli I, Hartstein M, et al. Rehabilitation of the dysmorphic lower eyelid from hyaluronic acid filler: what to do after a good periocular treatment goes bad. Aesthet Surg J. 2019;40:197–205. [DOI] [PubMed] [Google Scholar]