Abstract
Background
Recessive dystrophic epidermolysis bullosa (RDEB) is an incurable widespread blistering skin disorder caused by mutations in the gene encoding for type VII collagen (C7), the major component of anchoring fibrils.
Objectives
To evaluate the efficacy and safety of intravenous (IV) gentamicin readthrough therapy in patients with RDEB harbouring nonsense mutations. The primary outcomes were increased expression of C7 in patients’ skin and safety assessments (ototoxicity, nephrotoxicity, autoimmune response); secondary outcomes included measuring wound healing in target wounds and assessment by a validated Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI) scoring system.
Methods
An open-label pilot trial to assess two different IV gentamicin regimens between August 2018 and March 2020 with follow-up through to 180 days post-treatment was carried out. Three patients with RDEB with confirmed nonsense mutations in COL7A1 in either one or two alleles and decreased baseline expression of C7 at the dermal–epidermal junction (DEJ) of their skin participated in the study. Three patients received gentamicin 7.5 mg kg–1 daily for 14 days and two of the three patients further received 7.5 mg kg–1 IV gentamicin twice weekly for 12 weeks. Patients who had pre-existing auditory or renal impairment, were currently using ototoxic or nephrotoxic medications, or had allergies to aminoglycosides or sulfate compounds were excluded.
Results
After gentamicin treatment, skin biopsies from all three patients (age range 18–28 years) exhibited increased C7 in their DEJ. With both regimens, the new C7 persisted for at least 6 months post-treatment. At 1 and 3 months post-treatment, 100% of the monitored wounds exhibited > 85% closure. Both IV gentamicin infusion regimens decreased EBDASI total activity scores. Of the patients assessed with the EBDASI, all exhibited decreased total activity scores 3 months post-treatment. All three patients completed the study; no adverse effects or anti-C7 antibodies were detected.
Conclusions
IV gentamicin induced the readthrough of nonsense mutations in patients with RDEB and restored functional C7 in their skin, enhanced wound healing and improved clinical parameters. IV gentamicin may be a safe, efficacious, low-cost and readily available treatment for this population of patients with RDEB.
In this open-label pilot trial, two different intravenous (IV) gentamicin regimens induced the production of type VII collagen (C7), enhanced wound closure and improved clinical parameters without adverse side-effects in patients with recessive dystrophic epidermolysis bullosa (RDEB) carrying nonsense mutations. IV gentamicin induced new C7 at the dermal–epidermal junction of all patients at levels of 45–125% that of normal human skin. Therefore, gentamicin may provide a relatively safe and efficacious therapy for the 30% of patients with RDEB who harbour nonsense mutations.
Linked Article: Laimer Br J Dermatol 2024; 191:161–162.
What is already known about this topic?
Patients with recessive dystrophic epidermolysis bullosa (RDEB) suffer from widespread skin blisters that heal with scarring; patients are at a heightened risk of succumbing to aggressive squamous cell carcinomas.
To date, there are no approved curative systemic treatments for RDEB.
Prior in vitro experiments demonstrated that gentamicin increased type VII collagen (C7) in cultured RDEB cells and topical gentamicin induced C7 and improved wound healing in patients with RDEB with nonsense mutations.
What does this study add?
Intravenous (IV) gentamicin induces the production of functional C7 that persists for at least 6 months post-treatment in patients with RDEB carrying nonsense mutations.
IV gentamicin enhances wound closure in patients with RDEB, as demonstrated by all monitored wounds achieving > 85% closure at 1 and 3 months post-treatment.
IV gentamicin may be a safe, efficacious, low-cost and readily available treatment for patients with RDEB harbouring nonsense mutations.
Recessive dystrophic epidermolysis bullosa (RDEB) is a rare, life-threatening, autosomal recessive skin-blistering disease caused by mutations in COL7A1, which encodes type VII collagen (C7).1 C7 is the main component of anchoring fibrils (AFs), structures at the dermal–epidermal junction (DEJ) that tether the epidermis to the dermis of human skin.2,3 Owing to decreased C7 and AFs, patients with RDEB have widespread skin blisters that heal with scarring and the formation of milia.1
The current mainstays of treatment for RDEB are palliative interventions and supportive care.4–6 Various therapies have been attempted, including protein, gene, cell and readthrough therapies.7–12 More recently, a localized treatment with topical herpes simplex virus type 1-based gene therapy designed to restore C7 and improve wound healing was approved by the US Food and Drugs Administration (FDA).13 However, as yet, there are no approved curative systemic treatments for RDEB.
Nonsense mutations are point mutations that cause the formation of a premature termination codon (PTC) in the mRNA, resulting in a truncated protein that is not functional. Nonsense-mediated readthrough therapy (NMRT) is an innovative approach that exploits the biochemical ability of aminoglycosides to bind and conformationally alter ribosomes, permitting readthrough of PTCs and restoring the translation of a full-length functional protein.14,15 Recent in vitro and in vivo research has confirmed the capability of NMRT to produce the functional protein-of-interest in several genetic disorders.16–21
Over 800 distinct mutations have been identified in COL7A1, with approximately 30% of them being nonsense. We previously showed that gentamicin induced PTC readthrough and restored C7 in RDEB cells in vitro.22 In addition, topical or intradermal gentamicin generated new C7 and AFs in the DEJ of the skin of patients with RDEB, improved wound closure and reduced blister formation.23 Topical and intradermal gentamicin are local treatments that fail to address the widespread systemic manifestations of the disease. We hypothesized that intravenous (IV) gentamicin might systematically treat all blisters and erosions simultaneously. In this study, three patients with RDEB with nonsense mutations in COL7A1 were treated. Two IV gentamicin infusion schedules were implemented: daily IV infusions for 14 days (continuous schedule) and intermittent, twice-weekly IV infusions for 12 weeks (biweekly schedule). The results showed that IV gentamicin induced C7 in the DEJ, enhanced wound closure at selected test sites and improved patients’ clinical parameters without adverse side-effects.
Patients and methods
Patients and interventions
Gentamicin is a well-studied, FDA-approved antibiotic; our study involved an off-label use of this drug. The inclusion criteria were that patients with RDEB had to have nonsense mutations in COL7A1 in either one or two alleles, and an absence of or decreased C7 expression at their DEJ when compared with that of normal human skin (NHS). The NHS used in this study was obtained from tissue discarded during breast-reduction surgeries conducted by the University of Southern California Department of Plastic Surgery. The exclusion criteria were pre-existing renal or auditory impairment; allergies to aminoglycosides or sulfate compounds; pregnancy; and exposure to gentamicin within the past 6 weeks.
We treated three patients with RDEB who were previously characterized as having nonsense mutations (widespread blisters, erosions, scarring and milia formation) and associated medical complications such as anaemia and oesophageal strictures. Dosing regimens were designed based on prior investigations involving individuals with Duchenne muscular dystrophy and cystic fibrosis who harboured nonsense mutations and were administered IV gentamicin.20,24 Each patient received daily gentamicin infusions at a dosage of 7.5 mg kg–1 for 14 days. Following a 1–3-month washout period, two of the three patients further received twice-weekly gentamicin infusions at an identical dosage of 7.5 mg kg–1 for 12 weeks. Two open erosive wounds and two areas of intact skin were selected for monitoring in each patient before starting gentamicin infusions. Wound sites were selected based on investigator and patient preference; consideration was given to ensure that areas that were easy to photograph were selected. The wounds selected for treatment were not clinically infected and were typically chronic in nature.
The study was registered with ClinicalTrials.gov (NCT03392909).
Clinical and safety assessments
Each week, the patients completed a brief standardized telephone questionnaire that included several clinical instruments: the Skindex-16 quality of life (QoL) survey (a QoL measure for patients with skin diseases)25 and the Wong–Baker Faces Pain Rating Scale.26 A dermatologist assessed patients using Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI) scores at baseline and at the 1-, 3- and 6-month post-treatment follow-up visits. The patients also kept a wound-healing diary and photographed their selected test wounds for monitoring once a week.
The percentage of wound closure was assessed at 1, 3 and 6 months post-treatment using marked, matched photographs taken during clinical visits. Standardized digital photographs were taken from the monitored wounded sites, and open wound areas were determined with computer-assisted planimetry and ImageJ [National Institutes of Health (NIH), Bethesda, MD, USA]. The percentage of wound closure was graded as 85–100% (+++), 50–85% (++) and < 50% (+).23
A number of safety parameters were also assessed at baseline and at the 1-, 3- and 6-month post-treatment visits, including complete blood count, blood urea nitrogen (BUN), creatinine, calculated creatinine clearance, electrolytes, liver function tests (LFTs) and pure tone audiometry.
Assessment of type VII collagen in patients’ skin
Prior to the onset of the study, 6-mm punch biopsies were obtained from intact skin sites and subjected to quantitative immunofluorescence (IF) staining for the expression of baseline C7 at the DEJ, as previously described.23 At the 1-, 3- and 6-month follow-up visits, biopsies from healed wounds and intact skin sites were obtained and processed in an identical fashion. IF staining was performed with a monoclonal anti-C7 antibody (clone MAP185; Millipore, Billerica, MA, USA), as well as a rabbit polyclonal antibody, to the noncollagenous domain of C7 (NC1).23 Immunolabelled vertical sections of NHS (positive control), healed wounds and intact skin sites were photographed using the same camera and identical exposure times. Quantification of C7 expression at the DEJ was performed by computer-assisted image analysis using ImageJ (NIH) as described.23,27 NHS was included as a positive control and the expression of C7 was compared with the expression in NHS (100%). The NHS samples were matched in terms of age within the patients’ age range.
Assessment of anti-type VII collagen autoantibodies
Serum circulating anti-C7 antibodies were assessed by an enzyme-linked immunosorbent assay (ELISA) using recombinant NC1-coated plates, as previously described.28 To evaluate if there were any anti-C7 antibodies deposited in the patients’ skin, skin biopsy samples were subjected to direct IF (DIF) staining using fluorescein isothiocyanate-conjugated goat antihuman IgG (Sigma, St. Louis, MO, USA), as previously described.28–30
Results
Patients and study endpoints
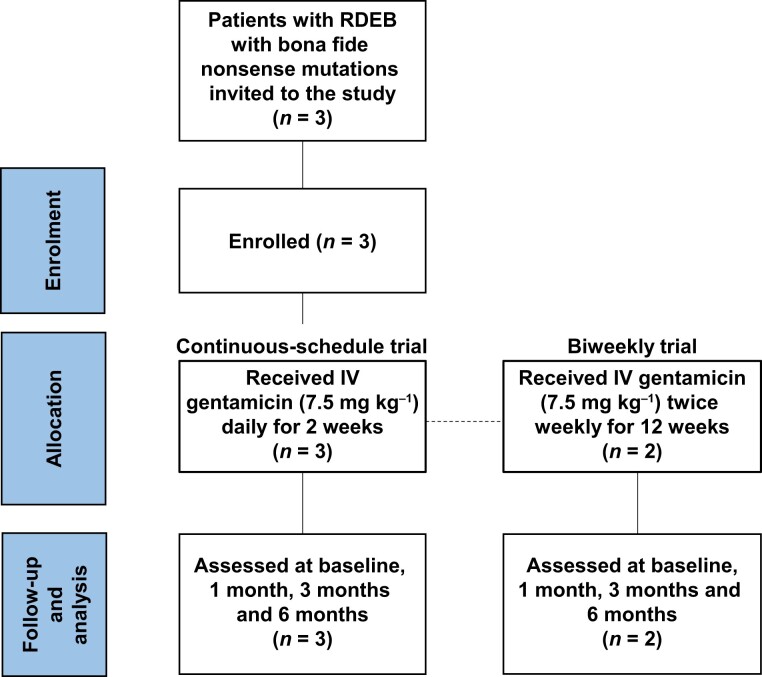
Three patients with RDEB (aged 18–28 years) with confirmed nonsense mutations in COL7A1 were recruited between August 2018 and July 2019. The study was completed in March 2020. All patients met the study inclusion criteria (Figure 1). Patient 1 (PT1) had homozygous nonsense mutations in COL7A1 (Table 1), while patients 2 and 3 (PT2 and PT3) each had heterozygous COL7A1 mutations with only one allele containing a nonsense mutation. IF staining with a polyclonal antibody to the N-terminal NC1 of C7 demonstrated that PT1 and PT2 expressed either nondetectable or minimal baseline levels of C7 at their DEJ. PT3 showed a moderate amount of C7 (50% of that seen in NHS) at baseline as detected by a monoclonal antibody to C7, which probably represented the C7 mutant protein product from the COL7A1 missense mutation (Table 1). All three patients (PT1–PT3) participated in the continuous schedule arm of the trial and were administered IV gentamicin at 7.5 mg kg–1 daily for 14 days. Later, PT2 and PT3 joined the biweekly schedule arm of the trial (henceforth denoted as PT2* and PT3*), wherein they were administered IV gentamicin at a dosage of 7.5 mg kg–1 twice weekly for a duration of 12 weeks.
Figure 1.
CONSORT flow diagram outlining the process of enrolling and completing the trial in patients with recessive dystrophic epidermolysis bullosa (RDEB). IV, intravenous.
Table 1.
Patient characteristics, mutations and baseline type VII collagen (C7) expression
| Characteristic | PT1 | PT2/PT2* | PT3/PT3* | NHS |
|---|---|---|---|---|
| Sex | M | F | F | |
| Age (years) | 19 | 28 | 18 | |
| Allele 1/allele 2 | R578X/R578X | R613X/IVS85-1G > A | R185X/G1764A | –/– |
| C7 at DEJ (%)a | 0 | 5 | 50 | 100 |
Patients 1–3 (PT1–PT3) participated in the continuous schedule part of the trial. Later, PT2 and PT3 joined the biweekly schedule part of the trial (denoted as PT2* and PT3*). DEJ, dermal–epidermal junction; F, female; M, male; NHS, normal human skin. aAssessed by immunofluorescence using a rabbit polyclonal antibody to C7 for PT1/PT2 and with a monoclonal antibody to C7 for PT3. Expression levels at the DEJ were calculated from a comparison with NHS (see ‘Patients and methods’). NHS was used for comparison with patient samples.
Intravenous gentamicin induced new type VII collagen
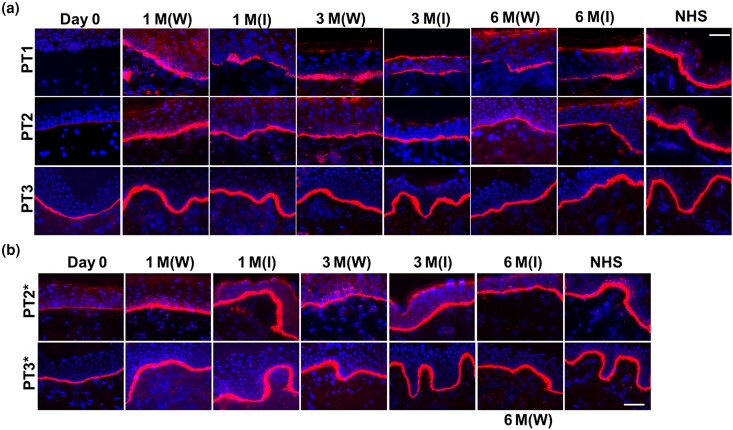
A primary endpoint of the study was expression of C7, which was assessed at baseline and after gentamicin treatment (at 1, 3 and 6 months post-treatment). As shown in Figure 2(a), skin biopsies obtained from healed wounds and intact skin sites 1 month after IV gentamicin on the continuous schedule were subjected to IF using a polyclonal NC1 antibody (PT1 and PT2) or a monoclonal antibody targeting the NC1 domain of C7 (PT3). C7 was not detectable in PT1 on day 0, but after treatment with gentamicin, new C7 was expressed in both healed wounds and intact skin – at levels of 45% and 38%, respectively – of that in NHS. The results were better for PT2, who had minimal expression of C7 on day 0 but had post-treatment expression of C7 in healed wounds at a level of 91% that of NHS and at a level of 75% for intact skin. For PT3, who had moderate expression of C7 on day 0, healed skin wounds demonstrated C7 expression after gentamicin treatment that was 125% that of NHS. Additionally, PT3’s intact skin demonstrated C7 expression of 114% vs. NHS. Skin biopsies taken from both healed wounds and intact skin sites at 3 and 6 months post-treatment demonstrated the persistence of the newly created C7 at the DEJ in all three patients.
Figure 2.
Intravenous (IV) gentamicin generated new type VII collage (C7) in the skin of patients with recessive dystrophic epidermolysis bullosa (RDEB). Immunofluorescence staining of skin biopsy specimens from all three patients with RDEB (PT1, PT2, PT3) taken from intact skin sites prior to treatment (day 0) and from healed periwound sites (W) and intact skin (I) at 1, 3 and 6 months after gentamicin treatment using a polyclonal antibody to C7 (PT1, PT2) or a monoclonal antibody to C7 (PT3). (a) PT1–PT3 were treated with continuous-schedule IV gentamicin for 2 weeks and (b) PT2* and PT3* were treated with biweekly gentamicin for 3 months. Day 0 biopsies for those following the biweekly schedule were retaken prior to initiating the second arm of the trial. Note that within the selected wound sites, as well as the intact skin site, gentamicin induced continuous and sustained expression of C7 at the dermal–epidermal junction of all three patients for both treatment schedules. All images were obtained using the same camera and identical exposure times. Scale bars = 50 μm. M, month; NHS, normal human skin.
In the biweekly schedule arm, 1 month after IV gentamicin, PT2* had increased C7 expression in healed wounds at a level of 90% of that observed in NHS. In intact skin, C7 expression increased to 107% of that observed in NHS. In PT3*, the level of C7 expression in healed wounds increased to 123% of that observed in NHS, while in intact skin it reached 116% of the level seen in NHS (Figure 2b). At 3 and 6 months post-treatment, gentamicin-induced C7 was sustained.
Intravenous gentamicin improved wound closure
Prior to gentamicin treatment, all three patients were evaluated by a dermatologist who selected two open erosive wounds (n = 6) and two intact skin areas (n = 6) as test sites to be monitored for wound closure or progression and new blister formation throughout the study. Open wound areas were calculated using ImageJ (NIH) analysis of standardized wound photographs taken at baseline and at the 1-, 3- and 6-month post-treatment clinic visits. All selected wounds had been present for at least 1 year prior to test site selection. The chronicity of these wounds suggested that these test sites were not prone to spontaneous wound closure. Representative images of monitored wounds before and after the patients received IV gentamicin are provided in Figure S1 (see Supporting Information). When following the continuous schedule IV gentamicin part of the study, all six monitored wounds demonstrated a minimum of 85% closure at 1 and 3 months post-treatment (Table 2). At the 6-month follow-up, three of the six wounds maintained at least 85% closure. There were no instances of the development of new wounds in any of the intact sites for the duration of the study.
Table 2.
Wound closure of monitored test sites during a trial of continuous and biweekly intravenous gentamicin in patients with recessive epidermolysis bullosa
| Location | PT1 | PT2 | PT3 | PT2* | PT3* | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Back | Buttocks | Upper arm | Back | Calf | Shin | Left back |
Lower back | Knee | Calf | |
| Baseline wound area (cm2) | 27.9 | 26.9 | 7.1 | 3.8 | 6.1 | 4.0 | 5.6 | 20.1 | 20.4 | 9.3 |
| Wound history (years)a | > 1 | > 2 | > 1 | > 2 | > 1 | > 1 | > 1 | > 1 | > 1 | > 1 |
| Wound closureb | ||||||||||
| 1 month | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| 3 month | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| 6 month | +++ | +++ | + | + | + | +++ | + | ++ | +++ | +++ |
Patients 1–3 (PT1–PT3) participated in the continuous schedule part of the trial. Later, PT2 and PT3 joined the biweekly schedule part of the trial (denoted as PT2* and PT3*). Percentage of wound closure: 85–100% (+++); 50–85% (++); < 50% (+). PT, patient. aApproximate age of recurrent wounds based on the patients’ recollection. bPercentage of wound closure based on clinical photographs taken at day 0, month 1 and month 3 (see ‘Patients and methods’).
When following the biweekly schedule IV gentamicin, all four monitored wounds demonstrated a minimum of 85% closure at 1 and 3 months post-treatment (Table 2). Two of the four wounds showed > 85% closure at 6 months post-treatment, and all intact sites remained closed throughout the trial period.
Intravenous gentamicin improved clinical parameters
Secondary study outcomes included assessment for clinical improvement and improvement in QoL using the EBDASI, the Skindex-16 and the Wong–Baker Faces Pain Rating Scale.25,26,31 Each clinical instrument was completed at baseline and at the 1-, 3- and 6-month post-treatment clinic visits.
The EBDASI is a clinically validated tool used to grade objectively the severity of disease in patients with EB. It consists of two subscales: the damage score reflects permanent, disease-induced damage such as scarring, contractures or milia formation, while the activity score reflects the more modifiable elements of the disease that may be amenable to treatment.
On the continuous schedule, compared with baseline, all three patients demonstrated a decrease in EBDASI total activity scores at the 1- and 3-month post-treatment visits (Table 3). In concordance with their reduced disease activity, the responses of PT1–PT3 to the Skindex-16 questionnaire showed a trend toward amelioration of physical symptoms (irritation, burning, stinging) and emotional symptoms (frustration, worry, embarrassment; Table 3).
Table 3.
Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI) and quality of life survey results for patients with recessive dystrophic epidermolysis bullosa participating in a trial of continuous and biweekly intravenous gentamicin
| Clinical score | Timepoint | PT1 | PT2 | PT3 | PT2* | PT3* |
|---|---|---|---|---|---|---|
| EBDASI total score (activity, damage) | Day 0 | 47, 109 | 66, 135 | 23, 107 | 52, 148 | 25, 126 |
| 1 month | 27, 136 | 52, 135 | 20, 109 | 64, 141 | 22, 121 | |
| 3 month | 45, 118 | 22, 139 | 17, 109 | 43, 144 | 22, 110 | |
| 6 month | 34, 121 | 43, 121 | 25, 126 | 60, 156 | 26, 117 | |
| Skindex-16: Symptoms | Day 0 | 13 | 15 | 15 | 16 | 6 |
| 1 month | 1 | 12 | 9 | 10 | 4 | |
| 3 month | 4 | 9 | 4 | 10 | 6 | |
| 6 month | 0 | 10 | 6 | 8 | 8 | |
| Skindex-16: Emotions | Day 0 | 2 | 6 | 34 | 6 | 12 |
| 1 month | 1 | 2 | 25 | 0 | 12 | |
| 3 month | 1 | 3 | 17 | 1 | 12 | |
| 6 month | 0 | 2 | 12 | 1 | 17 | |
| Skindex-16: Functioning | Day 0 | 2 | 1 | 8 | 4 | 5 |
| 1 month | 0 | 1 | 5 | 1 | 7 | |
| 3 month | 0 | 2 | 5 | 2 | 5 | |
| 6 month | 0 | 4 | 5 | 2 | 5 |
Patients 1–3 (PT1–PT3) participated in the continuous schedule part of the trial. Later, PT2 and PT3 joined the biweekly schedule part of the trial (denoted as PT2* and PT3*).
On the biweekly schedule, PT2* and PT3* demonstrated decreases in their EBDASI total activity and damage scores at the 3-month post-treatment visit vs. baseline. While PT2* demonstrated improved Skindex-16 scores in each subcategory (symptoms, emotions and function), the QoL scores of PT3* did not change significantly. Improvement in EBDASI total activity scores was observed in both the continuous-schedule (PT2 and PT3) and biweekly study arms (PT2* and PT3*), but only the biweekly schedule resulted in improvement in EBDASI total damage scores (Table 3).
Intravenous gentamicin did not produce adverse effects
Another primary study endpoint was the absence of adverse effects to IV gentamicin, in particular ototoxicity and nephrotoxicity. No detectable changes from baseline or abnormalities were noted on pure tone audiometry, BUN or creatinine clearance in any of the patients on either the continuous or biweekly schedules. Additional safety parameters, such as complete blood count, electrolyte level and LFTs, were closely monitored throughout the study (Tables S1, S2; see Supporting Information). No notable changes were detected in these parameters.
Additionally, patients were monitored for the development of new C7 autoantibodies, a potential adverse effect of any RDEB treatment that introduces new full-length C7 to the patient’s immune system. Epidermolysis bullosa acquisita (EBA), a well-documented acquired autoimmune bullous disease, is known to be associated with the presence of autoantibodies targeting C7.29,30 Patients with EBA produce pathogenic anti-C7 antibodies that bind to and perturb the function of their AFs. To monitor for the potential induction of C7 autoantibodies, sera from patients were collected at day 0 and at 1, 3 and 6 months post-treatment and subjected to ELISA plates coated with C7. None of the three patients showed any increase in anti-C7 IgG after IV gentamicin treatment on either the continuous or bi-weekly schedules (Figure S2a; see Supporting Information). Furthermore, DIF staining performed on patient skin biopsies did not show any anti-C7 deposits in the DEJ (Figure S2b; see Supporting Information). The induction of new C7 by gentamicin did not elicit an autoimmune response.
Discussion
In this study, three patients with RDEB harbouring nonsense mutations were administered IV gentamicin continuously for 14 days, and two of three patients further received IV gentamicin twice weekly for 12 weeks. With either regimen, IV gentamicin treatment resulted in increased C7 levels at the DEJ of both healed wounds and unwounded intact skin samples at the 1-, 3- and 6-month post-treatment visits. The induction of newly generated C7 was associated with the closure of monitored wound sites. No adverse effects occurred in any of the patients, and no patient generated anti-C7 autoantibodies, despite the induction of newly created C7 protein.
Patients with RDEB have reduced C7 at the DEJ of their skin. Satisfactory dermal–epidermal adherence requires C7 levels at least 35% of that found in NHS.32,33 The therapeutic efficacy of gentamicin-induced PTC readthrough relies on both the quantity and functionality of the C7 generated. Our prior in vitro experiments demonstrated that gentamicin increased C7 in cultured RDEB keratinocytes and fibroblasts with nonsense mutations.22 In the current study, PT1 and PT2 were found to have baseline C7 between 0% and 5% the levels of NHS. One month after continuous-schedule IV gentamicin, there was a marked increase in C7 expression in both patients, with levels ranging between 45% and 91% that of NHS. PT3’s baseline expression level of 50% that of NHS probably represented the detection of mutant C7 protein produced by a missense allele. Nevertheless, PT3 also experienced a striking rise in C7 expression to 125% at the 1-month post-treatment follow-up. Most importantly, increased expression of C7 was also detected in the intact skin sites, suggesting that IV gentamicin could prevent future blistering and wounding. Consistent with our previous study of the application of topical gentamicin to RDEB skin wounds, we found that IV gentamicin induced C7 that was durable for 6 months. Collagens are large, stable macromolecules with long half-lives.34,35 With data indicating a robust response to short-term gentamicin treatment and the marked stability of C7, we envision that IV gentamicin could be delivered as a short-term pulse therapy every 3–6 months.
Considering that most open chronic erosive lesions in patients with RDEB are colonized with ambient bacteria, any agent that decreases bacterial counts could theoretically improve wound closure.36 Hence, gentamicin’s ability to improve wound closure could be secondary to its antimicrobial properties rather than its NMRT capabilities. Yet, none of the patients at any point in this study demonstrated frank signs of infection. The majority of our cohort exhibited increased C7 expression, enhanced wound closure and improved EBDASI total activity scores at 3 months post-treatment. Thus, the probable major mechanism by which gentamicin improved clinical wound healing was through the induction of new, functional, properly located and stable C7 at the DEJ.
This study was designed with two arms. In the first arm (continuous schedule), all three patients received daily IV gentamicin for 14 days. PT2* and PT3* were re-enrolled in the second arm (biweekly schedule) and received twice-weekly infusions for 12 weeks. We observed that while on the biweekly schedule, PT2* and PT3* demonstrated stronger expression of C7 at the 1- and 3-month post-treatment visits vs. the continuous schedule. Furthermore, the biweekly schedule offered greater convenience and flexibility for patients. However, a solid conclusion on which schedule is more efficacious was limited by the small sample size.
Several aminoglycosides have been used in vitro for genetic diseases caused by nonsense mutations, but gentamicin has demonstrated superiority over other agents in the aminoglycoside class. Furthermore, gentamicin is the only aminoglycoside that has been used to induce PTC readthrough in clinical trials for various genetic diseases caused by nonsense mutations.14,15 Additionally, gentamicin therapy has many advantages over other treatments, including gene- and cell-based treatments. Firstly, patients with RDEB are not required to be exposed to live cells, exogenous DNA or RNA, or viral vectors. Secondly, gentamicin, which is widely available commercially, offers a relatively safe, affordable and logistically simple treatment option. Lastly, gentamicin has been extensively studied and its potential side-effects are well known, making it feasible for continuous or biweekly IV administration in an outpatient clinic or at home. Although this study did not enrol paediatric patients, our prior clinical trial using similar doses of IV gentamicin in paediatric patients with junctional epidermolysis bullosa did not demonstrate any adverse effects.37 Therefore, we are hopeful that using continuous or biweekly IV gentamicin will be equally safe for paediatric patients with RDEB.
In summary, this study demonstrated that IV gentamicin can suppress nonsense mutations in patients with RDEB and generate sufficient levels of C7 in skin to improve wound healing and reduce EBDASI activity scores. Future research goals include optimization of systemic gentamicin treatment, with a particular focus on the frequency of administration and dosage. We believe that gentamicin may provide a relatively safe and efficacious therapy for the 30% of patients with RDEB who harbour nonsense mutations and that this treatment may hold great promise for other inherited dermatoses caused by nonsense mutations.
Supplementary Material
Acknowledgements
We thank the patients for granting permission to publish this information.
Contributor Information
David T Woodley, Department of Dermatology, The Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Michelle Hao, Department of Dermatology, The Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Andrew Kwong, Department of Dermatology, The Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Brandon Levian, Department of Dermatology, The Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Jon Cogan, Department of Dermatology, The Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Yingping Hou, Department of Dermatology, The Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Daniel Mosallaei, Department of Dermatology, The Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Elana Kleinman, Department of Dermatology, The Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Kate Zheng, Department of Dermatology, The Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Claire Chung, Department of Dermatology, The Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Gene Kim, Department of Dermatology, The Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
David Peng, Department of Dermatology, The Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Mei Chen, Department of Dermatology, The Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Funding sources
National Institutes of Health, Epidermolysis Bullosa Research Partnership, Epidermolysis Bullosa Medical Research Foundation, Directed Medical Research Program, and VA Merit Award.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and the Supporting Information.
Ethics statement
The study protocol was approved by the Institutional Review Board (IRB) of the University of Southern California and all investigations were conducted according to the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients prior to the onset of the study. Written informed consent was received for the use of photographs from all patients prior to the onset of the study and the record of informed consent has been retained.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s website.
References
- 1. Pfendner EG, Lucky AW. Dystrophic epidermolysis bullosa. Available at: http://www.ncbi.nlm.nih.gov/books/NBK1304 (last accessed 12 April 2020).
- 2. Uitto J, Christiano AM. Molecular genetics of the cutaneous basement membrane zone. Perspectives on epidermolysis bullosa and other blistering skin diseases. J Clin Invest 1992; 90:687–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parente MG, Chung LC, Ryynänen J et al. Human type VII collagen: cDNA cloning and chromosomal mapping of the gene. Proc Natl Acad Sci U S A 1991; 88:6931–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fine J-D, Hintner H. Life with Epidermolysis Bullosa (EB): Etiology, Diagnosis, Multidisciplinary Care and Therapy. Springer; , 2009. [Google Scholar]
- 5. Li AW, Prindaville B, Bateman ST et al. Inpatient management of children with recessive dystrophic epidermolysis bullosa: a review. Pediatr Dermatol 2017; 34:647–55. [DOI] [PubMed] [Google Scholar]
- 6. Zhou X, Zhang Y, Zhao M et al. Surgical management of hand deformities in patients with recessive dystrophic epidermolysis bullosa. J Plast Surg Hand Surg 2020; 54:33–9. [DOI] [PubMed] [Google Scholar]
- 7. Woodley DT, Remington J, Huang Y et al. Intravenously injected human fibroblasts home to skin wounds, deliver type VII collagen, and promote wound healing. Mol Ther J Am Soc Gene Ther 2007; 15:628–35. [DOI] [PubMed] [Google Scholar]
- 8. Woodley DT, Keene DR, Atha T et al. Injection of recombinant human type VII collagen restores collagen function in dystrophic epidermolysis bullosa. Nat Med 2004; 10:693–5. [DOI] [PubMed] [Google Scholar]
- 9. Chen M, Kasahara N, Keene DR et al. Restoration of type VII collagen expression and function in dystrophic epidermolysis bullosa. Nat Genet 2002; 32:670–5. [DOI] [PubMed] [Google Scholar]
- 10. Hou P-C, Del Agua N, Lwin SM et al. Innovations in the treatment of dystrophic epidermolysis bullosa (DEB): current landscape and prospects. Ther Clin Risk Manag 2023; 19:455–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wagner JE, Ishida-Yamamoto A, McGrath JA et al. Bone marrow transplantation for recessive dystrophic epidermolysis bullosa. N Engl J Med 2010; 363:629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Siprashvili Z, Nguyen NT, Gorell ES et al. Safety and wound outcomes following genetically corrected autologous epidermal grafts in patients with recessive dystrophic epidermolysis bullosa. JAMA 2016; 316:1808–17. [DOI] [PubMed] [Google Scholar]
- 13. Guide SV, Gonzalez ME, Bağcı IS et al. Trial of Beremagene Geperpavec (B-VEC) for dystrophic epidermolysis bullosa. N Engl J Med 2022; 387:2211–19. [DOI] [PubMed] [Google Scholar]
- 14. Linde L, Kerem B. Introducing sense into nonsense in treatments of human genetic diseases. Trends Genet 2008; 24:552–63. [DOI] [PubMed] [Google Scholar]
- 15. Bidou L, Allamand V, Rousset J-P, Namy O. Sense from nonsense: therapies for premature stop codon diseases. Trends Mol Med 2012; 18:679–88. [DOI] [PubMed] [Google Scholar]
- 16. Barton-Davis ER, Cordier L, Shoturma DI et al. Aminoglycoside antibiotics restore dystrophin function to skeletal muscles of mdx mice. J Clin Invest 1999; 104:375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bedwell DM, Kaenjak A, Benos DJ et al. Suppression of a CFTR premature stop mutation in a bronchial epithelial cell line. Nat Med 1997; 3:1280–4. [DOI] [PubMed] [Google Scholar]
- 18. Heier CR, DiDonato CJ. Translational readthrough by the aminoglycoside geneticin (G418) modulates SMN stability in vitro and improves motor function in SMA mice in vivo. Hum Mol Genet 2009; 18:1310–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Keeling KM, Brooks DA, Hopwood JJ et al. Gentamicin-mediated suppression of Hurler syndrome stop mutations restores a low level of alpha-L-iduronidase activity and reduces lysosomal glycosaminoglycan accumulation. Hum Mol Genet 2001; 10:291–9. [DOI] [PubMed] [Google Scholar]
- 20. Wagner KR, Hamed S, Hadley DW et al. Gentamicin treatment of Duchenne and Becker muscular dystrophy due to nonsense mutations. Ann Neurol 2001; 49:706–11. [PubMed] [Google Scholar]
- 21. Kuschal C, DiGiovanna JJ, Khan SG et al. Repair of UV photolesions in xeroderma pigmentosum group C cells induced by translational readthrough of premature termination codons. Proc Natl Acad Sci U S A 2013; 110:19483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cogan J, Weinstein J, Wang X et al. Aminoglycosides restore full-length type VII collagen by overcoming premature termination codons: therapeutic implications for dystrophic epidermolysis bullosa. Mol Ther 2014; 22:1741–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Woodley DT, Cogan J, Hou Y et al. Gentamicin induces functional type VII collagen in recessive dystrophic epidermolysis bullosa patients. J Clin Invest 2017; 127:3028–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sermet-Gaudelus I, Renouil M, Fajac A et al. In vitro prediction of stop-codon suppression by intravenous gentamicin in patients with cystic fibrosis: a pilot study. BMC Med 2007; 5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chren MM, Lasek RJ, Sahay AP, Sands LP. Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg 2001; 5:105–10. [DOI] [PubMed] [Google Scholar]
- 26. Garra G, Singer AJ, Taira BR et al. Validation of the Wong–Baker FACES Pain Rating Scale in pediatric emergency department patients. Acad Emerg Med 2010; 17:50–4. [DOI] [PubMed] [Google Scholar]
- 27. Kwong A, Cogan J, Hou Y et al. Gentamicin induces laminin 332 and improves wound healing in junctional epidermolysis bullosa patients with nonsense mutations. Mol Ther 2020; 28:1327–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Woodley DT, Cogan J, Wang X et al. De novo anti-type VII collagen antibodies in patients with recessive dystrophic epidermolysis bullosa. J Invest Dermatol 2014; 134:1138–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Woodley DT, Burgeson RE, Lunstrum G et al. Epidermolysis bullosa acquisita antigen is the globular carboxyl terminus of type VII procollagen. J Clin Invest 1988; 81:683–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Woodley DT, Briggaman RA, O’Keefe EJ et al. Identification of the skin basement-membrane autoantigen in epidermolysis bullosa acquisita. N Engl J Med 1984; 310:1007–13. [DOI] [PubMed] [Google Scholar]
- 31. Loh CCH, Kim J, Su JC et al. Development, reliability, and validity of a novel Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI). J Am Acad Dermatol 2014; 70:89–97. [DOI] [PubMed] [Google Scholar]
- 32. Tidman MJ, Eady RA. Evaluation of anchoring fibrils and other components of the dermal-epidermal junction in dystrophic epidermolysis bullosa by a quantitative ultrastructural technique. J Invest Dermatol 1985; 84:374–7. [DOI] [PubMed] [Google Scholar]
- 33. Kern JS, Loeckermann S, Fritsch A et al. Mechanisms of fibroblast cell therapy for dystrophic epidermolysis bullosa: high stability of collagen VII favors long-term skin integrity. Mol Ther 2009; 17:1605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burgeson RE. Type VII collagen, anchoring fibrils, and epidermolysis bullosa. J Invest Dermatol 1993; 101:252–5. [DOI] [PubMed] [Google Scholar]
- 35. Kühl T, Mezger M, Hausser I et al. Collagen VII half-life at the dermal-epidermal junction zone: implications for mechanisms and therapy of genodermatoses. J Invest Dermatol 2016; 136:1116–23. [DOI] [PubMed] [Google Scholar]
- 36. Alexeev V, Huitema L, Phillips T et al. T-cell activation and bacterial infection in skin wounds of recessive dystrophic epidermolysis bullosa patients. Exp Dermatol 2022; 31:1431–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mosallaei D, Hao M, Antaya RJ et al. Molecular and clinical outcomes after intravenous gentamicin treatment for patients with junctional epidermolysis bullosa caused by nonsense variants. JAMA Dermatol 2022; 158:366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and the Supporting Information.