Abstract
Global human immunodeficiency virus type 1 (HIV-1) diversity may require engineering vaccines to express antigens representing strains prevalent in the target population of vaccine testing. The majority (90%) of incident infections in Thailand are genetic subtype E, with a small percentage of subtype B infections in the intravenous drug user populations. We have evaluated and compared the binding and HIV-1 neutralizing properties of serum antibodies induced in baboons by CHO cell-expressed monomeric gp120 derived from a CCR5-using (R5) subtype E primary HIV-1CM235 or a CXCR4-using (X4) subtype B T-cell line-adapted (TCLA) HIV-1SF2 isolate. In contrast to the subtype-specific HIV-1 neutralizing antibodies induced with recombinant HIV-1SF2 gp120 (rgp120SF2), rgp120CM235 immunization induced antibodies capable of neutralizing both subtype E and subtype B TCLA HIV-1 isolates. However, neither immunogen induced antibodies capable of neutralizing primary HIV-1 isolates. Antibody induced by rgp120CM235 preferentially bound natively folded gp120 and retained strong cross-reactivity against multiple gp120 strains within subtype E as well as subtype B. In contrast, antibody responses to rgp120SF2 were directed predominantly to linear epitopes poorly exposed on native gp120 and had more limited cross-recognition of divergent gp120. Fine epitope mapping revealed differences in antibody specificities. While both rgp120CM235 and rgp120SF2 induced antibodies to regions within C1, V1/V2, V3, and C5, unique responses were induced by rgp120CM235 to multiple epitopes within C2 and by rgp120SF2 to multiple epitopes within C3, V4, and C4. These data demonstrate that strain and/or phenotypic differences of HIV-1 subunit gp120 immunogens can substantially alter antibody binding specificities and subsequent HIV-1 neutralizing capacity.
Most human immunodeficiency virus type 1 (HIV-1) subunit vaccine candidates are based on genes from prototype T-cell line-adapted (TCLA) subtype B viruses. Examples are gp120 and gp160 immunogens based on HIV-1 strain IIIB, MN, or SF2. Since the HIV-1 epidemic in southeast Asia is largely caused by subtype E viruses (35, 43, 56–58), it may be important to evaluate vaccines expressing antigens from subtype E for use in this region. Subtype E HIV-1 is antigenically distinct from subtype B; sera (39, 40) and neutralizing monoclonal antibodies (MAbs) (48, 78) derived from subtype B-infected donors preferentially neutralize viruses from the same subtype, though other studies have not identified such an association between HIV-1 serum neutralization serotype and genetic subtype (29, 33). HIV-1 sera from subtype B- and E-infected individuals bind preferentially to HIV-1 gp120 and gp160 from subtypes B and E, respectively (39, 80). However, while gp120 from subtype B and subtype E may be distinct antigenically, it remains to be determined whether as immunogens they are capable of inducing cross-subtype functional immune responses. An example of discordance between HIV-1 gp120 antigenic and immunogenic properties was demonstrated by the ability of column-immobilized gp120 to remove primary isolate-neutralizing antibody activity from HIV-1 serum and its inability to elicit such antibodies in animals (70).
Previous subunit HIV-1 envelope vaccines using monomeric forms of gp120 or gp160 are immunogenic in small animals, primates, and humans, but the antibody responses, though capable of neutralizing TCLA HIV-1 isolates, have limited neutralizing activity against primary HIV-1 isolates (4, 25, 30, 41, 42, 67, 85); however, recent studies using a resting cell assay obtained significant neutralization of several X4-using primary HIV-1 isolates by sera from individuals immunized with monomeric recombinant HIV-1SF2 gp120 (rgp120SF2) (10, 88). These results may be attributable to the inefficiency of these monomeric gp120 vaccines to elicit antibodies specific for conserved, discontinuous epitopes, since the majority of antibodies are focused primarily to linear epitopes poorly accessible on cell surface expressed gp120-gp41 (81). Monomeric gp120 or gp160 vaccines based on TCLA isolates, therefore, may lack structural properties critical for the ability to induce broadly reactive and neutralizing antibody. These structural properties may be related to the adaptation of the HIV-1 envelope strain, since TCLA and primary isolates have been demonstrated to have significant phenotypic differences with respect to coreceptor usage (1, 14, 15, 18) and susceptibility to antibody- or serum-mediated neutralization (2, 7, 13, 45, 63, 65). Immunization with monomeric gp120 from strains MN and SF2 protected chimpanzees against homologous and heterologous primary isolate HIV-1SF2 challenge (5, 17), and a vaccine containing rgp120SF2 protected rhesus macaques against challenge with the closely related SHIVSF13 (26). However, several individuals enrolled in clinical trials of candidate monomeric gp120 subunit vaccines became HIV-1 infected despite receiving the full vaccination regimen (12, 31, 44), indicating that these vaccines are less than 100% effective.
There are several potently neutralizing MAbs which map to regions accessible on monomeric gp120 or gp41 (8, 11, 21, 23, 52, 53, 60, 75, 77, 78). The neutralizing epitopes, present on monomeric gp120, are not currently immunogenic when presented in the context of a vaccine. The majority of the broadly anti-gp120 neutralizing MAbs are directed to conformational epitopes that have been particularly difficult to elicit with monomeric HIV-1 subunit vaccines. Studies designed to correlate antibody binding and neutralizing capacity have shown poor correlation with binding to monomeric gp120 and superior correlation with binding to oligomeric forms of HIV-1 envelope (19, 45, 64), though this correlation is not complete for all antibodies (20). This attribute is likely due to highly antigenic, but not functional, epitopes that are more accessible on gp120 and less accessible in the context of membrane-expressed oligomeric gp120-gp41 (19, 45, 50, 64, 69, 72). Presentation of gp120 as part of an uncleaved oligomeric gp140 protein resulted in the induction of antibodies capable of neutralizing divergent TCLA isolates as well as some susceptible primary HIV-1 isolates (83).
In this study, we compared the binding and neutralizing properties of serum antibodies induced in baboons immunized with rgp120 from either a subtype B X4 TCLA (HIV-1SF2) or a subtype E R5 primary (HIV-1CM235) HIV-1 isolate. Sera collected after a series of immunizations were evaluated for antibody binding and neutralization properties. The two immunogens induced antibody responses that were readily distinguished. The X4 TCLA gp120 induced antibodies specific for multiple linear epitopes within gp120 that are poorly exposed on native gp120 and neutralized only subtype B TCLA HIV-1 isolates. In contrast, the R5 primary gp120CM235 induced antibodies preferentially reactive with native gp120, bound to multiple subtype B and E gp120 strains, and neutralized both subtype B and subtype E TCLA isolates. These data demonstrate that HIV-1 envelope glycoprotein structural and antigenic properties, associated with HIV-1 strain and/or phenotype, have substantial impact on vaccine immunogenicity in nonhuman primates.
MATERIALS AND METHODS
Materials.
The rgp120SF2 subunit protein was produced in CHO cells and has been previously described (24); rgp120CM235 was prepared by similar methods. Both proteins are correctly glycosylated and bind CD4, and preparations are >90% pure. HIV-1SF2 is a TCLA isolate that uses the X4 coreceptor. HIV-1CM235 is a primary isolate that uses the R5 coreceptor and was originally isolated by cocultivation in peripheral blood mononuclear cells (PBMC) from a subtype E HIV-1-infected individual living in Chiang Mai, Thailand (43). MF-59, a squalene-water emulsion containing 5% squalene, 0.5% Tween 80, and 0.5% Span 85 (79), was used as the adjuvant for both rgp120SF2 and rgp120CM235. Denaturation of gp120SF2 and gp120CM235 was performed by reduction and carboxymethylation as described elsewhere (37). Briefly, the proteins were chemically denatured with 6 M guanidine, reduced in 10 mM dithiothreitrol under N2, carboxymethylated with 50 mM iodoacetamide, and dialyzed back into phosphate-buffered saline (PBS) before use. Peptides from the third hypervariable (V3) region of gp120 (V3SF2 and V3CM242) were synthesized by GenoSys (The Woodlands, Tex.) to >80% purity as assessed by amino acid analysis, high-pressure liquid chromatography, and mass spectrometry. The subtype E strain CM235 was selected based on its similarity with consensus subtype E throughout gp120 with the exception of the V3 loop region. The V3 peptide sequence for strain CM242 was used in the construction of rgp120CM235 since the V3 loop corresponding to CM242 was more representative of the Thai E V3 consensus sequence at the time of isolation. The native CM235 and CM242 sequences differ in two amino acids within the V3 loop (43).
Baboon immunizations.
Five baboons were immunized intramuscularly (i.m.) at 0, 1, 2, and 6 months with 50 μg of rgp120SF2 (Chiron Corporation, Emeryville, Calif.) formulated in MF-59 adjuvant (Chiron). Additionally, 10 baboons were immunized i.m. at 0, 1, and 6 months with 50 μg of rgp120CM235 (Chiron) formulated in MF-59 adjuvant. All baboon immunizations were performed at the primate center at the Southwest Foundation for Biomedical Research (San Antonio, Tex.). Sera were collected 2 weeks after the 6-month rgp120SF2 or rgp120CM235 immunization for analysis. Sera collected 2 weeks after the third rgp120SF2 immunization (month 2) were also evaluated for neutralizing and binding antibody properties. No significant differences in binding or neutralizing antibody were detected in sera collected after the third or fourth immunization. Therefore, all data reported are from sera collected after the 6-month (fourth) rgp120SF2 immunization. Table 1 summarizes the vaccine formulation, dose, schedule, and antibody titer for each baboon in the two groups.
TABLE 1.
Immunization schedule, dose, and antibody endpoint binding titers against homologous and cross-subtype monomeric rgp120 and V3 peptides
| Baboon | Immunogen | Dose (μg); schedule (mo) | Serum antibody endpoint binding titer (ELISA)a
|
|||
|---|---|---|---|---|---|---|
| rgp120
|
V3 peptide
|
|||||
| SF2 | CM235 | SF2 | CM235 | |||
| Clade B | ||||||
| B54 | rgp120SF2/MF-59 | 50; 0, 1, 2, 6 | 51,200 | 51,200 | 6,400 | <100 |
| B55 | rgp120SF2/MF-59 | 50; 0, 1, 2, 6 | 204,800 | 51,200 | 51,200 | <100 |
| B56 | rgp120SF2/MF-59 | 50; 0, 1, 2, 6 | 51,200 | 12,800 | 25,600 | <100 |
| B57 | rgp120SF2/MF-59 | 50; 0, 1, 2, 6 | 102,400 | 12,800 | ND | <100 |
| B58 | rgp120SF2/MF-59 | 50; 0, 1, 2, 6 | 25,600 | 12,800 | 3,200 | <100 |
| Geometric mean | 67,600 | 22,300 | ||||
| Clade E | ||||||
| B62 | rgp120CM235/MF-59 | 50; 0, 1, 6 | 12,800 | 102,400 | <100 | 800 |
| B63 | rgp120CM235/MF-59 | 50; 0, 1, 6 | 12,800 | 51,200 | <100 | 200 |
| B64 | rgp120CM235/MF-59 | 50; 0, 1, 6 | 12,800 | 51,200 | <100 | 400 |
| B65 | rgp120CM235/MF-59 | 50; 0, 1, 6 | 51,200 | 409,600 | <100 | 1,600 |
| B66 | rgp120CM235/MF-59 | 50; 0, 1, 6 | 25,600 | 102,400 | 100 | 1,600 |
| B67 | rgp120CM235/MF-59 | 50; 0, 1, 6 | 25,600 | 102,400 | <100 | 800 |
| B68 | rgp120CM235/MF-59 | 50; 0, 1, 6 | 25,600 | 409,600 | 100 | 400 |
| B69 | rgp120CM235/MF-59 | 50; 0, 1, 6 | 3,200 | 12,800 | <100 | 100 |
| B70 | rgp120CM235/MF-59 | 50; 0, 1, 6 | 25,600 | 51,200 | <100 | 200 |
| B71 | rgp120CM235/MF-59 | 50; 0, 1, 6 | 25,600 | 204,800 | <100 | 3,200 |
| Geometric mean | 18,100 | 95,500 | ||||
Antibody endpoint binding titers of preimmune baboon sera against each of the antigens were <100. ND, not determined.
Measurement of serum antibody binding titers by EIA.
To measure binding to the immunogen, an enzyme immunoassay (EIA) was used as described previously (83). Briefly, rgp120SF2 (0.63 μg/ml), rgp120CM235 (0.63 μg/ml), or V3 peptide V3SF2 (subtype B) or V3CM242 (subtype E) (1 μg/ml) in PBS (pH 7.4; with 0.01% thimerosal) was coated overnight at 4°C onto Immulon 2 microtiter plates. Plates were washed twice with wash buffer (PBS with 0.1% Tween 20 [pH 7.4]) prior to incubation with twofold dilutions of baboon sera (diluted in wash buffer with 5% skim milk [pH 7.4]) for 1 h at 37°C. Plates were washed three times with wash buffer and incubated with horseradish peroxidase-conjugated goat anti-human immunoglobulin G (diluted 1:4,000 in wash buffer with 5% skim milk, pH 7.4); Southern Biotechnologies, Birmingham, Ala.). After a 1-h incubation at 37°C, plates were washed three times, after which the substrate ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid); Kirkegaard & Perry, Gaithersburg, Md.] was added. The reaction was stopped with 0.5% sodium dodecyl sulfate after 30 min at 37°C. Serum endpoint titers were determined as the highest serum dilution with enzyme-linked immunosorbent assay (ELISA) optical density (OD) signals greater than twice the mean plus two times the standard deviations of the individual preimmune baboon sera (typically >0.10 OD).
Native/denatured gp120 binding ratios determined by SPR.
To determine the native/denatured gp120 binding ratio, surface plasmon resonance (SPR) (BIAcore 2000; BIAcore Inc., Piscataway, N.J.) was used as described previously (81–83). Immobilizations of proteins (rgp120SF2, rgp120CM235, reduced, carboxymethylated [rcm] gp120SF2, and rcmgp120CM235) to the BIAcore biosensor dextran matrix were performed with 100 mM N-hydroxysuccinimide and 400 mM N-ethyl-N′-(3-diethylaminopropyl) carbodiimide hydrochloride (EDC) chemical activation and coupling through free amine groups on the proteins. Unreacted EDC-esters were deactivated by reacting with an injection of 1 M ethanolamine. Immobilized rgp120 retains binding to CD4 and antibodies mapping to conformation epitopes, demonstrating minimal loss in protein structure upon covalent coupling to the biosensor matrix (82). Pre- and postimmune baboon sera and HIV-1 sera (1:100) were diluted in HBS running buffer (10 mM HEPES, 150 mM NaCl, 3.4 mM EDTA, 0.05% BIAcore surfactant P20 [pH 7.4]), and 30-μl aliquots were injected through the immobilized protein matrices at a flow rate of 5 μl/min. The net difference in baseline signal (in response units [RU]) and signal after completion of antibody injection was taken to represent the binding value of a particular sample. Regeneration was performed with two 5-μl pulses of 60 mM H3PO4 for both the SF2 and CM235 rgp120 and rcmgp120 matrices. Binding of the individual preimmune sera was subtracted from that of each of the postimmune sera to yield the corrected serum binding value. Binding of preimmune sera to each protein was <20 RU. Corrected serum binding values to both rgp120 and rcmgp120 were then divided to yield the native/denatured gp120 binding ratio.
Linear epitope mapping.
The entire sequences of both immunizing proteins, rgp120SF2 and rgp120CM235, excluding the signal sequences, were modeled with 12-mer peptides overlapping by eight amino acids, 118 peptides each. Peptides were synthesized on the heads of synthetic pins with cleavable diketopiperazine linkages (Chiron Mimotopes, San Diego, Calif.) by using 9-fluorenylmethoxycarbonyl chemistry and cleaved from the pins as previously described (38). Peptides were covalently linked to biotin at the N terminus by a short peptide linker (Ser-Gly-Ser-Gly) and are denoted by the N-terminal amino acid. Peptides (typically ≥80% purity) were used without further purification as antigens in an ELISA. Briefly, plates were coated with streptavidin (0.25 μg/well) overnight and then peptides (0.1 μg/well) for 1 h. Pre- and postimmune sera were diluted 1:1,000 in 5% nonfat dry milk in PBS and incubated with peptides for 2 h at room temperature. After washing, anti-human secondary antibody was added at 1:1,000 for 1 h. Appropriate substrate was added, and plates were read kinetically. All assays were carried out in at least duplicate. Median serum reactivity against 20 background peptides bound to streptavidin was subtracted from all readings. Readings above 3 OD/min and absent in the preimmune sera were considered significant.
Monomeric gp120 capture assay.
Culture supernatants of TCLA viruses from subtype B (IIIB and SF2) and subtype E (NPO3, 9466, 9461, and 42368) were harvested from acutely infected H9 cells. Primary HIV-1 (92/US/660, SF13, 92/US/717, and 9031) and subtype E (9466, CM235, CMU06, and 8868) isolates were isolated from PBMC of subjects infected with subtype B and E viruses, respectively. At peak p24 antigen production, supernatants were harvested and lysed with Empigen BB (1.0%) for 1 h at room temperature. Lysates were diluted in PBS with 5% skim milk and 0.1% Tween 20 and captured by sheep antibodies (D7324) to the C terminus of gp120 (49) adsorbed to wells of Immulon 2 microtiter plates (2.5 μg/ml in sodium bicarbonate buffer [pH 9.6] overnight). Test sera were diluted in PBS with 5% skim milk, 0.1% Tween 20 and 5% normal goat serum, titrated down the plate (twofold), and detected with appropriate horseradish peroxidase-labeled secondary antibody as described above (83). HIV-1 serum pools from 25 subtype B-infected and 25 subtype E-infected individuals were used as positive controls and to control for the amount of gp120 captured from each viral stock. Pooled normal human serum was used as a negative control for the HIV-1 serum pools, and preimmune baboon sera were used as negative controls for the baboon immune sera. Endpoint titers were determined as described above. Binding ratios were determined by dividing the endpoint titer of each subtype B or subtype E baboon serum by the endpoint titer of the B or E HIV-1 serum pool, respectively.
HIV-1 neutralization assay.
Neutralization assays, using H9 target cells for TCLA viruses or stimulated PBMC targets for primary isolates, were performed as previously described (39, 41). Briefly, test sera and virus inoculum (100 50 tissue culture infective doses) were preincubated for 30 min prior to addition of target cells. After overnight incubation, cells were washed and resuspended in appropriate culture media. Virus growth was monitored by ELISA measurement of p24 antigen in culture supernatants, and neutralization was determined during the early virus growth phase (days 4 to 6). Pre- and postimmune baboon sera were directly compared in all assays at a dilution of 1:10 in the presence of virus and a final dilution of 1:20 after the addition to target cells. Data represented as fold reduction were derived by the ratio of p24 antigen in preimmune sera to that in postimmune sera. Thus, a 10-fold reduction in p24 antigen indicates 90% neutralization. Human sera from subjects infected with subtype E and B HIV-1 were used as positive controls. All sera were complement depleted by heat inactivation at 56°C for 40 min prior to use. Subtype B TCLA viruses MN, IIIB, SF2, and RF were obtained from the NIH AIDS Research and Reference Reagent Program. The subtype E TCLA viruses were adapted to chronic growth in H9 cells and verified to be neutralization sensitive to soluble CD4 and a subtype E-derived anti-V3 MAb (22). The corresponding primary subtype E isolate HIV-9461 was a gift from Jay Levy (University of California, San Francisco), and HIV-NP03 and 42368 were provided by researchers at the Armed Forces Research Institute of the Medical Sciences in Bangkok, Thailand. The primary isolate CM235 (homologous to the vaccine strain) is a CCR5-using isolate that could not be adapted to growth in H9 cells.
RESULTS
Immunogenicity of SF2 and CM235 in baboons.
Five baboons (B54 to B58) were immunized i.m. at 0, 1, 2, and 6 months with 50 μg of rgp120SF2, and 10 baboons (B62 to B71) were immunized i.m. at 0, 1, and 6 months with rgp120CM235. Both SF2 and CM235 gp120 were formulated with MF-59 adjuvant, and sera collected after the 6-month immunization were studied for binding to homologous and heterologous CHO monomeric gp120 and V3 peptides by ELISA (Table 1). Sera from all baboons bound to both SF2 and CM235 rgp120 but preferentially bound to gp120 from the strain homologous to the immunizing protein. SF2-immunized baboon ELISA titers to rgp120SF2 were up to eightfold higher than those to rgp120CM235. CM235-immunized baboon sera bound 2- to 16-fold more strongly to rgp120CM235 than to rgp120SF2. All baboons seroconverted to their homologous V3, but the binding was more type specific. In contrast to reactivity against heterologous gp120, no cross-reactivity to the heterologous V3 peptide was observed, with the exception of weak binding to V3 of SF2 by sera B66 and B68. These data suggest antibody cross-recognition of gp120 in regions outside of the V3 region.
Binding of baboon immune sera to rgp120SF2 and rgp120CM235 was directly compared to binding of subtype B and E HIV-1 serum pools by SPR (Table 2). Direct comparisons were possible since SPR measurements, unlike those by ELISA, do not require secondary antibodies for antibody detection and eliminate potential differential binding efficiencies of conjugated secondary antibodies to human and baboon immunoglobulin. Pooled HIV-1 sera from individuals infected with subtype E (n = 25) or subtype B (n = 25) HIV-1 were used for comparison. The US B HIV-1 serum pool binding (692 RU) was similar to the mean of the rgp120SF2-immunized baboon sera (715 RU), with two of the baboon sera (B55 and B57) showing higher antibody reactivity. The Thai E pool was >2-fold more reactive than the mean of the gp120CM235-immunized baboons, with no single baboon sera reaching antibody reactivity levels comparable to the pool. These data demonstrate that while antibody levels elicited by immunization by rgp120SF2 were comparable to those for subtype B HIV-1 infection, antibody levels elicited by immunization with rgp120CM235 were weaker than the average titers induced during subtype E HIV-1 infection.
TABLE 2.
Binding of sera from SF2 and CM235 gp120-immunized baboons and of HIV-1 serum pools to rgp120 from clades B and E
| Samplea | Binding (RU)b
|
|
|---|---|---|
| rgp120SF2 | rgp120CM235 | |
| SF2-immunized baboons | ||
| US B pool | 692 | |
| B54 | 447 | |
| B55 | 1,539 | |
| B56 | 502 | |
| B57 | 812 | |
| B58 | 276 | |
| Meanc | 715 | |
| CM235-immunized baboons | ||
| Thai E pool | 1,802 | |
| B62 | 847 | |
| B63 | 552 | |
| B64 | 687 | |
| B65 | 1,300 | |
| B66 | 986 | |
| B67 | 841 | |
| B68 | 1,072 | |
| B69 | 1,041 | |
| B70 | 120 | |
| B71 | 683 | |
| Meand | 1,118 | |
B54 to B58 were immunized with rgp120SF2, and B62 to B71 were immunized with rgp120CM235, as described in Table 1. US B and Thai E pools are pools of individual sera (n = 25 each) from HIV-1-infected individuals known to be infected with clade B and clade E HIV-1, respectively.
Values for individual serum samples represent corrected binding after subtraction of background from preimmune sera as described in Materials and Methods.
Arithmetic mean of the five SF2-immunized baboons.
Arithmetic mean of the 10 CM235-immunized baboons.
Binding to native and denatured gp120.
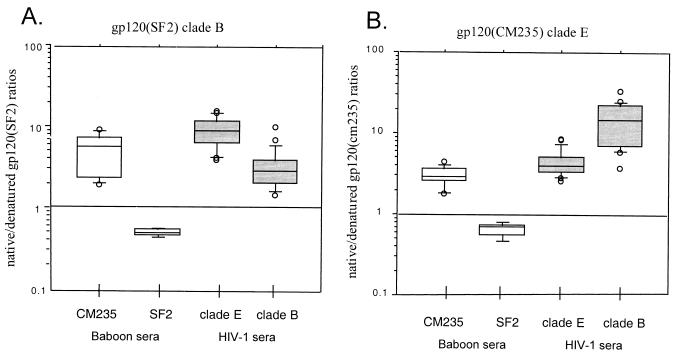
Sera from SF2 and CM235 gp120-immunized baboons were screened by SPR for reactivity against native and denatured forms of HIV-1 gp120 (81–83). Previous studies have shown that serum antibodies from human vaccinees receiving IIIB strains of HIV-1 gp120 or gp160 preferentially bound denatured gp120 independent of the CD4-binding competency of the immunogen (81), in contrast to the predominant antibody response to native gp120 elicited during natural HIV-1 infection (46, 81). Native/denatured gp120 antibody binding ratios to rgp120SF2 and rgp120CM235 for baboon sera and HIV-1 serum pools are shown in Fig. 1. Individual sera from 25 subtype B and 25 subtype E HIV-1-infected individuals were included as comparisons and bound preferentially to natively folded gp120 (2- to 30-fold) from both strains SF2 and CM235. The native/denatured binding ratios for both subtype B and E HIV-1 sera to the cross-subtype gp120 were higher than to the homologous subtype gp120. HIV-1 sera (subtype E) had mean ratios of 4.5 ± 1.8 against CM235 and 9.5 ± 3.9 against SF2, while HIV-1 sera (subtype B) had mean ratios of 3.4 ± 2.0 against SF2 and 14.2 ± 8.7 against CM235. This finding suggests a relative enrichment of binding specific for conformational epitopes within the cross-subtype-reactive antibody population. In contrast, sera from SF2-immunized baboons preferentially bound denatured gp120 from both the homologous SF2 gp120 (0.54 ± 0.06) and CM235 gp120 (0.64 ± 0.09), with comparable ratios against the two proteins. Sera from CM235-immunized baboons, however, preferentially bound natively folded gp120 from both SF2 and CM235, and the ratio increased from 2.9 ± 1.5 against the homologous CM235 to 6.6 ± 4.6 against the cross-subtype SF2 gp120, also suggesting that the cross-reactive epitopes are predominantly directed toward conformational epitopes. These ratios are within twofold of those obtained with the subtype E HIV-1 sera. These data demonstrate qualitative differences in the specificities elicited by these two immunogens with the CM235 capable of eliciting a greater fraction of antibodies specific for gp120 conformational epitopes.
FIG. 1.
Sera from baboons immunized with rgp120CM235 or rgp120SF2 were collected 2 weeks after the 6-month immunization for evaluation. Sera from individuals infected with subtype E (n = 25) or subtype B (n = 25) HIV-1 isolates were included as comparisons. The sera evaluated are listed along the x axis. Sera were evaluated for binding to native and denatured forms of rgp120SF2 (A) and rgp120CM235 (B). Data are plotted for individual sera as the ratio of native/denatured gp120 binding as determined by SPR.
Peptide epitope mapping.
Sera from SF2- and CM235-immunized baboons were screened against linear peptides (12-mers) encompassing the entire gp120 sequence from SF2 and CM235 gp120. Peptide-specific binding by the SF2 and CM235 baboon sera to the homologous as well as the cross-subtype set of peptides is summarized in Table 3. Sera from the SF2-immunized baboons responded strongly to epitopes throughout all conserved regions within gp120 (C1 to C5) as well as V2 to V5. The CM235-immunized baboons also responded strongly to several epitopes, but these were fewer in number and located within C1, V2, C2, V3, and C5. Regions immunogenic on both the CM235 and SF2 gp120 included C1, V2, C2, V3, and C5. The most striking differences in peptide specificities were within the C3, V4, and C4 regions. Sera from the SF2-immunized baboons recognized multiple peptides within C3, V4, and C4 (n = 14), while sera from the CM235-immunized baboons were minimally reactivity in this region. Sera from CM235-immunized baboons recognized multiple peptides within C2; in particular, peptide 234 was very reactive with CM235 baboon sera but not with the SF2 baboon sera. A summary of total homologous and cross-subtype peptide reactivities is shown in Table 3 for the CM235- and SF2-immunized baboons. The SF2-immunized baboons recognized a total of 38 homologous peptides, while the CM235-immunized baboons recognized a total of 17 homologous peptides. The total numbers of cross-subtype peptides recognized by both the CM235 and SF2 gp120 sera decreased to 8 and 14, respectively. The presence of several reactive cross-subtype peptides was consistent with the cross-subtype gp120 reactivity observed by ELISA (Table 1). For both immunogens, most of the cross-subtype peptide recognition was within C1, C5, and a peptide spanning the tip of the V3 loop (peptide 306). These data indicate that sera from SF2-immunized baboons reacted with a larger number of gp120 peptides, and this increased linear epitope recognition can be related to preferential serum recognition of denatured gp120 (Fig. 1). In contrast, sera from CM235-immunized baboons recognized fewer gp120 peptides and preferentially bound conformational epitopes within gp120.
TABLE 3.
Comparison of linear epitope specificities of SF2- and CM235-immunized baboons
| gp120 region | Total peptidesb | Peptidesa
|
|||
|---|---|---|---|---|---|
| Baboons immunized with SF2
|
Baboons immunized with CM235
|
||||
| SF2 | CM235 | SF2 | CM235 | ||
| C1 | 25 | 58, 90, 102, 106 | 86, 90, 102, 106 | 30, 102 | 30, 102 |
| V1/V2 | 17 | 162, 166, 170, 174 | 170 | — | 166, 170, 174 |
| C2 | 25 | 194, 266, 274, 278 | 262, 266 | — | 202, 234, 266 |
| V3 | 9 | 302, 306, 310, 314 | 306, 310 | 306 | 306, 310, 314 |
| C3 | 17 | 334, 338, 342, 354, 358, 370, 386 | — | — | — |
| V4 | 5 | 394, 406 | — | — | — |
| C4 | 11 | 410, 414, 430, 434, 442 | — | 430 | — |
| V5 | 3 | 462 | — | — | 462, 466 |
| C5 | 11 | 470, 474, 478, 482, 486, 494, 498 | 470, 474, 478, 482, 486 | 470, 474, 478, 498 | 470, 474, 494, 498 |
| Totalc | 123 | 38 | 14 | 8 | 17 |
Peptides to which at least 40% of baboon sera from each vaccine group are reactive, represented by the starting amino acid number for each 12-amino-acid-length peptide. —, no peptide recognized in the region.
Total number of peptides contained in each of the gp120 regions and screened for serum reactivity.
Total number of reactive peptides for each baboon group.
Binding of sera to heterologous and cross-subtype strains of gp120.
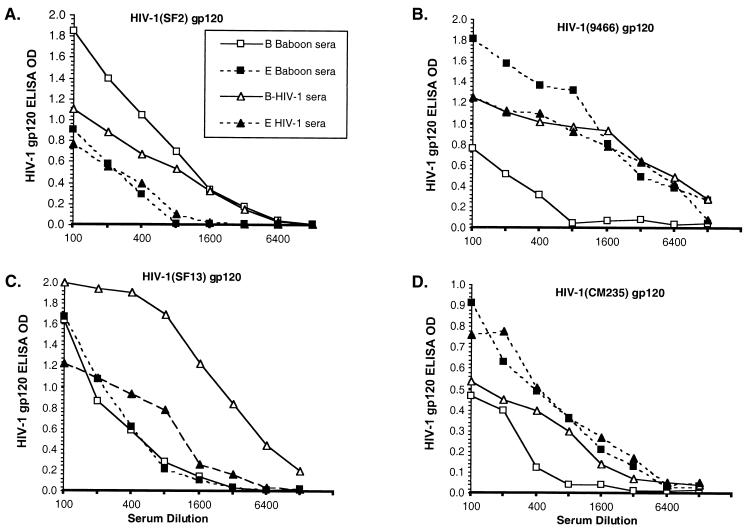
The ability of sera from SF2- and CM235-immunized baboons to bind to gp120 from divergent HIV-1 isolates was evaluated. Monomeric gp120 was captured from the supernatant of detergent-treated acutely infected cells of TCLA isolates from subtype B SF2 (Fig. 2A) and subtype E 9466 (Fig. 2B) as well as primary isolates from subtype B SF13 (Fig. 2C) and subtype E CM235 (Fig. 2D), using antibodies specific for the C terminus of gp120. The serum binding from SF2- and CM235-immunized baboons to divergent intra- and intersubtype gp120 was compared to that of subtype B and E HIV-1 serum pools. The levels of binding of the B and E HIV-1 serum pools to the captured gp120 were controls for the amount of gp120 captured and the degree of cross-reactive antibodies within HIV-1 serum. Binding of sera from SF2-immunized baboons is comparable to, or better than, that of the US B pool against the homologous TCLA SF2 (Fig. 2A), but binding to primary HIV-1 (SF13) and to TCLA E (9466) and primary E (CM235) is approximately 4- to 16-fold lower than that of the US B pool. In contrast, CM235 baboon sera bound comparably to the Thai E pool for all gp120s studied.
FIG. 2.
Examples of a single subtype B-immunized baboon and a subtype E-immunized baboon along with the pooled subtype B HIV-1 sera and subtype E HIV-1 sera binding to gp120 from TCLA SF2, subtype B (A), TCLA 9466, subtype E (B) primary SF13, subtype B (C), and primary CM235, subtype E (D). Sera were run at eight twofold dilutions, and the OD at each dilution is plotted. Preimmune baboon sera and normal human sera negative controls were <0.10 OD at all dilutions.
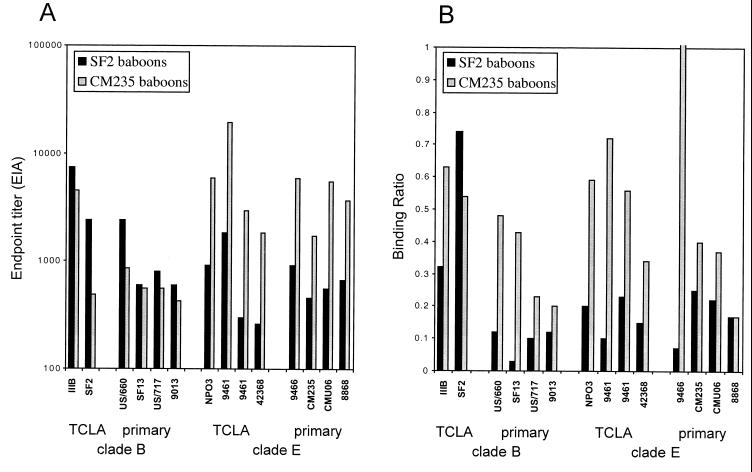
Baboon serum binding endpoint titers to homologous, heterologous, and cross-subtype gp120 are summarized in Fig. 3. Serum titers for the SF2-immunized baboons binding to rgp120SF2 are comparable to the serum titers for the CM235-immunized baboons binding to rgp120CM235 (Fig. 3A). Reactivities against the subtype B gp120s are within two to threefold for the SF2 and CM235 baboon sera with the exception of SF2 gp120, where the SF2-immunized baboons had fivefold-stronger reactivity. The CM235 baboon sera, however, consistently bound 3- to 12-fold more strongly than the SF2 baboon sera to all subtype E gp120s. These data are plotted as ratios in Fig. 3B, normalized for the reactivity of the US B and Thai E HIV-1 serum pools. For each captured gp120, the SF2 and CM235 baboon serum endpoint titers were divided by the endpoint titers of the US B and Thai E serum pools, respectively. The SF2-immunized baboons had the highest ratio against the SF2 gp120 (0.75), with decreased ratios (0.02 to 0.32) for the other gp120s. The CM235-immunized baboons had a ratio of approximately 0.4 against the homologous CM235 gp120, with ratios ranging between 0.17 and 0.72 for all other gp120s. These data demonstrate the presence of measurable amounts of cross-reactive antibodies in both the CM235 and SF2 baboon sera. However, the CM235 serum cross-recognition pattern was comparable to the Thai E HIV-1 serum pool, while the SF2 baboon serum recognition profile was more restricted and type specific for homologous SF2 gp120.
FIG. 3.
Binding to gp120 from divergent HIV-1 isolates by sera from rgp120SF2- and rgp120CM235-immunized baboons. Monomeric gp120 was captured from the supernatant of detergent-treated acutely infected cells. TCLA isolates from subtypes B (IIIB and SF2) and E (NPO3, 9466, 9461, and 42368) and primary isolates from subtypes B (US/660, SF13, US/717, and 9013) and E (9466, CM235, CMU06, and 8868) were evaluated. Data are presented as serum endpoint titer (determined by EIA) against each gp120 (A) and ratio of baboon serum binding to the HIV-1 serum pool from the same subtype (B). The endpoint titers and binding ratios are means of the 5 rgp120SF2 and 10 rgp120CM235 immune sera. Mean endpoint titers for individual preimmune baboon sera binding to each of the gp120 were <1:100 in panel A.
HIV-1 neutralizing antibody.
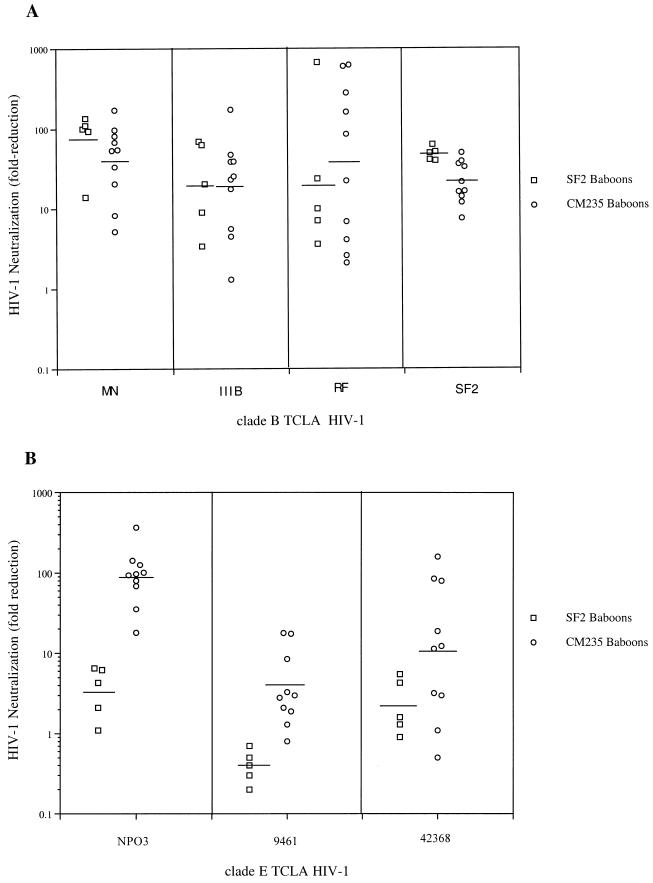
To determine if the differences in serum antibody specificities have an impact on functional neutralizing antibody, sera from SF2- and CM235-immunized baboons were evaluated for the ability to neutralize TCLA HIV-1 isolates from both subtype B (Fig. 4) and E subtype (Fig. 4). Sera collected before and after the 6-month immunization with gp120 were evaluated, and neutralization was defined as a 10-fold or greater reduction in p24 antigen by immune sera, using the individual paired preimmune sera as the reference. Sera from all five SF2-immunized baboons neutralized the homologous HIV-1SF2, with a mean fold reduction of 48.8. SF2 baboon sera neutralized heterologous TCLA HIV-1: MN, five of five (mean, 91.1); IIIB, four of five (mean, 15.9); and RF, three of five (mean, 14.4). In contrast, none of the SF2 baboon sera neutralized subtype E TCLA NPO3, 9461, or 42368 (Fig. 4B). Sera from CM235-immunized baboons neutralized subtype E TCLA viruses: NPO3, 10 of 10 (mean, 113.0); 42368, 6 of 10 (mean, 37.6); and 9461, 2 of 10 (mean, 5.9). The subtype E-immunized baboons displayed neutralizing activity versus subtype B TCLA isolates: SF2, 9 of 10 (mean, 24.4); MN, 8 of 10 (mean 59.6); IIIB, 7 of 10 (mean, 33.7); and RF, 6 of 10 (mean, 178.5). The lack of cross-subtype neutralization by SF2-immunized baboons is probably not due to relative resistance of the subtype E TCLA to serum neutralization, since the NPO3 and 42368 isolates appear susceptible to neutralization by subtype E sera. Neutralization data are summarized in Table 4. Both rgp120SF2 and rgp120CM235 immunization of baboons induced antibodies capable of neutralizing TCLA HIV-1 within the same subtype. However, only rgp120CM235 immunization resulted in substantial intersubtype neutralization. These data represent the first example of an immunogen inducing cross-subtype neutralizing antibody responses. When sera were also evaluated for neutralization of the primary HIV-1CM235, no significant neutralization was observed (data not shown).
FIG. 4.
Sera from immunized baboons were tested for the ability to neutralize subtype B (A) and subtype E (B) TCLA isolates. Subtype B TCLA isolates included MN, IIIB, RF, and SF2; subtype E TCLA isolates included NPO3, 9461, and 42368. Data are plotted as fold reduction in p24 antigen for each HIV-1 isolate in the presence of pre- and postimmune baboon sera. The horizontal bar indicates the geometric mean of the rgp120SF2 or rgp120CM235 immune sera. Sera were tested at a dilution of 1:10 in the presence of virus (1:20 in the presence of virus and cells).
TABLE 4.
Comparison of sera from SF2 and CM235 gp120-immunized baboons to neutralize TCLA HIV-1 from clades B and E
| Immunogen | No. of sera with >90% HIV-1 neutralization/no. testeda
|
|
|---|---|---|
| Clade B | Clade E | |
| gp120SF2 | 16/20 (80b) | 0/15 (0) |
| gp120CM235c | 30/40 (75) | 18/30 (60) |
Four subtype B (MN, IIIB, RF, and SF2) and three subtype E (NPO3, 9461, and 42368) TCLA HIV-1 isolates were evaluated.
Percentage of sera with >90% HIV-1 neutralization activity against the panel of isolates.
None of the 10 CM235 baboon sera were able to neutralize the homologous primary isolate HIV-1CM235.
DISCUSSION
Immunization of baboons with a monomeric rgp120 based on a subtype E, R5 primary HIV-1 envelope induced antibodies capable of neutralizing TCLA HIV-1 from homologous and heterologous subtypes. This was in contrast to immunization obtained with an rgp120 based on a subtype B, X4 TCLA isolate which induced subtype-restricted TCLA HIV-1 neutralization. Cross-subtype HIV-1 neutralization by rgp120CM235-specific immune sera was associated with an antibody binding profile that included preferential recognition of conformational epitopes within gp120 and strong cross-recognition of divergent strains of gp120. This finding is consistent with a study demonstrating that HIV-1 serum antibodies directed toward conformational epitopes within gp120 were responsible for heterologous TCLA HIV-1 neutralization (71). The data presented here may indicate that strain-specific differences in antigenic structure can significantly affect the immunogenicity of rgp120. However, since the current rgp120 immunogens differed with respect to both coreceptor usage and subtype, the property responsible for these observed differences cannot be determined at this time. Despite the induction of antibodies specific for conserved, conformational epitopes and with neutralizing activity against intra- and intersubtype TCLA HIV-1 by the rgp120CM235 immunogen, no neutralization of the homologous primary HIV-1CM235 was obtained. In comparison to sera from subtype E HIV-1-infected individuals, antibody titers achieved with rgp120CM235 immunization were lower. The impact of higher antibody titer on primary HIV-1 isolate neutralization capacity remains to be determined.
Serum antibodies from rgp120CM235-immunized baboons were directed predominantly to conformational epitopes on the homologous rgp120CM235 as well as on the heterologous rgp120SF2. This preferential recognition of conformational gp120 epitopes is similar to profiles obtained in serum from naturally HIV-1-infected individuals (46, 81). This was in contrast to the responses to rgp120SF2 and to those obtained in previous studies evaluating other HIV-1 subunit envelope vaccines based on TCLA HIV-1 isolates (81). Lack of immune serum recognition of conformational epitopes in the previous study correlated with poor binding to cell surface-expressed HIV-1 envelope and poor neutralization of heterologous HIV-1 isolates (81). The ability of rgp120CM235 to elicit a substantially higher relative amount of antibody capable of recognizing conformational epitopes (5- to 10-fold) compared to rgp120SF2 may be related to increased structural stability of gp120 proteins from primary isolates compared to gp120 TCLA isolates. It has been demonstrated that primary and TCLA HIV-1 isolates have different antigenic properties with respect to differential exposure of epitopes and susceptibility to antibody-mediated neutralization (3, 6, 47, 55, 65, 72, 84, 85, 87). HIV-1 isolates adapted for growth in T-cell lines may have adopted a structural configuration with enhanced exposure of epitopes involved in CD4 or coreceptor interactions and resulting in a gp120 molecule with greater structural flexibility (83). Increased structural stability or rigidity of primary gp120 envelope immunogens may allow increased immune recognition of discontinuous epitopes such as the CD4 binding site. Preferential recognition of conformational gp120 epitopes was also observed previously with an oligomeric gp140IIIB immunogen, suggesting that gp140 oligomerization may more efficiently present conformational gp120 epitopes, perhaps by enhancing gp120 conformational stability. The HIV-1 envelope glycoprotein gp160 is known to exist as a multimer (trimer or tetramer) on the surface of a virion (16, 59, 66, 76). Immunization with the soluble CD4-rgp120IIIB complex also was effective at generating antibody responses to conformational epitopes in mice, suggesting CD4-induced stabilization of gp120 conformational structure (32). The data presented here suggest that conformational determinants within R5 primary gp120 antigens may be more efficiently presented for immune recognition in the absence of gp120-gp41 oligomerization or binding to CD4 than the X4 TCLA SF2 rgp120.
Preferential binding to denatured gp120 by rgp120SF2 immune sera was supported by linear epitope mapping studies. Immune sera from rgp120SF2-immunized baboons recognized twice as many gp120 peptides as sera from the rgp120CM235-immunized baboons. Regions immunogenic on both the CM235 and SF2 gp120 included C1, C2, V2, V3, V5, and C5. Sera from the SF2-immunized baboons strongly recognized additional peptides within C3, V4, and C4, while sera from the CM235-immunized baboons had minimal reactivity in this region. Many epitopes within C3 and C4 are predicted not to be exposed on native, monomeric gp120 and thereby inaccessible to antibody binding (50). The strongest linear epitope responses from the SF2 sera were directed to the V3 loop. This was also evident by the ELISA data presented in Table 1. Lack of recognition of the larger (30-amino-acid) cross-subtype V3 peptide by ELISA with recognition of the smaller (12-amino-acid) peptide (peptide 306) by PEPscan suggests that the smaller peptide poorly mimics the tip of the V3 loop in the context of the larger V3 peptide. The antibody titers for V3SF2 were much higher relative to rgp120SF2 than the corresponding relative V3CM242 and rgp120CM235 titers for the CM235-immunized baboons. This finding indicates increased accessibility and immunogenicity of the V3 loop in the X4 rgp120SF2 compared to the R5 rgp120CM235. It is possible that increased exposure and accessibility of the V3 region within the rgp120SF2 protein block antibody recognition of some of the immunogenic discontinuous epitopes. These data indicate many of the antibodies induced by rgp120SF2 immunization are specific for epitopes poorly accessible on the surface of native gp120, consistent with studies demonstrating that many immunogenic gp120 epitopes are poorly exposed on properly folded gp120 (50).
Cross-subtype neutralization by rgp120CM235 immune sera may be attributable to antibody specific for conserved, conformational epitopes. Antibodies directed toward V3 have been shown to potently neutralize TCLA HIV-1, though neutralization of primary HIV-1 is less efficient (11, 45, 84). Cross-subtype neutralization of subtype B TCLA by rgp120CM235 sera occurred, however, in the absence of detectable V3SF2- and low-level V3CM242-specific antibodies, indicating non-V3-mediated neutralization. SPR studies show that recognition of subtype B gp120 by CM235 baboon sera occurred predominantly by antibodies binding to conformational epitopes. In contrast, epitope mapping studies revealed that the cross-subtype gp120 peptide-specific responses of rgp120SF2 immune sera were directed to epitopes within C1 and C5 (data not shown). These regions are well exposed on monomeric forms of rgp120, but based on data from the crystal structure of HIV-1 gp120 (36, 86), mutagenesis analysis (27, 34), and antibody binding studies (50, 51), the C1 and C5 regions are involved in the gp120-gp41 noncovalent interaction and are expected to be poorly exposed on the membrane-expressed oligomeric gp120-gp41 complex. Recent data, however, have demonstrated the ability of V3- and C5-specific MAbs to bind free HIV-1 in a virus capture EIA (54). The lack of cross-subtype neutralization by the rgp120SF2 immune sera is thus attributable to the absence of subtype E V3 binding antibody together with the lack of antibodies specific for conserved, conformational epitopes. The absence of detectable neutralizing activity against primary HIV-1 isolates in rgp120CM235 immune sera indicates insufficient antibody specific for neutralizing epitopes on primary isolates. The majority of the antibody specificities may be directed toward neutralizing epitopes unique to TCLA HIV-1 and not to epitopes such as the CD4 binding site (8, 28, 60, 73) or gp120 epitopes exposed after CD4 binding known to be involved in binding interactions with coreceptor (62, 74).
The use of a gp120 immunogen based on an R5, primary HIV-1 isolate significantly altered the epitope specificities and conformational dependence of immune serum antibody compared to X4 TCLA gp120SF2. Structural features associated with the primary isolate gp120 may play a significant role in the immunogenicity of these proteins. Alteration of gp120 structure has previously been shown to affect resulting antigenicity and immunogenicity. Removal of the V1 and V2 domains altered the accessibility of a TCLA HIV-1 to neutralization by some MAbs (9). Removal of the V2 region from a primary HIV-1 isolate while not affecting in vitro replication greatly enhanced its susceptibility to HIV-1 serum-mediated neutralization (68). Finally, infection of rhesus macaques with a simian immunodeficiency virus isolate that had glycosylation sites within V1 removed induced serum antibodies to novel epitopes previously blocked by glycosylation and which potently neutralize the virus (61). HIV-1 subunit proteins based on R5 rather than X4 strains may also differentially expose or block particular epitopes. Further optimizations of HIV-1 subunit proteins based on strain, phenotype, or oligomerization may be required to induce potent primary isolate neutralization responses.
Thus, both genetic subtype or virus phenotype (primary versus TCLA) may influence the immunogenicity of soluble HIV-1 envelope proteins, indicating the importance of determining correlates linking protein structural elements, antigenicity, and immunogenicity. In this study, subtype E rgp120CM235 was as efficient as subtype B rgp120SF2 at inducing subtype B TCLA neutralizing antibody. This finding suggests the possibility of substituting a single subtype E rgp120 vaccine to induce neutralizing antibody against both subtype B and E in an area where both strains predominate, such as Thailand.
ACKNOWLEDGMENTS
We thank Susan Hegerich, Ann King, and Mark Louder (Henry M. Jackson Foundation) and Yide Sun and Keith Higgins (Chiron Corporation) for technical assistance; Kathy Brasky (Southwest Foundation for Biomedical Research, San Antonio, Tex.) for the care and immunizations of primates; Kathy Steimer for initiating studies with Thai E gp120 at Chiron; and the nursing staff, Charles Oster, and the Military Medical Consortium for Applied Retroviral Research for sera from HIV-1-infected volunteers and for care of these individuals.
This work was supported in part by cooperative agreement no. DAMD17-93-V-3004 between the U.S. Army Medical Research and Materiel Command and the Henry M. Jackson Foundation for the Advancement of Military Medicine.
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC-CCR5: a RANTES, MIP-1α MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Ashkenazi A, Smith D H, Marsters S A, Riddle L, Gregory T J, Ho D D, Capon D J. Resistance of primary isolates of human immunodeficiency virus type 1 to soluble CD4 is independent of CD4-rgp120 binding affinity. Proc Natl Acad Sci USA. 1991;88:7056–7060. doi: 10.1073/pnas.88.16.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beddows S, Louisirirotchanakul S, Cheingsong-Popov R, Easterbrook P J, Simmonds P, Weber J. Neutralization of primary and T-cell line adapted isolates of human immunodeficiency virus type 1: role of V3-specific antibodies. J Gen Virol. 1998;79:77–82. doi: 10.1099/0022-1317-79-1-77. [DOI] [PubMed] [Google Scholar]
- 4.Belshe R B, Graham B S, Keefer M C, Gorse G J, Wright P, Dolin R, Matthews T, Weinhold K, Bolognesi D P, Sposto R, Stablein D M, Twadell T, Berman P W, Gregory T, Izu A E, Walker M C, Fast P. Neutralizing antibodies to HIV-1 in seronegative volunteers immunized with recombinant gp120 from the MN strain of HIV-1. NIAID AIDS Vaccine Clinical Trials Network. JAMA. 1994;272:475–480. doi: 10.1001/jama.272.6.475. [DOI] [PubMed] [Google Scholar]
- 5.Berman P W, Murthy K K, Wrin T, Vennari J C, Cobb E K, Eastman D J, Champe M, Nakamura G R, Davison D, Powell M F, Bussiere J, Francis D P, Matthews T, Gregory T J, Obijeski J F. Protection of MN-rgp120-immunized chimpanzees from heterologous infection with a primary isolate of human immunodeficiency virus type 1. J Infect Dis. 1996;173:52–59. doi: 10.1093/infdis/173.1.52. [DOI] [PubMed] [Google Scholar]
- 6.Bou-Habib D C, Roderiquez G, Oravecz T, Berman P W, Lusso P, Norcross M A. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. J Virol. 1994;68:6006–6013. doi: 10.1128/jvi.68.9.6006-6013.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brighty D W, Rosenberg M, Chen I S, Ivey Hoyle M. Envelope proteins from clinical isolates of human immunodeficiency virus type 1 that are refractory to neutralization by soluble CD4 possess high affinity for the CD4 receptor. Proc Natl Acad Sci USA. 1991;88:7802–7805. doi: 10.1073/pnas.88.17.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton D R, Pyati J, Koduri R, Sharp S J, Thornton G B, Parren P W, Sawyer L S, Hendry R M, Dunlop N, Nara P L, Lamacchia M, Garratty E, Stiehm E R, Bryson Y J, Cao Y, Moore J P, Ho D D, Barbas C F. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 9.Cao J, Sullivan N, Desjardin E, Parolin C, Robinson J, Wyatt R, Sodroski J. Replication and neutralization of human immunodeficiency virus type 1 lacking the V1 and V2 variable loops of the gp120 envelope glycoprotein. J Virol. 1997;71:9808–9812. doi: 10.1128/jvi.71.12.9808-9812.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clements-Mann M L, Weinhold K, Matthews T J, Graham B S, Gorse G J, Keefer M C, McElrath M J, Hsieh R H, Mestecky J, Zolla-Pazner S, Mascola J, Schwartz D, Siliciano R, Corey L, Wright P F, Belshe R, Dolin R, Jackson S, Xu S, Fast P, Walker M C, Stablein D, Excler J L, Tartaglia J, Duliege A M, Sinangil F, Paoletti E. Immune responses to human immunodeficiency virus (HIV) type 1 induced by canarypox expressing HIV-1MN gp120, HIV-1SF2 recombinant gp120, or both vaccines in seronegative adults. NIAID AIDS Vaccine Evaluation Group. J Infect Dis. 1998;177:1230–1246. doi: 10.1086/515288. [DOI] [PubMed] [Google Scholar]
- 11.Conley A J, Gorny M K, Kessler J A, Boots L J, Ossorio-Castro M, Koenig S, Lineberger D W, Emini E A, Williams C, Zolla-Pazner S. Neutralization of primary human immunodeficiency virus type 1 isolates by the broadly reactive anti-V3 monoclonal antibody 447/52D. J Virol. 1994;68:6994–7000. doi: 10.1128/jvi.68.11.6994-7000.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connor R I, Korber B T M, Graham B S, Hahn B H, Ho D D, Walker B D, Neumann A U, Vermund S H, Mestecky J, Jackson S, Fenamore E, Cao Y, Gao F, Kalams S, Kunstman K J, McDonald D, McWilliams N, Trkola A, Moore J P, Wolinsky S M. Immunologic and virologic analyses of persons infected by human immunodeficiency virus type 1 while participating in trials of recombinant gp120 subunit vaccines. J Virol. 1998;72:1552–1576. doi: 10.1128/jvi.72.2.1552-1576.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daar E S, Li X L, Moudgil T, Ho D D. High concentrations of recombinant soluble CD4 are required to neutralize primary human immunodeficiency virus type 1 isolates. Proc Natl Acad Sci USA. 1990;87:6574–6578. doi: 10.1073/pnas.87.17.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzo P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 15.Drajic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 16.Earl P L, Doms R W, Moss B. Oligomeric structure of the human immunodeficiency virus type 1 envelope glycoprotein. Proc Natl Acad Sci USA. 1990;87:648–652. doi: 10.1073/pnas.87.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.el-Amad Z, Murthy K K, Higgins K, Cobb E K, Haigwood N L, Levy J A, Steimer K S. Resistance of chimpanzees immunized with recombinant gp120SF2 to challenge by HIV-1SF2. AIDS. 1995;9:1313–1322. doi: 10.1097/00002030-199512000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Feng Y, Broder C, Kennedy P E, Berger E A. HIV-1 entry co-factor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 19.Fouts T R, Binley J M, Trkola A, Robinson J E, Moore J M. Neutralization of the human immunodeficiency virus type 1 primary isolate JR-FL by human monoclonal antibodies correlates with antibody binding to the oligomeric form of the envelope glycoprotein complex. J Virol. 1997;71:2779–2785. doi: 10.1128/jvi.71.4.2779-2785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fouts T R, Trkola A, Fung M S, Moore J P. Interactions of polyclonal and monoclonal anti-glycoprotein 120 antibodies with oligomeric glycoprotein 120-glycoprotein 41 complexes of a primary HIV type 1 isolate: relationship to neutralization. AIDS Res Hum Retroviruses. 1998;14:591–597. doi: 10.1089/aid.1998.14.591. [DOI] [PubMed] [Google Scholar]
- 21.Gorny M K, Conley A J, Karwowska S, Buchbinder A, Xu J Y, Emini E A, Koenig S, Zolla-Pazner S. Neutralization of diverse human immunodeficiency virus type 1 variants by an anti-V3 human monoclonal antibody. J Virol. 1992;66:7538–7542. doi: 10.1128/jvi.66.12.7538-7542.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorny M K, Mascola J R, Israel Z R, Van Cott T C, Williams C, Balfe P, Hioe C, Brodine S, Burda S, Zolla-Pazner S. A human monoclonal antibody specific for the V3 loop of HIV type 1 clade E cross-reacts with other HIV type 1 clades. AIDS Res Hum Retroviruses. 1998;14:213–221. doi: 10.1089/aid.1998.14.213. [DOI] [PubMed] [Google Scholar]
- 23.Gorny M K, Moore J P, Conley A J, Karwowska S, Sodroski J, Williams C, Burda S, Boots L J, Zolla-Pazner S. Human anti-V2 monoclonal antibody that neutralizes primary but not laboratory isolates of human immunodeficiency virus type 1. J Virol. 1994;68:8312–8320. doi: 10.1128/jvi.68.12.8312-8320.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haigwood N L, Nara P L, Brooks E, Van Nest G A, Ott G, Higgins K W, Dunlop N, Scandella C J, Eichberg J W, Steimer K S. Native but not denatured recombinant human immunodeficiency virus type 1 gp120 generates broad-spectrum neutralizing antibodies in baboons. J Virol. 1992;66:172–182. doi: 10.1128/jvi.66.1.172-182.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanson C V. Measuring vaccine-induced HIV neutralization: report of a workshop. AIDS Res Hum Retroviruses. 1994;10:645–648. doi: 10.1089/aid.1994.10.645. [DOI] [PubMed] [Google Scholar]
- 26.Heeney J L, Teeuwsen V J P, van Gils M, Bogers W M J M, Morghen C D G, Radaelli A, Barnett S, Morein B, Akerblom L, Wang Y, Lehner T, Davis D. Beta-chemokines and neutralizing antibody titers correlate with sterilizing immunity generated in HIV-1 vaccinated macaques. Proc Natl Acad Sci USA. 1998;95:10803–10808. doi: 10.1073/pnas.95.18.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helseth E, Olshevsky U, Furman C, Sodroski J. Human immunodeficiency virus type 1 gp120 envelope glycoprotein regions important for association with the gp41 transmembrane glycoprotein. J Virol. 1991;65:2119–2123. doi: 10.1128/jvi.65.4.2119-2123.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho D D, McKeating J A, Li X L, Moudgil T, Daar E S, Sun N C, Robinson J E. Conformational epitope on gp120 important in CD4 binding and human immunodeficiency virus type 1 neutralization identified by a human monoclonal antibody. J Virol. 1991;65:489–493. doi: 10.1128/jvi.65.1.489-493.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ichimura H, Kliks S C, Visrutaratna S, Ou C Y, Kalish M L, Levy J A. Biological, serological, and genetic characterization of HIV-1 subtype E isolates from northern Thailand. AIDS Res Hum Retroviruses. 1994;10:263–269. doi: 10.1089/aid.1994.10.263. [DOI] [PubMed] [Google Scholar]
- 30.Kahn J O, Sinangil F, Baenziger J, Murcar N, Wynne D, Coleman R L, Steimer K S, Dekker C L, Chernoff D. Clinical and immunologic responses to human immunodeficiency virus (HIV) type 1SF2 gp120 subunit vaccine combined with MF59 adjuvant with or without muramyl tripeptide dipalmitoyl phosphatidylethanolamine in non-HIV-infected human volunteers. J Infect Dis. 1994;170:1288–1291. doi: 10.1093/infdis/170.5.1288. [DOI] [PubMed] [Google Scholar]
- 31.Kahn J O, Steimer K S, Baenziger J, Duliege A M, Feinberg M, Elbeik T, Chesney M, Murcar N, Chernoff D, Sinangil F. Clinical, immunologic, and virologic observations related to human immunodeficiency virus (HIV) type 1 infection in a volunteer in an HIV-1 vaccine clinical trial. J Infect Dis. 1995;171:1343–1347. doi: 10.1093/infdis/171.5.1343. [DOI] [PubMed] [Google Scholar]
- 32.Kang C Y, Hariharan K, Nara P L, Sodroski J, Moore J P. Immunization with a soluble CD4-gp120 complex preferentially induces neutralizing anti-human immunodeficiency virus type 1 antibodies directed to conformation-dependent epitopes of gp120. J Virol. 1994;68:5854–5862. doi: 10.1128/jvi.68.9.5854-5862.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kostrikis L G, Cao Y, Ngai H, Moore J P, Ho D D. Quantitative analysis of serum neutralization of human immunodeficiency virus type 1 from subtypes A, B, C, D, E, F, and I: lack of direct correlation between neutralization serotypes and genetic subtypes and evidence for prevalent serum-dependent infectivity enhancement. J Virol. 1996;70:445–458. doi: 10.1128/jvi.70.1.445-458.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kowalski M, Potz J, Basiripour L, Dorfman T, Goh W C, Terwilliger E, Dayton A, Rosen C, Haseltine W, Sodroski J. Functional regions of the envelope glycoprotein of human immunodeficiency virus type 1. Science. 1987;237:1351–1355. doi: 10.1126/science.3629244. [DOI] [PubMed] [Google Scholar]
- 35.Kunanusont C, Foy H M, Kreiss J K, Rerks-Ngarm S, Phanuphak P, Raktham S, Pau C P, Young N L. HIV-1 subtypes and male-to-female transmission in Thailand. Lancet. 1995;345:1078–1083. doi: 10.1016/s0140-6736(95)90818-8. [DOI] [PubMed] [Google Scholar]
- 36.Kwong P D, Wyatt R, Robinson J, Sweet R W, Sodroski J, Hendrickson W A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leonard C K, Spellman M W, Riddle L, Harris R J, Thomas J N, Gregory T J. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J Biol Chem. 1990;265:10373–10382. [PubMed] [Google Scholar]
- 38.Loomis-Price L D, Cox J H, Mascola J R, VanCott T C, Michael N L, Fouts T R, Redfield R R, Robb M L, Wahren B, Sheppard H W, Birx D L. Correlation between humoral responses to HIV-1 envelope and disease progression in early-stage infection. J Infect Dis. 1998;178:1306–1316. doi: 10.1086/314436. [DOI] [PubMed] [Google Scholar]
- 39.Mascola J R, Louder M K, Surman S R, VanCott T C, Yu X F, Bradac J, Porter K R, Nelson K E, Girard M, McNeil J G, McCutchan F E, Birx D L, Burke D S. Human immunodeficiency virus type 1 neutralizing antibody serotyping using serum pools and an infectivity reduction assay. AIDS Res Hum Retroviruses. 1996;12:1319–1328. doi: 10.1089/aid.1996.12.1319. [DOI] [PubMed] [Google Scholar]
- 40.Mascola J R, Louwagie J, McCutchan F E, Fischer C L, Hegerich P A, Wagner K F, Fowler A K, McNeil J G, Burke D S. Two antigenically distinct subtypes of human immunodeficiency virus type 1: viral genotype predicts neutralization serotype. J Infect Dis. 1994;169:48–54. doi: 10.1093/infdis/169.1.48. [DOI] [PubMed] [Google Scholar]
- 41.Mascola J R, Snyder S W, Weislow O S, Belay S M, Belshe R B, Schwartz D H, Clements M L, Dolin R, Graham B S, Gorse G J, Keefer M C, McElrath M J, Walker M C, Wagner K F, McNeil J G, McCutchan F E, Burke D S. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. J Infect Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- 42.Matthews T J. Dilemma of neutralization resistance of HIV-1 field isolates and vaccine development. AIDS Res Hum Retroviruses. 1994;10:631–632. doi: 10.1089/aid.1994.10.631. [DOI] [PubMed] [Google Scholar]
- 43.McCutchan F E, Hegerich P A, Brennan T P, Phanuphak P, Singharaj P, Jugsudee A, Berman P W, Gray A M, Fowler A K, Burke D S. Genetic variants of HIV-1 in Thailand. AIDS Res Hum Retroviruses. 1992;8:1887–1895. doi: 10.1089/aid.1992.8.1887. [DOI] [PubMed] [Google Scholar]
- 44.McElrath M J, Corey L, Greenberg P D, Matthews T J, Montefiori D C, Rowen L, Hood L, Mullins J I. Human immunodeficiency virus type 1 infection despite prior immunization with a recombinant envelope vaccine regimen. Proc Natl Acad Sci USA. 1996;93:3972–3977. doi: 10.1073/pnas.93.9.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore J P, Cao Y, Qing L, Sattentau Q J, Pyati J, Koduri R, Robinson J, Barbas C F, Burton D R, Ho D D. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120, and their neutralization is not predicted by studies with monomeric gp120. J Virol. 1995;69:101–109. doi: 10.1128/jvi.69.1.101-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore J P, Ho D D. Antibodies to discontinuous or conformationally sensitive epitopes on the gp120 glycoprotein of human immunodeficiency virus type 1 are highly prevalent in sera of infected humans. J Virol. 1993;67:863–875. doi: 10.1128/jvi.67.2.863-875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore J P, Ho D D. HIV-1 neutralization: the consequences of viral adaptation to growth on transformed T cells. AIDS. 1995;9:S117–S136. [PubMed] [Google Scholar]
- 48.Moore J P, McCutchan F E, Poon S W, Mascola J, Liu J, Cao Y, Ho D D. Exploration of antigenic variation in gp120 from clades A through F of human immunodeficiency virus type 1 by using monoclonal antibodies. J Virol. 1994;68:8350–8364. doi: 10.1128/jvi.68.12.8350-8364.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore J P, McKeating J A, Jones I M, Stephens P E, Clements G, Thomson S, Weiss R A. Characterization of recombinant gp120 and gp160 from HIV-1: binding to monoclonal antibodies and soluble CD4. AIDS. 1990;4:307–315. doi: 10.1097/00002030-199004000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Moore J P, Sattentau Q J, Wyatt R, Sodroski J. Probing the structure of the human immunodeficiency virus surface glycoprotein gp120 with a panel of monoclonal antibodies. J Virol. 1994;68:469–484. doi: 10.1128/jvi.68.1.469-484.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore J P, Sodroski J. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J Virol. 1996;70:1863–1872. doi: 10.1128/jvi.70.3.1863-1872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moore J P, Trikola A, Boots L J, Kessler J A, McCutchan F E, Mascola J, Ho D D, Robinson J, Conley A J. A human monoclonal antibody to a complex epitope in the V3 region of human immunodeficiency virus type 1 has broad reactivity within and outside of clade B. J Virol. 1995;69:122–130. doi: 10.1128/jvi.69.1.122-130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Ruker F, Katinger H. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nyambi P N, Gorny M K, Bastiani L, Van Der Groen G, Williams C, Zolla-Pazner S. Mapping of epitopes exposed on intact human immunodeficiency virus type 1 (HIV-1) virions: a new strategy for studying the immunological relatedness of HIV-1. J Virol. 1998;72:9384–9391. doi: 10.1128/jvi.72.11.9384-9391.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Brien W A, Mao S H, Cao Y, Moore J P. Macrophage-tropic and T-cell line-adapted chimeric strains of human immunodeficiency virus type 1 differ in their susceptibilities to neutralization by soluble CD4 at different temperatures. J Virol. 1994;68:5264–5269. doi: 10.1128/jvi.68.8.5264-5269.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ou C Y, Takebe Y, Luo C C, Kalish M, Auwanit W, Bandea C, De La Torre N, Moore J L, Schochetman G, Yamazaki S, Gayle H D, Young N L, Weniger B G. Wide distribution of two subtypes of HIV-1 in Thailand. AIDS Res Hum Retroviruses. 1992;8:1471–1472. doi: 10.1089/aid.1992.8.1471. [DOI] [PubMed] [Google Scholar]
- 57.Ou C Y, Takebe Y, Weniger B G, Luo C C, Kalish M L, Auwanit W, Yamazaki S, Gayle H D, Young N L, Schochetman G. Independent introduction of two major HIV-1 genotypes into distinct high-risk populations in Thailand. Lancet. 1993;341:1171–1174. doi: 10.1016/0140-6736(93)91001-3. [DOI] [PubMed] [Google Scholar]
- 58.Pau C P, Lee T S, Auwanit W, George J R, Ou C Y, Parekh B S, Granade T C, Holloman D L, Phillips S, Schochetman G, Young N L, Takebe Y, Gayle H D, Weniger B G. Highly specific V3 peptide enzyme immunoassay for serotyping HIV-1 specimens from Thailand. AIDS. 1993;7:337–340. doi: 10.1097/00002030-199303000-00005. [DOI] [PubMed] [Google Scholar]
- 59.Pinter A, Honnen W J, Tilley S A, Bona C, Zaghouani H, Gorny M, Zolla-Pazner S. Oligomeric structure of gp41, the transmembrane protein of human immunodeficiency virus type 1. J Virol. 1989;63:2674–2679. doi: 10.1128/jvi.63.6.2674-2679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Posner M R, Cavacini L A, Emes C L, Power J, Byrn R. Neutralization of HIV-1 by F105, a human monoclonal antibody to the CD4 binding site of gp120. J Acquired Immune Defic Syndr. 1993;6:7–14. [PubMed] [Google Scholar]
- 61.Reitter J N, Means R E, Desrosiers R C. A role for carbohydrates in immune evasion in AIDS. Nat Med. 1998;4:679–684. doi: 10.1038/nm0698-679. [DOI] [PubMed] [Google Scholar]
- 62.Rizzuto C D, Wyatt R, Hernandez-Ramos N, Sun Y, Kwong P D, Hendrickson W A, Sodroski J. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science. 1998;280:1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- 63.Robb M L, Polonis V, Vahey M, Gartner S, Michael N, Fowler A, Redfield R R. HIV neutralization assay using polymerase chain reaction-derived molecular signals. J Acquired Immune Defic Syndr. 1992;5:1224–1229. [PubMed] [Google Scholar]
- 64.Sattentau Q J, Moore J P. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J Exp Med. 1995;182:185–196. doi: 10.1084/jem.182.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sawyer L S, Wrin M T, Crawford-Miksza L, Potts B, Wu Y, Weber P A, Alfonso R D, Hanson C V. Neutralization sensitivity of human immunodeficiency virus type 1 is determined in part by the cell in which the virus is propagated. J Virol. 1994;68:1342–1349. doi: 10.1128/jvi.68.3.1342-1349.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schawaller M, Smith C E, Skehel J J, Wiley D C. Studies with cross-linking reagents on the oligomeric structure of the env-glycoprotein of HIV. Virology. 1989;172:367–369. doi: 10.1016/0042-6822(89)90142-6. [DOI] [PubMed] [Google Scholar]
- 67.Schwartz D H, Gorse G, Clements M L, Belshe R, Izu A, Duliege A M, Berman P, Twaddell T, Stablein D, Sposto R, Siliciano R, Matthews T. Induction of HIV-1 neutralising and syncytium-inhibiting antibodies in uninfected recipients of HIV-1IIIB rgp120 subunit vaccine. Lancet. 1993;342:69–73. doi: 10.1016/0140-6736(93)91283-r. [DOI] [PubMed] [Google Scholar]
- 68.Stamatatos L, Cheng-Mayer C. An envelope modification that renders a primary, neutralization-resistant clade B human immunodeficiency virus type 1 isolate highly susceptible to neutralization by sera from other clades. J Virol. 1998;72:7840–7846. doi: 10.1128/jvi.72.10.7840-7845.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stamatatos L, Cheng-Mayer C. Structural modulations of the envelope gp120 glycoprotein of human immunodeficiency virus type 1 upon oligomerization and differential V3 loop epitope exposure of isolates displaying distinct tropism upon virion-soluble receptor binding. J Virol. 1995;69:6191–6198. doi: 10.1128/jvi.69.10.6191-6198.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stamatos N M, Mascola J R, Kalyanaraman V S, Louder M K, Frampton L M, Birx D L, VanCott T C. Neutralizing antibodies from sera of human immunodeficiency virus type 1 infected individuals bind to monomeric gp120 and oligomeric gp140. J Virol. 1998;72:9656–9667. doi: 10.1128/jvi.72.12.9656-9667.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steimer K S, Scandella C J, Skiles P V, Haigwood N L. Neutralization of divergent HIV-1 isolates by conformation-dependent human antibodies to Gp120. Science. 1991;254:105–108. doi: 10.1126/science.1718036. [DOI] [PubMed] [Google Scholar]
- 72.Sullivan N, Sun Y, Li J, Hofmann W, Sodroski J. Replicative function and neutralization sensitivity of envelope glycoproteins from primary and T-cell line-passaged human immunodeficiency virus type 1 isolates. J Virol. 1995;69:4413–4422. doi: 10.1128/jvi.69.7.4413-4422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thali M, Furman C, Ho D D, Robinson J, Tilley S, Pinter A, Sodroski J. Discontinuous, conserved neutralization epitopes overlapping the CD4-binding region of human immunodeficiency virus type 1 gp120 envelope glycoprotein. J Virol. 1992;66:5635–5641. doi: 10.1128/jvi.66.9.5635-5641.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thali M, Moore J P, Furman C, Charles M, Ho D D, Robinson J, Sodroski J. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J Virol. 1993;67:3978–3988. doi: 10.1128/jvi.67.7.3978-3988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thali M, Olshevsky U, Furman C, Gabuzda D, Posner M, Sodroski J. Characterization of a discontinuous human immunodeficiency virus type 1 gp120 epitope recognized by a broadly reactive neutralizing human monoclonal antibody. J Virol. 1991;65:6188–6193. doi: 10.1128/jvi.65.11.6188-6193.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thomas D J, Wall J S, Hainfeld J F, Kaczorek M, Booy F P, Trus B L, Eiserling F A, Steven A C. gp160, the envelope glycoprotein of human immunodeficiency virus type 1, is a dimer of 125-kilodalton subunits stabilized through interactions between their gp41 domains. J Virol. 1991;65:3797–3803. doi: 10.1128/jvi.65.7.3797-3803.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tilley S A, Honnen W J, Racho M E, Hilgartner M, Pinter A. A human monoclonal antibody against the CD4-binding site of HIV-1 gp120 exhibits potent, broadly neutralizing activity. Res Virol. 1991;142:247–259. doi: 10.1016/0923-2516(91)90010-z. [DOI] [PubMed] [Google Scholar]
- 78.Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore J P, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Van Nest G A, Steimer K S, Haigwood N L, Burke R L, Ott G. Advanced adjuvant formulations for use with recombinant subunit vaccines. In: Brown F, Chanock R, Ginsberg H S, Lerner R, editors. Vaccines, modern approaches to new vaccines. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 57–62. [Google Scholar]
- 80.VanCott T C, Bethke F R, Artenstein A W, McCutchan F E, McNeil J G, Mascola J R, Redfield R R, Birx D L. Serotyping international HIV-1 isolates by V3 peptides and whole gp160 proteins using BIAcore. Methods. 1994;6:188–198. [Google Scholar]
- 81.VanCott T C, Bethke F R, Burke D S, Redfield R R, Birx D L. Lack of induction of antibodies specific for conserved, discontinuous epitopes of HIV-1 envelope glycoprotein by candidate AIDS vaccines. J Immunol. 1995;155:4100–4110. [PubMed] [Google Scholar]
- 82.VanCott T C, Bethke F R, Kalyanaraman V, Burke D S, Redfield R R, Birx D L. Preferential antibody recognition of structurally distinct HIV-1 gp120 molecules. J Acquired Immune Defic Syndr. 1994;7:1103–1115. [PubMed] [Google Scholar]
- 83.VanCott T C, Mascola J R, Kaminski R W, Kalyanaraman V, Hallberg P L, Burnett P R, Ulrich J T, Rechtman D J, Birx D L. Antibodies with specificity to native gp120 and neutralization activity against primary human immunodeficiency type 1 isolates elicited by immunization with oligomeric gp160. J Virol. 1997;71:4319–4330. doi: 10.1128/jvi.71.6.4319-4330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.VanCott T C, Polonis V R, Loomis L D, Michael N L, Nara P L, Birx D L. Differential role of V3-specific antibodies in neutralization assays involving primary and laboratory-adapted isolates of HIV type 1. AIDS Res Hum Retroviruses. 1995;11:1379–1391. doi: 10.1089/aid.1995.11.1379. [DOI] [PubMed] [Google Scholar]
- 85.Wrin T, Nunberg J H. HIV-1MN recombinant gp120 vaccine serum, which fails to neutralize primary isolates of HIV-1, does not antagonize neutralization by antibodies from infected individuals. AIDS. 1994;8:1622–1623. doi: 10.1097/00002030-199411000-00017. [DOI] [PubMed] [Google Scholar]
- 86.Wyatt R, Kwong P D, Desjardins E, Sweet R W, Robinson J, Hendrickson W A, Sodroski J G. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 87.Zhang Y J, Fredriksson R, McKeating J A, Fenyo E M. Passage of HIV-1 molecular clones into different cell lines confers differential sensitivity to neutralization. Virology. 1997;238:254–264. doi: 10.1006/viro.1997.8812. [DOI] [PubMed] [Google Scholar]
- 88.Zolla-Pazner S, Xu S, Burda S, Duliege A M, Excler J L, Clements-Mann M L. Neutralization of syncytium-inducing primary isolates by sera from human immunodeficiency virus (HIV)-uninfected recipients of candidate HIV vaccines. J Infect Dis. 1998;178:1502–1506. doi: 10.1086/314452. [DOI] [PubMed] [Google Scholar]