Abstract
Background:
Food protein–induced enterocolitis syndrome (FPIES) is a non-immunoglobulin E mediated food allergy that typically presents with repetitive emesis and may be associated with lethargy, marked pallor, hypotension, hypothermia, and/or diarrhea. Although many foods are known to cause FPIES, peanut-triggered FPIES is emerging due to changes in the feeding practice guidelines, which recommends early peanut introduction in infants.
Objective:
We aimed to characterize peanut-triggered acute FPIES cases in our pediatric population and to describe their attributes, treatment, and outcomes. We hypothesized that increases in the incidence of peanut-triggered FPIES coincided with implementation of the guidelines for early peanut introduction.
Methods:
A retrospective chart review was conducted of pediatric patients who presented to Phoenix Children's Hospital Emergency Department and subspecialty clinics during a 6-year period (January 2013 to September 2019).
Results:
Thirty-three cases of patients with acute FPIES were identified, five of which were peanut triggered. In those patients with peanut-triggered FPIES, the median age for peanut introduction was 7 months (range, 5–24 months). Two patients had positive peanut skin-prick test results. All five cases were identified in the past 2 years (2018 to 2019). No peanut-triggered reactions were documented in the preceding 4-year period (2013 to 2017).
Conclusion:
Peanut may be an emerging trigger of acute FPIES, coinciding with an earlier introduction of peanut in the infant diet after implementation of the new addendum guidelines for the prevention of peanut allergy. Oats and rice were the most common triggers of acute FPIES in our cohort. Further study will help clarify the significance and reproducibility of these findings.
INTRODUCTION
Food protein-induced enterocolitis syndrome (FPIES) is a non–immunoglobulin E (IgE) mediated food allergy. Acute FPIES usually manifests in infancy and is characterized by discrete episodes of delayed repetitive emesis on ingestion of the culprit food. It can also be associated with lethargy, marked pallor, hypotension, hypothermia, and/or diarrhea. The cumulative incidence of infantile FPIES varies among countries, with incidence rates of 0.015% in Australia,1 0.7% in Spain,2 and an estimated prevalence of 0.59% in the United States.3 Numerous food triggers of FPIES have been identified and are influenced by age, geographic location, and associated patterns of food introduction.4,5 The most commonly identified triggers in the United States are cow's milk and soy, followed by grains (especially rice and oats), eggs, fruits, and vegetables.4–6 In Europe, fish is the most frequently identified trigger among solid foods.2,7 Globally, peanut has infrequently been reported as a trigger of acute FPIES.6,8,9
When using the findings from the LEAP (Learning Early About Peanut Allergy) study10 and other publications on early peanut introduction, the National Institute of Allergy and Infectious Diseases (NIAID) Addendum Guidelines for the Prevention of Peanut Allergy11 were proposed in 2017. Peanut introduction was recommended as early as 4–6 months of age. Interestingly, in the LEAP sudy,10 one of the nine patients with a negative oral food challenge (OFC) who subsequently discontinued peanut consumption, did so due to peanut-triggered FPIES. After implementation of the NIAID guidelines, peanut-triggered acute FPIES has increasingly been described in the literature.8,9,12 We hypothesized that the number of cases of peanut-triggered FPIES had increased at our institution, which coincided with the implementation of early peanut introduction guidelines.
METHODS
Institutional review board (IRB) approval was obtained from Phoenix Children's Hospital to perform a retrospective review (IRB-19–407). Electronic medical records were searched for pediatric patients (0–17 years of age) who presented to the Phoenix Children's Hospital Emergency Department and subspecialty clinics (Gastroenterology and Allergy/Immunology) with diagnostic codes related to FPIES over a 6-year period (2013–2019). Cases were identified by using the International Classification of Diseases, Ninth Edition (ICD-9)13 code for allergic gastroenteritis and colitis (ICD-9 code K558.3) and International Classification of Diseases, Tenth Edition (ICD-10)14 code for FPIES (ICD-10 code K52.2). One additional FPIES case was included because it was known to our Allergy/Immunology clinic during the 6-year period but identified under ICD-10 code for unspecified allergic reaction (ICD-10 code T78.40XA). Patients with a confirmed diagnosis of acute FPIES based on the international consensus guidelines criteria were included.15 Patients who did not meet consensus guidelines criteria were excluded from the analysis.
RESULTS
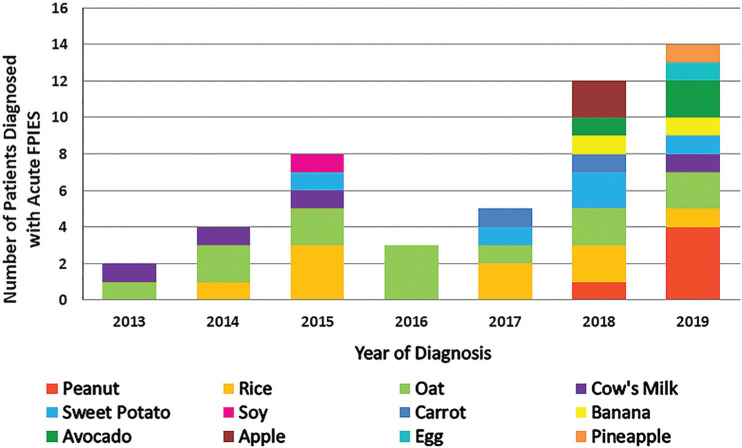
Five hundred and sixty-eight cases were identified. Of the 535 patients excluded, 432 had food-induced allergic proctocolitis, 31 had chronic FPIES, 52 had alternative primary diagnoses, 19 did not meet consensus criteria for acute FPIES, and 1 acute FPIES case was excluded despite, diagnosis in 2015 because symptoms started in 2008 (Online Supplemental Fig. 1). Thirty-three patients met criteria for a diagnosis of acute FPIES and underwent further analysis. Nineteen of the 33 patients had reactions driven by one food trigger, 11 had two triggers, 2 had three triggers, and 1 patient had four triggers. The most commonly identified triggers distributed by frequency were the following: oats, rice, sweet potato, peanut, cow's milk, avocado, apple, banana, eggs, and soy (Fig. 1). Five patients had peanut-triggered acute FPIES, described below (Table 1).
Figure 1.
Stacked column graph of the acute food protein–induced enterocolitis syndrome (FPIES) cases separated by year and causative food(s). One patient may have had more than one food trigger.
Table 1.
Clinical characteristics of patients with PN-triggered FPIES
| Patient No. | Year | Sex | Race | Ethnicity | FPIES Food Trigger(s) | IgE-mediated FA | AD | Age at PN Introduction, mo | Onset of Symptoms, hr | Clinical Features | Therapy | PN SPT Result | OFC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2018 | M | Caucasian | Non-Hispanic | PN | No | No | 5 | 3 | Vomiting, pallor, lethargy | Oral hydration | Negative* | No |
| 2 | 2019 | M | Caucasian | Non-Hispanic | PN, rice | Milk | No | 24 | 3 | Vomiting | Oral hydration | Negative (0-mm wheal, 3-mm flare) | No |
| 3 | 2019 | M | Caucasian | Non-Hispanic | PN, avocado | No | No | 7 | 4 | Vomiting, lethargy | Oral hydration | Negative (0-mm wheal, 0-mm flare) | Yes (failed at 15 mo) |
| 4 | 2019 | M | Asian | Non-Hispanic | PN | Egg | Yes | 6 | 1.5 | Vomiting, diarrhea, pallor | Oral hydration, IVF, oral ondansetron, IM epinephrine | Positive (4-mm wheal, 4-mm flare) | Yes (failed at 7 mo) |
| 5 | 2019 | F | Caucasian | Non-Hispanic | PN, avocado | Egg | Yes | 7 | 2 | Vomiting, pallor, lethargy | Oral hydration, oral ondansetron | Positive (6-mm wheal, 19-mm flare) | Yes (failed at 7 mo) |
FPIES = Food protein-induced enterocolitis syndrome; IgE = immunoglobulin E; FA = food allergy; AD = atopic dermatitis; PN = peanut; SPT = skin-prick test; OFC = oral food challenge; IVF = intravenous fluids; IM, intramuscular;
= refers to reported negative test (external testing).
Patient no. 1 was a 5-month-old boy fed peanut flour mixed with breast milk. On his third exposure, he experienced multiple episodes of prolonged emesis with associated pallor, beginning 3 hours after peanut ingestion. On repeated peanut exposure, he again developed delayed protracted emesis. Both episodes were treated with oral hydration only. A peanut skin-prick test (SPT) result was negative. Patient no. 2 was a 24-month-old boy with a history of acute FPIES to rice and IgE-mediated cow's milk allergy who developed repetitive emesis 3 hours after his first exposure to peanut butter. He was treated with oral hydration only. A peanut SPT result was negative (0-mm wheal, 3-mm flare). Patient no. 3 was a 7-month-old boy with FPIES to avocado who developed protracted emesis with associated lethargy 4 hours after his seventh exposure to peanut butter. Previous peanut exposure had been well tolerated. Two subsequent ingestions of peanut butter were marked by emesis beginning 2 hours after ingestion. All episodes were treated with oral hydration. The peanut SPT result (0-mm wheal, 0-mm flare) and specific IgE value were negative. At 15 months, the patient failed a peanut OFC due to emesis 4 hours after OFC initiation.
Patient no. 4 was a 6-month-old boy with a history of IgE-mediated egg allergy and mild atopic dermatitis. Due to his increased risk for peanut allergy, a peanut SPT was performed, and the result was found to be positive (4-mm wheal, 4-mm flare) with a negative peanut-specific IgE result. One and a half hours after his first peanut ingestion, the patient developed prolonged emesis. He was treated with oral hydration. The patient underwent supervised graded OFC with a total of 2.1 g of peanut protein. Ninety minutes after the last dose, the patient developed prolonged emesis, with associated pallor. He was treated with intramuscular epinephrine without clinical improvement. He was transferred to the emergency department where he was treated with intravenous fluids and oral ondansetron, with symptom resolution. Patient no. 5 was a 7-month-old girl with a history of acute FPIES to avocado, IgE-mediated egg allergy, and mild atopic dermatitis. Peanut had not yet been introduced, and due to an increased risk of peanut allergy, a peanut SPT was performed, and the result was found to be positive (6-mm wheal, 19-mm flare). Supervised peanut OFC was performed. The patient consumed a total of 2.3 g of peanut protein, with one brief episode of emesis 45 minutes after the last peanut dose. After completing OFC, 2.5 hours later, the patient developed recurrent emesis, with associated lethargy and pallor. She was treated with oral ondansetron and oral hydration.
Four of the five patients were introduced peanut between 5 and 7 months of age, with two of these four patients at high risk for IgE-mediated peanut allergy due to known egg allergy.11 Per parental preference, peanut exposure was delayed in patient no. 2. A clinical diagnosis was made on average 1.4 months after the first reaction (range, 0–4 months). In patients with acute FPIES to triggers other than peanut (n = 28), the mean age for the first reaction was 6.2 months (range, 1–10 months). The diagnosis in these patients was made on average 5 months after symptom onset (range, 0–34 months).
DISCUSSION
Most patients with acute FPIES have negative IgE testing results to the inciting food.16 Atypical FPIES is defined by the presence of positive IgE sensitization to the culprit food,15,16 and its frequency varies by populations and triggers (4–24%).5,6 Interestingly, two of our patients had egg allergy and, therefore, underwent peanut SPT, results of which were positive. Robbins et al.9 previously reported three cases of peanut-triggered FPIES, all with peanut IgE sensitization. Cow's milk–triggered FPIES has demonstrated an association between IgE sensitization and disease persistence,5 with evidence that a minority of these patients may evolve to an IgE-mediated phenotype over time.17 The role of peanut sensitization in persistent FPIES or progression to IgE-mediated disease remains incompletely defined.
A personal history of atopy was noted in three patients, all with IgE-mediated food allergy (cow's milk or unbaked eggs) and two with concomitant mild atopic dermatitis. This trend towards atopy has previously been reported, with atopic co-morbidities being more prevalent in children with acute FPIES than those without.18 Nonetheless, direct causation has not been identified for this association, which suggests, perhaps, a predisposition to both types of reactions: IgE and non-IgE mediated. Three patients of our five peanut-triggered cases had an additional culprit food, including avocado (n = 2) and rice (n = 1). Overall, 90.9% of our 33 patients with acute FPIES had one to two food triggers, and only one had four triggers (3.1%). These findings were consistent with large FPIES cohorts in which 91% of the patients reacted to up to two foods.5
FPIES culprit foods vary significantly with geographic distribution,1,2,5,6 and align with the first foreign proteins introduced into the infant diet. In Spain and Italy, where fish is among one of the first food groups introduced, it is recognized as one of the most common FPIES triggers. Conversely, the development of acute FPIES to cow's milk or cereal grains after the age of 12–18 months is uncommon.13 Our findings likely represent patterns of food introduction in our area, the southwest United States. Among the 33 patients, the most common culprit foods were oats (29.1%) and rice (20.8%). These are emerging triggers that are now surpassing previously recognized cow's milk and soybean in the United States.5,6 In a recent study of 74 FPIES cases at a referral center in Texas, rice was the most common individual food trigger (53%).4 Oats were our most commonly identified food trigger, which possibly reflects earlier introduction of oats into the diet as a consequence of pediatric consensus recommendations to use oats rather than rice for formula thickening in infant gastroesophageal reflux disease due to concerns with regard to inorganic arsenic content of American rice.19 We also identified a wide variety of other triggers, as follows: sweet potato, cow's milk, avocado, apple, banana, eggs, and soybean, all foods recommended by the American Academy of Pediatrics for complementary feeding during solid food introduction.20
LIMITATIONS
There are a number of limitations to our retrospective review. First, our study relied on ICD-9 and ICD-10 codes to identify patients. It is likely that cases were missed due to incorrect diagnostic coding. Five peanut-triggered cases were diagnosed in 2018–2019 and none in the preceding 4-year period (2013 to 2017). A comparison of the incidence of peanut-triggered FPIES before and after publication of the NIAID addendum guidelines11 in 2017 found no statistically significant difference. Given the small numbers involved, our study was likely underpowered to detect a statistically significant difference in the incidence rate of peanut-triggered FPIES. Interestingly, 53% of our total acute FPIES cohort was also diagnosed in the past 2 years (2018–2019) of the 6-year study period. Potential explanations for this include one or more of the following: (1) increased awareness of acute FPIES among the pediatric medical community because the first FPIES international consensus was published in 201713; (2) expansion of the clinicial practice within the Division of Allergy and Immunology at Phoenix Children's Hospital over the study period, with resultant ascertainment bias; and (3) a true increase in the incidence of FPIES with peanut as an emerging food trigger.
CONCLUSION
This study highlighted the importance of recognizing the potential emerging role of peanut as an acute FPIES trigger. Systematic review and meta-analysis of published FPIES cohorts may help confirm the role of peanut among the U.S. population as an acute FPIES trigger. Further study is needed to evaluate the role of preexisting atopic disease in risk stratification and to better characterize the role of IgE sensitization in prolonged disease and subsequent development of IgE-mediated peanut allergy.
Footnotes
The authors have no conflicts of interest to declare pertaining to this article. Catherine M. Freeman and Juan Carlos Murillo are co-first authors
No external funding sources reported
Supplemental data available at www.IngentaConnect.com
REFERENCES
- 1.Mehr S, Frith K, Barnes EHet al. Food protein-induced enterocolitis syndrome in Australia: a population-based study, 2012-2014. J Allergy Clin Immunol. 2017; 140:1323–1330. [DOI] [PubMed] [Google Scholar]
- 2.Alonso SB, Ezquiaga JG, Berzal PTet al. Food protein-induced enterocolitis syndrome: increased prevalence of this great unknown-results of the PREVALE study. J Allergy Clin Immunol. 2019; 143:430–433. [DOI] [PubMed] [Google Scholar]
- 3.Nowak-Wegrzyn A, Warren CM, Brown-Whitehorn Tet al. Food protein-induced enterocolitis syndrome in the US population-based study. J Allergy Clin Immunol. 2019; 144:1128–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackman AC, Anvari S, Davis CMet al. Emerging triggers of food protein-induced enterocolitis syndrome: lessons from a pediatric cohort of 74 children in the United States. Ann Allergy Asthma Immunol. 2019; 122:407–411. [DOI] [PubMed] [Google Scholar]
- 5.Caubet JC, Ford LS, Sickles Let al. Clinical features and resolution of food protein-induced enterocolitis syndrome: 10-year experience. J Allergy Clin Immunol. 2014; 134:382–389. [DOI] [PubMed] [Google Scholar]
- 6.Ruffner MA, Ruymann K, Barni Set al. Food protein-induced enterocolitis syndrome: insights from review of a large referral population. J Allergy Clin Immunol Pract. 2013; 1:343–349. [DOI] [PubMed] [Google Scholar]
- 7.Ludman S, Harmon M, Whiting Det al. Clinical presentation and referral characteristics of food protein-induced enterocolitis syndrome in the United Kingdom. Ann Allergy Asthma Immunol. 2014; 113:290–294. [DOI] [PubMed] [Google Scholar]
- 8.Wang KY, Lee J, Cianferoni Aet al. Food protein-induced enterocolitis syndrome food challenges: experience from a large referral center. J Allergy Clin Immunol Pract. 2019; 7:444–450. [DOI] [PubMed] [Google Scholar]
- 9.Robbins KA, Ackerman OR, Carter CAet al. Food protein-induced enterocolitis syndrome to peanut with early introduction: a clinical dilemma. J Allergy Clin Immunol Pract. 2018; 6:664–666. [DOI] [PubMed] [Google Scholar]
- 10.Du Toit G, Roberts G, Sayre PHet al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015; 372:803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Togias A, Cooper SF, Acebal MLet al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Allergy Clin Immunol. 2017; 139:29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James M,Anvari S, Anagnostou A.. Development of peanut allergy despite early introduction: a real-world case series in the United States. Pediatr Allergy Immunol. 2020; 31:589–592. [DOI] [PubMed] [Google Scholar]
- 13.US National Center for Health Statistics. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Available from: https://www.cdc.gov/nchs/icd/icd9cm.htm.
- 14.US National Center for Health Statistics. International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) Available from: http://www.cdc.gov/nchs/icd/icd10cm.htm. [PubMed]
- 15.Nowak-Weôgrzyn A, Chehade M, Groetch MEet al. International consensus guidelines for the diagnosis and management of food protein-induced enterocolitis syndrome: executive summary-Workgroup Report of the Adverse Reactions to Foods Committee, American Academy of Allergy. Asthma & Immunology. J Allergy Clin Immunol. 2017; 139:1111–1126.e4. [DOI] [PubMed] [Google Scholar]
- 16.Anvari S, Davis CM.. Food protein-induced enterocolitis syndrome. J Food Allergy. 2020; 2:48–54, doi: 10.2500/jfa.2020.2.200011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kessel A, Dalal I.. The pendulum between food protein-induced enterocolitis syndrome and IgE-mediated milk allergy. Acta Paediatr. 2011; 100:e183-e185. [DOI] [PubMed] [Google Scholar]
- 18.Ruffner MA, Wang KY, Dudley JWet al. elevated atopic comorbidity in patients with food protein-induced enterocolitis. J Allergy Clin Immunol Pract. 2020; 8:1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AAP group offers advice to reduce infants' exposure to arsenic in rice. AAP Arsenic in Rice Expert Work Group. AAP News. 2014; 35:13; accessed October 31, 2020. Available online at https://www.aappublications.org/content/35/11/13.1#:;:text=AAP%20group%20offers%20advice%20to%20reduce%20infants'%20exposure%20to%20arsenic%20in%20rice,-AAP%20Arsenic%20in&text=Arsenic%20recently%20has%20been%20reported,of%20bladder%20and%20lung%20cancer. [Google Scholar]
- 20.American Academy of Pediatrics. Infant Food and Feeding. Available online at https://www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/HALF-Implementation-Guide/Age-Specific-Content/Pages/Infant-Food-and-Feeding.aspx; accessed October 29, 2020.