Abstract
The dynamics of the establishment of, and reactivation from, gammaherpesviruses latency has not been quantitatively analyzed in the natural host. Gammaherpesvirus 68 (γHV68) is a murine gammaherpesvirus genetically related to primate gammaherpesviruses that establishes a latent infection in infected mice. We used limiting dilution reactivation (frequency of cells reactivating γHV68 in vitro) and limiting dilution PCR (frequency of cells carrying γHV68 genome) assays to compare γHV68 latency in normal (C57BL/6) and B-cell-deficient (MuMT) mice. After intraperitoneal (i.p.) inoculation, latent γHV68 was detected in the spleen, bone marrow, and peritoneal cells. Both B-cell-deficient and C57BL/6 mice established latent infection in peritoneal cells after either i.p. or intranasal (i.n.) inoculation. In contrast, establishment of splenic latency was less efficient in B-cell-deficient than in C57BL/6 mice after i.n. inoculation. Analysis of reactivation efficiency (reactivation frequency compared to frequency of cells carrying γHV68 genome) revealed that (i) regardless of route or mouse strain, splenic cells reactivated γHV68 less efficiently than peritoneal cells, (ii) the frequency of cells carrying γHV68 genome was generally comparable over the course of infection between C57BL/6 and B-cell-deficient mice, (iii) between 28 and 250 days after infection, cells from B-cell-deficient mice reactivated γHV68 10- to 100-fold more efficiently than cells from C57BL/6 mice, (iv) at 7 weeks postinfection, B-cell-deficient mice had more genome-positive peritoneal cells than C57BL/6 mice, and (v) mixing cells (ratio of 3 to 1) that reactivated inefficiently with cells that reactivated efficiently did not significantly decrease reactivation efficiency. Consistent with a failure to normally regulate chronic γHV68 infection, the majority of infected B-cell-deficient mice died between 100 and 200 days postinfection. We conclude that (i) B cells are not required for establishment of γHV68 latency, (ii) there are organ-specific differences in the efficiency of γHV68 reactivation, (iii) B cells play a crucial role in regulating reactivation of γHV68 from latency, and (iv) B cells are important for controlling chronic γHV68 infection.
Murine gammaherpesvirus 68 (γHV68) was isolated from a vole, and it infects outbred and inbred mice. The genomic sequence of γHV68 is available and confirms its close relationship with other gammaherpesviruses (22), including Epstein-Barr virus (EBV) and Kaposi’s sarcoma herpesvirus (also called human herpesvirus 8). γHV68 can acutely infect multiple organs of mice, including the spleen, liver, lung, kidney, adrenal glands, heart, and thymus (13, 18). Infection has been associated with splenomegaly, pneumonitis, and a fatal arteritis in mice lacking responsiveness to gamma interferon (18, 20, 24, 25). An association of γHV68 with the development of lymphomas has been reported (17). It has been shown that γHV68 can establish a latent infection in the spleen (18, 19, 24), and B cells have been implicated as the predominant latent cell type in hematopoietic cells in vivo (19). Because of its genomic structure, association with lymphomas, and evidence that it establishes a latent infection in B lymphocytes, γHV68 has been suggested as a murine model for EBV and Kaposi’s sarcoma herpesvirus (10, 11, 15, 19, 22).
To further examine the role of B cells in γHV68 infection and latency, we analyzed γHV68 infection of B-cell-deficient mice. These mice are deficient in mature B cells by virtue of a homozygous mutation in the transmembrane exon of the μ heavy-chain gene (9). Previously, we have shown that γHV68 can establish latency in B-cell-deficient mice (24), thus demonstrating that B lymphocytes are not required for establishment of latency by γHV68. We extend these observations here, demonstrating a role for B cells in regulating γHV68 latency, as measured by a quantitative limiting dilution reactivation assay. Further studies, comparing the relationship between viral reactivation and the frequency of γHV68-genome positive cells in vivo, provide evidence for organ-specific differences in γHV68 latency and for a critical role for B cells in regulating γHV68 reactivation from latency.
MATERIALS AND METHODS
Mice, infections, and organ harvests.
B-cell-deficient mice backcrossed onto a C57BL/6 background (C57BL/6J-Igh-6tm1Cgn mice) were purchased from The Jackson Laboratory (Bar Harbor, Maine). Mice were then bred and maintained at Washington University, St. Louis, Mo., in accordance with all university and federal guidelines. C57BL/6 mice were purchased from The Jackson Laboratory. Mice were infected with 106 PFU of γHV68 in Dulbecco modified Eagle medium (DMEM)–10% fetal calf serum either in a 1-ml volume for intraperitoneal (i.p.) inoculation or 10-μl volume for intranasal (i.n.) inoculation. γHV68 WUMS strain (ATCC VR1465) was used for all infections. The viral stock was passaged once on NIH 3T12 cells for amplification. Three separate first-round passages of the ATCC VR1465 stock were used in these studies. At various times postinfection, mice were sacrificed by cervical dislocation after metofane anesthesia, and various organs were harvested and analyzed for latent virus. All organs were harvested in DMEM–10% fetal calf serum. Resident peritoneal exudate cells (PECs) were harvested by peritoneal lavage with 10 ml of medium (6). Spleens and thymuses were disrupted in a tissue homogenizer, using a protocol that preserves cell viability, and filtered over Nitex to remove splenic stroma (1). Bone marrow cells were harvested from the femurs and tibias of mice, by flushing with several milliliters of medium (5, 12). Erythrocytes were lysed with ammonium chloride, and cells were washed and resuspended in DMEM–10% fetal calf serum.
Limiting dilution ex vivo reactivation assay to detect latent virus.
MEF (mouse embryonic fibroblast) cells were obtained from BALB/c mice and maintained as previously described (24). Limiting dilution analysis to detect reactivation from latency was performed as previously described (24). Briefly, serial twofold dilutions of test cells harvested from mice were plated onto indicator MEF cells in 96-well tissue culture plates. The wells were scored microscopically for viral cytopathic effect (CPE) after 3 weeks. A maximum of 100,000 cells was plated per well, as greater numbers of cells were toxic to the MEF monolayer. To measure the presence of preformed infectious virus in the test cell populations, the cells were killed prior to plating by mechanical disruption in 1/3× DMEM in the presence of 0.5-mm-diameter silica beads in a Mini-Beadbeater-8 (Biospec Products, Bartlesville, Okla.). Controls for the specificity and sensitivity of this assay are presented in Results. No difference in reactivation from latency was observed for MEF cells derived from BALB/c or from C57BL/6 mice.
Detection of γHV68 DNA by nested PCR.
Nested PCR to detect the ORF (open reading frame) 50 gene of γHV68 was shown to have a sensitivity of one copy of γHV68 DNA. The sequences of the outer PCR primers used were 5′-AACTGGAACTCTTCTGTGGC-3′ and 5′-GGCCGCAGACATTTAATGAC-3′, which amplify a 586-bp product. The sequences of the inner PCR primers used were 5′-CCCCAATGGTTCATAAGTGG-3′ and 5′-ATCAGCACGCCATCAACATC-3′, which amplify a 382-bp product. Primers were synthesized by GIBCO BRL. Each PCR mixture contained 50 mM KCl, 10 mM Tris-HCl (pH 8.5), 0.1% Triton X-100, 1.5 mM MgCl2, 0.2 mM nucleotides, 1 ng of each primer per μl, and 1 U of Taq polymerase (Promega). PCRs were performed on a Perkin-Elmer 9600 GENEAMP thermocycler. The initial round of PCR was performed in a 20-μl total volume with 45 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s, followed by extension at 72°C for 5 min. The conditions for the second round of PCR were identical except that the reaction mixture was amplified for 25 cycles. For the second round, 1 μl of the first-round PCR was amplified in a total volume of 10 μl. Second-round PCR products were visualized by electrophoresis on a 2% agarose gel stained with ethidium bromide. Plasmid pBamHI N containing ORF 50 of γHV68, kindly provided by Stacey Efstathiou (4), was used to determine the sensitivity of the nested PCR for detection of γHV68 DNA. pBamHI N was quantitated spectrophotometrically and diluted in mouse liver DNA or tRNA (0.5 mg/ml) in Tris-EDTA. One microgram of total nucleic acid from serial 10-fold dilutions of pBamHI N in mouse liver DNA or tRNA was analyzed by nested PCR in a series of control PCRs.
Determination of the frequency of latently infected cells harboring the γHV68 genome.
To determine the frequency of cells carrying the γHV68 genome in spleen and PECs from latently infected mice, nested PCR (single-copy sensitivity) was performed on serial dilutions of cells, using a previously published method (12) or an adaptation of the previously published method described here. Briefly, test cells were diluted in an isotonic medium (150 mM KCl, 10 mM Tris-HCl [pH 7.5], 1.5 mM MgCl2) in a background of uninfected MEF cells. To keep the total cell number constant for each PCR, serial fourfold dilutions of cells ranging from 10,000 test cells to 2.5 test cells per PCR, with a total of 10,000 cells (MEF plus test cells) per PCR, were made. Twelve to 24 PCRs were analyzed per cell concentration. Five-microliter cell dilutions were added to PCR tubes containing 5 μl of lysis buffer (10 mM Tris-HCl [pH 8.5], 1.5 mM MgCl2, 1% Nonidet P-40, 1% Tween 20, 0.2 mg of proteinase K per ml) and were lysed overnight at 56°C. Proteinase K was inactivated at 95°C for 15 min, and 10 μl of adjusted PCR cocktail (25 mM KCl, 10 mM Tris-HCl [pH 9.0], 0.5% Triton X-100, 1.5 mM MgCl2, 2× deoxynucleoside triphosphates, primers, Taq polymerase) was added directly to each cell lysate, so that final PCR conditions were as described above. Nested PCR was performed as described above. The dilutional nested PCR method was shown to have a sensitivity of one copy of γHV68 DNA in a background of 10,000 cells, by adding dilutions of plasmid pBamHI N to 10,000 uninfected MEF cells prior to cell lysis. These controls for one-copy sensitivity, as well as negative controls of MEF cells alone or water alone, were included for each set of PCRs performed. In addition, one-copy sensitivity for γHV68 DNA was observed when 106 copies of pBamHI N plasmid DNA were added to naive spleen cells and subsequently diluted, demonstrating that target DNA was not destroyed during the lysis procedure.
RESULTS
PECs from C57BL/6 and B-cell-deficient mice harbor a high frequency of γHV68-infected cells after i.p. inoculation.
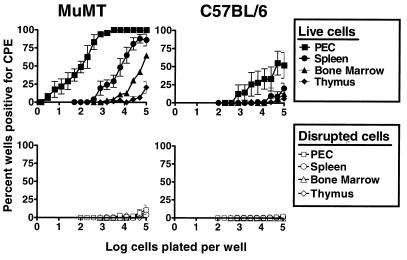
To quantitate the frequency of cells that reactivate γHV68, we have developed an ex vivo limiting dilution reactivation assay (24). In this assay, defined numbers of latently infected cells are plated on an indicator layer of MEF cells (which replicates reactivated γHV68). Viral CPE on the fibroblast monolayer is scored 2 to 3 weeks after plating of the test cells. Since reactivation from latency requires live cells, the presence of preformed infectious virus can be detected by disrupting live cells without inactivating preformed infectious virus (see below and reference 24). Using this assay, we surveyed hematopoietic cell reservoirs for the presence of latent γHV68 after i.p. inoculation of C57BL/6 and B-cell-deficient mice. Resident PECs harbored a high frequency of cells that reactivate γHV68 in both C57BL/6 and B-cell-deficient (MuMT) mice 42 days postinfection (Fig. 1). Indeed, the frequency of PECs that reactivate γHV68 was ∼50-fold higher than the frequency of splenocytes that reactivate γHV68 (Fig. 1). Low frequencies of cells that reactivate γHV68 were also detected in bone marrow cells from both C57BL/6 and B-cell-deficient mice, while an even lower frequency was detected in thymocytes. The profiles of γHV68 reactivation from different hematopoietic cell reservoirs were very similar in C57BL/6 and B-cell-deficient mice. However, the frequency of cells which reactivated γHV68 was consistently ∼100-fold higher in cell populations from γHV68-infected B-cell-deficient mice than in γHV68-infected C57BL/6 mice.
FIG. 1.
Survey of γHV68 latency in lymphoid organs from B-cell-deficient mice (MuMT) and C57BL/6 mice 42 days postinfection. Limiting dilution assay to detect reactivation from latency was performed with cells from the spleen, bone marrow, thymus, or resident PECs from B-cell-deficient or C57BL/6 mice. Shown are the percentages of wells that scored positive for viral CPE 3 weeks after plating, as a function of the number of cells plated per well; 24 wells were plated per each cell dilution in each experiment. Shown in the bottom panels are the results obtained when cells were killed by mechanical disruption prior to plating, which indicates that no preformed infectious virus was present in any of the samples tested. B-cell-deficient mice demonstrated latent virus in the same organ systems as C57BL/6 mice. The frequency of cells that reactivated latent virus from B-cell-deficient mice was about 100-fold higher than from C57BL/6 mice in all organs tested. Data represent averages of three to seven separate experiments. Cells from three to five mice per group were pooled and assayed per experiment. Error bars represent standard errors from the means.
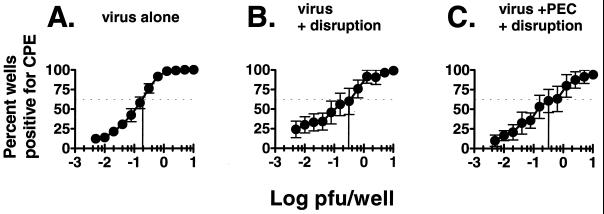
Notably, no evidence of preformed infectious virus was detected in the PEC population, as determined by plating mechanically disrupted cells (Fig. 1). To ensure that the reactivation assay was able to distinguish between the presence of preformed infectious γHV68 and reactivation from latency in the PEC population, we determined the sensitivity of the limiting dilution assay for detection of preformed infectious virus. A limiting dilution titration of γHV68 was carried out before and after mechanical disruption in the presence and absence of PECs (Fig. 2). Limiting dilution analysis of virus alone confirmed that this assay is ∼5-fold more sensitive than the standard plaque assay (i.e., detects 0.2 PFU of γHV68) [Fig. 2A and reference 24]). Mechanical disruption of virus alone (Fig. 2B) or in the presence of PECs (Fig. 2C) did not significantly inhibit detection of preformed infectious virus. Since we detected 0.2 PFU/well, and since no preformed infectious virus was detected when 104 to 105 PECs or spleen cells from latently infected organs were evaluated after mechanical disruption (Fig. 1), we concluded that there is <1 PFU of γHV68 per 5 × 104 to 5 × 105 cells. The frequency of cells reactivating γHV68 is much higher than this (see below), indicating that preformed infectious virus cannot explain results from the limiting dilution reactivation assay. This finding proved that we are detecting and quantitating latent γHV68 using this assay.
FIG. 2.
Detection of infectious virus is not affected by mechanical disruption of PECs. Serial twofold dilutions of a stock of γHV68 virus, which had a known titer as determined by plaque assay on NIH 3T12 fibroblasts, were plated on a monolayer of MEF cells in 96-well plates. The percentage of wells that scored positive for viral CPE is shown as a function of log PFU (as determined by plaque assay) plated per well. (A) Stock γHV68 virus; (B) γHV68 virus subjected to a mechanical disruption process which kills >99% of cells (see Materials and Methods); (C) γHV68 virus inoculated into PECs from C57BL/6 mice prior to mechanical disruption of cells. The vertical lines indicate the log PFU per well in which 63.2% of the wells scored positive. By Poisson distribution, 0.2 PFU could be detected with virus alone, and between 0.2 and 0.4 PFU could be detected after mechanical disruption alone or after mechanical disruption in the presence of PECs. Data represent averages of five separate experiments. Error bars represent standard errors from the means.
Clinical course of γHV68 infection in B-cell-deficient mice.
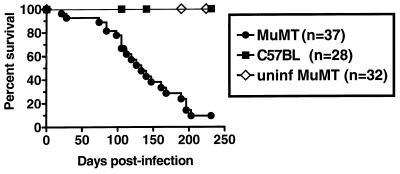
Intraperitoneal inoculation of C57BL/6 mice with 1 × 106 to 2 × 106 PFU of γHV68 does not lead to significant morbidity or mortality over the course of 1 year p.i. (reference 25 and data not shown). In contrast, as we have previously reported, a significant percentage of γHV68-infected B-cell-deficient mice develop an arteritis that affects the great elastic vessels (25). In addition, as shown here (e.g., Fig. 1) and previously (24), B-cell-deficient mice harbor a significantly higher frequency of cells that reactivate γHV68 than C57BL/6 mice. Given these abnormalities in B-cell-deficient mice, we evaluated mortality of B-cell-deficient compared to control mice over a prolonged period. Most γHV68-infected B-cell-deficient mice (but not uninfected B-cell-deficient mice) died between 100 and 250 days postinfection (Fig. 3). These data on mortality, combined with the presence of arteritis and higher frequencies of cells that reactivate γHV68 in B-cell-deficient mice, led us to perform a more complete evaluation of chronic infection in B-cell-deficient and C57BL/6 mice.
FIG. 3.
B-cell-deficient mice (MuMT) eventually succumb after infection with γHV68. The Kaplan-Meier survival curve of B-cell-deficient versus C57BL/6 mice after i.p. inoculation with 106 PFU of γHV68 shows that 94% of B-cell-deficient mice died 21 to 203 days p.i. The last two B-cell-deficient mice were sacrificed 250 days p.i. None of the C57BL/6 mice died. Uninfected (uninf) mouse controls were breeders sacrificed at 189 to 378 days of age. Data are the pooled results of three separate experiments.
Establishment of γHV68 latency in C57BL/6 and B-cell-deficient mice is not dependent on the route of inoculation.
Our previous studies, demonstrating the establishment of γHV68 latency in B-cell-deficient mice (24), apparently conflict with the data of Usherwood et al. (21), who reported that B-cell-deficient mice do not harbor splenic latent virus (although a more recent analysis by these investigators has provided evidence of latent infection in the lungs of B-cell-deficient mice [16]). Two potentially significant differences between the experimental system we have employed and that used by Usherwood et al. (21) are (i) the route of inoculation and (ii) the assay used to detect latent virus. While we have used the i.p. route of inoculation, Usherwood et al. (21) infected mice by i.n. inoculation. To determine whether route of inoculation affects the establishment of latency in C57BL/6 and B-cell-deficient mice, we compared the i.n. and i.p. routes of inoculation of C57BL/6 and B-cell-deficient mice on days 9, 15, and 49 postinfection.
(i) Intraperitoneal inoculation leads to establishment of latency in the spleen and peritoneum of both C57BL/6 and B-cell-deficient mice.
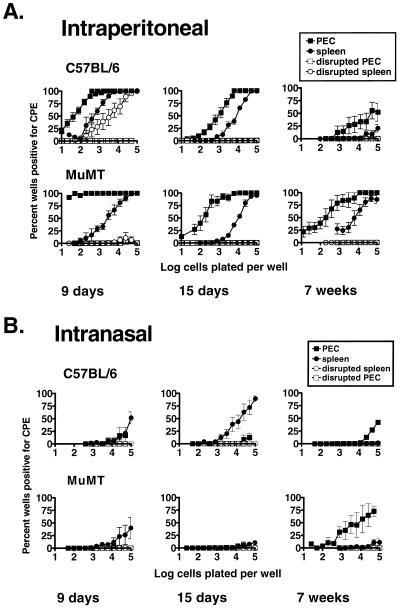
After i.p. inoculation, C57BL/6 mice had preformed infectious γHV68 in the spleen on day 9 postinfection (demonstrated by the presence of viral CPE in wells receiving mechanically disrupted cells) (Fig. 4A). Preformed infectious virus was cleared by day 15 postinfection (Fig. 4A; compare mechanically disrupted cells on days 9 and 15). In contrast, B-cell-deficient mice did not harbor infectious virus on day 9 or 15 postinfection (Fig. 4A). These results are consistent with our previous results (24), and with immunohistochemical staining for γHV68 antigens with a polyclonal antiserum (4a), demonstrating a critical role for B cells in the efficient establishment of acute infection of the spleen 9 days postinfection. Latent γHV68 was detected in PECs and splenocytes of both C57BL/6 and B-cell-deficient mice after i.p. inoculation. The frequency of cells reactivating γHV68 in PECs was higher than in the spleen at all times tested (Fig. 4A). The frequency of cells reactivating γHV68 from both PECs and splenocytes was higher from latently infected B-cell-deficient mice than from latently infected C57BL/6 mice (Fig. 1 and 4A).
FIG. 4.
Establishment of latency in B-cell-deficient mice is not dependent on the route of inoculation. γHV68 latency was determined after i.p. (A) and i.n. (B) inoculation. Mice were infected in parallel with 106 PFU of γHV68. Limiting dilution assay to detect reactivation from latency was performed on PECs and splenocytes isolated from B-cell-deficient (MuMT) and C57BL/6 mice 9 days, 15 days, and 7 weeks postinfection. Shown is the percentage of wells that scored positive for viral CPE as a function of the number of cells plated per well, 3 weeks after plating; 24 wells were plated per cell dilution per experiment. Shown in open symbols are the results obtained when cells were killed by mechanical disruption prior to plating, representing preformed infectious virus. Data represent averages of two separate experiments. Three mice per group were assayed per experiment. Error bars represent standard errors from the means.
(ii) Intranasal inoculation leads to establishment of latency in the peritoneum and spleen of C57BL/6 mice and in the peritoneum, but not the spleen, of B-cell-deficient mice.
Establishment of latency in the spleen and peritoneum differed between mice infected by the i.n. route and those infected i.p. (Fig. 4). No infectious virus was detected on day 9 postinfection in the spleens of C57BL/6 mice after i.n. inoculation (compare mechanically disrupted cells in Fig. 4). This may be because the peak of viral infection appeared to be earlier after i.n. inoculation than i.p. inoculation (18, 24) or because systemic infection is less efficient after i.n. inoculation. Latent virus was detected in C57BL/6 mouse spleens, but not in B-cell-deficient mouse spleens, on day 15 after i.n. inoculation (Fig. 4B). Although 9 days postinfection, a low frequency of cells that reactivate γHV68 was detected in both B-cell-deficient mouse and C57BL/6 spleens, the detection of latently infected cells was just above the limit of detection of the in vitro reactivation assay (100,000 splenocytes per well), and latent virus was less consistently observed in B-cell-deficient than C57BL/6 mice (note error bars). These data are consistent with those of Usherwood et al. (21) using a different assay to detect latent virus, in which it was found that B-cell-deficient mice do not establish detectable γHV68 latency in the spleen 7 to 35 days after i.n. inoculation.
Seven weeks after i.n. inoculation, PECs from both B-cell-deficient and C57BL/6 mice harbored latent virus, although establishment of latency in PEC was delayed after i.n. compared with i.p. inoculation (compare Fig. 4A and B). Thus, establishment of latency in peritoneal cells of both C57BL/6 and B-cell-deficient mice is not dependent on the route of inoculation. This finding demonstrated that peritoneal cells contain a cell type (present in B-cell-deficient mice and thus not a B cell) that harbors latent γHV68 in vivo.
Comparison of γHV68 reactivation as a function of time postinfection in C57BL/6 and B-cell-deficient mice reveals that B cells are important for controlling the frequency of cells reactivating latent γHV68.
After i.n. inoculation, the frequency of cells reactivating γHV68 detected in C57BL/6 spleens was lower at 7 weeks postinfection than at 15 days postinfection (Fig. 4B). Similarly, after i.p. inoculation, the frequency of cells that reactivated γHV68 from C57BL/6 PECs and spleen decreased over time (Fig. 4A). In contrast to the results obtained for C57BL/6 mice, the frequency of cells from B-cell-deficient mice that reactivated γHV68 from PECs and splenocytes did not decrease significantly over 7 weeks postinfection. To gain a more complete picture of the establishment of γHV68 latency in C57BL/6 and B-cell-deficient mice, the kinetics of γHV68 latency over 250 days of infection was determined by the ex vivo reactivation assay (Fig. 5 and 6). In addition, recrudescent γHV68 infection has been observed in major histocompatibility complex class II-deficient mice (3), and a sensitive assay for linear γHV68 genomes has detected lytic γHV68 replication in the lungs of γHV68-infected B-cell-deficient mice late after infection (16). These data, coupled to the fact that γHV68-infected B-cell-deficient mice die over time (Fig. 3), provided a rationale for determining whether preformed infectious γHV68 appeared in spleens and PECs at late times after infection with γHV68.
FIG. 5.
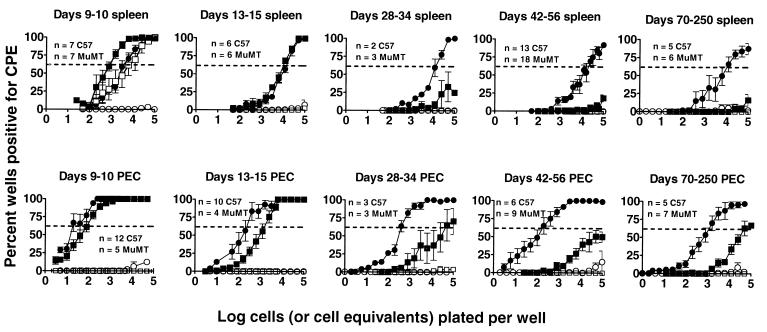
Time course of latency in B-cell-deficient (MuMT) and C57BL/6 mice, as detected by ex vivo reactivation assay. Limiting dilution assay to detect reactivation from latency was performed at various days postinfection from splenocytes and PECs of i.p.-inoculated mice. Data from similar days were pooled. ■, C57 cells; ●, MuMT cells; □ and ○, C57 and MuMT cells killed by mechanical disruption prior to plating, representing preformed infectious virus. Data represent averages of multiple separate experiments, as indicated (n). Three mice per group were assayed per experiment. Error bars represent standard errors from the means. Dotted lines indicate 63.2%, which was used to calculate the frequency of reactivating cells by Poisson distribution.
FIG. 6.
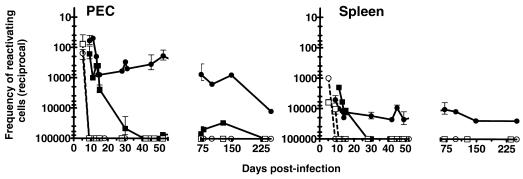
Estimation of the frequency of reactivating latently infected cells at various times after infection. The frequency of reactivating PECs or splenocytes from B-cell-deficient and C57BL/6 mice was calculated at various times postinfection for each individual experiment represented in Fig. 4. Reciprocal frequency indicates the number of cells plated per well when 63.2% of the wells scored positive for viral CPE (Poisson distribution). Symbols are as in Fig. 5. Infectious virus was cleared from PECs by day 9 postinfection in both B-cell-deficient and C57BL/6 mice and was cleared from the spleen by day 9 in B-cell-deficient mice and on day 11 in C57BL/6 mice. The frequency of latent cells cannot be determined on days prior to clearance of infectious virus. Each point represents at least one experiment. For days postinfection on which more than one experiment was performed, error bars represent standard errors from the means.
(i) Lack of preformed infectious γHV68 in the spleen and PECs of B-cell-deficient mice at late times after infection.
We determined whether detectable levels of preformed infectious virus were present in splenocytes and PECs of B-cell-deficient and C57BL/6 mice between 9 and 250 days after infection. Infectious virus was detected in the PEC population of both B-cell-deficient and C57BL/6 mice on day 5 postinfection but was cleared by day 9 postinfection, at which time viral latency could readily be detected in the PEC population (Fig. 5 and 6). After this initial time point, preformed infectious virus was detected very sporadically and at low levels (<1 PFU per 5 × 104 splenocytes or PECs) in both B-cell-deficient and C57BL/6 mice (Fig. 5). There was no consistent increase in the frequency of preformed infectious virus over time, and B-cell-deficient and C57BL/6 mice did not differ significantly. We concluded that recrudescent infection in spleen and PECs was not occurring in normal or B-cell-deficient mice.
(ii) Frequencies of PECs and splenocytes that reactivate latent γHV68 are very similar in C57BL/6 and B-cell-deficient mice at early times postinfection.
At days 9 and 10 postinfection, the frequency of cells reactivating γHV68 could not be determined in splenocytes harvested from C57BL/6 mice due to ongoing acute virus replication (Fig. 5 and 6; note the presence of preformed infectious virus detected in the disrupted cell samples). However, by 13 to 15 days postinfection, preformed infectious virus was cleared from the spleens of C57BL/6 mice and the frequencies of cells reactivating γHV68 were nearly identical in C57BL/6 and B-cell-deficient mice (Fig. 5 and 6). Similarly, at days 9 and 10 postinfection, the frequencies of PECs reactivating γHV68 were very similar in C57BL/6 and B-cell-deficient mice. Thus, patterns of establishment of latency were very similar at early times postinfection in C57BL/6 and B-cell-deficient mice, indicating that the absence of B cells does not grossly perturb this process.
(iii) The frequency of C57BL/6 PECs and splenocytes that reactivate γHV68 decreases dramatically over the first 4 weeks postinfection.
In C57BL/6 mice, the frequency of cells reactivating γHV68 decreased dramatically (about 500-fold in PECS and >10-fold in splenocytes) between days 15 and 42 postinfection and appeared to reach a low, steady-state level (Fig. 5 and 6). The estimated frequency of cells reactivating γHV68 in C57BL/6 spleen was about 1 in 10,000 cells on days 13 to 15 postinfection, which decreased to fewer than 1 in 100,000 cells (the limit of detection of the assay) by day 30 postinfection. The estimated frequency of latent cells in C57BL/6 PEC was about 1 in 100 cells on day 9 postinfection, which decreased to about 1 in 1,000 cells on days 13 to 15 postinfection and to about 1 in 50,000 cells at later times.
(iv) The frequency of B-cell-deficient mouse PECs and splenocytes that reactivate γHV68 remains fairly constant, even at late times postinfection.
The frequency of cells reactivating γHV68 from B-cell-deficient mice at most time points was much higher than the frequency observed in the equivalent cell populations harvested from C57BL/6 mice. The frequency of cells reactivating γHV68 from B-cell-deficient mice was relatively stable over time (Fig. 5 and 6). Approximately 1 in 4,000 B-cell-deficient mouse splenocytes harvested on day 9 postinfection reactivated virus, while ∼1 in 10,000 B-cell-deficient mouse splenocytes reactivated virus by day 150 postinfection.
With PECs harvested from infected B-cell-deficient mice, the frequency of reactivation from latency was significantly higher than with splenocytes. Approximately 1 in 100 PECs reactivated virus on day 9 postinfection. The frequency of reactivation remained between 1 in 100 to 1 in 1,000 cells through day 150 postinfection (Fig. 6). However, between days 150 and 250 postinfection there was a substantial drop in the observed frequency of reactivation to ∼1 in 10,000 cells reactivating γHV68. As discussed above, the majority of infected B-cell-deficient mice died between days 100 and 200 postinfection (by day 250 postinfection, 94% of infected B-cell-deficient mice were dead [Fig. 3]). Thus, B-cell-deficient mice analyzed on days 150 and 250 postinfection represent a subset of the animals originally infected. Thus, analysis of infected B-cell-deficient mice at late times postinfection may select for animals with the lowest levels of latent virus.
Latently infected C57BL/6 and B-cell-deficient mice harbor similar frequencies of γHV68 genome-positive cells.
Multiple mechanisms might explain the persistence of high frequencies of cells reactivating γHV68 in B-cell-deficient mouse tissues over time. We elected to test the hypothesis that the frequency of cells reactivating γHV68 is directly related to the frequency of γHV68 genome-positive cells present in tissues. We therefore determined the frequency of γHV68 genome-positive cells in PEC and splenocyte populations harvested from latently infected C57BL/6 and B-cell-deficient mice at various times postinfection (Fig. 7). The presence of viral genome was assessed by using a nested PCR assay to detect the presence of γHV68 gene 50 sequences in serial dilutions of cells from latently infected mice (see Materials and Methods). This assay has been shown to detect a single copy of the viral genome in a background of cellular DNA from 104 cells (26). In parallel, the frequency of cells that reactivate γHV68 in vitro was determined by the limiting dilution reaction assay (see Materials and Methods). Comparison of the frequency of genome-positive cells to the frequency of cells reactivating γHV68 allowed us to measure the efficiency of reactivation in different cell populations.
FIG. 7.
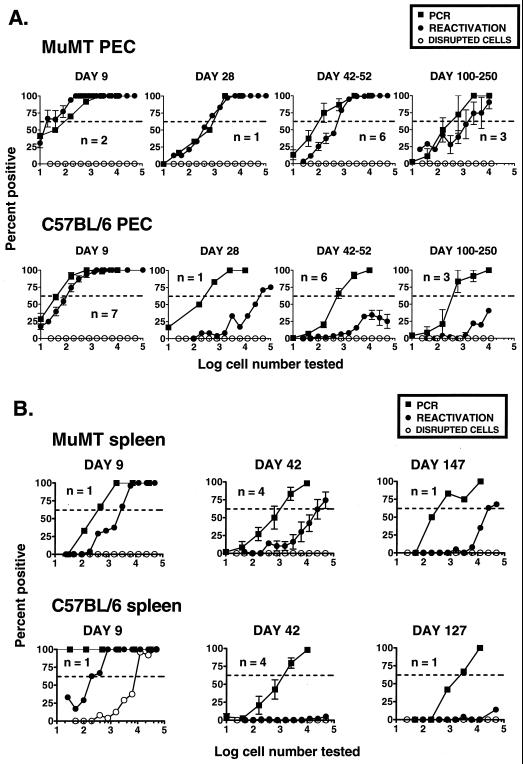
Comparison of the frequency of cells that harbor viral genome to the frequency of cells that reactivate virus in vitro, after i.p. inoculation. PECs (A) or splenocytes (B) from B-cell-deficient (MuMT) or C57BL/6 mice were analyzed by two different methods in parallel, at various times postinfection. Reactivation was quantitated by limiting dilution reactivation assay. The results of reactivation assay using disrupted cells, representing infectious virus, are shown in open symbols. The frequency of genome-positive cells was determined by dilutional nested PCR. Shown are the percentages of reactivation or PCR assays that scored positive as a function of the number of cells analyzed. For each cell number, 24 wells or 12 PCR assays were analyzed per experiment. The dotted line indicates 63.2%, which was used to calculate the frequency of reactivating or genome-positive cells by Poisson distribution. Data represent single experiments, or the average of multiple separate experiments, as indicated (n). Each experiment represents a pool of three mice. Error bars represent standard errors from the means.
(i) Correlation between viral genome load and frequency of γHV68 reactivation from B-cell-deficient mice and detection of organ-specific differences in reactivation efficiency.
When PECs from latently infected B-cell-deficient mice were analyzed, a very good correlation between the frequency of cells that reactivated γHV68 and the number of γHV68 genome-positive cells was observed over the time course from days 9 to 250 postinfection (Fig. 7A). This finding indicated that nearly all genome-positive PECs reactivated in the in vitro culture system. When splenocytes from the infected B-cell-deficient mice were analyzed, the frequency of γHV68 reactivation was consistently lower than the frequency of γHV68-genome positive cells over the entire time course (Fig. 7B). Notably, the frequency of splenocytes harboring viral genome remained fairly constant (∼1 in 400 cells) over this time interval, while the frequency of reactivation dropped from ∼1 in 5,000 to ∼1 in 20,000 cells (Fig. 7B). Thus, unlike latently infected PECs, it appears that only 2 to 10% of viral genome-positive splenocytes isolated from B-cell-deficient mice reactivated latent γHV68 in the in vitro reactivation assay.
(ii) Correlation between viral genome load and frequency of γHV68 reactivation from C57BL/6 mice.
We expected the decreased frequency of reactivation seen in PECs and splenocytes from C57BL/6 mice over time (Fig. 5 and 6) to be explained by decreased frequencies of genome-positive cells. However, quantitation of the frequency of genome-positive cells in C57BL/6 mice led to the surprising observation that these animals harbor nearly as many γHV68 genome-positive cells as do latently infected B-cell-deficient mice. Comparison of the frequencies of γHV68 genome-positive PECs and ex vivo reactivation revealed that the load of viral genome in C57BL/6 splenocytes and PECs remained very high over the time course examined, while the frequency of reactivation dropped dramatically (Fig. 5 to 7).
Notably, it was not possible to compare the frequencies of viral genome-positive and reactivating splenocytes at day 9 after infection of C57BL/6 mice due to the presence of ongoing virus replication in the spleen at this time point (Fig. 7B, disrupted cells). However, latency was established in C57BL/6 PECs by day 9 postinfection, as preformed infectious virus was not detected (Fig. 7A, disrupted cells). At early times postinfection (day 9), the frequency of genome-positive PECs and the frequency of reactivation correlated very closely for cells from C57BL/6 and B-cell-deficient mice (Fig. 7A). Thus, initial establishment of latency and initial efficiency of reactivation were similar for B-cell-deficient and C57BL/6 mice.
In marked contrast to results obtained 9 days afterinfection, by days 100 to 200 postinfection, in C57BL/6 mice the frequency of γHV68 genome-positive PECs was ∼1 in 200 cells whereas the frequency of reactivation was <1 in 10,000 cells (Fig. 7A). Similarly, when splenocytes from C57BL/6 mice were analyzed, the frequency of viral genome-positive cells (∼1 in 500 to 2,000 cells) was much greater at days 42 and 127 postinfection than the frequency of reactivating cells (<1 in 100,000 cells) (Fig. 7B). Thus, at late times after infection significantly less than 1% of γHV68 genome-positive PECs or splenocytes recovered from latently infected C57BL/6 mice reactivated in vitro. In contrast, in B-cell-deficient mice, there was a much closer correlation between the frequency of γHV68 genome-positive cells and reactivation frequency. In both C57BL/6 and B-cell-deficient mice, the efficiency of reactivation from γHV68 genome-positive splenocytes was lower than that from genome-positive PEC.
(iii) Relationship between genome load and γHV68 reactivation after i.n. inoculation.
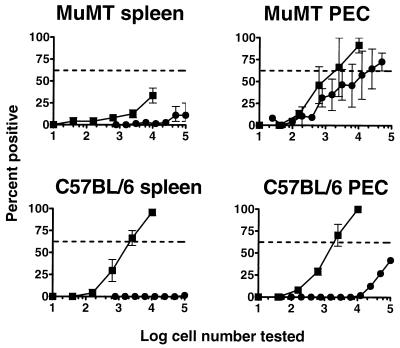
Since the frequency of reactivation from PECs and splenocytes was very low by 7 weeks after i.n. inoculation of C57BL/6 mice (Fig. 4B), we determined whether a disparity between the frequency of viral genome harboring cells and reactivation also exists after i.n. inoculation. Analysis of the viral genome load 7 weeks after i.n. inoculation revealed a large disparity between the frequency of genome-positive splenocytes and the frequency of reactivation in C57BL/6 mice, similar to that observed after i.p. inoculation (Fig. 8). Notably, the frequency of genome-positive splenocytes after i.n. inoculation (ca. 1 in 3,000 cells) was very similar to the frequency observed after i.p. inoculation (Fig. 7). As expected, when the frequency of viral genome-positive splenocytes in B-cell-deficient mice 7 weeks after i.n. inoculation was determined, very low frequencies of γHV68-positive cells were detected (Fig. 8). The latter is consistent with the failure to detect virus reactivation from the spleens of B-cell-deficient mice after i.n. inoculation (Fig. 3B and reference 21).
FIG. 8.
Comparison of the frequency of genome-positive cells to reactivation frequency ex vivo 7 weeks after i.n. inoculation. PECs and splenocytes from B-cell-deficient and C57BL/6 mice were analyzed by two different methods in parallel. Reactivation (●) was quantitated by limiting dilution reactivation assay. The frequency of genome-positive cells was determined by dilutional nested PCR (■). Shown are the percentages of reactivation or PCR assays that scored positive as a function of the number of cells analyzed. For each cell number, 24 wells or 12 PCR assays were analyzed per experiment. The dotted line indicates 63.2%, which was used to calculate the frequency of reactivating or genome-positive cells by Poisson distribution. Data represent averages of two separate experiments. Three mice per group were assayed per experiment. Error bars represent standard errors from the means.
Examination of PECs isolated from B-cell-deficient mice 7 weeks postinfection revealed both a relatively high frequency of genome-positive cells (∼1 in 2,000 cells) and a slightly lower frequency of γHV68 reactivating cells (∼1 in 10,000 cells). However, similar to the observations after i.p. inoculation, a much greater proportion of genome-positive PECs from B-cell-deficient mice than from C57BL/6 mice reactivated in vitro. As after i.p. inoculation, the frequency of viral genome-positive C57BL/6 PECs 7 weeks postinfection (∼1 in 2,000 cells) was substantially higher than the frequency of C57BL/6 PECs reactivating γHV68 (<1 in 100,000 cells). Thus, very similar relationships between viral genome load and reactivation were observed after either i.p. or i.n. inoculation.
(iv) Comparison of viral genome load in latently infected B-cell-deficient and C57BL/6 mice 42 to 50 days postinfection reveals a higher frequency of PECs harboring virus in B-cell-deficient mice than in C57BL/6 mice.
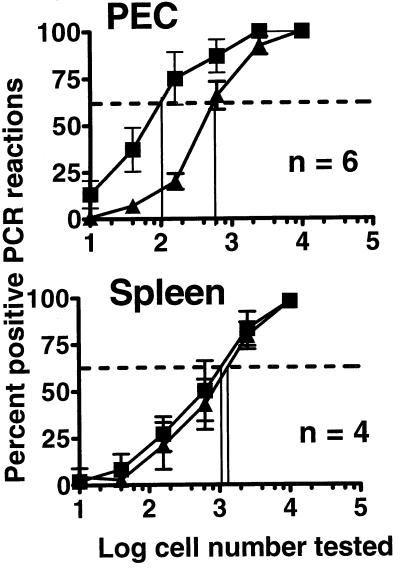
Data compiled from several independent experiments with cells harvested between days 42 and 50 after i.p. inoculation revealed that there were ca. 6-fold more γHV68 genome-positive PECs in B-cell-deficient mice than in C57BL/6 mice (Fig. 9). However, while a significant difference was observed in the frequency of viral genome-positive PECs in C57BL/6 and B-cell-deficient mice, no detectable difference was observed in the frequency of viral genome-positive cells in the splenocyte populations isolated from these strains of mice (Fig. 9). Thus, the frequency of viral genome-positive PECs and splenocytes in B-cell-deficient and C57BL/6 mice does not account for the large difference in the frequency of cells reactivating γHV68 in these mouse strains.
FIG. 9.
Comparison of the frequency of genome-positive cells from C57BL/6 and B-cell-deficient mice 7 weeks after i.p. inoculation. The frequency of genome-positive cells in PECs and splenocytes from B-cell-deficient (■) and C57BL/6 (▴) mice was determined by dilutional nested PCR. Data shown are results from Fig. 5 plotted so as to compare the frequency of genome-positive cells between B-cell-deficient and C57BL/6 mice 7 weeks postinfection. The vertical lines indicate the frequency of genome-positive cells for each population, defined by the cell number that scored positive 63.2% of the time (dotted lines). Data represent averages of multiple separate experiments (n). Each experiment represents a pool of three mice. Error bars represent standard errors from the means.
Mixing C57BL/6 and B-cell-deficient mouse latently infected PECs does not significantly decrease the frequency of cells reactivating γHV68.
Since viral genome load does not explain the large disparity in the frequency of cells from B-cell-deficient versus C57BL/6 mice that reactivate γHV68, we considered the possibility that a soluble factor (e.g., antibody) present in the C57BL/6 reactivation culture (and absent in the B-cell-deficient mouse reactivation culture) is involved in blocking detection of reactivated γHV68. To address this possibility, we performed mixing experiments in which PECs isolated from latently infected C57BL/6 mice were added to PECs isolated from latently infected B-cell-deficient mice in the limiting dilution reactivation assay (Fig. 10). We chose to use PECs for this analysis since (i) there was a very strong correlation between the frequency of viral genome-positive cells and the frequency of cells reactivating γHV68 in the PEC population isolated from B-cell-deficient mice and (ii) the frequency of reactivation from PECs was significantly higher than that from splenocytes, affording a more accurate assessment of the relationship between cells isolated from B-cell-deficient and C57BL/6 mice. We performed assays in which various ratios of B-cell-deficient mouse and C57BL/6 PECs were mixed, along with control assays in which B-cell-deficient mouse PECs or C57BL/6 PECs from latently infected mice were plated alone (Fig. 10). For this analysis, PECs from infected mice were isolated either 41 (Fig. 10A) or 127 to 148 (Fig. 10B) days postinfection. It should be noted that for the analyses shown in Fig. 10, in which C57BL/6 and B-cell-deficient mouse cells were mixed, the number of B-cell-deficient mouse cells plated per well (as opposed to the total number of cells per well plated) was plotted, allowing a direct comparison of reactivation efficiency between the different groups.
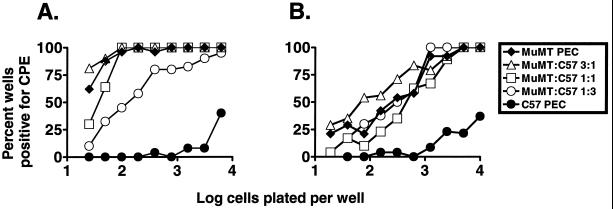
FIG. 10.
Mixing latently infected C57BL/6 PECs and B-cell-deficient (MuMT) PECs. Limiting dilution assay to detect reactivation from latency was performed with PECs from B-cell-deficient mice, C57BL/6 mice, or a mixture of the two at the ratios shown. Shown are the percentages of wells that scored positive for viral CPE 3 weeks after plating, as a function of the number of cells plated per well. For assays containing both C57BL/6 and B-cell-deficient PECs, the number of B-cell-deficient mouse PECs in each well was plotted versus the percentage of wells positive for CPE; 24 wells were plated per each cell dilution. PECs isolated 7 (A) and 18 to 21 (B) weeks postinfection from three B-cell-deficient or C57BL/6 mice were pooled for each experiment. Each panel represents a single experiment.
With PECs isolated from mice 41 days postinfection, a slight decrease in the frequency of cells reactivating γHV68 was observed at the highest ratio of C57BL/6 PECs to B-cell-deficient mouse PECs (Fig. 10A, 3:1 ratio). However, the frequency of C57BL/6 PECs reactivating γHV68 was still 50- to 100-fold lower than the reactivation frequency in cultures containing a 3:1 ratio of C57BL/6 to B-cell-deficient mouse PECs (Fig. 10A). Similarly, when PECs isolated from C57BL/6 mice 127 days postinfection were mixed with PECs isolated from B-cell-deficient mice 148 days postinfection, no decrease in the frequency of cells reactivating γHV68 was observed (Fig. 10B). These data argue against the presence of a soluble factor generated by C57BL/6 cells that inhibits reactivation of γHV68 genome-positive cells.
DISCUSSION
In this report we present a detailed analysis of γHV68 latency in C57BL/6 and B-cell-deficient mice, test the hypothesis that the frequency of genome-positive cells predicts the frequency of gammaherpesvirus reactivation, and identify both organ-specific and mouse strain-specific differences in the efficiency of γHV68 reactivation from latency. We demonstrate for the first time that regardless of route of inoculation, peritoneal cells are a rich source of cells latently infected with γHV68 and that bone marrow is a reservoir of cells harboring latent γHV68. Furthermore, our analysis confirms that B cells are not required for the establishment of γHV68 latency in hematopoietic cells (24) but demonstrate a key role of B cells in regulating γHV68 latency. In addition, it is now clear that our initial observation of higher frequencies of cells reactivating γHV68 in B-cell-deficient mice (24) largely reflects inefficient reactivation from C57BL/6 latently infected PEC and splenocyte populations. It is possible that the observed differences in the behavior of γHV68 latently infected cells isolated from C57BL/6 and B-cell-deficient mice reflect a disregulation of γHV68 latency in vivo, contributing to a chronic and ultimately fatal disease process in γHV68-infected B-cell-deficient mice.
Sites of γHV68 latency.
We found that resident PECs harbor a high frequency of cells latently infected with γHV68 in both C57BL/6 and B-cell-deficient mice. In addition, γHV68 latency was observed in the bone marrow. Studies here support our previous observation of γHV68 latency in B-cell-deficient mice (24) and provide strong evidence that some hematopoeitic cell type other than a mature B cell is a major reservoir for latent γHV68. It is interesting to speculate that the latent cell types in the peritoneum and the bone marrow are related and that the bone marrow may provide a source for circulating cells that carry latent γHV68. Related to this, we have identified macrophages (and, at a significantly lower level, B cells) as the major cell type harboring latent γHV68 in PECs from C57BL/6 mice (26). As macrophages are bone marrow derived, further experiments will need to address whether bone marrow-derived cells, perhaps macrophages or B cells, contribute to γHV68 latency in other organs.
There is evidence that lung epithelial cells may also be a site of γHV68 latency (16). However, the latter analysis did not unambiguously identify epithelial cells as a site of latent γHV68, since it relied on the ability to detect by in situ hybridization viral tRNA-like transcripts (argued as a criterion for latency), combined with the failure to detect by in situ hybridization viral transcripts encoding glycoprotein H (gH) and thymidine kinase (TK) (argued as proving a lack of lytic replication). Because the tRNAs are abundantly expressed during the viral lytic cycle (2, 27), and the relative sensitivities of in situ hybridization for viral tRNA-like transcripts versus gH and TK transcripts were not determined, these investigators may have simply detected tRNA-like transcripts in lytically infected epithelial cells that were not expressing sufficiently high levels of gH and TK transcripts to be detected. Indeed, linear viral genomes (diagnostic of ongoing viral replication) were detected in the lungs, but no gH/TK-positive cells were detected by in situ hybridization (2). This finding shows that in situ hybridization was not sensitive enough to detect lytic γHV68 replication even when the authors proved that such cells must exist.
Route of inoculation.
It has been supposed that i.n. inoculation is the natural route of infection for γHV68. Based on this argument, studies of γHV68 latency using i.p. inoculation have been criticized as unphysiologic. However, experimental data demonstrating respiratory spread of γHV68 have not been published. In fact, the only published transmission of γHV68 occurred when an uninfected dam ate infected pups (13). Thus, the natural route of γHV68 infection is not known. Aside from possible enteral infection, a sexual route of transmission, as well as transmission by biting or scratching, may contribute to γHV68 spread in the natural situation. Because the natural route of γHV68 has not been documented experimentally, we compared latency by using two different routes, i.p. and i.n. Regardless of route, the same organs examined carried latent γHV68. Of particular interest was the demonstration that peritoneal cells are a rich source of cells carrying latent γHV68. We were concerned that i.p. inoculation might contribute to the high frequency of peritoneal cells carrying latent γHV68. However, the fact that i.n. inoculation leads to latency in the peritoneum rules out the trivial possibility that i.p. inoculation falsely accentuates the significance of this reservoir. These data show that the route of inoculation is not the primary determinant of sites of γHV68 latency.
The previously reported critical role for B cells in efficient establishment of latency in the spleen (21) after i.n. inoculation was confirmed by the studies presented here. This result was previously interpreted to indicate that B cells were the sole site of latency within the lymphoid compartment (21). However, the fact that latency can be established in the spleen of B-cell-deficient mice after i.p. infection demonstrates that B cells are not required per se for generation of splenic latency. A role for B cells in trafficking γHV68 to the spleen has recently been demonstrated by Stewart et al. (16). Adoptive transfer of naive T-cell-depleted splenocytes from C57BL/6 mice into i.n.-inoculated latent B-cell-deficient mice resulted in an increase in γHV68 genome-positive cells in the spleens of the recipient B-cell-deficient mice. These results provide a likely explanation for our finding splenic latency in B-cell-deficient mice after i.p. but not i.n. inoculation. B cells likely play a key role in trafficking of latent cells to the spleen after i.n. inoculation, but this requirement is likely overcome by the more efficient establishment of systemic infection that occurs after i.p. inoculation. Notably, B cells were not required for establishment of latency in the peritoneum even after i.n. inoculation. The latter suggests that distinct mechanisms are involved in trafficking virus to the spleen and peritoneum.
Possible relationship between regulation of viral latency and clinical course of γHV68 infection.
It is clear that the clinical outcome of γHV68 infection in B-cell-deficient mice is very different from that in C57BL/6 mice (>90% of B-cell-deficient mice succumb to γHV68 infection between days 100 and 200 postinfection). While the cause of death of the γHV68-infected B-cell-deficient mice is unknown, the presence of a pronounced hemorrhagic exudative process in the lungs of these mice was noted (data not shown), which raises the possibility that they succumbed to a γHV68-associated pneumonia. This possibility is supported by the demonstration of lytic γHV68 replication (as evidenced by the presence of linear genomes) in the lungs of B-cell-deficient mice at late times after infection (16). In addition, as discussed in results, ∼70% of γHV68-infected B-cell-deficient mice have detectable arteritic lesions at the base of the aorta, although these lesions are not obstructive and may not contribute to mortality (25). We have shown by electron microscopy that the arteritic lesions in B-cell-deficient mice contain lytically replicating γHV68 (3a). The presence of lytic replication in two different organs (lungs and aorta) of B-cell-deficient mice late after infection could contribute to death of these animals. The relationship between the presence of continued viral replication in lung and aorta and high frequencies of splenocytes and PECs that reactivate γHV68 in the ex vivo assay is not known. However, it is interesting to speculate that continued reactivation may seed the lung or aorta, contributing to chronic pathology. It is also possible that continued replication of γHV68 at multiple sites alters the basic nature of γHV68 latency. For example, latently infected cells from both C57BL/6 and B-cell-deficient mice isolated early after infection reactivate efficiently. In normal mice, the efficiency of reactivation decreases, while the efficiency of reactivation is relatively stable in the B-cell-deficient mice. One might argue that the efficiently reactivating latent cells found in B-cell-deficient mice are actually recently infected (seeded from lung or aorta). If there is a continuous seeding of infectious virus into the latent reservoir in B-cell-deficient mice, one might predict that the frequency of genome-positive cells would be higher in B-cell-deficient mice than in C57BL/6 mice. The only evidence consistent with this was that at 42 to 50 days postinfection, the frequency of γHV68 genome-positive cells was ∼6 fold higher in B-cell-deficient mice than in C57BL/6 mice. The issue of how disregulation of latency and persistent production of infectious virus relate will require further studies.
Relationship between viral genome-positive cells and viral latency.
The availability of a small-animal model and quantitative assays both for cells that reactivate γHV68 and for cells that carry γHV68 genome allowed us to analyze both the dynamics of establishment of latency in vivo and the efficiency of reactivation in an ex vivo assay.
(i) Organ-dependent differences in the efficiency of γHV68 reactivation.
By quantitating both the frequency of cells that reactivate γHV68 and the frequency of cells that carry γHV68 genome, we were able to determine the parameters that regulate γHV68 latency. Two variables (organ and mouse strain) were important determinants of the efficiency of reactivation of γHV68. Analysis of PECs provided the clearest evidence that the majority of γHV68 genome-positive cells at this site are latently infected. In B-cell-deficient mice, the frequency of cells reactivating γHV68 in the PEC population correlated very closely with the frequency of γHV68 genome-positive cells. This close correlation was also true at early times (e.g., day 9) in the PEC population isolated from infected C57BL/6 mice. These data contrast with findings in splenocytes, which were consistently less efficient at reactivating γHV68. Thus, fewer genome-positive splenocytes reactivated in the ex vivo assay than genome-positive PECs, regardless of mouse strain from which the cells were isolated. This observation argues that either (i) a significant proportion of splenocytes carrying the γHV68 genome are not latently infected (i.e., they are unable to reactivate γHV68) or (ii) the necessary stimulus for efficient reactivation of latently infected splenocytes is not present in the ex vivo reactivation assay. Differentiation of these two possibilities will require (i) identification of the γHV68 latent gene program(s), (ii) determination of whether cells in different organs express different latent gene programs, and (iii) identification of specific signals that induce γHV68 reactivation. In the case of γHV68 latent gene expression, we have identified candidate viral genes that are expressed in latently infected PEC and/or splenocytes (23). Notably, distinct patterns of candidate latency-associated viral gene expression were observed with RNA isolated from latently infected PECs versus latently infected splenocytes, suggesting that there may indeed be distinct γHV68 latency programs present in these two organ systems.
(ii) Mouse strain-dependent differences in the efficiency of γHV68 reactivation.
In addition to organ-specific effects on the efficiency of reactivation, both PECs and splenocytes isolated from B-cell-deficient mice reactivated γHV68 more efficiently than comparable cell populations from C57BL/6 mice. This mouse strain-dependent difference is not due to differences in background genes, since we used B-cell-deficient mice backcrossed onto the C57BL/6 background and compared these mice to C57BL/6 mice. This finding supports the hypothesis that the presence or absence of mature B cells per se is a primary determinant of the nature of γHV68 latency. We think it likely that this is in addition to a role for B cells in trafficking γHV68, as outlined above. Either of two distinct scenarios might explain differences in reactivation between C57BL/6 and B-cell-deficient mice: (i) the presence of a B-cell-dependent (perhaps immunologic) process in vivo results in a form of γHV68 latency from which γHV68 does not efficiently reactivate ex vivo or (ii) the presence of a B-cell-dependent process or mediator in the C57BL/6 reactivation culture regulates γHV68 reactivation (either by inhibiting reactivation, or blocking detection of reactivated virus). Since mixing cells from C57BL/6 mice with efficiently reactivating cells from B-cell-deficient mice did not inhibit reactivation in the ex vivo reactivation assay, we favor the former hypothesis.
In the presence of the complete immune system, B-cell-dependent immunoselection may drive the appearance of a form of latency from which γHV68 reactivates inefficiently under the ex vivo culture conditions. The latter hypothesis is based on the observed behavior of EBV in vivo, where it is now clear that there are multiple latency programs (8, 14). In the case of EBV, it has been shown that viral gene expression, as well as the phenotype of the long-term latently infected B cell, is quite distinct from that of the EBV-immortalized lymphoblasts observed during the acute phase of infection (14). The working hypothesis in the case of EBV infection is that immune surveillance detects and destroys EBV-immortalized B cells, which express an array of latency-associated antigens involved in B-cell growth transformation, while the long-term latency reservoir appears to express few (or no) viral antigens and is not detected by the host immune response. If the situation for γHV68 is analogous, we may expect to find that genome-bearing cells in C57BL/6 mice express a different latency gene program than similar cells from B-cell-deficient mice. Analysis of this hypothesis will require (i) identification of cell types that carry latent γHV68, (ii) characterization of the anti-γHV68 immune response in B-cell-deficient mice, and (iii) definition of the latency gene program(s) used by this gammaherpesvirus.
A number of different B-cell-dependent processes might influence the nature of γHV68 latency. Since B cells are a site of viral latency (19, 26) and are likely involved in trafficking γHV68, presentation of latency-associated antigens by B cells may be pivotal to immune recognition of cells expressing certain latent γHV68 genes. Alternatively, the presence of immune antibody may alter latency via effects on intermittent reactivation and/or dissemination of virus. Evidence demonstrating a role for B cells in regulating latent γHV68 comes from studies characterizing virus replication in the lungs of γHV68-infected mice (16). Depletion of CD4 and CD8 T cells from C57BL/6 mice did not lead to detectable virus replication in the lungs of these animals, but depletion of CD4 and CD8 T cells from infected B-cell-deficient mice resulted in a dramatic increase in virus titer in the lungs of these animals (16). The latter could reflect a lack of antibody in B-cell-deficient mice. This possibility is supported by studies in the mouse cytomegalovirus system demonstrating a critical role for antibody in limiting dissemination of reactivated virus (7).
REFERENCES
- 1.Barkon M L, Haller B L, Virgin H W., IV Circulating IgG can play a critical role in clearance of intestinal reovirus infection. J Virol. 1996;70:1109–1116. doi: 10.1128/jvi.70.2.1109-1116.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowden R J, Simas J P, Davis A J, Efstathiou S. Murine gammaherpesvirus 68 encodes tRNA-like sequences which are expressed during latency. J Gen Virol. 1997;78:1675–1687. doi: 10.1099/0022-1317-78-7-1675. [DOI] [PubMed] [Google Scholar]
- 3.Cardin R D, Brooks J W, Sarawar S R, Doherty P C. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Dal Canto, A. J., S. H. Speck, and H. W. Virgin IV. Unpublished data.
- 4.Efstathiou S, Ho Y M, Minson A C. Cloning and molecular characterization of the murine herpesvirus 68 genome. J Gen Virol. 1990;71:1355–1364. doi: 10.1099/0022-1317-71-6-1355. [DOI] [PubMed] [Google Scholar]
- 4a.Gould, J. G., K. W. Weck, S. H. Speck, and H. W. Virgin IV. Unpublished data.
- 5.Heise M T, Connick M, Virgin H W. Murine cytomegalovirus inhibits interferon-gamma induced antigen presentation to CD4 T cells by macrophages via regulation of MHC class II associated genes. J Exp Med. 1998;187:1037–1046. doi: 10.1084/jem.187.7.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heise M T, Virgin H W., IV The T-cell-independent role of gamma interferon and tumor necrosis factor alpha in macrophage activation during murine cytomegalovirus and herpes simplex virus infection. J Virol. 1995;69:904–909. doi: 10.1128/jvi.69.2.904-909.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonjic S, Pavic I, Polic B, Crnkovic I, Lucin P, Koszinowski U H. Antibodies are not essential for the resolution of primary cytomegalovirus infection but limit dissemination of recurrent virus. J Exp Med. 1994;179:1713–1717. doi: 10.1084/jem.179.5.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kieff E. Epstein-Barr virus and its replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2343–2396. [Google Scholar]
- 9.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 10.Nash A A, Sunil-Chandra N P. Interactions of the murine gammaherpesvirus with the immune system. Curr Opin Immunol. 1994;6:560–563. doi: 10.1016/0952-7915(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 11.Nash A A, Usherwood E J, Stewart J P. Immunological features of murine gammaherpesvirus infection. Semin Virol. 1996;7:125–130. [Google Scholar]
- 12.Pollock J L, Presti R M, Paetzold S, Virgin H W. Latent murine cytomegalovirus infection in macrophages. Virology. 1997;227:168–179. doi: 10.1006/viro.1996.8303. [DOI] [PubMed] [Google Scholar]
- 13.Rajcani J, Blaskovic D, Svobodova J, Ciampor F, Huckova D, Stanekova D. Pathogenesis of acute and persistent murine herpesvirus infection in mice. Acta Virol. 1985;29:51–60. [PubMed] [Google Scholar]
- 14.Rickinson A B, Kieff E. Epstein-Barr virus. In: Fields B N, Knipe D M, Howley P M, editors. Virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2397–2446. [Google Scholar]
- 15.Simas J P, Efstathiou S. Murine gammaherpesvirus 68: a model for the study of gammaherpesvirus pathogenesis. Trends Microbiol. 1998;6:276–282. doi: 10.1016/s0966-842x(98)01306-7. [DOI] [PubMed] [Google Scholar]
- 16.Stewart J P, Usherwood E J, Ross A, Dyson H, Nash T. Lung epithelial cells are a major site of murine gammaherpesvirus persistence. J Exp Med. 1998;187:1941–1951. doi: 10.1084/jem.187.12.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sunil-Chandra N P, Arno J, Fazakerley J, Nash A A. Lymphoproliferative disease in mice infected with murine gammaherpesvirus 68. Am J Pathol. 1994;145:818–826. [PMC free article] [PubMed] [Google Scholar]
- 18.Sunil-Chandra N P, Efstathiou S, Arno J, Nash A A. Virological and pathological features of mice infected with murine gammaherpesvirus 68. J Gen Virol. 1992;73:2347–2356. doi: 10.1099/0022-1317-73-9-2347. [DOI] [PubMed] [Google Scholar]
- 19.Sunil-Chandra N P, Efstathiou S, Nash A A. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J Gen Virol. 1992;73:3275–3279. doi: 10.1099/0022-1317-73-12-3275. [DOI] [PubMed] [Google Scholar]
- 20.Usherwood E J, Ross A J, Allen D J, Nash A A. Murine gammaherpesvirus-induced splenomegaly: a critical role for CD4 T cells. J Gen Virol. 1996;77:627–630. doi: 10.1099/0022-1317-77-4-627. [DOI] [PubMed] [Google Scholar]
- 21.Usherwood E J, Stewart J P, Robertson K, Allen D J, Nash A A. Absence of splenic latency in murine gammaherpesvirus 68-infected B cell-deficient mice. J Gen Virol. 1996;77:2819–2825. doi: 10.1099/0022-1317-77-11-2819. [DOI] [PubMed] [Google Scholar]
- 22.Virgin H W, IV, Latreille P, Wamsley P, Hallsworth K, Weck K E, Dal Canto A J, Speck S H. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J Virol. 1997;71:5894–5904. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Virgin H W, IV, Presti R M, Li X-Y, Liu C, Speck S H. Three distinct regions of the murine gammaherpesvirus 68 genome are transcriptionally active in latently infected mice. J Virol. 1999;73:2321–2332. doi: 10.1128/jvi.73.3.2321-2332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weck K E, Barkon M L, Yoo L I, Speck S H, Virgin H W., IV Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. J Virol. 1996;70:6775–6780. doi: 10.1128/jvi.70.10.6775-6780.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weck K E, Dal Canto A J, Gould J D, O’Guin A K, Roth K A, Saffitz J E, Speck S H, Virgin H W. Murine gamma-herpesvirus 68 causes large-vessel arteritis in mice lacking interferon-gamma responsiveness: a new model for virus-induced vascular disease. Nat Med. 1997;3:1346–1353. doi: 10.1038/nm1297-1346. [DOI] [PubMed] [Google Scholar]
- 26.Weck K E, Kim S S, Virgin IV H W, Speck S H. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J Virol. 1999;73:3273–3283. doi: 10.1128/jvi.73.4.3273-3283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weck, K. E., H. W. Virgin IV, and S. H. Speck. Unpublished data.