Endosymbiotic Wolbachia bacteria in the worms are targets for a new therapeutic approach
Onchocerciasis, or river blindness, caused by the filaria Onchocerca volvulus, affects more than 17 million people in Africa, Latin America, and Yemen. The microfilaricidal agent ivermectin is the principal means of controlling the disease, through mass treatment. Wolbachia endosymbiotic bacteria in filarias have emerged as a new target for treatment with drugs that lead to long term sterilisation of adult female filarias. Participants at recent international meetings have agreed that anti-Wolbachia chemotherapy with doxycycline (currently for six weeks) could be used to treat infected individuals. This approach holds promise for new developments based on registered antibiotics that are affordable in resource poor settings, as extensive registration processes are not needed. Recent experimental findings also indicate that endotoxin-like molecules from Wolbachia have a role in the pathogenesis of the disease and in adverse reactions after treatment.
Summary points
Onchocerciasis is a major cause of blindness
Current strategies rely on mass distribution of ivermectin by a community directed distribution system
New chemotherapeutic strategies need to be developed to kill adult worms or sterilise them in the long term
Wolbachia symbiotic endobacteria in filarias are essential for fertility and offer novel targets for treatment
Anti-wolbachial treatment with 100 mg/day doxycycline for six weeks can be used to treat onchocerciasis
Wolbachia have been implicated in the induction of adverse effects of microfilaricidal drugs and may have a role in the development of pathology, including keratitis
Methods
This review is based on information from World Health Organization publications on onchocerciasis1,2; on recent conferences, such as the WHO-cosponsored Hamburg conference on filariasis in September 2001 and the conference on the eradicability of onchocerciasis organised by the Carter Center and the WHO in Atlanta, USA, in January 20023,4; and on our experimental work and human trials in Ghana. We also used recent literature on the topic.
Current concepts of control and treatment
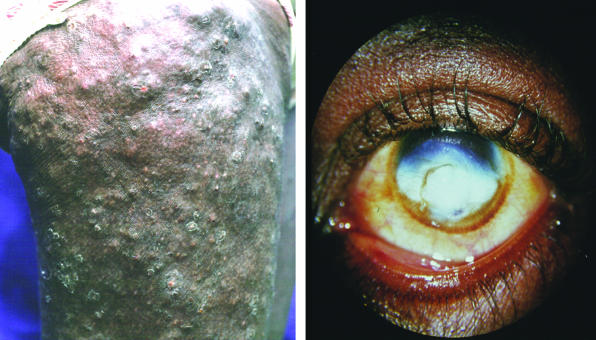
Onchocerciasis is the second most common cause of preventable blindness in sub-Saharan Africa; it has an estimated prevalence of 500 000 with visual impairment and 270 000 with blindness.2 The infection is transmitted by Simulium spp blackflies, which transfer infective larvas during a meal of blood. These larvas mature to female and male worms within a year and are located in subcutaneous nodules (onchocercomas). The females produce millions of microfilarias (first stage larvas) during their lifetime, which lasts up to 14 years.5 The microfilarias induce the pathology characteristic of the disease, including chronic dermatitis and skin atrophy, lymphadenitis and fibrosis, and ocular inflammation that can lead to blindness (fig 1).
Figure 1.
Major manifestations of onchocerciasis: chronic lichenification with papular dermatitis (left); sclerosing keratitis (right)
Major steps have been taken towards control of onchocerciasis, mainly owing to successful programmes involving active partnership between the WHO or Pan American Health Organization and various donor organisations (box B1 and fig A on bmj.com).6 Mass distribution of the microfilaricidal drug ivermectin is the principal intervention in onchocerciasis control.7 However, recent endemicity suggests that, at least in Africa, this approach may not stop transmission8,9 and that in areas where interruption of transmission has been achieved by vector control the infection will be reimported by (re)migration of people who still carry the infection.4 Ivermectin, although it acts rapidly to reduce the number of skin microfilarias, depletes them for only a few months, after which microfilarias reappear at levels of 20% or more of pretreatment numbers within a year.10 This microfilarial density seems to be sufficient for transmission to continue.11 The reason for this rather limited effect of ivermectin is that it does not kill the long lived adult worms and that its embryocidal activity seems to be mainly restricted to the late stages of microfilarial development, leaving early embryogenesis intact.5 Studies using albendazole showed only a very transient effect on early embryogenesis.10 Thus newly infected people will continue to enter the transmission cycle, and worldwide elimination of onchocerciasis may remain a distant goal not achievable without better drugs. Furthermore, should resistance to ivermectin arise in human onchocerciasis, as seen with intestinal helminths in animals,12 control activities would be severely compromised.
Box 1.
Programmes executed by the WHO for the elimination of onchocerciasis as a public health problem
Thus for both individual and community based treatment a chemotherapeutic approach that has a more sustained effect than ivermectin on microfilarial levels is needed. The ideal agent would have a deleterious effect on the adult filarias.
Wolbachia endobacteria—new targets for chemotherapy
Wolbachia are bacterial symbionts of the major human filarias, including Onchocerca volvulus, the parasitic nematode that causes onchocerciasis. They belong to the order of Rickettsiales and are found in the body wall (the hypodermis), in oocytes, in all embryonic stages, and in microfilarias (fig 2). Wolbachia spp in filarias seem to have evolved as symbionts essential for fertility of their nematode hosts and are transmitted transovarially to the next worm generation, in a similar way to mitochondria. Antibiotics have been used to deplete the endosymbionts from filarias and show the mutualistic symbiosis between filarial Wolbachia and their hosts. Depletion of Wolbachia resulted in the disruption of embryogenesis in female worms.13,14 A partial macrofilaricidal activity has also been reported in bovine onchocerciasis (O ochengi).15 These effects were strictly associated with depletion of the bacteria and were not observed in a filarial species that is devoid of Wolbachia. So far, tetracyclines, rifampicin, and chloramphenicol have shown activity against Wolbachia in vivo,13,w1-3 and azithromycin has shown in vitro activity.w4
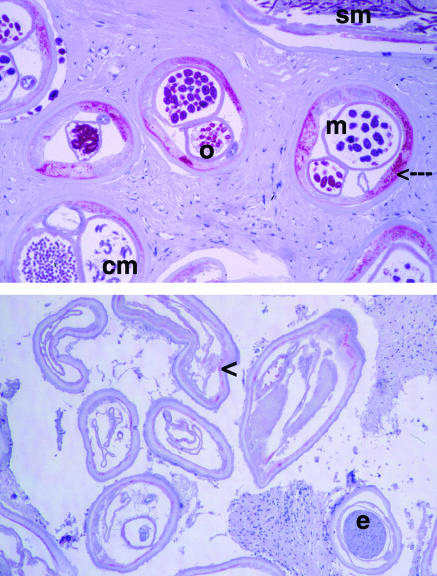
Figure 2.
Transverse sections of female Onchocerca volvulus worms showing the effect of doxycycline treatment on Wolbachia endobacteria (visualised by staining with antibodies against bacterial heat shock protein-60) and on worm embryogenesis. (Top) Onchocercoma from an untreated patient with worms containing numerous endobacteria (red, arrow) and intact embryogenesis (o=oocytes; m=morulae; cm=coiled microfilarias; sm=stretched microfilarias) (x90). (Bottom) Onchocercoma from a patient 11 months after treatment for six weeks with doxycycline (100 mg/day); worms without bacteria; embryos (e) are degenerated. The arrowhead depicts staining of mitochondria (smaller than the bacteria, different location) by anti-hsp-60 antibodies (x55)
The fact that doxycycline is already registered allowed for a quick transition to phase IIa studies on human onchocerciasis, which have been carried out in Ghana over the past three years. Doxycycline (100 mg/day) was given to 63 patients for six weeks under daily supervision by a physician. Activity of doxycycline against Wolbachia and adult worms was assessed from extirpated onchocercomas. Immunohistology with antibodies to Wolbachia proteins was used to assess the presence or absence of Wolbachia and monitor changes in embryogenesis (see fig 2). Nodules from 36 patients not treated with doxycycline served as controls.
Administration of 100 mg/day doxycycline for six weeks led to depletion of Wolbachia followed by an interruption of embryogenesis in worms, which could be observed for 18 months.16,17 In further trials using doxycycline at 200 mg/day for four or six weeks, interruption of embryogenesis was observed for 24 months (Büttner and Hoerauf, unpublished data). This is the longest period of sterility of female worms ever achieved by an antifilarial drug without severe side effects. Consistent with the blockade of embryogenesis, after additional treatment with ivermectin more than 90% of the patients who had received doxycycline showed an absence of microfilaridermia after 18 months, and the remaining patients showed very low numbers of microfilarias. In contrast, patients treated with ivermectin alone showed an increase in microfilarial counts as early as four months after administration of ivermectin.17
Preliminary results with other doxycycline regimens indicate that
100 mg or 200 mg doxycycline per day for two weeks does not eliminate the endobacteria or interrupt embryogenesis
200 mg doxycycline per day for four weeks may have the same efficacy as l00 mg for six weeks (Hoerauf and Büttner, unpublished data).
Wolbachia have emerged as a target for a chemotherapy that fulfils the priority research objective of long term sterilising activity. The long period of treatment (four weeks or more) and the known contraindications to doxycycline (pregnant or breastfeeding women, children up to 9 years of age) preclude its application for mass treatment at the moment. Further research is needed to shorten the length of the current regimen (potentially with other antibiotics or combinations). However, individual treatment of people with imported infection or those leaving an endemic area for a long time constitutes an indication for doxycycline (six weeks at 100 mg/day), as agreed at the conferences in Hamburg and Atlanta.3,4 Other indications could be in formerly endemic areas where a high level of control has already been achieved and re-emergence should be prevented.
Wolbachia as inducers of pathology
Clinical disease of the skin and the eye is associated with microfilarias. The presence of live microfilarias at either site does not seem to be associated with the development of much pathology. However, when the microfilarias die, especially after chemotherapy, the host response to degenerating worms can result in severe inflammatory responses that lead to progressive loss of vision and ultimately blindness. This was seen when patients were treated systemically with diethylcarbamazine, when the side effects, termed the Mazzotti reaction, could be especially severe.2 In contrast, after treatment with ivermectin microfilarias migrate to the lymph nodes and limited clinical pathology occurs.18
Until recently filarial products themselves were thought to be the major stimulus for the underlying inflammatory response. However, a recent study showed a significant association between adverse reactions after microfilaricidal treatment and elevated concentrations of Wolbachia DNA.19 Neutrophil recruitment to onchocercomas seems to depend almost exclusively on the presence of Wolbachia in the worms, as few neutrophils are detected at these sites in people treated with doxycycline.20 In addition, in vitro and in vivo studies have shown that neutrophils participate in the attack on microfilarias in concert with eosinophils and macrophages (fig B on bmj.com). Whereas the accumulation and activation of eosinophils seem to be triggered by filarial products, the role of neutrophil recruitment is again dependent on Wolbachia, and the activity of macrophages seems to depend on molecules derived from both filarias and Wolbachia.
A recent study clearly showed that endotoxin-like products of Wolbachia constitute a major proinflammatory stimulus in the eye. The study used a well established mouse model, in which parasite antigens are injected directly into the corneal stroma, and measured the effect on corneal inflammation (keratitis) by in vivo scanning confocal microscopy, which measures corneal thickness and haze, and by infiltration of neutrophils to the corneal stroma.21 Keratitis was induced by extracts of O volvulus worms recovered from untreated patients but not by worms from patients after depletion of Wolbachia with doxycycline. Further molecular analysis implicated bacterial lipopolysaccharide-like molecules in this process, mediated through its receptor on mammalian cells, Toll-like receptor 4.22 The authors concluded that the physical presence of neutrophils in the corneal stroma, together with release of neutrophil granular products and the presence of eosinophils, will disrupt normal corneal function, resulting in loss of corneal clarity. Figure 3 illustrates how Wolbachia contribute to keratitis, primarily by upregulating expression of neutrophil chemokines in the corneal stroma and vascular adhesion molecules on limbal vessels.23
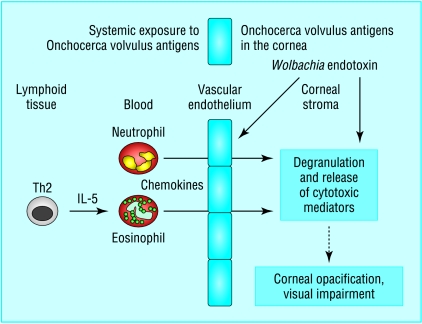
Figure 3.
The role of Wolbachia in the pathogenesis of river blindness (murine model). Subcutaneous immunisation with Onchocerca volvulus antigens, which is a model for chronic infection, stimulates the adaptive arm of the immune system, resulting in a predominant Th2 response, with elevated interleukin-4, interleukin-5, eosinophilia, and parasite-specific antibodies. After injection of parasite antigens into the cornea, production of neutrophil and eosinophil chemokines is upregulated in the cornea, and expression of vascular adhesion molecules (PECAM-1 and ICAM-1) on limbal vessels is elevated. Neutrophils and eosinophils are then recruited to the corneal stroma in a biphasic manner, with neutrophils infiltrating within 24 hours and eosinophils predominating after 72 hours. The physical presence of these cells, combined with degranulation and release of cytotoxic mediators, leads to disruption of normal corneal function, loss of corneal clarity, and visual impairment. Lipopolysaccharide-like molecules from Wolbachia endobacteria seem to induce expression of chemotactic cytokines and vascular adhesion molecules that mediate neutrophil recruitment to the corneal stroma. Adapted from Kaifi et al23
Taken together, these studies show that Wolbachia are major contributors to the immunopathology of onchocerciasis and thus represent targets not only for a novel chemotherapy but also for mediating pathological reactions (box B2).
Additional educational resources:
Useful books
World Health Organization. Onchocerciasis and its control. World Health Organ Tech Rep Ser 1995;852:1-103
Klei T, Rajan TV, eds. World class parasites: the filariae. New York: Kluwer Academic Press, 2002:1-181
Useful websites
World Health Organization—www.who.int/health_topics/onchocerciasis or www.who.int/health_topics/filariasis
Global Alliance to Eliminate Lymphatic Filariasis—www.filariasis.org
Carter Center—www.cartercenter.org/healthprograms/
Box 2.
Wolbachia derived immunopathological mechanisms of onchocerciasis
- Eosinophils, macrophages, and neutrophils are involved in the killing of microfilarias
- Accumulation and activation of neutrophils depend on Wolbachia endobacteria
- Wolbachia may be associated with severity of adverse reactions after chemotherapy of onchocerciasis
- Wolbachia are major triggers of keratitis in a mouse model
- Wolbachia, in addition to being targets for chemotherapy of onchocerciasis, could evolve into targets for mediating disease and adverse reactions
The future—anti-wolbachial chemotherapy?
The need for new drugs for onchocerciasis to achieve elimination of transmission and hence a public health problem is undisputed. However, the development of new compounds that do not generate profits once they have been registered is a problem. The big advantage of a chemotherapeutic approach that targets filarial Wolbachia is the possibility of using already registered antibiotics. Time is also an important factor, as final elimination from selected foci as well as the potential development of resistance to ivermectin mean that a complementary drug would have to be ready to use in less time than would be needed for full registration of a new compound.
Although the disadvantages of treating onchocerciasis with antibiotics seem to be the long course of treatment needed and the contraindications to doxycycline, the advantages of the approach targeting Wolbachia could outweigh its inherent problems. This novel development is in the early stages, and several compounds and regimens await testing in the field, either for treatment or for mediating pathology. Further research may provide better regimens, not only for onchocerciasis but also for lymphatic filariasis, where Wolbachia may also have a role in adverse reactions after chemotherapy.24,w5
Supplementary Material
Footnotes
Funding: European Commission (grants ICA4-CT-1999-10002 and ICA4-CT 2002-10051), German Research Foundation (DFG, grants Ho 2009/1-3 and 2009/5-1), Wellcome Trust (grant 062680), and Caritas Charity. Keratitis studies were funded by NIH grant EY10320 (EP).
Competing interests: None declared.
Two extra figures and additional references appear on bmj.com
References
- 1.World Health Organization. WHO Expert Committee on Onchocerciasis. World Health Organ Tech Rep Ser. 1987;752:1–167. [PubMed] [Google Scholar]
- 2.World Health Organization. Onchocerciasis and its control. World Health Organ Tech Rep Ser. 1995;852:1–103. [PubMed] [Google Scholar]
- 3.Hoerauf A, Walter RD, Remme H, Lazdins J, Fleischer B. Call to consolidate achievements for onchocerciasis and lymphatic filariasis control. Trends Parasitol. 2001;17:566–567. doi: 10.1016/s1471-4922(01)02192-4. [DOI] [PubMed] [Google Scholar]
- 4. Carter Center. Final report of the conference on the eradicability of onchocerciasis, Atlanta, 2002. www.cartercenter.org/documents/1047.pdf (accessed 5 Dec 2002).
- 5.Plaisier AP, van Oortmarssen GJ, Remme J, Habbema JD. The reproductive lifespan of Onchocerca volvulus in west African savanna. Acta Trop. 1991;48:271–284. doi: 10.1016/0001-706x(91)90015-c. [DOI] [PubMed] [Google Scholar]
- 6.Benton B, Bump J, Seketeli A, Liese B. Partnership and promise: evolution of the African river-blindness campaigns. Ann Trop Med Parasitol. 2002;96(suppl 1):S5–14. doi: 10.1179/000349802125000619. [DOI] [PubMed] [Google Scholar]
- 7.Richards FO, Boatin B, Sauerbrey M, Seketeli A. Control of onchocerciasis today: status and challenges. Trends Parasitol. 2001;17:558–563. doi: 10.1016/s1471-4922(01)02112-2. [DOI] [PubMed] [Google Scholar]
- 8.Richards F, Hopkins D, Cupp E. Programmatic goals and approaches to onchocerciasis. Lancet. 2000;355:1663–1664. doi: 10.1016/S0140-6736(00)02235-2. [DOI] [PubMed] [Google Scholar]
- 9.Abiose A, Homeida M, Liese B, Molyneux D, Remme H. Onchocerciasis control strategies. Lancet. 2000;356:1523–1524. doi: 10.1016/S0140-6736(05)73271-2. [DOI] [PubMed] [Google Scholar]
- 10.Awadzi K, Addy ET, Opoku NO, Plenge-Bönig A, Büttner DW. The chemotherapy of onchocerciasis XX: ivermectin in combination with albendazole. Trop Med Parasitol. 1995;46:213–220. [PubMed] [Google Scholar]
- 11.Alley WS, van Oortmarssen GG, Boatin BB, Nagelkerke NN, Plaisier AA, Remme HJ, et al. Macrofilaricides and onchocerciasis control, mathematical modelling of the prospects for elimination. BMC Public Health. 2001;1:12. doi: 10.1186/1471-2458-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prichard R. Genetic variability following selection of Haemonchus contortus with anthelmintics. Trends Parasitol. 2001;17:445–453. doi: 10.1016/s1471-4922(01)01983-3. [DOI] [PubMed] [Google Scholar]
- 13.Taylor MJ, Hoerauf A. A new approach to the treatment of filariasis. Curr Opin Infect Dis. 2001;14:727–731. doi: 10.1097/00001432-200112000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Bandi C, Trees AJ, Brattig NW. Wolbachia in filarial nematodes: evolutionary aspects and implications for the pathogenesis and treatment of filarial diseases. Vet Parasitol. 2001;98:215–238. doi: 10.1016/s0304-4017(01)00432-0. [DOI] [PubMed] [Google Scholar]
- 15.Langworthy S, Renz A, Mackenstedt U, Henkle-Dührsen K, Bronsvoort M, Tanya V, et al. Macrofilaricidal activity of tetracycline against the filarial nematode, Onchocerca ochengi: elimination of Wolbachia precedes worm death and suggests a dependent relationship. Proc R Soc Lond B Biol Sci. 2000;267:1063–1069. doi: 10.1098/rspb.2000.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoerauf A, Volkmann L, Hamelmann C, Adjei O, Autenrieth IB, Fleischer B, et al. Endosymbiotic bacteria in worms as targets for a novel chemotherapy in filariasis. Lancet. 2000;355:1242–1243. doi: 10.1016/S0140-6736(00)02095-X. [DOI] [PubMed] [Google Scholar]
- 17.Hoerauf A, Mand S, Adjei O, Fleischer B, Büttner DW. Depletion of Wolbachia endobacteria in Onchocerca volvulus by doxycycline and microfilaridermia after ivermectin treatment. Lancet. 2001;357:1415–1416. doi: 10.1016/S0140-6736(00)04581-5. [DOI] [PubMed] [Google Scholar]
- 18.Darge K, Lucius R, Monson MH, Behrendsen J, Büttner DW. Immunohistological and electron microscopic studies of microfilariae in skin and lymph nodes from onchocerciasis patients after ivermectin treatment. Trop Med Parasitol. 1991;42:361–367. [PubMed] [Google Scholar]
- 19.Keiser PB, Reynolds SM, Awadzi K, Ottesen EA, Taylor MJ, Nutman TB. Bacterial endosymbionts of Onchocerca volvulus in the pathogenesis of posttreatment reactions. J Infect Dis. 2002;185:805–811. doi: 10.1086/339344. [DOI] [PubMed] [Google Scholar]
- 20.Brattig N, Büttner DW, Hoerauf A. Neutrophil accumulation around Onchocerca worms and chemotaxis of neutrophils are dependent on Wolbachia endobacteria. Microbes Infect. 2001;3:439–446. doi: 10.1016/s1286-4579(01)01399-5. [DOI] [PubMed] [Google Scholar]
- 21.Hall LR, Pearlman E. Pathogenesis of onchocercal keratitis. Clin Microbiol Rev. 2002;12:445–453. doi: 10.1128/cmr.12.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.St André A, Blackwell NM, Hall LR, Hoerauf A, Brattig N, Taylor M, et al. A critical role for endosymbiotic Wolbachia bacteria and TLR4 signaling in the pathogenesis of river blindness. Science. 2002;295:1892–1895. doi: 10.1126/science.1068732. [DOI] [PubMed] [Google Scholar]
- 23.Kaifi JT, Diaconu E, Pearlman E. Distinct roles for PECAM-1, ICAM-1, and VCAM-1 in recruitment of neutrophils and eosinophils to the cornea in ocular onchocerciasis (river blindness) J Immunol. 2001;166:6795–6801. doi: 10.4049/jimmunol.166.11.6795. [DOI] [PubMed] [Google Scholar]
- 24.Cross HF, Haarbrink M, Egerton G, Yazdanbakhsh M, Taylor MJ. Severe reactions to filarial chemotherapy are associated with the release of Wolbachia endosymbionts into the blood. Lancet. 2001;358:1873–1875. doi: 10.1016/S0140-6736(01)06899-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.