Abstract
Introduction
We aimed to describe a clinical presentation of central serous retinopathy that poses a diagnostic and management dilemma.
Case Presentation
A 30-year-old male patient presented with bilateral vision loss and multifocal serous retinal detachments involving the posterior pole of both eyes. Optical coherence tomography revealed prominent bilateral bacillary layer detachments. The patient complained of recent headaches and tinnitus. However, the clinical exam did not reveal overt inflammation and the patient admitted to being under significant stress. The clinical presentation raised concerns for both central serous retinopathy (CSR) and Vogt-Koyanagi-Harada (VKH). Additional findings, including white fundus spots and focal areas of retinal vascular leakage, were seen in our patient. We highlight these because, while they have been described in CSR, they are not commonly discussed and could add to the diagnostic dilemma. After a conservative approach that avoided steroids, our patient showed marked improvement over the following month, supporting a diagnosis of CSR.
Conclusion
CSR can mimic VKH disease. A high level of suspicion is needed to avoid instituting steroid therapy that could induce a severe iatrogenic exacerbation of the disease.
Keywords: Masquerade, Bacillary layer detachment, Multifocal serous retinal detachments, Central serous chorioretinopathy, Central serous retinopathy, Central serous chorioretinopathy Vogt-Koyanagi-Harada
Introduction
Vogt-Koyanagi-Harada (VKH) disease is a T-cell-mediated autoimmune disease affecting melanocytes in pigmented tissues of the eyes, ear, skin, meninges, and hair [1]. Tyrosinase, an enzyme involved in melanin synthesis, maybe the autoantigen in VKH. Ocular involvement is characterized by an acute phase which presents as serous retinal detachments and varying degrees of bilateral granulomatous posterior inflammation or panuveitis [1, 2]. Other symptoms include headache, fever, confusion, and meningismus. Late systemic findings may include tinnitus, dysacusis, vertigo, vitiligo, and poliosis. Treatment involves immunosuppression with a combination of early high-dose steroids and often steroid-sparing immunomodulatory agents.
Meanwhile, central serous chorioretinopathy (CSCR) is a non-inflammatory disease that causes serous detachments of the retina. Gass proposed that increased permeability of the choriocapillaris causes increased hydrostatic pressure in the choroid, leading to hyperpermeability of the choriocapillaris, pigment epithelial detachments (PEDs), and defects in the RPE monolayer that allow fluid to leak under the retina [3, 4]. Exogenous corticosteroid use is one of the strongest risk factors for CSCR [5].
Given the wide range of disease severity and wide spectrum of clinical presentations in both VKH and CSCR, some patients may present with clinical and imaging findings that are at the intersection of these disease entities, posing a considerable diagnostic challenge. Both conditions can manifest with symptoms of blurred vision and metamorphopsia. On exam and retinal imaging, there is often serous retinal detachment in both entities. Yet, establishing the correct diagnosis promptly is imperative in order to choose and administer vision-saving therapies. A failure to distinguish between VKH and CSCR not only hinders timely intervention but also poses a significant risk, given that steroid therapy, an important early intervention for VKH, can greatly exacerbate CSCR. Hence, a nuanced understanding of the distinctive features of VKH and CSCR is paramount to ensure optimal patient outcomes. Here we present a case that illustrates an interesting clinical presentation and discuss aspects of the case that can be confusing and those that may be diagnostically helpful.
Case Presentation
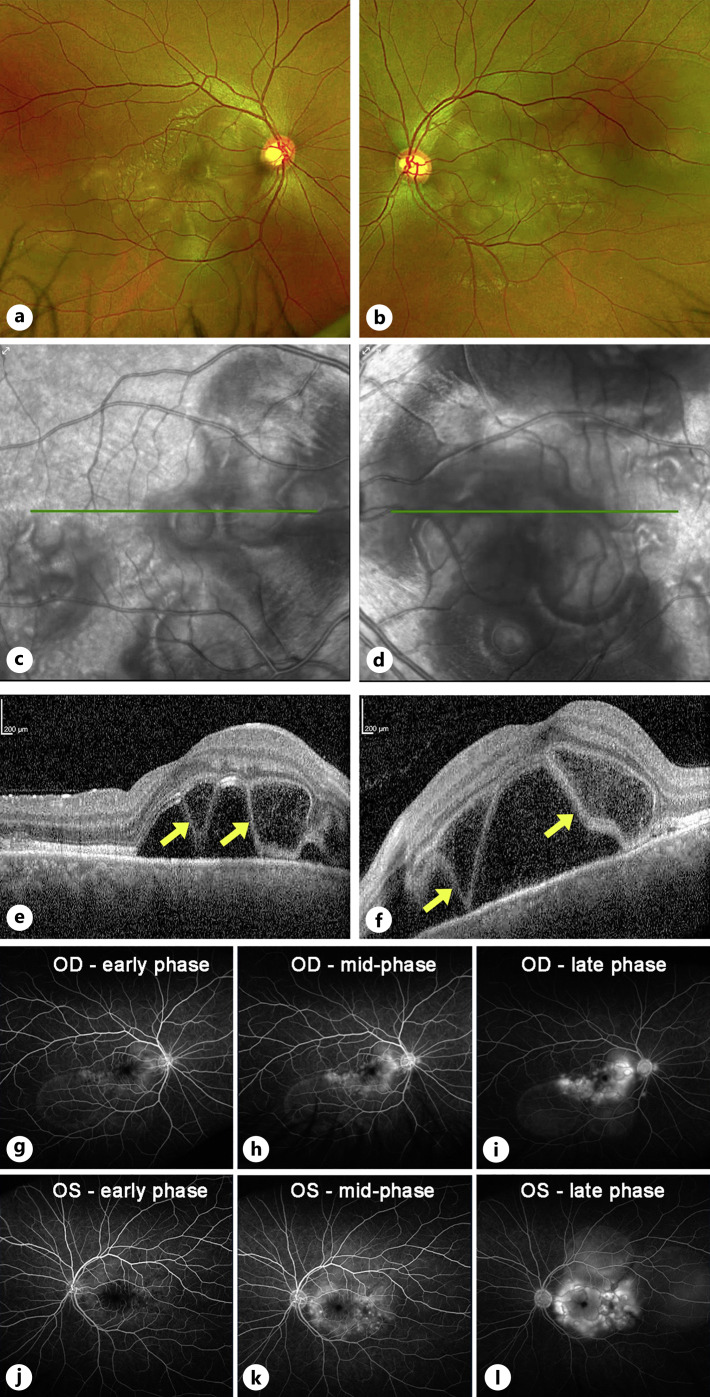
A 30-year-old Korean-American male with an unremarkable ocular history was referred to our retina clinic for a recent onset of blurred vision and metamorphopsia. The patient reported a sequential involvement starting with the left eye, followed 1 day later by symptoms in the right eye. Visual acuity was 20/40 OD and 20/200 OS. Intraocular pressures were normal. Anterior segment exam was only notable for trace AC cells in the left eye (2 cells/hpf). There was no conjunctival or scleral injection, and no keratic precipitates were seen. Posterior segment examination revealed trace cells in the anterior vitreous OU (1–3 cells/hpf) and bilateral multiloculated serous retinal detachments concentrated in the posterior poles. There was no evidence of optic disc edema or hyperemia, nor any vascular sheathing (Fig. 1a–d). Optical coherence tomography (OCT) revealed bilateral bacillary layer detachments (BALAD, yellow arrows in Fig. 1e, f). On initial fluorescein angiography (FA), multiple areas of ink-blot leakage were observed in the posterior poles of both eyes, culminating in multiloculated areas of pooling (Fig. 1g–l). There was no evidence or history of vitiligo, madarosis, poliosis, or alopecia. On review of systems, he endorsed mild headaches and tinnitus but denied eye pain, skin rashes, or use of steroids. Given the presentation, our initial differential diagnosis included VKH and CSCR. Since we had a significant clinical concern for CSCR, considering the risk of treating with steroids in that scenario, the decision was made to initiate a course of eplerenone (50 mg daily) plus topical dorzolamide three times daily OU and to withhold steroids.
Fig. 1.
Imaging at presentation. Color fundus photos of the right (a) and left (b) eyes demonstrate bilateral posterior pole multiloculated subretinal fluid pockets. There is no evidence of disc edema, hyperemia, or any vascular sheathing, and the media is clear. Infrared images (c, d) better highlight the bilateral multiloculated pockets of subretinal fluid. Optical coherence tomography (e, f) of the macula showed a prominent accumulation of outer retinal and subretinal fluid, along with areas of bacillary layer detachments (BALAD, yellow arrows). Fluorescein angiography demonstrated scattered areas of leakage within the macula and multiloculated areas of pooling in both the right (g–i) and left (j–l) eyes. No leakage is noted from the optic discs or vessels.
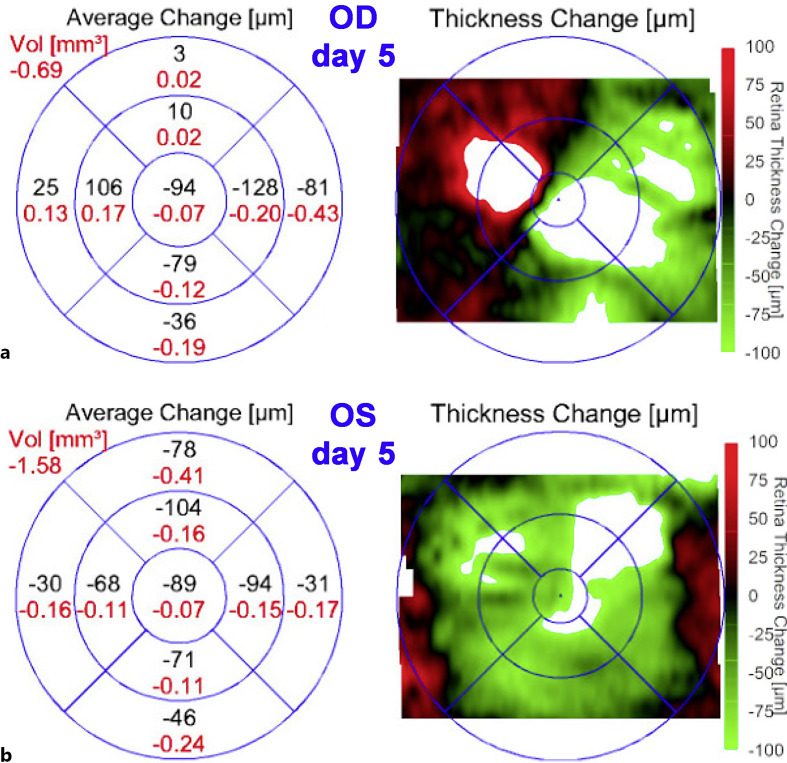
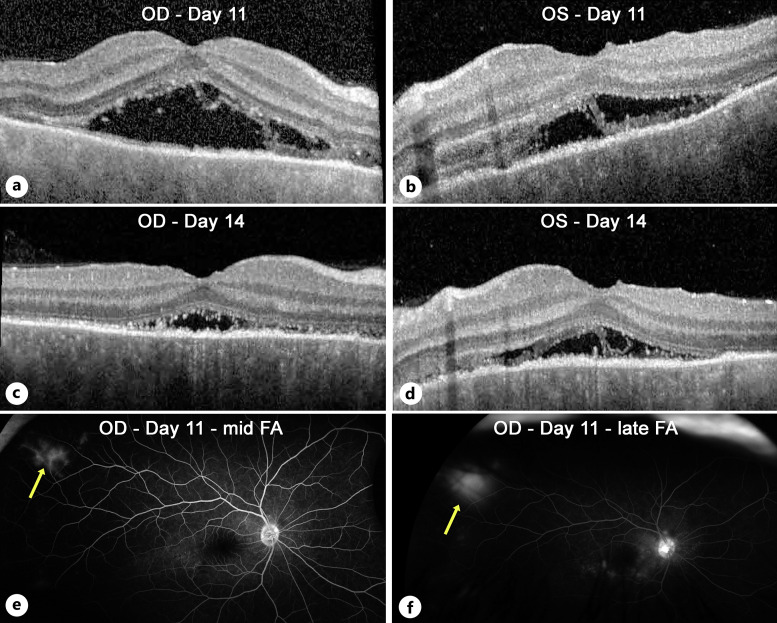
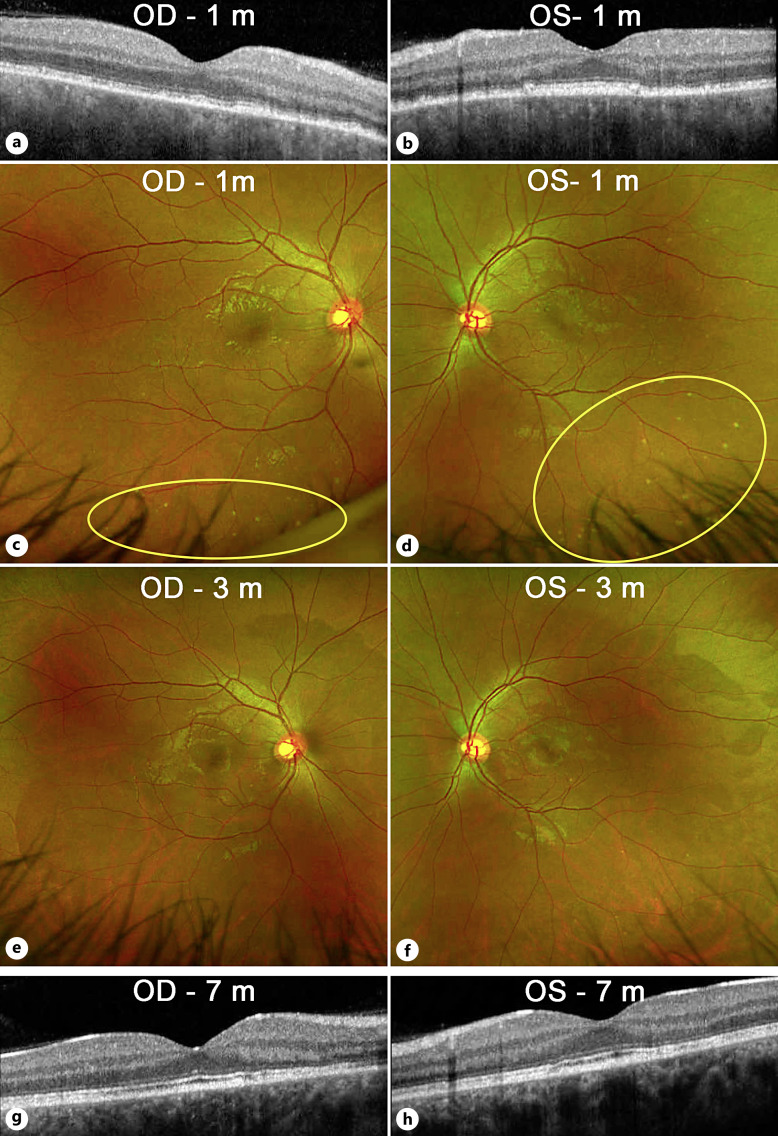
Five days later, there was some improvement in vision (20/25 OD and 20/200 OS). OCT showed decreased central fluid in the right eye, with some new foci of fluid nasally (Fig. 2a). The left eye showed a marked reduction in central thickness (Fig. 2b). While it was still difficult to firmly reject the possibility of VKH, we did not see any further evidence of inflammation or VKH-like symptoms, so we maintained our course of treatment and increased eplerenone to 50 mg twice daily. At day 11 of follow-up, OCT showed definite improvement (Fig. 3a, b) with a marked decrease in macular fluid, which was even more evident on day 14 (Fig. 3c, d). Yet, to add to the clinical uncertainty, repeat FA on day 11 showed a small peripheral area of vascular leakage OD (Fig. 3e, f). As the clinical picture showed improvement with the avoidance of steroids, we continued our approach. One month after the initial visit, the vision had improved to 20/25 OD and 20/20 OS. While there were obvious ellipsoid zone and RPE changes, OCT showed complete resolution of the macular fluid (Fig. 4a, b). Interestingly, color fundus photos showed for the first time the presence of white spots in the inferior midperiphery OU (Fig. 4c, d). These spots disappeared 2 months later (Fig. 4e, f). At the most recent follow-up 7 months after initial presentation, the patient had regained 20/20 vision OU (Fig. 4g, h). There was no recurrence of fluid despite the slow tapering of eplerenone over the last 3 months down to 25 mg daily. The ellipsoid zone changes persisted but showed some improvement.
Fig. 2.
OCT changes on day 5 compared to baseline. The left panels are diagrams showing the average change in retinal thickness (μm in black) and volume (mm3 in red) on day 5 compared to baseline for the right eye (a) and left eye (b). The right panels show macular maps of changes in retinal thickness for the right eye (a) and left eye (b). As shown by the color scale to the right, red represents thickening while green represents thinning compared to baseline.
Fig. 3.
OCT and FA changes by 2 weeks after presentation. OCT imaging demonstrated a significant decrease in macular fluid on day 11 (a, b), which became more significant by day 14 (c, d). Fluorescein angiography of the right eye showed a reduction in macular leakage compared to baseline but also highlighted an isolated area of peripheral vascular staining in the right eye that was visible in the mid-phase (e, yellow arrow) and progressively leaked up to the late phase (f, yellow arrow).
Fig. 4.
Imaging after the acute phase. a, b Optical coherence tomography of the macula at 1 month showed complete resolution of the macular fluid and bacillary layer detachments OU. Some residual ellipsoid zone, interdigitation zone, and RPE changes can be seen. c, d Color fundus photos showed resolution of the subretinal fluid blebs, but a new finding of white/yellow dots in the inferior midperiphery OU (yellow ovals). e, f By 3 months, these white dots had mostly resolved. g, h No recurrence of macular fluid was seen up to 7 months of follow-up on OCT. Some improvement of the ellipsoid changes was seen in both eyes.
Discussion
Several aspects of our patient’s clinical presentation pointed toward the possibility of VKH including the presence of bilateral multifocal serous retinal detachments on exam, the prominent bacillary layer detachments on OCT, and the multiloculated areas of pooling on FA OU. BALAD has recently been characterized as an OCT finding consisting of intraretinal fluid accumulation leading to splitting at the level of the inner segment myoid zone and has been described in a variety of diseases including both inflammatory and degenerative diseases of the macula. In a recent study describing OCT features of VKH, BALAD (referred to as membranous structures in that study) was found to have the highest positive predictive value (97.3%) for VKH of all the OCT features assessed [6]. Another group showed that it is a common tomographic finding in eyes with acute VKH disease and mostly observed in patients with more serious disease [7]. Importantly, the Standardization of Uveitis Nomenclature (SUN) working group, included BALAD (“septae” on OCT) as one of the criteria for diagnosis of acute VKH [2]. Finally, a study from South Korea [8] comparing 35 VKH patients to 25 patients with bilateral CSCR found that 54% of eyes with VKH showed bacillary layer detachments, while only 4% of CSCR eyes did. The same study also showed that on OCT, 60% of eyes with CSCR showed PEDs while none of the VKH eyes did. Both of these OCT characteristics would have suggested VKH in our case, since our patient did not have PEDs, but did have prominent BALAD in both eyes. Of note, although higher in VKH patients, subfoveal choroidal thickness may not be a useful distinguishing feature on OCT since it is elevated in both conditions (average of 628 microns in VKH and 465 microns in CSCR) [8].
The presence of trace cells in both the anterior and posterior segment exams was also a concern. While VKH is an inflammatory disease and is often associated with cells, in its 2021 criteria for VKH, the Standardization of Uveitis Nomenclature (SUN) working group [2] makes it clear that more than 50% of cases of early VKH present without vitreous haze or keratic precipitates. Also, one-third of cases have only 0 to +0.5 cells in the AC. They also establish that the most common fluorescein angiogram finding at this early stage is the presence of multiloculated areas of pooling, similar to those present in our patient.
Another confounding factor in our case was the fact that the patient complained of mild headaches and tinnitus. Upon further questioning, we found out that the patient had just been diagnosed with VKH by the referring ophthalmologist and had read about the association of headaches and tinnitus with VKH. He endorsed these symptoms on review of systems only after reading about VKH. Finally, the patient’s Korean descent was considered a risk factor for VKH since it is known that there is a significant incidence of VKH in East and Southeastern Asians, Asian Indians, Middle Easterners, Hispanics, and Native Americans. Interestingly, while VKH is common in both Japanese and Koreans, the HLA associations with VKH in these groups appear to be different.
Despite all of these clinical elements weighing in favor of a diagnosis of VKH, several factors raised concern for the possibility of CSCR. First, aside from a few (rare) cells seen on exam, the general appearance of the eyes was not suggestive of inflammation. There was no disc hyperemia on exam or disc leakage on FA. The study from Korea comparing VKH and bilateral CSCR patients found that disc hyperemia and disc leakage were seen in about 80% of eyes with VKH, but only 0–2% of CSCR eyes. In addition, the patient was a 30 y/o male on a stressful job. This put him in the highest demographic group for CSCR (gender, age, stress level, and self-reported type A personality). Finally, there are rare reports of BALAD in patients with CSCR [9], and of severe complications which occurred when patients with CSCR were misdiagnosed as VKH and treated with steroids [10].
Because of the diagnostic dilemma, the patient was started on eplerenone and dorzolamide, omitting steroids. While not firmly established, a few studies suggest that carbonic anhydrase inhibitors may be helpful in the treatment of active CSCR [11]. Several studies have shown signs of efficacy in treating both acute and chronic CSCR [12, 13]. Also, while prospective, double-blind, randomized, placebo-controlled studies [14] have concluded that there is a lack of efficacy of eplerenone in treating CSCR, follow-up comments in the journals Eye and Lancet [15, 16] found some limitations to these studies. It is possible that in certain scenarios, particularly in the acute phase of the disease and in patients under stress [13], eplerenone may be of benefit. Given the severity of our patient’s presentation and his self-declared high levels of stress, we opted to trial this medication. Without conclusive prospective evidence, it is unclear if the rapid improvement seen in our patient was due to natural history or was affected by either the eplerenone or the dorzolamide.
While certain populations and demographics are at higher risk for VKH and/or central serous retinopathy (CSR), both conditions occur worldwide. Even for uveitis specialists and retina specialists in tertiary referral centers who are more aware of these cases of CSCR masquerading as VKH, our case presents a unique constellation of findings that can be confusing. These include findings that are not often recognized as clinical presentations of CSCR, including mild areas of vascular leakage in the retinal periphery and subretinal white dots. Both of these have been reported as uncommon findings in CSCR. Yannuzzi et al. [17] published a case series in which they noted an atypical presentation of peripheral vascular leakage in CSCR. Subretinal white dots have rarely been described in CSCR and may mislead clinicians to suspect retinitis or other ocular conditions. These white dots are thought to represent outer retinal phagocytic cells engulfing degenerated photoreceptor outer segments [18].
There have been some case reports of patients with CSCR presenting with some features suspicious for VKH, but our case is of interest due to a combination of clinical factors that would typically be considered characteristic of VKH. Our patient was already referred with a diagnosis of VKH, due to the clinical presentation, including severe acute bilateral vision changes and bilateral multifocal serous detachments. But more striking was the presence of bilateral prominent bacillary layer detachments on OCT, a finding that is often used as a way to distinguish VKH from CSR [8]. Thus, this case exemplifies well the complex nature of the diagnostic distinction between an inflammatory condition requiring steroids and a non-inflammatory condition that would be severely worsened by steroids. The lack of disc hyperemia and frank inflammatory findings on the exam, the absence of disc leakage on FA, and the history of CSCR-compatible demographic factors may have been helpful. Also, awareness of atypical CSCR findings such as subretinal white dots and focal areas of peripheral leakage that could confuse the clinical picture and diagnosis is important. However, we believe the main lesson is that whenever there is reasonable clinical suspicion for CSCR, a conservative approach of very close observation should be followed. Our case report is very relevant to patients with atypical presentations of CSCR. Awareness of these presentations that masquerade as VKH is currently not widespread. Although we are not advocating for a sweeping change in the standard of care, we are highlighting the need for a careful and close clinical follow-up in these cases, particularly in the early stages, to rule out CSCR before committing to steroid therapy that could be vision threatening in these cases. Patients that better define themselves as having inflammatory VKH-like conditions (e.g., further symptomatology, new clinical or imaging findings, or lumbar puncture results) can still be recognized early enough to establish appropriate anti-inflammatory therapy if there is a very careful close follow-up in the early phases. Finally, as has been the case with other clinical conditions with a wide spectrum of presentations, it is possible that these rare and very severe cases of CSCR may actually represent a distinct entity. Thus, these cases need to be collected so that more careful evaluation may help determine if a new clinical entity should be described.
Acknowledgments
The authors thank all the clinical and supporting staff for their hard work and support in the care of the patient.
Statement of Ethics
Written informed consent was obtained from the patient for publication of the details of the clinical case and accompanying images. Ethical approval is not required for this study in accordance with local and national guidelines.
Conflict of Interest Statement
The authors have no conflicting interests that need to be disclosed.
Funding Sources
Research activities were in part supported by a National Eye Institute Visual Science Core Grant P30 EY030413 (UTSW Department of Ophthalmology), an Unrestricted grant from Research to Prevent Blindness (UTSW Department of Ophthalmology), and a private VanSickle Family Foundation Grant (R.L.U.-V.).
Author Contributions
Conceptualization and supervision: R.L.U.-V. and Y.-G.H. Writing – original draft preparation: M.D and R.L.U.-V. Writing – review and editing: D.S., P.C., Y.-G.H., and R.L.U.-V. All authors have read and agreed to the published version of the manuscript.
Funding Statement
Research activities were in part supported by a National Eye Institute Visual Science Core Grant P30 EY030413 (UTSW Department of Ophthalmology), an Unrestricted grant from Research to Prevent Blindness (UTSW Department of Ophthalmology), and a private VanSickle Family Foundation Grant (R.L.U.-V.).
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material (for all online suppl. material, see https://doi.org/10.1159/000538736). Further inquiries can be directed to the corresponding authors.
Supplementary Material.
References
- 1. Joye A, Suhler E. Vogt-Koyanagi-Harada disease. Curr Opin Ophthalmol. 2021;32(6):574–82. [DOI] [PubMed] [Google Scholar]
- 2. Standardization of Uveitis Nomenclature SUN Working Group . Classification criteria for vogt-koyanagi-harada disease. Am J Ophthalmol. 2021;228:205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaye R, Chandra S, Sheth J, Boon CJF, Sivaprasad S, Lotery A. Central serous chorioretinopathy: an update on risk factors, pathophysiology and imaging modalities. Prog Retin Eye Res. 2020;79:100865. [DOI] [PubMed] [Google Scholar]
- 4. Spaide RF, Gemmy Cheung CM, Matsumoto H, Kishi S, Boon CJF, van Dijk EHC, et al. Venous overload choroidopathy: a hypothetical framework for central serous chorioretinopathy and allied disorders. Prog Retin Eye Res. 2022;86:100973. [DOI] [PubMed] [Google Scholar]
- 5. Nicholson BP, Atchison E, Idris AA, Bakri SJ. Central serous chorioretinopathy and glucocorticoids: an update on evidence for association. Surv Ophthalmol. 2018;63(1):1–8. [DOI] [PubMed] [Google Scholar]
- 6. Liu XY, Peng XY, Wang S, You QS, Li YB, Xiao YY, et al. Features of optical coherence tomography for the diagnosis of vogt-koyanagi-harada disease. Retina. 2016;36(11):2116–23. [DOI] [PubMed] [Google Scholar]
- 7. Atas F, Kaya M, Saatci AO. Bacillary layer detachment in acute vogt-koyanagi-harada disease. Turk J Ophthalmol. 2022;52(6):400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shin WB, Kim MK, Lee CS, Lee SC, Kim H. Comparison of the clinical manifestations between acute vogt-koyanagi-harada disease and acute bilateral central serous chorioretinopathy. Korean J Ophthalmol. 2015;29(6):389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murillo SA, Medina SP, Romero RM, Murillo FH. Bacillary layer detachment in an atypical case of central serous chorioretinopathy associated with high hyperopia. Case Rep Ophthalmol. 2022;13(2):504–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nguyen NV, Khan F, Emig M, Yeh S. Management of atypical central serous chorioretinopathy mimicking vogt-koyanagi-harada disease. J Vitreoretin Dis. 2023;7(3):249–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liew G, Ho IV, Ong S, Gopinath B, Mitchell P. Efficacy of topical carbonic anhydrase inhibitors in reducing duration of chronic central serous chorioretinopathy. Transl Vis Sci Technol. 2020;9(13):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chatziralli I, Vlachodimitropoulou A, Daoula C, Vrettou C, Galani E, Theodossiadis G, et al. Eplerenone in the treatment of central serous chorioretinopathy: a review of the literature. Int J Retina Vitreous. 2018;4:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fraenkel D, Suffo S, Langenbucher A, Seitz B, Abdin AD. Eplerenone for treatment of chronic central serous chorioretinopathy. Eur J Ophthalmol. 2021;31(4):1885–91. [DOI] [PubMed] [Google Scholar]
- 14. Lotery A, Sivaprasad S, O'Connell A, Harris RA, Culliford L, Cree A, et al. Eplerenone versus placebo for chronic central serous chorioretinopathy: the VICI RCT. Efficacy Mech Eval. 2021;8(2):1–82. [PubMed] [Google Scholar]
- 15. Sacconi R, Borrelli E, Querques G. Eplerenone for chronic central serous chorioretinopathy. Lancet. 2020;396(10262):1556. [DOI] [PubMed] [Google Scholar]
- 16. Sadda SR. Lack of efficacy of eplerenone for treatment of active central serous chorioretinopathy. Eye. 2020;34(9):1489–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yannuzzi LA, Shakin JL, Fisher YL, Altomonte MA. Peripheral retinal detachments and retinal pigment epithelial atrophic tracts secondary to central serous pigment epitheliopathy. 1984. Retina. 2012;32(Suppl 1):1554–72. [DOI] [PubMed] [Google Scholar]
- 18. Spaide RF. Central serous chorioretinopathy. In: Holz FG, Spaide RF, editors. Medical retina essentials in Ophthalmology Springer; 2005. p. 84. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material (for all online suppl. material, see https://doi.org/10.1159/000538736). Further inquiries can be directed to the corresponding authors.