Abstract
Background
Evolutionarily, immune response is a complex mechanism that protects the host from internal and external threats. Pattern-recognition receptors (PRRs) recognize MAMPs, PAMPs, and DAMPs to initiate a protective pro-inflammatory immune response. PRRs are expressed on the cell membranes by TLR1, 2, 4, and 6 and in the cytosolic organelles by TLR3, 7, 8, and 9, NLRs, ALRs, and cGLRs. We know their downstream signaling pathways controlling immunoregulatory and pro-inflammatory immune response. However, the impact of PRRs on metabolic control of immune cells to control their pro- and anti-inflammatory activity has not been discussed extensively.
Summary
Immune cell metabolism or immunometabolism critically determines immune cells’ pro-inflammatory phenotype and function. The current article discusses immunometabolic reprogramming (IR) upon activation of different PRRs, such as TLRs, NLRs, cGLRs, and RLRs. The duration and type of PRR activated, species studied, and location of immune cells to specific organ are critical factors to determine the IR-induced immune response.
Key Message
The work herein describes IR upon TLR, NLR, cGLR, and RLR activation. Understanding IR upon activating different PRRs is critical for designing better immune cell-specific immunotherapeutics and immunomodulators targeting inflammation and inflammatory diseases.
Keywords: Immunometabolism, Infection, Inflammation, Toll-like receptors, NOD-like receptors, cGLRs, Retinoic acid-inducible gene-1-like receptors, Glycolysis, Oxidative phosphorylation
Introduction
Inflammation is a protective host immune response seen during acute trauma or microbes/pathogens and associated microbial/pathogen-associated molecular patterns (MAMPs/PAMPs) exposure to contain the damage or the pathogen responsible for the infection [1]. Furthermore, cellular injury also produces death or damage-associated molecular patterns (DAMPs). The recognition of different molecular patterns (MAMPs, PAMPs, and DAMPs) by pattern-recognition receptors (PRRs) initiates the NF-κB, inflammasome, and interferon (IFN)-releasing factors (IRFs)-dependent pro-inflammatory immune response. The inflammatory process involves a complex network of cellular and molecular signaling cascades to restore the tissue or organ homeostasis, repair, and regeneration. However, severe local or systemic acute inflammation may result in pathology, organ failure, and death, as seen during sepsis. Furthermore, persisting chronic inflammation may cause chronic inflammatory diseases, such as cancers, autoinflammation, and autoimmunity [2–5].
The cellular components of the immune system playing an active role in the inflammatory process involve endothelial cells, epithelial cells, monocytes/macrophages, mast cells, neutrophils, dendritic cells, innate lymphoid cells, mucosal-associated invariant T cells, natural killer cells, and different subsets of T cells [6–9]. These immune cells express different types of PRRs such as Toll-like receptors (TLRs), NOD-like receptors (NLRs), absent in melanoma-2 (AIM-2)-like receptors (ALRs), C-type lectin receptors (CLRs), Retinoic acid-inducible gene-1 (RIG-1)-like receptors (RLRs), and cGLRs, recognizing different PAMPs and DAMPs to initiate the protective pro-inflammatory cascade. However, the metabolic status of immune cells governs their pro- and anti-inflammatory function, which also depends on their location site.
Immunometabolism combines classical immunology with metabolism and can be classified into cellular and tissue immunometabolism [10]. Cellular immunometabolism deals with the impact of metabolic programming on the cellular fate and functions (pro- and anti-inflammatory) of immune cells. In contrast, tissue immunometabolism deals with the impact of immune cells on the tissue and systemic metabolism, governing the host’s adaptation to environmental changes [10]. The concept of systemic immunometabolism has also emerged, which supports the notion that different organs are specialized for specific metabolic tasks with the potential to impact systemic immune response [11]. For example, liver, adipose tissue (AT), and immune response have well-established crosstalk [12, 13]. Similarly, the gut-brain-microbiota axis impacts systemic and local immune response [11, 14, 15].
Immune cells (macrophages, DCs, neutrophils, and T cells) with a reprogramed immunometabolism from oxidative phosphorylation (OXPHOS) coupled with Krebs or tricarboxylic acid (TCA) cycle to increased glycolysis develop a pro-inflammatory phenotype and secrete pro-inflammatory cytokines and molecules, such as IL-1β, IL-6, IL-18, type 1 IFNs, and IFN-γ secretion [16]. The shift from OXPHOS to aerobic glycolysis in immune cells is required to meet the increased energy (adenosine triphosphate or ATP molecules) demand to clear the invading pathogen or DAMP by increasing their pro-inflammatory function. The increase in glycolytic enzymes, such as hexokinase 1 and 2 (HK 1 and 2), glyceraldehyde 3 phosphate dehydrogenase (GAPDH), pyruvate kinase isoenzyme M2 (PKM2) expression, and glucose transporters (GLUTs) such as GLUT1 are critical for a glycolysis shift [16, 17]. The details of immunometabolic reprogramming (IR) among different immune cells during inflammatory conditions, such as sepsis and cancer, have been discussed elsewhere [18–24]. Therefore, the present article discusses the impact of PRR activation on IR, which governs the pathogenesis of inflammation and inflammatory diseases.
TLRs in Inflammation and Immunity and Associated IR
TLRs are the first discovered PRRs critical for inflammation pathogenesis and recognition of different PAMPs and DAMPs [25, 26]. Humans and mice have ten and thirteen different functional TLRs (expressed extra and intracellularly) recognizing PAMPs/MAMPs and DAMPs discussed in detail elsewhere [26–29]. TLR signaling depends on myeloid differentiation primary-response protein 88 (MyD88) and TIR-domain-containing adapter-inducing interferon-β (TRIF) or TIR-domain-containing adapter molecule 1 to generate NF-κB and IRF-dependent pro-inflammatory immune response [30–33]. We will not discuss the signaling cascade associated with extracellular (TLR1, TLR2, TLR4, TLR5, and TLR6) and intracellular (TLR3, TLR7, TLR8, and TLR9) TLRs to generate the NF-κB and IRF-dependent pro-inflammatory cytokines and chemokines release as they are discussed in detail elsewhere [26–28, 34, 35]. Therefore, the primary focus of this section is to discuss the impact of TLR signaling on IR in response to the immunorecognition of PAMPs and DAMPs via TLRs.
TLR Signaling-Induced Downstream Pro-Inflammatory IR at Early Time Course (0–4 h of Stimulation)
The duration of TLR stimulation is critical for IR as it is governed by several metabolic pathways and controlling factors. For example, a recent study has indicated the impact of lipopolysaccharide (LPS) treatment on macrophage immunometabolism during early time course (0–4 h) [36]. The LPS-mediated TLR4 activation induces tumor necrosis factor receptor-associated factor 6 (TRAF6) and TANK-binding kinase 1 (TBK1) recruitment, activation, and their interaction in macrophages within 10 min, which disappears after 2 h [37]. The activated TBK1 phosphorylates TRAF6 bound signal transducer and activator of transcription 3 (STAT3) on serine 727 (Ser727) within 20 min of stimulation of macrophages with LPS [37]. The STAT3 phosphorylation non-canonically activates glycolysis, succinate production, and inflammatory cytokine (IL-1β) production by translocating to the mitochondria, which alters their metabolism and reactive oxygen species (ROS) production (Fig. 1) [37, 38]. The TLR2, TLR3, TLR7, and TLR9 activation also induces STAT3 Ser727 phosphorylation within 20 min of their activation. The STAT3 Ser727 phosphorylation occurs independently of STAT3 Y705 tyrosine (Tyr or Y) phosphorylation and mitochondrial electron transport chain (ETC) complex II integrity [39]. Notably, STAT3 Ser727 and Y705 phosphorylation are also critical for steady-state IL-10 production.
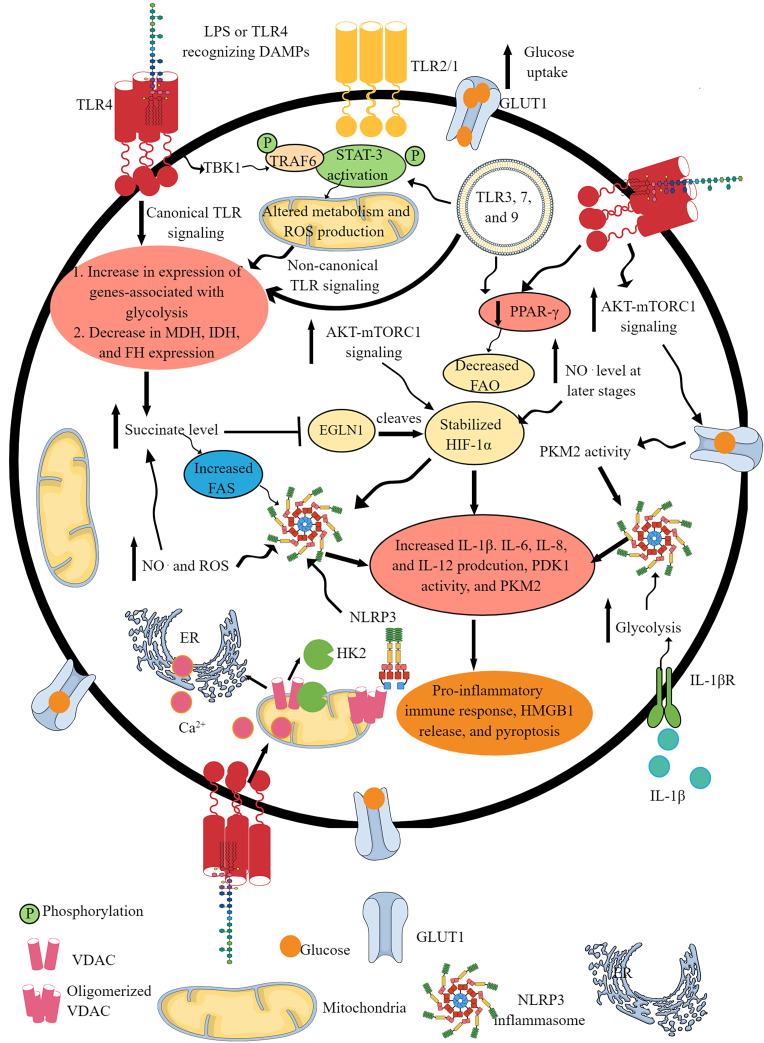
Fig. 1.
TLR and NLR signaling pathway activation-induced IR. Activation of different TLRs, such as TLR4, TLR2/1, TLR3, TLR7, and TLR9, shifts OXPHOS to glycolysis as indicated by the upregulation of glycolysis genes and downregulation of mitochondrial genes involved in OXPHOS and FAO. This IR occurs downstream of canonical and non-canonical (involves TBK1-TRAF6-STAT3 axis) TLR signaling pathways. The GLUT1 overexpression upon TLR activation further supports glycolysis by increasing glucose uptake. The TLR signaling decreases the PPAR-γ expression, which further decreases FAO to support the pro-inflammatory immune cell phenotype and function. TLR activation increases glucose uptake via increased mTOR-AKT signaling that also supports HIF-1α stabilization. The succinate accumulation upon pro-inflammatory TLR signaling activation further supports HIF-1α stabilization by inhibiting EGLN1. The NO. generation at later stages activates NLRP3 inflammasome activity and succinate accumulation. The TLR signaling-induced glycolysis, increased succinate level, HIF-1α stabilization and accumulation, PKM2, mammalian target of rapamycin complex 1, and AKT overactivity support NLRP3 inflammasome activation and IL-1β release. The HK2 dissociation from VDAC at the outer mitochondrial membrane during TLR signaling-induced glycolysis activates IP3 receptors in the ER to release Ca2+ in the cytosol – mitochondria uptake cytosolic Ca2+ molecules for VDAC oligomerization. The oligomerized VDACs aggregate with NLRP3 during its initial assembly to form the NLRP3 inflammasome complex. Furthermore, IL-1β released due to the NLRP3 inflammasome activity supports glycolysis through binding to IL-1βR. Thus, TLRs and NLRs (NLRP3) support each other’s pro-inflammatory function through IR.
Both, catalytic subunits succinate dehydrogenase A and B (SDHA and SDHB) of the SDH or complex II of the ETC are critical for hypoxia-inducible factor-1α (HIF-1α) stabilization and IL-1β production in macrophages [39]. Furthermore, the phosphorylation of serine and tyrosine are critical for maximum transcription by STAT3 [40]. Hence, TLR signaling-mediated canonical and non-canonical (STAT3-dependent) pathways are involved in glycolysis and IR to initiate the inflammatory immune response (Fig. 1). Additionally, TLR7-mediated mitochondrial RNA recognition also increases cytosolic fumarate level by suppressing fumarate hydratase for enhanced type 1 IFN (IFN-β) production [41]. Furthermore, TLR signaling (TLR4, TLR1/TLR2, TLR2/TLR6, TLR7/8, TLR9, and TLR3)-mediated TBK1, inhibitory kinase β kinase ε (IKKε), and AKT (protein kinase B) activation are critical for glycolysis during DC activation by promoting the glycolytic enzyme HK2 association with mitochondria [42].
The early course (2 h) of LPS treatment to macrophages induces a rapid glucose uptake to promote glycolysis and TCA cycle volume to over-generate citric acid or citrate. The GLUTs (GLUT1) mediate a rapid glucose uptake, and their expression increases upon TLR activation, giving a pro-inflammatory phenotype to immune cells, such as macrophages, DCs, and T cells (Fig. 1) [18, 43, 44]. For example, TLR4 activation in macrophages activates phosphatidyl inositol 3-kinase (PI3K) via B-cell adapter for PI3K (BACP, an adapter molecule with a functional N-terminal TIR homology domain), activating AKT and mechanistic or mammalian target of rapamycin complex 1 [44–46]. The activated AKT increases the GLUT1 endosomal recycling and its surface expression on macrophages and other immune cells (Fig. 1) [47]. BCAP critically regulates IL-1R-induced phosphoinositide 3-kinase (PI3K)-Akt-mTOR activation-induced pro-inflammatory Th17 immune cell differentiation in response to IL-1β [48].
MyD88 and TRIF-dependent coordinated downstream signaling is critical for complete adenosine triphosphate (ATP) citrate lyase (ACLY) activation in response to the LPS stimulation [36]. For example, MyD88 and TRIF exert an additive effect on immediate IR, such as increased glucose uptake and cytosolic acetyl-Coenzyme (Ac-CoA) and oxaloacetic acid or oxaloacetate (OAA) synthesis via ACLY upregulation to prime macrophages in response to early after TLR4 stimulation. Furthermore, the upregulated ACLY activity during glycolysis promotes glucose-dependent Ac-CoA incorporation into histones [36]. The ACLY translocation to the nucleus under inflammatory conditions, including sepsis in macrophages, induces NF-κB acetylation that supports their full activation and pro-inflammatory function, including the overexpression of SLC25A1, encoding citrate career and ACLY [49]. Furthermore, LPS-induced ACLY activity is critical for histone acetylation at the IL-12b gene locus and associated enhancer, indicating its role in facilitating enhancer chromatin accessibility [36]. AKT inhibitor (MK-2206) blunts LPS-inducible ACLY phosphorylation, indicating that the AKT signaling is also critical for its phosphorylation [36, 50]. However, ACLY inhibition does not affect the expression of early genes, such as CXCL1, CXCL2, IL-1α, and IL-1β, but downregulates the expression of the late genes, including IL-6, IL-12b, IL-18, IL-27, CXCL9, and CXCL10 [36]. Of note, ACLY inhibition stimulates the overexpression of anti-inflammatory genes, such as IL-10 and IL-1 receptor antagonist (IL-1RA). Thus, ACLY inhibition does not affect inflammation in general but affects it at a specific level by modulating innate immune response and inflammation upon TLR4 activation.
The histone acetylation in macrophages during early LPS treatment due to increased glycolysis enhances the induction and translation of critical pro-inflammatory genes. For example, induction of IL-1β and chemokine (C-X-C motif) ligand 1 (CXCL1) genes in macrophages occurs within 30 min of LPS treatment and peaks at 2 h [36]. At the same time, the transcription of IL-6 and IL-12b genes (secondary response genes) in macrophages (bone-marrow-derived macrophages or BMDMs) occurs at 2 h post-LPS treatment, which stays 4 h. Therefore, early IR in BMDMs upon LPS stimulation may alter pro-inflammatory gene induction. For example, macrophages’ mitochondrial potential and ROS production decrease early after LPS stimulation without mitochondrial mass alteration, which increases later. Furthermore, mitochondria are well-known inflammation regulators, as discussed elsewhere [51–53].
LPS induces a time-dependent transition of metabolism in macrophages (BMDMs) from basal to pro-inflammatory state, and lactate, succinate, and itaconate levels increase within 2 h, which are very high at 24 h (Table 1). Both oxidized and reduced glutathione levels decreased compared to early and late time courses after LPS stimulation. The initial mitochondrial ROS (mtROS) production is also low, indicating a tightly controlled redox balance. After 2 h of LPS stimulation in BMDMs, the adenosine diphosphate (ADP) ribose production is highest due to the poly (ADP ribose) polymerase (PARP, needs nicotinamide adenine dinucleotide (NAD+) as a cofactor, which also serves as a coenzyme for redox reactions) overactivity that is crucial for the pro-inflammatory phenotype of macrophages [54, 55]. The LPS treatment to macrophages increases nicotinamide phosphoribosyl transferase (NAMT, a key enzyme in NAD+ salvage), which maintains sufficient NAD+ pool for PARP and GAPDH activity and Warburg effect (a shift from OXPHOS to aerobic glycolysis to meet frequent energy requirement) to induce pro-inflammatory macrophage phenotype and function [56]. The Warburg effect was first reported by Otto Warburg in cancer cells in 1924 to meet high energy demand for maintaining uncontrolled growth and proliferation [57]. The Warburg effect involves increased glucose uptake and its conversion to lactate to meet high energy demand by cancer cells and immune cells during infections and inflammatory conditions [58–60].
Table 1.
Outline of immunometabolic reprograming upon activation of different PRRs
| PRRs | Immunometabolic shift/reprogramming | Metabolic pathway enzymes upregulated | Metabolites upregulated |
|---|---|---|---|
| TLR4, TLR2, TLR1, TLR3, TLR6, TLR7, TLR8, and TLR9 | OXPHOS to glycolysis | EGLN1, a prolyl hydroxylase, decreases that stabilizes HIF-1α | 1. Succinate levels increases till 48 h, subsides, and then increases at 72 h |
| HK2 activity increases to support glycolysis | |||
| FAO to FAS | ACOD1 activity increases | ||
| PDK1 activity increases | |||
| Increased glutaminolysis | ACC activity increases | 2. Itaconate level increases similarly | |
| Increased TCA or Krebs cycle | Inactive PKM2 expression increases | ||
| GAPDH malonylation is critical for TNF-α production | |||
| cGLRs (cGAS/STING signaling) | OXPHOS to glycolysis | Glycolysis enzymes expression and activity increases | Succinate and itaconate production increases in a time-dependent manner |
| NLRP3 | OXPHOS to glycolysis | Glycolysis enzyme upregulate, such as PKM2 activity increases | Succinate and itaconate production increases in a time-dependent manner |
| FAO to FAS | |||
| CLRs | OXPHOS to glycolysis | Glycolytic enzymes | Succinate and itaconate accumulation |
| RLRs | Inhibit glycolysis and support glucose utilization to PPP and HBP for type I and III IFN production | Enzymes supporting PPP and HBP | Decrease PEP, pyruvate, and lactate levels |
| Decrease succinate, fumarate, malate, and aconitate levels without affecting OAA levels |
Furthermore, de novo synthesis of NAD+ in macrophages is critical for macrophage-driven inflammatory conditions as a decreased NAD+/NADH ratio inhibits glycolysis due to an increase in intracellular NADH or a decrease in the intracellular NAD+ [61, 62]. The Mycobacterium tuberculosis (Mtb) infection depletes NAD+ in MICs to inhibit glycolysis, which lowers protective immunity in patients with tuberculosis by decreasing the early recruitment of different immune cells and IFN-γ production [63]. Macrophage TLR stimulation under high glucose conditions critically downregulates HIF-1α levels and induces their pyroptosis due to methylglyoxal (MGO, a side product of glycolysis) overexpression [64]. Hence, extracellular glucose levels determine macrophages’ fate and function, including pro-inflammatory cytokine release and pyroptosis upon TLR stimulation-dependent IR such as glycolysis.
TLR Signaling-Induced Downstream Pro-Inflammatory IR at Later Time Course (12–24 h or More)
The comprehensive metabolic map of macrophages stimulated with LPS or endotoxin for 24 h shows an increase in the genes associated with glycolysis and a decrease in the mitochondrial genes, such as TCA cycle genes, including malate dehydrogenase (MDH), isocitrate dehydrogenase (IDH), and FH (Fig. 1) [41, 65]. Furthermore, LPS also increases succinate (a Krebs cycle intermediate) levels through glutamine-dependent anaplerosis (mainly) and gamma-aminobutyric acid (GABA)-shunt pathway [41, 65]. However, increased citrate and FA levels indicate the diversion of the TCA cycle for biosynthetic or anabolic needs. The citrate upregulation upon treatment of macrophages with the combination of LPS and interferon (IFN)-γ indicates that TCA cycle fragmentation is critical for generating M1 macrophages [66]. However, aspartate-argininosuccinate shunt (AAS) induction due to aspartate-aminotransferase (AAT) activation compensates TCA cycle fragmentation to generate NO. and IL-6 upon LPS+IFN-γ treatment inducing M1 macrophage polarization. The details of citrate in IR and inflammation are discussed elsewhere [67, 68].
Furthermore, AAS induction upon LPS stimulation (acute and prolonged) in macrophages is supported by the increased argininosuccinate synthase 1 (ASS1) expression and also increases cytosolic fumarate and associated protein succination [41]. The process of succination involves a reaction between fumarate and cysteine residues of protein to produce S-(2-succinyl)cysteine (2SC) [69]. During acute LPS treatment to macrophages, the ASS1 induction is critical for fumarate accumulation, whereas FH suppression upon prolonged LPS stimulation depends on FH inhibition. Notably, overexpressed ASS1, causing fumarate accumulation, mildly regulates IL-10 and TNF-α production in LPS-stimulated macrophages. Furthermore, fumarate or FH-regulated IL-10-TNF-axis is active in human macrophages. Therefore, sustained FH expression and enzymatic activity controlling cytosolic fumarate level is a critical regulator of IL-10 and TNF-α production in macrophages upon TLR activation [41]. The argininosuccinate lyase (ASSL) cleaving argininosuccinate to fumarate also plays a role in fumarate accumulation, indicating the role of AAS in fumarate accumulation.
The dicarboxylic acid transporter transports excess succinate from mitochondria to cytosol. The cytosolic citrate stabilizes HIF-1α by inhibiting its hydroxylation through egg-laying abnormal (EGL)-9 family hypoxia-inducible factor 1 (EGLN1), serving as a prolyl hydroxylase 2 or PHD 2, recognizing its two conserved prolyl residues (Pro564 and Pro402) by von Hippel-Lindau tumor suppressor protein (pVHL) (Fig. 1; Table 1) [70–73]. For example, hydroxylated conserved prolyl residues of HIF-1α are recognized by pVHL due to the generation of the high-affinity pVHL binding site, causing HIF-1α polyubiquitination and proteasomal degradation [74]. EGLN1 catalyzes the posttranslational formation of 4-hydroxyproline in HIF-1α. Thus, LPS-induced glycolysis, elevated succinate (regulates HIF-1α and IL-1β axis) levels, and succinylation of several proteins increase IL-1β production to induce inflammation (Fig. 1) [65]. Furthermore, SDH oxidizes succinate. This phenomenon increases the reduced ubiquinone or ubiquinol (CoQH2) level, making it hard to efficiently consumed by complex III, causing reverse electron transport (RET, where electron transfer occurs in the reverse direction from CoQH2 to ubiquinone or CoQ) at complex I and increasing mtROS production, which further supports HIF-1α stabilization and IL-1β synthesis [75, 76]. The details of ETC-mediated immunometabolism or IR regulations have been discussed elsewhere [77].
Interestingly, extracellular succinate serves as metabokine and alarmin to modulate the immune response and alter the myeloid immune cell, such as macrophages and DCs function via binding to its cognate succinate receptor 1 [78, 79]. For example, extracellular succinate via succinate receptor 1 exerts pro-inflammatory action on macrophages in autoimmune diseases, such as rheumatoid arthritis [78]. However, it exerts anti-inflammatory action in the AT and tumor microenvironment, such as lung cancer, by promoting the generation of anti-inflammatory M2 macrophages [78, 80].
Similarly, citrate career transports excess citrate from mitochondria to the cytosol and supports lipogenesis via ACLY activity [67, 68]. LPS or TLR4 stimulation-induced ACLY breaks citrate into OAA and Ac-CoA, serving as a substrate for fatty acid synthesis (FAS) [81]. Furthermore, cytosolic citrate and ACLY support the generation of pro-inflammatory molecules, including ROS, NO., and prostaglandin E2 (PGE2) [81–83]. Thus, cytosolic citrate and succinate accumulation are critical for IR, supporting the pro-inflammatory phenotype of immune cells, including macrophages, upon TLR activation at early and late time courses.
The NO. overproduction further increases HIF-1α level, NLRP3 activity, and IL-1β production to support pro-inflammatory macrophage phenotype and function at later stages (72 h onward) (Fig. 1). However, NO. is not a critical/fundamental inducer of glycolysis as macrophages without NO. undergo glycolysis and support inflammatory phenotype and function. It is important to note that in this study, macrophages were stimulated overnight (16 h) with LPS and IFN-γ, which confirms earlier findings that early or within the first 2 h ROS or NO. is not a critical factor in pro-inflammatory IR but involve at a later stage [36, 84]. Even mitochondrial potential and ROS production in macrophages are reduced during early time points (30 min–2 h) of LPS stimulation [36]. However, NO. at later stages (48 h) reroutes pyruvate in inflammatory macrophages away from pyruvate dehydrogenase (PDH), suppresses their metabolism to mitochondrial aconitase (ACO2), and promotes glutamine-based anaplerosis [85]. Thus, NO. accumulation at later stages shuts mitochondrial ETC complexes and decreases the production of inflammatory mediators such as HIF-α and IL-1β.
The HIF-α stabilization (supporting glycolysis and lactate accumulation) causing its abundance induces the pyruvate dehydrogenase kinase 1 (PDK1) gene expression that phosphorylates PDH for its inhibition (Table 1) [86, 87]. The increased PDK1 inhibits the PDH flux via phosphorylation, increasing the pyruvate-derived Ac-CoA level for citrate overproduction [87]. The increased citrate production keeps in check the rate of reductive carboxylation of α-ketoglutarate (α-KG) and increases FAS and itaconate production. The PDK1 upregulation in CD8+T cells upon IL-2 stimulation in an mTOR-HIF-1α-axis dependent way also sustains glucose uptake and glycolysis. Furthermore, TLR7 activation induces IR to glycolysis in CD8+T cells to enhance their effector functions [88]. Thus, TLR signaling-mediated overexpressed PDK1 is critical for innate and adaptive immune cells’ IR to glycolysis. The PDH inhibition decreases the pyruvate oxidation to citrate in the mitochondria.
Citrate is required to synthesize itaconate and lipogenesis. The increased demand for itaconate and lipid molecules decreases citrate oxidation via the Krebs cycle. Furthermore, the unchanged PDK1 abundance maintains PDH flux even in the presence of HIF-1α, a critical node for TLR4 activation-mediated macrophage activation [87]. Hence, PDH targeting is a metabolic intervention to treat chronic inflammatory diseases. For example, LPS treatment or TLR4 activation increases glycolysis, glutamine uptake, and glutaminolysis to support the TCA cycle and lipogenesis or FAS to transform naïve macrophages into pro-inflammatory or M1 macrophages (Table 1) [18, 87]. Along with supporting the TCA cycle, glutaminolysis supports AAS metabolites, including fumarate and glutathione production [41]. During this process, activated TLRs (TLR2, 3, 4, and 7) reduce the peroxisome-proliferator-activated receptor-γ (PPAR-γ) expression in MICs (macrophages and DCs) in an NF-κB-dependent manner to maintain their pro-inflammatory phenotype and function (Fig. 1) [89]. For example, pro-inflammatory cytokines (TNF-α and IFN-γ) inhibit PPAR-γ expression in macrophages [90].
PPAR-γ activation critically regulates lipid/fat metabolism (increases fatty acid oxidation or FAO and uptake of oxidized lipid through CD36 overexpression) in immune cells (macrophages and DCs), and its activation induces anti-inflammatory phenotype (M2 or alternatively activated macrophages or AAMs or foam cells) and function [91–97]. Furthermore, PPAR-γ also controls the AAM or M2 phenotype and function by regulating glutaminolysis [98]. Therefore, TLRs and PPAR-γ have inverse crosstalk where activation of one inhibits the other, as discussed elsewhere [99, 100].
The pulmonary alveolar macrophages (PAMs) highly express PPAR-γ and exhibit higher FAO or β-oxidation of lipids and OXPHOS with relatively lower glycolysis levels than interstitial pulmonary macrophages (IPMs, exhibit high glycolysis) that affects their pro-inflammatory function upon PRR, such as TLR stimulation [101, 102]. Furthermore, glycolysis upregulation does not occur in tissue-resident PAMs as seen in BMDMs upon TLR4 activation, and glycolysis inhibition in PAMs does not affect their pro-inflammatory function [102]. However, HIF-1α stabilization upon TLR4 stimulation or hypoxia shifts OXPHOS of PAMs to glycolysis, which is not sufficient to produce glycolysis-dependent pro-inflammatory immune response but supports their survival under different pro-inflammatory conditions, including acute lung injury (ALI) [102, 103].
Human PAMs depend on OXPHOS but not glycolysis for pro-inflammatory immune response upon LPS exposure or TLR4 activation, as seen in MDMs [104]. A further study has shown that lung environment, including the relative absence of glucose in alveoli, is critical to determine the IR among PAMs upon different stimuli, such TLR activation and stimulation with IL-4 as pro-inflammatory stimuli, including LPS, TNF-α, and IFN-γ increase airway surface liquid (ASL) glucose levels [105–107]. Hence, tissue location and type of immune cells such as macrophages, DCs, and T cells may affect their IR upon TLR and other PRR activation, which needs further investigation. For example, TLR8 activation in human regulatory T cells (Tregs) inhibits glycolysis by inhibiting mTOR signaling, HIF-1α synthesis and stabilization, and glucose uptake, which reverses the immunosuppressive function of Tregs that can be used in different solid tumors such as melanoma as an immunotherapeutic approach [108].
Of note, the TCA cycle remodeling occurs in two stages upon LPS-induced TLR4 activation with the alteration of succinate and itaconate levels, which increase initially and then decrease at a later stage [109]. The pyruvate and oxoglutarate dehydrogenase complex (PDHC and OGDC are members of the mitochondrial α-ketoacid dehydrogenase family) inhibition decreases succinate and itaconate levels at a later stage (48 h). The dynamic changes in the lipoylation of PDHC and OGDC E2 subunits regulate acyl group transfer to CoA, and the PDHC E1 subunit phosphorylation controls PDHC and OGDC inhibition. The PDHC and OGDC inhibition at later stages (48 h) involves NO. or reactive nitrogen species (RNS), which covalently alter thiol groups on their lipoic arms to generate a series of adducts that block catalytic activity, including nitroxyl (HNO) [110, 111]. For example, S-Nitroso-CoA, a product of RNS and the E2 subunit’s natural substrate, can deliver these modifications to lipoic arms. This dynamic metabolic reprogramming-induced transient metabolic state favors HIF-1α stabilization during the early stages of TLR4 activation that subsides with time (after 48 h) and reactivates at 72 h (a second increase) with succinate and itaconate increase (Table 1) [109, 111]. Hence, the succinate and itaconate fluctuation upon TLR activation influences the dynamics of HIF-1α and pro-inflammatory phenotype upon continual and acute activation in MICs, such as macrophages and DCs. Thus, changing macrophage immunometabolism can regulate functional transitions during an immune response that may aggravate and subside depending on the external stimulus and HIF-1α availability.
Furthermore, LPS treatment increases the PKM2 (a critical metabolic regulator) expression in murine BMDMs (Fig. 1). However, this PKM2 primarily forms an enzymatically inactive dimer or monomer. Furthermore, inducing PKM2 tetramer (enzymatically active retains in the cytosol) formation by DASA-58 (a potential pyruvate kinase isozyme (PKM2) allosteric activator) and TEPP-46 (a PKM2 activator) treatment inhibits LPS-induced PKM2 nuclear translocation without affecting cytosolic PKM2 in BMDMs.
The LPS-induced Warburg effect in murine macrophages is critical for IL-1β release but not for TNF-α release, as indicated by the 2-deoxyglucose (2-DG, a glycolysis inhibitor) treatment [65]. However, macrophage TNF-α and IL-10 release upon LPS stimulation are under the control of FH expression/activity or fumarate levels [41]. The treatment of LPS-stimulated BMDMs and peritoneal macrophages with DASA-58 and TEPP-46 inhibits the production of pro-IL-1β without affecting TNF-α and IL-6 production as the Warburg effect is not critical for their production [17, 65]. Furthermore, the PKM2 activation in macrophages inhibits LPS-induced expression of proglycolytic and HIF-1α-dependent genes. M1 macrophages overexpress inactive PKM2 as activated PKM2 in LPS-stimulated macrophages boosts the anti-inflammatory M2 cytokine, IL-10 expression. Thus, LPS treatment to macrophages increases inactivated PKM2 in the cytosol and nucleus that support HIF-1α overexpression and increased IL-1β production. Furthermore, LPS increases the binding of PKM2 to the HIF-1α-specific binding site of IL-1β promoter, which gets inhibited in the presence of TEP-46 and DASA-58. Therefore, PKM2 activators inhibit LPS-induced glycolysis and succinate accumulation in macrophages to lower the inflammation and promote polarization of pro-inflammatory M1 to anti-inflammatory M2 macrophages.
In addition to TLR4, the activation of TLR2, 6, and 9 also increases PKM2 expression, HIF-1α, and pro-IL-1β expression. Hence, TLR2, 4, 6, and 9 activations reprogram macrophage immunometabolism to glycolysis by increasing PKM2 that binds to HIF-1α-specific binding site of IL-1β promoter to generate pro-inflammatory IL-1β cytokine without affecting TNF-α and IL-6 production. However, LPS stimulation of macrophages induces TNF-α production via malonylation of GAPDH that dissociates it from TNFα mRNA to promote its translation (Table 1) [112]. Thus, glycolysis is not directly involved in TNF-α production. However, an altered TCA cycle via a citrate-derived molecule called malonyl-CoA and FH inhibition causing fumarate accumulation are critical for TNF-α production. For example, in resting macrophages, GAPDH suppresses the translation of several inflammatory mRNAs, including the TNF-α one. Furthermore, activated PKM2 counteracts the LPS-mediated inflammatory events, mostly by inhibiting the Warburg effect or glycolysis in vivo [17]. The PKM2 activation strategy to decrease the inflammation may work during sterile inflammatory conditions but needs caution during infections as a decreased inflammatory response at the initial stages of infection may prove detrimental to the host due to infection dissemination that may later lead to sepsis [17]. The immunometabolic changes supporting IL-1β release and ROS production among macrophages upon LPS stimulation are late-stage inflammatory responses (12–24 h).
Furthermore, pyruvate transport to mitochondria through mitochondrial pyruvate carrier (MPC), followed by its utilization in the Krebs cycle, is dispensable [113]. Therefore, MPC is not required for pro-inflammatory IR and activation of M1 macrophages, given that MPC deletion in MICs does not affect their inflammatory function and macrophage polarization to M1 phenotype in murine endotoxemia. Furthermore, although mice and human microglia exhibit pro-inflammatory phenotypes and function upon LPS-mediated TLR4 signaling, their IR differs. For example, murine microglia overexpress HK2, whereas human microglia overexpress phosphofructokinase (PFK) [114, 115]. Hence, a species-specific investigation in IR downstream of PRR, such as TLR signaling, is critical for translational research for target-specific therapy.
Notably, TEPP-46 treatment not only inhibits macrophages pro-inflammatory function via activating PKM2 (enzymatically active PKM2 tetramer) but it also inhibits T cell glycolysis that is critical for pro-inflammatory cytokines, such as IL-17 and IFN-γ release [116]. For example, TLR4 signaling in T cells promotes their inflammatory functions, including in autoinflammation, such as experimental autoimmune encephalomyelitis (EAE) or multiple sclerosis (MS) [117]. However, TLR4 activation through TRIF adapter on effector CD4+T cells inhibits ERK1/2 activation that inhibits IFN-γ synthesis and release but increases IL-17A production upon subsequent T cell receptor (TCR) activation by inducing mitogen-activated protein kinase (MAPK) phosphatase 3 (MKP-3) and acts as a tonic inhibitor and inhibits inflammation in experimental colitis model [118]. Furthermore, co-stimulation of TLR4 and TCR does not exert the same inhibitory effect on ERK1/2 activation and IFN-γ production. Thus, TLR4-dependent pro- and anti-inflammatory action on T cells depends on T cell type and TCR stimulation. Hence, we need further studies on TLR-based IR among T cell types and other immune cells, including macrophages and DCs.
TLR-Dependent IR in Pro-Inflammatory M1 to Anti-Inflammatory M2 Macrophage Polarization
Acetyl-CoA carboxylase (ACC) is a lipid biosynthesis regulatory enzyme, which also regulates TLR4 stimulation-mediated early glycolysis and remodeling of macrophages’ lipidome to control the secretion of pro-inflammatory cytokines (IL-1β and IL-6) and molecules [119]. There are two forms of ACCs: (1) ACC1, which localizes in the cytosol, and (2) ACC2 residing on the outer mitochondrial membrane, but both convert Ac-CoA to malonyl-CoA for de novo FAS to produce long chain FAs [120]. For example, ACC deficient or ACC inhibitor (firsocostat) treated macrophages exhibit significantly decreased IL-1β, IL-6, and inducible nitric oxide synthase (iNOS) expression without affecting CD80 and CD86 expression at 6 h post-stimulation with TLR4 or TLR2 agonists. Furthermore, ACC deficiency shifts macrophages to a hyperglycolytic bioenergetic state. It increases GLUT1 expression compared to wild-type macrophages, which sets an upper limit of glycolytic rate in LPS-stimulated ACC−/− macrophages [119]. ACC deficiency does not affect SDH activity. Notably, ACC is dispensable for IL-4-mediated M2 macrophage polarization at early time course (first 6 h post-stimulation) as indicated by the similar levels of STAT6 phosphorylation at tyrosine-641 and arginase in control and IL-4 treated macrophages.
In macrophages stimulated with IL-4 for a longer duration (18–24 h), ACC1 upregulation is critical for M2 polarization, and its inhibition at this time course abrogates M2 polarization without affecting M1 macrophages [121]. Thus, duration and stimulation type determine the ACC1-dependent macrophage polarization. For example, granulocyte macrophages colony-stimulating factor-treated human monocyte-derived macrophages (hMDMs) exhibit ACC1 overexpression than murine BMDMs at 18–24 stimulation along with other M1 macrophage immunometabolic markers (GLUT1, PKM, G6PD, and SDHA). Furthermore, macrophage colony-stimulating factor treatment on hMDMs has an anti-inflammatory action, whereas granulocyte macrophage colony-stimulating factor (GM-CSF) exerts a pro-inflammatory effect through IR.
Interestingly, IL-4 treatment to macrophages via AKT-mammalian target of rapamycin complex 1 (mTORC1) signaling controls ACLY activity that induces M2 macrophage polarization through increased histone acetylation-dependent induction of a specific subset of M2 genes, regulating their proliferation and chemokine production [50]. Thus, ACLY activation in response to the anti-inflammatory (IL-4, a type II cytokine that supports M2 macrophages via IL-4 receptor alpha chain (IL-4Rα) that dimerizes to form type 1 signaling complex) and pro-inflammatory (LPS) stimuli is critical to determine macrophage phenotype and function [36, 50, 122]. Furthermore, IL-4 treatment to macrophages upregulates GLUT3 expression, critical for M2 macrophage polarization independently of glucose uptake, but induces IL-4/IL-4R complex endocytosis, which phosphorylates STAT6 for M2 polarization [123, 124]. Interestingly, IL-4 also induces overexpression of lipid (CD36, CPT1A) and amino acid (CD98) transporters on M2 macrophages [121].
It is important to note that the AAMs/M2 macrophage phenotype or their differentiation does not require glycolysis in intact OXPHOS [125]. For example, the activated arginase 2 (Arg2) regulates IL-10. IL-10 is an anti-inflammatory cytokine and downregulates inflammatory mediators, including succinate, HIF-1α, and IL-1β in M1 macrophages [126]. IL-10 in M1 macrophages regulates Arg2, is a mitochondrial microRNA-155 (miR-155). For example, Arg2 is critical for IL-10-induced mitochondrial dynamics and oxidative respiration modulation by increasing the SDH or complex II activity [126]. Thus, M1 to M2 macrophage polarization also involves IR from glycolysis to OXPHOS. Furthermore, GLUT3 upregulation is critical for macrophage polarization to M2 macrophages [123, 124]. GLUT3, without affecting glucose transport (uptake or efflux), thus the glucose metabolism induces Ras-mediated IL-4/IL-4 receptor (IL-4R) complex endocytosis that phosphorylates and dimerizes STAT6 for, inducing and maintaining M2 macrophage phenotype and function [123, 124]. Furthermore, exogenous metabolic cofactor coenzyme A (CoA) support IL-4-induced M2 macrophage polarization by providing a weak TLR4 signal through activating MyD88 downstream signaling [127]. The CoA-induced TLR4-mediated MyD88 signaling in macrophages primes them for increased receptivity for IL-4 signaling by reshaping chromatin accessibility for increased transcription of IL-4-associated genes [127]. Thus, host metabolic DAMPs, such as exogenous CoA, can prime macrophages to M2 phenotype by activating TLR4-MyD88 signaling to control inflammation. The detailed M2 macrophage IR has been discussed elsewhere [18, 128].
TLR Signaling-Induced Anti-Inflammatory Immunometabolites and Their Analogs to Control Pro-Inflammatory IR
Itaconate is an endogenous SDH inhibitor and increases succinate levels, and itaconate production decreases in immune-responsive gene 1 (Irg1)-deficient macrophages [129]. Irg1 is one of the most highly expressed enzymes in pro-inflammatory macrophages, and its enzymatic product, called cis-aconitate decarboxylase 1 (ACOD1), catalyzes the itaconate formation from cis-aconitate (a TCA cycle metabolite) in immune cells (Table 1) [129, 130]. Citroaconate inhibits ACOD1 or Irg1 activity, decreasing itaconate levels, and is a nuclear factor erythroid 2 (NFE2)-related factor 2 (NRF2) agonist [131]. Citroaconate (an endogenous ACOD1 inhibitor) exerts its antiviral and immunomodulatory action against influenza A virus (IAV) that is recognized by TLR7/8, RLRs, and nucleotide-binding domain and leucine-rich-repeat-containing protein 3 (NLRP3) by decreasing mtROS, IL-6, IL-1β, CXCL10, TNF-α, macrophage inflammatory protein-1β (MIP-1β) or chemokine (C-C motif) ligand 4 (CCL4), and IFN-induced protein with tetratricopeptide repeats 1 (IFIT1) and increasing CXCL8 or IL-8 and CCL5 levels [131, 132]. Notably, murine ACOD1 is more active than human ACOD1 in generating itaconate (5–10 times) in activated macrophages [133, 134]. Hence, ACOD1 is less prominent in controlling human inflammation than mice. ACOD1-depleted induced pluripotent stem cell-derived chimeric antigen receptor (CAR)-macrophages (CAR-iMACs) manifest increased ROS production, more potent phagocytosis, and enhanced cytotoxic functions against cancer cells due to reduced itaconate (an immunometabolite) production [135]. It would be interesting to observe similar findings in human CAR-MACs, although human ACOD1 is less potent in itaconate generation than murine macrophages.
Irg1 overexpression also promotes MHC-1 expression and genes involved in antigen processing, such as transporter-associated with antigen processing 1 (TAP1) and proteasome subunit beta type 9 (PSMB9) in macrophages by regulating STAT1/3 phosphorylation that depends on pentose phosphate pathway or shunt (PPP or PPS) and NADPH oxidase-mediated ROS production [136]. Notably, activated iNOS, which requires tetrahydrobiopterin (BH4) as a cofactor)-mediated NO. production upon LPS and IFN-γ stimulation in macrophages due to upregulated glycolysis, Krebs cycle remodeling, and mitochondrial respiration inhibition further support glycolysis by increasing citrate, succinate, and itaconate levels [84, 137]. Itaconate also inhibits ten-eleven translocation (TET) DNA dioxygenases in LPS-stimulated pro-inflammatory macrophages to limit inflammatory immune response in mice subjected to endotoxemia [138]. The details of itaconate in the host immunity and inflammation have been discussed elsewhere [139, 140].
The treatment of LPS-stimulated pro-inflammatory macrophages with dimethyl itaconate inhibits SDH, which is a part of complex II of the mitochondrial ETC. Thus altered mitochondrial respiration, as indicated by the decreased oxygen consumption rate, limits the IL-1β, IL-8, IL-6, IL-12, NO., and HIF-1α levels without affecting TNF-α levels [130]. Another study has indicated that 4-octyl itaconate (4-OI, a cell-permeable itaconate derivative) also inhibits GAPDH by alkylating its cysteine 22 residue to downregulate aerobic glycolysis in M1 macrophages in vitro and in vivo in a lethal endotoxemia model [141]. Notably, both studies have used itaconate chemical derivatives (DIM and 4-OI) in murine macrophages.
Mesaconate is an itaconate metabolite that does not inhibit SDH activity as potently as itaconate but exerts immunomodulatory action by inhibiting IL-6 and IL-12 production by inhibiting glycolysis and promoting CXCL10 production independently of NRF2 and activating transcription factor 3 (ATF3) activation in mouse BMDMs and hMDMs and white blood cells (WBCs) [131, 142]. It is important to note that mesaconate and itaconate did not inhibit IL-1β production and inflammasome activation in normal murine BMDMs, hMDMs, and WBCs upon LPS treatment or TLR4 activation [142]. However, itaconate or mesaconate pre-treated RAW264.7 macrophages (which have a cancerous origin and have high glycolysis to maintain their proliferation) show a decreased LPS-induced IL-1β, IL-6 and IL-10 expression and IL-6 and IL-10 production. In their study, the authors have observed similar findings with DMI and 4-OI as anti-inflammatory agents, which decrease IL-1β, IL-6, and TNF-α production upon pretreatment to LPS-stimulated or TLR4-activated macrophages. However, they are more cytotoxic [142]. Furthermore, with 1 mM DMI and 4-OI pretreatment to RAW264.7 macrophages stimulated with LPS, no itaconate production occurs and exerts a more drastic impact on other metabolites due to their high toxicity. Therefore, mesaconate has an anti-inflammatory activity equal to non-derivatives of itaconate, and itaconate chemical derivatives (DMI and 4-OI) do not exert similar anti-inflammatory action as itaconate itself and are more cytotoxic.
The anti-inflammatory action of 4-OI also involves NRF2 activation, which is dispensable for the itaconate and mesaconate’s anti-inflammatory action [142, 143]. 4-OI alkylates cysteine residues 151, 257, 288, 273, and 297 on Kelch-like ECH-associated protein 1 (KEAP1, a central player in antioxidant response) to enable NRF2 to promote the expression of downstream genes with antioxidant and anti-inflammatory functions [143, 144]. DMI and 4-OI pre- and posttreatment also inhibit NLRP3 inflammasome-mediated IL-1β production in macrophages, not shown by itaconate and mesaconate treatment [142]. Thus, itaconate and mesaconate inhibit IL-1β secretion but not pro-IL-1β formation [145]. DMI also inhibits NLRC4 inflammasome-dependent IL-1β production. Furthermore, LPS stimulation or TLR4 activation is dispensable for mesaconate production from the intracellular itaconate, indicating the independence of this metabolic pathway on a previous macrophage activation [142]. Along with inhibiting glycolysis, itaconate also inhibits the TCA cycle, but mesaconate only inhibits glycolysis.
The detailed analysis of the impacts of itaconate and mesaconate activity on TLR4-stimulated macrophages indicates their immunomodulatory action rather than a simple anti-inflammatory action [142, 145]. For example, itaconate and mesaconate treatment decreases the expression of most chemokines and various cytokines by decreasing the antigen (Ag) presentation potential and T-cell activation [142]. Mesaconate and itaconate increase CXCL10, IFN-β1, IL-23A, and IL-17RA expression, and both molecules exert immunomodulatory action in the murine endotoxemia model and increase their survival upon pretreatment. Thus, itaconate, including 4-OI and mesaconate, can target TLR-mediated inflammatory diseases, including autoimmune ones, such as systemic lupus erythematosus (SLE), where TLR7 and 9 are critical for inflammation and inflammatory organ damage such as SLE-associated nephritis by targeting altered TLR signaling-mediated pro-inflammatory IR in immune cells such as macrophages and B cells [146–149]. Furthermore, itaconate can target T-cell IR due to increased glycolysis, lipid biosynthesis, oxidative stress, and mTOR signaling in autoimmune and inflammatory diseases, including SLE [150]. For example, itaconate targeted aberrant glycolysis and OXPHOS in Th17 and Tregs polarizing T cells and adoptive transfer of itaconate-treated Th17 polarizing T cells ameliorates EAE, an animal model for human MS [151].
NLRs in IR
The details of NLR family members in innate immunity, inflammation, and their role as cell death sensors have been discussed elsewhere [152–154]. Therefore, we are not discussing them here. NLRP3 is the most studied and prominent inflammasome generating IL-1β, IL-18, gasdermin-D, and inducing pyroptosis. TLR (TLR 2, 3, 4, and 7) signaling mediated by MyD88 adapter protein-dependent NF-κB activation primes NLRP3 inflammasome activation via generating pro-IL-1β and other non-transcriptional and posttranslational modification mechanisms (Fig. 1) [155–158]. TLR signaling-induced glycolysis stabilizes HIF-1α and induces IL-1β and IL-6 release; accordingly, IR to glycolysis further stimulates NLRP3 inflammasome-dependent release of mature IL-1β for inflammation (Fig. 1). For example, TLR signaling-induced glycolysis involves HK 1 and 2 overexpression and dissociation from the outer mitochondrial membrane (Fig. 1) [159, 160]. The dissociation of HK2 from the voltage-dependent anion channel (VDAC) of the outer mitochondrial membrane activates inositol triphosphate (IP3) receptors to release calcium (Ca2+) from the ER (Fig. 1) [160]. Mitochondria uptake this cytosolic Ca2+ for VDAC oligomerization, forming macromolecule-sized pores in the outer mitochondrial membrane to release proteins and mitochondrial DNA [160]. Furthermore, the VDAC oligomers aggregate with NLRP3 during its initial assembly to form the NLRP3 inflammasome complex (Fig. 1).
During Gram-positive bacterial infection, HK serves as an innate immune receptor and recognizes N-acetylglucosamine released from the phagocytosed cytosolic peptidoglycan (PGN) as a PAMP to activate NLRP3 inflammasome, independently of potassium (K+) efflux [159, 161]. Thus, TLR signaling-induced IR (glycolysis induction and upregulation) is critical for NLRP3 inflammasome complex formation and activation (Fig. 1). Hence, NLRP3 inflammasome can sense altered glycolytic flux depending on the enzyme and glycolytic step [162]. Furthermore, the pro-inflammatory TLR signaling-induced ROS generation can also activate NLRP3 inflammasome (Fig. 1) [163]. Notably, the priming of immune cells such as macrophages with LPS induces c-Jun N- terminal protein kinase 1 (JNK1), which phosphorylates NLRP3 at S194, facilitating the self-association of NLRP3 to form NLRP3 oligomer [164]. However, another study indicates that LPS priming is dispensable in human monocytes but not in monocyte-derived macrophages for NLRP3 inflammasome activation in vitro [165].
The LPS-primed and ATP-stimulated macrophages activate PKM2 enzymatic activity that modulates eukaryotic translation initiation factor 2 alpha kinase 2 (EIF2AK2) phosphorylation to promote glycolysis for NLRP3 and AIM-2 inflammasomes dependent IL-1β, IL-18, and high mobility group box 1 protein (HMG-B1) release and pyroptosis [166, 167]. Furthermore, hyperglycemia might also increase PKM2 activity in macrophages to increase NLRP3 inflammasome activity to enhance plaque vulnerability in patients with diabetes mellitus and chronic heart disease [168]. NLRP3 inflammasome activation-induced IL-1β further increases glycolysis by increasing the glycolytic enzyme PFKFB3 as NLRP3- and IL-1R-deficient mice exhibit decreased glycolysis [169]. Thus, TLR-MyD88 signaling-dependent glycolysis is further increased by the NLRP3 inflammasome-dependent IL-1β through IL-1R signaling [170]. Hence, induction of glycolytic IR upon TLR, NLRP3, and cGAS/STING signaling activation supports each other to aggravate inflammation through the mechanisms discussed (Fig. 1; Table 1).
Furthermore, glycolysis inhibitors itaconate and 4-OI inhibit NLRP3-dependent IL-1β secretion in monocytes isolated from cryopyrin-associated periodic syndrome patients and decrease the inflammation in urate-induced murine peritonitis by inhibiting the NLRP3- and NIMA-related kinase 7 (NEK7, a shortest NEK protein among NEK family members) [171]. NEK7 is a serine-threonine kinase, a critical ROS, and K+-sensing (stimulus for NLRP3 inflammasome formation). It interacts directly with the curved leucine-rich repeat (LRR) domains of the NLRP3 for activating the canonical NLRP3 inflammasome signaling [172–176]. Furthermore, ROS and K+ efflux trigger chloride intracellular channel (CLIC) proteins CLIC1 and CLIC4. For example, mtROS induces CLIC translocation to the plasma membrane to induce chloride (Cl−) efflux, which drives NEK7 and NLRP3 inflammasome activation for IL-1β production [177, 178]. However, human monocytes use an alternative NLRP3 inflammasome activation pathway upon LPS stimulation without involving K+ efflux to release IL-1β [179]. Therefore, NEK7 activity is dispensable in LPS-primed human macrophages, where IKKβ activation recruits NLRP3 to phosphatidylinositol-4-phosphate (PIP4, a phospholipid enriched on the trans-Golgi network) [180].
The itaconate and 4-OI do not inhibit AIM-2 and NLRC4 inflammasome activation. However, prolonged priming of macrophages with LPS establishes tolerance to late NLRP3 inflammasome activation as itaconate acts synergistically with iNOS and prevents full caspase 1 (CASP1) activation and GSDMD processing through posttranslational modification [181]. This uncontrolled IR in inflammatory macrophages induces tolerance to the NLRP3 inflammasome activation, which may cause pyroptosis and aggravate inflammatory tissue damage. Dimethyl itaconate (DMI), 4-OI, dimethyl fumarate (DMF, an approved treatment for multiple sclerosis or MS), and Monomethyl fumarate (MMF) inhibit NLRP3 inflammasome activation in response to lysophosphatidylcholine (LPC), which activates TLR4 and TLR2/TLR1 signaling that via pro-inflammatory IR controls NLRP3 inflammasome priming and activation [182, 183]. However, it is noteworthy that in the presence of classic TLR activators, such as LPS, LPC suppresses some TLR-mediated intracellular pro-inflammatory events, including NF-κB translocation, iNOS expression, and NO. synthesis. In murine macrophages, it activates p38MAP kinase and JNK but blocks ERK activation, which is not seen in TLR-transfected human embryonic kidney (HEK)-293A cells. Thus, LPC alone serves as an inflammogen by activating TLR signaling and dependent NLRP3 inflammasome activation. However, in the presence of other TLR stimulatory agents, it counteracts some pro-inflammatory events downstream of TLR signaling. Hence, it will be interesting to investigate the altered IR in the presence of LPC and other TLR agonists with the potential to activate NLRP3 inflammasome.
DMF breaks into MMF, a ligand for a niacin receptor 1 (Niacr1) gene-encoded G protein-coupled receptor called GPR109a or hydroxycarboxylic acid receptor 2 (HCAR2). The MMF recognition by GPR109a in the lysosomes of immune cells inhibits NLRP3 inflammasome-dependent IL-1β production along with L-6, IL-12, and TNF-α [184]. Furthermore, GPR109a activation in macrophages and DCs induces anti-inflammatory molecules, such as IL-10 and aldehyde dehydrogenase 1 family member 1A (ALDH1A) expression [185]. Meanwhile, macrophages and DCs lacking GPR109a or Niacr1 overexpress pro-inflammatory IL-6. Butyrate, a short-chain fatty acid (SCFA) produced by gut microbiota, activates GPR109a in macrophages and DCs to exert anti-inflammatory action [185]. DMF and endogenous fumarate also inhibit NLRC4 and AIM-2 inflammasome activation along with NLRP3 inflammasome-induced cell death (pyroptosis) and lactate dehydrogenase (LDH) release by interacting with the cysteine residues of gasdermin-D (GSDMD), forming S-(2-succinyl)-cysteine, which prevents its interaction with caspases, limiting its processing, oligomerization, and capability to induce pyroptotic cell death [186]. Disulfiram (a USFDA-approved drug for alcoholism) also inhibits pyroptosis by covalently modifying human/mouse Cys191/Cys192 in GSDMD, which prevents pore formation to prevent the IL-1β release and death in mice with endotoxemia [187].
Butyrate inhibits NF-κB and NLRP3 inflammasome expression, activation, and function in innate immune cells, such as endothelial cells, macrophages, and adipocytes, during sterile inflammatory conditions, such as colitis-induced colon cancer [188–191]. However, butyrate potentiates the NLRP3 inflammasome activation and the production of antimicrobial proteins (AMPs, calprotectin) during bacterial infection with Escherichia coli, Enterococcus fecalis, Streptococcus gordonii, Staphylococcus aureus, and Bacillus subtilis to restrict their growth [192–194]. It is important to note that butyrate is not critical for increased antimicrobial action of macrophages at the time of infection. The butyrate treatment increases the antimicrobial action of macrophages by increasing the AMP production without affecting phagocytosis, inflammatory cytokine (IL-1β and TNF-α) production, and apoptosis [193].
Butyrate increases the antimicrobial function of macrophages by inhibiting histone deacetylase (HDAC) activity, specifically HDAC3 activity that occurs upstream of metabolic changes and antimicrobial response. Notably, butyrate-induced elevated antimicrobial action of macrophages is independent of GPR109a activation [193]. Butyrate treatment decreases the IL-10 production from macrophages in the presence of bacterial pathogens. The butyrate-treated lamina propria macrophages showed a decreased glycolysis, glycolytic capacity, and glycolytic reserve, as indicated by the decreased extracellular acidification rate due to reduced glucose concentration [193]. However, butyrate treatment did not alter mitochondrial OXPHOS in macrophages compared to controlled macrophages, but it increased their adenosine monophosphate (AMP) level. The elevated AMP induces AMPK (affected by AMP/ATP ratio) overexpression, which phosphorylates tuberous sclerosis complex 2 (TSC2, a tumor suppressor protein) [195, 196]. The phosphorylated TSC2 inhibits S6K and 4EBP1 phosphorylation, which inhibits the mechanistic target of rapamycin (mTOR, a master regulator of autophagy and glycolysis) activity [195–198]. mTOR is an energy-sensing pathway downstream of TSC2 [195]. Hence, NLRP3 inflammasome-dependent pro- and anti-inflammatory actions of butyrate vary with the nature (sterile and infectious) of inflammatory disease and need further investigation.
The increase in FAS upon TLR activation also increases NLRP3 inflammasome activation (Fig. 1; Table 1). For example, mice with reduced FAS exhibit a decreased NLRP3-mediated CASP1 activation. FA synthase (FASN), a key enzyme involved in FAS that catalyzes the palmitic acid and FASN or palmitic acid synthesis inhibition, blocks NLRP3 activation and the IL-1β and IL-18 production [199]. Thus, FASN-mediated FAS and NLRP3 palmitoylation are critical for NLRP3 inflammasome activation. Furthermore, IL-1β via IL-1Ra increases FAS to support the pro-inflammatory environment [200]. Thus, NLRP3 activation supports glycolysis and FAS to support pro-inflammatory IR in immune cells such as macrophages (Fig. 1; Table 1). Therefore, strategies to inhibit IL-6 and NLRP3 (both support glycolysis) in several acute inflammatory conditions, such as coronavirus disease 2019 (COVID-19), are emerging [201].
GB111-NH2 inhibits GAPDH and α enolase (glycolytic enzymes) and impairs NADH (decreased production) and mtROS production to induce NLRP3 activation-mediated IL-1β secretion and pyroptosis [202]. The succinate and pyruvate treatment inhibit GB111-NH2-induced NLRP3 activation. However, pyruvate supplementation did not affect ATP and nigericin-induced NLRP3 inflammasome activation [202]. Koningic acid (KA inhibits GAPDH in glycolysis) and ENOblock (EB inhibits α enolase in glycolysis) also inhibit NLRP3 inflammasome activation. Thus, immunometabolites, including succinate, pyruvate, and KA may have immunomodulatory action depending on the local tissue environment, PRR, and immune cells primarily involved in the inflammatory process. We need further studies in this direction.
cGAS-STING Signaling or cGLRs in Immune Cells and IR
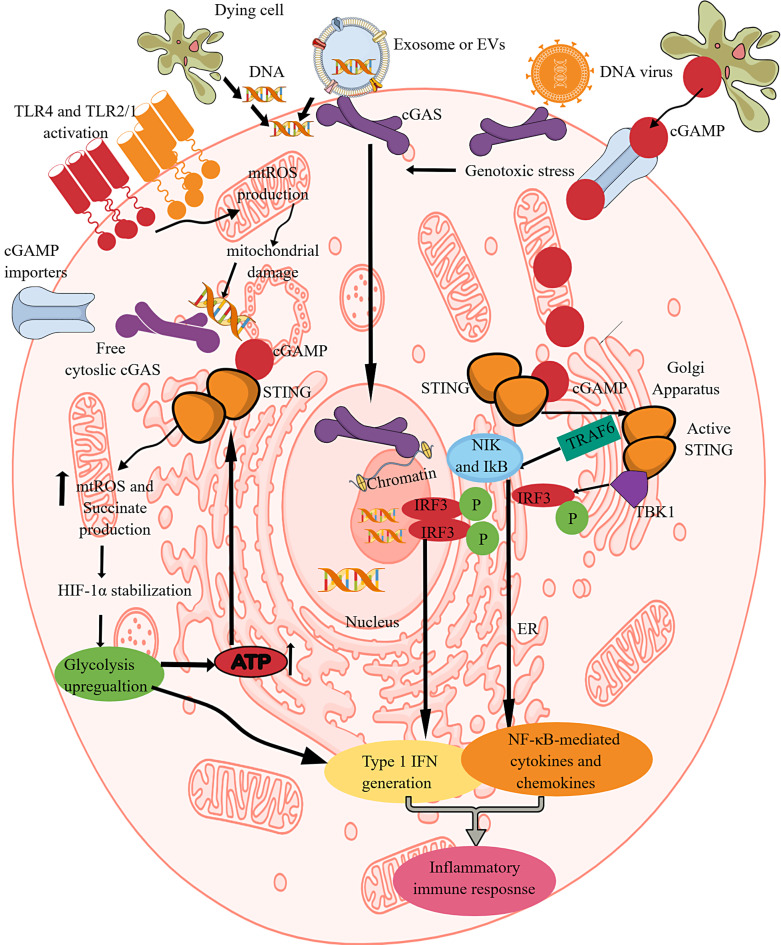
cGAS is a cytosolic PRR for double-stranded DNA (dsDNA) in the cytosol, which can be host and pathogen-derived. We and others have discussed the cGAS-STING signaling in detail elsewhere [203–207]. Briefly, cGAS catalyzes cytosolic dsDNA into cGAMP, which activates STING (an adapter molecule) (Fig. 2). The activated STING phosphorylates TBK1, which initiates NF-κB and interferon regulatory factor 3 (IRF3)-dependent downstream signaling to produce pro-inflammatory cytokines and type 1 IFNs (Fig. 2). Therefore, it is critical to discuss the impact of IR on cGAS-STING or cGLR signaling and vice versa. STING regulates glycolysis through HIF-1α stabilization by increasing the mtROS and succinate production that shifts macrophage OXPHOS towards increased aerobic glycolysis for their pro-inflammatory phenotype and function (enhanced NO. production by upregulating iNOS or NOS2 expression, inflammasome activation, and IL-1β release) (Table 1; Fig. 2) [208]. On the other hand, in tumor immune microenvironment, aerobic glycolysis in activated DCs drives STING signaling to facilitate their antitumor action [209]. Mechanistically, glycolysis-mediated ATP overproduction increases STING signaling that stabilizes HIF-1α, supporting glycolysis to exert protective pro-inflammatory action to clear cancer cells (Fig. 2). Thus, STING activation promotes glycolysis in macrophages and DCs as an anti-infection and anticancer defense (Table 1). The liver X receptor (LXR, a member of the nuclear hormone receptor family) activation in macrophages inhibits pro-inflammatory immune response through different mechanisms, such as inhibition of osteopontin, iNOS, cyclo-oxygenase-II, and IL-6 activity and production upon LPS-mediated TLR4, TNF-α, IL-1β, and IFN-γ activation [210–215]. The synthesis and activation of ADP-ribosylation factor-like 7 (ARL7) upon LXR activation have also been recognized as a metabolic and anti-inflammatory target [216]. However, LXR activation-mediated lipid metabolism in macrophages suppresses cGAS-STING activation by inducing the sphingomyelin phosphodiesterase acid-like 3A (SMPDL3A) expression, which degrades cGAMP and restricts STING activation and suppresses cGAS/STING signaling-dependent pro-inflammatory immune response [217]. Hence, we critically need further studies in this direction.
Fig. 2.
cGAS/STING (cGLR) signaling-dependent IR. cGLRs or cGAS/STING signaling is critical for recognizing the cytosolic dsDNA and generating type 1 IFNs and NF-κB-dependent cytokines. cGAS-mediated cytosolic dsDNA recognition by cGAS generates cGAMP. STING recognizes cGAMP and undergoes dimerization to become active. The activated STING activates TBK1 and TRAF6, which activate IRF3 and NF-κB-dependent type 1 IFNs and cytokines. This process also activates glycolysis by increasing mtROS production, succinate accumulation, and HIF-α stabilization. The increased glycolysis overproduces ATP molecules, which further increases STING activation. Furthermore, TLR activation induced mtROS production and mitochondrial damage, releasing the mitochondrial DNA into the cytosol that the cGAS recognizes to initiate the cGAS/STING signaling. Hence, TLR and cGAS/STING signaling support each other through IR or glycolysis.
The cytosolic dsDNA activates cGLRs (cGAS/STING signaling pathway), and exploring the impact of pro-inflammatory TLR signaling-induced IR on cGAS/STING signaling activation needs an investigation. For example, during polymicrobial sepsis, activated TLR1/TLR2 signaling increases intracellular hydrogen peroxide (H2O2) and mtROS production in leukocytes (Fig. 3) [218]. TLR4 activation induces ROS-mediated mitochondrial oxidative stress (Fig. 3) [219]. It is important to note that TLR3 and TLR9 activation do not result in ROS production. The mtROS-induced damaged mitochondrial DNA can progressively be released into the cytosol for activating the pro-inflammatory cGAS/STING signaling pathway (Fig. 3). TLR activation induces glycolysis that supports HIF-1α stabilization for releasing pro-inflammatory cytokines (IL-1β, IL-6, and IL-12). The cytosolic dsDNA-induced STING activation further stabilizes HIF-1α for pro-inflammatory phenotype and function of immune cells. STING activation induces macrophage itaconate production (Table 1) [220]. Furthermore, IL-6 supports aerobic glycolysis by supporting HK2 and 6-phoshofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB3) overexpression by activating STAT3 in different inflammatory conditions varying from infections to cancers [221–223]. Hence, IL-6 inhibition exerts its anti-inflammatory effect by targeting pro-inflammatory IR. We need further investigations to explore cGLRs-dependent IR and the impact of other PRR-signaling-induced IR of cGLR activity.
Fig. 3.
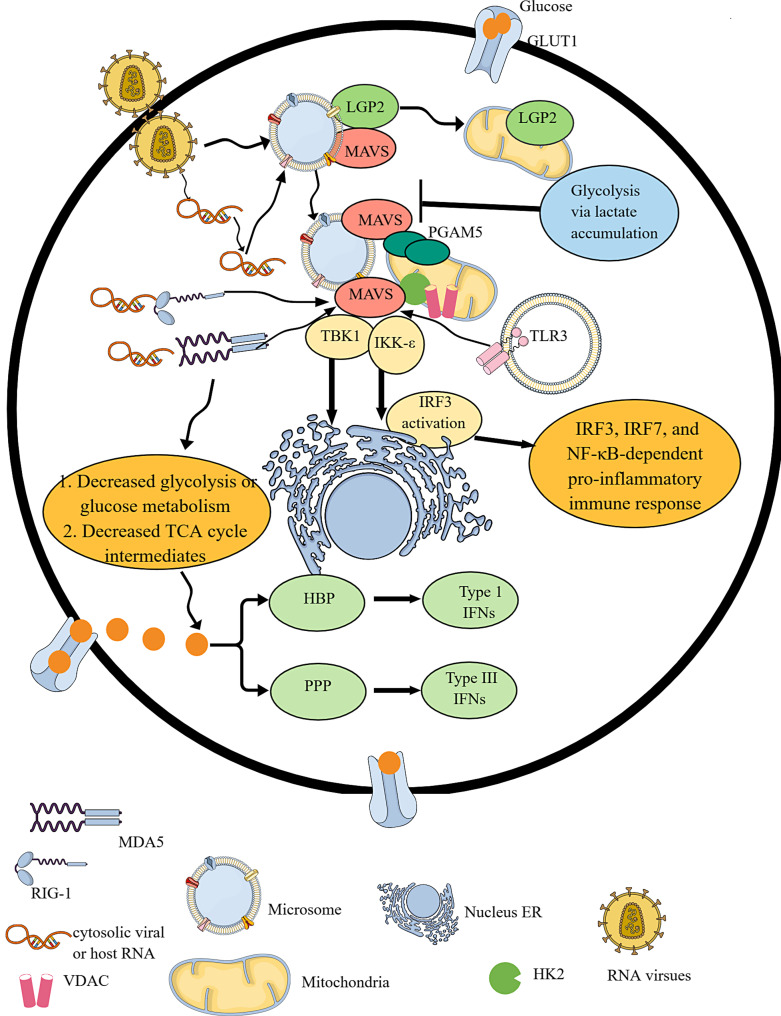
RLR signaling activation-mediated IR. RLR signaling activation involves the recognition of cytosolic RNA via RIG-1 and MDA5. During homeostasis, LGP2 is bound to the MAVS in the microsome. LGP2 moves to mitochondria upon viral infection, leaving MAVS free in the microsome. Thus, upon recognizing cytosolic RNA, RIG-1 and MDA5 interact with MAVS, which directly interacts with the oligomerized mitochondrial PGAM5. The RIG-1 and MDA5 interaction with MAVS interacting with oligomerized PGAM5 is critical for the downstream TBK1 and IRF3 phosphorylation-mediated type 1 IFN release. IRF3 activation occurs at the ER. The RIG-1 and MDA5 activation suppress glycolysis and the TCA cycle. Instead of glycolysis, cellular glucose undergoes PPP and HBP to generate type III and IFNs. Furthermore, glycolysis via increased lactate accumulation suppresses MAVS activity through binding to its TM domains. TLR3 activation also activates MAVS.
RLR Signaling Pathway in IR
RLRs are cytosolic PRRs for viral infections and recognize viral RNAs as PAMPs (Fig. 3). However, they can also sense host-derived RNAs as DAMPs and viral (herpes simplex virus 1, Epstein-Barr virus, vaccinia, and adenovirus) dsDNA as PAMPs to initiate type 1 IFN-dependent pro-inflammatory immune response [224]. The nuclear-resident RIG-1 also senses viral replication and induces antiviral immunity, which involves canonical or cytosolic RLR signaling as a signal to sense IAV replication in the nucleus for generating a cooperative induction of type 1 IFNs [225]. However, nuclear RIG-1 signaling remains inactive upon infection with cytoplasmic-replicating Sendai virus but signals upon nucleus-derived viral agonists, including pregenomic RNA of hepatitis B virus. RLR protein family has three known members: (1) RIG-1, also known as DDX58, (2) melanoma differentiation-associated protein 5 or MDA5 or IFIH1, and (3) laboratory for genetics and physiology 2 or LGP2. LGP2 is a positive regulator of RIG-1 and MDA5-dependent antiviral immune response and synergizes MDA5 in RLR-dependent antiviral immunity [226–228]. For example, LGP2 interacts with mitochondrial antiviral protein signaling (MAVS, which is anchored into mitochondria, mitochondria-associated endoplasmic reticulum membranes (MAMs), and peroxisomes via its transmembrane (TM) domain) in microsomes and blocks RIG-1/MAVS interaction in the resting stage, indicating that microsomes harbor RLR family members but not mitochondria (Fig. 3) [229]. The virus infection or cytosolic RNA induces LGP2 localization to mitochondria from the microsome and leaves MAVS free, which correlates well with IRF3 activation (Fig. 3). The viral or host-derived RNA recognition by RIG-1 and MDA5 (RLRs) activates the adapter protein MAVS via caspase activation and recruitment domains that oligomerize upon recognition of viral and host RNA [224, 230].
MAVS is also known by other names, such as caspase activation recruitment domain adapter-inducing IFN-β (CARDIF), interferon-beta promoter stimulator 1, and virus-induced signal adapter [231–234]. Virus-induced signal adapter or MAVS is involved in TLR3 and RLR-dependent antiviral immune response via interacting with TRIF and TRAF6 (Fig. 3) [233]. Subsequently, MAVS interacts and activates TBK1 and IKK-ε, which are critical components of the IRF3 and IRF7 signaling pathway to generate type 1 IFNs and NF-κB-dependent pro-inflammatory immune response (Fig. 3) [235, 236]. IRF3 activation occurs on the endoplasmic reticulum (ER)-derived membranes but not on the mitochondria (Fig. 3) [229]. Hence, ER-derived membranes are key RLR signaling platforms.
The LGP2-MAVS complex in the microsome negatively regulates RIG-1 activation during immune homeostasis that translocates to the mitochondria to release MAVS for facilitating antiviral RLR signaling-dependent immune response (Fig. 3). Upon dsRNA, poly(I: C) challenge, mitochondrial protein phosphoglycerate mutase family member 5 (PGAM5) overexpression, and oligomerization take place along with their direct interaction with MAVS, which is critical for downstream TBK1 and IRF3 phosphorylation-mediated type 1IFN production (Fig. 3) [237]. Furthermore, PGAM5-deficient cells are defective in clearing vesicular stomatitis virus (VSV) infection and type 1 IFN production. On the other hand, upon LPS stimulation, mitochondrial PGAM5 in macrophages dephosphorylate dynamin-related protein 1 (Drp1) to generate mtROS and promote pro-inflammatory M1 macrophage phenotype exhibiting glycolysis, generating pro-inflammatory cytokines and molecules downstream to NF-κB and MAPK pathways [238]. Thus, stimulus or PRR type determines the downstream immunoregulatory functioning of the PGAM5, including glycolysis support or MAVS-dependent type 1 IFN release. Details of RLR signaling in the infection and immunity have been discussed elsewhere [224, 239–242]. The following sections discuss RLR signaling-induced IR and the impact of IR induced by other PRRs on RLR signaling.
RIG-1 activation induces MAVS activation, which hijacks HK binding to MAVS to impair HK mitochondrial localization and activation [243]. The RLR activation decreases most metabolic intermediates downstream of glucose metabolism, such as phosphoenolpyruvate (PEP), pyruvate, and lactate levels, at the initial stages of type 1 IFN production (Fig. 3; Table 1). At this initial step, TCA intermediates, including succinate, fumarate, aconitate, and malic acid (malate), decrease due to reduced pyruvate levels without affecting OAA levels [243]. Thus, RLR signaling impairs glucose metabolism (Fig. 3; Table 1). Furthermore, 2-DG (inhibits HK to block glycolysis) increases RLR signaling-dependent type 1 IFN and IL-6 release. Hence, decreased glycolysis promotes RLR signaling-dependent antiviral or pro-inflammatory immune response due to decreased HK activity at the early stages of RLR signaling [243].
The HK2 (which stays bound to MAVS on the mitochondria) activity gets hijacked upon RLR signaling activation-mediated MAVS-RIG-1 recognition, causing an impaired HK2 localization in the mitochondria and its activation. The HK2 interacts with MAVS through VDAC1 (which is involved in glycolysis regulation) in the mitochondria [159, 243, 244]. MAVS in the mitochondria recruits NLRP3 (resting NLRP3 co-localizes ER membranes) there and facilitates its oligomerization for CASP1-dependent IL-1β production and NLRP3 inflammasome-dependent pro-inflammatory activities during glycolysis that further supports glycolysis [245, 246]. Furthermore, VDAC dysregulation or inhibition suppresses mitochondrial activity that inhibits ROS generation and NLRP3 inflammasome activation [247]. Thus, glycolysis may also inhibit RLR signaling due to the involvement of MAVS in the NLRP3 recruitment to mitochondria for NLRP3 inflammasome activation and dependent pro-inflammatory immune response. For example, cytosolic dsRNA activates NLRP3 inflammasome-dependent IL-1β production through mitochondrial MAVS that triggers membrane permeabilization and potassium (K+) efflux, which occurs independently of TLR3 and RLR (RIG-1 and MDA5) signaling [248]. Furthermore, hypoxia induces MAVS, NLRP3, and CASP1 overexpression, and increased production of IL-1β, IL-6, and IL-18 further supports that MAVS is critical for NLRP3 inflammasome during hypoxia and glycolysis [249]. NLRX1 overexpression inhibits MAVS-dependent NLRP3 inflammasome activation during hypoxia to prevent pro-inflammatory damage.
Anaerobic glycolysis via lactate production is a negative signal to repress RLR signaling-mediated MAVS activation and type 1 IFN and other NF-κB-dependent pro-inflammatory cytokines production (Fig. 3) [243]. Cells lacking PDH upon RLR activation show a robust decrease in TBK1 and IRF3 phosphorylation and MAVS aggregation due to lactate overproduction and accumulation. On the other hand, LDH decreasing lactate production and accumulation enhances TBK1 and IRF3 phosphorylation along with increased type 1 IFN production upon RLR stimulation. Hence, cellular lactate accumulation inhibits anaerobic glycolysis during RLR signaling in vitro and in vivo [243]. The accumulated lactate directly binds MAVS TM domains and inhibits its mitochondrial localization, association with RIG-1, and aggregation required for downstream TBK1 and IRF3 phosphorylation and activation to produce type I IFNs (Fig. 3).
The activated MAVS shifts glycolysis to the PPP or PPS and hexosamine biosynthesis pathway during RLR signaling (Fig. 3; Table 1) [250]. The activated MAVS associates with glucose-6-phosphate dehydrogenase (G6PD) and TRAF6 on peroxisomes during viral infections, activating RLR signaling to initiate PPP and type III IFN (IFN-λ) production. The MAVS association with glutamine-fructose-6-phosphate transaminase 2 (GFPT2, a rate-limiting enzyme of the HBP and generates fructose-6-phosphate (F6P) to the HBP end-product uridine diphosphate N-acetylglucosamine [UDP-GlcNAc]), TRAF2, and TRAF6 on MAMs activates HBP metabolism and type 1 IFN production during viral infections [250, 251]. Thus, VSV infection or poly (I:C) treatment increases glucose flux to PPP and HBP instead of glycolysis to generate RLR signaling-dependent type 1 and III IFNs (Fig. 3). Interestingly, peroxisome-located MAVS guides glucose flux to PPP for generating type III IFNs, and MAMs-associated MAVS supports glucose flux to HBP for type 1 IFN production [250]. The HBP-mediated O-linked β-N-acetylglucosamine (O-GlcNAc) signaling promotes antiviral RLR signaling by O-GlcNAcylation of MAVS on serine 366 for K63-linked ubiquitination of MAVS and subsequent activation of downstream TBK1 and IRF3 axis to generate type 1 IFNs [252, 253]. The HBP-mediated O-GlcNAc transferase (OGT), a key enzyme for protein O-GlcNAcylation also induces O-GlcNAcylation of the receptor-interacting protein kinase 3 (RIPK3, a serine-threonine kinase) on threonine 467 (T467) to prevent RIPK3-RIPK1 hetero- and RIPK3-RIPK3 homo-interaction to inhibit downstream innate immune response and necroptosis [254]. RIPK3 via CASP8 activation induces NLRP3-CASP1 inflammasome-dependent inflammatory signaling and pyroptosis [255, 256]. Thus, RLR signaling-induced HBP may suppress NLRP3 inflammasome activation and necroptosis to mount an effective type 1 IFN-dependent immune response. D-glucosamine, a dietary supplement, protects against lethal viral infections induced by the human influenza virus, coxsackievirus, VSV, and SARS-CoV-2 in mice through increasing MAVS O-GlcNAcylation [253, 257]. The MAVS O-GlcNAcylation downstream of the RLR signaling pathway may inhibit NLRP3 inflammasome activation to prevent cell death for effective antiviral immunity, which needs further investigation.
Furthermore, GLUT4 translocation to the plasma membrane from its intracellular compartment also inhibits RLR signaling by sequestering RLRs into the plasma membrane in muscle cells [258]. Insulin treatment and viral infections alter RLR activation by promoting the GLUT4 and RLR translocation to the plasma membrane [258]. UBXN9 (a ubiquitin-domain-containing protein) regulates GLUT4 translocation to the plasma membrane, and its disruption supports GLUT4 translocation to the plasma membrane from its intracellular compartment. However, in muscle cells, RLR signaling inhibition upon insulin treatment occurs independently of glycolysis but is dictated by GLUT4 translocation to the plasma membrane. UBXN proteins (UBXN1, UBXN9, and UBXN11) inhibit retrovirus and lentivirus production by regulating RLR signaling and canonical NF-κB signaling by stabilizing inhibitory κBα (IκBα) [259].
Furthermore, phorbol 12-myristate 13-acetate (PMA) and LPS stimulation of white blood cells or immune cells, such as monocytes/macrophages, B cells, and T cells, upregulate GLUT1, GLUT3, and GLUT4 expression that enhances glucose uptake to support IR for glycolysis from OXPHOS, which further increases in the presence of insulin [260]. However, in neutrophils, only GLUT1 and GLUT3 overexpression on the plasma membrane occurs upon LPS stimulation. Thus, activation of other PRRs (TLRs, NLRs, and cGLRs) supporting glycolysis may inhibit RLR signaling, and we need further studies in this direction. For example, succinate accumulation during TLR and NLR activation also inhibits RLR signaling by suppressing the MAVS aggregation required to activate the downstream MAVS-TBK1-IRF3 to generate type 1 IFNs CXCL10, and INF stimulated gene 15 (ISG15) during VSV infection [261]. This finding further supports the idea that immune metabolites (lactate and succinate) supporting glycolytic IR suppress RLR signaling-dependent immunity.
Adipocytes also regulate inflammation and immunity through different PRRs and release several adipokines and cytokines [262]. They also express RLRs, and GLUT4 overexpression in hyperplastic adipocytes can impair their RLR signaling-mediated antiviral immune response [263, 264]. Thus, obese people may exert a lower RLR-mediated antiviral and inflammatory immune response. For example, dysregulated RLR signaling during obesity abolishes ER stress-induced Type 1 IFN generation to promote obesity and insulin resistance [265]. Thus, we need further explorations in IR downstream to RLR signaling for understanding immune response under diverse conditions.
Future Perspective and Conclusion
PRRs are critical for the maintenance of immune homeostasis. Their activation plays a significant role in the recognition and clearance of potential MAMPs, PAMPs, and DAMPs through generating pro-inflammatory immune responses. However, IR governs pro- and anti-inflammatory stages or functions of immune cells. For example, transition from OXPHOS to glycolysis and FAO to FAS critically determines the pro-inflammatory functions of innate immune cells such as macrophages, neutrophils, DCs, and NK cells upon the activation of TLRs, NLRP3 inflammasomes, CLRs (recognizing fungal (Candida albicans) infection) and cGAS/STING (cGLR) signaling pathway to initiate the pro-inflammatory immune response (Table 1) [266]. Furthermore, molecules or cytokines (IL-1β) released through their corresponding receptors further support glycolysis in these immune cells for the continued pro-inflammatory response. For example, TNF-α also supports glycolysis through upregulating HK2 [267].
Furthermore, TNF-α treatment may support antiviral immune response by supporting PPP and HBP that work upon activation of RLRs to synthesize type 1 and III IFNs. The pro-inflammatory cytokine IL-6 also supports glycolysis [223]. Thus, targeting glycolysis through immunometabolic approaches, for example, 2-DG, can be effective downstream of TLR, NLRP3, ALRs, and cGLRs, which support inflammation through glycolysis. However, this strategy fails during RLR signaling pathway activation, which does not support glycolysis.
The lactate accumulation due to glycolysis during hypoxia seen in various inflammatory conditions, including cancers and infections, induces histone lactylation at lysine, which has different temporal dynamics from lactylation [268]. For example, histone lactylation in M1 macrophages induces M2-like genes (arginase 1 or ARG1) in M1 macrophages that are critical for inflammation resolution and wound healing but also support immunosuppressive TIME. P300 is a potential histone lactylation writer protein under hypoxic conditions supporting glycolysis [268]. Thus, lactate accumulation is critical to protect the tissue from aggravated injury during acute inflammation and supports chronic inflammatory conditions such as cancers. Thus, the PRR activation intensity and chronicity further reshape the IR to suppress or aggravate the inflammation to maintain immune homeostasis.
Furthermore, some inhibitory PRRs, which are anti-inflammatory in action and critical to maintaining immune homeostasis, exist [269]. Even TLR4 activation in the endosomes or phagosomes exerts anti-inflammatory action through TRAM and TRIF-dependent type 1 IFN generation and antagonizes the pro-inflammatory action of the cell membrane-bound TLR4 [270, 271]. Therefore, it will be interesting to investigate the impact of inhibitory PRRs on IR of PRR-regulated immunometabolism and to design novel strategies to target immunometabolism to maintain immune homeostasis.
HIF-1α stability is critical for glycolysis downstream to TLR and TCR signaling in CD8+T cells for their effector function. However, mitochondrial dysregulation under stressful conditions such as chronic infections and cancers prevents the HIF-α degradation through the ubiquitin-proteasome system (UPS), causing enhanced glycolysis precursor T-cell population (Tpex) to generate exhausted T cells [272–274]. Therefore, metabolic engineering of CAR T cells is a novel approach for enhanced stemness and functionality of Tpex cells for cancer immunotherapy. Furthermore, these metabolically engineered CAR T cells with lower glycolysis may have enhanced RLR signaling through LGP2 controls survival and fitness of antigen-specific CD8+T cells during peripheral T-cell expansion [275]. RLR signaling also determines the quality of polyfunctional T-cell response [276]. Hence, metabolically engineered CAR T cells or CAR macrophages with intact RLR signaling may prove better immunotherapeutic approaches for cancer with lower side effects. For example, patients undergoing CAR T-cell therapy are highly immunosuppressive and develop severe bacterial and viral (IAV) infections [277, 278]. RLR activation in mice attenuates TLR-mediated Th1 cell and Th17 immune response, inducing death at sublethal bacterial infection by suppressing the transcription of the gene encoding the p40 subunit of interleukin 12 (IL-12b) [279]. It is well known that RLR signaling (PPP and HBP) and TLR signaling (glycolysis) induce different IR to generate corresponding immune responses. Therefore, it is critical to understand detailed IR downstream to different PRRs for designing better immunotherapeutic approaches for infectious and inflammatory diseases, including autoimmunity and cancers. For example, along with mitochondria, lysosomes and peroxisomes coordinate cellular metabolic processes and expression of PRRs such as TLRs (TLR2, TLR4, and TLR6 upregulate along with cytosolic endosomal TLRs) and NLRP3 alters in these organelles upon microbial (bacteria and viruses, such as herpes simplex virus 1) stimuli in immune cells such as macrophages, indicating altered immunometabolism also dysregulates PRR expression [280–282].
Furthermore, different tissue macrophages differ in their characteristics; for example, large intestinal macrophages overexpress metabolic proteins than small intestinal macrophages, liver macrophages (Kupffer cells) highly express ACC1 and CPT1A than splenic macrophages, PAMs highly express CD36, but it is lower than that of colonic, splenic, liver, and peritoneal macrophages [121]. On the other hand, brain macrophages or microglia highly express GLUT1, indicating their higher reliance on glucose utilization due to their location in high glucose-consumption organs. Peritoneal macrophages highly express different pro-inflammatory metabolic markers (GLUT1, PKM, SDHA, G6PD, CPT1A, and ACC1) but have lower CD38 and CD98 expression than splenic and liver macrophages [121]. Therefore, timing, and PRR type activation critically depend on the immune cell type (macrophages, DCs, T cells, and B cells) and their tissue location. For example, macrophage-mediated efferocytosis depends on FAS; therefore, macrophages (peritoneal macrophages) overexpressing ACC1 at homeostasis will need lower doses of PRR-based immunometabolism modulator than macrophages (PAMs) with its lower expression. Due to higher metabolic instability or poor mitochondrial fitness in mature small intestinal macrophages (overexpressing PD-L1 marker, which is inversely associated with ACC1 expression), their PRR-based IR targeting needs caution [121]. The negative association between PD-L1 and ACC1 expression in intestinal macrophages indicates an association between PD-L1 and FAS in these macrophages. Therefore, these parameters are critical for future immunotherapeutics targeting immune cell-specific IR through PRRs for their success.
Conflict of Interest Statement
Authors declare no competing interest.
Funding Sources
Authors did not receive any funding for this work.
Author Contributions
Vijay Kumar conceived the idea, wrote the manuscript, and developed the figures. John H. Stewart IV has done the final editing.
Funding Statement
Authors did not receive any funding for this work.
References
- 1. Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016;15(8):551–67. [DOI] [PubMed] [Google Scholar]
- 2. Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Michels N, van Aart C, Morisse J, Mullee A, Huybrechts I. Chronic inflammation towards cancer incidence: a systematic review and meta-analysis of epidemiological studies. Crit Rev Oncol Hematol. 2021;157:103177. [DOI] [PubMed] [Google Scholar]
- 5. Pisetsky DS. Pathogenesis of autoimmune disease. Nat Rev Nephrol. 2023;19(8):509–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kumar V, Sharma A. Neutrophils: cinderella of innate immune system. Int Immunopharmacol. 2010;10(11):1325–34. [DOI] [PubMed] [Google Scholar]
- 7. Kumar V, Sharma A. Mast cells: emerging sentinel innate immune cells with diverse role in immunity. Mol Immunol. 2010;48(1–3):14–25. [DOI] [PubMed] [Google Scholar]
- 8. Kumar V. Innate lymphoid cells: new paradigm in immunology of inflammation. Immunol Lett. 2014;157(1–2):23–37. [DOI] [PubMed] [Google Scholar]
- 9. Kumar V, Ahmad A. Role of MAIT cells in the immunopathogenesis of inflammatory diseases: new players in old game. Int Rev Immunol. 2017;37(2):90–110. [DOI] [PubMed] [Google Scholar]
- 10. Man K, Kutyavin VI, Chawla A. Tissue immunometabolism: development, physiology, and pathobiology. Cell Metab. 2017;25(1):11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lercher A, Baazim H, Bergthaler A. Systemic immunometabolism: challenges and opportunities. Immunity. 2020;53(3):496–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Padmanabha D, Baker KD. Drosophila gains traction as a repurposed tool to investigate metabolism. Trends Endocrinol Metab. 2014;25(10):518–27. [DOI] [PubMed] [Google Scholar]
- 13. Kohlgruber AC, LaMarche NM, Lynch L. Adipose tissue at the nexus of systemic and cellular immunometabolism. Semin Immunol. 2016;28(5):431–40. [DOI] [PubMed] [Google Scholar]
- 14. Fung TC. The microbiota-immune axis as a central mediator of gut-brain communication. Neurobiol Dis. 2020;136:104714. [DOI] [PubMed] [Google Scholar]
- 15. Foster JA, Baker GB, Dursun SM. The relationship between the gut microbiome-immune system-brain Axis and major depressive disorder. Front Neurol. 2021;12:721126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang C-H, Curtis JD, Maggi LB Jr, Faubert B, Villarino AV, O’Sullivan D, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153(6):1239–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MA, Sheedy FJ, Gleeson LE, et al. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 2015;21(1):65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kumar V. Targeting macrophage immunometabolism: dawn in the darkness of sepsis. Int Immunopharmacol. 2018;58:173–85. [DOI] [PubMed] [Google Scholar]
- 19. Kumar V. Dendritic cells in sepsis: potential immunoregulatory cells with therapeutic potential. Mol Immunol. 2018;101:615–26. [DOI] [PubMed] [Google Scholar]
- 20. Kumar V. T cells and their immunometabolism: a novel way to understanding sepsis immunopathogenesis and future therapeutics. Eur J Cell Biol. 2018;97(6):379–92. [DOI] [PubMed] [Google Scholar]
- 21. Kumar V. Inflammation research sails through the sea of immunology to reach immunometabolism. Int Immunopharmacol. 2019;73:128–45. [DOI] [PubMed] [Google Scholar]
- 22. Kumar V. Natural killer cells in sepsis: underprivileged innate immune cells. Eur J Cell Biol. 2019;98(2–4):81–93. [DOI] [PubMed] [Google Scholar]
- 23. Kumar V. Immunometabolism: another road to sepsis and its therapeutic targeting. Inflammation. 2019;42(3):765–88. [DOI] [PubMed] [Google Scholar]
- 24. Kumar V, Stewart JH 4th. Immunometabolic reprogramming, another cancer hallmark. Front Immunol. 2023;14:1125874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388(6640):394–7. [DOI] [PubMed] [Google Scholar]
- 26. Vijay K. Toll-like receptors in immunity and inflammatory diseases: past, present, and future. Int Immunopharmacol. 2018;59:391–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kumar V. Toll-like receptors in the pathogenesis of neuroinflammation. J Neuroimmunol. 2019;332:16–30. [DOI] [PubMed] [Google Scholar]
- 28. Kumar V. Toll-like receptors in sepsis-associated cytokine storm and their endogenous negative regulators as future immunomodulatory targets. Int Immunopharmacol. 2020;89(Pt B):107087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kumar V, Barrett JE. Toll-like receptors (TLRs) in health and disease: an overview. Handb Exp Pharmacol. 2022;276:1–21. [DOI] [PubMed] [Google Scholar]
- 30. Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. [DOI] [PubMed] [Google Scholar]
- 31. Kawai T, Akira S. Signaling to NF-kappaB by toll-like receptors. Trends Mol Med. 2007;13(11):460–9. [DOI] [PubMed] [Google Scholar]
- 32. Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32(3):305–15. [DOI] [PubMed] [Google Scholar]
- 33. Fitzgerald KA, Kagan JC. Toll-like receptors and the control of immunity. Cell. 2020;180(6):1044–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kumar V. Going, Toll-like receptors in skin inflammation and inflammatory diseases. EXCLI J. 2021;20:52–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kawai T, Ikegawa M, Ori D, Akira S. Decoding Toll-like receptors: recent insights and perspectives in innate immunity. Immunity. 2024;57(4):649–73. [DOI] [PubMed] [Google Scholar]
- 36. Lauterbach MA, Hanke JE, Serefidou M, Mangan MSJ, Kolbe CC, Hess T, et al. Toll-like receptor signaling rewires macrophage metabolism and promotes histone acetylation via ATP-citrate lyase. Immunity. 2019;51(6):997–1011.e7. [DOI] [PubMed] [Google Scholar]
- 37. Balic JJ, Albargy H, Luu K, Kirby FJ, Jayasekara WSN, Mansell F, et al. STAT3 serine phosphorylation is required for TLR4 metabolic reprogramming and IL-1β expression. Nat Commun. 2020;11(1):3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gough DJ, Corlett A, Schlessinger K, Wegrzyn J, Larner AC, Levy DE. Mitochondrial STAT3 supports ras-dependent oncogenic transformation. Science. 2009;324(5935):1713–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gobelli D, Serrano-Lorenzo P, Esteban-Amo MJ, Serna J, Pérez-García MT, Orduña A, et al. The mitochondrial succinate dehydrogenase complex controls the STAT3-IL-10 pathway in inflammatory macrophages. iScience. 2023;26(8):107473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wen Z, Zhong Z, Darnell JE. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82(2):241–50. [DOI] [PubMed] [Google Scholar]
- 41. Hooftman A, Peace CG, Ryan DG, Day EA, Yang M, McGettrick AF, et al. Macrophage fumarate hydratase restrains mtRNA-mediated interferon production. Nature. 2023;615(7952):490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Everts B, Amiel E, Huang SC-C, Smith AM, Chang C-H, Lam WY, et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKɛ supports the anabolic demands of dendritic cell activation. Nat Immunol. 2014;15(4):323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Freemerman AJ, Johnson AR, Sacks GN, Milner JJ, Kirk EL, Troester MA, et al. Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-MEDIATED glucose metabolism drives a proinflammatory phenotype*. J Biol Chem. 2014;289(11):7884–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Song W, Li D, Tao L, Luo Q, Chen L. Solute carrier transporters: the metabolic gatekeepers of immune cells. Acta Pharm Sin B. 2020;10(1):61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sun Q, Chen X, Ma J, Peng H, Wang F, Zha X, et al. Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc Natl Acad Sci USA. 2011;108(10):4129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Troutman TD, Hu W, Fulenchek S, Yamazaki T, Kurosaki T, Bazan JF, et al. Role for B-cell adapter for PI3K (BCAP) as a signaling adapter linking Toll-like receptors (TLRs) to serine/threonine kinases PI3K/Akt. Proc Natl Acad Sci USA. 2012;109(1):273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Robey RB, Hay N. Is Akt the “Warburg kinase”? akt-energy metabolism interactions and oncogenesis. Semin Cancer Biol. 2009;19(1):25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Deason K, Troutman TD, Jain A, Challa DK, Mandraju R, Brewer T, et al. BCAP links IL-1R to the PI3K-mTOR pathway and regulates pathogenic Th17 cell differentiation. J Exp Med. 2018;215(9):2413–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Santarsiero A, Convertini P, Todisco S, Pierri CL, De Grassi A, Williams NC, et al. ACLY nuclear translocation in human macrophages drives proinflammatory gene expression by NF-κB acetylation. Cells. 2021;10(11):2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Covarrubias AJ, Aksoylar HI, Yu J, Snyder NW, Worth AJ, Iyer SS, et al. Akt-mTORC1 signaling regulates Acly to integrate metabolic input to control of macrophage activation. Elife. 2016;5:e11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bock FJ, Tait SWG. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol. 2020;21(2):85–100. [DOI] [PubMed] [Google Scholar]
- 52. Marchi S, Guilbaud E, Tait SWG, Yamazaki T, Galluzzi L. Mitochondrial control of inflammation. Nat Rev Immunol. 2023;23(3):159–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vringer E, Tait SWG. Mitochondria and cell death-associated inflammation. Cell Death Differ. 2023;30(2):304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liaudet L, Pacher P, Mabley JG, Virág L, Soriano FG, Haskó G, et al. Activation of poly(ADP-ribose) polymerase-1 is a central mechanism of lipopolysaccharide-induced acute lung inflammation. Am J Respir Crit Care Med. 2002;165(3):372–7. [DOI] [PubMed] [Google Scholar]
- 55. Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD(+) metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021;22(2):119–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cameron AM, Castoldi A, Sanin DE, Flachsmann LJ, Field CS, Puleston DJ, et al. Inflammatory macrophage dependence on NAD+ salvage is a consequence of reactive oxygen species–mediated DNA damage. Nat Immunol. 2019;20(4):420–32. [DOI] [PubMed] [Google Scholar]
- 57. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–14. [DOI] [PubMed] [Google Scholar]
- 58. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–64. [DOI] [PubMed] [Google Scholar]
- 60. Liberti MV, Locasale JW. The warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41(3):211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Minhas PS, Liu L, Moon PK, Joshi AU, Dove C, Mhatre S, et al. Macrophage de novo NAD+ synthesis specifies immune function in aging and inflammation. Nat Immunol. 2019;20(1):50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Baker SA, Rutter J. Metabolites as signalling molecules. Nat Rev Mol Cell Biol. 2023;24(5):355–74. [DOI] [PubMed] [Google Scholar]
- 63. Pacl HT, Chinta KC, Reddy VP, Nadeem S, Sevalkar RR, Nargan K, et al. NAD(H) homeostasis underlies host protection mediated by glycolytic myeloid cells in tuberculosis. Nat Commun. 2023;14(1):5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Aki T, Funakoshi T, Noritake K, Unuma K, Uemura K. Extracellular glucose is crucially involved in the fate decision of LPS-stimulated RAW264.7 murine macrophage cells. Sci Rep. 2020;10(1):10581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496(7444):238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jha AK, Huang SC, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42(3):419–30. [DOI] [PubMed] [Google Scholar]
- 67. Williams NC, O’Neill LAJ. A role for the Krebs cycle intermediate citrate in metabolic reprogramming in innate immunity and inflammation. Front Immunol. 2018;9:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Infantino V, Pierri CL, Iacobazzi V. Metabolic routes in inflammation: the citrate pathway and its potential as therapeutic target. Curr Med Chem. 2019;26(40):7104–16. [DOI] [PubMed] [Google Scholar]
- 69. Blatnik M, Thorpe SR, Baynes JW. Succination of proteins by fumarate: mechanism of inactivation of glyceraldehyde-3-phosphate dehydrogenase in diabetes. Ann N Y Acad Sci. 2008;1126:272–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Epstein ACR, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107(1):43–54. [DOI] [PubMed] [Google Scholar]
- 71. Berra E, Benizri E, Ginouvès A, Volmat V, Roux D, Pouysségur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 2003;22(16):4082–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7(1):77–85. [DOI] [PubMed] [Google Scholar]
- 73. Chowdhury R, Leung IK, Tian YM, Abboud MI, Ge W, Domene C, et al. Structural basis for oxygen degradation domain selectivity of the HIF prolyl hydroxylases. Nat Commun. 2016;7:12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ivan M, Kaelin WG. The EGLN-HIF O2-sensing system: multiple inputs and feedbacks. Mol Cell. 2017;66(6):772–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chouchani ET, Pell VR, Gaude E, Aksentijević D, Sundier SY, Robb EL, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515(7527):431–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mills EL, Kelly B, Logan A, Costa ASH, Varma M, Bryant CE, et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 2016;167(2):457–70.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zotta A, O’Neill LAJ, Yin M. Unlocking potential: the role of the electron transport chain in immunometabolism. Trends Immunol. 2024;45(4):259–73. [DOI] [PubMed] [Google Scholar]
- 78. Littlewood-Evans A, Sarret S, Apfel V, Loesle P, Dawson J, Zhang J, et al. GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis. J Exp Med. 2016;213(9):1655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zasłona Z, O’Neill LAJ. Cytokine-like roles for metabolites in immunity. Mol Cell. 2020;78(5):814–23. [DOI] [PubMed] [Google Scholar]
- 80. Krzak G, Willis CM, Smith JA, Pluchino S, Peruzzotti-Jametti L. Succinate receptor 1: an emerging regulator of myeloid cell function in inflammation. Trends Immunol. 2021;42(1):45–58. [DOI] [PubMed] [Google Scholar]
- 81. Infantino V, Iacobazzi V, Palmieri F, Menga A. ATP-citrate lyase is essential for macrophage inflammatory response. Biochem Biophys Res Commun. 2013;440(1):105–11. [DOI] [PubMed] [Google Scholar]
- 82. Infantino V, Convertini P, Cucci L, Panaro MA, Di Noia MA, Calvello R, et al. The mitochondrial citrate carrier: a new player in inflammation. Biochem J. 2011;438(3):433–6. [DOI] [PubMed] [Google Scholar]
- 83. Infantino V, Iacobazzi V, Menga A, Avantaggiati ML, Palmieri F. A key role of the mitochondrial citrate carrier (SLC25A1) in TNFα- and IFNγ-triggered inflammation. Biochim Biophys Acta. 2014;1839(11):1217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bailey JD, Diotallevi M, Nicol T, McNeill E, Shaw A, Chuaiphichai S, et al. Nitric oxide modulates metabolic remodeling in inflammatory macrophages through TCA cycle regulation and itaconate accumulation. Cell Rep. 2019;28(1):218–30.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Palmieri EM, Gonzalez-Cotto M, Baseler WA, Davies LC, Ghesquière B, Maio N, et al. Nitric oxide orchestrates metabolic rewiring in M1 macrophages by targeting aconitase 2 and pyruvate dehydrogenase. Nat Commun. 2020;11(1):698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8(9):705–13. [DOI] [PubMed] [Google Scholar]
- 87. Meiser J, Krämer L, Sapcariu SC, Battello N, Ghelfi J, D’Herouel AF, et al. Pro-inflammatory macrophages sustain pyruvate oxidation through pyruvate dehydrogenase for the synthesis of itaconate and to enable cytokine expression. J Biol Chem. 2016;291(8):3932–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li Q, Yan Y, Liu J, Huang X, Zhang X, Kirschning C, et al. Toll-like receptor 7 activation enhances CD8+ T cell effector functions by promoting cellular glycolysis. Front Immunol. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Necela BM, Su W, Thompson EA. Toll-like receptor 4 mediates cross-talk between peroxisome proliferator-activated receptor gamma and nuclear factor-kappaB in macrophages. Immunology. 2008;125(3):344–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nagy ZS, Czimmerer Z, Szanto A, Nagy L. Pro-inflammatory cytokines negatively regulate PPARγ mediated gene expression in both human and murine macrophages via multiple mechanisms. Immunobiology. 2013;218(11):1336–44. [DOI] [PubMed] [Google Scholar]
- 91. Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998;93(2):229–40. [DOI] [PubMed] [Google Scholar]
- 92. Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM. PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 2001;7(1):48–52. [DOI] [PubMed] [Google Scholar]
- 93. Szatmari I, Rajnavolgyi E, Nagy L. PPARgamma, a lipid-activated transcription factor as a regulator of dendritic cell function. Ann N Y Acad Sci. 2006;1088:207–18. [DOI] [PubMed] [Google Scholar]
- 94. Szatmari I, Rajnavolgyi E, Nagy L. PPARgamma, a lipid-activated transcription factor as a regulator of dendritic cell function. Ann N Y Acad Sci. 2006;1088(1):207–18. [DOI] [PubMed] [Google Scholar]
- 95. Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Klotz L, Hucke S, Thimm D, Classen S, Gaarz A, Schultze J, et al. Increased antigen cross-presentation but impaired cross-priming after activation of peroxisome proliferator-activated receptor gamma is mediated by up-regulation of B7H1. J Immunol. 2009;183(1):129–36. [DOI] [PubMed] [Google Scholar]
- 97. Nagy L, Szanto A, Szatmari I, Széles L. Nuclear hormone receptors enable macrophages and dendritic cells to sense their lipid environment and shape their immune response. Physiol Rev. 2012;92(2):739–89. [DOI] [PubMed] [Google Scholar]
- 98. Nelson VL, Nguyen HCB, Garcìa-Cañaveras JC, Briggs ER, Ho WY, DiSpirito JR, et al. PPARγ is a nexus controlling alternative activation of macrophages via glutamine metabolism. Genes Dev. 2018;32(15–16):1035–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Appel S, Mirakaj V, Bringmann A, Weck MM, Grünebach F, Brossart P. PPAR-gamma agonists inhibit toll-like receptor-mediated activation of dendritic cells via the MAP kinase and NF-kappaB pathways. Blood. 2005;106(12):3888–94. [DOI] [PubMed] [Google Scholar]
- 100. Dana N, Vaseghi G, Haghjooy Javanmard S. Crosstalk between peroxisome proliferator-activated receptors and toll-like receptors: a systematic review. Adv Pharm Bull. 2019;9(1):12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Huang L, Nazarova EV, Tan S, Liu Y, Russell DG. Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. J Exp Med. 2018;215(4):1135–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Woods PS, Kimmig LM, Meliton AY, Sun KA, Tian Y, O’Leary EM, et al. Tissue-resident alveolar macrophages do not rely on glycolysis for LPS-induced inflammation. Am J Respir Cell Mol Biol. 2020;62(2):243–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Woods PS, Kimmig LM, Sun KA, Meliton AY, Shamaa OR, Tian Y, et al. HIF-1α induces glycolytic reprograming in tissue-resident alveolar macrophages to promote cell survival during acute lung injury. Elife. 2022;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pereverzeva L, van Linge CCA, Schuurman AR, Klarenbeek AM, Ramirez Moral I, Otto NA, et al. Human alveolar macrophages do not rely on glucose metabolism upon activation by lipopolysaccharide. Biochim Biophys Acta, Mol Basis Dis. 2022;1868(10):166488. [DOI] [PubMed] [Google Scholar]
- 105. Garnett JP, Nguyen TT, Moffatt JD, Pelham ER, Kalsi KK, Baker EH, et al. Proinflammatory mediators disrupt glucose homeostasis in airway surface liquid. J Immunol. 2012;189(1):373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Baker EH, Baines DL. Airway glucose homeostasis: a new target in the prevention and treatment of pulmonary infection. Chest. 2018;153(2):507–14. [DOI] [PubMed] [Google Scholar]
- 107. Svedberg FR, Brown SL, Krauss MZ, Campbell L, Sharpe C, Clausen M, et al. The lung environment controls alveolar macrophage metabolism and responsiveness in type 2 inflammation. Nat Immunol. 2019;20(5):571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Li L, Liu X, Sanders KL, Edwards JL, Ye J, Si F, et al. TLR8-Mediated metabolic control of human treg function: a mechanistic target for cancer immunotherapy. Cell Metab. 2019;29(1):103–23.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Seim GL, Britt EC, John SV, Yeo FJ, Johnson AR, Eisenstein RS, et al. Two-stage metabolic remodelling in macrophages in response to lipopolysaccharide and interferon-γ stimulation. Nat Metab. 2019;1(7):731–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Palmieri EM, Holewinski R, McGinity CL, Pierri CL, Maio N, Weiss JM, et al. Pyruvate dehydrogenase operates as an intramolecular nitroxyl generator during macrophage metabolic reprogramming. Nat Commun. 2023;14(1):5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Seim GL, John SV, Arp NL, Fang Z, Pagliarini DJ, Fan J. Nitric oxide-driven modifications of lipoic arm inhibit α-ketoacid dehydrogenases. Nat Chem Biol. 2023;19(3):265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Galván-Peña S, Carroll RG, Newman C, Hinchy EC, Palsson-McDermott E, Robinson EK, et al. Malonylation of GAPDH is an inflammatory signal in macrophages. Nat Commun. 2019;10(1):338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ran L, Zhang S, Wang G, Zhao P, Sun J, Zhou J, et al. Mitochondrial pyruvate carrier-mediated metabolism is dispensable for the classical activation of macrophages. Nat Metab. 2023;5(5):804–20. [DOI] [PubMed] [Google Scholar]
- 114. Hu Y, Cao K, Wang F, Wu W, Mai W, Qiu L, et al. Dual roles of hexokinase 2 in shaping microglial function by gating glycolytic flux and mitochondrial activity. Nat Metab. 2022;4(12):1756–74. [DOI] [PubMed] [Google Scholar]
- 115. Sabogal-Guáqueta AM, Marmolejo-Garza A, Trombetta-Lima M, Oun A, Hunneman J, Chen T, et al. Species-specific metabolic reprogramming in human and mouse microglia during inflammatory pathway induction. Nat Commun. 2023;14(1):6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Angiari S, Runtsch MC, Sutton CE, Palsson-McDermott EM, Kelly B, Rana N, et al. Pharmacological activation of pyruvate kinase M2 inhibits CD4+ T cell pathogenicity and suppresses autoimmunity. Cell Metab. 2020;31(2):391–405.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Reynolds JM, Martinez GJ, Chung Y, Dong C. Toll-like receptor 4 signaling in T cells promotes autoimmune inflammation. Proc Natl Acad Sci USA. 2012;109(32):13064–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. González-Navajas JM, Fine S, Law J, Datta SK, Nguyen KP, Yu M, et al. TLR4 signaling in effector CD4+ T cells regulates TCR activation and experimental colitis in mice. J Clin Invest. 2010;120(2):570–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Yeudall S, Upchurch CM, Seegren PV, Pavelec CM, Greulich J, Lemke MC, et al. Macrophage acetyl-CoA carboxylase regulates acute inflammation through control of glucose and lipid metabolism. Sci Adv. 2022;8(47):eabq1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wang Y, Yu W, Li S, Guo D, He J, Wang Y. Acetyl-CoA carboxylases and diseases. Front Oncol. 2022;12:836058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Heieis GA, Patente TA, Almeida L, Vrieling F, Tak T, Perona-Wright G, et al. Metabolic heterogeneity of tissue-resident macrophages in homeostasis and during helminth infection. Nat Commun. 2023;14(1):5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ramalingam TR, Pesce JT, Sheikh F, Cheever AW, Mentink-Kane MM, Wilson MS, et al. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor alpha1 chain. Nat Immunol. 2008;9(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Yu D-M, Zhao J, Lee EE, Kim D, Mahapatra R, Rose EK, et al. GLUT3 promotes macrophage signaling and function via RAS-mediated endocytosis in atopic dermatitis and wound healing. J Clin Invest. 2023;133(21):e170706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zhang P, Miska J, Heimberger AB. GLUT3 regulates alternative macrophage signaling through a glucose transport–independent role. J Clin Invest. 2023;133(21):e174540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wang F, Zhang S, Vuckovic I, Jeon R, Lerman A, Folmes CD, et al. Glycolytic stimulation is not a requirement for M2 macrophage differentiation. Cell Metab. 2018;28(3):463–75.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Dowling JK, Afzal R, Gearing LJ, Cervantes-Silva MP, Annett S, Davis GM, et al. Mitochondrial arginase-2 is essential for IL-10 metabolic reprogramming of inflammatory macrophages. Nat Commun. 2021;12(1):1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Jones AE, Rios A, Ibrahimovic N, Chavez C, Bayley NA, Ball AB, et al. The metabolic cofactor Coenzyme A enhances alternative macrophage activation via MyD88-linked signaling. bioRxiv. 2024:2024.03.28.587096. [Google Scholar]
- 128. Van den Bossche J, O’Neill LA, Menon D. Macrophage immunometabolism: where are we (going)? Trends Immunol. 2017;38(6):395–406. [DOI] [PubMed] [Google Scholar]
- 129. Cordes T, Wallace M, Michelucci A, Divakaruni AS, Sapcariu SC, Sousa C, et al. Immunoresponsive gene 1 and itaconate inhibit succinate dehydrogenase to modulate intracellular succinate levels. J Biol Chem. 2016;291(27):14274–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Lampropoulou V, Sergushichev A, Bambouskova M, Nair S, Vincent EE, Loginicheva E, et al. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab. 2016;24(1):158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chen F, Elgaher WAM, Winterhoff M, Büssow K, Waqas FH, Graner E, et al. Citraconate inhibits ACOD1 (IRG1) catalysis, reduces interferon responses and oxidative stress, and modulates inflammation and cell metabolism. Nat Metab. 2022;4(5):534–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ichinohe T. Respective roles of TLR, RIG-I and NLRP3 in influenza virus infection and immunity: impact on vaccine design. Expert Rev Vaccin. 2010;9(11):1315–24. [DOI] [PubMed] [Google Scholar]
- 133. Michelucci A, Cordes T, Ghelfi J, Pailot A, Reiling N, Goldmann O, et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci USA. 2013;110(19):7820–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chen F, Lukat P, Iqbal AA, Saile K, Kaever V, van den Heuvel J, et al. Crystal structure of cis-aconitate decarboxylase reveals the impact of naturally occurring human mutations on itaconate synthesis. Proc Natl Acad Sci USA. 2019;116(41):20644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Wang X, Su S, Zhu Y, Cheng X, Cheng C, Chen L, et al. Metabolic Reprogramming via ACOD1 depletion enhances function of human induced pluripotent stem cell-derived CAR-macrophages in solid tumors. Nat Commun. 2023;14(1):5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Liu X, Wu XP, Zhu XL, Li T, Liu Y. IRG1 increases MHC class I level in macrophages through STAT-TAP1 axis depending on NADPH oxidase mediated reactive oxygen species. Int Immunopharmacol. 2017;48:76–83. [DOI] [PubMed] [Google Scholar]
- 137. Kelly B, O’neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25(7):771–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Chen LL, Morcelle C, Cheng ZL, Chen X, Xu Y, Gao Y, et al. Itaconate inhibits TET DNA dioxygenases to dampen inflammatory responses. Nat Cell Biol. 2022;24(3):353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. O’Neill LAJ, Artyomov MN. Itaconate: the poster child of metabolic reprogramming in macrophage function. Nat Rev Immunol. 2019;19(5):273–81. [DOI] [PubMed] [Google Scholar]
- 140. Peace CG, O’Neill LA. The role of itaconate in host defense and inflammation. J Clin Invest. 2022;132(2):e148548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Liao ST, Han C, Xu DQ, Fu XW, Wang JS, Kong LY. 4-Octyl itaconate inhibits aerobic glycolysis by targeting GAPDH to exert anti-inflammatory effects. Nat Commun. 2019;10(1):5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. He W, Henne A, Lauterbach M, Geißmar E, Nikolka F, Kho C, et al. Mesaconate is synthesized from itaconate and exerts immunomodulatory effects in macrophages. Nat Metab. 2022;4(5):524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Mills EL, Ryan DG, Prag HA, Dikovskaya D, Menon D, Zaslona Z, et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018;556(7699):113–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39(4):199–218. [DOI] [PubMed] [Google Scholar]
- 145. Swain A, Bambouskova M, Kim H, Andhey PS, Duncan D, Auclair K, et al. Comparative evaluation of itaconate and its derivatives reveals divergent inflammasome and type I interferon regulation in macrophages. Nat Metab. 2020;2(7):594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Horton CG, Farris AD. Toll-like receptors in systemic lupus erythematosus: potential targets for therapeutic intervention. Curr Allergy Asthma Rep. 2012;12(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Devarapu SK, Anders HJ. Toll-like receptors in lupus nephritis. J Biomed Sci. 2018;25(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Tang C, Wang X, Xie Y, Cai X, Yu N, Hu Y, et al. 4-Octyl itaconate activates Nrf2 signaling to inhibit pro-inflammatory cytokine production in peripheral blood mononuclear cells of systemic lupus erythematosus patients. Cell Physiol Biochem. 2018;51(2):979–90. [DOI] [PubMed] [Google Scholar]
- 149. Fillatreau S, Manfroi B, Dörner T. Toll-like receptor signalling in B cells during systemic lupus erythematosus. Nat Rev Rheumatol. 2021;17(2):98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Sharabi A, Tsokos GC. T cell metabolism: new insights in systemic lupus erythematosus pathogenesis and therapy. Nat Rev Rheumatol. 2020;16(2):100–12. [DOI] [PubMed] [Google Scholar]
- 151. Aso K, Kono M, Kanda M, Kudo Y, Sakiyama K, Hisada R, et al. Itaconate ameliorates autoimmunity by modulating T cell imbalance via metabolic and epigenetic reprogramming. Nat Commun. 2023;14(1):984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kumar V. Inflammasomes: pandora’s box for sepsis. J Inflamm Res. 2018;11:477–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Sundaram B, Tweedell RE, Prasanth Kumar S, Kanneganti TD. The NLR family of innate immune and cell death sensors. Immunity. 2024;57(4):674–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183(2):787–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Juliana C, Fernandes-Alnemri T, Kang S, Farias A, Qin F, Alnemri ES. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J Biol Chem. 2012;287(43):36617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. He Y, Hara H, Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016;41(12):1012–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Wolf AJ, Reyes CN, Liang W, Becker C, Shimada K, Wheeler ML, et al. Hexokinase is an innate immune receptor for the detection of bacterial peptidoglycan. Cell. 2016;166(3):624–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Baik SH, Ramanujan VK, Becker C, Fett S, Underhill DM, Wolf AJ. Hexokinase dissociation from mitochondria promotes oligomerization of VDAC that facilitates NLRP3 inflammasome assembly and activation. Sci Immunol. 2023;8(84):eade7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. O’Sullivan D, Kelly B, Pearce EL. When hexokinase gets that NAG-ing feeling. Cell Metab. 2016;24(2):198–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Olona A, Leishman S, Anand PK. The NLRP3 inflammasome: regulation by metabolic signals. Trends Immunol. 2022;43(12):978–89. [DOI] [PubMed] [Google Scholar]
- 163. Abais JM, Xia M, Zhang Y, Boini KM, Li PL. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal. 2015;22(13):1111–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Song N, Liu ZS, Xue W, Bai ZF, Wang QY, Dai J, et al. NLRP3 phosphorylation is an essential priming event for inflammasome activation. Mol Cell. 2017;68(1):185–97.e6. [DOI] [PubMed] [Google Scholar]
- 165. Gritsenko A, Yu S, Martin-Sanchez F, Diaz-Del-Olmo I, Nichols EM, Davis DM, et al. Priming is dispensable for NLRP3 inflammasome activation in human monocytes in vitro. Front Immunol. 2020;11:565924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Xie M, Yu Y, Kang R, Zhu S, Yang L, Zeng L, et al. PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation. Nat Commun. 2016;7:13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Liu D, Xiao Y, Zhou B, Gao S, Li L, Zhao L, et al. PKM2-dependent glycolysis promotes skeletal muscle cell pyroptosis by activating the NLRP3 inflammasome in dermatomyositis/polymyositis. Rheumatol. 2021;60(5):2177–89. [DOI] [PubMed] [Google Scholar]
- 168. Li Q, Leng K, Liu Y, Sun H, Gao J, Ren Q, et al. The impact of hyperglycaemia on PKM2-mediated NLRP3 inflammasome/stress granule signalling in macrophages and its correlation with plaque vulnerability: an in vivo and in vitro study. Metabolism. 2020;107:154231. [DOI] [PubMed] [Google Scholar]
- 169. Finucane OM, Sugrue J, Rubio-Araiz A, Guillot-Sestier MV, Lynch MA. The NLRP3 inflammasome modulates glycolysis by increasing PFKFB3 in an IL-1β-dependent manner in macrophages. Sci Rep. 2019;9(1):4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Tan Q, Huang Q, Ma YL, Mao K, Yang G, Luo P, et al. Potential roles of IL-1 subfamily members in glycolysis in disease. Cytokine Growth Factor Rev. 2018;44:18–27. [DOI] [PubMed] [Google Scholar]
- 171. Hooftman A, Angiari S, Hester S, Corcoran SE, Runtsch MC, Ling C, et al. The immunomodulatory metabolite itaconate modifies NLRP3 and inhibits inflammasome activation. Cell Metab. 2020;32(3):468–78.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. He Y, Zeng MY, Yang D, Motro B, Núñez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530(7590):354–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Schmid-Burgk JL, Chauhan D, Schmidt T, Ebert TS, Reinhardt J, Endl E, et al. A genome-wide CRISPR (clustered regularly interspaced short palindromic repeats) screen identifies NEK7 as an essential component of NLRP3 inflammasome activation. J Biol Chem. 2016;291(1):103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Shi H, Wang Y, Li X, Zhan X, Tang M, Fina M, et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat Immunol. 2016;17(3):250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Hughes MM, O’Neill LAJ. Metabolic regulation of NLRP3. Immunol Rev. 2018;281(1):88–98. [DOI] [PubMed] [Google Scholar]
- 176. Sharif H, Wang L, Wang WL, Magupalli VG, Andreeva L, Qiao Q, et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature. 2019;570(7761):338–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Domingo-Fernández R, Coll RC, Kearney J, Breit S, O’Neill LAJ. The intracellular chloride channel proteins CLIC1 and CLIC4 induce IL-1β transcription and activate the NLRP3 inflammasome. J Biol Chem. 2017;292(29):12077–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Tang T, Lang X, Xu C, Wang X, Gong T, Yang Y, et al. CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. Nat Commun. 2017;8(1):202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Gaidt MM, Hornung V. Alternative inflammasome activation enables IL-1β release from living cells. Curr Opin Immunol. 2017;44:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Schmacke NA, O’Duill F, Gaidt MM, Szymanska I, Kamper JM, Schmid-Burgk JL, et al. IKKβ primes inflammasome formation by recruiting NLRP3 to the trans-Golgi network. Immunity. 2022;55(12):2271–84.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Bambouskova M, Potuckova L, Paulenda T, Kerndl M, Mogilenko DA, Lizotte K, et al. Itaconate confers tolerance to late NLRP3 inflammasome activation. Cell Rep. 2021;34(10):108756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Carneiro AB, Iaciura BM, Nohara LL, Lopes CD, Veas EM, Mariano VS, et al. Lysophosphatidylcholine triggers TLR2-and TLR4-mediated signaling pathways but counteracts LPS-induced NO synthesis in peritoneal macrophages by inhibiting NF-κB translocation and MAPK/ERK phosphorylation. PLoS One. 2013;8(9):e76233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Hoyle C, Green JP, Allan SM, Brough D, Lemarchand E. Itaconate and fumarate derivatives inhibit priming and activation of the canonical NLRP3 inflammasome in macrophages. Immunology. 2022;165(4):460–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Straß S, Geiger J, Cloos N, Späth N, Geiger S, Schwamborn A, et al. Immune cell targeted fumaric esters support a role of GPR109A as a primary target of monomethyl fumarate in vivo. Inflammopharmacology. 2023;31(3):1223–39. [DOI] [PubMed] [Google Scholar]
- 185. Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40(1):128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Humphries F, Shmuel-Galia L, Ketelut-Carneiro N, Li S, Wang B, Nemmara VV, et al. Succination inactivates gasdermin D and blocks pyroptosis. Science. 2020;369(6511):1633–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Hu JJ, Liu X, Xia S, Zhang Z, Zhang Y, Zhao J, et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat Immunol. 2020;21(7):736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Wang X, He G, Peng Y, Zhong W, Wang Y, Zhang B. Sodium butyrate alleviates adipocyte inflammation by inhibiting NLRP3 pathway. Sci Rep. 2015;5(1):12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Yuan X, Wang L, Bhat OM, Lohner H, Li PL. Differential effects of short chain fatty acids on endothelial Nlrp3 inflammasome activation and neointima formation: antioxidant action of butyrate. Redox Biol. 2018;16:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Jiang L, Wang J, Liu Z, Jiang A, Li S, Wu D, et al. Sodium butyrate alleviates lipopolysaccharide-induced inflammatory responses by down-regulation of NF-κB, NLRP3 signaling pathway, and activating histone acetylation in bovine macrophages. Front Vet Sci. 2020;7:579674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Liu H, Bian Z, Zhang Q, Xiao Z, Cao Y, Sun X, et al. Sodium butyrate inhibits colitis-associated colorectal cancer through preventing the gut microbiota dysbiosis and reducing the expression of NLRP3 and IL-1β. J Funct Foods. 2021;87:104862. [Google Scholar]
- 192. Lobel L, Garrett WS. Butyrate makes macrophages “go nuclear” against bacterial pathogens. Immunity. 2019;50(2):275–8. [DOI] [PubMed] [Google Scholar]
- 193. Schulthess J, Pandey S, Capitani M, Rue-Albrecht KC, Arnold I, Franchini F, et al. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity. 2019;50(2):432–45.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Park OJ, Ha YE, Sim JR, Lee D, Lee EH, Kim SY, et al. Butyrate potentiates Enterococcus faecalis lipoteichoic acid-induced inflammasome activation via histone deacetylase inhibition. Cell Death Discov. 2023;9(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115(5):577–90. [DOI] [PubMed] [Google Scholar]
- 196. Howell JJ, Hellberg K, Turner M, Talbott G, Kolar MJ, Ross DS, et al. Metformin inhibits hepatic mTORC1 signaling via dose-dependent mechanisms involving AMPK and the TSC complex. Cell Metab. 2017;25(2):463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015;125(1):25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Braun C, Weichhart T. mTOR-dependent immunometabolism as Achilles’ heel of anticancer therapy. Eur J Immunol. 2021;51(12):3161–75. [DOI] [PubMed] [Google Scholar]
- 199. Leishman S, Aljadeed NM, Qian L, Cockcroft S, Behmoaras J, Anand PK. Fatty acid synthesis promotes inflammasome activation through NLRP3 palmitoylation. bioRxiv. 2023;2023.10.30:564549. [Google Scholar]
- 200. Matsuki T, Horai R, Sudo K, Iwakura Y. IL-1 plays an important role in lipid metabolism by regulating insulin levels under physiological conditions. J Exp Med. 2003;198(6):877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Paniri A, Akhavan-Niaki H. Emerging role of IL-6 and NLRP3 inflammasome as potential therapeutic targets to combat COVID-19: role of lncRNAs in cytokine storm modulation. Life Sci. 2020;257:118114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Sanman LE, Qian Y, Eisele NA, Ng TM, van der Linden WA, Monack DM, et al. Disruption of glycolytic flux is a signal for inflammasome signaling and pyroptotic cell death. Elife. 2016;5:e13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Kumar V. A STING to inflammation and autoimmunity. J Leukoc Biol. 2019;106(1):171–85. [DOI] [PubMed] [Google Scholar]
- 204. Kumar V. The trinity of cGAS, TLR9, and ALRs guardians of the cellular galaxy against host-derived self-DNA. Front Immunol. 2020;11:624597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Kumar V, Bauer C, Stewart JH. Targeting cGAS/STING signaling-mediated myeloid immune cell dysfunction in TIME. J Biomed Sci. 2023;30(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Kumar V, Bauer C, Stewart JH. Cancer cell-specific cGAS/STING Signaling pathway in the era of advancing cancer cell biology. Eur J Cell Biol. 2023;102(3):151338. [DOI] [PubMed] [Google Scholar]
- 207. Dvorkin S, Cambier S, Volkman HE, Stetson DB. New frontiers in the cGAS-STING intracellular DNA-sensing pathway. Immunity. 2024;57(4):718–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Gomes MTR, Guimarães ES, Marinho FV, Macedo I, Aguiar E, Barber GN, et al. STING regulates metabolic reprogramming in macrophages via HIF-1α during Brucella infection. Plos Pathog. 2021;17(5):e1009597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Hu Z, Yu X, Ding R, Liu B, Gu C, Pan XW, et al. Glycolysis drives STING signaling to facilitate dendritic cell antitumor function. J Clin Invest. 2023;133(7):e166031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9(2):213–9. [DOI] [PubMed] [Google Scholar]
- 211. Ogawa D, Stone JF, Takata Y, Blaschke F, Chu VH, Towler DA, et al. Liver x receptor agonists inhibit cytokine-induced osteopontin expression in macrophages through interference with activator protein-1 signaling pathways. Circ Res. 2005;96(7):e59–67. [DOI] [PubMed] [Google Scholar]
- 212. Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest. 2006;116(3):607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Pascual-García M, Valledor AF. Biological roles of liver X receptors in immune cells. Arch Immunol Ther Exp. 2012;60(4):235–49. [DOI] [PubMed] [Google Scholar]
- 214. Pascual-García M, Rué L, León T, Julve J, Carbó JM, Matalonga J, et al. Reciprocal negative cross-talk between liver X receptors (LXRs) and STAT1: effects on IFN-γ-induced inflammatory responses and LXR-dependent gene expression. J Immunol. 2013;190(12):6520–32. [DOI] [PubMed] [Google Scholar]
- 215. Schulman IG. Liver X receptors link lipid metabolism and inflammation. FEBS Lett. 2017;591(19):2978–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Hong C, Walczak R, Dhamko H, Bradley MN, Marathe C, Boyadjian R, et al. Constitutive activation of LXR in macrophages regulates metabolic and inflammatory gene expression: identification of ARL7 as a direct target. J Lipid Res. 2011;52(3):531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Hou Y, Wang Z, Liu P, Wei X, Zhang Z, Fan S, et al. SMPDL3A is a cGAMP-degrading enzyme induced by LXR-mediated lipid metabolism to restrict cGAS-STING DNA sensing. Immunity. 2023;56(11):2492–507.e10. [DOI] [PubMed] [Google Scholar]
- 218. Gong Y, Zou L, Feng Y, Li D, Cai J, Chen D, et al. Importance of Toll-like receptor 2 in mitochondrial dysfunction during polymicrobial sepsis. Anesthesiology. 2014;121(6):1236–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Ko MK, Saraswathy S, Parikh JG, Rao NA. The role of TLR4 activation in photoreceptor mitochondrial oxidative stress. Invest Ophthalmol Vis Sci. 2011;52(8):5824–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Sun P, Zhang Z, Wang B, Liu C, Chen C, Liu P, et al. A genetically encoded fluorescent biosensor for detecting itaconate with subcellular resolution in living macrophages. Nat Commun. 2022;13(1):6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Ando M, Uehara I, Kogure K, Asano Y, Nakajima W, Abe Y, et al. Interleukin 6 enhances glycolysis through expression of the glycolytic enzymes hexokinase 2 and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3. J Nippon Med Sch. 2010;77(2):97–105. [DOI] [PubMed] [Google Scholar]
- 222. Han J, Meng Q, Xi Q, Zhang Y, Zhuang Q, Han Y, et al. Interleukin-6 stimulates aerobic glycolysis by regulating PFKFB3 at early stage of colorectal cancer. Int J Oncol. 2016;48(1):215–24. [DOI] [PubMed] [Google Scholar]
- 223. Li YS, Ren HC, Cao JH. Roles of Interleukin-6-mediated immunometabolic reprogramming in COVID-19 and other viral infection-associated diseases. Int Immunopharmacol. 2022;110:109005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Rehwinkel J, Gack MU. RIG-I-like receptors: their regulation and roles in RNA sensing. Nat Rev Immunol. 2020;20(9):537–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Liu G, Lu Y, Thulasi Raman SN, Xu F, Wu Q, Li Z, et al. Nuclear-resident RIG-I senses viral replication inducing antiviral immunity. Nat Commun. 2018;9(1):3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Satoh T, Kato H, Kumagai Y, Yoneyama M, Sato S, Matsushita K, et al. LGP2 is a positive regulator of RIG-I- and MDA5-mediated antiviral responses. Proc Natl Acad Sci USA. 2010;107(4):1512–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472(7344):481–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Bruns AM, Horvath CM. LGP2 synergy with MDA5 in RLR-mediated RNA recognition and antiviral signaling. Cytokine. 2015;74(2):198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Esser-Nobis K, Hatfield LD, Gale M Jr. Spatiotemporal dynamics of innate immune signaling via RIG-I-like receptors. Proc Natl Acad Sci USA. 2020;117(27):15778–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Sun Q, Sun L, Liu HH, Chen X, Seth RB, Forman J, et al. The specific and essential role of MAVS in antiviral innate immune responses. Immunity. 2006;24(5):633–42. [DOI] [PubMed] [Google Scholar]
- 231. Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6(10):981–8. [DOI] [PubMed] [Google Scholar]
- 232. Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437(7062):1167–72. [DOI] [PubMed] [Google Scholar]
- 233. Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell. 2005;19(6):727–40. [DOI] [PubMed] [Google Scholar]
- 234. Schlee M, Hartmann G. Discriminating self from non-self in nucleic acid sensing. Nat Rev Immunol. 2016;16(9):566–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4(5):491–6. [DOI] [PubMed] [Google Scholar]
- 236. Goubau D, Deddouche S, Reis e Sousa C, Sousa C. Cytosolic sensing of viruses. Immunity. 2013;38(5):855–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Yu YQ, Zielinska M, Li W, Bernkopf DB, Heilingloh CS, Neurath MF, et al. PGAM5-MAVS interaction regulates TBK1/IRF3 dependent antiviral responses. Sci Rep. 2020;10(1):8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Bang BR, Miki H, Kang YJ. Mitochondrial PGAM5-Drp1 signaling regulates the metabolic reprogramming of macrophages and regulates the induction of inflammatory responses. Front Immunol. 2023;14:1243548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Loo YM, Gale M Jr. Immune signaling by RIG-I-like receptors. Immunity. 2011;34(5):680–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Ramos HJ, Gale M Jr. RIG-I like receptors and their signaling crosstalk in the regulation of antiviral immunity. Curr Opin Virol. 2011;1(3):167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Thoresen D, Wang W, Galls D, Guo R, Xu L, Pyle AM. The molecular mechanism of RIG-I activation and signaling. Immunol Rev. 2021;304(1):154–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Yoneyama M, Kato H, Fujita T. Physiological functions of RIG-I-like receptors. Immunity. 2024;57(4):731–51. [DOI] [PubMed] [Google Scholar]
- 243. Zhang W, Wang G, Xu ZG, Tu H, Hu F, Dai J, et al. Lactate is a natural suppressor of RLR signaling by targeting MAVS. Cell. 2019;178(1):176–89.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Roberts DJ, Miyamoto S. Hexokinase II integrates energy metabolism and cellular protection: akting on mitochondria and TORCing to autophagy. Cell Death Differ. 2015;22(2):364–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Park S, Juliana C, Hong S, Datta P, Hwang I, Fernandes-Alnemri T, et al. The mitochondrial antiviral protein MAVS associates with NLRP3 and regulates its inflammasome activity. J Immunol. 2013;191(8):4358–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Subramanian N, Natarajan K, Clatworthy MR, Wang Z, Germain RN. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell. 2013;153(2):348–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–5. [DOI] [PubMed] [Google Scholar]
- 248. Franchi L, Eigenbrod T, Muñoz-Planillo R, Ozkurede U, Kim YG, Arindam C, et al. Cytosolic double-stranded RNA activates the NLRP3 inflammasome via MAVS-induced membrane permeabilization and K+ efflux. J Immunol. 2014;193(8):4214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Li H, Zhang S, Li F, Qin L. NLRX1 attenuates apoptosis and inflammatory responses in myocardial ischemia by inhibiting MAVS-dependent NLRP3 inflammasome activation. Mol Immunol. 2016;76:90–7. [DOI] [PubMed] [Google Scholar]
- 250. He QQ, Huang Y, Nie L, Ren S, Xu G, Deng F, et al. MAVS integrates glucose metabolism and RIG-I-like receptor signaling. Nat Commun. 2023;14(1):5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Levine ZG, Walker S. The biochemistry of O-GlcNAc transferase: which functions make it essential in mammalian cells? Annu Rev Biochem. 2016;85:631–57. [DOI] [PubMed] [Google Scholar]
- 252. Li T, Li X, Attri KS, Liu C, Li L, Herring LE, et al. O-GlcNAc transferase links glucose metabolism to MAVS-mediated antiviral innate immunity. Cell Host Microbe. 2018;24(6):791–803.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Song N, Qi Q, Cao R, Qin B, Wang B, Wang Y, et al. MAVS O-GlcNAcylation is essential for host antiviral immunity against lethal RNA viruses. Cell Rep. 2019;28(9):2386–96.e5. [DOI] [PubMed] [Google Scholar]
- 254. Li X, Gong W, Wang H, Li T, Attri KS, Lewis RE, et al. O-GlcNAc transferase suppresses inflammation and necroptosis by targeting receptor-interacting serine/threonine-protein kinase 3. Immunity. 2019;50(4):1115–90.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Lawlor KE, Khan N, Mildenhall A, Gerlic M, Croker BA, D’Cruz AA, et al. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Commun. 2015;6(1):6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Speir M, Lawlor KE. RIP-roaring inflammation: RIPK1 and RIPK3 driven NLRP3 inflammasome activation and autoinflammatory disease. Semin Cell Dev Biol. 2021;109:114–24. [DOI] [PubMed] [Google Scholar]
- 257. Qi Q, Chen Q, Dong Y, Wang K, Wang J, Jin G, et al. Oral administration of D-glucosamine confers broad-spectrum protection against human coronaviruses including SARS-CoV-2. Signal Transduct Target Ther. 2023;8(1):250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Harrison AG, Yang D, Cahoon JG, Geng T, Karginov TA, Torrance BL, et al. The glucose transporter GLUT4 tethers RIG-I-like receptors to suppress antiviral immunity. J Immunol. 2023;210(1_Suppl ment):161.17–61.17. [Google Scholar]
- 259. Hu Y, O’Boyle K, Auer J, Raju S, You F, Wang P, et al. Multiple UBXN family members inhibit retrovirus and lentivirus production and canonical NFκΒ signaling by stabilizing IκBα. PLoS Pathog. 2017;13(2):e1006187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Maratou E, Dimitriadis G, Kollias A, Boutati E, Lambadiari V, Mitrou P, et al. Glucose transporter expression on the plasma membrane of resting and activated white blood cells. Eur J Clin Invest. 2007;37(4):282–90. [DOI] [PubMed] [Google Scholar]
- 261. Xiao Y, Chen X, Wang Z, Quan J, Zhao X, Tang H, et al. Succinate is a natural suppressor of antiviral immune response by targeting MAVS. Front Immunol. 2022;13:816378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Chan CC, Damen M, Alarcon PC, Sanchez-Gurmaches J, Divanovic S. Inflammation and immunity: from an adipocyte’s perspective. J Interferon Cytokine Res. 2019;39(8):459–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Shepherd PR, Gnudi L, Tozzo E, Yang H, Leach F, Kahn BB. Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J Biol Chem. 1993;268(30):22243–6. [PubMed] [Google Scholar]
- 264. Yu L, Yan K, Liu P, Li N, Liu Z, Zhu W, et al. Pattern recognition receptor-initiated innate antiviral response in mouse adipose cells. Immunol Cell Biol. 2014;92(2):105–15. [DOI] [PubMed] [Google Scholar]
- 265. Yang G, Lee HE, Seok JK, Kang HC, Cho YY, Lee HS, et al. RIG-I deficiency promotes obesity-induced insulin resistance. Pharmaceuticals. 2021;14(11):1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Domínguez-Andrés J, Arts RJW, ter Horst R, Gresnigt MS, Smeekens SP, Ratter JM, et al. Rewiring monocyte glucose metabolism via C-type lectin signaling protects against disseminated candidiasis. PLoS Pathog. 2017;13(9):e1006632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Ciesla J, Moreno I Jr, Munger J. TNFα-induced metabolic reprogramming drives an intrinsic anti-viral state. PLoS Pathog. 2022;18(7):e1010722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574(7779):575–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Rumpret M, von Richthofen HJ, Peperzak V, Meyaard L. Inhibitory pattern recognition receptors. J Exp Med. 2022;219(1):e20211463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Aksoy E, Taboubi S, Torres D, Delbauve S, Hachani A, Whitehead MA, et al. The p110δ isoform of the kinase PI(3)K controls the subcellular compartmentalization of TLR4 signaling and protects from endotoxic shock. Nat Immunol. 2012;13(11):1045–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Kagan JC. Defining the subcellular sites of innate immune signal transduction. Trends Immunol. 2012;33(9):442–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Yu YR, Imrichova H, Wang H, Chao T, Xiao Z, Gao M, et al. Disturbed mitochondrial dynamics in CD8+ TILs reinforce T cell exhaustion. Nat Immunol. 2020;21(12):1540–51. [DOI] [PubMed] [Google Scholar]
- 273. Scharping NE, Rivadeneira DB, Menk AV, Vignali PDA, Ford BR, Rittenhouse NL, et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat Immunol. 2021;22(2):205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Wu H, Zhao X, Hochrein SM, Eckstein M, Gubert GF, Knöpper K, et al. Mitochondrial dysfunction promotes the transition of precursor to terminally exhausted T cells through HIF-1α-mediated glycolytic reprogramming. Nat Commun. 2023;14(1):6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Suthar MS, Ramos HJ, Brassil MM, Netland J, Chappell CP, Blahnik G, et al. The RIG-I-like receptor LGP2 controls CD8+ T cell survival and fitness. Immunity. 2012;37(2):235–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Kandasamy M, Suryawanshi A, Tundup S, Perez JT, Schmolke M, Manicassamy S, et al. RIG-I signaling is critical for efficient polyfunctional T cell responses during influenza virus infection. PLoS Pathog. 2016;12(7):e1005754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Stewart AG, Henden AS. Infectious complications of CAR T-cell therapy: a clinical update. Ther Adv Infect Dis. 2021;8:20499361211036773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Wilson Dib R, Ariza-Heredia E, Spallone A, Chemaly RF. Respiratory viral infections in recipients of cellular therapies: a review of incidence, outcomes, treatment, and prevention. Open Forum Infect Dis. 2023;10(4):ofad166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Negishi H, Yanai H, Nakajima A, Koshiba R, Atarashi K, Matsuda A, et al. Cross-interference of RLR and TLR signaling pathways modulates antibacterial T cell responses. Nat Immunol. 2012;13(7):659–66. [DOI] [PubMed] [Google Scholar]
- 280. Gao Y, Chen Y, Zhan S, Zhang W, Xiong F, Ge W. Comprehensive proteome analysis of lysosomes reveals the diverse function of macrophages in immune responses. Oncotarget. 2017;8(5):7420–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Di Cara F, Savary S, Kovacs WJ, Kim P, Rachubinski RA. The peroxisome: an up-and-coming organelle in immunometabolism. Trends Cell Biol. 2023;33(1):70–86. [DOI] [PubMed] [Google Scholar]
- 282. Settembre C, Perera RM. Lysosomes as coordinators of cellular catabolism, metabolic signalling and organ physiology. Nat Rev Mol Cell Biol. 2024;25(3):223–45. [DOI] [PubMed] [Google Scholar]