Abstract
Elevated hippocampal perfusion has been observed in people at clinical high risk for psychosis (CHR-P). Preclinical evidence suggests that hippocampal hyperactivity is central to the pathophysiology of psychosis, and that peripubertal treatment with diazepam can prevent the development of psychosis-relevant phenotypes. The present experimental medicine study examined whether diazepam can normalize hippocampal perfusion in CHR-P individuals. Using a randomized, double-blind, placebo-controlled, crossover design, 24 CHR-P individuals were assessed with magnetic resonance imaging (MRI) on two occasions, once following a single oral dose of diazepam (5 mg) and once following placebo. Regional cerebral blood flow (rCBF) was measured using 3D pseudo-continuous arterial spin labeling and sampled in native space using participant-specific hippocampus and subfield masks (CA1, subiculum, CA4/dentate gyrus). Twenty-two healthy controls (HC) were scanned using the same MRI acquisition sequence, but without administration of diazepam or placebo. Mixed-design ANCOVAs and linear mixed-effects models were used to examine the effects of group (CHR-P placebo/diazepam vs. HC) and condition (CHR-P diazepam vs. placebo) on rCBF in the hippocampus as a whole and by subfield. Under the placebo condition, CHR-P individuals (mean [±SD] age: 24.1 [±4.8] years, 15 F) showed significantly elevated rCBF compared to HC (mean [±SD] age: 26.5 [±5.1] years, 11 F) in the hippocampus (F(1,41) = 24.7, pFDR < 0.001) and across its subfields (all pFDR < 0.001). Following diazepam, rCBF in the hippocampus (and subfields, all pFDR < 0.001) was significantly reduced (t(69) = −5.1, pFDR < 0.001) and normalized to HC levels (F(1,41) = 0.4, pFDR = 0.204). In conclusion, diazepam normalized hippocampal hyperperfusion in CHR-P individuals, consistent with evidence implicating medial temporal GABAergic dysfunction in increased vulnerability for psychosis.
Subject terms: Diagnostic markers, Drug development, Psychosis, Transporters in the nervous system
Introduction
Transformations in our understanding of the nature, etiology, and early course of psychotic disorders drove a marked re-orientation of mental health services toward early intervention in individuals at clinical high risk for psychosis (CHR-P), raising the prospect that prevention of psychosis onset may be a realistic goal [1, 2]. Despite this progress, current treatments have a minimal influence on transition to psychosis [3, 4], and there is no robust evidence to favor any single preventive intervention over another [5, 6]. A better understanding of the neurobiology underlying the CHR-P phenotype is critical for the much-needed development of interventions to prevent psychosis onset.
Postmortem, preclinical, genetic, and clinical neuroimaging evidence suggests that hippocampal abnormalities are central to the pathophysiology of psychosis [7–9], thus representing a potential therapeutic target. Hippocampal dysfunction in psychosis is proposed to arise from a disruption in neural excitatory/inhibitory balance, likely driven by GABAergic inhibitory interneuron dysfunction [10–14], although glutamatergic receptor dysfunction is also implicated [15, 16]. This imbalance would render the hippocampus dysrhythmic and hyperactive [17], and excessive glutamatergic output [18–21] from this region to the striatum, amygdala, and prefrontal cortex may underlie the development of positive, negative, and cognitive symptoms, respectively [22]. Such network properties are supported by tractography evidence, demonstrating for example monosynaptic projections from the ventral hippocampus to the striatum [23]. Preclinical findings in the well-validated methylazoxymethanol acetate (MAM) rodent model of neurodevelopmental disruption [24–26] indicate that a loss of hippocampal parvalbumin-expressing (PV+) inhibitory interneurons [27] leads to hippocampal hyperactivity as measured with electrophysiology [28, 29]. Other rodent models have also shown that selective reduction in PV mRNA expression [30] or knock-out of PV+ interneuron [28, 29] or a glutamate-metabolizing enzyme [31] are each sufficient to induce hippocampal hyperactivity. In MAM-treated rats, hippocampal hyperactivity leads to striatal hyperdopaminergia [25], which is a core neurobiological feature of positive symptoms in schizophrenia [22]. This hippocampal hyperactivity can be normalized by hippocampal chemical inactivation [26, 32], or by pharmacologically facilitating GABA signaling through direct hippocampal infusion of either a nonselective (the benzodiazepine midazolam) [33] or selective positive allosteric modulator (PAM) for α5-subunit-containing GABAA receptors [33, 34]. Furthermore, repeated administration of the benzodiazepine diazepam during the peripubertal period to MAM-treated rats prevented the development of psychosis-relevant features in adulthood [35–37], highlighting the prophylactic potential of GABA-enhancing compounds for psychosis.
In CHR-P individuals, hippocampal hyperactivity has been observed in vivo [38–42], which – as a result of neurovascular coupling – is associated with increased regional cerebral blood flow or volume (rCBF or rCBV) by arterial spin labeling (ASL) or gadolinium-contrast magnetic resonance imaging (MRI), respectively. Elevated hippocampal rCBF in CHR-P individuals has been associated with elevated striatal pre-synaptic dopamine synthesis capacity [43] and medial prefrontal cortex GABA concentrations [44]. Baseline elevated hippocampal rCBF or rCBV was also found to predict higher positive and negative symptom severity [38], poor functioning [38, 43], and transition to psychosis [38, 39, 44], and to normalize in those individuals who remit from the CHR-P state [40]. Within the hippocampus, hyperactivity is proposed to originate in the CA1 subfield and then extend to the subiculum and beyond [9, 38, 39, 42]. Hence, hippocampal rCBF may be a marker for symptom severity and psychosis onset in CHR-P individuals, which preclinical evidence suggests may be prevented by pharmacological enhancement of hippocampal GABA levels [35–37].
Mechanistic research focussed on whether a well-characterized, marketed GABA-enhancing compound such as a benzodiazepine can downregulate hippocampal hyperactivity in CHR-P individuals would provide fundamental evidence for the development of more hippocampal-specific GABA-enhancing compounds for the at-risk stage. Prior positron or xenon emission tomography (PET or XET) research demonstrated global [45–48] and temporal lobe [45] reductions in CBF under an acute non-sedating dose of a benzodiazepine in healthy controls. However, no such studies have investigated the hippocampus specifically, or included CHR-P individuals. In the present experimental medicine study, we compared the acute effects of a single dose of diazepam vs. placebo on ASL-derived rCBF in the hippocampus and its subfields in a sample of antipsychotic-naïve CHR-P individuals. To determine baseline alterations in the CHR-P group, rCBF measures from CHR-P individuals under placebo were first compared to those from a sample of healthy controls (HC). We hypothesized that, compared to HC, CHR-P individuals under placebo would show increased rCBF in the hippocampus and its subfields, which would be most apparent in the CA1 subregion [38, 39]. Additionally, based on preclinical evidence [34], we hypothesized that a single dose of diazepam would reduce hippocampal rCBF in CHR-P individuals compared to placebo, and that this would be observed across all subfields due to their similar levels of GABAA receptor expression [49, 50]. For completeness, supplementary analyses examined broader effects of diazepam on voxel-wise grey matter (GM) rCBF and associations between baseline levels of symptoms/functioning and hippocampal rCBF in CHR-P individuals.
Materials and methods
Participants
Twenty-four CHR-P individuals, aged 18–32, were recruited from OASIS (Outreach and Support in South London), an early-intervention service within the South London and Maudsley NHS Foundation Trust, UK [51]. CHR-P criteria was determined using the Comprehensive Assessment of At-Risk Mental State (CAARMS) [52]. All individuals were required to be experiencing current attenuated psychotic symptoms, defined as having a severity and frequency score of ≥3 on P1-P4 of the CAARMS. Exclusion criteria included a psychosis/neurological disorder diagnosis, previous/current exposure to antipsychotics, current exposure to psychotropic medications with direct GABAergic/glutamatergic action (except for antidepressants, see Supplementary Methods for list of drug types), pregnancy/breastfeeding, IQ < 70, and any contraindication to MRI scanning. A flowchart of study participation, outlining participant dropouts, is displayed in Supplementary Fig. 1.
To validate hippocampal hyperactivity in this sample of CHR-P individuals, we utilized data from 22 HC from a previous study [53], acquired with the same MRI scanner, scanning sequences, and acquisition parameters as the CHR-P sample (see Supplementary Methods). HC data were reanalyzed with the same preprocessing steps as the CHR-P data. While HC participants were not assessed with the CAARMS, they scored very low on self-report questionnaires of schizotypy (measuring psychotic-like experiences) and were assessed with the Mini International Neuropsychiatric Inventory in order to exclude personal history of neurological/psychiatric disorders. Further details on recruitment and inclusion/exclusion criteria are described in the original publication [53].
Study design and procedure
This experimental medicine study had full ethical approval from the National Health Service UK Research Ethics Committee and was carried out at King’s College London. While the study received ethical clearance as ‘not a Clinical Trial of an Investigational Medicinal Product’ by the EU directive 2001/20/EC, it was registered on clinicaltrials.gov (NCT06190483). All participants provided written informed consent. Using a randomized, double-blind, placebo-controlled, crossover design, the 24 CHR-P participants underwent two MRI sessions, once under a single oral dose of diazepam (5 mg) and once under oral placebo (50 mg ascorbic acid), with a minimum 3-week washout period between visits. In the assessment visit, demographic information, basic medical history, and clinical/cognitive assessments were collected (CAARMS [52], Global Functioning Social and Role scales [54], Hamilton Anxiety and Depression scales [55, 56], Wechsler Adult Intelligence Scale III [57], Trail Making Test A & B [58]). At each scanning visit, the diazepam/placebo capsule was administered 60 min prior to MRI scanning, so that the MRI session coincided with peak diazepam plasma levels [59]. Further study procedure details can be found in the Supplementary Methods.
MRI acquisition
MRI scanning for all participants was conducted at the Centre for Neuroimaging Sciences, King’s College London, using a General Electric MR750 3.0 T MR scanner with an 8-channel head coil. rCBF was measured using a 3D pseudo-continuous ASL sequence (multi-shot 3D Fast Spin Echo Stack of Spirals) as used in previous CHR-P studies from our group [40, 41, 53], and T1-weighted SPGR and T2-weighted FRFSE images were also acquired. Further acquisition details can be found in Supplementary Methods.
Image processing
Generation of hippocampal/subfield and total GM masks
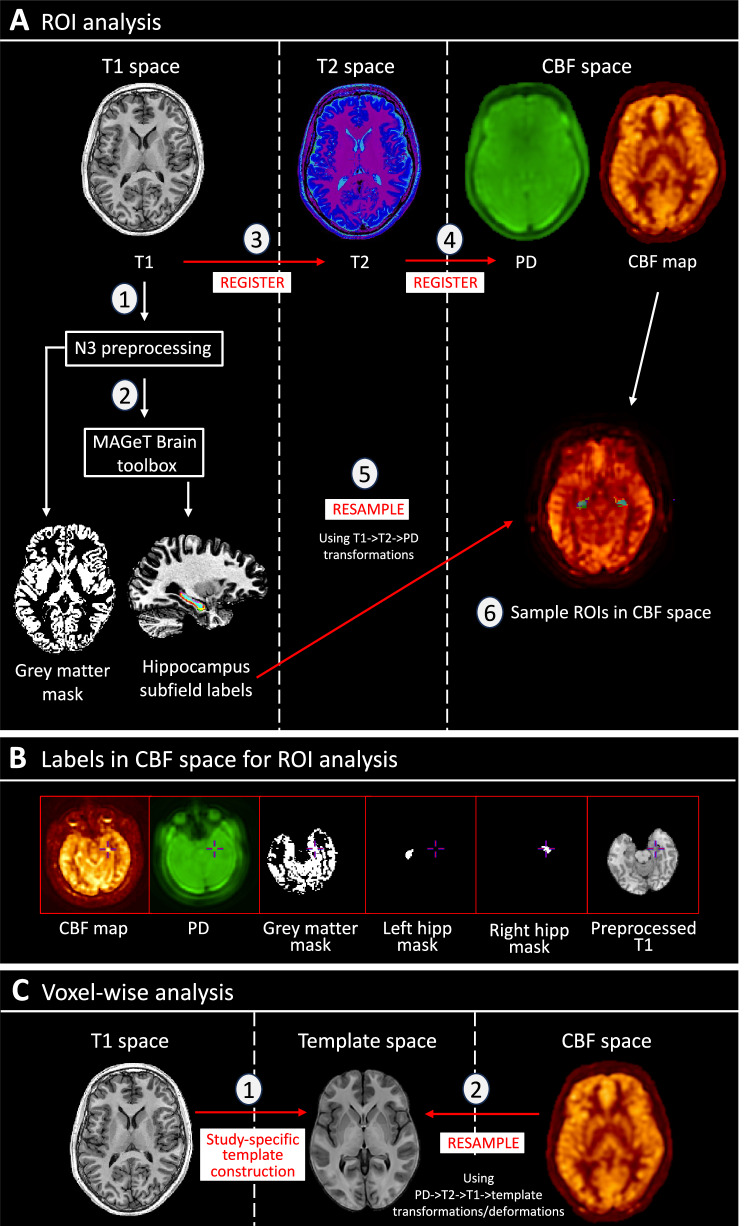
Structural scans were preprocessed using the N3 algorithm [60]. Hippocampus/subfield masks were generated for each participant from their preprocessed structural T1 scan by using the MAGeT Brain (multiple automatically generated templates of different brains) toolbox [61–63] (Fig. 1). This toolbox has been validated to generate hippocampus and subfield segmentations in Alzheimer’s disease and psychosis cohorts, with greater accuracy than other available toolboxes including Freesurfer 7 and FSL FIRST [62]. This is largely due to an intermediate template step which allows incorporating the neuroanatomical variability of the dataset into the segmentation of each participant, reducing registration and resampling errors, thereby yielding more accurate results. Hippocampal subfields CA2/3 were not included due to the limitations in reliably sampling these smaller regions within the spatial resolution and low signal-to-noise ratio of ASL [64]. Total GM masks were made through binarizing GM segmentations.
Fig. 1. ASL preprocessing and analysis pipeline.
A Diagram showing pipeline for region-of-interest (ROI) analysis. T1 images were preprocessed (1) and run through the MAGeT Brain toolbox (2), generating masks for grey matter, whole hippocampus, and hippocampal subfields. Using T1- > T2- > PD transformations calculated from registration (3 & 4), these masks were resampled (5) to CBF space (single resampling step) to allow for sampling of rCBF in native space (6). B Demonstration of optimum registration between CBF map, masks, and T1 images. C Schematic showing steps for voxel-wise analysis for CHR-P diazepam vs. placebo. A study-specific template was generated (1) from participant-averages (averaged structural scans from both drug conditions), and the CBF maps were resampled (2) to common space (single resampling step calculated from PD- > T2- > T1-> template transformations/deformations). hipp hippocampus, PD proton density, rCBF regional cerebral blood flow, ROI region-of-interest.
ASL sampling
Masks were registered and resampled to the individuals respective CBF map using ANTs/2.5.0 (https://github.com/ANTsX/ANTs), and the mean rCBF value was extracted per region-of-interest (ROI) per hemisphere in native space using minc-toolkit-v2/1.9.18 tools (https://bic-mni.github.io/; Fig. 1; Supplementary Methods).
Region-of-interest (ROI) analysis
All ROI analyses were completed in R 4.2.2 (https://www.r-project.org/). Individual models assessed the effect of group (CHR-P placebo/diazepam vs. HC) or condition (CHR-P diazepam vs. placebo) on rCBF per ROI (total GM, whole hippocampus, CA1, subiculum, CA4/DG). Each model included rCBF values per hemisphere, on the basis that bilateral sampling of the same region in the same subject reflects a repeated measurement. Therefore, a group/condition*hemisphere term was included to investigate whether the effect of group or condition on rCBF significantly differed within a region based on hemisphere. Significance was set at pFDR < 0.05, corrected for multiple comparisons [65].
CHR-P placebo/diazepam vs. HC
Two-way mixed analysis of covariance models (ANCOVA; package stats/3.6.2) assessed rCBF differences in CHR-P placebo/diazepam compared to HC, covarying for age, sex, and global CBF. Supplementary analyses covaried for demographic characteristics which differed between groups (IQ, ethnicity) or known to affect rCBF (daily current cigarette use [66] and ROI GM volume [67]).
CHR-P diazepam vs. CHR-P placebo
Diazepam-induced changes in rCBF compared to placebo were assessed using linear mixed-effects models (package lme4/1.1-34) with participant ID as a random effect (intercept). Given the within-subject design and widespread expression of GABAA receptors with benzodiazepine binding sites across the brain [49], global CBF was not included as a covariate. Supplementary analyses investigated potential confounding effects of global CBF, age, sex, order of scan conditions, and number of days between scans on the results.
Exploratory/supplementary analyses
Voxel-wise grey matter rCBF analysis
For completeness, we explored the effects of diazepam vs. placebo on voxel-wise GM rCBF (pFDR < 0.05). The two-level modelbuild toolkit (github.com/CoBrALab/optimized_ants MultivariateTemplateConstruction) was used to generate a study-specific anatomical template (Fig. 1; Supplementary Methods). CBF maps were resampled into common-space and smoothed with a 6-mm FWHM Gaussian kernel. A voxel-wise linear mixed-effects model (R-3.5.1, RMINC-1.5.2.2, lme4 1.1–21) was used to investigate the effect of condition (CHR-P diazepam vs. placebo) on rCBF, with participant ID as random effect and masked using a study-averaged GM mask. The above procedure was repeated for investigating voxel-wise group differences in rCBF between CHR-P placebo and HC, using the one-level model template build and running a voxel-wise linear model, covarying for global CBF, age, and sex.
Baseline clinical characteristics and hippocampal rCBF change
Supplementary Pearson’s correlation analyses assessed whether baseline clinical characteristics (positive, negative, cognitive, anxiety, and depression symptom severity and social and role functioning) were associated with mean percent change in bilateral hippocampal rCBF under diazepam vs. placebo (see Supplementary Methods for further details on composition of clinical scores). Confounding effects of global CBF on these results were explored using partial Pearson’s correlations. Outlier detection was performed on significant correlations using Cook’s distance. Significance was set at p < 0.05, and multiple comparison corrections were not performed as these analyses were exploratory.
Results
Participant demographic and clinical characteristics are detailed in Table 1. HC individuals had a significantly higher IQ and differed in terms of ethnicity compared to CHR-P, driven by an above-average mean IQ [68] and a high proportion of white ethnicity in the HC group. There were no significant differences in change between pre- and post-scan Bodily Symptom Scale [69] scores between the placebo and diazepam conditions (Supplementary Table 1).
Table 1.
Demographic and clinical characteristics.
| CHR-P (n = 24) | HC (n = 22) | t/χ2 | p | |
|---|---|---|---|---|
| Age (years; mean ±SD) | 24.1 ± 4.8 | 26.5 ± 5.1 | 1.7 | 0.09 |
| Sex (male/female; n) | 9/15 | 11/11 | 0.4 | 0.49 |
| Ethnicity (n) | 13.9 | 0.007 | ||
| White | 11 | 16 | – | – |
| Asian | 2 | 6 | – | – |
| Black | 6 | 0 | – | – |
| Mixed or multiple | 4 | 0 | – | – |
| Other | 1 | 0 | – | – |
| IQ (WAIS-III short version [68]; mean ±SD) | 97.6 ± 21.6 | 119.3 ± 16.7 | 4.6 | < 0.001 |
| Current daily cigarette use, n (%) | 8 (33) | 2 (9) | 3.7 | 0.055 |
| Current alcohol use, n (%) | 18 (75) | 19 (90) | 1.8 | 0.177 |
| Current cannabis use, n (%) | 7 (29) | 4 (19) | 0.6 | 0.431 |
| CAARMS [52] score (mean ±SD) | ||||
| Positive symptoms | 46.4 ± 12.9 | NA | – | – |
| Negative symptoms (n = 21) | 29.1 ± 24.7 | NA | – | – |
| Total (n = 21) | 75.9 ± 29.9 | NA | – | – |
| Global functioning score [54] (mean ± SD) | ||||
| Social | 6.4 ± 1.5 | NA | – | – |
| Role | 6.1 ± 1.8 | NA | – | – |
| Hamilton scale score (mean ± SD) | ||||
| Anxiety [55] (n = 22) | 17.1 ± 8.5 | NA | – | – |
| Depression [56] (n = 21) | 13.9 ± 6.9 | NA | – | – |
| Current antidepressant medication, n (%) | 9 | NA | – | – |
| Current or prior antipsychotic medication, n (%) | 0 | NA | – | – |
| Current benzodiazepine/hypnotic medication, n (%) | 0 | NA | – | – |
p statistics which are < 0.05 are in bold and this denotes a significant difference between groups.
CAARMS comprehensive assessment of at-risk mental states, CHR-P clinical high-risk for psychosis, HC healthy control, IQ intelligent quotient, WAIS Weschler adult intelligence scale.
ROI analysis
CHR-P placebo vs. HC
Global CBF
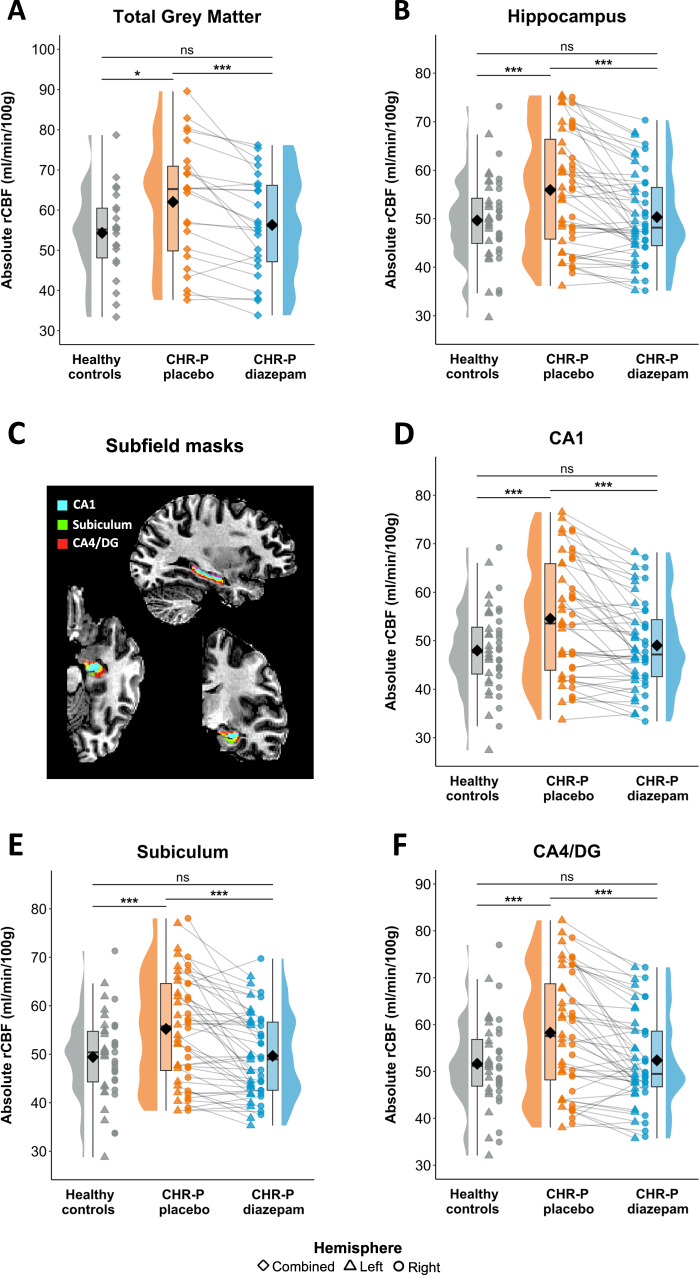
Mean CBF in total brain GM (ml/100 g/min) was significantly higher (F(1,42) = 5.2, pFDR = 0.014, Cohen’s d = 0.59) in CHR-P individuals under placebo (62.1 ± 14.9) compared to HC (54.3 ± 10.9; Fig. 2A).
Fig. 2. Region-of-interest rCBF findings.
Absolute rCBF for HC and CHR-P participants (under placebo and diazepam) for A total grey matter, B hippocampus, and C hippocampus subfields, including D CA1, E subiculum, and F CA4/DG. CHR-P clinical high risk for psychosis, DG dentate gyrus, rCBF regional cerebral blood flow, ns non-significant; * < 0.05; *** < 0.001.
Hippocampus and subfield rCBF
After controlling for global CBF, age, and sex, CHR-P participants in the placebo condition had significantly higher rCBF compared to HC in the hippocampus (F(1,41) = 24.7, pFDR < 0.001, Cohen’s d = 0.60), which did not differ between hemispheres (F(1,44) = 1.6, pFDR = 0.217; Fig. 2B). Similar results were found for all subfields: CA1 (group: F(1,41) = 25.8, pFDR < 0.001, Cohen’s d = 0.62; group*hemisphere: F(1,44) = 1.1, pFDR = 0.364; Fig. 2D), subiculum (group: F(1,41) = 25.7, pFDR < 0.001, Cohen’s d = 0.59; group*hemisphere: F(1,44) = 1.2, pFDR = 0.274; Fig. 2E), and CA4/DG (group: F(1,41) = 20.4, pFDR < 0.001, Cohen’s d = 0.59; group*hemisphere: F(1,44) = 3.2, p = 0.082; Fig. 2F). These results did not change when adding covariates of no-interest (IQ, ethnicity, current daily cigarette use, ROI GM volume; Supplementary Table 2).
CHR-P diazepam vs. CHR-P placebo
Global CBF
In CHR-P participants, mean CBF in total brain GM was significantly lower (t(23) = −4.3, pFDR < 0.001, Cohen’s d = −0.88) under diazepam (56.3 ± 12.7) compared to placebo (62.1 ± 14.9; Fig. 2A).
Hippocampus and subfield rCBF
Diazepam significantly reduced rCBF in the hippocampus (t(69) = −5.1, pFDR < 0.001, Cohen’s d = −0.83), which did not differ between hemispheres (t(69) = 0.9, pFDR = 0.366; Fig. 2B). This effect was observed across all subfields: CA1 (condition: t(69) = −5.1, pFDR < 0.001, Cohen’s d = −0.83; condition*hemisphere: t(69) = 0.8, pFDR = 0.403; Fig. 2D), subiculum (condition: t(69) = −4.9, pFDR < 0.001, Cohen’s d = −0.76; condition*hemisphere: t(69) = 1.1, pFDR = 0.303; Fig. 2E), and CA4/DG (condition: t(69) = −4.7, pFDR < 0.001, Cohen’s d = −0.79; condition*hemisphere: t(69) = 0.8, p = 0.405; Fig. 2F). These results did not change after controlling for global CBF, age, sex, order of scan conditions, or number of days between scans (Supplementary Table 2).
CHR-P diazepam vs. HC
Global CBF
There was no significant difference (F(1,42) = 0.5, pFDR = 0.209, Cohen’s d = 0.17) in mean total brain GM CBF in CHR-P in the diazepam condition (56.3 ± 12.7) compared to HC (54.3 ± 10.9; Fig. 2A).
Hippocampus and subfield rCBF
There was no significant difference in rCBF between CHR-P under diazepam compared to HC in the hippocampus (F(1,41) = 0.4, pFDR = 0.204, Cohen’s d = 0.08; Fig. 2B), CA1 (F(1,41) = 0.9, pFDR = 0.153, Cohen’s d = 0.12; Fig. 2D), subiculum (F(1,41) = 0.8, pFDR = 0.272, Cohen’s d = 0.03; Fig. 2E), or CA4/DG (F(1,41) = 0.3, pFDR = 0.201; Fig. 2F, Cohen’s d = 0.08).
Exploratory/supplementary analyses
Voxel-wise grey matter rCBF analysis
CHR-P placebo vs. HC
Several cortical regions showed higher (e.g., inferior/dorsolateral frontal gyrus and temporal pole) and lower (e.g., inferior parietal and middle occipital gyrus) rCBF in the CHR-P placebo condition compared to HC (pFDR< 0.05; Supplementary Fig. 2; Supplementary Table 3).
CHR-P diazepam vs. CHR-P placebo
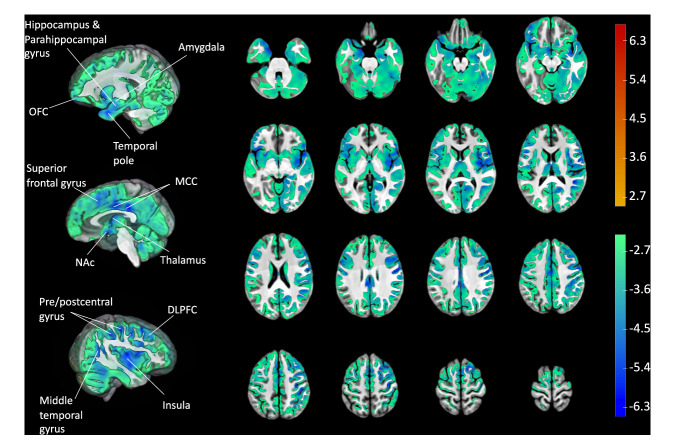
There was a global pattern of reduced rCBF under diazepam vs. placebo (Fig. 3; Supplementary Table 4). Peak voxels (all pFDR < 0.01) were located in temporal (temporal pole, parahippocampal gyrus, hippocampus, amygdala, middle temporal gyrus), parietal (pre/post central gyrus, middle cingulate), frontal (dorsolateral prefrontal cortex, ventromedial orbitofrontal cortex, insula, superior frontal gyrus), and occipital (lingual gyrus, occipital gyrus) regions, cerebellum, and subcortical regions (thalamus, putamen, caudate, and nucleus accumbens).
Fig. 3. Voxel-wise grey matter rCBF findings.
T-statistic map of drug condition (diazepam vs. placebo) effect on grey matter rCBF at the whole-brain level in CHR-P individuals from voxel-wise linear mixed effects models, thresholded and displayed at 5% FDR. Peak regions with t-statistic > 5 have been labeled. Color bars denote t-statistics which reflect 5% FDR threshold (i.e., ±2.498) and less (i.e., up to ±6.68) for both contrasts (diazepam < placebo in blue/green and placebo < diazepam in yellow/red). N.B. there were no significant voxels at 5% FDR threshold for placebo < diazepam. DLPFC dorsolateral prefrontal cortex, MCC middle cingulate cortex, NAc nucleus accumbens, OFC orbitofrontal cortex, rCBF regional cerebral blood flow.
Clinical characteristics and hippocampal rCBF change
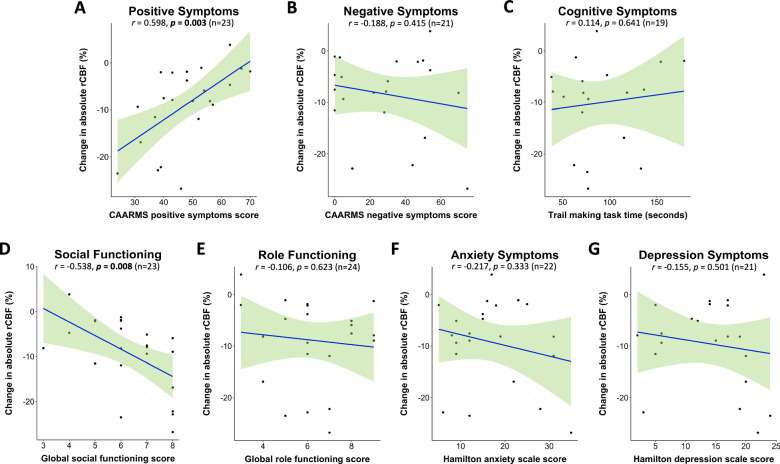
Exploratory analyses revealed significant associations between baseline clinical characteristics and change in hippocampal rCBF, such that higher baseline positive symptom severity (r = 0.494, p = 0.014) and poorer social functioning (r = −0.416, p = 0.043) correlated with less reduction in hippocampal rCBF following diazepam vs. placebo. Removal of outliers strengthened these correlations (positive symptoms: r = 0.598, p = 0.003; Fig. 4A; social functioning: r = −0.538, p = 0.008; Fig 4D). There were no significant correlations between diazepam-induced change in whole hippocampal rCBF and baseline negative (r = −0.188, p = 0.415; Fig. 4B), cognitive (r = 0.114, p = 0.641; Fig. 4C), anxiety (r = −0.217, p = 0.333; Fig. 4F), or depression (r = −0.155, p = 0.501; Fig. 4G) symptoms or role functioning (r = −0.106, p = 0.623; Fig. 4E). Most of these results remained unchanged when adding global CBF as covariate (Supplementary Table 5). There were no significant associations between baseline symptoms/functioning and hippocampal rCBF under placebo (Supplementary Table 6).
Fig. 4. Association between baseline clinical characteristics and diazepam-induced hippocampal rCBF changes.
Pearson correlations between change in absolute hippocampal rCBF by diazepam vs. placebo and baseline clinical characteristics (at assessment visit): A positive symptoms (n = 23), B negative symptoms (n = 21), C cognitive functioning (n = 19), D social functioning (n = 23), E role functioning (n = 24), F anxiety symptoms (n = 22), and G depression symptoms (n = 21). N.B. for panels D & E a higher score denotes less impairment, whilst for all other panels a higher score/time denotes higher symptom severity. Shaded light green areas reflect 95% confidence intervals. The number of participants differs between panels due to the removal of outliers or missing data. rCBF regional cerebral blood flow.
Discussion
In our study, CHR-P individuals under placebo showed significantly higher hippocampal rCBF compared to HC. Following diazepam challenge, hippocampal rCBF in CHR-P individuals was significantly reduced compared to placebo and normalized to HC levels. This effect was also evident across all hippocampal subfields. These results are consistent with data from psychosis-relevant preclinical models and lend new empirical support for the development and investigation of more hippocampal-selective GABA-enhancing compounds as a potential therapeutic strategy in early psychosis.
Our finding of elevated hippocampal rCBF in CHR-P (under placebo) compared to HC is consistent with previous studies in CHR-P individuals [38–42]. Although we also identified higher global CBF, as found previously [40], hippocampal rCBF remained significantly elevated after controlling for global CBF, suggesting this region is particularly hyperactive. However, it is important to note we did not observe elevated hippocampal rCBF at the whole-brain level. Hippocampal rCBF was still significantly higher in CHR-P (under placebo) vs. HC on the ROI level after controlling for GM volume within that region, suggesting it was not driven by putative underlying GM differences in CHR-P individuals [70]. We hypothesized that elevations would be most pronounced in the CA1 subfield, based on prior rCBV studies in CHR-P individuals [38, 39, 42] and current models of psychosis pathophysiology [7]. However, in the ROI analysis, we observed similarly elevated rCBF across CA1, subiculum, and CA4/DG. Our finding may be related to the reduced spatial resolution of ASL compared to gadolinium-contrasted MRI as used in prior studies [38, 39, 42]. In addition, we sampled rCBF across the whole length of each subfield, instead of only anterior portions as done in those previous studies [38, 39, 42], hence it is possible that posterior portions also showed hyperactivity. Indeed, prior ASL studies in CHR-P [40, 41] and high schizotypy [53] individuals reported peak increases within the body/tail of the hippocampus. Additionally, our study used rCBF which is more tightly coupled to neuronal activity than rCBV [71], sampled in native vs. common space thereby circumventing normalization errors [72], and sampled with individual hippocampal/subfield masks thereby affording higher accuracy given the neuroanatomical diversity among individuals [73].
In line with our second hypothesis, diazepam significantly reduced hippocampal and subfield rCBF vs. placebo in CHR-P individuals, to the extent that rCBF was no longer significantly different to HC. This finding aligns with predictions from preclinical studies [34]. In the MAM rodent model, hippocampal hyperactivity is associated with a local reduction of GABAergic PV+ interneurons [28–30], and increasing GABAergic inhibition by hippocampal infusion of an α5-GABAA PAM normalizes hippocampal hyperactivity [34]. Importantly, repeated oral administration of diazepam in peripubertal MAM rats prevents local PV+ interneuron loss [36] and the emergence of a hyperdopaminergic state at adulthood [35]. A likely mechanism is downregulation of amygdala-hippocampal overdrive, which causes PV+ interneuron loss in the hippocampus and, consequently, hippocampal hyperactivity. This is supported by findings that direct hippocampal infusion of the benzodiazepine midazolam normalizes increased dopamine neuron firing in the VTA of adult MAM rats [33]. Our finding of diazepam-induced reductions in rCBF across all hippocampal subfields aligns with the pharmacology of benzodiazepines and GABAA receptor distribution [50, 74]. Benzodiazepines are PAMs of the GABAA receptor via the benzodiazepine site, composed of an α1-3/α5 and gamma subunit [75]. Whilst GABAA receptors are expressed on several cell types and sites [76], most commonly benzodiazepine binding facilitates greater hyperpolarization of post-synaptic glutamatergic pyramidal cells and reduced pyramidal cell firing [75], resulting in reduced metabolic requirements and, therefore, reduced rCBF [77]. α1-3/α5-GABAA receptors (and therefore benzodiazepine sites) are highly expressed across the hippocampus [49, 50], and although there are slight differences in the levels of α1-3/α5 receptor subunits across hippocampal subfields [74], hippocampal subfields are highly interconnected [75]. Therefore, it is intuitive that diazepam was associated with a similar magnitude of reduction in rCBF across subfields.
Complementary voxel-wise analyses revealed rCBF reductions across multiple other cortical and subcortical regions in the diazepam condition. The largest reductions were seen in the pre/post central gyrus and inferior frontal regions, areas with the highest benzodiazepine receptor binding sites [49] and which receive projections from the hippocampus [18, 19]. Large rCBF reductions were also seen in the striatum, ventromedial PFC, and amygdala, which together with the hippocampus compose a cortico-limbic-striatal circuit proposed to be central to the pathophysiology of psychosis [22]. Whilst the hippocampus projects directly to these regions [78–80], it is not possible to determine whether reductions in rCBF here are due to primary drug effects (i.e., due to local increases in GABAergic inhibition), or secondary, downstream effects deriving from drug-induced reductions in hippocampal hyperactivity. The reductions in rCBF observed in further cortical regions may be related to the ubiquitous binding profile of benzodiazepines. α1–α3 receptor subunits, implicated in benzodiazepine-related side effects such as sedation and addiction, show widespread cortical distribution [81]. Conversely, α5-GABAA receptors are preferentially expressed in the hippocampus [50, 74], and are not associated with such side effects [81]. PET studies have demonstrated that compared to healthy controls, antipsychotic-free patients with schizophrenia showed reduced hippocampal binding of an α5-GABAA selective ligand [82], but along with CHR-P individuals [83], show no differences with less specific α1-3,5-GABAA ligands [84–87]. In line with this, evidence from the MAM model shows (i) α5 but not α1-3-GABAA receptors are reduced in the subiculum and CA1 [88], (ii) overexpression of the α5, but not α1, subunit normalizes hippocampal hyperactivity [32], and (iii) an α5-GABAA PAM was able to normalize hippocampal hyperactivity [34] and attenuate VTA dopaminergic firing to a greater extent than the non-specific benzodiazepine midazolam [33]. Taken together, pharmacological agents with high selectivity for α5-GABAA receptors may be able to regulate hippocampal hyperactivity more specifically in psychosis while potentially avoiding some of the unwanted side effects of less-selective benzodiazepines. While several α5-GABAA PAMs exist [89, 90], none have yet proceeded to clinical development for psychosis.
Finally, exploratory analyses suggested that diazepam-induced reductions in hippocampal rCBF were smallest in CHR-P participants with higher baseline positive symptoms severity and poorer social functioning, although no significant associations were observed between symptoms/functioning and hippocampal rCBF under placebo. Interpretation of these findings is limited by low power for examining correlations between clinical and imaging variables, and differences in timing between clinical and MRI measurements (i.e., 1st and 2nd scan were ~2 and ~6 weeks after assessment visit). Nonetheless, current theories propose persistent hyperactivity (i.e., excessive glutamate release) in the hippocampus of CHR-P individuals may lead to atrophy of neuropil and PV+ inhibitory interneurons [7]. Therefore, diazepam may not be able to downregulate rCBF as effectively in CHR-P individuals with a more severe clinical profile and with a potentially greater degree of GABAergic dysfunction. Following development of a primary psychotic disorder, rCBF alterations appear to change with disorder progression and antipsychotic treatment. Whilst higher temporal lobe rCBF has also been reported in drug-naïve first episode psychosis individuals [91], no significant temporal rCBF differences were found in individuals with prior [92] or current [93, 94] antipsychotic-treatment, and people with chronic schizophrenia show temporal hypoperfusion [95]. In this regard, preclinical work in MAM-treated rats revealed that antipsychotic exposure blocked the therapeutic effects of GABA-enhancing compounds on psychosis-relevant phenotypes [96]. Taken together, this evidence suggests that, for individuals with a primary psychotic disorder, GABA-enhancing compounds may only be efficacious for regulating hippocampal hyperactivity when administered prior to initiating antipsychotic treatment.
Overall, this experimental medicine study presents first-in-human evidence of successful down-regulation of hippocampal hyperactivity by pharmacological modulation of the GABAergic system in CHR-P individuals. We used a robust gold-standard study design, with an adequately powered sample based on prior methodological research [97] and retrospective power analysis: with an effect size range of Cohen’s d = 0.76–0.88 and sample size of n = 24 we had an achieved power of 97–99%. All participants in our study were antipsychotic-naïve, avoiding the known effects of antipsychotics on rCBF or the GABAergic system [96, 98, 99]. Subjective effects related to sleep/sedation did not differ between the placebo and diazepam conditions, indicating that these were unlikely to affect the observed rCBF differences between conditions. We used advanced computational neuroimaging methods to segment the hippocampal subfields with high accuracy and maintained this level of accuracy by sampling rCBF with participant-specific masks in native space. We used ASL, a highly suitable neuroimaging measure for investigating the effects of a GABAergic drug challenge on hippocampal function, given it measures rCBF in a fully quantitative, non-invasive manner.
This study also has some limitations. Firstly, despite segmenting all hippocampal subfields, we could not reliably sample the smaller CA2/3 subfield due to the spatial resolution of ASL. Additionally, we were not able to restrict sampling of the hippocampal subfields to only the anterior sections (which are relevant in the pathophysiology of psychosis [7]) due to limitations of the segmentation methodology. Secondly, unlike the CHR-P group, the HC group were scanned in the absence of a placebo condition, which may have impacted rCBF. Thirdly, our study sample was smaller than previous studies comparing hippocampal rCBF between HC and CHR-P [40, 41], hence our analysis comparing HC and CHR-P individuals under the diazepam condition may have been underpowered to detect a significant difference. Finally, we did not include data on specific clinical outcomes (such as transition to psychosis) because the study was not designed nor powered for this.
Conclusions
This study provides first evidence that a single dose of a non-specific GABA-enhancing drug like diazepam can significantly reduce hippocampal and subfield hyperactivity in CHR-P individuals and normalize it to HC levels. Diazepam-associated reductions in rCBF were also observed in other cortico-limbic-striatal regions, supporting further network-based investigations of whether diazepam can modulate this circuit in CHR-P individuals. Furthermore, the results validate the use of ASL and native-space hippocampal and subfield sampling as viable biomarker endpoints for the development of more hippocampal-selective GABA-enhancing compounds for psychosis prevention.
Supplementary information
Acknowledgements
We would like to thank all our participants who took the time to participate in this study, as well as the OASIS clinical team members and the radiographers at the Centre for Neuroimaging Sciences.
Author contributions
NRL: Methodology, Software, Formal analysis, Investigation, Data curation, Writing – original draft, Visualization, Project administration; Funding acquisition. AK: Investigation, Writing – review & editing, Project administration; GAD: Software, Formal analysis, Writing – review & editing; SK: Investigation, Writing – review & editing; PBL: Investigation, Writing – review & editing; LJ: Investigation, Writing – review & editing; TR: Investigation, Writing – review & editing; AD: Investigation, Writing – review & editing; MAN: Investigation, Writing – review & editing; CC: Investigation, Writing – review & editing; TA: Formal analysis, Writing – review & editing; FZ: Software, Resources, Writing – review & editing; TS: Resources, Writing – review & editing; ADM: Resources, Writing – review & editing; PFP: Resources, Writing – review & editing; AAG: Writing – review & editing; SCRW: Resources, Writing – review & editing; PM: Conceptualization, Resources, Writing – review & editing; AE: Methodology, Writing – review & editing, Supervision; MMC: Software, Methodology, Writing – review & editing, Supervision; GM: Conceptualization, Methodology, Writing – review & editing, Supervision, Funding acquisition.
Funding
This independent research was funded by the Wellcome Trust & The Royal Society (grant number 202397/Z/16/Z to GM), and was partly funded and carried out at the National Institute for Health and Care Research (NIHR) Maudsley Biomedical Research Centre (BRC). The views expressed are those of the authors and not necessarily those of the Wellcome Trust & The Royal Society, the NIHR, or the Department of Health and Social Care. For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) license to any Accepted Author Manuscript version arising from this submission. NRL is supported by an MRC DTP PhD studentship. LAJ is supported by an MRC Clinical Research Training Fellowship (MR/T028084/1). PFP is supported by the European Union funding within the MUR PNRR Extended Partnership initiative on Neuroscience and Neuropharmacology (Project no. PE00000006 CUP H93C22000660006 “MNESYS, A multiscale integrated approach to the study of the nervous system in health and disease”). TJR Is supported by an MRC Clinical Research Training Fellowship (MR/W015943/1). AAG is supported by the National Institute of Mental Health (USPHS MH57440).
Data availability
Data will be made freely available upon publication (10.6084/m9.figshare.24763839), including (i) mean rCBF values per subject per ROI per hemisphere per condition, and (ii) coding scripts for the MRI preprocessing pipeline (run in Unix/shell) and generation of figures (run in R).
Competing interests
GM has received consulting fees from Boehringer Ingelheim. AE has received consulting fees from Leal Therapeutics. AAG has received funds from Lundbeck, Pfizer, Lilly, Roche, Janssen, Alkermes, Newron, Takeda and Merck. SCRW has recently received research funding from Boehringer Ingelheim and GE Healthcare to perform investigator-led research. All other authors have nothing to disclose.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-024-01864-9.
References
- 1.Fusar-Poli P. Integrated mental health services for the developmental period (0 to 25 Years): a critical review of the evidence. Front Psychiatry. 2019;10:355. doi: 10.3389/fpsyt.2019.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mcgorry PD, Killackey E, Yung A. Early intervention in psychosis: concepts, evidence and future directions. World Psychiatry. 2008;7:148–56. doi: 10.1002/j.2051-5545.2008.tb00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fusar-Poli P, Davies C, Solmi M, Brondino N, De Micheli A, Kotlicka-Antczak M, et al. Preventive treatments for psychosis: umbrella review (Just the Evidence) Front Psychiatry. 2019;10:764. doi: 10.3389/fpsyt.2019.00764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosnjak Kuharic D, Kekin I, Hew J, Rojnic Kuzman M, Puljak L. Interventions for prodromal stage of psychosis. Cochrane Database Syst Rev. 2019 doi: 10.1002/14651858.CD012236.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies C, Cipriani A, Ioannidis JPA, Radua J, Stahl D, Provenzani U, et al. Lack of evidence to favor specific preventive interventions in psychosis: a network meta-analysis. World Psychiatry. 2018 doi: 10.1002/wps.20526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devoe DJ, Farris MS, Townes P, Addington J. Interventions and transition in youth at risk of psychosis: a systematic review and meta-analyses. J Clin Psychiatry. 2020;81:9326. doi: 10.4088/JCP.17r12053. [DOI] [PubMed] [Google Scholar]
- 7.Lieberman JA, Girgis RR, Brucato G, Moore H, Provenzano F, Kegeles L, et al. Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention. Mol Psychiatry. 2018 doi: 10.1038/mp.2017.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knight S, McCutcheon R, Dwir D, Grace AA, O’Daly O, McGuire P, et al. Hippocampal circuit dysfunction in psychosis. Transl Psychiatry. 2022;12:344. doi: 10.1038/s41398-022-02115-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo J, Rothman DL, Small SA. Why hippocampal glutamate levels are elevated in schizophrenia. JAMA Psychiatry. 2023;80:274–5. doi: 10.1001/jamapsychiatry.2022.3849. [DOI] [PubMed] [Google Scholar]
- 10.Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;44:88–97. doi: 10.1016/S0006-3223(98)00138-3. [DOI] [PubMed] [Google Scholar]
- 11.Heckers S, Stone D, Walsh J, Shick J, Koul P, Benes FM. Differential hippocampal expression of glutamic acid decarboxylase 65 and 67 messenger RNA in bipolar disorder and schizophrenia. Arch Gen Psychiatry. 2002;59:521–9. doi: 10.1001/archpsyc.59.6.521. [DOI] [PubMed] [Google Scholar]
- 12.Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res. 2002;55:1–10. doi: 10.1016/S0920-9964(01)00188-8. [DOI] [PubMed] [Google Scholar]
- 13.Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, et al. Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res. 2011;131:165–73. doi: 10.1016/j.schres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang AY, Lohmann KM, Yang CK, Zimmerman EI, Pantazopoulos H, Herring N, et al. Bipolar disorder type 1 and schizophrenia are accompanied by decreased density of parvalbumin- and somatostatin-positive interneurons in the parahippocampal region. Acta Neuropathol. 2011;122:615–26. doi: 10.1007/s00401-011-0881-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh T, Poterba T, Curtis D, Akil H, Al Eissa M, Barchas JD. Rare coding variants in ten genes confer substantial risk for schizophrenia. Nature. 2022;604:509–16. doi: 10.1038/s41586-022-04556-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trubetskoy V, Pardiñas AF, Qi T, Panagiotaropoulou G, Awasthi S, Bigdeli TB, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022;604:502–8. doi: 10.1038/s41586-022-04434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heckers S, Konradi C. GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophr Res. 2015 doi: 10.1016/j.schres.2014.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev. 2007;56:1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groenewegen HJ, der Zee EVV, te Kortschot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23:103–20. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- 20.Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behav Brain Res. 2006;174:215–24. doi: 10.1016/j.bbr.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 21.Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comparat Neurol. 1991;313:574–86. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- 22.Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 2016 doi: 10.1038/nrn.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–39. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry. 2006 doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007 doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lodge DJ, Grace AA. Hippocampal dysfunction and disruption of dopamine system regulation in an animal model of schizophrenia. Neurotox Res. 2008 doi: 10.1007/BF03033801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009 doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilani AI, Chohan MO, Inan M, Schobel SA, Chaudhury NH, Paskewitz S, et al. Interneuron precursor transplants in adult hippocampus reverse psychosis-relevant features in a mouse model of hippocampal disinhibition. Proc Natl Acad Sci USA. 2014;111:7450–5. doi: 10.1073/pnas.1316488111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiemes A, Serrano Navacerrada ME, Kim E, Randall K, Simmons C, Rojo Gonzalez L, et al. Erbb4 deletion from inhibitory interneurons causes psychosis-relevant neuroimaging phenotypes. Schizophr Bull. 2022;49:569–80. doi: 10.1093/schbul/sbac192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boley AM, Perez SM, Lodge DJ. A fundamental role for hippocampal parvalbumin in the dopamine hyperfunction associated with schizophrenia. Schizophr Res. 2014 doi: 10.1016/j.schres.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lander SS, Khan U, Lewandowski N, Chakraborty D, Provenzano FA, Mingote S, et al. Glutamate dehydrogenase–deficient mice display schizophrenia-like behavioral abnormalities and CA1-specific hippocampal dysfunction. Schizophr Bull. 2019;45:127–37. doi: 10.1093/schbul/sby011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donegan JJ, Boley AM, Yamaguchi J, Toney GM, Lodge DJ. Modulation of extrasynaptic GABAA alpha 5 receptors in the ventral hippocampus normalizes physiological and behavioral deficits in a circuit specific manner. Nat Commun. 2019;10:2819. doi: 10.1038/s41467-019-10800-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perez SM, McCoy AM, Prevot TD, Mian MY, Carreno FR, Frazer A, et al. Hippocampal α5-GABAA receptors modulate dopamine neuron activity in the rat ventral tegmental area. Biol Psychiatry Global Open Sci. 2023;3:78–86. doi: 10.1016/j.bpsgos.2021.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gill KM, Lodge DJ, Cook JM, Aras S, Grace AA. A novel α5GABA a r-positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du Y, Grace AA. Peripubertal diazepam administration prevents the emergence of dopamine system hyperresponsivity in the MAM developmental disruption model of schizophrenia. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du Y, Grace AA. Loss of parvalbumin in the hippocampus of MAM schizophrenia model rats is attenuated by peripubertal diazepam. Int J Neuropsychopharmacol. 2016 doi: 10.1093/ijnp/pyw065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du Y, Grace AA. Amygdala Hyperactivity in MAM Model of Schizophrenia is Normalized by Peripubertal Diazepam Administration. Neuropsychopharmacology. 2016;41:2455–62. doi: 10.1038/npp.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009 doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I, et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013 doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen P, Chaddock CA, Egerton A, Howes OD, Bonoldi I, Zelaya F, et al. Resting hyperperfusion of the hippocampus, midbrain, and basal ganglia in people at high risk for psychosis. Am J Psychiatry. 2016 doi: 10.1176/appi.ajp.2015.15040485. [DOI] [PubMed] [Google Scholar]
- 41.Allen P, Azis M, Modinos G, Bossong MG, Bonoldi I, Samson C, et al. Increased resting hippocampal and basal ganglia perfusion in people at ultra high risk for psychosis: replication in a second cohort. Schizophr Bull. 2018 doi: 10.1093/schbul/sbx169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Provenzano FA, Guo J, Wall MM, Feng X, Sigmon HC, Brucato G, et al. Hippocampal pathology in clinical high-risk patients and the onset of schizophrenia. Biol Psychiatry. 2020;87:234–42. doi: 10.1016/j.biopsych.2019.09.022. [DOI] [PubMed] [Google Scholar]
- 43.Modinos G, Richter A, Egerton A, Bonoldi I, Azis M, Antoniades M, et al. Interactions between hippocampal activity and striatal dopamine in people at clinical high risk for psychosis: relationship to adverse outcomes. Neuropsychopharmacology. 2021;46:0. doi: 10.1038/s41386-021-01019-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Modinos G, Şimşek F, Azis M, Bossong M, Bonoldi I, Samson C, et al. Prefrontal GABA levels, hippocampal resting perfusion and the risk of psychosis. Neuropsychopharmacology. 2018;43:2652–9. doi: 10.1038/s41386-017-0004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mathew RJ, Wilson WH, Daniel DG. The effect of nonsedating doses of diazepam on regional cerebral blood flow. Biological Psychiatry. 1985;20:1109–16. doi: 10.1016/0006-3223(85)90010-1. [DOI] [PubMed] [Google Scholar]
- 46.Mathew RJ, Wilson WH. Evaluation of the effects of diazepam and an experimental anti-anxiety drug on regional cerebral blood flow. Psychiatry Res Neuroimaging. 1991;40:125–34. doi: 10.1016/0925-4927(91)90004-A. [DOI] [PubMed] [Google Scholar]
- 47.Matthew E, Andreason P, Pettigrew K, Carson RE, Herscovitch P, Cohen R, et al. Benzodiazepine receptors mediate regional blood flow changes in the living human brain. Proc Natl Acad Sci USA. 1995;92:2775–9. doi: 10.1073/pnas.92.7.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moresco RM, Tettamanti M, Gobbo C, Del Sole A, Ravasi L, Messa C, et al. Acute effect of 3-(4-acetamido)-butyrril-lorazepam (DDS2700) on brain function assessed by PET at rest and during attentive tasks. Nucl Med Commun. 2001;22:399–404. doi: 10.1097/00006231-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Nørgaard M, Beliveau V, Ganz M, Svarer C, Pinborg LH, Keller SH, et al. A high-resolution in vivo atlas of the human brain’s benzodiazepine binding site of GABAA receptors. Neuroimage. 2021;232:117878. doi: 10.1016/j.neuroimage.2021.117878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. GABAA receptor subunits in the rat hippocampus I: Immunocytochemical distribution of 13 subunits. Neuroscience. 1997;80:987–1000. doi: 10.1016/S0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]
- 51.Fusar-Poli P, Spencer T, De Micheli A, Curzi V, Nandha S, Mcguire P. Outreach and support in South-London (OASIS) 2001-20: Twenty years of early detection, prognosis and preventive care for young people at risk of psychosis. Eur Neuropsychopharmacol. 2020;39:111–22. doi: 10.1016/j.euroneuro.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yung AR, Yuen HP, McGorry PD, Phillips LJ, Kelly D, Dell’Olio M, et al. Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. Aust N Z J Psychiatry. 2005 doi: 10.1111/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]
- 53.Modinos G, Egerton A, McMullen K, McLaughlin A, Kumari V, Barker GJ, et al. Increased resting perfusion of the hippocampus in high positive schizotypy: a pseudocontinuous arterial spin labeling study. Human Brain Mapp. 2018 doi: 10.1002/hbm.24231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carrión RE, Auther AM, McLaughlin D, Olsen R, Addington J, Bearden CE, et al. The global functioning: Social and role scales-further validation in a large sample of adolescents and young adults at clinical high risk for psychosis. Schizophr Bull. 2019;45. 10.1093/schbul/sby126 [DOI] [PMC free article] [PubMed]
- 55.Hamilton M. Hamilton anxiety rating scale (HAM-A) J Med. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 56.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960 doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Velthorst E, Levine SZ, Henquet C, de Haan L, van Os J, Myin-Germeys I, et al. To cut a short test even shorter: Reliability and validity of a brief assessment of intellectual ability in Schizophrenia - A control-case family study. Cogn Neuropsychiatry. 2013 doi: 10.1080/13546805.2012.731390. [DOI] [PubMed] [Google Scholar]
- 58.Tombaugh TN. Trail making test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004 doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 59.Greenblatt DJ, Allen MD, MacLaughlin DS, Harmatz JS, Shader RI. Diazepam absorption: effect of antacids and food. Clin Pharmacol Ther. 1978;24. 10.1002/cpt1978245600 [DOI] [PubMed]
- 60.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 61.Chakravarty MM, Steadman P, van Eede MC, Calcott RD, Gu V, Shaw P, et al. Performing label‐fusion‐based segmentation using multiple automatically generated templates. Hum Brain Mapp. 2012;34:2635–54. doi: 10.1002/hbm.22092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pipitone J, Park MT, Winterburn J, Lett TA, Lerch JP, Pruessner JC, et al. Multi-atlas segmentation of the whole hippocampus and subfields using multiple automatically generated templates. NeuroImage. 2014;101:494–512. doi: 10.1016/j.neuroimage.2014.04.054. [DOI] [PubMed] [Google Scholar]
- 63.Winterburn JL, Pruessner JC, Chavez S, Schira MM, Lobaugh NJ, Voineskos AN, et al. A novel in vivo atlas of human hippocampal subfields using high-resolution 3T magnetic resonance imaging. NeuroImage. 2013;74:254–65. doi: 10.1016/j.neuroimage.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 64.Lindner T, Bolar DS, Achten E, Barkhof F, Bastos-Leite AJ, Detre JA, et al. Current state and guidance on arterial spin labeling perfusion MRI in clinical neuroimaging. Magn Reson Med. 2023;89:2024–47. doi: 10.1002/mrm.29572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 66.Zubieta JK, Heitzeg MM, Xu Y, Koeppe RA, Ni L, Guthrie S, et al. Regional cerebral blood flow responses to smoking in tobacco smokers after overnight abstinence. AJP. 2005;162:567–77. doi: 10.1176/appi.ajp.162.3.567. [DOI] [PubMed] [Google Scholar]
- 67.Niu X, Guo Y, Chang Z, Li T, Chen Y, Zhang X, et al. The correlation between changes in gray matter microstructure and cerebral blood flow in Alzheimer’s disease. Front Aging Neurosci. 2023;15:1205838. doi: 10.3389/fnagi.2023.1205838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silva MA. Development of the WAIS-III: a brief overview, history, and description. 2008.
- 69.Zuardi AW, Cosme RA, Graeff FG, Guimarães FS. Effects of ipsapirone and cannabidiol on human experimental anxiety. J Psychopharmacol. 1993;7:82–88. doi: 10.1177/026988119300700112. [DOI] [PubMed] [Google Scholar]
- 70.Ho NF, Holt DJ, Cheung M, Iglesias JE, Goh A, Wang M, et al. Progressive decline in hippocampal CA1 volume in individuals at ultra-high-risk for psychosis who do not remit: findings from the longitudinal youth at risk study. Neuropsychopharmacology. 2017;42:1361–70. doi: 10.1038/npp.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hyder F, Sanganahalli BG, Herman P, Coman D, Maandag NJ, Behar KL, et al. Neurovascular and neurometabolic couplings in dynamic calibrated fMRI: transient oxidative neuroenergetics for block-design and event-related paradigms. Front Neuroenergetics. 2010;2. Accessed October 26, 2023. https://www.frontiersin.org/articles/10.3389/fnene.2010.00018 [DOI] [PMC free article] [PubMed]
- 72.Robbins S, Evans AC, Collins DL, Whitesides S. Tuning and comparing spatial normalization methods. Medical Image Analysis. 2004;8:311–23. doi: 10.1016/j.media.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 73.Yang Q, Cai S, Chen G, Yu X, Cattell RF, Raviv TR, et al. Fine scale hippocampus morphology variation cross 552 healthy subjects from age 20 to 80. Front Neurosci. 2023;17:1162096. doi: 10.3389/fnins.2023.1162096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hörtnagl H, Tasan RO, Wieselthaler A, Kirchmair E, Sieghart W, Sperk G. Patterns of mRNA and protein expression for 12 GABAA receptor subunits in the mouse brain. Neuroscience. 2013;236:345–72. doi: 10.1016/j.neuroscience.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Engin E, Benham RS, Rudolph U. An emerging circuit pharmacology of GABAA receptors. Trends Pharmacol Sci. 2018;39:710–32. doi: 10.1016/j.tips.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kullmann DM, Ruiz A, Rusakov DM, Scott R, Semyanov A, Walker MCPresynaptic. extrasynaptic and axonal GABAA receptors in the CNS: where and why? Prog Biophys Mol Biol. 2005;87:33–46. doi: 10.1016/j.pbiomolbio.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Venkat P, Chopp M, Chen J. New insights into coupling and uncoupling of cerebral blood flow and metabolism in the brain. Croat Med J. 2016;57:223–8. doi: 10.3325/cmj.2016.57.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Friedman DP, Aggleton JP, Saunders RC. Comparison of hippocampal, amygdala, and perirhinal projections to the nucleus accumbens: combined anterograde and retrograde tracing study in the Macaque brain. J Comp Neurol. 2002;450:345–65. doi: 10.1002/cne.10336. [DOI] [PubMed] [Google Scholar]
- 79.Rosene DL, Van Hoesen GW. Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science. 1977;198:315–7. doi: 10.1126/science.410102. [DOI] [PubMed] [Google Scholar]
- 80.Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: II. Cortical afferents. J Comparat Neurol. 2003;466:48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- 81.Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov. 2011;10:685–97. doi: 10.1038/nrd3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marques TR, Ashok AH, Angelescu I, Borgan F, Myers J, Lingford-Hughes A, et al. GABA-A receptor differences in schizophrenia: a positron emission tomography study using [11C]Ro154513. Mol Psychiatry. 2021;26:2616–25. doi: 10.1038/s41380-020-0711-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kang JI, Park HJ, Kim SJ, Kim KR, Lee SY, Lee E, et al. Reduced binding potential of GABA-A/benzodiazepine receptors in individuals at ultra-high risk for psychosis: an [18 F]-fluoroflumazenil positron emission tomography study. Schizophr Bull. 2014;40:548–57. doi: 10.1093/schbul/sbt052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Busatto GF, Pilowsky LS, Costa DC, Ell PJ, David AS, Lucey JV, et al. Correlation between reduced in vivo benzodiazepine receptor binding and severity of psychotic symptoms in schizophrenia. Am J Psychiatry. 1997;154:56–63. doi: 10.1176/ajp.154.1.56. [DOI] [PubMed] [Google Scholar]
- 85.Abi-Dargham A, Laruelle M, Krystal J, D’Souza C, Zoghbi S, Baldwin RM, et al. No evidence of altered in vivo benzodiazepine receptor binding in schizophrenia. Neuropsychopharmacology. 1999;20:650–61. doi: 10.1016/S0893-133X(98)00107-9. [DOI] [PubMed] [Google Scholar]
- 86.Lee JS, Lee JD, Park HJ, Oh MK, Chun JW, Kim SJ, et al. Is the GABA system related to the social competence improvement effect of aripiprazole? An (18)F-fluoroflumazenil PET study. Psychiatry Investig. 2013;10:75–80. doi: 10.4306/pi.2013.10.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Frankle WG, Cho RY, Prasad KM, Mason NS, Paris J, Himes ML, et al. In vivo measurement of GABA transmission in healthy subjects and schizophrenia patients. Am J Psychiatry. 2015;172:1148–59. doi: 10.1176/appi.ajp.2015.14081031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kiemes A, Gomes FV, Cash D, Uliana DL, Simmons C, Singh N, et al. GABAA and NMDA receptor density alterations and their behavioral correlates in the gestational methylazoxymethanol acetate model for schizophrenia. Neuropsychopharmacol. 2022;47:687–95. doi: 10.1038/s41386-021-01213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jacob TC. Neurobiology and therapeutic potential of α5-GABA type a receptors. Front Mol Neurosci. 2019;12:179. doi: 10.3389/fnmol.2019.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maramai S, Benchekroun M, Ward SE, Atack JR. Subtype selective γ-aminobutyric acid type A receptor (GABAAR) modulators acting at the benzodiazepine binding site: an update. J Med Chem. 2020;63:3425–46. doi: 10.1021/acs.jmedchem.9b01312. [DOI] [PubMed] [Google Scholar]
- 91.Chen J, Xue K, Yang M, Wang K, Xu Y, Wen B, et al. Altered coupling of cerebral blood flow and functional connectivity strength in first-episode schizophrenia patients with auditory verbal hallucinations. Front Neurosci. 2022;16. 2024. https://www.frontiersin.org/journals/neuroscience/articles/10.3389/fnins.2022.821078 [DOI] [PMC free article] [PubMed]
- 92.Selvaggi P, Jauhar S, Kotoula V, Pepper F, Veronese M, Santangelo B, et al. Reduced cortical cerebral blood flow in antipsychotic-free first-episode psychosis and relationship to treatment response. Psychol Med. 2023;53:5235–45. doi: 10.1017/S0033291722002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Squarcina L, Perlini C, Peruzzo D, Castellani U, Marinelli V, Bellani M, et al. The use of dynamic susceptibility contrast (DSC) MRI to automatically classify patients with first episode psychosis. Schizophr Res. 2015;165:38–44. doi: 10.1016/j.schres.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 94.Mäntylä T, Kieseppä T, Suvisaari J, Raij TT. Delineating insight-processing-related functional activations in the precuneus in first-episode psychosis patients. Psychiatry Res Neuroimaging. 2021;317:111347. doi: 10.1016/j.pscychresns.2021.111347. [DOI] [PubMed] [Google Scholar]
- 95.Percie du Sert O, Unrau J, Gauthier CJ, Chakravarty M, Malla A, Lepage M, et al. Cerebral blood flow in schizophrenia: a systematic review and meta-analysis of MRI-based studies. Prog Neuro-Psychopharmacol Biol Psychiatry. 2023;121:110669. doi: 10.1016/j.pnpbp.2022.110669. [DOI] [PubMed] [Google Scholar]
- 96.Gill KM, Cook JM, Poe MM, Grace AA. Prior antipsychotic drug treatment prevents response to novel antipsychotic agent in the methylazoxymethanol acetate model of schizophrenia. Schizophr Bull. 2014 doi: 10.1093/schbul/sbt236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang DJJ, Chen Y, Fernández-Seara MA, Detre JA. Potentials and challenges for arterial spin labeling in pharmacological magnetic resonance imaging. J Pharmacol Exp Ther. 2011;337:359–66. doi: 10.1124/jpet.110.172577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hawkins PCT, Wood TC, Vernon AC, Bertolino A, Sambataro F, Dukart J, et al. An investigation of regional cerebral blood flow and tissue structure changes after acute administration of antipsychotics in healthy male volunteers. Hum Brain Mapp. 2017;39:319–31. doi: 10.1002/hbm.23844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peris-Yague A, Kiemes A, Cash D, Cotel MC, Singh N, Vernon AC, et al. Region-specific and dose-specific effects of chronic haloperidol exposure on [3H]-flumazenil and [3H]-Ro15-4513 GABAA receptor binding sites in the rat brain. Eur Neuropsychopharmacol. 2020;41:106–17. doi: 10.1016/j.euroneuro.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made freely available upon publication (10.6084/m9.figshare.24763839), including (i) mean rCBF values per subject per ROI per hemisphere per condition, and (ii) coding scripts for the MRI preprocessing pipeline (run in Unix/shell) and generation of figures (run in R).