Abstract
Infection of human B cells with Epstein-Barr virus (EBV) results in activation of the cell cycle and cell growth. To interpret the mechanisms by which EBV activates the cell, we have assayed many proteins involved in control of the G0 and G1 phases of the cell cycle and regulation of apoptosis. In EBV infection most of the changes, including the early induction of cyclin D2, are dependent on expression of EBV genes, but an alteration in the E2F-4 profile was partly independent of viral gene expression, presumably occurring in response to signal transduction activated when the virus binds to its receptor, CD21. By comparing the expression of genes controlling apoptosis, including those encoding several members of the BCL-2 family of proteins, the known relative resistance of EBV-immortalized B-cell lines to apoptosis induced by low serum was found to correlate with expression of both BCL-2 and A20. A20 can be regulated by the NF-κB transcription factor, which is known to be activated by the EBV LMP-1 protein. Quantitative assays demonstrated a direct temporal relationship between LMP-1 protein levels and active NF-κB during the time course of infection.
When Epstein-Barr virus (EBV) infects resting human B lymphocytes, it drives the cells into the cell cycle and maintains cell division. The lymphoblastoid cell lines (LCLs) that arise from this type of EBV infection are relatively resistant to apoptosis caused by deprivation of serum growth factors. Cells of this LCL type are produced in vivo upon primary infection of humans but are then eliminated by the immune response, asymptomatically in infants but in adults in the course of the disease known as infectious mononucleosis. In the absence of normal immune surveillance, cells of the LCL type can develop into lymphomas (reviewed in reference 61). Genetic analysis of EBV has demonstrated several viral genes that are required for initiation and maintenance of growth. These include the genes that encode the nuclear proteins EBNA-1, EBNA-2, EBNA-LP, EBNA-3A, and EBNA-3C and the plasma membrane protein LMP-1 (reviewed in references 22 and 23). Some of the biochemical functions of these proteins are now becoming clear. EBNA-1 is required for EBV plasmid maintenance, EBNA-2 causes transcription activation through several interactions (including the Notch pathway), and EBNA-LP is able to cooperate with EBNA-2 in regulation of some genes. EBNA-3C causes cells to progress through cell cycle check points in both G1 and G2/M by an unknown mechanism, and the partly related EBNA-3A protein has effects on gene regulation (15). The LMP-1 protein activates signalling through several transduction pathways, including TRAF- and TRADD-mediated activation of NF-κB (10, 19, 30, 47, 64) and activation of SAP/JNK1 kinase, leading to c-Jun phosphorylation (20, 34). The EBV immortalization genes are not all expressed simultaneously upon infection; EBNA-LP and EBNA-2 are the first to be expressed, followed by the remaining EBNA proteins and then LMP-1.
We have studied the mechanism by which the resting B cells which EBV infects are driven into the cell cycle and the expression of genes that may control apoptosis during the infection of B cells. We and others have shown previously that binding of the virus to its receptor on the B-cell surface (CD21) not only mediates uptake of the virus but also results in signal transduction (71, 72), which preactivates the cell, enabling expression of transfected genes and giving an early transient activation of NF-κB (75). In B cells preactivated by exposure to purified gp340 (a form of the EBV surface glycoprotein which mediates virus binding to CD21), we showed that transfection of the first two viral genes known to be expressed during infection (EBNA-LP and EBNA-2) resulted in induction of the RNA encoding an early marker of cell cycle entry, cyclin D2 (72). Cyclin-dependent kinases (cdks) control cell cycle progression partly through the E2F family of transcription factors and the pocket proteins, Rb, p107, and p130, that can bind the various E2F complexes (65). Here we study the expression of these proteins during the time course of EBV infection, when EBV drives the cells into cycle, and describe modifications to the E2F profile that are dependent upon virus infection, some of which require viral protein synthesis. Current models for entry of resting cells into the cell cycle in response to serum stimulation imply a key early role for E2F4 and p130 proteins in governing the G0-to-G1 transition (45, 73, 78); it has so far been unclear whether activation of the cells by EBNA-LP and EBNA-2 is mediated through a direct effect on E2F-pocket protein complexes or through activation of cyclin expression. Here we show that the timing of changes we have detected in E2F and pocket proteins and the induction of cyclin D2 protein upon EBV infection are consistent with a cyclin D2-cdk complex mediating activation of the cell cycle. Once the cells are established in cycle, the EBNA-2, EBNA-3C, and LMP-1 proteins are thought to control cell growth (32, 35, 56).
Prevention of apoptosis is now recognized to be as important as proliferation in determining the overall increase in cell number in various cell types. In certain circumstances B lymphocytes are particularly prone to apoptosis, which is the main mechanism by which cells bearing self-reactive and low-affinity immunoglobulins are eliminated, particularly through Fas-mediated apoptosis (3). EBV-immortalized B cells are relatively resistant to apoptosis induced by low serum. Resting B cells contain relatively high levels of BCL-2 protein, similar to LCL cells, and BCL-2 has been shown to protect B cells against apoptosis. BCL-2 expression can also be induced by the EBV LMP-1 protein (28). In fact, BCL-2 is merely the prototypical member of a family of related proteins which are key regulators of apoptosis (16, 17, 48, 58, 59, 83). The family also includes BCL-XL and MCL-1 (which, like BCL-2, suppress apoptosis) and BAX, BAD, and BAK, which promote cell death (7, 13, 24, 33, 49, 60, 84). These proteins form various heterodimers and homodimers, and it is likely that the interactions among them are critical to BCL-2 family functions (58, 67, 68). BAX is central to the BCL-2 family of proteins, since it interacts with several antiapoptotic family members, including BCL-2, BCL-XL, and MCL-1. The BCL-2 homology (BH) domains 1 and 2 define the family, and BH1 and BH2 of BCL-2 are required for interaction with BAX and for suppression of apoptosis (85). In contrast, BAX BH3 domain is required for association with BCL-2 and promotion of apoptosis (86). In addition to dimerization, further complexity is present, since multiple isoforms of some family members are generated via alternate splicing, and these may be functionally distinct. For example, the alternate BCL-X form, BCL-XS, lacks BH1 and BH2 and promotes apoptosis (7). The balance of the levels of these various proteins, rather than the simple expression of BCL-2, is a key aspect of the regulation of apoptosis, but there is little knowledge of their levels in EBV-immortalized B cells and their relative importance. A further regulator of apoptosis present in LCLs is the A20 protein. A20 is a ring finger protein which suppresses apoptosis and can be induced by LMP-1 (26, 39, 50, 51, 66). We have measured the levels of these apoptosis-regulatory gene products in a panel of cell lines, including LCLs and Burkitt’s lymphomas (BLs), with or without EBV immortalizing-gene expression. The results suggest that, in addition to BCL-2, the A20 antiapoptotic protein may also have a role in preventing the sensitivity of LCLs to apoptosis induced by low serum. The A20 promoter can be regulated by LMP-1 through its NF-κB site (39), and we have directly measured the induction of active nuclear NF-κB during primary infection, showing that it correlates with LMP-1 expression.
MATERIALS AND METHODS
Purification of B cells, EBV, and virus infections.
Primary B cells from peripheral blood were isolated as described previously (12, 72). Buffy coats were centrifuged over Ficoll-Paque (Pharmacia LKB) gradients, and CD19-positive lymphocytes were immunoselected with pan-B Dynabeads M450 (Dynal). The beads were removed by competition with Detachabeads (Dynal), and the cells were resuspended at 106/ml in RPMI 1640 supplemented with penicillin, streptomycin, and 15% heat-inactivated fetal calf serum. The cells were incubated for 40 h prior to infection with EBV or treatment with 30 ng of PMA (phorbol-12-myristate-13-acetate) as indicated. UV radiation inactivation of EBV prior to infection was performed in a Stratalinker with 9 J of UV radiation. Tonsil B cells were purified by negative selection. Single-cell suspensions prepared from fresh tonsils were centrifuged over Ficoll-Paque. T cells were removed by rosetting with 5-2-aminoethylisothiouronium bromide (AET)-treated sheep erythrocytes followed by centrifugation over Percoll (specific gravity, 1.08; Pharmacia Biotech). The dense resting B cells were further isolated by centrifugation over Percoll at a specific gravity of 1.074 and resuspended at 106/ml in RPMI 1640 supplemented with penicillin, streptomycin, and 15% heat-inactivated fetal calf serum. The isolated cells were analyzed by flow cytometry for purity and DNA content with fluorescein isothiocyanate (FITC)-conjugated anti-CD20 and propidium iodide, respectively. The cells were infected with the B95-8 strain of EBV as described previously (28a).
Cell lines and apoptosis assay.
BL41, DG75 (6), BL40, and BL2 (40) are EBV-negative BL cell lines. Akata (76) and Elijah are EBV-positive BL cell lines that have retained a group I phenotype (62). Mutu III clone 148, BL72 (40), and WewakI (62) are EBV-positive BL cell lines that have acquired a group III phenotype. Namalwa (37), Raji (57), and BL74 (40) are EBV-positive BL cell lines with the intermediate group II phenotype. BL36-LCL, PF-LCL, LCL3, IB4 (36), and X50-7 (82) are in vitro EBV-immortalized LCLs. The cell lines were maintained in RPMI 1640 medium (Gibco-BRL) supplemented with 10 to 15% (vol/vol) fetal calf serum and antibiotics.
To test the sensitivity of cell lines to Fas-induced apoptosis, the cells were diluted to 106 per ml and treated with 100 or 500 ng of an anti-Fas immunoglobulin M (IgM) class antibody (no. 05-201; Upstate Biotechnology Inc.)/ml or were left untreated as a control. The anti-Fas antibody had κ light chains so, as an additional control, cells were treated with equivalent concentrations of an unrelated IgM(κ) antibody (Pharmingen 03081D) that had been dialyzed to remove azide. After 24 h, cell viability was determined by trypan blue exclusion as a measure of cell membrane permeability. Cell genomic DNA was analyzed as previously described (4).
Cell proliferation measured by [3H]thymidine incorporation.
Uninfected or EBV-infected cells (2 × 105) were pulse labelled for 2 h with 1 μCi of [3H]thymidine per well and harvested onto glass fiber filters with a cell harvester (Skatron Ltd., Lier, Norway). The filters were air dried, and scintillations were counted.
Immunoblotting, immunofluorescence, and antibodies.
Radioimmunoprecipitation assay lysates were prepared and quantitated, and immunoblotting was performed as previously described (9). Proteins were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. After being blocked with 10% milk powder in phosphate-buffered saline, the membranes were probed with the following antibodies (all antibodies were mouse monoclonal antibodies unless otherwise indicated).
The antibodies used were a 1/10 dilution of anti-EBNA-LP monoclonal JF 186 (25), 1/500 dilution of anti-EBNA-2 monoclonal antibody PE2 (Dako), 1/10 dilution of anti-LMP-1 monoclonal S12 (41), 1/1,000 dilution of rabbit polyclonal anti-p107 (C-18; Santa Cruz Biotechnology), 1/1,000 dilution of rabbit polyclonal anti-pRB (C-15; Santa Cruz Biotechnology), 1/1,000 dilution of rabbit polyclonal anti-p130 (C-20; Santa Cruz Biotechnology), 1/80 dilution of anti-cyclin D2 (G132-43; Pharmingen), 1/500 dilution of anti-cyclin E (HE12; Santa Cruz Biotechnology), 1/700 dilution of anti-E2F-1 (KH-95; Santa Cruz Biotechnology), 1/1,000 dilution of rabbit polyclonal anti-E2F-4 (C-108; Santa Cruz Biotechnology), 1/1,000 dilution of rabbit polyclonal anti-p27 (C-19; Santa Cruz Biotechnology), 1/1,000 dilution of rabbit polyclonal anti-cdk2 (M2; Santa Cruz Biotechnology), 1/500 dilution of rabbit polyclonal anti-cdk4 (C-22; Santa Cruz Biotechnology), and 1/1,000 dilution of rabbit polyclonal anti-cdk6 (C-21; Santa Cruz Biotechnology).
Three rabbit BAX antibodies were used. BAX N-20 (Santa Cruz Biotechnology) was used at 0.4 μg/ml, BAX P-19 (Santa Cruz Biotechnology) was used at 0.5 μg/ml, and BAX 13666E (Pharmingen) was used at a dilution of 1:1,000. The BCL-X-specific rabbit antibody S-18 (Santa Cruz Biotechnology) was used at 0.4 μg/ml, the BCL-2 antibody C124 (Dako) was used at 1 μg/ml, the BAK-specific goat antibody G-23 (Santa Cruz Biotechnology) was used at 0.5 μg/ml, and the BAG-1-specific rabbit antibody C-16 (Santa Cruz Biotechnology) was used at 0.5 μg/ml.
The secondary antibodies were horseradish peroxidase-conjugated donkey anti-rabbit Ig or sheep anti-mouse Ig (both Amersham) or horseradish peroxidase-conjugated rabbit anti-goat Ig (Santa Cruz Biotechnology). Bound immunocomplexes were detected by enhanced chemiluminescence (Amersham).
For immunofluorescent detection of EBNA-LP, cytospins were prepared from uninfected and EBV-infected cells 30 h after infection and fixed in 1:1 methanol-acetone. The cells were stained with JF186 (1:10 dilution) followed by FITC-conjugated sheep anti-mouse IgG (Sigma) or the secondary antibody alone. Positive cells were counted by fluorescent microscopy.
RNase protection assay and Northern blotting.
Northern blotting and RNase protection assays were performed as described previously (4, 71, 72) with cytoplasmic RNA. The A20 probe for Northern blotting contained nucleotides 337 to 1614 of the cDNA (50), kindly provided by V. M. Dixit. For the A20 RNAse protection assay, nucleotides 2218 to 1705 of the cDNA were subcloned to make the probe. The E2F-4 RNAse protection probe contained nucleotides 531 to 246 of the E2F-4 cDNA (5) and was made by in vitro transcription with SP6 RNA polymerase from the plasmid pcDNA3-E2F4ΔApaI (a kind gift of E. Lam) linearized with BstXI.
Gel retardation assay.
A double-stranded oligonucleotide, AGTTGAGGGGACTTTCCCAGGC, was end-labelled with T4 polynucleotide kinase. Nuclear extracts were prepared as described previously (54). For each assay, 5 μg of LCL3 extract protein or an equivalent amount of extract (primary B-cell infections) was incubated in a final reaction volume of 20 μl containing 40 mM NaCl, 10 mM Tris-Cl (pH 7.5), 1 mM EDTA, 1 mM 2-mercaptoethanol, 4% (vol/vol) glycerol, 1 mg of bovine serum albumin/ml, 0.1 mg of poly(dIC)/ml, and 50 ng of 32P-labelled oligonucleotide for 30 min at 25°C. Samples were electrophoresed on 4% polyacrylamide gels in 0.5× Tris-borate-EDTA.
RESULTS
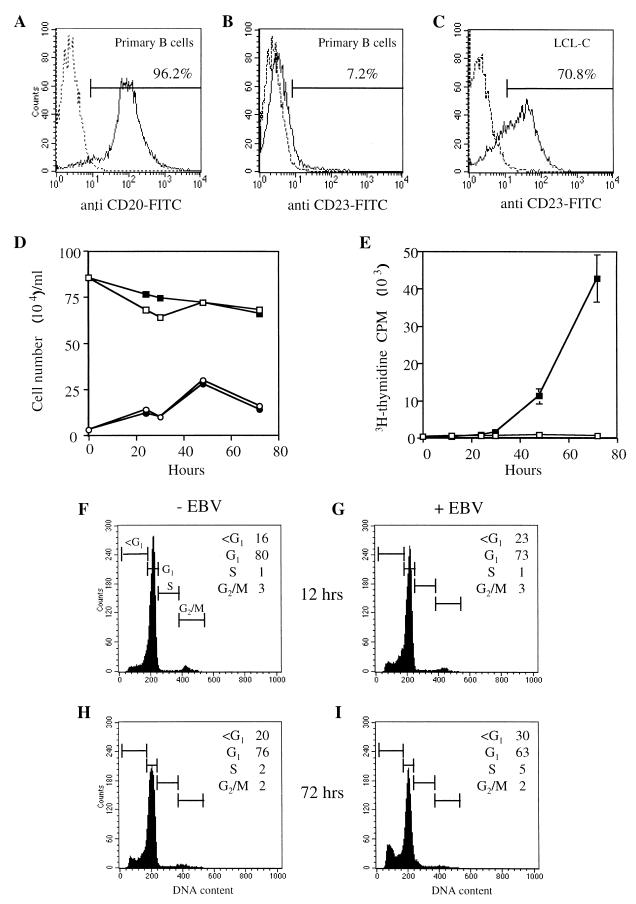
In the experiments we have published previously on the early events after EBV infection of B cells, the B cells were purified by positive selection of peripheral blood lymphocytes on CD19 beads (12, 72). CD19 is part of the membrane complex containing the EBV receptor (CD21). There was, therefore, the possibility that the selection process might have affected the signal transduction and G0 status of the purified B cells, even though our standard procedure involved an overnight incubation in medium after the purification to allow any transduction effects of the purification process to dissipate. Although most parameters (very low [3H]thymidine incorporation, 2 N DNA content, and cell surface and intracellular markers) were as expected for resting cells (12, 72), we have found some variation in the level of hypophosphorylated p130 in the purified B cells. This seemed to be more a consequence of variation in the batches of buffy coats used for B-cell purification than a result of the positive selection protocol. Nevertheless, the purification procedure was modified, increasing the recovery period to 40 h. With this revised purification procedure, the cells were confirmed as predominately resting B cells, since they were CD20 positive and CD23 negative, lacked measurable [3H]thymidine incorporation, and lacked G2- or S-phase cells in fluorescence-activated cell sorter analysis (Fig. 1 and data not shown). Furthermore, p130 levels in the purified-B-cell population were consistently normal and similar to those of resting B cells purified from tonsils by a negative selection protocol (Fig. 2). Other types of resting (G0) cells have been shown to contain an E2F factor made up of E2F-4 complexed with a DP protein bound to the transcriptionally repressive pocket protein p130 (45, 73, 78). The resting B cells were shown also to express E2F-4 (Fig. 2). The purified B cells are thus in G0 by all criteria that we have been able to measure.
FIG. 1.
Primary B cells isolated by CD19 positive selection were stained with FITC-conjugated antibodies recognizing (A) CD20 or (B) CD23 and analyzed by flow cytometry. An LCL immortalized with B95-8 EBV (C) was used as a positive control for CD23 staining. The percentage of positive cells was determined by using linear gates set to include 1% positive cells on unstained samples (dotted lines). (D) Numbers of viable (squares) and dead (circles) cells were determined by trypan blue exclusion in uninfected (open symbols) and EBV-infected (filled symbols) cultures. (E) DNA synthesis was measured by [3H]thymidine incorporation in uninfected (open squares) and EBV infected (filled squares) cells. Uninfected (F and H) and EBV-infected (G and I) cells containing less than 2 N DNA and those in G1, S, and G2/M phases of the cell cycle were analyzed by propidium iodide staining at 12 (F and G) and 72 (H an I) h after the addition of virus.
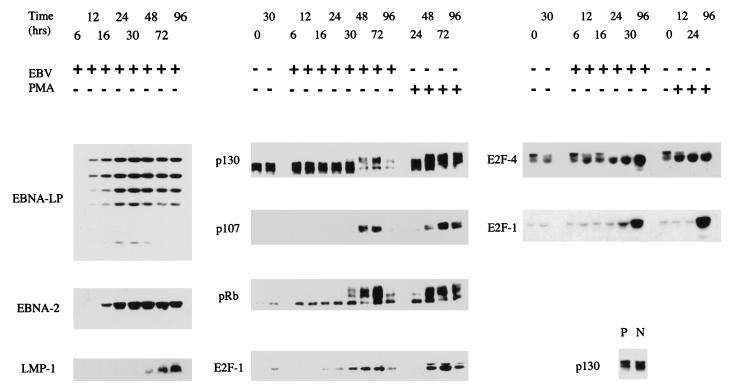
FIG. 2.
Purified B cells positively selected from peripheral blood were infected with EBV or treated with PMA (30 ng/ml) in a time course, and aliquots were assayed by Western blotting for the proteins indicated. Purified B cells negatively selected from tonsils (N) were also compared with the positively selected B cells from peripheral blood (P) for p130 expression (lower right panel).
Purified resting cells were infected with EBV, and the expression of viral and cell proteins at various times after infection was monitored by Western blotting extracts of the cells. As we have shown previously (72), the cells begin to enter S phase about 48 to 72 h after infection under these conditions (Fig. 1). Cell death in the cultures was found to be relatively low: about 70% of the cells remained viable by trypan blue exclusion (Fig. 1), consistent with the fluorescence-activated cell sorter analysis of propidium iodide-stained cells, which showed about 30% of the cells with a sub-G1 DNA content at 72 h after the addition of virus. The viral proteins EBNA-LP, EBNA-2, and LMP-1 were assayed. As shown previously (72), EBNA-LP and EBNA-2 are the earliest viral proteins that can be detected (Fig. 2). These transcription factors are assumed to modify the activity of cell genes or proteins, but the relevant early cell targets have not yet been identified. Across the same time course of infection, the E2F-1 and E2F-4 proteins were induced, with E2F-1 being undetectable or present at only a very low level in the uninfected cells at the start of the experiments (Fig. 2). E2F-4 was present as multiple bands; the slower-migrating forms are thought perhaps to represent phosphorylated forms of the protein (78, 79). The level of the slower-migrating forms of E2F-4 was reduced during the infection time course, but the overall level of E2F-4 detected by Western blotting was increased (Fig. 2). The levels of the pRb and p107 pocket proteins were very low or undetectable in the uninfected cells, but both p107 and pRb were induced upon infection, with phosphorylated forms of pRb appearing about 30 h after infection. In contrast, p130 levels did not increase, but slower-migrating forms, presumably with different phosphorylation states, appeared later, about 48 h after the addition of virus. PMA treatment of the B cells (which drives the cells into division) induced changes in E2F-1, E2F-4, and the pocket proteins similar to those of EBV infection (Fig. 2).
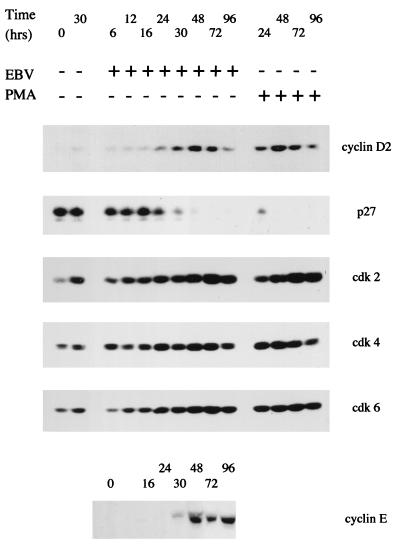
We demonstrated previously that cyclin D2 RNA was induced by EBV as early as 24 h after infection in our system (72), and this has now been confirmed at the protein level by Western blotting (Fig. 3). Cyclin E was found to be induced at about the same time the level of p27 protein declined abruptly, perhaps consistent with targeted degradation of p27, as has been reported in other systems of cell cycle entry (55, 69, 80). Although we previously reported induction of cdk-2, -4, and -6 proteins from very low levels upon EBV infection (12), it is now clear with improved detection methods that there is detectable cdk-2, -4, and -6 in the uninfected cells but that all these cdks are induced further upon EBV infection. Some of the induction of cdk-2 also occurred when the cells were cultured in the absence of EBV (Fig. 3). Again, PMA treatment of the B cells resulted in changes to the profile of cyclin D2, p27, and cdks similar to those in EBV infection.
FIG. 3.
Purified B cells from peripheral blood were infected with EBV or treated with PMA (30 ng/ml) in a time course, and aliquots were assayed by Western blotting for the proteins indicated. +, present; −, absent.
To distinguish which of these effects was dependent on viral gene expression, the levels of cyclin D2, p130, E2F-4, and p27 proteins were measured at 24 and 48 h after infection with EBV or UV-inactivated EBV (Fig. 4A). After UV inactivation, the virus particles remain but viral gene expression is prevented (the effectiveness of the UV inactivation was verified by monitoring expression of EBNA-LP, which was prevented by the UV treatment). The results (Fig. 4A) show that the induction of cyclin D2 protein, the altered gel mobility (presumed hyperphosphorylation) of p130, and the reduction in p27 levels were all dependent on viral gene expression, since the effects were prevented by UV inactivation of the virus. The changes in the migration pattern of E2F-4 upon EBV infection were also analyzed, comparing the effects of UV-inactivated EBV with those of wild-type virus. An increase in the level of E2F-4 protein was prevented by UV inactivation of the virus, but the reduction in amount of the slower-migrating forms of E2F-4 was only partly affected by the UV treatment of the virus, indicating that part of that change in E2F-4 might be in response to the signal transduction occurring when virus binds to the B cell (Fig. 4A). As shown previously for cyclin D2 (72), the induction of E2F-4 expression also occurred at the RNA level (Fig. 4B), at a time consistent with its being a consequence of EBNA-2 and EBNA-LP expression from the EBV genome.
FIG. 4.
(A) Purified B cells from peripheral blood were infected with EBV (lanes 2 and 4) or UV-inactivated EBV (lanes 3 and 5) or left uninfected (lane 1). Extracts of the cells were prepared at the indicated times after infection and assayed by Western blotting. (B) RNA was extracted from cells infected as for panel (A) and assayed by RNase protection analysis for E2F-4 RNA. The undigested probe (1% of the input) is shown in track P, and track Y is the negative control RPA with yeast RNA. The band at 286 nucleotides (arrowhead) indicates the presence of E2F-4 RNA. Samples 48 (1) and 48 (2) are the 48-h time points from two parallel infections. Size markers (track M) were an MspI digest of pBR322 DNA end repaired with Klenow DNA polymerase. U.V. EBV, UV-inactivated EBV.
Regulation of apoptosis during EBV infection is another important aspect of the transition from the resting state to immortalized LCL. There are likely to be changing apoptotic signals during the process of infection, since there is induction of cell proteins that are able to cause apoptosis, such as E2F-1 (Fig. 2) and c-Myc (1), as growth is activated. BCL-2 (perhaps induced by LMP-1) has already been implicated in the control of apoptosis in LCLs and group III BL cells (27, 28), but the involvement of other pathways has been suggested (44). It is the balance of pro- and antiapoptotic proteins that is important, so we examined the levels of several proteins that regulate apoptosis in LCLs. To provide a framework in which the relationship between the EBV gene expression in EBV-immortalized LCLs and sensitivity to apoptosis might be interpreted, the protein levels in LCLs were compared to those in other B-cell lines with and without EBV gene expression. The most suitable lines for comparison were BL cells, since there are examples of these cell lines both containing and lacking EBV and there is also the opportunity to compare group I EBV-positive BL cells (which express only EBNA-1 of the six viral proteins shown to be involved in LCL immortalization) to group III EBV-positive BL cells, which have a pattern of EBV gene expression similar to that of the LCLs.
EBV-negative and group I EBV-positive BL cell lines are relatively sensitive to apoptosis induced by ionomycin, stimulation via cell surface Ig molecules, or serum deprivation compared to group III BL lines (8, 27, 28, 31, 81). Additionally, although both group III BL cell lines and LCLs express the Fas receptor, only LCLs are sensitive to ligation of the Fas receptor (21). However, this study measured DNA synthesis and did not directly examine effects of Fas on apoptosis. To confirm that decreased DNA synthesis in Fas-treated LCLs was due to induction of apoptosis, we treated Fas receptor-positive group III BL cell lines and LCLs with an agonistic Fas antibody. Consistent with earlier results (21), anti-Fas induced cell death in LCLs but not in group III BL cell lines (Fig. 5A), and analysis of genomic DNA demonstrated DNA degradation characteristic of apoptosis (Fig. 5B).
FIG. 5.
Sensitivity of LCLs and resistance of BL cells to Fas-induced apoptosis. (A) Cell viability was assayed by trypan blue exclusion after 24 h of treatment with anti-Fas antibody at 100 (crosshatch) or 500 (solid) ng/ml or with control antibody (open). Error bars indicate standard deviation. (B) DNA laddering was assayed in IB4 and LCL3 cells treated for 8 h with 100 (tracks 1) or 500 (tracks 2) ng of anti-Fas antibody or 500 ng of control antibody (tracks 3)/ml or with no additions (tracks 4).
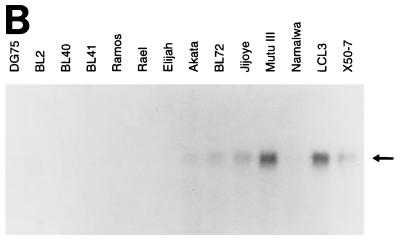
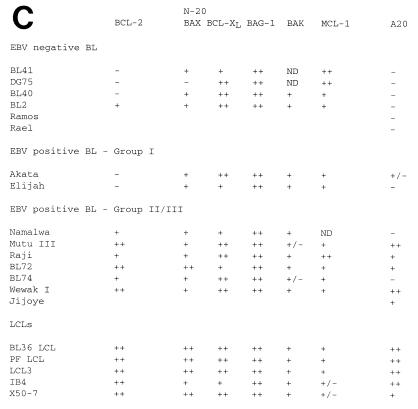
To relate these differences in apoptosis sensitivity to the endogenous levels of apoptosis-regulatory molecules, we examined expression of BCL-2, BAX, BCL-XL, BAK, MCL-1, and BAG-1 isoforms by immunoblotting (Fig. 6A), and the results are summarized in Fig. 6C. Expression of the antiapoptotic A20 gene was measured by Northern blotting (Fig. 6B) and compared to the protein data (Fig. 6C). Reactivity with the P-19 antibody was also determined in the cell lines (Fig. 6A) (see below). Consistent with previous data (27, 28), expression of BCL-2 was higher in LCLs and group III BL cell lines than in EBV-negative and group I BL cell lines. The sizes of the proteins on the Western blots indicated that BAX was entirely the α form, with no evidence of β, γ, or δ splicing, and BCL-X was entirely the L form, with no evidence of BCL-XS. We have recently clarified the nature of the two BAG-1 isoforms (53) and shown that the absence of BAX protein in DG75 cells is due to a frameshift mutation in the BAX gene (9). Of the other genes assayed which can contribute to the overall threshold of apoptosis, only the A20 gene showed a correlation with the resistance to apoptosis induced by low serum characteristic of LCLs: in this panel of cell lines, it correlated almost as well as BCL-2 expression. However, neither the endogenous A20 nor BCL-2 expressed in LCLs is sufficient to make the EBV-immortalized LCLs resistant to Fas-induced apoptosis (Fig. 6), in contrast to the group III BL lines, which have EBV gene expression similar to that of LCLs but are relatively resistant to Fas-induced apoptosis.
FIG. 6.
(A) Expression of BCL-2 family proteins in BL cell lines and LCLs. Lysates were prepared from the indicated cell lines, and equal amounts of protein were analyzed for expression of BCL-2 family proteins by immunoblotting. The position of migration of each protein is indicated. N20 data for BAX expression in these cell lines is shown in reference 9. (B) Analysis of A20 RNA expression in BL cell lines and LCLs. Cytoplasmic RNA isolated from the indicated cell lines was analyzed by Northern blotting with the A20 cDNA as a probe. Equal loading of the gel tracks was confirmed by ethidium bromide staining of the rRNA bands on the gel before transfer (data not shown). (C) Summary of BCL-2 family protein expression and A20 RNA expression in the panel of cell lines studied in this paper. Lines marked ND were not tested for the indicated protein or RNA. A20 expression was determined by Northern blotting, except for Raji, IB4, and BL36LCL, which were assayed by RNase protection. Group III BL cell lines and LCLs are resistant to apoptosis induced by cross-linking surface immunoglobulin, ionomycin, and serum depletion relative to group I and EBV-negative BL cell lines. In addition, LCLs are sensitive to Fas-induced apoptosis, whereas group III BL cell lines are resistant. Group I BL cell lines and EBV-negative BL lines do not express Fas and are therefore resistant to Fas-induced apoptosis.
The promoter for the A20 gene can be regulated through two adjacent NF-κB sites 45 to 66 bp upstream of the transcription start (38), so it seemed likely that this transcription factor (which can be induced by LMP-1) would be what determines A20 expression in LCLs. Recent work indicates that during the time course of early EBV infection there is a very early induction of NF-κB followed by a later, larger induction, presumably caused by the LMP-1 protein. The early transient induction of NF-κB correlated with the signal transduction activated by the binding of EBV to its receptor on the cell, CD21 (75). We analyzed the time course of expression of different components of the NF-κB complex and its inhibitory I-κB proteins during EBV infection (Fig. 7). LMP-1 continued to accumulate even up to 144 h after infection, but the p65, p50, p52, c-Rel, Rel-B, and Iκ-Bα proteins were all induced to their steady-state levels by 48 h after infection. The level of Iκ-Bβ was higher in the uninfected cells than in the EBV-infected cells but also achieved a steady-state level by 48 h after infection. LMP-1 expression in our system is usually detected at 48 to 72 h, so the components of the NF-κB factor were therefore all present at their steady-state levels by the time LMP-1 was expressed.
FIG. 7.
Purified B cells positively selected from peripheral blood were infected with EBV in a time course (hours post infection), and aliquots were assayed by Western blotting for the proteins indicated. +, present; −, absent; ×, empty lane.
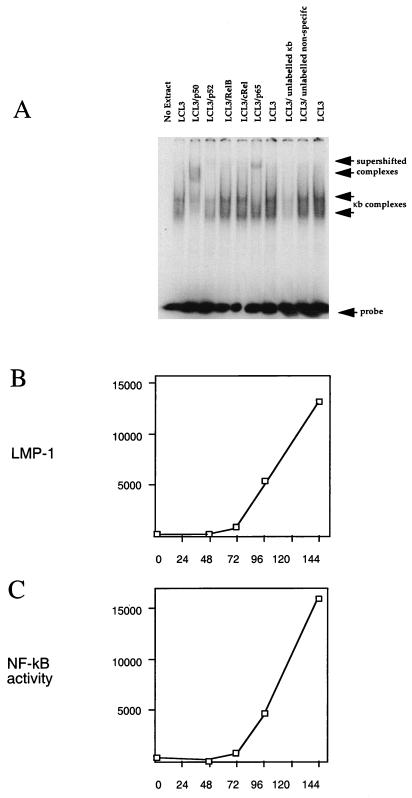
Since NF-κB exists as an inactive complex with I-κB in the cytoplasm, awaiting activation in response to signal transduction, we wished to compare the time course of induction of the LMP-1 protein and the level of activated NF-κB. The level of activated NF-κB was determined by gel retardation assays of an oligonucleotide containing a consensus NF-κB binding site. The specificity of the assay was established by using nuclear extracts of LCL3 cells (Fig. 8A); the broad band of retarded κB complexes was dependent on the addition of cell extract and was competed by an oligonucleotide containing a κB site but not by an unrelated control oligonucleotide. Further evidence for specificity was provided by supershifting the complexes with antibodies to components of the NF-κB factor. The faster-migrating part of the complex was supershifted by an antibody to p50, the slower-migrating part of the complex was supershifted by an antibody to p65, and the level of the whole complex was reduced by an antibody to p52. Antibodies to RelB and cRel did not appear to affect the complexes, and major components of the two complexes may be p50-p50 and p50-p65. This assay was then applied to the time course of EBV infection by using nuclear extracts from purified B cells at various times after infection. The level of activated NF-κB (Fig. 8C) and the level of LMP-1 (Fig. 8B) correlated in time, consistent with LMP-1 being responsible for the major increase in activated NF-κB observed during primary infection. The NF-κB site in the oligonucleotide used for the determination of activated NF-κB in the gel retardation assay was identical to the NF-κB site in the A20 promoter 57 to 66 nucleotides upstream of the transcription start in the published A20 sequence (38). The induction of NF-κB in these B cells purified from peripheral blood would thus account for the expression of A20 in the LCLs.
FIG. 8.
(A) Establishment of specificity of gel retardation assay for NF-κB complexes with LCL3 cell extracts by competition with 100-fold excess of unlabelled NF-κB oligonucleotide or a control oligonucleotide of unrelated sequence. NF-κB complexes (arrows) were also tested in supershift assays with antibodies to p50, p52, RelB, cRel, and p65 (Santa Cruz Biotechnology antibodies sc-114, sc-848, sc-226, sc-70, and sc-109, respectively) as indicated (1 μg per assay). (B) Purified B cells positively selected from peripheral blood were infected with EBV in a time course (hours post infection), and aliquots were assayed by Western blotting for LMP-1. The LMP-1 signal was quantified by scanning the autoradiograph, and the results are plotted in arbitrary units. (C) Nuclear extracts were prepared from the same time course of infection as for panel B and used in a gel retardation assay for NF-κB performed as for panel A. The active NF-κB, indicated by the amount of specifically shifted probe, was quantified by scanning the autoradiograph, and the results are plotted in arbitrary units.
DISCUSSION
We have assayed many proteins involved in regulation of the cell cycle and apoptosis during EBV infection. The most straightforward interpretation of our data is that the changes represent the consequences of EBV infection leading to cell proliferation, but it is important to appreciate that there is a heterogeneity of response at the cellular level to the addition of virus to the B lymphocytes. Characterization of the cells showed a high degree of homogeneity. For example, the cells were selected with the B-cell marker CD19 and then also verified to be 96% positive for the B-cell marker CD20; they were a resting population, as shown by the lack of [3H]thymidine incorporation and lack of CD23 activation marker. It is possible that there is further heterogeneity in the B-cell preparation with consequent heterogeneity of response to the virus, but there is no evidence for this. Under the conditions used, about 27% of the cells became positive for EBNA-LP expression by immunofluorescence. We are not able to track individual cells through the long time course of immortalization, so we cannot tell at this stage which of the cells will eventually grow out. Over the first 72 h of infection, when most of the changes occur, there is relatively little cell death and negligible proliferation, so the changes are not likely to be accounted for by population effects involving subgroups of cells containing the individual marker proteins; but since we have not yet followed the proteins studied here by Western blotting by immunofluorescence (most of the antibodies used are not suitable for immunofluorescence studies), it has not been possible to link the changes directly to expression of EBV proteins in individual cells. It is also important to remember that the virus preparation is very likely to be heterogeneous, since there is no opportunity to plaque purify EBV (there is no plaque assay) and there is little knowledge of the infectivity per virus particle.
In spite of these reservations, the results we have described are highly consistent with the entry of the cells into the cell cycle and prevention of apoptosis as a consequence of virus infection. Since EBV infects resting B cells, the cells must first enter the cell cycle. It is clear that some activation of the resting cell occurs as a result of the binding of the gp350 surface glycoprotein on the virus particle to the receptor on the cell, CD21 (72, 75). Signal transduction from this results in tyrosine phosphorylation of CD19 and some effects of the virus infection in the cell can be prevented with inhibitors of various protein kinases (71). A small, transient activation of NF-κB has also been demonstrated to result from virus binding (75), and we have shown here that part of the reduction in mobility of E2F-4 occurs with UV-inactivated EBV, implying that viral gene expression is not necessary for this effect. Other responses to EBV infection that we have measured in this study all required viral gene expression.
One of the most remarkable aspects of the data is that the changes in protein profiles are so clear and complete in the time course studied. For example, p27 virtually disappears by 72 h after infection and there are major changes in the phosphorylation profiles of p130 and pRb which at first sight seem to be inconsistent with only about 27% of the cells becoming positive for EBNA-LP, our early marker for virus infection. Potential explanations for this include more than 27% of the cells being infected (perhaps there are large variations in the degree of immunofluorescence upon infection) or activation of other cells by those that have achieved viral gene expression. It is noticeable that the cells clump together after the addition of virus, giving the opportunity for cell-to-cell communication. Alternatively, there might be soluble factors secreted as a consequence of early viral gene expression that can then cause changes in the uninfected cells.
We have also studied regulation of apoptosis in EBV-negative and -positive BL-derived cell lines and EBV-immortalized LCLs. Phenotypically distinct BL cell lines and LCLs are differentially sensitive to induction of apoptosis by some agents. EBV-negative and group I EBV-positive BL lines are relatively sensitive to ionomycin, stimulation via surface Ig molecules, or serum deprivation compared to group III BL cell lines (8, 27, 28, 31, 81). Additionally, although both group III BL lines and LCLs express the Fas receptor (21), only LCLs are sensitive to Fas-induced apoptosis (EBV-negative and group I BL lines do not express Fas receptor and are therefore insensitive to Fas ligation). Group III BL cells express high levels of BCL-2, whereas group I and EBV-negative lines do not, and it has been concluded that this contributes to the resistance of group III BL lines to apoptosis induced by ionomycin, Ig, and serum withdrawal (27, 28). However, control of apoptosis is thought to be determined by the relative levels of pro- and antiapoptotic BCL-2-related proteins, and in those experiments the endogenous levels of other BCL-2 family proteins were not measured. Indeed, BCL-2-independent events also play a role in resistance to apoptosis triggered by these agents in some BL lines (44), and the resistance of group III BL lines to Fas-induced apoptosis relative to LCLs cannot be determined by BCL-2, since both these groups of lines express equivalent amounts of BCL-2 (18, 28). This is also consistent with the inability of BCL-2 expression to suppress Fas-induced apoptosis in some systems (14, 18, 29, 43, 46, 52, 70, 77).
Some of the proteins known to be induced by EBV infection, such as E2F-1 and c-Myc, are able to induce apoptosis in some circumstances. The time course of changes in the E2F-1 and E2F-4 proteins has been described in this paper, and our analysis of the expression and properties of Myc family proteins during EBV infection will be published elsewhere. To provide a framework for investigation of the control of apoptosis in LCLs and during EBV infection, we studied the expression of the BCL-2 family of proteins and other pro- and antiapoptotic genes. Expression of BCL-2 and A20 both correlated well with resistance to apoptosis. Attention has previously focused on BCL-2 in this context, but our results and several recent reports of effects of A20 in lymphoid and epithelial cells indicate that A20 is also likely to be important. It is well established that the A20 gene can be activated by NF-κB through two adjacent NF-κB binding sites in the promoter (39), and BCL-2 has also been reported to be induced by LMP-1 (28). NF-κB activity, measured by using a standard NF-κB site identical in sequence to one of those in the A20 promoter, showed an excellent temporal correlation between active, nuclear NF-κB and LMP-1 expression during infection, indicating that LMP-1 is likely to be the major determinant of NF-κB activity in the LCL cells. The Namalwa and BL74 cell lines, which were negative for A20, have the group II pattern of EBV gene expression and do not express LMP-1, although both expressed BCL-2.
It was notable in the Western blots of BCL-2 family protein expression (Fig. 6A) that the pattern of expression of BAX detected with the N20 antibody was quite different from the protein expression detected with the P-19 antibody, which has also been described as detecting human BAX (Santa Cruz Biotechnology). The protein from some cell lines detected by P-19 also showed altered migration on the sodium dodecyl sulfate gel (Fig. 6A). We considered the possibility that the two antibodies might be detecting hitherto-unknown different forms of BAX in these cells or that the specificity of one of the antibodies (probably P-19) was described incorrectly. This is significant because a considerable amount of earlier work (2, 42, 63, 74) has used P-19 to monitor human BAX protein levels. Upon investigation (Fig. 6A and data not shown), it became clear that the P-19 antibody cross-reacts in Western blotting experiments with another protein of a size similar to that of human BAX. The authenticity of the N20 specificity for Bax was verified by showing that a third anti-BAX antibody (13666E) gave the same pattern of reactivity as N20 in a panel of relevant cell lines and the Bax protein recognized in the cell extracts was the same size as the protein produced by transfection of a Bax cDNA (data not shown). It therefore seems that antibody P-19 does not recognize endogenous human Bax. We have, however, repeated the experiments in the study in this laboratory that used P-19 (2) with the N20 antibody and confirmed the conclusions of the study (data not shown).
The first two viral genes known to be expressed upon infection are EBNA-LP and EBNA-2. These two proteins have been shown to cooperate in the induction of the cyclin D2 RNA, which is an early marker of cell cycle entry that is induced after infection (72). Although EBNA-2 and EBNA-LP can activate transcription of various promoters, it is not yet clear whether the promoter for cyclin D2 is regulated directly by these proteins. Deletion analysis of the cyclin D2 promoter, seeking elements that may control its response to serum growth factors, has revealed both positive and negative regulatory regions upstream of the transcription start (11). One of the consequences of activation of cdk activity is phosphorylation of pocket proteins, which bind and regulate the E2F complex. The E2F family of transcription factors are thought to be major determinants of cell cycle progression, and different E2F family members and complexes with various pocket proteins are characteristic of the resting and cycling cells (65). Thus, E2F-4–DP-1–p130 complexes are found in resting cells (45, 73), but the dominant G1 E2F complex in cycling cells contains E2F-1 with DP-1 and Rb or p107. Clearly, changes in the E2F profile and changes in cyclins are both early events in the cell cycle entry caused by EBV infection. Since only the RNA of cyclin D2 had been measured in previous experiments (72) and little study had been made of the E2F proteins (12), it was unclear whether the earliest effects of EBNA-2 and EBNA-LP on the cell cycle entry process were likely to be mediated through changing the balance of E2F complex proteins or through cyclin D2. In this study, in which we have analyzed the time course of changes to E2F factors, pocket proteins, cyclins, cdks, and cdk inhibitors, the induction of cyclin D2 is the earliest event we have observed that is dependent on viral gene expression (presumably of EBNA-2 and EBNA-LP). Identification of the initial target genes in the cell for EBV immortalizing proteins will thus now focus on whether the cyclin D2 promoter can be activated directly by EBNA-2 and EBNA-LP or whether there may be other intermediate steps. There is no consensus binding site for RBP-Jk in the first 1,000 nucleotides upstream of the reported transcription starts in the sequence of the cyclin D2 promoter (11), but EBNA-2 is known to be able to activate transcription through other DNA binding proteins; by analyzing the mechanism of induction of cyclin D2 RNA and screening for other genes that might be induced soon after infection, we hope to identify the direct cell targets that EBV modulates when it infects human B cells and, with such high efficiency, causes them to proliferate.
ACKNOWLEDGMENTS
We thank V. M. Dixit and E. Lam for plasmids used in this work.
REFERENCES
- 1.Alfieri C, Birkenbach M, Kieff E. Early events in Epstein-Barr virus infection of human B lymphocytes. Virology. 1991;181:595–608. doi: 10.1016/0042-6822(91)90893-g. . (Erratum, 185:946.) [DOI] [PubMed] [Google Scholar]
- 2.Allday M J, Inman G J, Crawford D H, Farrell P J. DNA damage in human B cells can induce apoptosis, proceeding from G1/S when p53 is transactivation competent and G2/M when it is transactivation defective. EMBO J. 1995;14:4994–5005. doi: 10.1002/j.1460-2075.1995.tb00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashkenazi A, Dixit V. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 4.Askew D S, Ashmun R A, Simmons B C, Cleveland J L. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991;6:1915–1922. [PubMed] [Google Scholar]
- 5.Beijersbergen R L, Kerkhoven R M, Zhu L, Carlee L, Voorhoeve P M, Bernards R. E2F-4, a new member of the E2F gene family, has oncogenic activity and associates with p107 in vivo. Genes Dev. 1994;8:2680–2690. doi: 10.1101/gad.8.22.2680. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Bassat H, Goldblum N, Mitrani S, Goldblum T, Yoffey J M, Cohen M M, Bentwich Z, Ramot B, Klein E, Klein G. Establishment in continuous culture of a new type of lymphocyte from a “Burkitt like” malignant lymphoma (line D.G.-75) Int J Cancer. 1977;19:27–33. doi: 10.1002/ijc.2910190105. [DOI] [PubMed] [Google Scholar]
- 7.Boise L H, Gonzalez-Garcia M, Postema C E, Ding L, Lindsten T, Turka L A, Mao X, Nunez G, Thompson C B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 8.Bonnefoy-Berard N, Genestier L, Flacher M, Revillard J P. The phosphoprotein phosphatase calcineurin controls calcium-dependent apoptosis in B cell lines. Eur J Immunol. 1994;24:325–329. doi: 10.1002/eji.1830240208. [DOI] [PubMed] [Google Scholar]
- 9.Brimmell M, Mendiola R, Mangion J, Packham G. BAX frameshift mutations in cell lines derived from human haemopoetic malignancies are associated with resistance to apoptosis and microsatellite instability. Oncogene. 1998;16:1803–1812. doi: 10.1038/sj.onc.1201704. [DOI] [PubMed] [Google Scholar]
- 10.Brodeur S, Cheng G, Baltimore D, Thorley-Lawson D. Localization of the major NF-kappa B activating site and the sole TRAF3 binding site of LMP-1 defines two distinct signaling motifs. J Biol Chem. 1997;272:19777–19784. doi: 10.1074/jbc.272.32.19777. [DOI] [PubMed] [Google Scholar]
- 11.Brooks A, Shiffman D, Chan C, Brooks E, Milner P. Functional analysis of the human cyclin D2 and D3 promoters. J Biol Chem. 1996;271:9090–9099. doi: 10.1074/jbc.271.15.9090. [DOI] [PubMed] [Google Scholar]
- 12.Cannell E, Farrell P, Sinclair A. Epstein-Barr virus exploits the normal cell pathway to regulate Rb activity during the immortalisation of primary B cells. Oncogene. 1996;13:1413–1421. [PubMed] [Google Scholar]
- 13.Chittenden T, Harrington E A, O’Connor R, Flemington C, Lutz R J, Evan G I, Guild B C. Induction of apoptosis by the Bcl-2 homologue. Bak Nature. 1995;374:733–736. doi: 10.1038/374733a0. [DOI] [PubMed] [Google Scholar]
- 14.Chiu V K, Walsh C M, Liu C C, Reed J C, Clark W R. Bcl-2 blocks degranulation but not fas-based cell-mediated cytotoxicity. J Immunol. 1995;154:2023–2032. [PubMed] [Google Scholar]
- 15.Cludts I, Farrell P. Multiple functions within the Epstein-Barr virus EBNA-3A protein. J Virol. 1998;72:1862–1869. doi: 10.1128/jvi.72.3.1862-1869.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cory S. Regulation of lymphocyte survival by the bcl-2 gene family. Annu Rev Immunol. 1995;13:513–543. doi: 10.1146/annurev.iy.13.040195.002501. [DOI] [PubMed] [Google Scholar]
- 17.Craig R W. The bcl-2 gene family. Semin Cancer Biol. 1995;6:35–43. doi: 10.1006/scbi.1995.0005. [DOI] [PubMed] [Google Scholar]
- 18.Debatin K M, Krammer P H. Resistance to APO-1 (CD95) induced apoptosis in T-ALL is determined by a BCL-2 independent anti-apoptotic program. Leukemia. 1995;9:815–820. [PubMed] [Google Scholar]
- 19.Devergne O, Hatzivassiliou E, Izumi K M, Kaye K M, Kleijnen M F, Kieff E, Mosialos G. Association of TRAF1, TRAF2, and TRAF3 with an Epstein-Barr virus LMP1 domain important for B-lymphocyte transformation: role in NF-κB activation. Mol Cell Biol. 1996;16:7098–7108. doi: 10.1128/mcb.16.12.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eliopoulos A, Young L. Activation of the cJun N-terminal kinase (JNK) pathway by the Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) Oncogene. 1998;16:1731–1742. doi: 10.1038/sj.onc.1201694. [DOI] [PubMed] [Google Scholar]
- 21.Falk M H, Trauth B C, Debatin K M, Klas C, Gregory C D, Rickinson A B, Calender A, Lenoir G M, Ellwart J W, Krammer P H, Bornkamm G. Expression of the APO-1 antigen in Burkitt lymphoma cell lines correlates with a shift towards a lymphoblastoid phenotype. Blood. 1992;79:3300–3306. [PubMed] [Google Scholar]
- 22.Farrell P. Epstein-Barr virus immortalizing genes. Trends Microbiol. 1995;3:105–109. doi: 10.1016/s0966-842x(00)88891-5. [DOI] [PubMed] [Google Scholar]
- 23.Farrell P. Signal transduction from the Epstein-Barr virus LMP-1 transforming protein. Trends Microbiol. 1998;6:175–177. doi: 10.1016/s0966-842x(98)01262-1. [DOI] [PubMed] [Google Scholar]
- 24.Farrow S N, White J H, Martinou I, Raven T, Pun K T, Grinham C J, Martinou J C, Brown R. Cloning of a bcl-2 homologue by interaction with adenovirus E1B 19K. Nature. 1995;374:731–733. doi: 10.1038/374731a0. [DOI] [PubMed] [Google Scholar]
- 25.Finke J, Rowe M, Kallin B, Ernberg I, Rosen A, Dillner J, Klein G. Monoclonal and polyclonal antibodies against Epstein-Barr virus nuclear antigen 5 (EBNA-5) detect multiple protein species in Burkitt’s lymphoma and lymphoblastoid cell lines. J Virol. 1987;61:3870–3878. doi: 10.1128/jvi.61.12.3870-3878.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fries K L, Miller W E, Raab-Traub N. Epstein-Barr virus latent membrane protein 1 blocks p53-mediated apoptosis through the induction of the A20 gene. J Virol. 1996;70:8653–8659. doi: 10.1128/jvi.70.12.8653-8659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gregory C D, Dive C, Henderson S, Smith C A, Williams G T, Gordon J, Rickinson A B. Activation of Epstein-Barr virus latent genes protects human B cells from death by apoptosis. Nature. 1991;349:612–614. doi: 10.1038/349612a0. [DOI] [PubMed] [Google Scholar]
- 28.Henderson S, Rowe M, Gregory C, Croom-Carter D, Wang F, Longnecker R, Kieff E, Rickinson A. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991;65:1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- 28a.Hollyoake M, Stühler A, Farrell P J, Gordon J, Sinclair A J. The normal cell cycle activation program is exploited during the infection of quiescent B lymphocytes by Epstein-Barr virus. Cancer Res. 1995;55:4784–4787. [PubMed] [Google Scholar]
- 29.Itoh N, Tsujimoto Y, Nagata S. Effect of bcl-2 on Fas antigen-mediated cell death. J Immunol. 1993;151:621–627. [PubMed] [Google Scholar]
- 30.Izumi K, Kieff E. The EBV oncogene product LMP-1 engages the TRADD protein to mediate B lymphocyte growth transformation and activate NF-κB. Proc Natl Acad Sci USA. 1997;94:12592–12597. doi: 10.1073/pnas.94.23.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly K, Knox K A. Differential regulatory effects of cAMP-elevating agents on human normal and neoplastic B cell functional response following ligation of surface immunoglobulin and CD40. Cell Immunol. 1995;166:93–102. doi: 10.1006/cimm.1995.0011. [DOI] [PubMed] [Google Scholar]
- 32.Kempkes B, Spitkovsky D, Jansen-Durr P, Ellwart J W, Kremmer E, Delecluse H J, Rottenberger C, Bornkamm G W, Hammerschmidt W. B-cell proliferation and induction of early G1-regulating proteins by Epstein-Barr virus mutants conditional for EBNA2. EMBO J. 1995;14:88–96. doi: 10.1002/j.1460-2075.1995.tb06978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiefer M C, Brauer M J, Powers V C, Wu J J, Umansky S R, Tomei L D, Barr P J. Modulation of apoptosis by the widely distributed Bcl-2 homologue Bak. Nature. 1995;374:736–739. doi: 10.1038/374736a0. [DOI] [PubMed] [Google Scholar]
- 34.Kieser A, Kilger E, Gires O, Ueffing M, Kolch W, Hammerschmidt W. Epstein-Barr virus latent membrane protein-1 triggers AP-1 activity via the c-Jun N-terminal kinase cascade. EMBO J. 1997;16:6478–6485. doi: 10.1093/emboj/16.21.6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kilger E, Kieser A, Baumann M, Hammerschmidt W. Epstein-Barr virus-mediated B-cell proliferation is dependent upon latent membrane protein 1, which simulates an activated CD40 receptor. EMBO J. 1998;17:1700–1709. doi: 10.1093/emboj/17.6.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King W, Thomas-Powell A L, Raab-Traub N, Hawke M, Kieff E. Epstein-Barr virus RNA. V. Viral RNA in a restringently infected, growth-transformed cell line. J Virol. 1980;36:506–518. doi: 10.1128/jvi.36.2.506-518.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein G, Dombos L. Relationship between the sensitivity of EBV-carrying lymphoblastoid lines to superinfection and the inducibility of the resident viral genome. Int J Cancer. 1973;11:327–337. doi: 10.1002/ijc.2910110210. [DOI] [PubMed] [Google Scholar]
- 38.Krikos A, Laherty C, Dixit V. Transcriptional activation of the tumor necrosis factor inducible zinc finger protein A20 is mediated by κB elements. J Biol Chem. 1992;267:17971–17976. [PubMed] [Google Scholar]
- 39.Laherty C D, Hu H M, Opipari A W, Wang F, Dixit V M. The Epstein-Barr virus LMP1 gene product induces A20 zinc finger protein expression by activating nuclear factor kappa B. J Biol Chem. 1992;267:24157–24160. [PubMed] [Google Scholar]
- 40.Lenoir G, Vuillaume M, Bonnardel C. The use of lymphomatous and lymphoblastoid cell lines in the study of Burkitt’s lymphoma. In: Lenoir G, O’Conor G, Olweny C, editors. Burkitt’s lymphoma: a human cancer model. Lyon, France: IARC Publications; 1985. pp. 309–318. [PubMed] [Google Scholar]
- 41.Mann K P, Staunton D, Thorley-Lawson D A. Epstein-Barr virus-encoded protein found in plasma membrane in transformed cells. J Virol. 1985;55:710–720. doi: 10.1128/jvi.55.3.710-720.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meijerink J P, Smetsers T F, Sloetjes A W, Linders E H, Mensink E J. Bax mutations in cell lines derived from hematological malignancies. Leukemia. 1995;9:1828–1832. [PubMed] [Google Scholar]
- 43.Memon S A, Moreno M B, Petrak D, Zacharchuk C M. Bcl-2 blocks glucocorticoid- but not Fas- or activation-induced apoptosis in a T cell hybridoma. J Immunol. 1995;155:4644–4652. [PubMed] [Google Scholar]
- 44.Milner A E, Johnson G D, Gregory C D. Prevention of programmed cell death in Burkitt lymphoma cell lines by bcl-2-dependent and -independent mechanisms. Int J Cancer. 1992;52:636–644. doi: 10.1002/ijc.2910520424. [DOI] [PubMed] [Google Scholar]
- 45.Moberg K, Starz M, Lees J. E2F-4 switches from p130 to p107 and pRb in response to cell cycle reentry. Mol Cell Biol. 1996;16:1436–1449. doi: 10.1128/mcb.16.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moreno M B, Memon S A, Zacharchuk C M. Apoptosis signaling pathways in normal T cells: differential activity of Bcl-2 and IL-1 beta-converting enzyme family protease inhibitors on glucocorticoid- and Fas-mediated cytotoxicity. J Immunol. 1996;157:3845–3849. [PubMed] [Google Scholar]
- 47.Mosialos G, Birkenbach M, Yalamanchili R, VanArsdale T, Ware C, Kieff E. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell. 1995;80:389–399. doi: 10.1016/0092-8674(95)90489-1. [DOI] [PubMed] [Google Scholar]
- 48.Oltvai Z N, Korsmeyer S J. Checkpoints of dueling dimers foil death wishes. Cell. 1994;79:189–192. doi: 10.1016/0092-8674(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 49.Oltvai Z N, Milliman C L, Korsmeyer S J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 50.Opipari A, Boguski M, Dixit V. The A20 cDNA induced by tumor necrosis factor alpha encodes a novel type of zinc finger protein. J Biol Chem. 1990;265:14705–14708. [PubMed] [Google Scholar]
- 51.Opipari A, Jr, Hu H M, Yabkowitz R, Dixit V M. The A20 zinc finger protein protects cells from tumor necrosis factor cytotoxicity. J Biol Chem. 1992;267:12424–12427. [PubMed] [Google Scholar]
- 52.Owen-Schaub L B, Radinsky R, Kruzel E, Berry K, Yonehara S. Anti-Fas on nonhematopoietic tumors: levels of Fas/APO-1 and bcl-2 are not predictive of biological responsiveness. Cancer Res. 1994;54:1580–1586. [PubMed] [Google Scholar]
- 53.Packham G, Brimmell M, Cleveland J. Mammalian cells express two differently localized Bag-1 isoforms generated by alternative translation initiation. Biochem J. 1997;328:808–813. doi: 10.1042/bj3280807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Packham G, Lahti J, Fee B, Gawn J, Coustan-Smith E, Campana D, Douglas L, Kidd V, Ghosh S, Cleveland J. Fas activates NF-κB and induces apoptosis in T cell lines by signaling pathways distinct from those activated by TNF-α. Cell Death Differ. 1997;4:130–139. doi: 10.1038/sj.cdd.4400217. [DOI] [PubMed] [Google Scholar]
- 55.Pagano M, Tam S, Theodoras A, Beer-Romero P, Sal G, Chau V, Yew P, Draetta G, Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 56.Parker, G., R. Touitou, and M. Allday. Submitted for publication.
- 57.Pulvertaft R. A study of malignant tumours in Nigeria in short term tissue culture. J Clin Pathol. 1965;18:261–273. doi: 10.1136/jcp.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reed J. Double identity for proteins of the Bcl-2 family. Nature. 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 59.Reed J C, Miyashita T, Takayama S, Wang H G, Sato T, Krajewski S, Aime-Sempe C, Bodrug S, Kitada S, Hanada M. BCL-2 family proteins: regulators of cell death involved in the pathogenesis of cancer and resistance to therapy. J Cell Biochem. 1996;60:23–32. doi: 10.1002/(SICI)1097-4644(19960101)60:1%3C23::AID-JCB5%3E3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 60.Reynolds J E, Yang T, Qian L, Jenkinson J D, Zhou P, Eastman A, Craig R W. Mcl-1, a member of the Bcl-2 family, delays apoptosis induced by c-Myc overexpression in Chinese hamster ovary cells. Cancer Res. 1994;54:6348–6352. [PubMed] [Google Scholar]
- 61.Rickinson A B, Kieff E. Epstein-Barr Virus. In: Fields B N, Knipe P M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2397–2446. [Google Scholar]
- 62.Rowe M, Rowe D T, Gregory C D, Young L S, Farrell P J, Rupani H, Rickinson A B. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt’s lymphoma cells. EMBO J. 1987;6:2743–2751. doi: 10.1002/j.1460-2075.1987.tb02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saegusa M, Takano Y, Hashimura M, Shoji Y, Okayasu I. The possible role of bcl-2 expression in the progression of tumors of the uterine cervix. Cancer. 1995;76:2297–2303. doi: 10.1002/1097-0142(19951201)76:11<2297::aid-cncr2820761118>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 64.Sandberg M, Hammerschmidt W, Sugden B. Characterization of LMP1’s association with TRAF1, TRAF2, and TRAF3. J Virol. 1997;71:4649–4656. doi: 10.1128/jvi.71.6.4649-4656.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sardet C, LeCam L, Fabrizio E, Vidal M. E2Fs and the retinoblastoma protein family. In: Yaniv M, Ghysdael J, editors. Oncogenes as transcriptional regulators. Basel, Switzerland: Birkhauser Verlag; 1997. pp. 1–57. [Google Scholar]
- 66.Sarma V, Lin Z, Clark L, Rust B M, Tewari M, Noelle R J, Dixit V M. Activation of the B-cell surface receptor CD40 induces A20, a novel zinc finger protein that inhibits apoptosis. J Biol Chem. 1995;270:12343–12346. doi: 10.1074/jbc.270.21.12343. [DOI] [PubMed] [Google Scholar]
- 67.Sato T, Hanada M, Bodrug S, Irie S, Iwama N, Boise L H, Thompson C B, Golemis E, Fong L, Wang H G, Reed J C. Interactions among members of the Bcl-2 protein family analyzed with a yeast two-hybrid system. Proc Natl Acad Sci USA. 1994;91:9238–9242. doi: 10.1073/pnas.91.20.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sedlak T W, Oltvai Z N, Yang E, Wang K, Boise L H, Thompson C B, Korsmeyer S J. Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc Natl Acad Sci USA. 1995;92:7834–7838. doi: 10.1073/pnas.92.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sheaff R J, Groudine M, Gordon M, Roberts J M, Clurman B E. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464–1478. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- 70.Shima Y, Nishimoto N, Ogata A, Fujii Y, Yoshizaki K, Kishimoto T. Myeloma cells express Fas antigen/APO-1 (CD95) but only some are sensitive to anti-Fas antibody resulting in apoptosis. Blood. 1995;85:757–764. [PubMed] [Google Scholar]
- 71.Sinclair A J, Farrell P J. Host cell requirements for efficient infection of quiescent primary B lymphocytes by Epstein-Barr virus. J Virol. 1995;69:5461–5468. doi: 10.1128/jvi.69.9.5461-5468.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sinclair A J, Palmero I, Peters G, Farrell P J. EBNA-2 and EBNA-LP cooperate to cause G0 to G1 transition during immortalization of resting human B lymphocytes by Epstein-Barr virus. EMBO J. 1994;13:3321–3328. doi: 10.1002/j.1460-2075.1994.tb06634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith E, Leone G, Degregori J, Jakoi L, Nevins J. The accumulation of an E2F-p130 transcriptional repressor distinguishes a G0 cell state from a G1 cell state. Mol Cell Biol. 1996;16:6965–6976. doi: 10.1128/mcb.16.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Strobel T, Swanson L, Korsmeyer S, Cannistra S. Radiation-induced apoptosis is not enhanced by expression of either p53 or BAX in SW626 ovarian cancer cells. Oncogene. 1997;14:2753–2758. doi: 10.1038/sj.onc.1201132. [DOI] [PubMed] [Google Scholar]
- 75.Sugano N, Chen W, Roberts M, Cooper N. Epstein-Barr virus binding to CD21 activates the initial viral promoter via NF-kappa B induction. J Exp Med. 1997;186:731–737. doi: 10.1084/jem.186.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takada K, Horinouchi K, Ono Y, Aya T, Osato T, Takahashi M, Hayasaka S. An Epstein-Barr virus-producer line Akata: establishment of the cell line and analysis of viral DNA. Virus Genes. 1991;5:147–156. doi: 10.1007/BF00571929. [DOI] [PubMed] [Google Scholar]
- 77.Takayama S, Sato T, Krajewski S, Kochel K, Irie S, Millan J A, Reed J C. Cloning and functional analysis of BAG-1: a novel Bcl-2-binding protein with anti-cell death activity. Cell. 1995;80:279–284. doi: 10.1016/0092-8674(95)90410-7. [DOI] [PubMed] [Google Scholar]
- 78.Vairo G, Livingston D, Ginsberg D. Functional interaction between E2F-4 and p130: evidence for distinct mechanisms underlying growth suppression by different retinoblastoma protein family members. Genes Dev. 1995;9:869–881. doi: 10.1101/gad.9.7.869. [DOI] [PubMed] [Google Scholar]
- 79.Van der Sman, J., N. S. B. Thomas, and E. Lam. Modulation of E2F complexes during G0 to S phase transition in human primary B lymphocytes. J. Biol. Chem., in press. [DOI] [PubMed]
- 80.Vlach J, Hennecke S, Amati B. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27. EMBO J. 1997;16:5334–5344. doi: 10.1093/emboj/16.17.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang H, Grand R J, Milner A E, Armitage R J, Gordon J, Gregory C D. Repression of apoptosis in human B-lymphoma cells by CD40-ligand and Bcl-2: relationship to the cell-cycle and role of the retinoblastoma protein. Oncogene. 1996;13:373–379. [PubMed] [Google Scholar]
- 82.Wilson G, Miller G. Recovery of Epstein-Barr virus from nonproducer neonatal human lymphoid cell transformants. Virology. 1979;95:351–358. doi: 10.1016/0042-6822(79)90490-2. [DOI] [PubMed] [Google Scholar]
- 83.Yang E, Korsmeyer S J. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996;88:386–401. [PubMed] [Google Scholar]
- 84.Yang E, Zha J, Jockel J, Boise L H, Thompson C B, Korsmeyer S J. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 85.Yin X, Oltvai Z, Korsmeyer S. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature. 1994;369:321–323. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]
- 86.Zha H, Aime-Sempe C, Sato T, Reed J C. Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J Biol Chem. 1996;271:7440–7444. doi: 10.1074/jbc.271.13.7440. [DOI] [PubMed] [Google Scholar]