Abstract
Purpose
Evidence suggests that high-risk lifestyle behaviors exacerbate the health of cancer survivors and increase cancer mortality. This study examined the prevalence of lifestyle-related risk factors among female breast cancer survivors by duration of survivorship in the United States.
Methods
We analyzed data from 7,443 women aged ≥18-years who participated in the 2009 Behavioral Risk Factor Surveillance System and reported having ever-diagnosed breast cancer. Adjusted prevalence with 95 % confidence interval for lifestyle-related risk factors (including current smoking, excessive alcohol drinking, obesity, engaging in physical activity ≥150 min/week, and consuming fruits and vegetables ≥5 times/day) was estimated using log-linear regression while controlling for confounders.
Results
Overall, the prevalence estimates for lifestyle-related risk factors were 10.2 % for current smoking, 6.8 % for excessive alcohol drinking, 24.7 % for obesity, 53.8 % for engaging in physical activity ≥150 min/week, and 33.9 % for consuming fruits and vegetables ≥5 times/day among female breast cancer survivors. After adjustment for covariates, with increasing years of survivorship, a linearly increasing trend was observed for current smoking (P=0.038), and quadratic trends were observed for excessive alcohol drinking (P<0.001) and obesity (P=0.048). The adjusted prevalence estimates for engaging in physical activity ≥150-min/week and consuming fruits and vegetables ≥5 times/day did not vary significantly by duration of survivorship.
Conclusion
Continuing efforts on counseling and encouraging breast cancer survivors to adopt healthy lifestyles are needed to improve their health.
Implications for cancer survivors
Understanding the trends of modifiable lifestyle-related risk factors among breast cancer survivors with varying duration of survivorship may assist health care providers to provide appropriate counseling for breast cancer patients to improve their health. Clinical and public health intervention programs should seek to maximize the number of recommended healthy behaviors especially in those women who are at high risk for failing to comply with the healthy lifestyle guidelines.
Keywords: Breast cancer, Cancer duration, Health-related behaviors, Lifestyles, BRFSS
Introduction
High-risk lifestyle behaviors such as smoking, excessive alcohol drinking, physical inactivity, and consuming a poor diet have been linked to elevated incidence rates of cancer (including breast cancer) [1–3], poor prognosis after cancer diagnosis [4–8], and higher risk for cancer mortality [9–11]. In contrast, among cancer patients, adoption of healthy lifestyles is associated with improved survival, reduced risks for recurrent and second cancers, reduced cancer mortality, and improved quality of life [12–20]. For example, compared with women who were physically inactive both before and after diagnosis of breast cancer, women diagnosed with breast cancer who increased physical activity after diagnosis had a 45 % lower risk for death; but women who decreased physical activity after diagnosis had a threefold higher risk for death [18]. Women diagnosed with breast cancer who consumed diets with a higher score on the Healthy Eating Index had a 60 % reduced risk for all-cause mortality and 88 % reduced risk for breast cancer mortality [4]. Thus, understanding the behavioral risk factors related to cancer prognosis is important for public health and health care professionals to implement effective interventions to achieve optimal survivorship. To date, evidence has shown the percentages of cancer survivors who adhere to lifestyle behavior recommendations remain low. Blanchard et al. reported that only 37.1 % of breast cancer survivors met physical activity recommendation (i.e., engage in at least 150 min of moderate-to-strenuous or 60 min of strenuous physical activity per week), 18.2 % met the fruit and vegetable consumption recommendation (i.e., consume at least five servings of fruits and vegetables per day), and 88.1 % met the smoking recommendation (i.e., not smoke) [21]. In addition, significant racial and ethnic differences in behavioral risk factors among breast cancer survivors have been reported [22]. In these studies, however, how prevalent the lifestyle-related risk factors are among breast cancer patients through the time course of cancer survivorship is unknown. Given that lifestyle modification elicits significant impact on prognosis among cancer survivors [23–25] and women living with breast cancer are likely to be interested in lifestyle modification to decrease cancer recurrence and mortality risk, more in-depth studies are necessary to extend our understanding on how modifiable health-related risk factors vary across the course of cancer survivorship. This is important for developing effective intervention programs for breast cancer patients to improve their survivorship and increase their physical and emotional wellbeing. Therefore, using data from a large, population-based sample of US adult women, we examined the prevalence of lifestyle-related risk factors among female breast cancer survivors by duration of survivorship.
Methods
We analyzed the data from the 2009 Behavioral Risk Factor Surveillance System (BRFSS). The BRFSS uses state-based, random-digit-dialed telephone surveys to collect health information including health and lifestyle behaviors, preventive health practices, health care access, and related chronic conditions among non-institutionalized adults aged ≥18-years. The BRFSS sampling methodology has been described elsewhere [26], and BRFSS data have been found to provide valid and reliable estimates of population prevalence consistent with those derived from national household surveys in the US [27, 28]. The median response rate was 52.5 %, and the median cooperation rate (the percentage of eligible residents contacted by BRFSS officials who completed the survey) was 75.0 % for the 2009 BRFSS. The BRFSS is a state-based population survey; the BRFSS data are publically accessible (http://www.cdc.gov/BRFSS/) and can be retrieved by individual states.
Cancer diagnosis was assessed using the following questions: (1) “Have you ever been told by a doctor, nurse, or other health professional that you had cancer?”, (2) “How many different types of cancer have you had?”, (3) “At what age were you told that you had cancer (for the first diagnosis of cancer if having more than one type of cancer)?”, and (4) “What type of cancer was it (for the most recent diagnosis if having more than one type of cancer)?”. We limited our analytic sample to women who reported being diagnosed with breast cancer only; women with breast cancer who also had other types of cancer were excluded because their survival years since breast cancer diagnosis could not be determined. The years of survivorship were calculated as participants’ age subtracted by the age at cancer diagnosis, and was categorized as <1, 1–4, 5–9, 10–14, 15–19, 20–24, 25–29, and ≥30 years.
We examined lifestyle-related risk factors like smoking and alcohol drinking as well as healthy behaviors like engaging in physical activity and eating a healthy diet rich in fruits and vegetables. Current smoking was defined as participants who had smoked at least 100 cigarettes during their lifetime and were still smoking at the time the survey was conducted [29]. Alcohol drinking was assessed by asking female respondents how many days per week or per month they had had at least one drink (equivalent to a 12-ounce beer, a 5-ounce glass of wine, or a drink with one shot of liquor) of any alcoholic beverages during the past 30 days, how many drinks they had on average on the days when they drank, and how many times they had four or more drinks on an occasion. Excessive alcohol drinking was defined as women who consumed a daily average of any alcohol beverages of >1drink or had ≥1 episode of consuming at least four drinks on one occasion during the previous 30 days [30]. Obesity was defined as having a body mass index of ≥30 kg/m2. Physical activity was assessed by asking participants whether, in a usual week, they did moderately intensive activities (such as brisk walking, bicycling, gardening, or anything else that causes small increases in breathing or heart rate) or vigorously intensive activities (such as running, aerobics, heavy yard work, or anything else that causes large increases in breathing or heart rate) for at least 10 min at a time. If the answer was “yes”, they were further asked about how many days per week and how much total time per day they engaged in the activities. We then calculated the average minutes per week (min/week) for these activities with a conversion of 1 min of vigorous-intensity activities equivalent to 2 min of moderate-intensity activities. Following the 2008 Physical Activity Guidelines [31], participants were categorized as engaging in moderate–intensive physical activity ≥150 min/week or not. Fruit and vegetable consumption was assessed by asking participants how many times per day (or per week, per month, per year) they drank fruit juices or ate fruit, green salad, potatoes (excluding French fries, fried potatoes, or potato chips), carrots, or any other vegetables. Following the American Cancer Society guidelines [32], participants were categorized as consuming fruit and vegetables either ≥5 times/day or not.
The potential confounding factors for our analyses included age (18–44, 45–64, and ≥65 years), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and other), education (<high school diploma, high school graduate, some college/technical school, and ≥college graduate), routine health check-up during the past year (yes or no), health insurance coverage (yes or no), and the number of chronic conditions including diabetes, heart disease, stroke, hyper-tension, asthma, arthritis, and disability. These conditions were assessed by asking participants whether they had ever been told by a healthcare professional that they had the conditions or still had asthma at the time when the survey was conducted. Disability was assessed by asking participants whether they were limited in any way in any activities because of physical, mental, or emotional problems or whether they were required to use special equipment such as a cane, a wheelchair, a special bed, or a special telephone because of any health problem; participants answering ‘yes’ to either question were defined as having a disability. The number of chronic conditions (including disability) was summed, and participants were categorized as having 0, 1, 2, and ≥3 chronic conditions.
Statistical analysis
The unadjusted and adjusted prevalence estimates with 95 % confidence intervals for lifestyle-related risk factors were weighted to the census 2009 US population and were then estimated using log-linear regression models with robust variance estimator without and with controlling for study covariates. Trends in the prevalence of lifestyle-related risk factors were tested using orthogonal contrasts. We conducted the analyses using SAS (version 9.2, SAS Institute, Cary, NC) and SUDAAN software (release 10.0.1, Research Triangle Institute, Research Triangle Park, NC) to account for the multistage, complex sampling design.
Results
Of 10,314 women diagnosed with breast cancer who participated in the BRFSS, we excluded 1,396 women who also had other types of cancers. After we further excluded participants who responded “don’t know/not sure” or had missing responses to any of the study variables, there were 7,443 female breast cancer survivors who were eligible for our analysis. Participants’ median age was 64.2 years, and the median survivorship time was 7.4 years. By race/ethnicity, 78.6 % of breast cancer survivors were non-Hispanic white, 10.4 % non-Hispanic black, 6.6 % Hispanic, and 4.4 % other racial/ethnic group. Approximately 65.0 % of breast cancer survivors had attained an educational level of greater than a high school diploma; 95.7 % reported having health insurance coverage; and 84.8 % had a routine medical check-up during the past year. About 28.9 % reported having one chronic condition; 23.4 % had two conditions; and 25.8 % had at least three conditions.
Overall, the weighted, unadjusted prevalence estimates for lifestyle-related risk factors among breast cancer survivors were 10.2 % (95 % CI, 8.9–11.5 %) for current smoking, 6.8 % (95 % CI, 5.9–7.9 %) for excessive alcohol drinking, 24.7 % (95 % CI, 22.9–26.5 %) for obesity, 53.8 % (95 % CI, 51.6–56.0 %) for engaging in physical activity ≥150 min/week, and 33.9 % (95 % CI, 31.9–36.0 %) for consuming fruits and vegetables ≥5 times/day (Table 1). With increasing levels of education, current smoking, and obesity decreased significantly whereas excessive alcohol drinking, engaging in physical activity ≥150 min/week, and consuming fruits and vegetables ≥5 times/day increased significantly (P≤0.001 for all); however, the opposite trends were observed with increasing number of chronic conditions (P<0.05 for all except for current smoking, Table 1).
Table 1.
Weighted prevalence (%) of lifestyle-related risk factors among female breast cancer survivors stratified by selected characteristics, BRFSS 2009
| No. | Current smoking | Excessive drinking | Obesity | Engaging in physical activity ≥150 min/week | Consuming fruits and vegetables ≥5 times/day | |
|---|---|---|---|---|---|---|
| Overall | 7,443 | 10.2 (8.9, 11.5) | 6.8 (5.9, 7.9) | 24.7 (22.9, 26.5) | 53.8 (51.6, 56.0) | 33.9 (31.9, 36.0) |
| Age (years) | ||||||
| 18–44 | 246 | 12.3 (7.9, 18.4) | 12.6 (7.4, 20.6) | 34.0 (24.9, 44.5) | 60.2 (49.8, 69.7) | 34.4 (25.3, 44.8) |
| 45–64 | 2,832 | 12.3 (10.4, 14.6) | 8.7 (7.2, 10.6) | 24.5 (21.8, 27.6) | 60.8 (57.3, 64.1) | 34.6 (31.3, 38.0) |
| ≥65 | 4,365 | 8.0 (6.5, 9.9) | 4.5 (3.7, 5.5) | 23.7 (21.5, 26.1) | 47.0 (44.1, 49.9) | 33.2 (30.7, 35.9) |
| Race/ethnicity | ||||||
| Non-Hispanic white | 6,356 | 10.0 (8.9, 11.3) | 7.6 (6.6, 8.8) | 22.9 (21.2, 24.7) | 55.1 (53.0, 57.1) | 34.3 (32.3, 36.3) |
| Non-Hispanic black | 501 | 11.9 (6.7, 20.4) | 4.5 (2.0, 9.8) | 42.0 (33.1, 51.4) | 42.9 (33.8, 52.4) | 32.8 (24.5, 42.4) |
| Hispanic | 260 | 6.9 (3.1, 14.7) | 3.6 (1.3, 9.9) | 24.0 (15.5, 35.3) | 48.3 (34.1, 62.7) | 35.0 (23.4, 48.7) |
| Other | 326 | 13.1 (7.5, 21.8) | 2.6 (1.2, 5.4) | 15.6 (9.7, 24.3) | 64.4 (52.0, 75.2) | 27.9 (18.3, 40.2) |
| Education | ||||||
| <High school | 534 | 18.3 (13.0, 25.1) | 2.2 (1.2, 4.0) | 38.7 (31.0, 47.1) | 37.2 (29.5, 45.7) | 21.3 (14.7, 29.7) |
| High school graduate | 2,292 | 12.8 (10.2, 16.0) | 3.8 (2.7, 5.3) | 29.8 (26.4, 33.5) | 45.6 (41.8, 49.6) | 24.7 (21.5, 28.2) |
| Some college/technical | 2,139 | 11.6 (9.6, 14.1) | 7.1 (5.5, 9.1) | 26.8 (23.5, 30.5) | 53.7 (50.0, 57.4) | 35.0 (31.5, 38.6) |
| College graduate | 2,478 | 5.6 (4.1, 7.5) | 9.7 (8.0, 11.9) | 16.6 (14.2, 19.4) | 62.9 (58.7, 66.9) | 42.3 (38.4, 46.2) |
| Routine check-up visit | ||||||
| Yes | 6,320 | 9.8 (8.5, 11.3) | 6.7 (5.7, 7.8) | 24.9 (23.0, 26.9) | 53.7 (51.4, 56.0) | 34.4 (32.3, 36.7) |
| No | 1,123 | 12.2 (9.4, 15.7) | 7.6 (5.2, 11.0) | 23.2 (18.6, 28.6) | 54.3 (47.0, 61.4) | 30.9 (25.6, 36.7) |
| Health insurance | ||||||
| Yes | 7,156 | 9.5 (8.3, 10.9) | 6.9 (6.0, 8.0) | 24.6 (22.8, 26.5) | 53.9 (51.8, 56.1) | 34.2 (32.1, 36.2) |
| No | 287 | 25.0 (15.7, 37.3) | 4.7 (2.3, 9.7) | 25.4 (16.3, 37.2) | 50.4 (32.7, 67.9) | 28.0 (17.2, 42.0) |
| No. of chronic condition | ||||||
| 0 | 1,378 | 9.6 (7.0, 12.8) | 9.8 (7.5, 12.6) | 10.8 (8.4, 13.8) | 66.5 (60.2, 72.3) | 38.1 (33.0, 43.5) |
| 1 | 2,060 | 8.6 (6.9, 10.8) | 7.7 (5.8, 10.0) | 20.5 (17.4, 23.9) | 61.9 (57.9, 65.9) | 34.2 (30.5, 38.1) |
| 2 | 1,878 | 12.1 (9.2, 15.8) | 6.7 (5.2, 8.5) | 23.5 (20.2, 27.2) | 53.2 (49.0, 57.3) | 31.2 (27.5, 35.1) |
| ≥3 | 2,127 | 10.6 (8.6, 13.0) | 3.5 (2.4, 5.2) | 42.2 (38.4, 46.1) | 34.3 (30.9, 38.0) | 32.4 (28.7, 36.3) |
| Years of survivorship | ||||||
| <1 | 327 | 7.8 (4.1, 14.2) | 4.0 (1.9, 8.1) | 27.9 (20.6, 36.6) | 57.3 (47.9, 66.2) | 30.1 (22.5, 39.1) |
| 1–4 | 1,922 | 9.6 (7.8, 11.9) | 6.9 (5.3, 8.9) | 25.4 (22.0, 29.1) | 54.8 (50.5, 59.0) | 32.3 (28.5, 36.4) |
| 5–9 | 1,813 | 11.0 (8.7, 13.9) | 8.1 (6.2, 10.5) | 24.5 (21.3, 28.0) | 56.2 (52.0, 60.2) | 36.4 (32.4, 40.5) |
| 10–14 | 1,327 | 10.4 (7.8, 13.8) | 8.4 (6.3, 11.1) | 25.6 (21.3, 30.5) | 52.9 (48.0, 57.6) | 32.4 (28.0, 37.1) |
| 15–19 | 771 | 7.4 (4.7, 11.4) | 7.7 (4.9, 11.9) | 25.7 (19.6, 32.9) | 52.5 (43.6, 61.3) | 38.5 (31.1, 46.5) |
| 20–24 | 539 | 11.7 (7.7, 17.3) | 5.9 (2.8, 12.0) | 25.2 (19.0, 32.7) | 51.1 (42.7, 59.5) | 30.9 (24.4, 38.1) |
| 25–29 | 282 | 10.3 (5.7, 18.0) | 4.0 (1.8, 8.3) | 19.3 (13.4, 27.2) | 53.2 (44.0, 62.2) | 36.0 (27.1, 45.9) |
| ≥30 | 462 | 13.4 (6.2, 26.6) | 0.9 (0.5, 1.7) | 17.3 (10.6, 27.0) | 44.7 (35.5, 54.2) | 32.0 (23.4, 42.2) |
After multivariate adjustment for covariates including age, race/ethnicity, education, routine health check-up, health insurance coverage, and the number of chronic conditions, with increasing years of survivorship, a linearly increasing trend was observed for the prevalence of current smoking (P=0.038), and quadratic trends were observed for the prevalence of excessive alcohol drinking (P<0.001) and obesity (P=0.048; Fig. 1). Compared with women who were recently diagnosed with breast cancer (within a year), those who survived for at least 30 years had an adjusted prevalence ratio of 2.03 (95 % CI, 0.98–5.25) for current smoking, 0.36 (95 % CI, 0.13–0.99) for excessive alcohol drinking, and 0.57 (95 % CI, 0.35–0.93) for obesity. The adjusted prevalence estimates for engaging in physical activity ≥150-min/week and consuming fruits and vegetables ≥5 times/day did not vary much by years survived since cancer diagnosis (Fig. 1).
Fig. 1.
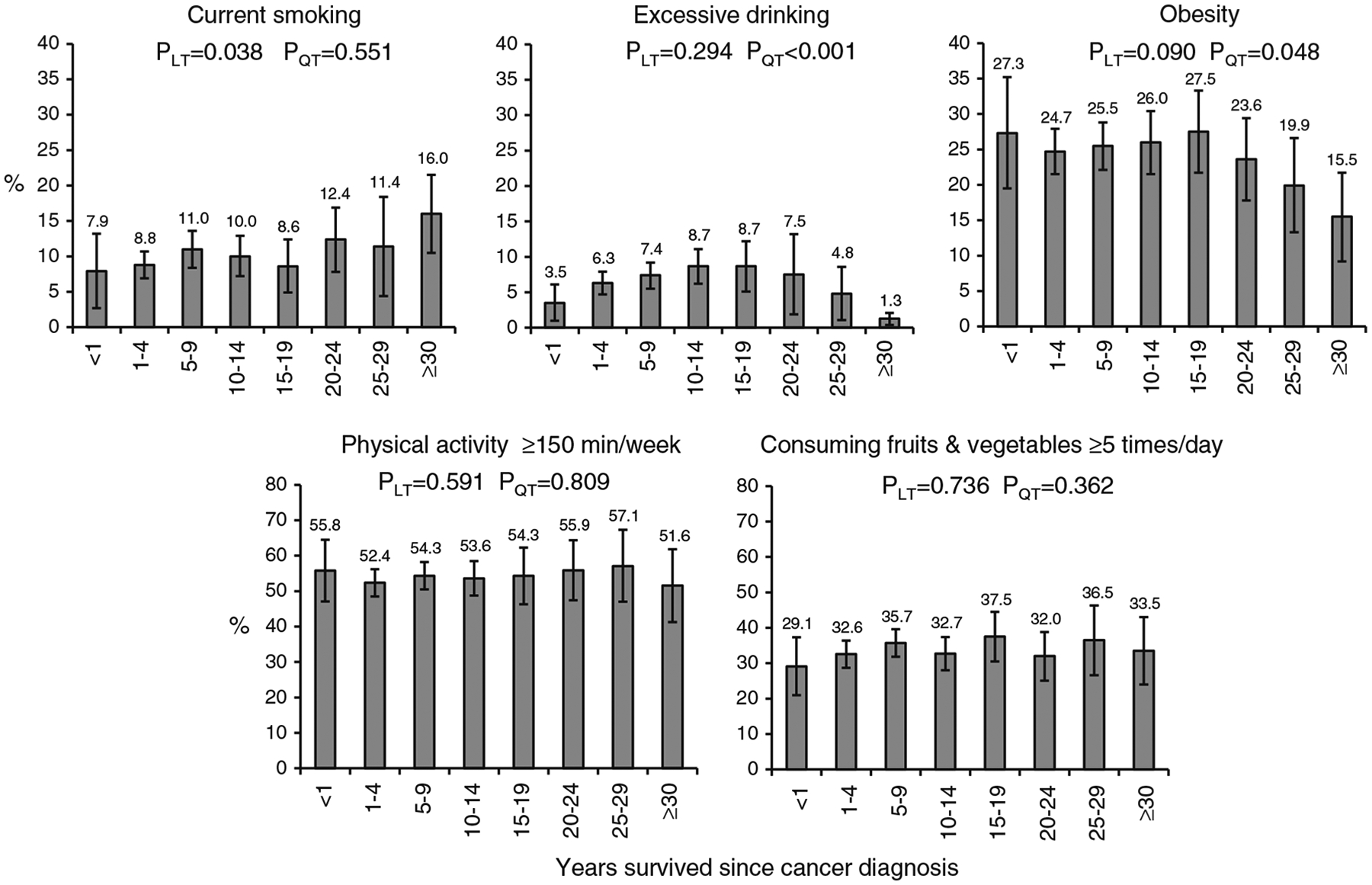
Adjusted prevalence estimates (with 95 % confidence intervals) for lifestyle-related risk factors by years survived since cancer diagnosis among female breast cancer survivors, BRFSS 2009. Adjusted for age, race/ethnicity, education, routine health check-up, health insurance coverage, and the number of chronic conditions. LT linear trend, QT quadratic trend
Discussion
The strength of the present study is that our data were derived from a large, population-based sample of women who reported being breast cancer survivors, which provided a unique opportunity for us to report nationally representative estimates for the prevalence of modifiable lifestyle-related risk factors across different survivorship periods after cancer diagnosis. To our knowledge, this has not been previously described in the literature. Our results showed that among long-term breast cancer survivors (≥30 years after cancer diagnosis), favorable changes were observed for excessive alcohol drinking and obesity; however, there was about twofold increase in the prevalence of current smoking compared to those who were diagnosed with cancer recently. The prevalence of meeting recommendations for physical activity participation and fruit/vegetable consumption did not vary much by duration of survivorship, which is consistent with previous findings [32, 33].
Our findings have several important implications in the development of effective long-term care programs for breast cancer patients. First, our study showed an overall low prevalence of meeting lifestyle recommendations among breast cancer survivors, suggesting more effects from clinical care providers and public health professionals are needed to encourage cancer survivors to adhere to lifestyle recommendations. Second, our results showed some variations in health-related risk factors across the time course of survivorship period. This suggests the lifestyle education and intervention programs may emphasize different health behavioral risk factors at varying periods of survivorship; particularly, efforts should be made to promote their physical activity participation and healthy eating style across all survivorship periods, to reduce obesity and risky alcohol drinking among those who have relatively short survivorship periods after cancer diagnosis, and to reduce smoking or promote smoking cessation among those who have longer survivorship periods.
The number of women surviving breast cancer has increased despite a relatively stable incidence rate in the USA [34]. This increase may be attributed to many factors including early detection, effective treatment and subsequent follow-up care, and aging population [35]. In 2007, about 2.6 million US women were breast cancer survivors [36], which is predicted to increase to 3.4 million by 2015 [37]. To reduce the risk of cancer recurrence and mortality and improve health outcomes among cancer survivors, the American Cancer Society recently updated the guidelines on nutrition and physical activity which include avoiding tobacco products, achieving and maintaining a healthy weight, staying physically active (at least 150 min of moderate intensity or 75 min of vigorous intensity or their combination each week—preferably spread throughout the week), eating a healthy diet (high in fruits, vegetables, and whole grains and low in processed meat and red meat), and limiting alcohol intake (no more than one drink per day for women) [32, 33]. However, limited evidence from studies examining behavioral risk factors among small samples (i.e., varied from n=386 to n=2,885) of female breast cancer survivors showed that about 12–14 % of them were currently smoking [21, 38, 39] and 5 % were risky drinkers (defined as an average of ≥10 alcoholic beverages per week) [39], which is consistent with our findings that about one-tenth or fewer of breast cancer survivors reported currently smoking or excessive alcohol drinking. Our results further revealed that about 25 % of breast cancer survivors were obese, which has not been reported previously. For physical activity, our results showed a much higher prevalence of engaging in physical activity at recommended levels than the findings of the previous studies (54% versus 20–37 %) among breast cancer survivors [21, 39]. This may have resulted from different physical activity guidelines applied to assess physical activity. In the present study, we applied the new 2008 DHHS physical activity guidelines [31], in which the requirement for frequency (at least 5 days a week) of physical activity participation has been removed and the equivalent combination of moderate and vigorous physical activity was counted. These modifications have led to a high prevalence of meeting physical activity recommendations in the general population [40]. The percentage of breast cancer survivors who reported consuming at least five times/day of fruits and vegetables was much higher than that reported by Blanchard et al (34 versus 18 %) [21], which was based on the assessment of consuming at least five servings per day of fruits and vegetables. Nonetheless, our results in combination with previous findings demonstrate that breast cancer survivors’ adherence to lifestyle recommendations remains low and achieving recommended levels of lifestyle behavior modification is a distant target in these patients. Increasing efforts (such as effective counseling and health education through physicians or health care professionals) are needed to increase their knowledge about the benefits healthy lifestyles confer, particularly among subpopulations with elevated rates of unhealthy behaviors at varying stages of the survivorship period.
Our study has several limitations. First, all responses in the BRFSS were self-reported and, thus, subject to recall bias. Second, the severity of breast cancer and its treatments were not assessed due to lack of data. Third, the 2009 BRFSS survey excluded adults who had been institutionalized or hospitalized and those with only mobile telephones. Because these adults are more likely to have severe physical or mental illness (for those who were institutionalized or hospitalized) or to be of low socioeconomic status (for those with only mobile telephones), this exclusion may have led us to underestimate the prevalence of lifestyle-related behaviors among women with breast cancer. Finally, the BRFSS sampling frame did not allow to capture all cancer survivors to participate in the survey, therefore, we may not have a full picture of the health-related risk factors among breast cancer survivors, so generalizability of the results is limited.
In conclusion, to promote the overall health of breast cancer survivors through the survivorship period after cancer diagnosis, clinical and public health intervention programs should seek to maximize the number of recommended healthy behaviors especially in those women who are at high risk for failing to comply with the healthy lifestyle guidelines. Nationally prioritized lifestyle and nutrition programs may benefit all women including breast cancer survivors. In addition, the potential barriers that prevent women from improving their lifestyle behaviors need to be investigated.
Footnotes
Disclosure of interests All authors have no financial support or competing interest to declare.
Disclaimer The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Contributor Information
Guixiang Zhao, Division of Behavioral Surveillance, Public Health Surveillance Program Office, Office of Surveillance, Epidemiology and Laboratory Services, Centers for Disease Control and Prevention, 2500 Century Parkway, Mailstop E-97, Atlanta, GA 30345, USA.
Chaoyang Li, Division of Behavioral Surveillance, Public Health Surveillance Program Office, Office of Surveillance, Epidemiology and Laboratory Services, Centers for Disease Control and Prevention, 2500 Century Parkway, Mailstop E-97, Atlanta, GA 30345, USA.
Catherine A. Okoro, Division of Behavioral Surveillance, Public Health Surveillance Program Office, Office of Surveillance, Epidemiology and Laboratory Services, Centers for Disease Control and Prevention, 2500 Century Parkway, Mailstop E-97, Atlanta, GA 30345, USA
Jun Li, Division of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Xiao Jun Wen, Division of Behavioral Surveillance, Public Health Surveillance Program Office, Office of Surveillance, Epidemiology and Laboratory Services, Centers for Disease Control and Prevention, 2500 Century Parkway, Mailstop E-97, Atlanta, GA 30345, USA.
Arica White, Division of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Lina S. Balluz, Division of Behavioral Surveillance, Public Health Surveillance Program Office, Office of Surveillance, Epidemiology and Laboratory Services, Centers for Disease Control and Prevention, 2500 Century Parkway, Mailstop E-97, Atlanta, GA 30345, USA
References
- 1.Khan N, Afaq F, Mukhtar H. Lifestyle as risk factor for cancer: evidence from human studies. Cancer Lett. 2010;293(2):133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nahleh Z, Bhatti NS, Mal M. How to reduce your cancer risk: mechanisms and myths. Int J Gen Med. 2011;4:277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein CJ, Colditz GA. Modifiable risk factors for cancer. Br J Cancer. 2004;90(2):299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.George SM, Irwin ML, Smith AW, et al. Postdiagnosis diet quality, the combination of diet quality and recreational physical activity, and prognosis after early-stage breast cancer. Cancer Causes Control. 2011;22(4):589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwan ML, Weltzien E, Kushi LH, Castillo A, Slattery ML, Caan BJ. Dietary patterns and breast cancer recurrence and survival among women with early-stage breast cancer. J Clin Oncol. 2009;27(6):919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li CI, Daling JR, Porter PL, Tang MT, Malone KE. Relationship between potentially modifiable lifestyle factors and risk of second primary contralateral breast cancer among women diagnosed with estrogen receptor-positive invasive breast cancer. J Clin Oncol. 2009;27(32):5312–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pierce JP, Stefanick ML, Flatt SW, et al. Greater survival after breast cancer in physically active women with high vegetable–fruit intake regardless of obesity. J Clin Oncol. 2007;25(17):2345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doyle C, Kushi LH, Byers T, et al. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA Cancer J Clin. 2006;56(6):323–53. [DOI] [PubMed] [Google Scholar]
- 9.Khaw KT, Wareham N, Bingham S, Welch A, Luben R, Day N. Combined impact of health behaviours and mortality in men and women: the EPIC-Norfolk prospective population study. PLoS Med. 2008;5(1):e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kvaavik E, Batty GD, Ursin G, Huxley R, Gale CR. Influence of individual and combined health behaviors on total and cause-specific mortality in men and women: the United Kingdom health and lifestyle survey. Arch Intern Med. 2010;170(8):711–8. [DOI] [PubMed] [Google Scholar]
- 11.van Dam RM, Li T, Spiegelman D, Franco OH, Hu FB. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ. 2008;337:a1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alfano CM, Smith AW, Irwin ML, et al. Physical activity, long-term symptoms, and physical health-related quality of life among breast cancer survivors: a prospective analysis. J Cancer Surviv. 2007;1(2):116–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedenreich CM, Gregory J, Kopciuk KA, Mackey JR, Courneya KS. Prospective cohort study of lifetime physical activity and breast cancer survival. Int J Cancer. 2009;124(8):1954–62. [DOI] [PubMed] [Google Scholar]
- 14.Holick CN, Newcomb PA, Trentham-Dietz A, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(2):379–86. [DOI] [PubMed] [Google Scholar]
- 15.Holmes MD, Kroenke CH. Beyond treatment: lifestyle choices after breast cancer to enhance quality of life and survival. Womens Health Issues. 2004;14(1):11–3. [DOI] [PubMed] [Google Scholar]
- 16.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293(20):2479–86. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28(3):753–65. [DOI] [PubMed] [Google Scholar]
- 18.Irwin ML, Smith AW, McTiernan A, et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol. 2008;26(24):3958–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ford ES, Zhao G, Tsai J, Li C. Low-Risk Lifestyle Behaviors and All-Cause Mortality: Findings From the National Health and Nutrition Examination Survey III Mortality Study. Am J Public Health. 2011;101(10):1922–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCullough ML, Patel AV, Kushi LH, et al. Following cancer prevention guidelines reduces risk of cancer, cardiovascular disease, and all-cause mortality. Cancer Epidemiol Biomarkers Prev. 2011;20(6):1089–97. [DOI] [PubMed] [Google Scholar]
- 21.Blanchard CM, Courneya KS, Stein K. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol. 2008;26(13):2198–204. [DOI] [PubMed] [Google Scholar]
- 22.White A, Pollack LA, Smith JL, Thompson T, Underwood JM, Fairley T. Racial and ethnic differences in health status and health behavior among breast cancer survivors—Behavioral Risk Factor Surveillance System, 2009. J Cancer Surviv. 2013;7(1):93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demark-Wahnefried W, Platz EA, Ligibel JA, et al. The role of obesity in cancer survival and recurrence. Cancer Epidemiol Bio-markers Prev. 2012;21(8):1244–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellsworth RE, Valente AL, Shriver CD, Bittman B, Ellsworth DL. Impact of lifestyle factors on prognosis among breast cancer survivors in the USA. Expert Rev Pharmacoecon Outcomes Res. 2012;12(4):451–64. [DOI] [PubMed] [Google Scholar]
- 25.Loprinzi PD, Cardinal BJ, Winters-Stone K, Smit E, Loprinzi CL. Physical activity and the risk of breast cancer recurrence: a literature review. Oncol Nurs Forum. 2012;39(3):269–74. [DOI] [PubMed] [Google Scholar]
- 26.Mokdad AH, Stroup DF, Giles WH. Public health surveillance for behavioral risk factors in a changing environment. Recommendations from the Behavioral Risk Factor Surveillance Team. MMWR Recomm Rep. 2003;52(9):1–12. [PubMed] [Google Scholar]
- 27.Nelson DE, Holtzman D, Bolen J, Stanwyck CA, Mack KA. Reliability and validity of measures from the Behavioral Risk Factor Surveillance System (BRFSS). Soc Prev Med. 2001;46 Suppl 1:S3–S42. [PubMed] [Google Scholar]
- 28.Nelson DE, Powell-Griner E, Town M, Kovar MG. A comparison of national estimates from the National Health Interview Survey and the Behavioral Risk Factor Surveillance System. Am J Public Health. 2003;93(8):1335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Tabacco Control State Highlights. Data Sources, Definitions and Interpretation. Available at http://www.cdc.gov/tobacco/data_statistics/state_data/state_highlights/2010/pdfs/data.pdf. Accessed 24 May 2013. [Google Scholar]
- 30.US Department of Agriculture and US Department of Health and Human Services. Dietary Guidelines for Americans 2010. Chapter 3-Foods and Food Components to Reduce. Available at http://www.cnpp.usda.gov/Publications/DietaryGuidelines/2010/PolicyDoc/Chapter3.pdf. 7th Edition. Washington, DC: US Government Printing Office. Accessed 24 May 2013. [Google Scholar]
- 31.US Depatment of Health and Human Services. 2008 Physical Activity Guidelines for Americans. Hyattsville, MD: US Department of Health and Human Services; 2008. Available at http://www.health.gov/paguidelines/guidelines/default.aspx. Accessed 28 November 2012. [Google Scholar]
- 32.Kushi LH, Doyle C, McCullough M, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62(1):30–67. [DOI] [PubMed] [Google Scholar]
- 33.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–74. [DOI] [PubMed] [Google Scholar]
- 34.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations), National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2009_pops09/, based on November 2011 SEER data submission, posted to the SEER web site, April 2012. Accessed 28 November 2012. [Google Scholar]
- 35.Hewitt ME, Greenfield S, Stovall E. Committee on Cancer Survivorship: Improving Care and Quality of Life. (2006) From cancer patient to cancer survivor: lost in transition. Washington, D.C: National Academies Press; 2012. [Google Scholar]
- 36.Centers for Disease Control and Prevention. Cancer survivors—United States, 2007. MMWR Morb Mortal Wkly Rep. 2011;60(9):269–72. [PubMed] [Google Scholar]
- 37.De AR, Tavilla A, Verdecchia A, et al. Breast cancer survivors in the United States: geographic variability and time trends, 2005–2015. Cancer. 2009;115(9):1954–66. [DOI] [PubMed] [Google Scholar]
- 38.Bellizzi KM, Rowland JH, Jeffery DD, McNeel T. Health behaviors of cancer survivors: examining opportunities for cancer control intervention. J Clin Oncol. 2005;23(34):8884–93. [DOI] [PubMed] [Google Scholar]
- 39.Coups EJ, Ostroff JS. A population-based estimate of the prevalence of behavioral risk factors among adult cancer survivors and noncancer controls. Prev Med. 2005;40(6):702–11. [DOI] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention. Prevalence of self-reported physically active adults—United States, 2007. MMWR Morb Mortal Wkly Rep. 2008;1297(48). [PubMed] [Google Scholar]


