Abstract
Background And Objective.
Cannabis Use Disorder (CUD) has no FDA approved treatment. Serotonin-2c (5HT2c) agonists have preclinical and human laboratory evidence for potential efficacy for CUD. We assessed the tolerability and effects of lorcaserin (5HT2c agonist) on CUD.
Methods.
In a 10-week, open label, uncontrolled trial, the tolerability of lorcaserin was tested in outpatients with CUD. Adverse events (AE) were assessed weekly. Cannabis use was assessed twice weekly by the Timeline follow-back and quantitative urine metabolites.
Results.
17 participants enrolled, and 14 received medication. Participants’ average age was 35 years; majority were male (N=12). The medication was well tolerated in males. There were no serious adverse events (SAE). The most common AE’s were headache/migraine (N=4, all females), anorexia (N=3), and irritability (N=2). Participants decreased their frequency of cannabis use significantly (p < 0.001), adjusted for baseline use. By the end of the trial, participants decreased by 1.76 (SE=0.47) cannabis using days/week. Average daily amount of cannabis and urine THC metabolite levels did not change significantly.
Conclusions:
Lorcaserin was well tolerated in males but not females suggesting possible sex differences. Future trials of other 5HT2c agonists (lorcaserin was withdrawn at the request of the FDA) should consider longer dose titration phases.
Trial Registration:
Keywords: cannabis use disorder, lorcaserin, sex differences, serotonin 2c agonist, tolerability
Introduction
Cannabis is the most commonly used drug in the United States.(2019 NSDUH Annual National Report | CBHSQ Data, no date) Social and policy trends demonstrate increasing acceptance and use of cannabis with decreasing perception of harm.(Hasin et al., 2017; 2019 NSDUH Annual National Report | CBHSQ Data, no date) Large epidemiological datasets demonstrate increases in the number of people utilizing cannabis, greater numbers of individuals with problematic cannabis use and those who meet DSM-5 criteria for Cannabis Use Disorder (CUD), particularly during the COVID-19 era.(Hasin and Walsh, 2021) There are no FDA approved medications for the treatment of CUD, and few individuals seeking treatment for CUD undergoing behavioral interventions are able to achieve sustained abstinence.(Hughes et al., 2016; Carroll et al., 2012; Stephens, Roffman and Curtin, 2000) Given the limited treatment options available to clinicians, further research is needed in medication development.
Preclinical literature has demonstrated that serotonin-2c (5HT2c) receptor agonists alter neurobiological systems and behaviors relevant to substance use. Electrophysiological, (V et al., 2002; Di Matteo et al., 1999; Di Matteo et al., 2000; Gobert et al., 2000) neurochemical, (Boothman et al., 2006; Bubar and Cunningham, 2007; Liu et al., 2007;Pozzi et al., 2002) and behavioral (Grottick, Fletcher and Higgins, 2000; Higgins, Sellers and Fletcher, 2013; Somerville et al., 2007) evidence in animal models supports that 5HT2c receptor activation exerts inhibitory control over the mesocorticolimbic dopamine system and DA-dependent behaviors that are key in the underlying processes of addiction. 5HT2c receptor agonists have also been shown to reduce drug-seeking behavior, drug administration, and impulsive behavior in animal studies.(Higgins and Fletcher, 2015; Higgins et al., 2013) Administration of 5HT2c receptor agonists block Δ9-tetrahydrocannabinol (THC, the main psychoactive component of cannabis) induced conditioned place preference in addition to decreasing the firing rate of ventral tegmental area dopaminergic neurons following exposure to THC in a rat model.(Higgins, Fletcher and Shanahan, 2020) Both of these inhibitory effects were reversed when a 5HT2c receptor antagonist was used in pretreatment.(Higgins, Fletcher and Shanahan, 2020) These data support 5HT2c agonists’ role in suppressing addiction-related behavior with THC in animal models,(Higgins, Fletcher and Shanahan, 2020) as cannabis use associated behaviors appear to be under inhibitory control of 5HT2c-receptors.
In 2012 the FDA approved lorcaserin (Belviq™), a 5HT2c receptor agonist, in the treatment of chronic weight management, making this class of drug available for the first time in the clinical treatment of patients. The substance use research community has a strong interest in the potential of 5HT2c-receptor agonists in the treatment of CUD. An inpatient, human laboratory study of lorcaserin 10mg twice daily administered for a total of 13 days that was placebo-controlled and counterbalanced within-subjects demonstrated significant decreases in cannabis self-administration and cravings in 15 chronic, frequent, non-treatment seeking cannabis users.(Arout et al., 2021) Given these promising findings, we investigated the clinical potential of lorcaserin by assessing its tolerability in the treatment of CUD in a real-world clinic setting over a 10-week, outpatient, uncontrolled, open-label trial. We obtained preliminary data on changes in cannabis use patterns.
Methods
Subjects
This trial was conducted at the Substance Treatment and Research Service (STARS), a research clinic at Columbia University Irving Medical Center and the New York State Psychiatric Institute in New York, NY. Participant recruitment began in January 2017 and concluded in September 2018, with data collection completed in November 2018. All potential participants received a comprehensive medical and psychiatric evaluation including a physical exam, laboratory testing including pregnancy testing for females, urine testing for toxicology and to confirm pregnancy status, an electrocardiogram, the Mini-International Neuropsychiatric Interview (MINI) for DSM-5, the Hamilton Depression Rating Scale, and other self-report measures. Cannabis use for the 28 days before entry in the study was measured using the timeline follow-back method. Participants were recruited by internal and external clinical referrals, and to the research clinic in general by local advertising across a variety of media outlets and locations (radio, print, television, internet and subway).
Participants ranged from ages 18–60, met DSM-5 criteria for current CUD, were seeking treatment, positive for THC in urine toxicology at consent, had body mass index (BMI) ≥ 18.5 and were judged capable of giving informed consent and complying with study procedures. Participants were excluded if they had a lifetime history of the DSM-5 diagnosis of schizophrenia, schizoaffective disorder, or bipolar disorder; had another DSM-5 disorder that was unstable or felt likely to require intervention or current DSM-5 diagnosis for an eating disorder; were prescribed or taking medications contra-indicated with lorcaserin, including ergotamine (Cafergot, Ergomar) or dihydroergotamine (Migranal), 5HT2B receptor agonists like cabergoline, or medications metabolized by CYP2D6 (thioridazine, tamoxifen, metoprolol, aripiprazole, codeine, etc.); had a known history of allergy, intolerance or hypersensitivity to lorcaserin; were pregnant or lactating; had unstable medical conditions; had evidence of liver or renal dysfunction by laboratory assessment; had a current DSM-5 diagnosis of substance dependence other than cannabis or nicotine dependence; were legally mandated to participate in a substance use disorder treatment program; or were assessed to be a risk for suicide or homicide. The study protocol was reviewed and approved by the Institutional Review Board of the New York State Psychiatric Institute, and all participants provided written informed consent.
Treatment
All participants were assigned to treatment with oral lorcaserin (target dose of 20mg XR daily) under open-label conditions accompanied by weekly medication management sessions with a study physician over 8 weeks of the 10-week study (weeks 2–9). After 1 week of baseline assessments, the first dose of medication (10mg × 1) was given on study day 8 of week 2, followed by 20mg by mouth daily beginning on day 9 and to be continued throughout the study until 1 day decrease to 10mg (week 9, study day 63) and then discontinuation. Baseline assessments without medication exposure were collected during week 1. Participants were followed for an additional week (study week 10), following discontinuation of the medication. Dosing was flexible based on individual tolerability of the medication.
During the study, all participants also received a standardized medication management treatment, where they met weekly with a study psychiatrist to perform study assessments and monitor for both adverse events and medication effects, while meeting twice weekly with research staff to perform further assessments. Grounds for early discontinuation of participation included worsening of psychiatric symptoms or substance use to an extent that placed the participant at risk for self-destructive behavior or other harm, pregnancy, other adverse events deemed to place the subject at increased risk with continued treatment, including a decrease in BMI below 18.5. Participants could decline to take the medication and continue to participate with study procedures for the duration of the protocol.
At each visit participants received $5 for travel expenses as well as cash incentive payments starting at $2.50 and increasing by $2.50 for every consecutive made study visit during the protocol, up to $25 per visit. The maximum compensation was $417.50 for completing all procedures and visits.
Measures
Baseline demographic data was collected on all participants. The primary outcome was tolerability as measured by adverse events during the study which were assessed weekly with a version of the SAFTEE by a study physician, recording both the number and type of adverse events and any serious adverse events if they occurred. Exploratory measures for the effect on cannabis use was assessed by self-report confirmed by urine toxicology. Using the timeline follow-back method, self-report of cannabis use was obtained for each day of the study period. Urine was collected twice weekly and measured for both THC and creatinine concentrations to obtain creatinine-corrected quantitative tetrahydrocannabinol-9-carboxylic acid levels (CN-THCCOOH), by dividing the concentration urine THC in ng/mL by the concentration of urine creatinine in mg/dL and multiplying by 100 to yield a value in ng THC/mg creatinine.
Statistical Analysis
Summary statistics of baseline demographics of the sample were computed using frequencies, means, standard deviations, medians, and interquartile range. The means, standard deviations, medians, and interquartile ranges of daily cannabis use in grams and in dollars, cannabis using days, and urine THC concentration were also computed. Adverse effects were summarized counting the number of participants who reported each adverse effect at least once during the study. Dose reductions and discontinuation of medication due to adverse effects were also summarized. To analyze whether cannabis use decreases with each week in the study, longitudinal mixed effects models with a random intercept for participant and an autoregressive correlation structure (AR1) to account for the within subject correlation was used. For the outcomes of cannabis use in grams, dollars, and using days, predictors included the corresponding value at baseline (28 days prior to week 1) and the effect of week (1–10). For the outcome of urine THC concentration, the lognormal distribution was used to model the skewed distribution. The model for urine THC concentration did not adjust for baseline because no urines were collected before week 1. If the effect of week was significant, a contrast was performed to estimate the change from week 1 to 10. All analyses were conducted in SAS® and all statistical tests were 2-tailed at a significance level of 5%.
Results
Participants
Demographic and baseline clinical characteristics of enrolled participants (N=17) are shown in Table 1. The sample was predominantly male (70.6%), in their mid-thirties (mean age = 35.1), unmarried (58.8%), and of diverse religious and racial backgrounds, with the majority of participants (58.8%) identifying as either Black, Asian, Hawaiian/Pacific Islander or Other. The majority of the sample (52.9%) were currently not employed.
Table 1.
Demographic and baseline clinical characteristics of the participants
| Characteristics | n | Mean (SD) or % |
|---|---|---|
| Age (years) | 17 | 35.1 (10.4) |
| Gender | ||
| Male | 12 | 70.6% |
| Female | 5 | 29.4% |
| Race | ||
| Hispanic | 1 | 5.9% |
| Black | 5 | 29.4% |
| Caucasian | 7 | 41.2% |
| Asian | 2 | 11.8% |
| Hawaiian / Pacific Islander | 1 | 5.9% |
| Other | 1 | 5.9% |
| Ethnicity | ||
| Not Hispanic | 15 | 88.2% |
| Hispanic | 2 | 11.8% |
| Education (years) | 16 | 14.6 (3.2) |
| Religion | ||
| Protestant | 2 | 12.5% |
| Catholic | 3 | 18.8% |
| Jewish | 2 | 12.5% |
| Islamic | 1 | 6.3% |
| Other | 6 | 37.5% |
| None | 2 | 12.5% |
| Marital Status | ||
| Single | 10 | 58.8% |
| Married | 5 | 29.4% |
| Divorced | 1 | 5.9% |
| Widowed | 1 | 5.9% |
| Current Employment | ||
| Full-Time | 6 | 35.3% |
| Part-Time | 2 | 11.8% |
| Homemaker | 2 | 11.8% |
| Temporarily unemployed | 3 | 17.6% |
| Unemployed | 3 | 17.6% |
| Other | 1 | 5.9% |
| Income Group | ||
| Under $30K | 2 | 16.7% |
| $30K-$100K | 4 | 33.3% |
| Over $100K | 6 | 50.0% |
Note: Missing data in education (N=16), religion (N=16) and income (N=12).
Adverse Events
Among the 17 participants enrolled, 3 participants dropped prior to starting medication. Of the 14 participants who received lorcaserin, 8 participants completed the full medication administration schedule (through week 9) and completed all 10 weeks of the study. One additional participant received all 8 weeks of medication (9 participants received all 8 weeks of medication) but did not attend sessions in weeks 9 and 10. Mean study retention was 7.3 weeks (SD=3.3 weeks). Forty-seven percent (8/17) of participants reported any adverse effects during the trial (see Table 2). The most common adverse effect was headache or migraine (23.5%) which was experienced in 4 patients, followed by anorexia (17.6%) and then irritability (11.8%). The three participants who discontinued study medication due to adverse effects were all female and all endorsed headache as the primary reason for discontinuation. One additional female participant who experienced the adverse effect of headache was receptive to and underwent a dose reduction (decreased to 10mg daily). The headache resolved on the reduced dose and ultimately, this participant returned to the full dose (20mg/day) over time and continued in the trial through end of study.
Table 2:
Adverse events by number of subjects reporting the symptom at least once (N=8)
| Adverse Event | Frequency | Percent |
|---|---|---|
| Anorexia | 3 | 37.50 |
| Headache | 3 | 37.50 |
| Irritability | 2 | 25.00 |
| Chills | 1 | 12.50 |
| Chlamydia | 1 | 12.50 |
| Congestion (nasal) | 1 | 12.50 |
| Decreased appetite | 1 | 12.50 |
| Diarrhea | 1 | 12.50 |
| Ear infection | 1 | 12.50 |
| Fever | 1 | 12.50 |
| Gonorrhea | 1 | 12.50 |
| Insomnia | 1 | 12.50 |
| Itchiness | 1 | 12.50 |
| Left hip pain | 1 | 12.50 |
| Lightheaded | 1 | 12.50 |
| Migraine | 1 | 12.50 |
| Mild tingling/euphoria | 1 | 12.50 |
| Nausea | 1 | 12.50 |
| Toothache | 1 | 12.50 |
| Vomiting/nausea | 1 | 12.50 |
| Yeast infection | 1 | 12.50 |
Cannabis Use
The observed means, standard deviations, medians and interquartile ranges of cannabis use across measures by study week are listed in Table 3 for participants completing each week. The days of cannabis use per week decreased significantly (F1,92 = 13.80, beta estimate = −0.195, p < .001) with each week in the trial, while adjusting for the number of using days in the 4 weeks prior to beginning the study (F1,92 = 9.02, p < .01). A contrast was performed with the mean level of baseline cannabis use set to 21.8 days (during past 4 weeks prior to study entry) to compare the change in cannabis using days from week 1 to week 10. At week 1, the estimated average cannabis using days per week for participants was 4.8 days (SE = 0.51) compared to 3 days (SD = 0.57) at week 10. By the end of the 10-week trial participants significantly decreased on average by approximately 1.76 (SD = 0.47) cannabis using days per week.
Table 3.
Summary statistics of cannabis use by study week
| Daily Cannabis Use (grams) | Daily Cannabis Use (dollars) | Cannabis using days | Urine THC/Creatinine ratio (ng THC/mg creatinine) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week | N | Mean | SD | Median | IQR | Mean | SD | Median | IQR | Mean | SD | Median | IQR | Mean | SD | Median | IQR |
| 1 | 14 | 2.54 | 2.27 | 2.21 | 0.85–3.00 | 11.14 | 9.62 | 7.67 | 5.89–15.00 | 5.00 | 2.63 | 6.00 | 3.00–7.00 | 208.72 | 315.32 | 119.50 | 89.70–195.61 |
| 2 | 14 | 1.85 | 1.51 | 1.33 | 1.00–2.43 | 12.72 | 16.91 | 7.00 | 6.43–12.00 | 4.93 | 2.40 | 6.00 | 3.00–7.00 | 148.74 | 119.09 | 120.64 | 62.09–190.61 |
| 3 | 13 | 2.08 | 1.59 | 1.45 | 1.00–3.00 | 11.17 | 7.58 | 10.28 | 5.36–15.00 | 3.62 | 2.26 | 4.00 | 1.00–5.00 | 192.06 | 221.35 | 134.43 | 69.15–203.25 |
| 4 | 11 | 1.99 | 1.70 | 1.80 | 1.00–2.00 | 11.20 | 10.13 | 7.50 | 5.00–14.00 | 3.64 | 2.87 | 3.00 | 1.00–7.00 | 155.73 | 140.74 | 103.49 | 53.37–204.35 |
| 5 | 11 | 1.81 | 2.05 | 1.46 | 0.28–2.00 | 9.71 | 7.36 | 6.50 | 5.00–16.00 | 3.82 | 2.60 | 4.00 | 1.00–7.00 | 235.09 | 145.87 | 214.14 | 155.91–257.46 |
| 6 | 10 | 2.34 | 1.87 | 1.75 | 1.00–3.43 | 12.31 | 8.42 | 8.14 | 7.08–16.50 | 3.50 | 2.80 | 2.50 | 2.00–7.00 | 181.92 | 124.09 | 152.76 | 101.82–223.21 |
| 7 | 9 | 2.73 | 2.21 | 2.45 | 1.00–3.29 | 14.52 | 9.43 | 9.46 | 8.00–25.71 | 4.11 | 3.26 | 5.00 | 0.00–7.00 | 214.83 | 201.69 | 165.13 | 83.84–345.82 |
| 8 | 9 | 2.57 | 1.44 | 2.80 | 1.00–4.00 | 16.93 | 14.06 | 10.00 | 7.29–33.60 | 3.67 | 2.55 | 4.00 | 3.00–5.00 | 500.80 | 673.38 | 172.34 | 156.09–845.50 |
| 9 | 8 | 2.21 | 1.54 | 1.75 | 1.00–3.86 | 11.77 | 7.18 | 8.00 | 7.00–17.14 | 4.38 | 2.56 | 4.00 | 3.00–7.00 | 132.54 | 58.41 | 148.08 | 88.05–148.45 |
| 10 | 8 | 2.00 | 1.19 | 2.00 | 1.00–3.17 | 11.77 | 5.84 | 9.00 | 7.57–18.00 | 3.63 | 2.50 | 3.50 | 2.00–5.50 | 245.76 | 345.98 | 119.60 | 75.97–206.22 |
SD = standard deviation, IQR = interquartile range.
The average daily cannabis use in grams did not significantly change with each week in the trial (F1,77 = 2.05, NS), while adjusting for baseline use (F1,77 = 12.2, p < .001). Similarly, the average daily cannabis use in dollars did not significantly change with each week in the trial (F1,72 = 0.07, NS), while adjusting for baseline use (F1,60 = 50.1, p < .001). Urine THC concentration did not change significantly with each week in the study (F1,63 = 1.11, NS).
Discussion
This 10-week, outpatient, open-label, uncontrolled, fixed-flexible dosing pilot study tested lorcaserin up to 20 mg daily for 8-weeks in the treatment of CUD. Our pilot study demonstrates that most participants exposed to lorcaserin found it tolerable, unless they experienced headache. There were no severe adverse events and no early terminations by study physician. The side effect of headache, however, was an obstacle to continuing in the study. Of the 4 participants who experienced headache, all were females. One of these female participants was agreeable to a dose reduction (10mg) which led to resolution in headache and eventually she returned to the full daily dose (20mg) and completed the study. The other 3 female participants declined to trial a lower dose of the medication, selecting discontinuation of study medication. Our finding of headache being a common side effect is supported by the previous inpatient human laboratory study of lorcaserin in daily cannabis users, where 4 of the 15 participants also endorsed headache.(Arout et al., 2021) Unfortunately, the study does not comment on sex differences as it relates to the occurrence of adverse effects, though 4 of the 15 participants enrolled in the previous inpatient study were females.(Arout et al., 2021) The authors of the human laboratory study concluded that lorcaserin was well tolerated as patients did not discontinue study medication or drop-out. There are key differences between the two studies that likely contributed to differences in tolerability as it relates to drop-out and medication discontinuation including duration of medication administration (13 days vs. 56 days), setting (inpatient vs. outpatient), subject population (non-treatment seeking vs treatment seeking), and amount of cash compensation for study participation. Both studies had similar rates of enrollment for male and female participants, with approximately 20–25% of the sample being female. Unfortunately, sex equity enrollment is challenging to achieve in CUD clinical research trials and similar rates are observed in other trials.(Brezing and Levin, 2018)
The adverse events seen in this open label pilot suggest a possible role of sex differences contributing to the tolerance or intolerance of this medication and risk for headache. Our findings also suggest that, particularly for female patients, our study had too rapid of a dose escalation with only one trial dose of 10mg before increasing to the target dose of 20mg per day which may have contributed to the experience of adverse events. A notable methodological consideration in future studies of 5HT2c agonists should include a slower medication dose escalation schedule which may allow for greater tolerability and avoid the experience of adverse events, discontinuation of medication, and drop-out we saw in our small pilot.
Our preliminary, uncontrolled findings of the study medication’s effects on cannabis use outcomes showed a reduction in the average number of days per week of cannabis use, but no reduction in the mean creatinine-corrected urine THC concentration, and no reduction in the average reported grams or cash value of cannabis used. This differential observation in improvements across measures of cannabis use outcomes is consistent with other treatment trials for CUD.(Lintzeris et al., 2019) With regards to urine THC concentrations, open label pilot studies, such as this one, provide less of an ability to detect statistical differences. Additionally, given the time it takes for chronic, frequent cannabis users to clear THC from their urine, it may have been difficult over an 8-week study period to detect a reduction in this measure(Lowe et al., 2009). This is consistent with previous studies of medication in the treatment of CUD (Notzon et al., 2018).
There are several factors likely contributing to differential outcomes in self-report on the Timeline Followback for cannabis, including the expansive nature of available THC-containing cannabis products (edibles, vaping, traditional plant matter), their differential costs, and the irrelevance of weight as a measure of quantity of use for some (e.g.: all products except plant matter). It is important to identify a universal, primary outcome measure of self-report of cannabis use given the increasing relevance non-abstinent reduction in use as an endpoint for treatment as compared to abstinence.(Levin et al., 2021) Concordance in the field on this measure is important to have consistency across treatment trials. Frequency of use appears to be the most clinically relevant measure for overall cannabis “load” and when accounted for over time, likely has progressive benefits even in seemingly modest reductions in days of use per week.(Lintzeris et al., 2019)
Despite the promising clinical findings in this small pilot, on February 13, 2020, the FDA issued a Drug Safety Communication that requested the manufacturer of lorcaserin voluntarily withdraw the medication, which it did, due to long term safety trial data that demonstrated an increased occurrence of malignancy in patients treated for weight loss, with one additional malignancy observed per 470 patients over one year. (Belviq Recall | FDA Requests Withdrawal Over Cancer Risk, no date; Sharretts et al., 2020) There was no difference in the incidence of cancer over the initial months of treatment with the finding associated with longer duration of treatment. The FDA did not recommend any special screening for patients who have taken lorcaserin.
Medications with initial approval for weight loss, that also have the potential in the treatment of substance use disorders, have demonstrated challenges due to unacceptable safety profiles. Fenfluramine and its derivatives functioned as agonists at peripheral serotonin 2B receptors which resulted in pulmonary hypertension and valvular heart disease.(Bohula et al., 2018) Rimonabant, an endocannabinoid receptor antagonist, demonstrated unacceptably high rates of serious neuropsychiatric side effects, including suicidal ideation.(Christensen et al., 2007) Lorcaserin has followed a similar pathway with its safety data precluding any further investigation. While lorcaserin itself cannot be studied, our pilot findings do suggest that future 5HT2c receptor agonists with a more favorable safety profile warrant investigation for their role in the treatment of CUD. However, the small sample size, as well as the open-label and uncontrolled design of the current study, limit the conclusions. The high frequency of clinic visits and support provided by staff during the trial may also account for the changes in cannabis use seen without a placebo control arm.
Conclusions
Lorcaserin is no longer an available treatment option for CUD. Future 5HT2c-receptor agonists with better safety profiles should be evaluated in the treatment of CUD with implementation of slower dose titration schedules and attention to adverse effects, such as headache, in addition to the role sex differences may play in making some individuals vulnerable to medication intolerability. While there are limitations to small, open-label, uncontrolled pilot studies, investigating the feasibility of a new medication approach using this methodology is an efficient way to assess dosing and tolerability in a real-world clinic setting. Frequency of use in the current complex cannabis landscape is likely a more reliable and clinically relevant measure than weight or dollar amounts.
Figure 1:
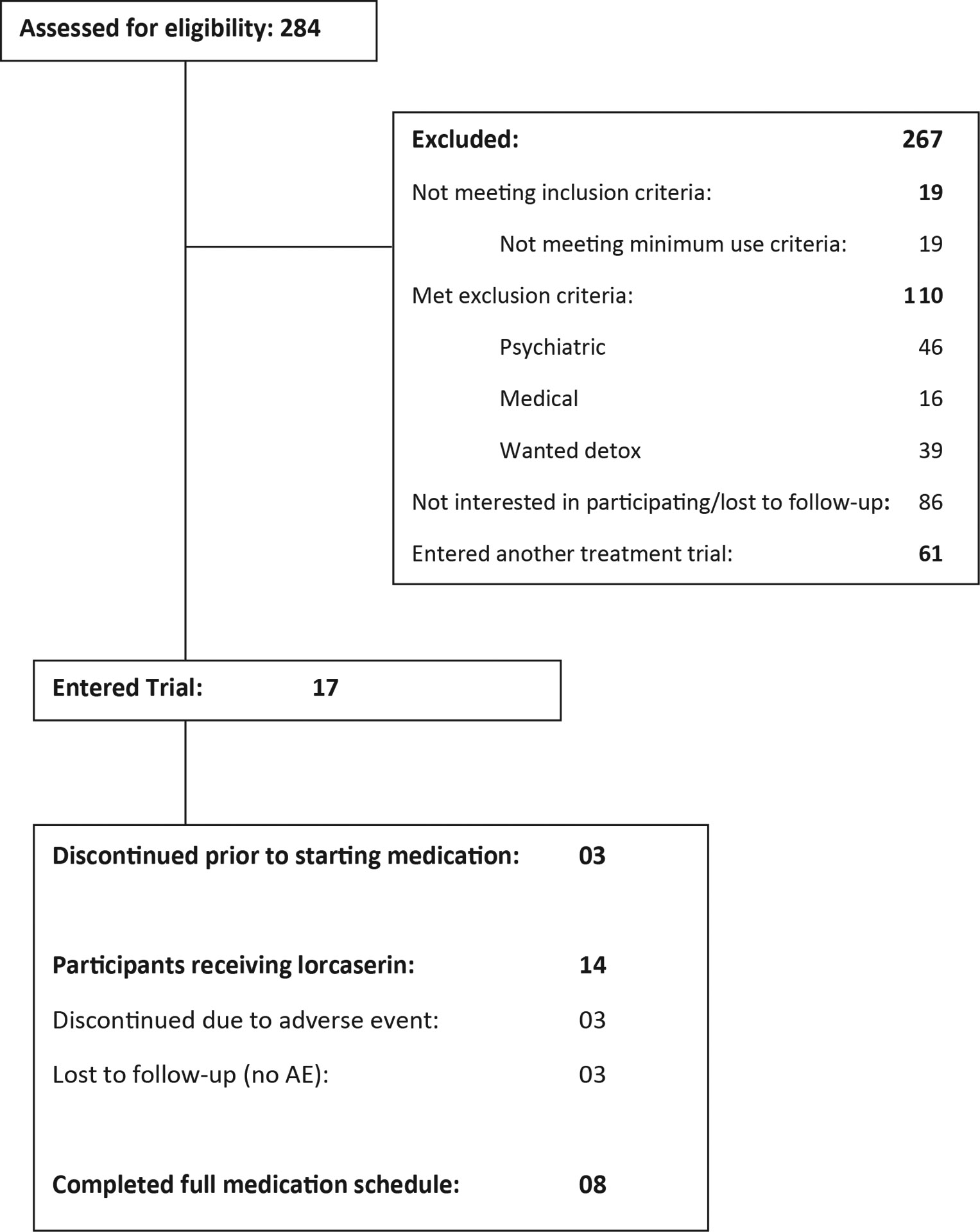
CONSORT Flow Diagram for study
Acknowledgments
Dr. Brezing was supported by NIDA K23DA045080, and the research was supported by a pilot grant from the Dartmouth P30 Center for Technology and Behavioral Health (P30 DA029926) and medication for this study was supported by Dr. Levin’s NIDA funded K24 DA029647-08. Drs. Mariani, Levin, Naqvi, Mitra, and Pavlicova reported no biomedical financial interests or potential conflicts of interest. Also, Ms. Mahony, Ms. Choi, Mr. Brooks, and Mr. Sibai reported no biomedical financial interests or potential conflicts of interest. The authors alone are responsible for the content and writing of this paper. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 2019 NSDUH Annual National Report | CBHSQ Data (no date). Available at: https://www.samhsa.gov/data/report/2019-nsduh-annual-national-report (Accessed: 15 June 2021).
- Arout CA et al. (2021) ‘5HT-2C agonist lorcaserin decreases cannabis self-administration in daily cannabis smokers’, Addiction Biology, 26(4), p. e12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recall Belviq | FDA Requests Withdrawal Over Cancer Risk (no date) Drugwatch.com. Available at: https://www.drugwatch.com/belviq/recall/ (Accessed: 11 January 2022).
- Bohula EA et al. (2018) ‘Cardiovascular safety of lorcaserin in overweight or obese patients’, New England Journal of Medicine, 379(12), pp. 1107–1117. [DOI] [PubMed] [Google Scholar]
- Boothman L et al. (2006) ‘In vivo evidence that 5-HT2C receptors inhibit 5-HT neuronal activity via a GABAergic mechanism’, British Journal of Pharmacology, 149(7), pp. 861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezing CA and Levin FR (2018) ‘The Current State of Pharmacological Treatments for Cannabis Use Disorder and Withdrawal’, Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 43(1), pp. 173–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubar MJ and Cunningham KA (2007) ‘Distribution of serotonin 5-HT2C receptors in the ventral tegmental area’, Neuroscience, 146(1), pp. 286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM et al. (2012) ‘Combining cognitive behavioral therapy and contingency management to enhance their effects in treating cannabis dependence: less can be more, more or less’, Addiction, 107(9), pp. 1650–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen R et al. (2007) ‘Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials’, Lancet (London, England), 370(9600), pp. 1706–1713. [DOI] [PubMed] [Google Scholar]
- Di Matteo V et al. (1999) ‘SB 242084, a selective serotonin2C receptor antagonist, increases dopaminergic transmission in the mesolimbic system’, Neuropharmacology, 38(8), pp. 1195–1205. [DOI] [PubMed] [Google Scholar]
- Di Matteo V et al. (2000) ‘Biochemical and electrophysiological evidence that RO 60–0175 inhibits mesolimbic dopaminergic function through serotonin(2C) receptors’, Brain Research, 865(1), pp. 85–90. [DOI] [PubMed] [Google Scholar]
- Gobert A et al. (2000) ‘Serotonin(2C) receptors tonically suppress the activity of mesocortical dopaminergic and adrenergic, but not serotonergic, pathways: a combined dialysis and electrophysiological analysis in the rat’, Synapse (New York, N.Y.), 36(3), pp. 205–221. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Fletcher PJ and Higgins GA (2000) ‘Studies to investigate the role of 5-HT(2C) receptors on cocaine- and food-maintained behavior’, The Journal of Pharmacology and Experimental Therapeutics, 295(3), pp. 1183–1191. [PubMed] [Google Scholar]
- Hasin D and Walsh C (2021) ‘Trends over time in adult cannabis use: A review of recent findings’, Current Opinion in Psychology, 38, pp. 80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS et al. (2017) ‘US Adult Illicit Cannabis Use, Cannabis Use Disorder, and Medical Marijuana Laws: 1991–1992 to 2012–2013’, JAMA psychiatry, 74(6), pp. 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA et al. (2013) ‘Evaluation of chemically diverse 5-HT₂c receptor agonists on behaviours motivated by food and nicotine and on side effect profiles’, Psychopharmacology, 226(3), pp. 475–490. [DOI] [PubMed] [Google Scholar]
- Higgins GA and Fletcher PJ (2015) ‘Therapeutic Potential of 5-HT2C Receptor Agonists for Addictive Disorders’, ACS chemical neuroscience, 6(7), pp. 1071–1088. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Fletcher PJ and Shanahan WR (2020) ‘Lorcaserin: A review of its preclinical and clinical pharmacology and therapeutic potential’, Pharmacology & Therapeutics, 205, p. 107417. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Sellers EM and Fletcher PJ (2013) ‘From obesity to substance abuse: therapeutic opportunities for 5-HT2C receptor agonists’, Trends in Pharmacological Sciences, 34(10), pp. 560–570. [DOI] [PubMed] [Google Scholar]
- Hughes JR et al. (2016) ‘Attempts to stop or reduce daily cannabis use: An intensive natural history study’, Psychology of Addictive Behaviors: Journal of the Society of Psychologists in Addictive Behaviors, 30(3), pp. 389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin FR et al. (2021) ‘Lorcaserin treatment for extended-release naltrexone induction and retention for opioid use disorder individuals: A pilot, placebo-controlled randomized trial’, Drug and Alcohol Dependence, 219, p. 108482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintzeris N et al. (2019) ‘Nabiximols for the Treatment of Cannabis Dependence’, JAMA Internal Medicine, 179(9), pp. 1242–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S et al. (2007) ‘Serotonin2C receptor localization in GABA neurons of the rat medial prefrontal cortex: implications for understanding the neurobiology of addiction’, Neuroscience, 146(4), pp. 1677–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe RH et al. (2009) ‘Extended urinary Delta9-tetrahydrocannabinol excretion in chronic cannabis users precludes use as a biomarker of new drug exposure’, Drug and Alcohol Dependence, 105(1–2), pp. 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notzon DP et al. (2018) ‘Open-label pilot study of injectable naltrexone for cannabis dependence’, The American Journal of Drug and Alcohol Abuse, 44(6), pp. 619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi L et al. (2002) ‘Stimulation of 5-hydroxytryptamine (5-HT(2C)) receptors in the ventrotegmental area inhibits stress-induced but not basal dopamine release in the rat prefrontal cortex’, Journal of Neurochemistry, 82(1), pp. 93–100. [DOI] [PubMed] [Google Scholar]
- Sharretts J et al. (2020) ‘Cancer Risk Associated with Lorcaserin - The FDA’s Review of the CAMELLIA-TIMI 61 Trial’, The New England Journal of Medicine, 383(11), pp. 1000–1002. [DOI] [PubMed] [Google Scholar]
- Somerville EM et al. (2007) ‘5-HT2C receptor activation inhibits appetitive and consummatory components of feeding and increases brain c-fos immunoreactivity in mice’, European Journal of Neuroscience, 25(10), pp. 3115–3124. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Roffman RA and Curtin L (2000) ‘Comparison of extended versus brief treatments for marijuana use’, Journal of Consulting and Clinical Psychology, 68(5), pp. 898–908. [PubMed] [Google Scholar]
- V DM et al. (2002) ‘Role of serotonin(2C) receptors in the control of brain dopaminergic function.’, Pharmacology, Biochemistry, and Behavior, 71(4), pp. 727–734. [DOI] [PubMed] [Google Scholar]


