Abstract
Coxsackievirus B3 infection causes significant cardiac inflammation in male, but not female, B1.Tg.Eα mice. This gender difference in disease susceptibility correlates with selective induction of CD4+ Th1 (gamma interferon-positive) cell responses in animals with testosterone, whereas estradiol promotes preferential CD4+ Th2 (interleukin-4 positive [IL-4+]) cell responses. Differences in immune deviation of CD4+ T cells cannot be explained by variation in B7-1 or B7-2 expression. Infection significantly upregulated both molecules, but no differences were detected between estradiol- and testosterone-treated groups. Significantly increased numbers of activated (CD69+) T cells expressing the γδ T-cell receptor were found in male and testosterone-treated male and female mice. In vivo depletion of γδ+ cells by using monoclonal antibodies inhibited myocarditis and resulted in a shift from a Th1 to Th2 response phenotype. Taken together, our results indicate that testosterone promotes a CD4+ Th1 cell response and myocarditis by promoting increased γδ+ cell activation.
Often, autoimmune diseases have a definite gender bias. Systemic lupus erythematosis, rheumatoid arthritis, multiple sclerosis, thyroiditis, and non-insulin-dependent diabetes mellitus all dominate in women (15, 22, 28, 42). Despite this gender dominance, distinct pathophysiological mechanisms may be involved. Systemic lupus erythematosis which is characterized by autoantibodies and an association with Th2 cell responses, usually affects younger women. In contrast, rheumatoid arthritis, a disease more common after menopause and associated with cell-mediated injury of joints, correlates with a deficiency in Th2-like cytokines (42). These clinical observations are consistent with reports that estrogens enhance humoral responses and suppress cellular immunity (3, 9, 33, 42). However, the effects of estrogens on immunity are far from clear-cut, as estrogens have also been reported to suppress autoantibody response (39), and testosterone therapy enhances Th2 cell responses in experimental allergic encephalomyelitis (7). Why different investigators report diametrically opposed hormonal effects in autoimmune disease models is not clear. Possibly, how hormones affect immunity depends on which organ systems are involved (35), on the nature of the antigen-specific lymphocytes, on the hormone dose used, or on counterbalancing interactions between different hormones in vivo.
Estrogens have multiple effects within the immune response. These hormones suppress class II major histocompatibility complex (MHC) antigen expression in transplanted allogeneic coronary arteries (29), which probably explains the suppressive effects of this hormone on antigen presentation (43). Both estrogens and testosterone induce cytokine expression. Estrogens promote gamma interferon (IFN-γ), interleukin-1β (IL-1β), IL-10, and IL-5 gene expression (6, 11, 13, 35, 40). Testosterone, while having no effect on IL-1β expression, is even more effective than estrogen in IL-5 induction. Often, the effect of the hormone on cytokine levels is dose dependent and might be biphasic (augmenting cytokine production at certain doses while suppressing production at other doses) (6, 13). Finally, hormones modulate expression of apoptotic factors and lymphoid cell death (10, 12, 38).
Clinically, myocarditis is a male-dominant disease, with a 2:1 ratio over females (44). Of females afflicted by this disease, most are peripartum. Experimental studies using coxsackievirus B3 (CVB3) infection of mice show gender bias similar to that observed clinically (17, 30–32). Recently, we have shown that susceptibility to CVB3-induced experimental myocarditis is dependent on preferential induction of CD4+ Th1 cell responses (19, 21). Furthermore, immune bias toward the Th1 phenotype requires activated T cells expressing the γδ T-cell receptor (TcR) (19). CVB3 infection of female mice results in preferential Th2 cell responses, but in vivo treatment of females with androgens can shift the dominant CD4+ T-cell response toward the Th1 phenotype and promote significant myocarditis (20). The present investigation extends these initial studies by showing that testosterone enhances γδ+ T-cell activation in vivo and that these effectors are responsible for the gender bias in this disease model.
MATERIALS AND METHODS
Mice.
B1.Tg.Eα mice are genetically modified animals in which MHC class II IE expression is restored by introduction of the Eαk gene into C57BL/6 mice (25, 37). These animals were obtained from Chella David (Department of Immunology, Mayo Clinic, Rochester, Minn.). Previous studies in this laboratory have shown that B1.Tg.Eα male mice are highly susceptible to CVB3-induced myocarditis, that disease depends on a Th1 cell response, and that γδ+ T cells regulate the CD4+ Th cell phenotype (21a). Males, 5 to 7 weeks of age, were infected by intraperitoneal (i.p.) injection of 104 PFU of CVB3 in 0.5 ml of phosphate-buffered saline (PBS) (23). Mice were euthanized by i.p. injection of 120 mg of sodium pentobarbital per kg of body weight in 0.5 ml of PBS.
Virus titer.
Hearts were homogenized in medium. Cellular debris was removed by centrifugation at 1,045 × g for 10 min. The supernatant was titered by the plaque-forming assay on HeLa cell monolayers as described previously (23).
Histology.
Hearts were removed, fixed in 10% buffered formalin, paraffin embedded, sectioned, and stained with hematoxylin and eosin. Stained sections were used for image analysis in transmitted light mode with an Olympus BX50 compound light microscope (4× objective lens; numerical aperture, 0.13). True color digital images (640 by 480 pixels) were captured with a Sony DXC-960MD/LLP video camera connected via an RS170 cable to a video frame grabber on a Sun SPARCstation 5. Image processing and analysis were accomplished with IMIX software (Princeton Gamma Tech, Inc., Princeton, N.J.). Final percent cardiac inflammation was calculated by dividing the area of injury by the total area of the heart cross section.
Hormones.
17β-Estradiol and 4-androsten-17β-ol-one (testosterone) were purchased in powder form from Sigma. The hormones were initially dissolved to 10 mg/ml in ethanol and subsequently diluted to 20 μg/ml in PBS. Control animals received PBS with 0.5% ethanol without hormone. Animals were injected i.p. with 0.5 ml. For tissue culture, water-soluble estradiol and testosterone were purchased from Sigma and diluted directly in medium to the desired concentration.
Serum hormone concentrations.
Mice were bled by cardiac puncture. Blood was allowed to clot and then centrifuged at 1,045 × g for 10 min, and serum was retrieved. Levels of testosterone and estradiol were determined by radioimmunoassay through the Fletcher-Allen Health Center clinical laboratories.
Antibodies.
Fluorochrome-conjugated antibodies and Fc-Block (anti-FcγIII/II receptor; clone 2.4G2) were purchased from Pharmingen (San Diego, Calif.). These were fluorescein isothiocyanate (FITC)–anti-CD4 (clone GK1.5), phycoerythrin (PE)–anti-CD69 (clone H1.2F3), FITC–anti-γδ TcR (clone GL3), PE–anti-mouse IL-4 (clone 11B11), and PE–anti-mouse IFN-γ (clone XMG1.2). Immunoglobulin isotype controls were PE-rat immunoglobulin G1 (IgG1) (clone R3-34), FITC- and PE-rat IgG2a (clone R35-95), and FITC- and PE-hamster IgG (clone A19-3). For antibody depletion studies, hybridoma clone GL3-3A making monoclonal anti-γδ TcR antibody was grown as ascites as described in detail previously (41). Animals were injected i.p. with 100 μg of Sepharose G-100-purified antibody in 0.5 ml of PBS on days −2 and −1 relative to infection.
Flow cytometry.
Mice were euthanized by i.p. injection of sodium pentobarbital (120 mg/kg in PBS). The spleens were removed, pressed through fine mesh screens to produce single-cell suspensions, and washed in RPMI 1640 medium containing penicillin (100 U/ml), streptomycin (100 μg/ml), and 5% fetal bovine serum. The cells were centrifuged at 377 × g for 10 min, the pellet was resuspended in erythrocyte lysing solution (Sigma Chemical Co., St. Louis, Mo.), and the remaining lymphoid cells were washed once with medium and counted by trypan blue exclusion. Hearts were retrieved, minced finely with scissors, and subjected to three sequential digestions with 10 ml of 0.4% collagenase II (Worthington Biochemical Co., Freehold, N.J.) for 12 min at 37°C. Lymphoid cells were isolated by centrifugation of the dissociated cell population on Histopaque (Sigma) at 1,048 at g for 10 min. Approximately 105 lymphocytes were incubated with a 1:100 dilution of Fc-Block and a 1:100 dilution of fluorochrome-conjugated antibody for 20 min at 4°C. Control cells were incubated with fluorochrome-labeled isotype immunoglobulin. The cells were washed in PBS containing 1% bovine serum albumin and 0.01% sodium azide (buffer) and then resuspended in 2% paraformaldehyde. For intracellular cytokine staining, a modification of the method of Picker et al. (34) was used. Briefly, 106 splenocyte were cultured in medium containing brefeldin A (10 μg/ml), phorbol myristate acetate (50 ng/ml), and ionomycin (500 ng/ml) (all from Sigma) for 4 h at 37°C in 5% CO2. The cells were washed, incubated with a 1:100 dilution of Fc-Block and a 1:100 dilution of FITC–anti-CD4 antibody for 20 min at 4°C, and then washed and incubated in 2% paraformaldehyde for 10 min. The membranes were permeabilized by incubation in buffer containing 0.5% saponin. The cells were incubated with 1:100 dilutions of anticytokine or isotype immunoglobulins for 20 min at 4°C, washed in buffer containing saponin and then in buffer without saponin, and resuspended in 2% paraformaldehyde. Staining cells were analyzed by using a Coulter Epics Elite instrument with a single excitation wavelength (488 nm) and band filters for PE (575 nm) or FITC (525 nm). Each sample population was classified for cell size (forward scatter) and complexity (side scatter) and gated on a population of interest; then 10,000 cells were evaluated. Criteria for positive staining were established based on the intensity of the isotype controls. The results were expressed as the percentage of cells within a size/complexity gate that stained positively for each marker after subtraction of the stained cells in the isotype control cultures.
Statistics.
Data were evaluated by Student’s t test.
RESULTS
Modulation of myocarditis severity with exogenous sex-associated hormones.
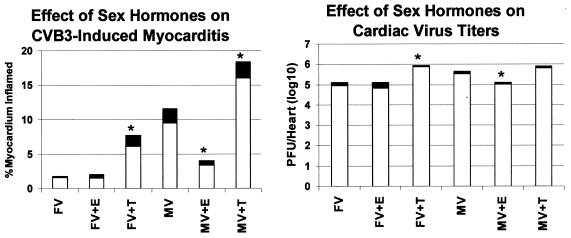
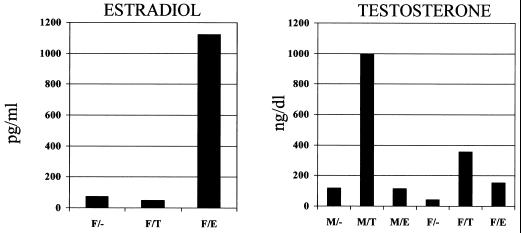
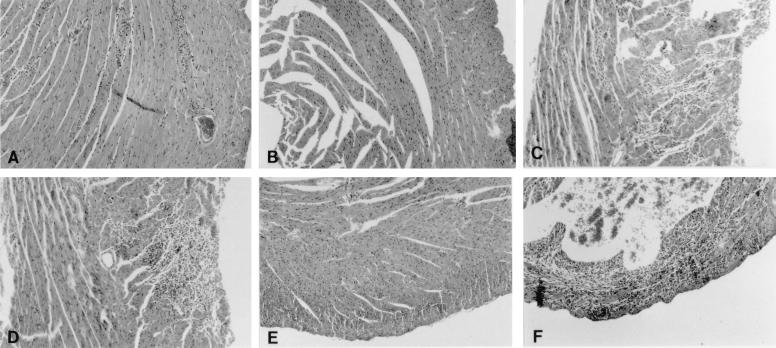
Male and female B1.Tg.Eα mice were injected ip with 10 μg of estradiol and testosterone, or with buffer alone, and then injected ip with 104 PFU of CVB3 3 days later. Animals were killed 7 days after infection, and hearts were evaluated for myocarditis and virus titer (Fig. 1). We also determined hormone levels in the plasma in uninfected male and female mice injected with either PBS control or hormones 2 days earlier (Fig. 2). Serum testosterone levels increased in both male and female mice given testosterone, although the effect was most pronounced in males. Minimal cardiac inflammation was detected in females or females given estradiol. Testosterone treatment increased the percentage of the myocardium inflamed approximately threefold compared to females not given hormone (6.1 and 1.6%, respectively). Males develop significantly more severe cardiac inflammation than females, but treating males with estradiol prior to infection suppresses myocarditis (9.5 and 3.4% of the myocardium inflamed, respectively). Testosterone treatment of males further enhances disease (9.5% in untreated males and 16% in testosterone-treated males). Cardiac virus titers were highest in males and both male and female mice treated with testosterone. Titers were somewhat lower in females and estradiol-treated animals. Figure 3 shows representative photomicrographs of hormone-treated and untreated male mice after infection.
FIG. 1.
Sex steroid treatment of CVB3-infected mice. Male (M) and female (F) B1.Tg.Eα mice were injected i.p. with 10 μg of hormone [17β-estradiol (E) or 4-androsten-17β-ol-one (T)]. Animals were infected with 104 PFU of CVB3 (V) 2 days later and were killed 7 days after infection. Hearts were removed and divided in half; one portion was titered for virus by the plaque-forming assay on HeLa cells, while the remainder was fixed in 10% formalin, paraffin embedded, sectioned, and stained by hematoxylin and eosin. The percentage of the myocardium undergoing inflammation was determined by image analysis. Results represent mean (clear bar) ± standard deviation (black bar) of four mice per group in one of three experiments. ∗, significantly different from non-hormone-treated group at P ≤ 0.05.
FIG. 2.
Serum hormone levels in testosterone and estradiol-treated mice. Male (M) and female (F) mice were injected i.p. with 10 μg of either testosterone (M/T and F/T) or estradiol (M/E and F/E). Controls received PBS instead of hormone (M/- and F/-). Animals were euthanized with sodium pentobarbital 2 days later and bled. Serum was evaluated by radioimmunoassay for hormone (estradiol or testosterone) levels.
FIG. 3.
Myocarditis in hormone-treated male and female mice. (A) CVB3-infected female; (B) infected female treated with estradiol; (C) infected female treated with testosterone; (D) infected male; (E) infected male treated with estradiol; (F) infected male treated with testosterone. Magnification, ×40.
CD4+ Th cell responses in hormonally treated CVB3-infected mice.
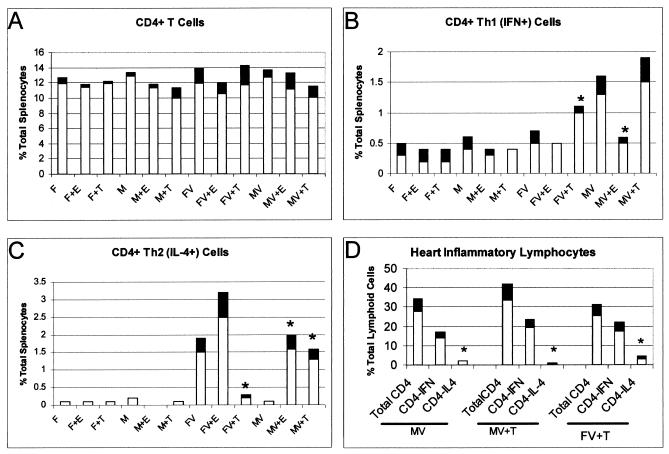
Spleens were removed from both uninfected and infected mice. Cells were evaluated for total CD4+ cells and for CD4+ cells producing either IFN-γ (Th1-like) or IL-4 (Th2-like) cytokines (Fig. 4). Total percentages of CD4+ T cells were similar in spleens of all mice. Infected males not treated with hormone had predominantly IFN-γ-producing CD4+ T cells. Numbers of Th1-like cells were higher in both infected males and females given testosterone than in uninfected animals, infected animals given estradiol, or infected females. The most striking differences were noted in the Th2 cell populations, which were significantly increased in infected females, in infected males and females given estradiol, and, surprisingly in infected males given testosterone.
FIG. 4.
Th1 and Th2 cells in the spleens and hearts of uninfected and CVB3-infected (V) male (M) and female (F) mice treated with either estradiol (E) or testosterone (T). Animals were treated with hormone and infected as described for Fig. 1. Splenic lymphocytes were retrieved and stained for CD4 cell surface marker and for intracellular IFN-γ and IL-4. Lymphocytes from the hearts of male and testosterone-treated animals were also evaluated for cytokine expression. Such evaluations were not done on female and estradiol-treated mice because of very low lymphocyte recovery from hearts with minimal inflammation. Results represent mean (clear bar) ± standard deviation (black bar) of four separate experiments. ∗ , significantly different from non-hormone-treated mice at P ≤ 0.05.
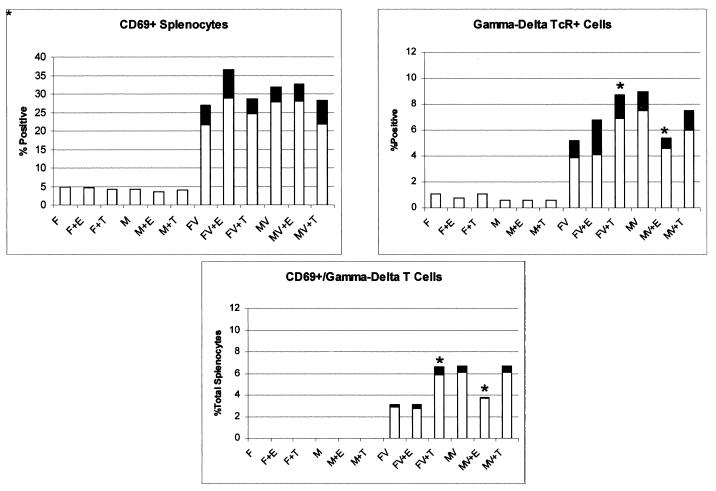
Infection dramatically enhanced B7 expression in the spleen, but no reproducible differences were noted between females or males and between various hormone-treated groups (data not shown). Previously published studies (19) demonstrated that T cells expressing the γδ TcR promoted CVB3-induced myocarditis by favoring Th1 cell responses. To determine whether differences in γδ T-cell responses occur between male and female mice, splenocytes were stained for the early activation marker, CD69, and for γδ TcR (Fig. 5). Both percentages of total CD69+ and γδ+ T cells increased in the spleen after infection, but again, no reproducible differences were detected between males, females, and hormone-treated groups. However, CD69+ γδ+ (doubly labeled) populations were significantly greater in infected males and infected animals given testosterone than in infected females and infected animals given estradiol. Thus, there is a correlation between recently activated γδ+ T cells and myocarditis severity. Treatment of infected males and testosterone-treated infected animals with 100 μg of anti-γδ TcR antibody on each of 2 days prior to infection substantially inhibited myocarditis compared to mice given isotype immunoglobulin (Fig. 6). Flow analysis of γδ+ T cells in the spleen indicated greater than 95% elimination of this population by antibody treatment (7.1% of splenocytes in untreated animals were γδ+, compared to 0.3% in antibody-treated mice).
FIG. 5.
γδ+ and CD69+ (early activation marker) splenocytes in uninfected and infected (V) male (M) and female (F) mice treated with estradiol (E) or testosterone (T). Results represent mean (clear bar) ± standard deviation (black bar) of four separate experiments. ∗, significantly different from non-hormone-treated mice at P ≤ 0.05.
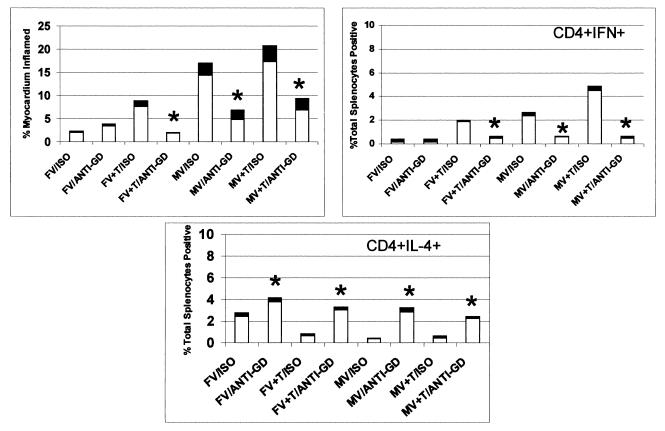
FIG. 6.
Depletion of γδ+ cells protects against CVB3-induced myocarditis and alters Th1/Th2 CD4+ cell balance. Male (M) and female (F) mice were injected i.p. with 100 μg of either hamster IgG (isotype control; ISO) or hamster anti-mouse γδ TcR monoclonal antibody (clone GL3-3A; anti-GD) and then infected with CVB3 (V). Some animals were also treated with 10 μg of testosterone (T) 2 days before infection. All animals were killed 7 days after infection and evaluated for myocarditis and IFN-γ+ or IL-4+ CD4+ T cells. Results represent mean (clear bar) ± standard deviation (black bar) of five mice. ∗, significantly different from non-hormone-treated mice at P ≤ 0.05.
DISCUSSION
This report shows that activation of γδ+ T cells differs between CVB3-infected male and female mice and is influenced by sex-associated hormones. Why γδ+ T-cell responses vary in this manner was not determined. Two possibilities seem most likely. First, since virus titers also were slightly higher in animals with testosterone than in animals with estrogen, the enhanced γδ+ T-cell response might reflect the elevation in virus titers. Previous studies demonstrated that CVB3 infection stimulates 70-kDa heat shock protein expression and that the γδ+ T cells react to these molecules (16, 18). Therefore, increases in virus concentrations should correspond to increased γδ+ T-cell activation. Whether the differences in virus titers between hormonally treated groups is sufficient to explain the marked differences in the levels of γδ+ T-cell response is unclear. A second possibility is that the hormones modulate γδ+ T-cell responses by these steroids’ known ability to control cytokine expression.
The present study used hormone injections into nongonadectomized animals. Evaluation of plasma hormone levels indicate that the injections alter circulating hormone levels. However, it is possible that hormone treatment also alters other chemicals within the body, such as glucocorticoids, which might affect myocarditis induction (8). Additionally, this study used a single concentration of hormone which has been shown by other investigators to alter hormone levels in vivo. Either increasing or decreasing the exogenous hormone concentration might alter the effect on viral pathogenesis.
The enhanced γδ+ T-cell response in the presence of testosterone promotes immune deviation of CD4+ T cells to a Th1 phenotype. All testosterone-treated groups showed excellent percentages of IFN-γ-producing CD4+ T cells in the spleen and usually had few IL-4-producing (Th2) lymphocytes. The exception to this observation was male mice treated with exogenous testosterone, which had both increased Th1 and Th2 cells in the spleen. At high concentrations of testosterone, estrogen-like effects can sometimes occur (2); thus, it is possible that the increase in Th2-like cells results from excess androgens and conversion of some of the hormone to estrogen.
Males and testosterone-treated animals of both sexes also had more IFN-γ+ than IL-4+ cells in the heart 7 days after infection. This finding indicates that the prevalence of Th1-like cells during myocarditis is not restricted to peripheral lymphoid organs. However, evaluation of Th phenotypes in the hearts of myocarditis-resistant mice was not possible due to the limited numbers of T cells present in the myocardium of these animals.
Variations in expression of B7-1 and B7-2 have been used to explain prominent Th1 and Th2 cell responses in autoimmunity models (24, 26, 27). Although CVB3 infection greatly upregulates both of these molecules compared to uninfected animals, no differences between B7-1 and B7-2 were observed among the hormone-treated animals. Additionally, while estrogen has been reported to suppress MHC class II antigen expression (29), we found no reproducible effect on either IA or IE expression in the spleen (data not shown). Thus, it is unlikely that hormones modulate Th cell responses through their effects on antigen presentation.
Depletion of γδ+ T-cell populations in all testosterone-treated mice resulted in both suppression of myocarditis and decreased Th1 cell response. This finding not only confirms the importance of γδ+ T cells in CVB3 pathogenesis but also provides additional evidence that the γδ+ T cells modulate CD4+ T-cell responses. Should the hormone directly alter CD4+ T-cell activity, one might have expected to observe changes in hormonally treated but uninfected control mice. One potential problem with antibody depletion of the γδ+ T cells should be considered. Antibody binding to the TcR can activate T cells. Although flow analysis at the end of the experiment demonstrated that greater than 95% of γδ+ T cells were eliminated from antibody-treated mice, it is possible that these cells were activated before their depletion. Thus, there is the possibility that antibody modulation of Th cell responses results from activation of specific γδ+ T cells rather than from their elimination.
γδ+ T cells often accumulate at the sites of inflammation during autoimmune and infectious diseases. In experimental models of arthritis, γδ+ cells are beneficial to the host since disease is aggravated with their elimination. In experimental CVB3-induced myocarditis, however, elimination of γδ+ T cells ameliorates disease. The mechanism by which γδ+ T cells affect CVB3-induced disease is not clear. These lymphocytes are potent cytokine producers and might affect immune deviation in the CD4+ cell population by altering the local cytokine environment (4). Alternatively, γδ+ T cells might alter CD4+ T-cell responses through other mechanisms. γδ+ T cells express high levels of FasL (36), making these cells highly cytolytic.
Although everyone agrees that sex steroids influence cytokine and Th responses, the literature is confusing as to what the specific effects are. Much of the work in this area has been done with neurological disease models, specifically either experimental allergic or Theiler’s virus-induced encephalomyelitis (1, 6, 9, 13, 14). In each model, estrogen promotes disease susceptibility through induction of antigen-specific Th1 cell responses, while testosterone favors Th2 cell differentiation. Other investigators find either that estrogens do not modulate Th cell responses (5) or that varying estrogen/progesterone balances in vivo can favor either Th1 or Th2 responses (42). Most likely, as discussed by Lockshin (28), sexual dimorphism in disease is a complex issue and depends on many factors besides hormones. Additionally, since hormones alter various aspects of the immune system, including antigen presentation, MHC antigen expression, and cell apoptosis, these steroids may not have uniform effects in all antigen-specific immune responses. In the present system, the hormones most likely modulate immunity through alterations in γδ+ T-cell activation. How the steroids alter γδ+ T-cell responses has not been determined. One possibility is that the effect on γδ+ T cells is the indirect consequence of alterations in virus replication in vivo. Since androgens enhance virus titers in the heart (31) and γδ+ T cells react to heat shock proteins induced by infection (16), increases in virus titers should enhance γδ+ T-cell responses. A second possibility is that the hormones directly influence γδ+ T-cell responses. In either case, estradiol and testosterone may affect myocarditis and CD4+ T-cell responses differently than in other organs if γδ+ T-cell responses are not important in those other disease models.
ACKNOWLEDGMENTS
This work was supported by grants HL58583 (S.A.H.) and AI33470 (M.K.N.) from the National Institutes of Health and by grant-in-aid 9750081 (S.A.H.) from the American Heart Association.
REFERENCES
- 1.Bebo B F J, Zelinka-Vincent E, Adamus G, Amundson D, Vandenbark A A, Offner H. Gonadal hormones influence the immune response to PLP 139-151 and the clinical course of relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 1998;84:122–130. doi: 10.1016/s0165-5728(97)00214-2. [DOI] [PubMed] [Google Scholar]
- 2.Burak W E J, Quinn A L, Farrar W B, Brueggemeir R W. Androgens influence estrogen-induced responses in human breast carcinoma cells through cytochrome P450 aromatase. Breast Cancer Res Treat. 1997;44:57–64. doi: 10.1023/a:1005782311558. [DOI] [PubMed] [Google Scholar]
- 3.Carlsten H, Nilsson N, Jonsson R, Backman K, Holmdahl R, Tarkowski A. Estrogen accelerates immune complex glomerulonephritis but ameliorates T cell-mediated vasculitis and sialadenitis in autoimmune MLR lpr/lpr mice. Cell Immunol. 1992;144:190–202. doi: 10.1016/0008-8749(92)90236-i. [DOI] [PubMed] [Google Scholar]
- 4.Chomarat P, Kjeldsen-Kragh J, Quayle A, Natvig J, Miossec P. Different cytokine production profiles of gamma delta T cell clones: relation to inflammatory arthritis. Eur J Immunol. 1994;24:2087–2091. doi: 10.1002/eji.1830240923. [DOI] [PubMed] [Google Scholar]
- 5.Clerici E, Bergamasco E, Ferrario E, Villa M L. Influence of sex steroids on the antigen-specific primary antibody response in vitro. J Clin Lab Immunol. 1991;34:71–78. [PubMed] [Google Scholar]
- 6.Correale J, Arias M, Gilmore W. Steroid hormone regulation of cytokine secretion by proteolipid protein-specific CD4+ T cell clones isolated from multiple sclerosis patients and normal control subjects. J Immunol. 1998;161:3365–3374. [PubMed] [Google Scholar]
- 7.Dalal M, Kim S, Voskuhl R R. Testosterone therapy ameliorates experimental autoimmune encephalomyelitis and induces a T helper 2 bias in the autoantigen-specific T lymphocyte response. J Immunol. 1997;159:3–6. [PubMed] [Google Scholar]
- 8.Da Silva J A P, Peers S H, Perretti M, Willoughby D A. Sex steroids affect glucocorticoid response to chronic inflammation and to interleukin-1. J Endocrinol. 1993;136:389–397. doi: 10.1677/joe.0.1360389. [DOI] [PubMed] [Google Scholar]
- 9.Erbach G T, Bahr J M. Enhancement of in vivo humoral immunity by estrogen: permissive effect of a thymic factor. Endocrinology. 1991;128:1352–1358. doi: 10.1210/endo-128-3-1352. [DOI] [PubMed] [Google Scholar]
- 10.Evans M J, MacLaughlin S, Marvin R D, Abdou N I. Estrogen decreases in vitro apoptosis of peripheral blood mononuclear cells from women with normal menstrual cycles and decreases TNF-alpha production in SLE but not in normal cultures. Clin Immunol Immunopathol. 1997;82:258–262. doi: 10.1006/clin.1996.4300. [DOI] [PubMed] [Google Scholar]
- 11.Fox H S, Bond B L, Parslow T G. Estrogen regulates the IFN-gamma promoter. J Immunol. 1991;146:4362–4367. [PubMed] [Google Scholar]
- 12.Garcia-Segura L M, Cardona-Gomez P, Naftolin F, Chowen J A. Estradiol upregulates Bcl-2 expression in adult brain neurons. Neuroreport. 1998;9:593–597. doi: 10.1097/00001756-199803090-00006. [DOI] [PubMed] [Google Scholar]
- 13.Gilmore W, Weiner L P, Correale J. Effect of estradiol on cytokine secretion by proteolipid protein-specific T cell clones isolated from multiple sclerosis patients and normal control subjects. J Immunol. 1997;158:446–451. [PubMed] [Google Scholar]
- 14.Hill K E, Pigmans M, Fujinami R S, Rose J W. Gender variations in early Theiler’s virus induced demyelinating disease: differential susceptibility and effects of IL-4, IL-10 and combined IL-4 with IL-10. J Neuroimmunol. 1998;85:44–51. doi: 10.1016/s0165-5728(97)00263-4. [DOI] [PubMed] [Google Scholar]
- 15.Homo-Delarche F, Fitzpatrick F, Christeff N, Nunez E A, Bach J F, Dardenne M. Sex steroids, glucocorticoids, stress and autoimmunity. J Steroid Biochem Mol Biol. 1991;40:619–637. doi: 10.1016/0960-0760(91)90285-d. [DOI] [PubMed] [Google Scholar]
- 16.Huber S. Heat-shock protein induction in adriamycin and picornavirus-infected cardiocytes. Lab Investig. 1992;67:218–224. [PubMed] [Google Scholar]
- 17.Huber S, Job L, Auld K. Influence of sex hormones on coxsackie B3 virus infection in Balb/c mice. Cell Immunol. 1982;67:173–179. doi: 10.1016/0008-8749(82)90210-6. [DOI] [PubMed] [Google Scholar]
- 18.Huber S, Moraska A, Choate M. T cells expressing the gamma delta T-cell receptor potentiate coxsackievirus B3-induced myocarditis. J Virol. 1992;66:6541–6546. doi: 10.1128/jvi.66.11.6541-6546.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huber S, Mortensen A, Moulton G. Modulation of cytokine expression by CD4+ T cells during coxsackievirus B3 infections of BALB/c mice initiated by cells expressing the γδ+ T-cell receptor. J Virol. 1996;70:3039–3045. doi: 10.1128/jvi.70.5.3039-3044.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huber S, Pfaeffle B. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J Virol. 1994;68:5126–5132. doi: 10.1128/jvi.68.8.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huber S, Polgar J, Schultheiss P, Schwimmbeck P. Augmentation of pathogenesis of coxsackievirus B3 infections in mice by exogenous administration of interleukin-1 and interleukin-2. J Virol. 1994;68:195–206. doi: 10.1128/jvi.68.1.195-206.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.Huber, S. A., J. E. Stone, D. H. Wagner, Jr., J. Kupperman, L. Pfeiffer, C. David, R. L. O’Brien, G. S. Davis, and M. K. Newell. γδ+ T cells regulate major histocompatability complex class II (IA and IE)-dependent susceptibility to coxsackievirus B3-induced autoimmune myocarditis. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 22.Jansson L, Holmdahl R. Estrogen-mediated immunosuppression in autoimmune diseases. Inflamm Res. 1998;47:290–301. doi: 10.1007/s000110050332. [DOI] [PubMed] [Google Scholar]
- 23.Knowlton K, Jeon E, Berkley N, Wessely R, Huber S. A mutation in the puff region of VP2 attenuates the myocarditic penotype of an infectious cDNA of the Woodruff variant of coxsackievirus B3. J Virol. 1996;70:7811–7818. doi: 10.1128/jvi.70.11.7811-7818.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuchroo V K, Das M P, Brown J A, Ranger A M, Zamvil S S, Sobel R A, Weiner H L, Nabavi N, Glimcher L H. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 25.LeMeur M, Gerlinger P, Benoit C, Mathis D. Correcting an immune-response deficiency by creating Ea gene transgenic mice. Nature. 1985;316:38–42. doi: 10.1038/316038a0. [DOI] [PubMed] [Google Scholar]
- 26.Lenschow D, Herold K, Rhee L, Patel B, Koons A, Qin H, Fuchs E, Singh B, Thompson C, Bluestone J. CD28/B7 regulation of Th1 and Th2 subsets in the development of autoimmune diabetes. Immunity. 1996;5:285. doi: 10.1016/s1074-7613(00)80323-4. [DOI] [PubMed] [Google Scholar]
- 27.Lenschow D, Ho S, Sattar H, Rhee L, Gray G, Navavi N, Herold K, Bluestone J. Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J Exp Med. 1995;181:1145. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lockshin M D. Why women? J Am Med Womens Assoc. 1998;53:4–8. [PubMed] [Google Scholar]
- 29.Lou H, Kodama T, Zhao Y J, Maurice P, Wang Y N, Katz N, Foegh M L. Inhibition of transplant coronary arteriosclerosis in rabbits by chronic estradiol treatment is associated with abolition of MHC class II antigen expression. Circulation. 1996;94:3355–3361. doi: 10.1161/01.cir.94.12.3355. [DOI] [PubMed] [Google Scholar]
- 30.Lyden D, Huber S. Aggreavation of coxsackievirus group B type 3-induced myocarditis and increase in cellular immunity to myocyte antigens in pregnant BALB/c mice and animals treated with progesterone. Cell Immunol. 1984;87:462–472. doi: 10.1016/0008-8749(84)90015-7. [DOI] [PubMed] [Google Scholar]
- 31.Lyden D, Olszewski J, Feran M, Job L, Huber S. Coxsackievirus B3-induced myocarditis. Effects of sex steroids on viremia and infectivity of cardiocytes. Am J Pathol. 1987;126:432–438. [PMC free article] [PubMed] [Google Scholar]
- 32.Lyden D, Olszewski J, Huber S. Variation in susceptibility of BALB/c mice to coxsackievirus group B type 3-induced myocarditis with age. Cell Immunol. 1987;105:332–339. doi: 10.1016/0008-8749(87)90081-5. [DOI] [PubMed] [Google Scholar]
- 33.Nikolaevich K N, Ivanovich S J, Victorovich S S. Major reproduction hormones as regulators of cell-to-cell interactions in humoral immune responses. Brain Behav Immun. 1991;5:149–161. doi: 10.1016/0889-1591(91)90013-z. [DOI] [PubMed] [Google Scholar]
- 34.Picker L J, Singh M K, Zdraveski Z, Treer J R, Maino V C. Demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood. 1995;86:1408–1419. [PubMed] [Google Scholar]
- 35.Ruh M F, Bi Y, D’Alonzo R, Bellone C J. Effect of estrogens on IL-1beta promoter activity. J Steroid Biochem Mol Biol. 1998;66:203–210. doi: 10.1016/s0960-0760(98)00042-9. [DOI] [PubMed] [Google Scholar]
- 36.Suda T, Okazaki Y, Naito T, Yokota N, Arai S, Ozaki K, Nakao K, Nagata S. Expression of the Fas ligand in cells of the T lineage. J Immunol. 1995;154:3806–3813. [PubMed] [Google Scholar]
- 37.Taneja V, Hansen J, Smart M, Griffiths M, Luthra H, David C. Expression of H-2E molecule mediates protection to collagen induced arthritis in HLA-DQ8 transgenic mice: role of cytokines. Int Immunol. 1997;9:1213–1219. doi: 10.1093/intimm/9.8.1213. [DOI] [PubMed] [Google Scholar]
- 38.Toda I, Wickham L A, Sullivan D A. Gender and androgen treatment influence the expression of proto-oncogenes and apoptotic factors in lacrimal and salivary tissue of MRL/lpr mice. Clin Immunol Immunopathol. 1998;86:59–71. doi: 10.1006/clin.1997.4466. [DOI] [PubMed] [Google Scholar]
- 39.Waksman Y, Hod I, Friedman A. Therapeutic effects of estradiol benzoate on development of collagen-induced arthritis (CIA) in the Lewis rat are mediated via suppression of the humoral response against denatured collagen type II (CII) Clin Exp Immunol. 1996;103:376–383. doi: 10.1111/j.1365-2249.1996.tb08290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Campbell H D, Young I G. Sex hormones and dexamethasone modulate interleukin-5 gene expression in T lymphocytes. J Steroid Biochem Mol Biol. 1993;44:203–1. doi: 10.1016/0960-0760(93)90080-g. 210. [DOI] [PubMed] [Google Scholar]
- 41.Weller A, Simpson K, Herzum M, Van Houten N, Huber S. Coxsackievirus B3 induced myocarditis: virus receptor antibodies modulate myocarditis. J Immunol. 1989;143:1843–1850. [PubMed] [Google Scholar]
- 42.Wilder R L. Hormones, pregnancy, and autoimmune diseases. Ann N Y Acad Sci. 1998;840:45–50. doi: 10.1111/j.1749-6632.1998.tb09547.x. [DOI] [PubMed] [Google Scholar]
- 43.Wira C R, Rossoll R M. Antigen-presenting cells in the female reproductive tract: influence of sex hormones on antigen presentation in the vagina. Immunology. 1995;84:505–508. [PMC free article] [PubMed] [Google Scholar]
- 44.Woodruff J. Viral myocarditis. Am J Pathol. 1980;101:425–483. [PMC free article] [PubMed] [Google Scholar]