Abstract
Burn pits are a method of open-air waste management that was common during military operations in Iraq, Afghanistan, and other regions in Southwest Asia. Veterans returning from deployment have reported respiratory symptoms, potentially from exposure to burn pit smoke, yet comprehensive assessment of such exposure on pulmonary health is lacking. We have previously shown that exposure to condensates from burn pit smoke emissions cause inflammation and cytotoxicity in mice. In this study, we explored the effects of burn pit smoke condensates on human airway epithelial cells (HAECs) to understand their impact on cellular targets in human lung. HAECs were cultured at the air-liquid interface (ALI) and exposed to burn pit waste smoke condensates (plywood, cardboard, plastic, mixed, and mixed with diesel) generated under smoldering and flaming conditions. Cytotoxicity was evaluated by measuring transepithelial electrical resistance (TEER) and lactate dehydrogenase (LDH) release; toxicity scores were quantified for each exposure. Pro-inflammatory cytokine release and modulation of gene expression were examined for cardboard and plastic condensate exposures. Burn pit smoke condensates generated under flaming conditions affected cell viability, with flaming mixed waste and plywood exhibiting the highest toxicity scores. Cardboard and plastic smoke condensates modulated cytokine secretion with GM-CSF and IL-1β altered in more than one exposure group. Gene expression of detoxifying enzymes (ALDH1A3, ALDH3A1, CYP1A1, CYP1B1, NQO1 etc), mucins (MUC5AC, MUC5B), and cytokines was affected by several smoke condensates. Particularly, expression of IL6 was elevated following exposure to all burn pit smoke condensates and polycyclic aromatic hydrocarbon acenaphthene was positively associated with the IL-6 level in the basolateral media of HAECs. These observations demonstrate that exposure to smoke condensates of materials present in burn pits adversely affects HAECs and that aberrant cytokine secretion and altered gene expression profiles following burn pit material smoke exposure could contribute to development of airway disease.
Keywords: Burn pit, particulate matter, smoldering, flaming, human airway epithelial cells, IL-8, transepithelial electrical resistance measurement, cytotoxicity, unsupervised machine learning
Graphical Abstract

INTRODUCTION
Open burning of garbage was widely used during military operations in Iraq, Afghanistan, and Southwest Asia creating a mixture of airborne hazards1. According to the Airborne Hazards and Open Burn Pit Registry (AHOBPR), open-air burn pits constitute the most common deployment-related exposures in veterans, occurring in more than 90% of cases2. The Department of Defense (DoD) prohibited the disposal of waste in open-air burn pits in 2010 through a Directive-Type Memorandum (09-032), unless the base commander determines the unavailability of alternatives. Although incinerators were added to the bases for waste management to reduce emission since 2006, burn pits were still present until 20143. Veterans deployed in Southwest Asia and Afghanistan were typically exposed to more particulate matter than the United States National Ambient Air Quality Standards, and suffer from several deployment-related lung diseases, including asthma, bronchiolitis and other respiratory disorders 4–10. Burn pit exposure has been associated with respiratory diagnoses, including symptoms such as shortness of breath, chronic sinus infection, runny nose, cough, respiratory allergy and wheezing2. A growing body of research continues to uncover the relationship between burn pit smoke exposure and adverse health effects. For example, epidemiological studies identified associations between burn pit smoke exposure during deployment and chronic respiratory symptoms11, 12. Accordingly, legislative efforts are already underway to address the issue of burn pit smoke exposure associated health complications13, including the Veterans Burn Pits Exposure Recognition Act in 2021 and the Promise to Address Comprehensive Toxics (PACT) Act in 202213, 14.
However, evidence directly linking burn pit smoke emission and respiratory toxicity is lacking. Previously, we have shown that smoke generated from the incineration of burn pit waste materials contains carbon dioxide, carbon monoxide, methane (CH4), volatile organic compounds (VOCs), nitrogen oxides (NOx) and particulate matter (PM) ranging between 0.3 to 2.8 μm15. Recently, the importance of waste material and combustion conditions to cause respiratory toxicity was noted in a nose-only mouse model of exposure, with PMs identified as the key player driving the effects16. Smoke condensates were mutagenic, possibly owing to the presence of polycyclic aromatic hydrocarbons (PAHs) and aromatic amines15. Further analysis of the PMs revealed an array of PAHs including highly toxic nitrated- and oxygenated-species15. Exposure of the condensates through oropharyngeal aspiration in mice caused lung inflammation and altered pulmonary function, with a clear influence of the burn pit waste material type and combustion temperature on lung. As such, smoke condensates from flaming conditions of several burn pit waste materials caused greater neutrophil infiltration in lungs and elevated levels of interleukin-6 and macrophage inflammatory protein-2 in bronchoalveolar lavage fluid15.
Human airway epithelial cells (HAECs) line human lung bronchi and perform critical barrier and physiological functions17, 18. A compromised HAEC layer is associated with several lung diseases, including chronic obstructive pulmonary disease19, asthma and allergy20, 21. Short-term exposure to combustible tobacco smoke causes decreased epithelial barrier function, inflammation, cytotoxicity, and alterations in gene and protein expression patterns in cultured airway epithelial cells22. Although long-term effects of burn pit smoke exposures are yet to be well understood, studies investigating the effects of acute cellular cytotoxicity or markers of cellular stress caused by PM of burn pit emissions on HAECs are needed to elucidate the potential impact of these exposures on pulmonary health. In the study presented here, we examined the cellular effects of model burn pit smoke PMs using the well-differentiated air-liquid interface cultures of HAECs to understand the impact of exposure to burn pit smoke on human airways.
EXPERIMENTAL PROCEDURES
Primary human airway epithelial cell isolation and culture
Human lungs deemed unsuitable for transplantation were obtained under the auspices of the University of North Carolina Biomedical Institutional Review Board approved protocol #03-1396. All donors had no reported smoking history and were free of prior chronic lung disease. Authorized representatives provided informed consent for research use. Primary human airway epithelial cells were obtained from the trachea and bronchi as previously described23, 24. Passage-one cells were thawed and seeded directly on collagen coated 6.5 mm Transwell inserts (Costar #3470). Cells were differentiated at the air-liquid interface (ALI) for 28-35 days until use. Cultures from one male and one female donor were used for the initial analysis of cytotoxicity. Cultures established from three female and three male donors were used for subsequent experiments determining changes in cytokine release and gene expression.
Combustion of burn pit waste materials and collection of smoke condensates
Details describing the different types of waste materials used to generate condensates were reported previously15, 16. Briefly, five different waste types were used to generate particulate matter (PM) using a quartz-tube furnace connected to a multi-stage cryotrap system25. These waste materials were selected based on previously reported data on military waste26 and included plywood, cardboard, plastic, mixed waste, and a combination of mixed waste and diesel fuels. Accordingly, we used plywood and cardboard of military grade from ActionPak, Inc., Bristol, PA. Plastic waste material was sourced from Edwards Industrial Surplus, Robards, KY, and was a mixture of low-density polyethylene, high-density polyethylene, polyethylene terephthalate, and polystyrene pellets. We also included a group of mixed waste, comprising 49 percent weight of cardboard, 27 percent weight of plastic and 24 percent weight of plywood. To simulate the addition of accelerant during waste incineration in burn pits, we included another group where 10 percent weight of diesel fuel (Air Methods and Characterization Division at the U.S. EPA) was added to the mixed waste. Waste materials were burned under two conditions, smoldering and flaming, based on the modified combustion efficiency (MCE) which is calculated as the ratio of emitted CO2 concentrations to emitted CO2 and CO concentrations (ΔCO2 / (ΔCO2 + ΔCO) x 100); MCE more than 95% and 65-85% were respectively considered to be flaming and smoldering combustion conditions27. The incineration temperatures were approximately 500 °C for smoldering and 640 °C for flaming condition. Collection of smoke condensate and detailed smoke properties have been reported previously15. Stocks of burn pit waste smoke condensates were sonicated to resuspend the floating particles, then diluted in sterile PBS to obtain the required particulate concentration. Stock solutions of 1 mg/ml were sonicated and vortexed, and diluted to obtain a secondary stock with a particulate concentration of 33μg in 35μl, which is added to the apical side of the transwell (diameter 6.5 mm, area 0.33 cm2) to account for an exposure of 100 μg/cm2; serially diluted suspensions of the secondary stock were prepared to obtain a particulate content of 16.5, 8.25 and 0.33 μg in a 35 μl volume to apically expose HAECs to smoke condensates with a particulate matter exposure dose of 50, 25 and 1 μg/cm2 respectively. Vehicle control cultures were treated with sterile PBS only.
Exposure of HAECs to burn pit condensates and sample collection
For the initial screening of different burn pit condensates from two combustion temperatures, HAECs were exposed to four particulate material concentrations (1, 25, 50 and 100 μg/cm2) for 24 hours and were evaluated for markers of cytotoxicity and cytokine release. For cytotoxicity, transepithelial electrical resistance (TEER) was measured using an EVOM2 epithelial voltohmmeter (World Precision Instruments). In addition, the apical surface was washed with PBS and the cell free supernatant (600 g x 5 min) was assayed for lactate dehydrogenase (LDH) activity the same day using the Roche Cell Cytotoxicity Kit (Cat#11644793001) following the manufacturer’s instructions. Basolateral media was stored at −20 °C for enzyme-linked immunosorbent assay (ELISA) of IL-8 (BD OptEIA, Cat#555244) following the manufacturer’s protocol.
Distribution Analysis of TEER, LDH, and IL-8 Changes from Exposures
For statistical analysis comparing TEER, LDH and IL-8 from doses of all five waste materials under two burn conditions at four doses of particulate matter, we used GraphPad Prism (version 8.0.1.244). Three technical replicates from one male and one female donor were used for each exposure group. Friedman’s one-way repeated measure non-parametric Analysis of Variance test with Dunn’s multiple comparison for comparison among the groups. At the highest concentration of condensates, missing data points (less than 7% of all values) were imputed using the mean of the technical replicates of the specific donor at the given exposure condition. An adjusted p-value of <0.05 was considered significant.
Toxicity score calculation of burn pit condensates
To compare the cytotoxic effects of condensates from different waste materials, we calculated and assigned a toxicity score to each burn pit waste condensate. We used the changes in TEER and the release of LDH from the cells following exposure to four ascending condensate doses (1, 25, 50 and 100 μg/cm2) for 24 hours and calculated the area under curve (AUC) using BMDExpress 3.20 software28, corresponding to each parameter (TEER and LDH). AUC values were derived from the best curve fit model, tested across the following models: Hill, power, linear, polynomial 2, polynomial 3, exponential 3, exponential 5. Best curve fit models were selected based upon the highest Akaike Information Criteria. A toxicity score was assigned to each group for each parameter (TEER and LDH) and an overall Toxicity Score (TS) was calculated using the formula provided in Figure 1. For TEER measurements, the absolute value of the negative AUC was used to calculate the TS. Finally, the toxicity scores of each parameter were added together for each group, to obtain a combined TS for each exposure.
Figure 1. Toxicity scores were calculated for each exposure group.
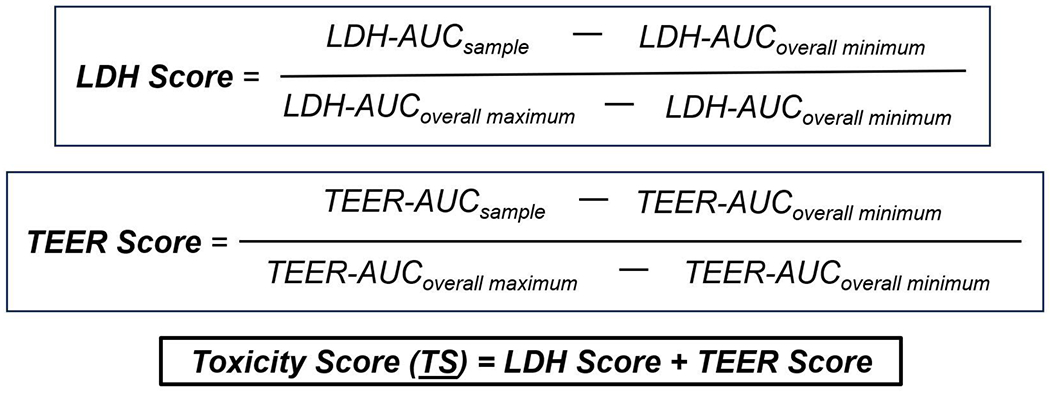
We plotted the fold changes of lactic acid dehydrogenase (LDH) and transepithelial electrical resistance (TEER) changes at the four doses for each exposure compared to control to measure area under the curve (AUC) and estimated respective scores for each group as described in the methods. Toxicity score (TS) was calculated by combined LDH and TEER scores for each condensate for each condition (smoldering and flaming).
Assessment of cytokines and chemokines
Basolateral ALI culture media from three male and three female donors was collected 24 hours after apical exposure to 1 (low) and 25 (high) μg/cm2 dose smoldering and flaming cardboard and plastic emission condensates. The V-PLEX Human Cytokine 30-plex assay, performed on a MESO QuickPlex SQ 120 instrument (Meso Scale Discovery) was used to evaluate 30 mediators of inflammatory response and immune system regulation. Of these 30 mediators, only 17 cytokines showed measurable levels in the basolateral media of HAECs in all or most replicates and were reported in the final data.
Gene expression measurement
Primary HAEC ALI culture lysates were obtained from the six donors 4 and 24 hours after exposure to 1 (low) and 25 (high) μg/cm2 dose smoldering and flaming cardboard and plastic emission condensates (Ambion PureLink™ RNA kit, 12183025) as described previously29. Gene expression was measured using an integrated fluidic circuit based multi-plex qRT-PCR system (Standard BioTools Inc.). Relative expression of 19 selected genes was normalized to beta (β)-actin expression. These genes were selected to account for inflammatory, detoxifying, and innate defense responses of HAECs following exposure to condensates.
Data Organization and Processing of Cytokine and Gene Expression Data
All cytokine and gene expression data were processed, and subsequent analyses were carried out in R software (v 4.1.3.). Gene expression data contained 1 to 6 replicates per sample, which were averaged to condense the data to have 1 data point for each sample. Variance stabilizing normalization (VSN) was performed on both data types using the vsn package30. Next, data imputation was performed on the mRNA data that had missing data points using Quantile Regression Imputation of Left-Censored data (QRILC) within the imputeLCMD package31. Since the mRNA missing values were likely attributable to low expression or missing not at random (MNAR), QRILC was selected to impute missing gene measures as it generates data from the left side of a normal distribution. Typically subjects or biomarkers with less than 75% of observed data within each sample condition would be removed prior to imputation, however this would have hindered our ability to perform downstream individual statistical analyses. Overall, the imputed data accounted for less than 11% of the records used in subsequent analyses.
Statistical Analysis of Cytokine and Gene Expression Changes
To determine if there were statistically significant differences across the burn conditions (control, smoldering, or flaming) in individual cytokines and genes, Friedman’s tests were employed to perform a three-group comparison. In addition, this analysis was further stratified by condensate (plastic or cardboard) and dose [low (1 μg/cm2) or high (25 μg/cm2)] to investigate whether the modulations seen across the burn conditions were affected by the condensate and/or the concentration of the condensate sample. Given the sample size (n = 6, from three male and three female donors), Friedman’s tests followed by Nemenyi’s post hoc tests were chosen, which are non-parametric versions of an ANOVA and a Tukey’s post hoc, respectively. Nemenyi’s post hoc tests were run using the PMCMRplus package 32. P-values were adjusted for multiple tests (referred to as “Padj” values) using false-discovery rate (FDR) q-values33. P value< 0.1 was considered significant. Biomarkers that were significantly modulated under multiple sample conditions were visualized in a Venn diagram using the ggvenn 34 package.
Clustering Analysis of Cytokines and Gene Expression
To further investigate the impact of each condensate (plastic or cardboard) and burn conditions (control, smoldering, or flaming) on cytokine and gene expression profiles after exposure, principal component analysis (PCA) was performed to understand if these sample conditions yield similar expression profiles at an aggregate level ignoring the donor-to-donor variations, similar to our previous study 35. This subset of sample conditions was prioritized to enhance resulting visualizations while focusing on conditions of high importance. PCA is an unsupervised machine learning technique that seeks to compress the variance from an original dataset into the fewest number of principal components 36, 37. PCA findings can be visualized via plots, wherein each axis represents a component that captures large amounts of variance in the data; in this case, the first two principal components were plotted within a 2D plot to visualize potential patterns amongst sample conditions. PCA was performed using the factoextra 38 package.
Next, the effects of these sample conditions were further investigated on individual cytokines and genes. To do so, these expression profiles were visualized using a heat map showing average concentrations for each biomarker after exposure to each sample condition using the pheatmap 39 package. The biomarkers in the heat map were grouped together using hierarchical clustering to group together cytokines or genes based on the average distance between them 36. This clustering approach was implemented to identify groups of cytokines with concerted response profiles across exposure conditions, as previously described 40, 41.
Correlation analysis between polyaromatic hydrocarbons (PAHs) and cytokine levels
We used the PAH levels present in the smoldering and flaming condenstaes15 and the cytokine levels to perform Spearman’s rank correlation tests using the psych package42 in R (v 4.3.2) 43. The undetermined values of PAHs were replaced with the detection limit (2.36 μg/g of particulate matter). The measured correlation coefficients (r) were plotted using the corrplot package44.
Data Availability
All data have been deposited within the University of North Carolina -Center for Environmental Medicine, Asthma, and Lung Biology Dataverse 45. All script used in this manuscript are publicly available through the University of North Carolina -Center for Environmental Medicine, Asthma, and Lung Biology Github46.
RESULTS
Smoke condensates of burn pit materials are cytotoxic
Burn pit waste incineration results in exposure to toxic chemicals and gaseous fumes, which may potentially result in an array of adverse health outcomes. To understand the pulmonary impact of these exposures, we generated smoke condensates from five different burn pit waste materials: plywood; cardboard; plastic; mixed waste (combination of plywood, cardboard and plastic); and mixed waste containing diesel as a surrogate for the commonly used accelerant jet fuel to facilitate combustion15. We examined the acute effects of four different doses of these ten condensates under smoldering and flaming combustion conditions compared to a control group (treated with phosphate buffered saline) to explore the relative toxicity in HAECs.
When LDH activity in apical washes were evaluated as a marker of cytotoxicity, there were significant increases at the highest particulate dose (100 μg/cm2) of almost all smoke condensates (Figure 2B). Flaming condensates also caused significantly elevated LDH release at the lower dose of 50 μg/cm2. Furthermore, mixed waste smoke condensate from smoldering condition also caused significantly increased LDH release at a lower dose (50 μg/cm2) (Figure 2B). When the doses were compared between smoldering and flaming smoke condensates for each waste material, flaming plywood, plastic and diesel containing mixed waste condensates caused significantly elevated LDH release at the dose of 100 μg/cm2 (Figure 2B). These results confirm our previous observations on in vivo models that the flaming condition produces more toxic emission and plastic waste is potentially more deleterious to cellular viability15.
Figure 2. Exposure to condensate of burn pit materials’ smoke caused inflammation and cytotoxicity.
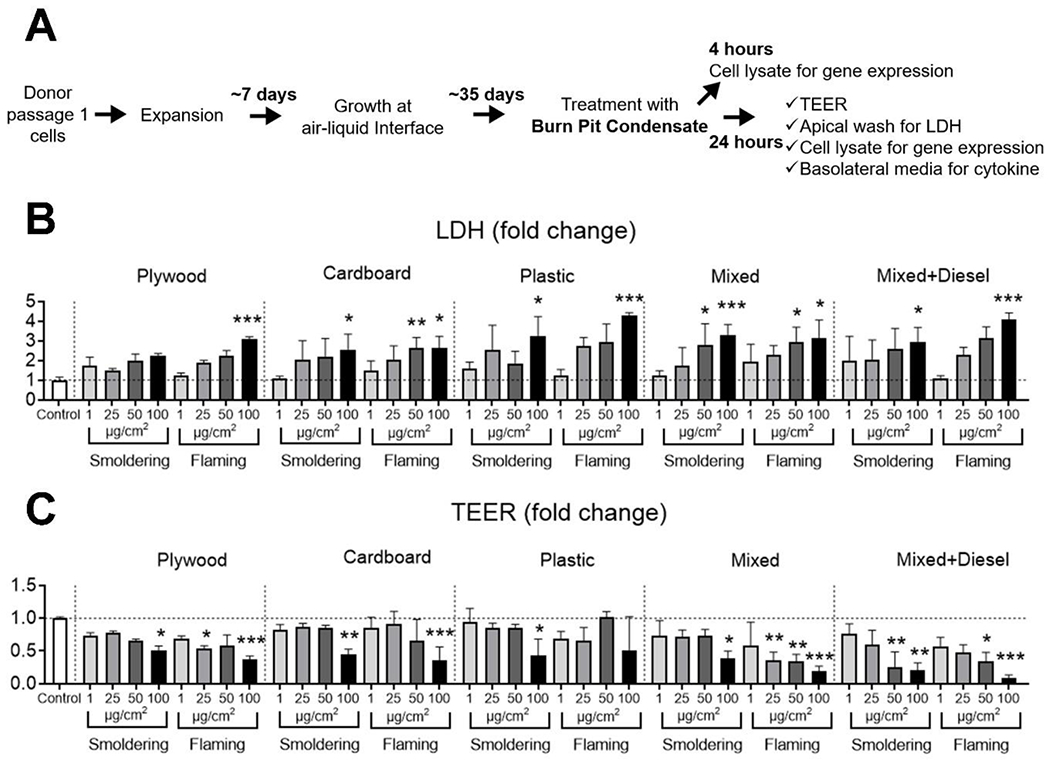
(A) Scheme shows the study plan followed to culture primary HAECs and subsequent treatment with smoke condensates and collection of samples after exposure. HAECs cultured at air-liquid interface (ALI) were apically treated with smoke condensates at four doses (1, 25, 50 and 100 μg/cm2) for 24 hours and cultures were examined for apical lactate dehydrogenase (LDH) release and changes in trans-epithelial electrical resistance (TEER). Changes in LDH release (B) and TEER measurements (C) across the doses for the exposure groups are shown as bar diagrams. Data is shown as fold change compared to the control group treated with phosphate buffered saline; * = p<0.05, ** = p<0.01 and *** = p<0.001 compared to control; n=6 technical replicates, equal number of cultures from both male and female donors were combined for each exposure. The Friedman one-way test with Dunn’s multiple comparisons was used to compare among the groups, with Wilcoxon signed rank test used for comparison between doses.
TEER measurement of HAEC ALI cultures is a non-destructive method to evaluate epithelial barrier integrity following treatment47. When HAEC ALI cultures were exposed to smoke condensates at the four particulate doses, significant TEER reduction was noted for the highest dose (100 μg/cm2) of almost all smoke condensates, (Figure 2C). Flaming mixed waste condensate also decreased TEER at 25 and 50 μg/cm2 doses. Additionally, diesel containing mixed waste decreased TEER at 50 μg/cm2 dose under both smoldering and flaming conditions (Figure 2C). Interestingly, the flaming smoke condensate of mixed waste significantly decreased TEER values at 25, 50 and 100 μg/cm2 doses compared to the smoldering condensate exposure (Figure 2C).
Flaming smoke condensates exhibited higher Toxicity Scores than smoldering condensates
To understand the relative cytotoxic effects of different burn pit smoke condensates, a toxicity score (TS) was calculated and assigned to each smoke condensate, combining both the TEER decrease and the elevated levels of LDH in apical secretions in each exposure group. The highest TS was from condensates under flaming conditions. Overall, the highest toxicity was exhibited by flaming mixed waste (TS=2.00) and plywood smoldering had the lowest toxicity (TS = 0.03) (Table 1). Flaming diesel-containing mixed waste was the second-most toxic exposure category with a score of 1.08. The lowest toxicity scores were dominated by four smoldering condensates: diesel-containing mixed waste, mixed waste, plastic, and plywood wastes with TSs respectively of 0.14, 0.10, 0.05 and 0.03. IL-8 secretion from HAECs in the basolateral media was elevated following exposure to the burn pit waste condensates. However, the changes did not reach statistical significance compared to PBS-treated control ALI cultures for most exposure groups (Figure 3).
Table 1. Toxicity score of each burn pit smoke condensate.
LDH release and changes in TEER in each exposure group was used to measure “Toxicity Score” for each burn pit waste smoke condensate; higher score indicates intensified toxicity.
| Burn Pit Waste Material | Toxicity Score |
|---|---|
| Mixture Flaming | 2.00 |
| Mixture+Diesel Flaming | 1.08 |
| Plywood Flaming | 0.78 |
| Plastic Flaming | 0.23 |
| Cardboard Smoldering | 0.16 |
| Cardboard Flaming | 0.15 |
| Mixture+Diesel Smoldering | 0.14 |
| Mixture Smoldering | 0.10 |
| Plastic Smoldering | 0.05 |
| Plywood Smoldering | 0.03 |
Figure 3. Secretion of IL-8 in basolateral media by HAECs following burn pit smoke condensate exposure.
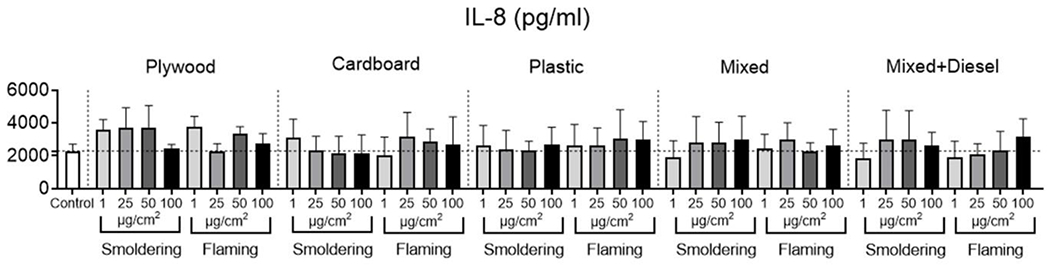
Cells were exposed to burn pit smoke condensates from burn pit waste materials generated at two different temperatures namely smoldering and flaming, at four doses (1, 25, 50 and 100 μg/cm2) for 24 hours and basolateral media was assessed for IL-8 level. IL-8 levels in basolateral media in different exposure groups as indicated in the graphs at the specified doses. Data is given as fold change compared to control; n=6 technical replicates, equal number of cultures from both male and female donors were combined for each exposure. No significant changes were noted in any exposure groups compared to vehicle control.
Based on these results and previous observations15, 16, 25, two concentrations (1 and 25 μg/cm2) of smoke condensates of cardboard and plastic wastes produced under smoldering and flaming conditions were selected to analyze changes in selected genes and cytokines/chemokines (Figure 2A). This decision was also based on the toxicity score of these waste smoke condensates (Table 1), with both cardboard and plastic waste condensates having minimal overt cytotoxicity.
Exposure to burn pit condensates caused differential gene expression in HAECs
After identifying differential modulation of cytokine levels, to better to characterize the immediate impact of smoke condensate exposure on HAECs we examined the expression of 19 selected genes following exposure to smoke condensates at two time points: 4 hours and 24 hours. Following 4 hours of exposure to the high particulate dose (25 μg/cm2) of smoke condensates, all genes under investigation were differentially modulated in at least one exposure group based on statistical distribution analyses using Friedman’s tests (Table S2). When comparing control samples to smoldering or flaming cardboard samples, 16 genes were identified as significantly different (p value < 0.1) as detailed further in Table S3. All genes were downregulated except for IL6. There were no significant differences between exposure to smoldering and flaming cardboard emissions (Table S3). When comparing control samples to smoldering plastic samples, only five genes (HMOX1, ALDH3A1, IL1β, IL6, and MCP1) were significantly modulated. All genes were upregulated except for IL6, IL1β, and ALDH3A1 (Table S3). When comparing control samples to flaming plastic samples, seven genes (HMOX1, CSF2, IL6, IL8, TXNRD1, CYP1A1, and CYP1B1) were significantly modulated. All genes were downregulated except for IL6 and CSF2 (Table S3). When comparing smoldering samples to flaming plastic samples, nine genes were significantly downregulated except for CSF2 (detailed further in Table S3).
When gene expression at 24-hour time point was considered, seventeen genes were identified as significantly different after exposure to incinerating cardboard (Table S2). When comparing control samples to smoldering cardboard samples, two genes (ALDH3A1 and MUC5AC) were significantly modulated at the low dose and 15 were modulated at the high dose (detailed further in Table S3). All significantly altered genes were downregulated except for IL6 at the high dose (Table S3). When comparing control samples to flaming cardboard samples, one gene (CYP1A1) was decreased at the low dose and 13 genes were further modulated at the high dose (detailed further in Table S3). All genes were downregulated except for IL6. There were no significant differences between exposure to smoldering and flaming cardboard emissions (Table S3).
Similarly, seven unique genes exposed to burning plastic were significantly different (Table S2). When comparing control samples to smoldering samples, four genes (CXCL1, GCLM, IL6, and NQO1) were significantly modulated at a low dose. With the exception of IL6, all genes were downregulated (Table S3). When comparing control samples to flaming samples, 4 genes (HMOX1, IL6, ALDH1A3, and CYP1A1) were modulated at a low dose and 2 genes (CYP1A1 and CYP1B1) were modulated at a high dose. Excluding HMOX1 and IL6, all significantly altered genes were downregulated. There were no significant differences between smoldering and flaming samples. Interestingly, IL6 was upregulated in all four condensate smoke exposures and burn temperatures. In addition, more genes were significantly altered from cardboard than plastic condensates irrespective of incineration temperature and exposure duration.
Smoke condensates caused distinct clusters of gene expression patterns following 4- and 24-hours of exposure
To observe the overall gene expression pattern across each waste material, burn condition, and exposure duration, PCA was performed (Figure 4). The analysis was able to capture most (86.4%) of the variance from the gene expression data within the first two eigenvectors, supporting the characterization of the clustering patterns using the two-dimensional plot. Cells exposed to incinerating cardboard clustered similarly to control samples regardless of the burn temperature or the length of exposure. All plastic smoke exposures clustered away from control and cardboard exposures; however, plastic flaming smoke exposure groups had the most similar gene expression profiles (Figure 4). These results indicate that the waste material has the most substantial impact on gene expression profiles of HAECs, irrespective of incineration temperature or length of exposure.
Figure 4. Clustering of aggregate gene expression patterns resulting from burn pit smoke condensate exposure to HAEC.
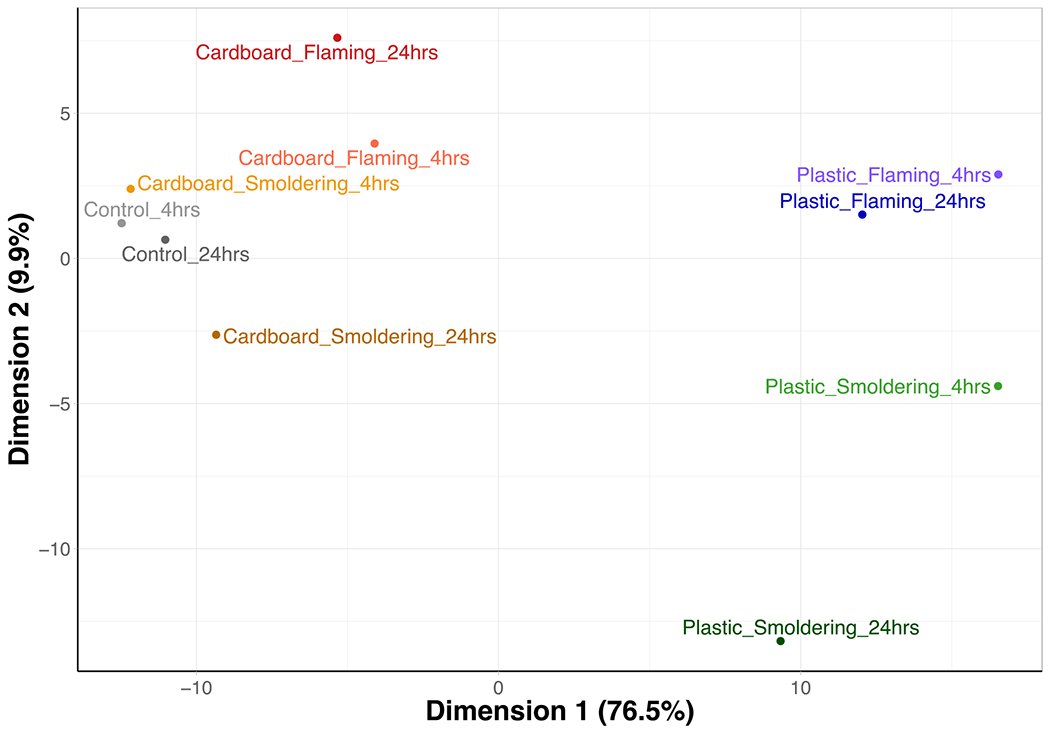
HAECs were treated with burn pit smoke condensates for 4- and 24-hours, and cell lysates were collected to probe for selected genes expression. Principal component analysis (PCA) of overall gene expression within cells following burn pit smoke condensate exposure at the higher dose (25 μg/cm2) is shown. Condensates were generated from cardboard and plastic waste smoke, incinerated at smoldering and flaming conditions; n=6, ALI cultures from equal number of female and male donors were combined for each exposure group.
Modulations of gene expression patterns seen at the aggregate level using PCA were reflected at the individual gene level as reflected using heat maps (Figure 5). Cells exposed to cardboard smoke were still the most similar to control group and plastic smoke exposure was distinctly different from the control group. Hierarchical clustering of the genes identified three clusters of similarity based on gene expression, which were largely consistent between both time points. The first cluster after 4 hours of exposure had more muted responses, but was upregulated in cardboard flaming samples (Figure 5A). The first cluster after 24 hours of exposure was upregulated in most treatment groups except for plastic smoldering (Figure 5B). Genes that fell into this first cluster at both time points included: IL6, CSF2, CXCR1 and MCP1. The second cluster consisted of one gene that was downregulated across the treatment groups. This gene was HMOX1 at the 4hr duration and CYP1A1 at the 24hr duration. Lastly, the third cluster contained the rest of the genes that were downregulated in both exposure durations detailed further in Figure 5.
Figure 5. Individual gene expression clusters across burn pit smoke condensate exposure groups.
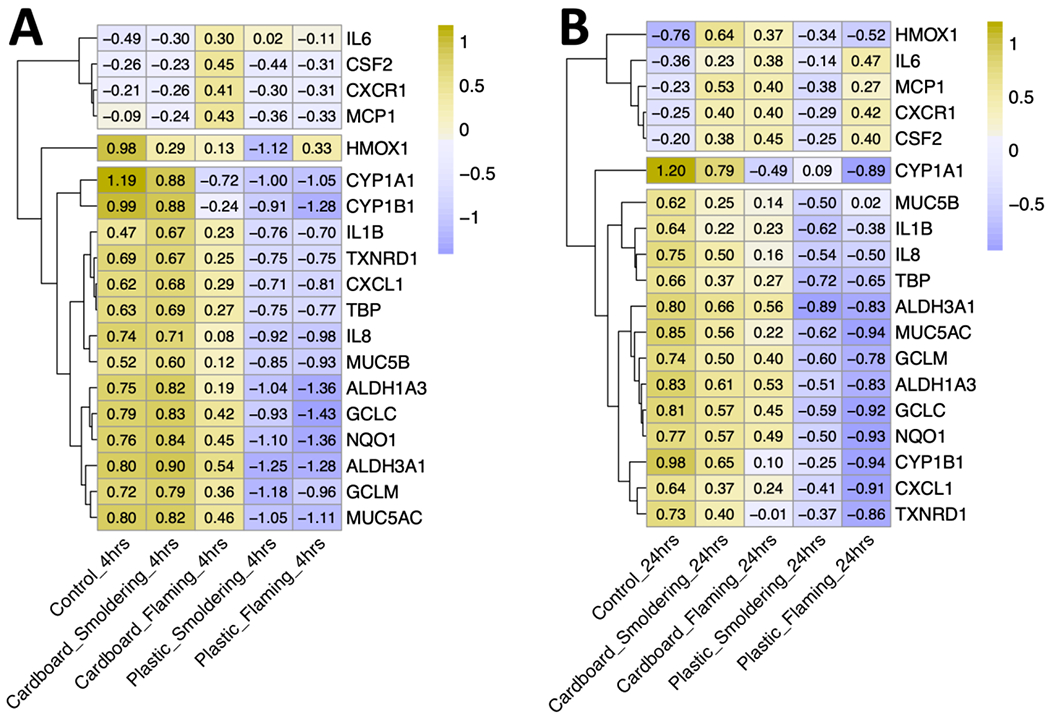
Heatmap visualizing the scaled relative expression of individual genes at the higher dose (25 μg/cm2) across the four treatment groups post 4- (A) and 24-hours (B) of exposure. Condensates were generated from cardboard and plastic waste smoke, incinerated at smoldering and flaming conditions; n=6, ALI cultures from equal number of female (N = 3) and male (N= 3) donors were combined for this analysis. Hierarchical clustering of genes on the y axis were based on scaled expression levels. Exposure groups on the x axis are arranged from highest on the left to lowest on the right average scaled expression.
Smoke condensate of cardboard incineration caused more alterations in gene expression
Gene expression changes that were statistically significant (P value <0.1) between each treatment group compared to control combining both doses for both 4- and 24-hour time points were compared using Venn diagrams to understand the overlapping and unique changes (Figure 6). At both time points, cardboard condensate caused significant changes in more genes than plastic. Specifically, after 4 hours of exposure cardboard altered seventeen genes combining both smoldering and flaming conditions compared to plastic, which altered ten genes (Figure 6a). Fourteen genes altered by both burn conditions of cardboard include: ALDH1A3, ALDH3A1, CXCL1, CYP1A1, CYP1B1, GCLC, GCLM, IL6, IL8, MUC5AC, MUC5B, NQO1, TBP and TXNRD1. On the other hand, plastic condensates altered the expression of two genes in both burn conditions: HMOX1 and IL6 (Figure 6a).
Figure 6. Gene expression changes commonly modulated across smoke condensate exposures.
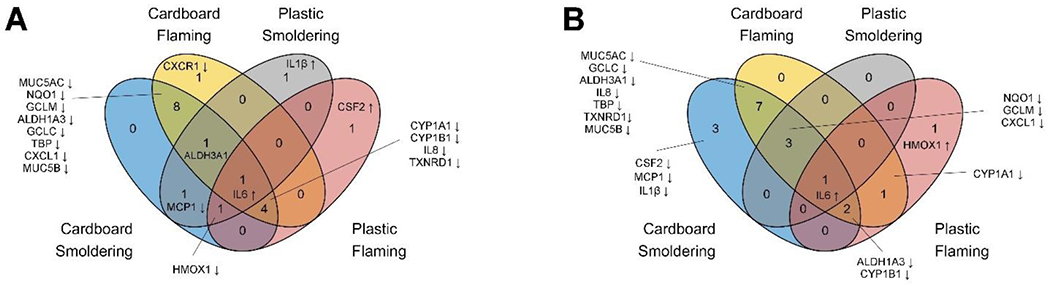
Venn diagram showing number of genes significantly altered (P Value < 0.1) by the smoke condensates compared to the respective controls, post 4- (A) and 24-hours (B) of exposure. Genes altered in cardboard and plastic waste smoke condensate exposure groups under smoldering and flaming combustion conditions are shown in the Venn diagram. The names of the genes are indicated in the figure with up- and downward directed arrows representing increase and decrease in expression level. ALDH3A1 was respectively decreased and increased in cardboard and plastic smoke exposure groups.
After 24-hours of exposure, seventeen genes were modulated by cardboard combining both smoldering and flaming conditions compared to eight genes modulated by plastic (Figure 6b). Thirteen genes altered between smoldering and flaming condensates include: ALDH1A3, ALDH3A1, CXCL1, GCLC, CYP1A1, CYP1B1, GCLM, IL6, IL8, MUC5B, NQO1, TBP and TXNRD1. In comparison, plastic smoke condensates caused significant alteration in eight genes: NQO1, CYP1A1, CYP1B1, GCLM, IL6, CXCL1, HMOX1 and ALDH1A3. Collectively, these Venn diagrams demonstrate that more genes were altered based on the source material rather than incineration temperature. In addition, there were more genes commonly altered based on condensate material rather than incineration temperature in HAECs. Interestingly, IL-6 expression was elevated in all exposure groups, irrespective of the source material or the temperature of incineration. When the number of genes altered at the lower (1 μg/cm2) and higher (25 μg/cm2) doses of each waste material were compared (table S3), more changes were noted at the higher dose, indicating a higher impact of smoke condensate exposure on HAEC gene expression pattern.
Smoke condensate-mediated effects on cytokine secretion from HAECs
The effect from burn pit smoke condensate exposure in vivo, especially on inflammatory response, was reported in our previous study where neutrophil infiltration, increase in interleukin-6 and macrophage inflammatory protein-2 levels, and decrease in breathing frequency were noted in mice15. To understand the inflammatory response of the HAECs from acute exposure to the burn pit smoke condensates, we collected the basolateral media following 24 hours of exposure to cardboard and plastic waste combustion smoke condensates and quantified changes in cytokine and chemokine levels.
Overall, seven cytokines were identified as significantly different (p value < 0.1) after exposure to burning cardboard based on statistical distribution analyses using Friedman’s tests (Table S4). When comparing control samples to smoldering cardboard samples, two cytokines, IL-13 and TNF-α at the low dose (1 μg/cm2) were significantly different based on Nemenyi’s post hoc tests. When comparing control samples to flaming cardboard samples, two cytokines (IL-10 and TNF-α) at the low dose and three cytokines (GM-CSF, IL-1β, and TNF-α) at the high dose (25 μg/cm2) were significantly modulated. There were no significant differences between smoldering and flaming samples. All altered cytokines were downregulated except for GM-CSF (Table S5).
Similarly, five cytokines were significantly different in HAECs exposed to burning plastic (Table S4). When comparing control samples to smoldering plastic samples, these included three cytokines (GM-CSF, IL-2, and TNF-α) at the low dose and two cytokines (IL-7 and TNF-α) at the high dose. There were no significant differences between control and flaming smoke condensates. When comparing smoldering samples to flaming plastic samples, one cytokine (IL-1β) was significantly different at a low dose. Significantly modulated cytokines, GM-CSF, IL-1β, and IL-7 were upregulated, while IL-2 and TNF-α were downregulated. (Table S5). Interestingly, TNF-α was downregulated in smoldering cardboard and plastic HAECs and GM-CSF was upregulated in lower doses of cardboard and plastic HAECs.
Cytokine secretion based on waste material and combustion condition
To elucidate potential clustering of samples based on condensate, combustion temperature, and exposure duration, PCA was performed. The analysis was able to capture most (97.7%) of the variance from the cytokine expression data within the first two eigenvectors, supporting the characterization of the clustering patterns using the two-dimensional plot. Although cardboard under flaming conditions appears to be separated from the rest of the samples, this figure shows that exposure to an incinerating waste material regardless of combustion temperature and duration had little impact on aggregate cytokine secretion in HAECs (Figure 7A). The muted cytokine expression responses were seen at the individual levels are reflected in the heat map. However, hierarchical clustering of these cytokines identified three groups of similarity. For the first group, all cytokines were downregulated following exposure to smoke condensates (Figure 7B). This group consisted of TNF-α, IL-10, IL-1β, IL-13, IL-6, TARC, IL-2, Eotaxin-3, and IP-10. The second cytokine cluster, which included Il-8, IL-15, and MDC, was upregulated following exposure to smoke condensates, except for flaming plastic. The third cytokine cluster had varying expression levels resulting from condensate smoke exposure and consisted of VEGF, IL-7, MCP1, GM-CSF and IL-1α (Figure 7B). Although hierarchical clustering of the cytokines revealed more differences across the exposure groups, they were not reflected at the aggregate-level or in the individual statistical analyses.
Figure 7. Clustering analyses of cytokine secretion by HAECs following burn pit smoke condensate exposure.
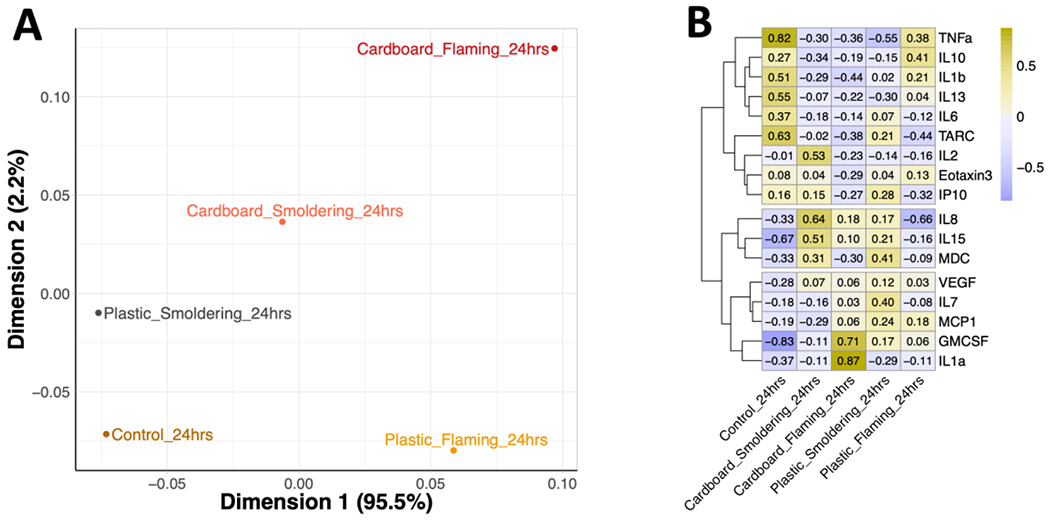
HAECs were treated with burn pit smoke condensates for 24 hours, and basolateral media was examined for cytokine levels. (A) Principal component analysis (PCA) of overall changes in basolateral cytokines secretion following burn pit smoke condensate exposure at the higher dose (25 μg/cm2) is shown. (B) Heatmap visualizing the scaled relative expression of individual cytokines at the higher dose (25 μg/cm2) for each exposure group. Hierarchical clustering of cytokines on the y axis was based on scaled expression levels. Condensates were generated from cardboard and plastic waste smoke, incinerated at smoldering and flaming conditions; n=6, ALI cultures from equal number of female and male donors were combined for this analysis.
Cytokine secretion from HAECs was influenced by PAHs content of the burn pit smoke condensates
We further explored any potential association between the cytokine levels in the basolateral media with the sixteen PAHs from the United States Environmental Protection Agency’s priority list (Figure 8). In our previous study we have identified these PAHs in the burn pit smoke condensates15. Spearman’s rank correlation analysis revealed strong negative correlation between the levels of IL-15, IP-10 and MDC with all sixteen PAHs, indicating a robust suppression of these cytokine secretions by HAECs. Apart from these, TARC level was also impacted by twelve PAHs. Interestingly, acenaphthene level in burn pit smoke condensates was positively correlated with IL-6 and IL-1β, and showed an opposite impact on Eotaxin-3. IL-1β was also positively correlated with naphthalene, fluorene and dibenzo[ah]anthracene levels. Positive correlation was also noted between TNF-α and dibenzo[ah]anthracene level.
Figure 8. Spearman’s rank correlation between cytokine levels and PAH concentrations.
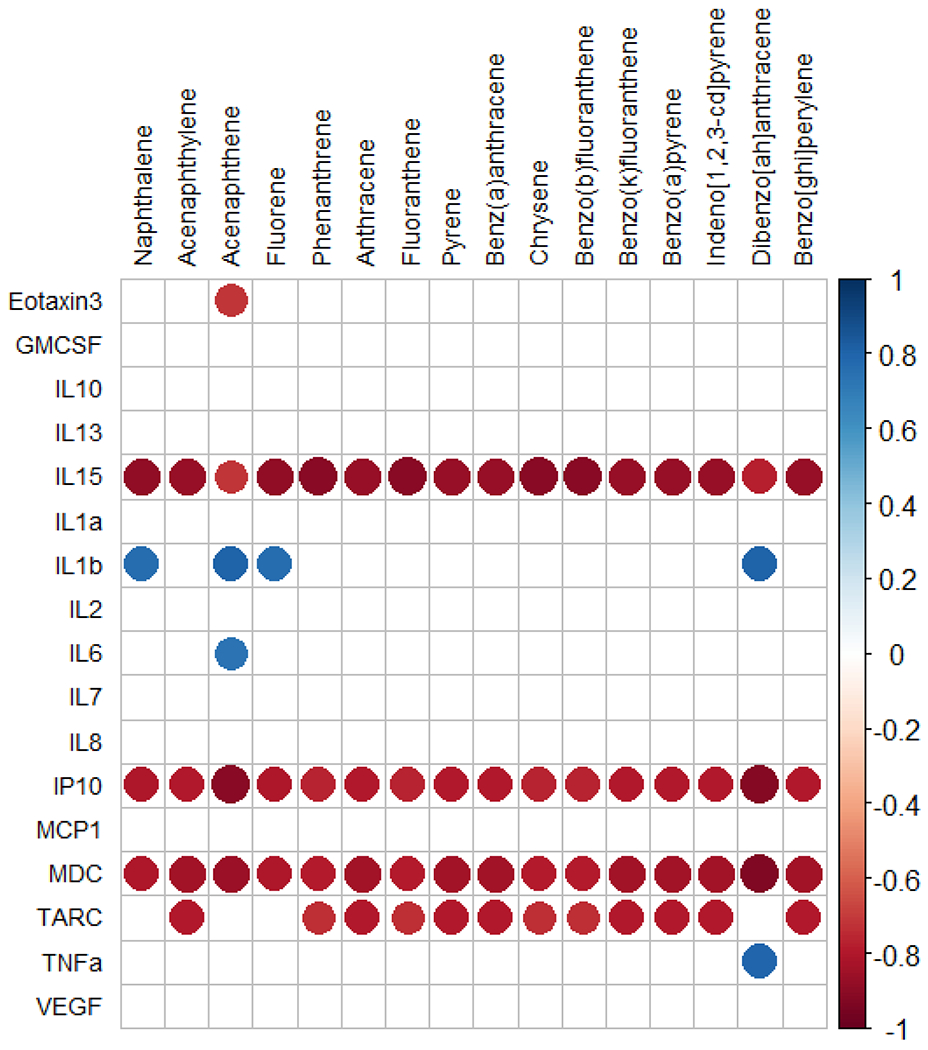
The cytokine levels in the basolateral media of HAECs were correlated with 16 PAHs present in the burn pit smoke condensates. Only significant associations (p<0.05) are shown. Positive and negative correlations are depicted as blue and red dots, respectively.
DISCUSSION
Airborne hazards derived from burn pit smoke pose a significant health risk to military personnel. The US Veterans Affairs Department now recognizes several respiratory morbidities associated with burn pit smoke exposure as deployment related diseases. While the military has taken measures to reduce burn pit exposure, veterans returning from deployment have reported respiratory symptoms, potentially from exposure to burn pit smoke. However, studies linking burn pit smoke with pulmonary toxicity are still lacking. Exposure to burn pit smoke can be high for personnel actively involved in maintaining the pit, and lower for other personnel on the base but further removed from the site. Hence, burn pit smoke exposure-mediated health implications are expected to derive from both acute and chronic exposure to low- and high-levels of toxic fumes and particulates. We have previously shown that exposures to condensates from burn pit smoke emissions cause inflammation and cytotoxicity in the lungs of mice 15, 48. In this study we explored the effect of burn pit smoke condensates on primary human airway epithelial cells (HAECs), to understand the effects on cellular targets in the human lung. We found that exposure to burn pit smoke condensate causes toxicity in airway epithelial cells, which differed depending on the type of waste and the combustion temperature. Exposures to condensates generated under flaming condition generated a higher overall TS. Of the two waste materials tested for inflammatory response and gene regulation, smoke from cardboard waste under both smoldering and flaming conditions had a greater impact on cytokine release and changes in gene expression associated with inflammation, metabolism, and cellular stress.
In our previous studies we demonstrated that compared to smoldering smoke, flaming smoke condensates caused intensified adverse effects on pulmonary health, likely linked to their more complex and condensed chemical composition. Flaming smoke condensate of plastic significantly increased neutrophil infiltration in lungs and increased macrophage inflammatory protein-2 in bronchoalveolar lavage fluid after 4- and 24-hours of exposure15. We further noted that the PAHs 9-nitroanthracene and fluorene are directly associated with the neutrophil infiltration within lungs. Similarly, we also noted that exposure to condensates from flaming conditions induced a greater TS. Flaming smoke condensates exhibited the highest TSs for all waste condensates, confirming that higher temperature and modified combustion efficiency of waste incineration results in more toxic components and exaggerated toxic effects. When the specific waste material is considered, mixed waste, diesel-containing mixed waste, and plastic dominated the higher TSs indicating smoke from incineration of these materials at a higher temperature may cause significant adverse pulmonary effects. In contrast, smoke condensates from smoldering conditions exhibited moderate to lower TSs in the hierarchy, suggesting that combustion temperature and type of burn pit fire affects adverse cellular effects.
Pulmonary responses to burn pit smoke condensates largely depend on the source of waste material and the incineration condition. Interestingly, both cytokine secretion and gene expression patterns noted for cardboard smoke condensates were distinctly different from plastic smoke condensates. We previously utilized oropharyngeal aspiration of burn pit smoke condensates in CD-1 mice to understand their impacts in vivo15. These analyses identified that flaming smoke condensate of cardboard is rich in PAHs, with the composition being different from that of plastic. Specifically, cardboard smoke condensate contains higher levels of benz[a]anthracene-7,12-quinone and inorganic elements15 including copper, and silicon which are closely related to protein and albumin leakage within lung. In a follow up study, we utilized a weighted gene co-expression network analysis (WGCNA) to understand the relationship between the chemical content of burn pit smoke condensates and respiratory implications48. When more than 50 chemicals were analyzed across eight smoke condensates, distinct chemical groups were identified which co-existed; flaming plastic smoke condensate was rich in high levels of PAHs and nitro-PAHs, whereas flaming plywood and cardboard smoke condensates were rich in inorganic elements48. Specifically, cardboard smoke condensate was rich in inorganic elements, including aluminum, calcium, chromium, copper, iron, potassium, magnesium, sodium, sulfur, silica and zinc15, 48. Our current observations suggest that IL-15, IP-10, MDC and TARC cytokines are negatively correlated with the PAHs present in the smoke condensates. Interestingly, acenaphthene is positively correlated with IL-6 and IL-1β, while negatively associated with eotaxin-3. Of note, acenaphthene has already been reported to increase IL-6 expression in a Zebrafish model49. Previous literature reported adverse effects of several inorganic elements on cellular health. For example, zinc and copper caused release of IL-6 from cardiomyocytes and cardiac fibroblasts at micromolar concentration50. Expression of MCP1 has been shown to be elevated by iron51 and silica52; of note, flaming cardboard caused upregulation of MCP1 within 4 hours of exposure. Hence, we hypothesize that the enhanced effects cardboard smoke condensate on inflammatory cytokine secretion in HAECs are driven by PAHs and inorganic elements that are not as abundant in plastic smoke condensates.
Data from the Millennium Cohort Study did not identify any direct relationship between burn pit exposure and adverse health implications, such as chronic “multisymptom illness”53 and newly reported lupus and rheumatoid arthritis54. Self-reported respiratory symptoms and newly reported asthma, chronic bronchitis and emphysema were initially not associated with burn pit exposure; however, increased reporting of symptoms from deployers residing close to the burn pit location has since changed that assumption55. Hence, studies on controlled exposure of burn pit smoke using in vitro models are necessary to establish clear cause-and-effect relationships and uncover potential waste components driving toxicity and adverse health effects. Well-differentiated HAECs cultured at the ALI present a relevant human in vitro model to understand the effects of these toxic smoke mixtures on airway epithelium. In our study, we observed a greater number of changes caused by cardboard smoke, but the impact was greater for plastic smoke in terms of inflammatory response and gene expression. Condensates from flaming cardboard smoke increased the gene expression of several pro-inflammatory mediators (IL6, MCP1, CXCR1, and CSF2). Of note, the importance of CSF2 in inflammation and autoimmunity is widely recognized because of its role in differentiation and proliferation of granulocytes and macrophages from progenitor cells56. Furthermore, vast literature is available on the critical role played by IL-6 in asthma and other inflammatory lung diseases57. In fact, Rivera et al reported that deployment increased the development of asthmatic symptoms in military personnel58. Elevated levels of cytokines play a determining role in the pathogenesis of lung diseases, including chronic obstructive pulmonary disease (COPD) 59, 60 and lung cancer61, 62. Hence, to summarize, veterans exposed to smoke from burn pit waste combustion may be susceptible to the development of pulmonary diseases.
Limitations
Our study has several limitations. First, although we included HAECs from male and female donors, our relatively small sample size prohibited the differentiation between sex-specific cellular effects from burn pit smoke condensate exposure. In addition, the limited sample size overall likely diminished the statistical power of the distribution analyses. Future studies will expand upon this design and specifically address sex as a biological variable in responses to these exposure conditions while having a more robust sample size. Second, we have utilized short-term acute exposure duration, which may not reflect a real-world scenario, and long-term repeated exposure may provide more insights into the health effects from these exposures. Third, our previous study indicated a wide range of particulate concentrations in the actual emissions depending on the waste material source and combustion conditions. When the mass of the waste is considered, plastic incineration at smoldering condition generated largest amount of PMs15, 16 . For our experiments, we normalized the exposures to particle concentration, which may intensify or underestimate the effects of specific burn pit smoke. Furthermore, the modulation of cytokine secretion and gene expression observed under these controlled experimental conditions may not reflect the real-world circumstances. In fact, exposure to particulate matter from different source materials at high concentrations may exhibit similar cellular responses and respiratory symptoms, yet the pulmonary and systemic health effects of burn pit smoke exposure will depend on several independent and combined factors. Lastly, some discrepancies were noted between the cytokine levels and the expression patterns of the corresponding gene. For example, IL-6 cytokine level in basolateral media was reduced in all exposure groups, yet expression of the corresponding gene was increased. MCP-1 level was elevated in HAECs exposed to plastic smoke condensates, however its gene expression was increased by both smoldering and flaming smoke condensates of cardboard at 24 hours of exposure. Similar differential regulation was also noted for IL-8 and GM-CSF too, suggesting complex mechanisms involved in burn pit smoke condensate-mediated effects. These discrepancies could stem from different optimal timepoints for assessing mRNA and protein levels of these cytokines and/or directionality of cytokine secretion, preferentially releasing cytokines towards the apical or basolateral compartment as we have described before63.
Conclusions
Observations from our current study indicate that burn pit waste smoke condensates cause toxicity in HAECs. The high toxicity corresponding to flaming smoke condensates confirms that exposure to chemicals originating from waste combustion at a high temperature may have more severe adverse effects on pulmonary health. Acute exposure to smoke condensate modulated the secretion of cytokines from HAECs depending on both source material and incineration temperature, with smoke generated from burning military-grade cardboard causing a significant pro-inflammatory response and smoke generated from plastic inducing significant cytotoxicity and suppression of stress response and metabolizing genes. Hence, our current observations from short-term exposure of smoke condensates indicate the need for more comprehensive studies on the pulmonary effects of burn pit smoke exposure. Using the current in vitro exposure model of HAECs, we are starting to understand and rank the potential toxicity of model emission mixtures of military burn pits. Future studies will further highlight other cellular effects of these smoke condensates on pulmonary health.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs. James Samet and Andrew Johnstone for their careful review of this manuscript. The research described in this article has been reviewed by the Center for Public Health and Environmental Assessment, U.S. Environmental Protection Agency and approved for publication. Approval does not signify that the contents necessarily reflect the views or the policies of the Agency nor does mention of trade names or commercial products constitute endorsement or recommendation for use. The graphical abstract of this manuscript was created with BioRender.com.
FUNDING INFORMATION
The study reported in this manuscript was funded by FY17 Peer Reviewed Medical Research Program (Award No. W81XWH-18-1-0731) of the United States Department of Defense (I.J.), intramural research program of the Office of Research and Development of the U.S. Environmental Protection Agency (M.I.G.), and a grant (R03ES032539) from National Institute of Environmental Health Sciences (Y.H.K.). The provision of cells and media for these studies was made possible by support of the Tissue Procurement and Cell Culture Core at the Marsico Lung institute by NIH grant DK065988 and Cystic Fibrosis Foundation grant BOUCHE19R0.
ABBREVIATIONS
- HAEC
human airway epithelial cells
- ALI
air-liquid interface
- PM
particulate material
- TEER
transepithelial electrical resistance
- LDH
lactate dehydrogenase
- PAHs
polyaromatic hydrocarbons
- PCA
principal component analysis
- TS
toxicity score
- AUC
area under curve
- COPD
chronic obstructive pulmonary disease
Footnotes
SUPPORTING INFORMATION
Demographics details of the primary cell donors (Table S1); Comparison of gene expression using Friedman’s tests (Table S2); Comparison of gene expression Nemenyi’s post hoc tests (Table S3); Comparison of cytokine expression using Friedman’s tests (Table S4); Comparison of cytokine expression Nemenyi’s post hoc tests (Table S5).
REFERENCES
- 1.Wiedinmyer C; Yokelson RJ; Gullett BK, Global emissions of trace gases, particulate matter, and hazardous air pollutants from open burning of domestic waste. Environ Sci Technol 2014, 48 (16), 9523–30. [DOI] [PubMed] [Google Scholar]
- 2.Davis CW; Rabin AS; Jani N; Osterholzer JJ; Krefft S; Hines SE; Arjomandi M; Robertson MW; Sotolongo AM; Falvo MJ; Post-Deployment Cardiopulmonary Evaluation, N., Postdeployment Respiratory Health: The Roles of the Airborne Hazards and Open Burn Pit Registry and the Post-Deployment Cardiopulmonary Evaluation Network. Fed Pract 2022, 39 (8), 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woskie SR; Bello A; Rennix C; Jiang L; Trivedi AN; Savitz DA, Burn Pit Exposure Assessment to Support a Cohort Study of US Veterans of the Wars in Iraq and Afghanistan. J Occup Environ Med 2023, 65 (6), 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berman R; Rose CS; Downey GP; Day BJ; Chu HW, Role of Particulate Matter from Afghanistan and Iraq in Deployment-Related Lung Disease. Chem Res Toxicol 2021, 34 (12), 2408–2423. [DOI] [PubMed] [Google Scholar]
- 5.Falvo MJ; Sotolongo AM; Osterholzer JJ; Robertson MW; Kazerooni EA; Amorosa JK; Garshick E; Jones KD; Galvin JR; Kreiss K; Hines SE; Franks TJ; Miller RF; Rose CS; Arjomandi M; Krefft SD; Morris MJ; Polosukhin VV; Blanc PD; D’Armiento JM, Consensus Statements on Deployment-Related Respiratory Disease, Inclusive of Constrictive Bronchiolitis: A Modified Delphi Study. Chest 2023, 163 (3), 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garshick E; Abraham JH; Baird CP; Ciminera P; Downey GP; Falvo MJ; Hart JE; Jackson DA; Jerrett M; Kuschner W; Helmer DA; Jones KD; Krefft SD; Mallon T; Miller RF; Morris MJ; Proctor SP; Redlich CA; Rose CS; Rull RP; Saers J; Schneiderman AI; Smith NL; Yiallouros P; Blanc PD, Respiratory Health after Military Service in Southwest Asia and Afghanistan. An Official American Thoracic Society Workshop Report. Ann Am Thorac Soc 2019, 16 (8), e1–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garshick E; Blanc PD, Military deployment-related respiratory problems: an update. Curr Opin Pulm Med 2023, 29 (2), 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King MS; Eisenberg R; Newman JH; Tolle JJ; Harrell FE Jr.; Nian H; Ninan M; Lambright ES; Sheller JR; Johnson JE; Miller RF, Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan. N Engl J Med 2011, 365 (3), 222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris MJ; Dodson DW; Lucero PF; Haislip GD; Gallup RA; Nicholson KL; Zacher LL, Study of active duty military for pulmonary disease related to environmental deployment exposures (STAMPEDE). Am J Respir Crit Care Med 2014, 190 (1), 77–84. [DOI] [PubMed] [Google Scholar]
- 10.Morris MJ; Rawlins FA; Forbes DA; Skabelund AJ; Lucero PF, Deployment-related Respiratory Issues. US Army Med Dep J 2016, (2-16), 173–8. [PubMed] [Google Scholar]
- 11.Liu J; Lezama N; Gasper J; Kawata J; Morley S; Helmer D; Ciminera P, Burn Pit Emissions Exposure and Respiratory and Cardiovascular Conditions Among Airborne Hazards and Open Burn Pit Registry Participants. J Occup Environ Med 2016, 58 (7), e249–55. [DOI] [PubMed] [Google Scholar]
- 12.Sharkey JM; Abraham JH; Clark LL; Rohrbeck P; Ludwig SL; Hu Z; Baird CP, Postdeployment Respiratory Health Care Encounters Following Deployment to Kabul, Afghanistan: A Retrospective Cohort Study. Mil Med 2016, 181 (3), 265–71. [DOI] [PubMed] [Google Scholar]
- 13.Furlow B, US Congress considers new health-care bill for burn pit-exposed veterans. Lancet Oncol 2022, 23 (3), e102. [DOI] [PubMed] [Google Scholar]
- 14.Wang X; Doherty TA; James C, Military burn pit exposure and airway disease: Implications for our Veteran population. Ann Allergy Asthma Immunol 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YH; Warren SH; Kooter I; Williams WC; George IJ; Vance SA; Hays MD; Higuchi MA; Gavett SH; DeMarini DM; Jaspers I; Gilmour MI, Chemistry, lung toxicity and mutagenicity of burn pit smoke-related particulate matter. Part Fibre Toxicol 2021, 18 (1), 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vance SA; Kim YH; George IJ; Dye JA; Williams WC; Schladweiler MJ; Gilmour MI; Jaspers I; Gavett SH, Contributions of particulate and gas phases of simulated burn pit smoke exposures to impairment of respiratory function. Inhal Toxicol 2023, 35 (5-6), 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rezaee F; Georas SN, Breaking barriers. New insights into airway epithelial barrier function in health and disease. Am J Respir Cell Mol Biol 2014, 50 (5), 857–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiemstra PS; Tetley TD; Janes SM, Airway and alveolar epithelial cells in culture. Eur Respir J 2019, 54 (5). [DOI] [PubMed] [Google Scholar]
- 19.Schulz C; Wolf K; Harth M; Kratzel K; Kunz-Schughart L; Pfeifer M, Expression and release of interleukin-8 by human bronchial epithelial cells from patients with chronic obstructive pulmonary disease, smokers, and never-smokers. Respiration 2003, 70 (3), 254–61. [DOI] [PubMed] [Google Scholar]
- 20.Stewart CE; Torr EE; Mohd Jamili NH; Bosquillon C; Sayers I, Evaluation of differentiated human bronchial epithelial cell culture systems for asthma research. J Allergy (Cairo) 2012, 2012, 943982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hellings PW; Steelant B, Epithelial barriers in allergy and asthma. J Allergy Clin Immunol 2020, 145 (6), 1499–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghosh A; Abdelwahab SH; Reeber SL; Reidel B; Marklew AJ; Garrison AJ; Lee S; Dang H; Herring AH; Glish GL; Kesimer M; Tarran R, Little Cigars are More Toxic than Cigarettes and Uniquely Change the Airway Gene and Protein Expression. Sci Rep 2017, 7, 46239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fulcher ML; Randell SH, Human nasal and tracheo-bronchial respiratory epithelial cell culture. Methods Mol Biol 2013, 945, 109–21. [DOI] [PubMed] [Google Scholar]
- 24.Fulcher ML; Gabriel S; Burns KA; Yankaskas JR; Randell SH, Well-differentiated human airway epithelial cell cultures. Methods Mol Med 2005, 107, 183–206. [DOI] [PubMed] [Google Scholar]
- 25.Kim YH; Warren SH; Krantz QT; King C; Jaskot R; Preston WT; George BJ; Hays MD; Landis MS; Higuchi M; DeMarini DM; Gilmour MI, Mutagenicity and Lung Toxicity of Smoldering vs. Flaming Emissions from Various Biomass Fuels: Implications for Health Effects from Wildland Fires. Environ Health Perspect 2018, 126 (1), 017011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aurell J; Barnes M; Gullett BK; Holder A; Eninger R, Methodology for characterizing emissions from small (0.5–2 MTD) batch-fed gasification systems using multiple waste compositions. Waste Manag 2019, 87, 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urbanski S, Wildland fire emissions, carbon, and climate: Emission factors. Forest Ecology and Management 2014, 317, 51–60. [Google Scholar]
- 28.BMDExpress 3.20 software. https://github.com/auerbachs/BMDExpress-3/releases/tag/3.20.
- 29.Brocke SA; Billings GT; Taft-Benz S; Alexis NE; Heise MT; Jaspers I, Woodsmoke particle exposure prior to SARS-CoV-2 infection alters antiviral response gene expression in human nasal epithelial cells in a sex-dependent manner. Am J Physiol Lung Cell Mol Physiol 2022, 322 (3), L479–L494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.VSN package of Bioconductor. https://bioconductor.org/packages/release/bioc/html/vsn.html.
- 31.imputeLCMD package. https://www.rdocumentation.org/packages/imputeLCMD/versions/2.0/topics/impute.QRILC.
- 32.PMCMRplus: Calculate Pairwise Multiple Comparisons of Mean Rank Sums. https://cran.r-project.org/web/packages/PMCMRplus/PMCMRplus.pdf.
- 33.qvalue package of Bioconductor. https://www.bioconductor.org/packages/release/bioc/html/qvalue.html.
- 34.Yan L; Yan ML, Package ‘ggvenn’. 2023.
- 35.Payton AD; Perryman AN; Hoffman JR; Avula V; Wells H; Robinette C; Alexis NE; Jaspers I; Rager JE; Rebuli ME, Cytokine signature clusters as a tool to compare changes associated with tobacco product use in upper and lower airway samples. Am J Physiol Lung Cell Mol Physiol 2022, 322 (5), L722–L736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Payton A; Roell KR; Rebuli ME; Valdar W; Jaspers I; Rager JE, Navigating the bridge between wet and dry lab toxicology research to address current challenges with high-dimensional data. Front Toxicol 2023, 5, 1171175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furihata C; Watanabe T; Suzuki T; Hamada S; Nakajima M, Collaborative studies in toxicogenomics in rodent liver in JEMS. MMS; a useful application of principal component analysis on toxicogenomics. Genes Environ 2016, 38, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kassambara A. J. R. p. v., Factoextra: extract and visualize the results of multivariate data analyses. 2016, 1. [Google Scholar]
- 39.Kolde R, pheatmap: Pretty Heatmaps. Implementation of heatmaps that offers more control over dimensions and appearance. 2015.
- 40.Hickman E; Smyth T; Cobos-Uribe C; Immormino R; Rebuli ME; Moran T; Alexis NE; Jaspers I, Expanded characterization of in vitro polarized M0, M1, and M2 human monocyte-derived macrophages: Bioenergetic and secreted mediator profiles. PLoS One 2023, 18 (3), e0279037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hickman E; Payton A; Duffney P; Wells H; Ceppe AS; Brocke S; Bailey A; Rebuli ME; Robinette C; Ring B; Rager JE; Alexis NE; Jaspers I, Biomarkers of Airway Immune Homeostasis Differ Significantly with Generation of E-Cigarettes. Am J Respir Crit Care Med 2022, 206 (10), 1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.psych package v2.4.1. https://rdocumentation.org/packages/psych/versions/2.4.1.
- 43.Perryman AN; Kim HH; Payton A; Rager JE; McNell EE; Rebuli ME; Wells H; Almond M; Antinori J; Alexis NE; Porter NA; Jaspers I, Plasma sterols and vitamin D are correlates and predictors of ozone-induced inflammation in the lung: A pilot study. PLoS One 2023, 18 (5), e0285721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.corrplot package. https://rdocumentation.org/packages/corrplot/versions/0.92.
- 45.UNC-CEMALB Dataverse. https://dataverse.unc.edu/privateurl.xhtml?token=14ccc245-37e7-492a-b75a-2fe416e12f08.
- 46.UNC-CEMALB Github. https://github.com/UNC-CEMALB/P1011_Emission-Mixtures.
- 47.Srinivasan B; Kolli AR; Esch MB; Abaci HE; Shuler ML; Hickman JJ, TEER measurement techniques for in vitro barrier model systems. J Lab Autom 2015, 20 (2), 107–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim YH; Rager JE; Jaspers I; Gilmour MI, Computational Approach to Link Chemicals in Anthropogenic Smoke Particulate Matter with Toxicity. Chem Res Toxicol 2022, 35 (12), 2210–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang J; Wang K; Guo J; Huang Y; Wei Y; Jia K; Peng Y; Lu H, Study on the mechanism of liver toxicity induced by acenaphthene in zebrafish. Ecotoxicol Environ Saf 2023, 249, 114441. [DOI] [PubMed] [Google Scholar]
- 50.Ansteinsson V; Refsnes M; Skomedal T; Osnes JB; Schiander I; Lag M, Zinc- and copper-induced interleukin-6 release in primary cell cultures from rat heart. Cardiovasc Toxicol 2009, 9 (2), 86–94. [DOI] [PubMed] [Google Scholar]
- 51.Mitchell RM; Lee SY; Randazzo WT; Simmons Z; Connor JR, Influence of HFE variants and cellular iron on monocyte chemoattractant protein-1. J Neuroinflammation 2009, 6, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barrett EG; Johnston C; Oberdorster G; Finkelstein JN, Silica-induced chemokine expression in alveolar type II cells is mediated by TNF-alpha-induced oxidant stress. Am J Physiol 1999, 276 (6), L979–88. [DOI] [PubMed] [Google Scholar]
- 53.Powell TM; Smith TC; Jacobson IG; Boyko EJ; Hooper TI; Gackstetter GD; Phillips CJ; Smith B; Millennium Cohort Study, T., Prospective assessment of chronic multisymptom illness reporting possibly associated with open-air burn pit smoke exposure in Iraq. J Occup Environ Med 2012, 54 (6), 682–8. [DOI] [PubMed] [Google Scholar]
- 54.Jones KA; Smith B; Granado NS; Boyko EJ; Gackstetter GD; Ryan MA; Phillips CJ; Smith TC; Millennium Cohort Study, T., Newly reported lupus and rheumatoid arthritis in relation to deployment within proximity to a documented open-air burn pit in Iraq. J Occup Environ Med 2012, 54 (6), 698–707. [DOI] [PubMed] [Google Scholar]
- 55.Smith B; Wong CA; Boyko EJ; Phillips CJ; Gackstetter GD; Ryan MA; Smith TC; Millennium Cohort Study, T., The effects of exposure to documented open-air burn pits on respiratory health among deployers of the Millennium Cohort Study. J Occup Environ Med 2012, 54 (6), 708–16. [DOI] [PubMed] [Google Scholar]
- 56.Hamilton JA, GM-CSF in inflammation. J Exp Med 2020, 217 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rincon M; Irvin CG, Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int J Biol Sci 2012, 8 (9), 1281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rivera AC; Powell TM; Boyko EJ; Lee RU; Faix DJ; Luxton DD; Rull RP; Millennium Cohort Study, T., New-Onset Asthma and Combat Deployment: Findings From the Millennium Cohort Study. Am J Epidemiol 2018, 187 (10), 2136–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barnes PJ, The cytokine network in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2009, 41 (6), 631–8. [DOI] [PubMed] [Google Scholar]
- 60.Chung KF, Cytokines in chronic obstructive pulmonary disease. Eur Respir J Suppl 2001, 34, 50s–59s. [PubMed] [Google Scholar]
- 61.Liu Q; Li A; Tian Y; Wu JD; Liu Y; Li T; Chen Y; Han X; Wu K, The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev 2016, 31, 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheng ZH; Shi YX; Yuan M; Xiong D; Zheng JH; Zhang ZY, Chemokines and their receptors in lung cancer progression and metastasis. J Zhejiang Univ Sci B 2016, 17 (5), 342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jaspers I; Flescher E; Chen LC, Respiratory epithelial cells display polarity in their release of the chemokine IL-8 after exposure to ozone. Inflamm Res 1997, 46 Suppl 2, S173–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data have been deposited within the University of North Carolina -Center for Environmental Medicine, Asthma, and Lung Biology Dataverse 45. All script used in this manuscript are publicly available through the University of North Carolina -Center for Environmental Medicine, Asthma, and Lung Biology Github46.


