Abstract
BACKGROUND AND OBJECTIVES:
Infant weight patterns predict subsequent weight outcomes. Rapid infant weight gain, defined as a >0.67 increase in weight-for-age z-score (WAZ) between two time points in infancy, increases obesity risk. Higher oxidative stress, an imbalance between antioxidants and reactive oxygen species, has been associated with low birthweight and paradoxically also with later obesity. We hypothesized that prenatal oxidative stress may also be associated with rapid infant weight gain, an early weight pattern associated with future obesity.
METHODS:
Within the NYU Children’s Health and Environment Study prospective pregnancy cohort, we analyzed associations between prenatal lipid, protein, and DNA urinary oxidative stress biomarkers and infant weight data. Primary outcome was rapid infant weight gain (>0.67 increase in WAZ) between birth and later infancy at the 8 or 12 month visit. Secondary outcomes included: very rapid weight gain (>1.34 increase in WAZ), low (<2500 g) or high (≥4000 g) birthweight, and low (< −1 WAZ) or high (>1 WAZ) 12 month weight.
RESULTS:
Pregnant participants consented to the postnatal study (n = 541); 425 participants had weight data both at birth and in later infancy. In an adjusted binary model, prenatal 8-iso-PGF2α, a lipid oxidative stress biomarker, was associated with rapid infant weight gain (aOR 1.44; 95% CI: 1.16, 1.78, p = 0.001). In a multinomial model using ≤0.67 change in WAZ as a reference group, 8-iso-PGF2α was associated with rapid infant weight gain (defined as >0.67 but ≤1.34 WAZ; aOR 1.57, 95% CI: 1.19, 2.05, p = 0.001) and very rapid infant weight gain (defined as >1.34 WAZ; aOR 1.33; 95% CI: 1.02, 1.72, p < 0.05) Secondary analyses detected associations between 8-iso-PGF2α and low birthweight outcomes.
CONCLUSIONS:
We found an association between 8-iso-PGF2α, a lipid prenatal oxidative stress biomarker, and rapid infant weight gain, expanding our understanding of the developmental origins of obesity and cardiometabolic disease.
INTRODUCTION
Infant weight and growth patterns predict obesity risk across the life course [1]. Rapid infant weight gain is defined as a greater than a 0.67 increase in weight-for-age z-score between two time points in infancy [2]. This growth pattern is common among infants born with low birthweight, and has an overall cited prevalence of up to 30% of all infants from birth to 2 years regardless of birthweight status [3, 4]. Scientists have found that rapid infant weight gain, most notably in the first year of life, increases odds of later obesity by two-to-threefold as well as lifetime cardiometabolic risk [2, 5, 6]. Evidence shows that psychosocial stressors and toxic environmental exposures during pregnancy increase odds of rapid infant weight gain [7], reinforcing the sensitivity of the prenatal period to cardiometabolic programming across the life course. Understanding whether prenatal biomarkers associated with later obesity are also associated with rapid infant weight gain would contribute to scientific efforts to elucidate the developmental biology of early obesity risk.
Oxidative stress occurs when levels of damaging free radicals exceed the balance of protective antioxidants [8]. Excess prenatal oxidative stress has been associated with small for gestational age and low birthweight outcomes [9, 10]. Paradoxically, later in life, oxidative stress is tightly associated with future obesity; scientists refer to adult obesity as “a state of chronic oxidative stress” [11]. Given that oxidative stress is associated with low weight at the beginning of life but with obesity later in life, it may have relationships with early growth patterns known to increase obesity risk. Researchers modeling weight trajectories in childhood have already shown that increased oxidative stress is associated with a “low-high” weight trajectory across multiple years of childhood [12]. Whether prenatal oxidative stress is also associated with rapid infant weight gain, a “low-high” pattern in infancy known to be associated with later obesity, remains underexplored.
Prenatal oxidative stress can be detected in the urine with biomarkers, which are lipid, protein, and DNA molecules that respond to increased stress and are modified by reactive oxygen species in vivo [13, 14]. Lipid biomarkers, generated by the oxidation of polyunsaturated fatty acids, measure lipid peroxidation and adipose inflammation common in obesity [11]. The lipid biomarker 8-iso-prostaglandin F2α (8-iso-PGF2α) has been established as the “gold standard” biomarker of oxidative stress, and is the subject of over 200 publications [12, 14, 15]. Protein biomarkers, generated by the oxidation of protein side chains, are prominent in type 2 diabetes pathology and gestational diabetes, and they signal inactivation of proteins that protect from atherosclerosis [16, 17]. DNA biomarkers, generated by oxidation of DNA bases like guanosine, indicate cellular damage in highly progressed disease, and has been implicated in cancerous and genetic outcomes [14]. Less is known about whether the developmental origins of rapid infant weight gain involve oxidative damage to lipid, protein, or DNA molecules [13, 18].
To close these gaps in understanding, we assessed associations between prenatal oxidative stress in pregnant participants and rapid infant weight gain in their infants in the New York University Children’s Health and Environment Study (NYU CHES), a longitudinal birth cohort with racial, ethnic, and socioeconomic diversity in New York City [19]. Leveraging lipid, protein, and DNA oxidative stress measured in prenatal urine samples, we hypothesized that higher levels of the lipid biomarker 8-iso-PGF2α would be associated with rapid infant weight gain.
METHODS
Study population
NYU CHES is a prospective pregnancy cohort examining the impact of environmental exposures on children’s health and development [19]. Between March 2016 and April 2019, NYU CHES recruited 2000 participants who were 18 years and older, less than 18 weeks pregnant, and planned to deliver at one of 3 NYU-affiliated hospitals: NYU Langone Hospital—Manhattan, serving a higher income population; NYU Langone―Brooklyn, serving a primarily immigrant community; and Bellevue Hospital, a public hospital serving populations with economic disadvantage. Participants were excluded if their pregnancy was medically threatened. All participants provided written informed consent, and the Institutional Review Board of the NYU Grossman School of Medicine approved this study.
Of those recruited, 1120 participants provided urine samples at prenatal study visits. The study randomly selected 680 participants to measure oxidative stress biomarkers in their urine (Supplementary Fig. 1). Of the 680 participants selected, 674 had live births, and 541 consented to the postnatal phase of the study. To calculate rapid infant weight gain, we needed one infant weight data point at birth (n = 541) and a second weight data point at either the 8 month (n = 352) or at 12 month (n = 318) visit. Our analytic sample included 425 mother-infant pairs with the requisite weight data, comprising 78% of the 541 infants who consented to the postnatal study. This sample would have adequate power as per effect sizes detected in prior studies of oxidative stress biomarkers and weight outcomes [12, 20, 21]. We confirmed that participants included in this analysis were comparable with all NYU CHES participants: similar racial sampling (~50% Hispanic, ~30% White), rate of public insurance (~50%), marital status (~10% single), and maternal age (mean of 31 years old) [19]. Using bivariate analyses of sociodemographics, we also confirmed that those followed in the postnatal study reflected those in the prenatal study.
Independent variable: prenatal oxidative stress biomarkers
Pregnant participants provided spot urine samples during prenatal visits in early (<18 weeks), mid (18–25 weeks), and late (>25 weeks) pregnancy. Mean gestational ages at urine collection were 10.8 (standard deviation [SD] = 3.4), 20.8 (SD = 2.2), and 29.3 (SD = 3.6) weeks. Each sample was aliquoted into polyethylene containers and stored in −80 °C refrigerators. Physiological fluctuations can cause variation in biomarker measurement within and between participants, so we analyzed intraclass correlation coefficients to describe how strongly the three values across pregnancy resembled one other. Given that the intraclass correlation coefficients of our biomarkers were of moderate size (0.43–0.66), we averaged the values from three urine samples across pregnancy to define oxidative stress levels with more precision than using a single urine sample [22].
By using high performance liquid chromatography coupled with tandem mass spectrometry, our team measured lipid, protein, and DNA biomarkers in urine samples at the Wadsworth Laboratory at the New York State Department of Health. Lipid biomarkers included F2-isoprostanes, formed from the peroxidation of arachidonic acid (a specific polyunsaturated fatty acid) and Malondialdehyde [MDA], an abundant aldehyde formed from the peroxidation of polyunsaturated fatty acids generally [14]. Four bioactive F2-isoprostanes were measured (8-iso-prostaglandin F2α [8-iso-PGF2α], 11β − prostaglandin F2α [11 − PGF2α], 15(R) − prostaglandin F2α [15 − PGF2α], 8 − iso,15(R) − prostaglandin F2α [8,15 − PGF2α]). Of these, 8-iso-PGF2α is noteworthy for its stability, specificity, and reproducibility in response to pro-oxidant stimulation [23]. The protein biomarker, o,o’−dityrosine [diY], represents oxidated amino acids (tyrosine) and the DNA biomarker 8 − hydroxy − 2’−deoxyguanosine[8 − OHdG] is an oxidated purine nucleoside (guanosine) [14].
Our laboratory participated in multiple external quality assurance evaluations and procedures, including the Centers for Disease Control’s Biomonitoring Quality Assurance Support Program [24]. The limits of detection (LOD) of our biomarker measurements met acceptable values also described in the literature [14]. We applied standard approaches to address samples with biomarker measurements below LOD, by imputing the LOD value of that oxidative stress biomarker divided by the square root of 2 [25]. The percent below LOD for each of the biomarkers included: 8-iso-PGF2α - 22.8%, 11 − PGF2α- 27.7%, 15 − PGF2α – 19.6%, 8,15 − PGF2α−6.9%, MDA – 0%, diY- 0%, 8−OHdG- 0%. Per established methods, urinary levels were adjusted for creatinine using the following equation: 1000*(urinary level of biomarker/molar weight of biomarker)*(molar weight of creatinine/urinary levels of creatinine) [26, 27]. Oxidative stress biomarker concentrations were skewed, so our primary analyses were log transformed for improved model fit as done in prior studies [12]. To improve interpretability, we also calculated effect estimates per standard deviation increase in biomarker concentration.
Infant weights
Prenatal oxidative stress biomarkers from pregnant participants were linked to the weight data of the infants they birthed. Information on birthweight and infant sex was obtained from the electronic health record. Weight at the 8 month visit was collected using a questionnaire where parents were asked to recall the weight from the preceding child well visit. Parents were first asked, “What was the date of your baby’s most recent well-child visit or checkup?” followed by, “What was his/her weight at that visit?” Weight at the 12 month visit was obtained by study team members at an in-person study visit. A trained examiner measured the child’s weight to the nearest 10 g using a calibrated infant scale. The mean of 3 weight measurements was used for the 12 month weight.
Rapid infant weight gain
Sex- and gestational age-specific z-scores for birthweight were calculated from the International Fetal and Newborn Growth Consortium for the 21st Century standard [28]. Sex-specific z-scores were calculated using World Health Organization growth charts and by the child’s precise age in months at the visit [29]. At both the 8 and 12 month visit, we measured change in weight-for-age z-score from birth. Our dichotomous primary outcome was rapid infant weight gain, defined as greater than 0.67 unit increase in weight-for-age z-score, which has been replicated in multiple studies to be associated with later obesity [2, 5, 30]. We also conducted analyses with very rapid infant weight gain – defined as >1.34 unit increase in weight-for-age z-score – which confers a particularly high risk of overweight and obesity [31].
Infant weight outcomes
Our secondary outcomes included birthweight and infant weight at 12 months. We defined low birthweight as less than 2500 g per World Health Organization standards [32] and high birthweight using the macrosomia definition of greater than 4000 g [33]. At the 12 month study visit, we defined high weight as greater than one z-score (>1 SD above the population mean), and low weight as less than negative one z-score (<1 SD below the population mean) [34].
Other characteristics
Sociodemographic factors like race/ethnicity, marital status, maternal age at enrollment, parity, and insurance type were collected by prenatal questionnaires and from electronic health records. Pre-pregnancy weight and height from electronic health records were used to calculate pre- pregnancy body mass index (BMI).
Statistical analysis
We used descriptive statistics to summarize baseline characteristics, prenatal oxidative stress biomarkers, and weight outcomes. Using independent sample unadjusted t-tests, we described baseline characteristics and oxidative stress biomarkers by rapid infant weight gain status. Binary adjusted logistic regressions were used to examine whether lipid, protein, and DNA oxidative stress biomarkers were associated with rapid infant weight gain. We selected confounders a priori associated with our predictor and outcome, referencing prior work on early child obesity and oxidative stress [12]. Models were adjusted for maternal pre-pregnancy BMI, maternal race/ethnicity, insurance status (public vs. private), marital status (single vs. married or living as married), maternal age at enrollment, parity (nulliparous yes vs. no), child sex, and child age at visit. As per prior work on oxidative stress and early child weights, gestational age at birth was considered likely to be on the causal pathway and not included as a covariate [12]. We adjusted for multiple comparisons accounting for lipid, protein, and DNA biomarkers by using a conservative Bonferroni correction and a p-value cutoff of 0.017 [35]. We also conducted two additional sensitivity analyses, one restricting our sample to only include weights collected at the 12 month study visit (n = 318) and another to only include infants with normal birthweight (n = 356).
For oxidative stress biomarkers associated with rapid infant weight gain at the Bonferroni-corrected threshold of significance, we used multinomial logistic regressions to examine whether significant oxidative stress biomarkers were associated with rapid (defined in this model here as an increase in weight-for-age z-score >0.67 but <1.34) and very rapid infant weight gain (defined as an increase in weight-for-age z-score >1.34), using ≤0.67 change in weight-for-age z-score as a reference group (3 categories). For our secondary outcomes, we used multinomial logistic regressions to examine whether significant oxidative stress biomarkers were associated with birthweight (low/high) extracted from the electronic medical record or weight outcomes (low/high) from the 12 month study weights, using weight within 1 standard deviation as a reference group (3 categories). Finally, we explored effect modification by examining subgroups within key sociodemographic variables: sex (male vs. female), race and ethnicity (membership in a racial or ethnic minority group vs. White), insurance status (public vs. private). We created models with interaction terms between the oxidative stress biomarker and the sociodemographic variable and examined stratified models. We used Stata/SE version 15.1 (Stata Corp, College Station, TX) to perform statistical analyses.
RESULTS
Our final sample included 425 mother-child infant dyads (Table 1). This was a diverse sample as 54.5% of mothers identified as Hispanic, 9.0% as non-Hispanic Asian, and 5.1% as non-Hispanic Black. Over half of families (56.5%) were on public insurance. Mean gestational age was full-term (39.1 weeks, SD = 1.8) and mean birthweight was in the healthy range (3.3 kg, SD = 1.8). Table 1 shows that in unadjusted analyses, mothers whose infants had rapid infant weight gain differed by race (more likely to identify as Hispanic or Black), public insurance status (more likely to be on public insurance), recruitment site (more likely to be from Bellevue Hospital), gestational age (earlier gestational age), and birthweight (lower birthweight). In a population of mothers with a mean pre- pregnancy BMI in the overweight range (26.5, SD = 5.9), infants had a median weight-for-age z-score of 0.5 (69.2nd percentile) at the 12 month visit (Table 2), and 39.2% of infants had rapid infant weight gain at either the 8 month or the 12 month visit.
Table 1.
Sample characteristics.
| Variable, n (%) or Mean (SD) | Total (N = 425) | Rapid Infant Weight Gaina | |
|---|---|---|---|
| No (n = 258) | Yes (n = 167)b | ||
| Maternal Characteristics | |||
| Maternal age (years) | 31.9 (5.6) | 32.2 (5.1) | 31.5 (6.2) |
| Pre-pregnancy BMIc | 26.5 (5.9) | 26.2 (6.0) | 27.0 (5.8) |
| Nulliparous | 195 (46.1) | 122 (47.3) | 93 (55.7) |
| Gestational Diabetes | 76 (17.9) | 48 (18.6) | 28 (16.8) |
| Race/Ethnicityc | |||
| Hispanic | 234 (55.1) | 122 (47.3)*** | 112 (67.1)*** |
| White (non-Hispanic) | 128 (30.1) | 101 (39.2)*** | 27 (16.2)*** |
| Black (non-Hispanic) | 18 (4.2) | 6 (2.3)* | 12 (7.2)* |
| Asian (non-Hispanic) | 34 (8.0) | 21 (8.1) | 13 (7.8) |
| Multirace/Other | 11 (2.6) | 5 (3.1) | 3 (1.8) |
| Public Insurance | 238 (56.0) | 129 (50.0)** | 109 (65.3)** |
| Marital Statusc | |||
| Married/Living as Married | 382 (89.9) | 233 (90.3) | 159 (89.2) |
| Divorced/Separated | 5 (1.2) | 2 (0.8) | 3 (1.8) |
| Single/Widowed | 38 (8.9) | 8.9 (23) | 15 (9.0) |
| Recruitment Site | |||
| Bellevue Hospital | 117 (27.5) | 56 (21.7)*** | 61 (36.5)*** |
| NYU Manhattan Hospital | 183 (43.1) | 132 (51.2)*** | 55 (30.5)*** |
| NYU Brooklyn Hospital | 125 (29.4) | 70 (27.1) | 51 (32.9) |
| Child Birth Characteristics | |||
| Assigned female sex | 210 (49.4) | 127 (49.2) | 83 (49.7) |
| Gestational age at birth | 39.1 (1.8) | 39.6 (1.2)*** | 38.3 (2.2)*** |
| Birthweight (grams) | 3277.4 (544.2) | 3464.7 (437.1)*** | 2988.1 (567.4)*** |
| Low birthweight (<2500 g) | 36 (8.5) | 5 (2.0)*** | 31 (18.6)*** |
| High birthweight (≥4000 g) | 31 (7.3) | 26 (10.2)** | 5 (3.0)** |
| Birthweight weight for age z-score | −0.02 (1.2) | 0.38 (0.87)*** | −0.66 (1.3)*** |
BMI body mass index, SD standard deviation
Rapid infant weight gain defined as >0.67 weight for age z-score between birth and weight at either 8 or 12 month visits,
Independent sample t-tests:
p < 0.05,
p < 0.01,
p < 0.001;
Missingness for each covariate was as follows: pre-pregnancy BMI (n = 1); race/ethnicity (n = 1); marital status (n = 1);
Table 2.
Weight outcomes by study visit.
| Study Visit | n | Age at Visit (months) 50th (25th, 75th) | Weight (kg) 50th (25th, 75th) | Weight-for-Age z-score 50th (25th, 75th) | % Rapid Weight Infant Gain |
|---|---|---|---|---|---|
| Birth | 425 | – | 3.3 (3.0, 3.6) | 0.1 (−0.6, 0.7) | – |
| 8 Month | 352 | 8.3 (8.1, 8.7) | 8.3 (7.4, 9.1) | −0.2 (−0.9, 0.7) | 27.4% |
| 12 Month | 318 | 12.7 (12.1, 14.3) | 10.3 (9.3, 11.2) | 0.5 (−0.08, 1.1) | 42.5% |
Rapid infant weight gain defined as >0.67 change in weight for age z-score from birth.
Prenatal oxidative stress biomarkers and rapid infant weight gain
Table 3 displays descriptive statistics of prenatal oxidative stress biomarkers by rapid infant weight gain. In pregnant participants who had infants with rapid infant weight gain, unadjusted t-tests describe mean levels of 8-iso-PGF2α that were significantly higher (0.2 μmol mol Cr−1, SD = 0.4) than in pregnant participants who had infants without rapid infant weight gain (0.08 μmol mol Cr−1, SD = 0.1). Using increments modeled in prior work with oxidative stress biomarkers [12], a ln-unit increase in 8-iso-PGF2α was associated with increased odds of rapid infant weight gain (aOR 1.44; 95% CI: 1.16, 1.78) with a p-value of 0.001. Similarly, a standard deviation increase in 8-iso-PGF2α level was associated with increased odds of rapid infant weight gain (aOR 2.09; 95% CI: 1.18, 3.69) with a p-value of 0.01. Results from both models met the Bonferroni-corrected significance threshold. We conducted sensitivity analyses examining associations between each ln-unit increase of 8-iso-PGF2α and rapid infant weight gain using a sample restricted to 12 month study weights (aOR 1.38; 95% CI: 1.08, 1.75) and a sample restricted to infants with normal birthweights (aOR 1.33; 95% CI: 1.06, 1.69), with similar results. We did not detect associations between other oxidative stress biomarkers and rapid infant weight gain.
Table 3.
Prenatal oxidative stress biomarkers and rapid infant weight gain.
| Adjusted concentrations (μmol mol Cr−1) | Descriptive Statistics (N = 425) | Adjusted Analyses (n = 423)a | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | By RIWG Status | aOR (95% CI)a | p | |||
| No | Yes | |||||
| Lipid | 8-iso-PGF2α | 0.1 (0.3) | 0.08 (0.1)* | 0.2 (0.4)* | 1.44 (1.16, 1.78)* | 0.001 |
| 11 − PGF2α | 0.2 (0.4) | 0.1 (0.2) | 0.2 (0.5) | 1.05 (0.89, 1.24) | 0.54 | |
| 15 − PGF2α | 0.2 (0.3) | 0.2 (0.3) | 0.1 (0.2) | 0.91 (0.76, 1.10) | 0.33 | |
| 8,15 − PGF2α | 0.3 (0.6) | 0.3 (0.5) | 0.3 (0.6) | 0.89 (0.71, 1.12) | 0.34 | |
| MDA | 33.4 (76.6) | 30.8 (16.7) | 38.8 (120.5) | 1.16 (0.80, 1.69) | 0.42 | |
| Protein | diY | 0.8 (0.6) | 0.8 (0.4) | 0.8 (0.8) | 0.99 (0.65, 1.49) | 0.95 |
| DNA | 8−OHdG | 1.6 (4.3) | 1.4 (0.6) | 1.9 (6.9) | 1.25 (0.81, 1.95) | 0.31 |
Rapid infant weight gain (RIWG) defined as >0.67 increase in weight-for-age z-score between birth and 8–12 months;
p < 0.01;
ln-transformed for analyses for model fit; Adjusted for maternal BMI, race/ethnicity, insurance type, single marital status, maternal age, parity, child sex, child age at visit.
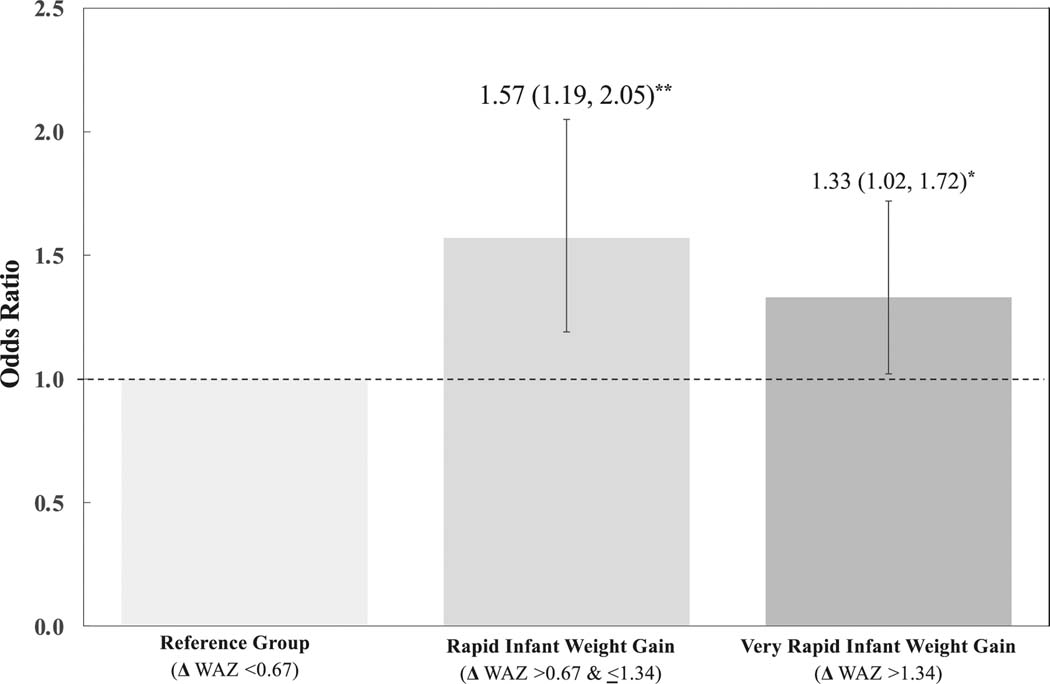
Figure 1 displays a multinomial model showing that 8-iso-PGF2α was associated with 1.57 greater odds of rapid infant weight gain (defined here as >0.67 and <1.34 increase in weight- for-age z-score; 95% CI: 1.19, 2.05) and a 1.33 increased odds of very rapid infant weight gain (defined as >1.34 increase in weight-for-age z-score; 95% CI: 1.02, 1.72), using ≤0.67 change in weight-for-age z-score as the reference group. We detected similar results when examining standard deviation increases in 8-iso-PGF2α (Rapid infant weight gain: aOR 2.07; 95% CI: [1.16, 3.68]; Very rapid infant weight gain: aOR 2.07 [1.17, 3.70]).
Fig. 1. Multinomial model of 8-iso-PGF2α and rate of infant weight gain.
Three-category multinomial logistic regressions (aOR [95% CI]) showing an increase in odds ratio per ln-unit increase in 8-iso-PGF2α for rapid infant weight gain and very rapid infant weight gain using ≤0.67 change in weight-for-age z-score as the reference group. Adjusted for maternal BMI, race/ethnicity, insurance type, single marital status, maternal age, parity, child sex, child age at visit; *p < 0.05; **p < 0.01.
8-iso-PGF2α lipid oxidative stress biomarker and weight outcomes
In adjusted models of 8-iso-PGF2α and infant weight outcomes, each ln-unit increase in 8-iso-PGF2α was associated with a decrease of 48.3 g in birthweight (95% CI: −95.6, −1.0). Similarly, each standard deviation increase in 8-iso-PGF2α was associated with a decrease of 53.2 g (95% CI: −106.1, −0.40) in birthweight. Table 4 displays results of our multinomial models examining associations between 8-iso-PGF2α and secondary infant weight outcomes. Each ln-unit increase in 8-iso-PGF2α was associated with increased odds of low birthweight (aOR 1.45, 95% CI: 1.04, 2.03), but a standard deviation increase in 8-iso-PGF2α was not significant in a similar model. We did not find associations between 8-iso-PGF2α and weight outcomes at 12 months (Table 4).
Table 4.
Multinomial models of 8-iso-PGF2α and secondary infant weight outcomes.
| Per Ln-unit increase PGF2α of 8-iso-PGF2αa | Per SD increase of 8-iso-PGF2α | ||
|---|---|---|---|
| Birthweight aOR (95% CI) | 2500–4000 g | REFERENCE | |
| Low: <2500 g | 1.45 (1.04, 2.03)* | 1.34 (0.98. 1.82) | |
| High: >4000 g | 1.03 (0.70, 1.52) | 0.73 (0.25, 2.09) | |
| 12 Month aOR (95% CI) | Weight between −1 & 1 SD of WAZ | REFERENCE | |
| Low: < −1 SD of WAZ | 0.92 (0.72, 1.17) | 0.76 (0.44, 1.30) | |
| High: >1 SD of WAZ | 0.91 (0.61, 1.38) | 0.68 (0.20, 2.38) |
Three-category multinomial logistic regression models adjusted for maternal BMI, race/ethnicity, insurance type, single marital status, maternal age, parity, child sex, child age at visit;
aOR adjusted odds ratio, WAZ weight-for-age z-score, SD standard deviation;
p < 0.05;
ln-transformed for analyses for model fit.
In our investigation of effect modification by child sex, race/ethnicity, and insurance type, we did not find statistically significant interactions (Supplementary Table 1).
DISCUSSION
In a diverse prospective pregnancy cohort from New York City, we found that increased urinary concentrations of a lipid oxidative stress biomarker in a pregnant participant were associated with increased odds of a greater than 0.67 increase in weight-for-age z-score between birth and later infancy in their infant, which has not been described previously. This study also replicates prior modest associations between oxidative stress and low birthweight outcomes [12, 21]. By detecting a potential longitudinal relation- ship between prenatal oxidative stress and rapid infant weight gain, we extend prior work showing that oxidative stress biomarkers are associated with low weight at the beginning of life but with obesity later in life. These findings frame future lines of inquiry to delineate the potential roles of lipid-derived oxidative stress in the developmental biology of obesity risk across the life course.
This is one of the first studies to examine rapid infant weight gain alongside lipid, protein, and DNA urinary oxidative stress biomarkers. Our analysis identified associations between 8-iso-PGF2α and rapid infant weight gain, expanding prior work with comparable effect sizes showing modest associations between 8-iso-PGF2α and weight outcomes in children and adults [12, 36]. Among the F2 prostaglandins, 8-iso-PGF2α is a particularly durable biomarker of oxidative stress and may be more specific than MDA, which reflects lipid peroxidation of polyunsaturated fatty acids more generally. Rapid infant weight gain increases obesity risk but in of itself is not a disease status. Therefore, it may not be associated with the oxidative damage to protein or DNA molecules characteristic of advanced cellular pathology. Future studies should continue to clinically correlate lipid, protein, and DNA biomarkers longitudinally to follow the progression of cardiometabolic processes across the life course.
Low weight early in life and poor cardiometabolic outcomes later in life have been explained using the “thrifty phenotype” hypothesis, which theorizes that early undernutrition may activate metabolic settings that favor later weight gain [37, 38]. A prior study from The Infant Development and the Environment Study also identified 8-iso-PGF2α as associated with a two-to-three fold increase in the odds of a “low-high” weight trajectory from birth to 3 years of age relative to a normal weight trajectory [12]. We found that 8-iso-PGF2α had modest associations with both rapid infant weight gain and low birthweight, reinforcing this “low-high” pattern in infancy. Decreased fetal growth may occur due to decreased blood and nutrient flow in pregnancy, increasing free radicals and reactive oxygen species from perfusion injury. Resultant oxidative stress may trigger inflammation in fetal adipose tissue, favoring weight gain later on [11]. Our findings show that this association does not only happen in the setting of “catch-up growth” triggered by low birthweight (<2500 g), as sensitivity analyses with only normal birthweights yielded similar results and overall less than 10% of infants had a low birthweight in this cohort. Future studies should expand upon this work by examining the role of birthweight or fetal size in mediating associations between prenatal oxidative stress levels and infant growth patterns.
The “fetal overnutrition hypothesis” theorizes that exposure to maternal obesity prenatally increases lifetime risk of obesity. For example, developing adipose tissue and vasculature may be particularly susceptible to lipid oxidative stress signaling from excessive maternal adipose tissue during fetal periods of rapid growth [11, 39]. Maternal obesity is also associated with an increased risk of gestational diabetes [33, 40], which confers increased risk of high birthweight, another infant weight outcome associated with future obesity [41]. Scientists have found associations between gestational diabetes and oxidative stress biomarkers, including 8-iso-PGF2α, but primarily in protein biomarkers [17]. In our sample, while about 18% of pregnant participants had gestational diabetes, only 7% infants had high birthweight while almost 40% met rapid infant weight gain standards. Despite shared associations with oxidative stress, our findings may not reflect a high birthweight pathway but rather they support future work to continue disentangling lipid, protein, and DNA sources of prenatal oxidative stress in the context of maternal obesity.
Increased oxidative stress has been associated with multiple potential sources, including psychosocial stress (e.g., socioeconomic disadvantage), toxic environmental exposures (e.g., air pollution, toxic metals), and unhealthy lifestyle practices (e.g., dietary patterns lacking antioxidants, cigarette smoke) [15, 42]. Our descriptive characteristics reflect socioeconomic differences by rapid infant weight gain status like race/ethnicity and insurance status. Researchers have previously noted that rapid infant weight gain may account for between 15–70% of obesity disparities between children identifying as White and their peers from racial and ethnic minority groups [43]. Of note, our sample included a high percentage of participants who self-identified as Hispanic, a historically marginalized group with higher rates of obesity [44]. Supplemental analyses showed an interaction trend (p = 0.12) between race/ethnicity and the association between 8-iso-PGF2α and rapid infant weight gain. Future cohort studies should include an in-depth examination of racial and ethnic disparities in rapid infant weight gain in the context of exposures to established sources of oxidative stress like psychosocial and environmental stressors [7, 45–47]. Understanding these disparities could eventually inform prenatal interventions applying a life course framework [48] to target modifiable health practices (e.g., dietary patterns [49], smoking [50]) and leverage community-derived cultural assets to potentially mitigate levels of oxidative stress during vulnerable time periods
We found that 8-iso-PGF2α was associated with very rapid infant weight gain (>1.34 increase in weight-for-age z-score), but that the effect size was smaller or equivocal to the odds of rapid infant weight gain between >0.67 and <1.34 weight-for-age z-score. Pediatric researchers have already identified numerous feeding practices and styles that increase risk for rapid infant weight gain, particularly in the first year of life [51, 52]. These early feeding practices may have joint effects with potential risk related to prenatal oxidative stress, increasing the magnitude of rapid infant weight gain (e.g., very rapid) and subsequent risk for obesity. Current work assessing the utility of oxidative stress biomarkers for risk stratification are in early developmental stages [13, 18]. Considering their use in early child obesity will rely on future studies to delineate how oxidative stress levels may interact with modifiable infant feeding practices.
These results must be interpreted in context of the study’s limitations: the quality of weight data at the 8 month visit and followup loss. The 8 month weight variable relied on parent report of the child’s weight from the most recent well child check, which is subject to recall bias. Still, our findings are supported by robust weight data used in this study: birthweight was recorded using the electronic health record, and 12 month visit weights were measured with precision by a trained research team. Seventy-five percent of weights from the 8 month visit also had a 12 month visit in-person weight. Another limitation was followup loss, as this study was conducted in a sample of mother-child dyads with complete available data. While using a subsample increases risk of selection bias, we compared our subsample with the full cohort and found similar baseline characteristics, supporting representation of the full cohort.
CONCLUSION
We detected an association between prenatal 8-iso-PGF2α – a lipid oxidative stress biomarker—and rapid infant weight gain outcomes. Our findings contribute to a body of work showing how prenatal oxidative stress biomarkers have associations with infant weight and growth patterns known to increase obesity and cardiometabolic risk. Understanding prenatal oxidative stress may have implications for understanding the developmental origins of cardiometabolic disease and inform strategies for the primary prevention of obesity.
Supplementary Material
ACKNOWLEDGEMENTS
We thank all of the NYU CHES participants and staff for their important contributions. This work was supported by the institutional funds of NYU Grossman School of Medicine as well as the NIH Office of the Director (UG3/UH3OD023305). CD-L acknowledges support from training grants by the National Center for Advancing Translational Sciences, National Institutes of Health 2KL2TR001446-06 and the Life Course Intervention Research Network (Health Resources and Services Administration) UA6MC32492.
Footnotes
COMPETING INTERESTS
The authors declare no competing interests.
ADDITIONAL INFORMATION
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41366-023-01302-8.
DATA AVAILABILITY
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Ward ZJ, Long MW, Resch SC, Giles CM, Cradock AL, Gortmaker SL. Simulation of growth trajectories of childhood obesity into adulthood. N Engl J Med. 2017;377:2145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng M, Lamb KE, Grimes C, Laws R, Bolton K, Ong KK, et al. Rapid weight gain during infancy and subsequent adiposity: a systematic review and meta-analysis of evidence. Obes Rev. 2018;19:321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bichteler A, Gershoff ET. Identification of children’s BMI trajectories and prediction from weight gain in infancy. Obesity. 2018;26:1050–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320:967–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arisaka O, Ichikawa G, Koyama S, Sairenchi T. Childhood obesity: rapid weight gain in early childhood and subsequent cardiometabolic risk. Clin Pediatr Endocrinol. 2020;29:135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ong KK, Loos RJF. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. 2006;95:904–8. [DOI] [PubMed] [Google Scholar]
- 7.Felder JN, Epel E, Coccia M, Cordeiro A, Laraia B, Adler N, et al. Prenatal maternal objective and subjective stress exposures and rapid infant weight gain. J Pediatr. 2020;222:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higdon JV, Frei B. Obesity and oxidative stress: a direct link to CVD? Arterioscler Thromb Vasc Biol. 2003;23:365–7. [DOI] [PubMed] [Google Scholar]
- 9.Loy SL, Sirajudeen KNS, Hamid, Jan JM. The effects of prenatal oxidative stress levels on infant adiposity development during the first year of life. J Dev Orig Health Dis. 2014;5:142–51. [DOI] [PubMed] [Google Scholar]
- 10.Weber D, Stuetz W, Bernhard W, Franz A, Raith M, Grune T, et al. Oxidative stress markers and micronutrients in maternal and cord blood in relation to neonatal outcome. Eur J Clin Nutr. 2014;68:215–22. [DOI] [PubMed] [Google Scholar]
- 11.Vincent HK, Innes KE, Vincent KR. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes, Obes Metab. 2007;9:813–39. [DOI] [PubMed] [Google Scholar]
- 12.Arogbokun O, Rosen E, Keil AP, Milne GL, Barrett E, Nguyen R, et al. Maternal oxidative stress biomarkers in pregnancy and child growth from birth to age 6. J Clin Endocrinol Metab. 2021;106:1427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho E, Karimi Galougahi K, Liu CC, Bhindi R, Figtree GA. Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biol. 2013;1:483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez MP, Kannan K. Simultaneous analysis of seven biomarkers of oxidative damage to lipids, proteins, and DNA in urine. Environ Sci Technol. 2018;52:6647–55. [DOI] [PubMed] [Google Scholar]
- 15.Eick SM, Geiger SD, Alshawabkeh A, Aung M, Barrett E, Bush NR, et al. Enviromental influences on Child Health Outcomes. Associations between social, biologic, and behavioral factors and biomarkers of oxidative stress during pregnancy: findings from four ECHO cohorts. Sci Total Environ. 2022;835:155596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol. 2019;11:45–63. [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Yin Q, Li N, Ouyang Z, Zhong M. Plasma markers of oxidative stress in patients with gestational diabetes mellitus in the second and third trimester. Obstet Gynecol Int. 2016;2016:e3865454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frijhoff J, Winyard PG, Zarkovic N, Davies SS, Stocker R, Cheng D, et al. Clinical relevance of biomarkers of oxidative stress. Antioxid Redox Signal. 2015;23:1144–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trasande L, Ghassabian A, Kahn LG, Jacobson MH, Afanasyeva Y, Liu M, et al. The NYU Children’s Health and Environment Study. Eur J Epidemiol. 2020;35:305–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallardo JM, Gómez-López J, Medina-Bravo P, Juárez-Sánchez F, Contreras-Ramos A, Galicia-Esquivel M, et al. Maternal obesity increases oxidative stress in the newborn. Obesity. 2015;23:1650–4. [DOI] [PubMed] [Google Scholar]
- 21.Kim YJ, Hong YC, Lee KH, Park HJ, Park EA, Moon HS, et al. Oxidative stress in pregnant women and birth weight reduction. Reprod Toxicol. 2005;19:487–92. [DOI] [PubMed] [Google Scholar]
- 22.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28:505–13. [DOI] [PubMed] [Google Scholar]
- 24.About the Program | National Biomonitoring Program | CDC [Internet]. 2021. [cited 2022 Nov 14]. Available from: https://www.cdc.gov/biomonitoring/about.html.
- 25.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- 26.Martinez-Moral MP, Kannan K. How stable is oxidative stress level? An observational study of intra- and inter-individual variability in urinary oxidative stress biomarkers of DNA, proteins, and lipids in healthy individuals. Environ Int. 2019;123:382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Brien KM, Upson K, Cook NR, Weinberg CR. Environmental chemicals in urine and blood: improving methods for creatinine and lipid adjustment. Environ Health Perspect. 2016;124:220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st). International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384:857–68. [DOI] [PubMed] [Google Scholar]
- 29.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. [DOI] [PubMed] [Google Scholar]
- 30.Druet C, Stettler N, Sharp S, Simmons RK, Cooper C, Davey Smith G, et al. Prediction of childhood obesity by infancy weight gain: an individual-level meta-analysis. Paediatric Perinat Epidemiol. 2012;26:19–26. [DOI] [PubMed] [Google Scholar]
- 31.Wang G, Johnson S, Gong Y, Polk S, Divall S, Radovick S, et al. Weight gain in infancy and overweight or obesity in childhood across the gestational spectrum: a prospective birth cohort study. Sci Rep Nat Publ Group. 2016;6:29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cutland CL, Lackritz EM, Mallett-Moore T, Bardají A, Chandrasekaran R, Lahariya C, et al. Low birth weight: case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine. 2017;35:6492–6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ouzounian JG, Hernandez GD, Korst LM, Montoro MM, Battista LR, Walden CL, et al. Pre-pregnancy weight and excess weight gain are risk factors for macrosomia in women with gestational diabetes. J Perinatol. 2011;31:717–21. [DOI] [PubMed] [Google Scholar]
- 34.Mei Z, Grummer-Strawn LM. Standard deviation of anthropometric Z-scores as a data quality assessment tool using the 2006 WHO growth standards: a cross country analysis. Bull World Health Organ. 2007;85:441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen SY, Feng Z, Yi X. A general introduction to adjustment for multiple comparisons. J Thorac Dis. 2017;9:1725–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singhal A. Long-term adverse effects of early growth acceleration or catch-up growth. Ann Nutr Metab. 2017;70:236–40. [DOI] [PubMed] [Google Scholar]
- 38.Barker D, Osmond C. Infant mortality, childhood nutrition, ischaemic heart dis- ease in England and Wales. Lancet. 1986;327:1077–81. [DOI] [PubMed] [Google Scholar]
- 39.Cusick SE, Georgieff MK. The role of nutrition in brain development: the golden opportunity of the “First 1000 Days”. J Pediatr. 2016;175:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao D, Chang Q, Wu QJ, Gao SY, Zhao H, Liu YS, et al. Relationship between Maternal central obesity and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of cohort studies. J Diabetes Res. 2020;2020:e6303820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cnattingius S, Villamor E, Lagerros YT, Wikström AK, Granath F. High birth weight and obesity—a vicious circle across generations. Int J Obes. 2012;36:1320–4. [DOI] [PubMed] [Google Scholar]
- 42.Leni Z, Künzi L, Geiser M. Air pollution causing oxidative stress. Curr Opin Toxicol. 2020;20:1–8. [Google Scholar]
- 43.Isong IA, Rao SR, Bind MA, Avendaño M, Kawachi I, Richmond TK. Racial and ethnic disparities in early childhood obesity. Pediatrics. American Academy of Pediatrics; 2018. Jan [cited 2020 Nov 23]. 141. Available from: http://pediatrics.aappublications.org/content/141/1/e20170865. [DOI] [PMC free article] [PubMed]
- 44.Ogden CL, Fryar CD, Martin CB, Freedman DS, Carroll MD, Gu Q, et al. Trends in obesity prevalence by race and hispanic origin—1999–2000 to 2017–2018. JAMA. 2020;324:1208–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baker JL, Michaelsen KF, Rasmussen KM, Sørensen TIA. Maternal prepregnant body mass index, duration of breastfeeding, and timing of complementary food intro- duction are associated with infant weight gain. Am J Clin Nutr. 2004;80:1579–88. [DOI] [PubMed] [Google Scholar]
- 46.Valvi D, Mendez MA, Garcia-Esteban R, Ballester F, Ibarluzea J, Goñi F, et al. Prenatal exposure to persistent organic pollutants and rapid weight gain and overweight in infancy. Obesity. 2014;22:488–96. [DOI] [PubMed] [Google Scholar]
- 47.Mendez MA, Garcia -Esteban R, Guxens M, Vrijheid M, Kogevinas M, Goñi F, et al. Prenatal organochlorine compound exposure, rapid weight gain, and overweight in infancy. environmental health perspectives. Environ Health Perspect. 2011;119:272–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halfon N, Forrest CB, Lerner RM, Faustman EM, editors. Handbook of Life Course Health Development. Cham (CH): Springer; 2018. [cited 2022 Feb 23]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK543707/. [PubMed] [Google Scholar]
- 49.Messito MJ, Mendelsohn AL, Katzow MW, Scott MA, Vandyousefi S, Gross RS. Prenatal and pediatric primary care-based child obesity prevention program: a randomized trial. Pediatrics. 2020;146:e20200709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.White O, Roeder N, Blum K, Eiden RD, Thanos PK. Prenatal effects of nicotine on obesity risks: a narrative review. Int J Environ Res Public Health. 2022;19:9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pesch MH, Pont CM, Lumeng JC, McCaffery H, Tan CC. Mother and infant predictors of rapid infant weight gain. Clin Pediatr (Phila). 2019;58:1515–21. [DOI] [PubMed] [Google Scholar]
- 52.Rotevatn TA, Melendez-Torres GJ, Overgaard C, Peven K, Hyldgaard Nilsen J, Bøggild H, et al. Understanding rapid infant weight gain prevention: a systematic review of quantitative and qualitative evidence. Eur J Public Health. 2020;30:703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.