Abstract
The regulation of postprandial muscle protein synthesis (MPS) with or without physical activity has been an intensely studied area within nutrition and physiology. The leucine content of dietary protein and the subsequent plasma leucinemia it elicits postingestion is often considered the primary drivers of the postprandial MPS response. This concept, generally known as the leucine “trigger” hypothesis, has also been adopted within more applied aspects of nutrition. Our view is that recent evidence is driving a more nuanced picture of the regulation of postprandial MPS by revealing a compelling dissociation between ingested leucine or plasma leucinemia and the magnitude of the postprandial MPS response. Much of this lack of coherence has arisen as experimental progress has demanded relevant studies move beyond reliance on isolated amino acids and proteins to use increasingly complex protein-rich meals, whole foods, and mixed meals. Our overreliance on the centrality of leucine in this field has been reflected in 2 recent systematic reviews. In this perspective, we propose a re-evaluation of the pre-eminent role of these leucine variables in the stimulation of postprandial MPS. We view the development of a more complex intellectual framework now a priority if we are to see continued progress concerning the mechanistic regulation of postprandial muscle protein turnover, but also consequential from an applied perspective when evaluating the value of novel dietary protein sources.
Keywords: protein turnover, dietary protein, protein metabolism
Introduction
Possessing an adequate quality and quantity of skeletal muscle tissue underlies functional capacity, performance, and metabolic health across the lifespan. Skeletal muscle protein mass is regulated via dynamic fluctuations in muscle protein synthesis (MPS) and muscle protein breakdown (MPB) rates. In the postabsorptive state, MPB rates exceed that of MPS, effectuating a net loss of muscle protein. In weight-stable individuals, this is offset, on roughly a daily basis, by the dietary protein-induced transient stimulation of MPS and suppression of MPB, permitting a net gain of muscle protein, the “anabolic response” to food intake. Within the regulation of the anabolic response to food intake, the indispensable amino acids (IAAs) appear primarily responsible [1], and of the IAAs, the branched-chain amino acid leucine (which is highly abundant in skeletal muscle) has attracted the most attention as the proposed primary driver of postprandial anabolism. This is due to leucine’s unique ability to potently upregulate translation initiation, therefore acting as the initial “trigger” for stimulating MPS rates. Consequently, the leucine content of a bolus of dietary protein and the subsequent plasma leucinemia following its digestion and absorption have typically been considered the primary drivers of the postprandial MPS response. From this, the “leucine threshold” or “leucine trigger” hypotheses emerged, and we use the latter term herein. Although, to our knowledge, no agreed definition exists, the leucine trigger hypothesis is essentially the concept that following protein ingestion, leucine must appear in circulation with sufficient rapidity and quantity to affect intracellular anabolic signaling to control postprandial MPS. The hypothesis holds that, to some degree, plasma leucinemia and MPS are proportionate and that exposure of the muscle cell to leucine is central to the regulation of postprandial MPS. Within this, there seems to be an assumption that plasma leucine is largely reflective of the availability within the muscle. Aside from the mechanistic study of metabolism, this hypothesis has also been adopted within more applied aspects of nutrition, with such leucine variables often being used to evaluate the anabolic value of novel or more complex protein sources, at least from a muscle-centric perspective [2].
The hours (or days) following a bout of exercise are of particular interest from an MPS perspective due to the synergistic manner by which muscle contraction and dietary protein promote net muscle protein accretion. A bout of exercise independently stimulates MPS, peaking ∼2 to 6 h postexercize [3,4]) and remaining elevated for ∼24 to 48 h [[5], [6], [7]], though MPB is also stimulated for around 24 h [5,7]. As such, in the postabsorptive state, muscle protein net balance remains negative postexercize [5], but protein (or amino acid) ingestion before [8,9], immediately after [[10], [11], [12], [13]], or ≤24 h after [[14], [15], [16]], potentiates MPS and, somewhat, inhibits MPB [17] promoting a positive net protein balance. Over time, these effects represent the basis of maximizing the muscle adaptive response to prolonged exercise training [18].
The reason for writing this perspective is our view that we have reached an important junction concerning the utility, or at least primacy, of plasma leucine variables predicting postprandial MPS rates. Recent evidence from multiple research laboratories provides a contemporary lens through which to (re)view historical data supporting this thesis. We believe that current research questions are driving a more nuanced picture, which brings into question the extent to which leucine (alone) can explain or predict the magnitude of the postprandial MPS response. We also wish to highlight the consequential nature of considering this updated view when we seek to use it to scrutinize the quality of the novel and understudied dietary proteins, an increasingly crucial modern research direction.
Origins of the hypothesis
Early in vitro and animal models collectively identified leucine as being distinctive in its ability to stimulate MPS by acting upon mammalian target of rapamycin complex 1 (mTORC1) (and independently of it [19]) and its downstream effectors, a role not shared by other amino acids [[20], [21], [22], [23], [24], [25], [26], [27]]. These studies repeatedly corroborated the idea that leucine is unique among the amino acids in its capacity to modulate mTOR and eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1) phosphorylation while also affecting the most potent amplification of ribosomal protein S6 kinase beta-1 (p70S6K1) signaling, thereby exerting effective control of translation initiation, the rate-limiting step in MPS [28,29]. In vivo, mTORC1 activation is necessary for the stimulation of MPS by IAA [30], of which leucine is also the key regulator. As such, at least at the molecular level, there is evidence that leucine is the most potent nutritional anabolic signal, from which emerged the assumption of its primary importance in controlling postprandial MPS in humans. Nonetheless, it is worth noting that, even within these mechanistic models, there were other amino acids implicated in anabolic signaling pathways [[31], [33]] as well as amino acid-independent signaling pathways [34,35].
Rodent studies were in line with this extrapolation, showing that the leucine content of an ingested dietary protein was proportional to the magnitude of the postprandial MPS response [36]. This work was confirmed in humans, where the proportional relationship between ingested leucine and the MPS response became consequential for applied questions such as identifying dose responses or differences between protein sources. For instance, Tang et al. [12] investigated the MPS response to ingestion of whey, soy, or casein proteins in young men in resting and postexercized muscles. The whey protein condition provided the highest (2.3 g) and most digestible (more rapid and higher peak plasma leucinemia, as well as greater total leucinemia) leucine content and, in turn, provoked the greatest MPS response. The soy and casein boluses both contained 1.8 g leucine, but the more rapidly digested soy protein elicited greater leucinemia and, resultantly, MPS response compared with the casein condition. Proportionality between ingested leucine content, parameters of plasma leucinemia, and MPS was also seen by other contemporaneous studies [[37], [39]]. The concept was further refined by work demonstrating that rapidity of protein digestion and intestinal leucine absorption was key, rather than total peripheral leucine availability, solidifying leucine’s unique role as a signal, rather than only a limiting substrate as is the case with other amino acids [40]. Further, the relationship appeared to exist in both resting and exercised muscle, albeit the latter is often proposed to sensitize the muscle; that is, lower the threshold required [41]. These elegant observations provided an important and impactful framework for understanding the regulation of resting and postexercize postprandial muscle protein turnover and developing more refined dietary protein recommendations.
Leucine; signal and substrate
The “leucine trigger” hypothesis was conceptualized from the work outlined above, elegantly encompassing the interaction between nutrition (i.e., the amount of ingested leucine) and physiology (i.e., digestion, absorption, splanchnic extraction, etc.) that subsequently determines the extent to which that source becomes available to the muscle. It is worth considering the 2 distinct anabolic roles fulfilled by leucine, acting as both a precursor and a signaling molecule, with differing exogenous quantities perhaps required for each purpose. To elaborate, the WHO daily recommendation for leucine is 39 mg/kg/d [42] (∼2.7 g/d for a 70 kg individual), which provides an approximation of the minimal amount of leucine that needs to be ingested on a daily basis to provide adequate precursor for protein synthesis, balancing losses. On a per-meal basis, this equates to 0.9 g (assuming 3 meals a day). Although this requirement likely fulfills substrate requirements, it may not be optimal for signaling purposes, and the per meal “signaling requirement” is often proposed to be 2–3 g of ingested leucine (∼35 mg/kg body mass), implying this latter role may be more limited from a dietary intake perspective. Although the quantity of leucine ingested as a “signal” strongly predicts the appearance in plasma [43], it is unclear to what extent it predicts a rise in extra/intra (myo)cellular leucine, and it should also be considered that most studies do not allow the disentanglement of leucine’s signaling role from leucine-independent signaling pathways involved in translation initiation.
The proposed underlying mechanism for the leucine trigger hypothesis is that postprandial peripheral leucine availability must be elevated sufficiently to increase intramyocellular leucine, sensed by Sestrin2 [44], which initiates a kinase signaling cascade that phosphorylates/dephosphorylates the downstream effectors p70S6K and 4EBP-1, activating translation initiation [45,46]. Minor disagreements exist as to which leucinemic variable may be of most relevance. For example, are intra- (or extra-) cellular anabolic receptors [47], transporters [48], and/or signaling pathways more responsive to postprandial plasma or intracellular leucine being available rapidly (i.e., sensitive to a subjective peak), to the maximum extent [i.e., an absolute (and debatable) concentration], overall exposure (i.e., prolonged elevations of peripheral leucine) or some combination of the above. An intracellular (rather than extracellular [47]) leucine sensor, such as Sestrin2 [44,49], is now generally regarded as the locus of regulation, but the relationship between intracellular leucine concentrations and MPS is still poorly elucidated. Indeed, our understanding is undermined by an incomplete picture (due to minimal data) of how the postprandial flux (as opposed to concentrations) of intracellular leucine triggers an elevation in postprandial MPS in vivo. The natural abundance of leucine in muscle tissue and its perpetual recycling may further cloud our ability to untangle leucine’s role and highlight that commonly measured plasma measures are somewhat removed from the locus of regulation.
Experimental considerations
To some degree, the inconsistencies in the literature surrounding the pre-eminence of leucine variables which we now come on to discuss, may have emerged from methodological restrictions associated with human studies. These include limits to the number (and therefore timing) of muscle biopsy collections, which restrict the temporal resolution of the data (at least compared with that of in vitro or rodent studies). To illustrate, in vivo, evidence in humans has demonstrated that even small (0.75–3 g) quantities of leucine-enriched IAAs and branched-chain amino acids alone are capable of robustly stimulating MPS rates [32,[50], [51], [52], [53]]. In these cases, however, MPS typically rises only transiently compared with larger boluses, delivering what is essentially an optimal magnitude response but for a suboptimal duration [32,52]. It is likely that such studies reveal leucine’s potent signaling capacity to elevate MPS with relatively small increases in intracellular leucine but also reflect the reliance on endogenous amino acids to act as substrate in the absence of a full complement of exogenous amino acids. A prolonging of high magnitude MPS rates, thereby maximizing net muscle protein accretion, is consequently contingent on adequate availability of a full complement of dietary-derived amino acids within a whole protein source to provide both signal and substrate [52]. In support, adding leucine to suboptimal amounts of protein has been shown to elevate MPS acutely [32,[54], [56]] and in more chronic settings [55,57], even in older adults displaying anabolic resistance [[54], [56]].
Collectively, the admittedly reductive leucine trigger narrative has logically resulted from a weight of coherent in vitro and in vivo studies and has provided a simple and effective framework through which to understand the regulation of postprandial muscle protein turnover. In turn, the more intellectual focus of the work discussed has been applied in a similarly reductive capacity (i.e., based solely on leucine content and subsequent bioavailability postingestion) to contemporary issues within human and sports nutrition such as evaluating the anabolic properties of lesser studied alternative protein sources. Given the ever-changing research climate, defining the precise utility of the leucine trigger hypothesis is, therefore, not only intellectually interesting but also highly consequential from an applied perspective.
Recent evaluations concerning the primacy of leucine
Given the interpretative difficulties contained within single studies discussed above, 2 recent systematic reviews have attempted to amalgamate decades of data to evaluate the extent to which ingested leucine dictates MPS [43,58]. Zaromskyte et al. [58] took a qualitative approach, broadly concluding that the evidence supported a role for the magnitude of postprandial leucinemia in regulating the MPS response to ingested protein. Worthy of note, however, is their approach revealed only around half of the studies included were supportive of the hypothesis, and its utility was restricted primarily to older adults (mostly postexercize) and only to the ingestion of isolated protein sources [58]. Intrigued by these findings, we recently conducted a quantitative systematic review [43]. This allowed us to report direct correlations between the key variables, which we interpreted as the distinct predictive capacities of the leucine trigger hypothesis on postprandial (and postexercize) MPS rates (notwithstanding the possibility of reverse causation). In agreement, and building on the findings of Zaromskyte et al. [58], we reported that the dose of leucine ingested was significantly, but only mildly, predictive of the postexercize MPS response (r2 = 0.05), and this relationship was driven by older subjects (r2 = 0.18) and absent in younger adults (r2 = 0.01). In older adults, this relationship was particularly strong in the early postprandial period (0–2 h) (r2 = 0.64), whereas it was absent in younger individuals (r2 = 0.006), perhaps speaking to the increased importance of leucine’s signaling role in older adults. When looking at each of the candidate postprandial plasma leucine variables, none were quantitatively associated with the postexercize postprandial MPS response. We concluded that the leucine content of a dietary protein can only explain part of the variability in postexercize postprandial MPS and likely under specific experimental conditions and/or within particular populations. Of relevance, we included several more studies that had used protein-rich whole-food sources in our analysis compared with that of Zaromskyte et al. [58]. Similar inclusion criteria in this latter study may, therefore, have weakened the observed relationships they did report.
It is interesting to consider the reasons why we [43] and others [58,59] have noted stronger evidence for the leucine trigger within older compared with younger adults. It is well established that older adults display a reduced sensitivity to the anabolic effects of protein [60]. It is, therefore, possible that the apparently tighter relationship between leucine variables and MPS in older adults simply reflects a rightwards shift in the dose-response curve, allowing the selected doses to provide a greater spread of MPS responses, thereby bringing out a higher resolution of data to reveal correlations. Alternatively, leucine as a trigger may play a greater regulatory role in older muscle due to it being less efficiently digested and absorbed [61], preferentially extracted within splanchnic tissues [62,63], less effectively delivered and transported into muscle [64,65] and/or blunted sensitivity of intracellular sensors or signaling proteins [66,67]. From the viewpoint of more widely evaluating the leucine trigger hypothesis, it is, therefore, important to bear in mind that experimental design may influence interpretations of any given dataset, and that the relationship between leucine and MPS subtly changes through the lifespan.
Despite the different approaches, there was general agreement between these 2 recent systematic reviews insofar as the leucine trigger hypothesis may explain less of the variability in postprandial MPS rates than perhaps was expected. This supports the idea that the regulation of postprandial muscle protein turnover may be more complex than assumed and prompts a detailed examination of recent and historical literature viewed through this lens. This is particularly important if we are to move toward understanding and investigating what other candidate postprandial nutritional (or metabolic, cardiovascular, or endocrine) factors may have been overlooked by our assumed knowledge and now require deeper consideration.
Evidence from studies using protein-rich whole foods and mixed meals
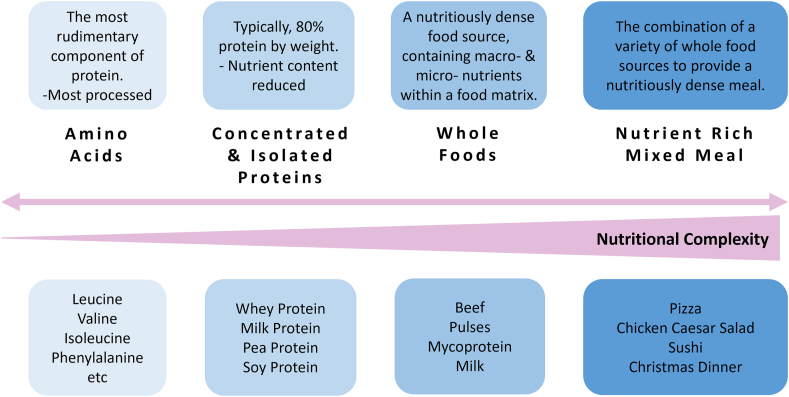
With the majority of data concerning postprandial muscle protein anabolism derived from experiments using protein isolates (or even crystalline amino acids), it is worth considering that this is a rare format for protein to be consumed day-to-day. More usual is to ingest protein within a whole-food matrix, either alone or as part of a mixed meal, which introduces further complexity to understanding digestion, absorption, and postprandial nutrient metabolism compared with isolated proteins (this concept is schematically illustrated in Figure 1). Moreover, ingesting protein within complex whole foods is typically accompanied by a higher energy content and myriad potential macro- or micro-nutrient interactions [68,69], even at the level of small metabolites (e.g., bioactive peptides [70]), all of which may modulate the postprandial anabolic milieu. This escalating complexity of whole foods and meals offers an explanation for the inability of Zaromskyte et al. [58] and ourselves [43] to notice a link between leucine load and MPS following their ingestion. Further resolution to this explanation, mechanistically, is currently lacking, not least due to how few studies have investigated the anabolic response to ingestion of whole foods or mixed meals. Nevertheless, the increasing appreciation [[71], [73]] of both this regulatory complexity and its translational importance has resulted in a recent uptick in experimental interest.
FIGURE 1.
A framework for the categorization of different protein forms, taken from the most basic level through increasing levels of nutritional complexity (greater energy content, macro- and micronutrient density, complex food matrices, bioactive peptides, etc.). With increasing nutritional complexity plasma leucine appears to become less predictive of postprandial muscle protein synthesis rates, and seemingly other leucine-independent factors become more prominent in regulating the anabolic response to feeding.
An additional impetus for our desire to re-evaluate the broader leucine trigger data was our own work examining the MPS response to the ingestion of the fungal-derived protein-rich whole-food mycoprotein (Fusarium venenatum), where our hypotheses were repeatedly falsified with regards to leucine. Initially, we compared the MPS response following ingestion of mycoprotein and milk protein isolate in rested and exercised tissue, matching the 2 protein sources on leucine content (2.5 g) [74]. Although the leucine load ingested was identical, the nutrients in mycoprotein are encapsulated within a fibrous cell wall, and the hyphal cell structure of mycoprotein remains largely intact during digestion, such that the extraction of amino acids is contingent on proteases permeating the cell membrane, hydrolyzing protein structures, and releasing amino acids in the small intestine [75]. This, together with additional energetic and macronutrient load, results in a slower and lower postprandial rise of amino acids into the circulation compared with milk protein isolate [76]. Therefore, the leucine trigger hypothesis would imply that mycoprotein would stimulate MPS rates to a lesser extent than milk protein isolate. Consistent with our previous work [76], we observed mycoprotein to be more slowly digested and absorbed, delivering a lower and slower pattern of leucinemia. However, contrary to the leucine trigger hypothesis, mycoprotein stimulated a greater postprandial increase in MPS rates compared with milk protein concentrate [74]. This surprising contradiction obliged us to look beyond leucine for mechanistic insight. Though we were unable to pin down the precise explanation, we reasoned that leucine-independent factors may have been at play, and the whole-food nature and greater structural complexity of mycoprotein compared with the milk protein concentrate offered candidate mechanisms. The mycoprotein beverage also contained significantly more energy and slightly more protein than the milk protein beverage, and whereas the prevailing wisdom would suggest that this does not modulate the anabolic response, the contrary remains plausible.
Our follow-up work minimized the number of extraneous variables present when comparing different protein sources; we compared higher and lower doses of mycoprotein with the latter matched in leucine content by the addition of crystalline leucine [77], and a protein isolate extracted from mycoprotein to its whole-food form [78]. In both cases, this engineered divergent postprandial leucine kinetics (from the same protein source) and still supported the conclusion that leucine-independent mechanisms must, at least in part, govern the MPS to (whole-food) protein ingestion. In the latter case, we actually proposed a directional hypothesis that essentially predicted against the leucine trigger hypothesis and in favor of a whole-food effect, demonstrating how is conceptualization of the regulation of MPS had begun to shift. We have recently also reported a lack of support for the leucine hypothesis when investigating the MPS response to ingesting whole-food protein-rich algal species [79], pea protein [80], and plant-based protein blends [80].
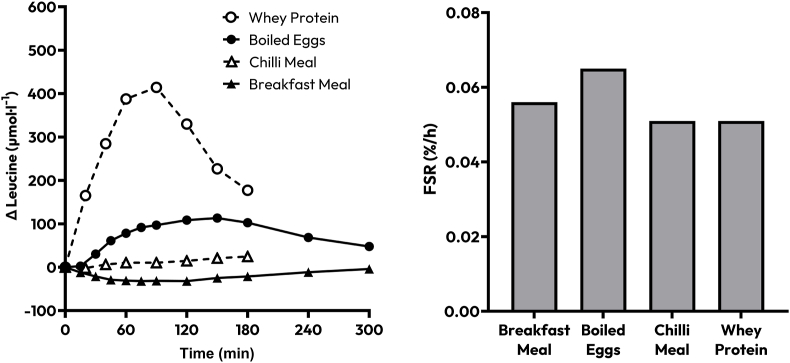
Other research groups have also recently failed to observe coherent connections between plasma leucine and MPS responses to the ingestion of diverse protein-rich whole foods, such as whole egg compared with egg white [71], cooked compared with raw egg [81], cheese compared with milk protein concentrate [82], mealworms compared with milk protein concentrate [83], and whole salmon compared with the sum of its isolated constituent nutrients [72]. In each case, plasma leucine appearance in circulation, and on one occasion [82] total plasma leucine availability, was dissimilar between conditions but did not provoke different MPS responses. In a similar vein, data investigating the effect of mixed meal ingestion on the anabolic response to feeding has also returned a lack of coherence between leucinemic variables and MPS (summarized in Figure 2). Gwin et al. [84] observed plasma leucine concentrations that were many magnitudes lower following the ingestion of the mixed macronutrient meal (∼20-fold lower when expressed as postprandial AUC) compared with 2 isolated protein conditions (whey protein and IAA-enriched whey protein) and yet observed equivalent MPS across conditions. Similarly, Fuchs et al. [81] observed that a negligible-protein (5 g) breakfast meal eliciting no postprandial increase in plasma leucine concentrations supported a remarkably robust increase in MPS rates. This would suggest that the disassociation between leucinemia and MPS may be further amplified when considering protein ingestion within the context of a mixed meal. It also emphasizes that placing an absolute numeric value on any trigger is not possible when comparing across studies.
FIGURE 2.
Approximate data extracted and redrawn from Abou Sawan et al. [85], 20211 and Hermans et al. [82], 20222 displaying the approximate increase in postprandial leucine concentrations (left) and postprandial muscle protein synthesis rates (right), following the ingestion of 2 different “mixed-meals” (chilli1 and a breakfast of a croissant, butter, and orange juice2), a whole-food (boiled eggs2), and a refined protein source (whey protein1). FSR, fractional synthetic rate.
Thus, although modern research into the MPS response to protein-rich whole foods and/or mixed meals has reliably been inconsistent with the leucine trigger hypothesis, it is difficult to square this with the clear utility of the concepts in vitro, in animals and in isolated protein human feeding trials. Reconciliation has been attempted by suggesting that a “whole food” effect may stimulate MPS via leucine (or amino acid) independent mechanisms or lower the leucine threshold required (i.e., increased sensitivity of the intracellular anabolic machinery to the presence of leucine), perhaps due to the action of 1 or more of the multitude of other nutrients present [73]. Indeed, perhaps leucine-independent anabolic signaling becomes more prominent when more nutritionally dense whole foods are ingested. Although the mechanisms underpinning this proposed potentiation are yet to be elucidated, an enhanced colocalization of mTORC1-Rheb complexes with the lysosome was observed following the ingestion of whole egg compared with egg white, which was correlated with the MPS response [85]. This perhaps offers a glimpse as to how whole foods might amplify the anabolic response to protein ingestion. Nonetheless, evidence for a whole-food effect has not been consistent, with the aforementioned work of West et al. [78] concerning mycoprotein within or without its whole-food matrix as a clear example. It is likely that the leucine hypothesis does not require abandonment but, rather, refining, considering these more complex experimental physiologic environments that will continue to emerge as this research area evolves.
Reconsidering protein and macronutrient coingestion studies
As summarized, it has been the advancement of research on whole foods that has prompted many to question the simplicity of the connection between dietary leucine content, plasma leucine and MPS. However, in wishing to refine the theory to conserve its utility, it is worth re-examining simpler nutritional interventions. Coingesting protein with carbohydrate or fat, in any significant quantity, has been reliably shown to decrease postprandial plasma leucine concentrations, although whether this occurs due to a decrease in the rate of appearance or an increased rate of disappearance from circulation is unclear from most studies. However, the work of Gorissen et al. [86] would suggest that carbohydrate coingestion delayed the rate of exogenous amino acid appearance rather than increasing the rate of disappearance. Equally reliable is the observation that coingestion does not impair the postprandial MPS response [[86], [87], [88], [89], [90]], offering a clear example of a dissociation between plasma leucine concentrations (and perhaps the exogenous rate of appearance) and MPS. Such data did not prompt more scrutiny over the importance of leucine alone, but this is likely due to the theory being widely accepted at the time and no consensus over a numeric value put to leucine concentrations. Also, these research questions were posed to investigate the possibility of macronutrient coingestion potentiating MPS. Therefore, the results were largely viewed as a lack of effect rather than a “rescuing” of MPS, which may be an alternative interpretation.
Mechanistically, the coingestion data demonstrate either a lack of coherence with the leucine trigger or, in keeping with the proposed “whole food effect,” suggests other factors might be lowering the pressure required on the trigger, thereby rescuing the MPS response. However, given the relative simplicity of these nutritional interventions compared with providing whole food, it is worth considering contributing factors that are largely leucine-independent. These may include caloric load providing additional substrate for energy metabolism in the fasted state (sparing protein for MPS), postprandial insulin concentrations [91,92], and/or direct signaling by carbohydrate or fat (metabolites) [34,85,93]. Indeed, although only a modest increase in insulin is required to facilitate postprandial MPS [92], it might be that the role insulin plays in modulating the anabolic response to feeding changes with escalating nutritional complexity. Regardless, this would suggest that even within this relatively simple nutritional context, other factors independent of leucine can influence the regulation of postprandial muscle protein anabolism.
Simple protein isolate/concentrate ingestion
Viewing from this more critical standpoint, even when we scrutinize the leucine trigger concept within studies employing ingestion of protein isolates alone (i.e., largely free of other macronutrients, additional energy, or food matrices), we can still find instances suggesting more complex regulation. For example, first considering dairy protein, Mitchell et al. [94] observed disparate plasma leucinemia following the ingestion of milk and whey protein concentrates but no differences in MPS rates. Similarly, Chan et al. [95] compared milk protein to a more digestible form of milk protein that was otherwise identical, and despite (as intended) manipulating plasma leucine kinetics between conditions, no difference in MPS rates was observed. Similar data have now been generated from several studies using ingestion of concentrated plant-dairy protein blends compared to dairy proteins alone in both young [96,97] and older individuals [98]. Furthermore, the ingestion of plant-based proteins, despite being mostly isolated from their characteristically different nutrient matrices, has been shown to elicit lower postprandial leucinemia compared to dairy proteins, yet mostly with comparable MPS responses [99,100]. It is also clear that example studies in line with the importance of leucine can be cherry-picked in a similar manner (e.g., [12,38,101]), but the lack of agreement between leucine and MPS in the aforementioned examples is difficult to interpret. It is possible that in all these example cases, the “threshold” for stimulating MPS rates was exceeded and that all the proteins provided were adequately leucinemic to maximize the MPS response, making the relationship impossible to observe. However, if this were the case, it would bring into question the practical utility of the leucine trigger hypothesis in any meal (19–30 g spans the above studies) amount of protein, even when isolated.
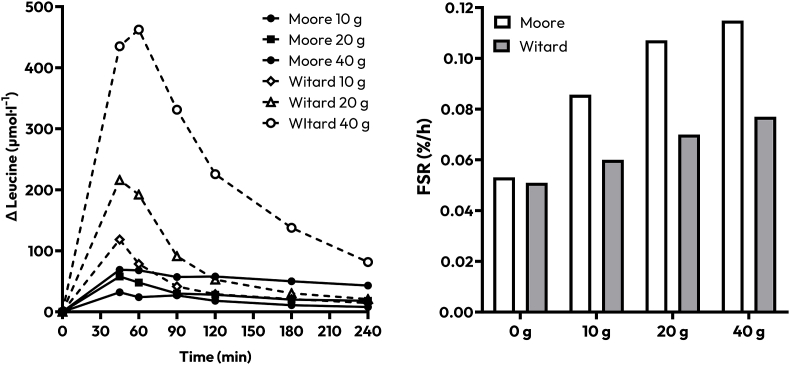
To add an additional layer of analysis, and as alluded to throughout, it is worth considering within the isolated protein studies if it is possible to locate more precisely where (in terms of concentration rather than subcellular location) a threshold may reside. Here, it is interesting to highlight an intriguing lack of agreement between 2 seminal studies from which much of our understanding of the dose-response relationship between protein intake and MPS in young individuals is derived. It is important to preface the “dose-response” aspect of the following works by acknowledging that more recent work has implied that the notion that postexercize MPS rates are maximized with relatively small boluses of protein (∼20 g) is overly simplistic [102]. However, the works of Moore et al. [10] and Witard et al. [103] concurred that 20 g of protein optimally stimulates MPS at rest (Witard et al. [103]) and following a bout of resistance exercise (both studies), at least over a ∼4 h period. It is reasonable to conclude that continuing rises in plasma leucine beyond this threshold would, therefore, (at least within their experimental setups) not beget greater MPS if a plateau has already been reached. This is in line with the principle that leucine thresholds may simply be exceeded in most studies not coherent with the trigger hypothesis. However, the leucine concentrations observed in each study to achieve the identified maximal stimulation of MPS are markedly different. Peak plasma leucine concentrations in the work of Moore et al. [10] reached ∼146 and ∼167 μmol/L–1 following the ingestion of 20 g and 40 g albumin protein, respectively. By contrast, Witard et al. [103] observed peak leucine values of ∼400 and 600 μmol/L–1 after the ingestion of 20 g and 40 g whey protein, respectively. In fact, the ingestion of 10 g of whey protein elicited plasma leucine concentrations that surpassed peak leucinemia with 40 g of albumin protein (Figure 3). This would suggest that neither the relative, in line with our systematic review [43] nor the absolute change in postprandial leucinemia regulate the proportionality of the MPS response, even within this controlled paradigm. A number of variations in the designs of the 2 studies (e.g., the tracers utilized, protein fraction measured, analytical techniques applied, different subject cohorts providing biological variation, etc.) likely contribute to these differences. However, the magnitude of absolute differences in values does illustrate the difficulty in ever quantifying an absolute plasma leucine threshold (notwithstanding biological variability would undoubtedly affect any threshold). These examples force our hand to acknowledge that the relationship between plasma leucinemia and postprandial MPS is more nuanced than previously thought by showing a lack of proportionality even within some of the “cleanest” comparisons that we have in the literature.
FIGURE 3.
Approximate data extracted and redrawn from Moore et al. [10], 2009 and Witard et al. [103], 2014 displaying the approximate increase in postprandial leucine concentrations (left) and postprandial, postexercize muscle protein synthesis rates (right), following the ingestion of varying doses (0, 10, 20, 40 g) of albumin and whey proteins, respectively. FSR, fractional synthetic rate.
Future directions
Studies to inform a clearer view of the role of exogenous leucine in the regulation of MPS may start by employing designs that allow us to delineate the relationship not only between plasma but also extra- and intramyocellular leucine (flux) and MPS in greater detail. It is quite possible that plasma leucine, intracellular leucine, and leucine flux are more disassociated than assumed, and the confusion is purely our reliance on a wealth of data concerning an inappropriate proxy (plasma leucine). It would be intriguing to examine the extent to which a bolus of amino acids devoid of leucine is capable of stimulating MPS, elucidating how essential exogenous leucine is in vivo and/or what proportion of the MPS response it might control. Understanding the mechanisms through which whole foods might potentiate the MPS response to protein ingestion and lower the onus on leucine per se is crucial. This might initially be pursued by establishing whether the “whole food effect” is a consistent phenomenon across multiple food sources and then identifying candidate nutrient(s) with anabolic potential that can be studied in a more reductive approach. There would also be value in investigating the postprandial MPS response at different meals throughout the day (rather than the research-typical first meal of the day), and the response across subsequent successive meals. This would allow us to ascertain whether the regulation of MPS by leucine differs diurnally and whether the response at a given meal is modulated by prior leucine ingestion.
In conclusion, in the present work, we aimed to advance the argument that compelling evidence now exists implying a dissociation between ingested leucine, postprandial plasma leucinemic variables, and the magnitude of the MPS response. This, in turn, brings into question the centrality of exogenous leucine for the stimulation of postprandial MPS. Although leucine clearly plays a unique role in stimulating translation initiation, and sufficient data exist to show that leucine variables partly explain postprandial muscle protein anabolism in vivo, it is clear that the relationship is not as tight as once envisioned. Human data does not refute a strong regulating effect of leucine on mTORC1; however, the translation of this mechanistic framework to the quantification of MPS in humans does not appear linear. One reason for this lack of clarity may be an overreliance on plasma leucine variables, which, despite being synonymous with the hypothesis, offers a relatively static measure that renders the muscle itself a “black box,” and therefore, less than an optimal proxy for the leucine trigger. There is a comparative lack of data considering intracellular leucine and leucine flux, which may elucidate more of the complexities associated with the regulation of MPS by leucine across the nutritional contexts discussed.
We have now observed this lack of coherence in our own work across 4 separate studies [74,[77], [79]], but this has been reported in parallel with work from multiple laboratories under a variety of conditions [[81], [84],86,95,100] and, resultantly, by 2 recent systematic reviews [43,58]. Though this observation is difficult to reconcile with the original depictions of the leucine trigger concept, it is clear that much of the discrepancy has been brought about by studies delving into the ingestion of more complex, yet practically relevant nutrient boluses (i.e., macronutrient coingestion, protein-rich whole foods, energy-rich mixed meals). Rates of postprandial leucine appearance in the plasma are modulated by the digestion and absorption kinetics of a given food/meal, which provoke markedly different plasma leucine concentrations. Nonetheless, there is clear evidence that even foods and meals that induce mild plasma leucinemia following ingestion are capable of robustly stimulating MPS. It is, therefore, difficult to identify an absolute plasma leucine concentration range that needs to be reached to maximize MPS. This does not preclude leucine as an important factor in determining postprandial MPS; it may yet still represent the single most important factor. However, it does illustrate the greater complexity of physiologic regulation in vivo in humans in response to nutritionally relevant meals.
We propose that a re-evaluation of the primary role of leucine in the regulation of the anabolic response in human muscle tissue to food intake is warranted, with a more nuanced conceptual framework required to facilitate future work to consider and incorporate other candidate mechanisms and physiologically relevant nutritional contexts. This is of key contemporary applied relevance. We, as a scientific community, are currently often using the leucine content of a dietary protein source as a de facto proxy to predict its anabolic potential, which may lead to a devaluation of prospective alternative protein sources and undermine the need for proper human experimentation. The identification of multiple other candidate nutrient anabolic mechanisms that act dependently through, or independently of, leucine may be an important mindset we have to adopt such that we do not miss the wood for the trees when attempting to generate a more sophisticated understanding of the regulation of postprandial muscle protein turnover in an era of furthering sustainable human nutrition.
Acknowledgments
We thank Freyja Haigh for her input designing and conceptualizing Figure 1.
Author contributions
The authors’ responsibilities were as follows – AJM, BTW: had the original idea for the perspective and wrote the manuscript; SW, FBS: provided intellectual input through regular scientific discussions and manuscript drafts, and all authors: read and approved the final manuscript.
Conflict of interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Benjamin Wall reports a relationship with Marlow Foods Ltd that includes: consulting or advisory, funding grants, and travel reimbursement. Francis Stephens reports a relationship with Marlow Foods Ltd that includes: consulting or advisory, funding grants, and travel reimbursement. Alistair Monteyne reports a relationship with Marlow Foods Ltd that includes: funding grants and travel reimbursement. Sam West reports a relationship with Marlow Foods Ltd that includes: funding grants and travel reimbursement. Benjamin Wall reports a relationship with BODi that includes: funding grants. Francis Stephens reports a relationship with BODi that includes: consulting or advisory and funding grants. Benjamin Wall reports a relationship with VDF FutureCeuticals Inc that includes: funding grants and travel reimbursement. Francis Stephens reports a relationship with VDF FutureCeuticals Inc that includes: funding grants and travel reimbursement. Benjamin Wall reports a relationship with PVolve that includes: funding grants. Francis Stephens reports a relationship with PVolve that includes: funding grants. Benjamin Wall reports a relationship with Science in Sport that includes: funding grants. Benjamin Wall reports a relationship with PepsiCo Inc that includes: speaking and lecture fees and travel reimbursement. Benjamin Wall reports a relationship with Danone Nutricia Research that includes: speaking and lecture fees and travel reimbursement. Francis Stephens reports a relationship with Ritual that includes: funding grants. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
The authors reported no funding received for this study.
References
- 1.Tipton K.D., Gurkin B.E., Matin S., Wolfe R.R. Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. J Nutr. Biochem. 1999;10(2):89–95. doi: 10.1016/S0955-2863(98)00087-4. [DOI] [PubMed] [Google Scholar]
- 2.Phillips S.M. The impact of protein quality on the promotion of resistance exercise-induced changes in muscle mass. Nutr. Metab. (Lond.). 2016;13(1):64. doi: 10.1186/s12986-016-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Vliet S., Beals J.W., Holwerda A.M., Emmons R.S., Goessens J.P., Paluska S.A., et al. Time-dependent regulation of postprandial muscle protein synthesis rates after milk protein ingestion in young men. J Appl. Physiol. (1985). 2019;127(6):1792–1801. doi: 10.1152/japplphysiol.00608.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore D.R., Tang J.E., Burd N.A., Rerecich T., Tarnopolsky M.A., Phillips S.M. Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J Physiol. 2009;587(4):897–904. doi: 10.1113/jphysiol.2008.164087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biolo G., Maggi S.P., Williams B.D., Tipton K.D., Wolfe R.R. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am. J Physiol. 1995;268(3 Pt 1):E514–E520. doi: 10.1152/ajpendo.1995.268.3.E514. [DOI] [PubMed] [Google Scholar]
- 6.Burd N.A., West D.W., Moore D.R., Atherton P.J., Staples A.W., Prior T., et al. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J Nutr. 2011;141(4):568–573. doi: 10.3945/jn.110.135038. [DOI] [PubMed] [Google Scholar]
- 7.Phillips S.M., Tipton K.D., Aarsland A., Wolf S.E., Wolfe R.R. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am. J Physiol. 1997;273(1 Pt 1):E99–E107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- 8.Tipton K.D., Elliott T.A., Cree M.G., Aarsland A.A., Sanford A.P., Wolfe R.R. Stimulation of net muscle protein synthesis by whey protein ingestion before and after exercise. Am. J Physiol. Endocrinol. Metab. 2007;292(1):E71–E76. doi: 10.1152/ajpendo.00166.2006. [DOI] [PubMed] [Google Scholar]
- 9.Burke L.M., Hawley J.A., Ross M.L., Moore D.R., Phillips S.M., Slater G.R., et al. Preexercise aminoacidemia and muscle protein synthesis after resistance exercise, Med. Sci. Sports Exerc. 2012;44(10):1968–1977. doi: 10.1249/MSS.0b013e31825d28fa. [DOI] [PubMed] [Google Scholar]
- 10.Moore D.R., Robinson M.J., Fry J.L., Tang J.E., Glover E.I., Wilkinson S.B., et al. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J Clin. Nutr. 2009;89(1):161–168. doi: 10.3945/ajcn.2008.26401. [DOI] [PubMed] [Google Scholar]
- 11.Pennings B., Koopman R., Beelen M., Senden J.M., Saris W.H., Van Loon L.J. Exercising before protein intake allows for greater use of dietary protein–derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am. J Clin. Nutr. 2011;93(2):322–331. doi: 10.3945/ajcn.2010.29649. [DOI] [PubMed] [Google Scholar]
- 12.Tang J.E., Moore D.R., Kujbida G.W., Tarnopolsky M.A., Phillips S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl. Physiol. 2009;107(3):987–992. doi: 10.1152/japplphysiol.00076.2009. 1985. [DOI] [PubMed] [Google Scholar]
- 13.Brook M.A., Scaife P., Bass J.J., Cegielski J., Watanabe S., Wilkinson D.J., et al. A collagen hydrolysate/milk protein-blend stimulates muscle anabolism equivalently to an isoenergetic milk protein-blend containing a greater quantity of essential amino acids in older men. Clin. Nutr. 2021;40(6):4456–4464. doi: 10.1016/j.clnu.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliot T.A., Cree M.G., Sanford A.P., Wolfe R.R., Tipton K.D. Milk Ingestion Stimulates Net Muscle Protein Synthesis following resistance exercise. Med. Sci. Sports Exerc. 2006;38(4):667–674. doi: 10.1249/01.mss.0000210190.64458.25. [DOI] [PubMed] [Google Scholar]
- 15.Holwerda A.M., Kouw I.W., Trommelen J., Halson S.L., Wodzig W.K., Verdijk L.B., et al. Physical activity performed in the evening increases the overnight muscle protein synthetic response to presleep protein ingestion in older men. J Nutr. 2016;146(7):1307–1314. doi: 10.3945/jn.116.230086. [DOI] [PubMed] [Google Scholar]
- 16.Kim I.Y., Schutzler S., Schrader A., Spencer H.J., Azhar G., Ferrando A.A., et al. The anabolic response to a meal containing different amounts of protein is not limited by the maximal stimulation of protein synthesis in healthy young adults. Am. J Physiol. Endocrinol. Metab. 2016;310(1):E73–E80. doi: 10.1152/ajpendo.00365.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biolo G., Tipton K.D., Klein S., Wolfe R.R. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am. J Physiol. 1997;273(1 Pt 1):E122–E129. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- 18.Morton R.W., Murphy K.T., McKellar S.R., Schoenfeld B.J., Henselmans M., Helms E., et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J Sports Med. 2018;52(6):376–384. doi: 10.1136/bjsports-2017-097608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stipanuk M.H. Leucine and protein synthesis: mTor and beyond, Nutr. Rev. 2007;65(3):122–129. doi: 10.1111/j.1753-4887.2007.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 20.Anthony J.C., Anthony T.G., Layman D.K. Leucine supplementation enhances skeletal muscle recovery in rats following Exercise1,2. J Nutr. 1999;129(6):1102–1106. doi: 10.1093/jn/129.6.1102. [DOI] [PubMed] [Google Scholar]
- 21.Anthony J.C., Anthony T.G., Kimball S.R., Vary T.C., Jefferson L.S. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F Formation1. J Nutr. 2000;130(2):139–145. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- 22.Anthony J.C., Yoshizawa F., Anthony T.G., Vary T.C., Jefferson L.S., Kimball S.R. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130(10):2413–2419. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- 23.Crozier S.J., Kimball S.R., Emmert S.W., Anthony J.C., Jefferson L.S. Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J Nutr. 2005;135(3):376–382. doi: 10.1093/jn/135.3.376. [DOI] [PubMed] [Google Scholar]
- 24.Suryawan A., Jeyapalan A.S., Orellana R.A., Wilson F.A., Nguyen H.V., Davis T.A. Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am. J Physiol. Endocrinol. Metab. 2008;295(4):E868–E875. doi: 10.1152/ajpendo.90314.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buse M.G., Reid S.S. Leucine. A possible regulator of protein turnover in muscle. J Clin. Invest. 1975;56(5):1250–1261. doi: 10.1172/JCI108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J.B., Jefferson L.S. Influence of amino acid availability on protein turnover in perfused skeletal muscle. Biochim. Biophys. Acta. 1978;544(2):351–359. doi: 10.1016/0304-4165(78)90103-4. [DOI] [PubMed] [Google Scholar]
- 27.Hong S.O., Layman D.K. Effects of leucine on in vitro protein synthesis and degradation in rat skeletal muscles. J Nutr. 1984;114(7):1204–1212. doi: 10.1093/jn/114.7.1204. [DOI] [PubMed] [Google Scholar]
- 28.Atherton P.J., Smith K., Etheridge T., Rankin D., Rennie M.J. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids. 2010;38(5):1533–1539. doi: 10.1007/s00726-009-0377-x. [DOI] [PubMed] [Google Scholar]
- 29.Deldicque L., Sanchez Canedo C., Horman S., De Potter I., Bertrand L., Hue L., et al. Antagonistic effects of leucine and glutamine on the mTOR pathway in myogenic C2C12 cells. Amino Acids. 2008;35(1):147–155. doi: 10.1007/s00726-007-0607-z. [DOI] [PubMed] [Google Scholar]
- 30.Dickinson J.M., Fry C.S., Drummond M.J., Gundermann D.M., Walker D.K., Glynn E.L., et al. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr. 2011;141(5):856–862. doi: 10.3945/jn.111.139485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng D., Yang Q., Wang H., Melick C.H., Navlani R., Frank A.R., et al. Glutamine and asparagine activate mTORC1 independently of Rag GTPases. J Biol. Chem. 2020;295(10):2890–2899. doi: 10.1074/jbc.AC119.011578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Churchward-Venne T.A., Burd N.A., Mitchell C.J., West D.W., Philp A., Marcotte G.R., et al. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J Physiol. 2012;590(11):2751–2765. doi: 10.1113/jphysiol.2012.228833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chantranupong L., Scaria S.M., Saxton R.A., Gygi M.P., Shen K., Wyant G.A., et al. The CASTOR proteins are arginine sensors for the mTORC1 pathway. Cell. 2016;165(1):153–164. doi: 10.1016/j.cell.2016.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castellano B.M., Thelen A.M., Moldavski O., Feltes M., van der Welle R.E., Mydock-McGrane L., et al. Lysosomal cholesterol activates mTORC1 via an SLC38A9–Niemann-Pick C1 signaling complex. Science. 2017;355(6331):1306–1311. doi: 10.1126/science.aag1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gwinn D.M., Shackelford D.B., Egan D.F., Mihaylova M.M., Mery A., Vasquez D.S., et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell. 2008;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norton L.E., Wilson G.J., Layman D.K., Moulton C.J., Garlick P.J. Leucine content of dietary proteins is a determinant of postprandial skeletal muscle protein synthesis in adult rats. Nutr. Metab. (Lond.). 2012;9(1):67. doi: 10.1186/1743-7075-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koopman R., Crombach N., Gijsen A.P., Walrand S., Fauquant J., Kies A.K., et al. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am. J Clin. Nutr. 2009;90(1):106–115. doi: 10.3945/ajcn.2009.27474. [DOI] [PubMed] [Google Scholar]
- 38.Pennings B., Boirie Y., Senden J.M., Gijsen A.P., Kuipers H., van Loon L.J. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J Clin. Nutr. 2011;93(5):997–1005. doi: 10.3945/ajcn.110.008102. [DOI] [PubMed] [Google Scholar]
- 39.Burd N.A., Yang Y., Moore D.R., Tang J.E., Tarnopolsky M.A., Phillips S.M. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br. J Nutr. 2012;108(6):958–962. doi: 10.1017/S0007114511006271. [DOI] [PubMed] [Google Scholar]
- 40.West D.W., Burd N.A., Coffey V.G., Baker S.K., Burke L.M., Hawley J.A., et al. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise1–4. Am. J Clin. Nutr. 2011;94(3):795–803. doi: 10.3945/ajcn.111.013722. [DOI] [PubMed] [Google Scholar]
- 41.D’Hulst G., Masschelein E., De Bock K. Resistance exercise enhances long-term mTORC1 sensitivity to leucine. Mol. Metab. 2022;66 doi: 10.1016/j.molmet.2022.101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joint WHO/FAO/UNU Expert Consultation, Protein and amino acid requirements in human nutrition. World Health Organ. Tech. Rep. Ser. 2007;935:1–265. back cover. [PubMed] [Google Scholar]
- 43.Wilkinson K., Koscien C.P., Monteyne A.J., Wall B.T., Stephens F.B. Association of postprandial postexercise muscle protein synthesis rates with dietary leucine: A systematic review. Physiol. Rep. 2023;11(15) doi: 10.14814/phy2.15775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolfson R.L., Chantranupong L., Saxton R.A., Shen K., Scaria S.M., Cantor J.R., et al. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science. 2016;351(6268):43–48. doi: 10.1126/science.aab2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bar-Peled L., Sabatini D.M. Regulation of mTORC1 by amino acids. Trends Cell Biol. 2014;24(7):400–406. doi: 10.1016/j.tcb.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Betz C., Hall M.N. Where is mTOR and what is it doing there? J Cell Biol. 2013;203(4):563–574. doi: 10.1083/jcb.201306041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bohé J., Low A., Wolfe R.R., Rennie M.J. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: A dose–response study. J Physiol. 2003;552(1):315–324. doi: 10.1113/jphysiol.2003.050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dickinson J.M., Rasmussen B.B. Amino acid transporters in the regulation of human skeletal muscle protein metabolism. Curr. Opin. Clin. Nutr. Metab. Care. 2013;16(6):638–644. doi: 10.1097/MCO.0b013e3283653ec5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glynn E.L., Fry C.S., Drummond M.J., Timmerman K.L., Dhanani S., Volpi E., et al. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J Nutr. 2010;140(11):1970–1976. doi: 10.3945/jn.110.127647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilkinson D.J., Bukhari S.S., Phillips B.E., Limb M.C., Cegielski J., Brook M.S., et al. Effects of leucine-enriched essential amino acid and whey protein bolus dosing upon skeletal muscle protein synthesis at rest and after exercise in older women. Clin. Nutr. 2018;37(6 Pt A):2011–2021. doi: 10.1016/j.clnu.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Churchward-Venne T.A., Breen L., Di Donato D.M., Hector A.J., Mitchell C.J., Moore D.R., et al. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial. Am. J Clin. Nutr. 2014;99(2):276–286. doi: 10.3945/ajcn.113.068775. [DOI] [PubMed] [Google Scholar]
- 52.Fuchs C.J., Hermans W.J., Holwerda A.M., Smeets J.S., Senden J.M., van Kranenburg J., et al. Branched-chain amino acid and branched-chain ketoacid ingestion increases muscle protein synthesis rates in vivo in older adults: a double-blind, randomized trial. Am. J Clin. Nutr. 2019;110(4):862–872. doi: 10.1093/ajcn/nqz120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jackman S.R., Witard O.C., Philp A., Wallis G.A., Baar K., Tipton K.D. Branched-Chain amino acid Ingestion Stimulates Muscle Myofibrillar Protein Synthesis following resistance exercise in humans. Front Physiol. 2017;8:390. doi: 10.3389/fphys.2017.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wall B.T., Hamer H.M., de Lange A., Kiskini A., Groen B.B., Senden J.M., et al. Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clin. Nutr. 2013;32(3):412–419. doi: 10.1016/j.clnu.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 55.Devries M.C., McGlory C., Bolster D.R., Kamil A., Rahn M., Harkness L., et al. Leucine, not total protein, content of a supplement is the primary determinant of muscle protein anabolic responses in healthy older women. J Nutr. 2018;148(7):1088–1095. doi: 10.1093/jn/nxy091. [DOI] [PubMed] [Google Scholar]
- 56.Holwerda A.M., Paulussen K.J., Overkamp M., Goessens J.P., Kramer I.F., Wodzig W.K., et al. Leucine coingestion augments the muscle protein synthetic response to the ingestion of 15 g of protein following resistance exercise in older men. Am. J Physiol. Endocrinol. Metab. 2019;317(3):E473–E482. doi: 10.1152/ajpendo.00073.2019. [DOI] [PubMed] [Google Scholar]
- 57.Murphy C.H., Saddler N.I., Devries M.C., McGlory C., Baker S.K., Phillips S.M. Leucine supplementation enhances integrative myofibrillar protein synthesis in free-living older men consuming lower- and higher-protein diets: a parallel-group crossover study. Am. J Clin. Nutr. 2016;104(6):1594–1606. doi: 10.3945/ajcn.116.136424. [DOI] [PubMed] [Google Scholar]
- 58.Zaromskyte G., Prokopidis K., Ioannidis T., Tipton K.D., Witard O.C. Evaluating the leucine trigger hypothesis to explain the post-prandial regulation of muscle protein synthesis in young and older adults: A systematic review. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.685165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katsanos C.S., Kobayashi H., Sheffield-Moore M., Aarsland A., Wolfe R.R. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am. J Physiol. Endocrinol. Metab. 2006;291(2):E381–E387. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 60.Wall B.T., Gorissen S.H., Pennings B., Koopman R., Groen B.B., Verdijk L.B., et al. Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PLOS ONE. 2015;10(11) doi: 10.1371/journal.pone.0140903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gorissen S.H., Trommelen J., Kouw I.W., Holwerda A.M., Pennings B., Groen B.B., et al. Protein type, protein dose, and age modulate dietary protein digestion and phenylalanine absorption kinetics and plasma phenylalanine availability in humans. J Nutr. 2020;150(8):2041–2050. doi: 10.1093/jn/nxaa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Volpi E., Mittendorfer B., Wolf S.E., Wolfe R.R. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am. J Physiol. 1999;277(3):E513–E520. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- 63.Boirie Y., Gachon P., Beaufrère B. Splanchnic and whole-body leucine kinetics in young and elderly men. Am. J Clin. Nutr. 1997;65(2):489–495. doi: 10.1093/ajcn/65.2.489. [DOI] [PubMed] [Google Scholar]
- 64.Timmerman K.L., Lee J.L., Fujita S., Dhanani S., Dreyer H.C., Fry C.S., et al. Pharmacological vasodilation improves insulin-stimulated muscle protein anabolism but not glucose utilization in older adults. Diabetes. 2010;59(11):2764–2771. doi: 10.2337/db10-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rasmussen B.B., Fujita S., Wolfe R.R., Mittendorfer B., Roy M., Rowe V.L., et al. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006;20(6):768–769. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cuthbertson D., Smith K., Babraj J., Leese G., Waddell T., Atherton P., et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19(3):422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 67.Fry C.S., Drummond M.J., Glynn E.L., Dickinson J.M., Gundermann D.M., Timmerman K.L., et al. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis, Skelet. Muscle. 2011;1(1):11. doi: 10.1186/2044-5040-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moughan P.J. Holistic properties of foods: a changing paradigm in human nutrition. J Sci. Food Agric. 2020;100(14):5056–5063. doi: 10.1002/jsfa.8997. [DOI] [PubMed] [Google Scholar]
- 69.Jacobs D.R., Gross M.D., Tapsell L.C. Food synergy: an operational concept for understanding nutrition. Am. J Clin. Nutr. 2009;89(5):1543S–1548S. doi: 10.3945/ajcn.2009.26736B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Vliet S., Bain J.R., Muehlbauer M.J., Provenza F.D., Kronberg S.L., Pieper C.F., et al. A metabolomics comparison of plant-based meat and grass-fed meat indicates large nutritional differences despite comparable Nutrition Facts panels. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-93100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Vliet S., Shy E.L., Abou Sawan S.S., Beals J.W., West D.W., Skinner S.K., et al. Consumption of whole eggs promotes greater stimulation of postexercise muscle protein synthesis than consumption of isonitrogenous amounts of egg whites in young men. Am. J Clin. Nutr. 2017;106(6):1401–1412. doi: 10.3945/ajcn.117.159855. [DOI] [PubMed] [Google Scholar]
- 72.Paulussen K.J., Barnes T.M., Askow A.T., Salvador A.F., McKenna C.F., Scaroni S.E., et al. Underpinning the food matrix regulation of postexercise myofibrillar protein synthesis by comparing salmon ingestion with the sum of its isolated nutrients in healthy young adults. J Nutr. 2023;153(5):1359–1372. doi: 10.1016/j.tjnut.2023.02.037. [DOI] [PubMed] [Google Scholar]
- 73.Burd N.A., Beals J.W., Martinez I.G., Salvador A.F., Skinner S.K. Food First approach to enhance the regulation of post-exercise skeletal muscle protein synthesis and remodeling. Sports Med. 2019;49(1):59–68. doi: 10.1007/s40279-018-1009-y. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Monteyne A.J., Coelho M.O., Porter C., Abdelrahman D.R., Jameson T.S., Jackman S.R., et al. Mycoprotein ingestion stimulates protein synthesis rates to a greater extent than milk protein in rested and exercised skeletal muscle of healthy young men: a randomized controlled trial. Am. J Clin. Nutr. 2020;112(2):318–333. doi: 10.1093/ajcn/nqaa092. [DOI] [PubMed] [Google Scholar]
- 75.Colosimo R., Warren F.J., Finnigan T.J., Wilde P.J. Protein bioaccessibility from mycoprotein hyphal structure: in vitro investigation of underlying mechanisms. Food Chem. 2020;330 doi: 10.1016/j.foodchem.2020.127252. [DOI] [PubMed] [Google Scholar]
- 76.Dunlop M.V., Kilroe S.P., Bowtell J.L., Finnigan T.J., Salmon D.L., Wall B.T. Mycoprotein represents a bioavailable and insulinotropic non-animal-derived dietary protein source: a dose–response study. Br. J Nutr. 2017;118(9):673–685. doi: 10.1017/S0007114517002409. [DOI] [PubMed] [Google Scholar]
- 77.Monteyne A.J., Coelho M.O., Porter C., Abdelrahman D.R., Jameson T.S., Finnigan T.J., et al. Branched-Chain Amino Acid Fortification Does Not Restore Muscle Protein Synthesis Rates following Ingestion of Lower- Compared with Higher-Dose mycoprotein. J Nutr. 2020;150(11):2931–2941. doi: 10.1093/jn/nxaa251. [DOI] [PubMed] [Google Scholar]
- 78.West S., Monteyne A.J., Whelehan G., Abdelrahman D.R., Murton A.J., Finnigan T.J., et al. Mycoprotein ingestion within or without its whole-food matrix results in equivalent stimulation of myofibrillar protein synthesis rates in resting and exercised muscle of young men. Br. J Nutr. 2023;130(1):20–32. doi: 10.1017/S0007114522003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van der Heijden I., West S., Monteyne A.J., Finnigan T.J., Abdelrahman D.R., Murton A.J., et al. Algae ingestion increases resting and exercised myofibrillar protein synthesis rates to a similar extent as mycoprotein in young adults. J Nutr. 2023;153(12):3406–3417. doi: 10.1016/j.tjnut.2023.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.West S., Monteyne A.J., Whelehan G., van der Heijden I., Abdelrahman D.R., Murton A.J., et al. Ingestion of mycoprotein, pea protein, and their blend support comparable postexercise myofibrillar protein synthesis rates in resistance-trained individuals. Am. J Physiol. Endocrinol. Metab. 2023;325(3):E267–E279. doi: 10.1152/ajpendo.00166.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fuchs C.J., Hermans W.J., Smeets J.S., Senden J.M., van Kranenburg J., Gorissen S.H., et al. Raw eggs to support postexercise recovery in healthy young men: did rocky get it right or wrong? J Nutr. 2022;152(11):2376–2386. doi: 10.1093/jn/nxac174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hermans W.J., Fuchs C.J., Hendriks F.K., Houben L.H., Senden J.M., Verdijk L.B., et al. Cheese ingestion increases muscle protein synthesis rates both at rest and during recovery from exercise in healthy, young males: A randomized parallel-group trial. J Nutr. 2022;152(4):1022–1030. doi: 10.1093/jn/nxac007. [DOI] [PubMed] [Google Scholar]
- 83.Hermans W.J., Senden J.M., Churchward-Venne T.A., Paulussen K.J., Fuchs C.J., Smeets J.S., et al. Insects are a viable protein source for human consumption: from insect protein digestion to postprandial muscle protein synthesis in vivo in humans: a double-blind randomized trial. Am. J Clin. Nutr. 2021;114(3):934–944. doi: 10.1093/ajcn/nqab115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gwin J.A., Church D.D., Hatch-McChesney A., Allen J.T., Wilson M.A., Varanoske A.N., et al. Essential amino acid-enriched whey enhances post-exercise whole-body protein balance during energy deficit more than iso-nitrogenous whey or a mixed-macronutrient meal: a randomized, crossover study. J Int. Soc. Sports Nutr. 2021;18(1):4. doi: 10.1186/s12970-020-00401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abou Sawan S., van Vliet S., West D.W., Beals J.W., Paluska S.A., Burd N.A., et al. Whole egg, but not egg white, ingestion induces mTOR colocalization with the lysosome after resistance exercise, Am. J Physiol. Cell Physiol. 2018;315(4):C537–C543. doi: 10.1152/ajpcell.00225.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gorissen S.H., Burd N.A., Hamer H.M., Gijsen A.P., Groen B.B., van Loon L.J. Carbohydrate coingestion delays dietary protein digestion and absorption but does not modulate postprandial muscle protein accretion. J Clin. Endocrinol. Metab. 2014;99(6):2250–2258. doi: 10.1210/jc.2013-3970. [DOI] [PubMed] [Google Scholar]
- 87.Staples A.W., Burd N.A., West D.W., Currie K.D., Atherton P.J., Moore D.R., et al. Carbohydrate does not augment exercise-induced protein accretion versus protein alone. Med. Sci. Sports Exerc. 2011;43(7):1154–1161. doi: 10.1249/MSS.0b013e31820751cb. [DOI] [PubMed] [Google Scholar]
- 88.Hamer H.M., Wall B.T., Kiskini A., de Lange A., Groen B.B., Bakker J.A., et al. Carbohydrate co-ingestion with protein does not further augment post-prandial muscle protein accretion in older men. Nutr. Metab. (Lond.). 2013;10(1):15. doi: 10.1186/1743-7075-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koopman R., Beelen M., Stellingwerff T., Pennings B., Saris W.H., Kies A.K., et al. Coingestion of carbohydrate with protein does not further augment postexercise muscle protein synthesis. Am. J Physiol. Endocrinol. Metab. 2007;293(3):E833–E842. doi: 10.1152/ajpendo.00135.2007. [DOI] [PubMed] [Google Scholar]
- 90.Gorissen S.H., Burd N.A., Kramer I.F., van Kranenburg J., Gijsen A.P., Rooyackers O., et al. Co-ingesting milk fat with micellar casein does not affect postprandial protein handling in healthy older men. Clin. Nutr. 2017;36(2):429–437. doi: 10.1016/j.clnu.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 91.Abdulla H., Smith K., Atherton P.J., Idris I. Role of insulin in the regulation of human skeletal muscle protein synthesis and breakdown: a systematic review and meta-analysis. Diabetologia. 2016;59(1):44–55. doi: 10.1007/s00125-015-3751-0. [DOI] [PubMed] [Google Scholar]
- 92.Greenhaff P.L., Karagounis L.G., Peirce N., Simpson E.J., Hazell M., Layfield R., et al. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am. J Physiol. Endocrinol. Metab. 2008;295(3):E595–E604. doi: 10.1152/ajpendo.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yasuda M., Tanaka Y., Kume S., Morita Y., Chin-Kanasaki M., Araki H., et al. Fatty acids are novel nutrient factors to regulate mTORC1 lysosomal localization and apoptosis in podocytes. Biochim. Biophys. Acta. 2014;1842(7):1097–1108. doi: 10.1016/j.bbadis.2014.04.001. PMID 24726883. [DOI] [PubMed] [Google Scholar]
- 94.Mitchell C.J., McGregor R.A., D’Souza R.F., Thorstensen E.B., Markworth J.F., Fanning A.C., et al. Consumption of milk protein or whey protein results in a similar increase in muscle protein synthesis in middle aged men. Nutrients. 2015;7(10):8685–8699. doi: 10.3390/nu7105420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chan A.H., D’Souza R.F., Beals J.W., Zeng N., Prodhan U., Fanning A.C., et al. The degree of aminoacidemia after dairy protein ingestion does not modulate the postexercise anabolic response in young men: A randomized controlled trial. J Nutr. 2019;149(9):1511–1522. doi: 10.1093/jn/nxz099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reidy P.T., Walker D.K., Dickinson J.M., Gundermann D.M., Drummond M.J., Timmerman K.L., et al. Protein blend ingestion following resistance exercise promotes human muscle protein synthesis. J Nutr. 2013;143(4):410–416. doi: 10.3945/jn.112.168021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pinckaers P.J., Kouw I.W., Gorissen S.H., Houben L.H., Senden J.M., Wodzig W.K., et al. The muscle protein synthetic response to the ingestion of a plant-derived protein blend does not differ from an equivalent amount of milk protein in healthy young males. J Nutr. 2023;152(12):2734–2743. doi: 10.1093/jn/nxac222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Borack M.S., Reidy P.T., Husaini S.H., Markofski M.M., Deer R.R., Richison A.B., et al. Soy-dairy protein blend or whey protein isolate ingestion induces similar postexercise muscle mechanistic target of rapamycin complex 1 signaling and protein synthesis responses in older men. J Nutr. 2016;146(12):2468–2475. doi: 10.3945/jn.116.231159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pinckaers P.J., Kouw I.W., Hendriks F.K., van Kranenburg J.M., de Groot L.C., Verdijk L.B., et al. No differences in muscle protein synthesis rates following ingestion of wheat protein, milk protein, and their protein blend in healthy, young males. Br. J Nutr. 2021;126(12):1832–1842. doi: 10.1017/S0007114521000635. [DOI] [PubMed] [Google Scholar]
- 100.Pinckaers P.J., Hendriks F.K., Hermans W.J., Goessens J.P., Senden J.M., van Kranenburg J.M., et al. Potato protein ingestion increases muscle protein synthesis rates at rest and during recovery from exercise in humans. Med. Sci. Sports Exerc. 2022;54(9):1572–1581. doi: 10.1249/MSS.0000000000002937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang Y., Churchward-Venne T.A., Burd N.A., Breen L., Tarnopolsky M.A., Phillips S.M. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr. Metab. (Lond.). 2012;9(1):57. doi: 10.1186/1743-7075-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Trommelen J., van Lieshout G.A., Nyakayiru J., Holwerda A.M., Smeets J.S., Hendriks F.K., et al. The anabolic response to protein ingestion during recovery from exercise has no upper limit in magnitude and duration in vivo in humans, Cell Rep. Med. 2023;4(12) doi: 10.1016/j.xcrm.2023.101324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Witard O.C., Jackman S.R., Breen L., Smith K., Selby A., Tipton K.D. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am. J Clin. Nutr. 2014;99(1):86–95. doi: 10.3945/ajcn.112.055517. [DOI] [PubMed] [Google Scholar]