Abstract
Background and Aims:
A single-nation study reported that pretreatment HBV viral load is associated with on-treatment risk of HCC in patients who are HBeAg-positive without cirrhosis and with chronic hepatitis B initiating antiviral treatment. We aimed to validate the association between baseline HBV viral load and on-treatment HCC risk in a larger, multinational cohort.
Approach and Results:
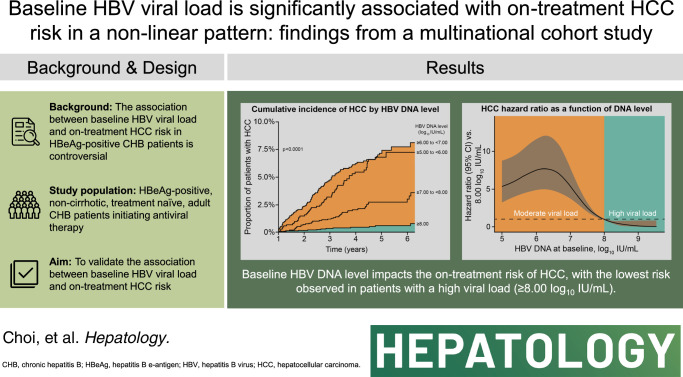
Using a multinational cohort from Korea, Hong Kong, and Taiwan involving 7545 adult patients with HBeAg-positive, without cirrhosis and with chronic hepatitis B who started entecavir or tenofovir treatment with baseline HBV viral load ≥5.00 log10 IU/mL, HCC risk was estimated by baseline viral load. HBV viral load was analyzed as a categorical variable. During continuous antiviral treatment (median, 4.28 y), HCC developed in 200 patients (incidence rate, 0.61 per 100 person-years). Baseline HBV DNA level was independently associated with on-treatment HCC risk in a nonlinear pattern. HCC risk was lowest with the highest baseline viral load (≥8.00 log10 IU/mL; incidence rate, 0.10 per 100 person-years), but increased sharply as baseline viral load decreased. The adjusted HCC risk was 8.05 times higher (95% CI, 3.34–19.35) with baseline viral load ≥6.00 and <7.00 log10 IU/mL (incidence rate, 1.38 per 100 person-years) compared with high (≥8.00 log10 IU/mL) baseline viral load (p<0.001).
Conclusions:
In a multinational cohort of adult patients with HBeAg-positive without cirrhosis and with chronic hepatitis B, baseline HBV viral load was significantly associated with HCC risk despite antiviral treatment. Patients with the highest viral load who initiated treatment had the lowest long-term risk of HCC development.
INTRODUCTION
Chronic hepatitis B virus infection (CHB) is the most important global cause of HCC, the most common primary cancer of the liver.1,2 Liver cancer is the third leading cause of cancer-related death worldwide and rapidly fatal in the majority of patients, with 905,677 new cases and 830,180 deaths in 2020.1 Without antiviral treatment, up to 25% of patients with CHB develop HCC in their lifetime.2 It has been consistently shown that long-term antiviral treatment is tolerable and reduces HCC risk and mortality in patients with CHB.3,4 Nonetheless, globally, only 2.2% of patients with CHB received antiviral treatment in 2019.5
One reason for the severely limited treatment coverage for CHB infection is that current clinical practice guidelines restrict antiviral treatment primarily to patients with confirmed hepatic necroinflammation, that is, elevated alanine aminotransferase (ALT), regardless of serum HBV DNA levels.3,4,6 Within these guidelines, patients in the initial phase of HBV infection with positive HBeAg and high serum HBV DNA levels (≥8.00 log10 IU/mL) may not qualify for treatment, often needing to wait until ALT levels become elevated.
Serum HBV DNA levels become rapidly undetectable after the initiation of antiviral treatment in patients with CHB.3 Therefore, baseline HBV viral load has been regarded as an insignificant predictor of on-treatment HCC risk in patients with CHB.7–9 However, a recent single-nation study found that baseline viral load was inversely associated with HCC risk in patients who are HBeAg-positive with CHB during antiviral treatment.10 The on-treatment risk of HCC was 6 times higher in patients initiating treatment with baseline HBV viral loads of 5.00–5.99 log10 IU/mL versus patients with baseline viral load ≥8.00 log10 IU/mL. The increased risk of HCC in patients with decreased viral load was reducible, but not completely reversible, with antiviral treatment.10 These findings suggest that adult patients who are HBeAg-positive without cirrhosis with high viral load may have to be treated regardless of ALT levels, as treatment in this early phase could prevent HCC risk from irreversibly increasing. However, the findings from this single-nation study were not readily generalizable.
This study aimed to further investigate the association between baseline HBV viral load and HCC risk in adult patients with HBeAg-positive without cirrhosis initiating antiviral treatment in a larger, multinational data set.
METHODS
Study population
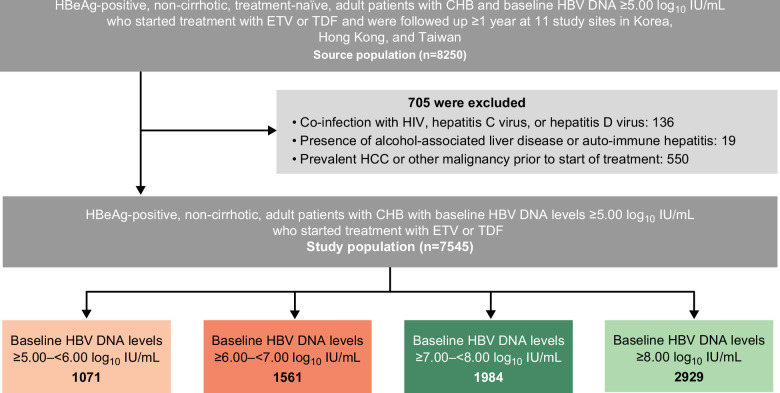
The source population (n=8250) for the present study was derived from a historical cohort of adult patients with HBeAg-positive without cirrhosis, treatment-naïve, and with CHB with baseline HBV viral load ≥5.00 log10 IU/mL who initiated either entecavir or tenofovir disoproxil fumarate (TDF) treatment and were followed up for ≥1 year, from 11 study sites in Korea, Hong Kong, and Taiwan (Figure 1, Supplemental Table S1, http://links.lww.com/HEP/I218, Supplemental Table S2, http://links.lww.com/HEP/I218).
FIGURE 1.
Patient flow. Abbreviations: CHB, chronic hepatitis B; ETV, entecavir; TDF, tenofovir disoproxil fumarate.
Patients were ineligible for inclusion if they had HBV viral load <5.00 log10 IU/mL at baseline; were missing HBV viral load; were HBsAg-negative or HBeAg-negative at baseline or had either status missing; were coinfected with HIV, HCV, or HDV; exhibited evidence of alcohol-associated liver disease or autoimmune hepatitis, prevalent HCC, cirrhosis (or status missing), or any other malignancy before baseline; had received a liver transplant before or within 12 months of treatment initiation; or developed HCC within 12 months of treatment initiation.
The presence of cirrhosis was determined using a composite objective definition to account for variation between study sites. Patients with site-defined cirrhosis and Child-Pugh score ≥6 were classified as cirrhotic; in addition, patients with baseline platelet count <100,000/µL were classified as cirrhotic.
Outcome
The outcome of interest was HCC incidence. The index date was the date of initiating entecavir or TDF, and patients were followed up with regular HCC surveillance every 6 months from the index date to the date of HCC development, liver transplantation, or death. Histologic examination or characteristic imaging features (nodule >1 cm with arterial hypervascularization and portal/delayed-phase washout) on dynamic CT or MRI were used to diagnose HCC.11,12
Statistical analyses
Patients were grouped for analysis into 4 tiers of baseline HBV viral load. Baseline characteristics were summarized with continuous variables presented as the median (Q1, Q3) and categorical or discrete variables presented as n (%). Full details on statistical analyses are provided in the Supplemental Appendix, http://links.lww.com/HEP/I218.
In summary, all analyses aimed to compare on-treatment risk of HCC across HBV DNA viral load tiers. Missing data were evaluated and then multiply imputed using predictive mean matching. Variables included in the imputation process are specified in Supplemental Table S3, http://links.lww.com/HEP/I218. The proportional hazards (PH) assumption was inspected for one multiply imputed dataset by means of Schoenfeld residuals (Supplemental Figure S1). Patients were censored at 6.5 years’ follow-up to meet the PH assumption.
Cumulative HCC incidence curves were stratified by baseline HBV viral load and compared using the log-rank test. A multivariable Cox PH model was fitted to assess the association between baseline HBV viral load and HCC risk with the time to event of HCC as the outcome of interest. The model adjusted for all variables specified in Supplemental Table S3, http://links.lww.com/HEP/I218. Propensity score weighting (PSW) and propensity score matching (PSM) analyses were conducted to reduce bias by balancing baseline characteristics between patients of the different viral load tiers; the same variables were adjusted for in the propensity score models as in the primary multivariable analysis, with the exception of viral load.
On-treatment HCC risk by baseline HBV viral load was modeled using a penalized spline regression.
Sensitivity analyses were additionally conducted. A nested case-control analysis aimed to show the robustness of the primary results. A further multivariable Cox PH analysis excluded patients from the Choi et al study13 to validate the findings from Choi et al without bias resulting from the inclusion of the same group of patients in both analyses.
Stratified multivariable Cox PH analyses were conducted for baseline age (one which stratified patients into 2 groups: <50 years and ≥50 years and one which stratified patients into 4 groups: <40 years, ≥40 years and <50 years, ≥50 years and <60 years, and ≥60 years), modified Platelet Age GEnder-HBV (mPAGE-B) level (<11 and ≥11),14 and platelet count (<150,000/µL and ≥150,000/µL) to assess the impact of these characteristics on the results.
Three subgroup multivariable Cox PH analyses with age (<50 y and ≥50 y; or <40 y, ≥40 y and <50 y, ≥50 y and <60 y, and ≥60 y) or mPAGE-B score (<11 and ≥11) as a categorical fixed effect were conducted to determine the moderation effect between HBV viral load and age or mPAGE-B score as categorical variables.
R version 4.2.1 was used for all statistical analyses.15 All tests were two-sided, and a significance level of p<0.05 was used across the analyses.
RESULTS
Patient disposition and baseline characteristics
After the application of eligibility criteria, data from 7545 patients were included in the analysis. Patients were grouped into 4 tiers of baseline HBV viral load: ≥8.00 log10 IU/mL, ≥7.00 and <8.00 log10 IU/mL, ≥6.00 and <7.00 log10 IU/mL, and ≥5.00 and <6.00 log10 IU/mL, with over 1000 patients in each tier (Table 1 and Figure 1). Median (Q1, Q3) age for all patients was 44 (35, 53) years and most were male (61.6%). Median (Q1, Q3) follow-up time was 4.3 (2.7, 5.0) years. Baseline characteristics were similar across HBV viral load tiers except for age, platelet counts, and ALT; patients in lower viral load tiers were older and had lower platelet counts and ALT levels, which were well-balanced after PSW and PSM with standardized mean differences <0.1 for all variables (Supplemental Table S4, http://links.lww.com/HEP/I218 and Supplemental Table S5, http://links.lww.com/HEP/I218).
TABLE 1.
Baseline characteristics of the study population of 7545 patients
| HBV viral load (log10 IU/mL) | |||||
|---|---|---|---|---|---|
| Variable | Whole cohort (n=7545) | ≥5.00 to <6.00 (n=1071) | ≥6.00 to <7.00 (n=1561) | ≥7.00 to <8.00 (n=1984) | ≥8.00 (n=2929) |
| Age (y) | 44 (35, 53) | 50 (42, 57) | 48 (38, 56) | 42 (34, 52) | 40 (33, 50) |
| Sex (male) | 4648 (61.6) | 666 (62.2) | 984 (63.0) | 1139 (57.4) | 1859 (63.5) |
| Platelet count (1000 platelets/µL) | 187 (154, 226) | 175 (142, 214) | 175 (143, 213) | 187 (154, 227) | 197 (166, 235) |
| ALT (U/L)a | 111 (62, 231) | 66 (42, 126) | 83 (50, 161) | 128 (75, 266) | 135 (84, 280) |
| Albumin (g/dL) | 4.1 (3.8, 4.4) | 4.2 (3.9, 4.4) | 4.1 (3.9, 4.4) | 4.1 (3.8, 4.4) | 4.1 (3.8, 4.4) |
| Bilirubin (mg/dL) | 0.8 (0.6, 1.1) | 0.8 (0.6, 1.1) | 0.8 (0.6, 1.1) | 0.8 (0.6, 1.1) | 0.8 (0.6, 1.1) |
| Creatinine (mg/dL) | 0.9 (0.7, 1.0) | 0.9 (0.7, 1.0) | 0.9 (0.7, 1.0) | 0.8 (0.7, 1.0) | 0.8 (0.7, 1.0) |
| Diabetes | 828 (11.0) | 154 (14.4) | 219 (14.0) | 208 (10.5) | 247 (8.4) |
| Hypertension | 1333 (17.7) | 240 (22.4) | 349 (22.4) | 342 (17.2) | 402 (13.7) |
| mPAGE-B scoreb | 9 (7, 12) | 10 (8, 12) | 10 (8, 12) | 9 (6, 11) | 8 (6, 11) |
| Patients receiving each treatment | ETV: 5564 (73.7) | ETV: 809 (75.5) | ETV: 1198 (76.8) | ETV: 1434 (72.3) | ETV: 2123 (72.5) |
| TDF: 1981 (26.3) | TDF: 262 (24.5) | TDF: 363 (23.3) | TDF: 550 (27.7) | TDF: 806 (27.5) | |
| Follow-up time (years) | 4.3 (2.7, 5.0) | 4.2 (2.8, 5.0) | 4.2 (2.6, 5.0) | 4.4 (2.7, 5.0) | 4.3 (2.6, 5.1) |
Continuous variables are summarized using median (Q1, Q3), categorical variables are summarized using n (%); missing data for each variable were excluded when producing these summaries.
ALT was adjusted for as a categorical variable, split into the following levels based on ULN (35 U/L for males, 25 U/L for females): <1 × ULN (n=449 [6.0%]); ≥1 to <2 × ULN (n=1434 [19.0%]); ≥2 × ULN (n=5662 [75.0%]).
Includes age, sex, platelet count, and albumin.
Abbreviations: ALT, alanine aminotransferase; ETV, entecavir; mPAGE-B, modified Platelet Age GEnder-HBV; TDF, tenofovir disoproxil fumarate; ULN, upper limit of normal.
HCC risk
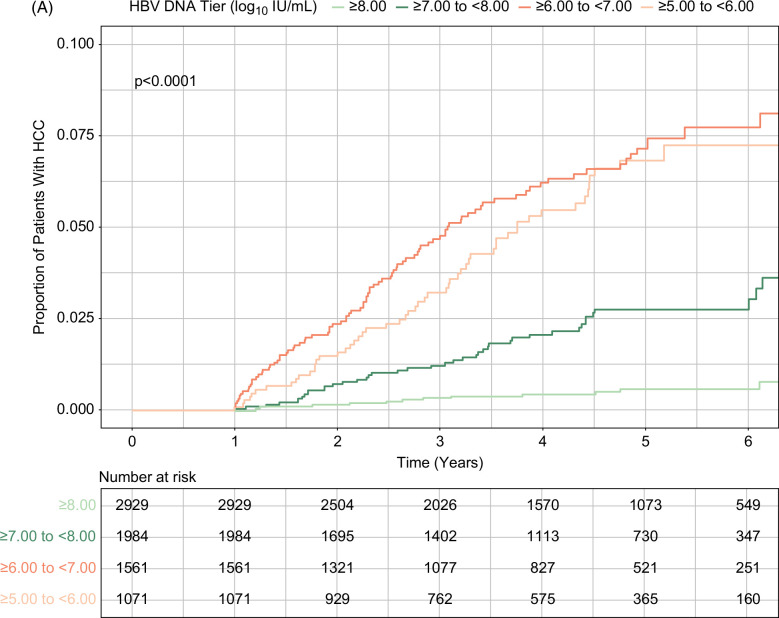
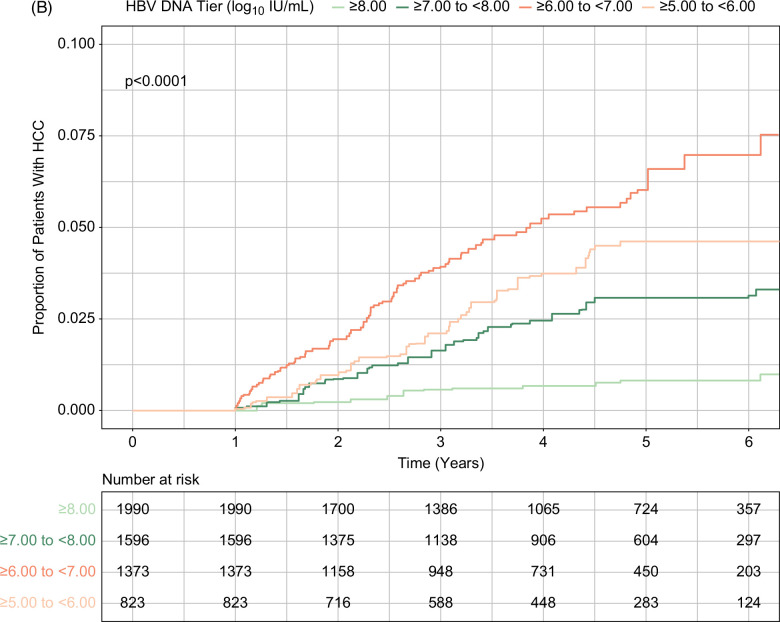
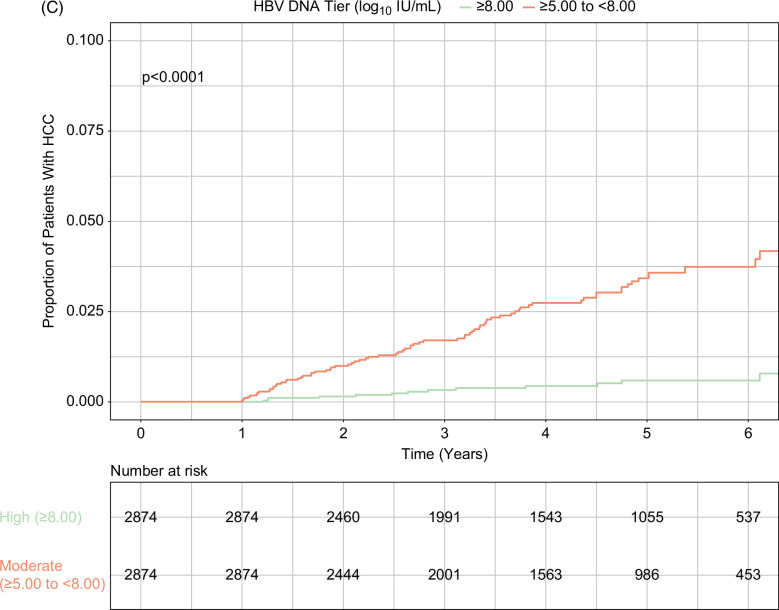
In total, 200 patients developed HCC over the course of the study, with an incidence rate of 0.61 per 100 person-years (95% CI, 0.53–0.70). HCC incidence rates were 0.10 (95% CI, 0.50–0.17), 0.48 (95% CI, 0.35–0.65), 1.38 (95% CI, 1.11–1.69), and 1.17 (95% CI, 0.88–1.53) in patients with HBV viral load ≥8.00 log10 IU/mL, ≥7.00 and <8.00 log10 IU/mL, ≥6.00 and <7.00 log10 IU/mL, and ≥5.00 and <6.00 log10 IU/mL, respectively (Table 2). Cumulative incidence of HCC was significantly different by baseline viral load tiers; it was lowest in patients with baseline viral load ≥8.00 log10 IU/mL and highest in those with baseline viral load ≥5.00 and <8.00 log10 IU/mL (p<0.001; Figure 2A). This result was consistent in the PSW and PSM populations, in which baseline viral load ≥8.00 log10 IU/mL again had the lowest cumulative HCC incidence (Figures 2B, C).
TABLE 2.
Univariable and multivariable analyses showing the association of HBV viral load, and on-treatment risk of HCC
| Variables | Incidence rate (per 100 PYs) | HR | 95% CI (lower) | 95% CI (upper) | p |
|---|---|---|---|---|---|
| Univariable analysis | |||||
| HBV viral load tier (log10 IU/mL) | |||||
| ≥8.00 | 0.10 | 1.00 (reference) | — | — | — |
| ≥7.00 and <8.00 | 0.48 | 4.81 | 2.04 | 11.30 | 0.0004 |
| ≥6.00 and <7.00 | 1.38 | 13.39 | 5.73 | 31.28 | <0.0001 |
| ≥5.00 and <6.00 | 1.17 | 11.20 | 5.44 | 23.05 | <0.0001 |
| Multivariable analysis | |||||
| HBV viral load tier (log10 IU/mL) | |||||
| ≥8.00 | 0.10 | 1.00 (reference) | — | — | — |
| ≥7.00 and <8.00 | 0.48 | 4.04 | 1.68 | 9.73 | 0.002 |
| ≥6.00 and <7.00 | 1.38 | 8.05 | 3.34 | 19.35 | <0.001 |
| ≥5.00 and <6.00 | 1.17 | 6.23 | 3.10 | 12.51 | <0.001 |
| Age, y, per 1-year increase | — | 1.57 | 1.27 | 1.95 | <0.001 |
| Sex | — | ||||
| Female | 1.00 (reference) | — | — | — | |
| Male | 2.19 | 1.35 | 3.56 | 0.002 | |
| Platelet count, ×1000/μL, per 1000/μL increase | — | 0.56 | 0.48 | 0.65 | <0.001 |
| ALT levels, U/La | — | ||||
| <1 × ULN | 1.00 (ref) | — | — | — | |
| ≥1 to <2 × ULN | 1.89 | 1.18 | 3.02 | 0.008 | |
| ≥2 × ULN | 0.86 | 0.53 | 1.40 | 0.55 | |
The multivariable model adjusted for all baseline characteristics except for follow-up time and mPAGE-B score.
ALT was adjusted for as a categorical variable, split into the following levels based on ULN (35 U/L for males, 25 U/L for females): <1 × ULN; ≥1 to <2 × ULN; ≥2 × ULN.
Abbreviations: PY, person-year; ULN, upper limit of normal.
Figure 2.
Cumulative incidence of HCC during treatment by baseline HBV viral load levels. (A) Unweighted analysis. (B) PSW analysis. (C) PSM analysis (high vs. moderate viral load tiers). High and moderate viral loads indicated baseline serum HBV DNA levels ≥8.00 log10 IU/mL and ≥5.00 to <8.00 log10 IU/mL, respectively. Abbreviations: PSM, propensity score matching; PSW, propensity score weighting.
By multivariable Cox PH analyses, baseline HBV DNA level was independently associated with on-treatment HCC risk. Patients with moderate baseline viral load (≥5.00 and <8.00 log10 IU/mL) had between 4 and 8 times higher on-treatment HCC risk than those with high baseline viral load (≥8.00 log10 IU/mL; p<0.01; Table 2), which was consistently observed in the PSW and PSM analyses (Supplemental Table S6, http://links.lww.com/HEP/I218 and Supplemental Table S7, http://links.lww.com/HEP/I218). In contrast, elevated baseline ALT levels (≥2 × upper limit of normal) were not significantly associated with HCC risk (Table 2).
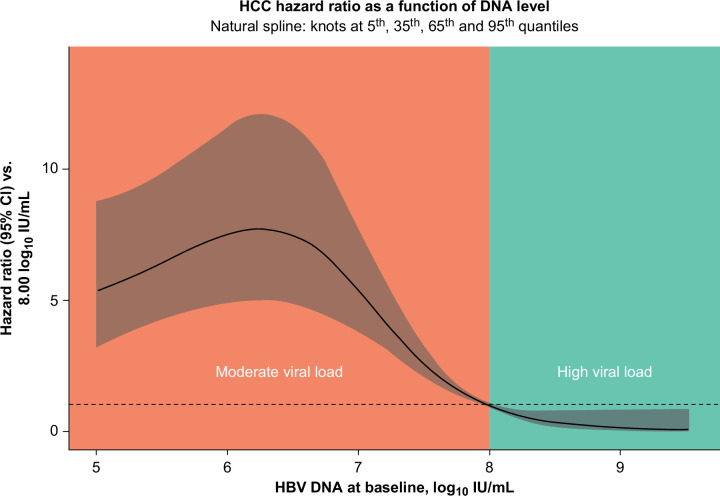
A penalized spline regression curve also showed that the on-treatment risk of HCC increased sharply as baseline HBV viral loads decreased below 8.00 log10 IU/mL and flattened below 6.00 log10 IU/mL (Figure 3).
FIGURE 3.
Penalized spline regression showing adjusted HR for the risk of HCC by baseline HBV DNA levels. The penalized spline regression model adjusted for HBV viral load.
Sensitivity analyses
These results were corroborated in the nested case-control study (Supplemental Table S8, http://links.lww.com/HEP/I218). An increased HCC risk was shown as adjusted odds ratios of 4.41 (95% CI, 2.23–8.75), 8.42 (95% CI, 4.29–16.55), and 6.65 (95% CI, 3.34–13.25) for patients with HBV viral load ≥7.00 and <8.00 log10 IU/mL, ≥6.00 and <7.00 log10 IU/mL, and ≥5.00 and <6.00 log10 IU/mL, respectively, compared with those with HBV viral load ≥8.00 log10 IU/mL. Baseline ALT levels again were not significantly associated with HCC risk.
Results excluding patients from the Choi et al study aligned with those of the full study cohort. Patients with moderate baseline viral load (≥5.00 and <8.00 log10 IU/mL) had between 3 and 7 times higher on-treatment HCC risk than those with high baseline viral load (≥8.00 log10 IU/mL; p<0.01; Supplemental Table S9, http://links.lww.com/HEP/I218).
Stratified analyses
Analyses stratified by baseline age (<50 y and ≥50 y), mPAGE-B score (<11 and ≥11), and platelet count (<150,000/µL and ≥150,000/µL) consistently showed that regardless of these patient factors, high baseline viral load (≥8.00 log10 IU/mL) was significantly associated with the lowest on-treatment risk of HCC (Supplemental Table S10, http://links.lww.com/HEP/I218).
The stratified analysis assessing HCC risk within the 4 age groups (<40 y, ≥40 y and <50 y, ≥50 y and <60 y, and ≥60 y) found that, in the 3 latter age groups, patients with baseline HBV viral load ≥5.00 and <7.00 log10 IU/mL had a significantly higher risk of HCC development than those with baseline viral load ≥8.00 log10 IU/mL (p<0.05), in line with the primary findings (Supplemental Table S10, http://links.lww.com/HEP/I218). Among the youngest group of patients (<40 y), the association between baseline HBV viral load and HCC risk was not statistically significant, although it should be noted that the number of HCC events occurring for each viral load tier was very low in this age group.
Multivariable analyses with age or mPAGE-B as a categorical fixed effect found that point estimates varied across the subgroups (age <50 y and ≥50 y; age <40 y, ≥40 y and <50 y, ≥50 y and <60 y, and ≥60 y; mPAGE-B score <11 and ≥11). However, p values were not significant, indicating no moderation effect between HBV viral load and age or mPAGE-B score as categorical variables (ie, the risk of HCC in each HBV DNA tier was not significantly different across all age subgroups and both mPAGE-B subgroups; Supplemental Table S11, http://links.lww.com/HEP/I218).
DISCUSSION
We found that pretreatment baseline HBV viral load is significantly associated with HCC risk despite antiviral treatment in a large multinational cohort of patients who are HBeAg-positive without cirrhosis and with CHB from 11 study sites in Korea, Hong Kong, and Taiwan. Patients initiating antiviral treatment with a high baseline viral load (≥8.00 log10 IU/mL) had the lowest on-treatment risk of HCC. In contrast, risk increased sharply as baseline viral load decreased, which persisted during the whole antiviral treatment period. Adjusted on-treatment HCC risk was 4–8 times higher with baseline viral load ≥5.00 and <8.00 log10 IU/mL versus baseline viral load ≥8 log10 IU/mL. These findings were consistent in the PSW, PSM, and nested case-control analyses, and in the analysis which excluded patients from the Choi et al study. Furthermore, the findings were corroborated in the analyses of patients stratified by age, mPAGE-B score, and platelet count.
Most patients with CHB have positive HBeAg and high serum levels of HBV DNA (≥8 log10 IU/mL) at the initial phase of infection and slowly progress to decline in HBV DNA levels if untreated.3,4,6 Therefore, our results strongly suggest that initiating antiviral treatment at an earlier point when patients have high baseline viral load would maintain the lowest risk of HCC over the duration of treatment.
Our findings are consistent with a recent single-nation study from Korea,10 which found that patients who are HBeAg-positive with CHB who initiated treatment with moderate viral load (5.00–7.99 log10 IU/mL) had a significantly higher risk of HCC than those who started the treatment with higher baseline viral load. As the number of patients and HCC events included in our analyses were far greater, the present study provides more robust results than the aforementioned study in Korea. Furthermore, our findings are consistent with a study that demonstrated that HCC risk was highest with baseline HBV DNA levels of 6.00–7.00 log10 IU/mL in untreated patients who are HBeAg-positive without cirrhosis and with CHB.16
The REVEAL cohort reported that higher baseline serum HBV DNA levels are associated with an increased risk of HCC in untreated patients without cirrhosis and with CHB,17 a finding that apparently stands in contrast to our analysis. However, the REVEAL cohort included mostly patients who are HBeAg-negative (85%) who predominantly had ALT levels within the normal range, and the highest tier of HBV DNA in the analysis was 106 copies/mL (~5 log10 IU/mL). Therefore, our findings among patients who are HBeAg-positive who initiated antiviral treatment with higher baseline HBV DNA levels (≥5.00 log10 IU/mL) and elevated ALT are complementary rather than contradictory to the REVEAL cohort study.
Several mechanisms may explain the association between higher baseline HBV viral load and lower HCC risk. First, with the prolonged duration of the HBeAg-positive phase of CHB, HBV viral DNA integrations into the host genome accumulate, causing chromosomal damage and instability and leading to the functional loss of tumor-suppressor genes or activation of tumor-promoting genes associated with hepatocarcinogenesis.18–21 Second, the immune response mounted in the HBeAg-positive phase of CHB causes the destruction of infected hepatocytes by T cells and consequent clonal expansion of hepatocytes selected for viral resistance, resulting in a progressive decline in serum HBV DNA levels but also potentially increasing the likelihood of carcinogenesis.22,23 Indeed, lower HBV viral load levels have been found to be a risk factor for a significantly greater degree of hepatic inflammation.24 These data collectively suggest that patients accumulate HCC risk with decreasing HBV viral loads during CHB infection.
Current guidelines for patients who are HBeAg-positive recommend treatment only in patients with elevated ALT levels or evidence of significant histologic disease despite high HBV DNA levels.3,4 Although liver biopsy is the gold standard for assessing the degree of histologic disease, it is rarely conducted in clinical practice and thus elevated ALT levels are often used to inform treatment decision-making. However, ALT levels are not always consistent with the degree of liver inflammation in patients with CHB.24–27 One study found that nearly a third of patients with detectable HBV DNA and normal ALT levels without significant fibrosis still had significant liver inflammation.28 Thus, ALT is not a reliable or sensitive indicator for histologic disease in these patients. In such patients with normal ALT, moderate HBV DNA levels are independent predictors of significant liver inflammation,24,25,28 a finding in line with our results, where patients with moderate HBV DNA levels also had the highest risk of HCC.
The impact of treating patients with normal/minimally raised ALT levels has been investigated in several studies. A clinical trial reported a significant benefit of TDF treatment in reducing the risk of liver fibrosis progression and the number of transcriptionally active distinct HBV-host DNA integrations in patients with elevated HBV DNA and minimally raised ALT.29 A historical cohort study in patients who are HBeAg-positive with CHB reported that untreated patients with normal ALT levels had a significantly higher risk of HCC and death or transplantation than treated patients with high ALT levels, suggesting that treatment initiation early in the disease course for certain patients with CHB could prevent unnecessary deaths.9 A multicenter cohort study corroborated this finding, demonstrating that while initiating treatment in patients who are HBeAg-positive with moderate HBV DNA viral load (5.00–7.99 log10 IU/mL) lowers long-term HCC risk, the risk still remains higher than that of patients initiating treatment with high baseline viral load (ie, earlier in the disease course).10 Other studies have demonstrated that expanding the treatment criteria to include patients who are immune-tolerant with CHB infection is highly cost-saving, by preventing premature death and productivity loss among economically active patients.30,31 Together, these findings point to the benefit of initiating antiviral treatment early with high viral load in patients who are HBeAg-positive with CHB, regardless of ALT levels.
Our findings complement the results of a retrospective cohort study that analyzed 855 patients who are treatment-naïve with CHB in the indeterminate phase between 1992 and 2021 at 14 sites within the United States, Europe, and Asia.32 The analysis found that antiviral therapy reduces HCC risk by 70% for patients with CHB in the indeterminate phase, suggesting that patients who are not indicated for treatment under current guidelines may nonetheless benefit substantially from treatment. Indeed, an editorial article discussing the aforementioned study highlighted the need for renewal and potential expansion of international HBV treatment guidelines.33
This study has several limitations. First, due to the observational nature of the study design, our findings are potentially subject to bias and confounding. To overcome such limitations, we applied strict inclusion criteria and implemented statistical methods to minimize the possibility of confounding. Moreover, the study cohort was large, which enabled adjustment for baseline factors across HBV DNA tiers. The sensitivity analyses, including PSW, PSM, and nested case-control analyses, corroborated the findings of the multivariable analysis. Considering that HCC incidence in patients without cirrhosis and with CHB is relatively low, a randomized clinical trial would be unrealistic to establish the benefit of early treatment initiation in patients with CHB. Therefore, our large-size historical cohort study is a valid option to address this issue. All the study sites were in East Asia, which may limit the generalizability of these findings to patients with CHB globally. Furthermore, as liver biopsy is rarely performed before the initiation of antiviral treatment in patients with CHB, and the majority of patients included in the study did not have FibroScan data available, we were unable to assess the change in fibrosis stage over the course of the study and its association with on-treatment HCC risk. Fibrosis data should be reported separately in future studies. In addition, the criteria used to determine the presence of cirrhosis may have failed to detect Child-Pugh A5 compensated cirrhosis. Patients with confirmed coinfections of HIV, HCV, or HDV and those with evidence of alcohol-associated liver disease were excluded from the analysis; however, the presence of those characteristics could not be systematically tested and verified, and patients missing data for those characteristics may have been included. Information regarding the genotype of included patients was not available, preventing the assessment of potential variation in genotype between the viral load groups. Similarly, information on patients’ HBV DNA levels during the year before treatment initiation was not available; future research evaluating the association between the stability of HBV DNA levels before treatment and HCC risk would be valuable. Another limitation is the lack of detailed information regarding the discontinuation of antiviral therapy during the follow-up. However, in Korea and Hong Kong, but not Taiwan, the majority of patients remain on antiviral therapy without discontinuation until achieving HBsAg seroclearance. Moreover, it is important to note that this study was analyzed based on the intention-to-treat principle.
Finally, most patients included in the present study had elevated ALT levels, likely due to the treatment and reimbursement guidelines in the countries from which the study population was drawn.34 Thus, patients in this analysis were likely not in the very earliest phase of CHB infection. However, a study in patients without cirrhosis and with untreated CHB with normal ALT levels produced results consistent with our findings, reporting that HCC risk was highest with baseline HBV DNA levels of 6–7 log10 IU/mL, and lowest with >8 log10 IU/mL, independent of other predictive factors.16 Notably, an aforementioned study by Choi et al, which compared the risk of HCC between patients with treated and untreated CHB, stratified according to baseline viral load, highlighted the importance of early initiation of antiviral treatment in patients with high viral load.10 The HCC risk that accumulates while waiting for ALT levels to rise is never fully reversible. Taking these previous findings into consideration, our results indicate that treating patients earlier in the disease course, before the elevation in ALT levels, could maintain an even lower risk of HCC than observed in the present analysis.
The present study found that high baseline HBV viral load was associated with the lowest HCC risk and decreasing baseline viral load was associated with increasing on-treatment HCC risk in patients who are HBeAg-positive without cirrhosis initiating entecavir or TDF. These findings suggest that even with prolonged antiviral treatment, the accumulation of the risk of HCC, indirectly indicated by the declining HBV viral load, cannot be entirely reversed. Given that currently recommended first-line anti-HBV treatments have potent efficacy, high long-term safety profiles, high genetic barrier to resistance, and lowered cost, the early initiation of antiviral treatment when patients have high HBV viral load may need to be considered to maintain the lowest risk of HCC in adult patients with HBeAg-positive without cirrhosis and with CHB.
Supplementary Material
DATA AVAILABILITY STATEMENT
Owing to protections around the sharing of private health data, individual patient data are not permitted to be shared or made publicly available. The statistical analysis plan is available upon request from the corresponding author.
AUTHOR CONTRIBUTIONS
Substantial contributions to study conception and design; substantial contributions to analysis and interpretation of the data; drafting the article or revising it critically for important intellectual content; final approval of the version of the article to be published: all authors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
ACKNOWLEDGMENTS
The authors thank the patients, the investigators, and their teams who took part in this study. The authors also acknowledge Isabel Haber, BS, from Costello Medical, Boston, MA, USA, for medical writing and editorial assistance based on the authors’ input and direction.
FUNDING INFORMATION
This study was sponsored by Gilead Sciences. Support for third-party writing assistance for this article, provided by Isabel Haber, BS, Costello Medical, Boston, MA, was funded by Gilead Sciences in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
CONFLICTS OF INTEREST
Terry Cheuk-Fung Yip consults, advises, is on the speakers’ bureau, and received grants from Gilead. W. Ray Kim advises Gilead, Inovio, and Roche. Leland J. Yee is employed by and owns stock in Gilead. Craig Brooks-Rooney is employed by Costello Medical. Tristan Curteis has other interests with Gilead. Laura J. Clark has other interests with Gilead. Zarena Jafry is employed by Costello Medical. Yi-Hsiang Huang consults, advises, and received grants from Gilead. He consults, advises, and is on the speakers’ bureau for Eisai and MSD. He advises and received grants from Bristol-Meyers Squibb. He advises AbbVie, AstraZeneca, Eli Lilly, Ipsen, Ono, and Roche. Cheng-Yuan Peng advises Bristol-Myers Squibb and Gilead. Grace Lai-Hung Wong advises, is on the speakers’ bureau for, and received grants from Gilead. She advises and is on the speakers’ bureau for Janssen. She advises AstraZeneca. She is on the speakers’ bureau for Abbott, AbbVie, Ascletis, Bristol-Myers Squibb, Echosens, Furui, and Roche. Young-Suk Lim advises and received grants from Gilead. The remaining authors have no conflicts to report.
Footnotes
Abbreviations: ALT, alanine aminotransferase; CHB, chronic hepatitis B; mPAGE-B, modified Platelet Age GEnder-HBV; PH, proportional hazard; PSM, propensity score matching; PSW, propensity score weighting; TDF, tenofovir disoproxil fumarate.
Won-Mook Choi is the first author.
Grace Lai-Hung Wong and Young-Suk Lim are the co-corresponding authors.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.hepjournal.com.
Contributor Information
Won-Mook Choi, Email: dr.choi85@gmail.com.
Terry Cheuk-Fung Yip, Email: terryfungyip@gmail.com.
W. Ray Kim, Email: wrkim@stanford.edu.
Leland J. Yee, Email: leland.yee4@gilead.com.
Craig Brooks-Rooney, Email: craig.brooks-rooney@costellomedical.com.
Tristan Curteis, Email: Tristan.Curteis@costellomedical.com.
Laura J. Clark, Email: laura.clark@costellomedical.com.
Zarena Jafry, Email: zarena.jafry@costellomedical.com.
Chien-Hung Chen, Email: e580306@ms31.hinet.net.
Chi-Yi Chen, Email: 5137ccy@gmail.com.
Yi-Hsiang Huang, Email: yhhuang@vghtpe.gov.tw.
Young-Joo Jin, Email: jyj412@hanmail.net.
Dae Won Jun, Email: gongori1004@gmail.com.
Jin-Wook Kim, Email: jwook2112@gmail.com.
Neung Hwa Park, Email: nhparkmd@gmail.com.
Cheng-Yuan Peng, Email: cypeng@mail.cmuh.org.tw.
Hyun Phil Shin, Email: drshp@khu.ac.kr.
Jung Woo Shin, Email: Sheenj@hanmail.net.
Yao-Hsu Yang, Email: r95841012@cgmh.org.tw.
Grace Lai-Hung Wong, Email: wonglaihung@gmail.com.
Young-Suk Lim, Email: wcyl_02@costellomedical.com.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2. Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010;42:S206–S214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lampertico P, Agarwal K, Berg T, Buti M, Janssen HLA, Papatheodoridis G, et al. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. [DOI] [PubMed] [Google Scholar]
- 4. Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cui F, Blach S, Manzengo Mingiedi C, Gonzalez MA, Sabry Alaama A, Mozalevskis A, et al. Global reporting of progress towards elimination of hepatitis B and hepatitis C. Lancet Gastroenterol Hepatol. 2023;8:332–342. [DOI] [PubMed] [Google Scholar]
- 6. Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Papatheodoridis G, Dalekos G, Sypsa V, Yurdaydin C, Buti M, Goulis J, et al. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol. 2016;64:800–806. [DOI] [PubMed] [Google Scholar]
- 8. Arends P, Sonneveld MJ, Zoutendijk R, Carey I, Brown A, Fasano M, et al. Entecavir treatment does not eliminate the risk of hepatocellular carcinoma in chronic hepatitis B: Limited role for risk scores in Caucasians. Gut. 2015;64:1289–1295. [DOI] [PubMed] [Google Scholar]
- 9. Kim G-A, Lim Y-S, Han S, Choi J, Shim JH, Kim KM, et al. High risk of hepatocellular carcinoma and death in patients with immune-tolerant-phase chronic hepatitis B. Gut. 2018;67:945–952. [DOI] [PubMed] [Google Scholar]
- 10. Choi W-M, Kim G-A, Choi J, Han S, Lim Y-S. Increasing on-treatment hepatocellular carcinoma risk with decreasing baseline viral load in HBeAg-positive chronic hepatitis B. J Clin Invest. 2022;132:e154833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. [DOI] [PubMed] [Google Scholar]
- 12. Galle RR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul J-L, et al. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- 13. Choi J, Kim HJ, Lee J, Cho S, Ko MJ, Lim Y-S. Risk of hepatocellular carcinoma in patients treated with entecavir vs tenofovir for chronic hepatitis B: A Korean nationwide cohort study. JAMA Oncol. 2019;5:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim JH, Kim YD, Lee M, Jun BG, Kim TS, Suk KT, et al. Modified PAGE-B score predicts the risk of hepatocellular carcinoma in Asians with chronic hepatitis B on antiviral therapy. J Hepatol. 2018;69:1066–1073. [DOI] [PubMed] [Google Scholar]
- 15. R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing Vienna; 2022. [Google Scholar]
- 16. Kim G-A, Han S, Choi GH, Choi J, Lim Y-S. Moderate levels of serum hepatitis B virus DNA are associated with the highest risk of hepatocellular carcinoma in chronic hepatitis B patients. Aliment Pharmacol Ther. 2020;51:1169–1179. [DOI] [PubMed] [Google Scholar]
- 17. Chen C-J, Yang H-I, Su J, Jen C-L, You S-L, Lu S-N, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. [DOI] [PubMed] [Google Scholar]
- 18. Mason WS, Gill US, Litwin S, Zhou Y, Peri S, Pop O, et al. HBV DNA integration and clonal hepatocyte expansion in chronic hepatitis B patients considered immune tolerant. Gastroenterology. 2016;151:986–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tu T, Budzinska MA, Vondran FW, Shackel NA, Urban S. Hepatitis B virus DNA integration occurs early in the viral life cycle in an in vitro infection model via sodium taurocholate cotransporting polypeptide-dependent uptake of enveloped virus particles. J Virol. 2018;92:e02007–e02017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tu T, Budzinska MA, Shackel NA, Urban S. HBV DNA integration: Molecular mechanisms and clinical implications. Viruses. 2017;9:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao L-H, Liu X, Yan H-X, Li W-Y, Zeng X, Yang Y, et al. Genomic and oncogenic preference of HBV integration in hepatocellular carcinoma. Nat Commun. 2016;7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tu T, Zhang H, Urban S. Hepatitis B virus DNA integration: In vitro models for investigating viral pathogenesis and persistence. Viruses. 2021;13:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mason WS, Liu C, Aldrich CE, Litwin S, Yeh MM. Clonal expansion of normal-appearing human hepatocytes during chronic hepatitis B virus infection. J Virol. 2010;84:8308–8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang J, Yan X, Zhu L, Liu J, Qiu Y, Li Y, et al. Significant histological disease of patients with chronic hepatitis B virus infection in the grey zone. Aliment Pharmacol Ther. 2023;57:464–474. [DOI] [PubMed] [Google Scholar]
- 25. Wang H, Xue L, Yan R, Zhou Y, Wang MS, Cheng MJ, et al. Comparison of histologic characteristics of Chinese chronic hepatitis B patients with persistently normal or mildly elevated ALT. PLoS One. 2013;8:e80585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gui HL, Wang H, Yang YH, Wu YW, Zhou HJ, Guo SM, et al. Significant histopathology in Chinese chronic hepatitis B patients with persistently high–normal alanine aminotransferase. J Viral Hepat. 2010;17(s1):44–50. [DOI] [PubMed] [Google Scholar]
- 27. Lai M, Hyatt BJ, Nasser I, Curry M, Afdhal NH. The clinical significance of persistently normal ALT in chronic hepatitis B infection. J Hepatol. 2007;47:760–767. [DOI] [PubMed] [Google Scholar]
- 28. Liu J, Wang J, Yan X, Xue R, Zhan J, Jiang S, et al. Presence of liver inflammation in Asian patients with chronic hepatitis B with normal ALT and detectable HBV DNA in absence of liver fibrosis. Hepatol Commun. 2022;6:855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hsu Y-C, Chen C-Y, Chang I-W, Chang C-Y, Wu C-Y, Lee T-Y, et al. Once-daily tenofovir disoproxil fumarate in treatment-naive Taiwanese patients with chronic hepatitis B and minimally raised alanine aminotransferase (TORCH-B): A multicentre, double-blind, placebo-controlled, parallel-group, randomised trial. Lancet Infect Dis. 2021;21:823–833. [DOI] [PubMed] [Google Scholar]
- 30. Kim H-L, Kim G-A, Park J-A, Kang H-R, Lee E-K, Lim Y-S. Cost-effectiveness of antiviral treatment in adult patients with immune-tolerant phase chronic hepatitis B. Gut. 2021;70:2172–2182. [DOI] [PubMed] [Google Scholar]
- 31. Lim Y-S, Ahn SH, Shim J-J, Razavi H, Razavi-Shearer D, Sinn DH. Impact of expanding hepatitis B treatment guidelines: A modelling and economic impact analysis. Aliment Pharmacol Ther. 2022;56:519–528. [DOI] [PubMed] [Google Scholar]
- 32. Huang DQ, Tran A, Yeh ML, Yasuda S, Tsai PC, Huang CF, et al. Antiviral therapy substantially reduces hepatocellular carcinoma risk in chronic Hepatitis B patients in the indeterminate phase. Hepatology. 2023;78:1558–1568. [DOI] [PubMed] [Google Scholar]
- 33. Lok J, Dusheiko G. Editorial: Re-assessing antiviral treatment criteria for chronic hepatitis B. Hepatology. 2023;78:1332–1333. [DOI] [PubMed] [Google Scholar]
- 34. Chien R-N, Kao J-H, Peng C-Y, Chen C-H, Liu C-J, Huang Y-H, et al. Taiwan consensus statement on the management of chronic hepatitis B. J Formos Med Assoc. 2019;118(1 pt 1):7–38. [DOI] [PubMed] [Google Scholar]