ABSTRACT
The literature holds few descriptions on immune response findings for laryngeal cryptococcosis. Immunology has been more extensively described in cases involving the central nervous system and the lungs, although many of these studies were conducted in animal models. We aimed to analyze the clinical and immunological characteristics of three patients with laryngeal cryptococcosis. We observed a weak participation of the innate immune response, whereas adaptive immunity showed the predominance of a Th2-type response over a Th1-type response. Most cases occur in male older adults with immunosuppressive conditions, of which HIV infection was absent. Hoarseness configured the main symptom. We found a disease that was restricted to the larynx and possibly the lungs by contiguity. Patients with hoarseness and lesions in nasal endoscopy should be investigated for cryptococcosis by a biopsy of the larynx, including with negative serum cryptococcal antigen. The immunological aspects of our findings of laryngeal involvement resembled those in the most commonly affected systems.
KEYWORDS: Laryngeal cryptococcosis, Cryptococcosis, Immunopathology
INTRODUCTION
Cryptococcal infections are prevalent worldwide 1 . Cryptococcus sp., including Cryptococcus neoformans and Cryptococcus gattii, are ubiquitous and are the main causes of cryptococcosis in humans 2 . C. neoformans is more commonly found in immunocompromised patients, whereas C. gattii is more frequently associated with immunocompetent hosts 3 .
Cryptococcus sp. have multiple immune system evasion mechanisms, and dissemination of the fungus may occur virtually to all organs, but the central nervous system (CNS) and the lungs are the main affected systems 4 . It is widely recognized that the host’s primary defense mechanism for solving cryptococcosis involves cell-mediated immunity, which suppresses the growth of yeasts in the lungs 5 . While T helper (Th)1 cells are crucial in initiating a protective immune response against cryptococcal infection, Th2 cells produce interleukin IL-4, IL-13, and IL-5. These cytokines have a detrimental effect on immune responses to cryptococcosis infections 6 . Nonetheless, some studies in humans have found no correlation between Th2 immune responses and disease amelioration 7,8 .
Laryngeal cryptococcosis is rare, with few reported cases 9-13 . Its main symptom involves persistent or progressive hoarseness and lesions that predominantly occur on the true vocal cords 9 . The literature holds few descriptions about immune response findings to laryngeal cryptococcosis. Immunology is more extensively described in cases involving the CNS and the lungs, although many of these studies were conducted in animal models. Due to the uncommon involvement of the larynx, immune responses are likely to differ, especially considering that the main risk factor in case series involves immunocompetent patients using inhaled corticosteroids 9-12,14,15 . We aimed to analyze the clinical and immunological characteristics of three patients with laryngeal cryptococcosis.
CASE REPORTS
Case 1
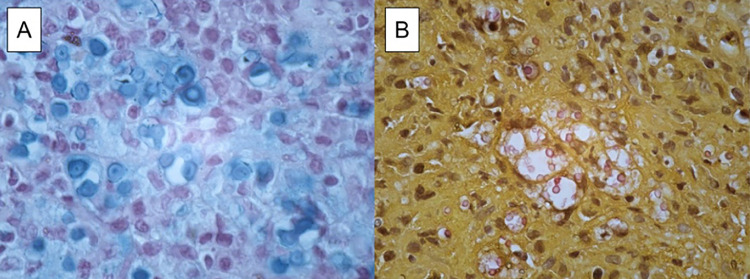
A 67-year-old Brazilian man underwent a liver transplant in March 2022 due to cryptogenic biliary cirrhosis. He has a history of hypertension, diabetes mellitus, and smoking. His immunosuppressive drugs include prednisone, tacrolimus, and mycophenolate mofetil. He was admitted to the infectious disease ward in December 2022 with a six-month history of sudden and progressive hoarseness, without associated symptoms and with a normal physical examination. Bronchoscopy showed an irregular vegetative-infiltrative lesion affecting the middle third of his left vocal cord and spanning the ipsilateral ventricular band and posterior commissure, without significant limiting airflow. Histological analysis showed a chronic granulomatous infiltrate with multiple giant cells, and Grocott and Alcian Blue stains were positive for Cryptococcus spp. (Figure 1A).
Figure 1. A) Alcian Blue staining shows the acidic mucin in the Cryptococcus spp. capsule (Case 1); B) Mucicarmine stain is positive for Cryptococcus spp. (Case 2).
This patient showed unremarkable laboratory findings, including negative HIV serology. Fungal serology for Histoplasma, Paracoccidioides, and serum Cryptococcus Antigen (CrAg) Latex Agglutination Test (LA) were negative. Chest computed tomography (CT) showed ground-glass opacities, foci of consolidation, and centrilobular micronodules in the posterior segment of the right upper and lower lobes, more pronounced on the right. Additionally, he had a well-delimited non-calcified pulmonary nodule in the lingular superior segment measuring 0.9 cm. Brain CT was normal. The examination of cerebrospinal fluid (CSF) was performed, and showed normal results. Direct mycological examination and fungal culture were negative. The serum and CSF Cryptococcus antigen tests were also negative.
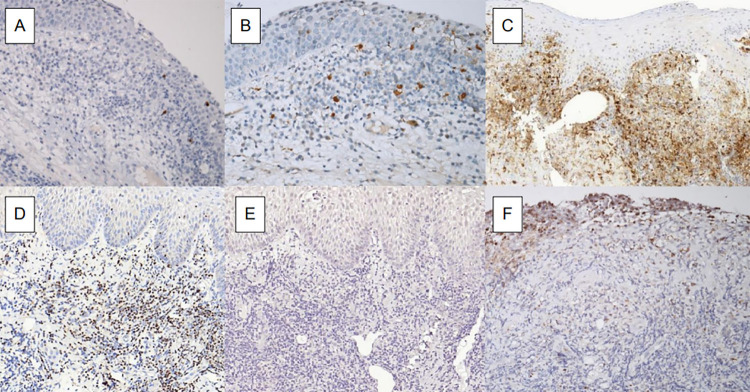
The analysis of the innate immune system showed immunohistochemical reactions for CD68 with sparse macrophages in areas of lymphocyte predominance, and the reaction for CD56 was very scarce, indicating low dendritic cell density. The adaptive immunity was evinced by immunohistochemical reactions for CD3, CD4, and CD8, showing a predominance of CD4 positive T-cells and the transcription factor GATA3 (Figure 2D), a marker of Th2-activated lymphocytes. Markers TNF-alpha and IFN-gamma obtained negative results (Figure 2E), interpreted as a lack of a Th1 inflammatory response (Table 1).
Figure 2. Immunohistochemical expression of: A) CD1a; B) S100 protein; C) CD4; D) GATA-3; E) IFN-γ; and F) IL-17.
Table 1. Clinical characteristics and immunology of patients with laryngeal cryptococcosis.
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Characteristic | |||
| Age (years) | 67 | 69 | 81 |
| Sex | Male | Male | Male |
| Main comorbidities | Liver transplant | Diabetes mellitus | Pulmonary Langerhans cell histiocytosis. |
| Inhaled corticosteroids | No | No | Yes |
| HIV infection | No | No | No |
| Clinical findings | Hoarseness | Hoarseness, dysphagia, shortness of breath, productive cough, and weight loss | Hoarseness, cough, weight loss |
| Type of lesion | Vegetative | Granulomatous | Granulomatous |
| Serum cryptococcal antigen | Negative | Negative | Negative |
| Treatment | Liposomal amphotericin-B, followed by fluconazole | Liposomal amphotericin-B, followed by fluconazole | Fluconazole |
| Outcomes | Clinical improvement | Clinical stability | Clinical improvement |
| Immunology | |||
| CD1a | Negative | Very scarce | Very scarce |
| S100 protein | Negative | Negative | Scarce |
| CD68 | Abundant | Abundant | Abundant |
| CD56 | Very scarce | Very scarce | Very scarce |
| IL-17 | Negative | Scarce | Scarce |
| Tbet | Negative | Negative | Mild |
| TNF-α | Scarce | Mild | Mild |
| IFN-γ | Negative | Scarce | Negative |
| GATA3 | Mild | Mild | Mild |
The patient was treated with Liposomal Amphotericin-B (L-AmB) at a dose of 3 mg/kg/day for one week, followed by 450 mg of fluconazole daily. He was discharged after two weeks of hospitalization. After six months of treatment, the patient reported complete improvement of hoarseness. The patient is awaiting a new bronchoscopy to conclude the treatment.
Case 2
A 69-year-old Brazilian man worked as a farmer and had hypertension, diabetes, and benign prostatic hyperplasia. He was a former smoker and had a history of alcoholism. His last occupation was as a lumberman. He was admitted to the infectious diseases ward with a seven-month history of progressive hoarseness, dysphagia, shortness of breath, productive cough, and weight loss.
Bronchoscopy showed a granulomatous lesion on his right vocal fold with almost complete airway obstruction. The patient underwent urgent tracheostomy, and the lesion was biopsied. Histological analysis showed encapsulated yeast-like structures on mucicarmine stain, compatible with Cryptococcus spp. (Figure 1B).
This patient showed unremarkable laboratory findings, including negative HIV serology. Fungal serology for Histoplasma, Paracoccidioides, and serum CrAg LA were also negative. Chest CT showed ground-glass opacities, foci of lung consolidation, and grossly nodular lesions in the lower lobes and lingula.
We observed a negative S100 protein-positive cell and very few CD1a and CD56, which reflects the weak participation of his innate immune response. The expression of IL-17 was negative in the lymphoid tissue. The adaptive immunity showed the predominance of CD4 positive T-cells (Figure 2C) and of transcription factor GATA3. TNF-α and IFN-γ markers showed mild and scarce levels, respectively, which were interpreted as lack of a Th1 inflammatory response (Table 1).
The patient was treated with L-AmB at a dose of 3 mg/kg/day for two weeks, followed by fluconazole 600 mg/day for two months. At the last appointment, the patient showed partial improvement of the lesion after an initial six-month of antifungal treatment.
Case 3
An 81-year-old Brazilian man with pulmonary Langerhans cell histiocytosis was admitted to the infectious disease ward with a two -month history of progressive hoarseness and cough. He used inhaled corticosteroids.
Nasal endoscopy showed a granulomatous lesion in the anterior commissure and middle thirds of the vocal folds, interarytenoid area, and ventricular bands. Histological analysis showed encapsulated yeast-like structures on mucicarmine and Grocott stains. This finding suggests Cryptococcus spp.
This patient showed unremarkable laboratory findings, including negative HIV serology. Fungal serology for Histoplasma, Paracoccidioides, and serum CrAg LA were also negative. Chest CT showed nodular opacities in the middle and upper lobes associated with conglomeration of pulmonary fibrosis, mediastinal and hilar lymph node enlargement, and bronchiectasis.
We observed a weak participation of the innate immune response with CD1a and S100 protein (Figures 2A and 2B). Adaptive immunity showed a predominance of a Th2-type responses when compared with Th1-type responses due to the predominant expression of the transcription factor GATA3 (Table 1). The lymphoid tissue scarcely expressed IL-17 (Figure 2F).
The patient was treated with fluconazole 600 mg/day for two months. He showed slight clinical improvement. Nasal endoscopic examination showed partial improvement in the lesion on the right vocal fold. The treatment schedule will continue for six months.
DISCUSSION
This study performed an immunopathological characterization of laryngeal cryptococcosis. We observed that a weak participation of the innate immune response and the predominance of a Th2-type response in adaptive immunity are significant to understand the immunological aspects of the disease. The main symptom of hoarseness is also important for clinical diagnosis. Additionally, our finding of a disease primarily restricted to the larynx (with a possible extension to the lungs by contiguity) suggests the localized nature of the infection and its potential progression.
In laryngeal cryptococcosis, a minority of patients was immunocompromised, and the main immunosuppression condition was HIV infection 9 . Although all patients in our study had some degree of immunosuppression, none lived with an HIV infection.
Regarding immunocompetent hosts, inhaled corticosteroid use configures a risk factor 16 . This can explain why the cryptococcal infection is more localized in the larynx. No patient had a disseminated disease. All cases probably included pulmonary findings by contiguity, however bronchoalveolar lavage was not performed. Worrallet al. showed that no patients had CNS involvement 9 .
In a way, this localized involvement may explain the low sensitivity of the serum CrAg (sensitivity = 39%) in diagnosing cryptococcosis 9 . All patients in our study had a negative antigen test. Diagnosis was made by histopathology in all cases, with visualization of cryptococcal yeasts.
The primary virulence factors of Cryptococcus neoformans, including urease, laccase, and capsules, play a pivotal role in promoting the aggregation of immature dendritic cells and inducing nonprotective Th2 immune reactions. By adaptive immunity, CD4+ T cells facilitate the transcription and expression of relevant cytokines and chemokines, coordinating fungal clearance and protecting naive mice. Tregs, a subset of CD4+ T cells, critically mediate peripheral tolerance and modulate immune responses. Tregs play both positive and negative roles in fungal infections. They disrupt the dynamic balance of Th1/Th2/Th17 cells, reduce the expression of Th2 cytokines, and inhibit the differentiation of Th17 cells. Treg-deficient mice show an increase in the production of immunoglobulin E, eosinophils, and Th2 cytokines such as IL-4, IL-5, and IL-13. The expression of the transcription factor GATA3 is up- or downregulated during Th2 or Th1 cell differentiation, respectively. Furthermore, GATA3 induces Th2 differentiation and represses Th1 differentiation. Beyond CD4+ T cells, CD8+ T cells also exert a significant role in host immunity against C. neoformans infections by producing tumor necrosis factor gamma (TNF-γ), which inhibits and eliminates pathogens by direct contact 17 . Regarding immune responses, in the absence of macrophages and dendritic cells responsible for innate immunity, polymorphonuclear leukocytes and B cells accumulate in the tissue but are unable to control the fungal infection. Their increasing presence is associated with excess damage to the host 18,19 . Furthermore, the increased expression of Th1 cytokines, such as TNF-α and IFN-γ, enhances fungal control. However, C. neoformans can active shift the Th1-Th2 balance toward a Th2 profile, and the predominant Th2 cells can promote fungal growth and dissemination 8,17,18 . Our study showed that laryngeal cryptococcosis was also immunologically Th2-dominant.
The immunopathology of cryptococcosis in CNS and pulmonary disorders has been more extensively studied. Regarding immunology, our findings of laryngeal involvement resembled to those observed in other common sites. Nonetheless, our case series is quite limited and all cases showed possible pulmonary involvement due to contiguity. Further studies are necessary to enhance the immunological characterization of laryngeal cryptococcosis.
CONCLUSION
In conclusion, our study described the immunopathological features of laryngeal cryptococcosis, which included a weak participation of the innate immune response and a predominance of a Th2-type response. Laryngeal involvement demonstrated a localized disease with diagnosis primarily based on histology. We recommend that patients presenting hoarseness and lesions during nasal endoscopy should be investigated for cryptococcosis through biopsy of the larynx, even if serum cryptococcal antigen testing yields negative results.
Footnotes
FUNDING: The authors received no financial support for the research, authorship, and/or publication of this article.
REFERENCES
- 1.Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2016;30:179–206. doi: 10.1016/j.idc.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gushiken AC, Saharia KK, Baddley JW. Cryptococcosis. Infect Dis Clin North Am. 2021;35:493–514. doi: 10.1016/j.idc.2021.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zavala S, Baddley JW. Cryptococcosis. Semin Respir Crit Care Med. 2020;41:69–79. doi: 10.1055/s-0039-3400280. [DOI] [PubMed] [Google Scholar]
- 5.Huffnagle GB, Yates JL, Lipscomb MF. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. J Exp Med. 1991;173:793–800. doi: 10.1084/jem.173.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price MS, Perfect JR. Host defenses against cryptococcosis. Immunol Invest. 2011;40:786–808. doi: 10.3109/08820139.2011.605196. [DOI] [PubMed] [Google Scholar]
- 7.Neal LM, Qiu Y, Chung J, Xing E, Cho W, Malachowski AN, et al. T cell-restricted notch signaling contributes to pulmonary Th1 and Th2 immunity during cryptococcus neoformans infection. J Immunol. 2017;199:643–655. doi: 10.4049/jimmunol.1601715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Firacative C, Gressler AE, Schubert K, Schulze B, Müller U, Brombacher F, et al. Identification of T helper (Th)1- and Th2-associated antigens of Cryptococcus neoformans in a murine model of pulmonary infection. 2681Sci Rep. 2018;8 doi: 10.1038/s41598-018-21039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Worrall DM, Lerner DK, Naunheim MR, Woo P. Laryngeal cryptococcosis: an evolving rare clinical entity. Ann Otol Rhinol Laryngol. 2019;128:472–479. doi: 10.1177/0003489419826131. [DOI] [PubMed] [Google Scholar]
- 10.Jeng JY, Tomblinson CM, Ocal IT, Vikram HR, Lott DG. Laryngeal cryptococcosis: literature review and guidelines for laser ablation of fungal lesions. Laryngoscope. 2016;126:1625–1629. doi: 10.1002/lary.25749. [DOI] [PubMed] [Google Scholar]
- 11.Mittal N, Collignon P, Pham T, Robbie M. Cryptococcal infection of the larynx: case report. J Laryngol Otol. 2013;127(Suppl 2):S54–S56. doi: 10.1017/S0022215113000522. [DOI] [PubMed] [Google Scholar]
- 12.Gordon DH, Stow NW, Yapa HM, Bova R, Marriott D. Laryngeal cryptococcosis: Clinical presentation and treatment of a rare cause of hoarseness. Otolaryngol Head Neck Surg. 2010;142(Suppl 1):S7–S9. doi: 10.1016/j.otohns.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 13.Quintero O, Trachuk P, Lerner MZ, Sarungbam J, Pirofski LA, Park SO. Risk factors of laryngeal cryptococcosis: a case report. Med Mycol Case Rep. 2019;24:82–85. doi: 10.1016/j.mmcr.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nadrous HF, Ryu JH, Lewis JE, Sabri AN. Cryptococcal laryngitis: case report and review of the literature. Ann Otol Rhinol Laryngol. 2004;113:121–123. doi: 10.1177/000348940411300207. [DOI] [PubMed] [Google Scholar]
- 15.Isaacson JE, Frable MA. Cryptococcosis of the larynx. Otolaryngol Head Neck Surg. 1996;114:106–109. doi: 10.1016/S0194-59989670293-0. [DOI] [PubMed] [Google Scholar]
- 16.Wong DJ, Stanley P, Paddle P. Laryngeal cryptococcosis associated with inhaled corticosteroid use: case reports and literature review. 63Front Surg. 2017;4 doi: 10.3389/fsurg.2017.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Shao J, Dai M, Fang W, Yang YL. Adaptive immunology of cryptococcus neoformans infections: an update. 1174967Front Immunol. 2023;14 doi: 10.3389/fimmu.2023.1174967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campuzano A, Wormley FL. Innate immunity against Cryptococcus, from recognition to elimination. 33J Fungi (Basel) 2018;4 doi: 10.3390/jof4010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voelz K, May RC. Cryptococcal interactions with the host immune system. Eukaryot Cell. 2010;9:835–846. doi: 10.1128/EC.00039-10. [DOI] [PMC free article] [PubMed] [Google Scholar]