Abstract
In recent years, the use of probiotics and their metabolites, known as postbiotics as natural preservatives has received increasing attention in the food industry. This study aimed to prepare and characterize postbiotics of Lactiplantibacillus sakei and to investigate its application as an anti-Listeria solution on beef fillets using an aerosolization technique. The functional groups, including organic acids, polysaccharides and other minor metabolites, were identified by Fourier transform infrared (FTIR) in the postbiotics. The 2, 2′-diphenyl-1-picrylhydrazyl radical scavenging activity of the postbiotics was reported as 0.82 mg mL-1. The antimicrobial test using the agar well diffusion method revealed a zone of inhibition of 27.00 ± 1.20 mm. Application of an aerosolized postbiotics solution resulted in a significant reduction in Listeria monocytogenes counts on beef fillets, reaching 3.30 log10 CFU g-1 over a 15-day storage period at 4.00 ± 1.00 ˚C. The results of this study revealed that the postbiotics of L. sakei was an effective antimicrobial additive for controlling foodborne pathogens in beef fillets and aerosolization is a promising method for developing an antimicrobial coating on meat to enhance meat safety.
Key Words: Aerosolization, Active packaging, Food shelf life, Lactiplantibacillus sakei, Postbiotics
Introduction
Meat and meat products constitute a major part of human food and contain valuable nutrients for growth and health. At the same time, meat is prone to microbial contamination, leading to discoloration and off-flavor of meat, introducing significant health risks to consumers by facilitating the transmission of important foodborne pathogens, including Listeria monocytogenes and Escherichia coli.1 Consumers are becoming more concerned about the safety and health of food and they are less interested in chemical additives which increase interest in employing biological preservatives and increasing information about the use of beneficial bacteria and their metabolites as antimicrobials in the food industry.2 Biopreservation uses natural materials with the origin of bacteria, fungi, plants, animals, enzymes, essential oils and secondary metabolites of bacteria (postbiotics) to increase the shelf life and maintain the quality of food.3,4
Several microorganisms, especially lactic acid bacteria (LAB), produce a large variety of antibacterial compounds and many efforts have been made to use LAB as bio-preservatives.5 The LAB are gram-positive and catalase-negative microorganisms which consist of different genera mainly Lactobacillus, Streptococcus, Lactococcus, Leuconostoc, and Bifidobacterium.6 The main antimicrobial mechanism of LAB is related to their unique activity in producing antimicrobial compounds (mostly postbiotics).7 Postbiotics are an emerging area of interest in the field of food science and biopreservation which refer to the metabolic byproducts or components produced by probiotics during their fermentation process.8,9 In addition to organic acids and bacteriocins, postbiotics comprise a diverse range of compounds including free fatty acids and amino acids that collectively enhance their antimicrobial and antioxidant properties.8 The use of postbiotics is an inventive solution to overcome drawbacks such as low survival compatibility and limited growth of probiotic bacteria that are used directly in the antimicrobial coating, film and food.5 The main components of postbiotics include various compounds such as enzymes, bacteriocins, bacteriocin-like inhibitory compounds, organic acids and low molecular weight metabolites with high antimicrobial properties on foodborne micro-organisms.6,10 The type of probiotic in which the postbiotics is prepared, the characteristics of the target bacteria and the concentration of metabolites can affect antibacterial properties of postbiotics.9
Postbiotics are available in both freeze-dried (lyophilized) and fresh (liquid) forms and there are various approaches to incorporating postbiotics into meat such as applying them directly to food or integrating them into meat formulations. In this regard, postbiotics have been recently used in meat and meat products including dipping in frankfurters,11 direct addition in fresh mutton sausage,12 direct addition in cooked sausage,13 dipping in chicken drumsticks,14 in edible film in buffalo meat patties7 and direct addition in ground beef.15 Additionally, they can be used by spraying onto the surfaces of fresh or ready-to-eat meat products.3,10,15 Aerosolization is a novel technique in antimicrobial coating. It involves turning liquid solutions into tiny airborne particles. This method is commonly used as a disinfectant method, especially in treating respiratory ailments with pharmaceuticals. Nebulizers are commonly used devices in aerosol systems. They generate particles with a diameter of approximately 5.00 m or larger.16 Aerosolization offers various benefits such as being easy to use, effectively covering surfaces, enhancing the penetration of antimicrobial substances, and causing minimal impact on the taste and texture of food due to the development of a thin coating.17 Notably, volatile compounds such as carboxylic acids, alcohols and hydrocarbons which contribute to the antimicrobial activity of postbiotics may be lost during aerosolization demanding careful consideration.18,19
Lactiplantibacillus sakei has received a lot of attention for its role in meat products and its ability to prevent the growth and colonization of L. monocytogenes in red meat products especially during cold storage.20,21 The L. sakei postbiotics have been used in different meat in edible film and coating forms.5,14,22 The L. monocytogenes has been thoroughly researched in relation to using postbiotics in food, owing to its significance in meat safety and sensitivity to postbiotic ingredients.5,8,10,11,13-15,22 In this study, anti-Listeria performances of postbiotics of L. sakei was assessed on beef fillets by aerosolization, a method that could potentially be used for commercial purposes due to its effectiveness.
Materials and Methods
Preparation of postbiotics. The L. sakei (PTCC 1712) was purchased from the Iranian Research Organization for Science and Technology (Tehran, Iran). For the preparation of postbiotics, the bacteria were cultivated in de Man Rogosa and Sharpe (MRS) broth (Ibresco, Karaj, Iran) for 48 hr at 37.00 ± 1.00 ˚C in a CO2 incubator (Sina Lab, Tehran, Iran). Then, the suspension was separated by centrifugation (Farzaneh Arman Co., Isfahan, Iran) at 4,000 g for 10min and postbiotics solution was collected, and filtered using a sterile syringe polytetrafluoro-ethylene filter (0.22 μm; Millipore Inc., Burlington, USA). Then, the solution was freeze-dried (Zist Farayand Tajhiz Sahand, Tabriz, Iran); freezing temperature of – 40.00 ˚C, pump pressure of 100 mTorr, and shelf temperature of – 60.00 ˚C; and kept at – 20.00 ˚C.15
Fourier transform infrared (FTIR) analysis. The chemical structural properties and surface groups of the postbiotics were investigated at the wavelength of 4,000 - 450 cm-1 using the FTIR spectroscopy (Nexus® 670; Thermo Nicolet Instrument, Madison, USA), and data processing was done on Omnic Software (version 6.0; Thermo Electron, Madison, USA).
Antioxidant activity. The 2, 2′-diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging assay was used to evaluate the total antioxidant capacity of postbiotics.23 One mL of DPPH solution (4.00 mg per 100 mL-1 methanol) was added to 1.00 mL of different postbiotics concentrations (0.001, 0.01, 0.50, 1.00, 2.00, and 4.00 mg mL-1). The mixture was shaken and maintained at room temperature for 45 min in the dark. The absorbance of the samples was measured at 517 nm using a spectrophoto-meter (Novaspec II; Pharmacia, Muttenz, Switzerland). Butylated hydroxyl toluene (Sigma, St. Louis, USA) was used as control in this assay and the percentage of free radical scavenging effect (%RS) was calculated using the following equation:
%RS = ([A blank – A sample ] / A blank ) × 100
where, Ablank and Asample indicate the absorption rates of the DPPH solution (control) and the postbiotics containing DPPH solution, respectively. The results were assessed as half maximal inhibitory concentration (IC50) values from the plot of the %RS concentration.
Antimicrobial activity. The antimicrobial activity of postbiotics was determined using the well diffusion method. An inoculum of 8.00 log10 CFU mL-1 of L. monocytogenes suspension was spread on the surface of Mueller-Hinton agar (Ibresco, Tehran, Iran) plates with a sterilized swab. Wells (8.00 mm diameter) were punched into the agar and filled with 100 μL of 40.00 % concentration of postbiotics powder. The plates were then incubated for 24 hr at 37.00 ± 1.00 ˚C. The diameter of the clear zone of inhibition (ZOI in mm) around the wells was measured with a digital caliper (Mitutoyo Corp., Kawasaki, Japan) in triplicate.24
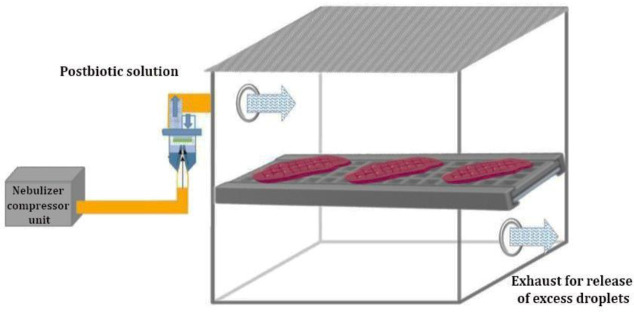
Anti- Listeria performance of postbiotics on beef fillet. Fresh beef fillets were purchased directly from a butchery (Urmia, Iran) and transferred to the laboratory of the Faculty of Veterinary Medicine, Urmia University under cold and hygienic conditions. Under an aseptic condition, fillet beef was cut into slices ( 5.00 g) and was inoculated by immersion into L. monocytogenes suspension ( 5.00 log10 CFU mL-1) for 1 min and drained for 15 min.24 The fillet slices were aerosolized with 10.50 mL of postbiotics solution (20.00 and 40.00%) for 30 min at a rate of 0.35 mL per min by atomizer nebulizer (3A Health Care, Lonato del Garda, Italy; Fig. 1) and packaged in sterile polyethylene box and stored at 4.00 ± 1.00 ˚C for 15 days.25 Inoculated fillets without postbiotics were applied as controls. In the interval of days 0.00, 3.00, 6.00, 9.00, 12.00 and 15.00, 50.00 g of beef fillet was homogenized (Cole-Parmer Canada, Quebec, Canada) with 450 mL of peptone water (0.10% w/v) using a stomacher (Seward Medical Ltd., London, UK) at 260 rpm for 4 min. Then, serial dilutions of suspensions were prepared and spread on PALCAM Listeria selective agar (Oxford Agar; Merck, Darmstadt, Germany). Colony count was performed after 48hr incubation at 35.00 ± 1.00 ˚C and calculated as log10 CFU per g.24,26
Fig. 1.
Schematic of fabricated chamber for aerosolization of postbiotics on beef fillet.
Statistical analysis. Analysis of variance (ANOVA) was used to examine data using GraphPad Prism (version 8.01; GraphPad Software Inc., San Diego, USA). Tukey's multiple-range test was also performed to determine whether there were any significant differences (p < 0.05) between the samples. All experiments were performed in triplicates.
Results
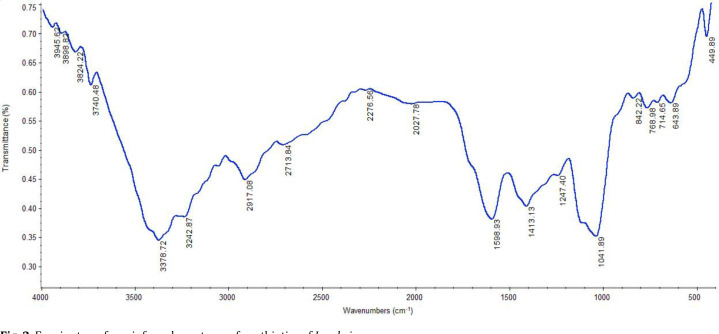
FTIR analysis. To characterize the various functional groups of the compounds in L. sakei postbiotics, the mid-infrared spectrum of the lyophilized sample was applied (Fig. 2). As identified from the spectrum, the absorption peaks at 3,378, 3,242, 2,917, 1,598, 1,413, 1,247, and 1,041 cm-1 were observed.
Fig. 2.
Fourier transform infrared spectrum of postbiotics of L. sakei.
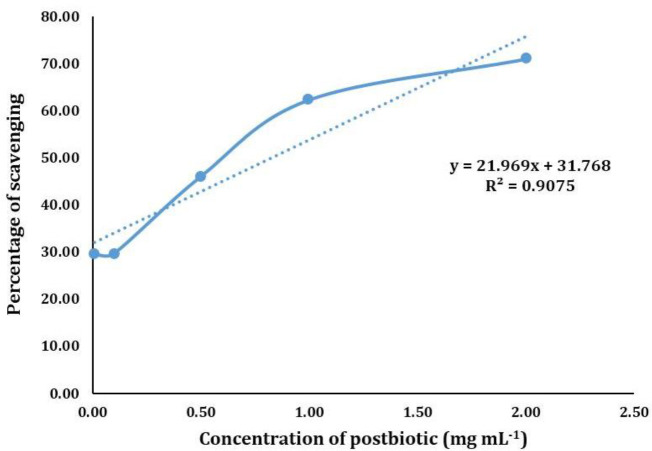
Antioxidant and antibacterial activity. The DPPH radical scavenging activity is commonly reported as IC50. In this research, IC50 was 0.82 mg mL-1 (Fig. 3). Additionally, postbiotics revealed antimicrobial activity on L. monocytogenes which was ZOI of 27.00 ± 1.20 mm.
Fig. 3.
The antioxidant activity 2, 2′-diphenyl-1-picrylhydrazyl (DPPH) of postbiotics of Lactiplantibacillus sakei.
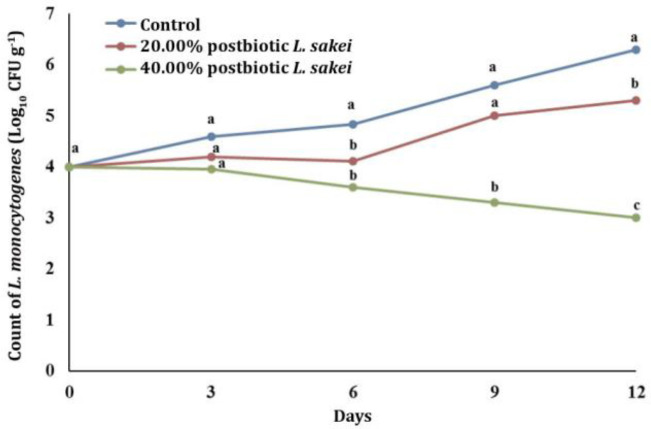
Anti- Listeria performance of postbiotics on beef fillet. Figure 4 shows the inhibitory effects of two concentrations of postbiotics (20.00 and 40.00%) on L. monocytogenes in beef fillets during 15 days of storage at 4.00 ˚C. Accordingly, during storage, L. monocytogenes in all treatments was increased and 40.00% postbiotics were the most effective Anti-Listeria treatments reducing the number of bacteria from 6.20 log10 CFU g-1 in the control group to 3.30 log10 CFU g-1 (p < 0.05) after 15 days. However, the 20.00% postbiotic treatment showed an insignificant decrease in bacterial counts compared to the control group.
Fig. 4.
The effect of different concentration of postbiotics of Lactiplantibacillus sakei on Listeria monocytogenes on beef fillet. On each sampling day, values with different letters are significantly different (p ≤ 0.05).
Discussion
The FTIR has been recommended for obtaining qualitative information about the metabolites of postbiotics.27 In this sense, Shafipour Yordshahi et al. used FTIR to investigate lyophilized postbiotics from Lactobacillus plantarum.26 The FTIR technique was also used to characterize the biosurfactants and production of exopolysaccharides during the growth of LAB.28 The obtained FTIR spectrum revealed that the functional molecules including biopolymers such as organic acids, polysaccharides and some others are in the postbiotics. Despite the fact that the chemical composition of postbiotics is still not completely defined, it has been found that L. sakei may produce several metabolites such as phosphoric acid, citric acid, butanoic acid, propionic acid and specific sugars in culture conditions.5 Variations in the postbiotic composition can be attributed to the specific microbial strains employed, the culturing conditions and the composition of the culture media which collectively exert the most significant influence on the types of metabolites produced.29,30 As identified from the spectrum, the absorption bands at 3,378 and 3,242 cm-1 can be assigned to the bonded/non-bonded O-H groups and N-H stretching vibration, respectively, and the peak centered at 2,917 cm-1 was attributed to sp3 C–H stretching vibrations.5 The spectral band found at 1598 cm-1 can be related to the stretching of the carbonyl bond (C=O) with a look at carboxylic acids. Moreover, the peaks at 1,413 and 1,247 cm-1 correspond to the aromatic ring vibration, CH2 scissoring vibration/COOH bending, and the sharp peak at 1041 cm-1 is characterized as sugars index bands (C– H bending vibration).31
The IC50 is defined as the antioxidant concentration required to reduce 50.00% of the initial DPPH concentration.32 In this study, the IC50 of L. sakei postbiotics was 0.82 mg mL-1. To date, there have been no reports on the DPPH activity of L. sakei postbiotics. Studies have proven that the various strains of bacteria and growth media can induce differences in the composition, levels of the compounds and properties of postbiotics.33 Hamad et al. reported the antioxidant activity as 37.45 μg mL-1 for the postbiotics of Pediococcus acidilactici,34 while, İncili et al. reported the DPPH activity of P. acidilactici postbiotics to 1,291.02 mg L-1.33 Antioxidant activity of postbiotics of LAB is linked to the type and concentration of phenolic compounds including gallic acid.11
The antimicrobial activities of LAB are related to various major compounds such as lactic acid, bacteriocin and hydrogen peroxide as well as minor compounds (i.e., bacteriocin-like compounds, cyclopentane, benzoic acid, and pyrrolo [1, 2-a] pyrazine-1, 4-dione) that are secreted during the growth of bacteria.5 In addition, LAB can produce various organic acids and a significant antimicrobial mechanism of postbiotics is the alteration of the bacterial cell membrane caused by organic acids. The synthesis of cell walls by prokaryotes is affected by organic acids and they can also disrupt the production of proteins.15 It seems that some postbiotic metabolites such as fatty acids may facilitate organic acids to enter the cell membranes. This causes the release of cellular substances and ions endangering the survival of bacterial cells especially gram-positive bacteria.5,35 Given that L. sakei postbiotics consist of several fatty acids, it is expected to have a great antibacterial effect.36 The ZOI of L. sakei postbiotics on L. monocytogenes was 27.00 ± 1.20 mm. Similar results have been reported in other studies, for example, Arrioja-Bretón et al. reported ZOI of 20.00 mm for L. sakei postbiotics.24 In another study, Bajpai et al. tested the antibacterial activities of lyophilized postbiotics of Pedioccoccus pentosaceus 4I1 on L. monocytogenes and reported ZOI around 18.00 mm.37 Our research showed significant inhibitory activity against L. monocytogenes by postbiotics of L. sakei.
Aerosolization of 40.00% postbiotics of L. sakei on beef fillet caused a significant decrease in L. monocytogenes counts (p < 0.05) by 3.30 log10 compared to the control group during the storage period, however, 20.00% postbiotics concentration did not significantly decrease the count of L. monocytogenes. Based on NACMCF studies, reductions of 2.00 log10 in the bacterial population in food inactivation studies have practical applications.38 Several studies have reported the anti-bacterial activity of L. sakei postbiotics. The postbiotics of Lacticaseibacillus casei incorporated into whey protein films were found to be effective in controlling pathogens and reducing 1.40 log10 of L. monocytogenes in fresh beef in 5 days.22 In a study, the anti-microbial activity of postbiotics of L. sakei, which were incorporated in bacterial nanocellulose, was examined in buffalo meat. Results showed 2.30 - 2.60 log CFU g-1 reduction in the L. monocytogenes count during storage for 9 days at 8.00 ˚C.4 Hartmann et al. found that the 10.00% concentration of postbiotics produced by different LAB strains a significant decrease in the L. monocytogenes counts as 3.30 log10 in ground beef.39 İncili et al. showed that 10.00% and 50.00% postbiotics of L. plantarum concentrations caused a significant decrease as 1.00 log10 and 2.10 log10, respectively, in the count of L. monocytogenes, compared to the control group.11 In another study, Ünlü et al. used the bacteriocin-containing freeze-dried postbiotics powders obtained from different LAB strains treatment onto the hot-dog surface and showed a reduction as 2.00 - 3.00 log10 in L. monocytogenes counts within 4 weeks.40 In addition, Arrioja-Bretón et al. found that the marinade of beef samples containing ten-fold concentrated postbiotics decreased the L. monocytogenes counts to 3.14 log10 at refrigeration temperature during 14 hr.24 The possible reasons for these variations among the results of studies were due to the use of different strains of LAB, the type and method of application of postbiotics and differences in food matrices.
In conclusion, this study was conducted to prepare postbiotics of L. sakei and evaluate their antibacterial performance in meat by aerosolization technique. Based on the results, these postbiotics had effective antioxidant capacity and implementing the aerosolization technique improved the anti-Listeria performance of postbiotics. We found that 40.00% postbiotics treatment effectively showed antibacterial activity and reduced L. monocytogenes counts by more than 3.00 log10 CFU mL-1 on beef fillets during storage at 4.00 ˚C for 15 days. It was concluded that the aerosolization technique could be successfully applied for the antimicrobial packaging of meat in which a lower amount of antimicrobials is used.
Acknowledgments
This research was funded by the Faculty of Veterinary Medicine, Urmia University, Urmia, Iran.
Conflict of interest
The authors declare no conflict of interest regarding this study.
References
- 1.Ghorbani M, Moradi M, Tajik H, et al. Carbon dots embedded bacterial cellulose membrane as active packaging: toxicity, in vitro release and application in minced beef packaging. Food Chem. 2024;433:137311. doi: 10.1016/j.foodchem.2023.137311. [DOI] [PubMed] [Google Scholar]
- 2.Barcenilla C, Ducic M, López M, et al. Application of lactic acid bacteria for the biopreservation of meat products: A systematic review. Meat Sci. 2022;183:108661. doi: 10.1016/j.meatsci.2021.108661. [DOI] [PubMed] [Google Scholar]
- 3.Mohammadi R, Moradi M, Tajik H, et al. Potential application of postbiotics metabolites from bio-protective culture to fabricate bacterial nanocellulose based antimicrobial packaging material. Int J Biol Macromol. 2022;220:528–536. doi: 10.1016/j.ijbiomac.2022.08.108. [DOI] [PubMed] [Google Scholar]
- 4.Pisoschi AM, Pop A, Georgescu C, et al. An overview of natural antimicrobials role in food. Eur J Med Chem. 2018;143:922–935. doi: 10.1016/j.ejmech.2017.11.095. [DOI] [PubMed] [Google Scholar]
- 5.Rasouli Y, Moradi M, Tajik H, et al. Fabrication of anti-Listeria film based on bacterial cellulose and Lactobacillus sakei-derived bioactive metabolites; application in meat packaging. Food Biosci. 2021;42:101218. [Google Scholar]
- 6.Zheng J, Wittouck S, Salvetti E, et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol. 2020;70(4):2782–2858. doi: 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]
- 7.Amiri S, Kazemi S. Concept and potential applications of postbiotics in the food industry [Persian] J Food Sci Technol. 2022;19(126):87–101. [Google Scholar]
- 8.İncili GK, Akgöl M, Karatepe P, et al. Quantification of bioactive metabolites derived from cell-free super-natant of Pediococcus acidilactici and screening their protective properties in frankfurters. Probiotics Anti-microb Proteins. 2023 doi: 10.1007/s12602-023-10147-6. doi: 10.1007/s12602-023-10147-6. [DOI] [PubMed] [Google Scholar]
- 9.de Toledo Guimarães J, Barros C, Sharafi H, et al. Postbiotics preparation for use in food and beverages. In: da Cruz AG, Silva MC, Pimentel TC, et al., editors. Probiotic foods and beverages. 1st ed. Berlin, Germany: Springer Nature ; 2023. pp. 223–242. [Google Scholar]
- 10.Sharafi H, Divsalar E, Rezaei Z, et al. The potential of postbiotics as a novel approach in food packaging and biopreservation: a systematic review of the latest developments. Crit Rev Food Sci Nutr. 2023 doi: 10.1080/10408398.2023.2253909. doi: 10.1080/10408398.2023.2253909. [DOI] [PubMed] [Google Scholar]
- 11.İncili GK, Karatepe P, Akgöl M, et al. Impact of chitosan embedded with postbiotics from Pediococcus acidilactici against emerging foodborne pathogens in vacuum-packaged frankfurters during refrigerated storage. Meat Sci. 2022;188:108786. doi: 10.1016/j.meatsci.2022.108786. [DOI] [PubMed] [Google Scholar]
- 12.Da Costa RJ, da Silva AP, da Fonseca RN, et al. Characterization of Enterococcus faecium EO1 isolated from mutton and activity of bacteriocin-like substances in the control of Listeria monocytogenes in fresh mutton sausage. LWT. 2021;141:110954. [Google Scholar]
- 13.De Lima AL, Guerra CA, Costa LM, et al. A natural technology for vacuum-packaged cooked sausage preservation with potentially postbiotic-containing preservative. Fermentation. 2022;8(3):106. [Google Scholar]
- 14.İncili GK, Karatepe P, Akgöl M, et al. Characterization of lactic acid bacteria postbiotics, evaluation in-vitro antibacterial effect, microbial and chemical quality on chicken drumsticks. Food Microbiol. 2022;104:104001. doi: 10.1016/j.fm.2022.104001. [DOI] [PubMed] [Google Scholar]
- 15.Moradi M, Tajik H, Mardani K, et al. Efficacy of lyophilized cell-free supernatant of Lactobacillus salivarius (Ls-BU2) on Escherichia coli and shelf life of ground beef. Vet Res Forum. 2019;10(3):193–198. doi: 10.30466/vrf.2019.101419.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan DJ, Cruz-Romero MC, Hernandez AB, et al. A novel method to deliver natural antimicrobial coating materials to extend the shelf-life of European hake (Merluccius merluccius) fillets. Food Packag Shelf Life. 2020;25:100522. [Google Scholar]
- 17.Jiang Y, Fan X, Li X, et al. Inactivation of Salmonella Typhimurium and quality presevation of cherry tomatoes by in-package aerosolization of anti-microbials. Food Control. 2017;73:411–420. [Google Scholar]
- 18.İncili GK, Akgöl M, Karatepe P, et al. Inhibitory effect of bioactive compounds derived from freeze-dried paraprobiotic of Pediococcus acidilactici against food-borne pathogens: In-vitro and food model studies. Food Res Int. 2023;170:113045. doi: 10.1016/j.foodres.2023.113045. [DOI] [PubMed] [Google Scholar]
- 19.İncili GK, Akgöl M, Karatepe P, et al. Whole-cell postbiotics: an innovative approach for extending the shelf life and controlling major foodborne pathogens in chicken breast fillets. Food Bioprocess Tech. 2023;16:1502–1524. [Google Scholar]
- 20.Chakchouk-Mtibaa A, Smaoui S, Ktari N, et al. Biopreservative efficacy of bacteriocin bacFL31 in raw ground turkey meat in terms of microbiological, physicochemical, and sensory qualities. Biocontrol Sci. 2017;22(2):67–77. doi: 10.4265/bio.22.67. [DOI] [PubMed] [Google Scholar]
- 21.Zagorec M, Champomier-Vergès M-C. Lactobacillus sakei: A starter for sausage fermentation, a protective culture for meat products. Microorganisms. 2017;5(3) doi: 10.3390/microorganisms5030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beristain-Bauza SDC, Mani-López E, Palou E, et al. Antimicrobial activity of whey protein films supplemented with Lactobacillus sakei cell-free supernatant on fresh beef. Food Microbiol. 2017;62:207–211. doi: 10.1016/j.fm.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 23.N Kocabey, M Yılmaztekin, Hayaloglu AA. Effect of maceration duration on physicochemical characteristics, organic acid, phenolic compounds and antioxidant activity of red wine from Vitis vinifera L. Karaoglan. J Food Sci Technol. 2016;53(9):3557–3565. doi: 10.1007/s13197-016-2335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arrioja-Bretón D, Mani-López E, Palou E, et al. Antimicrobial activity and storage stability of cell-free supernatants from lactic acid bacteria and their applications with fresh beef. Food Control. 2020;115:107286. [Google Scholar]
- 25.Shen C, Lemonakis L, Etienne X, et al. Evaluation of commercial antimicrobials against stress-adapted Campylobacter jejuni on broiler wings by using immersion and electrostatic spray and an economic feasibility analysis. Food Control. 2019;103:161–166. [Google Scholar]
- 26.Shafipour Yordshahi A, Moradi M, Tajik H, et al. Design and preparation of antimicrobial meat wrapping nanopaper with bacterial cellulose and postbiotics of lactic acid bacteria. Int J Food Microbiol. 2020;321:108561. doi: 10.1016/j.ijfoodmicro.2020.108561. [DOI] [PubMed] [Google Scholar]
- 27.Moradi M, Molaei R, Guimarães JT. A review on preparation and chemical analysis of postbiotics from lactic acid bacteria. Enzyme Microb Technol. 2021;143:109722. doi: 10.1016/j.enzmictec.2020.109722. [DOI] [PubMed] [Google Scholar]
- 28.Trabelsi I, Ben Slima S, Chaabane H, et al. Purification and caracterization of a novel exopolysaccharides produced by Lactobacillus sp, Ca6. Int J Biol Macromol. 2015;74:541–546. doi: 10.1016/j.ijbiomac.2014.12.045. [DOI] [PubMed] [Google Scholar]
- 29.Satpute SK, Kulkarni GR, Banpurker AG, et al. Biosurfactant/s from Lactobacilli species: prpoperties, challenges and potential biomedical application. J Basic Microbiol. 2016;56(11):1140–1158. doi: 10.1002/jobm.201600143. [DOI] [PubMed] [Google Scholar]
- 30.Chang HM, Foo HL, Loh TC, et al. Comparative studies of inhibitory and antioxidant activities, and organic acids compositions of postbiotics produced by probiotic Lactiplantibacillus plantarum strains isolated from malaysian foods. Front Vet Sci. 2021;7:602280. doi: 10.3389/fvets.2020.602280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Šebek J, Knaanie R, Albee B, et al. Spectroscopy of the C-H stretching vibrational band in selected organic molecules. J Phys Chem A. 2013;117(32):7442–7452. doi: 10.1021/jp4014674. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Morales F, Alonso-Castro AJ, Zapata-Morales JR, et al. Use of standardized units for a correct interpretation of IC50 values obtained from the inhibition of the DPPH radical by natural antioxidants. Chem Pap. 2020;74:3325–3334. [Google Scholar]
- 33.İncili GK, Karatepe P, Akgöl M, et al. Characterization of Pediococcus acidilactici postbiotic and impact of postbiotic-fortified chitosan coating on the microbial and chemical quality of chicken breast fillets. Int J Biol Macromol. 2021;184:429–437. doi: 10.1016/j.ijbiomac.2021.06.106. [DOI] [PubMed] [Google Scholar]
- 34.Hamad GM, Abdelmotilib NM, Darwish AMG, et al. Commercial probiotic cell-free supernatants for inhibition of Clostridium perfringens poultry meat infection in Egypt. Anaerob. 2020;62:102181. doi: 10.1016/j.anaerobe.2020.102181. [DOI] [PubMed] [Google Scholar]
- 35.Diop MB, Alvarez VB, Guiro AT, et al. Efficiency of neutralized antibacterial culture supernatant from bacteriocinogenic lactic acid bacteria supplemented with salt in control of microorganisms present in senegalese artisanally handled fish by immersion preservative technology during guedj seafood processing at 10°C and 30°C. J Food Microbiol Saf Hyg. 2016;1:102 . [Google Scholar]
- 36.Wang B, Song Q, Zhao F, et al. Production optimization, partial characterization and properties of an exopolysaccharide from Lactobacillus sakei L3. Int J Biol Macromol. 2019;141:21–28. doi: 10.1016/j.ijbiomac.2019.08.241. [DOI] [PubMed] [Google Scholar]
- 37.Bajpai VK, Han JH, Rather IA, et al. characterization and antibacterial potential of lactic acid bacterium Pedioccoccus pentosaceus 4I1 isolated from freshwater fish Zacco koreanus. Front Microbiol. 2016;7:2037. doi: 10.3389/fmicb.2016.02037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Advisory Committee on Microbiological Criteria for Foods. Parameters for determining inoculated pack/challenge study protocols. J Food Prot. 2010;73(1):140–202. doi: 10.4315/0362-028x-73.1.140. [DOI] [PubMed] [Google Scholar]
- 39.Hartmann HA, Wilke T, Erdmann R. Efficacy of bacteriocin-containing cell-free culture supernatants from lactic acid bacteria to control Listeria monocytogenes in food. Int J Food Microbiol. 2011;146(2):192–199. doi: 10.1016/j.ijfoodmicro.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 40.Ünlü G, Nielsen B, Ionita C. Inhibition of Listeria monocytogenes in hot dogs by surface application of freeze-dried bacteriocin-containing powders from lactic acid bacteria. Probiotics Antimicrob Proteins. 2016;8(2):102–110. doi: 10.1007/s12602-016-9213-2. [DOI] [PubMed] [Google Scholar]