Abstract
The importance of each of the two interferon (IFN) systems in impeding herpesvirus replication and in stimulating virus-specific lymphocytes to control an acute systemic infection is not completely understood. To further our knowledge, pseudorabies virus, attenuated by deletion of the glycoprotein E gene to impair its neurovirulence and by deletion of the thymidine kinase gene (gE−TK−PRV), was used to infect wild-type 129Sv/Ev and congenic mice with immune system-associated genetic deficiencies. Mice with mature B and T lymphocytes but lacking either one or both functional receptors for members of each of the two IFN families were infected with gE−TK−PRV. At 3 and 7 but not 14 days after infection, replicating gE−TK−PRV could be isolated only from livers or spleens of mice lacking the receptors for both IFN families, and these mice survived the infection. Therefore, functional IFN receptors were not required to induce a protective immune response against an acute infection with gE−TK−PRV. Furthermore, PRV-specific antibodies of all immunoglobulin G isotypes were produced in these mice. Mice without mature B and T lymphocytes and lacking either one or both functional receptors for members of each of the two IFN families were also infected with gE−TK−PRV. Three days after infection, replicating virus could be isolated only from mice lacking both mature B and T lymphocytes and functional IFN receptors, and these mice were not able to clear the virus. We present evidence that mice with an intact gamma IFN system but without mature B and T cells were able to prevent systemic dissemination of gE−TK−PRV.
Pseudorabies virus (PRV) is a member of the subfamily Alphaherpesvirinae. Besides the pig, considered the reservoir host of PRV, a variety of other species including mice and rats can naturally be infected with this virus. After initial replication at the entry site, the virus gains access to the central nervous system by transsynaptical cell-to-cell spread and retrograde axonal transport within neurons. Using lymphatic or hematogenic pathways, PRV may also disseminate systemically (4, 23, 29). In mice, wild-type PRV strains are associated with a neurovirulent phenotype. Within days after infection, almost 100% of the animals die. Neurovirulence of PRV is based on effective neuroinvasiveness and neurotoxicity that has been associated with the viral glycoproteins E (gE) and I (gI), which form heterodimers for proper function (2, 17, 42). For efficient replication in nondividing cells, such as neurons, the viral thymidine kinase (TK) is also important. Mice exposed to a gE− and TK− double deletion mutant (gE−TK−PRV) control the infection (2) and react with a potent virus-specific immune response (18). Therefore, gE−TK−PRV can be used to infect wild-type or congenic mice with immune system-associated genetic deficiencies in order to study the contributions of the innate and specific immune system against PRV infections.
The two interferon (IFN) systems are important components of innate immunity and can influence viral replication either directly by antiviral activity or indirectly by modulation of the immune response (4, 9, 21). The direct antiviral activity attributed to alpha/beta IFN (IFN-α/β) and gamma/IFN (IFN-γ) includes the induction of proteins and enzymes that inhibit virus multiplication by impairing accumulation of virus-specific mRNA and proteins. Indirect effects of IFNs include the induction of lymphokines, chemokines, and monokines that may attract and activate macrophages and NK cells (10).
IFNs also have pleiotropic effects on specific lymphocytes. IFN-γ is thought to be a key regulatory cytokine in Th-1-like immune responses (33, 34, 37). In mice, both IFN-γ production and a Th-1-like immune response appear to be associated with an immunoglobulin (Ig) isotype switch to virus-specific IgG2a or IgG3 (7, 37, 38).
With mice congenitally deficient in the IFN-γ gene, the apparent redundancy for IFN-γ in controlling acute virus infection was shown for herpes simplex virus type 1 (HSV-1) (47), murine gammaherpesvirus (31), influenza virus (13), Sendai virus (24), vesicular stomatitis virus (VSV), and Semliki Forest virus (33). For an immune response against other viruses, the IFN-α/β receptor, which binds IFN-α/β, or the IFN-γ receptor, which binds IFN-γ, appeared to be required, and the function of the IFN receptors was nonredundant. This was shown for lymphocytic choriomeningitis virus (LCMV), vaccinia virus, ectromelia virus, and mouse hepatitis virus (15, 17, 26, 36, 44). Limited data are available from virus-infected mice with a deletion of both copies of the IFN-α/β and IFN-γ receptor genes. Infection of these mice with less than 100 infective VSV or LCMV particles led to overwhelming virus replication and inadequate immune response (44).
Passive immunization of B-cell-deficient mice with PRV-specific antibodies (Abs) contributed to protection following virus challenge (33). PRV-specific cytolytic T cells can be elicited after virus infection (49), but cytolytic T cells do not appear to be essential for immunity (3). By contrast, depletion of CD4+ T cells partially abrogates PRV-induced protection (3). Previously, we have shown that gE−TK−PRV did not lead to systemic infection in mice with disrupted IFN-γ receptor, but the precise contribution of the innate and specific immune system to impeding virus spread was not analyzed in detail (33).
In a first set of experiments described here, mice with mature B and T cells but lacking either one or both functional receptors for members of each of the two IFN families were infected with gE−TK−PRV. The data showed that functional IFN receptors were not required to induce a protective immune response against an acute gE−TK−PRV infection. In a second set of experiments, mice without mature B and T cells (6) and again lacking either one or both functional receptors for each of the two IFN families were also infected with gE−TK−PRV. We present evidence that mice with an intact IFN-γ system but without mature B and T cells were able to prevent systemic dissemination of gE−TK−PRV.
MATERIALS AND METHODS
Mice.
Inbred 129Sv/Ev (H-2b) (wt129) mice were used throughout this study. Congenic mice with gene-targeted disruptions of the IFN-α/β receptor (A129) or IFN-γ receptor (G129) and mice with disrupted IFN-α/β and IFN-γ receptors (AG129) were used (15, 26) (Table 1). Mice with deleted recombination-activating gene 2 (RAG-2) (6) were crossed with A129 or AG129 mice to obtain homozygous AR129 (IFN-α/β receptor and RAG-2 deficient) and AGR129 (IFN-α/β and IFN-γ receptor and RAG-2 deficient) (17) mice, respectively. The altered genome of the newly bred mice was analyzed by PCR (6, 26). Homozygous AR129 or AGR129 (19) mice had a normal litter size, and the offspring remained healthy for more than 1 year under specific-pathogen-free conditions. All mice were bred and kept under specific-pathogen-free conditions at the Institut für Labortierkunde, University of Zürich.
TABLE 1.
Overview of mouse strains used
| Mouse strain | IFN-α/β system | IFN-γ system | Mature B and T cells |
|---|---|---|---|
| wt129a | Functional | Functional | Present |
| A129 | Nonfunctional | Functional | Present |
| G129 | Functional | Nonfunctional | Present |
| AG129 | Nonfunctional | Nonfunctional | Present |
| RAG-2 deficientb | Functional | Functional | Absent |
| AR129 | Nonfunctional | Functional | Absent |
| AGR129 | Nonfunctional | Nonfunctional | Absent |
Mice (129Sv/Ev; H-2b) of the same MHC haplotype were used.
Mice with both copies of RAG-2 deleted are unable to produce mature specific B and T cells (6).
Virus.
A PRV strain with deletions of genes encoding gE and TK (18) was obtained commercially (Intervet, Boxmer, The Netherlands). Aliquots of the same virus batch were used throughout. Before use, infective virus was determined as described below (“Virus titration”).
Infection of mice and collection of blood and organs.
Mice of both sexes, 6 to 8 weeks of age, were infected intraperitoneally with 5 × 105 50% tissue culture infective doses of gE−TK−PRV suspended in 200 μl of RPMI medium (Gibco BRL, Life Technologies, Basel, Switzerland). At 1, 3, and 7 and, in some cases, 14, 21, and 28 days postinfection (dpi) with gE−TK−PRV, animals were sacrificed. Blood was drawn and allowed to clot to obtain serum. Livers, lungs, kidneys, brains, one-fourth of the spleens, and clotted blood cells were snap frozen in liquid nitrogen and kept at −80°C. The rest of the spleen was taken and used for splenocyte restimulation experiments (see below).
Virus titration.
The snap-frozen organs and the blood cell pellets were allowed to thaw, and the organs were homogenized by mortar, pestle, and sterile sand. Tenfold dilutions starting at 10−2 of the centrifugation-clarified supernatant were titrated on MDBK cells as described elsewhere (25). Plaques were counted 64 h after seeding, and virus titers were expressed as log10 PFU per organ or cell sample. The detection limit of gE−TK−PRV was 102 PFU/organ or cell sample.
Splenocyte restimulation assay.
For the analysis of the cytokine production in vitro, erythrocyte-depleted splenocytes (1.5 × 106 cells/ml) were cultured in 24-well plates (Nunc, Breda, The Netherlands) as described before (32). Supernatants of the cultures were harvested 48 h after cell seeding and stored at −20°C.
Analysis of cytokine levels.
For the analysis of in vivo cytokine production of interleukin-1β (IL-1β), IFN-γ, IL-2, IL-4, IL-6, IL-10, and IL-12-p40, sera were analyzed at 1, 3, and 7 dpi with a commercial enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, Minn.). Similarly, cytokine concentrations in supernatants from splenocyte cultures were determined with the same system.
Determination of PRV-specific serum Ab titers.
Titers of PRV-specific total Ig and of PRV-specific IgG isotypes (IgG1, IgG2a, IgG2b, and IgG3) in serum were determined by ELISA essentially as previously described (32). Briefly, 96-well flat-bottomed plates (Nunc) were coated with 100 μl of predetermined inactivated PRV particles as antigen suspended in NaHCO3 (0.05 M, pH 9.6) and incubated overnight at 4°C. Subsequently, plates were frozen at −20°C for at least 24 h or until use. After thawing, the plates were washed with tap water. The serum samples were diluted twofold starting at 1/16 in phosphate-buffered saline (0.04 M), 0.1% Tween 20, and 1% bovine serum albumin (Sigma Chemical Co., St. Louis, Mo.) and incubated for 1 h at 37°C. After incubation, the plates were washed again with tap water. Ig (total)- and IgG isotype-specific Abs directly coupled to horseradish peroxidase (Southern Biotechnology Associates, Inc., Birmingham, Ala.) appropriately diluted in the same buffer as the sera were added for 30 min at 37°C. The IgG isotype specificity of the horseradish peroxidase-coupled Ab was verified as described elsewhere (5). After being washed with tap water, the substrate was allowed to react for 30 min at room temperature. The reaction was stopped with 2 M sulfuric acid and read at 450 nm. The Ig titers of the sera were defined as the reciprocal of the highest dilution with an absorbency twice that of the background.
RESULTS
gE−TK−PRV is systemically disseminated in AG129 mice but not in wt129, A129, or G129 mice.
Groups of three to four mice (Table 1) were infected with gE−TK−PRV and monitored clinically. To analyze systemic virus dissemination, gE−TK−PRV titers were determined in liver and spleen tissue at 3, 7, and, in some cases, 14 or 28 dpi. As expected from previous experiments, no virus could be detected at any time point in liver or spleen tissue from wt129 mice or G129 mice (33) or A129 mice (Table 2).
TABLE 2.
Determination of gE−TK−PRV titers in liver and spleen tissue
| Mouse strain(s) | dpia | Virus titer in tissue:
|
|
|---|---|---|---|
| Liver | Spleen | ||
| wt129, A129, G129, RAG-2 | 3 | <102 | <102 |
| 7 | <102 | <102 | |
| AG129 | 3 | 2.4 × 102 ± 2.1 × 102 | 2 × 102 ± 1.9 × 102b |
| 7 | 1.6 × 102 ± 0.8 × 102c | 1.5 × 102 ± 1.1 × 102b | |
| AR129 | 3 | <102 | <102 |
| 7 | <102 | <102 | |
| AGR129 | 3 | 4 × 102 ± 2 × 102 | 3.2 × 102 ± 1.5 × 102 |
| 7 | 5.3 × 106 ± 2.4 × 106 | 2.4 × 104 ± 2.1 × 104 | |
At 3 and 7 days after infection with gE−TK−PRV, organs were analyzed for the presence of virus. Values are means from two groups of three mice.
Four of six animals had detectable amounts of virus.
Five of six animals had detectable amounts of virus.
gE−TK−PRV-inoculated AG129 mice appeared clinically healthy and survived infections for up to 4 weeks. However, in this mouse strain virus could be recovered from spleen and liver tissue (Table 2) at 3 and 7 dpi. Two weeks after virus infection, no virus could be isolated from any organ of the 14 AG129 mice tested. Therefore, neither of the two IFN systems appeared to be required to clear systemic gE−TK−PRV.
RAG-2-deficient and AR129 mice but not AGR129 mice control systemic infection of gE−TK−PRV.
To analyze gE−TK−PRV replication in the absence of mature major histocompatibility complex (MHC)-restricted T cells and functional B cells, RAG-2-deficient mice were inoculated with gE−TK−PRV, and systemic virus dissemination was analyzed as described above (Tables 1 and 2). No virus could be isolated from livers or spleens of RAG-2-deficient mice at 3, 7, or 28 dpi. Thus, in the absence of specific lymphocytes, the innate immune system was able to prevent systemic spread of gE−TK−PRV.
To test the contribution of the IFN systems to the control of virus replication in RAG-2-deficient mice, AR129 and AGR129 mice (Table 1) were infected with gE−TK−PRV and monitored clinically. AR129 mice remained healthy throughout the analyzed period of 4 weeks after infection. By contrast, AGR129 mice were moribund at 7 dpi and were euthanized.
Virus titers in liver and spleen tissue were analyzed at 3, 7, and, in some cases, 14 and 28 dpi (Table 2). No virus could be detected in organs of AR129 mice at any time point. By contrast, at 3 dpi, 102 to 103 PFU of gE−TK−PRV could be isolated from livers and spleens of infected AGR129 mice. Virus titer in the AGR129 mice reached 106 to 107 PFU of gE−TK−PRV/liver or spleen at 7 dpi. About one-third of the animals were viremic at this time point, and virus could be isolated from whole blood as well as kidney, lung, and brain (data not shown). Histological evidence for tissue damage found in liver, pancreas, and lung tissue of AGR129 mice was further indicative of the presence of virus in these organs (data not shown). Therefore, the absence of functional receptors for IFN-α/β and IFN-γ in combination with the lack of mature T and B cells led to uncontrolled systemic virus replication in AGR129 mice. By contrast, the functional IFN-γ system in AR129 mice was sufficient to prevent systemic dissemination of gE−TK−PRV.
Determination of cytokines present in serum of gE−TK−PRV-infected mice.
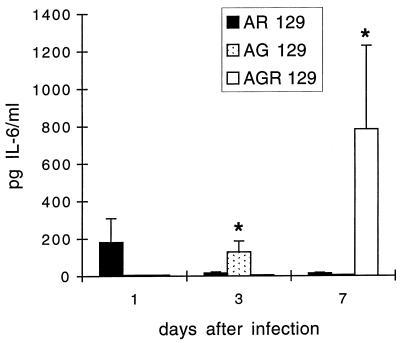
To analyze the early immune response against gE−TK−PRV infections ex vivo, the amounts of the proinflammatory cytokines IL-6, IL-1β, and tumor necrosis factor alpha (TNF-α) were analyzed in the sera at various time points after infection of mice. In three separate infection experiments, significant concentrations of IL-6 were detected in the sera from 10 of a total of 12 AR129 mice after 24 h, in all AG129 mice at 3 dpi, and in all AGR129 mice at 7 dpi (Fig. 1). Mock-infected animals had no detectable serum IL-6 (data not shown). It is possible that IFN-γ produced locally by AR129 mice with a functional IFN-γ receptor but not in AGR129 mice without a functional IFN receptor caused a rapid induction of acute-phase proteins including IL-6 (1, 28, 41).
FIG. 1.
Serum IL-6 levels in AR129, AG129, and AGR129 mice (left, middle, and right bars, respectively) infected with gE−TK−PRV. Serum IL-6 level was determined by ELISA at 1, 3, and 7 dpi. Values are means + standard deviations (error bars) from either 6 or 12 mice. Asterisks indicate statistical significance of values from different time points.
IL-1β (75 ± 40 pg/ml) was found only in sera from AGR129 mice at 7 dpi. Low but significant amounts of TNF-α were found at 7 dpi in the sera from three of six AGR129 mice analyzed (data not shown).
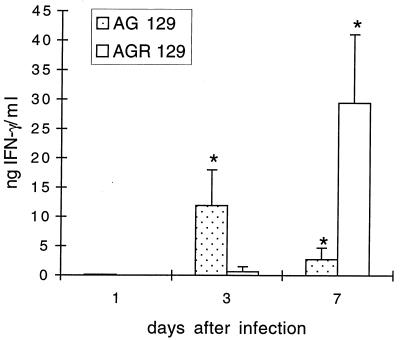
Among the lymphocyte-associated cytokines, IL-2, IL-4, IL-10, IFN-γ, and IL-12-p40 were analyzed. The latter two cytokines were detected in the sera of AG129 and AGR129 mice but not in those of the other mouse strains investigated. In AG129 mice, the serum IFN-γ concentration was maximal (12 ng/ml) at 3 dpi and declined thereafter. In AGR129 mice, IFN-γ was first detected at 3 dpi and reached more than 25 ng/ml of serum at 7 dpi (see Fig. 2). IL-12-p40 (785 ± 250 pg/ml) was found in AGR129 mice at 7 dpi. None of the cytokines analyzed was detected in mock-infected animals (data not shown). Thus, a rough correlation was found between viral titers in organs and cytokine levels in sera. With low amounts of virus present in organs, only IL-6 and IFN-γ were detected. With increasing viral titers, additional cytokines as well as more abundant quantities of the cytokines were detected. (Fig. 1 and 2; Table 2). Importantly, cytokines were detected early (day 1) in AR129 mice with an intact IFN-γ system but relatively late (days 3 to 7) in AG129 and AGR129 mice without functional IFN receptors.
FIG. 2.
Serum IFN-γ levels in AG129 and AGR129 mice infected with gE−TK−PRV. Serum IFN-γ level was determined by ELISA at 1, 3, and 7 dpi. Values are means + standard deviations (error bars) from either three or 6 mice. Asterisks indicate statistical significance of values from different time points.
Cytokine production of in vitro-restimulated spleen cells analyzed at 3 and 7 days after gE−TK−PRV infection.
In order to specifically analyze the cytokines secreted by splenocytes from the different mice, in vitro restimulation assays were performed at various time points after infection (Table 2). Around 800 pg of IFN-γ per ml was detected in spleen cell cultures from wt129 mice set up at 3 dpi. Significantly more IFN-γ was present in cultures of splenocytes from A129, G129, or AG129 mice analyzed at the same time point. Seven days after infection, 7 ng of IFN-γ per ml was produced by spleen cell cultures of wt129 mice. At this time point, similar amounts of IFN-γ ranging from 3 to 6 ng/ml were produced by spleen cells of A129 and AG129 mice but 10 times less was produced by spleen cells from G129 mice. In splenocyte cultures of AR129 or AGR129 mice devoid of specific lymphocytes, no significant amounts of IFN-γ were detected at any time point analyzed, even though large amounts of IFN-γ were detected in sera of AGR129 mice. Hence, comparable high amounts of IFN-γ were produced from splenocytes of gE−TK−PRV-infected wt129, A129, G129, and AG129 mice at day 3 and day 7. This indicates that the production of IFN-γ did not depend on intact receptors for IFN (Table 3).
TABLE 3.
Determination of IFN-γ and IL-4 levels in supernatants of in vitro-restimulated splenocytesa
| Mouse strain | IFN-γ
|
IL-4
|
IFN-γ/IL-4 ratio for gE−TK−PRV
|
|||||
|---|---|---|---|---|---|---|---|---|
| Mock (day 7) | gE−TK−PRV
|
Mock (day 7) | gE−TK−PRV
|
|||||
| Day 3 | Day 7 | Day 3 | Day 7 | Day 3 | Day 7 | |||
| wt129 | <10 | 835 ± 132 | 6,926 ± 904 | <10 | <10 | 101 ± 7 | NC | 68.5 |
| A129 | 15 ± 13 | 4,754 ± 810 | 6,293 ± 459 | 78 ± 62 | 16.3 ± 2.5 | 59.5 | 297 | 107.6 |
| G129 | 60 ± 16 | 3,825 ± 277 | 691 ± 284 | 70 ± 68 | 11.7 ± 3.7 | 54 ± 31 | 326 | 12.7 |
| AG129 | 33 ± 18 | 5,678 ± 2,800 | 2,920 ± 757 | 12.7 ± 7.5 | <10 | 49 ± 28 | NC | 59.6 |
| RAG-2 | <10 | <10 | ND | <10 | <10 | NC | NC | NC |
| AR129 | <10 | <10 | ND | <10 | <10 | NC | NC | NC |
| AGR129 | <10 | <10 | ND | <10 | <10 | NC | NC | NC |
Spleen cell restimulation assays were performed 3 and 7 days after infection with gE−TK−PRV. IFN-γ and IL-4 levels (picograms per milliliter) were determined by ELISA 48 h after in vitro restimulation. Values are means from spleen cells of three mice each either infected with gE−TK−PRV or mock infected. NC, values could not be calculated; ND, not determined.
In contrast to the production of IFN-γ, minimal amounts of IL-4 were found in the same culture supernatants at day 3 (Table 3). In general, somewhat more IL-4 was found at day 7. The IFN-γ/IL-4 ratio calculated was at least 10 but was as high as 300 at day 3. Therefore, the disruption of one or both IFN receptor genes did not alter the high IFN-γ/IL-4 ratio.
Other cytokines were found in spleen cell cultures of some mouse strains but were present in only low amounts. TNF-α was detected in similar levels in wt129, A129, G129, and AG129 mice at day 3 (90 to 110 pg/ml) and day 7 (110 to 160 pg/ml). In general, less than 100 pg of IL-12-p40 per ml was detected in any cell culture analyzed.
The lymphocyte-associated cytokines IL-2 (97.3 ± 25.4 pg/ml) and IL-10 (274.8 ± 127 pg/ml) were produced in cell cultures set up from spleens of 12 wt129 mice at 7 dpi. With spleen cells from the other mouse strains, only low amounts of IL-2 and IL-10 were occasionally seen (data not shown). Thus, among the cytokines analyzed, IFN-γ was the main cytokine secreted by splenocytes at 3 and 7 dpi, independent of the presence or absence of the two IFN receptors.
The production of PRV-specific IgG2a is independent of IFN receptors.
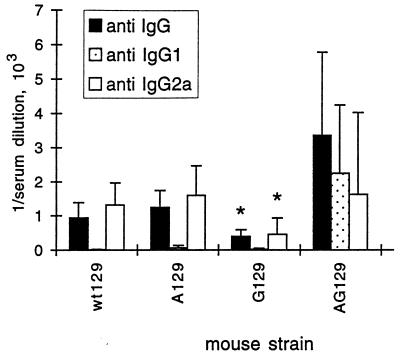
Sera of mice taken at 3, 7, 14, or 28 dpi were analyzed for PRV-specific Ab by ELISA (Fig. 3). At day 3, little if any PRV-specific Ab was detected in any mouse strain analyzed. At day 7, significantly more total IgG Ab against PRV was produced by wt129 mice than by G129 mice. By contrast, AG129 mice produced more IgG Ab against the antigen than did wt129 mice. Only half of the wt129, A129, and G129 mice had detectable IgG1 Ab against PRV, whereas all AG129 mice had high levels of IgG1 against PRV with a mean titer of 1/2,000. At day 7, wt129 mice as well as all IFN-receptor-deficient mice with mature B and T cells responded with IgG2a Ab specific to PRV. wt129 mice produced significantly more IgG2a Ab against PRV than did G129 mice. This positive regulatory role for IFN-γ in Ab formation has been noted earlier (33). AG129 mice produced more IgG2a Ab against the PRV antigen than did wt129 mice (Fig. 3), possibly as a result of the higher antigenic load. Little if any PRV-specific Ab of the IgG2b or IgG3 subclass was detected in any serum analyzed at 7 dpi.
FIG. 3.
PRV-specific Ab titers of mice infected with gE−TK−PRV at 7 dpi. Titers of total Ig, IgG1, and IgG2a (left, middle, and right bars of each strain, respectively) specific to PRV of the mouse strains indicated were determined by ELISA. Values are means + standard deviations (error bars) from either five or nine mice. Asterisks indicate statistical significance of values from different mouse strains compared to wt129.
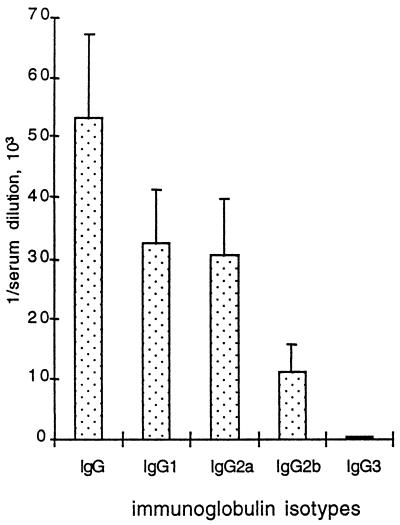
At 14 or 28 dpi, all mice with functional RAG (5) produced maximal PRV-specific Ab titers of all IgG isotypes with a preference for IgG2a and IgG2b as previously reported (33). In AG129 mice, the titers of IgG1 and IgG2a Abs remained similar (Fig. 4). Sera from AR129 or AGR129 mice remained negative in all ELISAs as expected from mice with deletion of RAG-2 (5). Thus, the onset of Ab production between days 3 and 7 and the spectrum of Ig isotype patterns produced were not fundamentally influenced by the absence of either IFN system, although differences in antigen load should be considered.
FIG. 4.
PRV-specific Ab titers of AG129 mice infected with gE−TK−PRV at 14 dpi. Titers of total Ig, IgG1, IgG2a, IgG2b, and IgG3 specific to PRV in the sera were determined by ELISA. Values are means + standard deviations (error bars) from three mice.
DISCUSSION
Components of both the innate and specific immune systems of mice were analyzed for their contribution to prevention of systemic dissemination of a highly attenuated pseudorabies virus, gE−TK−PRV.
AR129 mice have a functional IFN-γ system but lack mature MHC-restricted T and mature B lymphocytes because of a deletion of both copies of RAG-2 (6) (Table 1). After infection of AR129 mice with gE−TK−PRV, no virus could be isolated from any organ of these animals (Table 2). In the absence of PRV-specific lymphocytes, the control of virus replication in these mice could either be due to a direct antiviral effect of the IFN-γ system or be mediated indirectly by the innate immune system (14, 30, 42). IFN-γ was not detected in serum of gE−TK−PRV-infected AR129 mice, but we cannot exclude the possibility that this cytokine was produced locally in concentrations too small to be detected systemically. It is possible that IFN-γ and the acute-phase protein IL-6 induced rapidly within 1 dpi with gE−TK−PRV were able to control virus replication directly (Fig. 1). This has also been shown for other viral infections (1, 20, 28, 48). Alternatively, protection may have been mediated indirectly by cells of the innate immune system such as NK cells. NK cells have been shown to be effective in the defense against HSV-1 or murine cytomegalovirus. However, the following considerations argue against a massive activation of NK cells in AR129 mice infected with gE−TK−PRV. NK cell activity was described as being associated with early production of serum IL-1, IL-6, IL-12, IFN-γ, and TNF-α (28, 30, 43). With the exception of IL-6, no significant cytokine production was found in serum of AR129 mice, and none was detected in splenocyte cultures set up at 3 dpi (Table 3). Thus, the amount of gE−TK−PRV used in the present infection experiments may have failed to trigger a detectable NK cell activation in spleens of AR129 mice. Although NK cell activity in these mice has not been specifically tested after PRV infection, NK cells in AR129 mice are functional, as previously shown by their capacity for eliminating tumors (11). Therefore, our data suggest that the control of gE−TK−PRV replication by the IFN-γ system was due either to its direct local antiviral effect or, indirectly, to the induction of proinflammatory cytokines such as IL-6 or a combination of both (20). Preliminary experiments indicate that the IFN-α/β system (GR129 mice) was equally capable of preventing systemic dissemination of gE−TK−PRV (40).
In contrast to AR129 mice, AGR129 mice lack both IFN receptors and mature B and T cells. AGR129 mice appeared unable to control dissemination of gE−TK−PRV (Tables 1 and 2). High levels of IFN-γ were found in the sera of gE−TK−PRV-infected AGR129 mice at days 3 and 7, and some IL-6, IL-1β, IL-12-p40, and TNF-α were found at day 7 pi. It was shown that TNF-α and TNF-β could both directly inhibit virus replication and act in synergy with IFN-γ (46). However, our data indicate that in the absence of functional IFN receptors, TNF-α alone did not have a detectable antiviral effect in AGR129 mice. As suggested by others, the proinflammatory cytokines produced late after infection had no apparent antiviral effect when extensive virus replication had occurred (26, 28). Moreover, IFN molecules in mice devoid of functional IFN-α/β and IFN-γ receptors have no biological effect (16, 26).
The titers of gE−TK−PRV in spleens or livers from AGR129 mice without and AG129 mice with mature B and T cells were similar at 3 dpi with gE−TK−PRV (Table 2). Interestingly, significant amounts of IL-6 in these mice were detected only at 3 dpi. By contrast, in AR129 mice with an intact IFN-γ system, high levels of IL-6 and possibly other acute-phase proteins were present in sera at 1 dpi. Importantly, the presence of these molecules early after infection was associated with the control of PRV replication (Table 2). Therefore, the inability of mice devoid of functional IFN receptors to rapidly induce acute-phase proteins or other components of innate immunity could not be compensated for by mature B and T cells or their products within the first 3 days after infection.
By 3 dpi, the first virus-specific Ab could be detected in some AG129 mice. By 7 dpi, high titers of PRV-specific IgG1 and IgG2a were present in all AG129 mice, reaching maximal titers of virus-specific Abs of all Ig isotypes at 14 dpi (Fig. 4). Sera from AG129 mice had virus-neutralizing activity as determined by in vitro plaque inhibition assays (data not shown).
By 7 dpi, marginal virus titers were still detected in peripheral organs of AG129 mice, whereas in AGR129 mice the viral replication reached titers of 106 PFU per organ, and these mice had to be euthanized at this time point. By contrast, AG129 mice remained healthy, and no virus was detected in any organ analyzed by virological and histological methods 14 dpi (data not shown). We conclude that specific immune cells devoid of IFN receptors in AG129 mice are capable of clearing replicating gE−TK−PRV and that the Ab produced by these mice may partly be responsible for the clearance of replicating virus. Protection against virulent PRV by prior administration of serum from hyperimmune animals to G129 mice has been demonstrated elsewhere (33) and was also shown for HSV-2 (27). While the two IFN systems can possibly compensate for each other during acute PRV infections, their contribution in chronic viral infections still needs to be addressed (12, 22, 45).
wt129 mice survived infections with 2 × 106 PFU of VSV, whereas A129 or AG129 mice infected with the same virus were unable to mount a protective immune response, even when exposed to less than 100 infective particles (44). Furthermore, LCMV was capable of replicating persistently and unrestrictedly in both A129 and AG129 mice, whereas wt129 mice could clear the virus. Thus, the outcomes of VSV and LCMV infections in A129 or AG129 mice are markedly different from the outcome of an inoculation with the highly attenuated gE−TK−PRV. One explanation appears to be the higher efficiency with which VSV or LCMV can replicate in the IFN-receptor-deficient host compared to gE−TK−PRV. When AG129 mice were infected with 106 PFU of VSV, the virus titers were 106 PFU in spleens or livers 4 dpi (26). With a similar infective dose of the gE−TK−PRV in AGR129 or AG129 mice, only 102 to 103 PFU of gE−TK−PRV per organ was found within the same time frame. Although AGR129 or AG129 mice appeared unable to control gE−TK−PRV replication before 3 dpi, specific lymphocytes induced and Abs produced in AG129 mice were capable of inhibiting virus replication between days 3 and 14. After infection of A129 mice with VSV, the virus replication was overwhelming and the Ab production was inadequate to raise efficient antiviral protection. By contrast, antiviral protection by VSV-specific Ab given before systemic virus infection of A129 mice could be shown (39).
In mice, Ig isotype switch is an important readout to identify the type of immune response induced. Interestingly, a similar Ig isotype profile of PRV-specific Ab was produced in all mice with mature B and T cells analyzed, including AG129 mice. Yet, G129 mice showed impaired humoral responses as observed previously (33). Furthermore, AG129 mice infected with HSV-1 were able to clear replicating HSV-1 and produced virus-specific IgG2a (40). This is in contrast to LCMV-infected AG129 mice that were unable to switch to IgG2a, the dominant Ig isotype present in wt129 littermates after LCMV infection (46).
gE−TK−PRV-infected AR129 or GR129 mice (40) were able to control systemic spread of the virus, and a potent humoral immune response was induced in mice with intact RAG. Attenuated but replication-competent live virus, as used in the present experiments, is far more effective in inducing an immune response than are inactivated vaccines, indicating a link between some elements of virus replication and immunogenicity (33, 35). Herpesvirus replication can directly induce a plethora of gene products associated with immune system enhancement including IFN or IFN regulatory molecules, cytokines, chemokines, and their receptors (48). On the other hand, IFNs are potent amplifiers of acute-phase proteins and can regulate cells involved in early as well as in late immune responses (1, 8, 28). Each of the two IFN systems appears capable of controlling the spread of gE−TK−PRV, indicating the potency and need for early upregulation of proinflammatory signals to confine PRV replication. Indeed, the absence of the IFN receptors in AGR129 mice has devastating effects. In the absence of specific immunity, cytokines may eventually accumulate to high levels but they appear ineffective in preventing systemic dissemination of an apparently unrestricted replication of PRV.
ACKNOWLEDGMENTS
We thank Beat Scheier for expert technical assistance and Gottfried Alber (University of Leipzig), Sigrid Baumann, Cornel Fraefel, and Norbert Stäuber, from our Institute, for critical reading of the manuscript and for helpful discussion.
The study was supported by the Kanton of Zürich, Switzerland, and by a grant from the BBW (no. 96.0046-1, EU concerted action; BIO4-CT96-0398).
REFERENCES
- 1.Akira S, Kishimoto T. IL-6 and NF-IL6 in acute-phase response and viral infection. Immunol Rev. 1992;127:25–50. doi: 10.1111/j.1600-065x.1992.tb01407.x. [DOI] [PubMed] [Google Scholar]
- 2.Babic N, Klupp B, Brack A, Mettenleiter T C, Ugolini G, Flamand A. Deletion of glycoprotein gE reduces the propagation of pseudorabies virus in the nervous system of mice after intranasal inoculation. Virology. 1996;219:279–284. doi: 10.1006/viro.1996.0247. [DOI] [PubMed] [Google Scholar]
- 3.Bianchi A T J, Moonen-Leusen H W M, van Milligen F J, Savelkoul H F J, Zwart R J, Kimman T G. A mouse model to study immunity against pseudorabies virus infection: significance of CD4+ and CD8+ cells in protective immunity. Vaccine. 1998;16:1550–1558. doi: 10.1016/s0264-410x(98)00044-9. [DOI] [PubMed] [Google Scholar]
- 4.Boehm U, Klamp T, Groot M, Howard J C. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 5.Boyle J S, Koniaras C, Lew A M. Influence of cellular location of expressed antigen on the efficacy of DNA vaccination: cytotoxic T lymphocyte and antibody responses are suboptimal when antigen is cytoplasmic after intramuscular DNA immunization. Int Immunol. 1997;9:1897–1906. doi: 10.1093/intimm/9.12.1897. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Lansford R, Stewart V, Young F, Alt F W. RAG-2-deficient blastocyst complementation: an assay of gene function in lymphocyte development. Proc Natl Acad Sci USA. 1993;90:4528–4532. doi: 10.1073/pnas.90.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coutelier J P, van der Logt J T, Heessen F W, Warnier G, Van Snick J. IgG2a restriction of murine antibodies elicited by viral infections. J Exp Med. 1987;165:64–69. doi: 10.1084/jem.165.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Der S D, Zhou A, Williams B R, Silverman R H. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc Natl Acad Sci USA. 1998;95:15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Waal Malefyt R. The role of type I interferons in the differentiation and function of Th1 and Th2 cells. Semin Oncol. 1997;24:S9–94. S998. [PubMed] [Google Scholar]
- 10.Farber J M. HuMig: a new human member of the chemokine family of cytokines. Biochem Biophys Res Commun. 1993;192:223–230. doi: 10.1006/bbrc.1993.1403. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez, N. C., A. Lozier, C. Flament, P. Ricciardi-Castagnoli, D. Bellet, M. Suter, M. Perricaudet, E. Maraskovsky, and L. Zitvogel. Dendritic cells directly trigger NK cell functions: a cross-talk relevant in innate antitumor immune response in vivo. Nat. Med., in press. [DOI] [PubMed]
- 12.Geiger K D, Nash T C, Sawyer S, Krahl T, Patstone G, Reed J C, Krajewski S, Dalton D, Buchmeier M J, Sarvetnick N. Interferon-gamma protects against herpes simplex virus type 1-mediated neuronal death. Virology. 1997;238:189–197. doi: 10.1006/viro.1997.8841. [DOI] [PubMed] [Google Scholar]
- 13.Graham M B, Dalton D K, Giltinan D, Braciale V L, Stewart T A, Braciale T J. Response to influenza infection in mice with a targeted disruption in the interferon gamma gene. J Exp Med. 1993;178:1725–1732. doi: 10.1084/jem.178.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heise M T, Virgin H W. The T-cell-independent role of gamma interferon and tumor necrosis factor alpha in macrophage activation during murine cytomegalovirus and herpes simplex virus infections. J Virol. 1995;69:904–909. doi: 10.1128/jvi.69.2.904-909.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 16.Huez G, Silhol M, Lebleu B. Microinjected interferon does not promote an antiviral response in Hela cells. Biochem Biophys Res Commun. 1983;110:155–160. doi: 10.1016/0006-291x(83)91273-1. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs L, Rziha H J, Kimman T G, Gielkens A L, Van Oirschot J T. Deleting valine-125 and cysteine-126 in glycoprotein gI of pseudorabies virus strain NIA-3 decreases plaque size and reduces virulence in mice. Arch Virol. 1993;131:251–264. doi: 10.1007/BF01378630. [DOI] [PubMed] [Google Scholar]
- 18.Kimman T G, De Wind N, De Bruin T, de Visser Y, Voermans J. Inactivation of glycoprotein gE and thymidine kinase or the US3-encoded protein kinase synergistically decreases in vivo replication of pseudorabies virus and the induction of protective immunity. Virology. 1994;205:511–518. doi: 10.1006/viro.1994.1672. [DOI] [PubMed] [Google Scholar]
- 19.Klein M A, Frigg R, Flechsig E, Raeber A J, Kalinke U, Bluethmann H, Bootz F, Suter M, Zinkernagel R M, Aguzzi A. A crucial role for B cells in neuroinvasive scrapie. Nature. 1997;390:687–690. doi: 10.1038/37789. [DOI] [PubMed] [Google Scholar]
- 20.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 21.Landolfo S, Gribaudo G, Angeretti A, Gariglio M. Mechanisms of viral inhibition by interferons. Pharmacol Ther. 1995;65:415–442. doi: 10.1016/0163-7258(95)98599-l. [DOI] [PubMed] [Google Scholar]
- 22.Liu T, Tang Q, Hendricks R L. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J Virol. 1996;70:264–271. doi: 10.1128/jvi.70.1.264-271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mettenleiter T C. Molecular biology of pseudorabies (Aujeszky’s disease) virus. Comp Immunol Microbiol Infect Dis. 1991;14:151–163. doi: 10.1016/0147-9571(91)90128-z. [DOI] [PubMed] [Google Scholar]
- 24.Mo X Y, Tripp R A, Sangster M Y, Doherty P C. The cytotoxic T-lymphocyte response to Sendai virus is unimpaired in the absence of gamma interferon. J Virol. 1997;71:1906–1910. doi: 10.1128/jvi.71.3.1906-1910.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulder W A, Jacobs L, Priem J, Kok G L, Wagenaar F, Kimman T G, Pol J M. Glycoprotein gE-negative pseudorabies virus has a reduced capability to infect second- and third-order neurons of the olfactory and trigeminal routes in the porcine central nervous system. J Gen Virol. 1994;75:3095–3106. doi: 10.1099/0022-1317-75-11-3095. [DOI] [PubMed] [Google Scholar]
- 26.Müller U, Steinhoff U, Reis L F, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 27.Parr E L, Parr M B. Immunoglobulin G is the main protective antibody in mouse vaginal secretions after vaginal immunization with attenuated herpes simplex virus type 2. J Virol. 1997;71:8109–8115. doi: 10.1128/jvi.71.11.8109-8115.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramshaw I A, Ramsay A J, Karupiah G, Rolph M S, Mahalingam S, Ruby J C. Cytokines and immunity to viral infections. Immunol Rev. 1997;159:119–135. doi: 10.1111/j.1600-065x.1997.tb01011.x. [DOI] [PubMed] [Google Scholar]
- 29.Roizman B, Batterson W. Herpesviruses and their replication. In: Fields B N, Knipe D M, editors. Fundamental virology. New York, N.Y: Raven Press; 1986. pp. 607–636. [Google Scholar]
- 30.Ruzek M C, Miller A H, Opal S M, Pearce B D, Biron C A. Characterization of early cytokine responses and an interleukin (IL)-6-dependent pathway of endogenous glucocorticoid induction during murine cytomegalovirus infection. J Exp Med. 1997;185:1185–1192. doi: 10.1084/jem.185.7.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarawar S R, Cardin R D, Brooks J W, Mehrpooya M, Hamilton-Easton A M, Mo X Y, Doherty P C. Gamma interferon is not essential for recovery from acute infection with murine gammaherpesvirus 68. J Virol. 1997;71:3916–3921. doi: 10.1128/jvi.71.5.3916-3921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schijns V E, Haagmans B L, Horzinek M C. IL-12 stimulates an antiviral type 1 cytokine response but lacks adjuvant activity in IFN-gamma-receptor-deficient mice. J Immunol. 1995;155:2525–2532. [PubMed] [Google Scholar]
- 33.Schijns V E, Haagmans B L, Rijke E O, Huang S, Aguet M, Horzinek M C. IFN-gamma receptor-deficient mice generate antiviral Th1-characteristic cytokine profiles but altered antibody responses. J Immunol. 1994;153:2029–2037. [PubMed] [Google Scholar]
- 34.Scott P, Kaufmann S H. The role of T-cell subsets and cytokines in the regulation of infection. Immunol Today. 1991;12:346–348. doi: 10.1016/0167-5699(91)90063-Y. [DOI] [PubMed] [Google Scholar]
- 35.Siegrist C A, Saddallah F, Tougne C, Martinez X, Kovarik J, Lambert P H. Induction of neonatal TH1 and CTL responses by live viral vaccines: a role for replication patterns within antigen presenting cells? Vaccine. 1998;16:1473–1478. doi: 10.1016/s0264-410x(98)00111-x. [DOI] [PubMed] [Google Scholar]
- 36.Smith A L, Barthold S W, de Souza M S, Bottomly K. The role of gamma interferon in infection of susceptible mice with murine coronavirus, MHV-JHM. Arch Virol. 1991;121:89–100. doi: 10.1007/BF01316746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snapper C M, Paul W E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 38.Snapper C M, Rosas F, Moorman M A, Jin L, Shanebeck K, Klinman D M, Kehry M R, Mond J J, Maliszewski C R. IFN-gamma is a potent inducer of Ig secretion by sort-purified murine B cells activated through the mlg, but not the CD40, signaling pathway. Int Immunol. 1996;8:877–885. doi: 10.1093/intimm/8.6.877. [DOI] [PubMed] [Google Scholar]
- 39.Steinhoff U, Muller U, Schertler A, Hengartner H, Aguet M, Zinkernagel R M. Antiviral protection by vesicular stomatitis virus-specific antibodies in alpha/beta interferon receptor-deficient mice. J Virol. 1995;69:2153–2158. doi: 10.1128/jvi.69.4.2153-2158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suter, M. Unpublished data.
- 41.Tanaka T, Akira S, Yoshida K, Umemoto M, Yoneda Y, Shirafuji N, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T. Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell. 1995;80:353–361. doi: 10.1016/0092-8674(95)90418-2. [DOI] [PubMed] [Google Scholar]
- 42.Tirabassi R S, Townley R A, Eldridge M G, Enquist L W. Characterization of pseudorabies virus mutants expressing carboxy-terminal truncations of gE: evidence for envelope incorporation, virulence, and neurotropism domains. J Virol. 1997;71:6455–6464. doi: 10.1128/jvi.71.9.6455-6464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van den Broek M F, Muller U, Huang S, Aguet M, Zinkernagel R M. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J Virol. 1995;69:4792–4796. doi: 10.1128/jvi.69.8.4792-4796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weck K E, Dal Canto A J, Gould J D, O’Guin A K, Roth K A, Saffitz J E, Speck S H, Virgin H W. Murine gamma-herpesvirus 68 causes severe large-vessel arteritis in mice lacking interferon-gamma responsiveness: a new model for virus-induced vascular disease. Nat Med. 1997;3:1346–1353. doi: 10.1038/nm1297-1346. [DOI] [PubMed] [Google Scholar]
- 46.Wong G H, Goeddel D V. Tumour necrosis factors alpha and beta inhibit virus replication and synergize with interferons. Nature. 1986;323:819–822. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]
- 47.Yu Z, Manickan E, Rouse B T. Role of interferon-gamma in immunity to herpes simplex virus. J Leukoc Biol. 1996;60:528–532. doi: 10.1002/jlb.60.4.528. [DOI] [PubMed] [Google Scholar]
- 48.Zhu H, Cong J P, Mamtora G, Gingeras T, Shenk T. Cellular gene expression altered by human cytomegalovirus: global monitoring with oligonucleotide arrays. Proc Natl Acad Sci USA. 1998;95:14470–14475. doi: 10.1073/pnas.95.24.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zuckermann F A, Zsak L, Mettenleiter T C, Ben-Porat T. Pseudorabies virus glycoprotein gIII is a major target antigen for murine and swine virus-specific cytotoxic T lymphocytes. J Virol. 1990;64:802–812. doi: 10.1128/jvi.64.2.802-812.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]