Abstract
This study explores the genetic and immunologic factors involved in the differences in duration of transgene expression following in vivo transduction with recombinant adenoviruses. Different strains of mice (C3H/HeJ [C3H], C57BL/6J [B6], BALB/cJ [Balb/c], C.B10-H2b/LiMcdJ [Balb.B], CB6F1/J [(Balb/c × B6)F1], B6C3F1/J [(B6 × C3H)F1], and BALB/cj SCID) received 5 × 109 PFU of the first-generation adenovirus, which expresses human α1-antitrypsin (Ad/RSVhAAT). While all strains studied showed similar patterns of anti-adenovirus antibody formation, only Balb/c and C3H mice developed significant levels of anti-hAAT antibodies by 8 weeks posttransduction. In addition, while all strains had quantitatively comparable amounts of adenovirus genomes and hAAT mRNA transcripts in the liver 9 days posttransduction, only Balb/c mice had undetectable adenovirus vector genomes and hAAT mRNA in the liver 40 days posttransduction. Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling staining of liver sections from control and Ad/RSVhAAT-infected mice 5, 9, and 40 days posttransduction suggested that apoptosis was involved in the rapid elimination of transduced hepatocytes in Balb/c mice. Persistent expression of hAAT protein observed in BALB/cj SCID mice suggests that antigen-dependent immunity was essential for this apoptotic process in transduced Balb/c hepatocytes. In contrast to Balb/c mice, the loss of expression in C3H mice did not correlate with the loss of vector genomes or hAAT mRNA. Instead, the anti-hAAT antibodies in C3H but not Balb/c mice were found to interfere with detection of hAAT in the serum. In Balb.B and B6 mice, vector genome, hAAT mRNA transcripts, and hAAT protein levels persisted for at least 40 days posttransduction. This persistence correlated with poor anti-hAAT antibody formation and minimal hepatocyte toxicity. The expression of hAAT in (Balb/c × B6)F1 pups was found to be intermediate between the duration observed in the parental strains, while in (C3H × B6)F1 pups hAAT expression was similar to that seen in the B6 parents, which together support polygenic control of the immune responses in these mice. In summary, these findings suggest that there are three different profiles and at least two defined immune system-mediated mechanisms resulting in the loss of hAAT expression in mice and that different strains differ in the capacity to utilize these mechanisms.
Studies using immunodeficient mouse strains (1, 2, 34, 38) as well as the coadministration of immunosuppressive agents (11, 12, 22, 35) clearly implicate the host immune system in the loss of transgene expression following transduction with recombinant adenovirus vectors. While various humoral and cytotoxic immune responses have been identified following transduction of different strains of mice with recombinant adenovirus vectors expressing erythropoietin (27), human factor IX (hFIX) (18, 37), human α1-antitrypsin (hAAT) inhibitor (1, 20), and bacterial β-galactosidase (18, 33) genes, it remains unclear which genetic components from a strain contribute to the variability observed for a particular transgene protein.
First-generation adenovirus vectors do not express E1a proteins, which are dominant antigens for cytotoxic T-lymphocyte (CTL) formation in C57BL/6J (B6) mice but not in BALB/cJ (Balb/c) mice (12). However, epitopes from other viral proteins can drive the formation of CTLs in C3H/HeJ (C3H), B6, and BALB/c mice (26). The development of CTLs, possibly toward adenovirus late gene protein epitopes as a result of leaky late protein expression, has been offered as an explanation for the limited expression of lacZ in CBA mice following transduction with adenovirus (33). Furthermore, CTL responses toward transgene proteins, in addition to those directed against adenovirus proteins, have now been identified for many transgenes, including those encoding hFIX (18), bacterial β-galactosidase (25), chloramphenicol acetyltransferase (25), cytosine deaminase (25), low-density lipoprotein receptor (13), and human thrombopoietin (25), following administration of recombinant adenovirus vectors into immunocompetent mice. The relevance of antitransgene CTLs is suggested by reports of the loss of transgene expression in vivo following adoptive transfer of β-galactosidase and/or virus-specific CTLs (9, 36). However, the CTL response and loss of adenovirus-mediated transgene expression are not consistently correlated. For example, B6 mice given a recombinant adenovirus transducing hAAT showed persistent transgene expression even following reinoculation and CTL boosting with a different adenovirus vector not expressing hAAT (30).
Antibodies developed against the transgene protein products expressed from recombinant adenovirus vectors have previously been described (27). The identification of anti-hAAT antibodies following the transduction of C3H mice with the first-generation virus Ad/RSVhAAT (20, 22) has recently been reported and found to correlate with the disappearance of hAAT in the serum (20). In addition, it was shown that preimmunization with hAAT protein can prevent the appearance of detectable hAAT in the blood (20). Conversely, in hAAT transgenic mice, which are immunologically tolerant to hAAT but not to adenovirus, adenovirus-mediated hAAT expression was prolonged (20). Similarly, the development of anti-hFIX antibodies was found to correlate with a loss of expression, despite the persistence of viral DNA in Balb/c, CBA, CD1, and C3H mice given an intravenous administration of recombinant adenovirus expressing hFIX (18). Together, these studies demonstrate that both antibody-mediated and CTL-mediated immune responses may affect the duration of transgene expression following administration of first-generation adenovirus vectors, but the genetic components involved in these responses remain unknown.
Previously, it was reported from this laboratory that the duration of hAAT expression appeared not to segregate with the murine H-2 haplotype (1), based on the short hAAT expression found in C3H (H-2k) mice and the prolonged expression in C3H congenic B10.BR (H-2k) mice. To extend these observations into H-2 congenic mice on a Balb/c background, studies described herein examine the expression of hAAT in Balb/c congenic H-2b (C.B10-H2b/LiMcdJ [Balb.B; H-2b]) mice compared to that in Balb/c (H-2d) mice. Additional experiments were performed to evaluate hAAT expression, viral DNA, transgene mRNA, and transgene/adenovirus antibody formation in immunocompetent (Balb/c, C3H, and B6) and Balb.B mice. Evaluation of hAAT expression in F1 offspring supports the finding that at least two different murine loci, from within and outside the major histocompatibility (MHC) locus, are involved in the variable response seen following transduction with Ad/RSVhAAT.
MATERIALS AND METHODS
Animal studies.
Animal studies were performed in accordance with the institutional guidelines set forth by the University of Washington. Female C3H, B6, Balb/c, Balb.B, CB6F1/J [(Balb/c × B6)F1], B6C3F1/J [(C3H × B6)F1], and BALB/cj SCID (Balb/c SCID) mice were obtained from Jackson Laboratories (Bar Harbor, Maine) at 5 to 6 weeks of age and housed in specific-pathogen-free facilities. Mice were injected with recombinant adenovirus diluted to 200 μl in Dulbecco modified Eagle medium (Gibco BRL, Gaithersburg, Md.) by tail vein injection as previously described (16). Blood samples for serum protein analysis were obtained by the retro-orbital technique. Mice found to have serum hAAT levels less than 500 ng/ml for serum samples taken 3 to 7 days postinjection and thought to have received poor injections were removed from the study. Portal vein catheter placement into C3H mice was performed 1 day prior to adenovirus infusions as previously reported (28). The animals were sacrificed by cervical dislocation.
Recombinant adenoviruses.
Construction of the recombinant E1-deficient adenovirus type 5 vector Ad/RSVhAAT has been previously described (10). Ad/RSVhAAT expresses hAAT serum protein under the control of the Rous sarcoma virus long terminal repeat promoter. The recombinant adenovirus was grown in large scale in 293 cells and then purified and concentrated on two cesium chloride gradients (7). Each virus preparation was assayed for wild-type contamination (1) and quantified spectrophotometrically (optical density at 260 nm) and by plaque assay (1, 7). No endotoxin was identified in any of the virus preparations by using a commercially available endotoxin testing kit (Sigma, St. Louis, Mo.).
Analysis of murine serum.
Serum hAAT levels were determined by enzyme-linked immunosorbent assay (ELISA) (11) on duplicate samples, using multiple dilutions to ensure that readings were made on the linear portion of the standard curve. Those hAAT values below the sensitivity of the test were recorded as 25 ng/ml. A Sigma diagnostic kit was used for colorimetric determination of the activity of serum glutamic pyruvic transaminase (SGPT) with 10 μl of the serum (Sigma procedure 505).
The anti-hAAT antibody levels were determined by ELISA as previously described (22). Recorded scores are those duplicated on two separate samples. A second analysis was performed in those instances when the determinations were variant. Serum neutralizing antiadenovirus antibody analysis was performed as previously described (11).
Southern analysis.
DNA was prepared from 100 mg of snap-frozen murine liver samples (representing tissue from two to three lobes) as previously described (28). DNA concentrations were determined spectrophotometrically. Routinely, 2.5 μg of genomic DNA was digested overnight with HindIII as directed by the manufacturer (Bethesda Research Laboratories, Bethesda, Md.), electrophoresed on a 0.8% agarose gel, transferred to Hybond N+ (Amersham, Chicago, Ill.), and then hybridized in RapidHyb solution as recommended by the manufacturer (Amersham). Radiolabeling of hAAT and murine metallothionein (MMT) fragments was performed after isolation of the fragments from pAd.RSVhAAT (10) and pmMMT-I (28), respectively, with a Random primer labeling kit (Boehringer Mannheim, Indianapolis, Ind.) incorporating [α-32P]dCTP at >60% efficiency. As 1× and 10× copy number controls, 3.3 or 33 pg of pSP.RSVhAAT (4-kb) plasmid DNA was spiked into 2.5 μg of herring sperm DNA prior to restriction enzyme digestion. The relative amount of adenovirus DNA was determined by analysis on a model 400S PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.) and reported as a ratio between the specific signal and the MMT housekeeping gene signal which was obtained after stripping the blot and rehybridizing with radiolabeled MMT gene fragment.
RPA.
The hAAT template used to make the probe for the RNase protection assay (RPA) was prepared by reverse transcription-PCR of murine liver RNA from a mouse expressing hAAT following transduction with Ad/RSVhAAT, using forward (5′-CTGAGTTCGCCTTCAGCCTAT) and reverse (5′-TACCTAATACGAC TCAC TATAGGGAGAAAGCC T TCATGGGATC TGAGCC ) primers. The latter primer contains the bacteriophage T7 promoter used for labeling the probe. The L32 template was obtained from PharMingen (San Diego, Calif.), and the hAAT and L32 probes were prepared by using the PharMingen in vitro transcription kit. The RPA assay was performed with the PharMingen RPA kit. Quantification of the hAAT and L32 bands was performed on a model 400S PhosphorImager (Molecular Dynamics).
In vitro anti-hAAT antibody interference assays.
Equal amounts of serum (approximately 20 μl) were pooled from four C3H and three Balb/c mouse samples (Fig. 1) taken from 140 to 230 days posttransduction and called C3H immune and Balb/c immune sera, respectively. There was no detectable hAAT protein in the C3H or Balb/c immune and preimmune pools. Anti-hAAT antibody levels were undetectable for the preimmune pools and the +++ and ++ C3H and Balb/c immune pools, respectively. Briefly, the in vitro interference assay was performed by preparing hAAT standard, human calibrator serum 4 (Atlantic Antibodies, Stillwater, Minn.), in 100-μl aliquots of TBST (10 mM Tris, 100 mM NaCl, 0.05% Tween 20 [pH 7.5]) containing 5% milk, followed by the addition of 1 μl of saline or Balb/c preimmune, Balb/c immune, C3H preimmune, or C3H immune serum diluted 1:100 to 1:50,000 (final concentration). All tubes were then incubated at 4°C for 1 h, and the concentration of hAAT was determined by ELISA (11).
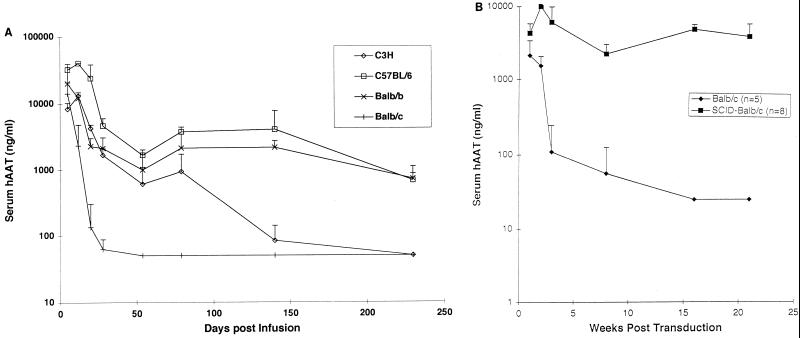
FIG. 1.
Serum hAAT concentrations following administration of Ad/RSVhAAT. Shown are the group average serum hAAT concentrations at various times following transduction of Balb/c (n = 4), Balb.B (n = 4), B6 (n = 3) (C57BL/6), and C3H (n = 3), mice (A) and of Balb/c (n = 5) and Balb/c SCID (n = 8) mice (B) with 5 × 109 PFU of Ad/RSVhAAT. The vertical bars represent standard deviations.
Biological assays.
Splenocytes were prepared as previously described (11) and determined to be >90% viable by trypan blue staining prior to the start of the experiments. The details of the proliferation assays were previously described. Gamma interferon (IFN-γ) concentrations were determined by ELISA as previously described (11).
In situ cell death detection assay.
Liver sections from Balb/c and Balb.B mice before and at various times after administration of 5 × 109 PFU of Ad/RSVhAAT were stored at −80°C in Tissue-Tek O.C.T. compound (Sakura Finetek USA Inc., Torrance, Calif.) and used to make the thin-section slides for the in situ cell death detection assay, performed with a fluorescein in situ cell death detection kit from Boehringer Mannheim. Following the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining, slides were briefly (for 10 s) counterstained with 4′,6-diamidino-2-phenylindole (DAPI) or Evans blue stain as suggested by the manufacturer. Representative samples were photographed on a Nikon VFM camera through a EX465-495 filter attached to an Eclipse E800 Microscope (Nikon, Melville, N.Y.) with a 20× objective and a mercury lamp. Exposure time was for 2 min. As a positive control, liver was similarly prepared from a Balb/c mouse 2 h after intraperitoneal injection with 10 μg (a lethal dose) of Jo-2 anti-Fas antibody (PharMingen).
RESULTS
Expression of Ad/RSVhAAT in various mouse strains.
The serum concentration of hAAT protein was determined in various strains of mice following tail vein injection of 5 × 109 PFU of Ad/RSVhAAT (Fig. 1A). While all groups (n = 3 to 4/group for one of three separate experiments) showed an initial decline in the serum concentration of hAAT, which appeared not to be mediated by antigen-specific responses (14, 32), persistence of expression thereafter varied between strains. Prolonged expression (for >9 months) was seen in both B6 (H-2b) and Balb.B (H-2b) mice, intermediate hAAT expression was seen in C3H (H-2k) mice, and very short hAAT expression (>2-log decrease by 20 days) was seen in Balb/c (H-2d) mice. The intermediate duration of hAAT protein expression observed in the C3H mice from this study was similar in profile but slightly longer than we originally reported (1). Prolonged expression seen in the Balb.B mice suggested that in the BALB background, the short duration of hAAT expression appeared to be dependent on the (H-2d) MHC locus since these mice are otherwise congenic. We previously reported (1) and found in a follow-up experiments (not shown in Fig. 1) that the duration of hAAT expression appeared not to segregate with the murine H-2 haplotype (1), since we observed short expression in C3H (H-2k) mice and prolonged expression in B10.BR (H-2k) mice. Together, these findings suggest that at least two loci, one within (on the Balb/c background) and one outside (on the B10 background) the MHC region, contribute to the variable duration of hAAT expression seen in different strains of mice.
To further determine if the short duration of hAAT expression seen on the Balb background was dependent on the H-2d MHC locus, Ad/RSVhAAT-mediated expression in T- and B-cell-deficient Balb/c SCID mice was evaluated (Fig. 1B). Prolonged expression of hAAT was observed for Balb/c SCID mice compared to wild-type Balb/c mice, which supports the hypothesis that duration of hAAT expression in the Balb background was dependent on functional T and B cells.
Anti-hAAT antibody formation in C3H mice hinders the ability to detect hAAT in murine sera following transduction with Ad/RSVhAAT.
Anti-hAAT and/or antiadenovirus antibodies have been identified in a number of mouse strains, but the factors influencing their formation are not known. To address this question, we determined the levels of adenovirus neutralizing antibodies and performed semiquantitative identification of anti-hAAT immunoglobulin G (IgG) for each of the mice represented in Fig. 1A (Table 1). While antiadenovirus neutralizing antibody titers for mice at 8 and 12 weeks were similar among the four strains tested, there were considerable differences in the formation of anti-hAAT antibodies. Levels of hAAT antibody were high in C3H mice but either undetectable or equivocal in Balb.B and B6 mice. Balb/c mice showed an intermediate level of anti-hAAT antibody formation.
TABLE 1.
Anti-hAAT and adenovirus neutralizing antibodies for mice represented in Fig. 1A
| Strain | Day 56 serum hAAT (ng/ml) | Serum anti-hAAT IgG at 8 wka
|
Adenovirus neutralizing antibody titerb
|
||
|---|---|---|---|---|---|
| Dilution of 1:1,000 | Dilution of 1:10,000 | 8 wk | 12 wk | ||
| C3H | 773, 994, <50 | +++, +++, +++ | +, +, ++ | 16, 64, 16 | 16, 64, 16 |
| B6 | 1,440, 2,049, 1,553 | −, +, − | −, −, − | ≤16, 16, 64 | 64, 64, 64 |
| Balb/c | <50, <50, <50, <50 | ++, ++, ++, + | +, +, −, − | —, 16, 64, 16 | —, 16, 16, 64 |
| Balb/b | 1,113, 1,642, 400, 1,039 | −, −, −, − | −, −, −, − | ≤16, 64, 16, — | 16, 64, 16, — |
Qualitatively reported for each animal in comparison to a standard curve of anti-hAAT monoclonal antibody (MAb) as follows: −, not detected; +, detected at level similar to that of standard MAb diluted >1:1,000; ++, detected at level similar to that of standard MAb diluted ≥1:100; or +++, detected at level greater than the lowest dilution of MAb standard. The order of the data is the same within each group. —, insufficient sample to perform the test.
Expressed as the median reciprocal (1/x) dilution yielding 75% neutralization. Values of ≤16 indicate that antibodies were detected but resulted in less than 75% neutralization at a 1:16 dilution.
An in vitro interference assay was developed to determine if the anti-hAAT antibodies identified in Balb/c and C3H mice interfered with ELISA-based detection of serum hAAT protein. Briefly, pooled sera from C3H and Balb/c mice (controls and samples taken >40 days after transduction with Ad/RSVhAAT) were mixed at various dilutions in duplicate with 100 ng of hAAT standard and preincubated for 2 h at 4°C. The amount of hAAT protein was then determined by the standard ELISA and plotted in Fig. 2. Strikingly, while C3H preimmune, Balb/c preimmune, and Balb/c 40-day immune serum pools had only a slight effect on the detection of hAAT, the C3H 40-day immune serum pool greatly inhibited detection of hAAT (50% detection at a 1:500 dilution). This finding indicated that anti-hAAT antibody formation in C3H mice hindered the ability to detect hAAT in C3H mice, and the detection of anti-hAAT IgG did not always correlate with the ability of that antibody to interfere with detection of hAAT, presumably because the spectrum of anti-hAAT antibody formed in C3H mice was of greater avidity and therefore more efficient at interfering with the ELISA.
FIG. 2.
In vitro serum interference of hAAT detection assay. Control hAAT serum at 100 ng/ml was incubated 2 h at 4°C with dilutions ranging from 1:100 to 1:50,000 of Balb/c immune, Balb/c preimmune, C3H immune, or C3H preimmune serum pools prepared as described in Materials and Methods. An incubation with sample buffer served as the control. The hAAT concentration as determined by ELISA in duplicate was plotted against serum dilution. The error bars represent the standard errors of the means.
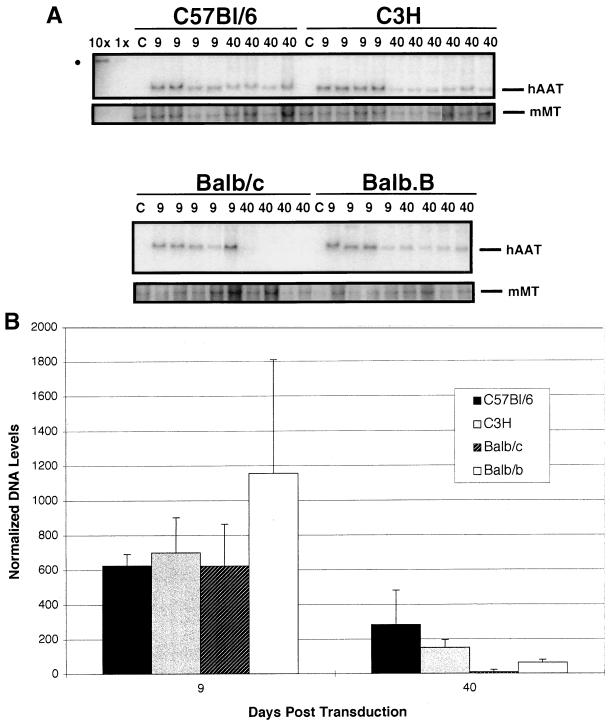
The identification of anti-hAAT antibodies capable of interfering with detection of serum hAAT in C3H mice following transduction with Ad/RSVhAAT suggested that the Ad/RSVhAAT genome and hAAT transcription may still be present in murine hepatocytes even though hAAT protein is no longer detectable in the serum. The persistence of Ad/RSVhAAT genome compared to the murine housekeeping MMT gene for Balb/c, C3H, Balb.B, and B6 mice was determined by Southern blotting (Fig. 3A) at 9 and 40 days after transduction with 5 × 109 PFU of Ad/RSVhAAT. The level of serum hAAT protein for B6, Balb/c, and Balb.B mice at 40 days posttransduction was similar to that shown in Fig. 1, and all of the C3H mice had serum hAAT concentrations of less than 150 ng/ml. Graphic representation of the amount of recombinant adenovirus genomic DNA normalized to the amount of MMT signal (Fig. 3B) showed that at 9 days posttransduction, all animals had similar amounts of recombinant adenovirus DNA, in the range of at least 10 copies per hepatocyte (10× lane), consistent with data previously reported (12, 23, 24, 28). By 40 days posttransduction, the B6, C3H, and Balb.B mice all demonstrated the presence of adenovirus DNA, while none of the four Balb/c mice showed a signal at this time. Persistence of hAAT genomes in all the C3H mice (Fig. 3A) supports the hypothesis that a noncytotoxic mechanism, such as the formation of anti-hAAT antibodies, limited the detection of hAAT in C3H mice. The two- to eightfold decline in hAAT genomes in C3H, Balb.B, and B6 mice (Fig. 3B) was consistent with the gradual and more variable loss of hAAT expression during the first 40 to 100 days (Fig. 1A). In addition, the loss of signal in Balb/c mice between 9 and 40 days posttransduction suggested that the rapid loss of transgene expression in Balb/c mice correlated with a loss of genomes, possibly by a cytotoxic process.
FIG. 3.
Persistence of Ad/RSVhAAT DNA genomes in various murine strains. (A) Southern blot of 2.5 μg of mouse liver DNA prepared from B6 (C57Bl/6), C3H, Balb/c, and Balb.B mice at 9 or 40 days after transduction with 5 × 109 PFU of Ad/RSVhAAT. Bars on the right indicate positions of the unique adenovirus DNA fragment which contains hAAT and the MMT gene fragment. The dot on the left corresponds to signals for the control fragment (10 and 1 hAAT cDNA copies per cell). The image is a dye sublimation print of the PhosphorImager file used for quantification which was labeled and aligned with Canvas 5.0 (Deneba Software, Miami, Fla.). (B) Graphic representation of average intensities of the signals in each of the groups from the blot following detection on a PhosphorImager. The error bars represent standard deviations.
To determine if the amount of adenovirus DNA correlated with the level of transgene mRNA expression, total RNA prepared from the mice (Fig. 4) was analyzed for hAAT transcript in an hAAT-specific RPA (Fig. 4). A control probe for the L32 housekeeping gene transcript was used simultaneously for normalization. While the Balb/c hAAT mRNA signal declined to undetectable concentrations between 9 and 40 days postinfusion, during the same period the hAAT transcript level in B6 mice showed no decline, and only a modest, three- to fivefold, decline was found in C3H and Balb.B mice. Thus, while there was good correlation in adenovirus DNA, hAAT mRNA, and hAAT protein expression over time for Balb.B, Balb/c, and B6 mice, C3H mice continued to express mRNAs from the recombinant adenovirus vector even though a very small amount of or no hAAT protein was detectable in the blood.
FIG. 4.
Persistence of hAAT mRNA transcripts in various mouse strains. (A) RPA of 2.5 μg of mouse liver RNA prepared from B6, C3H, Balb.B, and Balb/c mice at 5, 9, and 40 days after transduction with 5 × 109 PFU of Ad/RSVhAAT. Indicated to the left are the protected fragment sizes for hAAT and L32 (dashes) and the probe fragments for hAAT (upper dot) and L32 (lower dot). The mouse strain and days after transduction with adenovirus are indicated above the lanes, which include 1,000 cpm of both L32 and hAAT mixed probes (P), mouse L32 control RNA (C), and a yeast tRNA (Y) negative control. The image is a dye sublimation print of a representative PhosphorImager file used for quantification which was labeled with Canvas 5.0 (Deneba Software). (B) Graphic representation of the group average hAAT mRNA transcript intensities normalized to the L32 signal at 5, 9, and 40 days after transduction with Ad/RSVhAAT in B6 (C57Bl/6), C3H, Balb/c, and Balb.B mice. Shown is one of at least three experiments with similar results. The vertical bars represent standard deviations.
Inadvertent subcutaneous injection significantly enhances anti-hAAT antibody formation in C3H mice.
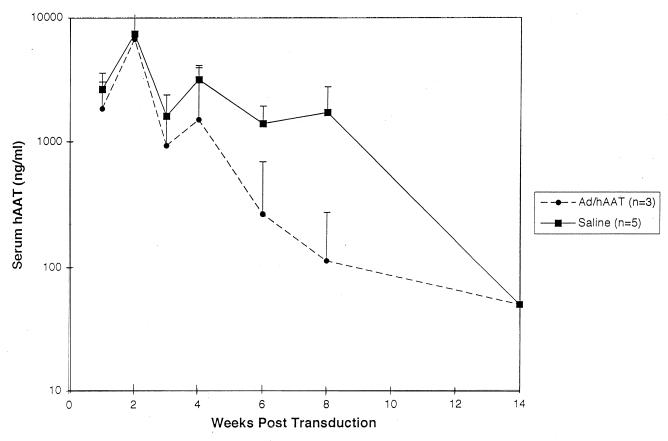
The scatter in persistence of hAAT expression in C3H mice led us to determine if subcutaneous extravasation of vector which may occur during tail vein infusion had an effect on the rate and/or avidity of anti-hAAT antibody formation and thus on the duration of hAAT expression in C3H mice. To address this question, 3 × 109 PFU of Ad/RSVhAAT was administered to C3H mice via a portal vein catheter at the same time as either saline or 2 × 109 PFU of Ad/RSVhAAT was administered subcutaneously at the base of the tail. Shown in Fig. 5, the duration of serum hAAT expression was prolonged in those mice which received subcutaneous saline injections compared to mice receiving Ad/RSVhAAT by the same route. In addition, anti-hAAT antibody formation in the group receiving subcutaneous Ad/RSVhAAT at 6 weeks was more robust than in the control mice (Table 2). The slight difference in total Ad/RSVhAAT administered (3 × 109 PFU in controls and 5 × 109 PFU in the subcutaneous administration group) has been reported to have a minimal effect on the duration of hAAT expression (20), which was in good accord with that routinely seen in our laboratory (unpublished data). The robust formation of anti-hAAT antibody with decreased hAAT expression following subcutaneous administration of Ad/RSVhAAT was in accord with previous data that showed intravenous administration of an antigen to be less immunogenic than subcutaneous administration (1a). Taken together, these findings suggest that the variability in duration of hAAT expression in C3H mice seen when previous data (1) and those of this study are compared (Fig. 1A) may correlate with the subcutaneous administration of Ad/RSVhAAT resulting in accelerated anti-hAAT antibody formation.
FIG. 5.
Effect of subcutaneous adenovirus administration on duration of hAAT expression. Shown are the group average serum hAAT concentrations at various times following transduction of two groups of C3H mice with 3 × 109 PFU of Ad/RSVhAAT via a portal vein catheter and subcutaneous administration of either saline or 2 × 109 PFU of Ad/RSVhAAT at the base of the tail. Shown is one of three separate experiments with similar results. The vertical bars represent standard deviations.
TABLE 2.
Anti-hAAT antibodies in C3H mice after subcutaneous adenovirus administrationa
| Treatment | 6 wk
|
12 wk
|
||
|---|---|---|---|---|
| Serum hAAT (ng/ml) | Anti-hAAT IgG (dilution of 1:5,000) | Serum hAAT (ng/ml) | Anti-hAAT IgG (dilution of 1:5,000) | |
| Ad/RSVhAAT | 760, 25, 25 | ++, +++, +++ | 25, 25, 25 | +++, +++, +++ |
| Saline | 1398, 2005, 1739, 1258, 610 | +, −, +, +, ++ | 25, 25, 25, 25, 25 | +++, ++, +++, ++, ++ |
For details, see the footnotes to Table 1.
A rapid cytotoxic response toward transduced hepatocytes in Balb/c mice limits the duration of hAAT expression.
The results shown in Fig. 3 indicated that the rapid loss of hAAT expression in Balb/c mice correlated with the loss of Ad/RSVhAAT genome, suggesting that a cytotoxic process may mediate the loss of expression in this strain. To evaluate the extent of liver toxicity following administration of Ad/RSVhAAT, serum SGPT levels were determined (Fig. 6) for the mice represented in Fig. 1A. As previously reported (14, 15), there was a similar small peak in SGPT levels within the first 24 to 50 h after transduction of all strains tested (Fig. 6). A second prolonged peak in SGPT elevation occurring after day 5, known to be related to antigen-dependent immunity (15), was more variable between strains; Balb/c mice showed the greatest degree of liver injury, and C3H and B6 mice showed the least. The Balb.B mice appeared to have an intermediate amount of liver injury. These results support the hypothesis that the rapid decline of serum hAAT expression in Balb/c mice could be related to a cytotoxic process. In addition, the minimal toxicity in C3H mice supports the hypothesis that the formation of anti-hAAT antibodies, rather than a cytotoxic process, limited ability to detect hAAT expression in C3H mice. The persistent hAAT expression seen in both B6 and Balb.B mice was associated with only weak cytotoxicity and poor anti-hAAT antibody formation. The loss of Ad/RSVhAAT genomic DNA (Fig. 3) and hAAT mRNA transcripts (Fig. 4) in Balb/c mice by 40 days posttransduction suggested a cytotoxic process for the loss of expression.
FIG. 6.
Liver-specific transaminase levels as a measure of liver cytotoxicity. SGPT levels were determined for samples taken from each of the mice represented in Fig. 1A. Shown is the average SGPT level for each strain at various time points following transduction with 5 × 109 PFU of Ad/RSVhAAT. The error bars represent standard deviations.
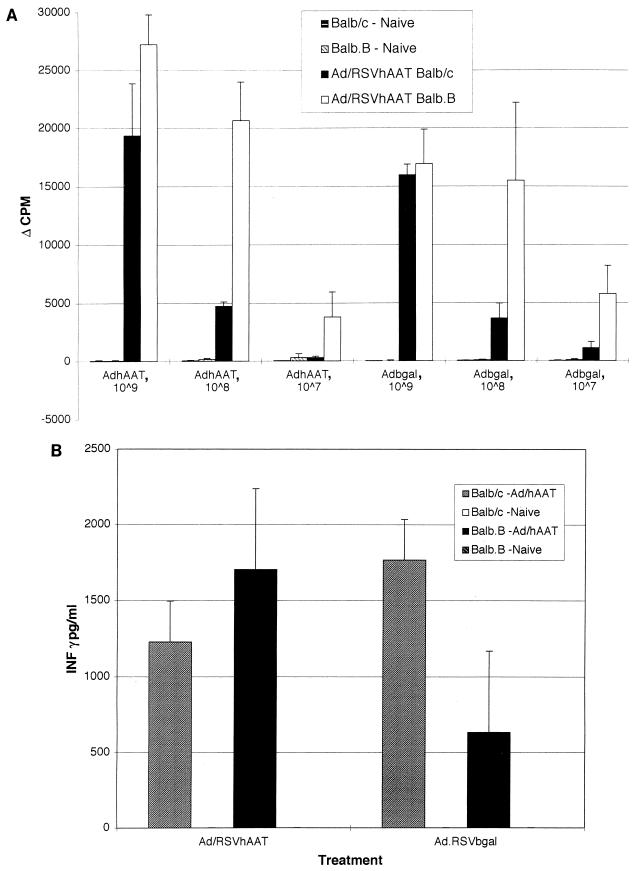
To determine if the loss of vector DNA and hAAT mRNA in Balb/c mice was associated with a difference in T-cell response to the vector and/or hAAT protein, we examined T-cell proliferative responses and IFN-γ secretion in splenocytes isolated from Balb.B (H-2b) and Balb/c (H-2d) mice 18 days after transduction with 5 × 109 PFU of Ad/RSVhAAT (Fig. 7A and B, respectively). The T-cell proliferation (Fig. 7A) and IFN-γ production in response to Ad/RSVhAAT and Ad/RSVβgal were robust and similar in Balb/c and Balb.B mice (Fig. 7B). Stimulation with hAAT protein resulted in no detectable T-cell proliferation or IFN-γ secretion. The similar levels of proliferation and IFN-γ secretion in response to adenovirus proteins with splenocytes isolated from both Balb/c and Balb.B mice, which clearly showed differences in the duration of hAAT protein (Fig. 1A), suggested that similar to the formation of CTL (9, 30, 36), these responses did not correlate with the duration of hAAT protein expression.
FIG. 7.
Splenocyte proliferation and IFN-γ production following infusion of Ad/RSVhAAT in Balb/c and Balb.B mice. Splenocytes were obtained from mice 15 days after the administration of 5 × 109 PFU of Ad/RSVhAAT or from mice not previously exposed to adenovirus. The cells were cultured in replicates of four with medium alone, anti-murine CD3 antibody, or the indicated amounts of UV-inactivated Ad/RSVhAAT (AdhAAT), Ad/RSVβgal (Adbgal), serum containing hAAT (calibrator serum 4), or serum from an hAAT-deficient patient (null) for 72 h. Following this incubation, [3H]thymidine was added and the cells were incubated for an additional 24 h. After this final incubation, the cells were harvested, and [3H]thymidine uptake was determined and plotted as Δcpm between unstimulated and stimulated samples (A). The error bars represent standard deviations. Proliferation results for the different groups of splenocytes for unstimulated and anti-CD3-stimulated cells were similar in all groups and are not shown. No proliferation was seen following incubation with calibrator or null patient serum (not shown). Secretion of IFN-γ (B) was determined for the same splenocytes cultured in replicates of four with medium alone, anti-murine CD3 antibody, or the indicated amounts of UV-inactivated Ad/RSVhAAT or Ad/RSVβgal for 72 h. After this incubation, cell supernatants were pooled from each replicate, and the amount of IFN-γ was determined in triplicate by ELISA. The error bars represent standard errors of the means. No IFN-γ secretion was detected in the naive mice or mice treated with calibrator or null patient serum (not shown). The results for naive and unstimulated cells with the anti-CD3 antibody were similar and are not shown.
Identification of apoptotic hepatic nuclei in Balb/c but not Balb.B mice following transduction with Ad/RSVhAAT.
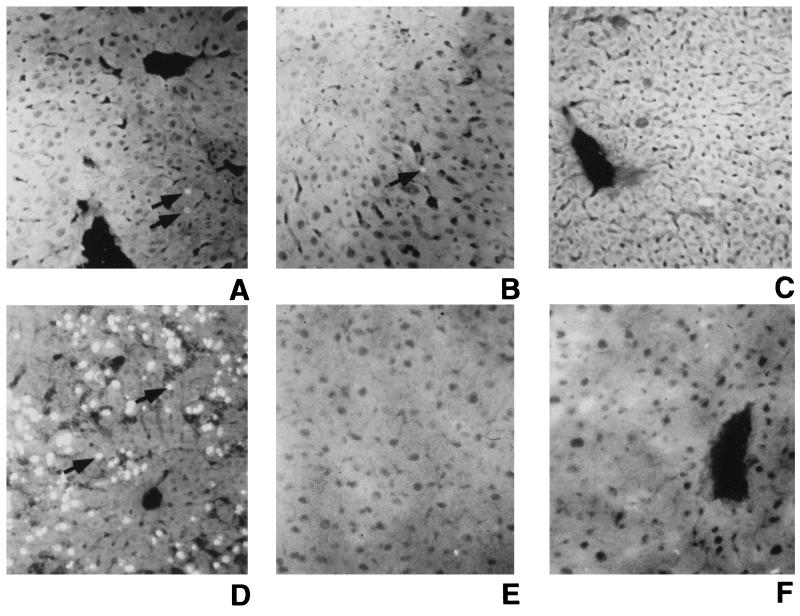
Recent reports indicate that end-organ cytotoxicity can be mediated by induced coexpression of Fas and Fas ligand, two molecules known to directly activate apoptosis (6). Others have demonstrated that apoptosis is evident following transduction of mice with recombinant adenovirus vectors (4). Specifically, Gao et al. (4) showed low-level apoptosis in hepatocytes isolated from C3H mice along with the generation of β-galactosidase-specific CTLs following administration of a first-generation adenovirus vector expressing β-galactosidase. However, it remained unclear whether these CTLs mediated the apoptosis. To determine if rapid loss of hAAT expression following transduction with 5 × 109 PFU of Ad/RSVhAAT was mediated by apoptosis, sections of livers from Balb/c and Balb.B mice were examined for DNA fragmentation by TUNEL assay. Representative liver sections from one of at least two similarly treated mice are shown in Fig. 8. Sections from Balb/c mice 5 and 9 days posttransduction (Fig. 8A and B, respectively) showed fluorescein isothiocyanate (FITC)-stained apoptotic nuclei in most high-power fields, while liver sections from Balb/c mice 40 days posttransduction and from Balb.B mice 9 days posttransduction showed no identifiable apoptotic nuclei (Fig. 8C and E, respectively). Naive Balb/c (Fig. 8F) and Balb.B (data not shown) mice showed no identifiable FITC-stained, apoptotic nuclei. A positive control liver section from a B6 mouse 2 h after administration of a lethal dose of anti-Fas antibody showed ≤50% FITC-stained apoptotic nuclei (Fig. 8D). Taken together, these findings suggest that the cytotoxic process associated with the rapid loss of vector DNA, hAAT RNA transcript, and hAAT protein expression in Balb/c mice may be mediated by the apoptotic loss of transduced hepatocytes.
FIG. 8.
Identification of apoptotic hepatocyte nuclei following transduction with Ad/RSVhAAT. Liver sections from Balb/c mice 5, 9, and 40 days after transduction with 5 × 109 PFU of Ad/RSVhAAT (A to C, respectively), a Balb/c positive control mouse 2 h after administration of 10 mg of Jo-1 anti-Fas antibody (D), a Balb.B mouse 9 days after transduction with 5 × 109 PFU of Ad/RSVhAAT (E), and a naive Balb/c negative control mouse (F) were TUNEL stained with FITC to visualize apoptotic nuclei. Shown are images of representative sections, visualized by fluorescence microscopy with a 20× objective, that were assembled and labeled with Canvas 5.0 (Deneba Software). The arrows indicate FITC-positive apoptotic nuclei.
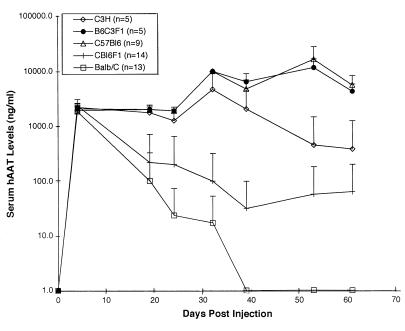
Duration of hAAT expression in Balb/c, C3H, B6, and two F1 murine strains.
To help determine what effect codominantly expressed MHC class I or II molecules within the MHC locus have on the variable duration of hAAT expression seen in wild-type Balb/c, C3H, and B6 mice, we evaluated the duration of hAAT expression in (Balb/c × B6)F1 and (C3H × B6)F1 mice and in parental strains. As seen in Fig. 9, the duration of hAAT expression for (Balb/c × B6)F1 mice was found to be intermediate to that of the two parental strains, demonstrating that the codominant expression of H-2d MHC class I molecules was not sufficient to result in a rapid loss of hAAT expression in the F1 offspring similar to that seen in the homozygotic Balb/c parental strain. We suggest that this could result from either multigene involvement or a gene dosage effect. In addition, the prolonged expression in the (C3H × B6)F1 mice was similar to that seen in the B6 parents, which suggested that the factors involved in the formation of anti-hAAT antibodies in C3H mice were recessive and/or the factors involved in the prolonged expression in B6 mice were dominant. Both of these support findings of this and prior studies that multiple genes were involved in determining the duration of hAAT expression in mice (1).
FIG. 9.
Serum hAAT concentrations following administration of Ad/RSVhAAT. Shown are the group average serum hAAT concentrations at various times following transduction of C3H, (C3H × B6)F1 (B6C3F1), B6 (C57Bl6), Balb/c × B6)F1 (CBl6F1), and Balb/c mice with 5 × 109 PFU of Ad/RSVhAAT. The vertical bars represent standard deviations.
DISCUSSION
This study has identified at least two different mechanisms by which the immune system for different strains of mice responds following transduction with first-generation adenovirus vectors. The first mechanism is exemplified in Balb/c mice, which showed a rapid loss of transgene expression, loss of adenovirus DNA and hAAT mRNA, and liver toxicity and apoptosis associated with hAAT antibody production. A second mechanism is seen in C3H mice, which showed a variable loss of transgene expression, retention of adenovirus DNA and hAAT mRNA, and the production of antibody to hAAT which blocked hAAT detection and may inhibit its function. Finally, in addition to the prior two mechanisms, Balb.B and B6 mice showed a gradual decline in expression over a period of months, formation of antibody to adenovirus, minimal hAAT antibody formation, and retention of adenovirus DNA and hAAT mRNA, suggesting a potential third immune clearance mechanism. While the basis for the loss of gene expression in the latter group is not clear, it may reflect an inefficient but progressive response to adenovirus protein.
In Balb/c mice, the rapid loss of hAAT expression, adenovirus genome, and hAAT mRNA and the development of significant liver cytotoxicity following transduction with Ad/RSVhAAT all suggest a clearance mechanism which may involve the destruction of hepatocytes transduced with adenovirus. The presence of apoptotic nuclei in Balb/c mice during the time of peak liver cytotoxicity (days 5 to 9) supports this hypothesis and implicates an apoptotic pathway in the process. While the apoptotic destruction of hepatocytes is consistent with classic CTL cytolysis, there are several other possible ligand/receptor pairs which can mediate apoptosis, including tumor necrosis factor alpha (TNF-α)/TNF receptor 1, Fas ligand/Fas, and Trail ligand/DR4. Indirect evidence supporting the role of these pathways in the clearance of wild-type adenovirus infection comes from the identification of a number of E3 and E1b proteins capable of inhibiting TNF-mediated cell lysis as well as Fas-mediated apoptotic pathways (31). While the precise role of CTLs in the clearance of Balb/c hepatocytes transduced with Ad/RSVhAAT remains unclear, identification of the particular apoptotic pathways involved in the rapid clearance of Ad/RSVhAAT seen in Balb/c mice should facilitate the development of testable strategies to prolong recombinant adenovirus expression in Balb/c mice.
This proposed mechanism for the clearance of hepatocytes transduced with recombinant adenovirus expressing hAAT is consistent with the previous report of Michou et al. (18) in which they demonstrate the complete loss of a recombinant adenovirus genome with the lacZ transgene by 45 days after transduction in Balb/c mice. Contrary to our study, they also found loss of this recombinant adenovirus in B6 mice and persistence of a recombinant adenovirus genome expressing hFIX in both Balb/c and B6 mice. This persistence of hFIX adenovirus DNA in both Balb/c and B6 mice is in good accord with early studies of Yao et al. (37) as well and emphasizes the role of the transgene product as a target for host immune response.
While we now report that the loss of detectable hAAT protein in C3H mice appears to reflect production of anti-hAAT antibody which blocks detection rather than a cytotoxic elimination of vector, it has been previously reported that the loss of transgene expression in C3H mice correlated with an early progressive decline of adenovirus DNA in the liver (12). This previously reported finding is consistent with the early decline in adenovirus genomes seen in all mouse strains examined in the present study. The lower rate of decline in hAAT protein expression and the persistence of adenovirus genomes for more than 40 days posttransduction that we report here include time points beyond those of the prior study.
hAAT antibodies have previously been found following transduction of CBA and C3H mice with recombinant adenovirus vectors (expressing E1 and E1/E2) which express hAAT (20). In CBA mice, this antibody formation was shown to correlate with persistent recombinant adenovirus genome up to 16 weeks after transduction with 108 PFU of Ad/hAATΔE1E2. The results presented here demonstrating hAAT antibody formation and persistent adenovirus genomes in C3H mice following transduction with 5 × 109 PFU are in good accord with this previous study and extend the observation to include the persistence of hAAT transcript and the ability of antibodies formed in C3H mice to inhibit hAAT detection by ELISA. In addition, the persistence of adenovirus DNA, hAAT RNA, and minimal hepatic injury reported here suggest that the anti-hAAT antibodies formed in C3H mice do not cause direct cytotoxicity (5) or hAAT transcript instability as proposed by others with respect to transcripts of hepatitis B virus (3, 8). Careful identification and characterization of the host factors involved in the formation of these anti-hAAT antibody responses will provide insight into the development of useful gene therapy systems for the treatment of patients with genetic diseases involving null mutations.
We have reported the successful prolongation of hAAT expression in C3H mice by coadministration of CTLA4Ig alone (11, 12) or both CTLA4Ig and anti-CD40 ligand antibody (MR1) (12). The prolonged expression of hAAT protein seen following treatment with murine CTLA4Ig and/or MR1 (anti-CD40 ligand antibody), two agents known to interfere with CD4 lymphocyte activation, is in good accord with anti-hAAT antibody formation being the predominant immune response seen in C3H mice following transduction with Ad/RSVhAAT. Indeed, a decrease in the formation of anti-hAAT and antiadenovirus antibodies has been reported for C3H mice receiving CTLA4Ig (22). This may also explain how these immunomodulatory agents could have variable effects in other strains of mice.
The mechanism for the gradual loss of hAAT transgene expression in B6 and Balb.B mice over time was not evaluated further in this study. While anti-hAAT antibody production appears not to account for elimination of expression in these strains, we cannot exclude a less robust process similar to that observed in Balb/c mice.
Finally, the intermediate duration of expression seen in the (Balb/c × B6)F1 mice and the prolonged duration of expression seen in (C3H × B6)F1 mice are both consistent with either a gene dosage effect of one dominantly expressed locus or the interaction of multiple genes. Given that previous studies from this laboratory (1) and the findings presented here implicate the involvement of at least two loci, one outside and another within the MHC, in antibody formation and prolonged transgene expression in Balb.B mice, we favor the latter hypothesis. The polygenic nature of the immune processes in mice, which modulate the nature and severity of such complex traits as the autoimmune disease systemic lupus erythematosis, has been reported (19, 29), and the results support a role for factors both within and outside the H-2 locus contributing to the disease process.
In summary, we have identified at least two different ways in which the murine immune system responds following intravenous transduction with the first-generation virus Ad/RSVhAAT. A good understanding of these immune responses is critical to the appropriate interpretation of many previous gene therapy studies and to the design of future studies. In addition, the characterization of these immune factors in mice may be useful in understanding the results seen in the polymorphic human population.
ACKNOWLEDGMENTS
This work was supported by NIH grant DK 51807. D.B.S. was the recipient of a National Hemophilia Association’s Judith Graham Pool fellowship and individual NRSA grant 16-7985.
We thank Julie Spyridis and Leonard Meuse for virus preparation and technical assistance. We appreciate the contribution of null serum from a patient with α1-antitrypsin deficiency by Mark Brantley at the NIH.
REFERENCES
- 1.Barr D, Tubb J, Ferguson D, Scaria A, Lieber A, Wilson C, Perkins J, Kay M A. Strain related variations in adenovirally mediated transgene expression from mouse hepatocytes in vivo: comparison between immunocompetent and immunodeficient inbred strains. Gene Ther. 1995;2:151–155. [PubMed] [Google Scholar]
- 1a.Borriello F, Sethna M P, Boyd S D, Schweitzer A N, Tivol E A, Jacoby D, Strom T B, Simpson E M, Freeman G J, Sharpe A H. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–313. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 2.Dai Y, Schwarz E M, Gu D, Zhang W W, Sarvetnick N, Verma I M. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc Natl Acad Sci USA. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franco A, Guidotti L, Hobbs M, Pasquetto V, Chisari F. Pathogenic effector function of CD4-positive T helper 1 cells in hepatitis B virus transgenic mice. J Immunol. 1997;159:2001–2008. [PubMed] [Google Scholar]
- 4.Gao G P, Yang Y, Wilson J M. Biology of adenovirus vectors with E1 and E4 deletions for liver-directed gene therapy. J Virol. 1996;70:8934–8943. doi: 10.1128/jvi.70.12.8934-8943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geffner J. Antibody-dependent cell mediated cytotoxicity. London, England: Academic Press; 1992. [Google Scholar]
- 6.Giordano C, Stassi G, De Maria R, Todaro M, Richiusa P, Papoff G, Ruberti G, Bagnasco M, Testi R, Galluzzo A. Potential involvement of Fas and its ligand in the pathogenesis of Hashimoto’s thyroiditis. Science. 1997;275:960–963. doi: 10.1126/science.275.5302.960. [DOI] [PubMed] [Google Scholar]
- 7.Graham F, Prevec L. Adenovirus-based expression vectors and recombinant vaccines. Bio/Technology. 1992;20:363–390. doi: 10.1016/b978-0-7506-9265-6.50022-1. [DOI] [PubMed] [Google Scholar]
- 8.Guidotti L, Ishikawa T, Hobbs M, Matzke B, Schreiber R, Chisari F. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan J M, Armentano D, Sparer T E, Wynn S G, Peterson P A, Wadsworth S C, Couture K K, Pennington S E, St. George J A, Gooding L R, Smith A E. Characterization of factors involved in modulating persistence of transgene expression from recombinant adenovirus in the mouse lung. Hum Gene Ther. 1997;8:45–56. doi: 10.1089/hum.1997.8.1-45. [DOI] [PubMed] [Google Scholar]
- 10.Kay M A, Graham F, Leland F, Woo S L. Therapeutic serum concentrations of human alpha-1-antitrypsin after adenoviral-mediated gene transfer into mouse hepatocytes. Hepatology. 1995;21:815–819. [PubMed] [Google Scholar]
- 11.Kay M A, Holterman A-X, Meuse L, Gown A, Ochs H, Linsley P, Wilson C B. Long-term hepatic adenovirus-mediated gene expression in mice following CTLA4Ig administration. Nat Genet. 1995;11:191–197. doi: 10.1038/ng1095-191. [DOI] [PubMed] [Google Scholar]
- 12.Kay M A, Meuse L, Gown A M, Linsley P, Hollenbaugh D, Aruffo A, Ochs H D, Wilson C B. Transient immunomodulation with anti-CD40 ligand antibody and CTLA4Ig enhances persistence and secondary adenovirus-mediated gene transfer into mouse liver. Proc Natl Acad Sci USA. 1997;94:4686–4691. doi: 10.1073/pnas.94.9.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozarsky K F, Jooss K, Donahee M, Strauss J F, Wilson J M. Effective treatment of familial hypercholesterolemia in the mouse model using adenovirus-mediated gene transfer of the VLDL receptor gene. Nat Genet. 1996;13:54–62. doi: 10.1038/ng0596-54. [DOI] [PubMed] [Google Scholar]
- 14.Lieber A, He C-Y, Meuse L, Schowalter D, Kirillova I, Winther B, Kay M A. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J Virol. 1997;71:8798–8807. doi: 10.1128/jvi.71.11.8798-8807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lieber A, He C-Y, Kirillova I, Kay M A. Recombinant adenoviruses with large deletions generated by Cre-mediated excision exhibit different biological properties compared with first-generation vectors in vitro and in vivo. J Virol. 1996;70:8944–8960. doi: 10.1128/jvi.70.12.8944-8960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lieber A, Vrancken Peeters M J, Kay M A. Adenovirus-mediated transfer of the amphotropic retrovirus receptor cDNA increases retroviral transduction in cultured cells. Hum Gene Ther. 1995;6:5–11. doi: 10.1089/hum.1995.6.1-5. [DOI] [PubMed] [Google Scholar]
- 17.Lusky M, Christ M, Rittner K, Dieterle A, Dreyer D, Mourot B, Schultz H, Stoeckel F, Pavirani A, Mehtali M. In vitro and in vivo biology of recombinant adenovirus vectors with E1, E1/E2A, or E1/E4 deleted. J Virol. 1998;72:2022–2032. doi: 10.1128/jvi.72.3.2022-2032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michou A I, Santoro L, Christ M, Julliard V, Pavirani A, Mehtali M. Adenovirus-mediated gene transfer: influence of transgene, mouse strain and type of immune response on persistence of transgene expression. Gene Ther. 1997;4:473–482. doi: 10.1038/sj.gt.3300412. [DOI] [PubMed] [Google Scholar]
- 19.Morel L, Rudofsky U, Longmate J, Schiffenbauer J, Wakeland E. Polygenic control of susceptibility to murine systemic lupus erythematosis. Immunity. 1994;1:219–229. doi: 10.1016/1074-7613(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 20.Morral N, O’Neal W, Zhou H, Langston C, Beaudet A. Immune responses to reporter proteins and high viral dose limit duration of expression with adenoviral vectors: comparison of E2a wildtype and E2a deleted vectors. Hum Gene Ther. 1997;8:1275–1286. doi: 10.1089/hum.1997.8.10-1275. [DOI] [PubMed] [Google Scholar]
- 21.Rawle F C, Knowles B B, Ricciardi R P, Brahmacheri V, Duerksen-Hughes P, Wold W S M, Gooding L R. Specificity of the mouse cytotoxic T lymphocyte response to adenovirus type 5: E1A is immunodominant in H-2b, but not in H-2d or H-2k mice. J Immunol. 1991;146:3977–3984. [PubMed] [Google Scholar]
- 22.Schowalter D B, Meuse L, Wilson C B, Linsley P S, Kay M A. Constitutive expression of murine CTLA4Ig from a recombinant adenovirus vector results in prolonged transgene expression. Gene Ther. 1997;4:853–860. doi: 10.1038/sj.gt.3300466. [DOI] [PubMed] [Google Scholar]
- 23.Schowalter D B, Tubb J C, Liu M, Wilson C B, Kay M A. Heterologous expression of adenovirus E3-gp19K in an E1a deleted adenovirus vector inhibits MHC I expression in vitro, but does not prolong transgene expression in vivo. Gene Ther. 1997;4:351–360. doi: 10.1038/sj.gt.3300398. [DOI] [PubMed] [Google Scholar]
- 24.Smith T A, Mehaffey M G, Kayda D B, Saunders J M, Yei S, Trapnell B C, McClelland A, Kaleko M. Adenovirus mediated expression of therapeutic plasma levels of human factor IX in mice. Nat Genet. 1993;5:397–402. doi: 10.1038/ng1293-397. [DOI] [PubMed] [Google Scholar]
- 25.Song W, Kong H-L, Traktman P, Crystal R G. Cytotoxic T lymphocyte responses to proteins encoded by heterologous transgenes transferred in vivo by adenovirus vectors. Hum Gene Ther. 1997;8:1207–1217. doi: 10.1089/hum.1997.8.10-1207. [DOI] [PubMed] [Google Scholar]
- 26.Sparer T E, Wynn S G, Clark D J, Kaplan J M, Cardoza L M, Wadsworth S C, Smith A E, Gooding L R. Generation of cytotoxic T lymphocytes against immunorecessive epitopes after multiple immunizations with adenovirus vector is dependent on haplotype. J Virol. 1997;71:2277–2284. doi: 10.1128/jvi.71.3.2277-2284.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tripathy S, Black H, Goldwasser E, Leiden J M. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 28.Vrancken-Peeters M J T F D, Lieber A, Perkins J, Kay M A. A method for multiple portal vein infusions in mice: quantitation of adenovirus-mediated hepatic gene transfer. BioTechniques. 1996;20:278–285. doi: 10.2144/96202rr05. [DOI] [PubMed] [Google Scholar]
- 29.Vyse T, Todd J. Genetic analysis of autoimmune disease. Cell. 1996;85:311–318. doi: 10.1016/s0092-8674(00)81110-1. [DOI] [PubMed] [Google Scholar]
- 30.Wadsworth S C, Zhou H, Smith A E, Kaplan J M. Adenovirus vector-infected cells can escape adenovirus antigen-specific cytotoxic T-lymphocyte killing in vivo. J Virol. 1997;71:5189–5196. doi: 10.1128/jvi.71.7.5189-5196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wold W. Adenovirus genes that modulate the sensitivity of virus-infected cells to lysis by TNF. J Cell Biochem. 1993;53:329–335. doi: 10.1002/jcb.240530410. [DOI] [PubMed] [Google Scholar]
- 32.Worgall S, Wolff G, Falck-Pedersen E, Crystal R. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum Gene Ther. 1997;8:37–44. doi: 10.1089/hum.1997.8.1-37. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, Nunes F A, Berencsi K, Furth E E, Gonczol E, Wilson J M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Nunes F A, Berencsi K, Gonczol E, Engelhardt J F, Wilson J M. Inactivation of E2a in recombinant adenoviruses improves the prospect for gene therapy in cystic fibrosis. Nat Genet. 1994;7:362–369. doi: 10.1038/ng0794-362. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y, Wilson J. CD40 ligand-dependent T cell activation: requirement of B7-CD28 signaling through CD40. Science. 1996;273:1862–1864. doi: 10.1126/science.273.5283.1862. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Wilson J M. Clearance of adenovirus-infected hepatocytes by MHC class I-restricted CD4+ CTLs in vivo. J Immunol. 1995;1555:2564–2570. [PubMed] [Google Scholar]
- 37.Yao S-N, Farjo A, Roessler B J, Davidson B L, Kurachi K. Adenovirus-mediated transfer of human factor IX gene in immunodeficient and normal mice: evidence for prolonged stability and activity of the transgene in liver. Viral Immunol. 1996;9:141–153. doi: 10.1089/vim.1996.9.141. [DOI] [PubMed] [Google Scholar]
- 38.Zsengeller Z K, Wert S E, Hull W M, Hu X, Yei S, Trapnell B C, Whitsett J A. Persistence of replication-deficient adenovirus-mediated gene transfer in lungs of immune-deficient (nu/nu) mice. Hum Gene Ther. 1995;6:457–467. doi: 10.1089/hum.1995.6.4-457. [DOI] [PubMed] [Google Scholar]