Abstract
Background
Induction with daratumumab-based regimens followed by autologous stem cell transplantation is the current standard for newly diagnosed multiple myeloma (NDMM) patients eligible for intensive chemotherapy. However, concerns emerged regarding potential negative effects following daratumumab-based treatment on CD34+ mobilization. We here compared CD34+ mobilization and clonogenic potential between daratumumab and non-daratumumab based therapy without upfront plerixafor administration among patients affected by NDMM.
Materials and methods
Clinical, mobilization and clonogenic data from 41 consecutively enrolled NDMM patients were analyzed. Patients underwent collection of autologous CD34+ by apheresis at the ASST Grande Ospedale Metropolitano Niguarda, Milan, Italy, from January 2021 to March 2023. Clonogenicity analysis was performed on BFU-E and CFU-GM.
Results
Seventy-five percent of daratumumab-treated patients underwent >1 apheresis, compared to 24% of non-daratumumab patients (p=0.0017). Daratumumab-treated patients had significantly lower CD34+ count (mean 38 vs 79/μL, respectively; p=0.0011), with a median CD34+ harvest of 3.98×106/kg (range 1.68–9.18) vs 6.87×106/kg (range 1.63–16.85) in non-daratumumab-treated (p=0.0006). In multivariate analysis the likelihood of undergoing >1 apheresis was significantly higher in older patients (OR 1.2, 95% CI 1–1.4, Z=2.10, p=0.03) and daratumumab-treated patients (OR 15, 95% CI 2.8–129, p=0.004). Moreover, daratumumab-based induction therapy demonstrated an independent negative association with BFU-E colony formation (p=0.0148), even when accounting for patient age and CD34+ levels.
Discussion
Our findings underscore the impact of daratumumab-based treatment on CD34+ mobilization in a real-life, upfront plerixafor-free population of NDMM patients. Higher probability of requiring multiple apheresis occurred among daratumumab-treated patients. Interestingly, the observation that daratumumab might negatively impact BFU-E colony formation, independent of CD34+ cell count, offers novel biological perspectives. Appropriate strategies should be adopted by the Apheresis teams to mitigate these potential negative effects.
Keywords: multiple myeloma, anti-CD38 monoclonal antibody, mobilization, collection, apheresis
INTRODUCTION
Multiple myeloma (MM) is a hematologic malignancy with a worldwide incidence of 160,000 cases and a global mortality of 106,000 patients for the year 20181. It is characterized by presence of abnormal clonal plasma cells in the bone marrow, with potential for uncontrolled growth causing destructive bone lesions, kidney injury, anemia and hypercalcemia2. The introduction into clinical practice of daratumumab, a humanized monoclonal antibody targeting CD38, has revolutionized the therapeutic landscape for MM, particularly in the setting of transplant-eligible patients. The updated 2021 European Haematology Association (EHA) and the European Society of Medical Oncology (ESMO) guidelines recommend the triplet proteasome inhibitor bortezomib plus dexamethasone with the addition of lenalidomide (VRd) or thalidomide (VTd) plus daratumumab as first options in the treatment of newly diagnosed multiple myeloma (NDMM) followed by autologous stem cell transplant (ASCT) and lenalidomide maintenance3.
Nonetheless, as daratumumab became increasingly integrated into routine clinical practice, concerns about its potential negative impact on hematopoietic stem cell (HSC) mobilization yields emerged4–7.
Considering that CD38 is expressed on both MM cells and CD34+ HSCs, investigations have been conducted to elucidate the underlying biological factors contributing to these observations. Nevertheless, in vitro studies evaluating the effects of daratumumab on mobilized CD34+ progenitor cells from MM patients demonstrated no evidence of toxicity8.
In this setting, the aim of our study is to evaluate the impact of daratumumab on autologous CD34+ mobilization yields and on CD34+ clonogenicity in a real-life population of daratumumab and non-daratumumab treated patients, who did not receive pre-emptive upfront therapy with plerixafor as mobilizing agent.
MATERIALS AND METHODS
Study population
This study is a retrospective case-control comparison of daratumumab and non-daratumumab treated patients, conducted in a real-life, upfront plerixafor-free MM population. Data from 41 consecutive newly diagnosed MM (NDMM) patients, balanced in numbers between daratumumab and non-daratumumab induction therapies, have been included. Patients underwent mobilization and collection of autologous CD34+ by apheresis at ASST Grande Ospedale Metropolitano (GOM) Niguarda from January 2021 to March 2023. This retrospective observational study protocol was approved by the Comitato Etico Milano Area 3 upon notification by the Principal Investigator (RCr) and informed consent is provided by patients for the related procedures and treatments. Inclusion criteria stipulated participants must be above 18 years of age, have a confirmed MM diagnosis at ASST GOM Niguarda, Milano/Ospedale Manzoni, Lecco. Patients who received plerixafor upfront for their first mobilization were excluded from the study. As of our local policy, plerixafor on-demand is not envisaged for NDMM patients9.
METHODS
The following data were retrospectively collected for each patient:
demographic and baseline characteristics: sex, age at mobilization, weight (expressed in kg), disease status at mobilization (as defined by International Myeloma Working Group-IMWG - Uniform Response Criteria for Multiple Myeloma10), MM characteristics;
treatment characteristics: induction therapy (either daratumumab-based or non daratumumab-based), mobilization strategy (cyclophosphamide monotherapy at a dose of 2–3 g/m2 was administered as mobilizing regimen to all patients unless contraindicated and granulocyte colony-stimulating factor [G-CSF] at a dose of 5 μg/kg/d), previous radiotherapy (as dichotomous variable “yes/no”), number of weeks from the end of the last induction therapy cycle to CD34+ mobilization, need for plerixafor administration following mobilization failure (i.e., failing to reach the set target of 6×106 CD34+ cells/kg);
CD34+ mobilization yields: number of CD34+ before cell harvest (i.e., CD34+ count, expressed in CD34/μL) number of harvested CD34+ ×106/kg, total number of apheresis needed to reach the set target (i.e., 6×106 CD34+/kg);
engraftment time: median time to absolute neutrophil count (ANC) recovery (defined as ANC >0,5×109L for three consecutive days without the support of G-CSF) and to platelets (PLT) recovery (defined as PLT >20×109L for three consecutive days without transfusion support).
Collection by apheresis
Daily monitoring of peripheral CD34+ levels was performed from day +10 following the initiation of the mobilization regimen. Apheresis procedures were initiated as soon as the CD34+ count exceeded 20/μL and they continued until the target cell count was reached or as dictated by the CD34+ kinetics if a downward trend was observed. Collection was realized employing Spectra Optia™ (Terumo BCT, Lakewood CO, USA) by connecting the patient through peripheral or central venous access, the latter whenever peripheral veins were not appropriate for the procedure. ACD-A was used as anticoagulant in the apheresis kit and calcium gluconate was administered to patient as intravenous infusion during collection, to prevent ACD-induced hypocalcemia. The overall volume of blood processed depended upon the initial CD34+ value before collection and the locally established collection efficiency. Local policy allows for processing a maximum of three total blood volumes per day.
Statistical analysis
The primary objective of this analysis was to ascertain whether patients undergoing daratumumab-based induction therapy had an increased probability of requiring more apheresis procedures compared to those not receiving daratumumab. Secondary objectives, pre-specified before data analysis, included the evaluation of any differences in CD34+ count levels at initial mobilization, the association of CD34+ levels greater than 20/μL as a predictor for target achievement, any differences in median CD34+ harvest and in clonogenic tests. The predefined target was set at 6×106 CD34+/kg and was assessed as a nominal dichotomous variable. Comparative analyses were performed using the Mann-Whitney U test for continuous variables and the Chi-square or Fisher’s exact test for categorical variables. Sensitivity and specificity of CD34+/μL >20/μL as a predictor were analysed using Receiver Operating Characteristic (ROC) analysis. The impact of daratumumab-based therapy on the primary objective, adjusted for other potential confounding factors, was evaluated through multiple logistic regression.
The need for one or more apheresis sessions was regarded as a nominal dichotomous variable. The influence of independent variables on continuous outcomes was assessed using multiple linear regression. Failure to achieve the target was defined when the predefined set target of 6×106 CD34+/kg was not obtained.
Clonogenicity essays
Clonogenicity assessment was conducted on stored harvested CD34+ cells from both groups, wherein quantification of colony forming unit-granulocyte-macrophage (CFU-GM) and burst forming unit-erythroid (BFU-E) at a rate of ×104/kg was performed. The impact of daratumumab on clonogenicity was evaluated in a multivariate analysis considering both patient age and CD34+ values. CFU-GM, BFU-E and CD34+ were evaluated as continuous variables in multiple linear regression.
RESULTS
Patient cohort description
A total of 41 consecutive patients with NDMM, who underwent collection of autologous CD34+ by apheresis between January 2021 and March 2023 at ASST GOM Niguarda, were analyzed. Median age was 62 years (range, 39 to 72), and 71% (No.=29) of the cohort was male. Twenty patients received daratumumab-based induction therapy (48.8%), while 21 (52.5%) patients belonged to the non-daratumumab group. Patients received a median of 4 cycles (range, 4 to 11 cycles) of induction chemotherapy, with a median of 4 cycles (range, 4 to 6) in the daratumumab group and 5 cycles (range, 4 to 11) in the non-daratumumab group respectively. Median number of weeks from the last daratumumab administration to mobilization was 6 (range, 4 to 10), while a median of 7 weeks (range, 2 to 13) passed from the last non daratumumab-based chemotherapy cycle to mobilization. Baseline patient and disease characteristics were well balanced between the two induction therapy groups, Table I. No statistically significant differences were reported between the two groups (daratumumab and non-daratumumab based induction therapy) when considering patients who did and did not receive radiotherapy before mobilization (p=0.40) and when considering platelet level before mobilization (i.e., below or above the value of 170×109L, p=0.20), Online supplementary Figure S1.
Table I.
Baseline patients and disease characteristics as divided in the two induction therapy groups: daratumumab-based and non daratumumab-based therapy
| Characteristics | Daratumumab group (No.=20) | Non-daratumumab group (No.=21) |
|---|---|---|
| Age, yr, median (range) | 62 (44–72) | 60 (39–71) |
| Male sex, No. (%) | 15 (75) | 14 (66) |
| Weight, kg, No. (%) | 74 (49–110) | 73 (54–106) |
| Subtype, No. (%) | ||
| IgG | 15 (75) | 13 (62) |
| IgA | 3 (15) | 5 (24) |
| IgE | 0 | 0 |
| Light chain | 2 (10) | 2 (9) |
| Non secretory | 0 | 1 (5) |
| Previous RT, No. (%) | 1 (5) | 4 (19) |
| Induction therapy type, No. (%) | ||
| Dara-VTD | 17 (85) | 0 |
| Dara-VCD | 2 (10) | 0 |
| Dara-RD | 1 (5) | 0 |
| VTD | 0 | 16 (76) |
| Other | 0 | 5 (24) |
| Number of full CHT cycles, median (range) | 4 (4–6) | 5 (4–11) |
| Number of weeks from the end of the last CHT cycle to mobilization, median (range) | 6 (4–10) | 7 (2–13) |
| BM infiltration before mobilization | ||
| absent, No. (%) | 5 (25) | 6 (28) |
| <30%, No. (%) | 15 (75) | 13 (62) |
| >30%, No. (%) | 0 | 2 (9) |
| Hb level before mobilization | ||
| 8–13 g/dL, No. (%) | 20 (100) | 21 (100) |
| <8 g/dL, No. (%) | 0 | 0 |
| WBC level before mobilization | ||
| <5×109L, No. (%) | 2 (10) | 4 (19) |
| >5×109L, No. (%) | 18 (90) | 17 (81) |
| PLT level before mobilization | ||
| <170×109L, No. (%) | 12 (60) | 17 (81) |
| >170×109L, No. (%) | 8 (40) | 4 (19) |
RT: radiotherapy; CHT: chemotherapy; BM: bone marrow; Hb: hemoglobin; WBC: white blood cells; PLT: platelets.
Moreover, no statistically significant difference emerged in the baseline response rates at the time of mobilization (p=0.34), Online supplementary Figure S2.
Autologous CD34+ mobilization yields
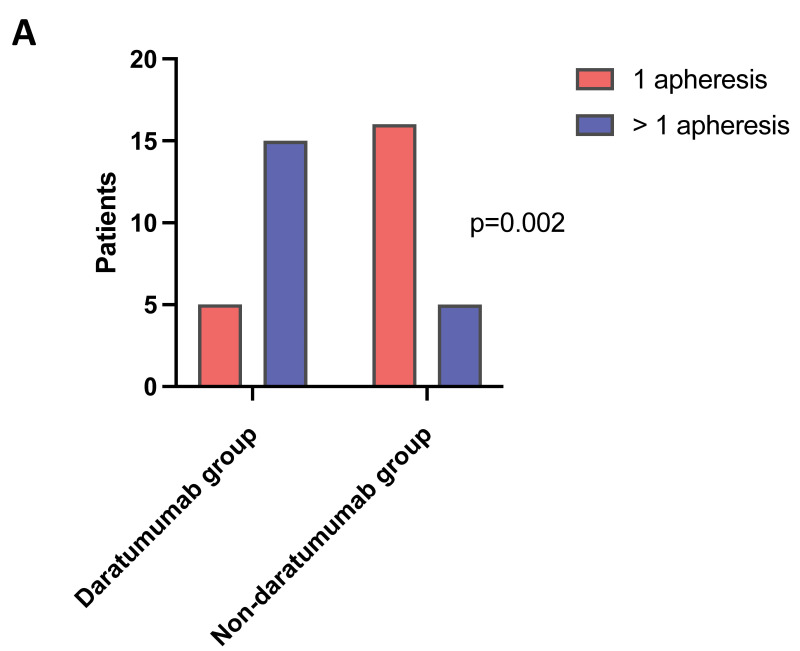
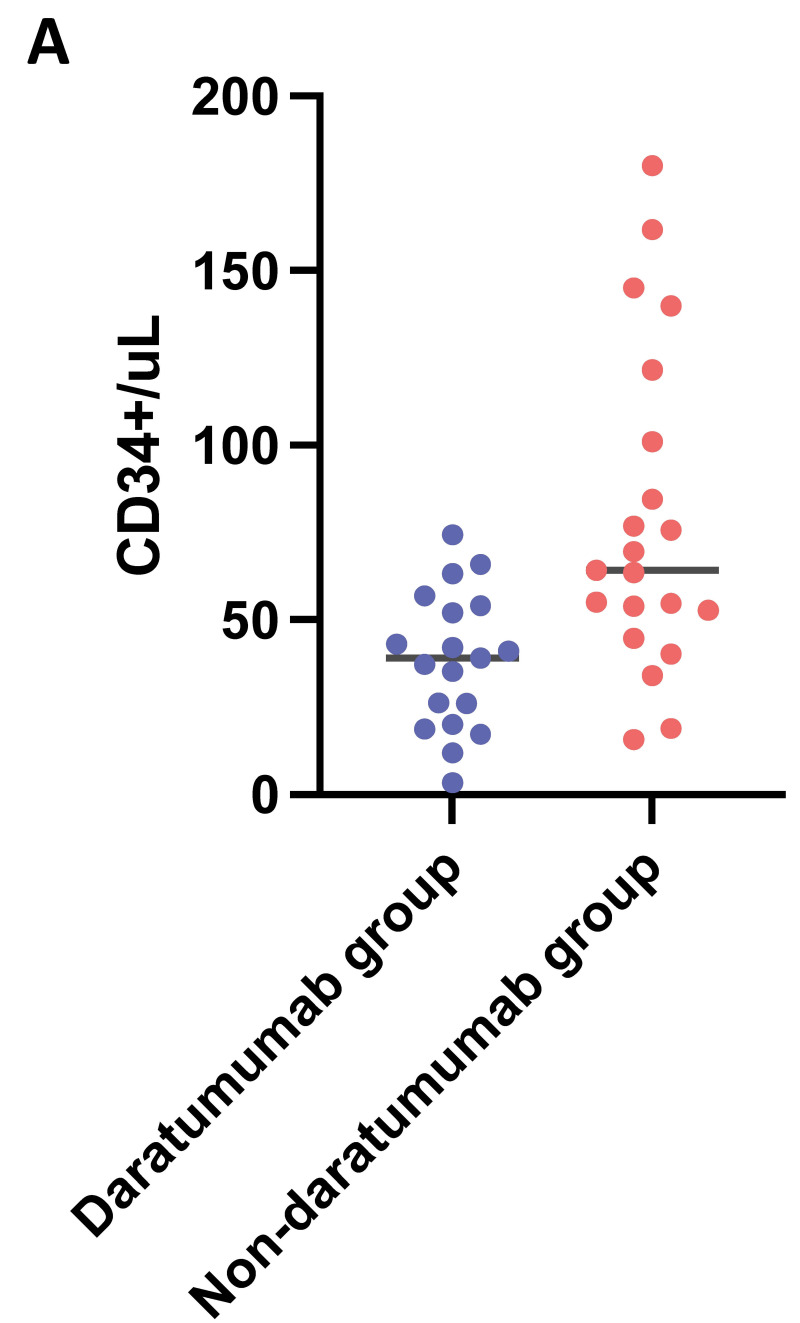
Seventy-five percent (No.=15) of the daratumumab-treated patients had to undergo >1 apheresis, compared to only 24% (No.=5) in the non-daratumumab group (p=0.0017), Figure 1A. A total of three blood volumes for each apheresis were processed for most patients, unless lower volumes were sufficient to reach the target, upon pre-apheresis calculation according to the local known collection efficiency. The total blood volumes processed during each apheresis and the CD34+ cells collected are shown in the Supplemental Table SI. In the same table are detailed the patients receiving apheresis through central venous catheter vs peripheral access (No.=6 vs No.=35 respectively). A correlation emerged between induction therapy (daratumumab-based and non daratumumab-based) and the number of apheresis needed to reach the set target (i.e., 6×106 CD34+/kg). Sixty-two percent (No.=13) of patients in the non-daratumumab group reached the set CD34+ target at first collection, compared to only 20% (No.=4) in the daratumumab group. The differences between the two groups were statistically significant (p=0.01), Figure 1B. Moreover, patients who received induction therapy containing daratumumab had significantly lower CD34+ count than non-daratumumab patients (mean 38 vs 79/μL, difference between means 40.4; 95% confidence interval (CI) 17–63 respectively; p=0.0011), Figure 2A. Interestingly, according to a ROC analysis, the CD34+ count value of 54.49/μL appeared to be the most effective predictor of target achievement probability within our cohort, with a sensibility of 82% and specificity of 76%, Figure 2B. Median CD34+ harvest was 3.98×106/kg (range 1.68–9.18) in patients who received induction therapy with daratumumab, while 6.87×106/kg (range 1.63–16.85) in non-daratumumab treated patients. Plerixafor at a dose of 0.24 mg/kg was administered as a rescue strategy to 25% (No.=5) of patients who underwent >1 apheresis, 80% of them (i.e., 4/5) being daratumumab patients.
Figure 1. Number of apheresis and CD34+ target according to induction therapy.
A) Number of patients who had to undergo 1 (red) vs >1 (blue) apheresis sessions in order to reach the set CD34+ target, divided into the two induction therapy groups (daratumumab-based and non daratumumab-based). B) Number of patients who reached the set CD34+ target (i.e., 6×106/kg) divided into the two induction therapy groups (daratumumab-based and non daratumumab-based).
Figure 2. Pre-apheresis CD34+ count, induction therapy group and CD34+ target.
Count of CD34+/μL in the two induction therapy groups: daratumumab-based (blue) and non daratumumab-based (red). A) Results from a ROC analysis considering the count of CD34+/μL and the ability to reach the set CD34+ target (target reached red, target not reached blue). B) The CD34+ count value of 54.49/μL appeared to be the most effective predictor of target achievement probability, with a sensitivity of 82% and specificity of 76%.
Previous radiotherapy and low number of platelets before mobilization did not have an impact on the number of apheresis needed to reach the set CD34+ target (p=0.34 and p=0.17, respectively), Online supplementary Figure S3. Furthermore, also the grade of response (i.e., VGPR or better) before CD34+ mobilization resulted not to have any statistically significant impact both on the number of apheresis needed to reach the set CD34+ target and on the collected CD34+/μL (p=0.20 and p=0.06, respectively), Online supplementary Figure S4.
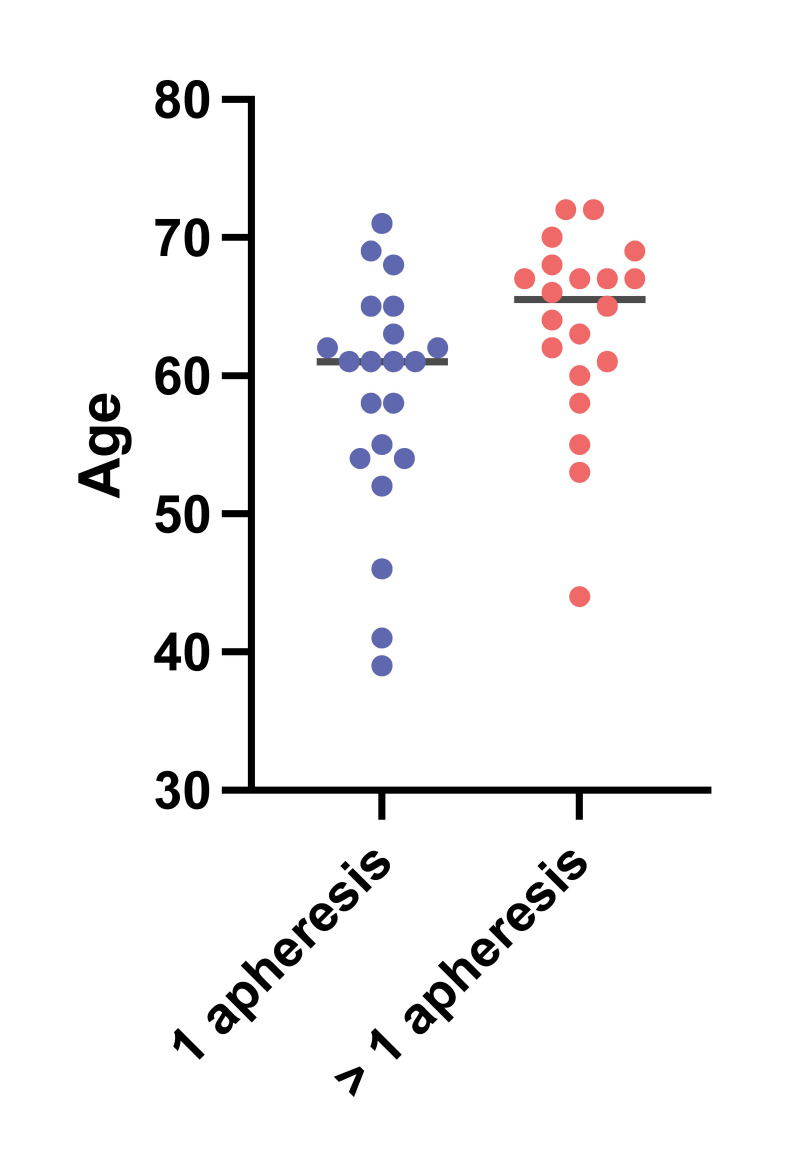
A multiple logistic regression analysis was performed considering age, sex, weight, induction therapy type (daratumumab and non daratumumab-based therapy) and disease status at mobilization. According to this analysis the probability of undergoing >1 apheresis was significantly increased in older patients (odds ratio [OR] 1.2, 95% CI 1.03–1.40, Z=2.10, p=0.03) −Figure 3− and in patients who had received daratumumab-based induction therapy (OR 15, 95% CI 2.8–129, p=0.004). In multivariate analysis, daratumumab induction therapy was significantly associated with a lower CD34+ count (p=0.0056).
Figure 3.
Probability of undergoing 1 apheresis (blue) vs >1 apheresis (red) with respect to age
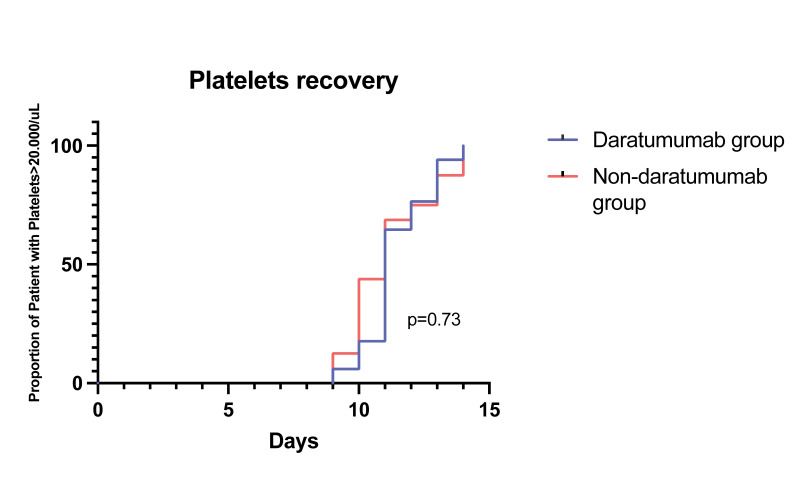
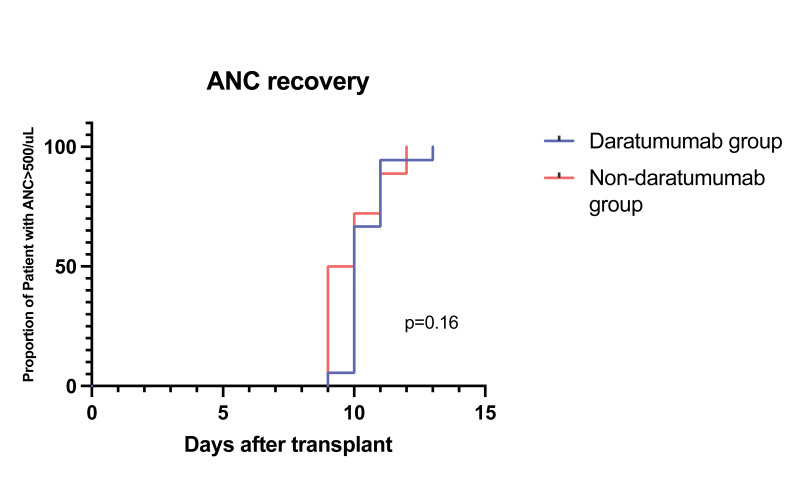
After autologous stem cell transplantation, the median time for neutrophils engraftment was 9.5 days in the daratumumab group and 10 days in the non-daratumumab group (p=0.16). The median time for platelet engraftment was 10.5 days in the daratumumab group and 11 days in the non-daratumumab group (p=0.73), Figure 4.
Figure 4. Median time for neutrophils and platelets engraftment divided into the two induction therapy groups.
Daratumumab-based (orange) and non-daratumumab based (light blue). ANC: absolute neutrophil count.
Clonogenicity data
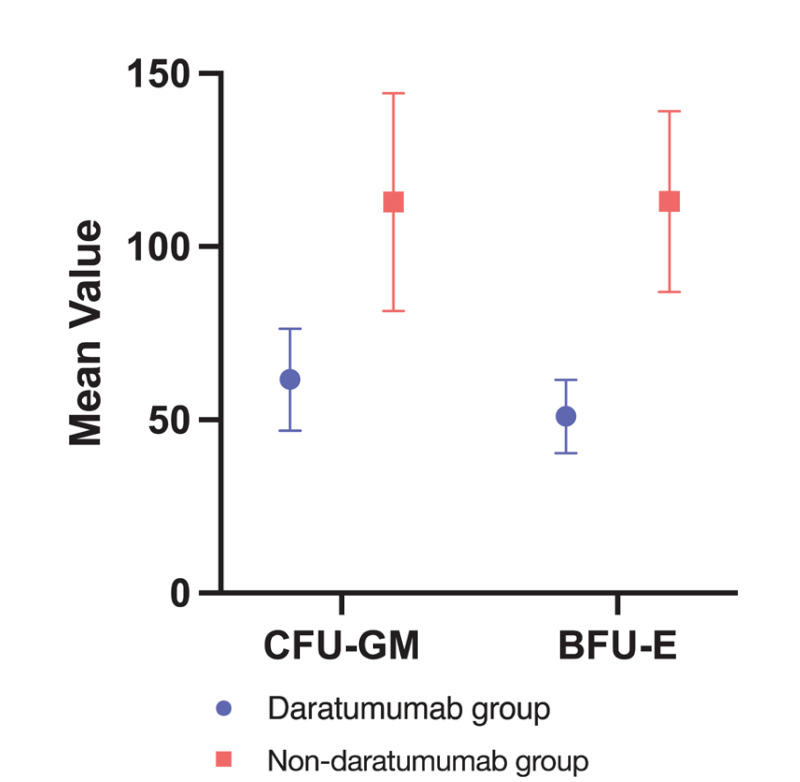
In order to evaluate the clonogenicity of the collected HSCs, CFU-GM and BFU-E were performed to all apheresis products. Overall, the median CFU-GM was 63.6 (95% CI 48.7–95.8)×104/kg, the median BFU-E was 60.7 (95% CI 53.1–77.40)×104/kg. Considering the single apheresis products separated in the two groups, median CFU-GM in the daratumumab-treated group was 48.01 (95% CI 29.4–79.4)×104/kg, while BFU-E was 49.8 (95% CI 33.1–60.7)×104/kg; they were 112.01 (95% CI 61.6–137.1)×104/kg and 107.17 (95% CI 76.3–135.4)×104/kg, respectively, in the non daratumumab-treated group, Figure 5.
Figure 5. CFU-GM and BFU-E mean value with 95% CI per the two induction therapy groups.
Daratumumab-based (blue) and non daratumumab-based (red). CFU-GM: colony forming unit - granulocyte macrophage; BFU-E: burst forming unit - erythroid; CI: confidence interval.
Considering the established associations between age, CD34+ content, and the outcomes of clonogenic tests, our analysis assessed the influence of daratumumab on clonogenicity through a multivariate analysis that simultaneously considered patient age and CD34+ ×106/kg. This approach aimed at evaluating the specific impact of daratumumab while accounting for the interrelationship between these two variables.
Notably, a lower incidence of BFU-E colony formation was observed in samples collected following the daratumumab-based regimen compared to those from the non-daratumumab group; indeed, a statistically significant difference between induction regimens has been observed (p=0.0148, no multicollinearity indexes were found among variables: variance inflation factor range 1.1–1.3). Conversely, no statistically significant difference emerged concerning CFU-GM colonies (p=0.7071).
DISCUSSION
To the best of our knowledge, our study represents the first investigation assessing the impact of the anti-CD38 monoclonal antibody daratumumab-based therapy on CD34+ mobilization yields and clonogenic potential in NDMM patients who did not receive upfront plerixafor administration, nor on-demand. The results of our study reveal a statistically significant difference in autologous HSC mobilization yields between the two examined groups, with the daratumumab-treated group experiencing significant impairment in the mobilization process. Moreover, we report a negative effect of daratumumab-based therapy on BFU-E colony formation. While existing data do not consistently align regarding the adverse impact of anti-CD38 monoclonal antibody on CD34+ mobilization yields, it is crucial to acknowledge that most of these studies employed upfront plerixafor administration in conjunction with G-CSF. A relevant example can be drawn from the randomized phase 3 CASSIOPEIA study, designed to compare the effects of Dara-VTd and VTd regimens on HSC mobilization and collection outcomes. Notably, 21.7% of the Dara-VTD-treated patients and 7.9% of those receiving VTd underwent upfront plerixafor administration as a preemptive mobilization strategy. Within this context, the Dara-VTd arm exhibited distinctive characteristics, including a diminished mean count of harvested HSCs and a heightened reliance on plerixafor for mobilization purposes4,11. Indeed, although certain investigations did not demonstrate a statistically significant impact of daratumumab on HSC mobilization yields, they reported a higher need of plerixafor rescue within the daratumumab-treated group6. Findings from the GRIFFIN12 and MASTER13 trials indicated that daratumumab-treated patients receiving upfront plerixafor displayed numerically greater HSC yields compared to those who received plerixafor in an on-demand manner for rescue purposes7.
In line with our study, a recently published Swedish retrospective study, which did not employ upfront plerixafor administration, observed that the addition of daratumumab to the treatment regimen resulted in a substantially lower HSC yield, hence necessitating an increased mean and median duration of apheresis procedures, as compared to non daratumumab-based therapies. The study also reported an increased need for the rescue use of plerixafor to effectively facilitate HSC mobilization in these patients5. However, no analysis on clonogenicity potential was performed.
These findings emphasize the need for investigations encompassing larger patient cohorts to validate the observed results and, in the long run, enhance the mobilization strategy for individuals subjected to daratumumab-based therapy14.
Our study demonstrates a statistically significant variation in HSC mobilization yields within the two analyzed groups in an upfront plerixafor-free setting, hence allowing for a more untainted analysis of outcomes. The daratumumab-administered group required an increased number of apheresis sessions to attain the predefined target, along with reduced pre-apheresis CD34+ count and CD34+ harvest levels. Importantly, despite the encountered challenges in the mobilization process, no statistically significant disparities were detected in neutrophil or platelet engraftment between the two groups after autologous stem cell transplantation. Conversely to some findings in the literature15,16, our study did not reveal any statistically significant effects associated with the administration of radiotherapy before stem cell mobilization or having a low platelet count (i.e., PLT count <170×109L) at the time of apheresis. This might be due to the low number of patients in our cohorts, not allowing these two variables to emerge as significant. We believe that strategies to mitigate the risk of failure to achieve the set CD34+ target would be worthy of use. Increasing the G-CSF dosage, i.e., to 10 μg/kg/day, or optimizing the mobilization strategy by administering higher doses of cyclophosphamide might be both feasible and effective in enhancing the HSC mobilization. Recently, Liberatore et al.14 reported optimal HSC collection after daratumumab-based induction with cyclophosphamide 4 g/m2 as mobilization strategy. Another viable option could be the “on-demand” administration of plerixafor to all patients undergoing daratumumab-based therapy. Despite its specific indications, on-demand plerixafor has been successfully employed, also in other clinical contexts9. Implementing these strategies alongside daratumumab-based treatment may help overcome mobilization challenges by trying to avoid multiple collections as well as reducing the mobilization failures. The achievement of the CD34+ target is indeed crucial to allow for high-dose therapy followed by ASCT, thus for the completion of the first-line therapeutic program in NDMM patients.
Our observation of impaired mobilization and collection of autologous HSCs following daratumumab-based therapy could potentially be attributed to the inherently low expression of CD38 on CD34+ HSCs17. The precise mechanism by which the anti-CD38 monoclonal antibody daratumumab influences HSCs mobilization yields has not been fully elucidated yet. However, several plausible factors warrant consideration. Firstly, daratumumab interaction with the CD38 molecule on HSCs may potentially impede their mobilization process. Notably, the disruption of interactions between myeloma cells and the bone marrow microenvironment, induced by daratumumab, may result in interference with adhesion molecules within the bone marrow milieu. This phenomenon could consequently impact HSC capacity to effectively mobilize from the bone marrow and enter circulation18,19. Furthermore, daratumumab immune-mediated properties, exemplified by processes like antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), possess the capacity to trigger the elimination of CD38-expressing cells20, which include HSCs, influencing their mobilization dynamics.
To gain insights into the biological factors contributing to our observed findings we analyzed clonogenicity data of BFU-E and CFU-GM. Interestingly, statistically significant disparities between induction regimens were observed in the context of BFU-E colony formation, with a negative impact of daratumumab. A previous study investigating the influence of daratumumab on CD34+ mobilization and the composition of erythroid and myeloid progenitor stem cell products, as measured by the number of BFU-E, CFU-GM, and colony-forming unit cell (CFU-C), revealed comparable colony formation regardless of daratumumab use21. However, it is important to note that in this study 88% of daratumumab-treated patients received plerixafor prior to mobilization. This could potentially offset the effects of daratumumab and lenalidomide on graft and stem cell collection, as stated by the authors. Also, published in vitro studies evaluating the effects of daratumumab on mobilized CD34+ progenitor cells from myeloma patients demonstrated no evidence of toxicity and no effects on progenitor cell assays8.
Our results stand as the first report of a possible impact of CD38+ monoclonal antibody daratumumab on HSC clonogenic potential. One plausible elucidation might come from a previous work wherein heightened influences of CD34+CD38++ cell subpopulation on BFU-E formation were reported. Specifically, a subset of CD34+ cells characterized by elevated CD38 expression (CD34+CD38++ cells) exhibited a pronounced enrichment in BFU-E quantities while concurrently manifesting a relative depletion in CFU-GM counts, in comparison to a control CD34+ cell population17. This observation may suggest that HSCs that have engaged with anti-CD38 monoclonal antibody, presumably leading to a reduced CD38 expression, may potentially exhibit diminished capacity to generate BFU-E colonies.
Our study has some limitations, firstly being a retrospective single-center observational study, hence involving a small number of patients, despite well balanced between the two groups. Secondly, the impact of anti-CD38 monoclonal antibodies other than daratumumab was not assessed. Although Isatuximab has recently been approved in combination therapies for the treatment of relapsed-refractory MM22,23 and many clinical trials have been conducted to evaluate its activity in NDMM24, no analysis on mobilization with this drug has been performed so far.
Nonetheless, our study is among the first efforts to explore autologous CD34+ cell mobilization subsequent to daratumumab and non-daratumumab-based induction therapies, employing a mobilization strategy devoid of upfront plerixafor administration, nor on-demand. Our findings confirm previous observation on impaired CD34+ mobilization among daratumumab-treated patients and detect for the first time a significant impact on the generation of BFU-E colonies, standing as the first documentation of a negative impact attributed to the anti-CD38 monoclonal antibody daratumumab on the clonogenic potential of HSCs in vitro. Reassuringly, despite these observed effects, there were no adverse consequences on neutrophil and platelet engraftment after ASCT.
CONCLUSIONS
The current study is among the first efforts to explore autologous CD34+ cell mobilization subsequent to daratumumab and non daratumumab-based induction therapies, employing a mobilization strategy devoid of upfront plerixafor administration. In our investigation, patients subjected to induction treatment involving the anti-CD38 monoclonal antibody demonstrated an increased likelihood of multiple apheresis for successful collection, with subsequent potential discomfort for the patients. Furthermore, to the best of our knowledge, present results stand as the first documentation of a negative impact attributed to the CD38-targeting monoclonal antibody daratumumab on the clonogenic potential of HSCs.
In light of these observations, while anti-CD38 monoclonal antibody therapy does indeed elicit effects on both mobilization yields and clonogenic potential, neutrophil and platelet engraftment after ASCT was not affected. Nevertheless, our study emphasizes that in a setting that does not employ upfront plerixafor administration as a mobilization strategy, as defined in Europe by the EMA label, patients treated with daratumumab have a higher probability of requiring multiple apheresis compared to non daratumumab-treated patients. Apheresis teams should adopt appropriate measures to mitigate this risk, trying to avoid the associated adverse effects and discomfort endured throughout the mobilization process.
Supplementary Information
ACKNOWLEDGMENTS
We thank all the healthcare workers of the Hematology Division, the Cellular Therapy Laboratory and the Immunohematology and Transfusion Medicine Service at the ASST Grande Ospedale Metropolitano Niguarda (Milano, Italy), of the Onco-hematology Division at the Ospedale Manzoni (Lecco, Italy) and their patients and families.
Footnotes
AUTHORS’ CONTRIBUTIONS: AZ and RCr designed the study, collected data and wrote the manuscript. IC supervised methodology and performed the statistical analyses. LP performed the clonogenicity assays. SP collected data. AMC, MA, LB, PB, GG, PM, MLP, ER, MS, CVV, EBV, CGP, SR, RCa managed the patients or performed the apheresis procedures. All Authors revised the manuscript and approved the final version.
The Authors declare no conflicts of interest.
REFERENCES
- 1.Ludwig H, Novis Durie S, Meckl A, Hinke A, Durie B. Multiple myeloma incidence and mortality a round the globe; interrelations between health access and quality, economic resources, and patient empowerment. Oncologist. 2020;25:e1406–e1413. doi: 10.1634/theoncologist.2020-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cowan AJ, Green DJ, Kwok M, Lee Sarah, Coffey David G, Holmberg Leona A, et al. Diagnosis and management of multiple myeloma: a review. JAMA. 2022;327:464–477. doi: 10.1001/jama.2022.0003. [DOI] [PubMed] [Google Scholar]
- 3.Dimopoulos MA, Moreau P, Terpos E, Mateos MV, Zweegman S, Cook G, et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2021;32:309–322. doi: 10.1016/j.annonc.2020.11.014. Erratum in: Ann Oncol 2022 Jan; 33:117. [DOI] [PubMed] [Google Scholar]
- 4.Hulin C, Offner F, Moreau P, Roussel M, Belhadj K, Benboubker L, et al. Stem cell yield and transplantation in transplant-eligible newly diagnosed multiple myeloma patients receiving daratumumab + bortezomib/thalidomide/dexamethasone in the phase 3 CASSIOPEIA study. Haematologica. 2021;106:2257–2260. doi: 10.3324/haematol.2020.261842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemonakis K, Tatting L, Lisak M, Carlson K, Crafoord J, Blimark CH, et al. Impact of daratumumab-based induction on stem cell collection parameters in Swedish myeloma patients. Haematologica. 2023;108:610–614. doi: 10.3324/haematol.2022.281610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thurlapati A, Roubal K, Davis JA, Shah SZ, Smith D, McGann M, et al. Stem cell mobilization for multiple myeloma patients receiving daratumumab-based induction therapy: a real-world experience. Transplant Cell Ther. 2023;29:340e1–340.e4. doi: 10.1016/j.jtct.2023.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Chhabra S, Callander N, Watts NL, Costa LJ, Thapa B, Kaufman JL, et al. Stem cell mobilization yields with daratumumab- and lenalidomide-containing quadruplet induction therapy in newly diagnosed multiple myeloma: findings from the MASTER and GRIFFIN trials. Transplant Cell Ther. 2023;29:174e1–174.e10. doi: 10.1016/j.jtct.2022.11.029. [DOI] [PubMed] [Google Scholar]
- 8.Ma X, Wong SW, Zhou P, Chaulagain CP, Doshi P, Klein AK, et al. Daratumumab binds to mobilized CD34+ cells of myeloma patients in vitro without cytotoxicity or impaired progenitor cell growth. Exp Hematol Oncol. 2018;7:27. doi: 10.1186/s40164-018-0119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milone G, Conticello C, Leotta S, Michieli MG, Martino M, Marco ALD, et al. Plerixafor on-demand in association with low-dose cyclophosphamide and G-CSF in the mobilization of patients with multiple myeloma: High effectiveness, low toxicity, and affordable cost. Leuk Res Rep. 2020;14:100227. doi: 10.1016/j.lrr.2020.100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durie BG, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K, et al. International Myeloma Working Group. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. Erratum in: Leukemia 2006; 20: 2220. Erratum in: Leukemia. 2007; 21: 1134. [DOI] [PubMed] [Google Scholar]
- 11.Moreau P, Attal M, Hulin C, Arnulf B, Belhadj K, Benboubker L, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomized, open-label, phase 3 study. Lancet. 2019;394:29–38. doi: 10.1016/S0140-6736(19)31240-1. Erratum in: Lancet. 2019. [DOI] [PubMed] [Google Scholar]
- 12.Voorhees PM, Kaufman JL, Laubach J, Sborov DW, Reeves B, Rodriguez C, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020;136:936–945. doi: 10.1182/blood.2020005288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa LJ, Chhabra S, Medvedova E, Dholaria BR, Schmidt TM, Godby KN, et al. Daratumumab, carfilzomib, lenalidomide, and dexamethasone with minimal residual disease response-adapted therapy in newly diagnosed multiple myeloma. J Clin Oncol. 2022;40:2901–2912. doi: 10.1200/JCO.21.01935. [DOI] [PubMed] [Google Scholar]
- 14.Liberatore C, Perini T, Passeri C, Ferla V, Fioritoni F, Girlando V, et al. Higher cyclophosphamide dose grants optimal stem-cell collection after daratumumab-based induction in multiple myeloma. Haematologica. 2023;108:3502–3505. doi: 10.3324/haematol.2023.283452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sauer S, Erdmann K, Jensen AD, Wennmann M, Pavel P, Jordan K, et al. Local radiation therapy before and during induction delays stem cell mobilization and collection in multiple myeloma patients. Transplant Cell Ther. 2021;27:876e1–876.e11. doi: 10.1016/j.jtct.2021.06.025. [DOI] [PubMed] [Google Scholar]
- 16.Chen Yuan, Gao Shan, Wang Yutong, Lu Minqiu, Chu Bin, Shi Lei, et al. Pre-mobilization platelet count predicts stem cell yield during mobilization in patients with multiple myeloma. Cancer Pathogenesis and Therapy. 2023;1:40–45. doi: 10.1016/j.cpt.2022.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snoeck HW, Lardon F, Lenjou M, Nys G, Van Bockstaele DR, Peetermans ME. Differential regulation of the expression of CD38 and human leukocyte antigen-DR on CD34+ hematopoietic progenitor cells by interleukin-4 and interferon-gamma. Exp Hematol. 1993;21:1480–1486. [PubMed] [Google Scholar]
- 18.Phipps C, Chen Y, Gopalakrishnan S, Tan D. Daratumumab and its potential in the treatment of multiple myeloma: overview of the preclinical and clinical development. Ther Adv Hematol. 2015;6:120–127. doi: 10.1177/2040620715572295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghose J, Viola D, Terrazas C, Caserta E, Troadec E, Khalife J, et al. Daratumumab induces CD38 internalization and impairs myeloma cell adhesion. Oncoimmunology. 2018;7:e1486948. doi: 10.1080/2162402X.2018.1486948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nijhof IS, Casneuf T, van Velzen J, van Kessel B, Axel AE, Syed K, Groen RW, et al. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood. 2016;128:959–970. doi: 10.1182/blood-2016-03-703439. [DOI] [PubMed] [Google Scholar]
- 21.Ash.confex.com [Internet] Impact of daratumumab on stem cell collection, graft composition and engraftment among multiple myeloma patients undergoing autologous stem cell transplant. [Accessed on 9/20/2023.]. Available at: https://ash.confex.com/ash/2020/webprogram/Paper142115.html.
- 22.Van de Donk NWCJ, Usmani SZ. CD38 antibodies in multiple myeloma: mechanisms of action and modes of resistance. Front Immunol. 2018;9:2134. doi: 10.3389/fimmu.2018.02134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Podar K, Leleu X. Relapsed/refractory multiple myeloma in 2020/2021 and Beyond. Cancers (Basel) 2021;13:5154. doi: 10.3390/cancers13205154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ocio EM, Bringhen S, Martinez-Lopez J, San-Miguel J, Oliva S, Rodriguez-Otero P, et al. Phase 1b study of isatuximab in combination with bortezomib, cyclophosphamide, and dexamethasone in newly diagnosed, transplant-ineligible multiple myeloma patients. Hemasphere. 2023;7:e829. doi: 10.1097/HS9.0000000000000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ocio EM, Rodríguez Otero P, Bringhen S, et al. Updates from a phase Ib study of isatuximab (Isa), bortezomib (V) and dexame-thasone (D) plus cyclophosphamide (C) or lenalidomide (R) in transplant-ineligible, newly diagnosed multiple myeloma (NDMM) J Clin Oncol. 2020;38(Suppl 15):8529. doi: 10.1200/JCO.2020.38.15_suppl.8529. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.