Abstract
Background
Several types of transfusion-related registries have been developed to improve patient outcomes and blood banks. In Korea, a transfusion program functioning as a blood group antibody database and a reference laboratory has been in operation since July 2013. This study was conducted to determine the current status of blood group antigens and antibodies in Korea and propose a model for registries in the field of transfusion medicine.
Materials and methods
Cases with unexpected red cell antibodies were registered online in the voluntary transfusion registry. Specific antigen-negative frequencies were calculated based on the recorded data. To determine the frequencies of RhCE antigens, data added via the Blood Information Sharing System were also analyzed. Data added to the registries between July 2013 and June 2022 were included in the analysis.
Results
Among 9,048 antibody cases registered from 29 hospitals, anti-E alone was identified most commonly, followed by anti-E and c, anti-C and e, anti-Lea, and anti-M (2,202, 1,792, 757, 618, and 383 cases, respectively). The frequencies of E-, E-c-, C-e-, Le(a-), and M- were 49.1%, 41.6%, 9.1%, 69.4%, and 21.8%, respectively.
Discussion
The distributions of antibodies and antigen frequencies were estimated through the transfusion registry. Antigen frequencies were calculated based on the results of antigen typing of red blood cell components performed at the time of issuing. The online transfusion registry serving as a blood group antibody database is useful for determining the frequencies of blood group antigens and antibodies.
Keywords: registry, alloantibody, antigen frequency
INTRODUCTION
Several types of transfusion-related registries have been developed worldwide, including antibody registries, platelet donor registries, rare donor programs, and hemovigilance registries. For example, a regional network was established in Lombardy, Italy, in 2005 to prepare rare blood units for transfusion to patients with complex immune profiles, including combinations of antibodies against clinically significant antigens or antibodies against high-prevalence antigens1,2.
Apart from rare donor programs, registries for unexpected red cell antibodies are also necessary. Patients with clinically significant alloantibodies should be transfused with red blood cell (RBC) units lacking the corresponding antigens to avoid hemolytic transfusion reaction. Titers of antibodies may decrease over time and even fall below the limits of detection3. Transfusion of corresponding antigen-positive units to patients with this antibody “evanescent” state can cause delayed hemolytic transfusion reaction (DHTR)4. To avoid DHTR, antigen-negative blood should be transfused in cases with a history of antibody, even if no antibody is currently detected. However, if a patient undergoes transfusion at a different facility, the antibody history may not be available. To overcome this, in the Netherlands, a nationwide antibody registry, The Transfusion Register of Irregular Antibodies and Cross (X)-match Problems (TRIX), was implemented in 20075,6. A recent meta-analysis showed that a national antibody registry helped blood banks detect patients with evanescent alloantibodies and reduced the risk of DHTR4.
The Korean Rare Blood Program (KRBP), which is a patient-centered transfusion registry, has been operating in Korea since July 2013. The KRBP is in fact divided into two separate registries: a transfusion registry named KRBP database and a case registry named KRBP case archive. The transfusion registry is a large database that provides prevalence data for blood group antigens and antibodies. While the transfusion registry mainly includes routinely identifiable red cell antibodies, the case registry consists of challenging cases, such as ABO subgroups, RhD variants, unexpected antibodies unidentifiable with routine tests, and cases with neonatal alloimmune thrombocytopenia. Such challenging cases are registered in the case registry along with the reason for the request, and the KRBP reference laboratory for blood group immunogenetics resolved the cases through molecular analysis and specialized immunohematological tests. Over the past decade, >9,000 antibodies were registered in the transfusion registry, and >800 cases were registered in the case registry and resolved through further analysis.
Our voluntary transfusion registry in Korea was analyzed in regard to the red cell antibodies that were reported. This study was conducted to determine the current status of blood group antigens and antibodies in Korea, and propose a model for registries in the field of transfusion medicine based on our 10-year experience of managing transfusion registries.
MATERIALS AND METHODS
Design of the transfusion registry
The transfusion registry is a database for red cell antibodies accumulated by voluntary registration. Blood bank managers across Korea were invited to voluntarily register data online (http://bloodgroupimmunogenetics.org) that satisfy all of the following three conditions: i) antibodies were detected, ii) antibody identification was performed, and iii) RBCs were transfused. Recorded data included the specificities of identified antibodies, methods of antibody screening and identification, and numbers of RBC units requested, antigen-typed, and obtained. The date of request, date of the test, and name of the hospital were also recorded. Date of birth was recorded but the patient’s name was obscured with special characters; there was no personally identifiable information (PII) such as patient’s full name, identification number, and contact information. Although individuals were not identifiable, the data could be distinguished from each other through the above information. Samples obtained and tested on different days, even for the same patient, were considered independent cases and registered separately. Invalid data were corrected or excluded from analysis and curated data accumulated over 10 years (July 2013–June 2022) were analyzed. This study was approved by the institutional review board of Seoul National University Bundang Hospital in 2017 and is being extended on an annual basis (approval number: B-1705-396-109).
Distribution of unexpected antibodies and calculation of specific antigen-negative frequencies
Antibodies recorded in the transfusion registry were sorted according to their specificities. The number of specific antigen-typed RBC units was recorded. The number of corresponding antigen-negative RBC units actually obtained was also recorded. The specific antigen-negative frequencies were calculated using the following equation:
Then, the average number of required units to be antigen-typed to obtain one specific antigen-negative unit was calculated by taking the reciprocal of the specific antigen-negative frequency and rounding up to the next integer.
Frequencies of RhCE antigens
To determine the true frequencies of RhCE antigens, the frequencies from several references other than the above calculation were analyzed. In addition to ABO and RhD types, the Korean Red Cross Blood Services (KRCBS) has provided the RhCE (C, E, c, and e) types of RBC units through the Blood Information Sharing System (BISS) since June 20167. Users can query information on blood products supplied to their blood banks. Data added to BISS between June 2016 and June 2022 and accessible via the BISS account of our blood bank, were analyzed. The frequencies of RhCE antigens differ significantly between D+ and D− individuals8, and the frequency of D− individuals is very low in Korea (0.15–0.30%)9. Therefore, the frequencies of RhCE antigens among D+ individuals were analyzed and compared with several references.
Survey of transfusion practices and registries
To understand the current status and requirements of blood banks in Korea, a survey was conducted among the KRBP workshop participants. An online survey link was distributed via e-mail to participants of the KRBP workshop between 27th September and 12th October 2022, and the responses were collected. The survey items included the number of screening cells and availability of screening cells with a specific phenotype. The survey results were analyzed by facility and individual.
RESULTS
Overview of the transfusion registry and frequencies of red cell antibodies and antigens
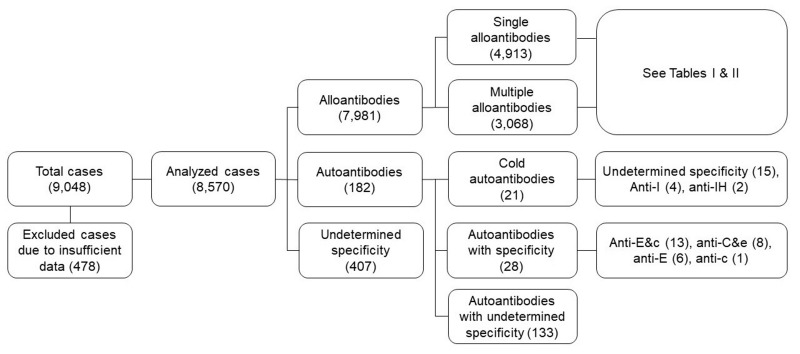
A total of 9,048 cases from 29 general hospitals across the country were registered in the transfusion registry (Figure 1). Among the participating hospitals, 19 superior general hospitals were included; the median number of the beds in these hospitals was 1,048. Among all cases with alloantibodies, the most common alloantibodies were as follows: anti-E (4,272 cases, 53.5%), anti-c (1,935 cases, 24.2%), anti-C (912 cases, 11.4%), anti-e (905 cases, 11.3%), anti-Lea (738 cases, 9.2%), and anti-M (420 cases, 5.3%) (Table I).
Figure 1. Schematic of analyzed cases from the transfusion registry.
The 9,048 total cases included 7,981 of alloantibodies and 182 of autoantibodies. See Tables I and II for detailed alloantibody results.
Table I.
Specificities and numbers of cases with alloantibodies
| Specificity | Cases |
|---|---|
| Single antibodies | (No.=4,913) |
| Anti-E | 2,202 |
| Anti-Lea | 618 |
| Anti-M | 383 |
| Anti-Jka | 323 |
| Anti-Fyb | 283 |
| Anti-Leb | 278 |
| Anti-P1 | 202 |
| Anti-Dia | 116 |
| Anti-c | 113 |
| Anti-Jkb | 94 |
| Anti-S | 87 |
| Anti-C | 67 |
| Anti-e | 59 |
| Anti-D | 31 |
| Anti-Xga | 15 |
| Anti-N | 13 |
| Anti-Lua | 11 |
| Anti-Fya | 7 |
| Anti-K | 4 |
| Anti-Lub | 4 |
| Anti-Dib | 2 |
| Anti-k | 1 |
| Two antibodies | (No.=2,862) |
| Anti-E, c | 1,792 |
| Anti-C, e | 757 |
| Anti-Lea, Leb | 62 |
| Anti-E, Fyb | 42 |
| Anti-E, Jka | 32 |
| Anti-e, Fyb | 19 |
| Anti-E, Jkb | 16 |
| Anti-Lea, P1 | 16 |
| Anti-E, M | 15 |
| Anti-Fyb, Lea | 14 |
| Anti-E, K | 12 |
| Anti-E, Lea | 7 |
| Anti-D, E | 6 |
| Anti-C, c | 6 |
| Anti-C, Jka | 5 |
| Anti-Jka, S | 5 |
| Anti-D, C | 4 |
| Anti-E, P1 | 4 |
| Anti-E, S | 4 |
| Anti-C, E | 3 |
| Anti-C, Fyb | 3 |
| Anti-E, Xga | 3 |
| Anti-e, Lea | 3 |
| Anti-P1, M | 3 |
| Anti-C, Lea | 2 |
| Anti-E, Leb | 2 |
| Anti-c, e | 2 |
| Anti-Fya, Lea | 2 |
| Anti-Jka, Lea | 2 |
| Anti-Lea, S | 2 |
| Anti-E, Dia | 2 |
| Anti-C, Jkb | 1 |
| Anti-C, Leb | 1 |
| Anti-E, Kpa | 1 |
| Anti-c, Lea | 1 |
| Anti-e, Jka | 1 |
| Anti-e, Jkb | 1 |
| Anti-f, Lea | 1 |
| Anti-K, M | 1 |
| Anti-Fyb, Jka | 1 |
| Anti-Lea, N | 1 |
| Anti-Lea, Lua | 1 |
| Anti-Lua, Lub | 1 |
| Anti-Dia, S | 1 |
| Anti-E, Dib | 1 |
| Anti-E, Wra | 1 |
| Three antibodies | (No.=197) |
| Anti-C, e, Jka | 37 |
| Anti-E, c, Jka | 33 |
| Anti-E, c, Jkb | 30 |
| Anti-E, c, Lea | 24 |
| Anti-E, c, N | 9 |
| Anti-E, c, M | 8 |
| Anti-E, c, S | 8 |
| Anti-C, e, Jkb | 7 |
| Anti-E, c, Leb | 7 |
| Anti-E, c, Fyb | 6 |
| Anti-C, e, Dia | 5 |
| Anti-C, e, S | 4 |
| Anti-C, e, M | 3 |
| Anti-E, Jkb, M | 3 |
| Anti-C, e, Lea | 2 |
| Anti-E, c, P1 | 2 |
| Anti-E, c, Dia | 2 |
| Anti-C, e, Fyb | 1 |
| Anti-C, K, Jsa | 1 |
| Anti-E, Jka, S | 1 |
| Anti-c, e, S | 1 |
| Anti-Fya, M, S | 1 |
| Anti-Fyb, Jka, S | 1 |
| Anti-Fyb, Dia, Lua | 1 |
| Fourantibodies | (No.=9) |
| Anti-E, Jkb, Lea, M | 3 |
| Anti-E, c, Lea, Leb | 2 |
| Anti-C, E, c, e | 1 |
| Anti-C, e, K, Jkb | 1 |
| Anti-C, e, Fyb, S | 1 |
| Anti-E, c, Fyb, Jka | 1 |
The top 20 most commonly identified alloantibodies were listed. Antigen typing was performed on hundreds to thousands of RBC units for each antigen and antigen-negative frequencies were calculated (Table II).
Table II.
Top-20 alloantibodies and corresponding antigen(s)-negative frequencies
| Antibody (anti-) | Cases | Proportion | Antigen-typed cases | Antigen-typed units | Obtained units | Antigen(s)-egative frequency | Units required for typing* |
|---|---|---|---|---|---|---|---|
| E | 2,202 | 27.6% | 1,305 | 4,194 | 3,027 | 72.2%** | 2 |
| E, c | 1,792 | 22.5% | 1,343 | 4,251 | 2,957 | 69.6%** | 2 |
| C, e | 757 | 9.5% | 555 | 1,880 | 1,091 | 58.0%** | 2 |
| Le a | 618 | 7.7% | 110 | 451 | 313 | 69.4% | 2 |
| M | 383 | 4.8% | 197 | 1,902 | 415 | 21.8% | 5 |
| Jk a | 323 | 4.0% | 215 | 1,741 | 491 | 28.2% | 4 |
| Fy b | 283 | 3.5% | 228 | 1,001 | 743 | 74.2% | 2 |
| Le b | 278 | 3.5% | 62 | 319 | 163 | 51.1% | 2 |
| P1 | 202 | 2.5% | 137 | 731 | 421 | 57.6% | 2 |
| Di a | 116 | 1.5% | 37 | 135 | 98 | 72.6% | 2 |
| c | 113 | 1.4% | 99 | 425 | 283 | 66.6%** | 2 |
| Jk b | 94 | 1.2% | 67 | 811 | 185 | 22.8% | 5 |
| S | 87 | 1.1% | 61 | 272 | 180 | 66.2% | 2 |
| C | 67 | 0.8% | 29 | 94 | 46 | 48.9%** | 3 |
| Le a , Le b | 62 | 0.8% | 17 | 132 | 46 | 34.9% | 3 |
| e | 59 | 0.7% | 36 | 82 | 64 | 78.1%** | 2 |
| E, Fy b | 42 | 0.5% | 25 | 108 | 83 | 76.9%** | 2 |
| C, e, Jk a | 37 | 0.5% | 11 | 450 | 28 | 6.2%** | 17 |
| E, c, Jk a | 33 | 0.4% | 22 | 162 | 68 | 42.0%** | 3 |
| E, Jk a | 32 | 0.4% | 26 | 191 | 54 | 28.3%** | 4 |
Mean number of units needed to be typed in order to find one compatible unit.
Antigen(s)-negative frequencies including C, E, c, and/or e were overestimated because many blood banks preselected C-, E-, c-, and/or e-negative units through the Blood Information Sharing System (BISS) and performed retyping (see Table III).
RhCE antigen frequencies
In the BISS, information was available regarding 213,691 RBC units (Table III). RhCE frequencies were comparable between the BISS and the previous study using genotyping8. The antigen-negative frequencies based on the transfusion registry in the present study were overestimated (Table II).
Table III.
RhCE-negative frequencies from several data sources
| Study period | Present study - BISS June 2016 ~ June 2022 |
Present study - KRBP July 2013 ~ June 2022 |
Previous study* Not provided |
||||
|---|---|---|---|---|---|---|---|
| No. | Frequency | Required units** | No. | Frequency | No. | Frequency | |
| C- | 27,295/213,691 | 12.8% | 8 | 46/94 | 48.9% | 39/305 | 12.8% |
| E- | 104,984/213,691 | 49.1% | 3 | 3,027/4,194 | 72.2% | 159/305 | 52.1% |
| c- | 89,651/213,691 | 42.0% | 3 | 283/425 | 66.6% | 127/305 | 41.6% |
| e- | 19,539/213,691 | 9.1% | 11 | 64/82 | 78.1% | 35/305 | 11.5% |
| C-e- | 19,379/213,691 | 9.1% | 11 | 1,091/1,880 | 58.0% | 35/305 | 11.5% |
| E-c- | 88,993/213,691 | 41.6% | 3 | 2,957/4,251 | 69.6% | 126/305 | 41.3% |
Reference No. 8. Hong YJ et al., Ann Hematol, 2016.
Average number of units required for typing to obtain one antigen(s)-negative unit.
BISS: blood information sharing system; KRBP: Korean Rare Blood Program.
Survey results
Responses were collected from 124 workshop participants in 73 facilities which was more than the number of hospitals participating in the transfusion registry. For antibody screening, more than three quarters of facilities used two cells. Di(a+) cells were available in less than a quarter of facilities. More than 90% of individuals stated that a database including patient identifiable information is necessary (Table IV).
Table IV.
Survey results from 73 facilities and 124 participants
| Survey item | No. (%) |
|---|---|
| 1. Responses by facilities (No.=73) | |
| A) No. of screening cells | |
| 2 cells | 55 (75.3%) |
| 3 cells | 11 (15.1%) |
| Not applicable | 7 (9.6%) |
| B) Specific screening cell available | |
| Di(a+) | 18 (24.7%) |
| Mi(a+) | 2 (2.7%) |
| Not applicable | 55 (75.3%) |
| 2. Responses by participants (No.=124) | |
| A) Database requires patient identifier | |
| Necessary | 115 (92.7%) |
| Not necessary | 9 (7.3%) |
| B) Reference when searching antigen frequency | |
| Textbook10 | 102 (82.3%) |
| Relevant articles | 72 (58.1%) |
| BISS (for C, E, c, e) | 46 (37.1%) |
| Own database | 9 (7.3%) |
BISS: Blood Information Sharing System.
DISCUSSION
Overview of the study
In the present study, data accumulated on the Korean transfusion registry over 10 years were analyzed. This study provides statistics for the blood group antibodies, including antibody specificity and specific antigen-negative frequency. Of the 29 general hospitals that participated in the transfusion registry, 19 were superior general hospitals accredited by the Ministry of Health and Welfare in Korea, with a median of >1,000 beds. Many hospitals in densely populated urban areas, such as Seoul, Busan, and Gyeonggi, participated. Although there is a limitation derived from voluntary registration, the statistics provided by this study approximate the current status of blood group antibodies in Korea, considering the size and regional distribution of the participating hospitals.
Frequencies of RhCE antigens
The present (and several previous) studies showed that antibodies to RhCE antigens are commonly identified in Korea11,12. To predict the probability of obtaining antigen(s)-negative units, it is necessary to determine the true frequencies of RhCE antigens. In the present study, C-, E-, c-, and e- frequencies were calculated through the BISS, which has provided blood banks with RhCE types of RBC units since June 2016 (Table III). The frequencies were calculated using large amounts of data, and the results showed similar frequencies to the previous study8. The RhCE-negative frequencies calculated through the transfusion registry were overestimated because many blood banks preselected antigen-negative units with reference to the BISS and retyped them. Therefore, RhCE frequencies based on the BISS currently represent the most reliable data, and it is recommended that they be referred to when searching for RhCE frequencies in the Korean population.
Current status and considerations for antibody screening
According to the United States Food and Drug Administration, for antibody screening, reagent RBCs with no fewer than two donor sources should be used and should include the following antigens: D, C, E, c, e, K, k, Fya, Fyb, Jka, Jkb, Lea, Leb, P1, M, N, S, s13,14. Among 73 facilities that attended KRBP workshop, 75.3% used cells from two sources and 15.1% used cells from three sources for antibody screening, and Di(a+) and Mi(a+) cells were available in 24.7% and 2.7% of facilities, respectively (Table IV). Anti-Dia was the 10th most commonly identified alloantibody, accounting for 1.5% of all alloantibodies (Table II). Dia antigen is a low-prevalence antigen that differs in frequency according to ethnicity. The gene frequency in East Asians is 1–5%, but DI*A alleles have never been found in individuals of unmixed European descent15. Considering the clinical significance of anti-Dia, it is recommended that Di(a+) cells be added to routine antibody screening in Korea and other Asian countries. Meanwhile, the Mia antigen (MNS7), previously known as Miltenberger subsystem of MNS blood group, is a low-prevalence antigen except in Chinese (7%) and Southeast Asian (10%) populations16. In Korea, foreigners can donate blood if they meet the following conditions: lived in Korea for >1 year; can communicate in Korean or through an interpreter; and have identification cards verifying residency in Korea17. As the number of immigrants from these countries is increasing each year, the chances of Mia antigen exposure and anti-Mia formation will increase18. There have been two cases of anti-Mia in Korea19,20. Blood banks should prepare for a time when Mi(a+) cells become necessary.
Red cell antibodies in Eastern countries
There are several previous studies related to red cell antibodies in Eastern countries. The common point of the present study in Korea and the previous studies in China, Thai, India, and Iran was that the anti-E, followed by antibodies against other RhCE antigens, was the most common21–24. There were differences between Eastern countries. The frequency of the K antigen was 12% in Iranian Jews and as high as 25% in Arabs, whereas it was very rare in Asians25. Unlike in China21, Thailand22, and Korea (Table I), anti-K was the second most common antibody in India and was considered important due to high immunogenicity, as in Western countries23,26. Another difference between Eastern countries lies in MNS system: Mia (mentioned in the paragraph above) and Mur antigens. While the Mur antigens have very low frequency in most ethnic groups, the frequency in China and Thailand was is 6–9%25 and anti-Mur was identified21. These racial differences should be taken into account when analyzing blood types of immigrants.
Features of registries in the field of transfusion medicine
To derive a model for registries in the field of transfusion medicine, features of several transfusion-related registries and databases were reviewed (Table V)1,2,5,6,27–37.
Table V.
Features and available information of transfusion-related registries and databases
| Classification | Features and available information | Reference |
|---|---|---|
| Regional registry or centralized transfusion service | Patient identifying records among hospitals - Prevention of DHTR - Additional detection of WBIT errors |
5, 6, 27, 28, 29, 30, 31 |
| Databases including transfusion reactions | Transfusion reactions Donor-to-recipient information |
29, 36, 37 |
| Cloud-based search engine | Rapid access to historical antigen information for blood products in hospital inventories | 32, 33 |
| Rare donor program | Information on rare donors (e.g. high-incidence antigen-negative) Cryopreservation of rare units |
1, 2 |
| Transfusion registry in Korea | Antibody statistics - Frequently identified antibodies - Geographic distribution - Specific antigen-negative frequency - Average units that need to be antigen-typed |
Present study |
| Web-based system in Korea | RhCE phenotype of units in inventory RhCE phenotype frequency |
34 |
| D-- donor registry in Korea | D-- donor | 34, 35 |
DHTR: delayed hemolytic transfusion reaction; WBIT: wrong blood in tube.
Several registries used patient identifying records; TRIX in the Netherlands5,6, Centralized Transfusion Services (CTS) in Pittsburgh27,28, and the centralized transfusion service database (CTS-D) in Seattle29. Patient records, such as ABO and Rh type, unexpected antibodies, and special needs, are tracked when a patient visits more than one hospital within the CTS system. When using the historical ABO type data in the CTS, the detection rate of wrong blood in tube (WBIT) errors was 38% higher than when it was not used28. The study based on the CTS-D revealed that the proportion of patients with clinically significant antibodies was greater in the group using more than one hospital than in the group using one hospital (7.11 vs 3.97%)29.
Registries with patient-trackable data also prevent DHTR. A web-based regional registry was developed to prevent DHTR due to antibody evanescence and fragmentation of records among hospitals. During the first year of operation, this registry prevented four cases of possible DHTR30. As another example, in the United States of America, a case was reported in which antibody screening was negative but a history of anti-Jka was identified in the local antibody registry. The patient was transfused with a total of 16 Jk(a-) units and no hemolytic transfusion reaction occurred31.
Facilitating the detection of specific antigen-negative blood units in the hospital inventory streamlines the blood banks. A cloud-based search engine enabled blood banks to access antigen information of the blood products and to prepare antigen-negative units rapidly32,33. In Korea, using BISS (which provides data on the RhCE types of blood units), the RhCE phenotype of a specific blood unit in the inventory can be searched and selected using the blood unit identification number. In addition, rare donors with the D--phenotype (lack of RhCE antigens) have been found and a patient with the D-- phenotype transfused with RBC units from the D--phenotype donor34,35.
As shown by these studies, registries that can resolve the interhospital fragmentation of records will be able to improve their transfusion services. The majority of KRBP workshop participants (92.7%) agreed on the necessity of a patient identifying database (Table IV). In future, it will be necessary to establish a database including PII to track the history of antibodies.
Limitations
The present study had several limitations. Firstly, although the blood banks may have been able to save time by referring to the specific antigen-negative frequencies and the number of units required to obtain compatible blood units in Tables II and III, this was not investigated. Secondly, participation in the transfusion registry was voluntary. A significant proportion of large hospitals participated; smaller hospitals were less likely to participate. If the practical benefits of the registry are publicized through research and promotion, the participation rate of hospitals would increase. Lastly, due to the absence of PII, antibodies from the same patient may be registered in duplicate, especially in patients receiving frequent transfusions along with pre-transfusion tests including antibody identification. Antibody identification interval in the same patient ranges from 1 to 6 months in Korea depending on the hospital. Although it is several months apart, there may be overlapping registrations.
CONCLUSIONS
In conclusion, distributions of antibodies and antigen frequencies were estimated through a website-based transfusion registry functioning as a blood group database. The various features of the registries discussed in this study will serve as references for individuals operating transfusion-related registries.
ACKNOWLEDGEMENTS
We are grateful to all the blood banks participating in the Korean Rare Blood Program (KRBP) for their cooperation and for adding data to the transfusion registry.
Footnotes
ETHICAL CONSIDERATION: This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital in 2017 and is being extended on an annual basis (approval number: B-1705-396-109). Exemption of written informed consent was approved by the Institutional Review Board (above). The research was conducted ethically, with all study procedures being performed in accordance with the requirements of the World Medical Association’s Declaration of Helsinki.
AUTHORS’ CONTRIBUTIONS: DWS wrote the first draft of the manuscript. JH and EYS collected, analyzed, and interpreted the data. YJH and KUP conceptualized and designed the study, and revised the manuscript critically. All Authors were involved in preparing the final manuscript and approved the submitted version.
The Authors declare no conflicts of interest.
Commented by doi 10.2450/BloodTransfus.753
FUNDING: This work was supported by the Research Fund for Operation of the Korean Rare Blood Program (12228045600) from the National Institute of Organ, Tissue, and Blood Management, Ministry of Health and Welfare, Republic of Korea.
REFERENCES
- 1.Morelati F, Arnaboldi P, Barocci F, Bodini U, Boiani E, Bresciani S, et al. Strategies for the transfusion of subjects with complex red cell immunisation: the Bank of rare blood donors of the Region of Lombardy. Blood Transfus. 2007;5:217–226. doi: 10.2450/2007.0016-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Revelli N, Villa MA, Paccapelo C, Manera MC, Rebulla P, Migliaccio AR, et al. The Lombardy Rare Donor Programme. Blood Transfus. 2014;12(Suppl 1):s249–s255. doi: 10.2450/2013.0182-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tormey CA, Hendrickson JE. Transfusion-related red blood cell alloantibodies: induction and consequences. Blood. 2019;133:1821–1830. doi: 10.1182/blood-2018-08-833962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powell Z, Jiang N, Shrestha R, Jackson DE. Would a national antibody register contribute to improving patient outcomes? Blood Transfus. 2022;20:132–142. doi: 10.2451/2021.0421-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Gammeren AJ, van den Bos AG, Som N, Veldhoven C, Vossen RCRM, Folman CC. A national Transfusion Register of Irregular Antibodies and Cross (X)-match Problems: TRIX, a 10-year analysis. Transfusion. 2019;59:2559–2566. doi: 10.1111/trf.15351. [DOI] [PubMed] [Google Scholar]
- 6.Hauser RG, Hendrickson JE, Tormey CA. TRIX with treats: the considerable safety benefits of a transfusion medicine registry. Transfusion. 2019;59:2489–2492. doi: 10.1111/trf.15449. [DOI] [PubMed] [Google Scholar]
- 7.Korean Red Cross [Internet] Blood information 198 (June 2016 issue) - Korean Red Cross Blood Services. [Accessed on 29/3/2023]. Available at: https://www.bloodinfo.net/file_util.do?action=download&display_filename=%ED%98%88%EC%9%A1%EC%A0%95%EB%B3%B4%206%EC%9B%94%ED%98%B8(%EC%B5%9C%EC%A2%85).pdf&filepath=upload_board/33/51/336851/50785/ [in Korean.]
- 8.Hong YJ, Chung Y, Hwang SM, Park JS, Kwon JR, Choi YS, et al. Genotyping of 22 blood group antigen polymorphisms and establishing a national recipient registry in the Korean population. Ann Hematol. 2016;95:985–991. doi: 10.1007/s00277-016-2645-7. [DOI] [PubMed] [Google Scholar]
- 9.Yang HS, Chun S, Lee SA, Kwon JR, Choi YS, Kim JN, et al. Transfusion atrategy of RhD-negative/variant patients in the Korean population. Lab Med Online. 2017;7:89–93. doi: 10.3343/lmo.2017.7.3.89. [DOI] [Google Scholar]
- 10.Han KS, Park KU, Song EY. Transfusion Medicine. 4th ed. Seoul: Korea Medical Book Publisher; 2014. [Google Scholar]
- 11.Chung Y, Kim JS, Youk HJ, Kim H, Hwang SH, Oh HB, et al. Relative immunogenicity of blood group antigens: first report in a Korean population. Transfus Apher Sci. 2022;62:103585. doi: 10.1016/j.transci.2022.103585. [DOI] [PubMed] [Google Scholar]
- 12.Shin DW, Kim H, Chung Y, Kim JN, Hong YJ, Park KU, et al. Establishment and utilization of a transfusion recipient registry in Korea: estimating the frequencies of specific antigen-negative blood units. Am J Clin Pathol. 2018;150:154–161. doi: 10.1093/ajcp/aqy044. [DOI] [PubMed] [Google Scholar]
- 13.Cohn CS, Delaney M, Johnson ST, Katz LM. Technical Manual. 20th ed. Bethesda, MD: AABB; 2020. [Google Scholar]
- 14.www.ecfr.gov [Internet] 21 CFR 660. 33. Testing of source material. (up to date as of 12/02/2022) [Accessed on 29/3/2023]. Available from: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-F/part-660/subpart-D/section-660.33/
- 15.Figueroa D. The Diego blood group system: a review. Immunohematology. 2013;29:73–81. [PubMed] [Google Scholar]
- 16.Agrawal S, Chowdhry M. A case report on anti-Mia antibody in a multi-transfused patient from India. Transfus Apher Sci. 2019;58:625–627. doi: 10.1016/j.transci.2019.08.027. [DOI] [PubMed] [Google Scholar]
- 17.blog.naver.com/blood_info [Internet] Donor Eligibility for Foreign Donors - Official Blog of Korean Red Cross Blood Services (updated in March 4th, 2021) [Accessed on 29/3/2023]. Available from: https://blog.naver.com/blood_info/222264284028/ [in Korean.]
- 18.Kim H, Shin KH, Kim HH, Lee HJ. Perceptions and experiences of migrants in Korea regarding blood donation in association with sociodemographic status. Ann Lab Med. 2022;42:258–267. doi: 10.3343/alm.2022.42.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SE, Kim J, Yoon MS, Song EY, Han KS. A case of hemolytic disease of the newborn due to anti-Mia antibody. Korean J Blood Transfus. 2004;15:231–235. [in Korean.] [Google Scholar]
- 20.Kim HH, Park TS, Oh SH, Lee EY, Son HC. Naturally-occurring anti-Mia in a 16-year-old Korean man: a case study and a review of the literature. Korean J Lab Med. 2004;24:146–148. [in Korean.] [Google Scholar]
- 21.Xu P, Li Y, Yu H. Prevalence, specificity and risk of red blood cell alloantibodies among hospitalised Hubei Han Chinese patients. Blood Transfus. 2014;12:56–60. doi: 10.2450/2013.0013-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nathalang O, Sriwanitchrak P, Tubrod J, Kupatawintu P. Antibody elutions in Thai patients with a positive direct antiglobulin test. Blood Transfus. 2011;9:306–310. doi: 10.2450/2010.0051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta R, Singh DK, Singh B, Rusia U. Alloimmunization to red cells in thalassemics: emerging problem and future strategies. Transfus Apher Sci. 2011;45:167–170. doi: 10.1016/j.transci.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Kosaryan M, Mahdavi MR, Roshan P, Hojjati MT. Prevalence of alloimmunisation in patients with beta thalassaemia major. Blood Transfus. 2012;10:396–397. doi: 10.2450/2012.0072-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reid ME, Lomas-Francis C, Olsson ML. The Blood Group Antigen FactsBook. 3rd ed. Academic Press; 2012. [Google Scholar]
- 26.Pujani M, Pahuja S, Dhingra B, Chandra J, Jain M. Alloimmunisation in thalassaemics: a comparison between recipients of usual matched and partial better matched blood. An evaluation at a tertiary care centre in India. Blood Transfus. 2014;12(Suppl 1):s100–s104. doi: 10.2450/2012.0154-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yazer M. The Pittsburgh centralized transfusion model: less is more. Transfusion. 2007;47(Suppl 2):164S–168S. doi: 10.1111/j.1537-2995.2007.01378.x. discussion 182S–183S. [DOI] [PubMed] [Google Scholar]
- 28.MacIvor D, Triulzi DJ, Yazer MH. Enhanced detection of blood bank sample collection errors with a centralized patient database. Transfusion. 2009;49:40–43. doi: 10.1111/j.1537-2995.2008.01923.x. [DOI] [PubMed] [Google Scholar]
- 29.Delaney M, Dinwiddie S, Nester TN, Aubuchon JA. The immunohematologic and patient safety benefits of a centralized transfusion database. Transfusion. 2013;53:771–776. doi: 10.1111/j.1537-2995.2012.03789.x. [DOI] [PubMed] [Google Scholar]
- 30.Schwickerath V, Kowalski M, Menitove JE. Regional registry of patient alloantibodies: first-year experience. Transfusion. 2010;50:1465–1470. doi: 10.1111/j.1537-2995.2010.02629.x. [DOI] [PubMed] [Google Scholar]
- 31.Mathur G, Wilkinson MB, Island ER, Menitove JE, Tilzer L. A case for a national registry of red blood cell antibodies. Vox Sang. 2022;117:738–740. doi: 10.1111/vox.13250. [DOI] [PubMed] [Google Scholar]
- 32.Denomme GA, Reinders S, Bensing KM, Piefer C, Schanen M, Curnes J, et al. Use of a cloud-based search engine of a centralized donor database to identify historical antigen-negative units in hospital inventories. Transfusion. 2020;60:417–423. doi: 10.1111/trf.15638. [DOI] [PubMed] [Google Scholar]
- 33.Knier M, Schanen M, Piefer C, Bensing KM, Marchan M, Anani WQ, et al. How to use a cloud-based search engine of a centralized donor database to identify historical antigen-negative units in hospital inventories. Transfusion. 2020;60:414–416. doi: 10.1111/trf.15641. [DOI] [PubMed] [Google Scholar]
- 34.Lee MK, Jung SY, Kim JU, Kim JP, Kim DH, Park JR. -D-/-D- Phenotype frequency among Korean donors. Korean J Blood Transfus. 2018;29:182–187. doi: 10.17945/kjbt.2018.29.2.182. [DOI] [Google Scholar]
- 35.Han JH, Kwon SY, Jekarl DW. Transfusion of a D--phenotype patient using the Rare Blood Donor Registry of the Korean Red Cross Blood Services. Blood Transfus. 2021;19:91–92. doi: 10.2450/2020.0264-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edgren G, Rostgaard K, Vasan SK, Wikman A, Norda R, Pedersen OB, et al. The new Scandinavian Donations and Transfusions database (SCANDAT2): a blood safety resource with added versatility. Transfusion. 2015;55:1600–1606. doi: 10.1111/trf.12986. [DOI] [PubMed] [Google Scholar]
- 37.Kleinman S, Glynn SA. Database research in transfusion medicine: The power of large numbers. Transfusion. 2015;55:1591–1595. doi: 10.1111/trf.13139. [DOI] [PubMed] [Google Scholar]