Abstract
Thrombocytopenia (defined as a platelet count <150×109/L) is a common condition in preterm neonates and may occur in 18–35% of all infants admitted to the Neonatal Intensive Care Unit (NICU). Neonatal platelet functionality in terms of reactivity is often described as reduced compared to adults, even in healthy, term neonates. However, this platelet “hyporeactivity” does not correspond to a global functional impairment of the normal delicately balanced neonatal hemostatic system. The extent to which neonatal thrombocytopenia and platelet hyporeactivity contribute to the bleeding risk in preterm neonates remains unknown. Prophylactic platelet transfusions are often administered to them to reduce the risk of bleeding. However, recent literature indicates that adopting a higher platelet transfusion threshold than a lower one results in significantly higher death rates or major bleeding and can be harmful. Although the mechanism by which this occurs is not entirely clear, a mismatch between adult transfused platelets and the neonatal hemostatic system, as well as volume overload, are speculated to be potentially involved. Therefore, future research should consider novel transfusion products that may be more suitable for premature neonates. Blood products derived from umbilical cord blood (UCB) are promising, as they might perfectly match neonatal blood features. Here, we discuss the current knowledge about UCB-derived products, focusing on UCB-derived platelet concentrates and their potential for future clinical application. We will discuss how they may overcome the potential risks of transfusing adult-derived platelets to premature infants while maintaining efficacy.
Keywords: newborn, platelets, thrombocytopenia, transfusion, cord blood
INTRODUCTION
Thrombocytopenia (defined as a platelet count <150×109/L) is a common finding in preterm neonates, as it may occur in 18–35% of infants admitted to the Neonatal Intensive Care Unit (NICU) and 73% of extremely low birth weight infants (ELBW)1,2. In term neonates, thrombocytopenia is quite rare, with a prevalence of 1–5%1,3. Thrombocytopenia can be divided into early- and late-onset, depending on the onset time before or after the first 72 hours of life. Early-onset thrombocytopenia is often associated with intrauterine growth retardation (IUGR), maternal hypertension, or pre-eclampsia3–5. In a well-appearing term neonate, early-onset thrombocytopenia is usually due to fetal neonatal alloimmune thrombocytopenia (FNAIT) or autoimmune thrombocytopenia (maternal systemic lupus erythematosus, immune thrombocytopenia)3,6–8. Late-onset thrombocytopenia is often due to sepsis or necrotizing enterocolitis and may be isolated or part of disseminated intravascular coagulation (DIC)3,4,9,10.
Regarding platelet function, neonatal platelet “hyporeactivity” does not correspond to global hemostatic functional impairment in normal conditions11–13. On the contrary, this feature seems to be an integral part of a delicately balanced neonatal hemostatic system10,14,15. The extent to which thrombocytopenia and platelets hyporeactivity, which are more frequent in preterm infants, contribute to the bleeding risk in this population remains unclear, including the impact on the incidence of intraventricular hemorrhage10,14. Prophylactic platelet transfusions are often administered to preterm neonates to reduce the risk of bleeding15–17. However, recent studies demonstrated that adopting a higher vs a lower threshold to transfuse platelets does not prevent bleeding and can be associated with harmful consequences, such as death and major bleeding10,18–20. Therefore, neonatologists should try to lower the transfusion threshold in line with those used in the studies. Research should focus on identifying thrombocytopenic patients with the highest bleeding risk and optimizing transfusion products for preterm neonates. Blood products derived from umbilical cord blood (UCB) might have a future role in this context.
In this narrative review, we will address the current knowledge and the unsolved questions about the differences between neonatal and adult platelets, neonatal thrombocytopenia, platelet transfusions, and their relationship with bleeding. In addition, we will discuss the current gaps in knowledge about transfusion risk/benefit ratio and explore the possible future of neonatal transfusion medicine, focusing on cord blood-derived products as a promising source of blood products for neonates.
PLATELET COUNT AND FUNCTION: WHAT ARE THE DIFFERENCES BETWEEN NEONATES AND ADULTS?
Hemostasis in fetal and neonatal life is a complex and dynamic process, with several differences compared to adult life11. At birth, the hemostatic system still needs to be wholly developed, and platelet numbers and functional impairment have been broadly described11.
Platelet count gradually increases during fetal life and usually reaches values similar to those in post-natal life (>150×109/L) in the third trimester of pregnancy. Thrombocytopenia (a platelet count <150×109/L) is hence a rare finding in term newborns1,3. In contrast, thrombocytopenia is more common in preterm neonates and may occur in up to 18–35% of infants admitted to NICU and 73% of ELBW infants (Table I)1,2. These features may be attributed to the differences in megakaryocytopoiesis and thrombocytopoiesis among neonates and adults. Thrombopoietin is higher in term and preterm neonates than adults, and megakaryocyte progenitors proliferate faster, producing larger megakaryocyte colonies.
Table I.
Differences between neonatal and adult platelets
| Neonates | Adults | |
|---|---|---|
| Platelet count |
|
|
| Megakaryocytopoiesis and thrombocytopoiesis features | ||
| Platelet function | ||
| Hemostatic features |
MKs: megakaryocytes; ELBW: extremely low birth weight; TPO: thrombopoietin; MCV: mean corpuscular volume; HCT: hematocrit; NICU: Neonatal Intensive Care Unit; vWf: von Willebrand factor.
These megakaryocytes are smaller, have lower ploidy, and may be more immature than those derived from adult progenitors. Otherwise, neonatal megakaryocytes are cytoplasmically more active than adult ones, meaning that megakaryocytopoiesis is characterized by the rapid proliferation of megakaryocytes with full cytoplasmic maturation at low ploidy levels. This mechanism is thought to provide the ability to expand bone marrow rapidly and blood volume without determining thrombocytosis (Table I)1,21.
Platelet function also differs between neonates and adults. Neonatal platelets have been described as hyporeactive compared to adult ones11–13. Cord blood-derived platelets showed reduced responsiveness to agonists such as adenosine 5’-diphosphate (ADP), epinephrine, collagen, thrombin, and thromboxane1,21,22. This probably depends on fewer adrenergic receptors, different pathways downstream from the receptors, and impaired calcium mobilization1,21,22. The agonist-induced secretion of platelet granule content is reduced in both term and preterm newborns, especially due to the lower content of dense granules and the reduced exocytosis of alpha granules10 (Table I).
However, this platelet hyporeactivity would not correspond to global functional hemostatic impairment, as healthy full-term newborns do not show clinical evidence of hemostatic disruption or a tendency to bleed. This clinical observation has been supported by bleeding and closure times which are even shortened in newborns compared to adults12,13,18,21. This paradoxical finding may be attributed to higher hematocrit levels, mean corpuscular volume, von Willebrand factor (vWf) concentrations, and longer vWf polymers12,13,18. All these factors provide adequate primary hemostasis and counterbalance platelet hyporeactivity in newborns (Table I)12,13,18,21.
However, in preterm neonates, platelet hyporeactivity seems to be more pronounced, especially among the most premature ones (<30 weeks of gestational age), with an inverse correlation with gestational age1,12,21,23. Similarly, closure and bleeding times appear to be inversely correlated with gestational age, but even in most preterm newborns, their values are not longer than in adults1,18,24. These alterations in neonates are predominantly present during the first week of life, with conflicting results on the exact timing of “normalization” in platelet function1,12,18,23,25–27. How thrombocytopenia and platelet hyporeactivity, typical of ELBW neonates, contribute to the bleeding risk in this population remains unknown10,14. This feature seems to be an integral part of a delicate balanced hemostatic system rather than a developmental deficiency10,14. In summary, the primary hemostasis differs in term and preterm newborns from adults but shows a delicate balance to prevent bleeding and thrombosis10,15.
Other parameters may help evaluate the bleeding risk beyond platelet count and function. The immature platelet fraction (IPF) represents the younger and reticulated platelets released from the bone marrow in response to a thrombopoietic stimulus6. Similarly to reticulocytes, IPF can represent the production rate and the state of thrombocytopoiesis. IPF can be expressed as a percentage of the circulating platelets (IPF%) or as an absolute number (IPF#)6. IPF can be measured automatically with the cell blood count without additional sampling. Reference ranges for non-thrombocytopenic newborns have already been established, with gestational age and post-natal day stratification. In general, IPF% increases in the first 2 weeks of life and returns to baseline values at the end of the first month. IPF% and IPF# are higher in premature infants, consistently with higher thrombopoietic activity during fetal life. IPF is significantly higher in thrombocytopenic newborns, with an inverse correlation to the severity of thrombocytopenia28,29. In addition, IPF could help predict the evolution of thrombocytopenia, as an increase above 8% predicts an increase in the platelet count on the following day6,28. As such, this parameter may be used in transfusion decision-making.
Moreover, IPF# may help discern thrombocytopenia derived from increased peripheral consumption from reduced production6. For example, thrombocytopenic preterm infants with necrotizing enterocolitis or sepsis showed decreased IPF#, as the pathophysiology of thrombocytopenia might be explained by reduced bone marrow platelet production6,30. The NEOHAT-2 study, an ongoing multicenter trial (NCT04598750), is currently evaluating the role of IPF as a predictor of bleeding risk in preterm newborns.
THROMBOCYTOPENIA AND BLEEDING IN NEONATES : IS THERE A CLEAR ASSOCIATION?
Approximately 6–11% of neonates admitted to NICU, mostly preterm, experience major bleeding, including intracranial hemorrhage (primarily intraventricular hemorrhage [IVH])9,10,31–33. These bleedings are multifactorial events, with several risk factors with established roles and others with a less clear impact on the bleeding risk. Thrombocytopenia, such as respiratory and hemodynamic instability, is often considered a risk factor for bleeding2,31–33.
In preterm neonates, a causal relationship between thrombocytopenia and IVH has never clearly been demonstrated9,32–36. They are often temporally associated, but this does not establish a causal association3,31,33,34,37. Neonates with severe thrombocytopenia often do not experience bleeding episodes, and inversely, up to 25–38% of premature newborns with IVH are not thrombocytopenic3,33,37. In the PlaNet-study, 91% of the neonates enrolled, despite thrombocytopenia <60×109/L, had only minor bleeding or did not have bleeding at all32. Notably, both IVH and thrombocytopenia are usually asymptomatic and detected by routine tests. This feature makes it even more challenging to establish a causal relationship between them since a cranial ultrasound is often performed once severe thrombocytopenia is detected (to rule out IVH) and, vice-versa, a platelet count is measured once an IVH is detected (to rule out thrombocytopenia).
Moreover, the progression of IVH does not correlate with the severity of thrombocytopenia and platelet count trend32,34. The situation gets even more complicated in simultaneous events such as sepsis, which may determine both thrombocytopenia and IVH without a direct connection. Lastly, a severe IVH may lead to platelet consumption and severe thrombocytopenia, making it often very challenging, if not impossible, to determine which occurred first: a classic “chicken or the egg” discussion.
If thrombocytopenia itself is not a sufficient marker of bleeding risk, other new factors should be considered to identify patients who would benefit from a platelet transfusion, i.e., IPF or functional tests such as PFA-10038,39.
PLATELET TRANSFUSIONS IN NEONATES : DO THEY GENUINELY PREVENT BLEEDING?
In this context of thrombocytopenia and platelet “hyporeactivity”, neonatologists often prescribe platelet transfusions, especially in preterm neonates, hoping to prevent or reduce the risk of bleeding. Approximately 80% of platelet transfusions and up to 98% are administered prophylactically to newborns without bleeding, but evidence about their usefulness is limited and controversial15–17.
Platelet transfusions are given to over 75% of thrombocytopenic newborns with a platelet count <50×109/L, with different thresholds across hospitals and countries9,32,33. In Europe, a platelet threshold of 25×109/L is usually adopted for stable infants without evidence of bleeding. In the United States, thresholds are generally higher, with transfusions given when the platelet count falls below 50×109/L or even 100×109/L9,40. This variability of transfusion practice worldwide is due to the lack of consensus and considerable variation in international guidelines40,41. A recent survey from Neonatal Transfusion Network led in European NICUs showed that 47–57% of NICUs use platelet count thresholds above 25×109/L for stable non-bleeding infants42.
A recent rial of platelet transfusion threshold, the PlaNeT-2 trial, randomized preterm neonates to receive platelet transfusion with a threshold of 25×109/L (restrictive group) or 50×109/L (liberal group). In the liberal group, 90% of neonates received at least one transfusion, against 53% of neonates in the restrictive group. Surprisingly, the rate of death or major bleeding within 28 days and the incidence of bronchopulmonary dysplasia were significantly lower in the restrictive group19. The authors of this trial suggested then adopting a threshold of 25×109/L platelets to transfuse non-bleeding infants and a threshold of 50×109/L in bleeding infants, irrespective of the clinical condition19. However, some limitations have to be noticed: only 37% of the infants were randomized by day 5 from birth, which is the higher bleeding risk period, and almost 40% received one or more transfusions before randomization22,43.
Another randomized trial reported no difference between the effects of two transfusion thresholds on time needed for a patent ductus arteriosus to close in preterm neonates. Moreover, the liberal transfusion group (transfused when platelet count was <100×109/L) had a higher incidence of IVH than the restrictive group (cut-off for transfusion <20×109), respectively 41 vs 9.1%20,22,44–46. That considerable difference was not confirmed for high-grade IVH. Interestingly, the cumulative volume administered was an independent predictor of IVH, with a 4.5% odd for every extra mL/kg transfused. The authors assumed that this higher rate of IVH may be due to a sudden increase in circulating volume during the transfusion, resulting in cerebral blood flow fluctuation20.
These studies demonstrate that using a higher threshold to transfuse platelets does not prevent bleeding and can even be associated with harmful consequences and possibly increase the risk of bleeding10,18–20. Therefore, the dogma that prophylactic platelet transfusions prevent bleeding in this patient group may be more intricate than we have always assumed.
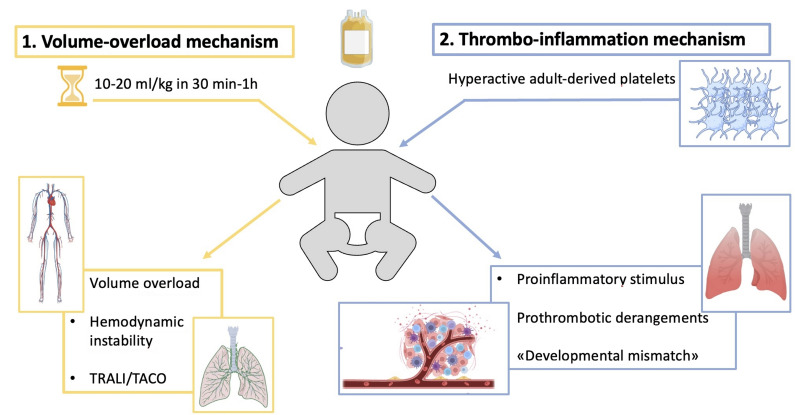
The exact explanation for the contra-intuitive results of the PlaNet-2 trial still needs to be discovered. Two mechanisms have been suggested to explain this unexpected result based on either transfusion volume overload or transfusion-related inflammatory reaction.
Volume overload mechanism. The volume of a neonatal platelet transfusion is usually 10–20 mL/kg, approximately three times the standard volume per kg transfused to an adult receiving a unit of platelets (depending on patient weight). The volume transfused to neonates corresponds to about 15–20% of the circulating blood volume of a preterm newborn22. Platelet concentration in the transfusion bags is about 800–1,600×109/L. In contrast to red blood cell (RBC) transfusions, administered slowly over 3–4 hours (approximately 5 mL/kg/hour), platelet transfusions are administered relatively rapidly over 30 minutes to 1 hour (about 10–20 mL/kg/hour), and sometimes even more quickly. This rapid volume expansion in an otherwise normovolemic neonate may have detrimental effects on a background of vascular fragility and hemodynamic instability, typical of most preterm neonates, and may, in turn, contribute to the pathogenesis of IVH (Figure 1)22. Transfusion-related acute lung injury (TRALI) and transfusion-associated circulatory overload (TACO) are other transfusion-associated adverse effects. They are not well-defined in newborns. The pathogenesis is still unclear, but the clinical presentation includes acute respiratory distress with hypoxemia and pulmonary edema. Pediatric patients might be at higher risk of TRALI and TACO, but data on newborns are scanty, with these conditions probably under-reported in this population due to the lack of a clear definition47.
Thrombo-inflammation mechanism. Platelets have an important immunologic and inflammatory role, as they may determine immune activation mediated by cytokines and chemokines through surface receptors or the release of the granular content14. Neonatal platelets exhibit a reduced ability compared to adult platelets in activating immune cells mediated by the reduced levels of P-selectin. In this context, platelet transfusions from adult donors may act like a pro-inflammatory and pro-thrombotic trigger and lead to the “developmental mismatch” between neonatal and adult blood14,19. The “developmental mismatch” involves the disruption of the normal neonatal balance, in which a relative procoagulant blood phenotype probably counterbalances the relative defective platelets functionality. Pro-inflammatory reactions secondary to the transfusion, platelet-derived reactive oxygen species, and proangiogenic factors might damage the lung capillary bed, contributing to bronchopulmonary dysplasia, as found in the PlaNeT-2 trial19. Furthermore, when adult “hyperreactive” platelets are transfused into neonatal blood, they may lead to thrombotic complications and fail to prevent bleeding episodes48 (Figure 1).
Figure 1. The two possible harmful pathogenetic effects of transfusing adult-derived platelets into neonatal blood are represented.
TACO: transfusion-associated circulatory overload; TRALI: transfusion-related acute lung injury. Created with BioRender.com.
An in vitro study showed that if cord blood or adult-derived platelets are mixed with cord or adult thrombocytopenic blood, the adult-derived platelets were more reactive than cord-derived platelets in response to various agonists, whichever type of blood they were mixed with49. However, when adult platelets were placed into cord blood, PFA-100 closure times were shorter and clot strength higher than when they were placed into neonatal blood49. Moreover, adult donor animal-derived platelets injected in the fetal yolk sac circulation in a murine model determined spontaneous platelet adhesion and platelet aggregate formation at multiple sites of the yolk sac. This supports the hypothesis that this “developmental mismatch” may disrupt the hemostatic balance of newborns in a prothrombotic direction22,50.
In conclusion, through the two mechanisms mentioned above (volume overload and thrombo-inflammation), adult-derived platelet transfusion may increase the risk of bleeding, thrombosis and other adverse outcomes.
In summary, given all the differences between neonatal and adult platelet activity and functionality, the potential for an adverse impact of transfusing adult platelets to preterm infants has to be considered. Therefore, in addition to possible further lowering of the transfusion threshold, future research might focus on novel transfusion products which are potentially less harmful and more suitable for preterm neonates.
INNOVATIVE STRATEGIES: WHAT IS THE ROLE OF CORD BLOOD-DERIVED PRODUCTS NOW AND IN THE NEAR FUTURE ?
In recent years, new technologies have been searched to improve the quality of platelet products for neonates, such as modification of adult platelets by pre-treatment with specific neonatal-mimicking inhibitors or age-matched in vitro production of platelet concentrates48.
In this context, blood products derived from UCB have been studied and developed for several clinical applications51–54. UCB is mainly used for hematopoietic stem cell transplantation for children and adults with hematological diseases. However, in about 80% of cases, donated units are discarded due to insufficient stem cells51,54. In these cases, novel clinical applications have been considered.
Due to the high hemoglobin content, UCB has been considered a source of RBCs for transfusion in newborns. It has been studied in many research projects, both as whole blood and as RBCs concentrate, for autologous or allogeneic use, in patients of any age. Autologous UCB transfusions have been studied for perinatal transfusion needs54–62. These studies enrolled newborns who required cardiac surgery soon after birth or premature newborns54–62. Results have been mainly positive, with a hematocrit or hemoglobin increase similar to allogeneic transfusions and no reported transfusion-related side effects54–62. In a study by Kotowshi et al., prematurity-related complications (bronchopulmonary dysplasia, IVH, necrotizing enterocolitis, respiratory distress syndrome) were reduced in the group transfused with autologous transfusions62. Clinical application of autologous UCB transfusions is limited, as their volume is usually insufficient to satisfy the whole requirement of anemia of prematurity. In the studies by Brune, Jansen and Khodabux, the need for additional allogeneic transfusions has been reported between 50% and 70% of enrolled patients, the more likely the lower the birth weight or the gestational age were56,63,64. Autologous UCB transfusion can be more suitable for patients requiring surgery after birth or for priming the cardiovascular bypass54,61. These results have been mainly reported before the spreading of strategies that limit iatrogenic blood losses, such as delayed cord clamping, so that they might be outdated nowadays54,61.
Allogeneic umbilical cord blood transfusions were then explored for anemia of prematurity, as they contain mainly fetal hemoglobin (HbF), similar to premature newborns55. Adult Hb, provided with adult-derived RBC transfusions, has a lower oxygen affinity and a higher release of oxygen to tissues. This mechanism may increase reactive oxygen species, contributing to the pathogenesis of retinopathy of prematurity, bronchopulmonary dysplasia, endothelial dysfunction, and necrotizing enterocolitis65–70.
In this context, allogeneic UCB transfusions are promising71. The first clinical trials showed safety without adverse events and a similar increase in hematocrit compared to adult-derived RBC72,73. In one study by Hassal et al., performed in a low-income setting, the authors showed the feasibility of transfusing allogeneic RBCs in anemic pediatric patients and demonstrated the absence of transfusion-related adverse reactions73.
A study by Bianchi et al. that enrolled ELBW infants within the first four weeks of life demonstrated an equivalent increase in hematocrit following either an adult or CB transfusion. At four weeks of life, patients required an equal number of transfusions on average. Furthermore, no acute transfusion responses or adverse transfusion-related events were reported in this study72. An Italian research group is currently leading a large multicenter randomized trial to compare the effect of adult-derived RBCs transfusions vs UCB-derived RBCs transfusions on retinopathy of prematurity in extremely low gestational age newborns (NCT05100212). Further research is needed to explore the use of this product.
UMBILICAL CORD BLOOD -PLATELET DERIVATIVES
Cord blood platelet concentrate has been obtained from whole cord blood with a standard protocol in Italy and Spain51–53,74. Platelet-rich plasma, derived from cord blood, has been successfully used as a regenerative product, as platelets may promote wound healing. Platelet-rich plasma contains a significant concentration of platelets, about 1×106/mL, which may secrete their content in the local environment, especially growth factors, but also interleukins and chemokines, all stored in platelet alpha granules. These factors stimulate the proliferation and differentiation of cells, enhance chemotaxis to the damaged area, and tissue regeneration54,75,76. Platelet-rich plasma-derived products from the UCB are richer in growth factors and poorer in inflammatory and immunomodulatory factors than those derived from adult peripheral blood54,77. These features reflect the relative immaturity of the innate immune system of healthy-term newborns54,78.
Platelet-rich plasma can be used as platelet gel, lysate, or serum. In the case of platelet gel, fibrinogen polymerization is needed to form a three-dimensional semisolid fibrin gel. Repeated freeze-thaw procedures are performed in the case of lysate54. Serum-containing platelet factors require blood collection without anticoagulant, so the clotted blood is hard centrifuged and recovered. Platelet serum and lysate are usually diluted in a saline solution.
UCB platelet gel has been successfully used in dystrophic epidermolysis bullosa skin and oral lesions, mucositis and esophagitis after stem cell transplantation, diabetic foot ulcers, recurrent perianal fistula, and for pressure ulcers in newborns79–87. In addition, UCB-derived serum has been used mainly in ocular surface lesions, dry eye, and mucocutaneous lesions graft-versus-host-disease-related51–54.
FUTURE PERSPECTIVES: PLATELETS FROM UMBILICAL CORD BLOOD
Similarly to RBCs, platelet concentrates derived from UCB might be a promising component for transfusing thrombocytopenic newborns. However, standard protocols to get this product have yet to be developed, and more studies are required to explore this topic.
Megakaryocytes ex-vivo production, as a source of platelets, has been explored in the past few years to overcome some limits of platelet concentrates, such as the limited shelf life and the development of alloimmune platelet transfusion refractoriness88,89. Megakaryocytes can be obtained from self-renewable embryonic, induced pluripotent stem cells or immortalized or chemically defined megakaryocyte cell lines. UCB has been successfully used as a stem cell source89–95. In vitro, expansion of stem cells in static conditions is more efficient from UCB than from peripheral blood, as in von Willebrand factor-coated microfluidic flow chambers, with phenotypic features quite similar between UCB and peripheral blood89,91.
From one CD34+ cell, up to 1×104 megakaryocytes can be obtained. With a system simulating the microenvironment of megakaryocyte maturation and platelet release, 70–80 platelets per megakaryocyte can be generated. This system might overcome the main problem of the clinical application of these megakaryocytes, which is the low rate of platelet generation92–95. Optimization of the induction system and quality and quantity of platelet production still have to be studied, as well as platelet ultrastructure and function89,92–95. However, a few clinical trials have already been performed with this technique: megakaryocytic progenitor cells were administered with positive effects to thrombocytopenic adults without adverse effects nor graft versus host disease in a 1-year follow-up, without ABO blood group and HLA type matching92–95. In the iPLAT1 trial, the authors managed to administer induced pluripotent stem cells-derived platelets, without any adverse reaction. Interestingly, they did not observe an increase in the platelet count, but larger platelets, as the ones derived by the stem cells are, were detected by flow cytometry in peripheral blood88,96.
In this context, it could be interesting and promising to achieve UCB platelet concentrate production and evaluate its biological activity, especially regarding platelet functionality and storage properties. Such a novel product may overcome all the potential risks of transfusing adult-derived platelets in premature infants and be a safer alternative for our neonates. Indeed, in a UCB-derived product, platelet function and immunologic profile are expected to be more similar to the neonatal ones compared to an adult-derived product. This “physiology matching” leads to the biological plausibility of using UCB-derived platelets to treat thrombocytopenic newborns. The reduced ability of neonatal platelets in activating an immune response might be an advantage of a UCB-derived product, as it would reduce inflammation, which is a major determinant of hemostatic imbalance and unfavorable outcomes. As an illustration, proinflammatory triggers play a significant role in the multifactorial pathogenesis of bronchopulmonary dysplasia97. Platelet products have already been reported to contain pro-inflammatory bioreactive components98,99. Evidence about the relationship between platelet biogenesis and pulmonary development is rising, as well as a possible platelet implication in alveolarization and lung regeneration100,101.
Of course, the achievement of such a product would probably be complex, as many factors must be considered. The UCB bags, even if adequately designed for cord blood collection, might need adjustment due to the volume of the cord blood itself and the amount of citrate phosphate dextrose needed.
Moreover, filtration, usually performed to eliminate the white cells, might also decrease the platelet’s number, despite filters being “platelet sparing”. The starting volume would probably be quite low, as well as the platelet count in the UCB. This might be an issue, considering that the high platelet number in adult-derived platelet concentrates (800–1,600×109/L) might be challenging to obtain. Pooling some AB0-matched cord blood together could be an option to overcome the volume problem, but it is associated with an increased risk of immune reactions. In fact, although the AB0 compatibility greatly decreases the risk of acute hemolytic reactions, minor antigens, different across donors, might still be present. They might be responsible for incompatibility reactions between the donor and the recipient.
Nowadays platelets are usually stored in platelet addictive solution-E (PAS-E), and previously they were re-suspended in plasma, but which one is the best solution for UCB-derived products is unknown.
Once faced with all these problems, platelet storage properties, such as activation, apoptotic and metabolic markers, must be assessed and compared to adult-derived platelet transfusions to check viability and establish administration time and expiry date.
In addition, safety must be evaluated in terms of bacterial contamination, as they are stored at room temperature. Once a validated product suitable for neonatal transfusions is obtained, clinical safety and efficacy studies will be required, including comparing this novel product to the traditional adult-derived platelet concentrate.
CONCLUSIONS
Thrombocytopenia in preterm newborns is a common issue in NICU. However, primary hemostasis is not defined just by platelet count, and other parameters should be considered, such as functional studies and IPF. Thrombocytopenia has traditionally been considered one of the most critical risk factors for bleeding, but a causal association between low platelet count and IVH, the most typical major bleeding of prematurity, has never been demonstrated. As recent studies showed that lower transfusion thresholds are associated with reduced risk of bleeding and death, transfusing platelets prophylactically with a platelet count above 25×109/L is a practice that should be avoided. A shared international neonatal platelet guideline is needed to optimize worldwide neonatal practice on transfusion thresholds.
Recent studies suggest that the current treatment of neonatal thrombocytopenia with prophylactical adult-derived platelet products may not be the best option for newborns. In this context, the development of novel cord blood-derived transfusion products, potentially more similar to the physiologic hemostatic system of the newborn, although challenging, should be considered as a future direction for research.
ACKNOWLEDGMENTS
We thank the “Griffini-Miglierina Foundation” for kindly supporting this study.
Footnotes
AUTHORSHIP CONTRIBUTIONS: VC, GC, GR, SG, FM, TK, SF, SS HN, ED, and EL contributed to the review conception and design. VC, GC, and EL wrote the first draft. GC, GR, SG, FM, TK, SF, SS HN, ED, and EL provided extensive critical revision. All Authors critically revised, read and approved the final version for submission.
The Authors declare no conflicts of interest.
FUNDING: This study was (partially) funded by Griffini-Miglierina Foundation. This study was (partially) funded by the Italian Ministry of Health -Current Research IRCCS.
References
- 1.Ferrer-Marin F, Stanworth S, Josephson C, Sola-Visner M. Distinct differences in platelet production and function between neonates and adults: implications for platelet transfusion practice. Transfusion. 2013;53:2814–2821. doi: 10.1111/trf.12343. quiz 2813. [DOI] [PubMed] [Google Scholar]
- 2.Christensen RD, Henry E, Wiedmeier SE, Stoddard RA, Sola-Visner MC, Lambert DK, et al. Thrombocytopenia among extremely low birth weight neonates: data from a multihospital healthcare system. J Perinatol. 2006;26:348–353. doi: 10.1038/sj.jp.7211509. [DOI] [PubMed] [Google Scholar]
- 3.Gunnink SF, Vlug R, Fijnvandraat K, van der Bom JG, Stanworth SJ, Lopriore E. Neonatal thrombocytopenia: etiology, management and outcome. Expert Rev Hematol. 2014;7:387–395. doi: 10.1586/17474086.2014.902301. [DOI] [PubMed] [Google Scholar]
- 4.Ulusoy E, Tufekci O, Duman N, Kumral A, Irken G, Oren H. Thrombocytopenia in neonates: causes and outcomes. Ann Hematol. 2013;92:961–967. doi: 10.1007/s00277-013-1726-0. [DOI] [PubMed] [Google Scholar]
- 5.Sola-Visner M, Saxonhouse MA, Brown RE. Neonatal thrombocytopenia: what we do and don’t know. Early Hum Dev. 2008;84:499–506. doi: 10.1016/j.earlhumdev.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Cremer M, Sallmon H, Kling PJ, Buhrer C, Dame C. Thrombocytopenia and platelet transfusion in the neonate. Semin Fetal Neonatal Med. 2016;21:10–18. doi: 10.1016/j.siny.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 7.de Vos TW, Winkelhorst D, de Haas M, Lopriore E, Oepkes D. Epidemiology and management of fetal and neonatal alloimmune thrombocytopenia. Transfus Apher Sci. 2020;59:102704. doi: 10.1016/j.transci.2019.102704. [DOI] [PubMed] [Google Scholar]
- 8.Winkelhorst D, Oostweegel M, Porcelijn L, Middelburg RA, Zwaginga JJ, Oepkes D, et al. Treatment and outcomes of fetal/neonatal alloimmune thrombocytopenia: a nationwide cohort study in newly detected cases. Br J Haematol. 2019;184:1026–1029. doi: 10.1111/bjh.15216. [DOI] [PubMed] [Google Scholar]
- 9.Fustolo-Gunnink SF, Huisman EJ, van der Bom JG, van Hout FMA, Makineli S, Lopriore E, et al. Are thrombocytopenia and platelet transfusions associated with major bleeding in preterm neonates? A systematic review. Blood Rev. 2019;36:1–9. doi: 10.1016/j.blre.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Davenport P, Sola-Visner M. Hemostatic Challenges in Neonates. Front Pediatr. 2021;9:627715. doi: 10.3389/fped.2021.627715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strauss T, Sidlik-Muskatel R, Kenet G. Developmental hemostasis: primary hemostasis and evaluation of platelet function in neonates. Semin Fetal Neonatal Med. 2011;16:301–304. doi: 10.1016/j.siny.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Bednarek FJ, Bean S, Barnard MR, Frelinger AL, Michelson AD. The platelet hyporeactivity of extremely low birth weight neonates is age-dependent. Thromb Res. 2009;124:42–45. doi: 10.1016/j.thromres.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Deschmann E, Sola-Visner M, Saxonhouse MA. Primary hemostasis in neonates with thrombocytopenia. J Pediatr. 2014;164:167–172. doi: 10.1016/j.jpeds.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 14.Davenport P, Sola-Visner M. Immunologic effects of red blood cell and platelet transfusions in neonates. Curr Opin Hematol. 2022;29:297–305. doi: 10.1097/MOH.0000000000000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boix H, Sanchez-Redondo MD, Cernada M, Fernandez MGE, Gonzalez-Pacheco N, Martin A, et al. Recommendations for transfusion of blood products in neonatology. An Pediatr (Engl Ed) 2022;97:60e61–60e68. doi: 10.1016/j.anpede.2022.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Dohner ML, Wiedmeier SE, Stoddard RA, Null D, Jr, Lambert DK, Burnett J, et al. Very high users of platelet transfusions in the neonatal intensive care unit. Transfusion. 2009;49:869–872. doi: 10.1111/j.1537-2995.2008.02074.x. [DOI] [PubMed] [Google Scholar]
- 17.Cetinkaya M, Atasay B. Editorial: Transfusions in the neonatal period. Front Pediatr. 2022;10:982918. doi: 10.3389/fped.2022.982918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Vecchio A, Latini G, Henry E, Christensen RD. Template bleeding times of 240 neonates born at 24 to 41 weeks gestation. J Perinatol. 2008;28:427–431. doi: 10.1038/jp.2008.10. [DOI] [PubMed] [Google Scholar]
- 19.Curley A, Stanworth SJ, Willoughby K, Fustolo-Gunnink SF, Venkatesh V, Hudson C, et al. Randomized trial of platelet-transfusion thresholds in neonates. N Engl J Med. 2019;380:242–251. doi: 10.1056/NEJMoa1807320. [DOI] [PubMed] [Google Scholar]
- 20.Kumar JDS, Sundaram V, Saini SS, Sharma RR, Varma N. Platelet transfusion for PDA closure in preterm infants: a randomized controlled trial. Pediatrics. 2019;143:e20182565. doi: 10.10.1542/peds.2018-2565. [DOI] [PubMed] [Google Scholar]
- 21.Sola-Visner M. Platelets in the neonatal period: developmental differences in platelet production, function, and hemostasis and the potential impact of therapies. Hematology Am Soc Hematol Educ Program. 2013;2012:506–511. doi: 10.10.1182/asheducation-2012.1.506. [DOI] [PubMed] [Google Scholar]
- 22.Ferrer-Marín F, Sola-Visner M. Neonatal platelet physiology and implications for transfusion. Platelets. 2022;33:14–22. doi: 10.1080/09537104.2021.1962837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sitaru AG, Holzhauer S, Speer CP, Singer D, Obergfell A, Walter U, et al. Neonatal platelets from cord blood and peripheral blood. Platelets. 2005;16:203–210. doi: 10.1080/09537100400016862. [DOI] [PubMed] [Google Scholar]
- 24.Saxonhouse MA, Garner R, Mammel L, Li Q, Muller KE, Greywoode J, et al. Closure times measured by the platelet function analyzer PFA-100 are longer in neonatal blood compared to cord blood samples. Neonatology. 2010;97:242–249. doi: 10.1159/000253755. [DOI] [PubMed] [Google Scholar]
- 25.Linder NSB, Levin E, Sirota L, Vishne TH, Tamarin I, Dardik R, Lubin D, Savion N, Varon D. Deposition of whole blood platelets on extracellular matrix under flow conditions in preterm infants. Arch Dis Child Fetal Neonatal. 2002;86:F127–130. doi: 10.1136/fn.86.2.f127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ucar T, Gurman C, Arsan S, Kemahli S. Platelet aggregation in term and preterm newborns. Pediatr Hematol Oncol. 2005;22:139–145. doi: 10.1080/08880010590907230. [DOI] [PubMed] [Google Scholar]
- 27.Hézard NPG, Schlegel N, Amory C, Leroux B, Nguyen P. Unexpected persistence of platelet hyporeactivity beyond the neonatal period: a flow cytometric study in neonates, infants and older children. Thromb Haemost. 2003;90:116–123. [PubMed] [Google Scholar]
- 28.Cremer M, Paetzold J, Schmalisch G, Hammer H, Loui A, Dame C, et al. Immature platelet fraction as novel laboratory parameter predicting the course of neonatal thrombocytopenia. Br J Haematol. 2009;144:619–621. doi: 10.1111/j.1365-2141.2008.07485.x. [DOI] [PubMed] [Google Scholar]
- 29.MacQueen BC, Christensen RD, Henry E, Romrell AM, Pysher TJ, Bennett ST, et al. The immature platelet fraction: creating neonatal reference intervals and using these to categorize neonatal thrombocytopenias. J Perinatol. 2017;37:834–838. doi: 10.1038/jp.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cremer M, Weimann A, Szekessy D, Hammer H, Buhrer C, Dame C. Low immature platelet fraction suggests decreased megakaryopoiesis in neonates with sepsis or necrotizing enterocolitis. J Perinatol. 2013;33:622–626. doi: 10.1038/jp.2013.21. [DOI] [PubMed] [Google Scholar]
- 31.Baer VL, Lambert DK, Henry E, Christensen RD. Severe thrombocytopenia in the NICU. Pediatrics. 2009;124:e1095–e1100. doi: 10.1542/peds.2009-0582. [DOI] [PubMed] [Google Scholar]
- 32.Stanworth SJ, Clarke P, Watts T, Ballard S, Choo L, Morris T, et al. Prospective, observational study of outcomes in neonates with severe thrombocytopenia. Pediatrics. 2009;124:e826–834. doi: 10.1542/peds.2009-0332. [DOI] [PubMed] [Google Scholar]
- 33.von Lindern JS, Hulzebos CV, Bos AF, Brand A, Walther FJ, Lopriore E. Thrombocytopaenia and intraventricular haemorrhage in very premature infants: a tale of two cities. Arch Dis Child Fetal Neonatal Ed. 2012;97:F348–352. doi: 10.1136/fetalneonatal-2011-300763. [DOI] [PubMed] [Google Scholar]
- 34.von Lindern JS, van den Bruele T, Lopriore E, Walther FJ. Thrombocytopenia in neonates and the risk of intraventricular hemorrhage: a retrospective cohort study. BMC Pediatr. 2011;11:16. doi: 10.1186/1471-2431-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villamor-Martinez E, Fumagalli M, Mohammed Rahim O, Passera S, Cavallaro G, Degraeuwe P, et al. Corrigendum: chorioamnionitis is a risk factor for intraventricular hemorrhage in preterm infants: a systematic review and meta-analysis. Front Physiol. 2019;10:102. doi: 10.3389/fphys.2019.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villamor-Martinez E, Fumagalli M, Alomar YI, Passera S, Cavallaro G, Mosca F, et al. Cerebellar hemorrhage in preterm infants: a meta-analysis on risk factors and neurodevelopmental outcome. Front Physiol. 2019;10:800. doi: 10.3389/fphys.2019.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerday E, Baer VL, Lambert DK, Paul DA, Sola-Visner MC, Pysher TJ, et al. Testing platelet mass versus platelet count to guide platelet transfusions in the neonatal intensive care unit. Transfusion. 2009;49:2034–2039. doi: 10.1111/j.1537-2995.2009.02253.x. [DOI] [PubMed] [Google Scholar]
- 38.Deschmann E, Saxonhouse MA, Feldman HA, Norman M, Barbian M, Sola-Visner M. Association of bleeding scores and platelet transfusions with platelet counts and closure times in response to adenosine diphosphate (CT-ADPs) among preterm neonates with thrombocytopenia. JAMA Netw Open. 2020;3:e203394. doi: 10.1001/jamanetworkopen.2020.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deschmann ESM, Feldman HA, Norman M, Barbian M, Sola-Visner M. Association between in vitro bleeding time and bleeding in preterm infants with thrombocytopenia. JAMA Pediat. 2019;173:393–394. doi: 10.1001/jamapediatrics.2019.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cremer M, Sola-Visner M, Roll S, Josephson CD, Yilmaz Z, Buhrer C, et al. Platelet transfusions in neonates: practices in the United States vary significantly from those in Austria, Germany, and Switzerland. Transfusion. 2011;51:2634–2641. doi: 10.1111/j.1537-2995.2011.03208.x. [DOI] [PubMed] [Google Scholar]
- 41.Sparger KA, Assmann SF, Granger S, Winston A, Christensen RD, Widness JA, et al. Platelet Transfusion Practices Among Very-Low-Birth-Weight Infants. JAMA Pediatr. 2016;170:687–694. doi: 10.1001/jamapediatrics.2016.0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scrivens A, Reibel NJ, Heeger L, Stanworth S, Lopriore E, New HV, et al. Survey of transfusion practices in preterm infants in Europe. Arch Dis Child Fetal Neonatal Ed. 2023;108:360–366. doi: 10.1136/archdischild-2022-324619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sola-Visner MC. Platelet transfusions in neonates - Less is more. N Engl J Med. 2019;380:287–288. doi: 10.1056/NEJMe1813419. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez-Luis G, Ghiradello S, Bas-Suarez P, Cavallaro G, Mosca F, Clyman RI, et al. Platelet counts and patent ductus arteriosus in preterm infants: an updated systematic review and meta-analysis. Front Pediatr. 2021;8:613766. doi: 10.3389/fped.2020.613766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simon SR, van Zogchel L, Bas-Suarez MP, Cavallaro G, Clyman RI, Villamor E. Platelet counts and patent ductus arteriosus in preterm infants: a systematic review and meta-analysis. Neonatology. 2015;108:143–151. doi: 10.1159/000431281. [DOI] [PubMed] [Google Scholar]
- 46.González-Luis G, Ghirardello S, Bas-Suárez P, Cavallaro G, Mosca F, Clyman RI, et al. Corrigendum: platelet counts and patent ductus arteriosus in preterm infants: an updated systematic review and meta-analysis. Front Pediatr. 2021;9:694606. doi: 10.3389/fped.2021.694606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore CM, Curley A. Platelet transfusion thresholds in neonatal medicine. Early Hum Dev. 2019;138:104845. doi: 10.1016/j.earlhumdev.2019.104845. [DOI] [PubMed] [Google Scholar]
- 48.Margraf A, Nussbaum C, Sperandio M. Ontogeny of platelet function. Blood Adv. 2019;3:692–703. doi: 10.1182/bloodadvances.2018024372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferrer-Marin F, Chavda C, Lampa M, Michelson AD, Frelinger AL, 3rd, Sola-Visner M. Effects of in vitro adult platelet transfusions on neonatal hemostasis. J Thromb Haemost. 2011;9:1020–1028. doi: 10.1111/j.1538-7836.2011.04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Margraf A, Nussbaum C, Rohwedder I, Klapproth S, Kurz ARM, Florian A, et al. Maturation of platelet function during murine fetal development in vivo. Arterioscler Thromb Vasc Biol. 2017;37:1076–1086. doi: 10.1161/ATVBAHA.116.308464. [DOI] [PubMed] [Google Scholar]
- 51.Rebulla P, Pupella S, Santodirocco M, Greppi N, Villanova I, Buzzi M, et al. Multicentre standardisation of a clinical grade procedure for the preparation of allogeneic platelet concentrates from umbilical cord blood. Blood Transfus. 2016;14:73–79. doi: 10.2450/2015.0122-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samarkanova D, Rodriguez L, Vives J, Coll R, Tahull E, Azqueta C, et al. Cord blood-derived platelet concentrates as starting material for new therapeutic blood components prepared in a public cord blood bank: from product development to clinical application. Blood Transfus. 2020;18:208–216. doi: 10.2450/2020.0305-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Samarkanova D, Cox S, Hernandez D, Rodriguez L, Casaroli-Marano RP, Madrigal A, et al. Cord blood platelet rich plasma derivatives for clinical applications in non-transfusion medicine. Front Immunol. 2020;11:942. doi: 10.3389/fimmu.2020.00942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orlando N, Pellegrino C, Valentini CG, Bianchi M, Barbagallo O, Sparnacci S, et al. Umbilical cord blood: Current uses for transfusion and regenerative medicine. Transfus Apher Sci. 2020;59:102952. doi: 10.1016/j.transci.2020.102952. [DOI] [PubMed] [Google Scholar]
- 55.Bianchi M, Papacci P, Valentini CG, Barbagallo O, Vento G, Teofili L. Umbilical cord blood as a source for red-blood-cell transfusion in neonatology: a systematic review. Vox Sang. 2018;113:713–725. doi: 10.1111/vox.12720. [DOI] [PubMed] [Google Scholar]
- 56.Khodabux CM, von Lindern JS, van Hilten JA, Scherjon S, Walther FJ, Brand A. A clinical study on the feasibility of autologous cord blood transfusion for anemia of prematurity. Transfusion. 2008;48:1634–1643. doi: 10.1111/j.1537-2995.2008.01747.x. [DOI] [PubMed] [Google Scholar]
- 57.Chasovskyi K, Fedevych O, McMullan DM, Mykychak Y, Vorobiova G, Zhovnir V, et al. Tissue perfusion in neonates undergoing open-heart surgery using autologous umbilical cord blood or donor blood components. Perfusion. 2015;30:499–506. doi: 10.1177/0267659114550234. [DOI] [PubMed] [Google Scholar]
- 58.Chasovskyi K, Fedevych O, Vorobiova G, Zhovnir V, Maksimenko A, Boychenko O, et al. Arterial switch operation in the first hours of life using autologous umbilical cord blood. Ann Thorac Surg. 2012;93:1571–1576. doi: 10.1016/j.athoracsur.2012.01.104. [DOI] [PubMed] [Google Scholar]
- 59.Choi ES, Cho S, Jang WS, Kim WH. Cardiopulmonary bypass priming using autologous cord blood in neonatal congenital cardiac surgery. Korean Circ J. 2016;46:714–718. doi: 10.4070/kcj.2016.46.5.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yavuz BA, Okulu E, Arsan S, Akin IM, Atasay B, Erdeve O. Is autologous cord blood transfusion effective and safe in preterm infants? Turk J Pediatr. 2017;59:352–354. doi: 10.24953/turkjped.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 61.Strauss RG, Widness JA. Is there a role for autologous/placental red blood cell transfusions in the anemia of prematurity? Transfus Med Rev. 2010;24:125–129. doi: 10.1016/j.tmrv.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kotowski M, Litwinska Z, Klos P, Pius-Sadowska E, Zagrodnik-Ulan E, Ustianowski P, Rudnicki J, Machalinski B. Autologous cord blood transfusion in preterm infants - could its humoral effect be the key to control prematurity-related complications? A preliminary study. J Physiol Pharmacol. 2017;68:921–927. [PubMed] [Google Scholar]
- 63.Brune T, Garritsen H, Hentschel R, Louwen F, Harms E, Jorch G. Efficacy, recovery, and safety of RBCs from autologous placental blood: clinical experience in 52 newborns. Transfusion. 2003;43:1210–1216. doi: 10.1046/j.1537-2995.2003.00503.x. [DOI] [PubMed] [Google Scholar]
- 64.Jansen M, Brand A, von Lindern JS, Scherjon S, Walther FJ. Potential use of autologous umbilical cord blood red blood cells for early transfusion needs of premature infants. Transfusion. 2006;46:1049–1056. doi: 10.1111/j.1537-2995.2006.00841.x. [DOI] [PubMed] [Google Scholar]
- 65.Bellach L, Eigenschink M, Hassanein A, Savran D, Salzer U, Müllner EW, et al. Packed red blood cell transfusion in preterm infants. Lancet Haematol. 2022;9:e615–e626. doi: 10.1016/s2352-3026(22)00207-1. [DOI] [PubMed] [Google Scholar]
- 66.Lust C, Vesoulis Z, Jackups R, Jr, Liao S, Rao R, Mathur AM. Early red cell transfusion is associated with development of severe retinopathy of prematurity. J Perinatol. 2019;39:393–400. doi: 10.1038/s41372-018-0274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stutchfield CJ, Jain A, Odd D, Williams C, Markham R. Foetal haemoglobin, blood transfusion, and retinopathy of prematurity in very preterm infants: a pilot prospective cohort study. Eye (Lond) 2017;31:1451–1455. doi: 10.1038/eye.2017.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hellstrom W, Martinsson T, Hellstrom A, Morsing E, Ley D. Fetal haemoglobin and bronchopulmonary dysplasia in neonates: an observational study. Arch Dis Child Fetal Neonatal Ed. 2021;106:88–92. doi: 10.1136/archdischild-2020-319181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hellstrom W, Martinsson T, Morsing E, Granse L, Ley D, Hellstrom A. Low fraction of fetal haemoglobin is associated with retinopathy of prematurity in the very preterm infant. Br J Ophthalmol. 2022;106:970–974. doi: 10.1136/bjophthalmol-2020-318293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amelio GS, Provitera L, Raffaeli G, Tripodi M, Amodeo I, Gulden S, et al. Endothelial dysfunction in preterm infants: the hidden legacy of uteroplacental pathologies. Front Pediatr. 2022;10:1041919. doi: 10.3389/fped.2022.1041919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Teofili L, Papacci P, Orlando N, Bianchi M, Molisso A, Purcaro V, et al. Allogeneic cord blood transfusions prevent fetal haemoglobin depletion in preterm neonates. Results of the CB-TrIP study. Br J Haematol. 2020;191:263–268. doi: 10.1111/bjh.16851. [DOI] [PubMed] [Google Scholar]
- 72.Bianchi M, Giannantonio C, Spartano S, Fioretti M, Landini A, Molisso A, et al. Allogeneic umbilical cord blood red cell concentrates: an innovative blood product for transfusion therapy of preterm infants. Neonatology. 2015;107:81–86. doi: 10.1159/000368296. [DOI] [PubMed] [Google Scholar]
- 73.Hassall OW, Thitiri J, Fegan G, Hamid F, Mwarumba S, Denje D, et al. Safety and efficacy of allogeneic umbilical cord red blood cell transfusion for children with severe anaemia in a Kenyan hospital: an open-label single-arm trial. Lancet Haematol. 2015;2:e101–e107. doi: 10.1016/s2352-3026(15)00005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Querol S, Samarkanova D. Rapid review: next generation of cord blood banks; transplantation and beyond. Transfusion. 2019;59:3048–3050. doi: 10.1111/trf.15466. [DOI] [PubMed] [Google Scholar]
- 75.Mussano F, Genova T, Munaron L, Petrillo S, Erovigni F, Carossa S. Cytokine, chemokine, and growth factor profile of platelet-rich plasma. Platelets. 2016;27:467–471. doi: 10.3109/09537104.2016.1143922. [DOI] [PubMed] [Google Scholar]
- 76.Nurden AT. Platelets, inflammation and tissue regeneration. Thromb Haemost. 2011;105(Suppl 1):S13–33. doi: 10.1160/THS10-11-0720. [DOI] [PubMed] [Google Scholar]
- 77.Parazzi V, Lavazza C, Boldrin V, Montelatici E, Pallotti F, Marconi M, et al. Extensive characterization of platelet gel releasate from cord blood in regenerative medicine. Cell Transplant. 2015;24:2573–2584. doi: 10.3727/096368915X687471. [DOI] [PubMed] [Google Scholar]
- 78.Teofili L. Cord blood platelet lysate: in vitro evaluation to support the use in regenerative medicine. Mediterr J Hematol Infect Dis. 2019;11:e2019021. doi: 10.4084/mjhid.2019.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gelmetti A, Greppi N, Guez S, Grassi F, Rebulla P, Tadini G. Cord blood platelet gel for the treatment of inherited epidermolysis bullosa. Transfus Apher Sci. 2018;57:370–373. doi: 10.1016/j.transci.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 80.Tadini G, Guez S, Pezzani L, Marconi M, Greppi N, Manzoni F, et al. Preliminary evaluation of cord blood platelet gel for the treatment of skin lesions in children with dystrophic epidermolysis bullosa. Blood Transfus. 2015;13:153–158. doi: 10.2450/2014.0160-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tadini G, Pezzani L, Ghirardello S, Rebulla P, Esposito S, Mosca F. Cord blood platelet gel treatment of dystrophic recessive epidermolysis bullosa. BMJ Case Rep. 2015;2015:bcr2014207364. doi: 10.1136/bcr-2014-207364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sindici E, Astesano S, Fazio L, Dragonetti A, Pugliese M, Scully C, et al. Treatment of oral lesions in dystrophic epidermolysis bullosa: a case series of cord blood platelet gel and low-level laser therapy. Acta Derm Venereol. 2017;97:383–384. doi: 10.2340/00015555-2512. [DOI] [PubMed] [Google Scholar]
- 83.Sindici E, Basiglio L, Cafaro A, Fazio L, Dragonetti A, Pugliese M, et al. The photobiomodulation therapy together with the use of cord blood platelet gel could be safely suggested as primary treatment for oral lesions in patients with inherited epidermolysis bullosa. Photodermatol Photoimmunol Photomed. 2020;36:318–321. doi: 10.1111/phpp.12548. [DOI] [PubMed] [Google Scholar]
- 84.Sindici E, Giuliano B, Astesano S, Fazio L, Dragonetti A, Pugliese M, et al. Cord blood platelet gel alone or in combination with photobiomodulation therapy for the treatment of oral ulcerations in patients with epidermolysis bullosa: a pilot clinical comparative study. Photodermatol Photoimmunol Photomed. 2018;34:269–272. doi: 10.1111/phpp.12366. [DOI] [PubMed] [Google Scholar]
- 85.Piccin A, Rebulla P, Pupella S, Tagnin M, Marano G, Di Pierro AM, et al. Impressive tissue regeneration of severe oral mucositis post stem cell transplantation using cord blood platelet gel. Transfusion. 2017;57:2220–2224. doi: 10.1111/trf.14205. [DOI] [PubMed] [Google Scholar]
- 86.Volpe P, Marcuccio D, Stilo G, Alberti A, Foti G, Volpe A, et al. Efficacy of cord blood platelet gel application for enhancing diabetic foot ulcer healing after lower limb revascularization. Semin Vasc Surg. 2017;30:106–112. doi: 10.1053/j.semvascsurg.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 87.Ferrario S, Zorz A, Sorrentino G, Villa S, Cavalli R, Mosca F, et al. Cord blood platelet gel as a treatment of occipital pressure injuries in newborns: report of two cases. Children (Basel) 2021;8:1079. doi: 10.3390/children8121079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sugimoto N, Kanda J, Nakamura S, Kitano T, Hishizawa M, Kondo T, et al. iPLAT1: the first-in-human clinical trial of iPSC-derived platelets as a phase 1 autologous transfusion study. Blood. 2022;140:2398–2402. doi: 10.1182/blood.2022017296. [DOI] [PubMed] [Google Scholar]
- 89.Six KR, Sicot G, Devloo R, Feys HB, Baruch D, Compernolle V. A comparison of haematopoietic stem cells from umbilical cord blood and peripheral blood for platelet production in a microfluidic device. Vox Sang. 2019;114:330–339. doi: 10.1111/vox.12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Balduini A, d’Apolito M, Arcelli D, Conti V, Pecci A, Pietra D, et al. Cord blood in vitro expanded CD41 cells: identification of novel components of megakaryocytopoiesis. J Thromb Haemost. 2006;4:848–860. doi: 10.1111/j.1538-7836.2006.01802.x. [DOI] [PubMed] [Google Scholar]
- 91.van den Oudenrijn S, von dem Borne AE, de Haas M. Differences in megakaryocyte expansion potential between CD34(+) stem cells derived from cord blood, peripheral blood, and bone marrow from adults and children. Exp Hematol. 2000;28:1054–1061. doi: 10.1016/s0301-472x(00)00517-8. [DOI] [PubMed] [Google Scholar]
- 92.Xi J, Zhu H, Liu D, Nan X, Zheng W, Liu K, et al. Infusion of megakaryocytic progenitor products generated from cord blood hematopoietic stem/progenitor cells: results of the phase 1 study. PLoS One. 2013;8:e54941. doi: 10.1371/journal.pone.0054941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xie X, Yao H, Han X, Yue W, Pei X. Therapeutic use of red blood cells and platelets derived from human cord blood stem cells. Stem Cells Transl Med. 2021;10(Suppl 2):S48–S53. doi: 10.1002/sctm.20-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guan X, Qin M, Zhang Y, Wang Y, Shen B, Ren Z, et al. Safety and efficacy of megakaryocytes induced from hematopoietic stem cells in murine and nonhuman primate models. Stem Cells Transl Med. 2017;6:897–909. doi: 10.5966/sctm.2016-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ito Y, Nakamura S, Sugimoto N, Shigemori T, Kato Y, Ohno M, et al. Turbulence activates platelet biogenesis to enable clinical scale ex vivo production. Cell. 2018;174:636–648e618. doi: 10.1016/j.cell.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 96.Chen SJ, Sugimoto N, Eto K. Ex vivo manufacturing of platelets: beyond the first-in-human clinical trial using autologous iPSC-platelets. Int J Hematol. 2023;117:349–355. doi: 10.1007/s12185-022-03512-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jobe AH. Animal models, learning lessons to prevent and treat neonatal chronic lung disease. Front Med (Lausanne) 2015;2:49. doi: 10.3389/fmed.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weyrich AS, Lindemann S, Zimmerman GA. The evolving role of platelets in inflammation. J Thromb Haemost. 2003;1:1897–1905. doi: 10.1046/j.1538-7836.2003.00304.x. [DOI] [PubMed] [Google Scholar]
- 99.Chioma R, Ghirardello S, Wlodarczyk K, Ulan-Drozdowska J, Spagarino A, Szumska M, et al. Association between the development of bronchopulmonary dysplasia and platelet transfusion: a protocol for a systematic review and meta-analysis. Front Pediatr. 2023;11:1049014. doi: 10.3389/fped.2023.1049014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Suzuki-Inoue K, Tsukiji N. Platelet CLEC-2 and lung development. Res Pract Thromb Haemost. 2020;4:481–490. doi: 10.1002/rth2.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tsukiji Nea. Platelets Play an Essential Role in Murine Lung Development through Clec-2/podoplanin. Interaction Blood. 2018;132(11):1167–1179. doi: 10.1182/blood-2017-12-823369. [DOI] [PubMed] [Google Scholar]