Abstract
The Epstein-Barr virus (EBV) latent membrane protein 2 (LMP2) is expressed in latently EBV-infected B cells, where it forms patches in the plasma membrane and interferes with B-cell receptor signal transduction through dominant-negative effects on protein kinases. LMP2 transcripts are detected in nasopharyngeal carcinoma, an epithelial-cell malignancy. In this study the function of LMP2A in epithelial cells was investigated. LMP2A was found to coprecipitate with protein kinase activities and to become phosphorylated in in vitro kinase assays. Analysis of LMP2A deletion mutants demonstrated that tyrosines implicated in interacting with Src family kinase SH2 domains and the SH2 domain of Csk, as well as the LMP2A immunoreceptor tyrosine-based activation motif, are important for its phosphorylation in epithelial cells. LMP2A tyrosine phosphorylation was triggered by cell adhesion to extracellular-matrix (ECM) proteins. Src family kinases, whose involvement in cell-ECM signaling and LMP2A phosphorylation in B lymphocytes has been well established, were found not to be responsible for LMP2A phosphorylation in epithelial cells. Instead, coexpression of Csk, a negative Src regulator, and LMP2A led to an increase in LMP2A phosphorylation both in nonadherent cells and upon cell adhesion. Csk also phosphorylated LMP2A in vitro. These results suggest that LMP2A has a different role in epithelial cells, where it interacts with cell adhesion-initiated signaling pathways. Although tyrosine phosphorylation of LMP2A occurs in both cell types, different protein kinases seem to be used: Src family kinases in B lymphocytes and Csk in epithelial cells.
Epstein-Barr virus (EBV) is a ubiquitous human B-lymphotropic herpesvirus capable of infecting both lymphoid and epithelial cells. EBV establishes latency in B cells, with periodic reactivation of virus and reinfection of oropharyngeal epithelia. EBV is the causative agent of infectious mononucleosis and is also associated with several human cancers, such as the lymphoid malignancies Burkitt’s lymphoma, posttransplant lymphoma, and Hodgkin’s disease, as well as nasopharyngeal carcinoma (NPC), an epithelial malignancy (25, 38–41, 51). EBV latency is characterized by the absence of viral replication and the expression of specific subsets of genes. Several types of latency can be distinguished according to the pattern of viral genes that are expressed. Type I latency, found in Burkitt’s lymphoma, has the most restricted pattern of gene expression, with expression of EBNA1, transcripts from the BamHI A region of the genome (5), and the EBV-encoded RNAs (EBERs), small nonpolyadenylated RNAs of unknown function. In immortalized B-cell lines (type III latency) the nuclear antigens (EBNAs 1 through 6), EBERs, BamHI A transcripts, and three latent membrane proteins, LMP1, LMP2A, and LMP2B, are expressed. In type II latency, characteristic of NPC, EBNA1, the EBERs, BamHI A transcripts, LMP1, and LMP2 are expressed (25).
The focus of this study is the LMP2 gene of EBV. Two forms of LMP2, LMP2A and LMP2B, originate from two mRNAs that initiate from different promoters and are transcribed across the fused terminal repeats of the viral episome (45). They share eight common exons but differ in exon 1, which is noncoding in LMP2B and codes for the amino terminus of LMP2A. The proteins are highly hydrophobic, with 12 transmembrane domains, and aggregate in the plasma membranes of latently infected B-lymphocytes together with LMP1 (30). LMP2A has been suggested to regulate viral reactivation from latency by negatively interfering with B-cell receptor signal transduction. The LMP2A amino terminus physically interacts with tyrosine kinases of the Src family, preferably with Lyn and Fyn, the hemopoietic-cell-specific kinase Syk, and the mitogen-activated protein kinase ERK1 (6, 18–20, 29, 37). LMP2A patches in the plasma membrane and acts as a decoy receptor complex. The LMP2A amino terminus is thought to modulate the activity of these signaling molecules, rendering them unresponsive to B-cell receptor-mediated activation events, as surface immunoglobulin (sIg) cross-linking fails to activate Lyn and Syk protein kinases in LMP2A-expressing B cells. Further-downstream events affected by LMP2A are inhibition of elevation of intracellular calcium levels, prevention of mitogen-activated protein kinase activation, and a block in the induction of expression of the immediate-early viral transactivator BZLF1, leading to suppression of viral replication in response to sIg cross-linking (32–35). The hydrophobic 119-amino-acid cytoplasmic amino terminus of LMP2A contains eight tyrosine residues, several of which are in the context of potential docking motifs for SH2 domains of cellular signaling molecules (31, 46). Most notable is the immunoreceptor tyrosine-based activation motif (ITAM) surrounding tyrosines 74 and 85. ITAMs are characterized by the consensus sequence YXX(L/I)(X6–8)YXX(L/I) and are found in associated molecules of the B- and T-cell receptor signaling complexes as well as the Fc receptor complexes (42, 50). Phosphorylated ITAMs serve as docking sites for the tandem SH2 domains of the tyrosine kinases Syk and ZAP70 in B and T cells, respectively. The inhibition of B-cell receptor-mediated signal transduction by LMP2A has recently been shown to depend on the LMP2A ITAM (19) and on tyrosine 112, found in the context of a YEEA motif capable of binding to the SH2 domains of Src family kinases (20). Other potential SH2 domain docking sites include a YSPA sequence surrounding tyrosine 31, predicted to bind to the SH2 domain of PLCγ2, and a YSPR sequence at tyrosine 101, which could constitute a binding site for the SH2 domain of Csk, a protein kinase that negatively regulates Src family kinase activity (31, 46).
The function of LMP2 in epithelial cells has not been determined. LMP2 is transcribed in NPC (4, 8), and NPC patients have elevated titers of antibodies to both LMP2A and LMP2B (28), suggesting that LMP2 is expressed during some stage of progression to disease. Additionally, LMP2 transcripts have been detected in the permissively infected hairy leukoplakia lesion, a human immunodeficiency virus-associated epithelial lesion found on the lateral border of the tongue (49).
This study addresses the properties of LMP2A in epithelial cells. Phosphorylation of LMP2A was analyzed, and the effects of the interaction of epithelial cells with their extracellular matrix (ECM) on LMP2A were investigated. The role of Src family kinases in epithelial cells was also determined. The data suggest that the role of LMP2 in epithelial cells is distinct from that in B cells, involving functional interactions with different signaling molecules and signaling pathways.
MATERIALS AND METHODS
Cell lines and establishment of derivatives.
H1299 non-small-cell lung carcinoma cells and 293 human kidney epithelial cells were routinely maintained in Dulbecco’s modified Eagle medium (DMEM-H) supplemented with 10% fetal bovine serum and antibiotics. Cells were subcultured three times per week. Transfections of H1299 cells were performed by using the Lipofectin reagent (Gibco-BRL) according to the manufacturer’s specifications. H1299 cell lines expressing LMP2A and vector control cell lines were established by selection in DMEM-H containing 200 μg of hygromycin B (Boehringer Mannheim)/ml following transfection with an expression construct (pMEP4) containing the LMP2A cDNA or vector alone. Stable pools were designated H1299-LMP2A and H1299-pMEP4, respectively. Transient transfections into 293 human kidney epithelial cells were performed by standard calcium phosphate transfection procedures.
Plasmids and expression constructs.
For transient transfections pSG5 (Stratagene)-derived expression constructs were used. pLMP2AHA, as well as the LMP2A deletion mutants pLMP2AΔ21-36, pLMP2AΔ21-64, pLMP2AΔ21-85, and pLMP2AΔ80-112, have been described previously (18, 20). In addition to the deletion, LMP2AΔ80-112 contains a tyrosine-to-phenylalanine substitution at position 74. All cDNAs are tagged with a hemagglutinin (HA) epitope at the carboxy terminus.
For the establishment of cell lines, the wild-type LMP2A cDNA was subcloned into the pMEP4 vector (Invitrogen) containing the hygromycin resistance gene. This places the gene of interest under the control of the mouse metallothionein promoter, which can be induced by the addition of heavy-metal cations. In the experiments described here, induction was performed by the addition of 10 μM CdCl2 for 18 to 21 h.
Antibodies and reagents.
For immunoprecipitation and detection of HA-tagged proteins, the monoclonal anti-HA antibody 12CA5 or a polyclonal anti-HA antiserum (Santa Cruz) was used. The phosphotyrosine-specific antibodies PY20 and 4G10 were purchased from Santa Cruz and Upstate Biotechnology Inc., respectively. A monoclonal antibody to Csk and a polyclonal anti-Csk antiserum were purchased from Transduction Labs and Santa Cruz, respectively. ECM proteins were obtained from Collaborative Biomedical Products through the University of North Carolina ACT core facility.
Cell adhesion experiments.
Tissue culture dishes were coated with ECM proteins at concentrations of 10 μg/ml for laminin 1, collagen, and poly-d-lysine and 20 μg/ml for fibronectin by incubating 100- or 60-mm dishes with appropriate dilutions of ECM protein in phosphate-buffered saline solution (PBS) at 4°C overnight, followed by two washes with PBS. Adherent cells were trypsinized briefly, the trypsin was inactivated with 0.5 mg of soybean trypsin inhibitor (Sigma)/ml, and the cells were centrifuged and resuspended in serum-free or complete medium. Cells were incubated in suspension at 37°C for 30 min to 1 h with rocking before plating. After plating for various amounts of time, media and nonadherent cells were aspirated, the plates were gently washed with cold PBS, and the cells were lysed in Nonidet P-40 (NP-40) lysis buffer (1% NP-40, 150 mM NaCl, 50 mM Tris-HCl [pH 7.4], 2 mM EDTA, 10 μg [each] of leupeptin and aprotinin/ml, 2 mM phenylmethylsulfonyl fluoride, and 1 mM sodium vanadate). Lysates were incubated on ice for 10 min, and debris was removed by centrifugation in a microcentrifuge at 4°C at 10,000 × g for 15 min. Protein concentrations were determined by the Bio-Rad protein assay.
Immunoprecipitations and in vitro kinase assays.
Typically, aliquots of cell lysates containing 500 to 1,000 μg of total protein were diluted to 500 μl with NP-40 buffer (as above). Lysates were precleared by incubation with 20 μl of protein G-Sepharose beads for 1 h at 4°C. Supernatants were incubated with appropriate dilutions of antibody for 1 to 4 h at 4°C before immune complexes were captured by the addition of 20 μl of protein G-Sepharose beads for an additional hour. Immune complexes were washed three times with 500 μl of NP-40 buffer. For Western analysis, 25 μl of sodium dodecyl sulfate (SDS) sample buffer was added to the immune complexes, and the proteins were denatured by boiling for 5 min and separated by SDS-polyacrylamide gel electrophoresis (PAGE).
For in vitro kinase assays, the immune complexes were washed five times in NP-40 buffer and twice in kinase buffer (20 mM HEPES [pH 7.3] plus 5 mM MgCl2). Kinase reactions were performed in 20 μl of kinase buffer plus 10 μM ATP and 2.5 μCi of [γ-32P]ATP. Reactions were allowed to proceed for 15 min at room temperature and were stopped by dilution with 1 ml of cold NP-40 buffer. The reaction products were washed three more times with NP-40 buffer before 25 μl of SDS sample buffer was added. The reaction products were denatured by boiling for 5 min and analyzed by SDS-PAGE.
Immunoblot analysis.
Proteins were transferred to an Immobilon membrane (Millipore) in a Bio-Rad transfer unit overnight. Ponceau S staining of the membrane was performed to ensure equal loading and transfer of proteins. Nonspecific reactivity was blocked by incubating the membrane with Tris-buffered saline solution containing 0.1% Tween 20 and 5% nonfat dry milk for 1 h at room temperature. Tyrosine-phosphorylated proteins were detected with a cocktail of the monoclonal antibodies 4G10 (1:1,000) and PY20 (1:300). The 12CA5 anti-HA monoclonal antibody (ascites fluid) was used at a 1:300 dilution. Appropriate anti-mouse or anti-rabbit secondary antibodies conjugated to horseradish peroxidase (Amersham) were used at a dilution of 1:1,000. Detection was performed with the Pierce Super Signal detection system according to the manufacturer’s instructions.
GST-fusion protein precipitations.
The glutathione S-transferase (GST)-LMP2 fusion protein was constructed by in-frame fusion of exon 1A of the B95.8 LMP2 open reading frame to GST in the vector pGEX2TK (Pharmacia). GST and GST-LMP2 proteins were prepared as follows. Escherichia coli DH5α transformed with pGEX2TK or pGEXGST-LMP2 was grown overnight in 5-ml cultures, diluted 1:20 with Luria-Bertani medium the next day, and allowed to grow for 2 h. Then 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added for 3 h, and the cells were harvested by centrifugation and washed twice with PBS. Cell pellets were stored at −70°C until use. Cell pellets were resuspended in 1 ml of PBS and sonicated three times for 30 s. Debris was removed by centrifugation in a microcentrifuge for 15 min at 10,000 × g. GST proteins were captured by incubation with glutathione-Sepharose beads at 4°C for 45 min, and the complexes were washed three times with PBS. Induction of fusion proteins was monitored by SDS-PAGE and staining with Coomassie brilliant blue. Equal amounts of protein were used in precipitation experiments. Epithelial-cell lysates were prepared in NP-40 buffer as described above and precleared by incubation with glutathione-Sepharose beads for 2 h, followed by preclearing with GST-Sepharose beads for 2 h. Precipitation was carried out by incubation of precleared lysates with glutathione beads, GST-Sepharose beads, or GST-LMP2–Sepharose beads. In vitro kinase assays were performed as described above.
RESULTS
Inducible expression of LMP2A in epithelial cells.
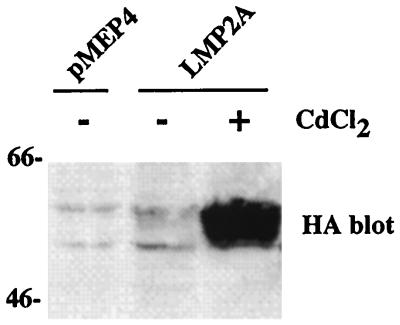
The LMP2A cDNA was placed under the control of a heavy-metal cation-inducible metallothionein promoter. The vector (pMEP4) also carries genes for hygromycin acetyltransferase and the EBV EBNA1 protein and contains the EBV origin of replication, allowing it to be maintained as an episome in transfected cells. Following transfection into H1299 non-small-cell lung carcinoma cells, transfectants were selected with hygromycin B and resistant pools were analyzed by immunoblotting for LMP2A expression after induction with CdCl2 (Fig. 1). In the absence of CdCl2, the metallothionein promoter had very little activity in this cell line.
FIG. 1.
Inducible expression of LMP2A in H1299 cells. H1299 cells were transfected with pMEP4 alone or pMEP4 containing the LMP2A cDNA tagged with an influenza HA epitope. Transfectants were selected with hygromycin B, and stable pools were established. LMP2A expression, controlled by the mouse metallothionein promoter, was induced by the addition of CdCl2. Western blotting was performed with the 12CA5 monoclonal anti-HA antibody.
LMP2A is associated with protein kinase activities in epithelial cells.
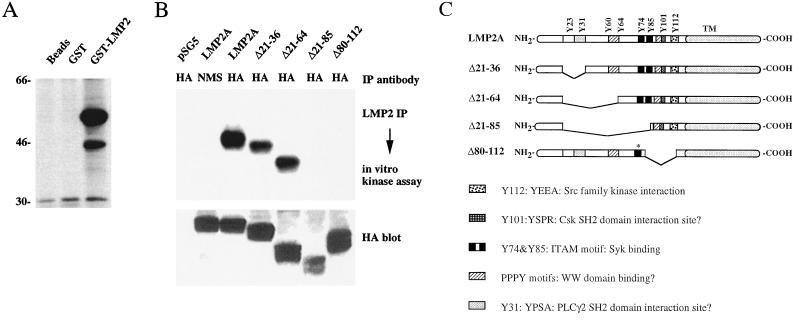
LMP2A is constitutively phosphorylated in lymphoblastoid cell lines and has been shown to interact with various tyrosine kinases such as the Src family kinases Lyn and Fyn, the hemopoietic-cell-specific kinase Syk, and the serine-threonine kinase ERK1 (6, 32, 37). To determine whether LMP2A is directly associated with protein kinases in epithelial cells, GST-fusion protein precipitation assays were performed. The LMP2A amino terminus was fused to GST, and this fusion protein, GST, or glutathione-Sepharose beads alone were incubated with epithelial-cell lysates. The GST-LMP2 fusion protein became heavily phosphorylated in in vitro kinase assays performed on the precipitated protein complexes. In control precipitations with GST protein or glutathione-Sepharose beads alone, no phosphorylated product was detected. Figure 2A shows results of a representative experiment performed in H1299 non-small-cell lung carcinoma cells. Identical results were obtained with other epithelial cell lines such as ME180 and C33A (data not shown). These data indicate that LMP2A is physically associated with protein kinase activities in epithelial cells and that the LMP2A amino terminus is sufficient for this interaction.
FIG. 2.
LMP2A is associated with protein kinase activities in epithelial cells. (A) GST-LMP2 interacts with protein kinases in H1299 lysates. GST and GST-LMP2 fusion proteins were incubated with H1299 cell extracts, and in vitro kinase assays with [γ-32P]ATP were performed on the precipitated protein complexes. Phosphorylated proteins were separated by SDS-PAGE and visualized by autoradiography. (B) Phosphorylation of WT LMP2A and deletion mutants after immunoprecipitation (IP) and an in vitro kinase assay. HA epitope-tagged LMP2A and deletion mutants were transiently transfected into H1299 cells, and the proteins were immunoprecipitated with the monoclonal anti-HA antibody (HA) or normal mouse serum (NMS), followed by an in vitro kinase assay with [γ-32P]ATP. Labeled proteins were visualized by autoradiography. The lower panel shows expression of the deletion mutants as detected by HA Western blotting. (C) Diagram of LMP2A deletion mutants. WT LMP2A and the deletion mutants used in this study are diagrammed with protein-protein interaction motifs. The asterisk in LMP2AΔ80-112 indicates a tyrosine-to-phenylalanine mutation at position 74. TM, LMP2A transmembrane domains.
To confirm these results in the context of the full-length LMP2A protein and to obtain information about phosphorylation and possible protein kinase interaction sites, wild-type (WT) LMP2A and several deletion mutants, all tagged with an HA epitope at the carboxy terminus, were transiently transfected into H1299 cells and the proteins were immunoprecipitated. In vitro kinase assays were carried out on the immune complexes, and the products were resolved by SDS-PAGE (Fig. 2B). WT LMP2A, as well as the mutants LMP2AΔ21-36 and LMP2AΔ21-64, became phosphorylated, while phosphorylation of the mutants LMP2AΔ21-85 and LMP2AΔ80-112 was not detected. Phosphorylated proteins were not detected in lysates of LMP2A-transfected cells in control precipitations using normal mouse serum or vector-transfected cells. These results confirm that LMP2A is constitutively associated with one or more protein kinase activities in epithelial cells.
A diagram of the various LMP2A mutants is shown in Fig. 2C. Mutants LMP2AΔ21-36 and LMP2AΔ21-64 became phosphorylated at levels comparable to that of WT LMP2A. Therefore, tyrosines 23, 31, 60, and 64, which are deleted in these two mutants, do not seem to represent major sites of tyrosine phosphorylation in LMP2A. LMP2AΔ21-85, which did not become phosphorylated, has the additional deletions of tyrosines 74 and 85. These two tyrosines are found within the ITAM of LMP2A and are important for its function in B cells (19). Notably, LMP2AΔ21-85 was phosphorylated in identical experiments in B lymphocytes, in contrast to our findings (20). In the other nonphosphorylated mutant, LMP2AΔ80-112, tyrosine 74 is mutated to phenylalanine and tyrosines 85, 101, and 112 are deleted. Therefore, the ITAM is mutated, as are a motif at tyrosine 101, YSPR, predicted to represent a docking site for the SH2 domain of Csk, and the YEEA motif surrounding tyrosine 112, an interaction site for the SH2 domain of Src family kinases. This mutational analysis implicates the ITAM as the major site for tyrosine phosphorylation, as both mutants in which this motif was deleted did not become phosphorylated in vitro. The predicted SH2 domain interaction sites at tyrosines 101 and 112 could be involved in mediating the association of protein kinases with LMP2A, although they do not seem to represent major phosphorylation sites, as they are retained in LMP2AΔ21-85, which did not become phosphorylated. Tyrosine 112 has been shown to be important for LMP2A phosphorylation in B cells. The results presented here suggest that tyrosine 112 has a different role in epithelial cells.
Tyrosine phosphorylation events in LMP2A-expressing cells in response to cell adhesion.
It was of interest to determine which epithelial-cell signaling pathways lead to LMP2A phosphorylation. Important aspects of epithelial-cell behavior are regulated by the interaction of cells with their ECM. These include control of cell shape, migration behavior, cellular proliferation, cell survival, and cell differentiation. The interaction of LMP2A with epithelial-cell signaling pathways that are activated by cell-ECM interactions was investigated. Changes in the tyrosine phosphorylation of several cellular proteins, such as focal adhesion kinase and paxillin, are among the earliest events observed upon cell adhesion (7, 26, 27).
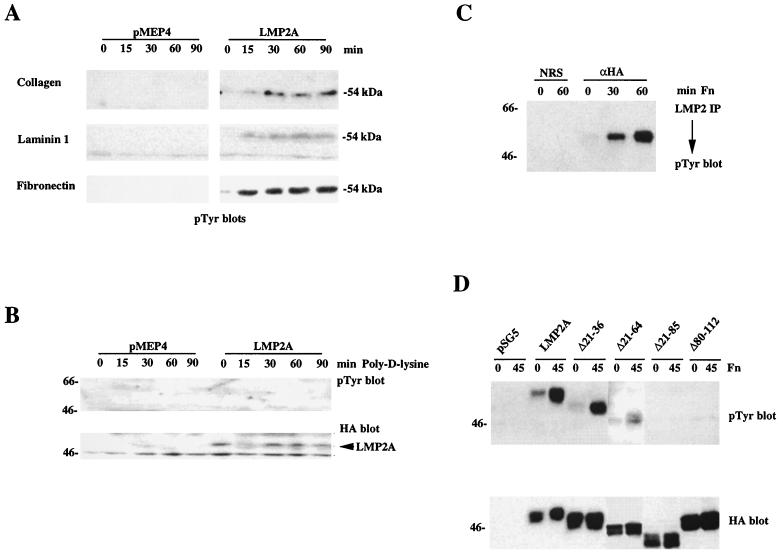
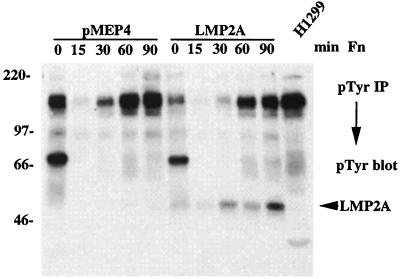
H1299-pMEP4 and H1299-LMP2A cells were induced with CdCl2, detached from the dishes, and replated on different ECM substrates, and changes in tyrosine phosphorylation after cell adhesion were monitored by Western blotting of whole-cell lysates. The major difference between LMP2A-expressing and vector control cells was the appearance of a heavily tyrosine-phosphorylated protein at 54 kDa, the molecular size of LMP2A (Fig. 3A). This effect was observed with several different ECM substrates such as collagen, fibronectin, and laminin 1 but did not occur when cells were nonspecifically adhering to poly-d-lysine (Fig. 3B). In all experiments Western blotting with the anti-HA monoclonal antibody confirmed that LMP2A was expressed at equal levels at all time points (Fig. 3B and data not shown). Phosphorylation of the 54-kDa protein did not increase when the cells were kept in suspension over the length of the time course (data not shown). These results indicate that cell adhesion to various ECM substrates triggers a signaling pathway that leads to tyrosine phosphorylation of LMP2A or of a cellular protein with a similar molecular weight that is induced by LMP2A expression. This event is probably mediated either through several cell surface receptors with different specificities or through a single receptor with pleiotropic specificities, since it occurred upon plating on different ECM substrates. However, the effect was not nonspecific, as an increase in LMP2A phosphorylation could not be detected after adhesion to poly-d-lysine, a substrate that promotes nonspecific cell adhesion through electrostatic interactions without involvement of cell surface receptors (Fig. 3B). Candidate receptors could be members of the integrin family. β1 integrins, for example, can bind to several different ECM proteins through heterodimer formation, depending on their α subunit, and could therefore account for the different ECM substrates that were active in our experiments. The exact nature of these receptors has not yet been determined and will be the subject of further studies.
FIG. 3.
Cell adhesion induces tyrosine phosphorylation of LMP2A in H1299 cells. (A) H1299-pMEP4 and H1299-LMP2A cells were induced with CdCl2, detached from tissue culture dishes, and replated onto collagen, laminin 1, and fibronectin for various amounts of time. Tyrosine phosphorylation was monitored by Western blotting. (B) Cell adhesion to poly-d-lysine does not induce LMP2A phosphorylation. H1299-pMEP4 and H1299-LMP2A cells were detached from the tissue culture dishes and plated on poly-d-lysine-coated dishes. (Upper panel) Phosphotyrosine blot of whole-cell lysates. (Lower panel) Detection of LMP2A protein by HA Western blotting. (C) Immunoprecipitation (IP) from lysates of LMP2A-expressing H1299 cells with polyclonal anti-HA antibodies or with normal rabbit serum (NRS) as a negative control after cell adhesion to fibronectin for various times. LMP2A phosphorylation was detected by anti-phosphotyrosine Western blotting. (D) Analysis of ECM-induced tyrosine phosphorylation of LMP2A deletion mutants. WT LMP2A and the LMP2A deletion mutants were transiently transfected into 293 cells, and the cells were serum starved and either kept in suspension or plated on fibronectin-coated plates for 45 min. (Upper panel) Tyrosine phosphorylation was monitored by immunoblotting. (Lower panel) Expression of the LMP2A deletion mutants as detected by anti-HA Western blotting. Mutants Δ21-36 and Δ21-64 became phosphorylated upon cell adhesion, whereas Δ21-85 and Δ80-112 did not.
Identification of LMP2A as the major phosphorylated protein in response to cell adhesion.
The previous experiments suggested that LMP2A becomes tyrosine phosphorylated in response to cell adhesion. However, it is possible that LMP2A induces the expression of a cellular protein with a molecular weight similar to that of LMP2A whose phosphorylation is induced upon cell-ECM interactions. To distinguish between these two possibilities, LMP2A was immunoprecipitated from lysates of CdCl2-induced H1299-LMP2A cells after plating on fibronectin, and tyrosine phosphorylation was monitored by Western blotting (Fig. 3C). Polyclonal anti-HA antibodies efficiently immunoprecipitated tyrosine-phosphorylated LMP2A, whereas nonspecific immunoprecipitation with normal rabbit serum did not yield any phosphorylated products. LMP2A was only slightly phosphorylated in nonadherent cells, shown at time zero, and the level of tyrosine phosphorylation increased rapidly over a time course of 60 min after plating (Fig. 3C). These data strongly suggest that the major tyrosine-phosphorylated protein observed in epithelial cells after cell adhesion is LMP2A.
To correlate constitutive tyrosine phosphorylation of LMP2A with that induced by adhesion to ECM proteins, the LMP2A deletion mutants were transiently transfected into 293 cells. Tyrosine phosphorylation of WT LMP2A and deletion mutants after stimulation of the cells with fibronectin was monitored by Western blotting. The LMP2A deletion mutants had the same phosphorylation pattern as that observed in vitro, with an adhesion-dependent increase in the tyrosine phosphorylation of LMP2AΔ21-36 and LMP2AΔ21-64 and an absence of phosphorylation of LMP2AΔ21-85 and LMP2AΔ80-112 (Fig. 3D).
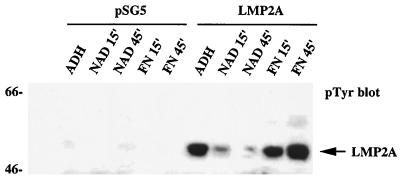
The relationship between the LMP2A phosphorylation status in adherent cells and its change upon cell adhesion was investigated in more detail. Serum-starved 293 cells transiently transfected with an LMP2A expression construct were detached from the culture dish and kept in suspension or plated on fibronectin for various amounts of time in the absence of serum. As detected by immunoblot analysis, LMP2A was phosphorylated in adherent cells, while the level of tyrosine phosphorylation decreased upon detachment of the cells and was nearly undetectable when the cells had been kept in suspension for 45 min. Replating onto fibronectin-coated dishes resulted in the expected rapid increase in LMP2A tyrosine phosphorylation (Fig. 4). These results indicate that LMP2A phosphorylation in epithelial cells is regulated in an adhesion-dependent manner, and they suggest the involvement of phosphatases as well as tyrosine kinases in this process.
FIG. 4.
LMP2A phosphorylation in adherent and nonadherent cells. Serum-starved transiently transfected 293 cells were lysed on the dish (ADH), kept in suspension for the indicated times (NAD), or replated on fibronectin for the indicated times (FN). Tyrosine phosphorylation in whole-cell lysates was monitored by immunoblotting.
To gain further insight into changes in phosphorylation levels of cellular proteins in LMP2A-expressing cells, tyrosine-phosphorylated proteins were immunoprecipitated from H1299 cell lysates at various times after adhesion to fibronectin and detected by immunoblotting with phosphotyrosine antibodies. The appearance of tyrosine-phosphorylated LMP2A protein was the only observable difference between vector and LMP2A-expressing cells. The levels and time courses of tyrosine phosphorylation of other proteins did not seem to be altered significantly (Fig. 5).
FIG. 5.
Tyrosine phosphorylation events in LMP2A-expressing cells. Tyrosine-phosphorylated proteins were immunoprecipitated from H1299-pMEP4 and H1299-LMP2A cell lysates after cell adhesion to fibronectin (Fn), and phosphorylated proteins were detected by anti-phosphotyrosine Western blotting. The rightmost lane shows a phosphotyrosine blot of an immunoprecipitation from adherent parental H1299 cells.
Src family kinases are not involved in LMP2A phosphorylation in epithelial cells, a role for Csk.
It has recently been demonstrated that Y112 is required for LMP2A phosphorylation and interaction with the Src family kinase Lyn in B cells (6, 20). The model proposes that the SH2 domain of Lyn binds to Y112, enabling Lyn to phosphorylate the ITAM, thereby creating docking sites for the tandem SH2 domains of Syk. Our mutational analysis of LMP2A and its phosphorylation in epithelial cells established a role for the ITAM in LMP2A phosphorylation. Tyrosines Y101 and Y112 could represent protein-protein interaction sites for LMP2A with protein kinases. Furthermore, both Src and Fyn are known to play important roles in cell adhesion signaling (2, 10, 13, 43). It was therefore of interest to investigate Src family kinases as plausible candidates for phosphorylating LMP2A in epithelial cells.
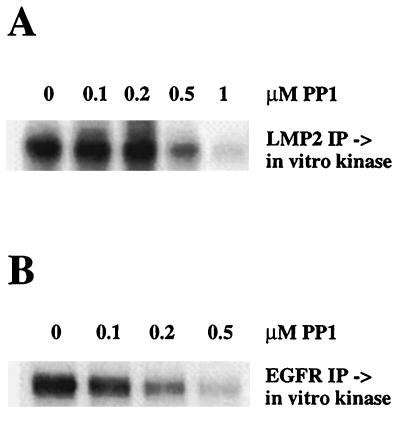
To determine if Src family kinases are responsible for in vitro phosphorylation of LMP2A, LMP2A was immunoprecipitated from CdCl2-induced H1299-LMP2A cells and in vitro kinase assays were performed in the presence of a Src family kinase-specific inhibitor, PP1 (22). The 50% inhibitory concentration (IC50) of PP1 for Src kinases has been reported as between 6 and 170 nM. PP1 was able to inhibit LMP2A phosphorylation only at concentrations greater than 500 nM (Fig. 6A). As a control for the specificity of the compound under our assay conditions, the epidermal growth factor receptor (EGFR) was immunoprecipitated from EGF-stimulated H1299 cells and autophosphorylation activity was assessed by an in vitro kinase assay in the presence of increasing concentrations of PP1. PP1 started to inhibit EGFR autophosphorylation at concentrations of 200 nM (Fig. 6B). The reported IC50 for EGFR phosphorylation has been reported as 250 nM (22). Therefore, PP1 is able to inhibit LMP2A phosphorylation in vitro only at concentrations that exceed its specificity for Src family kinases. Furthermore, we have not been able to detect a specific association of LMP2A and the Src family kinases Src, Fyn, and Yes, which are expressed in epithelial cells (data not shown). These data suggest that in epithelial cells, in contrast to results obtained in B lymphocytes, the phosphorylation of LMP2A in vitro is not dependent on Src family kinases.
FIG. 6.
Src family kinases do not phosphorylate LMP2A in vitro. (A) LMP2A was immunoprecipitated from H1299-LMP2A cells induced with CdCl2. In vitro kinase assays were performed in the presence of increasing concentrations of the Src family-specific inhibitor PP1. (B) The EGFR was immunoprecipitated from EGF-stimulated H1299 cells, and autophosphorylation activity was assessed by an in vitro kinase assay in the presence of increasing concentrations of PP1.
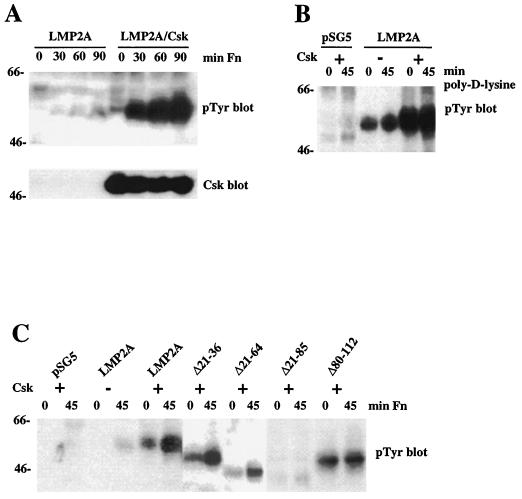
To further investigate the involvement of Src family kinases in LMP2A phosphorylation, LMP2A and Csk, a negative regulator of Src kinases (36), were coexpressed in H1299 or 293 human kidney epithelial cells. If Src kinases were responsible for the adhesion-dependent increase in LMP2A phosphorylation, the expression of Csk would be expected to exert an inhibitory effect. Surprisingly, it was found that Csk overexpression significantly increased the adhesion-dependent phosphorylation of LMP2A in H1299 cells (Fig. 7A). The level of LMP2A tyrosine phosphorylation in nonadherent cells induced by Csk overexpression was higher in 293 cells than in H1299 cells (Fig. 7B), most likely reflecting the increased transfection efficiency of 293 cells.
FIG. 7.
Coexpression of LMP2A and Csk leads to increased LMP2A phosphorylation after cell adhesion. (A) Transiently transfected LMP2A-expressing and LMP2A- and Csk-coexpressing H1299 cells were detached from tissue culture dishes and replated on fibronectin (Fn) for the indicated times. Tyrosine phosphorylation was assayed by Western blotting (upper panel). Expression of Csk was detected by anti-Csk Western blotting (lower panel). (B) Transiently transfected 293 cells, expressing Csk, LMP2A, or both LMP2A and Csk, were plated on poly-d-lysine-coated dishes, and tyrosine phosphorylation of LMP2A was monitored by Western blotting. No adhesion-dependent increase of tyrosine phosphorylation was detected. Note that constitutive LMP2A phosphorylation in nonadherent 293 cells is higher than that in H1299 cells. (C) Csk and LMP2A deletion mutants were cotransfected into 293 cells, detached from tissue culture dishes, and plated on fibronectin for the indicated times. Tyrosine-phosphorylated proteins were visualized by Western blotting. LMP2AΔ21-85 was not significantly phosphorylated, whereas WT LMP2A, LMP2AΔ21-36, and LMP2AΔ21-64 showed Csk- and adhesion-dependent increases in their phosphotyrosine contents. LMP2AΔ80-112 was phosphorylated, but an increase upon cell adhesion was not detected.
These results suggest that Src family kinases are not involved in the phosphorylation of LMP2A and that Csk may contribute to LMP2A phosphorylation in response to cell adhesion. In agreement with this hypothesis, nonspecific adhesion of LMP2A- and Csk-coexpressing 293 cells to poly-d-lysine did not result in an increase in LMP2A phosphorylation, although LMP2A was found to be phosphorylated to higher levels in nonadherent cells than in the absence of Csk (Fig. 7B).
To further characterize Csk-mediated phosphorylation of LMP2A, Csk and the LMP2A deletion mutants were coexpressed in 293 cells. After plating on fibronectin, tyrosine phosphorylation of LMP2 mutants was examined by Western blotting (Fig. 7C). Again, LMP2A, LMP2AΔ21-36, and LMP2AΔ21-64 became phosphorylated to higher levels, suggesting that their phosphorylation can be induced by Csk expression. LMP2AΔ21-85 was not phosphorylated, in agreement with the in vitro data. LMP2AΔ80-112, however, showed a significant level of tyrosine phosphorylation when Csk was coexpressed, in contrast to its behavior in vitro or when expressed alone and stimulated with fibronectin. Interestingly, this increase in phosphotyrosine content appeared not to be adhesion dependent (Fig. 7C), suggesting that an important feature of LMP2A is deleted in this mutant.
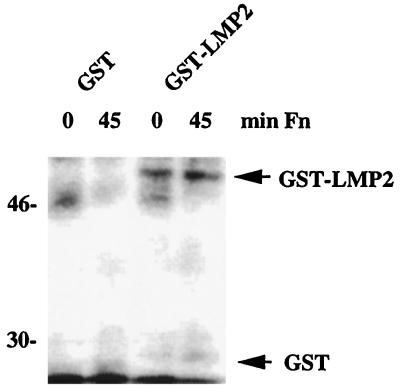
To determine whether LMP2A is a substrate for Csk, Csk was immunoprecipitated from 293 cells transiently transfected with a Csk expression construct. The immune complexes were incubated in in vitro kinase assays together with either GST or a GST-LMP2 fusion protein as the substrate. Although phosphorylation of GST was barely detectable, GST-LMP2 was efficiently phosphorylated (Fig. 8). In this assay no difference in the ability to phosphorylate GST-LMP2 was observed between Csk immunoprecipitated from nonadherent cells and Csk immunoprecipitated from cells stimulated with fibronectin. It has been suggested that Csk activity for Src kinases is dependent on the translocation of Csk to sites of Src activity and is most likely mediated by protein-protein interactions involving the Csk SH2 and SH3 domains (12, 17, 23, 47). If Csk acts on LMP2A in a similar manner, an adhesion-mediated Csk activation event that relies on Csk transport to LMP2A would not be detectable in this assay, because the GST-LMP2 fusion protein substrate is added in solution. These data suggest that Csk can phosphorylate LMP2A in vitro. It is also possible that a protein kinase coprecipitating with Csk is responsible for phosphorylation of the GST-LMP2 fusion protein.
FIG. 8.
In vitro phosphorylation of GST-LMP2 by Csk. 293 cells were transiently transfected with a Csk expression construct, and Csk was immunoprecipitated from unstimulated cells or cells plated on fibronectin (Fn) for 45 min. In vitro kinase assays were performed in the presence of GST or GST-LMP2 (used in equal amounts, as exogenous substrates) and [γ-32P]ATP. Reaction products were separated by SDS-PAGE and detected by autoradiography.
DISCUSSION
The EBV LMP2 gene is expressed in several diseases associated with EBV infection in both lymphoid and epithelial tissues. Most studies have focused on the role of LMP2 in B cells, the primary compartment of EBV latency. The role of LMP2 in epithelial cells has not been determined. The function of LMP2 in epithelial cells and its mode of action are likely to be different from those in B lymphocytes due to differences in the expression of signaling molecules and the presence and importance of different signaling pathways in these two cell types. In B cells, LMP2A interferes with signal transduction from the B-cell receptor complex, a cell surface receptor protein complex that is absent in epithelial cells. Furthermore, LMP2A has profound effects on the activation of the protein kinases Lyn and Syk in response to B-cell receptor cross-linking. Expression of both protein kinases is restricted to cells of the lymphoid lineage. Other Src family kinases, such as Src, Fyn, and Yes, are expressed in epithelial cells, whereas homologs of Syk in epithelial cells have not been identified.
The experiments described in this study demonstrate that LMP2A is tyrosine phosphorylated in epithelial cells. LMP2A was found to be constitutively phosphorylated in adherent cells and physically associated with protein kinase activities. Plating of LMP2A-expressing cells on various ECM proteins resulted in the rapid induction of LMP2A tyrosine phosphorylation. The observation that this induction of tyrosine phosphorylation occurred both in the presence and in the absence of serum (data not shown) strongly argues that LMP2A is phosphorylated by a kinase(s) that is activated upon cell-matrix interaction and is not dependent on growth factor receptor signaling. Loss of cell adhesion resulted in a rapid decrease in the phosphotyrosine content of LMP2A, suggesting the involvement of phosphatases in regulating this process and a requirement for cell anchorage in maintaining LMP2A tyrosine phosphorylation in epithelial cells. Cell-ECM interaction-induced LMP2A phosphorylation was observed with several different ECM substrates but was not detectable when the cells were plated on a nonspecific-adhesion-promoting substrate, poly-d-lysine. This indicates that either multiple different cell surface receptors can induce LMP2A phosphorylation or this event is mediated by a single promiscuous receptor. Integrins are heterodimeric cell surface receptors composed of α and β subunits, with different heterodimers demonstrating different specificities. The α5β1 receptor, for example, acts as a fibronectin receptor on keratinocytes, whereas the α2β1 integrin is a collagen receptor (1, 9). Furthermore, integrins are involved in the initiation and coordination of multiple signaling processes, with induction of tyrosine phosphorylation of cellular proteins being one of the earliest events after integrin engagement (7, 11, 24, 26, 27, 43). Therefore, integrins are likely candidate receptors to mediate the cell adhesion-dependent phosphorylation of LMP2A.
LMP2A in B cells is phosphorylated by Src family kinases which also play important roles in cell adhesion-mediated signaling (10, 13, 24, 43). However, the data presented in this study, using a Src-specific inhibitor, suggest that in epithelial cells, LMP2A does not seem to be phosphorylated by Src family kinases in vitro, since LMP2A phosphorylation was decreased only when inhibitor concentrations that are beyond the range specific for Src kinases were reached. This result was substantiated by the finding that expression of Csk, a negative regulator of Src kinases, did not lead to an inhibition of adhesion-mediated LMP2A phosphorylation. Unexpectedly, it was found that Csk significantly enhanced LMP2A phosphorylation in response to stimulation with fibronectin. The latter finding is particularly important because it argues against a nonspecific phosphorylation event mediated by Csk. Interestingly, coexpression of the focal adhesion kinase Fak, which is rapidly activated following cell adhesion, and LMP2A did not increase LMP2A phosphorylation (data not shown). Additionally, plating of LMP2A- and Csk-coexpressing cells on poly-d-lysine did not result in an adhesion-dependent increase in LMP2A phosphorylation, confirming the requirement for an appropriate ECM substrate. Taken together, these results indicate that Csk-mediated phosphorylation of LMP2A is not a nonspecific phenomenon due simply to overexpression of the kinase. In addition, Csk was able to phosphorylate a GST-LMP2 fusion protein in vitro, demonstrating that LMP2A is a substrate for Csk. However, it is also possible that another kinase, coprecipitating with Csk, might be responsible for phosphorylation of the GST-LMP2 fusion protein.
The role of Csk in cell adhesion-mediated signaling is not well understood. Csk has been demonstrated to physically interact with the cytoskeletal protein paxillin as well as the focal adhesion kinase Fak (48). These protein-protein interactions might be responsible for bringing Csk into the proximity of sites of Src activity, which is also found in focal adhesions. Csk phosphorylates Src at a C-terminal tyrosine, thereby creating a docking site within Src for the Src SH2 domain. The intramolecular interaction which occurs leads to a closed, inactive conformation (14). It will be important to determine the functional interactions among LMP2A, Csk, Src, and focal adhesion proteins, their phosphorylation, and their activities upon cell adhesion.
Investigation of LMP2A deletion mutants indicated that the same regions that are important for LMP2A function in B cells are important for its phosphorylation in epithelial cells. Mutants with deletions of the ITAM, Y112, and Y101, a possible Csk SH2 domain-binding site, did not become phosphorylated in vitro and upon cell adhesion. One difference from B lymphocytes was that the mutant LMP2AΔ21-85, which was found to be phosphorylated and associated with Src kinases in B cells (20), was not phosphorylated in epithelial cells. Studies in B cells further established tyrosine Y112 as important for LMP2A phosphorylation (20). This suggests a different role for the YEEA motif surrounding tyrosine Y112 in epithelial cells. In addition, LMP2AΔ80-112 was not phosphorylated in B cells but was found to be phosphorylated in epithelial cells when Csk was overexpressed. These results suggest that an interaction of the Csk SH2 domain at Y101 might be required to allow specific LMP2A phosphorylation at the ITAM when only endogenous Csk is present. In the presence of overexpressed Csk, LMP2A may be nonspecifically phosphorylated at other residues without a requirement for an interaction of the Csk SH2 domain with Y101 of LMP2A. The fact that an adhesion-dependent increase in phosphorylation of LMP2AΔ80-112 was not observed indicates that an important feature of LMP2A is deleted in this mutant. Interestingly, tyrosine 74, part of the ITAM, is found in the context of a YQP motif. The C-terminal tyrosines of Src kinases, such as Src, Lck, Fyn, and Yes, which are phosphorylated by Csk, are likewise found within a YQP sequence, although the surrounding residues bear little homology to the sequence surrounding Y74 in LMP2A. The analysis of point mutants will clarify if Y74 indeed represents a phosphorylation site for Csk. A physical interaction between Csk and LMP2A is likely to be very weak, since Csk protein was not detected in LMP2A immunoprecipitates (data not shown). However, a stable association of Csk with its known substrates Src and Lck has likewise not been detected (3, 44). The possibility that other unidentified kinases physically interact with LMP2A and phosphorylate it in vitro is not excluded.
Cell-ECM interactions play an important role in regulating epithelial-cell behavior. In squamous epithelium, for example, ECM receptors such as integrins are predominantly expressed in the basal proliferating cell layer, where contact with the basement membrane occurs. Differentiating cells detach from the basement membrane and form the upper layers of the epithelium. At the same time, integrin expression is downregulated and the cellular proliferative capacity is lost (1, 21). It is conceivable that LMP2A expression interferes with or enhances several aspects of ECM signaling, altering signals and keeping the epithelial cell in a state that is beneficial for the virus. Prevention of differentiation, for example, would allow the virus to persist and replicate in a cell that does not undergo the biochemical changes that take place during differentiation and result in metabolically dead cells that are sloughed off at the surface of the epithelium. It has been reported that expression of LMP1 in keratinocytes or keratinocyte cell lines can inhibit epithelial-cell differentiation (15, 16). It will be interesting to see if LMP2A can further modulate or alter epithelial-cell differentiation, possibly through a functional interplay with LMP1.
Cell-ECM interactions also play important roles in the regulation of cell migration and, in the case of neoplastic cells, in cell invasion and metastasis (43). These processes are profoundly affected by integrin expression and signaling. Epithelial malignancies often display altered levels of integrin expression, with up- or downregulation of specific members of the family, and in multiple systems these effects correlate with altered migration or invasive behavior. As NPC is a highly invasive cancer, it is possible that the expression of LMP2 in NPC contributes to the invasive phenotype by altering signals from the ECM. It will be important to determine the exact molecular processes that are affected by LMP2 expression in epithelial cells in order to understand LMP2 function in this cell type.
The results presented in this study strongly suggest that there is a function for LMP2 in epithelial cells and that, in analogy to the scenario in B cells, it seems to affect cellular signal transduction processes. The final outcome of impinging on these processes is likely to be different in B cells and epithelial cells, demonstrating how the virus can adapt to different cellular environments and alter cell behavior to its advantage, using the same molecule with a different function in a different context.
ACKNOWLEDGMENTS
We thank Jennifer Webster-Cyriaque and Keith Burridge for critical reading of the manuscript and William E. Miller and Michael D. Schaller for helpful discussions.
This work was supported by Public Health Service grants CA19014 and CA32979 from the National Institutes of Health to N.R.-T.
REFERENCES
- 1.Adams J C, Watt F M. Changes in keratinocyte adhesion during terminal differentiation: reduction in fibronectin binding precedes alpha 5 beta 1 integrin loss from the cell surface. Cell. 1990;63:425–435. doi: 10.1016/0092-8674(90)90175-e. [DOI] [PubMed] [Google Scholar]
- 2.Bockholt S M, Burridge K. An examination of focal adhesion formation and tyrosine phosphorylation in fibroblasts isolated from src−, fyn−, and yes− mice. Cell Adhesion Commun. 1995;3:91–100. doi: 10.3109/15419069509081279. [DOI] [PubMed] [Google Scholar]
- 3.Bougeret C, Delaunay T, Romero F, Jullien P, Sabe H, Hanafusa H, Benarous R, Fischer S. Detection of a physical and functional interaction between Csk and Lck which involves the SH2 domain of Csk and is mediated by autophosphorylation of Lck on tyrosine 394. J Biol Chem. 1996;271:7465–7472. doi: 10.1074/jbc.271.13.7465. [DOI] [PubMed] [Google Scholar]
- 4.Brooks L, Yao Q Y, Rickinson A B, Young L S. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J Virol. 1992;66:2689–2697. doi: 10.1128/jvi.66.5.2689-2697.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks L A, Lear A L, Young L S, Rickinson A B. Transcripts from the Epstein-Barr virus BamHI A fragment are detectable in all three forms of virus latency. J Virol. 1993;67:3182–3190. doi: 10.1128/jvi.67.6.3182-3190.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burkhardt A L, Bolen J B, Kieff E, Longnecker R. An Epstein-Barr virus transformation-associated membrane protein interacts with Src family tyrosine kinases. J Virol. 1992;66:5161–5167. doi: 10.1128/jvi.66.8.5161-5167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burridge K, Turner C E, Romer L H. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busson P, McCoy R, Sadler R, Gilligan K, Tursz T, Raab-Traub N. Consistent transcription of the Epstein-Barr virus LMP2 gene in nasopharyngeal carcinoma. J Virol. 1992;66:3257–3262. doi: 10.1128/jvi.66.5.3257-3262.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter W G, Wayner E A, Bouchard T S, Kaur P. The role of integrins alpha 2 beta 1 and alpha 3 beta 1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol. 1990;110:1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cary L A, Chang J F, Guan J L. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J Cell Sci. 1996;109:1787–1794. doi: 10.1242/jcs.109.7.1787. [DOI] [PubMed] [Google Scholar]
- 11.Clark E A, Brugge J S. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 12.Cloutier J F, Chow L M, Veillette A. Requirement of the SH3 and SH2 domains for the inhibitory function of tyrosine protein kinase p50csk in T lymphocytes. Mol Cell Biol. 1995;15:5937–5944. doi: 10.1128/mcb.15.11.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cobb B S, Schaller M D, Leu T H, Parsons J T. Stable association of pp60src and pp59fyn with the focal adhesion-associated protein tyrosine kinase, pp125FAK. Mol Cell Biol. 1994;14:147–155. doi: 10.1128/mcb.14.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper J A, Howell B. The when and how of Src regulation. Cell. 1993;73:1051–1054. doi: 10.1016/0092-8674(93)90634-3. [DOI] [PubMed] [Google Scholar]
- 15.Dawson C W, Rickinson A B, Young L S. Epstein-Barr virus latent membrane protein inhibits human epithelial cell differentiation. Nature. 1990;344:777–780. doi: 10.1038/344777a0. [DOI] [PubMed] [Google Scholar]
- 16.Fahraeus R, Rymo L, Rhim J S, Klein G. Morphological transformation of human keratinocytes expressing the LMP gene of Epstein-Barr virus. Nature. 1990;345:447–449. doi: 10.1038/345447a0. [DOI] [PubMed] [Google Scholar]
- 17.Ford C E, Furlong M T, Geahlen R L, Harrison M L. Signaling-induced association of a tyrosine-phosphorylated 36-kDa protein with p50csk. J Biol Chem. 1994;269:30378–30385. [PubMed] [Google Scholar]
- 18.Fruehling S, Lee S K, Herrold R, Frech B, Laux G, Kremmer E, Grasser F A, Longnecker R. Identification of latent membrane protein 2A (LMP2A) domains essential for the LMP2A dominant-negative effect on B-lymphocyte surface immunoglobulin signal transduction. J Virol. 1996;70:6216–6226. doi: 10.1128/jvi.70.9.6216-6226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fruehling S, Longnecker R. The immunoreceptor tyrosine-based activation motif of Epstein-Barr virus LMP2A is essential for blocking BCR-mediated signal transduction. Virology. 1997;235:241–251. doi: 10.1006/viro.1997.8690. [DOI] [PubMed] [Google Scholar]
- 20.Fruehling S, Swart R, Dolwick K M, Kremmer E, Longnecker R. Tyrosine 112 of latent membrane protein 2a is essential for protein tyrosine kinase loading and regulation of Epstein-Barr virus latency. J Virol. 1998;72:7796–7806. doi: 10.1128/jvi.72.10.7796-7806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuchs E, Byrne C. The epidermis: rising to the surface. Curr Opin Genet Dev. 1994;4:725–736. doi: 10.1016/0959-437x(94)90140-x. [DOI] [PubMed] [Google Scholar]
- 22.Hanke J H, Gardner J P, Dow R L, Changelian P S, Brissette W H, Weringer E J, Pollok B A, Connelly P A. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 23.Howell B W, Cooper J A. Csk suppression of Src involves movement of Csk to sites of Src activity. Mol Cell Biol. 1994;14:5402–5411. doi: 10.1128/mcb.14.8.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juliano R L, Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993;120:577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kieff E. Epstein-Barr virus and its replication. In: Fields B, Knipe D, Howley P, editors. Fields virology. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2343–2396. [Google Scholar]
- 26.Kornberg L, Earp H S, Parsons J T, Schaller M, Juliano R L. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem. 1992;267:23439–23442. [PubMed] [Google Scholar]
- 27.Kornberg L J, Earp H S, Turner C E, Prockop C, Juliano R L. Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of beta 1 integrins. Proc Natl Acad Sci USA. 1991;88:8392–8396. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lennette E T, Winberg G, Yadav M, Enblad G, Klein G. Antibodies to LMP2A/2B in EBV-carrying malignancies. Eur J Cancer. 1995;31A:1875–1878. doi: 10.1016/0959-8049(95)00354-l. [DOI] [PubMed] [Google Scholar]
- 29.Longnecker R, Druker B, Roberts T M, Kieff E. An Epstein-Barr virus protein associated with cell growth transformation interacts with a tyrosine kinase. J Virol. 1991;65:3681–3692. doi: 10.1128/jvi.65.7.3681-3692.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Longnecker R, Kieff E. A second Epstein-Barr virus membrane protein (LMP2) is expressed in latent infection and colocalizes with LMP1. J Virol. 1990;64:2319–2326. doi: 10.1128/jvi.64.5.2319-2326.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Longnecker R, Miller C L. Regulation of Epstein-Barr virus latency by latent membrane protein 2. Trends Microbiol. 1996;4:38–42. doi: 10.1016/0966-842x(96)81504-6. [DOI] [PubMed] [Google Scholar]
- 32.Miller C L, Burkhardt A L, Lee J H, Stealey B, Longnecker R, Bolen J B, Kieff E. Integral membrane protein 2 of Epstein-Barr virus regulates reactivation from latency through dominant negative effects on protein-tyrosine kinases. Immunity. 1995;2:155–166. doi: 10.1016/s1074-7613(95)80040-9. [DOI] [PubMed] [Google Scholar]
- 33.Miller C L, Lee J H, Kieff E, Burkhardt A L, Bolen J B, Longnecker R. Epstein-Barr virus protein LMP2A regulates reactivation from latency by negatively regulating tyrosine kinases involved in sIg-mediated signal transduction. Infect Agents Dis. 1994;3:128–136. [PubMed] [Google Scholar]
- 34.Miller C L, Lee J H, Kieff E, Longnecker R. An integral membrane protein (LMP2) blocks reactivation of Epstein-Barr virus from latency following surface immunoglobulin crosslinking. Proc Natl Acad Sci USA. 1994;91:772–776. doi: 10.1073/pnas.91.2.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller C L, Longnecker R, Kieff E. Epstein-Barr virus latent membrane protein 2A blocks calcium mobilization in B lymphocytes. J Virol. 1993;67:3087–3094. doi: 10.1128/jvi.67.6.3087-3094.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okada M, Nada S, Yamanashi Y, Yamamoto T, Nakagawa H. CSK: a protein-tyrosine kinase involved in regulation of src family kinases. J Biol Chem. 1991;266:24249–24252. [PubMed] [Google Scholar]
- 37.Panousis C G, Rowe D T. Epstein-Barr virus latent membrane protein 2 associates with and is a substrate for mitogen-activated protein kinase. J Virol. 1997;71:4752–4760. doi: 10.1128/jvi.71.6.4752-4760.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pathmanathan R, Prasad U, Sadler R, Flynn K, Raab-Traub N. Clonal proliferations of cells infected with Epstein-Barr virus in preinvasive lesions related to nasopharyngeal carcinoma. N Engl J Med. 1995;333:693–698. doi: 10.1056/NEJM199509143331103. . (See comments.) [DOI] [PubMed] [Google Scholar]
- 39.Raab-Traub N. Epstein-Barr virus and nasopharyngeal carcinoma. Semin Cancer Biol. 1992;3:297–307. [PubMed] [Google Scholar]
- 40.Raab-Traub N, Flynn K. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell. 1986;47:883–889. doi: 10.1016/0092-8674(86)90803-2. [DOI] [PubMed] [Google Scholar]
- 41.Raab-Traub N, Flynn K, Klein C, Pizza G, De Vinci C, Occhiuzzi L, Farneti G, Caliceti U, Pirodda E. EBV-associated malignancies. J Exp Pathol. 1987;3:449–456. [PubMed] [Google Scholar]
- 42.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. . (Letter.) [PubMed] [Google Scholar]
- 43.Rosales C, Juliano R L. Signal transduction by cell adhesion receptors in leukocytes. J Leukoc Biol. 1995;57:189–198. doi: 10.1002/jlb.57.2.189. [DOI] [PubMed] [Google Scholar]
- 44.Sabe H, Knudsen B, Okada M, Nada S, Nakagawa H, Hanafusa H. Molecular cloning and expression of chicken C-terminal Src kinase: lack of stable association with c-Src protein. Proc Natl Acad Sci USA. 1992;89:2190–2194. doi: 10.1073/pnas.89.6.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sample J, Liebowitz D, Kieff E. Two related Epstein-Barr virus membrane proteins are encoded by separate genes. J Virol. 1989;63:933–937. doi: 10.1128/jvi.63.2.933-937.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Songyang Z, Shoelson S E, McGlade J, Olivier P, Pawson T, Bustelo X R, Barbacid M, Sabe H, Hanafusa H, Yi T, Ren R, Baltimore D, Ratnofsky S, Feldman R A, Cantley L C. Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk, and Vav. Mol Cell Biol. 1994;14:2777–2785. doi: 10.1128/mcb.14.4.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Superti-Furga G, Fumagalli S, Koegl M, Courtneidge S A, Draetta G. Csk inhibition of c-Src activity requires both the SH2 and SH3 domains of Src. EMBO J. 1993;12:2625–2634. doi: 10.1002/j.1460-2075.1993.tb05923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tremblay L, Hauck W, Aprikian A G, Begin L R, Chapdelaine A, Chevalier S. Focal adhesion kinase (pp125FAK) expression, activation and association with paxillin and p50CSK in human metastatic prostate carcinoma. Int J Cancer. 1996;68:164–171. doi: 10.1002/(sici)1097-0215(19961009)68:2<169::aid-ijc4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 49.Webster-Cyriaque J, Raab-Traub N. Transcription of Epstein-Barr virus latent cycle genes in oral hairy leukoplakia. Virology. 1998;248:53–65. doi: 10.1006/viro.1998.9268. [DOI] [PubMed] [Google Scholar]
- 50.Weiss A, Littman D R. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 51.Young L S, Dawson C W, Clark D, Rupani H, Busson P, Tursz T, Johnson A, Rickinson A B. Epstein-Barr virus gene expression in nasopharyngeal carcinoma. J Gen Virol. 1988;69:1051–1065. doi: 10.1099/0022-1317-69-5-1051. [DOI] [PubMed] [Google Scholar]