Abstract
This study investigated how gene expression is affected by dietary fatty acids (FA) by using pigs as a reliable model for studying human diseases that involve lipid metabolism. This includes changes in FA composition in the liver, blood serum parameters and overall metabolic pathways. RNA-Seq data from 32 pigs were analyzed using Weighted Gene Co-expression Network Analysis (WGCNA). Our aim was to identify changes in blood serum parameters and gene expression between diets containing 3% soybean oil (SOY3.0) and a standard pig production diet containing 1.5% soybean oil (SOY1.5). Significantly, both the SOY1.5 and SOY3.0 groups showed significant modules, with a higher number of co-expressed modules identified in the SOY3.0 group. Correlated modules and specific features were identified, including enriched terms and pathways such as the histone acetyltransferase complex, type I diabetes mellitus pathway, cholesterol metabolism, and metabolic pathways in SOY1.5, and pathways related to neurodegeneration and Alzheimer’s disease in SOY3.0. The variation in co-expression observed for HDL in the groups analyzed suggests different regulatory patterns in response to the higher concentration of soybean oil. Key genes co-expressed with metabolic processes indicative of diseases such as Alzheimer’s was also identified, as well as genes related to lipid transport and energy metabolism, including CCL5, PNISR, DEGS1. These findings are important for understanding the genetic and metabolic responses to dietary variation and contribute to the development of more precise nutritional strategies.
Keywords: lipid metabolism, WGCNA, co-expression, RNA-seq, transcriptome, soybean oil, immune response, systems biology
1 Introduction
Fatty acids (FA) have an important role in controlling gene expression and regulating various signaling pathways by binding to specific transcription factors (Larsen et al., 2018). The ability to influence gene transcription impacts lipid metabolism, such as polyunsaturated fatty acids (PUFA) modulating low-density lipoprotein (LDL) and other genes related to lipid metabolism (Denke, 2006; Larsen et al., 2018). According to Larsen et al. (2018), a dietary FA alters plasma lipids, thereby affecting the risk of cardiovascular disease (CVD). Therefore, maintaining cholesterol balance is vital for a healthy life, including a healthy liver, which plays an important role in lipid metabolism and balance (Cohen, 2008).
The liver is responsible for eliminating excess cholesterol that is carried on lipoprotein particles such as high-density lipoprotein (HDL), intermediate-density lipoprotein (IDL), LDL, and remnants of chylomicrons (Maxfield and Tabas, 2005). In addition, very low-density lipoprotein (VLDL) is composed primarly of triglycerides that are assembled in the liver and then released into the bloodstream (Wondmkun, 2020; Zhou and Sun, 2021). Moreover, in lipid metabolism, compounds such as albumin, a predominant protein in mammalian blood plasma, function as carriers of compounds, including FA, facilitating their transport into cells (Vusse, 2009; Junk et al., 2010).
Pork consumption has economic importance and nutritional value for cultural consumers, including the FA profile, which has a direct impact on human health (OECD-FAO, 2021; Fanalli et al., 2022a). The consumption of unsaturated fatty acids, such as linoleic acid (LA, C18:2 cis 9, 12) and oleic acid (OA, C18:1 cis 9) has been related with health benefits, such as reduction of total and LDL cholesterol (Lunn and Theobald, 2006) and beneficial in anti-inflammatory and vascular activities (Sakurai et al., 2021), respectively. Furthermore, feeding pigs diets that are rich in vegetable oils containing unsaturated FA can lead to the production of healthier meat products, depending on changes in the animal’s lipid profile (Alencar et al., 2021; Fanalli et al., 2022c)
The chemical composition of blood serum in pigs reflects the health, nutritional status, and breeding conditions to which they have been exposed. Plasma protein levels have already been used to study the genetic control of disease resilience in pigs (Chen et al., 2023). As a result, the molecular contributions of variation on blood calcium (Ca) and phosphorus (P) levels and identifying putative genes and associated QTL regions (Reyer et al., 2019) and serum parameters for predict total Ca, P intake and identify biomarkers (Vötterl et al., 2021). However, studies on the biochemical parameters in pigs remain limited. Pigs are widely acknowledged as a reliable model for studying human diseases that involve lipid metabolism, such as diabetes, metabolic syndrome, obesity, and cardiovascular disease (Miller and Ullrey, 1987; Zhang et al., 2020; Pan et al., 2021; da Silva et al., 2023).
In this context, identifying gene networks associated with biological pathways helps us understand the mechanism of gene regulation in the liver of pigs fed diets with different levels of soybean oil. The Weighted Correlation Network Analysis (WGCNA) is widely used and has a crucial aspect of systems biology approaches (Kogelman et al., 2014). In our previous study, the FA profile influenced gene expression, resulting in the identification of 281 differentially expressed genes (DEG) among animals that consumed soybean oil at different levels (1.5% and 3%). This changed the composition of FA deposited in the liver, blood parameters, and metabolic pathways, as well as the network of processes in the liver of the animals (Fanalli et al., 2022c). Therefore, the aim of this study was to further investigate and analyze the relationships between the expressed genes and blood serum parameters. We investigated the systemic biological effects of different levels of soybean oil (1.5% and 3%) added to the diet using the WGCNA method.
2 Methods
Ethics statement
The experimental procedures involving animals are in accordance with the requirements of the Animal Care and Use Committee of Luiz de Queiroz College of Agriculture’s requirements (University of São Paulo, Piracicaba, Brazil; protocol: 2018.5.1787.11.6 and CEUA number: 2018–28) and are conducted in accordance with Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching (Fass, 2010).
2.1 Animal, and experimental diets
In this study, we used 32 immunocastrated male Large White breed pigs that were homozygous negative for the halothane gene (NN). Animals used in the study were genotyped for the malignant hypothermia mutation in the RYR1 gene (Fujii et al., 1991). The feeding trial included the growth and finishing phases (98 days); for more information on the animals, diet and experimental design, can be found in from Almeida et al. (2021); Fanalli et al. (2022b).
Throughout the experimental period, all pigs had ad libitum access to food and water. The experimental diets comprised formulations based on corn and soybean meal. The experimental diets consisted of corn–soybean meal growing–finishing diets, supplemented with either 1.5% soybean oil (SOY1.5) or 3% soybean oil (SOY3.0) These formulations were adjusted based on the growth and finishing phases, with the percentages representing the proportion of soybean oil in relation to the total diet formulation. The diets were formulated to meet or exceed requirements (Rostagno, 2011). In the SOY1.5 group, 16 animals received a diet containing 1.5% soybean oil, and in the SOY3.0 group, 16 animals received a diet containing 1.5% soybean oil (Supplementary Table S1).
2.2 Blood biochemical parameters
Phenotypic information included serum parameters obtained from animal blood. Blood samples were collected from the jugular vein 4 days before slaughter and immediately transferred to non-anticoagulant vacuum tubes (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ, United States) as described previously (Fanalli et al., 2022b). The parameters used for analysis were aspartate aminotransferase (AST), albumin, glucose, total protein, triglycerides, globulin, cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and very low-density lipoprotein (VLDL).
Analyzes included serum lipids and biochemistry were analyzed by the Mindray, BS120 (Guangdong, China). Quantification of total cholesterol and fractions was also performed by the enzymatic-colorimetric method, but by selective precipitation. This procedure was carried out using commercial kits following the manufacturer’s instructions. Blood serum glucose was quantified by the colorimetric enzymatic method according to Trinder (Trinder, 1969), using commercial kits according to the manufacturer’s recommended use. The analysis for the determination of total proteins was performed with commercial kits, following the protocol suggested by the manufacturer, using the Biureto method with some modifications (Fanalli et al., 2022b).
2.3 RNA-Seq and data analysis
Briefly, total RNA was extracted from the right hepatic lobe. All samples were then sequenced using the TruSeq PE Cluster Kit v4-cBot-HS kit (Illumina, San Diego, CA, United States). Clustering and sequencing were performed on the HiSeq 2500 instrument (Illumina, San Diego, CA, United States) using a TruSeq SBS Kit v4-HS (200 cycles) according to the manufacturer’s instructions (Fanalli et al., 2023).
The sample accession of the mRNA expression data of the PRJEB50513 [http://www.ebi.ac.uk/ena/data/view/PRJEB50513].
The RNA-Seq data quality control was checked using the FastQC, v.0.11.9 [https://www.bioinformatics.babraham.ac.uk/projects/fastqc]. To remove adapters and bases with low PHRED scores we use Trim Galore v. 0.6.5. The tagging of duplicate reads was performed using the Picard Mark Duplicates v. 1.8.x [https://broadinstitute.github.io/picard/]. The initial ten nucleotides of each read were removed.
Quality control and reads statistics were estimated using the RNA-Seq data analysis pipeline used by the EURO-FAANG group within the scope of the H2020 projects, namely, the BovReg project. The entire analysis of workflow was performed by a Nextflow manager (DI Tommaso et al., 2017). Briefly, after selecting high quality reads, they were mapped to the reference genome Sus scrofa 11.1 [http://www.ensembl.org/Sus_scrofa/Info/Index] using the Bowtie2 v.2.4.3 and RNA-Seq by Expectation Maximization (RSEM) v. 1.3.1 were used for the estimation of expression values (Li and Dewey, 2011).
2.4 Network analysis with weighted correlation network analysis (WGCNA)
The phenotypic data were centered and scaled (Li et al., 2018; Diniz et al., 2019), then a linear model was fitted in R (R Core Team, 2023), prior to analyze co-expression. An adjustment was performed using the equation: , where represents the adjusted/corrected phenotype, is the general mean vector for phenotypes, is the vector of phenotypes, is the incidence matrix for fixed effects, is the vector of fixed effects, and is the residual vector. Fixed effects including block and sire were used, therefore the block was defined by the weight at the time of exit from the nursery phase and at the time of entry into the experiment in the growth phase.
Parallel, gene co-expression networks were generated by using the WGCNA package in R (Langfelder and Horvath, 2008), with RNA-Seq data. The gene abundance was normalized by transcript per million (TPM) counts, to perform comparison among the samples using RSEM v.1.3.1 (Li and Dewey, 2011; Zhao et al., 2021).
The removed genes included unexpressed genes, defined as those with zero counts across all samples, and infrequently expressed genes, which were genes do not present in at least 50% of the samples.
The similarity among the gene expression profiles of samples in each of the diets was assessed by creating a similarity matrix through the calculation of Pearson’s correlations. Subsequently, the similarity matrix was converted into an adjacency matrix (A) using a β exponent, adhering to the free-scale topology (R 2 > 0.80) (Diniz et al., 2019; Silva-Vignato et al., 2019). We chose to use signed networks because they are better suited for capturing gene expression trends, including up and downregulation, and for classifying co-expressed gene modules (Langfelder and Horvath, 2008). To define the topological overlap matrix (TOM) modules based on dissimilarity (1-TOM) were used (Langfelder and Horvath, 2008). Modules were merged based on the dissimilarity between the eigengenes. We computed both the correlation matrix and the adjacency matrix, subsequently merging them into the topology matrix. Subsequently, we identified gene modules characterized by dissimilarities less than 0.25, equivalent to a correlation of 0.75, with a minimum module size set at 30 genes. Genes without clusters were grouped in the Grey module (Silva-Vignato et al., 2019).
Module-trait associations were assessed through the correlation between the module eigengene (ME) for each blood serum parameter, enabling the identification of modules correlated with specific traits. Gene significance (GS), which refers to the correlation between a gene and a trait, and module membership (MM), which refers to the correlation between an individual gene and the module eigengene, were calculated to validate the module-trait correlation (Zhang and Horvath, 2005; Zhang et al., 2022). For functional enrichment analysis, module genes were selected that showed significant associations (p-value <0.05) with at least one trait.
2.5 Functional enrichment analysis of co-expressed modules
Functional enrichment analysis was performed using the DAVID database (Sherman et al., 2022) (v. 2021). All nodes were considered after applying the weight threshold, with ME being significantly correlated with fatty acids using a significance criterion of p-value <0.05. The cut-off criterion for Gene Ontology (GO) terms (Ashburner et al., 2000) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways (Kanehisa and Goto, 2000; Kanehisa, 2019) was set at p-value <0.05. We selected the terms GO and KEGG pathway based on their relationship to metabolism, immunity, disease and FA.
2.6 Mining and detection of hub genes of co-expression modules
The weight threshold filter was used to retain higher weight connections. The weight assigned to each interaction reflects the degree of biological similarity between two proteins, ranging from 0 to 1. To maintain robustness, proteins with very low functional similarity were excluded from the analysis (Karaca, et al., 2024)
In all constructed networks, our goal was to use the most accurate edge quantification possible, while maintaining a threshold that minimizes disruption to the network, following Zhang’s recommendations (Zhang and Horvath, 2005).
Additionally, the stringApp plugin with STRINGFy function—STRING (Search Tool for the Retrieval of Interacting Genes/Proteins) app was used.
We identified the hub gene using on the following criteria: 1) MM vs. GS with a correlation greater than 0.20 and a p-value of <0.05; 2) we visualized the most significantly correlated genes with a WGCNA edge weight using Cytoscape (Shannon et al., 2003), utilizing the edges provided by the WGCNA “exportNetworkToCytoscape” function; 3) we applied the MCODE clustering algorithm (Wang et al., 2015) to identify densely connected subnetworks or modules. Default parameter settings, including “DegreeThreshold,” “NodeScoreThreshold,” “K-CoreThreshold” and “MaxDepth,” were used to extract a highly connected core subnetwork; 4) we obtained annotation information using the STRINGFy network function of the STRING app; 5) as a final step to identify the hub gene, we used the Maximal Clique Centrality (MCC) algorithm available in CytoHubba. This algorithm is a measure of the importance of nodes within a biological network (Chin et al., 2014).
3 Results
3.1 Construction and analysis of co-expression modules
We used RNA-Seq data obtained from liver samples from pigs fed diets containing two different levels of soybean oil. These data were used to construct a gene expression matrix consisting of 15,912 genes from 17 samples for the SOY1.5 group. During hierarchical clustering of the SOY1.5 samples, sample L7 was identified as an outlier and subsequently removed from the analysis, leaving a total of 16 samples. For the SOY3.0 group, we used 16 samples, as determined by statistical analysis in previous work, and generated a gene expression matrix comprising 16,098 genes (Fanalli et al., 2022b). Signed gene co-expression networks (i.e., modules) were identified with the WGCNA package in R. Supplementary Table S2 shows animal identification, and phenotypic values (blood serum parameters). The same sequential input relationship was followed for blood serum parameters. Following the scale-free network criteria, we select an appropriate weighting parameter for the adjacency function (Zhao et al., 2022).
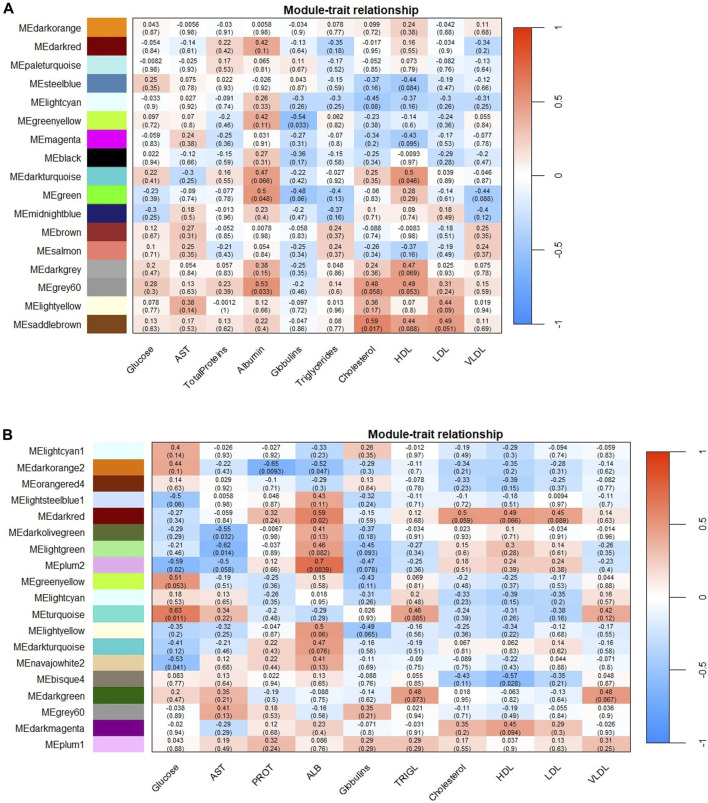
The Pearson’s correlation coefficient between the ME and their respective variables illustrates the correlation between the module and the phenotypic information (Figure 1).
FIGURE 1.
Module-trace associations between eigengene modules (ME) and the biochemical parameters studied. Panel (A) corresponds to SOY1.5 dietary treatment associations, while panel (B) corresponds to SOY3.0. Each row corresponds to an eigengene module, and each column to a biochemical parameter. Each cell contains the Pearson coefficient (number outside the parentheses) and the correction p-value (number in parentheses). The graphs are color-correlation coded according to the legend, where red represents a positive correlation and blue represents a negative correlation. AST: Aspartate aminotransferase; PROT: Total protein; ALB: Albumin, and TRIGL: Triglycerides.
As shown in Figure 1, we identified 5 ME for SOY1.5 and 10 ME for SOY3.0 (p-value <0.05) in the gene co-expression network analysis performed. All nodes identified in each color are in Table 1. This suggests that the addition of 3% soybean oil to the diet may cause changes due to the difference in oils.
TABLE 1.
Module Eigengene (ME) and nodes in groups SOY1.5 and SOY3.0.
| ME SOY3.0 group | Nodes | ME SOY1.5 group | Nodes |
|---|---|---|---|
| Lightcyan1 | 101 | Darkorange | 170 |
| Darkorange2 | 92 | Darkred | 211 |
| Orangered4 | 114 | Paleturquoise | 85 |
| Lightsteelblue1 | 101 | Steelblue | 121 |
| Darkred | 288 | Lightcyan | 273 |
| Darkolivegreen | 135 | Greenyellow | 464 |
| Lightgreen | 357 | Magenta | 511 |
| Plum2 | 71 | Black | 618 |
| Greenyellow | 442 | Darkturquoise | 200 |
| Lightcyan | 408 | Green | 696 |
| Turquoise | 1227 | Midnightblue | 277 |
| Lightyellow | 343 | Brown | 1396 |
| Darkturquoise | 255 | Salmon | 393 |
| Navajowhite2 | 56 | Darkgrey | 177 |
| Bisque4 | 75 | Grey60 | 251 |
| Darkgreen | 270 | Lightyellow | 246 |
| Grey60 | 406 | Saddlebrown | 139 |
| Darkmagenta | 132 | ||
| Plum1 | 118 |
In the SOY1.5 group, a significant association was observed between modules and traits such as albumin, cholesterol, globulin, and HDL (p-value <0.05). Specifically, albumin showed a positive correlation with both the Green module (r = 0.5) and the Grey60 module (r = 0.53), while cholesterol was positively correlated with the Saddlebrown module (r = 0.59). Furthermore, HDL also showed a positive correlation with the Darkturquoise module (r = 0.5). Additionally, globulins showed a negative correlation with the Greenyellow module (r = −0.54). In SOY3.0, significant module-trait associations were found for glucose, AST, total proteins, albumin, and HDL across 10 modules (p-value <0.05). Glucose showed a negative correlation with the Plum2 (r = −0.59) and Navajowhite2 (r = −0.53) and a positive correlation with Turquoise (r = 0.63). AST was negative correlation with the Darkolivegreen (r = −0.55) and Lightgreen (r = −0.62). The Darkorange2 was found to be associated with total proteins (r = −0.65). Albumin was positively correlated with the Darkred (r = 0.59) and Plum2 (r = 0.7) modules, and negative correlated with the Darkorange2 (r = −0.52). HDL was negatively correlated with the Bisque4 module (r = −0.57).
As mentioned above, the identified correlations can be used to support in-depth studies on the effects of oil levels in pig diets and how they obtained gene interactions may play different roles in regulation.
3.2 Functional enrichment analysis of genes in relevant modules
We used Cytoscape to visualize networks with high weight nodes and edges. We have organized the results into separate topics for each phenotype, to provide a comprehensive understanding of the underlying gene interactions and regulatory mechanisms in response to soybean oil inclusion in the diet (SOY1.5 or SOY3.0).
3.3 Analysis of albumin-associated modules and their differential enrichment across diets
For albumin phenotype, two modules were identified for SOY1.5 and three for SOY3.0. Among these, the Grey60 module in SOY1.5 was selected, containing nodes and edges with a weight >0.19. The Grey60 module was found to be associated with the albumin phenotype and comprised 755 nodes and 280,021 edges. After applying filtering, the resulting network was 112 nodes and 357 edges (Supplementary Table S3A) incorporating genes such as 3-hydroxyacyl-CoA dehydratase 1 (HACD1), glycogen phosphorylase, muscle-associated (PYGM), histone deacetylase 6 (HDAC6), formin-binding protein 4 (FNBP4), and protein phosphatase 1 regulatory subunit 12B (PPP1R12B), among others.
The enriched GO terms included histone acetyltransferase complex (GO:0000123) with genes such as acrosin binding protein (ACRBP) and OGT gene. Another enriched term was regulation of transcription (GO:0006357; GO:0006355) with genes like bromodomain-containing 2 (BRD2), zinc finger protein 630 (ZNF630), cyclin D binding myb-like transcription factor 1 (DMTF1), and notch receptor 3 (NOTCH3). Additionally, the DNA binding term (GO:0003677) was enriched and included genes such as methyl-CpG binding domain protein 6 (MBD6), nucleic acid binding protein 1 (NABP1), among others.
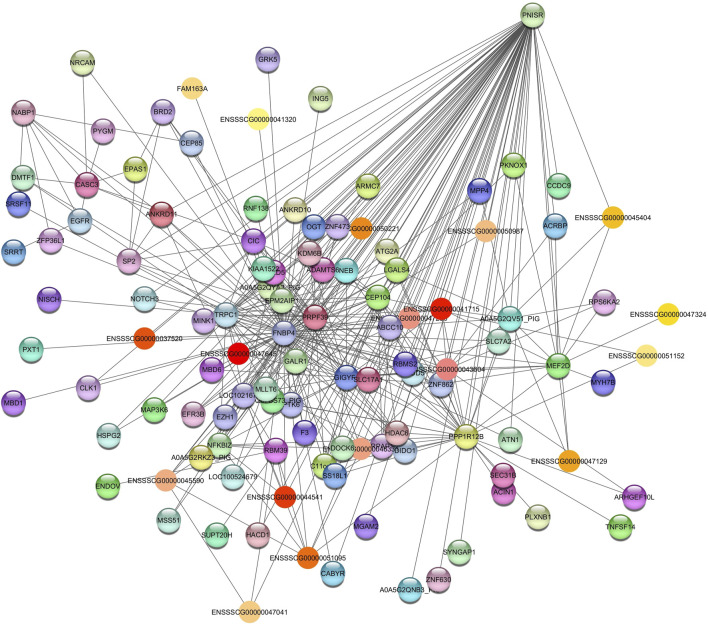
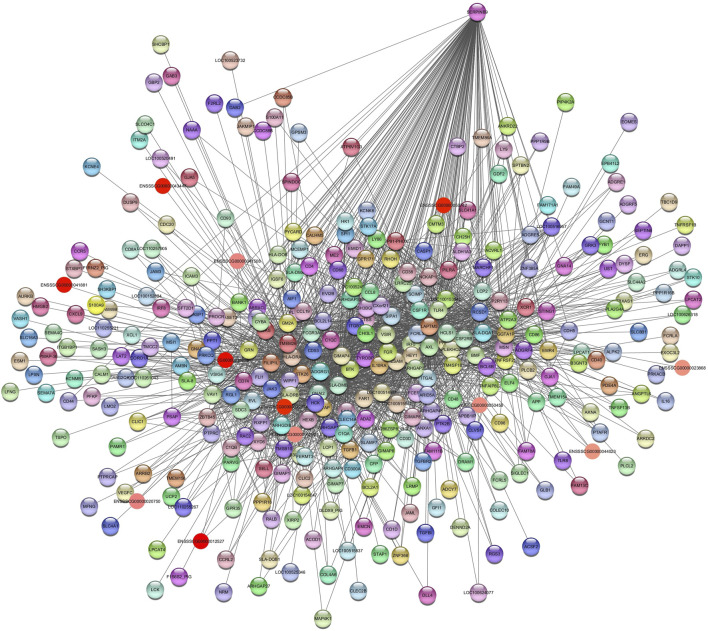
The hub gene identified was PNN Interacting Serine and Arginine Rich Protein (PNISR) for the Grey60 module (Figure 2). PNISR is a serine-arginine-rich protein that plays a role in the pre-mRNA splicing machinery (Zappaterra et al., 2020).
FIGURE 2.
Network view highlighting interactions of the hub gene PNISR. Interaction networks within the liver tissue from pigs fed a diet with 1.5% soybean oil inclusion (SOY1.5) were constructed using Cytoscape, followed by the stringApp plugin. The network associated with the Grey60 module is co-expressed with the albumin phenotype. Colored nodes represent genes/proteins included in the query.
Regarding the SOY1.5 group, the Green module, similar to the Grey60 module, showed a moderate correlation with the albumin phenotype. This module consists of 867 nodes and 373,625 edges. To ensure the relevance of our findings, we applied a filtering criterion with a weight threshold >0.18, resulting in a refined network comprising 95 nodes and 252 edges (Supplementary Table S3B). Genes included in this network are interleukin 16 (IL16), interleukin 10 receptor subunit alpha (IL10RA), C-X-C motif chemokine ligand 13 (CXCL13), FXYD domain containing ion transport regulator 2 (FXYD2).
The network showed enrichments related to GO terms, including immune response (GO:0006955) with genes such as C-C motif chemokine ligand 5 (CCL5), lymphocyte antigen 86 (LY86), and transport vesicle membrane (CD74), among others. Another significant GO term was positive regulation of ERK1 and ERK2 cascade (GO:0070374) with genes like CD4 molecule (CD4), caveolae associated (CAVIN3), arrestin beta 1 (ARRB1), arrestin beta 2 (ARRB2), and CD74. Additionally, negative regulation of NF-kappaB transcription factor activity (GO:0032088) was associated with genes such as ARRB1, ARRB2, and coronin 1A (CORO1A), along with other relevant terms.
Moreover, we observed significant enrichments in KEGG pathways, including hematopoietic cell lineage (ssc04640) with genes such as CD3 epsilon subunit of T-cell receptor complex (CD3E), CD4, MHC class II DR-alpha (HLA-DRA), and SLA-DQ beta1 domain (SLA-DQB1). The Th1 and Th2 cell differentiation pathway (ssc04658) exhibited the same genes. Additionally, the type I diabetes mellitus pathway (ssc04940) showed associations with genes such as HLA-DRA, SLA-DQA, SLA-DQB1, and MHC class II, DM beta (SLA-DMB), along with other pathways. In this context, the module displays genes with important relationships to immune response, cell signaling and disease.
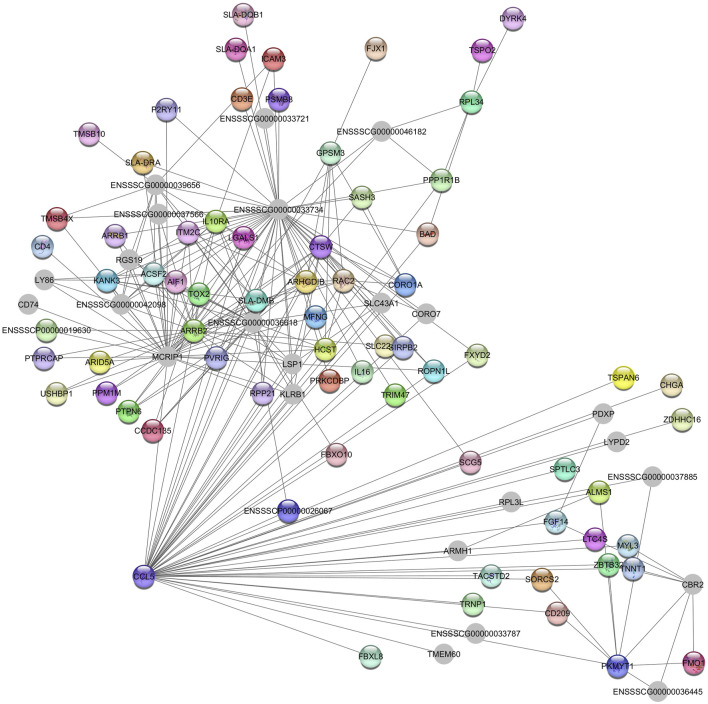
The hub gene identified in the Green module is CCL5, which is associated with immunoregulatory and inflammatory processes (Figure 3) (O’Leary et al., 2016).
FIGURE 3.
Network view highlighting interactions of the hub gene CCL5. Interaction networks within the liver tissue from pigs fed a diet with 1.5% soybean oil inclusion (SOY1.5) were constructed using Cytoscape, followed by the stringApp plugin. The network associated with the Green module is co-expressed with the albumin phenotype. Colored nodes represent genes/proteins included in the query.
For the SOY3.0 group, the Darkorange2, Darkred, and Plum2 modules were identified as associated with albumin.
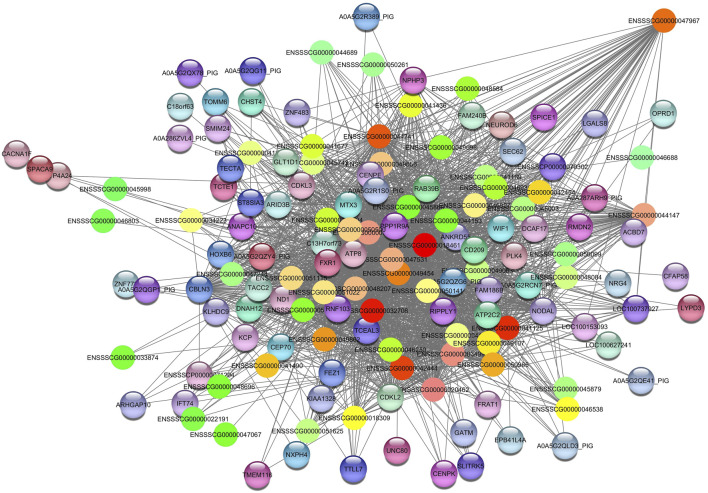
The Darkred module consists of 288 nodes and 35,408 edges. To ensure the relevance of our findings, we applied a filtering criterion with a weight threshold >0.14, resulting in a refined network comprising 142 nodes and 2047 edges (Supplementary Table S4A). The enrichment pathways of the Darkred module, such as the (ssc05022) pathways of neurodegeneration—multiple diseases, with genes such as calcium voltage-gated channel subunit alpha1 F (CACNA1F), NADH dehydrogenase subunit 1 (ND1), ATP synthase F0 subunit 8 (ATP8), dynein axonemal heavy chain 12 (DNAH12), RAB39B, member of the RAS oncogene family (RAB39B) and FRAT1 (FRAT regulator of WNT signaling pathway 1). Additionally, Alzheimer’s disease pathway (ssc05010) was also identified (Table 2). The hub gene identified in this module is ENSSCG00000047967 (Figure 4).
TABLE 2.
Pathways enriched in the Darkred module for SOY3.0.
| Top 10 pathways enriched in the Darkred module for SOY3.0 | p-value | Genes |
|---|---|---|
| ssc05022:Pathways of neurodegeneration - multiple diseases | 0.0028 | ENSSSCG00000018065, ENSSSCG00000024233, ENSSSCG00000012298, ENSSSCG00000020990, ENSSSCG00000032821, ENSSSCG00000018080 |
| GO:0007368∼determination of left/right symmetry | 0.0068 | ENSSSCG00000030998, ENSSSCG00000011632, ENSSSCG00000005124 |
| GO:0005813∼centrosome | 0.0126 | ENSSSCG00000010614, ENSSSCG00000024663, ENSSSCG00000026746, ENSSSCG00000008970, ENSSSCG00000015212, ENSSSCG00000005124 |
| GO:0005856∼cytoskeleton | 0.0159 | ENSSSCG00000029860, ENSSSCG00000023728, ENSSSCG00000015329, ENSSSCG00000010614, ENSSSCG00000037530 |
| GO:0007018∼microtubule-based movement | 0.0274 | ENSSSCG00000001705, ENSSSCG00000023296, ENSSSCG00000020990 |
| GO:0038092∼nodal signaling pathway | 0.0321 | ENSSSCG00000039399, ENSSSCG00000030998 |
| GO:0051382∼kinetochore assembly | 0.0349 | ENSSSCG00000016946, ENSSSCG00000023296 |
| ssc05014:Amyotrophic lateral sclerosis | 0.0423 | ENSSSCG00000018065, ENSSSCG00000024233, ENSSSCG00000020990, ENSSSCG00000018080 |
| ssc05010:Alzheimer disease | 0.0483 | ENSSSCG00000018065, ENSSSCG00000012298, ENSSSCG00000032821, ENSSSCG00000018080 |
FIGURE 4.
Network view highlighting interactions of the hub gene ENSSCG00000047967. Interaction networks within the liver tissue from pigs fed a diet with 3% soybean oil inclusion (SOY3.0) were constructed using Cytoscape, followed by the stringApp plugin. The network associated with the Darkred module is co-expressed with the albumin phenotype. Colored nodes represent genes/proteins included in the query.
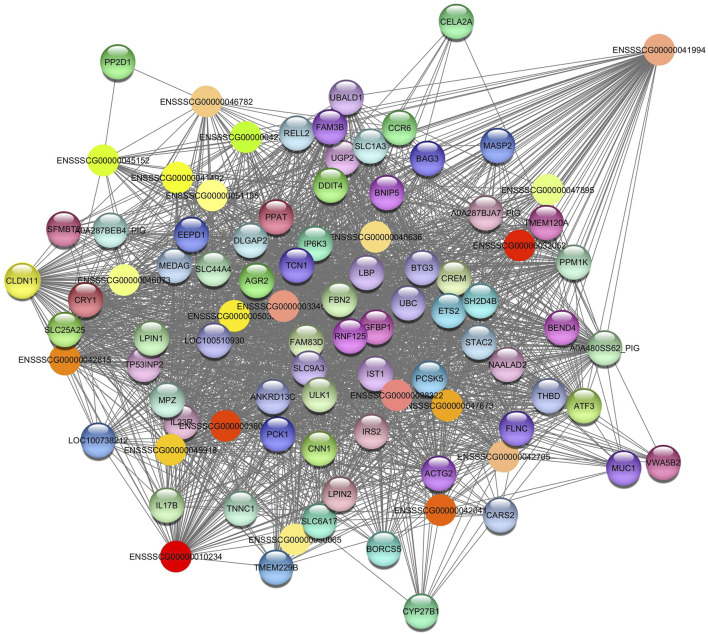
Darkorange2 module was identified in total protein and albumin. It showed a negative correlation in both phenotypes. For the Darkorange2, the hub gene identified was ENSSSCG00000041994 (Figure 5). The enriched pathways are related to fatty acid catabolic process (GO:0009062); gluconeogenesis (GO:0006094); response to insulin (GO:0032868); among others (Table 3). This module consists of 92 nodes and 3015 edges. To ensure the relevance of our findings, we applied a filtering criterion with a weight threshold >0.03, resulting in a refined network comprising 91 nodes and 2312 edges (Supplementary Table S4B).
FIGURE 5.
Network view highlighting interactions of the hub gene ENSSSCG00000041994. Interaction networks within the liver tissue from pigs fed a diet with 3% soybean oil inclusion (SOY3.0) were constructed using Cytoscape, followed by the stringApp plugin. The network associated with the Darkorange2 module is co-expressed with the total protein and albumin phenotypes. Colored nodes represent genes/proteins included in the query.
TABLE 3.
Pathways enriched in the Darkorange2 module for SOY3.0.
| Pathways enriched in the Darkorange2 module for SOY3.0 | p-value | Genes |
|---|---|---|
| GO:0006094∼gluconeogenesis | 0.0107 | ENSSSCG00000015595, ENSSSCG00000007507, ENSSSCG00000000164 |
| GO:0008104∼protein localization | 0.0292 | ENSSSCG00000002743, ENSSSCG00000022099, ENSSSCG00000009742 |
| GO:0008195∼phosphatidate phosphatase activity | 0.0434 | ENSSSCG00000008624, ENSSSCG00000038494 |
| GO:0009062∼fatty acid catabolic process | 0.0242 | ENSSSCG00000008624, ENSSSCG00000038494 |
| GO:0014823∼response to activity | 0.0272 | ENSSSCG00000005636, ENSSSCG00000000164 |
| GO:0032868∼response to insulin | 0.0057 | ENSSSCG00000016728, ENSSSCG00000003459, ENSSSCG00000000164 |
| GO:0032869∼cellular response to insulin stimulus | 0.0119 | ENSSSCG00000008624, ENSSSCG00000038494, ENSSSCG00000007507 |
| GO:0042594∼response to starvation | 0.0479 | ENSSSCG00000007507, ENSSSCG00000009742 |
| GO:0071549∼cellular response to dexamethasone stimulus | 0.0391 | ENSSSCG00000007507, ENSSSCG00000031888 |
| ssc04150:mTOR signaling pathway | 0.0199 | ENSSSCG00000008624, ENSSSCG00000038494, ENSSSCG00000031888, ENSSSCG00000009742 |
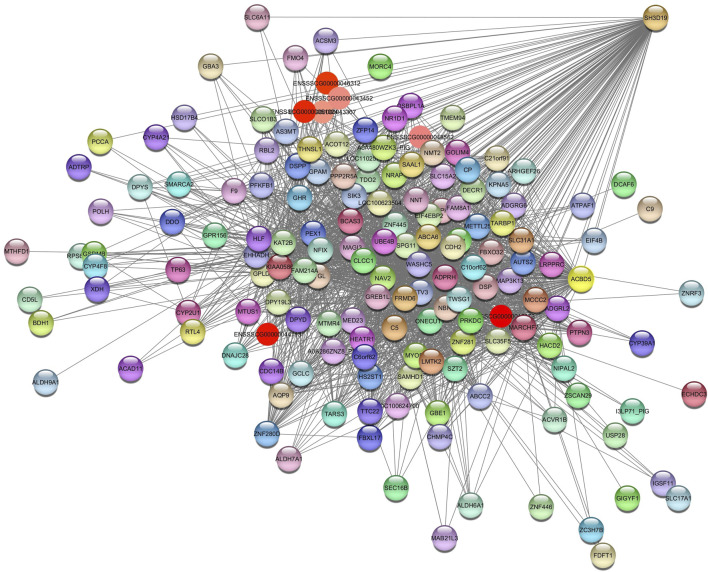
For the Plum2 module (Supplementary Table S4C), pathways were enriched, such as insulin-like growth factor receptor signaling pathway (GO:0048009), valine, leucine and isoleucine degradation (ssc00280), metabolic pathways (ssc01100), fatty acids degradation (ssc00071), acyl-CoA metabolic process (GO:0006637), and ssc04976 (bile secretion) (Supplementary Table S5). The hub gene identified is SH3 Domain Containing 19 (SH3D19) (Figure 6).
FIGURE 6.
Network view highlighting interactions of the hub gene SH3D19. Interaction networks within the liver tissue from pigs fed a diet with 3% soybean oil inclusion (SOY3.0) were constructed using Cytoscape, followed by the stringApp plugin. The network associated with the Plum2 module is co-expressed with the glucose and albumin phenotype. Colored nodes represent genes/proteins included in the query.
This module is related to glucose and albumin. It has a strong positive correlation with albumin and a negative correlation with glucose.
3.4 Hub genes and pathways related to AST
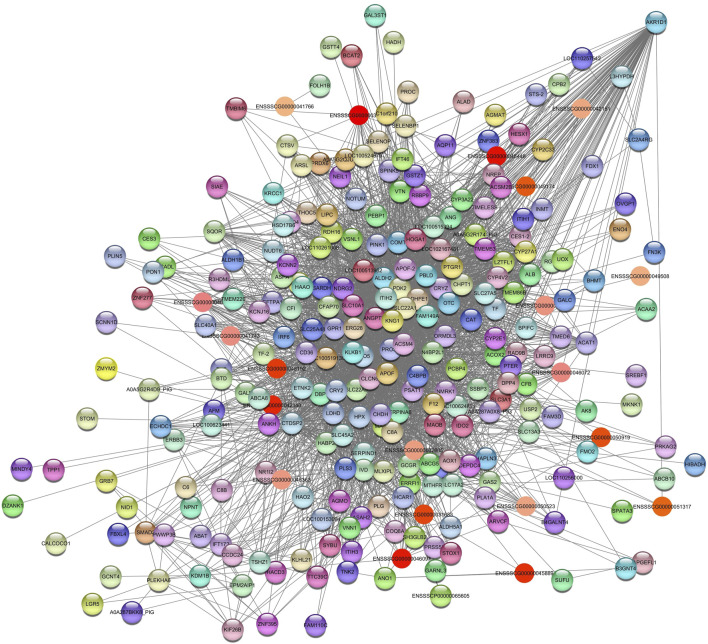
For AST, we identified the Darkolivegreen and Lightgreen modules in SOY3.0. After filtering for values >0.05, the network for Darkolivegreen module consisted of 6595 nodes and 2698 edges (Supplementary Table S6A). In the Lightgreen module, after applying a filter >0.10, the network retained 237 nodes and 2158 edges (Supplementary Table S6B). In the Lightgreen module, pathways were identified as (GO:0006629) lipid metabolic process with the genes LIPC, FMO5, COMT, PLA1A, AOX1, ACSM4, SLC27A5 and BCAT2. Additionally, pathways such a cholesterol homeostasis (GO:0042632); PPAR signaling pathway (ssc03320); fatty acid metabolism (ssc01212); gene expression (GO:0010467); fatty acid binding (GO:0005504); metabolic pathways (ssc01100) were enrichment. The hub gene aldo-keto reductase family 1 member D1 (AKR1D1) was identified (Figure 7).
FIGURE 7.
Network view highlighting interactions of the hub gene AKR1D1. Interaction networks within the liver tissue from pigs fed a diet with 3% soybean oil inclusion (SOY3.0) were constructed using Cytoscape, followed by the stringApp plugin. The network associated with the Lightgreen module is co-expressed with the AST phenotype. Colored nodes represent genes/proteins included in the query (small nodes indicate proteins with unidentified 3D structure, large nodes indicate those with known structures).
Deficiency of the enzyme encoded by this gene may contribute to liver dysfunction (Stelzer et al., 2016).
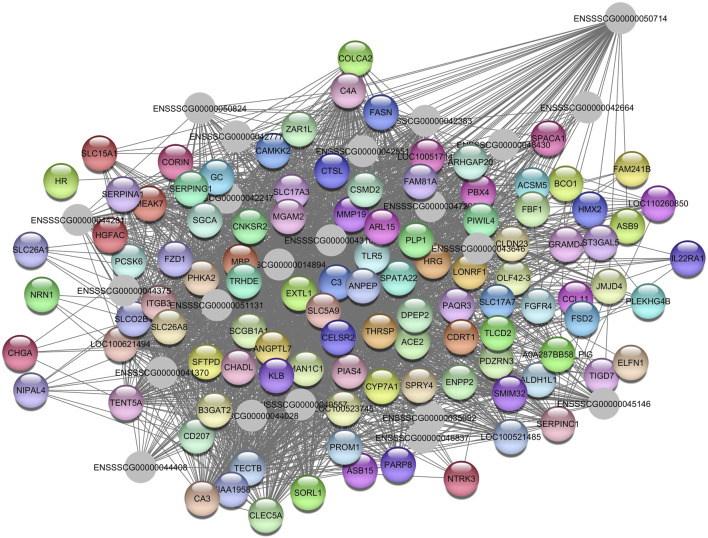
Regarding the Darkolivegreen module, the hub gene ENSSSCG00000050714 was identified (Figure 8), and the enriched pathways were inflammatory response (GO:0006954); metabolic pathways (ssc01100); regulation of triglyceride biosynthetic process (GO:0010866); innate immune response (GO:0045087); and transmembrane transport (GO:0055085).
FIGURE 8.
Network view highlighting interactions of the hub gene ENSSSCG00000050714. Interaction networks within the liver tissue from pigs fed a diet with 3% soybean oil inclusion (SOY3.0) were constructed using Cytoscape, followed by the stringApp plugin. The network associated with the Darkolivegreen module is co-expressed with the AST phenotype. Colored nodes represent genes/proteins included in the query.
3.5 Identifying modules and key genes associated with globulins
The Greenyellow module was found to be moderately associated with the globulin’s phenotype in SOY1.5. The network constructed from this module consisted of 466 nodes and 106,695 edges. After applying a filter to remove edges with a weight less than 0.17, the resulting network contained 144 nodes and 554 edges (Supplementary Table S7), which included genes such as OTU domain-containing ubiquitin aldehyde-binding protein 1 (OTUB1), leukotriene A4 hydrolase (LTA4H); niban apoptosis regulator 3 (NIBAN3); and eukaryotic translation initiation factor 4H (EIF4H).
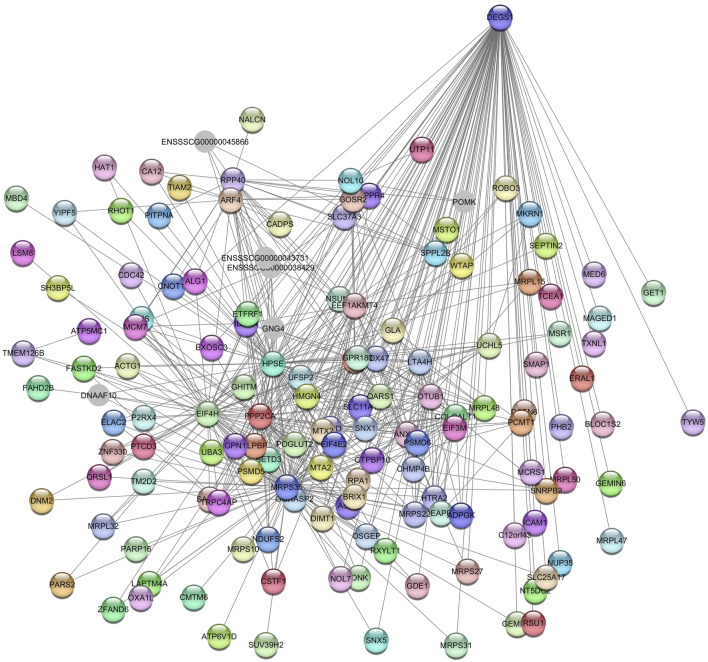
Enriched KEGG pathways included endocytosis (ssc04144), amyotrophic lateral sclerosis (ssc05014), and aminoacyl-tRNA biosynthesis (ssc00970), among others. The GO terms enriched included GTP binding (GO:0005525), rRNA methylation (GO:0031167), translation initiation factor activity (GO:0003743), and golgi apparatus (GO:0005794), among others. The gene identified as the hub gene is Delta 4-Desaturase, Sphingolipid 1 (DEGS1) (Figure 9).
FIGURE 9.
Network view highlighting interactions of the hub gene DEGS1. Interaction networks within the liver tissue from pigs fed a diet with 1.5% soybean oil inclusion (SOY1.5) were constructed using Cytoscape, followed by the stringApp plugin. The network associated with the Greenyellow module is co-expressed with the globulin phenotype. Colored nodes represent genes/proteins included in the query.
3.6 Identification of modules associated with cholesterol, and HDL
In the SOY1.5 group, the Saddlebrown module was associated with total cholesterol. The network contained 139 nodes and 9,580 edges. After filtering with a weight threshold of >0.13, the network was reduced to 141 nodes and 752 edges. Genes represents in this module include TNF receptor superfamily member 11b (TNFRSF11B), Fos proto-oncogene, AP-1 transcription factor subunit (FOS), solute carrier family 2 member 3 (SLC2A3), mitogen-activated protein kinase kinase kinase 8 (MAP3K8), vascular cell adhesion molecule 1 (VCAM1), and vascular endothelial growth factor C (VEGFC), among others.
The cholesterol-related hub gene identified in the Saddlebrown module is synaptonemal complex protein 3 (SYCP3). Suppression of SYCP3 affects the expression of genes associated with lipid metabolism. However, in the SOY1.5 group, the MM vs. GS for the module did not show a p-value <0.05.
On the other hand, HDL had modules in both SOY1.5 and SOY3.0. The Darkturquoise module was identified in SOY1.5, while the Bisque4 module were found in SOY3.0. The Darkturquoise module in the network consisted of 63 nodes and 252 edges (Supplementary Table S8A). Notable genes within this module include apolipoprotein E (APOE), apolipoprotein A2 (APOA2), fatty acid binding protein 1 (FABP1), and transcription factor like 5 (TCFL5).
The network was enriched for cholesterol metabolism (ssc04979), metabolic pathways (ssc01100). The GO terms such as high-density lipoprotein particle clearance (GO:0034384), tetrahydrobiopterin biosynthetic process (GO:0006729), positive regulation of cholesterol esterification (GO:0010873), high-density lipoprotein particle assembly (GO:0034380), phospholipid efflux (GO:0033700), low-density lipoprotein particle remodeling (GO:0034374), reverse cholesterol transport (GO:0043691), high-density lipoprotein particle remodeling (GO:0034375), mitochondrial membrane (GO:0031966), chylomicron (GO:0042627), and very-low-density lipoprotein particle (GO:0034361), among others.
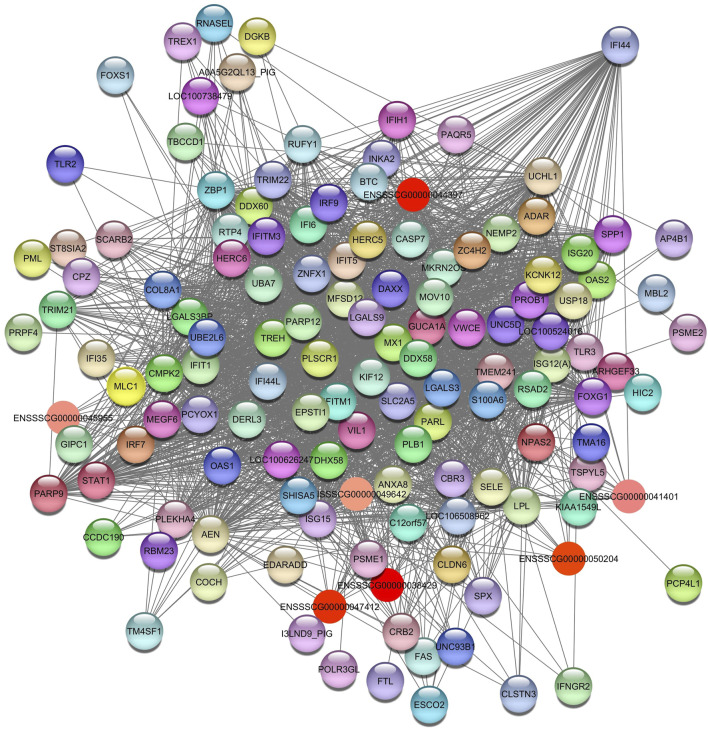
Relevant genes and pathways associated with the HDL in the liver were identified for SOY3.0 (Supplementary Table S8B). The enrichment analysis identified pathways such as positive regulation of I-kappaB kinase/NF-kappaB signaling (GO:0043123); response to interferon-gamma (GO:0034341); toll-like receptor signaling pathway (GO:0002224); ubiquitin protein ligase binding (GO:0031625); interleukin-27-mediated signaling pathway (GO:0070106); immune system process (GO:000237); and positive regulation of interleukin-6 production (GO:0032755). The hub gene identified in this pathway was interferon induced protein 44 (IF144) (Figure 10).
FIGURE 10.
Network view highlighting interactions of the hub gene IF144. Interaction networks within the liver tissue of pigs fed a diet with 3.0% soybean oil inclusion (SOY3.0) were constructed using Cytoscape, followed by the stringApp plugin. The network associated with the Bisque4 module is co-expressed with the HDL phenotype. Colored nodes represent genes/proteins included in the query.
3.7 Identification of modules and key genes associated with glucose
Three modules were identified for glucose: Plum2, Turquoise, Navajowhite2 for SOY3.0. As previously observed, the Plum2 module with the hub gene SH3D19, just as in the glucose phenotype.
In the Turquoise module (SSupplementary Table S9A), the enrichment pathways included immune response (GO:0006955), inflammatory response (GO:0006954), response to cholesterol (GO:0070723), negative regulation of I-kappaB kinase/NF-kappaB signaling (GO:0043124), positive regulation of interleukin-4 production (GO:0032753), positive regulation of interleukin-2 production (GO:0032743), positive regulation of angiogenesis (GO:0045766), and Th1 and Th2 cell differentiation (ssc04658). The hub gene identified is serpin family B member 9 (SERPINB9) (Figure 11).
FIGURE 11.
Network view highlighting interactions of the hub gene SERPINB9. Interaction networks within the liver tissue of pigs fed a diet with 3.0% soybean oil inclusion (SOY3.0) were constructed using Cytoscape, followed by the stringApp plugin. The network associated with the Turquoise module is co-expressed with the glucose phenotype. Colored nodes represent genes/proteins included in the query.
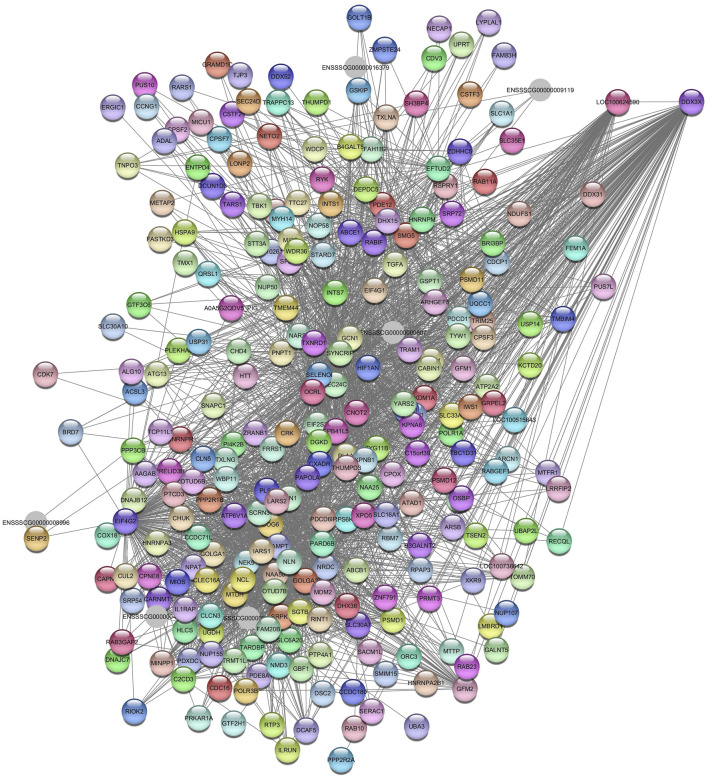
For the Navajowhite2 module (Supplementary Table S9B), we identified enriched pathways as spliceosome (ssc03040); NF-kappaB binding (GO:0051059); RNA binding (GO:0003723); RNA helicase activity (GO:0003724).
The DEAD-box helicase 3 X-linked (DDX3X) gene was identified as a hub gene in the Navajowhite2 module (Figure 12).
FIGURE 12.
Network view highlighting interactions of the hub gene DDX3X. Interaction networks within the liver tissue of pigs fed a diet with 3.0% soybean oil inclusion (SOY3.0) were constructed using Cytoscape, followed by the stringApp plugin. The network associated with the Najowhite2 module is co-expressed with the glucose phenotype. Colored nodes represent genes/proteins included in the query.
4 Discussion
The WGCNA algorithms, according to Xing et al. (2021), attempt to build a network with correlation values that match the properties of a scale-free network because they have biological significance. Thus, in this study, we identified the gene modules and the main signaling pathways that influence the blood serum parameters of pigs fed different levels of soybean oil from the networks constructed by the WGCNA. The variation in the number of modules identified in both SOY1.5 and SOY3.0 can be attributed to differences in the genes expressed in the liver, possibly due to regulatory mechanisms in response to oil intake. This would help to explain the differences in module content and enrichment results between the groups. In addition, these results support that regulation of gene expression plays a role in influencing pathways that may affect traits related to blood serum parameters.
A higher number of ME was observed in the SOY3.0 group. In our previous study, we observed differences in albumin levels between the SOY1.5 and SOY3.0 diet groups, with higher values observed in the SOY1.5 group (Fanalli et al., 2022b). Our results illustrate the changes in co-expression in the SOY1.5 and SOY3.0 groups in the diet, furthering our understanding of metabolic processes and their relevance to disease. These results are consistent with our previous findings indicating changes in gene expression when 3% soybean oil was added to the diet, showing differential expression (Fanalli et al., 2022c).
4.1 Co-expressed modules related to albumin
By examining the co-expression of albumin-related genes, we can understand the potential mechanisms and processes that are regulated by FA in the liver. Albumin is produced by the liver and is associated with a wide range of important physiological functions, such as immunomodulation and antioxidant effects (Spinella et al., 2016). Albumin may serve as an important modulator of innate immune responses to systemic inflammation (Spinella et al., 2016). Immune-related diseases are linked to the body’s defense against infection. In this context, the inclusion of immune nutrients, such as FA, has the potential to alter the body’s inflammatory and immune responses, as FA is involved in modulating macrophage functions (Radzikowska et al., 2019). This has been attributed to the immunomodulatory effects of PUFA, particularly those from the omega-3 series, which can influence clinical outcomes by modulating the immune system (Miles and Calder, 2015; Fanalli et al., 2022b). In inflammatory conditions, omega-3 FA can restore impaired barrier function and reduce the pro-inflammatory mediator production (Radzikowska et al., 2019).
Albumin is used as an indicator to evaluate dysfunction in the liver, pancreas, and overall nutritional status. It is more susceptible to reactive oxygen species (ROS) compared to other proteins, and the proportion of oxidized albumin serves as a marker for the degree of oxidative stress associated with various pathological conditions (Belinskaia et al., 2021). FA, especially PUFA, plays an important role in regulating the antioxidant properties of albumin, with PUFA significantly contributing to the pool of oxidizable biological compounds in plasma. In addition, these biomarkers associated with oxidative stress may hold prognostic and therapeutic significance for acute-on-chronic liver failure (ACLF). The gene CCL5, identified in this context, is correlated with immune response metabolic processes and immune response (Zhu and Jiang, 2023).
The hub gene identified in our study in the SOY1.5 group, that correlates with albumin, is CCL5 (RANTES) in diabetes-related renal pathophysiology, and serum albumin may play a relevant role (Nakajima et al., 2003). Furthermore, omega-6 and omega-3 have been observed in FA-related studies to play a role in regulating inflammatory responses through nuclear receptors that influence gene expression (Ogłuszka et al., 2017). In a study investigating the effects of diet on gene expression in pig muscle, an increase in dietary PUFA was related with a decrease in the expression of several chemokines, including CCL5. These chemokines are known to attract macrophages and monocytes to sites of inflammation, and the authors suggested that this decrease in the expression of these chemokines could be related to a reduction (Ogłuszka et al., 2017). This confirms our previous results where we observed greater PUFA deposition in the liver in the SOY1.5 group. Dietary changes can modulate gene expression, affecting inflammation, lipid metabolism and homeostasis.
On the other hand, in the SOY3.0 group, we observe more albumin-related and neurodegenerative disease-related cluster groups such as Darkred. Polyunsaturated fatty acids, including those of the omega-3 and omega-6 series, have been shown to improve cognitive function in individuals with neurodegenerative diseases (Avallone et al., 2019). These FA achieve this by modulating cellular properties and physiological processes (Avallone et al., 2019). Consequently, diets supplemented with PUFA-rich vegetable oils, such as rapeseed and soybean oil, may offer benefits to consumers. Fanalli et al. (2022c), who investigated differentially expressed genes in the same pig population, identified genes associated with network maps related to neurodegenerative diseases.
Some of the GO terms and KEGG pathways we identified in this study are enriched to general metabolism, human diseases such as neurodegenerative and infectious diseases, and the immune system. This demonstrates the importance of the study and its relevance to biological processes. In addition, related KEGG pathways Alzheimer’s disease and neurodegeneration are specifically associated with albumin.
4.2 Co-expressed modules related to glucose
Significant changes observed in the SOY3.0 group could potentially have a more profound effect on gene expression and regulatory networks, leading to a broader effect on lipid and glucose metabolism in porcine liver tissue. The difference in co-expression observed in relation to glucose may be related to the regulation of angiogenesis, suggesting a specific response to the diet with a higher concentration of soybean oil (SOY3.0). According to Fernandez and West, (2005), omega-3 are involved in reducing plasma TG by decreasing lipogenesis and VLDL secretion.
Significantly, the SOY3.0 group showed increased accumulation of OA. Both MUFA and PUFA have been implicated in the regulation of key regulators of hepatic gene transcription. These FA affect transcription factors that play a role in influencing the expression of genes central to glycolysis, de novo fatty acid synthesis, and FA oxidation. This regulatory effect on lipid metabolism can be either direct or indirect (Gnoni et al., 2010).
4.3 Co-expressed modules related to HDL
We have identified APOE associated with the SOY1.5 group co-expressed with HDL. In a study in which the APOE gene was knockout in Bama miniature pigs using the CRISPR-associated protein 9 (CRISPR/Cas9) system, increased levels of HDL cholesterol were observed in the pigs. When fed a high-fat, high-cholesterol diet, the pigs developed significant hypercholesterolemia and progressive atherosclerotic lesions (Fang et al., 2018).
Another gene identified is FABP1, a member of the FABP family, which plays a direct role in the conversion of fatty acids into eicosanoid intermediates, as well as in the stabilization of leukotriene, and is also a biomarker of liver injury or liver-damaging stress (Furuhashi and Hotamisligil, 2008; Ishimura et al., 2013). In human studies, this gene showed positive results with blood pressure, triglycerides and AST (Ishimura et al., 2013), while in this study we showed co-expression in modules related to HDL. Studies in young adults agreed that low HDL cholesterol levels and hypertriglyceridemia were associated with serum FABP1 levels, suggesting it as a possible circulating biomarker of adiposity and metabolic diseases associated with insulin resistance, involved in hepatic lipid binding and lipid metabolism (Shi et al., 2012). In the SOY1.5 group, the hub gene of the Darkturquoise module, DCXR, acts as an enzyme mediating the reductive metabolism of toxic reactive and toxic carbonyl compounds, already associated with diseases such as diabetes (Yang et al., 2017).
HDL plays a role in cytokine regulation, is part of the innate immune response and may have an atheroprotective effect by modulating the complement system (Yu et al., 2010). In addition to its antiatherogenic properties, it is associated with several immunomodulatory effects due to HDL ability to remove free cholesterol from the cell membrane. These findings are corroborated by the fact that the co-expressed hub gene is IF144, which is involved in the immune response. The main pathways enriched in this context were the immune system process and the positive regulation of interleukin-6 production. In people over 65 years of age, IL-6 is one of the factors that may influence the contribution of low HDL-C levels, as IL-6 has effects related to modifying the activity of triglyceride lipases (Zuliani et al., 2007). In relation to the SOY3.0 group, the correlation with this module was negative.
4.4 Key enrichment and hub gene related to globulins, cholesterol, AST and total proteins
We identified a negative correlation with globulins in the SOY1.5 group, along with enrichment in endocytosis, GTP binding, and translation initiation factor activity. In addition, we observed a module positively associated with cholesterol in the SOY1.5 group, with genes enriched in pathways such as cholesterol metabolism, and the SYCP3 gene was the hub gene identified, where knockdown of this gene affects the expression of genes related to lipid metabolism (Manunza et al., 2014).
We identified AST and total protein as modules present exclusively in the SOY3.0 group, suggesting specific modulations in important biological processes in response to the oil level.
The AKR1D1 hub gene, which has been identified in association with AST, is associated with steatosis and inflammation and regulates key metabolic processes. In vitro studies using human hepatoma cells have linked negative expression of AKR1D1 to Nonalcoholic fatty liver disease (NAFLD) (Nikolaou et al., 2019).
Modulation of co-expression by diets with different oil content: By applying WGCNA and analyzing the RNA-Seq data, we can examine how FA were specifically modulated in each diet, improving our understanding of what happened in the differential expression analysis (Fanalli et al., 2022c), where we identified several of them enriched in interesting pathways.
Briefly, in the SOY1.5 group, we identified genes with positive co-expression with albumin that may play specific roles in biological processes related to immune response, regulation of intracellular signaling cascades, and control of gene expression. Highlighting the diversity of genetic interactions and biological processes affected by the higher concentration of soybean oil, we identified positive co-expression in SOY3.0 that may be involved in specific biological processes such as Alzheimer’s disease, immune response, RNA binding, NF-kappaB binding. These effects differ from those observed in SOY1.5, where gene expression varied between groups, possibly due to the inclusion of oil at different concentrations and may be related to the effects of FA composition in modulating the properties of the membrane as well as some of the membrane lipids.
It has also been reported that membrane lipids are important for cellular maintenance and that changes or disorders in these lipids may have consequences in the brain, as the liver serves as a central organ for systemic metabolism (De Carvalho and Caramujo, 2018; Fanalli et al., 2022c).
The difference in co-expression observed in relation to HDL in the SOY1.5 and SOY3.0 groups suggests a different regulatory pattern for HDL in response to the higher concentration of soybean oil.
The results obtained illustrate how FA can modulate gene expression and biological processes in response to different dietary compositions, revealing a complex relationship between diet, blood phenotypes, and molecular pathways in pigs. Our study clearly demonstrates that different dietary FA profiles induce changes in tissue-specific expression profiles to alter the complex network of co-expression. By highlighting the importance of FA in health, our findings underscore the practicality of using serum biochemical parameter sampling. Further studies are warranted to explore potential applications for optimizing swine production and disease management.
5 Conclusion
We used a weighted gene co-expression network to investigate the effects of different diets on expression profiles. This resulted in altered gene interaction and co-expression patterns within the transcriptome. Our study also identified key pathways related to cardiovascular and neurodegenerative diseases, as well as immune responses, metabolic pathways and cholesterol metabolism, which showed correlations with biochemical parameters of pig blood serum. We identified key genes co-expressed with albumin such as CCL5, PNISR, ENSSCG00000047967, and globulins such as DEGS1. We also observed genes co-expressed with HDL such as DCXR and IF144. These correlations were observed in the context of two different levels of soybean oil supplementation, specifically 1.5% and 3%. These findings provide valuable insights into the complex effects of dietary FA profile on gene expression and metabolic pathways in pigs and contribute to our understanding of genetic and metabolic responses to dietary variation. These findings are also crucial for understanding the genetic and metabolic responses to dietary modification and contribute to the development of more precise nutritional strategies.
Acknowledgments
We thank the collaborative efforts between the University of São Paulo and Iowa State University. We also give thanks to DB Genética Suína.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Sao Paulo State Research Foundation (FAPESP, grant numbers: 2017/25180-2, 2018/15653-3, 2020/14148-3, 2021/06553-8, and 2022/10643-5), and Brazilian National Council for Scientific and Technological Development (CNPq, grant number: 301083/2018-5), which provided a researcher fellowship to AC. AA acknowledges Foundation for Science and Technology (FCT grant: 2021.02058.CEEDIND). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance Code 001.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by the experimental procedures involving animals are in accordance with the requirements of the Animal Care and Use Committee of Luiz de Queiroz College of Agriculture’s requirements (University of São Paulo, Piracicaba, Brazil; protocol: 2018.5.1787.11.6 and CEUA number: 2018-28) and are conducted in accordance with Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching (Fass, 2010). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
SF: Conceptualization, Formal Analysis, Writing–original draft, Writing–review and editing, Methodology. JG: Conceptualization, Formal Analysis, Methodology, Writing–original draft, Writing–review and editing. FdN: Formal Analysis, Writing–review and editing. IG: Formal Analysis, Writing–review and editing. HF: Writing–review and editing. GM: Conceptualization, Writing–review and editing. LC: Conceptualization, Writing–review and editing. JK: Writing–review and editing. AA: Formal Analysis, Writing–review and editing. AC: Conceptualization, Formal Analysis, Funding acquisition, Methodology, Resources, Supervision, Writing–original draft, Writing–review and editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2024.1394971/full#supplementary-material
References
- Alencar S. A. da S., Kiefer C., Nascimento K. M. R. de S., Viana L. H., Corassa A., Gomes M. de N. B., et al. (2021). Dietary soybean oil modulates fatty acid composition of pork. Trop. Anim. Health Prod. 53, 357. 10.1007/s11250-021-02804-1 [DOI] [PubMed] [Google Scholar]
- Almeida V. V., Silva J. P. M., Schinckel A. P., Meira A. N., Moreira G. C. M., Gomes J. D., et al. (2021). Effects of increasing dietary oil inclusion from different sources on growth performance, carcass and meat quality traits, and fatty acid profile in genetically lean immunocastrated male pigs. Livest. Sci. 248, 104515. 10.1016/J.LIVSCI.2021.104515 [DOI] [Google Scholar]
- Ashburner M., Ball C. A., Blake J. A., Botstein D., Butler H., Cherry J. M., et al. (2000). Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25, 25–29. 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avallone R., Vitale G., Bertolotti M. (2019). Omega-3 fatty acids and neurodegenerative diseases: new evidence in clinical trials. Int. J. Mol. Sci. 20, 4256. 10.3390/ijms20174256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belinskaia D. A., Voronina P. A., Batalova A. A., Goncharov N. V. (2021). Serum albumin, 65–75. [Google Scholar]
- Chen Y., Lonergan S., Lim K. S., Cheng J., Putz A. M., Dyck M. K., et al. (2023). Plasma protein levels of young healthy pigs as indicators of disease resilience. J. Anim. Sci. 101, skad014. 10.1093/jas/skad014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin C. H., Chen S. H., Wu H. H., Ho C. W., Ko M. T., Lin C. Y. (2014). cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 8, S11. 10.1186/1752-0509-8-S4-S11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D. E. (2008). Balancing cholesterol synthesis and absorption in the gastrointestinal tract. J. Clin. Lipidol. 2, S1–S3. 10.1016/j.jacl.2008.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva B. P. M., Fanalli S. L., Gomes J. D., de Almeida V. V., Fukumasu H., Freitas F. A. O., et al. (2023). Brain fatty acid and transcriptome profiles of pig fed diets with different levels of soybean oil. BMC Genomics 24, 91. 10.1186/s12864-023-09188-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Carvalho C. C. C. R., Caramujo M. J. (2018). The various roles of fatty acids. Molecules 23, 2583. 10.3390/molecules23102583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denke M. A. (2006). Dietary fats, fatty acids, and their effects on lipoproteins. Curr. Atheroscler. Rep. 8, 466–471. 10.1007/s11883-006-0021-0 [DOI] [PubMed] [Google Scholar]
- Diniz W. J. S., Mazzoni G., Coutinho L. L., Banerjee P., Geistlinger L., Cesar A. S. M., et al. (2019). Detection of co-expressed pathway modules associated with mineral concentration and meat quality in nelore cattle. Front. Genet. 10, 210. 10.3389/fgene.2019.00210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Tommaso P., Chatzou M., Floden E. W., Barja P. P., Palumbo E., Notredame C. (2017). Nextflow enables reproducible computational workflows. Nat. Biotechnol. 35, 316–319. 10.1038/nbt.3820 [DOI] [PubMed] [Google Scholar]
- Fanalli S. L., da Silva B. P. M., Petry B., Santana M. H. A., Polizel G. H. G., Antunes R. C., et al. (2022a). Dietary fatty acids applied to pig production and their relation to the biological processes: a review. Livest. Sci. 265, 105092. 10.1016/j.livsci.2022.105092 [DOI] [Google Scholar]
- Fanalli S. L., Silva B. P. M. da, Gomes J. D., Almeida V. V., Afonso J., Reecy J. M., et al. (2022b). Effect of dietary soybean oil inclusion on liver-related transcription factors in a pig model for metabolic diseases. Sci. Rep. 12, 10318. 10.1038/s41598-022-14069-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanalli S. L., Silva B. P. M. da, Gomes J. D., Almeida V. V. de, Freitas F. A. O., Moreira G. C. M., et al. (2022c). Differential gene expression associated with soybean oil level in the diet of pigs. Anim 12, 1632. 10.3390/ANI12131632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanalli S. L., Silva B. P. M. da, Gomes J. D., Durval M. C., Almeida V. V. de, Moreira G. C. M., et al. (2023). RNA-seq transcriptome profiling of pigs’ liver in response to diet with different sources of fatty acids. Front. Genet. 14, 1053021. 10.3389/fgene.2023.1053021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang B., Ren X., Wang Y., Li Z., Zhao L., Zhang M., et al. (2018). Apolipoprotein E deficiency accelerates atherosclerosis development in miniature pigs. DMM Dis. Model. Mech. 11, dmm036632. 10.1242/dmm.036632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass (2010). Guide for the care and use of agricultural animals in research and teaching. 3rd Edn. Champaign, IL: Federation of Animal Science Societies. [Google Scholar]
- Fernandez M. L., West K. L. (2005). Mechanisms by which dietary fatty acids modulate plasma lipids. J. Nutr. 135, 2075–2078. 10.1093/jn/135.9.2075 [DOI] [PubMed] [Google Scholar]
- Fujii J., Otsu K., Zorzato F., De Leon S., Khanna V. K., Weiler J. E., et al. (1991). Identification of a mutation in porcine ryanodine receptor associated with malignant hyperthermia. Science 253 (80-), 448–451. 10.1126/science.1862346 [DOI] [PubMed] [Google Scholar]
- Furuhashi M., Hotamisligil G. S. (2008). Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 7, 489–503. 10.1038/nrd2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnoni G. V., Natali F., Geelen M. J. H., Siculella L. (2010). Oleic acid as an inhibitor of fatty acid and cholesterol synthesis. Elsevier Inc., 1365–1373. 10.1016/B978-0-12-374420-3.00152-2 [DOI] [Google Scholar]
- Ishimura S., Furuhashi M., Watanabe Y., Hoshina K., Fuseya T., Mita T., et al. (2013). Circulating levels of fatty acid-binding protein family and metabolic phenotype in the general population. PLoS One 8, e81318. 10.1371/journal.pone.0081318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junk M. J. N., Spiess H. W., Hinderberger D. (2010). The distribution of fatty acids reveals the functional structure of human serum albumin. Angew. Chem. - Int. Ed. 49, 8755–8759. 10.1002/anie.201003495 [DOI] [PubMed] [Google Scholar]
- Kanehisa M. (2019). Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951. 10.1002/pro.3715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M., Goto S. (2000). KEGG: Kyoto Encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. 10.1093/nar/28.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaca C., Demir Karaman E., Leblebici A., Kurter H., Hulya E., Altug K., et al. (2024). New treatment alternatives for primary and metastatic colorectal cancer by an integrated transcriptome and network analyses. Sci. Rep. 14, 8762. 10.1038/s41598-024-59101-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogelman L. J. A., Cirera S., Zhernakova D. V., Fredholm M., Franke L., Kadarmideen H. N. (2014). Identification of co-expression gene networks, regulatory genes and pathways for obesity based on adipose tissue RNA Sequencing in a porcine model. BMC Med. Genomics 7, 57. 10.1186/1755-8794-7-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfelder P., Horvath S. (2008). WGCNA: an R package for weighted correlation network analysis. BMC Bioinforma. 9, 559. 10.1186/1471-2105-9-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S. V., Holven K. B., Ottestad I., Dagsland K. N., Myhrstad M. C. W., Ulven S. M. (2018). Plasma fatty acid levels and gene expression related to lipid metabolism in peripheral blood mononuclear cells: a cross-sectional study in healthy subjects. Genes Nutr. 13, 9. 10.1186/s12263-018-0600-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Dewey C. N. (2011). RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinforma. 12, 323. 10.1186/1471-2105-12-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Weng Q., Dong C., Zhang Z., Li R., Liu J., et al. (2018). A key gene, PLIN1, can affect porcine intramuscular fat content based on transcriptome analysis. Genes (Basel) 9, 194. 10.3390/genes9040194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn J., Theobald H. E. (2006). The health effects of dietary unsaturated fatty acids. Nutr. Bull. 31, 178–224. 10.1111/j.1467-3010.2006.00571.x [DOI] [Google Scholar]
- Manunza A., Casellas J., Quintanilla R., González-Prendes R., Pena R. N., Tibau J., et al. (2014). A genome-wide association analysis for porcine serum lipid traits reveals the existence of age-specific genetic determinants. BMC Genomics 15, 758. 10.1186/1471-2164-15-758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield F. R., Tabas I. (2005). Role of cholesterol and lipid organization in disease. Nature 438, 612–621. 10.1038/nature04399 [DOI] [PubMed] [Google Scholar]
- Miles E. A., Calder P. C. (2015). Fatty acids, lipid emulsions and the immune and inflammatory systems. World Rev. Nutr. Diet. 112, 17–30. 10.1159/000365426 [DOI] [PubMed] [Google Scholar]
- Miller E. R., Ullrey D. E. (1987). The pig as a model for human nutrition. Annu. Rev. Nutr. 7, 361–382. 10.1146/annurev.nu.07.070187.002045 [DOI] [PubMed] [Google Scholar]
- Nakajima K., Tanaka Y., Nomiyama T., Ogihara T., Ikeda F., Kanno R., et al. (2003). RANTES promoter genotype is associated with diabetic nephropathy in type 2 diabetic subjects. Diabetes Care 26, 892–898. 10.2337/diacare.26.3.892 [DOI] [PubMed] [Google Scholar]
- Nikolaou N., Gathercole L. L., Marchand L., Althari S., Dempster N. J., Green C. J., et al. (2019). AKR1D1 is a novel regulator of metabolic phenotype in human hepatocytes and is dysregulated in non-alcoholic fatty liver disease. Metabolism 99, 67–80. 10.1016/j.metabol.2019.153947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD-FAO (2021). OECD-FAO agricultural outlook 2021–2030. OECD-FAO Agric. Outlook 2021–2030, 163–177. [Google Scholar]
- Ogłuszka M., Szostak A., te Pas M. F. W., Poławska E., Urbański P., Blicharski T., et al. (2017). A porcine gluteus medius muscle genome-wide transcriptome analysis: dietary effects of omega-6 and omega-3 fatty acids on biological mechanisms. Genes Nutr. 12, 4. 10.1186/s12263-017-0552-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary N. A., Wright M. W., Brister J. R., Ciufo S., Haddad D., McVeigh R., et al. (2016). Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 44, D733–D745. 10.1093/nar/gkv1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z., Yao Y., Yin H., Cai Z., Wang Y., Bai L., et al. (2021). Pig genome functional annotation enhances the biological interpretation of complex traits and human disease. Nat. Commun. 12, 5848. 10.1038/s41467-021-26153-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzikowska U., Rinaldi A. O., Sözener Z. Ç., Karaguzel D., Wojcik M., Cypryk K., et al. (2019). The influence of dietary fatty acids on immune responses. Nutrients 11, 2990. 10.3390/nu11122990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2023). RStudio. Boston, MA URL. Boston, MA: Integrated Development for R. RStudio, PBC. [Google Scholar]
- Reyer H., Oster M., Wittenburg D., Murani E., Ponsuksili S., Wimmers K. (2019). Genetic contribution to variation in blood calcium, phosphorus, and alkaline phosphatase activity in pigs. Front. Genet. 10, 590. 10.3389/fgene.2019.00590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostagno H. S. (2011). Tabelas brasileiras para aves e suínos: Composição de alimentos e exigências nutricionais. Tabelas Bras. aves suínos Compos. Aliment. Exig. Nutr. 2, 186. [Google Scholar]
- Sakurai K., Shen C., Shiraishi I., Inamura N., Hisatsune T. (2021). Consumption of oleic acid on the preservation of cognitive functions in Japanese elderly individuals. Nutrients 13, 284. 10.3390/nu13020284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P., Markiel A., Ozier O., Baliga N. S., Wang J. T., Ramage D., et al. (2003). Cytoscape: a software Environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504. 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman B. T., Hao M., Qiu J., Jiao X., Baseler M. W., Lane H. C., et al. (2022). DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 50, W216–W221. 10.1093/nar/gkac194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Zhang Y., Gu W., Cui B., Xu M., Yan Q., et al. (2012). Serum liver fatty acid binding protein levels correlate positively with obesity and insulin resistance in Chinese young adults. PLoS One 7, e48777. 10.1371/journal.pone.0048777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Vignato B., Coutinho L. L., Poleti M. D., Cesar A. S. M., Moncau C. T., Regitano L. C. A., et al. (2019). Gene co-expression networks associated with carcass traits reveal new pathways for muscle and fat deposition in Nelore cattle. BMC Genomics 20, 32. 10.1186/s12864-018-5345-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinella R., Sawhney R., Jalan R. (2016). Albumin in chronic liver disease: structure, functions and therapeutic implications. Hepatol. Int. 10, 124–132. 10.1007/s12072-015-9665-6 [DOI] [PubMed] [Google Scholar]
- Stelzer G., Rosen N., Plaschkes I., Zimmerman S., Twik M., Fishilevich S., et al. (2016). The GeneCards suite: from gene data mining to disease genome sequence analyses. Curr. Protoc. Bioinforma. 54, 1. 10.1002/cpbi.5 [DOI] [PubMed] [Google Scholar]
- Trinder P. (1969). Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. Int. J. Lab. Med. 6, 24–27. 10.1177/000456326900600108 [DOI] [Google Scholar]
- Vötterl J. C., Klinsoda J., Hennig-Pauka I., Verhovsek D., Metzler-Zebeli B. U. (2021). Evaluation of serum parameters to predict the dietary intake of calcium and available phosphorus in growing pigs. Transl. Anim. Sci. 5, txab059. 10.1093/tas/txab059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vusse G. J. van der. (2009). Albumin as fatty acid transporter. Drug Metab. Pharmacokinet. 24, 300–307. 10.2133/dmpk.24.300 [DOI] [PubMed] [Google Scholar]
- Wang J., Zhong J., Chen G., Li M., Wu F. X., Pan Y. (2015). ClusterViz: a Cytoscape app for cluster analysis of biological network. IEEE/ACM Trans. Comput. Biol. Bioinforma. 12, 815–822. 10.1109/TCBB.2014.2361348 [DOI] [PubMed] [Google Scholar]
- Wondmkun Y. T. (2020). Obesity, insulin resistance, and type 2 diabetes: associations and therapeutic implications. Diabetes, Metab. Syndr. Obes. Targets Ther. 13, 3611–3616. 10.2147/DMSO.S275898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing K., Liu H., Zhang F., Liu Y., Shi Y., Ding X., et al. (2021). Identification of key genes affecting porcine fat deposition based on co-expression network analysis of weighted genes. J. Anim. Sci. Biotechnol. 12, 100. 10.1186/s40104-021-00616-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Jan Y. H., Mishin V., Heck D. E., Laskin D. L., Laskin J. D. (2017). Diacetyl/l -xylulose reductase mediates chemical redox cycling in lung epithelial cells. Chem. Res. Toxicol. 30, 1406–1418. 10.1021/acs.chemrestox.7b00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B. L., Wang S. H., Peng D. Q., Zhao S. P. (2010). HDL and immunomodulation: an emerging role of HDL against atherosclerosis. Immunol. Cell Biol. 88, 285–290. 10.1038/icb.2009.112 [DOI] [PubMed] [Google Scholar]
- Zappaterra M., Gioiosa S., Chillemi G., Zambonelli P., Davoli R. (2020). Muscle transcriptome analysis identifies genes involved in ciliogenesis and the molecular cascade associated with intramuscular fat content in Large White heavy pigs. PLoS One 15, e0233372. 10.1371/journal.pone.0233372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Horvath S. (2005). A general framework for weighted gene co-expression network analysis. Stat. Appl. Genet. Mol. Biol. 4, Article17. 10.2202/1544-6115.1128 [DOI] [PubMed] [Google Scholar]
- Zhang D., Hao W., Niu Q., Xu D., Duan X. (2022). Identification of the co-differentially expressed hub genes involved in the endogenous protective mechanism against ventilator-induced diaphragm dysfunction. Skelet. Muscle 12, 21. 10.1186/s13395-022-00304-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Cai Z., Lhomme M., Sahana G., Lesnik P., Guerin M., et al. (2020). Inclusion of endophenotypes in a standard GWAS facilitate a detailed mechanistic understanding of genetic elements that control blood lipid levels. Sci. Rep. 10, 18434. 10.1038/s41598-020-75612-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Liu H., Pan Y., Liu Y., Zhang F., Ao H., et al. (2022). Identification of potential candidate genes from Co-expression module analysis during preadipocyte differentiation in landrace pig. Front. Genet. 12, 753725. 10.3389/fgene.2021.753725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Li M. C., Konaté M. M., Chen L., Das B., Karlovich C., et al. (2021). TPM, fpkm, or normalized counts? A comparative study of quantification measures for the analysis of RNA-seq data from the NCI patient-derived models repository. J. Transl. Med. 19, 269. 10.1186/s12967-021-02936-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Sun X. (2021). Cholesterol metabolism: a double-edged sword in hepatocellular carcinoma. Front. Cell Dev. Biol. 9, 762828. 10.3389/fcell.2021.762828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Jiang H. (2023). Identification of oxidative stress-related biomarkers associated with the development of acute-on-chronic liver failure using bioinformatics. Sci. Rep. 13, 17073–17115. 10.1038/s41598-023-44343-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuliani G., Volpato S., Blè A., Bandinelli S., Corsi A. M., Lauretani F., et al. (2007). High interleukin-6 plasma levels are associated with low HDL-C levels in community-dwelling older adults: the InChianti study. Atherosclerosis 192, 384–390. 10.1016/j.atherosclerosis.2006.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.