Abstract
Current UK guidance on OSA management recommends only selective use of sleep studies - when there is diagnostic uncertainty, in children with comorbidities or to evaluate perioperative risk in those with suspected severe OSA. Routine use of sleep studies to confirm a diagnosis of obstructive sleep apnoea (OSA) in children before adenotonsillectomy is not currently recommended. We report the findings of a novel paediatric sleep service based on routine use of multi-channel sleep studies (MCSS) before adenotonsillectomy and present the results of a service evaluation assessing the impact of our practise on treatment outcomes and cost.
We conducted a retrospective study of 264 children with sleep disordered breathing seen in our centre between July 2018–June 2019, using medical records and a sleep study database to determine treatment outcomes and costs. Using responses from a questionnaire completed by otolaryngologists for a separate prospective study, we compare our costs with estimates of those associated with a standard UK model of care i.e. with selective use of sleep studies.
We estimate that our routine use of MCSS reduced the number of adenotonsillectomies by 44 % but at higher monetary costs than those estimated for the standard model of care. We note however, that reconfiguring our service to arrange a sleep study before the initial appointment, rather than after, would result in the service being cost neutral compared with the standard model. We also estimate that use of home multi-channel studies in our service would bring a significant cost saving (∼£50,000 - £80,000 per annum) compared to standard care.
Highlights
-
•
Sleep disordered breathing is frequently diagnosed by clinical evaluation.
-
•
The routine use of sleep studies to confirm SDB is not advised in UK guidance.
-
•
We estimate that our routine use of multi-channel studies reduced surgery by 44 %.
-
•
Our findings suggest this cost more than our estimate of standard care.
-
•
We estimate that we could achieve cost savings by using home multi-channel studies.
Abbreviations
- AHI
Apnoea-hypopnoea index
- BMI
Body mass index
- BTS
British Thoracic Society
- CCG
Clinical commissioning group
- CI
Confidence interval
- CPAP
Continuous positive airway pressure
- CRSS
Cardiorespiratory sleep study/studies
- ECG
Electrocardiogram
- EEG
Electroencephalogram
- ENT
Ear, nose and throat
- HRG
Healthcare resource groups
- HDU
High dependency unit
- MCSS
Multi-channel sleep studies
- NHS
National health service
- ODI3
3 % oxygen desaturation index
- ODI4
4 % oxygen desaturation index
- OSA
Obstructive sleep apnoea
- PSG
Polysomnography
- PS
Primary snoring
- SDB
Sleep-disordered breathing
- SMD
Submucous diathermy
- UARS
Upper airway resistance syndrome
1. Introduction
Sleep disordered breathing (SDB) is a spectrum of disorders ranging from primary snoring (PS) to obstructive sleep apnoea (OSA), estimated to affect 1–4% of children [1]. OSA can impair cognition and behaviour due to sleep fragmentation and brain hypoxia and can cause cardiovascular, metabolic and growth abnormalities [2,3]. History and examination findings cannot accurately distinguish children at either end of the SDB spectrum and, unlike polysomnography (PSG), the gold standard, have been shown to be poor discriminators of those needing intervention [4,5]. The British Thoracic Society (BTS) guideline for management of paediatric SDB evaluates a range of tools available for use in the diagnosis of SDB in children in the UK healthcare system where access to PSG is limited [6]. Our centre has developed a sleep study service which is novel for a UK secondary care centre and so not reflected in the current BTS guidance, which prompted us to evaluate our practice.
Paediatric sleep study services in the UK are typically based on the model of Htun et al. in which otolaryngologists make a clinical diagnosis of OSA and use pulse oximetry to stratify perioperative risk [7]. This model of care is consistent with current UK guidance which state that a sleep study is not required to confirm a diagnosis of OSA before adenotonsillectomy in children without co-morbidities [6,8]. In those without comorbidities but suspected to have severe OSA, preoperative pulse oximetry sleep monitoring is also confirmed to be of value in the BTS guidance.
In contrast, a systematic review of evidence from healthcare systems with better access to PSG recommends its use in all children with symptoms of SDB being considered for adenotonsillectomy as clinical parameters alone lack reliability to correctly classify disease [9]. It is likely that many children who undergo adenotonsillectomy for SDB based on clinical criteria alone could be safely managed with watchful waiting as many will not reach accepted PSG thresholds for intervention. Evidence from the CHAT study suggests that some children (aged 5–9 years) with mild OSA who reach the accepted clinical thresholds for intervention can be safely managed with watchful waiting [10]. It is arguable, therefore, that wider access to sleep studies that detect OSA with accuracy could allow the use of watchful waiting in more children where this is appropriate and cost effective.
We previously published an observational study describing our use of a multi-channel sleep system (MCSS) combining oximetry, video and pulse transit time to diagnose SDB in children in secondary care [11]. Using MCSS rather than oximetry alone, we identify twice as many children with SDB who might benefit from intervention. Our use of MCSS to diagnose SDB in children and guide surgical management decisions is novel for a secondary care centre in the UK National Health Service (NHS). This practice has been largely driven by a need to satisfy local Clinical Commissioning Group (CCG) funding requirements that children undergoing surgery for SDB either have a ‘strong clinical history’ or objective confirmation with a sleep study, based on other UK guidance which identifies tonsillectomy to be of limited value except in carefully defined clinical scenarios [12]. Our team of clinicians judged that combining clinical evaluation with MCSS findings would more accurately identify children with SDB needing surgery than using a ‘strong clinical history’ alone. We have undertaken this service evaluation to document the impact of our routine use of MCSS on treatment outcomes and cost in children with SDB.
Our working hypothesis is that MCSS facilitates more conservative management in children with SDB and reduces rates of adenotonsillectomy. We also hypothesise that there will be no significant cost difference between our practice of using MCSS to diagnose SDB and guide management compared with the more limited use of MCSS to stratify perioperative risk, as we assume that the costs of doing more MCSS is offset by a reduced rate of surgery.
2. Methods
We conducted the service evaluation in 2 parts, a retrospective review of sleep study data over 12 months (1 July 2018 to 30 June 2019 inclusive, at least 8 months before covid-19 pandemic in the UK) and a prospective study from July 2021–August 2022, the results of which are reported separately.
2.1. Inclusion
All children (<18 years of age) admitted between 1 July 2018 and 30 June 2019 for a supervised inpatient MCSS due to suspected obstructive SDB were eligible for inclusion. Inpatient MCSS is our default sleep study of choice. Children with co-morbidities such as Down syndrome or other chromosomal disorders, neurodevelopmental disorders, or obesity, were included if they did not have an exclusion criterion as below.
2.2. Exclusions
-
⁃Children referred for MCSS for reasons other than obstructive SDB or with a final diagnosis of ‘abnormal other’ (defined below) were excluded. Reasons for exclusion were:
-
oConfirmed or suspected central breathing control abnormality
-
oStridor due to congenital airway anomalies (e.g. laryngomalacia)
-
oAcute respiratory diagnoses e.g. asthma, pneumonia, bronchiolitis as a cause of desaturation episodes
-
oRoutine monitoring of infants with chronic lung disease or children needing long term oxygen therapy for chronic lung disease
-
oEpileptic seizures resulting in desaturation episodes
-
o
-
⁃
Studies were excluded if there was a technical fault resulting in failure of the video recording or if there was <4 h artefact-free oximetry data available.
-
⁃
Children whose treatment outcomes could not be ascertained from the available medical records were also excluded.
We used anonymised details from patient electronic records or case notes and our sleep study database, which is maintained for audit and service evaluation purposes, to determine demographics, sleep study findings and treatment outcomes. The cost of the MCSS service provision, outpatient costs and surgery were calculated using standard Healthcare Resource Groups (HRG) agreed tariffs (Table 1). We used patient electronic records to identify and code hospital attendance and subsequently apply the appropriate cost codes. The total costs per child were calculated by the summation of all clinic and surgical events attended using the hospital tariffs (Table 1) and sleep study costs. For estimated sleep study costs associated with alternative models of care we used the relevant HRG tariffs.
Table 1.
Hospital tariff used for clinical coding 2020–2021.
| Clinic | Cost (£) |
|---|---|
| Paediatrics | 379.00 |
| ENT | 225.00 |
| Sleep study | 606.00 |
| Surgery | |
| Adenoidectomy | 1278.99 |
| Adenotonsillectomy | 1891.28 |
| Tonsillectomy | 1579.78 |
| aSMD inferior turbinates | 143.00 |
Submucous diathermy.
In calculating costs, we checked that children seen for subsequent follow up were being assessed for SDB and not for other unrelated conditions such as conductive hearing loss. In children seen for both SDB and conductive hearing loss we did not include the follow up for hearing problems in the follow up totals. Similarly, if children had surgery to insert grommets (tympanostomy tubes) at the same time as adenotonsillectomy surgery, the costs of grommet insertion were not included in our calculations. However, we included the costs of adenoidectomy in children who first presented with SDB but later had adenoidectomy and grommet insertion due to hearing concerns, as we presume the surgery will have been of benefit for mild SDB if present.
For the prospective review, otolaryngologists completed a questionnaire to document their likely management decisions based on their clinical evaluation alone before requesting a sleep study. The questionnaire is available as an appendix with our report of the prospective study findings [13].
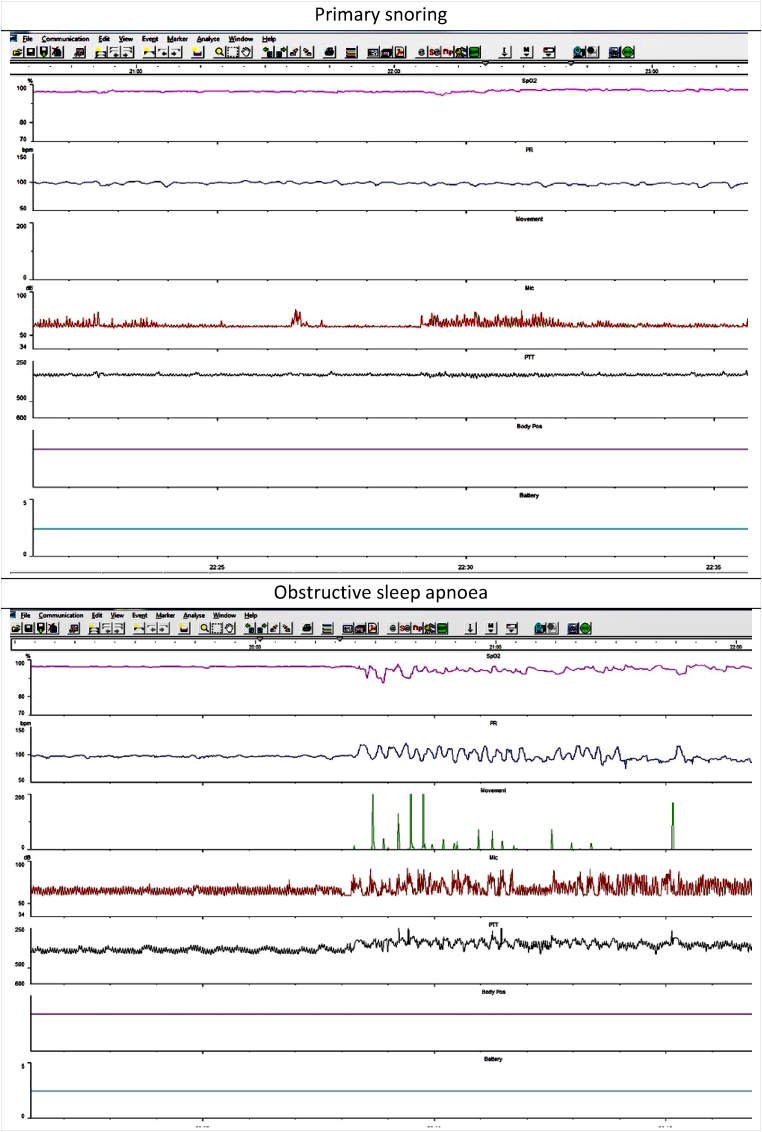
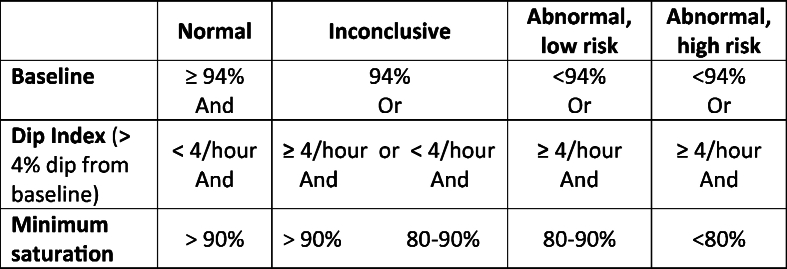
Multi-channel sleep studies were performed with Stowood Scientific Instruments VISI-3 sleep systems incorporating ECG, video, sound, movement, pulse transit time and oximetry data (Fig. 1). Video was recorded with a Sony EVI-D90P infra-red camera. The VISI-3 sleep system uses Masimo technology to obtain oximetry data with 2–4 s averaging times. Oximetry was measured with probes attached to a finger or toe. The following oximetry indices were recorded: mean saturation, minimum saturation, dip index defined as >4 % drop (i.e. 5 % or greater) in baseline saturation/hour and lasting for >5 but <180 s. Abnormal oximetry was defined as shown in Table 2 below
Fig. 1.
Example traces of primary snoring and obstructive sleep apnoea.
Table 2.
Oximetry risk criteria for OSA.
The VISI-3 sleep system uses two different mechanisms to assesses movement. The Black Shadow patient unit contains a microelectromechanical systems (MEMS) accelerometer. When worn by the patient, the movements detected by the accelerometer are converted into a numerical value and an accompanying trace which represents the magnitude of the movement.
Movement is also detected by the video camera with the VISI-3 system saving snapshots of the video to temporary memory once every second. A software program then analyses changes in individual pixels from the snapshots which are converted into a numerical value and a trace.
The movement trace obtained by video was the default measure used for this study as many children don't tolerate wearing the unit strapped to their trunk, rendering the MEMS accelerometer movement data unusable. The movement trace was used solely to determine sections of video worth a closer look. The Sony EVI-D90P infra-red camera has pan, tilt and zoom capabilities and sufficient resolution for chest wall movement and effort of breathing to be seen. The effort of breathing is not scored or quantified. The video is used simply to identify chest wall movement during a desaturation episode to help distinguish apnoeas due to impaired central breathing control from apnoeas due to obstruction.
Sound was recorded with a wall mounted microphone. The sound trace was used to identify sections of video to view more closely and to evaluate breathing sounds for evidence of obstruction.
Sleep study categories were determined by a clinician (MY) using oximetry, video and sound criteria as below. Our sleep reporting methods have been previously described [11]. Oximetry, heart rate, sound and movement traces were all used to identify sections of video for closer inspection. Fig. 1 gives two examples of a 20 min trace that would be used to identify sections of video to inspect more closely. The video was assessed for evidence of obstructive episodes (defined below). Following assessment of the oximetry data, sleep study montage and video, the reporting clinician assigned one of the following five categories: normal; primary snoring; upper airway resistance syndrome; obstructive sleep apnoea or abnormal other.
2.2.1. Sleep study category definitions
-
⁃
Normal: No snoring or obstructed breathing evident on video + normal or inconclusive oximetry (Table 2)
-
⁃
Primary snoring: Snoring but <3 obstructive episodes seen on video + normal or inconclusive oximetry
-
⁃
Upper airway resistance syndrome: Video and sound evidence of 3 or more discrete periods of obstructed breathing, associated arousals + normal or inconclusive oximetry
-
⁃
Obstructive sleep apnoea: Video and sound evidence of obstructed breathing, associated arousals + abnormal oximetry
-
⁃
Abnormal other: Abnormal oximetry findings without any associated video evidence of snoring or obstructed breathing.
2.2.2. Other definitions
-
⁃
‘Obstructed breathing’ - video and sound recordings document a brief pause in snoring but continued chest wall movement, followed by a gasp or other airway opening noise (i.e. a click or grunt). A period of obstructed breathing was considered to have occurred if the airway opening noise was accompanied by an arousal, indicating that a degree of increased respiratory effort was needed to overcome an obstructed airway. Only obstructive episodes with an associated arousal were counted.
-
⁃
An arousal was identified on video if movement of any body part was evident immediately after an obstructive event.
2.2.3. Treatment outcomes
Children were assessed to have one of five treatment outcomes.
-
1.
Watchful waiting or no treatment (with a plan for review after 4–6 months if needed)
-
2.
Medical therapy (nasal corticosteroids/leukotriene antagonists)
-
3.
Surgery (adenotonsillectomy, adenoidectomy, tonsillectomy, or other surgery)
-
4.
Nasal CPAP
-
5.
Other (e.g. weight management)
Children reported by parents to have ongoing SDB symptoms at review are referred to as having ‘persistent symptoms’ but such data were not collected systematically on all children in the study.
2.2.4. Follow up
Some children with moderate or severe OSA were offered a routine follow up appointment post-surgery; the remaining children were advised that they could request a post operative review if they had persistent symptoms 8 weeks after discharge. Children with mild OSA/UARS managed conservatively with watchful waiting or treated with nasal corticosteroids, were offered a review at least 4 months and usually 6 months after first seen and were then discharged if they reported improved or resolving symptoms. If parents reported that symptoms remained troublesome or had worsened since first seen, a repeat sleep study was offered, or a decision made to proceed to surgery. In children with persistent symptoms after tonsillectomy alone, adenoidectomy was considered; when adenoidectomy was not thought appropriate, or had already been done, medical therapy was offered; a repeat sleep study was also considered in those with persistent symptoms after surgery.
2.2.5. Data analysis
Data analysis was performed using MS Excel. Data were cleaned to include only those studies with 4 or more hours of oximetry recorded, then sorted by outcome or diagnosis. Descriptive statistics were performed. For the PTT swing data, comparison of variance between groups was undertaken using ANOVA and paired comparisons using t-tests (Two-Sample Assuming Unequal Variances).
2.2.6. Ethical considerations
The Research and Innovation department at the hospital Trust confirmed that ethical approval was not required for this service evaluation.
3. Results
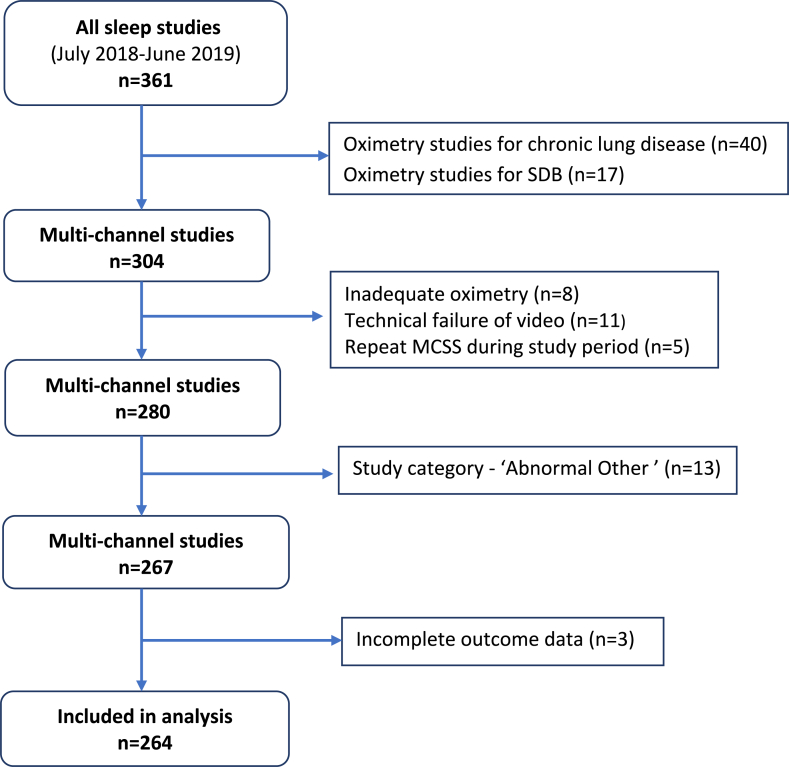
Total of 304 multi-channel sleep studies and 57 oximetry studies in 12-month study period.
-
o5 children had repeat MCSS during the 12-month study period
-
⁃2 had repeat studies due to previous technical failures
-
⁃3 had repeat studies for persistent symptoms and are shown in the flow diagram (Fig. 2) under the category of their first study
-
•First study showed PS → Repeat showed PS
-
•First study showed PS → Repeat showed severe OSA
-
•First study showed PS → Repeat showed OSA
-
•
-
⁃
- o 21 children had repeat studies between 1 July 2019–31 March 2022
-
⁃5 of these had a positive repeat study (1 OSA; 4 UARS) after an initial study that was normal or showed primary snoring
-
⁃
-
⁃13 sleep studies were excluded because the sleep study request or sleep study findings indicated a confirmed or suspected diagnosis of ‘Abnormal Other’
-
o5 sleep studies (4* children) either confirmed or were done to assess for central apnoeas (*one child had a repeat sleep study showing the same abnormality). There were 2 children with chromosomal abnormalities -SETD5 syndrome; 2q37 deletion and 7q33 duplication.
- o 1 child was investigated for a brief resolved unexplained event (BRUE)
- o 3 children had noisy breathing (stridor) due to laryngomalacia
- o 3 children had noisy breathing/desaturation episodes due to asthma, pneumonia and breathing pattern disorder
- o 1 child had desaturation episodes due to frontal lobe epilepsy
-
o
-
⁃
1 child had chronic lung disease requiring long term oxygen therapy with tracheostomy in situ, and had a study to assess the need for non-invasive ventilation.
- 3 children were excluded due to incomplete outcome data in their medical records
-
oOne had a sleep study confirming OSA, the other two had studies showing UARS.
-
o
-
⁃A total of 57 oximetry studies were done during the study period
- o 17 home oximetry studies were done in children with suspected SDB at parents' request. Difficulty with childcare arrangements or anticipated behaviour problems in a hospital environment were the commonest reasons cited for a home study request.
- o 40 oximetry studies were done to monitor infants or children needing long term oxygen therapy for a chronic lung disease (e.g. neonatal chronic lung disease; obliterative bronchiolitis)
Fig. 2.
Flow diagram of study participants showing exclusions.
Surgery was performed in 101 children (38 %); 2 had adenoidectomy initially but later tonsillectomy, 58 had adenotonsillectomy, 22 tonsillectomy and 22 adenoidectomy. One child had submucous diathermy (SMD) of inferior turbinates and adenoidectomy, another had SMD and reduction of inferior turbinates.
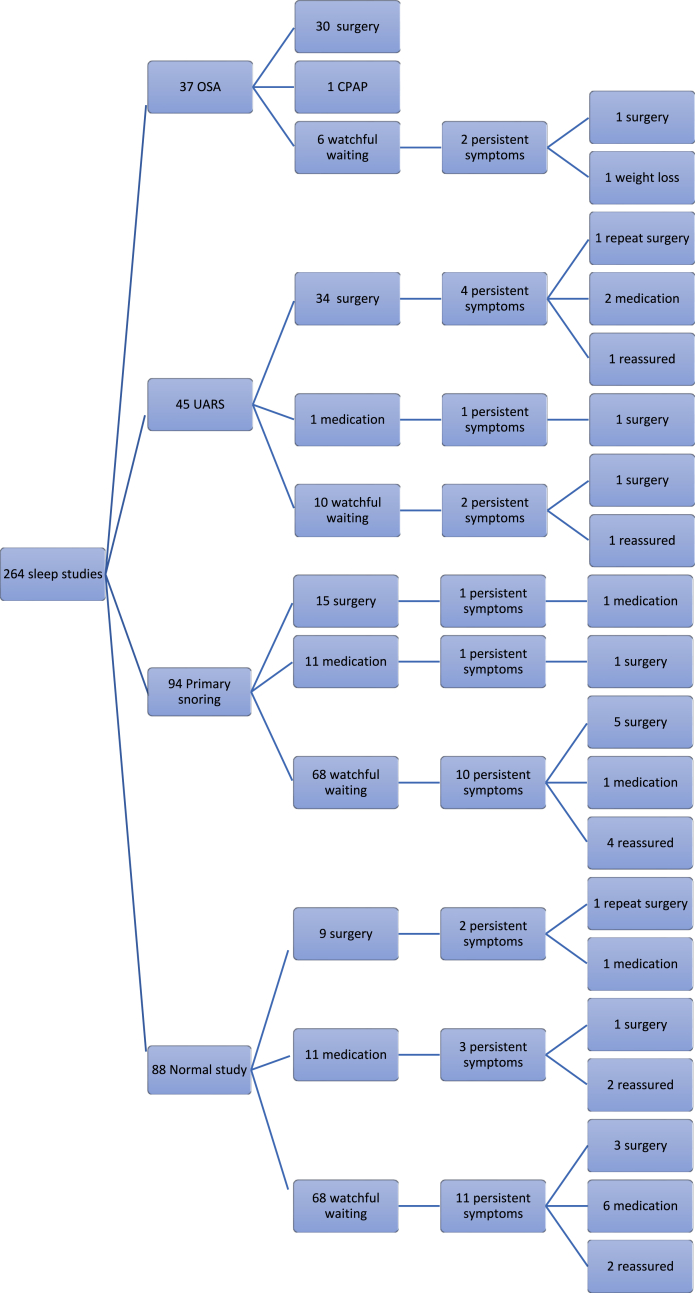
Table 3 shows the results of sleep study findings, demographics and referral origin. Fig. 3 shows treatment outcomes following the initial sleep study and after review for persistent symptoms.
Table 3.
Sleep study findings, demographics, and referral origin.
| Sleep study diagnosis | Males | Mean Age (years) | Body Mass Index Kg/m2 (n = 228) |
|---|---|---|---|
| Normal (n = 88) | 40 (45 %) | 6 (range 0–15, SD 3.6) | 17.1 (SD 2.6) |
| Primary snoring (n = 94) | 54 (57 %) | 5 (range 1–18, SD 3.0) | 16.9 (SD 2.2) |
| UARS (n = 45) | 25 (56 %) | 5 (range 1–14, SD 2.5) | 17.1 (SD 2.7) |
| OSA (n = 37) | 27 (73 %) | 6 (range 1–16, SD 4.0) | 20.1 (SD 7.3) |
| All (n = 264) | 147 (55 %) | 5 (range 0–18, SD 3.8) | 17.5 (SD 3.7) |
| Mean study duration | 8.9 h (SD 1.8 h) | ||
| Referral specialty | Number (%) | ||
| Otolaryngologist | 199 (75 %) | ||
| Paediatrician | 63(24 %) | ||
| General Practitioner | 2 (1 %) | ||
⁃ 6 children had a body mass index >2.5 SDS.
Fig. 3.
Flow chart of treatment outcomes following use of multi-channel study.
Table 4 shows the results of the sleep study outcomes and initial treatment.
Table 4.
Sleep study findings and initial treatment category.
| Study Outcomes (n = 264) |
Watchful waiting (n = 153) | Medication (n = 23) |
Surgery (n = 88) |
|---|---|---|---|
| Normal (n = 88) | 68 | 11 | 9 |
| Primary snoring (n = 94) | 68 | 11 | 15 |
| UARS (n = 45) | 10 | 1 | 34 |
| OSA (n = 37a) | 7 | 0 | 30 |
1 child with OSA was successfully treated with nasal CPAP.
The proportion of children reported to have persistent symptoms during the 12-month study period and for up to 3 years afterwards (July 2018–August 2022) are as follows.
-
•
Watchful waiting - 16 % (25/153)
-
•
Medication (nasal corticosteroids) - 22 % (5/23)
-
•
Surgery - 8 % (7/88).
Thirty five children underwent surgery despite a first sleep study that was normal or showed primary snoring. The following reasons for surgery were documented.
-
•
7 had a repeat sleep study showing deterioration (4 UARS; 3 OSA)
-
•
13 had tonsillectomies for recurrent tonsillitis
-
•
10 had adenoidectomies and insertion of grommets for hearing or speech difficulties
-
•5 had other indications for surgery
-
⁃Peritonsillar abscess
-
⁃Asymmetrical tonsils requiring histology
-
⁃Previous adenotonsillectomy for confirmed OSA with evidence of regrowth of adenoids and tonsils
-
⁃Sleep study suggestive of obstruction but didn't fully meet criteria for UARS
-
⁃Submucous diathermy and reduction of inferior turbinates
-
⁃
4. Other results
There were 26 children with OSA who were deemed to have a high perioperative risk (oxygen desaturation index >4/hour + oxygen saturation nadir <80 % on ≥1 occasion). Nineteen were referred to the nearby tertiary centre where 18 underwent surgery and 1 was treated with nasal CPAP following assessment by the long-term ventilation team. Three underwent surgery at our secondary care centre and 1 child relocated to another country and was lost to follow-up. Three children were scheduled for surgery but, due to COVID delays and/or parental choice, were managed conservatively. The medical record of one child imply spontaneous improvement of symptoms (based on parent report); for the remaining 2 children there is no further documentation of ongoing symptoms and it seems they were lost to follow up.
Thirty-four children (13 %) with symptoms of SDB were treated for OSA/nasal congestion with nasal steroids rather than surgery or watchful waiting; 23 were treated after an initial sleep study and a further 11 after reporting persistent symptoms at later follow-up.
-
⁃
25 children had skin prick tests (SPT) or specific IgE tests
-
⁃
12 had a confirmed diagnosis of allergic rhinitis with positive SPT or specific IgE results and in a further 12 children a diagnosis of allergic rhinitis was presumed (i.e. clinical diagnosis with negative or no SPT).
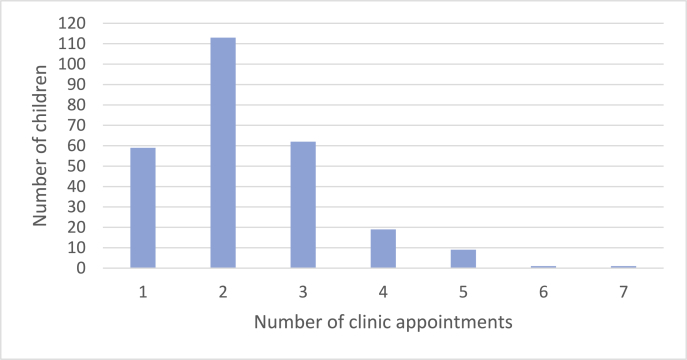
All children were seen in clinic before the sleep study and at least once after to discuss the outcome and further management. The mean number of total clinic appointments per patient was 2.3 (SD 1.1). Fig. 4 is a bar chart of the number of clinic appointments attended by children assessed in the retrospective study.
Fig. 4.
Total number of clinic appointments attended.
Table 5 shows the sleep study, outpatient follow up and surgery costs for children assessed in this retrospective review, as well as the estimated costs of alternative models of care associated with varying types of sleep study services.
Table 5.
Cost analysis of the current sleep service compared with estimated costs of alternative models of care with varying types of sleep studies, and assuming different rates of surgery (50 %, 68 %, or 80 %) in children assessed by clinical criteria alone.
| No of children | Total no of sleep studies | Sleep study costs (£) | Mean (SD) no of appts | Total appt costs (£) | Types of surgery (No of children) |
Total surgery costs (£) | Total costs (£) | |
|---|---|---|---|---|---|---|---|---|
| Actual retrospective study costs | 264 | 282 | 170,892.00 | 2.3 (1.1) | 142,748.00 | Tonsillectomy −22 Adenoidectomy −22 Adenotonsillectomy- 58 Other −2 |
172,873.18 | 486,513.18 |
| Estimated retrospective study costs with service changes (inpatient MCSS before 1st appt) | 264 | 282 | 170,892.00 | 1.3 | 80,683.65 | Tonsillectomy −22 Adenoidectomy −22 Adenotonsillectomy- 58 Other −2 |
172,873.18 | 424,448.83 |
| Estimated retrospective study costs with service changes and home MCSS | 264 | 282 | 98,136.00 | 1.3 | 80,683.65 | Tonsillectomy −22 Adenoidectomy −22 Adenotonsillectomy- 58 Other −2 |
172,873.18 | 351,692.83 |
| Estimated costs of a service model with home oximetry in 10 % and surgery in 68 % | 264 | 28 | 4760.00 | 1.5 | 93,096.52 | Tonsillectomy - 40 Adenoidectomy - 40 Adenotonsillectomy - 100 | 303,478.80 | 401,335.32 |
| Estimated costs of a service model with home oximetry in 30 % and surgery in 68 % | 264 | 84 | 14,280.00 | 1.7 | 118,292.55 | Tonsillectomy - 40 Adenoidectomy - 40 Adenotonsillectomy - 100 | 303,478.80 | 436,051.35 |
| Estimated costs of a service model with home oximetry in 10 % and surgery in 50 % | 264 | 28 | 4760.00 | 1.5 | 93,096.52 | Tonsillectomy −27 Adenoidectomy −27 Adenotonsillectomy- 78 | 224,706.63 | 322,563.15 |
| Estimated costs of a service model with home oximetry in 30 % and surgery in 80 % | 264 | 84 | 14,280.00 | 1.6 | 113,302.40 | Tonsillectomy - 47 Adenoidectomy - 47 Adenotonsillectomy - 117 | 355,641.95 | 481,224.35 |
A HRG coding tariff of £348 was used for estimating the costs of home multi-channel studies and £170 for home oximetry.
5. Discussion
The European, American and UK guidelines for managing children with SDB have important differences reflecting, among other differences, the availability of tools for diagnosing SDB in the respective healthcare systems [6,9,14,15]. Limited access to PSG or cardiorespiratory studies (CRSS) in the UK healthcare system has resulted in an established practise of diagnosing OSA by clinical evaluation alone in most cases. Sleep studies may be undertaken to support a diagnosis of OSA when there is diagnostic uncertainty or to stratify perioperative risk, particularly in children with co-morbidities, but are not routine for all before surgery.
Our centre has developed a service which is novel in UK secondary care, both in the type of sleep study used and criteria for use, which is not reflected in current guidance. This has led us to undertake a detailed service evaluation to document the outcomes and cost of our overall service. We demonstrate that our approach leads to more conservative management. A benefit of being able to deliver more conservative management is a reduced waiting time for those most in need of surgery and the possibility of cost saving. Based on the findings of our retrospective study, we estimate that our use of MCSS may have reduced the number of children undergoing surgery for SDB by 44 %.
Most UK secondary care centres assess children with SDB using oximetry alone, unlike tertiary centres which have access to CRSS and PSG as well, although access to PSG within the NHS is limited. We have previously described our use of a limited MCSS to diagnose SDB in children based on oximetry and video findings and shown that this identifies twice as many who may benefit from intervention than use of oximetry alone [11].
The value of using video to support a clinical diagnosis of OSA is increasingly recognised as parents often present video clips of their child demonstrating brief examples of sleep apnoea. The recently published BTS guidance acknowledges this by incorporating the use of a suggestive video clip as supportive information in the clinical evaluation [6]. The main limitation of using such video findings is the lack of widely accepted validated features needed to confirm a diagnosis of OSA. We have sought to do this with definitions described in our methods which we have also reported previously [11].
A study by Sivan et al. investigated the diagnostic accuracy of a 30-min sleep video recording in 58 children with symptoms of SDB who also had PSG [16]. Parents were asked to record videos using a defined format (including close up views of the head and naked trunk), which demonstrated periods of snoring, laboured breathing, or other patterns of breathing of concern. A clinician scored the videos for noisy breathing, movements, waking episodes, apnoea, chest retractions and mouth breathing. The authors found that they could identify SDB with a sensitivity of 0.94 (95 % confidence intervals: 0.81, 0.99] and specificity 0.68 [0.45, 0.86].
A key strength of our service is the use of two modalities which are potentially straightforward to report (and so are accessible to non-specialist paediatricians) yet which, used together, significantly improve accuracy in diagnosing SDB. We postulate that children with a video diagnosis of UARS will have either UARS or mild OSA (PSG defined AHI >1) and those with abnormal oximetry plus a video diagnosis of OSA are expected to correlate closely with children with a PSG-defined AHI >5 [11,16,17].
Previous studies suggested that oximetry has low sensitivity but high specificity for diagnosing SDB in children [17] but technological advances in modern oximeters have significantly improved their accuracy. A recent systematic review conducted for the new BTS guidance showed that, in a mixed population of children (including those with comorbidities), oximetry detects SDB with a sensitivity of 0.82 [0.76, 0.87] and specificity 0.75 [0.60, 0.85] [6]. Abnormal PSG was defined as follows: apnoea-hypopnoea index (AHI) > 1 (mild); AHI >5 (moderate); AHI >10 (severe).
However, the routine use of oximetry to determine which children should be offered surgery is not supported by current guidance [6,8]. UK tertiary centres cannot offer PSG or CRSS to all children with symptoms of SDB before surgery, so surgeons regularly decide whether to offer surgery based on clinical evaluation alone. This pragmatic approach is clearly justified in healthcare systems with limited resources. However, Wang et al. found the predictive accuracy of clinical suspicion to be just 30 % in 82 children with symptoms of SDB who were also evaluated with PSG (OSA defined as AHI>5), implying that using clinical evaluation alone, more children will undergo surgery than would be the case if PSG was used to identify those who need it [6]. Findings from the CHAT study, in which 464 children aged between 5 and 9 years with OSA were randomised to early adenotonsillectomy or watchful waiting, suggest that many school age children with mild OSA can be safely managed with ‘watchful waiting’ [10]. Nearly half the children in the watchful waiting group showed normalisation of the AHI score (<2), but this was most likely to occur in children with a baseline AHI that was at or below the median value (AHI <4.7).
Based on limited evidence, we suspect that MCSS detects SDB in children with high sensitivity and moderate specificity [16,18]. We note in this study that it identified SDB in 31 % of children presenting with a suggestive history, similar to the proportion observed with PSG. We acknowledge that more work is needed to validate this approach to diagnosing SDB in children as the only validation study of the VISI-sleep system against PSG involved a very small sample size. Van Someren et al. evaluated 10 children aged 2 months to 6 years and 4 months with both the Visilab system (oximetry, sound, video, and movement) and a conventional polysomnographic system (Oxcams), incorporating pulse oximetry, ECG, nasal airflow (thermistors), chest and abdominal movement (impedance), and video [18]. There were just two discrepancies in the final diagnosis between the two systems. One child deemed to have a normal study with the Visilab system had mild obstruction identified with PSG whilst another deemed to have obstruction with the Visilab system was shown to have mixed apnoea with PSG.
Given the limited validation of MCSS and the known night-to-night variability in all types of sleep studies, it is our practise to consider repeating a sleep study in children with persistent symptoms if the initial study was normal or demonstrated primary snoring only. We identified 7 children who had persistent symptoms, despite a reassuring first study, who had a repeat study which did confirm abnormalities for which they went on to have surgery. A further 28 children were offered surgery (tonsillectomy and/or adenoidectomy) despite a reassuring MCSS because of other indications such as recurrent tonsillitis or middle ear effusions associated with hearing or speech difficulties. One child underwent adenotonsillectomy based on clinical symptoms despite a sleep study result showing primary snoring (i.e. didn't meet criteria for UARS but was borderline). A small number of children with co-morbidities such as Down syndrome, were referred to the nearby tertiary centre for consideration of PSG because MCSS was abnormal but not conclusive in identifying OSA (i.e. ‘Abnormal other’).
In the UK, funding for adenotonsillectomy in children is restricted in some regions by clinical commissioning group guidelines and approval is only granted if certain criteria are met [12]. Clinicians treating children with suspected SDB in these regions therefore need to demonstrate a higher level of clinical suspicion (compared to other regions) or provide sleep study confirmation of OSA. The impact of these restrictions is that clinicians who have a low - moderate suspicion of OSA when assessing children with symptoms of SDB, are more likely to choose conservative management. UK secondary care centres do not have direct access to PSG and have limited opportunity to refer children without comorbidities to tertiary centres for this. A further constraint is by guidance stating that a sleep study is not required pre-operatively in children with suspected OSA without comorbidities [8] but those assessed in our Trust with a reassuring MCSS can still be considered for surgery if clinical suspicion remains high. In children managed conservatively, parents and clinicians generally achieve consensus that this is justified. In our experience, many parents are reluctant for their child to undergo surgery when there is a possibility that conservative management will achieve a similar outcome.
We undertook this retrospective review of our service over a full year ending at least 8 months before covid reached the UK to avoid the impact of pandemic-related disruption. We noted, however, that a few children in the later stages of the study were managed conservatively because of pandemic restrictions rather than by choice. Three children with MCSS findings of severe OSA were referred to tertiary services and were planned for surgery but were managed conservatively due to the COVID-19 disruptions or parental choice. We found documentation in the medical records for one of these children confirming a parent's report of spontaneous resolution of their symptoms.
Another reason for our choice of dates for the retrospective study was to allow identification of children with persistent symptoms during conservative management, as this could be considered a failure of the approach. The overall proportion of children with persistent symptoms was low - for those managed with ‘watchful waiting’ it was 16 % (25/153), for those offered medical therapies (predominantly nasal steroids) it was 22 % (5/23) and for those who had undergone surgery it was 8 % (7/88). We acknowledge that because this outcome was not obtained systematically, this outcome measure does not accurately represent the children who could be considered as treatment failures. We have reported it nevertheless as we consider it to be an important observation.
We identified 26 children with severe OSA and deemed at increased risk of perioperative complications based on criteria previously reported by a Multidisciplinary Working Party [19,20]. Of these, 19 were referred to the nearby tertiary centre for surgery (and/or a respiratory opinion) to ensure immediate access to paediatric high dependency unit facilities post operatively if needed. We have since adopted updated criteria for identifying which ‘high risk’ children to refer for surgery in a tertiary centre, based on more up to date outcome data [8] and anticipate that the changes will reduce the number of children needing tertiary referral.
The limited evidence available suggests that home multi-channel studies that measure airflow and respiratory effort along with other standard cardiorespiratory parameters can detect SDB with high sensitivity and moderate specificity, potentially at a lower cost by avoiding an inpatient stay [6]. Standard multi-channel studies (PSG or CRSS) require a high level of physiologist expertise and time to score and report which is beyond the capabilities of most secondary care centres to resource.
In children needing a sleep study solely to stratify perioperative risks, nocturnal oximetry is sufficient to provide the information required and is more cost effective. An example of this would be to determine whether a child undergoing a tonsillectomy can be managed safely in a secondary care centre with or without an overnight stay or would need referral to a tertiary centre with HDU facilities. However, there may be additional benefit in performing MCSS to assess for SDB in children where a decision has already been made to perform a tonsillectomy for recurrent tonsilitis, if confirmation of SDB would result in additional surgery i.e. an adenotonsillectomy instead of tonsillectomy alone.
The key to providing a sleep service with the capability of diagnosing SDB in children prior to surgery, is the ability to provide sufficient capacity to avoid undue delay to treatment. We suspect that our sleep service may be unusual as a secondary care centre in the NHS healthcare system to be able to achieve this. However, we think that our approach is feasible for other similar sized units and does not involve significant additional cost to the NHS compared to the other commonly used models of care.
5.1. Assumptions
For the cost analysis, we assumed that questionnaire responses in the prospective study about the likelihood of children requiring surgery was applicable to the larger group of children in the retrospective study. Our centre has a practise of managing a significant proportion of children conservatively, which may have introduced bias in the questionnaire responses. A higher percentage of children may be considered for surgery in other centres where the decision is made on clinical evaluation alone. It is also possible that the small sample size in the prospective study is skewed such that a higher proportion of children in the sample required surgery than would normally be the case.
The costs of our model of care would be roughly equivalent to a service in which 80 % of children assessed for SDB undergo surgery based on clinical criteria, assuming at least 30 % of children have an oximetry study to stratify perioperative risk. In the questionnaire responses in our prospective study, Otolaryngologists predicted that surgery was ‘likely’, ‘very likely’ or ‘definitely’ required in 68 % (28/41) of children based on their clinical assessment before a sleep study [13]. In a further 10 children they considered surgery was somewhat likely. For one of our costing scenarios, we assume that half of the children in whom surgery was considered ‘somewhat likely’ are offered surgery in addition to all those in whom it was considered ‘likely, ‘highly likely’ or ‘definitely’ which would be equivalent to 80 % (33/41) of the cohort. This represents a high proportion of children undergoing surgery but is plausible in a centre assessing children based on clinical criteria alone.
Our modelling of different sleep service models suggests that if 50 % of children in our retrospective study were deemed to require surgery based on a clinical evaluation (i.e. a much more conservative estimate than the 68 % obtained with the questionnaire responses), the use of MCSS would still reduce the number of children requiring surgery by almost 30 %. If this was achieved using home MCSS, the service would be cost neutral compared to the standard model of care with a 50 % rate of surgery. Compared to services where children with SDB are assessed on clinical criteria alone and where significantly more than 50 % undergo surgery, it is likely that the routine use of home MCSS would be associated with a cost saving.
We have assumed that at least 10 % of children with SDB assessed for surgery on clinical criteria alone would require a sleep study to assess perioperative risk. This is based on the BTS guideline recommendation that a sleep study is performed pre-operatively in children suspected to have severe OSA. There were 26 children (∼10 % of our cohort) who were suspected to be at increased perioperative risk and were therefore considered for referral to the nearby tertiary centre. We have therefore assumed in our modelling of costs that a minimum of 10 % of children assessed on clinical criteria alone will require a pre-operative sleep study to ensure adequate evaluation of this risk.
Given the predictive accuracy of clinical suspicion being of the order of 30 %, we assumed that sleep studies would be required in about 30 % of the cohort to identify the 10 % of children with severe OSA. We have therefore used a 30 % rate of oximetry studies and an 80 % rate of surgery in one model (Table 5), which we consider to be a high estimate of costs that could be incurred in centres relying solely on clinical evaluation for surgical decision making.
We assume that children evaluated without a preoperative sleep study in whom a decision is made to undergo surgery at the initial review, are likely to have one less appointment than those who have a sleep study before a decision is made about surgery. However, differences in follow up rates for both surgically and conservatively managed children is somewhat unpredictable with practice differing between centres. Some centres arrange routine follow up after surgery whereas other centres offer follow up for children with severe OSA or persistent symptoms.
In our modelling of costs, we estimate that at least 50 % of children managed according to the standard UK model will require at least one follow up appointment after an initial review. This assumes that 10 % of children who have a sleep study will require 2 appointments and that a further 10 % may require follow up due to persistent symptoms. It also assumes that 32 % of children managed conservatively (68 % undergoing surgery) will need at least one follow up appointment (mean 1.5 appointments/child). A higher follow up rate of 1.7 appointments is assumed for children assessed in a model of care in which 30 % have a sleep study and 68 % undergo surgery (this assumes 30 % who have a sleep study have 2 appointments, 10 % with persistent symptoms have 2 appointments and 32 % managed conservatively have 2 appointments). A rate of 1.6 appointments/child is assumed for children where 80 % undergo surgery and 30 % have a sleep study (this assumes 30 % who have a sleep study have 2 appointments, 10 % with persistent symptoms have 2 appointments and 20 % managed conservatively have 2 appointments). Also based on our data showing that 25 % of the cohort were seen in paediatric clinics which attract a higher tariff and 75 % in ENT clinics, we have estimated clinic costs by using a paediatric tariff for 25 % of the total and the lower ENT tariff for the remaining 75 % of the clinic costs.
Table 5 also includes a cost estimate of a planned modification to our sleep service to allow children referred from primary care to undergo a sleep study prior to their first appointment with Otolaryngologists, which we anticipate will significantly reduce costs. We also estimate that the routine use of home multi-channel studies would be associated with a cost saving compared to the standard model of care. It is possible that home MCSS will be associated with an increased rate of technical failures and therefore more repeat sleep studies which we have not considered in our costings.
5.2. Study limitations
A retrospective service evaluation of this kind does not provide data to demonstrate whether quality of life (QOL) outcomes is comparable for children managed conservatively and those treated with surgery. An ideal study would include a prospective evaluation of quality of life using a QOL questionnaire to assess children with equivalent disease severity, managed conservatively or with surgery.
We could not fully assess all relevant costs incurred in the provision of care for children with SDB, including costs of medication, follow up, and wider economic costs such as family time off work.
For the oximetry analysis we have used ODI4 as ODI3 was not recorded routinely in our earlier data collection in 2018. Whilst ODI3 is now preferred in paediatric practice [6,21], our data presented is limited by data collection at the onset of the study window.
A significant limitation of our findings is that the simplified type of MCSS used, combining oximetry and video to diagnose SDB, has only limited validation against PSG and further work is needed before this can be accepted into regular practice more widely. However, we note that this form of MCSS, is more accessible to non-specialist paediatricians than PSG or CRSS and therefore more suitable for use in secondary care.
It was not possible to identify the true treatment failure rate in this retrospective service evaluation. We have tried to assess this pragmatically by identifying children whose parents self-reported persistent symptoms at follow up review. However, not all children were reviewed and so these data were not collected systematically. We acknowledge that persistent symptoms may be an underestimate of the true number of children who can be considered as treatment failures. It is also possible that in the absence of objective measures or a carefully designed questionnaire administered prospectively, that some of the children with reported persistent symptoms may not represent true treatment failure.
5.3. Summary
We conclude that wider use of MCSS may increase safe, conservative management in children with SDB. In children with a negative MCSS, we could decrease morbidity by avoiding surgery with watchful waiting and/or medical treatment. For those with self-reported persistent symptoms, a repeat sleep study and/or surgery was offered as a safety net.
In our attempt to establish cost-effectiveness, we estimate that the overall cost of treatment based on our model of care was higher than would have been incurred with the standard model practised in most UK secondary care centres. This observation is potentially a reason why current UK guidance does not recommend the routine use of sleep studies to diagnose SDB in otherwise healthy children. Another reason is likely to be the lack of sufficient capacity in paediatric sleep services to ensure that all children with SDB who are being considered for surgery can get a study done in a timely manner. A third reason is that there is insufficient evidence to date to indicate improved or comparable outcomes in children assessed routinely with a sleep study compared to the standard UK model of care.
We consider that the findings of our cost analysis warrant further investigation with more robust studies to assess the cost-effectiveness of the routine use of multi-channel sleep studies in children before surgery, including those without comorbidities. We are aware of one such study that is currently underway [22]. In addition to evidence supporting the benefit and cost effectiveness of this approach, it would be necessary to assess the feasibility of increasing the capacity of sleep study provision for this approach to be viable within the UK healthcare system.
6. Conclusion
We have shown that the routine use of MCSS to guide surgical management decisions in children with SDB, facilitates conservative management whilst identifying those most in need of surgery and those at increased perioperative risk. We found that the proportion of children who self-report persistent symptoms after treatment or watchful waiting is low. It appears that our service costs more than the estimated cost of the standard model of care. However, we anticipate that planned changes to our service to allow sleep studies to be done before an initial appointment with otolaryngologists will result in the service being cost neutral. We conclude that the routine use of home MCSS to help guide management decisions may be associated with a cost saving compared to the standard UK model of care.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Michael Yanney: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Nicola Rowbotham: Writing – review & editing, Formal analysis, Data curation, Conceptualization. Christabella Ng: Writing – review & editing, Methodology, Formal analysis, Data curation. Muhammad Zulkifli: Methodology, Formal analysis, Data curation, Conceptualization. Ahmed Shehata: Data curation, Writing – review & editing. Alagappan Chidambaram: Writing – review & editing, Methodology, Data curation, Conceptualization. Paraskevi Tsirevelou: Writing – review & editing, Methodology, Data curation, Conceptualization. Neil Fergie: Writing – review & editing, Methodology, Data curation, Conceptualization. Pathik Thakkar: Formal analysis, Data curation. Emma Crookes: Methodology, Data curation. Roy Dean: Methodology, Data curation. Andrew Prayle: Writing – review & editing, Methodology, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/ personal relationships which may be considered as potential competing interests: Nicola Rowbotham reports a relationship with Cystic Fibrosis Trust that includes: travel reimbursement. Nicola Rowbotham reports a relationship with European Cystic Fibrosis Society that includes: travel reimbursement. Nicola Rowbotham reports a relationship with British Paediatric Respiratory Society that includes: travel reimbursement. Andrew Prayle reports a relationship with Vertex Pharmaceuticals Incorporated that includes: funding grants and travel reimbursement. Andrew Prayle reports a relationship with Cystic Fibrosis Trust that includes: funding grants. Andrew Prayle reports a relationship with Sir Jules Thorn Charitable Trust that includes: funding grants. Andrew Prayle reports a relationship with Nottingham University Hospitals NHS Trust that includes: funding grants. Andrew Prayle reports a relationship with UKRI Medical Research Council that includes: funding grants.
Acknowledgements
The authors wish to acknowledge the contribution of Dr Nabeel Ali who was the lead clinician during the establishment of the sleep service and helped conceptualise this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sleepx.2024.100115.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Lumeng J.C., Chervin R.D. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:242–252. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horne R.S.C., Roy B., Walter L.M., Biggs S.N., Tamanyan K., Weichard A., et al. Regional brain tissue changes and associations with disease severity in children with sleep disordered breathing. Sleep. 2018;41:1–10. doi: 10.1093/sleep/zsx203. [DOI] [PubMed] [Google Scholar]
- 3.Tan H.L., Gozal D., Kheirandish-Gozal L. Obstructive sleep apnea in children: a critical update. Nat Sci Sleep. 2013;5:109–123. doi: 10.2147/NSS.S51907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brietzke S.E., Katz E.S., Roberson D.W. Can history and physical examination reliably diagnose pediatric obstructive sleep apnea/hypopnea syndrome? A systematic review of the literature. Otolaryngol Head Neck Surg. 2004;131:827–832. doi: 10.1016/j.otohns.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Wang R.C., Elkins T.P., Keech D., Wauquier A., Hubbard D. Accuracy of clinical evaluation in pediatric obstructive sleep apnea. Otolaryngol Head Neck Surg. 1998;118:69–73. doi: 10.1016/S0194-5998(98)70377-8. [DOI] [PubMed] [Google Scholar]
- 6.Evans H.J., Gibson N.A., Bennett J. On behalf of the BTS paediatric sleep disorders Guideline Development Group, et al British Thoracic Society guideline for diagnosing and monitoring paediatric sleep-disordered breathing. Thorax. 2023;78:s1–s27. doi: 10.1136/thorax-2022-218938. [DOI] [PubMed] [Google Scholar]
- 7.Htun H.M., Lam M., Tse A., Kaimal K., Kumar B.N. Risk stratification of paediatric obstructive sleep apnoea (OSA) for adenotonsillectomy: how we do it at a UK district general hospital. J Otolaryngol ENT Res. 2017;8(6) doi: 10.15406/joentr.2017.08.00265. [DOI] [Google Scholar]
- 8.ENT UK Safe delivery of paediatric ENT surgery in the UK: a national strategy. A report of a combined Working Party of the British Association for Paediatric Otolaryngology (BAPO), ENT UK, the Royal College of Anaesthetists (RCOA) and the Association of Paediatric Anaesthetists of Great Britain and Ireland (APAGBI) https://www.entuk.org/_userfiles/pages/files/safe_delivery_paediatric_ent.pdf
- 9.Wise M.S., Nichols C.D., Grigg-Damberger M.M., Marcus C.L., Witmans M.B., Kirk V.G., D'Andrea L.A., Hoban T.F. Respiratory indications for polysomnography in children: an evidence-based review. Sleep. 2011;34(3) doi: 10.1093/sleep/34.3.389. 398A-398AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcus C.L., Moore R.H., Rosen C.L., Giordani B., Garetz S.L., Taylor H.G., et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368:2366–2376. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yanney M.P., Prayle A.P., Rowbotham N.J., Kurc M., Tilbrook S., Ali N. Observational study of pulse transit time in children with sleep disordered breathing. Front Neurol. 2020;11:316. doi: 10.3389/fneur.2020.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross E., Trotter M.I. Analysis of procedures of limited clinical value in ENT surgery. Bull Roy Coll Surg Engl. 2015;97:e29–e33. doi: 10.1308/rcsbull.2015.e29. [DOI] [Google Scholar]
- 13.Yanney M.P., Rowbotham N.J., Ng C., Zulkifli M., Shehata A., Chidambaram A., et al. Prospective evaluation of the impact of multi-channel studies on treatment outcomes in children with sleep disordered breathing. Sleep Med: X 7C (2024) 100111. doi: 10.1016/j.sleepx.2024.100111. [DOI] [PMC free article] [PubMed]
- 14.Kaditis A.G., Alonso Alvarez M.L., Boudewyns A., Alexopoulos E.I., Ersu R., Joosten K., et al. Obstructive sleep disordered breathing in 2- to 18-years old children: diagnosis and management. Eur Respir J. 2016;47:69–94. doi: 10.1183/13993003.00385-2015. [DOI] [PubMed] [Google Scholar]
- 15.Kaditis A.G., Alonso Alvarez M.L., Boudewyns A., Abel F., Alexopoulos E.I., Ersu R., et al. ERS statement on obstructive sleep disordered breathing in 1- to 23-months-old children. Eur Respir J. 2017;50:1–22. doi: 10.1183/13993003.00985-2017. [DOI] [PubMed] [Google Scholar]
- 16.Sivan Y., Kornecki A., Schonfeld T. Screening obstructive sleep apnoea syndrome by home videotape recording in children. Eur Respir J. 1996;9:2127–2131. doi: 10.1183/09031936.96.09102127. [DOI] [PubMed] [Google Scholar]
- 17.Brouillette R.T., Morielli A., Leimanis A., Waters K.A., Luciano R., Ducharme F.M. Nocturnal pulse oximetry as an abbreviated testing modality for pediatric obstructive sleep apnea. Pediatrics. 2000;105:405–412. doi: 10.1542/peds.105.2.405. [DOI] [PubMed] [Google Scholar]
- 18.van Someren V., Burmester M., Alusi G., Lane R. Are sleep studies worth doing? Arch Dis Child. 2000;83:76–81. doi: 10.1136/adc.83.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robb P.J., Bew S., Kubba H., Murphy N., Primhak R., Rollin A.M., Tremlett M. Tonsillectomy and adenoidectomy in children with sleep-related breathing disorders: consensus statement of a UK multidisciplinary working party. Ann R Coll Surg Engl. 2009 Jul;91(5):371–373. doi: 10.1308/003588409X432239. PMID: 19622257; PMCID: PMC2758429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Royal College of Paediatrics and Child Health . 2009. Working party on sleep physiology and respiratory control disorders in childhood. Standards for services for children with disorders of sleep physiology. London: RCPCH. [Google Scholar]
- 21.Selby A., Buchan E., Davies M., et al. Role of overnight oximetry in assessing the severity of obstructive sleep apnoea in typically developing children: a multicentre study. Arch Dis Child Published Online First. 22 January 2024 doi: 10.1136/archdischild-2023-326191. [DOI] [PubMed] [Google Scholar]
- 22.Oceja E., Rodríguez P., Jurado M.J., Luz Alonso M., del Río G., Villar M.Á., et al. Validity and cost-effectiveness of pediatric home respiratory polygraphy for the diagnosis of obstructive sleep apnea in children: rationale, study design, and methodology. Methods Protoc. 2021;4:9. doi: 10.3390/mps4010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.