Abstract
Human herpesvirus 8 (HHV-8) sensitivity to the nucleoside analog ganciclovir (GCV) suggests the presence of a virally encoded kinase that catalyzes the initial phosphorylation of GCV. Analysis of the HHV-8 genome identified two candidate kinases: proteins encoded by open reading frame (ORF) 21, with homology to the herpesvirus thymidine kinases (TK), and ORF 36, with homology to the herpesvirus phosphotransferases (PT). Experiments presented here show that both ORF 21 and ORF 36 encode GCV kinase activities as demonstrated by GCV phosphorylation and GCV-mediated cell death. In both regards the PT homologue ORF 36 was more active than the TK homologue ORF 21. ORF 21, but not ORF 36, weakly sensitized cells to killing by penciclovir. Neither ORF sensitized cells to killing by (E)-5-(2-bromovinyl)-2′-deoxyuridine.
Human herpesvirus 8 (HHV-8) is a gammaherpesvirus found in Kaposi’s sarcoma (KS), primary effusion lymphoma (PEL), and in some cases of multicentric Castleman’s disease (10, 14, 15). The spectrum of sensitivity of HHV-8 to antiviral agents most closely resembles that of human cytomegalovirus (HCMV) (2, 24, 45). HHV-8 replication in PEL-derived cell lines is inhibited by ganciclovir (GCV), foscarnet, and cidofovir and to a much lesser extent by penciclovir (PCV) and (E)-5-(2-bromovinyl)-2′-deoxyuridine (BVDU) (33, 41, 46). Acyclovir (ACV) is inactive. Retrospective studies of cohorts of AIDS patients suggest that both GCV and foscarnet reduce the risk of KS, while ACV has no similar protective effect (25, 43). The nucleoside analogs GCV, PCV, BVDU, and ACV are activated by phosphorylation (7, 40). Two herpesvirus kinase gene families have been identified that activate these compounds. The herpes simplex virus (HSV) thymidine kinase (TK) and HCMV UL97 are prototypes of the TK and phosphotransferase (PT) gene families, respectively. Alpha- and gammaherpesviruses encode members of both families, while betaherpesviruses encode only a PT.
HHV-8, like its relatives herpesvirus saimiri (HVS) and Epstein-Barr virus (EBV), encodes both TK (open reading frame [ORF] 21) and PT (ORF 36) family members (44, 52). ORF 21 and ORF 36 show sequence homology to other identified herpesvirus TKs and PTs, respectively (Tables 1 and 2). Comparative sequence analyses of 12 herpesvirus TKs have identified five conserved sites or domains (3, 29, 32, 38, 51). ORF 21 shows homology to other herpesvirus TKs at the putative ATP-binding pocket (site I), the predicted deoxythymidine and GCV recognition domains (sites III and IV), and the arginine-rich domain believed to bind substrate phosphoryl groups (site V) (Fig. 1A). Studies of HCMV UL97 (CMV PT) have identified two catalytic domains important in the phosphorylation of GCV. Mutations within these domains confer resistance (4, 19, 28, 58). The H-X-D-X-X-X-X-N (site II) motif is conserved among a large number of cellular and viral protein kinases and is believed to be a part of the catalytic domain involved in GCV binding and phosphate transfer (16, 35). Similarly, the highly conserved G-X-G-X-X-G (site I) sequence has been implicated in the binding of ATP required for GCV phosphorylation (42). ORF 36 shows amino acid similarity to HCMV UL97 at both of these sites (Fig. 1B). Other herpesviruses also have ORFs homologous to HCMV UL97 (HSV UL13, varicella-zoster virus [VZV] ORF 47, and EBV BGLF4), but their specificities for GCV have not been characterized (57). We examined HHV-8 ORFs 21 and 36 for their ability to activate GCV.
TABLE 1.
Sequence homologies of herpesvirus TKs
| TK | % Sequence homology with TK from:
|
|||
|---|---|---|---|---|
| VZV | HSV | HVS | EBV | |
| HHV-8 ORF 21 | 12.3 | 12.0 | 26.8 | 24.0 |
| EBV | 12.3 | 14.4 | 26.4 | |
| HVS | 14.1 | 11.2 | ||
| HSV | 24.6 | |||
TABLE 2.
Sequence homologies of herpesvirus PTs
| PT | % Sequence homology with PT from:
|
||||
|---|---|---|---|---|---|
| VZV | HSV | HVS | EBV | HCMV | |
| HHV-8 ORF 36 | 14.6 | 16.4 | 26.2 | 24.5 | 12.6 |
| HCMV | 12.9 | 12.7 | 12.5 | 14.5 | |
| EBV | 11.4 | 14.7 | 25.1 | ||
| HVS | 12.5 | 13.9 | |||
| HSV | 27.8 | ||||
FIG. 1.
Alignments of the HHV-8 sequences to the proposed TK and PT catalytic domains of other herpesviruses by the CLUSTAL method (MEGALIGN Lasergene software) (31). (A) HHV-8 ORF 21 is compared to identified TKs in HSV (UL23), VZV (ORF 46), EBV (BXLF1), and HVS (ORF 21) within five conserved domains, which comprise the hypothetical catalytic site (3, 38). (B) HHV-8 ORF 36 is compared to the PTs characterized in HCMV (UL97), HSV (UL13), and VZV (ORF 47) and to putative PTs identified in EBV (BGLF4) and HVS (ORF 36).
MATERIALS AND METHODS
ORF analysis.
BLASTN and BLASTP searches of the nonredundant GenBank database were performed by using DNA sequences within the unique region of the HHV-8 genome at nucleotides 35383 to 37125 (ORF 21) and 55976 to 57310 (ORF 36). Sequence bank accession numbers for ORF 21 and ORF 36 are gi|1136820 and gi|1718289, respectively (52). Amino acid sequences were aligned by using the CLUSTAL method (MEGALIGN Lasergene software package; DNASTAR Inc.) (31).
Cell lines.
JSC-1 is a recently established primary effusion lymphoma cell line that is dually infected with EBV and HHV-8. It is characterized in detail elsewhere (13). JSC-1 and BCBL-1 cells were grown in RPMI 1640 culture medium supplemented with 10% fetal bovine serum and gentamicin (50 μg/ml). 293T cells were derived from human kidney epithelial cells and are transformed with adenovirus E1A and E1B as well as the simian virus 40 (SV40) T antigen. 293T cells were grown in Dulbecco’s minimum essential medium (DMEM) supplemented with 10% fetal calf serum, a 0.1 mM concentration of nonessential amino acids, 100 U of penicillin per ml, and 250 μg of streptomycin per ml. TK-negative [TK(−)] cell lines LTK(−) and 143B were also maintained in serum-supplemented DMEM with 30 μg of bromodeoxyuridine per ml. Hypoxanthine aminopterin thymidine (HAT) selection of 143B- and LTK(−)-transfected cells was as previously described (53).
Compounds.
[8-3H]-GCV (specific activity, 14.6 Ci/mmol) was purchased from Moravek Biochemicals Inc. (Brea, Calif.). [methyl-3H]thymidine (specific radioactivity, 10 Ci/mmol) was obtained from Amersham Radiochemicals (Little Chalfont, England).
Northern blot analysis.
Total RNA was extracted by using the TRIzol reagent (Gibco BRL, Gaithersburg, Md.) (18). RNA (10 μg) was denatured in a solution containing 50% formamide, 17 mM MOPS (morpholinepropanesulfonic acid), and 2.2 M formaldehyde for 15 min at 60°C and chilled on ice. RNA was then fractionated by electrophoresis in 0.8% agarose gels containing 2.2 M formaldehyde and 20 mM MOPS and subsequently transferred to Hybond-N filters (Amersham, Arlington Heights, Ill.) in the presence of 10× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). Prehybridization and hybridization were performed at 42°C in a buffer containing 50% formamide, 5% sodium dodecyl sulfate (SDS), 1 mM EDTA, 0.5 M NaPO4 (pH 7.2), 1% bovine serum albumin, and 100 μg of salmon sperm DNA per ml.
ORF 21 and ORF 36 probes were generated from the full-length labeled DNA fragments of the cloned ORFs. Probes specific to sequences upstream and downstream from ORF 36 were generated from PCR-amplified fragments with the following primers: for ORF 34 (nucleotides 54684 to 55042), 5′-TTG AGC TCG CTC GTG TCC-3′ and 5′-GTC CAC TCC TCG GTA GCA-3′; and for ORF 37 (nucleotides 58522 to 58837), 5′-CTG TCA ACT GTA CCA TCG G-3′, 5′-GAT TGC TCA AGC AAC ATG C-3′. To eliminate hybridization to the ORF 37 transcript, an EcoRI-BamHI (350-bp) fragment generated from the 5′ end of the cloned ORF 36 was used. Synthesis of radiolabeled DNA probes was performed by primer extension with random oligonucleotide primers (random primed DNA labeling kit; Boehringer Mannheim, Indianapolis, Ind.) (53). After hybridization, filters were washed once in 3× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7])–0.1% SDS at 65°C and once in 0.3× SSPE–0.1% SDS at 60°C. Filters were exposed to Kodak XAR-5 film at −70°C with screens.
Cloning of herpesvirus kinases.
ORFs 21 and 36 were amplified by PCR with primers from the 5′ and 3′ ends of each coding sequence. These primers contained BglII sites within 5′ noncomplementary sequences. The primer sets were as follows: for HHV-8 TK (ORF 21), 5′-ATT CTC AGA TCT CGT ACC ATG GCA GAA GGC GGT T-3′ and 5′-GCC AAG ATC TGC TAG ACC CTG CAT GTC TC-3′; and for HHV-8 PT (ORF 36), 5′-AGT CAG ATC TAT GCG CTG GAA GAG AAT GGA G-3′ and 5′-GCC AAG ATC TTC TTC AGA AAA CAA GTC GCG-3′. PCR products of 1,750 bp (ORF 21) and 1,350 bp (ORF 36) were amplified with Vent polymerase for 39 cycles. The DNA template was genomic DNA isolated from the original PEL ascites fluid of an HIV-positive patient described previously (13). BglII-digested PCR products were cloned into the BglII site of the eukaryotic expression vector pHA-SG5 (17), made available by M. Hardwick. The resultant constructs, pSG5TK.17 and pSG5PT36B.16, express ORFs 21 and 36, respectively, with an N-terminal hemagglutinin (HA) fusion tag from the SV40 promoter. The orientation and fidelity of each construction were confirmed in each case by sequencing.
The HCMV UL97 coding region was amplified by PCR from cell lysates of HCMV AD169-infected human fibroblasts. Amplification of the 2,125-bp product was performed with PCR primers containing BglII sites within the following sequences: 5′-AGT CAG ATC TAT GTC CTC CGC ACT TCG-3′ and 5′-GCC AAG ATC TTC TTT ACT CGG GGA ACA GTT-3′. The UL97 coding region was similarly cloned into the BglII site of the pHA-SG5 vector, and the DNA sequence was confirmed by automated sequencing. A plasmid carrying the coding region of the EBV TK (37) was made available by J. R. Arrand and was subsequently subcloned at the BamHI site of the eukaryotic expression vector pCDNA3 (Invitrogen). The p106 plasmid (Stratagene) contains the HSV TK coding region expressed from its natural promoter.
Expression of herpesvirus kinases.
For transient expression assays, 293T cells were seeded on poly-d-lysine-coated plates in DMEM supplemented with serum. Transfections were performed at 70 to 80% confluency with DNA (2 μg) complexed with Lipofectamine (Life Technologies) in the absence of serum. After transfection, cells were incubated for 48 h and trypsinized for analysis.
Extraction of GCV-phosphorylated metabolites and analysis by HPLC.
293T cells, untransfected or transfected with ORF 21, ORF 36, or vector control plasmids, were seeded at 106 cells/ml and incubated with 8 μM [3H]GCV (specific activity, 1,000 dpm/pmol). Cells were incubated for 30 h, trypsinized, washed, and counted. Nucleotides were extracted from cells in 60% methanol at −70°C for 18 h. Dried cell extracts were stored at −70°C until analyzed.
Phosphorylated forms of GCV were separated by high-pressure liquid chromatography (HPLC) with a strong anion-exchange column (Whatman Partisil 10-SAX) according to a previously described procedure with minor modifications (23, 55). The HPLC system used a Hitachi pump, controller, and integrator and a Waters manual injector (Milford, Mass.). Cell extracts were reconstituted in 200 μl of HPLC-grade water, centrifuged at 12,000 × g for 5 min. Extracts from 6 × 105 to 7 × 105 cells were injected onto the column. Nucleotides were eluted by using a gradient of KH2PO4 buffer (pH 3.5) at a flow rate of 0.5 ml/min. The gradient consisted of 0.02 M KH2PO4 for 10 min followed by a linear shift to 1 M KH2PO4 for over 45 min, which was maintained for an additional 15 min. Fractions were collected every 1 min for the determination of radioactivity by scintillation counting. Relative peak retention times for GCV metabolites were as follows: GCV monophosphates (MP), 27 to 29 min; GCV diphosphates (DP), 40 to 43 min; and GCV triphosphates (TP), 69 to 72 min. For the calculation of the molar concentration of these metabolites, we assumed a mean cellular volume of 1 pl.
Western blot analysis.
Total cell lysates were extracted from 293T cells transfected with plasmids pSG5TK.17 and pSG5PT36B.16 following 48 h of expression. Proteins (25 μg) were solubilized in Laemmli buffer, separated on an SDS–7.5% polyacrylamide gel, and transferred to nitrocellulose membranes (53). The membranes were probed with an anti-HA mouse antibody (Boehringer Mannheim) at a dilution of 1:1,000, and the protein-antibody complex was detected by using an enhanced chemiluminescence Western blotting detection system (Amersham).
β-Galactosidase expression assays.
The same cell lysates from ORF 21- and ORF 36-expressing 293T cells that were used for the HPLC analysis and immunoblotting were also analyzed for β-galactosidase activity as described previously (1). The pβ-galac plasmid, in which β-galactosidase is constitutively expressed, was cotransfected with TK- and PT-encoding plasmids as an internal control to normalize transfection efficiency. Cell lysates (2 μg of protein) were incubated in 100-μl reaction mixtures, consisting of 32 mM Na2HPO4, 4.5 mM MgCl2, 0.8% beta-mercaptoethanol, and 80 mM chlorophenol red-β-d-galactopyranoside, at 37°C for 30 min, and absorbances at 560 nm were determined.
Assays for cell proliferation and viability.
The measurement of the cytotoxic effects of antiviral compounds was done according to previously described methods (34). Briefly, following transfection, 293T cells were seeded to poly-d-lysine-coated 96-well plates at 1.5 × 104 cells/well in 200 μl of the growth medium with and without various concentrations of a nucleoside analog: GCV, PCV, or BVDU. Cells were incubated for 4 days at 37°C in a humidified CO2-controlled atmosphere. Cytotoxic effects of the test compounds were then assessed by a colorimetric MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] dye reduction assay. MTT is a tetrazolium compound that is converted by mitochondrial enzymes into a purple formazan product with absorbance at 560 nm. Cells were incubated with 100 μl of MTT at 1 mg/ml in phosphate-buffered saline for 4 to 6 h at 37°C. The converted dye was then solubilized in acidic isopropanol (0.04 N HCl), and the optical density of each well was measured at 560 nm with a microplate spectrophotometer.
RESULTS
Phosphorylation of [3H]GCV by HHV-8 ORF 21 and ORF 36.
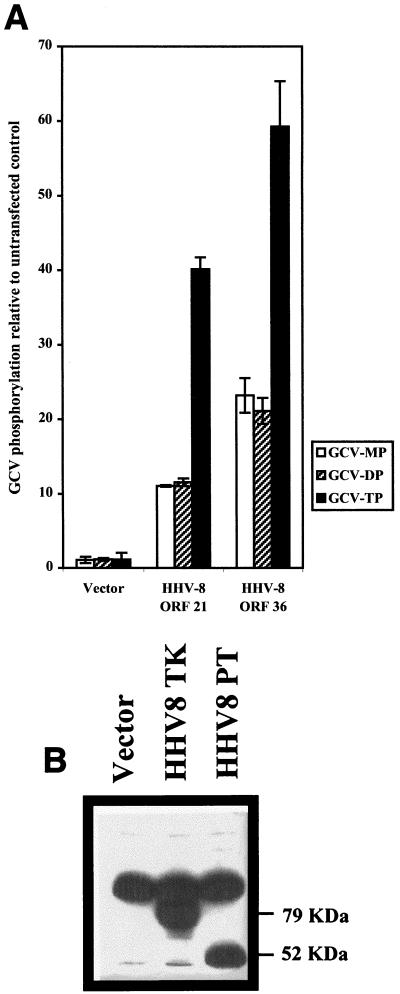
To determine whether the TK or PT homologues function as GCV kinases, we studied the phosphorylation of [3H]GCV following transient transfections of 293T cells with expression plasmids. HPLC analysis of extracts following incubation with 8 μM [3H]GCV (4 μCi/ml) confirmed previous reports that cellular enzymes inefficiently phosphorylate GCV compared to virally encoded enzymes (Fig. 2A) (26, 56). Extracts from untransfected cells or cells transfected with vector control plasmid showed low levels of the phosphorylated forms of GCV (less than 0.11 pmol/106 cells for each of the phosphorylated forms of GCV). ORF 21 and ORF 36 transfectants showed 11- and 23-fold higher GCV MP levels, 11- and 21-fold higher GCV DP levels, and 40- and 60-fold higher GCV TP levels than the vector control, respectively. The total amounts of phosphorylated GCV in ORF 21 and ORF 36 transfectants were 4.39 and 8.03 pmol of GCV/106 cells, respectively. The CMV PT was tested in parallel and yielded similar results (data not shown).
FIG. 2.
HPLC analysis of phosphorylated [3H]GCV products from TK- and PT-expressing cells. (A) 293T cells transfected with the vector control, ORF 21, or ORF 36-expressing plasmids were incubated with 8 μM [3H]GCV (specific activity, 1,000 dpm/pmol) for 30 h. Phosphorylated nucleosides were extracted and analyzed by HPLC (expressed in picomoles of GCV/106 cells [determined for each sample]). Histograms show GCV MP, GCV DP, and GCV TP levels in the vector, TK, and PT transfectants relative to untransfected 293T control cells. Data shown are the averages of triplicate measurements in single experiments. Each experiment was repeated three times, and results were consistent. Error bars indicate standard deviations. (B) Western blot analysis was performed on protein extracts from TK and PT transfectants by using an anti-HA antibody specific for HA fusion tags at the N terminus of expressed TK and PT proteins. Efficiencies of transfection for each sample were corrected with an internal β-galactosidase control. The HA antibody shows cross-reactivity to a 90- to 100-kDa protein present in all 293T cells tested.
In order to compare the relative phosphorylating activities of these HHV-8 kinases, we measured the levels of expression from each plasmid in parallel. Western blot analysis was carried out on extracts of 293T cells transfected with plasmids encoding HA-tagged ORF 21 and ORF 36 fusion proteins. Cells were cotransfected with a β-galactosidase expression plasmid so as to control for variation in transfection efficiency. With both genes expressed at comparable levels (Fig. 2B), ORF 36, as shown by HPLC analysis, was approximately twofold more active than ORF 21 in phosphorylating GCV.
Sensitivity of cells expressing HHV-8 kinases to killing by nucleoside analogs.
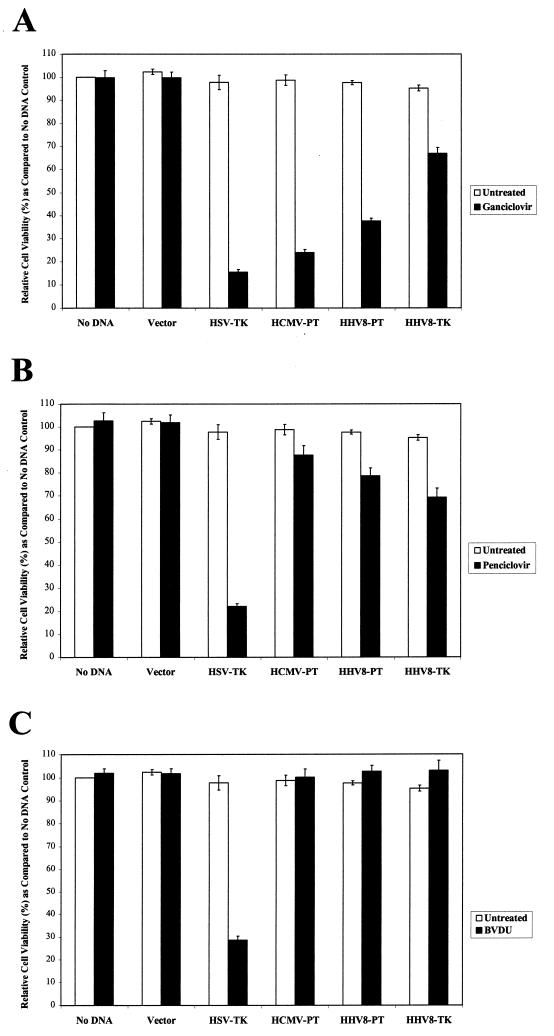
GCV TP is toxic to cells, because it inhibits the cellular DNA polymerase, is incorporated into growing DNA, or interferes with other cellular metabolic processes (9, 39). The concentration of GCV TP achieved in transfected cells as measured by HPLC exceeded the Ki of the cellular DNA polymerase (0.146 μM) by at least 1 order of magnitude (39). To determine the relative efficacies of the TK and PT in bringing about GCV-mediated cell death, 293T cells expressing HHV-8 TK and PT were examined for a decrease in cell viability following 4 days of GCV treatment. In parallel, 293T cells were transfected with plasmids encoding the HSV TK and HCMV UL97. Decreased cell viability, determined by MTT assay, was used as a measure of the kinase’s ability to sensitize cells to nucleoside analogs. As shown in Fig. 3A, all four herpesvirus-encoded kinases sensitize 293T cells to GCV-mediated cell killing, albeit to different degrees. Cell viabilities following GCV treatment decreased to 24% for HCMV UL97, 37% for HHV-8 PT, and 67% for HHV-8 TK. No cell killing was observed with GCV in the samples with no DNA or vector control samples. Inferences about the relative killing efficiency can be drawn for HCMV UL97 versus HHV-8 ORF 36 and HHV-8 ORF 21 in that each was expressed from similar HA fusion constructs that give comparable levels of HA expression as assessed by immunoblotting in parallel experiments. No conclusion can be drawn about HSV TK activity relative to the others insofar as the HSV TK was expressed from its natural promoter and was not an HA fusion protein.
FIG. 3.
Cytotoxic effects of GCV, PCV, and BVDU compounds in cells expressing HHV-8 ORFs 21 and 36 compared to those of other herpesvirus kinases. Following transfection, 293T cells were incubated in the absence or presence of GCV (25 μM) (A), PCV (25 μM) (B), or BVDU (25 μM) (C) for 4 days and assayed for cell viability. The data shown for each nucleoside analog are the means ± standard deviations of six separate experiments, and within each experiment, samples were performed in replicates of eight.
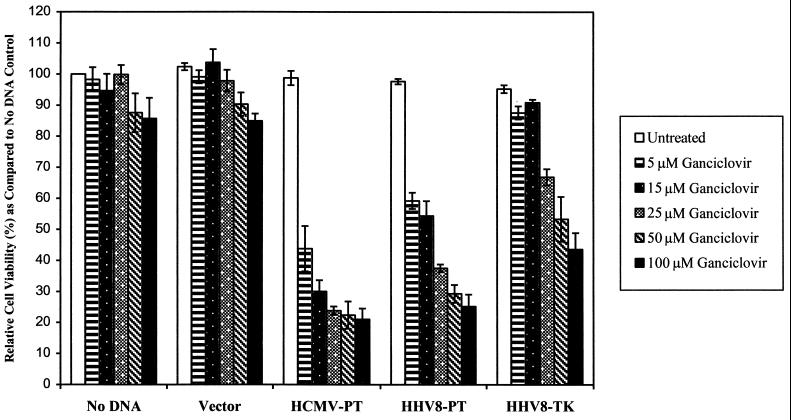
To examine the relative potency and specificity of GCV for HHV-8 encoded ORFs 21 and 36, we tested the kinases’ sensitizing effects at five different GCV concentrations (Fig. 4). At every concentration tested there was more killing in ORF 36-expressing cells than in those expressing ORF 21. At 5 μM, ORF 36 conferred GCV sensitivity with a decrease in cell viability to 59%. Cells expressing ORF 36 showed a progressive decrease in cell viability with higher concentrations of GCV, achieving maximal effects at 100 μM (cell viability decreased to 25%). Only at GCV concentrations above 25 μM were any decreases in cell viability as a result of ORF 21 expression observed. At the highest concentrations of GCV tested, some toxicity (<15%) was also observed for vector and controls with no DNA.
FIG. 4.
Dose response for GCV sensitivity in cells expressing HHV-8 ORFs 21 and 36. Transfectants were incubated with various concentrations (5, 15, 25, 50, and 100 μM) of GCV. HCMV UL97-expressing cells, samples with no DNA, and vector transfectants were included as negative controls. The means ± standard deviations determined in three separate experiments are shown. In each experiment, cell killing was assessed in eight replicate wells.
The expression of the HSV TK also sensitizes cells to killing with PCV and BVDU (5, 6). HCMV UL97 has no similar activity (11, 21, 22, 45, 60). We examined the sensitizing effects of HHV-8 ORFs 21 and 36 to PCV and BVDU with HSV TK and HCMV UL97 as controls (Fig. 3B and C). ORF 36 and ORF 21 were associated with marginal sensitivity to PCV (79 and 69% viable cells, respectively). Neither kinase was associated with sensitivity to BVDU. This spectrum of nucleoside sensitivities conferred by these HHV-8 homologues is consistent with the anti-HHV-8 activity reported for these nucleoside analogs in lytically induced PEL cell lines (46).
TK activity of the HHV-8 TK.
HAT selection in TK(−) cells and incorporation of [3H]thymidine were utilized to assess HHV-8 ORF 21 TK activity in mammalian cells (12, 37, 54). When plasmids encoding the HHV-8 ORF 21 sequence were transfected into TK(−) cell lines, including mouse LTK(−) and the human osteosarcoma 143B, no survival was observed in HHV-8 TK-transformed cells, whereas TK(−) cells transfected with plasmids carrying either the EBV or HSV TK gene survived selection in HAT medium and grew as HAT-resistant colonies within 2 to 3 weeks of transfection (Table 3).
TABLE 3.
TK activity in TK(−) cells
| Plasmid | HAT-resistant coloniesa |
|---|---|
| Vector | 0 |
| pCMV-HHV8 ORF 21 | 0 |
| pSV40-HHV8 ORF 21 | 0 |
| pHSV-HHV8 ORF 21 | 0 |
| pHSVTK | >180 |
| pEBVTK | >100 |
Data are the number of colonies per 100-mm dish for transfections performed in 143B TK(−) human osteosarcoma cells and mouse LTK(−) fibroblast cells. HHV8 ORF 21 was expressed and tested for TK activity with three different promoters, including CMV, SV40, and HSV TK natural promoters. Results are representative of at least five experiments.
Furthermore, when 143B TK(−) cells transfected with the HHV-8 TK were grown in the presence of [3H]thymidine, no incorporation of [3H]thymidine into cellular DNA was observed, whereas the incorporation of labeled thymidine into cellular DNA increased when 143B TK(−) cells were transfected with the EBV TK (data not shown).
Expression of HHV-8 kinase genes.
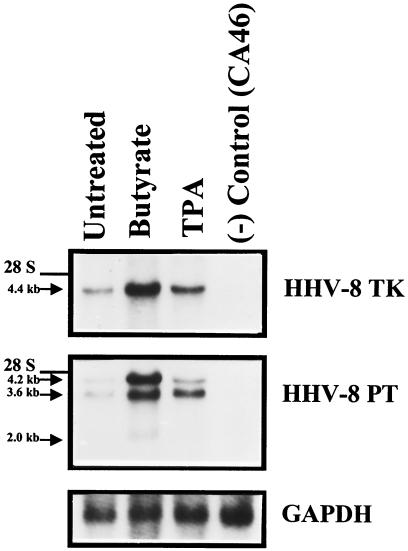
In PEL cell lines there was basal expression of both kinase genes as assessed by Northern blot hybridization of total cellular RNA. Following treatment with either sodium butyrate or phorbol ester, transcripts for these kinases increased dramatically (Fig. 5). Other herpesvirus TK and PT homologues are also expressed during the lytic stage of viral infection.
FIG. 5.
Expression of HHV-8-encoded TK and PT genes in infected cells in the presence or absence of butyrate and tetradecanoyl phorbol acetate (TPA) induction. JSC-1 cells were treated with sodium butyrate (1 mM) or TPA (20 ng/ml) for 24 h and harvested in parallel with control cells. Northern blot hybridization was performed on total RNA (10 μg) to determine the relative levels of PT and TK transcripts in induced and uninduced cells. Hybridizations to a human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe demonstrate a comparable loading of RNA in each lane. Several overlapping transcripts were detected with the full-length ORF 36 probe. Hybridization with probes corresponding to ORFs 34, 35, 37, and 38 allowed the assignment of the 4.4-kb band to a transcript carrying ORFs 34 and 35, a 2.0-kb band to the transcript carrying ORF 37, and a 3.6-kb band to the transcript carrying ORF 36 (data not shown).
DISCUSSION
Our results directly demonstrate the presence of two ORFs encoding GCV kinase activity in HHV-8. The ORF 36 and ORF 21 gene products phosphorylate GCV and lead to cell death. The activity of ORF 36 is significantly stronger than that of ORF 21. GCV kinase activity was first recognized in association with the HSV TK. In a variety of settings, the ability of this gene to sensitize cells to killing by GCV has been used for engineered suicide vectors in a variety of genetic manipulations. GCV kinase activity not associated with a herpesvirus TK family enzyme was first recognized in HCMV (36, 58). Homologues of HCMV UL97 have been recognized in all well-characterized herpesviruses, but these other genes have not been characterized functionally with regard to GCV. The studies presented here indicate that with regard to GCV activation, ORF 36 (the PT homologue) may be more important than ORF 21 (the TK homologue). This finding is consistent with the observation that the spectrum of sensitivity of HHV-8 to antiviral drugs is closer to that of HCMV than to that of HSV or VZV. We also note that other investigators have recently suggested that the PT homologue of EBV (BGLF4) may be more important than the TK homologue in the activation of GCV, although the activity of this enzyme has not been explicitly evaluated (27).
The patterns of viral gene expression for ORFs 21 and 36 are similar and resemble those of other lytic cycle genes. Low-level expression in the absence of inducers is consistent with a low level of lytic activity in primary effusion lymphoma cell lines, reflecting the presence of rare cells in the lytic cycle. The natural substrates for ORFs 21 and 36 are unknown, but other members of the herpesvirus TK family phosphorylate thymidine, deoxycytidine, or thymidylate. Several members of the PT family phosphorylate proteins, and these have also been referred to as protein kinases. HCMV UL97 is a serine/threonine kinase that autophosphorylates (30, 59). Since C-terminal mutations of UL97 alter its ability to phosphorylate GCV but do not affect its autophosphorylating functions, different substrate recognition sites may be associated with its protein activity versus its nucleoside kinase activity (8, 19, 20). Its alphaherpesvirus homologue, HSV UL13, phosphorylates the immediate-early regulatory protein α22, which in turn regulates posttranslational processing (49, 50). Similarly, the VZV homologue ORF 47 phosphorylates the ORF 62 regulatory protein (47, 48).
In general, antiherpesvirus antiviral agents have played an important part in the prevention or treatment of diseases associated with lytic viral infection, such as herpes encephalitis, varicella-zoster, and HCMV retinitis. However, the possibility that antiviral agents might be useful in the prevention of tumors is raised by observations that HHV-8 seroconversion precedes the development of KS and that the incidence of KS is lower in cohorts of HIV patients treated with antiviral agents that are effective in controlling HCMV infection. These findings suggest that at some stage in the pathogenesis of KS, the lytic replication of the viral genome is required. The identification of the viral enzyme responsible for the activation of GCV and thus the inhibition of lytic cycle replication should facilitate the investigation of prophylactic strategies.
ACKNOWLEDGMENTS
This work was supported by U.S. Public Health Service research grant PO1 CA15396 to R.F.A. R.F.A. is a Leukemia Society Scholar.
REFERENCES
- 1.Alam J, Cook J L. Reporter genes: application to the study of mammalian gene transcription. Anal Biochem. 1990;188:245–254. doi: 10.1016/0003-2697(90)90601-5. [DOI] [PubMed] [Google Scholar]
- 2.Alrabiah F A, Sacks S L. New antiherpesvirus agents. Their targets and therapeutic potential. Drugs. 1996;52:17–32. doi: 10.2165/00003495-199652010-00002. [DOI] [PubMed] [Google Scholar]
- 3.Balasubramanian N K, Veerisetty V, Gentry G A. Herpesviral deoxythymidine kinases contain a site analogous to the phosphoryl-binding arginine-rich region of porcine adenylate kinase; comparison of secondary structure predictions and conservation. J Gen Virol. 1990;71:2979–2987. doi: 10.1099/0022-1317-71-12-2979. [DOI] [PubMed] [Google Scholar]
- 4.Baldanti F, Silini E, Sarasini A, Talarico C L, Stanat S C, Biron K K, Furione M, Bono F, Palù G, Gerna G. A three-nucleotide deletion in the UL97 open reading frame is responsible for the ganciclovir resistance of a human cytomegalovirus clinical isolate. J Virol. 1995;69:796–800. doi: 10.1128/jvi.69.2.796-800.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balzarini J, Bohmann C, Walker R, De Clercq E. Comparative cytostatic activity of different antiherpetic drugs against herpes simplex virus thymidine kinase gene-transfected tumor cells. Mol Pharmacol. 1994;40:1253–1258. [PubMed] [Google Scholar]
- 6.Balzarini J, De Clercq E, Ayusawa D, Seno T. Murine mammary FM3A carcinoma cells transformed with the herpes simplex virus type 1 thymidine kinase gene are highly sensitive to the growth-inhibitory properties of (E)-5-(2-bromovinyl)-2′-deoxyuridine and related compounds. FEBS Lett. 1985;185:95–100. doi: 10.1016/0014-5793(85)80747-x. [DOI] [PubMed] [Google Scholar]
- 7.Bean B. Antiviral therapy: current concepts and practices. Clin Microbiol Rev. 1992;5:146–182. doi: 10.1128/cmr.5.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biron K K. Ganciclovir resistance of cytomegalovirus: mechanisms and prospects for rapid detection. Int Antivir News. 1994;2:117–118. [Google Scholar]
- 9.Biron K K, Stanat S C, Sorrell J B, Fyfe J A, Keller P M, Lambe C U, Nelson D J. Metabolic activation of the nucleoside analog 9-{[2-hydroxy-1-(hydroxymethyl)ethoxy]methyl}guanine in human diploid fibroblasts infected with human cytomegalovirus. Proc Natl Acad Sci USA. 1985;82:2473–2477. doi: 10.1073/pnas.82.8.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boshoff C, Whitby D, Hatznoannou T, Fisher C, van der Wait J, Hatzakis A, Weiss R, Schulz T. Kaposi’s-sarcoma-associated herpesvirus in HIV-negative Kaposi’s sarcoma. Lancet. 1995;345:1043–1044. doi: 10.1016/s0140-6736(95)90780-7. [DOI] [PubMed] [Google Scholar]
- 11.Boyd M R, Safrin S, Kern E R. Penciclovir: a review of its spectrum of activity, selectivity, and cross-resistance pattern. Antivir Chem Chemother. 1993;4:3–11. [Google Scholar]
- 12.Campione-Piccardo J, Rawls W E, Bacchetti S. Selective assay for herpes simplex viruses expressing thymidine kinase. J Virol. 1979;31:281–287. doi: 10.1128/jvi.31.2.281-287.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cannon, J. S., A. C. Hawkins, C. A. Griffin, Q. Tao, M. J. Borowitz, G. S. Hayward, and R. F. Ambinder. 1999. Unpublished data.
- 14.Cesarman E, Chang Y, Moore P S, Said J W, Knowles D M. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 15.Chang Y, Cesarman E, Pessin M S, Lee F, Culpepper J, Knowles D M, Moore P S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 16.Chee M, Lawrence G L, Barrell B G. Alpha-, beta-, and gammaherpesviruses encode a putative phosphotransferase. J Gen Virol. 1989;70:1151–1160. doi: 10.1099/0022-1317-70-5-1151. [DOI] [PubMed] [Google Scholar]
- 17.Cheng E H, Nicholas J, Bellows D S, Hayward G S, Guo H G, Reitz M S, Hardwick J M. A Bcl-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc Natl Acad Sci USA. 1997;94:690–694. doi: 10.1073/pnas.94.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 19.Chou S, Erice A, Jordan M C, Vercellotti G M, Michels K R, Talarico C L, Stanat S C, Biron K K. Analysis of the UL97 phosphotransferase coding sequence in clinical cytomegalovirus isolates and identification of mutations conferring ganciclovir resistance. J Infect Dis. 1995;171:576–583. doi: 10.1093/infdis/171.3.576. [DOI] [PubMed] [Google Scholar]
- 20.Chou S, Guentzel S, Michels K R, Miner R C, Drew W L. Frequency of UL97 phosphotransferase mutations related to ganciclovir resistance in clinical cytomegalovirus isolates. J Infect Dis. 1995;172:239–242. doi: 10.1093/infdis/172.1.239. [DOI] [PubMed] [Google Scholar]
- 21.De Clercq E. The antiviral spectrum of (E)-5-(2-bromovinyl)-2′-deoxyuridine. J Antimicrob Chemother. 1984;14:85–95. doi: 10.1093/jac/14.suppl_a.85. [DOI] [PubMed] [Google Scholar]
- 22.De Clercq E. In search of a selective antiviral chemotherapy. Clin Microbiol Rev. 1997;10:674–693. doi: 10.1128/cmr.10.4.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elion G B, Furman P A, Fyfe J A, De Miranda P, Beauchamp L, Schaeffer H J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl)guanine. Proc Natl Acad Sci USA. 1977;74:5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faulds D, Heel R C. Ganciclovir: a review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in cytomegalovirus infections. Drugs. 1990;39:597–638. doi: 10.2165/00003495-199039040-00008. [DOI] [PubMed] [Google Scholar]
- 25.Glesby M, Hoover D, Weng S, Graham N, Phair J, Detels R, Ho M, Saah A. Use of antiherpes drugs and the risk of Kaposi’s sarcoma: data from the multicenter AIDS cohort study. J Infect Dis. 1996;173:1477–1480. doi: 10.1093/infdis/173.6.1477. [DOI] [PubMed] [Google Scholar]
- 26.Golumbek P T, Hamzeh F M, Lietman P S, Pardoll D M. Herpes simplex 1 thymidine kinase gene is unable to completely eliminate live, nonimmunogenic tumor cell vaccines. J Immunother. 1992;12:224–230. doi: 10.1097/00002371-199211000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Gustafson E A, Chillemi A C, Sage D R, Fingeroth J D. The Epstein-Barr virus thymidine kinase does not phosphorylate ganciclovir or acyclovir and demonstrates a narrow substrate specificity compared to the herpes simplex virus type 1 thymidine kinase. Antimicrob Agents Chemother. 1998;42:2923–2931. doi: 10.1128/aac.42.11.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanson M N, Preheim L C, Chou S, Talarico C L, Biron K, Erice A. Novel mutation in the UL97 gene of a clinical cytomegalovirus strain conferring resistance to ganciclovir. Antimicrob Agents Chemother. 1995;39:1204–1205. doi: 10.1128/aac.39.5.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrison P T, Thompson R, Davison A J. Evolution of herpesvirus thymidine kinases from cellular deoxycytidine kinase. J Gen Virol. 1991;72:2583–2586. doi: 10.1099/0022-1317-72-10-2583. [DOI] [PubMed] [Google Scholar]
- 30.He Z, He Y S, Kim Y, Chu L, Ohmstede C, Biron K K, Coen D M. The human cytomegalovirus UL97 protein is a protein kinase that autophosphorylates on serines and threonines. J Virol. 1997;71:405–411. doi: 10.1128/jvi.71.1.405-411.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins D G, Sharp P M. Fast and sensitive multiple sequence alignments on a microcomputer. CABIOS. 1989;5:151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- 32.Honess R W, Craxton M A, Williams L L, Gompels U A. A comparative analysis of the sequence of the thymidine kinase gene of a gammaherpesvirus, herpesvirus saimiri. J Gen Virol. 1989;70:3003–3013. doi: 10.1099/0022-1317-70-11-3003. [DOI] [PubMed] [Google Scholar]
- 33.Kedes D H, Ganem D. Sensitivity of Kaposi’s sarcoma-associated herpesvirus replication to antiviral drugs. J Clin Investig. 1997;99:2082–2086. doi: 10.1172/JCI119380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larder B A, Darby G, Richman D. HIV with reduced sensitivity to zidovudine (AZT) isolated during prolonged therapy. Science. 1989;243:1731–1734. doi: 10.1126/science.2467383. [DOI] [PubMed] [Google Scholar]
- 35.Leader D P. Viral protein kinases and protein phosphatases. Pharmacol Ther. 1993;59:343–389. doi: 10.1016/0163-7258(93)90075-o. [DOI] [PubMed] [Google Scholar]
- 36.Littler E, Stuart A D, Chee M. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature. 1992;358:160–162. doi: 10.1038/358160a0. [DOI] [PubMed] [Google Scholar]
- 37.Littler E, Zeuthen J, McBride A A, Trost Sorensen E, Powell K L, Walsh-Arrand J E, Arrand J R. Identification of an Epstein-Barr virus-coded thymidine kinase. EMBO J. 1986;5:1959–1966. doi: 10.1002/j.1460-2075.1986.tb04450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lomonte P, Bublot M, Pastoret P-P, Thiry E. Location and characterization of the bovine herpesvirus type 4 thymidine kinase gene; comparison with thymidine kinase genes of other herpesviruses. Arch Virol. 1992;127:327–337. doi: 10.1007/BF01309595. [DOI] [PubMed] [Google Scholar]
- 39.Mar E-C, Chiou J-F, Cheng Y-C, Huang E-S. Inhibition of cellular DNA polymerase α and human cytomegalovirus-induced DNA polymerase by the triphosphates of 9-(2-hydroxyethoxymethyl)guanine and 9-(1,3-dihydroxy-2-propoxymethyl)guanine. J Virol. 1985;53:776–780. doi: 10.1128/jvi.53.3.776-780.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGuirt P V, Furman P A. Acyclovir inhibition of viral DNA chain elongation in herpes simplex virus infected cells. Am J Med. 1982;73:67–71. doi: 10.1016/0002-9343(82)90066-3. [DOI] [PubMed] [Google Scholar]
- 41.Medveczky M, Horvath E, Lund T, Medveczky P. In vitro antiviral drug sensitivity of the Kaposi’s sarcoma-associated herpesvirus. AIDS. 1997;11:1327–1332. doi: 10.1097/00002030-199711000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Michel D, Schaarschmidt P, Wunderlich K, Heuschmid M, Simoncini L, Muhlberger D, Zimmermann A, Pavic I, Mertens T. Functional regions of the human cytomegalovirus protein pUL97 involved in nuclear localization and phosphorylation of ganciclovir and pUL97 itself. J Gen Virol. 1998;79:2105–2112. doi: 10.1099/0022-1317-79-9-2105. [DOI] [PubMed] [Google Scholar]
- 43.Mocroft A, Youle M, Gazzard B, Morinek J, Halai R, Phillips A. Antiherpesvirus treatment and risk of Kaposi’s sarcoma in HIV infection. AIDS. 1996;10:1101–1105. [PubMed] [Google Scholar]
- 44.Moore P S, Gao S-J, Dominguez G, Cesarman E, Lungu O, Knowles D M, Garber R, Pellett P E, McGeoch D J, Chang Y. Primary characterization of a herpesvirus agent associated with Kaposi’s sarcoma. J Virol. 1996;70:549–558. doi: 10.1128/jvi.70.1.549-558.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neyts J, Andrei G, Snoeck R, Jähne G, Winkler I, Helsberg M, Balzarini J, De Clercq E. The N-7-substituted acyclic nucleoside analog 2-amino-7[(1,3-dihydroxy-2-propoxy)methyl]purine is a potent and selective inhibitor of herpesvirus replication. Antimicrob Agents Chemother. 1994;38:2710–2716. doi: 10.1128/aac.38.12.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neyts J, De Clercq E. Antiviral drug susceptibility of human herpesvirus 8. Antimicrob Agents Chemother. 1997;41:2754–2756. doi: 10.1128/aac.41.12.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ng T I, Grose C. Serine protein kinase associated with varicella-zoster virus ORF 47. Virology. 1992;191:9–18. doi: 10.1016/0042-6822(92)90161-h. [DOI] [PubMed] [Google Scholar]
- 48.Ng T I, Keenan L, Kinchington P R, Grose C. Phosphorylation of varicella-zoster virus open reading frame (ORF) 62 regulatory product by viral ORF 47-associated protein kinase. J Virol. 1994;68:1350–1359. doi: 10.1128/jvi.68.3.1350-1359.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Purves F C, Ogle W O, Roizman B. Processing of the herpes simplex virus regulatory protein α22 mediated by the UL13 protein kinase determines the accumulation of a subset of alpha and gamma mRNAs and proteins in infected cells. Proc Natl Acad Sci USA. 1993;90:6701–6705. doi: 10.1073/pnas.90.14.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Purves F C, Roizman B. The UL13 gene of herpes simplex virus 1 encodes the functions for posttranslational processing associated with phosphorylation of the regulatory protein alpha 22. Proc Natl Acad Sci USA. 1992;89:7310–7314. doi: 10.1073/pnas.89.16.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robertson G R, Walley J M. Evolution of the herpes thymidine kinase: identification and comparison of the equine herpesvirus I thymidine kinase gene reveals similarity to a cell-encoded thymidylate kinase. Nucleic Acids Res. 1988;16:11303–11317. doi: 10.1093/nar/16.23.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russo J J, Bohenzky R A, Chien M-C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Nucleotide sequence of Kaposi’s sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14868. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 54.Shiraki K, Mori H, Haya Y, Yamanishi K, Takahashi M. Regulation of thymidine kinase activity in mouse L cells biochemically transformed by varicella-zoster virus. Microbiol Immunol. 1989;33:693–698. doi: 10.1111/j.1348-0421.1989.tb02020.x. [DOI] [PubMed] [Google Scholar]
- 55.Slusher J T, Kuwahara S K, Hamzeh F M, Lewis L D, Kornhauser D M, Lietman P S. Intracellular zidovudine (ZDV) and ZDV phosphates as measured by a validated combined high-pressure liquid chromatography–radioimmunoassay procedure. Antimicrob Agents Chemother. 1992;36:2473–2477. doi: 10.1128/aac.36.11.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smee D F. Interaction of 9-(1,3-dihydroxy-2-propoxymethyl)guanine with cytosol and mitochondrial deoxyguanosine kinases: possible role in anti-cytomegalovirus activity. Mol Cell Biochem. 1985;69:75–81. doi: 10.1007/BF00225929. [DOI] [PubMed] [Google Scholar]
- 57.Smith R F, Smith T F. Identification of new protein kinase-related genes in three herpesviruses, herpes simplex virus, varicella-zoster virus, and Epstein-Barr virus. J Virol. 1989;63:450–455. doi: 10.1128/jvi.63.1.450-455.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sullivan V, Talarico C L, Stanat S C, Davis M, Coen D M, Biron K K. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature. 1992;358:162–164. doi: 10.1038/358162a0. [DOI] [PubMed] [Google Scholar]
- 59.Wolf D G, Honigman A, Lazarovits J, Tavor E, Panet A. Characterization of the human cytomegalovirus UL97 gene product as a virion-associated protein kinase. Arch Virol. 1998;143:1223–1232. doi: 10.1007/s007050050370. [DOI] [PubMed] [Google Scholar]
- 60.Zimmermann A, Michel D, Pavic I, Hampl W, Luske A, Neyts J, De Clercq E, Mertens T. Phosphorylation of aciclovir, ganciclovir, penciclovir, and S2242 by the cytomegalovirus UL97 protein: a quantitative analysis using recombinant vaccinia viruses. Antivir Res. 1997;36:35–42. doi: 10.1016/s0166-3542(97)00034-x. [DOI] [PubMed] [Google Scholar]