Abstract
Machine learning can be used to define subtypes of psychiatric conditions based on shared biological foundations of mental disorders. Here we analyzed cross-sectional brain images from 4,222 individuals with schizophrenia and 7038 healthy subjects pooled across 41 international cohorts from the ENIGMA, non-ENIGMA cohorts and public datasets. Using the Subtype and Stage Inference (SuStaIn) algorithm, we identify two distinct neurostructural subgroups by mapping the spatial and temporal ‘trajectory’ of gray matter change in schizophrenia. Subgroup 1 was characterized by an early cortical-predominant loss with enlarged striatum, whereas subgroup 2 displayed an early subcortical-predominant loss in the hippocampus, striatum and other subcortical regions. We confirmed the reproducibility of the two neurostructural subtypes across various sample sites, including Europe, North America and East Asia. This imaging-based taxonomy holds the potential to identify individuals with shared neurobiological attributes, thereby suggesting the viability of redefining existing disorder constructs based on biological factors.
Subject terms: Schizophrenia, Diagnostic markers
Machine learning can be used to identify subtypes of psychiatric disease. Here the authors identified two neurostructural subgroups in schizophrenia, each showing reproducibility and generalizability across different collection locations and illness stages, using the SuStain algorithm.
Introduction
Schizophrenia is one of the most severely disabling psychiatric disorders with a life-time prevalence of 1%; it affects approximately 26 million people worldwide1. The etiology of schizophrenia is still not fully understood. Current knowledge implicates multiple neurobiological mechanisms and pathophysiologic processes2,3. Furthermore, people diagnosed with schizophrenia show a substantial heterogeneity in clinical symptoms4, disease progression5, treatment response6, and other biological markers7,8. In addition, currently available treatments are not aligned with specific pathophysiological pathways/targets, which limits effectiveness of treatment selection9. Establishing a new taxonomy by identifying distinct subtypes based on neurobiological data could help resolve some of these heterogeneity-induced challenges. A key goal is to define biological subtypes, based on objective measures derived from imaging and other biomarkers10.
Artificial intelligence methods such as machine learning can be applied to brain imaging11 to categorize individuals based on their profiles of brain metrics, and holds the potential for revealing the underlying neurobiological mechanisms associated with disorder subtypes12. Machine learning algorithms are increasingly used to subtype brain disorders13–16. Prior studies have primarily focused on grouping individuals into distinct categories without considering disease progression17,18. A major obstacle to identifying distinct patterns of neuro-pathophysiological progression (referred to as progression subtypes) stems from the lack of sufficient longitudinal data covering the lifespan of the disorder. Recently, a data-driven machine learning approach known as Subtype and Stage Inference (SuStaIn) was introduced19. SuStaIn uses a large number of cross-sectional observations, derived from single time-point MRI scans, to identify clusters (subtypes) of individuals with common trajectory of disease progression (i.e., the sequence of MRI abnormalities across different brain regions) in brain disorders20–23. It should be noted that SuStaIn estimates the pseudo-longitudinal sequence (i.e., SuStaIn trajectory) based on only cross-sectional data. Therefore, the fitted SuStaIn trajectories do not directly reflect the actual pathophysiological progression of the illness. By applying SuStaIn to MRI data from individuals with schizophrenia, primarily collected from the Chinese population, we found that the progression of gray matter loss in schizophrenia can be better characterized through two distinct phenotypes: one characterized by a cortical-predominant progression, originating in the Broca’s area/fronto-insular cortex, and another marked by a subcortical-predominant progression, starting in the hippocampus22. Such brain-based taxonomies may reflect neurostructural subtypes with shared pathophysiological foundations, with relevance for neurobiological classification22. However, the generalizability of the two neurostructural subtypes to diverse populations outside of China, and external validation of the subgrouping is required before applying this knowledge to stratify clinical trials.
The Enhancing Neuro Imaging Genetics through Meta-Analysis (ENIGMA, http://enigma.ini.usc.edu) consortium is dedicated to conducting large-scale analyzes by pooling brain imaging data from research teams worldwide, using standardized image processing protocols. Previously, ENIGMA published findings revealing thinner cerebral cortex, smaller surface area, and altered subcortical volumes in schizophrenia compared to controls24,25. Here, we included structural MRI data obtained from 4291 individuals diagnosed with schizophrenia and 7078 healthy controls from 41 international cohorts from ENIGMA schizophrenia groups worldwide and other non-ENIGMA datasets (Supplementary Table 1–2). The large sample size allowed us to conduct systematic and comprehensive analyzes to verify the reproducibility and generality of neurostructural subtypes of schizophrenia across regions/locations and disease stages. This study’s aims were: (1) to validate the two neurostructural subtypes with distinct trajectories of neuro-pathophysiological progression in schizophrenia, (2) to verify the reproducibility and generality of the neurostructural subtypes, in subsamples across the world and across disease stages, and (3) to characterize subtype-specific signatures in terms of neuroanatomy and clinical symptomatic trajectory.
Together, these analyzes aim to create an easily accessible (with a single anatomical MRI), interpretable (based on ‘progressive’ pathology) and robustly generalizable (across ethnic, sex and language differences) taxonomy of subtypes that share common neurobiological mechanisms in schizophrenia. If proven effective, other complex neuropsychiatric disorders with high heterogeneity26,27, such as major depressive disorder, autism spectrum disorder, and obsessive-compulsive disorder, could also benefit from such a subtyping paradigm. This has the potential to transition the field of psychiatry from syndrome-based to both syndrome- and biology-based stratifications of mental disorders.
Results
Two biotypes with distinct pathophysiological progression trajectories
Distinct patterns of spatiotemporal progression of pathophysiological progression were identified using SuStaIn, based on cross-sectional MRI data from 4222 individuals diagnosed with schizophrenia (1683 females, mean age=32.4 ± 11.9 years) and 7038 healthy subjects (3440 females, mean age=33.0 ± 12.6 years) (Table 1). A 2-fold cross-validation procedure resulted in an optimal number of K = 2 clusters (subtypes) as determined by the largest Dice coefficient (Fig. 1a), indicating the best consistency of the subtype labeling across all individuals for a model in two independent schizophrenia populations. Figure 1b shows that only 1.2% of people were moved from subtype 1 to subtype 2, and 7.5% were moved from subtype 2 to subtype 1, indicating that 91.3% of individuals’ subtype labels were consistent between the SuStaIn classifications from two non-overlapping data folds. These findings suggest the presence of two stable schizophrenia biotypes with distinct ‘trajectories’ of pathophysiological progression (here, we put SuStaIn trajectory in quotes as it is not an actual longitudinal trajectory but rather a typical sequence of disease progression reconstructed from cross-sectional data).
Table 1.
Demographic and clinical characteristics in the primary sample including 4222 schizophrenia patients and 7038 healthy controls
| HC(n = 7038) | SCZ(n = 4222) | SCZ subtype1(n = 2622) | SCZ subtype2(n = 1600) | |||||
|---|---|---|---|---|---|---|---|---|
| n | mean(SD) | n | mean(SD) | n | mean(SD) | n | mean(SD) | |
| Sex (Female/Male) | 3440/3598 | - | 1683/2539 | - | 1044/1578 | - | 639/961 | - |
| Age (years) | 7038 | 33.0(12.6) | 4222 | 32.4(11.9) | 2622 | 32.4(11.8) | 1600 | 32.4(12.0) |
| Illness duration (years) | - | - | 2333 | 10.5(10.4) | 1442 | 10.4(10.5) | 891 | 10.5(10.4) |
| FES/Chronic/Unknown | - | - | 1112/1623/1477 | - | 696/1002/924 | - | 426/621/553 | - |
| PANSS Positive scale (P1-P7) | - | - | 2651 | 17.2(6.8) | 1622 | 17.3(3.9) | 1029 | 17.0(6.7) |
| PANSS Negative scale (N1-N7) | - | - | 2651 | 17.5(7.6) | 1622 | 17.6(7.6) | 1029 | 17.3(7.6) |
| PANSS General scale (G1-G16) | - | - | 2651 | 34.8(11.6) | 1622 | 35.2(11.6) | 1029 | 34.3(11.6) |
| PANSS Total score | - | - | 2651 | 69.5(22.4) | 1622 | 70.0(22.4) | 1029 | 68.6(22.5) |
| PANSS excitement dimension (P4, P7, G44, G14) | - | - | 1322 | 8.2(3.5) | 823 | 8.2(3.4) | 499 | 8.2(3.5) |
| PANSS depression/anxiety dimension (G1, G2, G3, G6, G15) | - | - | 1322 | 11.3(4.1) | 823 | 11.4(4.1) | 499 | 11.1(4.2) |
| PANSS cognitive dimension (P2, N5, G5, G10, G11) | - | - | 1322 | 10.6(4.0) | 823 | 10.5(4.0) | 499 | 10.6(4.0) |
Abbreviation: HC, healthy control; SCZ, schizophrenia; FES, first-episode schizophrenia; PANSS, Positive and Negative Syndrome Scale.
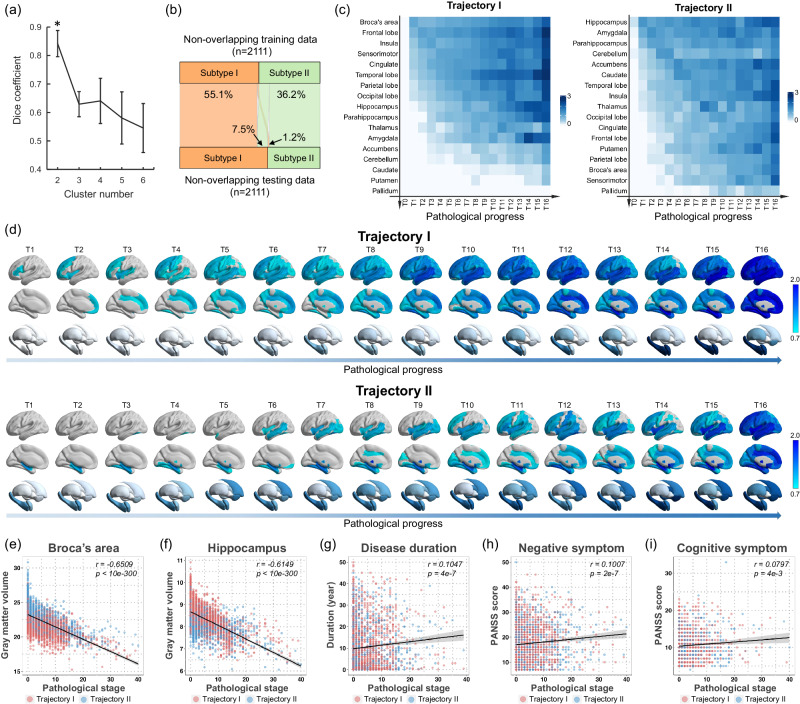
Fig. 1. Two pathophysiological progression trajectories in schizophrenia.
a Dice coefficient indicates that K = 2 is the optimal number (marked by asterisk) of subtypes with best consistency of the subtype labeling between two independent schizophrenia populations using non-overlap 2-folds cross-validation procedure. This procedure was repeated ten times (n = 10) to avoid the occasionality of one split. Data are presented as median values +/- standard deviation (SD). b The proportion of individuals whose subtype labels keep consistent by a non-overlap cross-validation procedure. c Sequences of regional volume loss across seventeen brain regions for each ‘trajectory’ via SuStaIn are shown in y-axis. The heatmap shows regional volume loss in which biomarker (y-axis) in a particular ‘temporal’ stage (T0-T16) in the ‘trajectory’ (x-axis). The Color bar represents the degree of gray matter volume (GMV) loss in schizophrenia relative to healthy controls (i.e., z score). d Spatiotemporal pattern of pathophysiological ‘trajectory’. The z-score images are mapped to a glass brain template for visualization. The spatiotemporal pattern of gray matter loss displays a progressive pattern of spatial extension along with later ‘temporal’ stages of pathological progression that are distinct between trajectories. e–f Pathological stages of SuStaIn are correlated with reduced gray matter volume of Broca’s area and hippocampus. g–i Pathological stages of SuStaIn are correlated with longer disease duration, worse negative symptoms and worse cognitive symptoms. Spearman correlation test is conducted for data analysis in figures (e–i). Two-sided p value is reported after multiple comparisons correction by FDR. The error bands in figures (e–i) represent 95% confidence interval. n = 4222 biologically independent samples in figures (e–f). n = 2333 biologically independent samples in figure (g). n = 2651 biologically independent samples in figure (h). n = 1322 biologically independent samples in figure (i).
Region of interest (ROI)-wise gray matter volume (GMV) z-scores, at each stage of the ‘trajectory’ for each subtype, show the sequence of regional volume loss across the 17 brain regions for each ‘trajectory’ (Fig. 1c). To visualize the spatiotemporal pattern of each ‘trajectory’, z-score whole brain images were mapped to a glass brain template (Fig. 1d). These maps show a progressive pattern of spatial expansion along with later ‘temporal’ stages of pathological progression distinct for each ‘trajectory’ (Supplementary Movie 1 and 2). Specifically, ‘trajectory’ 1 displayed an ‘early cortical-predominant loss’ biotype. It was characterized by an initial reduction in Broca’s area, followed by adjacent fronto-insular regions, then extending to the rest of the neocortex, and finally to the subcortex (Fig. 1d). Conversely, ‘trajectory’ 2 exhibited an ‘early subcortical-predominant loss’ biotype where volume loss began in the hippocampus, spread to the amygdala and parahippocampus, and then extended to the accumbens and caudate before affecting the cerebral cortex (Fig. 1d). The two ‘trajectories’ were highly consistent with our previous findings in a predominantly Chinese schizophrenia cohort22. We also re-estimated trajectories based on a validation dataset (N = 3120) that has removed the original data used in our previous SuStaIn study22. In the validation dataset, we replicated the two ‘trajectories’ that begin in either the Broca’s area or the hippocampus (Supplementary Fig. 1). We also observed a high similarity of ‘trajectory’ spatiotemporal pattern between the original dataset and the additional dataset (‘trajectory’ 1, r = 0.879, p < 0.001; ‘trajectory’ 2, r = 0.631, p < 0.001; Spearman correlation test). The phenotypic subtypes, based on the different pathophysiological ‘trajectories’, are thus replicated in a large cross-geography sample, confirming the presence of two different neuropathological pathways with different anatomical origins in schizophrenia22.
Trajectories are repeated in first-episode and medication-naïve samples
The sample size of this study was large enough to allow further exploratory analyses to identify pathophysiological progression trajectories in more homogeneous subsamples of schizophrenia. Here, we re-estimated the SuStaIn ‘trajectories’ based on a subsample of data from individuals with first-episode schizophrenia with illness duration less than two years (N = 1122; 513 females, mean age=25.4 ± 8.6 years), and a subsample of medication-naïve individuals with schizophrenia (N = 718, 353 females, mean age = 23.7 ± 7.8 years) (Supplementary Table 3). In both subsamples, we replicated the two ‘trajectories’ with either the Broca’s area or the hippocampus as the sites of origin (Supplementary Fig. 2), indicating that the two initiating regions - ranking ahead of other regional deficits—are the pathological effects of the disease itself, rather than medication-induced effects. Broca’s area and the hippocampus may, therefore, be candidate targets for intervention in schizophrenia, as these two brain regions were affected early in the disease process.
Trajectories are reproducible for samples from different parts of the world
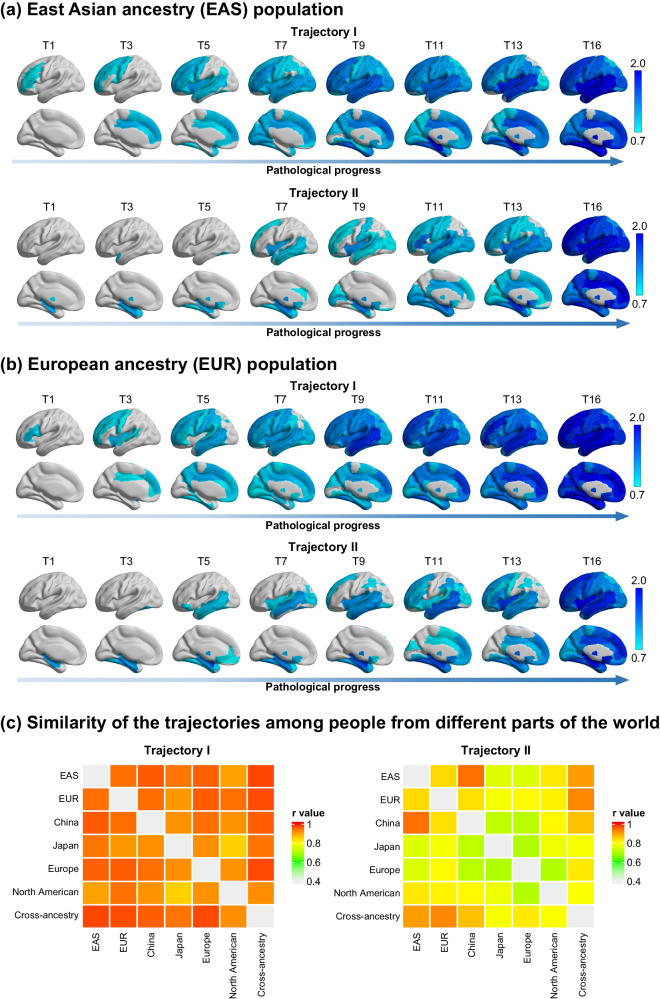
To examine whether the ‘trajectories’ were reproducible for samples from different parts of the world, we divided all samples into several sub-cohorts based on where the samples were obtained. Here, samples from China, Japan, South Korea and Singapore were classified into the East Asian ancestry (EAS) cohort. Samples from Europe, the United States, Canada and Australia were classified into the European ancestry (EUR) cohorts (Supplementary Table 4). In addition, Chinese, Japanese, European and North American cohorts were further classified by their site locations in terms of geographic distribution (Supplementary Table 4). Such a division was based on the similar ethnic or environmental factors for each country, region, or continent and the size of subsample, which need to be sufficient to conduct a reliable inference of the SuStaIn trajectory. We found that two ‘trajectories’ (the optimal number was also K = 2, which separately re-estimated in each cohort)—with Broca’s area leading and the hippocampus leading—were also repeated in EAS (Fig. 2a) and EUR (Fig. 2b) cohorts. In addition, the spatiotemporal pattern of each ‘trajectory’ showed strong, significant correlations between the EAS and EUR cohorts (‘trajectory’ 1, r = 0.948, p < 0.001; ‘trajectory’ 2, r = 0.842, p < 0.001; Spearman correlation test). This high level of similarity in the trajectories was also observed between cohorts from other locations (Fig. 2c). This suggests that the two biotypes with distinct ‘trajectories’ of pathophysiological progression in schizophrenia are robust, and their classification patterns are independent of macro-environmental or ethnogenetic factors.
Fig. 2. Trajectories are reproducibility for samples from different locations of the world.
Two sets of ‘trajectories’ are separately derived from two non-overlapping location cohorts, that are (a) East Asian ancestry (EAS) cohort, and (b) European ancestry (EUR) cohort. The Color bar represents the degree of gray matter volume (GMV) loss in schizophrenia relative to healthy controls (i.e., z-score). c The similarity of the spatiotemporal pattern of each ‘trajectory’ between any two of cohorts is shown by the heatmap. The color bar of the heatmap represents the similarity, which is quantified via the Spearman correlation coefficient between the trajectories from two cohorts. A total of six location cohorts are classified by where the sample locate at, including the EAS, EUR, China, Japan, Europe and North American. The whole sample is labeled as a cross-ancestry cohort.
Trajectories are associated with neurophysiological, pathological and neuropsychological progressions in schizophrenia
The SuStaIn calculated the probability of each patient belonging to a specific ‘trajectory’ and further assigned them to a sub-stage within that ‘trajectory’. Individuals who were assigned to the later stages of the ‘trajectory’ showed a significant correlation with less GMV of Broca’s area (Fig. 1e, r = 0.651, p < 0.0001) and hippocampus (Fig. 1f, r = 0.615, p < 0.0001). In addition, the later stages were correlated with longer disease duration (Fig. 1g, r = 0.105, p < 0.0001), worse negative symptoms (Fig.1h, r = 0.101, p < 0.0001) and worse cognitive symptoms (Fig.1i, r = 0.080, p = 0.004). These results suggest that the SuStaIn ‘trajectory’ reflects the underlying neural progression in schizophrenia.
Subtype-specific signatures in neuroanatomical pathology
To characterize subtype-specific neuroanatomical signatures, we assessed regional morphological measures using FreeSurfer in a subsample including 1840 individuals with schizophrenia and 1780 healthy controls. A total of 330 regional morphological measures in cortical thickness, cortical surface area, cortical volume, subcortical volume and subregion segmentation were quantified (see “Methods”).
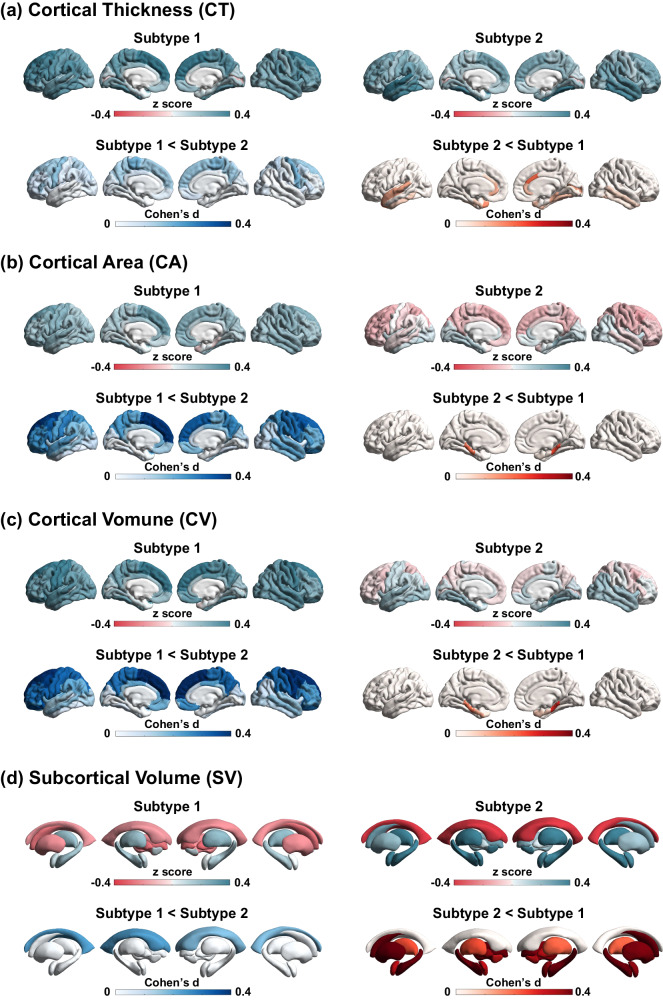
Regional morphological z-scores (i.e., normative deviations from healthy control group) for each subtype were computed and compared (Fig. 3). Morphological z-scores of all brain regions and inter-subtype comparisons are provided in Supplementary Table 5. Briefly, compared to healthy controls, average cortical volume/area reduction was only observed in subtype 1 (Supplementary Fig. 3a–b), though both subtype 1 and subtype 2 exhibited a moderate reduction in average cortical thickness (Supplementary Fig. 3c). Additionally, largest effects for cortical thickness/volume/area were located within the superior frontal regions for subtype 1 and in the superior/medial temporal regions for subtype2 (Supplementary Table 5). As for subcortical volume, larger effects for volumes of hippocampus, amygdala, thalamus, accumbens and brain stem were observed in subtype 2 compared to subtype 1 (Supplementary Fig. 3d–h). The hippocampal/amygdala subregions with the most significant reduction for subtype 2 were located in the molecular layer and cortico-amygdaloid transition area (Supplementary Fig. 4–5). Interestingly, we observed that, compared to healthy controls, the striatum (i.e., caudate, putamen) was larger among subtype 1 patients and smaller among subtype 2 patients (Supplementary Fig. 3i–j). The difference in the striatum between the two subtypes was also replicated in a subsample of medication-naive individuals with schizophrenia (Supplementary Table 6). The main findings of subtype-specific neuroanatomical signatures are described in Table 2. Taken together, subtype 1 exhibited greater deficits in cortical morphology but enlarged volume of the striatum, whereas subtype 2 displayed more severe volume loss in the subcortical regions, including the hippocampus, amygdala, thalamus, brain stem and striatum.
Fig. 3. Subtype-specific signatures in neuroanatomical pathology.
Brain morphological measures include (a) cortical thickness, (b) cortical surface area, (c) cortical volume, and (d) subcortical volume. For each morphological measure, regional z-scores (i.e., normative deviations from healthy control group) in each subtype are mapped to a brain template for visualization. Effect size of inter-subtype difference is quantified using Cohen’s d.
Table 2.
Main findings of subtype-specific neuroanatomical signatures
| Morphometry measures | Subtype-specific neuroanatomical signatures |
|---|---|
| Cortical Thickness/Volume/Area | a) Both subtype1 and subtype2 exhibit a moderate degree in the average cortical thickness reduction. |
| b) Reduction of average cortical volume/area is only observed in the subtype1. | |
| c) The worst reduction of cortical thickness/volume/area is located within the superior frontal regions for the subtype1, but in the superior/medial temporal regions for the subtype2. | |
| Subcortical Volume | a) Enlargement of lateral ventricle is found in both subtype1 and subtype2, but much larger in the subtype2. |
| b) Worse loss volumes of the hippocampus, amygdala, thalamus, and accumbent are observed in the subtype2, compared to the subtype1. | |
| c) Volumes of striatum (i.e., caudate, putamen) are increased in the subtype1, but decreased in the subtype2, compared to the healthy population. | |
| Hippocampus segmentation | a) Volume loss in hippocampal subregions is worse in the subtype2, compared to the subtype1. |
| b) The most significant volume loss is in the molecular layer for the subtype2. | |
| Amygdala segmentation | a) The subtype2 shows worse volume loss in amygdala subregions, compared to the subtype1. |
| b) The most significant decrease in volume is in the cortico-amygdaloid transition area for both the subtypes. | |
| Thalamus segmentation | a) The subtype2 shows worse volume loss in thalamus subregions, compared to the subtype1. |
| Brain stem segmentation | a) Volume loss of brain stem subregions is only observed in the subtype2. |
Clinical characterization of subtypes
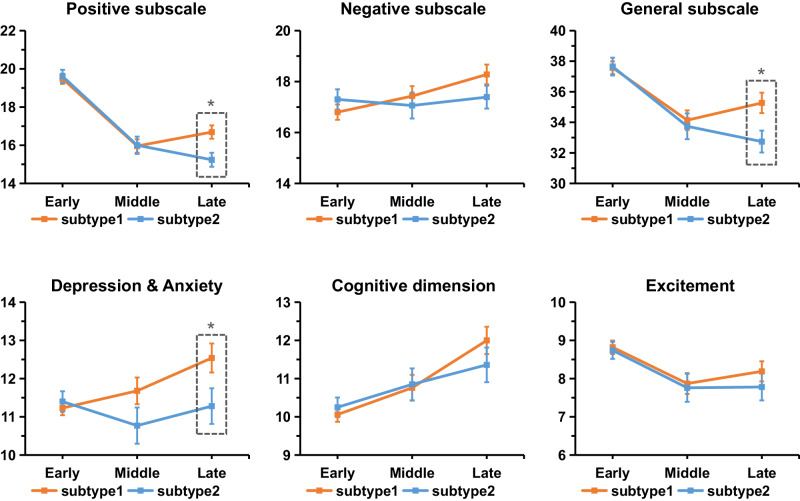
A total of 2622 (62.1%) individuals with schizophrenia were assigned to subtype 1 and the remaining 1600 patients (37.9%) were assigned to subtype 2. The two subtypes exhibit no significant difference in the age, sex, illness duration or PANSS scores (Table 1). To further characterize the psychotic symptomatic trajectory as the disease progresses for each subtype, we further defined three subgroups according to illness duration (early stage [<2 years], n = 926; middle stage [2–10 years], n = 578; late stage [>10 years], n = 682). The results suggested distinct trajectories of psychotic symptoms between the two subtypes (Fig. 4 and Table 3). Specifically, lower positive symptom severity was observed in late stage patients compared early stage patients in both subtypes (subtype 1, F = 37.4, p = 1.60e − 16; subtype2, F = 41.9, p = 4.68e − 18). With the increase of the disease course, subtype 1 showed a gradual worsening of negative symptoms (F = 4.6, p = 9.98e − 3), whereas the negative symptoms of subtype 2 remained stable across the three stages of the disease course (F = 0.1, p = 0.884). Additionally, a gradual worsening of depression/anxiety was only observed in subtype 1 (F = 5.9, p = 2.86e − 3). Inter-subtype comparisons showed that at the late stage (illness duration>10 years), subtype 1 exhibited worse positive symptoms (t = 2.9, p = 0.003), general psychopathology (t = 2.5, p = 0.010) and worse depression/anxiety (t = 2.1, p = 0.033) compared to subtype 2, after regressing out the effects of age, sex and SuStaIn stage.
Fig. 4. Symptomatic trajectories across three stages of disease duration.
Individuals of each subtype are divided into three subgroups according to their illness durations (early stage: ≤2 years; middle stage: 2−10 years; late stage: >10 years). Two sample t test was performed to compare the inter-subtype difference separately within each of the stages after regressing out the effects of age, sex and SuStaIn stage. * two-sided p < 0.05, uncorrected. At the late stage, subtype 1 exhibited worse positive symptom (t = 2.9, p = 0.003), general psychopathology (t = 2.5, p = 0.010) and worse depression/anxiety (t = 2.1, p = 0.033) compared to subtype 2. Data are presented as mean values +/- standard error (se). n = 579 (347), 362 (216), and 400 (282) biologically independent samples in the early stage, middle stage and late stage in subtype 1 (subtype 2) for positive, negative and general subscales. n = 377 (220), 144 (86), and 166 (109) biologically independent samples in the early stage, middle stage and late stage in subtype 1 (subtype 2) for depression & anxiety, cognitive dimension and excitement dimension.
Table 3.
Symptom scores for each subtype at different stages of disease duration
| Symptoms | Subtype 1 | F test | Subtype 2 | F test | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Early | Middle | Late | F | p | Early | Middle | Late | F | p | |
| PANSS Positive scale (P1–P7) | 19.5(6.4) | 16.0(6.7) | 16.7(7.0)* | 37.4 | 1.60E–16 | 19.6(6.4) | 16.0(6.7) | 15.2(6.2)* | 41.9 | 4.68E–18 |
| PANSS Negative scale (N1–N7) | 16.8(7.3) | 17.4(7.4) | 18.3(7.7) | 4.6 | 9.98E–03 | 17.3(7.4) | 17.1(7.5) | 17.4(7.5) | 0.1 | 0.884 |
| PANSS General scale (G1–G16) | 37.6(10.0) | 34.1(12.2) | 35.3(13.3)* | 10.6 | 2.80E–05 | 37.7(10.8) | 33.7(12.4) | 32.7(12.0)* | 15.6 | 2.30E–07 |
| PANSS Total score | 73.9(19.7) | 67.5(23.2) | 70.2(25.0)* | 9.3 | 9.40E–05 | 74.5(20.9) | 66.7(23.4) | 65.4(22.4)* | 15.7 | 2.05E–07 |
| PANSS excitement dimension (P4, P7, G44, G14) | 8.8(3.4) | 7.9(3.3) | 8.2(3.4) | 4.9 | 8.01E–03 | 8.7(3.3) | 7.8(3.4) | 7.8(3.7) | 4.12 | 0.017 |
| PANSS depression/anxiety dimension (G1, G2, G3, G6, G15) | 11.2(3.7) | 11.7(4.2) | 12.5(4.9)* | 5.9 | 2.86E–03 | 11.4(4.0) | 10.8(4.4) | 11.3(4.9)* | 0.7 | 0.511 |
| PANSS cognitive dimension (P2, N5, G5, G10, G11) | 10.1(3.7) | 10.8(4.0) | 12.0(4.6) | 13.5 | 1.74E–06 | 10.3(3.8) | 10.9(3.9) | 11.4(4.7) | 2.8 | 0.061 |
*indicates significant difference between the subtype1 and subtype2 using two sample t test (two-sided p < 0.05, uncorrected), after regressing out the effects of age, sex and SuStaIn stage. n = 579 (347), 362 (216), and 400 (282) biologically independent samples in the early, middle and late stage in subtype 1 (subtype 2) for PANSS positive, negative and general subscales and total score. n = 377 (220), 144 (86), and 166 (109) biologically independent samples in the early, middle and late stage in subtype 1 (subtype 2) for PANSS depression & anxiety, cognitive dimension and excitement dimension.
Generalization of SuStaIn subtyping and staging to unseen cohorts
We investigated whether the SuStaIn subtyping and staging can be generalized to unseen cohorts. A flowchart is shown in Supplementary Fig. 6a. Specifically, the Asian and Europe SuStaIn models were separately built based on the Asian ancestry cohorts and Europe ancestry cohorts, as described in 2.3. The two models were used for subtyping and staging those unseen samples. We compared whether those subtype and stage assignments match the result of the original model that has been built on all cohorts. We observed that most of the unseen individuals can keep the same subtype label with the original model (88.83% for the Asian model; 89.98% for the European model) (Supplementary Fig. 6b). In addition, there was a high consistency of individual staging between stages of unseen data and original model result (Asian model, r = 0.976, p < 0.001; Europe model, r = 0.979, p < 0.001, Spearman correlation test) (Supplementary Fig. 6c). These results indicates a high generalized ability of SuStaIn model to unseen data.
Discussion
Our study, applying a machine learning algorithm to brain MRI data from over 4000 individuals with schizophrenia, has revealed two distinct neurostructural subtypes based on patterns of neuro-pathological progression. These subtypes are reproducible and generalizable across different subsamples and illness stages, independent of macroeconomic and ethnic factors that differed across collection locations. Specific patterns of neuroanatomical pathology for each subtype were uncovered. Subtype 1 is characterized by early cortical-predominant loss that first occurs in the Broca’s area/fronto-insular cortex, and shows adverse signatures in cortical morphology and an enlarged striatum. In contrast, subtype 2 is marked by early subcortical-predominant loss that first appears in the hippocampus, and displays significant volume loss in subcortical regions, including the hippocampus, amygdala, thalamus, brain stem and striatum. Additionally, we observed distinct trajectories of specific symptoms clusters in these two subtypes: as disease progresses, subtype 1 exhibited a gradual worsening of negative and depression/anxiety symptoms, and less of a decline in positive symptoms compared to subtype 2.
Despite the growing body of evidence pointing to group-level gray matter volume deficits in various brain regions - especially in frontal and temporal regions - as well as altered subcortical volume in schizophrenia28, substantial individual variations persist within this population8,29. These inter-individual differences in brain structure may stem from two primary sources of variation. First, differences in underlying etiology and pathogenesis could result in varying clinical characteristics (referred to as phenotypic heterogeneity)3,30. Second, relative differences among subjects in the stage of dynamic progression (known as temporal heterogeneity) could further increase differences in the clinical presentation31,32. Such variations suggest that the pathological progression of schizophrenia might not be attributed to a single unified pathophysiological process. Indeed, our neurostructural subtypes uncovered two SuStaIn trajectories of gray matter loss through brain structural imaging. Several studies also reported dynamic patterns of accelerated gray matter loss over time in individuals with schizophrenia33,34. In addition, the staging of SuStaIn trajectory within the subtype reflects the underlying neurophysiological, pathological, and neuropsychological progressions in schizophrenia. Furthermore, we demonstrated that the phenotypic difference in the intrinsic neuro-pathophysiological trajectory was reproducible across samples worldwide, independent of macroeconomic and ethnic factors that differed across these sites.
The Broca’s area/fronto-insular cortex and hippocampus are identified separately in subtype 1 and subtype 2 as the first regions to show gray matter deficits. This is consistent with our prior finding based on individuals with schizophrenia primarily collected from the Chinese population22. Furthermore, the current study replicates the same two primary regions in a medication-naïve and a first-episode cohort, suggesting that these neuropathological changes are a reflection of the disease process, rather than medication effects. Broca’s area and the fronto-insular cortex have been extensively implicated in schizophrenia35, supporting Crow’s linguistic primacy hypothesis36 and a triple-network model of the disorder37. Abnormalities in Broca’s area and related regions have been linked with hallucinations in schizophrenia38,39. The early involvement of Broca’s area in the pathology could be related to the presence of these core symptoms of schizophrenia. Moreover, in individuals with psychosis, reductions in the inferior frontal cortex preceding the initial psychotic episode have been reported40,41. A prior study reported reduced dopamine release in the prefrontal cortex in patients with schizophrenia42. In relation to hippocampal pathology, research has emphasized the hippocampus as one of the initial regions to display volumetric loss in schizophrenia25,43. The hippocampus is thought to be involved in potential glutamatergic dysfunction in schizophrenia3. Decreased levels of the NMDA co-agonist D-serine were linked to neurobiological alterations similar to those seen in schizophrenia, including hippocampal volume loss44. Gray matter loss in schizophrenia is associated with medication, stress, drug use and inactivity45,46. In addition, schizophrenia is related to dopaminergic dysregulation, disturbed glutamatergic neurotransmission and increased proinflammatory status of the brain45. The causal interrelationships between these processes and gray matter loss are still unclear. These findings offer evidence regarding the specific neuroanatomical locations where gray matter loss is observed in the schizophrenia subtypes. These two potential origins could also offer a viewpoint on the pathological ‘spread’ of the disorder.
The subtyping method exhibits high potential for distinguishing neurostructural subtypes with shared pathophysiological foundations. Notably, subtype 1 displayed larger volume of the striatum, while subtype 2 demonstrated reduced volume. This was consistent with a previous study, which also identified two anatomical subtypes of schizophrenia: one shows enlarged volume in the basal ganglia; whereas the other shows widespread volumetric reduction in the cortical and some subcortical areas relative to healthy controls15. The striatum plays a key role in the dopamine system, which contributes to psychotic symptoms47. Nevertheless, studies of striatal pathology have reported inconsistent differences between patients and controls3. The variability of the striatum is greater in patients than in controls, which relates to overall structural morphometry28, dopamine D2 receptor and transporter levels48. This indicates that differences might exist within subgroups of the disorder3. Alterations in striatal activation are associated with reward-related deficits in schizophrenia49. A previous study suggests that disrupted putamen-cortices connectivity during reward-related processing is directly linked to structural changes in the putamen50. Despite the unclear causal relationship, this suggests that the differential effects on striatal volume between the two subtypes may be related to striatal dysfunction in schizophrenia. In addition, it is still uncertain whether the discrepancy in striatum between cases and controls indicates a primary pathology or an effect of antipsychotic treatment3. Interestingly, this study’s subtype-specific striatal differences were replicated in a subset of individuals who had not received antipsychotic treatment, suggesting that striatal variability persists even in those without antipsychotic treatment. In addition, a recent study reveals a more pronounced and widespread pattern of thinner cortex in deficit schizophrenia, a clinically defined subtype with primary, enduring negative symptoms, compared to non-deficit schizophrenia51. A recent work also reveals that the neuro-structural signature with cortical reduction was associated with progressive illness course, worse cognitive performance and elevated schizophrenia polygenic risk scores52. This also suggests the existence of distinct subtypes distinguished by unique neuroimaging features. Taken together, our neurostructural subtyping differentiated subgroups with unique pathological features, thereby enhancing our understanding of the neurobiological mechanisms underlying schizophrenia.
The two identified subtypes may have several potential therapeutic implications. While the underlying mechanisms associated with a subtype-specific symptomatic trajectory remain unclear, our research shows divergent long-term clinical outcomes between the two neurostructural subtypes. As the disease advanced, for subtype 1, the negative and depression/anxiety symptoms gradually worsened; for subtype 2 these symptoms remained stable. In addition, subtype 1 experienced worse positive symptoms than subtype 2 at the late stage of disease (i.e., duration > 10 years). This is consistent with a prior study that reported greater gray matter reduction in frontal regions in treatment-resistant compared with treatment-responsive individuals with schizophrenia53. Another intriguing aspect is that our prior research on treatment-resistant schizophrenia demonstrated that electroconvulsive therapy (ECT) can substantially enhance the volume of the hippocampus and insula; this is also associated with psychotic symptom alleviation54–56. Notably, these two brain regions were also identified as the ‘origins’ of gray matter loss separately in each subtype. This observation raises the possibility of exploring neuromodulation interventions, such as transcranial magnetic stimulation (TMS), to target these specific brain regions.
This study has several limitations. First, while the SuStaIn algorithm estimates pathophysiological trajectories from cross-sectional MRI data, it remains crucial to validate these outcomes with longitudinal data to verify the brain changes with disease progression over time. Second, the current study benefits from a large sample size, but the inclusion of data from various sites could potentially be influenced by confounding factors, including diverse cohorts, scanners, and locations. Harmonization methods have been employed to alleviate disparities across MRI acquisition protocols. Nonetheless, it remains essential to collect a sufficiently large sample from multi-centers under a standard imaging protocol and experimental paradigm. The lack of cognitive evaluation limits to examine the association of neurostructural biotype with cognitive impairment in schizophrenia. Third, a substantial portion of individuals with schizophrenia were likely to have received or currently use medications, and data from medication-naïve/free individuals were only available for a subset of the datasets. One important limitation is the assumption of progressive pathology in schizophrenia (discrete events of tissue loss or continuous downward drift), when applying SuStaIn. The few existing very long-term imaging studies in schizophrenia support this stance57 but selection bias cannot be fully overcome in the recruitment process for neuroimaging studies. Routine anatomical MRI for every person with psychosis seeking help, with periodic repeats, may provide better view of the validity of progressive pathology in the future. The selection of z-score waypoints and maximum z-score used in the SuStaIn algorithm should be careful based on prior information about degree of progress in different diseases. The computational complexity of the SuStaIn algorithm is highly time-consuming, which limits the exploration of spatiotemporal patterns of trajectories at finer spatial resolutions.
In summary, our study reveals two distinct neurostructural schizophrenia subtypes based on patterns of pathological progression of gray matter loss. We extend the reproducibility and generalizability of these brain imaging-based subtypes across illness stages, medication treatments and different sample locations worldwide, independent of macroeconomic and ethnic factors that differed across these sites. The identified subtypes exhibit distinct signatures of neuroanatomical pathology and psychotic symptomatic trajectories, highlighting the heterogeneity of the neurobiological changes associated with disease progress. This imaging-based taxonomy shows potential for the identification of homogeneous subsamples of individuals with shared neurobiological characteristics. This may be a first crucial step in the transition from only syndrome-based to both syndrome- and biology-based identification of mental disorder subtypes in the near future.
Methods
Study samples
This study analyzed cross-sectional T1-weighted structural MRI data from a total of 4,291 individuals diagnosed with schizophrenia (1,709 females, mean age=32.5 ± 11.9 years) and 7,078 healthy controls (3,461 females, mean age=33.0 ± 12.7 years). These datasets came from 21 cohorts of ENIGMA schizophrenia working groups from various countries around the world, 11 cohorts collected from Chinese hospitals over the last ~10 years, and 9 cohorts from publicly available datasets, i.e., HCP-EP58, JP-SRPBS59, fBIRN60, MCIC61, NMorphCH62, NUSDAST63, DS00003064, DS00011565 and DS00430266. The datasets came from various countries around the world. Details of demographics, geographic location, clinical characteristics, and inclusion/exclusion criteria for each cohort may be found in the Supplementary Information (Supplementary Table 1–2).
The severity of symptoms was evaluated by the Positive and Negative Syndrome Scale (PANSS)67, including a positive scale (total score of P1-P7), a negative scale (total score of N1-N7), a general psychopathology scale (total score of G1-G16) and total score. In addition, phenotypic characteristics were further quantified in three dimensions, such as cognitive (total score of P2, N5, G5, G10, G11), depression/anxiety (total score of G1, G2, G3, G6, G15) and excitement (total score of P4, P7, G44, G14) via a five-factor model of schizophrenia68.
All sites obtained approval from their local institutional review boards or ethics committees, and written informed consent from all participants and/or their legal guardians. The present study was carried out under the approve from the Medical Research Ethics Committees of Fudan University (Number: FE222711).
Image acquisition, processing and quality control
T1-weighted structural brain MRI scans were acquired at each study site. We used a standardized protocol for image processing using the ENIGMA Computational Anatomy Toolbox (CAT12) across multiple cohorts (https://neuro-jena.github.io/enigma-cat12/). These protocols enable region-based gray matter volume (GMV) measures for image data based on the automated anatomical (AAL3) atlas69. Further details of image acquisition parameters and quality control may be found in Supplementary Table 1–2.
Data harmonization
The ROI-wise GMV measures were first adjusted by regressing out the effects of sex, age, the square of age, site and total intracranial volume (TIV) using a regression model22. Subsequently, a harmonization procedure was performed using the ComBat algorithm for correcting multi-site data70. The adjusted values were transformed as z-scores (i.e., normative deviations) relative to the healthy control group. We multiplied these z-scores by -1 so that the z-score increases as regional GMV decreases. Finally, we removed these samples if they were marked as a statistical outlier (if any of their regional volumes >5 standard deviations away from the group-level average). After the quality control, 11,260 individuals were included, of which 4222 were schizophrenia patients (1683 females, mean age=32.4 ± 12.4 years) and 7038 healthy subjects (3440 females, mean age=33.0 ± 12.4 years).
Disease progress modeling
To uncover diverse patterns of pathophysiological progression from cross-sectional only MRI data and cluster individuals into groups (subtypes), we employed a machine learning approach—Subtype and Stage Inference (SuStaIn)19. The methodology of SuStaIn has been described in detail previously19. We also describe the applicability of SuStaIn algorithm to schizophrenia in Supplementary Materials. Here, we briefly describe the main parameter choices specific to the current study. The SuStaIn model requires an M × N matrix as input. M represents the number of cases (M = 4222). N is the number of biomarkers (N = 17). 17 gray matter biomarkers were previously used for SuStaIn modeling in schizophrenia22. Here, all of the AAL3 regions of whole brain were separated and merged into 17 regions of interest (ROIs)22, including frontal lobe, temporal lobe, parietal lobe, occipital lobe, insula, cingulate, sensorimotor, Broca’s area, cerebellum, hippocampus, parahippocampus, amygdala, caudate, putamen, pallidum, accumbens and thalamus (Supplementary Table 7). We further examine the relationship between regional volume and illness duration in patients with schizophrenia using the Spearman correlation test (Supplementary Fig. 7). To keep consistent with our previous study22, we used the z-score thresholds (z = 1, 2, 3) as “waypoints” of severity in the SuStaIn model. The maximum z-score in the SuStaIn algorithm was defined at z = 5 according to maximum z-score for each biomarker (Supplementary Table 8). We also performed a replication analysis with a reduced maximum z-score (z = 4) (Supplementary Fig. 8). We then ran the SuStaIn algorithm with 25 start points and 100,000 Markov Chain Monte Carlo (MCMC) iterations19 to estimate the most likely sequence that describes spatiotemporal pattern of pathophysiological progression (i.e., ‘trajectory’).
First, we used the Hopkins statistics to establish whether the data is clustered. A high value (H = 0.7756) shows a high clustering tendency at 90% confidence level, supporting a robust existence of clusters. SuStaIn can identify diverse trajectories of pathophysiological progression given a subtype number K. We fitted the model for K = 2-6 subtypes (‘trajectories’), separately. The optimal number of subtypes was determined according to the reproducibility of individual subtyping via a two-fold cross-validation procedure22. Specifically, all individuals were randomly split into two non-overlapping folds. This above procedure was repeated ten times. For each fold, we trained the SuStaIn model. For each individual, the trained SuStaIn model provides a subtype label. We measured the consistency of the subtype labeling across all individuals between two folds by using the Dice coefficient. The largest Dice coefficient was obtained for K = 2 (see Fig. 1a), indicating the best consistency based on cross-validation. Finally, the two-cluster model of SuStaIn was fitted to the entire sample. The most probable sequence (i.e., the order of biomarkers) was evaluated for each ‘trajectory’ via SuStaIn. For each individual, SuStaIn calculated the probability (ranging from 0 to 1) of belonging to each ‘trajectory’, and assigned the individual into a sub-stage of the maximum likelihood ‘trajectory’ through MCMC iterations. We also estimated the SuStaIn ‘trajectories’ based on a subsample from individuals with first-episode schizophrenia whose illness duration was less than two years (N = 1122, 513 females, mean age=25.4 ± 8.6 years), and a subsample of medication-naïve individuals with schizophrenia (N = 718, 353 females, mean age=23.7 ± 7.8 years).
Visualization of pathophysiological progression trajectory
To visualize the spatiotemporal patterns of pathophysiological progression, we calculated the mean z-score of regional GMV across individuals belonging to the same substage of each SuStaIn ‘trajectory’. The images of ROI-wise GMV z-scores were mapped into a glass brain template via visualization tools implemented in ENIGMA Toolbox (https://enigma-toolbox.readthedocs.io/en/latest/index.html) and BrainNetViewer (https://www.nitrc.org/projects/bnv/).
To examine whether the SuStaIn stage (a continuous indicator of the ‘temporal’ stage of SuStaIn ‘trajectory’) is associated with pathological processes and clinical characteristics in schizophrenia, we performed Spearman correlations between the SuStaIn stages and the degree of brain atrophy (i.e., regional GMV) in schizophrenia. We also examined whether SuStaIn stages were linked to disease duration, severity of symptoms, and phenotypic characteristics.
Neuroanatomical signatures using regional morphological measures
To further characterize the neuroanatomical signatures associated with each subtype, we conducted regional morphological analyzes in a subsample including 1840 individuals with schizophrenia and 1780 healthy controls. Brain morphological measures, such as cortical thickness, cortical surface area, cortical volume and subcortical volume, were quantified using FreeSurfer (version 7.3, http://surfer.nmr.mgh.harvard.edu/). A total of 68 × 3 regional measures for cortical thickness, cortical surface area and cortical volume were extracted based on the DK atlas71, along with 14 subcortical regions (bilaterally nucleus accumbens, amygdala, caudate, hippocampus, pallidum, putamen and thalamus) and 2 lateral ventricles. In addition, we performed an automated subregion segmentation (https://surfer.nmr.mgh.harvard.edu/fswiki/SubregionSegmentation) for the hippocampal substructures (n = 38 subregions)72, the nuclei of the amygdala (n = 18)73, the thalamic nuclei (n = 50)74, and the brain stem structures (n = 4)75, yielding a total of 110 subregional volumetric measures.
Regional morphological measures for each individual with schizophrenia were adjusted by regressing out the effects of sex, age, the square of age, TIV and site, and then transformed to z-scores (i.e., normative deviations from healthy control group). The mean regional morphological z-score across individuals belonging to each subtype was calculated, and mapped to brain templates for visualization of neuroanatomical signature deviation for each subtype relative to healthy population. To further manifest subtype-specific signature in neuroanatomical pathology, we compared the regional morphological z-scores between the two subtypes using two sample t-tests. Multiple comparisons were corrected by family wise error (FWE) correction.
Distinct symptom profiles between subtypes
To characterize the psychotic symptomatic trajectory with disease duration increases for each subtype, we further divided the individuals of each subtype into three subgroups according to their illness durations (early stage: <2 years; middle stage: 2-10 years; late stage: >10 years). The particular choice of bins was determined according to the distribution of illness duration (early stage n = 926, middle stage n = 578, late stage n = 682) and the size of subgroup enough to perform an inter-subtype comparison. We compared the difference of symptoms among the three stages of disease in each subtype using ANOVA. In addition, two sample t tests were performed to compare the inter-subtype differences separately within each of the stages after regressing out the effects of age, sex and SuStaIn stage.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
This work was supported by the grant from Science and Technology Innovation 2030-Brain Science and Brain-Inspired Intelligence Project (No. 2022ZD0212800 to YJ). This work was supported by National Natural Science Foundation of China (No. 82202242 to YJ, 82071997 to WC). This work was supported by the projects from China Postdoctoral Science Foundation (No. BX2021078 and 2021M700852 to YJ). This work was supported by the Shanghai Rising-Star Program (No. 21QA1408700 to WC) and the Shanghai Sailing Program (22YF1402800 to YJ) from Shanghai Science and Technology Committee. This work was supported by National Key R&D Program of China (No. 2019YFA0709502 to JF, 2022ZD0208500 to DY). This work is supported by the CAMS Innovation Fund for Medical Sciences (no. 2019-I2M-5-039 to CLuo). This work was supported by the grant from Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX01 to JF), ZJ Lab, Shanghai Center for Brain Science and Brain-Inspired Technology, and the grant from the 111 Project (No. B18015 to JF).
Author contributions
Y.J. and J.F. led the project. P.M.T., J.A.T., and T.G.M.E. provided crucial contributions to the organization and cooperation of the project via ENIGMA. Y.J. and J.F. take responsibility for the integrity of the data and the accuracy of the data analysis in the study. Study concept and design 1: Y.J. and J.F. Acquisition, analysis, interpretation of data 2: Y.J., C.Luo., J.W., L.P., X.C., S.X., J.Z., M.D., H.H., C.G., K.N., K.M., R.H., L.T.W., G.R., S.F.C., N.P., O.A.A., T.K., I.N., Fr.S., F.T.O., L.T., P.U., U.D., T.H., Dd.G., S.M., P.L., Y.T., T.Z., C.Li., W.Y., Y.Z., X.Y., E.Z., C.P.L., S.J.T., A.L.R., Da.G., G.P., J.B., A.K., E.P.C., R.S., P.F.C., M.A.G.L., G.S., F.P., D.V., N.B., J.C., Z.L., J.Y., A.S.G., O.U., B.B.B., A.U.D., K.R.M., V.D.C., K.S., M.G., Y.Q., Y.C.C., W.S.K., S.R.S., C.D., I.S.R., F.I., Ad.B., An.B., M.C., A.B., Si.C., G.P., M.T., M.T.M.P., M.K., F.G., S.K., T.V.R., S.R., M.H., W.W., Se.C., P.S., E.R., Fi.S., A.S., D.T., P.H., S.H., W.O., G.C., D.D.N., A.P., S.T., N.J., L.B.C., D.Y., J.A.T., T.G.M.E., W.C., and J.F. Drafting of the original manuscript 3: Y.J and L.P. Crucial advice to the revision of manuscript and the study 4: L.P., P.M.T., J.A.T., T.G.M.E., W.C., and J.F. All authors contributed edits and approved the contents of the manuscript.
Peer review
Peer review information
Nature Communications thanks Stephen Lawrie and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The raw image and clinical data are protected and are not available due to data privacy laws. The processed data are available through the following links. Data of NMorphCH, FBIRN and NUSDAST were obtained from the SchizConnect, a publicly available website (http://www.schizconnect.org/documentation#by_project). The NMorphCH dataset and NUSDAST dataset were download through a query interface at the SchizConnect (http://www.schizconnect.org/queries/new). The FBIRN dataset was download from https://www.nitrc.org/projects/fbirn/. The DS000115 dataset was download from OpenfMRI database (https://www.openfmri.org/). The DS000030 dataset was available at https://legacy.openfmri.org/dataset/ds000030/. The DS004302 dataset was available at https://openneuro.org/datasets/ds004302/versions/1.0.1. The HCP-EP dataset was available at https://www.humanconnectome.org/study/human-connectome-project-for-early-psychosis/. The Japanese SRPBS Multi-disorder MRI Dataset was available at https://bicr-resource.atr.jp/srpbsopen/. Requests for ENIGMA data can be applied via the ENIGMA Schizophrenia Working Group (https://enigma.ini.usc.edu/ongoing/enigma-schizophrenia-working-group/). The statistical data generated in this study are provided in the Supplementary Information/Source Data file. Source data are provided in this paper.
Code availability
SuStaIn algorithm is available on the UCL-POND GitHub (https://github.com/ucl-pond/). T1-weighted images were processed using the Computational Anatomy Toolbox for Standardized Processing of ENIGMA Data (https://neuro-jena.github.io/enigma-cat12/). A protocol for the current data processing is available at https://docs.google.com/document/d/1lb9v0v4j_OrgAKDh6_9fl3Hz2Wcfg46c/edit/. FreeSurfer (version 7.3, http://surfer.nmr.mgh.harvard.edu/) was used to quantify various morphological measures, such as cortical thickness, cortical surface area, cortical volume and subcortical volume. The visualization of ROI-wise z-score images was conducted using BrainNetViewer (https://www.nitrc.org/projects/bnv/). Other custom codes developed in the current study are available at GitHub (https://github.com/YuchaoJiang91/ENIGMA-SCZ-SuStaIn-Subtype).
Competing interests
LP reports personal fees from Janssen Canada, Otsuka Canada, SPMM Course Limited UK and the Canadian Psychiatric Association; book royalties from Oxford University Press; and investigator-initiated educational grants from Sunovion, Janssen Canada and Otsuka Canada, outside the submitted work. TK received unrestricted educational grants from Servier, Janssen, Recordati, Aristo, Otsuka, neuraxpharm. PH has received grants and honoraria from Novartis, Lundbeck, Mepha, Janssen, Boehringer Ingelheim, Neurolite outside of this work. OAA is a consultant to Cortechs.ai and received speakers honorarium from Lundbeck, Janssen, Sunovion. Other authors disclose no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lists of authors and their affiliations appear at the end of the paper.
Contributor Information
Jianfeng Feng, Email: jffeng@fudan.edu.cn.
ENIGMA Schizophrenia Consortium:
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-50267-3.
References
- 1.Organization W. H. The Global Burden Of Disease: 2004 Update. (World Health Organization, 2008).
- 2.Howes OD, Onwordi EC. The synaptic hypothesis of schizophrenia version III: a master mechanism. Mol. Psychiatry. 2023;28:1843–1856. doi: 10.1038/s41380-023-02043-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCutcheon RA, Krystal JH, Howes OD. Dopamine and glutamate in schizophrenia: biology, symptoms and treatment. World Psychiatry. 2020;19:15–33. doi: 10.1002/wps.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolfers T, et al. Mapping the heterogeneous phenotype of schizophrenia and bipolar disorder using normative models. JAMA Psychiatry. 2018;75:1146–1155. doi: 10.1001/jamapsychiatry.2018.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fusar-Poli P, et al. Heterogeneity of psychosis risk within individuals at clinical high risk: a meta-analytical stratification. JAMA Psychiatry. 2016;73:113–120. doi: 10.1001/jamapsychiatry.2015.2324. [DOI] [PubMed] [Google Scholar]
- 6.McCutcheon RA, et al. The efficacy and heterogeneity of antipsychotic response in schizophrenia: A meta-analysis. Mol. Psychiatry. 2021;26:1310–1320. doi: 10.1038/s41380-019-0502-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collado-Torres L, et al. Regional heterogeneity in gene expression, regulation, and coherence in the frontal cortex and hippocampus across development and schizophrenia. Neuron. 2019;103:203–216 e208. doi: 10.1016/j.neuron.2019.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brugger SP, Howes OD. Heterogeneity and homogeneity of regional brain structure in schizophrenia: a meta-analysis. JAMA Psychiatry. 2017;74:1104–1111. doi: 10.1001/jamapsychiatry.2017.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braff DL, Ryan J, Rissling AJ, Carpenter WT. Lack of use in the literature from the last 20 years supports dropping traditional schizophrenia subtypes from DSM-5 and ICD-11. Schizophr. Bull. 2013;39:751–753. doi: 10.1093/schbul/sbt068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The L. ICD-11: a brave attempt at classifying a new world. Lancet. 2018;391:2476. doi: 10.1016/S0140-6736(18)31370-9. [DOI] [PubMed] [Google Scholar]
- 11.Oren O, Gersh BJ, Bhatt DL. Artificial intelligence in medical imaging: switching from radiographic pathological data to clinically meaningful endpoints. Lancet Digit Health. 2020;2:e486–e488. doi: 10.1016/S2589-7500(20)30160-6. [DOI] [PubMed] [Google Scholar]
- 12.Rajpurkar P, Chen E, Banerjee O, Topol EJ. AI in health and medicine. Nat. Med. 2022;28:31–38. doi: 10.1038/s41591-021-01614-0. [DOI] [PubMed] [Google Scholar]
- 13.Wen J, et al. Multi-scale semi-supervised clustering of brain images: deriving disease subtypes. Med Image Anal. 2022;75:102304. doi: 10.1016/j.media.2021.102304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lalousis PA, et al. Heterogeneity and classification of recent onset psychosis and depression: a multimodal machine learning approach. Schizophr. Bull. 2021;47:1130–1140. doi: 10.1093/schbul/sbaa185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chand GB, et al. Two distinct neuroanatomical subtypes of schizophrenia revealed using machine learning. Brain. 2020;143:1027–1038. doi: 10.1093/brain/awaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Z, et al. A deep learning framework identifies dimensional representations of Alzheimer’s Disease from brain structure. Nat. Commun. 2021;12:7065. doi: 10.1038/s41467-021-26703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dwyer DB, et al. Brain subtyping enhances the neuroanatomical discrimination of schizophrenia. Schizophr. Bull. 2018;44:1060–1069. doi: 10.1093/schbul/sby008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo C, et al. Subtypes of schizophrenia identified by multi-omic measures associated with dysregulated immune function. Mol. Psychiatry. 2021;26:6926–6936. doi: 10.1038/s41380-021-01308-6. [DOI] [PubMed] [Google Scholar]
- 19.Young AL, et al. Uncovering the heterogeneity and temporal complexity of neurodegenerative diseases with Subtype and Stage Inference. Nat. Commun. 2018;9:4273. doi: 10.1038/s41467-018-05892-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogel JW, et al. Four distinct trajectories of tau deposition identified in Alzheimer’s disease. Nat. Med. 2021;27:871–881. doi: 10.1038/s41591-021-01309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young AL, et al. Characterizing the clinical features and atrophy patterns of MAPT-related frontotemporal dementia with disease progression modeling. Neurology. 2021;97:e941–e952. doi: 10.1212/WNL.0000000000012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang Y, et al. Neuroimaging biomarkers define neurophysiological subtypes with distinct trajectories in schizophrenia. Nat. Ment. Health. 2023;1:186–199. doi: 10.1038/s44220-023-00024-0. [DOI] [Google Scholar]
- 23.Jiang Y, et al. Identification of four biotypes in temporal lobe epilepsy via machine learning on brain images. Nat. Commun. 2024;15:2221. doi: 10.1038/s41467-024-46629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Erp TGM, et al. Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the enhancing neuro imaging genetics through meta analysis (ENIGMA) consortium. Biol. Psychiatry. 2018;84:644–654. doi: 10.1016/j.biopsych.2018.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Erp TG, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol. Psychiatry. 2016;21:585. doi: 10.1038/mp.2015.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okada N, et al. Subcortical volumetric alterations in four major psychiatric disorders: a mega-analysis study of 5604 subjects and a volumetric data-driven approach for classification. Mol. Psychiatry. 2023;28:5206–5216. doi: 10.1038/s41380-023-02141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koshiyama D, et al. White matter microstructural alterations across four major psychiatric disorders: mega-analysis study in 2937 individuals. Mol. Psychiatry. 2020;25:883–895. doi: 10.1038/s41380-019-0553-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howes OD, Cummings C, Chapman GE, Shatalina E. Neuroimaging in schizophrenia: an overview of findings and their implications for synaptic changes. Neuropsychopharmacology. 2023;48:151–167. doi: 10.1038/s41386-022-01426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alnaes D, et al. Brain heterogeneity in schizophrenia and its association with polygenic risk. JAMA Psychiatry. 2019;76:739–748. doi: 10.1001/jamapsychiatry.2019.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howes OD, Kapur S. A neurobiological hypothesis for the classification of schizophrenia: type A (hyperdopaminergic) and type B (normodopaminergic) Br. J. Psychiatry. 2014;205:1–3. doi: 10.1192/bjp.bp.113.138578. [DOI] [PubMed] [Google Scholar]
- 31.Jiang Y, et al. Progressive reduction in gray matter in patients with schizophrenia assessed with mr imaging by using causal network analysis. Radiology. 2018;287:729. doi: 10.1148/radiol.2018184005. [DOI] [PubMed] [Google Scholar]
- 32.Kirschner M, et al. Orbitofrontal-striatal structural alterations linked to negative symptoms at different stages of the schizophrenia spectrum. Schizophr. Bull. 2021;47:849–863. doi: 10.1093/schbul/sbaa169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson PM, et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc. Natl Acad. Sci. USA. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson PM, et al. Time-lapse mapping of cortical changes in schizophrenia with different treatments. Cereb. Cortex. 2009;19:1107–1123. doi: 10.1093/cercor/bhn152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fillman SG, et al. Elevated peripheral cytokines characterize a subgroup of people with schizophrenia displaying poor verbal fluency and reduced Broca’s area volume. Mol. Psychiatry. 2016;21:1090–1098. doi: 10.1038/mp.2015.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crow TJ. Is schizophrenia the price that Homo sapiens pays for language? Schizophr. Res. 1997;28:127–141. doi: 10.1016/S0920-9964(97)00110-2. [DOI] [PubMed] [Google Scholar]
- 37.Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J. Psychiatry Neurosci. 2012;37:17–27. doi: 10.1503/jpn.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGuire PK, Murray R, Shah G. Increased blood flow in Broca’s area during auditory hallucinations in schizophrenia. Lancet. 1993;342:703–706. doi: 10.1016/0140-6736(93)91707-S. [DOI] [PubMed] [Google Scholar]
- 39.Vercammen A, Knegtering H, den Boer JA, Liemburg EJ, Aleman A. Auditory hallucinations in schizophrenia are associated with reduced functional connectivity of the temporo-parietal area. Biol. Psychiatry. 2010;67:912–918. doi: 10.1016/j.biopsych.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 40.Del Re EC, et al. Baseline cortical thickness reductions in clinical high risk for psychosis: brain regions associated with conversion to psychosis versus non-conversion as assessed at one-year follow-up in the shanghai-at-risk-for-psychosis (SHARP) study. Schizophr. Bull. 2021;47:562–574. doi: 10.1093/schbul/sbaa127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pantelis C, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 42.Slifstein M, et al. Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: a positron emission tomographic functional magnetic resonance imaging study. JAMA Psychiatry. 2015;72:316–324. doi: 10.1001/jamapsychiatry.2014.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br. J. Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- 44.Balu DT, et al. Multiple risk pathways for schizophrenia converge in serine racemase knockout mice, a mouse model of NMDA receptor hypofunction. Proc. Natl Acad. Sci. USA. 2013;110:E2400–E2409. doi: 10.1073/pnas.1304308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kahn RS, Sommer IE. The neurobiology and treatment of first-episode schizophrenia. Mol. Psychiatry. 2015;20:84–97. doi: 10.1038/mp.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vita A, De Peri L, Deste G, Barlati S, Sacchetti E. The effect of antipsychotic treatment on cortical gray matter changes in schizophrenia: does the class matter? a meta-analysis and meta-regression of longitudinal magnetic resonance imaging studies. Biol. Psychiatry. 2015;78:403–412. doi: 10.1016/j.biopsych.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 47.McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia-an overview. JAMA Psychiatry. 2020;77:201–210. doi: 10.1001/jamapsychiatry.2019.3360. [DOI] [PubMed] [Google Scholar]
- 48.Brugger SP, et al. Heterogeneity of Striatal Dopamine Function in Schizophrenia: Meta-analysis of Variance. Biol. Psychiatry. 2020;87:215–224. doi: 10.1016/j.biopsych.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 49.Chase HW, Loriemi P, Wensing T, Eickhoff SB, Nickl-Jockschat T. Meta-analytic evidence for altered mesolimbic responses to reward in schizophrenia. Hum. Brain Mapp. 2018;39:2917–2928. doi: 10.1002/hbm.24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koch K, et al. Functional connectivity and grey matter volume of the striatum in schizophrenia. Br. J. Psychiatry. 2014;205:204–213. doi: 10.1192/bjp.bp.113.138099. [DOI] [PubMed] [Google Scholar]
- 51.Banaj N, et al. Cortical morphology in patients with the deficit and non-deficit syndrome of schizophrenia: a worldwide meta- and mega-analyses. Mol. Psychiatry. 2023;28:4363–4373. doi: 10.1038/s41380-023-02221-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chand GB, et al. Schizophrenia imaging signatures and their associations with cognition, psychopathology, and genetics in the general population. Am. J. Psychiatry. 2022;179:650–660. doi: 10.1176/appi.ajp.21070686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mouchlianitis E, McCutcheon R, Howes OD. Brain-imaging studies of treatment-resistant schizophrenia: a systematic review. Lancet Psychiatry. 2016;3:451–463. doi: 10.1016/S2215-0366(15)00540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang Y, Duan M, He H, Yao D, Luo C. Structural and functional MRI brain changes in patients with schizophrenia following electroconvulsive therapy: a systematic review. Curr. Neuropharmacol. 2022;20:1241–1252. doi: 10.2174/1570159X19666210809101248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J, et al. ECT-induced brain plasticity correlates with positive symptom improvement in schizophrenia by voxel-based morphometry analysis of grey matter. Brain Stimul. 2019;12:319–328. doi: 10.1016/j.brs.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 56.Jiang Y, et al. Insular changes induced by electroconvulsive therapy response to symptom improvements in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;89:254–262. doi: 10.1016/j.pnpbp.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 57.Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch. Gen. Psychiatry. 2011;68:128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lewandowski K. E., Bouix S., Ongur D., Shenton M. E. Neuroprogression across the Early Course of Psychosis. J Psychiatr Brain Sci 5, e200002 (2020). [DOI] [PMC free article] [PubMed]
- 59.Tanaka SC, et al. A multi-site, multi-disorder resting-state magnetic resonance image database. Sci. Data. 2021;8:227. doi: 10.1038/s41597-021-01004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keator DB, et al. The function biomedical informatics research network data repository. Neuroimage. 2016;124:1074–1079. doi: 10.1016/j.neuroimage.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gollub RL, et al. The MCIC collection: a shared repository of multi-modal, multi-site brain image data from a clinical investigation of schizophrenia. Neuroinformatics. 2013;11:367–388. doi: 10.1007/s12021-013-9184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alpert K, Kogan A, Parrish T, Marcus D, Wang L. The northwestern university neuroimaging data archive (NUNDA) Neuroimage. 2016;124:1131–1136. doi: 10.1016/j.neuroimage.2015.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kogan A, Alpert K, Ambite JL, Marcus DS, Wang L. Northwestern University schizophrenia data sharing for SchizConnect: A longitudinal dataset for large-scale integration. Neuroimage. 2016;124:1196–1201. doi: 10.1016/j.neuroimage.2015.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poldrack RA, et al. A phenome-wide examination of neural and cognitive function. Sci. Data. 2016;3:160110. doi: 10.1038/sdata.2016.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Repovs G, Barch DM. Working memory related brain network connectivity in individuals with schizophrenia and their siblings. Front Hum. Neurosci. 2012;6:137. doi: 10.3389/fnhum.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soler-Vidal J, et al. Brain correlates of speech perception in schizophrenia patients with and without auditory hallucinations. PLOS ONE. 2022;17:e0276975. doi: 10.1371/journal.pone.0276975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 68.Lindenmayer JP, Bernstein-Hyman R, Grochowski S. Five-factor model of schizophrenia. Initial validation. J. Nerv. Ment. Dis. 1994;182:631–638. doi: 10.1097/00005053-199411000-00006. [DOI] [PubMed] [Google Scholar]
- 69.Rolls ET, Huang C-C, Lin C-P, Feng J, Joliot M. Automated anatomical labelling atlas 3. Neuroimage. 2020;206:116189. doi: 10.1016/j.neuroimage.2019.116189. [DOI] [PubMed] [Google Scholar]
- 70.Pomponio R, et al. Harmonization of large MRI datasets for the analysis of brain imaging patterns throughout the lifespan. Neuroimage. 2020;208:116450. doi: 10.1016/j.neuroimage.2019.116450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Desikan RS, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 72.Iglesias JE, et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117–137. doi: 10.1016/j.neuroimage.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saygin ZM, et al. High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: manual segmentation to automatic atlas. Neuroimage. 2017;155:370–382. doi: 10.1016/j.neuroimage.2017.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iglesias JE, et al. A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology. Neuroimage. 2018;183:314–326. doi: 10.1016/j.neuroimage.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iglesias JE, et al. Bayesian segmentation of brainstem structures in MRI. Neuroimage. 2015;113:184–195. doi: 10.1016/j.neuroimage.2015.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The raw image and clinical data are protected and are not available due to data privacy laws. The processed data are available through the following links. Data of NMorphCH, FBIRN and NUSDAST were obtained from the SchizConnect, a publicly available website (http://www.schizconnect.org/documentation#by_project). The NMorphCH dataset and NUSDAST dataset were download through a query interface at the SchizConnect (http://www.schizconnect.org/queries/new). The FBIRN dataset was download from https://www.nitrc.org/projects/fbirn/. The DS000115 dataset was download from OpenfMRI database (https://www.openfmri.org/). The DS000030 dataset was available at https://legacy.openfmri.org/dataset/ds000030/. The DS004302 dataset was available at https://openneuro.org/datasets/ds004302/versions/1.0.1. The HCP-EP dataset was available at https://www.humanconnectome.org/study/human-connectome-project-for-early-psychosis/. The Japanese SRPBS Multi-disorder MRI Dataset was available at https://bicr-resource.atr.jp/srpbsopen/. Requests for ENIGMA data can be applied via the ENIGMA Schizophrenia Working Group (https://enigma.ini.usc.edu/ongoing/enigma-schizophrenia-working-group/). The statistical data generated in this study are provided in the Supplementary Information/Source Data file. Source data are provided in this paper.
SuStaIn algorithm is available on the UCL-POND GitHub (https://github.com/ucl-pond/). T1-weighted images were processed using the Computational Anatomy Toolbox for Standardized Processing of ENIGMA Data (https://neuro-jena.github.io/enigma-cat12/). A protocol for the current data processing is available at https://docs.google.com/document/d/1lb9v0v4j_OrgAKDh6_9fl3Hz2Wcfg46c/edit/. FreeSurfer (version 7.3, http://surfer.nmr.mgh.harvard.edu/) was used to quantify various morphological measures, such as cortical thickness, cortical surface area, cortical volume and subcortical volume. The visualization of ROI-wise z-score images was conducted using BrainNetViewer (https://www.nitrc.org/projects/bnv/). Other custom codes developed in the current study are available at GitHub (https://github.com/YuchaoJiang91/ENIGMA-SCZ-SuStaIn-Subtype).