Abstract
It has been shown previously that measles virus (MV) can be successfully used to express foreign proteins (M. Singh and M. A. Billeter, J. Gen. Virol. 80:101–106, 1998). To develop an inexpensive MV-based vaccine, we generated recombinant MVs that produce structural proteins of hepatitis B virus (HBV). A recombinant virus that expressed the HBV small surface antigen (HBsAg) was analyzed in terms of its replication characteristics, its genetic stability in cell culture, and its immunogenic potential in genetically modified mice. Although this virus showed a progression of replication slightly slower than that of the parental MV, it appeared to stably maintain the added genetic information; it uniformly expressed the appropriately glycosylated HBsAg after 10 serial passages. Genetically modified mice inoculated with this recombinant MV produced humoral immune responses against both HBsAg and MV proteins.
Hepatitis B virus (HBV) is a major cause of acute and chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Despite the availability of effective vaccines, hepatitis B remains a serious worldwide disease in that more than 250 million people are chronically infected with HBV. The majority of these individuals live in nonindustrialized countries. In southeast Asia, China, Oceania, and Africa, very high rates (5 to 20%) of prevalence of chronic HBV infection have been reported. In the United States, approximately 200,000 cases of new HBV infections occur each year (14).
A successful vaccine should be safe, efficacious, and cost-effective. An anti-HBV vaccine has been prepared from HBV surface antigen (HBsAg), initially purified from the plasma of chronic HBV carriers (13) and then produced by recombinant DNA technology in either Escherichia coli (17), Saccharomyces cerevisiae (19), or mammalian (CHO) cells (33). A complete vaccination course requires three intramuscular injections, and in most cases, long-lasting protective antibody levels are achieved only after the third injection (8). According to a World Health Organization report, the HBV vaccine costs more than the combined cost of six EPI (Expanded Programme on Immunization) vaccines (32). The high cost of HBV vaccine as well as the complex vaccination regimen tremendously hampers the success of vaccination programs aimed at controlling global HBV infection.
In an attempt to develop an inexpensive and effective HBV vaccine requiring only a single administration, a measles virus (MV) Edmonston vaccine strain-based vector that induces immunity against both MV and HBV was developed. HBsAg coding sequences were inserted in the MV genome, and a recombinant virus was obtained with our system for the rescue of MV from cloned DNA (22). This virus expressed HBsAg and induced humoral immune responses against both MV and HBsAg in genetically modified mice (20).
MATERIALS AND METHODS
Cells.
Cells were maintained as monolayers in Dulbecco’s modified Eagle’s medium supplemented with 5% fetal calf serum (FCS) for Vero (African green monkey kidney) cells, with 10% FCS for 293 (human embryonic kidney) cells, and with 10% FCS and 1.2 mg of G418 per ml for stably transfected 293-3-46 cells (22).
Plasmid constructions.
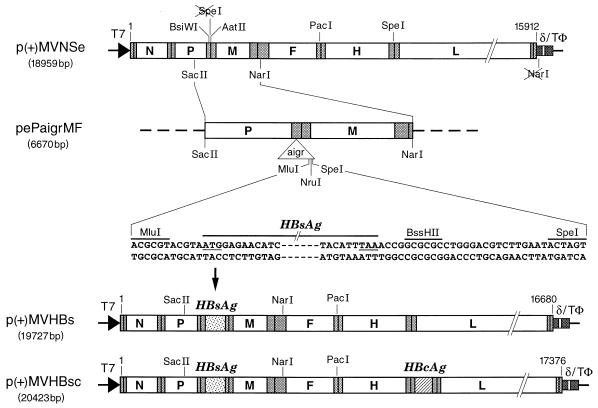
Plasmid p(+)MVNSe (29) carrying the antigenomic MV tag Edmonston B (MV-tag-Edm) sequence was slightly modified from p(+)MV (22) to contain only unique NarI and SpeI cleavage sites. Plasmid pHS2.5 (3) containing the full-length HBV genomic sequence of the ayw subtype (9) was used to PCR amplify the coding sequence (681 bp) of HBsAg with the primers 5′-ATCGACGCGTACGTAATGGAGAACATCACATCAGGAT-3′ and 5′-TGGCGCGCCGGTTTAAATGTATACCCAAAGACAA-3′ (MluI and BssHII recognition sites are underlined; the initiation codon in the first primer and the reverse complement of the termination codon in the second primer are in boldface letters). MluI- and BssHII-digested PCR products and complementary oligonucleotides (22-mers, forming a duplex containing BssHII and SpeI cohesive ends [see the sequences indicated in Fig. 1]) were ligated into the MluI and SpeI sites of pePaigrMF (see Fig. 1) to obtain plasmid pePMFHBs. Plasmid pePaigrMF was a derivative of plasmid pePMF2 (26) and contained an artificial intergenic region with unique cloning sites between the P and M genes of MV. A SacII-NarI fragment of pePMFHBs was used to replace the analogous segment in p(+)MVNSe, yielding p(+)MVHBs.
FIG. 1.
Cloning HBsAg and HBcAg ORFs in p(+)MVNSe antigenomic MV plasmid. ORFs of MV and HBV are shown as rectangles (not to scale) labeled with letters as follows: N, nucleocapsid; P, phosphoprotein; M, matrix; F, fusion; H, hemagglutinin; and L, large protein of MV. Stippled rectangles denote nontranslated regions, and vertical bars denote the nontranscribed intergenic trinucleotides. The triangle labeled “aigr” represents the artificial intergenic region, which consists of gene termination, intergenic, and gene start sequences followed by unique cloning sites. The flanking sequences together with start and stop codons (underlined) of the HBsAg ORF, plasmid names together with total sizes in base pairs, MV antigenomic nucleotide numbers (based on EMBL accession no. Z66517), and restriction sites are as indicated. T7 indicates the T7 RNA polymerase promoter, δ indicates the hepatitis delta virus ribozyme, and TΦ indicates the T7 RNA polymerase terminator.
Similarly, the coding sequences for the HBV core antigen (HBcAg) were amplified with the primers 5′-ATCGACGCGTACGTAATGGACATTGATCCTTAT-3′ and 5′-CCAGGCGCGCCGCTAACATTGAGATTCCCGAGAT-3′ (underlining and boldface are as described for the oligonucleotides noted above). The PCR products digested with MluI and BssHII and the oligonucleotides with BssHII and SpeI cohesive ends mentioned above were ligated into the MluI and SpeI sites of peFHaigrL, which contains an artificial intergenic region identical to that of pePaigrMF, to obtain peFHLHBc. A PacI-SpeI fragment of peFHLHBc was used to replace the analogous segment in p(+)MVNSe, yielding p(+)MVHBc. A SacII-NarI fragment of pePMFHBs was used to replace the analogous segment in p(+)MVHBc, yielding p(+)MVHBsc.
To generate plasmids containing the HBsAg and HBcAg coding regions under a cytomegalovirus promoter, the SnaBI-BglII fragment of pePMFHBs and the SnaBI-SpeI fragment of peFHLHBc (after being subcloned in pBluescript to obtain relevant flanking cloning sites) were individually inserted into the HincII and BglII sites of the pSCT vector (24). The resulting plasmids, pCMVHBs and pCMVHBc, were used to confirm the coding potential of the open reading frames (ORFs) in transient-expression assays.
All cloning procedures were performed basically as described previously (25), and sequences were confirmed by sequencing the entire inserted regions of the plasmids.
Rescue of recombinant MVs.
MV-tag-Edm and a mutant MV carrying HBsAg and HBcAg sequences were rescued with the 293-3-46 helper cell line as described previously (22). Briefly, 293-3-46 cells were seeded into a 35-mm-diameter well to reach ∼50 to ∼70% confluence 1 day before transfection. Five micrograms of either p(+)MVNSe, p(+)MVHBs, or p(+)MVHBsc together with 100 ng of pEMC-La (which bears the gene for MV polymerase L) was transfected by Ca2+ phosphate coprecipitation of DNA. Three days after transfection, three to four syncytia developed in cells transfected with antigenomic plasmids. Single syncytia were transferred to 35-mm-diameter wells of Vero cell culture plates, whose contents were subsequently expanded to 175 cm2 dishes. Viruses were harvested when they showed 80 to 90% cytopathic effects by scraping the cells into 3 ml of OptiMEM I (GIBCO BRL, Paisley, Scotland), followed by one round of freezing and thawing. These virus preparations were designated MV-tag-Edm, MVHBs, and MVHBsc passage 1 viruses according to the sequence used. The supernatants were clarified from cell debris and were kept at −80°C as crude virus stocks.
Virus characterization.
The rescued recombinant viruses were serially passaged 10 times in Vero cells at a multiplicity of infection (MOI) of 0.01. Passage 3, passage 7, and passage 10 viruses were used for further characterization. The virus titers were determined by endpoint dilution assays to calculate 50% tissue culture infectious dose (TCID50) values (16). Virus growth characteristics were analyzed by infecting Vero cell monolayers in 35-mm-diameter wells at an MOI of 0.01 and incubating the plates at 37°C. Infected cells were collected and lysed by freezing and thawing 4, 8, 12, 24, 36, 48, and 60 h postinoculation (hpi) to determine virus titers.
Plaque assays were carried out with Vero cell cultures in 35-mm-diameter wells (18). After 2 h of virus adsorption, the inoculum was removed and the cells were overlaid with 2 ml of Dulbecco’s modified Eagle’s medium containing 5% FCS and 1% SeaPlaque agarose. After 3 to 4 days, cultures were fixed with 1 ml of 10% trichloroacetic acid for 1 h and UV cross-linked for 30 min. After removal of the agarose overlay, cell monolayers were stained with crystal violet (0.3%, wt/vol, dissolved in 4% ethanol) to determine the number and sizes of plaques.
Expression of HBsAg and HBcAg by recombinant MVs. (i) Immunofluorescence.
Monolayers of Vero cells grown on tissue culture chamber slides (Life Technologies, Basel, Switzerland) were infected with either MVHBs, MVHBsc, or MV-tag-Edm for 24 h and fixed with 4% paraformaldehyde for 15 min, followed by permeabilization and blocking for 15 min with a solution containing 25 mM Tris-HCl (pH 7.5), 0.136 M NaCl, 2.6 mM KCl, 2% goat serum, 2% bovine serum albumin (fraction V), 0.1% gelatin, and 0.2% Saponin. The cells were consecutively processed with either goat polyclonal anti-HBsAg antibodies (ay; Chemicon, Temecula, Calif.) and donkey anti-goat antibody–fluorescein isothiocyanate (FITC) conjugate (Serotec Ltd., Oxford, England) or rabbit anti-HBcAg antibodies (a kind gift from Heinz Schaller) and anti-rabbit antibody–FITC conjugate (Chemicon). The numbers of fluorescing and nonfluorescing syncytia were counted.
(ii) ELISA.
The concentration of the secreted HBsAg in the culture supernatants of MVHBs was determined with a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Monolisa HBs; Sanofi Diagnostics, Paris, France) with known amounts of recombinant HBsAg for calibration.
(iii) Western immunoblotting.
Monolayers of Vero cells grown in six-well plates were infected at an MOI of 0.1 with either MVHBs, MVHBsc, or MV-tag-Edm. The cells were harvested 24 hpi and processed for sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis as described previously (28). HBsAg was visualized with goat polyclonal anti-HBsAg antibody as the first antibody and rabbit anti-goat antibody–horseradish peroxidase (HRPO) conjugate (Chemicon) as the second antibody, whereas HBcAg was visualized with rabbit anti-HBcAg antibody as the first antibody and swine anti-rabbit antibody–HRPO conjugate (DAKO A/S, Glostrup, Denmark) as the second antibody according to the enhanced chemiluminescence protocol (Amersham, Zurich, Switzerland).
Immunization of mice and characterization of humoral immune responses.
Genetically modified mice (Ifnar-CD46Ge; haplotype H-2bk) lacked the alpha/beta interferon (IFN type I) receptor and carried a large fragment of the human chromosome encompassing the CD46 gene (20). These mice were inoculated with 0.5 × 105 PFU of either MVHBs or MV-tag-Edm either intranasally (i.n.) or intraperitoneally (i.p.), with four mice per group. Additionally, UV-inactivated MVHBs was used to inoculate four mice per group i.n. or i.p., with the amount of inoculum used being the same as that used for the live virus. UV inactivation was carried out by exposing undiluted MVHBs to a UV source at a distance of 15 cm for 30 min. Mice were bled at weekly intervals postinoculation, and serum was separated and aliquoted for storage at −20°C until use.
Anti-MV antibody titers were determined by ELISA or neutralization tests. The MV ELISA was performed by coating 96-well plates with a 0.6-μg/ml solution of a commercially available MV (Edmonston, ATCC VR-24) antigen, a partially purified preparation from supernatants of infected Vero cells (VIRION Ltd., Zurich, Switzerland). The plates were consecutively incubated with various dilutions of mouse sera and rabbit anti-mouse immunoglobulin G–peroxidase conjugate (DAKO A/S) and then with the substrate, and optical density values at 492 nm (OD492) were measured. The OD values of more than 0.4 reflect clearly positive samples. Neutralizing antibody titers were determined in a plaque reduction neutralization assay (1) by incubating serum dilutions with 50 PFU of MV and were expressed as milli-international units per milliliter by World Health Organization standards. Anti-HBsAg antibody titers were determined with a commercially available quantitative ELISA kit (Monolisa anti-HBs; Sanofi Diagnostics) and expressed as milli-international units per milliliter.
RESULTS
Rescued recombinant MVs express HBsAg and HBcAg.
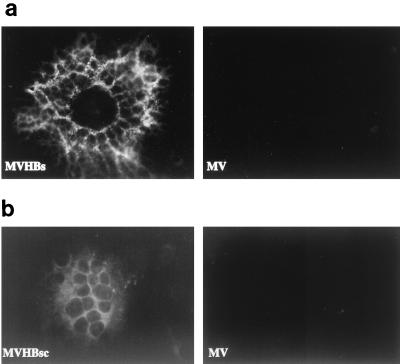
The ORF encoding HBsAg was inserted into p(+)MVNSe such that it was part of an additional transcription unit between the MV P and M genes (Fig. 1). Similarly, the ORF encoding HBcAg was also inserted into p(+)MVNSe, but between the MV H and L genes. The coding potential of both ORFs, subcloned individually into an expression plasmid containing a cytomegalovirus promoter, was first confirmed in transient-transfection assays by monitoring the expression of HBsAg and HBcAg by immunofluorescence (not shown). Subsequently, the single-insertion plasmid p(+)MVHBs and the dual-insertion plasmid p(+)MVHBsc were used for rescuing recombinant viruses with the helper cell line 293-3-46 (22). The recombinant viruses (MVHBs and MVHBsc) were amplified in Vero cells, and the syncytia obtained were screened for expression of HBsAg and HBcAg by immunofluorescence. All syncytia showed positive signals (Fig. 2, left images), whereas the syncytia of rescued MV-tag-Edm (Fig. 2, right images) showed no fluorescence, indicating that all syncytia induced by MVHBs or MVHBsc expressed HBsAg or HBcAg, respectively. The granular appearance of stained HBsAg, contrasting with the smooth appearance of HBcAg localized in the cytoplasm, reflects its localization in organelles of the secretory pathway. The syncytia induced by MVHBsc, when stained with antibodies directed against HBsAg, also exhibited a granular pattern (not shown).
FIG. 2.
Expression of HBsAg and HBcAg from recombinant MVs. Vero cells were infected with either MVHBs, MVHBsc, or MV-tag-Edm (indicated as MV) and processed for indirect immunofluorescence as described in Materials and Methods. HBVsAg-specific (a) and HBVcAg-specific (b) signals were detected with goat anti-HBsAg and rabbit anti-HBcAg antibodies, respectively, followed by anti-goat- and anti-rabbit antibody–FITC conjugate, respectively.
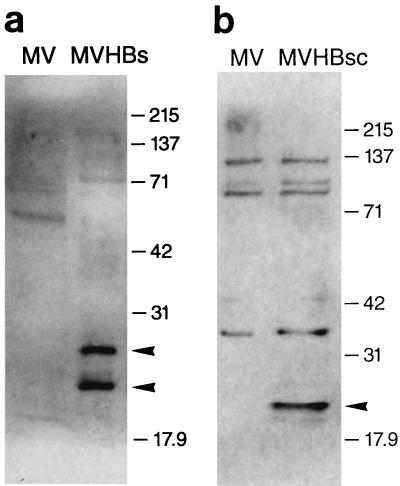
The expression of HBsAg from MVHBs and HBcAg from MVHBsc was further confirmed in Western immunoblots (Fig. 3). Vero cells were infected with recombinant MVHBs, MVHBsc, or MV-tag-Edm, and cell lysates were analyzed. The anti-HBsAg antibodies reacted with two proteins of approximately 27 and 24 kDa synthesized in MVHBs-infected Vero cells, whereas no such proteins were observed in MV-tag-Edm-infected cell lysates (Fig. 3a). The two proteins are synthesized in similar amounts and correspond to authentic glycosylated (27-kDa) and nonglycosylated (24-kDa) HBsAg (12). This shows that recombinant MVHBs can give rise to appropriately glycosylated HBsAg.
FIG. 3.
Western immunoblots of HBsAg and HBcAg expressed from recombinant MVs. Vero cells were infected with either MVHBs, MVHBsc, or MV-tag-Edm (indicated as MV), and total cellular proteins were harvested at 24 hpi and processed for Western blots as described in Materials and Methods. The nylon membranes with transferred proteins were developed with goat anti-HBsAg antibodies followed by anti-goat antibody–HRPO conjugate (a) and with rabbit anti-HBcAg antibodies followed by anti-rabbit antibody–HRPO conjugate (b), and proteins were visualized by enhanced chemiluminescence. Two protein bands of approximately 27 and 24 kDa observed in MVHBs cell lysates and a single band of approximately 21 kDa observed in MVHBsc cell lysates are marked with arrowheads. The molecular mass markers (in kilodaltons) are indicated.
The anti-HBcAg antibodies reacted with a protein of approximately 21 kDa synthesized in the MVHBsc-infected cells, whereas no such protein was detected in MV-tag-Edm-infected cell lysates (Fig. 3b). The HBcAg protein expressed from MVHBsc is similar in size to the authentic core protein of HBV (27).
Stability of HBsAg expression.
To determine whether the ORF encoding HBsAg is stably maintained in MVHBs, the recombinant virus was serially passaged 10 times in Vero cells at an MOI of 0.01; this amounts to an overall amplification factor of 3 × 1020. The recombinant viruses at passage 3, 7, and 10 were used to infect Vero cells, and the culture supernatants were collected, without disturbing the monolayers, when they showed 80 to 90% cytopathic effect. The clarified culture supernatants were used to quantify the amount of HBsAg with a commercially available ELISA kit. The recombinant virus mediated the synthesis of 7, 5.7, and 6.6 ng of HBsAg per ml of culture supernatant (total, 3 ml) from 106 cells after 3, 7, and 10 passages, respectively (results not shown). Thus, the expression of the inserted ORF was stable over 10 serial passages and the amount of HBsAg in the culture supernatants remained practically constant. Additionally, more than 97% of the syncytia elicited by the recombinant MV after 10 passages in cell culture were positive for HBsAg-specific immunofluorescence signals (Table 1).
TABLE 1.
Plaque characteristics of MVHBs and MV
| Virus | % of plaques positive by IFAa | Avg size of plaques (mm) |
|---|---|---|
| MVHBs at passage: | ||
| 3 | 98.3 | 0.67 |
| 7 | 97.3 | —b |
| 10 | 97.6 | — |
| MV | — | 0.93 |
IFA, indirect immunofluorescence assay.
—, not done.
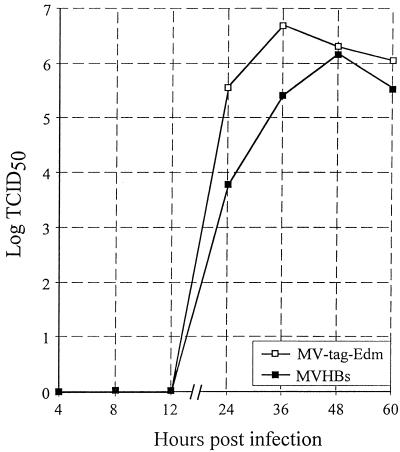
It is interesting that the replication of MVHBs was only slightly impaired: the recombinant virus reached peak titers of 6.18 × 106 TCID50s/ml 48 hpi, whereas MV-tag-Edm gave final titers of 6.7 × 106 TCID50s/ml 36 hpi (Fig. 4). The slightly slower progression of replication was also reflected in somewhat reduced plaque sizes. MVHBs produced plaques with an average diameter of 0.67 mm, while MV produced plaques with an average diameter of 0.93 mm (Table 1).
FIG. 4.
Growth curve comparison of MVHBs and MV-tag-Edm. Vero cells were infected with MVHBs or MV-tag-Edm at an MOI of 0.01, and the virus was harvested at the indicated time points. The virus titers, expressed as TCID50s per milliliter, are taken from one of three independent experiments; the variations (which were minor) are not shown.
Humoral immune response to rescued MVs.
The immunogenic potentials of rescued viruses (MVHBs and MV-tag-Edm) were monitored in genetically modified mice that lack the IFN type I receptor, express the human MV receptor CD46, and support MV replication (20). Four groups of four mice each were immunized with MVHBs or MV-tag-Edm i.n. or i.p., and antibody titers against MV and HBsAg were analyzed by ELISA 14 and 28 days postinoculation (dpi); antibody titers against MV were also determined by neutralization tests. Table 2 summarizes these results. All animals were negative for anti-MV or anti-HBsAg antibodies prior to immunization. The eight mice immunized with MVHBs mounted anti-HBsAg antibodies with titers ranging from 9.6 to 100.5 mIU/ml except mice 10 and 11, which also mounted poor immune responses against MV. Generally, the antibody response was stronger in mice immunized i.p. and in most mice titers increased between 14 and 28 dpi, suggesting that both i.n. and i.p. inoculated virus replicated in genetically modified mice, in line with findings reported by Mrkic et al. (20). Anti-MV antibody responses were detected in all mice and were higher 28 dpi. i.p. inoculation elicited a better response than i.n. inoculation. All four mice immunized i.n. with MVHBs failed to produce neutralizing anti-MV antibodies, suggesting that the MV carrying an additional gene may replicate less vigorously than standard MV.
TABLE 2.
Anti-HBsAg and anti-MV responses in mice inoculated with MV and MVHBs
| Virus | Route of inoculation | Animal | Anti-HBsAg antibody titer (mIU/ml)
|
Anti-MV antibody
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OD450 by ELISAa
|
Neutralization titer (mIU/ml)
|
||||||||||
| Preimmunization | 14 dpi | 28 dpi | Preimmunization | 14 dpi | 28 dpi | Preimmunization | 14 dpi | 28 dpi | |||
| MV | i.n. | 1 | 0 | 0 | 0 | 0.11 | 1.56 | 2.10 | <490 | <490 | 1,130 |
| 2 | —b | — | — | 0.10 | 1.06 | 1.06 | — | <320 | <370 | ||
| 3 | — | — | — | 0.08 | 1.16 | 1.33 | — | <400 | 570 | ||
| 4 | — | — | — | 0.11 | 1.53 | 1.76 | — | <380 | 1,630 | ||
| i.p. | 5 | 0 | 0 | 0 | 0.11 | 1.90 | 2.04 | <330 | 2,000 | 3,380 | |
| 6 | — | — | — | 0.14 | 1.65 | 2.00 | — | 1,740 | 8,370 | ||
| 7 | — | — | — | 0.23 | 1.90 | 2.06 | — | 1,130 | 3,050 | ||
| 8 | — | — | — | 0.22 | 1.75 | 2.04 | — | 480 | 2,990 | ||
| MVHBs | i.n. | 9 | 0 | 18.8 | 93.0 | 0.22 | 0.62 | 1.54 | <380 | <380 | <380 |
| 10 | 0 | 0 | 0 | 0.09 | 0.46 | 0.60 | — | <380 | <380 | ||
| 11 | 0 | 0 | 0 | 0.13 | 0.33 | 0.29 | — | <380 | <380 | ||
| 12 | 0 | 9.6 | 80.8 | 0.13 | 0.19 | 1.10 | — | <380 | <320 | ||
| i.p. | 13 | 0 | 27.5 | 100.5 | 0.13 | 1.70 | 2.01 | <380 | 900 | 2,300 | |
| 14 | 0 | 11.9 | 42.0 | 0.13 | 1.73 | 1.93 | — | 3,290 | 4,330 | ||
| 15 | 0 | 0 | 41.4 | 0.17 | 1.50 | 1.61 | — | 450 | 770 | ||
| 16 | 0 | 73.3 | 61.0 | 0.21 | 1.63 | 1.91 | — | 480 | 8,070 | ||
ELISA reading at a 1:100 dilution of serum.
—, not done.
To investigate whether the observed humoral immune responses were due to virus replication or simply reflected responses against the antigens in the inoculum, two groups of four mice were inoculated either i.n. or i.p. with UV-inactivated MVHBs to determine anti-HBsAg and anti-MV antibody titers 14 and 28 dpi. Virus inactivation was confirmed by inoculating Vero cell culture monolayers; even undiluted virus produced no syncytia. No anti-HBsAg response was observed in either i.n. or i.p. immunized mice (data not shown) except for a borderline response at 28 dpi (5.2 mIU/ml) in a single mouse. The inactivated virus failed to induce neutralizing antibodies regardless of the route of inoculation and produced moderate anti-MV ELISA antibody titers only after i.p. inoculation. This shows that in the absence of virus replication, the immune response is much weaker.
DISCUSSION
We describe here the generation of recombinant MVs that express HBsAg and HBcAg from reading frames inserted in additional transcription units into the MV genome. The HBsAg ORF was stably expressed from MVHBs even after 10 virus passages in cell culture. The HBsAg polypeptide was correctly glycosylated; about 20 ng of HBsAg was secreted in the culture supernatants of 106 cells. These amounts are comparable to HBsAg levels produced in other viral systems, e.g., adenovirus, where 20 to 250 ng of HBsAg from 106 cells was reported (6, 34).
The replication of the recombinant MVHBs was slightly slower than that of the parental MV-tag-Edm. Considering the gradient of gene expression of viruses belonging to the order Mononegavirales (2), insertion of additional genes in the MV genome is expected to result in slower viral growth kinetics. We did not investigate whether HBsAg, by itself, has a direct impact on MV replication. This does not appear likely, since similar slight decreases in growth kinetics have also been observed for recombinant MVs expressing either human interleukin-12 (28) or the indicator proteins chloramphenicol acetyltransferase (30), green fluorescent protein (11), and β-galactosidase (5). The rescued MVHBs appeared to faithfully maintain the inserted coding sequences over multiple passages in cell culture, although we cannot exclude the possibility that mutations which did not interfere with the capability of the artificially expressed proteins to react with the antibodies arose. We did not determine the sequences of the inserted HBs and HBc reading frames in the corresponding serially passaged MV recombinants; however, to date, whenever such sequence analyses have been performed on extensively passaged progeny of recombinant MVs found defective in the expression of inserted ORFs, the defect has always been due to a stop codon interrupting the otherwise unaltered ORF prematurely (unpublished observations). This suggests a relatively high fidelity of copying of MV RNA polymerase and essentially no RNA recombination by copy choice (10), which would lead to deletions.
To explore the potential of MVHBs as a vaccine vector, the humoral immune responses against HBsAg and MV proteins were monitored by immunizing genetically modified mice. These mice lack the IFN type I receptor and express human CD46, the best-characterized MV receptor (7, 21), with human-like tissue specificity (20). The majority of the mice inoculated with MVHBs produced anti-HBsAg antibodies with titers greater than 10 mIU/ml, a level sufficient in humans to confer protection against natural HBV infection (31). The pattern of responses against HBsAg and MV proteins in mice immunized with live and UV-inactivated MVHBs shows that virus replication is required to mount neutralizing antibodies; the UV-inactivated virus elicited low antibody titers detectable only by ELISA. Although these mice and those immunized with control MV-tag-Edm generally mounted anti-MV antibodies with titers equivalent to those of seroconverted humans (4, 15), the mice immunized i.n. responded poorly to both HBsAg and MV antigens. One explanation for the poor responses could be that the genetically modified mice used in our studies support only limited virus propagation in the respiratory tract, especially in the lungs (20). (The respiratory tract is the portal of entry of MV in humans [10, 23].) An alternative explanation may be that a larger number of macrophages in the peritoneal cavity than in the respiratory tract favor the rapid and efficient transport of MV infection to the lymphoid system. The genetic heterogeneity (mixed haplotype H-2bk) as well as the lack of IFN type I receptor in the mice used in this study might also have contributed to the observed variations in immune responses. Nevertheless, our experiments show that this mouse model is a useful tool for carrying out preliminary immunological studies of MV or MV-derived recombinants or mutants.
Our MV-tag-Edm-based system has several advantages over other viral vector systems for the delivery of foreign proteins for immunization purposes. First, it uses an MV strain which is already in use as a safe and efficacious vaccine (10). The production cost of MV vaccine is very low. Thus, delivery of immunogens of other pathogens such as HBV, human T-cell leukemia virus, human immunodeficiency virus, and malaria parasites by MV vectors would curtail expensive procedures involved in the production of vaccines against these pathogens. The current MV vaccine is well-known to induce a solid and lifelong immunity (10). Although the mechanisms of this potent immunogenicity are not clear, an MV-based vaccine vector is expected to induce long-lasting immunity simultaneously against MV and other expressed immunogens. As a preferred application beneficial particularly for developing countries, the use of MV vector cocktails delivering simultaneously several additional antigens could be envisaged instead of the routine MV vaccination in early childhood.
The present report together with those of our other studies shows that the MV genome can accommodate complex foreign gene sequences of various sizes such as ∼1.5 kb (HBsAg and HBcAg [this report]), 3.2 kb (human interleukin-12, including highly structured internal ribosome entry site sequences [28]), and ∼5 kb (simultaneously green fluorescent protein, β-galactosidase, and chloramphenicol acetyltransferase [29]), which together amount to nearly one-third of the size of the MV genome. The plasticity of MV and its ability to stably express foreign genes even after multiple passages suggest that it is a far better delivery system than other RNA virus vectors which are unable to accommodate and maintain foreign sequences due to tight restraint of RNA structure and encapsidation as well as high levels of recombination. Moreover, MV, an RNA virus with a cytoplasmic replication cycle without an intermediate DNA step (10), cannot cause insertional mutagenesis in the host genome. Nevertheless, a thorough investigation of the pathogenicity and protection potential of each MV-based recombinant should be performed with monkeys. The vector described here can be used for challenge studies with HBV in chimpanzees. Additionally, to prepare a complete vaccine against HBV, the pre-S proteins and HBcAg are envisaged to be simultaneously expressed from an MV vector. Towards this aim we have generated a recombinant MV that simultaneously expresses HBsAg and HBcAg.
ACKNOWLEDGMENTS
We are very grateful to J. Pavlovic and W. Bossart (Institute of Medical Virology, University of Zurich) for providing infrastructure and advice for animal experiments and for MV ELISAs, respectively, and to R. Glück, S. Brantschen, and P. Durrer (Swiss Serum and Vaccine Institute, Bern, Switzerland) for helpful discussions and MV neutralization tests.
This work was supported by the Schweizerische Nationalfonds (grants 31-43475-95 and 31-45900-95) and the NIH (grant R01 A135136).
REFERENCES
- 1.Albrecht P, Herrmann K, Burns G R. Role of virus strain in conventional and enhanced measles plaque neutralization test. J Virol Methods. 1981;3:251–260. doi: 10.1016/0166-0934(81)90062-8. [DOI] [PubMed] [Google Scholar]
- 2.Cattaneo R, Rebmann G, Schmid A, Baczko K, ter Meulen V, Billeter M A. Altered transcription of a defective measles virus genome derived from a diseased human brain. EMBO J. 1987;6:681–688. doi: 10.1002/j.1460-2075.1987.tb04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cattaneo R, Will H, Darai G, Pfaff E, Schaller H. Detection of an element of the SV40 late promoter in vectors used for expression studies in COS cells. EMBO J. 1983;2:511–514. doi: 10.1002/j.1460-2075.1983.tb01455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen R T, Markowitz L E, Albrecht P, Stewart J A, Mofenson L M, Preblud S R, Orenstein W A. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990;162:1036–1042. doi: 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- 5.Cornu T. Use of measles virus as a vector: stable expression of a supplementary large transcription unit or additional foreign viral glycoproteins. M.S. thesis. Zurich, Switzerland: University of Zurich; 1997. [Google Scholar]
- 6.Davis A R, Kostek B, Mason B B, Hsiao C L, Morin J, Dheer S K, Hung P P. Expression of hepatitis B surface antigen with a recombinant adenovirus. Proc Natl Acad Sci USA. 1985;82:7560–7564. doi: 10.1073/pnas.82.22.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dörig R E, Marcil A, Chopra A, Richardson C D. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 8.Ellis R W. New and improved vaccines against hepatitis B. In: Woodrow G, Levine M, editors. New generation vaccines. New York, N.Y: Marcel Dekker; 1990. pp. 439–447. [Google Scholar]
- 9.Galibert F, Mandart E, Fitoussi F, Tiollais P, Charnay P. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature. 1979;281:646–650. doi: 10.1038/281646a0. [DOI] [PubMed] [Google Scholar]
- 10.Griffin D E, Bellini W J. Measles virus. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1267–1312. [Google Scholar]
- 11.Hangartner L. Development of measles virus as a vector: expression of green fluorescent protein from different loci. M.S. thesis. Zurich, Switzerland: University of Zurich; 1997. [Google Scholar]
- 12.Heermann K H, Goldmann U, Schwartz W, Seyffarth T, Baumgarten H, Gerlich W H. Large surface proteins of hepatitis B virus containing the pre-s sequence. J Virol. 1984;52:396–402. doi: 10.1128/jvi.52.2.396-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilleman M R, McAleer W J, Buynak E B, McLean A A. The preparation and safety of hepatitis B vaccine. J Infect. 1983;7:3–8. doi: 10.1016/s0163-4453(83)96465-4. [DOI] [PubMed] [Google Scholar]
- 14.Hollinger F B. Hepatitis B virus. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2739–2807. [Google Scholar]
- 15.Hussey G D, Goddard E A, Hughes J, Ryon J J, Kerran M, Carelse E, Strebel P M, Markowitz L E, Moodie J, Barron P, Latief Z, Sayed R, Beatty D, Griffin D E. The effect of Edmonston-Zagreb and Schwarz measles vaccines on immune response in infants. J Infect Dis. 1996;173:1320–1326. doi: 10.1093/infdis/173.6.1320. [DOI] [PubMed] [Google Scholar]
- 16.Kärber G. Beitrag zur Kollektiven Behandlung pharmakologischer Reihenversuche. Arch Exp Pathol Pharmakol. 1931;162:480–483. [Google Scholar]
- 17.MacKay P, Pasek M, Magazin M, Kovacic R T, Allet B, Stahl S, Gilbert W, Schaller H, Bruce S A, Murray K. Production of immunologically active surface antigens of hepatitis B virus by Escherichia coli. Proc Natl Acad Sci USA. 1981;78:4510–4514. doi: 10.1073/pnas.78.7.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mann G F, Allison L M, Copeland J A, Agostini C F, Zuckerman A J. A simplified plaque assay system for measles virus. J Biol Stand. 1980;8:219–225. doi: 10.1016/s0092-1157(80)80037-0. [DOI] [PubMed] [Google Scholar]
- 19.McAleer W J, Buynak E B, Maigetter R Z, Wampler D E, Miller W J, Hilleman M R. Human hepatitis B vaccine from recombinant yeast. Nature. 1984;307:178–180. doi: 10.1038/307178a0. [DOI] [PubMed] [Google Scholar]
- 20.Mrkic B, Pavlovic J, Rülicke T, Volpe P, Buchholz C J, Hourcade D, Atkinson J P, Aguzzi A, Cattaneo R. Measles virus spread and pathogenesis in genetically modified mice. J Virol. 1998;72:7420–7427. doi: 10.1128/jvi.72.9.7420-7427.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naniche D, Varior-Krishnan G, Cervoni F, Wild T F, Rossi B, Rabourdin-Combe C, Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radecke F, Spielhofer P, Schneider H, Kaelin K, Christiansen G, Huber M, Dötsch C, Billeter M A. Rescue of infectious measles viruses from cloned DNA. EMBO J. 1995;14:5773–5784. doi: 10.1002/j.1460-2075.1995.tb00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riley E C, Murphy G, Riley R L. Airborne spread of measles in a suburban elementary school. Am J Epidemiol. 1978;107:421–432. doi: 10.1093/oxfordjournals.aje.a112560. [DOI] [PubMed] [Google Scholar]
- 24.Rusconi S, Severne Y, Georgiev O, Galli I, Wieland S. A novel expression assay to study transcriptional activators. Gene. 1990;89:211–221. doi: 10.1016/0378-1119(90)90008-f. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 26.Schneider H, Kaelin K, Billeter M A. Recombinant measles viruses defective for RNA editing and V protein synthesis are viable in cultured cells. Virology. 1997;227:314–322. doi: 10.1006/viro.1996.8339. [DOI] [PubMed] [Google Scholar]
- 27.Seifer M, Standring D N. Assembly and antigenicity of hepatitis B virus core particles. Intervirology. 1995;38:47–62. doi: 10.1159/000150414. [DOI] [PubMed] [Google Scholar]
- 28.Singh M, Billeter M A. A recombinant measles virus expressing biologically active human interleukin-12. J Gen Virol. 1998;80:101–106. doi: 10.1099/0022-1317-80-1-101. [DOI] [PubMed] [Google Scholar]
- 29.Singh M, Hangartner L, Cornu T, Christiansen G, Billeter M A. Abstracts of the 10th International Conference on Negative Strand Viruses. 1997. Measles virus as a vector system, abstr. 102; p. 101. [Google Scholar]
- 30.Spielhofer P. Generation of standard, variant and chimeric measles viruses from cloned DNA. Ph.D. thesis. Zurich, Switzerland: University of Zurich; 1995. [Google Scholar]
- 31.Szmuness W, Stevens C E, Zang E A, Harley E J, Kellner A. A controlled clinical trial of the efficacy of the hepatitis B vaccine (Heptavax B): a final report. Hepatology. 1981;1:377–385. doi: 10.1002/hep.1840010502. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization Website. October 1996, copyright date. [Online.] Hepatitis B vaccine. http://www.who.ch/gpv-documents/Newslets/H3Updat.htm. [30 March 1999, last date accessed.]
- 33.Yap I, Guan R, Chan S H. Recombinant DNA hepatitis B vaccine containing pre-S components of the HBV coat protein—a preliminary study on immunogenicity. Vaccine. 1992;10:439–442. doi: 10.1016/0264-410x(92)90391-v. [DOI] [PubMed] [Google Scholar]
- 34.Yuasa T, Kajino K, Saito I, Miyamura T. Preferential expression of the large hepatitis B virus surface antigen gene by an adenovirus-hepatitis B virus recombinant. J Gen Virol. 1991;72:1927–1934. doi: 10.1099/0022-1317-72-8-1927. [DOI] [PubMed] [Google Scholar]