Abstract
Vector-borne diseases, including dengue, leishmaniasis and malaria, may be more common among individuals whose occupations or behaviours bring them into frequent contact with these disease vectors outside of their homes. A systematic review was conducted to ascertain at-risk occupations and situations that put individuals at increased risk of exposure to these disease vectors in endemic regions and identify the most suitable interventions for each exposure. The review was conducted in accordance with PRISMA guidelines on articles published between 1945 and October 2021, searched in 16 online databases. The primary outcome was incidence or prevalence of dengue, leishmaniasis or malaria. The review excluded ecological and qualitative studies, abstracts only, letters, commentaries, reviews, and studies of laboratory-acquired infections. Studies were appraised, data extracted, and a descriptive analysis conducted. Bite interventions for each risk group were assessed. A total of 1170 articles were screened and 99 included. Malaria, leishmaniasis and dengue were presented in 47, 41 and 24 articles, respectively; some articles presented multiple conditions. The most represented populations were soldiers, 38% (43 of 112 studies); refugees and travellers, 15% (17) each; migrant workers, 12.5% (14); miners, 9% (10); farmers, 5% (6); rubber tappers and missionaries, 1.8% (2) each; and forest workers, 0.9% (1). Risk of exposure was categorised into round-the-clock or specific times of day/night dependent on occupation. Exposure to these vectors presents a critical and understudied concern for outdoor workers and mobile populations. When devising interventions to provide round-the-clock vector bite protection, two populations are considered. First, mobile populations, characterized by their high mobility, may find potential benefits in insecticide-treated clothing, though more research and optimization are essential. Treated clothing offers personal vector protection and holds promise for economically disadvantaged individuals, especially when enabling them to self-treat their clothing to repel vectors. Secondly, semi-permanent and permanent settlement populations can receive a combination of interventions that offer both personal and community protection, including spatial repellents, suitable for extended stays. Existing research is heavily biased towards tourism and the military, diverting attention and resources from vulnerable populations where these interventions are most required like refugee populations as well as those residing in sub-Saharan Africa.
Keywords: Dengue, Leishmaniasis, Malaria, Insecticide-treated clothing, Occupation, Systematic review
Graphical abstract
Highlights
-
•
A review of the evidence on optimal interventions targeting outdoor-biting vectors of dengue, leishmaniasis, and malaria.
-
•
Insecticide-treated clothing (ITC) provides personal protection against vector bites, akin to a treated net.
-
•
ITC can help outdoor occupations, tourism, refugee situations, and armed combat.
-
•
Combined with other interventions, ITC can provide 24-h protection against vector bites for people working outdoors.
1. Introduction
Vector-borne diseases, notably arboviral diseases, leishmaniasis and malaria, continue to disrupt everyday lives in endemic regions. These diseases cause substantial work absenteeism, increase morbidity and reduce productivity, with a substantial economic impact (Orem et al., 2012; Kioko, 2013; Lukwa et al., 2019). People may be exposed to vector bites at various times or locations depending on their daily activities (Monroe et al., 2019a). Some are temporarily exposed, while others are regularly exposed while working (Cotter et al., 2013). Those who work outdoors in disease-endemic areas may be simultaneously exposed to multiple disease vectors, including vectors of malaria, dengue or leishmaniasis (van den Bogaart et al., 2012; Aschale et al., 2019; Ahmed et al., 2021). All exposed to these vectors require vector bite protection that suits how, when and where they are exposed. Designing suitable interventions for such groups necessitates understanding the most vulnerable groups in endemic regions and the times and locations where populations are most likely to encounter vectors (Sturrock et al., 2013).
The most prevalent vector-borne diseases impacting people at work are dengue, leishmaniasis and malaria (GBD 2015 DALYs & HALE Collaborators, 2016). Lymphatic filariasis is also a prevalent vector-borne disease (GBD 2015 DALYs & HALE Collaborators, 2016) but is best controlled through preventative chemotherapy (Rebollo and Bockarie, 2017). Malaria, dengue and leishmaniasis pose a health threat to individuals in the tropics and subtropics who are refugees, are non-immune travellers, or spend most of their work time outdoors in endemic regions, including the military. These diseases place a heavy economic burden on not only individuals who get sick, but also those who care for the sick, their dependents, health care providers, businesses that rely on outdoor employment, and individuals who expect direct or indirect revenue from outdoor employment (Okwor and Uzonna, 2016; Castro et al., 2017; Conteh et al., 2021).
The distribution of dengue, malaria and leishmaniasis varies across occupations and outdoor activities. This review focuses on occupations associated with exposure to vectors resulting in higher frequency of infection and disease.
1.1. Dengue
Dengue is the most widespread arboviral (insect-transmitted virus) disease in humans (GBD 2015 DALYs & HALE Collaborators, 2016). The frequency and magnitude of dengue epidemics have increased dramatically in the past 40 years as both mosquito vectors and the four dengue virus serotypes have expanded geographically throughout the tropics and subtropics (Messina et al., 2019; Nature Education, 2014). The four dengue virus serotypes, DENV-1, DENV-2, DENV-3 and DENV-4, belong to the genus Flavivirus, family Flaviviridae (Westaway and Blok, 1997). Persons infected with one of these viruses acquire life-long immunity to that virus serotype but no cross-protective immunity to the other serotypes. Individuals living in dengue-endemic areas can be infected with all four dengue serotypes in their lifetime (Gubler, 1988). Risks posed by repeat dengue fever (DF) are much greater than those posed by the initial illness because the memory cells offer defence against reinfection only with the dengue serotype that caused the first illness (Sangkawibha et al., 1984; Wilken and Rimmelzwaan, 2020). The initial infection’s antibodies contribute to the transmission of the dengue virus and raise viremia when a person contracts a second dengue serotype. The immune system responds by making antibodies to the previous strain, allowing the new strain to proliferate. Consequently, higher viral titres are reached, worsening clinical symptoms and prognosis, including dengue haemorrhagic fever (Halstead, 2002). The global resurgence of dengue is attributed to urbanisation, transportation, human movement changes and behaviour (Brady and Hay, 2020).
Several vectors can transmit dengue, but the primary vectors are Aedes aegypti and Aedes albopictus (Gubler, 1998a).
1.1.1. Dengue vector biology and behaviour: Aedes aegypti
Aedes aegypti is the primary vector of dengue (Ramchurn et al., 2009), and all four dengue serotypes have been isolated from field-collected specimens (Gratz, 2004). This mosquito species transmits other arboviruses, including yellow fever, chikungunya, and Zika viruses (Leta et al., 2018; Powell, 2018), and might be a vector of Venezuelan equine encephalitis virus (Powell, 2018). Aedes aegypti is widely distributed: in the tropics and subtropics of Africa, Asia, South America, the Mediterranean region and some parts of the Middle East. The origin of the species is suggested to be in Africa (Mattingly, 1957).
Globalisation has been a factor in the successful spread of Ae. aegypti. The species thrives in densely populated areas without reliable water supplies or proper waste management and with poor sanitation (Honorio et al., 2009). Historically, Ae. aegypti has moved from continent to continent via ships, and this method of dispersal is thought to present the highest risk of introduction (Weaver and Reisen, 2010). Aedes aegypti lives in close association with humans, feeding solely on human blood in the daytime and living and breeding around human homes (Powell and Tabachnick, 2013). The mosquitoes often rest in dark rooms (e.g. inside bathrooms and under beds) (Waldetensai et al., 2021; Janaki et al., 2022) and breed in small water pools that collect in discarded refuse or water storage containers (Day, 2016; Msellemu et al., 2020). Their eggs survive desiccation, which helps maintain vector survival throughout the year (Carvalho and Moreira, 2017). When an Ae. aegypti female carries dengue, the virus can be passed to eggs and hatched larvae may carry the virus (vertical transmission); therefore, the hatched larvae carry the virus (Buckner et al., 2013; Ferreira-de-Lima and Lima-Camara, 2018).
1.1.2. Dengue vector biology and behaviour: Aedes albopictus
Aedes albopictus, also known as the Asian tiger mosquito, is among the world’s most invasive vector species because of its high ecological and physiological plasticity, including multiple breeding habitat types, drought-resistant eggs and adaptation to cold (Benelli et al., 2020). Aedes albopictus is competent for several arboviruses, including all four serotypes of dengue (Gratz, 2004), chikungunya and West Nile virus (Benedict et al., 2007; Muja-Bajraktari et al., 2022).
Aedes albopictus is an aggressive daytime biter (Benedict et al., 2007), and despite being an opportunistic feeder on a wide range of animal hosts, it prefers human blood (Benedict et al., 2007; Paupy et al., 2009). This opportunistic behaviour poses a serious health threat as the mosquito serves as a carrier, transmitting zoonotic infections to humans (Benedict et al., 2007). Although there are indications that Ae. albopictus is becoming partially endophilic (Genchi et al., 2009), it prefers to feed outdoors and is responsible for some recent disease outbreaks (Ramchurn et al., 2009; Rezza, 2012). It originated in Asia but has spread worldwide (Battaglia et al., 2022), and it will probably continue to spread until it has reached most of the tropics and subtropics and possibly also warmer temperate areas (Kraemer et al., 2019).
1.2. Leishmaniasis
Leishmaniasis comprises three protozoan parasitic diseases found in parts of the tropics, the subtropics and southern Europe and transmitted to humans by the bite of phlebotomine sand flies of the genus Phlebotomus or Lutzomya (Vega-Lopez and Ritchie, 2014; CDC, 2020a; WHO, 2022c). Three main forms of leishmaniasis infect people: cutaneous, visceral and mucocutaneous (WHO, 2022c). Cutaneous leishmaniasis is the most common; it causes skin lesions and ulcers on exposed parts of the body, leaving life-long scars and serious disability or stigma. Visceral leishmaniasis, also known as kala-azar, is the most serious form, leading to irregular episodes of fever, weight loss, enlargement of the spleen and liver, and anaemia (WHO, 2013a). Mucocutaneous leishmaniasis is severely disfiguring; it can lead to partial or total destruction of mucous membranes of the nose, mouth and throat (WHO, 2022c). Various species of an obligate intracellular parasite of the genus Leishmania are responsible for the disease (Akhoundi et al., 2016). This parasite dwells in cells of the monocytic phagocytic system of mammals and is transmitted by female sand flies (Steverding, 2017). More than 20 species of Leishmania are pathogenic to humans, and more than 30 species of sand flies function as vectors (Karimkhani et al., 2016) (Table 1). Akhoundi et al. (2016) provide a detailed review.
Table 1.
Manifestation and geographical distribution of leishmaniasis types. Only incriminated vectors are included, but there are numerous suspected vectors.
| Leishmaniasis type | Manifestation | Pathogen | Sand fly vector species | Endemicity |
|---|---|---|---|---|
| Cutaneous (the most common form of leishmaniasis) | Infections appear like any other skin lesion. | Old World: Leishmania major; L. tropica; L. aethiopica; L. infantum; L. donovani (CDC, 2020a) New World: L. mexicana; L. amazonensis; L. venezuelensis; L. infantum (syn. L. chagasi) |
Old World: Phlebotomus alexandri; Ph. arabicus; Ph. argentipes; Ph. longipes; Ph. martini; Ph. orientalis; Ph. papatasia; Ph. pedifer; Ph. sergentia New World: Lutzomyia anglesi; Lu. longipalpis; Lu. flaviscutella; Lu. nuneztovari; Lu. ovallesi; Lutzomyia group Olmeca |
Cutaneous infections are common in Afghanistan, Brazil, Iran, Peru, Saudi Arabia and Syria (Rahman et al., 2014); 90% of cutaneous leishmaniasis cases occur in Afghanistan, Brazil, Iran, Peru, Saudi Arabia and Syria (Soong, 2009). |
| Visceral (usually affects internal organs; also called kala-azar) | Fever, swelling of the liver and spleen, and anaemia. Fatality rate of 100% if not treated within two years (WHO, 2013a). | Old World: L. donovani; L. infantum New World: L. infantum (syn. L. chagasi) (CDC, 2020a) |
Old World: P. ariasi; P. argentipes; P. orientalis; P. perniciosus; Lu. cruzi; Lu. evansi; Lu. longipalpis | About 90% of cases occur in Bangladesh, Brazil, India, Nepal and Sudan (Thornton et al., 2010). Often transmitted in a peridomestic cycle in both the Old World (Bern et al., 2010; Rijal et al., 2019) and the New World (Sousa-Paula et al., 2020). |
| Mucocutaneous (Leishmania parasites may spread from the skin and cause sores in the mucous membranes of the nose). | Infection starts as a reaction at the bitten site and spreads into the mucous membrane; usually life-threatening. | L. infantum (syn. L. chagasi); L. braziliensis; L. (Viannia) panamensis; L. (V.) guyanensis; L. (Leishmania) amazonensis (CDC, 2020a) | Lu. wellcomei; Lu. carrerai; Lu. complexa; Lu. fischeri; Lu. gomezi; Lu. neivai; Lu. nuneztovari anglesi; Lu. ovallesi; Lu. panamensis; Lu. shawi; Lu. spinicrassa; Lu. whitmani; Lu. ylephiletor; Lu. yucumensis | About 90% of cases occur in Bolivia, Brazil and Peru (Casalle et al., 2020). It is almost always transmitted in a sylvatic transmission cycle. |
Note: Bold typeface indicates involvement in anthroponotic and peridomestic transmission.
Important in Syria.
Globally, leishmaniasis has more than doubled in prevalence, from 1,934,553 cases in 1990 to 4,166,621 in 2017; 0.9–1.6 million new cases occur each year (GBD 2015 DALYs & HALE Collaborators, 2016). The recent influx of refugees may have contributed to the rise. Leishmaniasis is among the top ten neglected tropical diseases in the world and the second most common vector-borne parasitic disease infecting individuals worldwide after malaria and is the third most common cause of morbidity after malaria and schistosomiasis in terms of disability-adjusted life years (DALYs) (GBD 2015 DALYs & HALE Collaborators, 2016). It is responsible for 20,000–30,000 deaths annually, and 350 million people at risk of infection, mostly in impoverished rural areas (Alvar et al., 2012; Mansueto et al., 2014; PAHO, 2017; Hotez, 2018). Leishmaniasis has a huge impact on affected countries, challenging public health services (Bacon et al., 2013), exacerbating poverty and decreasing worker productivity (Hotez et al., 2012). A large psychological burden results from disfiguration by skin lesions, and treatment is long and expensive (Sunyoto et al., 2019). In Nepal, the economic burden of leishmaniasis, including both direct and indirect costs, has been estimated at 11% of annual household income (Uranw et al., 2013).
1.2.1. Leishmaniasis vector biology
Phlebotomine sand flies are hematophagous insects and the natural vectors of leishmaniasis, Bartonella bacteria, sand-fly fever, and other bacterial and viral phlebovirus diseases (Maroli et al., 2013; Pons et al., 2016; Cecilio et al., 2022). About 50 species of Phlebotomus have been identified as potential leishmaniasis vectors (Kasap et al., 2013; Maroli et al., 2013; Medlock et al., 2014; Ayhan and Charrel, 2017). The vector lives in a variety of environments, from South American jungles to Middle Eastern deserts and on the Indian subcontinent. Sand flies are primarily active during dawn and dusk (CDC, 2020c; Durrani et al., 2012). They are primarily outdoor biters and very small, about one-fourth the size of mosquitoes (CDC, 2020a). They make no noise and have relatively painless bites (CDC, 2020c); thus, it is possible to be bitten by them unknowingly. Means for controlling the disease are situation-dependent (Balaska et al., 2021). Transmission can be classified broadly as sylvatic, for which personal bite protection tools are essential, and peridomestic (in and around human habitation), for which insecticides may be appropriate for control. Interventions include indoor residual spraying and the use of insecticide-treated nets for endophilic and anthroponotic sand flies (WHO, 2022d), and the use of deltamethrin-treated dog collars such as in Brazil to prevent zoonic transmission (Silva et al., 2019; Alves et al., 2020).
1.3. Malaria
Malaria is a parasitic disease caused by the coccidian protozoan of the genus Plasmodium and transmitted to humans by the bite of an infected female mosquito of the genus Anopheles, with about 70 species transmitting the disease (Sinka et al., 2012). Plasmodium falciparum is primarily responsible for severe illness in humans; Plasmodium vivax and, to a lesser degree, Plasmodium malariae and Plasmodium ovale also cause disease (Bruce-Chwatt, 1984). Parasite development is weather-dependent. Temperature is particularly critical; below 20°C (68 °F), P. falciparum cannot complete its growth cycle in the Anopheles mosquito and thus cannot be transmitted (CDC, 2020b).
Therefore, autochthonous malaria is most prevalent in the tropical regions causing high morbidity and mortality (GBD 2015 DALYs & HALE Collaborators, 2016). Nearly half the world’s population lives in areas at risk of malaria transmission, in 87 countries and territories. Approximately 249 million clinical episodes and 608,000 deaths were attributed to malaria in 2022, i.e. 8 million deaths increase over 2021 (WHO, 2023a) Most malaria transmission (95%) and deaths (95%) occur in the WHO African Region, with four African countries accounting for just over half of all malaria deaths worldwide (WHO, 2021a). Malaria exerts a high economic and social burden on endemic countries (Sachs and Malaney, 2002); even after substantial control, a 10% reduction in malaria case incidence is associated with a 1.8% increase in gross domestic product per capita (Sarma et al., 2019). The greatest impact on households is lost productivity (Devine et al., 2019).
Malaria in sub-Saharan Africa is mainly controlled using insecticide-treated nets (ITNs) and indoor residual spray (IRS) (WHO, 2021a) because of the indoor biting and resting behaviour of Afrotropical malaria vectors (Sinka et al., 2010a). Personal protection using topical repellents and insecticide-treated clothing (ITC) is recommended for bite prevention where outdoor biting and resting occur (Sinka et al., 2010b, 2011), although there is insufficient epidemiological evidence to recommend these interventions for public health (Maia et al., 2018a).
1.3.1. Malaria vector biology
The habitat for the aquatic stage in the life-cycle of Anopheles mosquitoes varies, ranging from small puddles to the edges of large permanent water bodies, depending on the species. Many species have adapted to breed in man-made habitats such as rice fields or wells (Sinka et al., 2010a, 2011). In sub-Saharan Africa, four species of mosquitoes (Anopheles gambiae (s.s.), Anopheles arabiensis, Anopheles funestus and Anopheles coluzzi) mediate 95% of global malaria (WHO, 2021a). These species have co-evolved with humans to be synanthropic (they tend to be found biting in and around human homes and feed almost exclusively on humans) (Sinka et al., 2010a). Most malaria vectors bite throughout the night, with some peaks past midnight (Dambach et al., 2018; Bedasso et al., 2022). Malaria in Central and South America is dominated by Anopheles albimanus, found mainly along the Atlantic and Pacific coasts of Central America, Venezuela, Colombia, Ecuador, Peru and the Caribbean, and by Anopheles pseudopunctipennis, which is also found in the Andes in Bolivia, at higher altitudes than other malaria vectors (Sinka et al., 2010b). Anopheles aquasalis is found in brackish coastal habitats throughout Central America and the Caribbean and in South America down to Ecuador on the Pacific coast and northern Argentina on the Atlantic coast. Malaria throughout the Amazon is mediated by Anopheles darlingi in the forest, as well as by Anopheles nuneztovari (s.s) in the north and the Anopheles albitarsis complex in disturbed forest or agricultural areas (Sinka et al., 2010b). These vectors tend to bite and rest outdoors and have varying degrees of anthropophagy depending on the ecology of the location, although An. darlingi is the most anthropophagic and tends to bite throughout the night with a peak biting activity in the evening (Zimmerman et al., 2013).
In Southeast Asia, there is a similar focus of forest malaria in the Mekong sub-region, mediated by Anpopheles dirus, Anopheles minimus and Anopheles maculatus complexes, with other species dominating in rice fields, such as Anopheles sinensis and Anopheles culicifacies (Sinka et al., 2011). These vectors bite humans and other animals (Sinka et al., 2011). In the urban settings of India, the Middle East and the Horn of Africa countries, Anopheles stephensi breeding mainly in contained waters, is an important vector of malaria. Anopheles fluviatilis is a major malaria vector in hill forests in India. In Papua New Guinea and Australasia, members of the Anopheles punctulatus complex mediate malaria. These species, especially Anopheles punctulatus, Anopheles koliensis and Anopheles farauti, are generally synanthropic and preferentially feed on humans, although they will feed on other hosts (Sinka et al., 2011). These mosquitoes bite through the night with a peak in the evening.
The aim of this review is to identify the different occupational risks of vector-borne disease propose appropriate intervention for populations at higher risk of exposure to vectors of dengue, leishmaniasis and malaria; identify knowledge gaps; and establish an evidence base for best-practice guidance.
2. Materials and methods
The methods for this systematic review were developed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher et al., 2009) (also see PRISMA checklist in Supplementary Table S1). The participants, interventions, comparators and outcomes (PICO) of the study are based on the Joanna Briggs Institute (JBI) protocol mnemonic “condition context, population” (CoCoPop) used in investigating the prevalence and incidence for systematic reviews (Munn et al., 2015). This review investigates vector-borne infections of dengue, leishmaniasis and malaria (condition) among outdoor workers, travellers and soldiers (population) who may be daily exposed to vector bites while fulfilling their day-to-day obligations in infection-endemic regions (context).
2.1. Definitions of outdoor occupations included in the review
2.1.1. Forest workers
Forest workers are those who conduct their work or other activities in and around forests. This category includes forest rangers, researchers, hunters, farmers and loggers who may enter and leave the forest daily or stay in it for some time. Because of the relatively low economic importance of forest work and its impact being largely on local populations, this occupation receives relatively little public health attention. Most forest workers are men aged 15–40 years (Davis et al., 2023), but may also include women (Östlund et al., 2020) and children.
2.1.2. Migrants
Migrants are persons who temporarily relocate to and live outside of their country or area of origin for economic reasons. Migrants are frequently involved in crop planting and harvesting, casual labour, construction and unskilled manual labour. They are primarily men, and often aged 15–40 years. Migrant workers may live in poor-quality accommodation, or their occupation may increase their exposure to disease vectors. Mobile migrant workers often lack immunity to locally circulating pathogens and may disseminate disease to new areas when they go home or to a different work location.
2.1.3. Deployed troops
Deployed troops are highly vulnerable to outdoor disease vectors because they are often deployed from another area and consequently lack immunity to locally circulating pathogens and spend much or all of their time outdoors. They include soldiers in active combat or training, security guards and sentries.
2.1.4. Refugees
A refugee is a person who “owing to a well-founded fear of being persecuted for reasons of race, religion, tribe, nationality, membership of a particular social group or political opinion, is outside the country of his nationality, and is unable to, or owing to such fear, is unwilling to avail himself of the protection of that country” (United Nations, 1989). There were about 27.1 million refugees and 53.2 million internally displaced people as of the end of 2022 (UNHCR, 2022a). Most refugees reside in the Middle East, South Asia or Africa. Two-thirds worldwide originate from the Syrian Arab Republic, Venezuela, Afghanistan, South Sudan and Myanmar, and 3 million Ethiopians are currently internally displaced (UNHCR, 2023). Not surprisingly, the overwhelming majority of refugees are hosted in neighbouring countries: Turkey, Lebanon and Jordan for Syrian refugees; Colombia for Venezuelans; Pakistan and the Islamic Republic of Iran for Afghans; Bangladesh and Malaysia for refugees from Myanmar; and Sudan, Ethiopia and Kenya for South Sudanese refugees (Devictor and Do, 2016; UNHCR, 2022a). Refugees and other displaced people are often more vulnerable to vector-borne disease because of poverty, poor living conditions and low immunity (Duffy et al., 1990; Boussery et al., 2001). The return of refugees or other displaced people to their homes also contributes to the spread of vector-borne disease (Sutherst, 2004; Abdul-Ghani et al., 2019).
2.1.5. Miners
Miners are people involved in the mining of metals and minerals such as gold, diamond and coal. Mining is commonly associated with vector bite exposure. Mining can be legal or unlawful, and its environmental disruption often increases the availability of breeding sites for vectors (Jones et al., 2018; Lowe, 2018). Miners comprise mobile populations of young adult men who often lack pre-existing immunity to locally circulating diseases. Miners may engage in shift work or open mining that exposes them to vector bites. They rarely come into contact with Aedes mosquitoes, which are more common in urban settings. Health problems occur because much informal mining activity is illegal and occurs in isolated areas without access to health services (Shanks and Wongsrichanalai, 2021). For miners, the risk of malaria is persistent, and the disease accompanies them as they move from one job site to another across many geographical areas, causing re-introduction of the disease (Cohen et al., 2012). Unfortunately, the problem is unresolved because health care systems struggle to reach miners working illegally in remote areas (Douine et al., 2017; Martins-Filho et al., 2023).
2.1.6. Tourists
It is predicted that by 2030 approximately 2 billion tourist trips to foreign countries will be taken worldwide each year (Paquet et al., 2022). Tourists are persons who travel for leisure or other purposes and stay in a country for 24 hours to 12 months. Travellers are vulnerable to vector bites and illnesses from vector-transmitted infections because most do not have immunity to diseases circulating in the host country. It has been estimated that 1.6 billion people travelled in 2020, with most of the trips in the tropics. Such a massive movement of people facilitates the spread of new and emerging infectious diseases (Odolini et al., 2012). The type of traveller may determine immediate or eventual infection outcome. Travellers who adopt local living standards can face a higher risk of endemic infection; these include individuals visiting friends and relatives and long-term expatriates (e.g. missionaries and business travellers). Others at higher risk are travellers staying in budget accommodation (e.g. local homes or hotels without screened windows) or camping. All long-term travellers have an inherently increased risk of infections because of prolonged exposure (Wu, 2019).
2.2. Article selection and inclusion criteria
Articles were selected by title. Abstracts of eligible papers were screened for inclusion and exclusion criteria (Table 2), and those meeting the criteria went on to full-text article review by one reviewer (DM). The review considered seasonal variance for at least one year because vector abundance and virus transmission vary seasonally. Dengue and malaria vectors are more prevalent during the rainy season (WHO, 2019a; Zheng et al., 2020), whereas sand flies are often abundant during the driest and/or coldest seasons of the year (Armed Forces Pest Management Board, 2015). Studies completed in less than a year may over- or underestimate infection in the region depending on season and vector abundance. If all criteria were met, data were extracted. EndNote reference manager software was used to store selected articles and remove duplicates.
Table 2.
Inclusion and exclusion criteria used the CoCoPop criteria for extraction of relevant articles.
| Category | Inclusion | Exclusion |
|---|---|---|
| Condition | Studies conducted in malaria-, dengue- and leishmaniasis-endemic regions, during disease outbreaks in non-endemic areas or in travellers returning from disease-endemic countries | Abstracts only, letters, commentaries, reports, and reviews |
| Context | Malaria, dengue and leishmaniasis acquired in endemic countries | Laboratory-acquired infection |
| Population | Military personnel, miners, forest workers, security personnel, open miners, gold miners in illegal operations, agriculture workers in forest-expanded areas, hunters, rubber tappers, humanitarian workers, outdoor recreation workers; religious gathering attendees; tourists returning from disease-endemic countries | Occupations/activities that do not expose individuals to outdoor biting vectors during the day or night |
| Persons aged 18 years and above | Participants below 18 years of age | |
| Participants spending substantial time outdoors (exposed to vector by activities and occupations) | Laboratory- and other experimental environment-acquired disease | |
| Diagnosis | Laboratory-confirmed diagnosis | Presumptive diagnosis |
| Study design | Conducted between 1945 and 2020 | Conducted before 1945 |
| Observational | Ecological, qualitative | |
| Non-randomized controlled trials | Case series containing < 10 patients |
The CoCoPop was used to define the inclusion criteria (Table 2) of the articles in this review. For a study to be included in the review, it had to meet all five criteria, i.e. condition, context, population, exposure type and study design.
2.3. Conditions and outcomes
Articles about the prevalence and incidence of malaria, dengue and leishmaniasis infections among risk groups with infection confirmed by laboratory tests (Table 3) were included in the review. The outcomes of interest were the incidence or prevalence of dengue, leishmaniasis or malaria infection or co-infection. We extracted estimates of dengue leishmaniasis and malaria prevalence and/or incidence according to how they were presented in the included studies. Prevalence was presented as a proportion or percentage, whereas incidence was presented in proportions and rates. Where prevalence was not directly indicated, percentage was computed as (n/N) × 100, where n is the number of individuals testing positive and N is the total number of individuals examined.
Table 3.
Confirmatory laboratory tests for diagnosis of dengue, leishmaniasis and malaria.
| Test type |
Dengue | Leishmaniasis | Malaria | |
|---|---|---|---|---|
| General test category | Specific tests | |||
| Virus detection/isolation | Vero and LLC-MK2 | ✔️ | ||
| Genetic probe assays | Biotinylated probes | ✔️ | ||
| DNA hybridisation | ✔️ | |||
| rRNA probes | ✔️ | |||
| RNA detection | Nucleic acid amplification test | ✔️ | ✔️ | |
| Nested PCR techniques | ✔️ | ✔️ | ||
| Antigen detection | NS1-based assays | ✔️ | ||
| Immunohistochemistry | ✔️ | |||
| Indirect fluorescence antibody test (IFAT) | ✔️ | |||
| Ks30 dipstick test | ✔️ | |||
| Serological tests | Direct agglutination test | ✔️ | ||
| ELISA | ✔️ | ✔️ | ✔️ | |
| MAC-ELISA | ✔️ | |||
| Protein G ELISA | ✔️ | |||
| IgM-based assays | ✔️ | ✔️ | ✔️ | |
| IgG-based assays | ✔️ | ✔️ | ✔️ | |
| IgA-based assays | ✔️ | ✔️ | ||
| IgE-based assays | ✔️ | |||
| Protein A (ProtA) | ✔️ | |||
| Hemagglutination inhibition | ✔️ | |||
| Immunofluorescence antibody testing | ✔️ | ✔️ | ||
| Plasmodium lactate dehydrogenase (pLDH) | ✔️ | |||
| ELISA IgG2 and IgG1 | ✔️ | |||
| Lymphocyte proliferation assay | ✔️ | |||
| LAMP | ✔️ | |||
| Neutralisation test | ||||
| Complement fixation | ||||
| Molecular methods | PCR | ✔️ | ||
| qPCR | ✔️ | |||
| qPCR-BM (bone marrow) | ✔️ | |||
| qPCR-blood | ✔️ | |||
| Microarrays | ✔️ | |||
| Flow cytometry assay | ✔️ | |||
| Automated blood cell counters | ✔️ | |||
| Mass spectrophotometry | ✔️ | |||
| Plasmodium-specific phospholipases A2 | ✔️ | |||
| Microscopy | Giemsa-stained blood film | ✔️ | ✔️ | |
| Fluorescent microscopy | ||||
| Rapid methods: Malaria antigen detection | Malaria rapid diagnostic test | ✔️ | ||
| Highly sensitive rapid diagnostic tests | ✔️ | |||
| ParaHIT | ✔️ | |||
| Paracheck | ✔️ | |||
| Quantitative buffy coat method | Becton Dickinson | ✔️ | ||
| LAMP | ✔️ | |||
| Immuno-chromatographic technique | ✔️ | ✔️ | ||
| ParaScreen | ✔️ | |||
| SD Bioline | ✔️ | |||
| Post-mortem diagnosis of malaria | Histopathology | ✔️ | ||
| Diagnosis of malaria in pregnancy | Placental histology | ✔️ | ||
| Complete blood count and chemistry profile | ✔️ | |||
| Plasmodium lactate dehydrogenase assay | Immunochromatographic dipstick assay | ✔️ | ||
| Ks30 dipstick test | ✔️ | |||
| Skin testing | Montenegro; delayed-type hypersensitivity | ✔️ | ||
Abbreviations: ELISA, enzyme-linked immunosorbent assay; IgG, immunoglobulin G; LAMP, loop-mediated isothermal amplification; MAC-ELISA, IgM antibody capture; PCR, polymerase chain reaction; qPCR, quantitative PCR.
2.4. Context, population and type of exposure
Articles included reported leishmaniasis, dengue and malaria infection or diseases among individuals who work outdoors or other risk groups in endemic countries. Articles that reported reintroduction of these infections in countries once considered to have achieved elimination were also considered.
This systematic review was restricted to people with occupational or other high risk of exposure in regions endemic for malaria, dengue and leishmaniasis and people who were infected in an endemic region and developed clinical signs in a non-endemic country. This population includes refugees, soldiers in combat or training, forest officers and loggers, game officers, rice farmers, people with night-shift duties such as security personnel or nurses, rubber tappers, fishers and miners. The participants’ age range, occupations or behaviour that exposes them to vector bites was recorded.
The review considered only exposure to bites of Aedes spp., Anopheles spp. and sand flies in natural environments, excluding laboratory exposures and mother-to-child transmission.
2.5. Study design: Risk of bias
Investigator DM used criteria developed by Loney et al. (1998) modified to assess each article for risk of bias (Supplementary Table S2). For each of nine equally weighted questions, a “yes” response was worth one point, while any other reply (“no,” “unclear” or “not applicable”) scored zero. A score of 8–9 was categorised as low risk of bias and a high-quality article; a score of 6–7 was categorised as moderate risk and medium quality; and a score of 5 or below was categorised as high risk and low quality. Articles with fewer than 5 points were excluded.
2.6. Literature search
The review focused on articles published between 1945 and 2020 (Supplementary Table S3). No language restrictions were applied. Reference lists of all identified studies and review articles from relevant references not identified by the electronic search were also explored. Experts in the field were contacted for information about ongoing or unpublished studies.
Electronic databases searched were BIOSIS, Cochrane Central, Elsevier, Embase, Global Health, Google Scholar, JSTOR, LILACS, Medicine Plus, MEDLINE, OpenGrey, Oxford University Research Archive (ORA), PubMed, the U.S. National Library of Medicine, Web of Science, and WHO Search.
The search terms used were (malaria OR dengue OR leishmaniasis) AND (soldiers OR refugees OR tourists OR rubber tappers OR loggers) (Supplementary Table S3). To include articles that reported infection from non-endemic countries, the search strategy was not limited to countries or regions of endemicity of infection of interest. The strategy succeeded in clearly identifying eligible studies (Wangroongsar et al., 2016; Douine et al., 2019; Arisco et al., 2021) from nine databases (Supplementary Table S4; the table indicates when each database was searched). Colleagues helped obtain 19 additional articles for the review.
2.7. Data extraction and effect measures
A custom Microsoft Excel spreadsheet was used to extract information from articles. In accordance with recommendations by Munn et al. (2020), the following data were extracted: condition, context (country of endemicity), population, study type, study duration, study year, incidence, prevalence, sample size (N), age range, diagnostic tool(s) and bibliographic reference. The data collection form was assessed and tested before data extraction began. Two reviewers worked independently. One extracted the data; the other checked and verified the collected data. When full articles were not available for data extraction, the author/investigator was contacted. Google Translator was used for articles in French, Portuguese and Turkish; no other non-English articles were obtained.
Effect measures extracted were incidence and prevalence. Incidence was presented as incidence rate or incidence rate ratio; prevalence was presented as prevalence proportions or obtained from presented odds ratios. No other effect measures were extracted.
2.8. Data synthesis
The review focused on assessing articles that presented prevalence and/or incidence of dengue, leishmaniasis and malaria infections. Data synthesis was of descriptive characteristics of the study and participants. Information collected was assessed based on the risk of disease to people whose occupations require them to spend most of their working hours outdoors in endemic regions of malaria, leishmaniasis and dengue.
3. Results
3.1. Study selection
The flow diagram (Fig. 1) presents the systematic approach used to select relevant articles throughout the research process, from the initial search, through various screening stages, to the final inclusion of 99 articles meeting the criteria for the study.
Fig. 1.
PRISMA flow diagram.
3.2. Populations presented
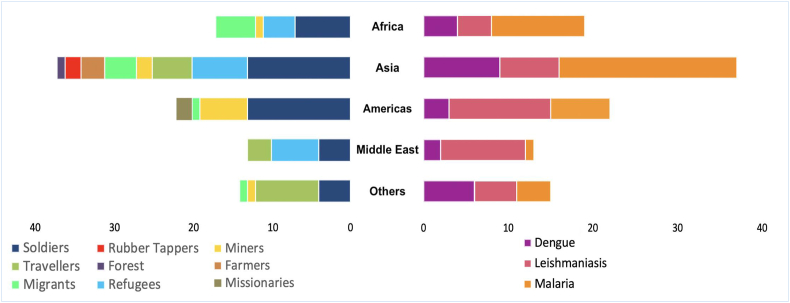
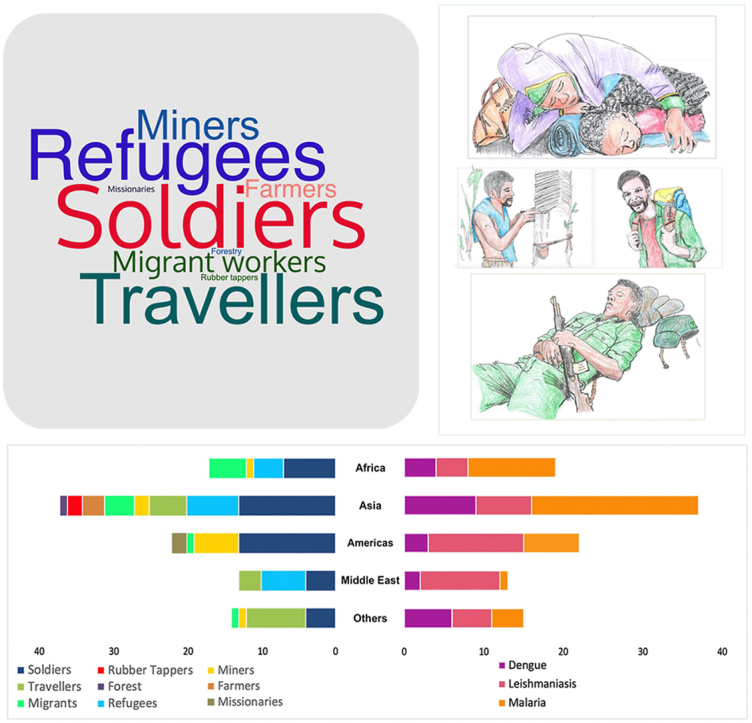
The 99 articles obtained (Fig. 1) describing 112 conditions are summarised in Table 4. Some articles presented more than one condition; other articles presented more than one population. The military population was the most represented, at 38% of conditions (43/112), followed by refugees, 15% (17/112); travellers, 15% (17/112); migrant workers, 12.5% (14/112); miners, 9% (10/112); farmers. 5% (6/112); rubber tappers and missionaries, 1.8% each (2/112); and forestry, with only 1 article. The word cloud in Fig. 2 presents the populations proportionally.
Table 4.
Characteristics of included studies.
| Condition/Context | Population | Study type | Study duration | Study years | Incidence | Prevalence | N | Age range (years) | Diagnostic tools | Appraisal score | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dengue | |||||||||||
| Africa | Soldiers | Prospective study | 24 m | 2011 | 330/25,458 | 25,458 | 19–56 | ELISA, RT-PCR | 7 | de Laval et al. (2013) | |
| Africa | Refugees | Active surveillance | 3 m | 2019 | 24% dengue fever; 54% dengue with warning signs; 22% severe dengue | – | 1–81 | RT-PCR | 5 | Ahmed et al. (2021) | |
| Africa | Refugees | – | 3 y | 1997–2006 | 15/38 | 38 | – | ELISA | 5 | Botros et al. (1989) | |
| Africa | Soldiers | Retrospective study | 36 m | 2008–2011 | 17.6/10,000 | 1000 | 19–56 | Serum, ELISA, microneutralisation assay | 7 | Hesse et al. (2017) | |
| Africa | Soldiers | Prospective study | 12 m | 1992–1993 | 43% virus isolation; 35% IgM-reactive | 129 | 19–25 | Serum | 6 | Sharp et al. (1995) | |
| Americas | Soldiers | Retrospective study | 36 m | 2008–2011 | 17.6/10,000 | 1000 | 19–56 | Serum, ELISA, microneutralisation assay | 7 | Hesse et al. (2017) | |
| Americas | Soldiers | Consecutive sample | 2 m | 1994 | 30/406 | 406 | – | ELISA, virus isolation | 6 | Trofa et al. (1997) | |
| Americas | Missionaries | Incidence | <1 m | 2010 | 25% | 28 | 16–69 | RT-PCR, IgM, MAC-ELISA | 5 | CDC (2011) | |
| Americas | Missionaries | 0 m | 2019 | 25% | 28 | 11–69 | rRT-PCR, MAC-ELISA | 5 | Sharp et al. (2012) | ||
| Asia | Travellers | Retrospective study | 48 m | 2001 | 5.6% | 696 | 15–54 | ELISA | 5 | Sung et al. (2003) | |
| Asia | Migrants | Screening | <1 m | 2003 | IgG: 80%; IgM: 0.5% | 600 | 20–39 | IgM/IgG | 6 | Perng et al. (2019) | |
| Middle East | Refugees | Incidence study | 12 m | 2016 | 27% | 436 | 1–70 | PCR | 5 | Alghazali et al. (2019) | |
| Southeast Asia | Soldiers | Outbreak study | 2 m | 1984 | 24/1000 | – | 20–43 | HI | 5 | Hayes et al. (1989) | |
| Southeast Asia | Soldiers | Prospective cohort | 5 m | 2015 | 6.60% | 585 | – | NT | 5 | Peragallo et al. (2003) | |
| Southeast Asia | Soldiers | Prospective cohort | 4 m | 2000 | 0.04% | 2500 | IgM & IgG | 7 | Kitchener et al. (2002) | ||
| Southeast Asia | Soldiers | Retrospective study | 36 m | 2008–2011 | 17.6/10,000 | 1000 | 19–56 | Serum, ELISA, microneutralisation assay | 7 | Hesse et al. (2017) | |
| Other | Soldiers | Prospective study | 24 m | 2011 | 330/25,458 | 25,458 | 19–56 | ELISA, RT-PCR | 7 | de Laval et al. (2013) | |
| Other | Travellers | Prospective study | 10 y | 2010 | 21/1000 | 24,920 | – | Serum, ELISA | 6 | Schwartz et al. (2008) | |
| Others | Travellers | Incidence | 36 m | 2005 | 0.34% | 63,000 | 11–70 | RT-PCR, IgG, IgM | 7 | Wichmann et al. (2007) | |
| Other | Travellers | Incidence | 70 m | 2010 | 10.1% | 594 | 40–49 | ELISA, IgG, IgM | 7 | Allwinn (2011) | |
| Other | Travellers | Retrospective study | 120 m | 2013 | 132 (21.5%) | 614 | 40–49 | NS1, RT-PCR | 7 | Trojanek et al. (2016) | |
| Southeast Asia | Travellers | Retrospective study | 0–16 m | 1999 | 107/292 | 292 | 19–60 | Indirect IFA | 6 | Lindback et al. (2003) | |
| Southeast Asia | Travellers | Retrospective study | 60 m | 2010 | Sri Lanka: 45.3/100,000; Bangladesh: 42.6/100,000; Thailand 13.6/100,000 | 925 | 0–76 | NS1, PCR, IgM | 7 | Rocklov et al. (2014) | |
| Southeast Asia | Travellers | Systematic records/retrospective study | 5 y | 2005 | 13/100,000 | 211 | – | Microscopy | 6 | Stienlauf et al. (2005) | |
| Southeast Asia | Migrant workers | Prospective study | 2 m | 2002 | 39/47 cases; 27/274 surveyed | 47 & 274 | – | ELISA | 5 | Seet et al. (2005) | |
| Southeast Asia | Travellers | Prospective cohort | 32 m | 2017 | 62% | 201 | 17–78 | RT-PCR, NS1 | 7 | Masyeni et al. (2018) | |
| Leishmaniasis | |||||||||||
| Africa | Farmers | Cross-sectional study | – | 2014 | 38% | 130 | – | ZCL lesion | 5 | Bellali et al. (2017) | |
| Africa | Migrants | Case-control studies | 36 m | 2011 | 39% | 376 | 14–38 | Positive DAT (Leishmania amastigotes) | 8 | Argaw et al. (2013) | |
| Africa | Migrants | Cross-sectional study | 3 m | 2016 | 9.6% | 178 | 1–29 | ICT | 8 | Aschale et al. (2019) | |
| Africa | Refugees | Outbreak study | – | – | 24.1% | 2714 | – | ELIZA, DAT | 5 | de Beer et al. (1991) | |
| Americas | Miners, migrants | Cross-sectional study | 9 m (wet season) | 2017/2018 | 73% | 168 | 16–75 | Smear, culture, PCR-RFLP | 7 | Loiseau et al. (2019) | |
| Americas | Soldiers | Retrospective study | 12 y | 1978 | 61% | 306 | – | Culture | 7 | Hepburn et al. (1993) | |
| Americas | Soldiers | Retrospective study | 2 y | 2011 | 1998: 29/990; 2004: 14/80 | 99 in 1998; 80 in 2004 | 18–48 | Serum, culture | 7 | van Thiel et al. (2011) | |
| Americas | Soldiers | Outbreak study | 6 m | 2020 | 3.5% (AR) | 858 | – | PCR | 7 | Henry et al. (2021) | |
| Americas | Soldiers | Retrospective study | 7 m | 2004 | 237/360 | 360 | – | Giemsa stain, PCR | 6 | Willard et al. (2005) | |
| Americas | Soldiers | Outbreak study, Incidence study | 6 m | Aug 2002–Jan 2003 | 16.9/100 (AR) | 71 | 19–37 | Microscopy, RDT | 6 | Berger et al. (2006) | |
| Americas | Soldiers | Outbreak study | 3 m | 1996 | Outbreak, 8 cases | 8 | – | Biopsy, smear, skin test | 5 | Silveira et al. (2002) | |
| Americas | Soldiers | Outbreak study | 1 m | 1986 | 77/303 (AR) | 303 | 18–23 | Microscopy | 7 | Ore et al. (2015) | |
| Americas | Soldiers | Not stated; suggests a prospective study | 12 m | 2002 | 25.3% | 352 | – | Montenegro skin test | 6 | Andrade et al. (2005) | |
| Americas | Soldiers | Incidence study | 3 m | 1995 | 89.6% | 48 | 19–20 | Giemsa stain, Montenegro intradermal reaction | 7 | de Oliveira Guerra et al. (2003) | |
| Americas | Soldiers | Prospective cohort | 6 y | 1981–1987 | 2.3/1000 | – | 15–56 | Scar | 7 | Dedet et al. (1989) | |
| Americas | Soldiers | Double-blind, placebo-controlled study | 3 m | 1995 | 2.8% active; 12.6% control | 86 | – | Microscopy, culture | 6 | Soto et al. (1995) | |
| Asia | Soldiers | Prospective cross-sectional survey | 12 m | 1985 | 50/5000 | 5000 | – | PCR, RDT, microscopy | 7 | Gunathilaka et al. (2020) | |
| Asia | Refugees | Cross-sectional study | 2 m | 1998 | 2.70% | 19,918 | – | Microscopy | 6 | Kolaczinski et al. (2004) | |
| Asia | Refugees | Cross-sectional study | 1 m | 1997 | 38% | 799 | 0–80 | Microscopy, culture, PCR | 7 | Rowland et al. (1999) | |
| Asia | Refugees | Cross-sectional, multicentric and observational study | – | 2015 | 8.3% | 421 | – | PCR | 6 | Douine et al. (2017) | |
| Asia | Travellers | Retrospective study | 168 m | 2016 | 60% (182) | 299 | 3–80 | Microscopy, skin smears | 8 | Sobirk et al. (2018) | |
| Middle East | Refugees | Retrospective study | 3.5 y | Jan 2011–Jun 2014 | 110 | 110 | 1–78 | Skin lesion | 6 | Inci et al. (2015) | |
| Middle East | Refugees | Retrospective study | 63 m | 2020 | 20 | – | 3–33 | Biopsy, PCR | 5 | Lindner et al. (2020) | |
| Middle East | Refugees | Retrospective study | 84 m | 2016 | 92.1% imported cases | 558 | 1–78 | PCR | 8 | Amr et al. (2018) | |
| Middle Easy | Refugees | Outbreak study | 1 m | 2012 | 74% of suspected cases | 1275 | – | Biopsy, PCR | 5 | Saroufim et al. (2014) | |
| Middle East | Refugees | Descriptive study | 6 y | 2010 & 2016 | 2.87/100,000 (IR) | – | Direct slit-skin smear | 5 | Kanani et al. (2019) | ||
| Middle East | Soldiers | Cross-sectional study | 1 m | 2013 | 13% | 225 | 19–63 | ELISA | 8 | Obwaller et al. (2018) | |
| Middle East | Soldiers | Retrospective study | 6 m | 2003 | 1.18% | 360 | – | PCR, biopsy, Giemsa-stained lesion smear | 5 | Willard et al. (2005) | |
| Middle East | Soldiers | Prospective cohort study | 2 y | 2015 | 19.5%; 64% bitten by sand fly | 200 | 24–61 | rK39, ELISA, IGRA, qPCR | 7 | Mody et al. (2019) | |
| Middle East | Soldiers | – | – | 2017 | 18% | 247 | – | ELISA, PCR, EDTA | 5 | Giladi et al. (1985) | |
| Middle East | Refugees | Incidence study | 3.5 y | 2010 | 18.5% | 416 | 0–60 | Giemsa-stained smear | 7 | Salman et al. (2014) | |
| Middle East | Travellers | Systematic records/retrospective study | 5 y | 2005 | 9% | 211 | – | Microscopy | 6 | Stienlauf et al. (2005) | |
| Other | Soldiers | Case series | 132 m | 2011 | 223 all cases | 223 | 2–86 | Giemsa stain, PCR | 8 | Wall et al. (2012) | |
| Other | Soldiers | Cross-sectional study | 1 m | 1985 | 13.30% | 225 | 19–63 | ELISA | 7 | Obwaller et al. (2018) | |
| Other | Travellers | Cross-sectional study | 120 m | 2008 | 64% | 286 | 11–77 | Smear biopsy, PCR | 6 | Solomon et al. (2011) | |
| Other | Travellers | Case series | 132 m | 2011 | 223 nested cases | 223 | 2–86 | Giemsa stain, PCR | 8 | Wall et al. (2012) | |
| Other | Travellers | Retrospective study | 96 m | 2003 | 79 | – | 19–30 | Microscopy, PCR | 5 | Lawn et al. (2004) | |
| Malaria | |||||||||||
| Africa | Farmers | Cross-sectional study | 1 m | 2020 | Microscopy: 14%; RDT: 17% | 1154 | 1–51+ | Microscopy | 7 | Mazigo et al. (2017) | |
| Africa | Migrants | Cross-sectional study | 3 m | 2019 | 55% | 773 | 27–52 | RDT, PCR | 7 | Martins et al. (2020) | |
| Africa | Migrants | Cross-sectional study | 3 m | 2016 | 22.4% | 178 | 15–65 | Microscopy | 8 | Aschale et al. (2019) | |
| Africa | Migrants | Cross-sectional study | 3 m | 2019 | 55% | 773 | 27–52 | RDT, PCR | 7 | Martins et al. (2020) | |
| Africa | Miners | Screening study | 4 m | 2014 | 216/1000 (AR) | 4053 | Microscopy, IFA | 6 | Li et al. (2015) | ||
| Africa | Soldiers | Retrospective study | 20 y | 2014 | 107/1000/year | 101 in 2005 | 17–48 | Biopsy | 7 | de Laval et al. (2014) | |
| Africa | Soldiers | Prospective study | 6 m | 1996 | 43% | 245 | 29–52 | – | 6 | Ennibi et al. (2012) | |
| Africa | Soldiers | Prospective cohort study | 6 m | 2015 | 1.8/10,000/week | 389 | – | RDT, microscopy | 6 | Wallace et al. (1996) | |
| Africa | Soldiers | Outbreak study | 6 m | 2013 | 18% (AR) | 439 | – | IFA | 5 | Sanchez et al. (2000) | |
| Africa | Soldiers | Incidence study | 6 m | 2002 | 71.3% | 72 | – | Smear or microscopy | 6 | Kawar et al. (2003) | |
| Africa | Migrants | Cross-sectional study | 0 m | 2013 | 12% overall | 592 | 18–65 | RDT | 7 | Schicker et al. (2015) | |
| Africa | Refugees | Community trial | 5 m | Jan 2001–Dec 2012 | OR 0.30 of treatment arm | 198 | – | PCR, microscopy | 6 | Kimani et al. (2006) | |
| Americas | Migrants | Case series | – | 2015 | 8/154 | 154 | – | Microscopy | 5 | Carreno-Almanzar et al. (2021) | |
| Americas | Migrants | Surveillance | 11 y | 2018 | 3% | – | < 5–65 > | Microscopy, RDT | 7 | Arisco et al. (2021) | |
| Americas | Miners | Passive surveillance | 12 y | 2016 | 15% | 203,773 | – | Microscopy | 7 | Sanchez et al. (2017) | |
| Americas | Miners | Case detection | 5 d | 2017 | 53.8% (AR) | 60% | 46 | – | RDT | 6 | Douine et al. (2019) |
| Americas | Miners | Surveillance | 11 y | 2018 | 3% | – | <5–65> | Microscopy, RDT | 7 | Arisco et al. (2021) | |
| Americas | Miners | Cross-sectional, multicentric, observational study | 6 m | 2020 | 22.3% (PCR prevalence) | 421 | – | NT | 6 | Douine et al. (2016) | |
| Americas | Miners | Cross-sectional study | 6 d | 2006 | 19% | 135 | 24–58 | ELISA, IgG | 6 | Silbergeld et al. (2002) | |
| Americas | Miners | Cross-sectional study | 1 m | 1996 | 48.3% | 205 | 20–63 | Blood testing/not mentioned | 7 | Pommier de Santi et al. (2016b) | |
| Americas | Soldiers | Double-blind, placebo-controlled study | 3 m | 1995 | 17% | 143 | – | Microscopy, PCR | 6 | Soto et al. (1995) | |
| Asia | Farmers | Cross-sectional study | 1 m | 2016 | 0.27% | 750 | 18–84 | Microscopy, RDT | 8 | Wangchuk et al. (2019) | |
| Asia | Farmers | Cross-sectional study | 2 m | 1996/1997 | 33.5% | 842 | – | Microscopy | 6 | Pluess et al. (2009) | |
| Asia | Migrants | Cross-sectional study | 5 m | 2016 | 3.8% | 309 | 27.70 ± 11.98 | RDT | 7 | George et al. (2019) | |
| Asia | Migrants | Cross-sectional study | 1 m | 2016 | 0.42% | 473 | 18–66 | Microscopy, RDT | 8 | Wangchuk et al. (2019) | |
| Asia | Refugees | Cross-sectional, multicentric, observational study | – | 2015 | 22.3% | 421 | – | RDT, PCR | 6 | Douine et al. (2017) | |
| Asia | Refugees | Cross-sectional malariometric surveys | 4 m | 2016 | Seroprevalence P. vivax (Jalala: 47.5%; Adezai: 17.6%); PCR P. vivax (Jalala: 15.6%; Kagan: 3.7%); P. falciparum (Jalala: 1.4%; Kagan: 0.8%) | 2522 | – | Blood sample | 6 | Wahid et al. (2016) | |
| Asia | Soldiers | Prospective cohort study | 12 m | 1993–2007 | 21/246 | 246 | – | Microscopy, PCR | 7 | Henderson et al. (1986) | |
| Asia | Soldiers | Prospective cohort study | 14 y | 2015 | Incidence 2.5/1000 in 1999 | – | – | Microscopy | 6 | Klein et al. (2009) | |
| Asia | Soldiers | Incidence study | 8 m | 2012 | 62% | – | 20–42 | Blood smear, RDT, PCR | 6 | Shaha et al. (2013) | |
| Asia | Soldiers | Case series | 4 m | 2002 | 52.4/1000 | 752 | 19–39 | Microscopy | 8 | Kotwal et al. (2005) | |
| Southeast Asia | Soldiers | Cross-sectional study | 1 m | 2017 | 11.2% | 313 | 24–40 | RDT, microscopy | 7 | Vilay et al. (2019) | |
| Southeast Asia | Soldiers | Prospective cohort study | 12 m | 1999 | 13% (AR); 5.2% (AR of redeployed) | – | – | Blood slide, PCR | 6 | Kitchener et al. (2003) | |
| Asia | Soldiers | Prospective cohort study | 8 m | 2015 | 82% | 11 | – | BinaxNOW® malaria kit, PCR | 5 | Klein et al. (2018) | |
| Asia | Soldiers in DMZ | Outbreak study | 12 m | 2010 | 70% | 3932 | 21–24 | Biopsy, PCR, microscopy, histology | 8 | Lee et al. (2002) | |
| Other | Migrants | Retrospective study | 240 m | 2015 | 89.3% | 3099 | 0–83 | Microscopy | 7 | Wangdahl et al. (2019) | |
| Other | Miners | Retrospective cohort study | 4 m | Sep 2010–Jan 2011 | Attack rate 26.5% | 272 | – | Blood smear, RDT, quantitative buffy coat | 5 | Pommier de Santi et al. (2016a) | |
| Other | Travellers | Retrospective study | 240 m | 2015 | 89.3% | 3099 | 0–83 | Microscopy | 7 | Wangdahl et al. (2019) | |
| Other | Travellers | Prospective cohort study | 5 y | 1985–1987 | Burma: 11.80/100,000; Korea: 0.25/10,000 | 2653 | – | Microscopy, RDT, PCR | 7 | Behrens et al. (2010) | |
| Other | Soldiers | Epidemiological review | 14 y | 1999 | 0.03/person/year (AR) | 213 | – | Microscopy, serum | 7 | Miller et al. (1999) | |
| Southeast Asia | Farmers | Cross-sectional study | 2 m | 2003 | 25% | 4306 | 16–60 | Microscopy | 7 | Erhart et al. (2005) | |
| Southeast Asia | Farmers | Prospective study | 12 m | 2005 | 780 cases; no denominator | 0.55–2.1% | 780 cases | – | Microscopy | 7 | Singhasivanon et al. (1999) |
| Southeast Asia | Forestry | Experimental study | 5 m | 2003–2008 | 479/1000 | 30/150 (∼20%) | 150 | – | Microscopy | 6 | Son et al. (2017) |
| Southeast Asia | Soldiers | Experimental non-randomised study | 5 m | 2017 | 62% | 118 | – | Microscopy, PCR | 5 | Brown et al. (1990) | |
| Southeast Asia | Rubber tappers | Incidence study | – | 2018 | 2.3% | 470 | – | RDT, ELISA, PCR | 6 | Jeffree et al. (2018) | |
| Southeast Asia | Rubber tappers | Incidence study | 7 m | 2000 | IRR 2.9 | 33 | _ | IgM or IgG by ELISA or isolation of dengue virus | 6 | Pattanasin et al. (2012) | |
| Southeast Asia | Refugees | Incidence study | 3 y | 1985 | 1983 (359/1000); 1984 (350/1000); 1985 (116/1000) | – | < 5–44 | Blood smear | 7 | Meek (1988) | |
| Middle East | Travellers | Systematic records/retrospective study | 5 y | 2005 | 26% | 211 | – | Microscopy | 6 | Stienlauf et al. (2005) | |
Abbreviations: Study duration: d, days; m, months; y, years. Diagnostic tools: AR, attack rate; ARR, attack rate ratio; DAT, direct agglutination test; EDTA, ethylenediaminetetraacetic acid; ELISA, enzyme-linked immunosorbent assay; HI, hemagglutination inhibition; ICT, immuno-chromatographic technique; IFA, immunofluorescence antibody testing; IR, incidence rate; IRR, incident rater ratio; IgG, immunoglobulin G; IgM, immunoglobulin M; IGRA, interferon-gamma release assay; MAC-ELISA, IgM antibody capture ELISA; NS1, non-structural protein 1; NT, neutralisation test; OR, odds ratio; PCR, polymerase chain reaction; qPCR, quantitative PCR; RDT, rapid diagnostic test; RFLP, restriction fragment length polymorphism; rRT-PCR, real-time reverse transcription PCR; RT-PCR, reverse transcription PCR; ZCL, zoonotic cutaneous leishmaniasis.
Fig. 2.
Word cloud of frequency of reported occupation or other risk groups (WordClouds.com).
3.3. Continent of endemicity
By continent of endemicity, Asia had the largest share, at 38% of 112 studies (from 99 articles), followed by the Americas (Central and South), with 21%, Africa, with only 18% and Middle East with 16%. “Others” in Fig. 3 represents studies involving numerous centres or compiling and consolidating data on cases from multiple locations.
Fig. 3.
Distribution of articles by continent of endemicity, representing the proportion of studies by population (left) and condition (right).
3.4. Study quality
The risk of bias was assessed using the information in Supplementary Table S2. Only 14 (14%) of the 99 articles obtained earned 8 “yes” responses, showing a very low probability of bias.
Biases in the studies included: (i) sample size: 11% of studies did not report the sample size; (ii) diagnosis: 1% of studies failed to report the diagnostic tools used; (iii) age of participants: 38% of studies failed to report participants’ ages; and (iv) study duration: 14% of studies had short study durations of less than one month; 23% had durations of more than one month but less than six months; and 12% had durations of more than six months but less than a year. Overall, 44% of the articles had the target duration of more than a year. Only five studies provided no information on the study duration. About 38% of the articles reviewed were from Asia.
3.5. Reporting biases
Malaria was most reported, and, unsurprisingly, the two neglected tropical diseases (NTDs) (leishmaniasis and dengue) were reported at a lower frequency. There were twice the number of articles about soldiers relative to other occupations (Fig. 3).
3.6. Disease and risk groups
3.6.1. Refugees
Although refugees were underrepresented in the literature, they make up a large risk group and often suffer high mortality from vector-borne diseases. Civil war and internal displacement of people in endemic countries contribute significantly to the spread of leishmaniasis. In Syria, a 10-fold increase to 270,000 cases of leishmaniasis occurred in 2016 (Hotez, 2018). The displacement of over 4.2 million Syrians into neighbouring Turkey, Lebanon and Jordan led to outbreaks of leishmaniasis where refugees and sand flies coexist (Koltas et al., 2014; Alhawarat et al., 2020; Ozbel et al., 2022). In Latin America, similarly, the incidence of leishmaniasis is far higher among internally displaced people (Villamizar-Pena et al., 2021). Armed conflict enables outbreaks of serious NTDs (Jacobson, 2011; Du et al., 2016) through a combination of factors, most notably, collapsed health-care infrastructure and population displacement. As populations migrate to endemic and non-endemic regions, they are exposed to infections for the first time or introduce diseases into new areas, respectively (Du et al., 2016).
Malaria is most commonly transmitted outdoors in forested tropical regions of South America (MacDonald and Mordecai, 2019) and Southeast Asia (Sandfort et al., 2020) and among refugee populations in sub-Saharan Africa (WHO, 2013b). One-third of global refugees are located in sub-Saharan Africa. At the same time, 90% of worldwide malaria-associated deaths occur in sub-Saharan Africa, about one-third taking place in complex humanitarian emergency settings; 18.7% of refugee deaths in 2021 were caused by malaria (UNHCR, 2022b). A major aspect of emergency settings is large-scale population displacement, often with a high proportion of children and women of childbearing age, who are most at risk of malaria death (Amodu et al., 2020; Salami et al., 2020).
Refugees were noted to have dengue outbreaks in camps in areas with frequent dengue virus circulation, including among Rohingya refugees in Bangladesh (WHO, 2022a). Because of the destabilisation of public health in Yemen by war, dengue outbreaks have also been recorded (Alghazali et al., 2019). Alarmingly, as dengue becomes more common in Africa (Brady and Hay, 2020), outbreaks have been recorded among refugees in Sudan (Ahmed et al., 2021) and Somalia (Botros et al., 1989).
Refugees can also introduce infections to the host population, as recently occurred in Turkey with displaced Syrian populations (Salman et al., 2014; Inci et al., 2015). Refugees who arrive with an infection can also transmit the infection to non-infected refugees, resulting in outbreaks (Abdul-Ghani et al., 2019; Ahmed et al., 2021). Large-scale epidemics can arise if the refugees come from a non-immune background, and vector-borne illness can become endemic if competent vector populations are present in the region where refugees are housed. The current conflict in Syria has led to a 485% increase in vector-borne diseases, including leishmaniasis and malaria, in Syria and its neighbouring countries (Tarnas et al., 2021). The arrival of Afghan refugees in Pakistan resulted in an increase in the global malaria burden (Rowland et al., 2002). When Somali refugees arrived in Oman, a malaria epidemic occurred in a previously malaria-free area (Baomar and Mohamed, 2000).
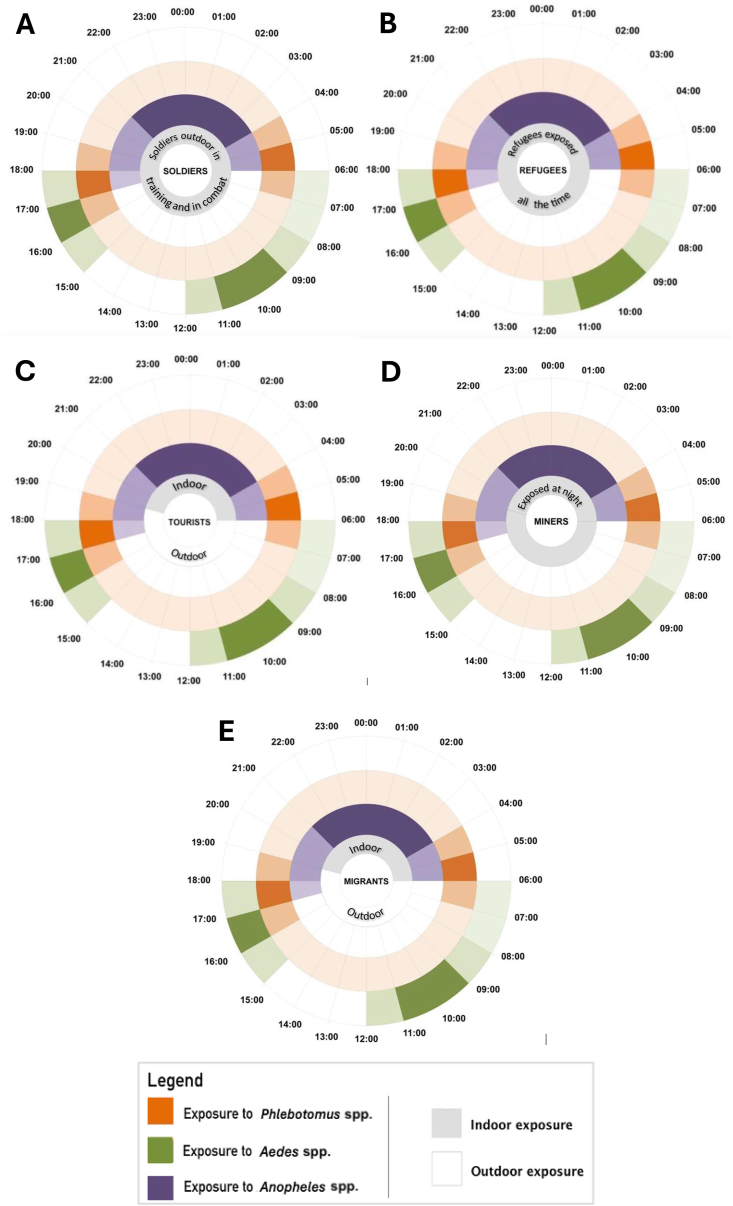
Refugees’ exposure is different when on the move and when they have reached a camp. Those in transit tend to sleep anywhere, keep moving during the day and night, and thus have round-the-clock exposure (Fig. 4). Those in camps have similar exposure to vectors as the host population, which may be during the evening or the night depending on local vectors, although transmission may be higher than among the host population and housing conditions are often of a low standard allowing vectors easier access to human hosts and making vector control more complicated (Messenger et al., 2023).
Fig. 4.
Occupational exposure to vectors for soldiers (A), refugees (B), tourists (C), miners (D) and migrant labourers (E).
3.6.2. Soldiers
Deployed troops now face a greater risk from vector-borne infections because vectors and the diseases they carry have changed geographically, qualitatively and quantitatively throughout time (Zapor and Moran, 2005). During the first Gulf War, among the 40 cases of leishmaniasis in U.S. soldiers recorded from Iraq, 12 were visceral leishmaniasis because of an unexpectedly high frequency of Leishmania tropica vascularisation (Pages et al., 2010). The majority of leishmaniasis cases have been documented since the year 2000, with about 80% of travel-acquired leishmaniasis in soldiers who travelled to endemic countries (Pavli and Maltezou, 2010). Therefore, according to available reports, soldiers are among the most reported and researched vulnerable groups to suffer from leishmaniasis (Pavli and Maltezou, 2010), although the burden is higher among refugees who remain for prolonged periods of time in endemic areas (Du et al., 2016). From 1942 to 1945, among the 1000 cases of cutaneous leishmaniasis recorded by the U.S. Army in all theatres, 630 occurred within 3 months in a single outbreak in the Karun River Valley of Iraq (Tesh, 1989). Leishmaniasis has been regularly reported by European and U.S. armed forces in training or operations in Central and South America (Takafuji et al., 1980; Berger et al., 2006).
During the post-World War II conflicts in Indochina, Malaysia and Korea, malaria impact on deployed forces was strongly reduced by the introduction of improved prophylactic drugs and improved vector control and personal protection measures (Pages et al., 2010). Resistance to chloroquine began to emerge during the Vietnam War, endangering military operations by causing malaria deaths that could not be sustained (Modell, 1968; Beadle and Hoffman, 1993). Malaria is and will continue to be a severe danger to troop health and battle preparedness (Sanders et al., 2005). For this reason, vector control and personal protection strategies are crucial to ensuring the operational readiness of armed forces, which are active in the area of outdoor disease control (Burkett et al., 2013). However, compliance of troops with personal protection or drug prophylaxis is generally low (Frances et al., 2003; Brisson and Brisson, 2012).
Dengue has affected soldiers in most major military conflicts. During World War II, there were high morbidity in areas such as Saipan, where nearly 30% of the troops contracted the disease in a period of three months (Pages et al., 2010). During the Vietnam War, about 15% of field evacuations with fevers of unknown origin were associated with dengue fever (Deller and Russell, 1967; Neel, 1991). Dengue fever has been considered to be a possible cause of febrile illness in troops deployed in tropical areas, including U.S. forces in Somalia (1992–1993) and Australian and Italian forces in East Timor (1999–2000) (Sharp et al., 1995; Kitchener et al., 2002; Pages et al., 2010; Gibbons et al., 2012). Dengue fever can cause a significant number of soldiers to become incapacitated because of its high morbidity rates. Soldiers in training and in combat and other people with a similar occupation have round-the-clock exposure (Fig. 4) to these disease vectors based on the region of endemicity. During night manoeuvres or in combat, soldiers can be infected with leishmaniasis (van Thiel et al., 2011) and malaria (Tuck et al., 2003). During the day, soldiers will be exposed to dengue vector bites if their operation is in a dengue-endemic region (Gibbons et al., 2012), especially in urban settings. Tools for a military setting need to be portable and require minimal compliance.
3.6.3. Travellers and tourists
Leisure travel in tropical and subtropical regions often involves a lot of outdoor activity that can lead to exposure to leishmaniasis vectors (Berens-Riha et al., 2009; Perez-Ayala et al., 2009). Cutaneous and mucocutaneous leishmaniasis are becoming more common among travellers involved in outdoor activities in endemic areas, and leishmaniasis is among the top 10 diseases affecting the skin in tourists and soldiers returning from endemic countries (Mansueto et al., 2014). Most travellers are unaware of leishmaniasis and the protective measures needed. A survey in Peru revealed that only 6% of 373 travellers to a rainforest area endemic for Leishmania braziliensis had heard of leishmaniasis (Bauer, 2002).
Malaria remains an important health threat to non-immune travellers with the explosive growth of global travel. Populations at high risk of acquiring malaria infections include previously semi-immune travellers who visit friends and relatives, business travellers and international tourists with destinations in sub-Saharan Africa and other malaria-endemic regions. In 2018, sub-Saharan Africa, a region with a high intensity of malaria transmission, received an estimated 56.6 million tourists, a 7% increase from the year before (World Bank, 2023). Travellers are important in malaria transmission, especially in pre-elimination settings, because they can reintroduce malaria (Ahmed et al., 2020). Most travel-related malaria cases are associated with poor compliance with existing bite prevention measures such as topical repellents and bednet use (Croft, 2014) or chemoprophylaxis (Tickell-Painter et al., 2017).
Dengue is endemic in most tropical and subtropical countries, which are popular tourist destinations, and thus is a frequent cause of febrile illness among travellers (Halstead and Wilder-Smith, 2019). It has overtaken malaria as the leading cause of febrile illness for those travelling to Southeast Asia (Wilder-Smith, 2012; Halstead and Wilder-Smith, 2019). Travellers not only are at significant risk of acquiring dengue but also contribute to its spread to non-endemic regions (Wilder-Smith, 2012). Between 2015 and 2019 infection rate (IR)/100,000 per year among travellers was 15.8% from Southeast Asia, 6.1% from Asia as a whole, 4.4% from the Caribbean, 3.9% from Central America, and 3.7% from Africa (Gossner et al., 2022). The proportion of febrile travellers returning from tropical and subtropical countries being diagnosed with dengue has increased from 2% in the early 1990s to 16% in 2005 and will likely continue to rise in line with the global increase in dengue (Messina et al., 2019) and also there have been a few reports of autochthonous outbreaks in this region (Gossner et al., 2022).
Tourists are exposed to dengue and leishmaniasis vectors during the day and malaria vectors at night (Fig. 4), and their risk of exposure is related to their activities, duration of stay and type of accommodation. Compared to other vector-transmitted infections when visiting endemic regions, dengue and malaria are most common among tourists (Wilder-Smith, 2012; Doltario et al., 2016). Malaria is more prevalent among men, indicating a relationship with more frequent night-time activity and higher compliance of women with personal protection (Lalani et al., 2016), whereas dengue was recorded equally for men and women (Schwartz et al., 2008).
3.6.4. Forest workers
This group includes those who labour in the forest as researchers, forest product harvesters, rubber tappers, or migratory workers. Their activities collectively display a similar exposure pattern to vectors involved with the transmission of dengue, leishmaniasis, and malaria within forested environments (Fig. 4). Because dengue is primarily an urban disease, it is less common among those in rural areas, whereas infections with arboviruses with a sylvatic cycle, such as yellow fever, are often associated with farming on the forest fringe (Kwagonza et al., 2018). Even so, a few studies have identified farming as a risk factor in Vietnam (Phuong et al., 2008), China and Ethiopia because of higher exposure to standing water and exposure to vectors when workers rest in the shade (Ferede et al., 2018; Li et al., 2021). Rubber tappers spend considerable time in rubber plantations, significantly increasing their risk of exposure to arbovirus vectors compared to people who periodically enter forests or remain in the village (Tangena et al., 2017). Among other populations reviewed, dengue was found to be most associated with semi-permanent settlements of poor-quality housing. Dengue outbreaks were observed among migrant workers in camps (Rabaa et al., 2013; Perng et al., 2019) and mine workers where mining created standing water suitable for Ae. aegypti breeding (Russell et al., 1996; Eisler, 2003).
In most areas of endemicity, leishmaniasis, specifically cutaneous leishmaniasis, is commonly considered an occupational hazard for forest workers, such as in the Amazon, where there is sylvatic transmission (Lainson and Shaw, 1992). Increased leishmaniasis risk has been recorded in French Guiana when miners go into the forests for work (Mondragon-Shem, 2022). In rural areas in North Africa, farmers are more likely to come into contact with sand flies (Phlebotomus papatasi) while conducting irrigation or tending to livestock close to rodent reservoirs of the disease (Bellali et al., 2017; Torres-Guerrero et al., 2017). Economic migrants tend to send money back to relatives in their home country, so it is not unusual for them to sleep in cheap accommodation in temporary houses or near livestock, which increases their probability of infection in Eastern Africa (Argaw et al., 2013).
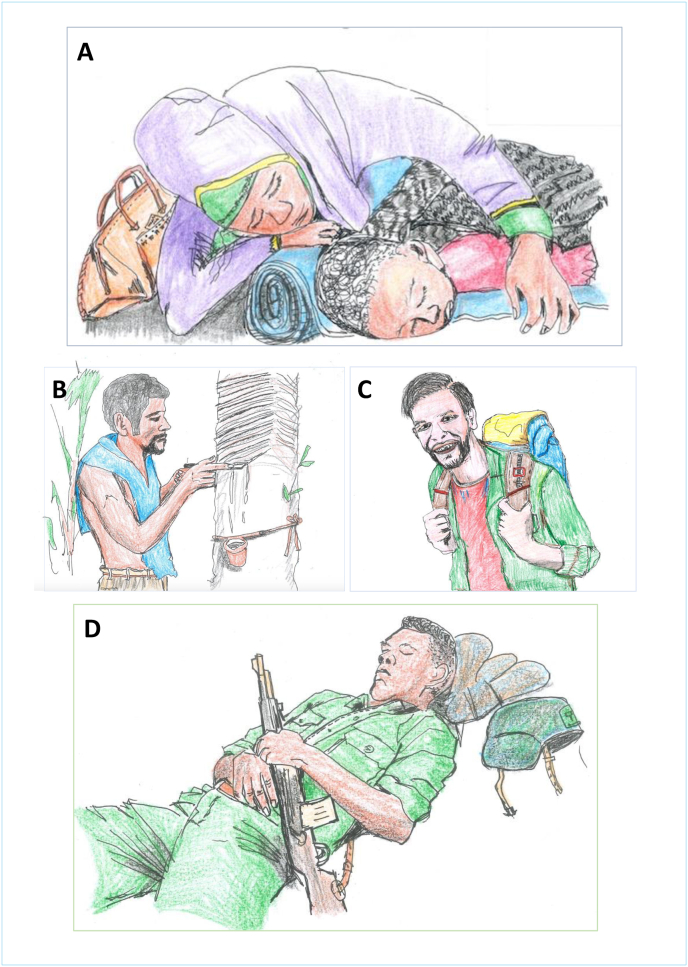
Increased risk of malaria exposure is seen among rubber tappers (Pattanasin et al., 2012; Jeffree et al., 2018). They commonly work beginning at 22:00 h or midnight and therefore have increased occupational exposure to sand fly vectors (Gradoni, 2018). The most common body part impacted by leishmaniasis in rubber tappers and other forest workers are the ears (Kaya and An, 2024). Among military personnel it is uncovered areas such as the lower arms and head (Lightburn et al., 2002). Rubber tappers work in wet regions in the tropics, which are hot and humid (ILO, 1998); they prefer to dress in clothing that does not totally cover the body, such as shorts and no shirt (Fig. 5). This helps them to cool, allowing evaporation of sweat. At night they sleep in temporary huts, which provide inadequate protection from vector bites (Pattanasin et al., 2012).
Fig. 5.
A A refugee woman and her child sleeping outdoors. B A rubber tapper wearing limited clothing in a humid climate. C A tourist on a trail expedition. D A soldier resting after intensive drilling.
One health challenge that forest workers face is the long distance to medical facilities, which can require days of travel time (Ekawati et al., 2020). They must therefore be adequately protected from vector bites before entering forests. Those who stay several days in the forest, such as forest rangers (Rahayu et al., 2020), and those who return daily after work may have different times of exposure to malaria vectors as those who go to the forests and spend more nights there, leading to a higher risk of malaria (Bannister-Tyrrell et al., 2019). Exposure to vector bites increases because of poor sleeping facilities like sleeping outdoors (Fig. 5), in makeshift huts or in improvised ground-level shelters, often in hammocks (von Seidlein et al., 2019). People working in the forests often spend weeks to months at work sites living in poor housing with minimal mosquito prevention tools (Ekawati et al., 2020).
3.6.5. Mobile populations: miners and migrant workers
Mobile populations, including migrant workers who move from their permanent residence to malaria-endemic areas for work or other purposes, may be a key cause of spread of malaria infection or even resistance to some antimalarials, as has occurred across the borders of Cambodia, Myanmar and Thailand (Kheang et al., 2018). Miners sleep in huts and makeshift houses and barns that are often not well protected against mosquitoes. Malaria cases represent the largest single portion of the disease burden among Papuan miners in Indonesia (Rodriguez-Fernandez et al., 2016). Frequent cross-border migration in search of employment increases the risk of malaria transmission, as reported among miners (Mondragon-Shem, 2022). It is common for illegal miners to come from non-endemic areas or to not be immune to malaria in the host area (Li et al., 2015). Mining-associated malaria endangers malaria elimination efforts (Hiwat et al., 2012), and miners need to be addressed as a group of particular concern.
Sand flies are usually active at dusk and dawn but can bite even during daytime (Fig. 4) when a host is in proximity (CDC, 2020c). Similarly, migrant workers travel from their home country for temporary, usually seasonal, work (Schicker et al., 2015; Wangroongsar et al., 2016). It has been frequently observed that their accommodation is impermanent or of poor quality and not built to keep away mosquitoes; this is the source of most infection among migrant workers, who at times sleep outside because of heat (Schicker et al., 2015) or stay out late and rise early to start work (Tadesse et al., 2021). Poor housing and early morning or evening outdoor exposure to vectors combine to make their exposure greater at night (Fig. 4).
3.6.6. Other risk groups
Men in endemic countries are more susceptible to malaria because of their greater likelihood of working night shifts (Susanna et al., 2012).
Based on time of day, there are four types of exposures: (i) round-the-clock exposure among mobile refugees and soldiers in combat; (ii) night-time occupational exposure among groups that work at night in malaria- or leishmaniasis-endemic areas without dengue transmission, e.g. rubber tappers; (iii) night-time exposure to malaria or leishmaniasis vectors because of poor housing, e.g. refugees in camps, miners and forest workers in temporary shelters; and (iv) daytime exposure among populations active outdoors during the day in urban dengue-endemic areas, e.g. tourists and all urban dwellers.
3.7. Proposed interventions to combat outdoor exposure
Of the 99 reviewed articles, 25 suggested means to prevent disease transmission among at-risk populations. Of these, 10 studies (about 10% of the total number of reviewed articles) suggested the use of insecticide-treated clothing (ITC) for prevention of outdoor exposure (Table 5). Prophylaxis was mentioned in 5 articles, vaccination in 4, and topical repellents in 6.
Table 5.
Reviewed intervention for outdoors occupation.
| Intervention | Advantages | Disadvantages |
|---|---|---|
| Chemoprophylaxis (Miller et al., 1999; Behrens et al., 2010; Li et al., 2015) | ‣ Available for malaria. ‣ Several types of prophylaxis available to choose from. ‣ Effective when the risk of acquiring infection is high (McBride, 2010). ‣ Cost per person per year for malaria prevention: US$0.53–5.97 (Conteh et al., 2021). |
‣ Possible reaction in users (Behrens et al., 2010). ‣ Needs repeated use (Deller and Russell, 1967). ‣ Not for prolonged use (McBride, 2010). ‣ Difficult to adhere (Kitchener et al., 2003). ‣ Intolerance (Kain et al., 2001; Boggild et al., 2007; Gawthrop and Ford, 2009). ‣ Linked to increased parasite resistance (Mika et al., 2008). ‣ Delays disease onset but not infection (Miller et al., 1999). |
| Insecticide-treated clothing (Soto et al., 1995; Kimani et al., 2006; Kittayapong et al., 2017; Klein et al., 2018; Obwaller et al., 2018) | ‣ Prevents bites from all major vectors. ‣ Application lasts for months. ‣ No need for reapplication. ‣ Can also kill vectors, providing possible mass protection if deployed to camps. ‣ Cost per person per year: US$5 (Tozan et al., 2014). |
‣ Reaction in some users, but uncommon (Young and Evans, 1998; Sharma et al., 2009). ‣ Efficacy reduced with time and washing (Orsborne et al., 2016; Kittayapong et al., 2017). ‣ There is a limited number of chemicals proved to be safe for treatment while these chemicals are resistant to some vectors. ‣ People may not wear treated clothing for long (Crawshaw et al., 2017). ‣ People may remove clothing when working in hot climates, e.g. tropical forests (Lightburn et al., 2002). |
| Topical repellents (Klein et al., 2018) | ‣ Readily available. ‣ Can be used on exposed skin if long clothing not worn. ‣ Cost per person per year: US$3.80 (Agius et al., 2020). |
‣ Needs daily regular reapplication (Gryseels et al., 2015; Crawshaw et al., 2017). ‣ Poor adherence (Gryseels et al., 2015). ‣ Mosquitoes may be diverted to unprotected people (Moore et al., 2007; Maia et al., 2013). |
| Indoor residual spraying (Jeffree et al., 2018) | ‣ Readily available. ‣ Highly effective against malaria (WHO, 2019b), dengue (Vazquez-Prokopec et al., 2017) and visceral leishmaniasis (Rijal et al., 2019); may also be effective against peridomestic cutaneous leishmaniasis (González et al., 2015). ‣ Cost per person per year: US$5.33 in Africa (Yukich et al., 2022). |
‣ Must be applied by a trained person (Malaria Consortium, 2021; Sadasivaiah et al., 2007). ‣ Useable only indoors. ‣ Logistically challenging. ‣ Suitable only for semi-permanent settlements (Messenger et al., 2023). |
| Immunisation/vaccination (Brown et al., 1990) | ‣ Prevents disease from developing or reduces severity. ‣ Protects around the clock and long term. ‣ Can be bundled with other vaccination programmes. ‣ Herd immunity: If enough people are immunised against an infection, it is more difficult for it to be spread to those not immunised. ‣ Vaccination helps reduce the social and psychological toll of illness on people and reduce the burden on hospitals and healthcare systems. ‣ Vaccine cost effectiveness similar for leishmaniasis (Bacon et al., 2013), malaria (Galactionova et al., 2017) and dengue in high-burden areas (Perera et al., 2019; Espana et al., 2021; Suwantika et al., 2021). |
‣ No available human vaccine for leishmaniasis (Zutshi et al., 2019); leishmaniasis vaccines in development (Claborn, 2010); canine vaccine available (Velez and Gallego, 2020). The first vaccine introduced is only for children and has to be used with other preventive methods (WHO, 2021c). ‣ Malaria has complex stages of the life-cycle but R21, the second malaria vaccine, targets the sporozoite stage only (WHO, 2023b). ‣ Licensed quadrivalent vaccine CYD-TDV available; indicated to people aged 9–45 years and residents of endemic regions with laboratory-confirmed previous dengue infection (Torres et al., 2019); not prequalified by WHO but approved in 19 countries (WHO, 2018; CDC, 2021a). ‣ Vaccine for all four dengue virus strains needed to prevent cross-reaction (CDC, 2022a). ‣ High cost and low uptake. |
| Spatial repellents (volatile pyrethroids, spatial emanators) | ‣ Protect multiple users in a space (Tambwe et al., 2021a). ‣ Multiple modes of action contribute to community and personal protection, i.e. inhibit blood-feeding, incapacitate, kill and reduce mosquito fertility (Bibbs and Kaufman, 2017; Tambwe et al., 2021b). ‣ Reduce malaria in Southeast Asia (Syafruddin et al., 2020) and Africa (WHO, 2023c) and dengue (Morrison et al., 2022). ‣ Cost: US$3 per household per year. |
‣ Regular replacement needed (Stevenson et al., 2018; Syafruddin et al., 2020). ‣ Proper disposal needed. ‣ Low temperature and high wind can reduce efficacy (Choi et al., 2016). ‣ Users may not know when the spatial repellent has worn out. ‣ Requires some degree of compliance. |
| Attractive targeted sugar bait | ‣ Attracts and kills mosquitoes and sand flies (Müller and Galili, 2016). ‣ Mortality up to 97% recorded (Fiorenzano et al., 2017). ‣ Can be used both indoors and outdoors against both male and female mosquitoes (Maia et al., 2018b). ‣ Can be used with multiple active ingredients for insecticide resistance management (NʼGuessan et al., 2007; Asidi et al., 2012). ‣ Minimal impact on non-target organisms (Müller et al., 2010; Khallaayoune et al., 2013; Müller and Galili, 2016). |
‣ Community-based intervention; mosquitoes may be killed in the vicinity away from the user and the user may not perceive the benefits (Maia et al., 2018b). ‣ May affect non-target insects when disposed of, including pollinators. ‣ Difficult to protect the baits for long periods from dust and rain (Müller and Galili, 2016). ‣ Cost has not been calculated. ‣ No evidence of clinical efficacy although trials ongoing. |
| Wolbachia endosymbionts | ‣ Can infect wide range of mosquitoes (Popovici et al., 2010; Vavre and Charlat, 2012). ‣ Drive into the population through cytoplasmic incompatibility, reducing need to reapply (Werren and Bartos, 2001). ‣ Have reduced dengue, chikungunya and Zika in multiple settings (Ant et al., 2022). ‣ ‣Cost-effective for endemic urban areas (Brady et al., 2020). |
‣ Community trust and acceptance of a mosquito-release program takes time (Ong, 2021). ‣ Sometimes Wolbachia endosymbionts are lost, and more releases are needed (Ant et al., 2022). |
| Gene drive using non-Mendelian inheritance to modify mosquitoes for population suppression or replacement with refractory strains (Alphey et al., 2020; Leung et al., 2022) | ‣ Expected to prevent the target infections from spreading, which will lower human morbidity and mortality, provided engineered mosquitos are present at sufficiently high frequencies (James, 2005). | ‣ Mosquitoes or parasites may evolve mechanisms to evade to genetic constructs (Wedell et al., 2019). ‣ Possible unknown consequences of incorporation of genetic material into unintended populations or species (Wedell et al., 2019). ‣ Public distrust of genetic modification (Collins, 2018). ‣ Research still in early stages. |
4. Discussion
Malaria has the highest rate of infection in Africa (WHO, 2022b, 2023c) among the three diseases under evaluation, which include dengue and leishmaniasis. The continent was underrepresented in studies that looked at occupational or outdoor exposure to malaria and other vector diseases. Nearly as many studies were conducted in Asia as in Africa and South America combined. Furthermore, leishmaniasis, which is also prevalent in the Horn of Africa, was rarely reported, and the majority of the limited studies that addressed leishmaniasis in Africa were of poor quality and did not meet the risk of bias threshold of 5 points (Supplementary Table S4). There are, therefore, very scarce data for leishmaniasis epidemiology in Africa. Other investigators have identified this gap of knowledge about prevalence and severity of the condition in Africa (Sunyoto et al., 2018) despite the negative consequences for those with untreated disease and the high risk among the large African refugee population (UNHCR, 2022a) from leishmaniasis and malaria (Amodu et al., 2020; Salami et al., 2020), with especially high mortality (18% of refugee deaths) from malaria (UNHCR, 2022b).
The literature search showed groups with the highest frequency of infections to be soldiers, refugees, travellers, migrants, miners, farmers, missionaries and forest workers, in that order. However, this appears to be publication bias. Soldiers make up a fraction of outdoor workers but happen to be the most studied for outdoor transmission of disease vectors. Military care for troops and adequate research funding have resulted in soldiers being relatively well studied in adequately designed studies and in extensive innovation in personal protection (Kitchen et al., 2009). Comparatively little data are available in the peer-reviewed literature on vector-borne disease for refugees despite their large population and relatively high burden of vector-borne disease. Other groups, such as fishers and farmers, are not well covered or clearly assessed and presented by robustly designed studies (Messenger et al., 2023).
Occupational exposure occurs among those practising subsistence agriculture and fishing, working mainly outdoors in fields and sleeping in poor housing (Monroe et al., 2019b; Swai et al., 2019), although this group is relatively poorly studied. Similarly, several articles described infection among travellers and tourists while visiting an endemic area or when they returned to their homes in places mostly non-endemic to these diseases. It may be assumed that this bias is due to the difference in health budgets and the strength of disease-reporting systems between endemic countries and countries from which the tourists originate.
4.1. Infection risk difference across groups
The risks that leishmaniasis, dengue and malaria pose to outdoor workers differ from those they pose to the general population, but outdoor workers remain a small population among the overall global burden of disease. By far the populations with the greatest risk of disease from outdoor exposure or poor housing are refugees and other displaced populations. Their number is climbing because of conflict, with 7 million refugees (UNHCR, 2022c) and 14 million displaced people (IDMC, 2022) in sub-Saharan Africa and 2.4 million refugees (UNHCR, 2022c) and 1.2 million displaced people (IDMC, 2022) in the Middle East in 2021.
In regions where forest malaria is common, the prevalence of malaria is often higher compared to the general population (MacDonald and Mordecai, 2019; Sandfort et al., 2020). Outdoor workers are exposed both while working outdoors and when they return home. However, the groups which bear the heaviest malaria burden are pregnant women (Pavli and Maltezou, 2010; Pell et al., 2011; Quaresima et al., 2021) and children under the age of five years accounted for around 80% of all malaria deaths worldwide in 2021 (WHO, 2023a); global estimates of other years show this age group accounts for about 90% of all malaria-related deaths (CDC, 2022b). A modelling study has estimated that 10% of malaria cases in Africa (10.6 million cases) are from outdoor transmission (Sherrard-Smith et al., 2019).
Very limited information is available for leishmaniasis because of the type of population it affects, usually, the very poor and marginalised; what information has been collected is inadequate and incomplete even for countries where the disease is prevalent (Alvar et al., 2012). The Global Burden of Disease study has shown that this disease prevalence has doubled in the last 30 years, and this growth is highly related to displaced populations.
Groups at risk of dengue and a high disease burden are still seen in tropical regions of Africa. Africa now has 16% of the worldʼs dengue cases, higher than previously thought because of underreporting and varied treatment-seeking behaviour. It is a major hidden burden (Endy et al., 2011; Kakkar, 2012). Dengue fever and dengue haemorrhagic fever are a concern globally, with the highest incidence among children aged 5–14 years in the Americas. Most (75%) of the population exposed to dengue lives in Asia-Pacific, with 1.3 billion at-risk individuals in 10 Southeast Asian countries. Dengue is a leading cause of hospitalisation and death in children in the region (Gubler, 2002; WHO, 2011). It is predominantly acquired outdoors, especially during the day, when the mosquitoes that transmit the virus are most active.
4.2. Control of outdoor transmission: vaccines and drugs
A large number of interventions are available for preventing vector-borne diseases transmitted outdoors (Williams et al., 2018). These may act on the individuals, who are mobile or be applied at a community level in temporary settlements such as mining camps or more permanent settlements such as refugee camps. Relatively inexpensive chemoprophylaxis (Tickell-Painter et al., 2017) and treatment (WHO, 2021b) are available for malaria, and a vaccine is available but not scalable for mobile populations (Alonso, 2021). Although a quadrivalent dengue vaccine has been developed, it is still not widely used and is not suitable for dengue-naïve individuals (Godói et al., 2017). Because of the geographical spread of dengue and dengue haemorrhagic fever and increased incidence in the last 20 years, management of the infection has become essential (Gubler, 1998b; Msellemu et al., 2020). Vector control is the primary means of dengue control, but even in countries with well-organised vector control, it is at best partially effective (Achee et al., 2015). There are no vaccines or drugs to prevent leishmaniasis (CDC, 2020a), and control depends on the setting, i.e. where the vector can be reliably located.
4.3. Transformative technologies: Modern interventions to control disease vectors
Wolbachia-based interventions and gene drive are recent advanced transformative technologies with the potential to revolutionise the control of arboviruses and malaria. Wolbachia spp. are naturally occurring bacteria that spread through a population through cytoplasmic incompatibility whereby the Wolbachia symbiont-carrying mosquito favours the spread of maternally transmitted Wolbachia spp. within the population (Shropshire et al., 2020). Wolbachia endosymbionts affect sperm, causing catastrophic effects on the fertilised embryo and ensuing lethality in crosses between symbiotic males and wild-type females, although the embryo will develop in the offspring of Wolbachia-carrying males and Wolbachia-carrying females. An additional benefit is that harbouring Wolbachia can prevent mosquitoes from transmitting diseases such as dengue, chikungunya and Zika (Ant et al., 2022). Wolbachia-based interventions, which are considered cost-effective for high-density urban populations, have been able to control arbovirus disease, as demonstrated by multiple trials in tropical cities (Ng, 2021; Ant et al., 2022; Velez et al., 2023).
Gene drive is a technology that allows researchers to rapidly spread a beneficial genetic trait through a population of insects. Engineered snippets of DNA are introduced into an organism’s genome to significantly increase the chance that a desired genetic trait will spread through a population faster than would normally happen through sexual reproduction through non-mendelian inheritance (Marshall et al., 2020). Gene-drive organisms are intended to spread a desired trait into a population containing two introduced linked sets of genetic modifications. The first set includes the genetic modifications that encode the new trait. The second set imparts the ability to “drive” the trait into a wild population with a far higher probability than would normally occur (Marshall et al., 2020). For malaria control, population suppression trains are being explored by manipulating sex ratios (Kyrou et al., 2018) and refractoriness to malaria by including antimicrobial peptides that kill Plasmodium spp. (Hoermann et al., 2021) so the mosquitoes cannot transmit malaria.
The advent of these technologies coincides with a decline in the efficacy of traditional vector-control methods, such as IRS, due to the emergence of insecticide resistance in mosquitoes (Tungu et al., 2023) and more difficult to implement because of population growth in malaria-endemic areas and tropical cities. As a result, there is a growing need for new tools to complement existing control measures and help reduce the spread of disease. Both gene drive and Wolbachia-based interventions are self-sustaining because they spread through a mosquito population; researchers can thus effectively reduce the transmission of disease without the repeated use of control measures and have the potential to be more sustainable in the long term. Both technologies will need to be integrated with other control measures, including community education, surveillance, standing water removal, and larvicide/insecticide use, and both require oversight to monitor programme effectiveness. Finally, it is important to carefully consider the potential risks and benefits of these technologies before deploying them on a large scale. Further research and development are needed to ensure that they are safe and effective and that their deployment does not have unintended consequences for the environment or human health. However, because of the nature of these technologies, they are more suitable for permanent settlement areas than for protecting those who work outdoors in rural or remote settings.
4.4. Control measures for people living in permanent settlements
Dengue epidemics among refugee populations are best tackled in the same way as in urban settlements because most refugees spend a period of several years in camps (Devictor and Do, 2016). For this reason, cost-effective source reduction, i.e. targeted prevention of breeding in highly productive mosquito breeding sites such as water storage containers (Andersson et al., 2015), is essential, and IRS could also be protective (Samuel et al., 2017), especially in outbreaks (Vazquez-Prokopec et al., 2010). Removal trapping may be useful because it is portable and relatively cheap, but evidence of impact on disease is currently limited (Oliver et al., 2021). There is also evidence of the efficacy of spatial repellents (SR) against dengue (Morrison et al., 2022).
Where peridomestic leishmaniasis transmission occurs, vectors are often found in and around houses. Currently, control ranges from none in Syria, where cases are escalating (Rehman et al., 2018), to highly organised and efficient control in some areas of India, resulting in a strong downward trend in cases (Kumar et al., 2020). Where peridomestic leishmaniasis transmission occurs, IRS is efficacious (Joshi et al., 2009), and ITNs with a small mesh size have been shown to reduce the disease incidence (Chowdhury et al., 2019).
Similarly, for malaria in Africa, where transmission is primarily indoors (Pluess et al., 2010) and ITNs have been shown to significantly reduce malaria incidence (Pryce et al., 2018), ITNs and IRS are the most effective tools against malaria (WHO, 2021b) and have been applied at scale with excellent results (Bhatt et al., 2015). Source reduction is often effective in water-limited areas (Bayoh et al., 2011) and may become more important now that An. stephensi, an invasive vector species that bites and rests outdoors has been observed in areas hosting internally displaced people (Allan et al., 2023). Where malaria vectors bite and rest outdoors, ITNs and IRS are still used although their efficacy may be compromised (Table 6), Additional control needs to be situation-specific, such as mass drug administration in areas of low and/or seasonal transmission (UNHCR, 2022b). Evidence is being collected for the public health benefit of ATSB although entomological trials are promising and additional tools are required to address mobile populations (Sandfort et al., 2020; Attractive Targeted Sugar Bait Phase III Trial Group, 2022; Ferreira et al., 2022).
Table 6.
Interventions against disease vectors of dengue, malaria and leishmaniasis by target vector.
| Intervention | Aedes spp. | Anopheles spp. | Sand flies |
|---|---|---|---|
| Indoor residual spray | ✔️ a | ✔️ a | ✔️ a |
| Insecticide-treated clothing | ✔️ b | ✔️ a | ✔️ a |
| Insecticide-treated bednets | NA | ✔️ a | ✔️ a |
| Insecticide-treated hammock nets | NA | ✔️ a | ? |
| Spatial repellent | ✔️ a | ✔️ a | ? |
| Topical repellent | ✔️ b | ✔️ c | ✔️ b |
Abbreviation: NA, not applicable.
Evidence of clinical efficacy used indoors but bite prevention demonstrated indoors and outdoors.
Bite prevention only.
Evidence of clinical efficacy in areas of forest malaria.
4.5. Personal protection methods for mobile populations
Individuals should always take steps to reduce contact with the vectors of dengue because they bite throughout the day; with leishmaniasis vectors, especially in poor housing; and in areas with sylvatic transmission and malaria vectors if outdoors at night.
Protection from sand fly- and Anopheles vectors requires similar measures. It is recommended to avoid contact with vectors by minimising nocturnal outdoor activities, especially from dusk to dawn, when sand flies and mosquitoes generally are the most active; to wear clothes that cover most of the body (Kiviat, 1993; Moore et al., 2012; CDC, 2020a); and to use an ITN while sleeping (WHO, 2021b). Where culturally appropriate, mobile outdoor populations may use hammock nets (Table 6) that reduce bites (Sochantha et al., 2010) and reduce malaria (Magris et al., 2007; Thang et al., 2009). If vectors are active during sleeping hours, people must rely on bite prevention by using topical mosquito repellents (Lupi et al., 2013), ITC (Banks et al., 2014), and volatile pyrethroid spatial repellents (Maia et al., 2018a). Insect repellents containing a minimum of 20% N, N-diethyl-3-methylbenzamide (DEET), 20% picaridin or 20% p-menthane-3,8-diol (PMD, 65% citriodiol) have been effective in preventing Anopheles and Aedes mosquito (Lupi et al., 2013) and sand-fly bites (Diaz, 2016), and there is some evidence of protection against forest malaria (Hill et al., 2007; Agius et al., 2020).
Similarly, protective clothing impregnated with permethrin is efficacious against all mosquito- and sand-fly bites (Jelinek, 2000; Wilder-Smith and Schwartz, 2005) and has good evidence of bite prevention (Banks et al., 2014) but not of disease protection against dengue (Kittayapong et al., 2017), with some evidence of prevention of malaria (Maia et al., 2018a) and leishmaniasis (Reyburn et al., 2000). For mobile populations that wear long clothing, the use of ITC is more sustainable and requires lower individual compliance with the intervention.
Spatial repellents (SR) function by evaporating into the air, establishing a protective zone to repel or eliminate insects (Ogoma et al., 2012). They can protect multiple people within the treated space (Tambwe et al., 2021a). The active ingredients, often volatile pyrethroids, impact the nervous system of insects, disrupting their normal functions and deterring them from landing or biting (Valbon et al., 2022). Activation of SR by an external energy source for dispersal characterizes it as an active emanator. Conversely, a passive emanator is an SR that gradually releases insecticides into the environment over time, operating independently without the requirement of external energy. Passive emanator spatial repellents are advantageous because they rely solely on natural air movement to emanate active ingredients from a dosed surface into a space so can be used in rural areas without electricity (Darbro et al., 2017).
Spatial repellents using a light, portable passive plastic emanator have been shown to protect against dengue (Morrison et al., 2022) and malaria (Maia et al., 2018a; Syafruddin et al., 2020; WHO, 2023d) in randomised control trials. A third trial showed a reduction in malaria although it was flawed due to no malaria transmission occurring in several clusters (Maia et al., 2018a; Syafruddin et al., 2020; WHO, 2023d). Evidence for bite prevention both indoors and outdoors for this intervention is extensive (IVCC, 2020). SR using passive emanators have also provided effective personal protection against vector-borne disease exposures of malaria in the forests (Charlwood et al., 2016; Tangena et al., 2018; Chen et al., 2023). However, evidence is limited for leishmaniasis since very little research has been done on this neglected disease, although operational trials are underway for leishmaniasis (The MENTOR Initiative, 2020) as well as malaria in displaced populations in Nigeria and Yemen and Uganda (Achee et al., 2023). SR may be used in addition to other interventions such as ITNs to provide additional layers of defence against mosquito bites (Dev, 2021) that in some instances can last for up to a year without the need for compliance (Swai et al., 2023a). This is an important feature, since topical repellents are generally not used frequently (Gryseels et al., 2015). Importantly, SR also cause mosquito mortality (Swai et al., 2023b) that contributes to community protection (Denz et al., 2021; Fairbanks et al., 2023).
4.6. Personal protection interventions versus community control
Research efforts should be directed towards devising novel interventions, including vaccines, with the potential to benefit a wider range of people. The focus of research on vector-borne diseases should also aim at scaling up innovations and interventions that will benefit the most vulnerable groups, including children and displaced populations.
Personal protection is essential for those who work outdoors in vector-prevalent regions because it provides a more effective way of controlling occupational exposure than community control interventions. Community interventions may provide good protection in areas of permanent or semi-permanent settlements but are ineffective when the population is mobile. Tailoring the intervention to fit the specific needs of individuals (Bancroft et al., 2022) and their duties may improve adherence and therefore provide more efficient protection. For those sleeping in unimproved housing, the addition of volatile pyrethroids may provide additional protection against mosquito bites (Fairbanks et al., 2023).
4.7. Insecticide-treated clothing (ITC)
It is a suitable intervention for mobile populations needing round-the-clock protection or outdoor protection during the day in dengue-endemic areas or at night in malaria- or leishmaniasis-endemic areas. ITC was the most frequently mentioned intervention and has evidence of protection for outdoor workers against mosquito and sand-fly bites (DeRaedt Banks et al., 2015; Londono-Renteria et al., 2015; Ghamari and Khoobdel, 2019) but also against malaria (Soto et al., 1995; Maia et al., 2018a) and leishmaniasis (Reyburn et al., 2000). This intervention may be effective for long-term users, such as refugees, soldiers in combat and forest rangers ranging from a month to a year or more because treated clothing has an excellent toxicity and safety profile for long-term use (Rossbach et al., 2010; EPA, 2023). Short-term users are those who will need the intervention for a few weeks or months; these include tourists, miners and migrant workers. For those sleeping at night in malaria- or leishmaniasis-endemic areas, the use of an ITN is also recommended (Chowdhury et al., 2019; WHO, 2021b).
Not all occupations will benefit equally from ITC. Acceptance of and adherence to ITC may depend upon occupation and cultural norms for body covering (Table 7). Occupations that require a uniform, such as military personnel and forest rangers, will benefit from ITC more than those not required to wear a uniform and who may prefer short clothing with less coverage, including miners, rubber tappers, migrant workers and loggers. Combining ITC with additional interventions such as topical or spatial repellents may be optimal for round-the-clock protection.
Table 7.
Suitable interventions for various occupations in varying situations.
| Occupation | Duration of exposure | Context | Proposed intervention | Local customs |
|---|---|---|---|---|
| Soldiers | Short | Training | ITC; Topical repellents | Follow orders |
| Long | Active combat | ITC; Topical repellents | ||
| Refugees | Short | War and natural calamities | ITC; Spatial repellents; | Clothes cover the whole body |
| Long | Host country | IRS; ITN; Improved housing; Spatial repellents | ||
| Tourists | Short | Tour | ITC | Customs and health-seeking behaviour |
| Long | Expatriate/Vacationing | ITC; IRS; ITN; Spatial repellents | ||
| Miners | – | Legal, formal | ITC; Topical repellents; Spatial repellents | Follow orders |
| – | Illegal, informal | ITC; ITN; Topical repellents; Spatial repellents | Anarchic | |
| Migrant workers | Short | Seasonal | Prophylaxis | Follow orders |
| Long | Permanent | IRS; ITN; Spatial repellents | Anarchic | |
| Farmers | Short | Seasonal | ITN; Spatial repellents | Anarchic |
| Long | Permanent | ITC | ||
| Forest workers | Short | Temporary | ITC; Topical repellents; Spatial repellents | Follow orders |
| Long | Permanent | ITC; Spatial repellents | Anarchic | |
| Rubber tappers | Short | Temporary | Spatial repellents; ITC | Follow orders |
| Long | Permanent | Spatial repellents; ITC | Anarchic | |
| Missionaries | Short | Sabbatical | Topical repellents; Spatial repellents; ITC | Health-seeking behaviour |
| Long | Mission | Improved housing; ITC; ITN; Spatial repellents |
Abbreviations: IRS, indoor residual spraying; ITC, insecticide-treated clothes; ITN, insecticide-treated nets.
ITC is not always optimally effective. Incorrect use, such as rolling up sleeves (Wallace et al., 1996), can undermine efficacy (Fig. 5). Some studies have suggested that improved wash resistance is needed (DeRaedt Banks et al., 2015; Kittayapong et al., 2017). When treated clothes are industrially impregnated, their efficacy lasts longer (DeRaedt Banks et al., 2015). The need for additional topical repellents for personal protection on soldiers in endemic regions during recreation, when uniforms are not used, has also been emphasised (Obwaller et al., 2018). Even so, ITC using pyrethroids is safe (National Research Council, 1994; Young and Evans, 1998), requires lower compliance than topical repellents and is relatively inexpensive, giving excellent cost benefits, especially to non-immune individuals (Soto et al., 1995). A useful feature of ITC is that it generally exerts some knockdown and kill of vectors (National Research Council, 1994) that will prevent vectors from moving from a protected to an unprotected individual (Moore et al., 2007). Killing, knockdown and feeding inhibition reduce vectorial capacity if applied at scale (Denz et al., 2021). This, combined with excellent bite prevention (Banks et al., 2014), user acceptance (Forgearini et al., 2016; Crawshaw et al., 2017) and some evidence of efficacy against malaria (Maia et al., 2018a) and leishmaniasis (Soto et al., 1995; Reyburn et al., 2000), makes self-treated clothing (Forgearini et al., 2016) optimal for those exposed to vectors round the clock, during the day in dengue transmission areas or while working at night (Li et al., 2015).
While ITC offer promise in shielding individuals outdoors, distinct from other vector control measures, they rely on insecticides akin to those utilized in interventions like IRS and ITNs. With vector resistance emerging against these insecticides, the efficacy of ITC is challenged. Therefore, it is imperative to explore the utilization of alternative insecticides that have not yet encountered vector resistance. By adopting such innovative strategies, the efficacy of ITC can be enhanced and ensure robust protection against the threat of vector-borne illnesses in outdoor environments. That is, continual efforts to improve and use utilize innovative personal protective tools remain imperative (Sharp et al., 1995; Wallace et al., 1996) until vaccines for malaria, dengue and leishmaniasis become available.
4.8. Study limitations
The review relied upon observational studies, which lack the experimental element of a random allocation of exposure or intervention, so the evidence presented depended on associations shown by the studies. Studies of risk factors typically cannot be randomised because they relate to innate human characteristics or habits and because randomisation exposes individuals to dangerous risks (Stroup et al., 2000). Because it is thus not possible to measure rates and patterns of disease using prospective designs (Ioannidis and Lau, 1999; Munn et al., 2020), retrospective non-randomised studies study designs are best used to assess relationships between possible exposure and disease outcomes to form hypotheses regarding risk or prevention (Munn et al., 2020). This is a challenge when conducting a systematic review of non-randomised studies because most reporting guidelines are designed for prospective randomised study designs (Shea et al., 2017; Oxman, 2018). Although meta-analytic techniques are routine in the synthesis of data from randomised controlled trials, there are no clear guidelines on how to best summarise frequency data such as incidence and prevalence estimates (Saha et al., 2008). Therefore, this systematic review of observational articles providing data on the incidence and prevalence of infections of dengue, leishmaniasis and malaria used a framework comparator CoCoPop mnemonic developed by Munn et al. (2015) specifically for prevalence and incidence reviews with additional criteria for study design, as per the PRISMA statement (Moher et al., 2009). The review synthesised the incidence and prevalence of studies that report infections and diseases resulting from occupational exposure to malaria, arbovirus and leishmaniasis vectors whilst adhering to the same basic principles of systematic review of other types of data (Moher et al., 2009). However, meta-analysis was not possible. By nature, observational studies are more at risk of confounding factors and various unavoidable sources of bias (Munn et al., 2020). Because of the weakness of non-randomised studies, this review provides weaker evidence than a review of prospective randomised trials.
5. Conclusion and recommendations
The review indicates that the timing and location of vector exposure are critical factors to consider for the control of vector-borne disease. These factors can be grouped as (i) round-the-clock outdoor exposure, (ii) daytime outdoor exposure, (iii) indoor night-time exposure or (iv) outdoor night-time exposure, and subdivided into the population categories: mobile and living in semi-permanent settlements of poor housing.
ITC is a favourable intervention for outdoor use, especially among mobile populations, and has the potential to protect soldiers, refugees without shelter and tourists, although its use can be impaired by partial adherence, particularly when short clothing is more commonly worn or where environmental conditions make the wearing of long clothing uncomfortable.
Those living in semi-permanent settlements, such as migrant workers (e.g. rubber tappers, gold miners) and refugees, will need multiple interventions tailored to their ecological setting to protect them from peridomestic transmission. IRS and ITNs or insecticide-treated hammock nets have the greatest weight of evidence for control of malaria, leishmaniasis and dengue in the peridomestic setting. For unimproved housing and possibly forest shelters SR holds excellent promise to provide protection against indoor biting disease vectors (Fairbanks et al., 2023). ITC and repellent may be needed for those with day- or night-time occupational exposure away from their living quarters. However, the evidence for ITC as a public health intervention is currently restricted to a handful of small studies, with most research conducted among military populations even though refugees are the population most at risk of severe morbidity or mortality from vector-borne disease. However, a number of operational trials are ongoing to evaluate the impact of SR for disease prevention among these vulnerable groups (Achee et al., 2023). Self-treatment of clothes should be encouraged as a disease vector intervention in dengue-, leishmaniasis- and malaria-endemic areas, particularly for individuals who cannot afford multiple outfits that need to be changed regularly. While the evidence for disease prevention is limited, it is known that insecticide-treated clothing and SR effectively prevent bites as well as contributing to mosquito mortality which will protect both users and non-users of these interventions (Fairbanks et al., 2023).
Although people occupationally exposed to vector-borne diseases, such as soldiers and travellers to endemic areas, may have a higher disease prevalence (Antinori et al., 2012; Wilder-Smith, 2012; Marasinghe et al., 2020), the local population in the endemic area still experiences the largest absolute number of infections (CDC, 2021b; WHO, 2022b). This highlights the need to address the issue of sustained control of vector-borne diseases in the local population in addition to supplemental control for occupationally exposed groups to control the spread of these diseases.
Funding
The completion of this review was made possible by the funds from the Deployed Warfighter Protection Research Program (DWFP; CFDA No. 12.360). The content of this review is the opinion of the authors and does not necessarily represent the views of AFPMB. Sarah Moore was partially supported by the Malaria Elimination Initiative (A134328).
Ethical approval
Not applicable.
CRediT authorship contribution statement
Daniel Msellemu: Conceptualization, Methodology, Writing – original draft, Formal analysis, Visualization, Writing – review & editing. Marcel Tanner: Writing – review & editing, Supervision. Rajpal Yadav: Methodology, Writing – review & editing. Sarah J. Moore: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The views expressed in this article are solely the responsibility of the authors and do not necessarily reflect the perspectives, choices, or policies of the institutions to which they are affiliated.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crpvbd.2024.100185.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Data availability
The data supporting the conclusions of this article are included within the article and its supplementary files.
References
- Abdul-Ghani R., Mahdy M.A.K., Al-Eryani S.M.A., Fouque F., Lenhart A.E., Alkwri A., et al. Impact of population displacement and forced movements on the transmission and outbreaks of Aedes-borne viral diseases: Dengue as a model. Acta Trop. 2019;197 doi: 10.1016/j.actatropica.2019.105066. [DOI] [PubMed] [Google Scholar]
- Achee N.L., Gould F., Perkins T.A., Reiner R.C., Jr., Morrison A.C., Ritchie S.A., et al. A critical assessment of vector control for dengue prevention. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achee N.L., Perkins T.A., Moore S.M., Liu F., Sagara I., Van Hulle S., et al. Spatial repellents: The current roadmap to global recommendation of spatial repellents for public health use. Curr. Res. Parasitol. Vector Borne Dis. 2023;3 doi: 10.1016/j.crpvbd.2022.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agius P.A., Cutts J.C., Han Oo W., Thi A., OʼFlaherty K., Zayar Aung K., et al. Evaluation of the effectiveness of topical repellent distributed by village health volunteer networks against Plasmodium spp. infection in Myanmar: A stepped-wedge cluster randomised trial. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A., Eldigail M., Elduma A., Dietrich I., Ali Y., Weaver S.C. First report of epidemic dengue fever and malaria co-infections among internally displaced persons in humanitarian camps of North Darfur, Sudan. Int. J. Infect. Dis. 2021;108:513–516. doi: 10.1016/j.ijid.2021.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S., Reithinger R., Kaptoge S.K., Ngondi J.M. Travel is a key risk factor for malaria transmission in pre-elimination settings in sub-Saharan Africa: A review of the literature and meta-analysis. Am. J. Trop. Med. Hyg. 2020;103:1380–1387. doi: 10.4269/ajtmh.18-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhoundi M., Kuhls K., Cannet A., Votypka J., Marty P., Delaunay P., et al. A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLoS Negl. Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghazali K.A., Teoh B.T., Loong S.K., Sam S.S., Che-Mat-Seri N.A., Samsudin N.I., et al. Dengue outbreak during ongoing civil war, Taiz, Yemen. Emerg. Infect. Dis. 2019;25:1397–1400. doi: 10.3201/eid2507.180046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhawarat M., Khader Y., Shadfan B., Kaplan N., Iblan I. Trend of cutaneous leishmaniasis in Jordan from 2010 to 2016: Retrospective study. JMIR Public Health Surveill. 2020;6 doi: 10.2196/14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan R., Weetman D., Sauskojus H., Budge S., Hawail T.B., Baheshm Y. Confirmation of the presence of Anopheles stephensi among internally displaced peopleʼs camps and host communities in Aden city, Yemen. Malar. J. 2023;22:1. doi: 10.1186/s12936-022-04427-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allwinn R. Significant increase in travel-associated dengue fever in Germany. Med. Microbiol. Immunol. 2011;200:155–159. doi: 10.1007/s00430-011-0185-2. [DOI] [PubMed] [Google Scholar]
- Alonso P.L. Malaria: a problem to be solved and a time to be bold. Nat. Med. 2021;27:1506–1509. doi: 10.1038/s41591-021-01492-6. [DOI] [PubMed] [Google Scholar]
- Alphey L.S., Crisanti A., Randazzo F.F., Akbari O.S. Opinion: Standardizing the definition of gene drive. Proc. Natl. Acad. Sci. USA. 2020;117:30864–30867. doi: 10.1073/pnas.2020417117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvar J., Velez I.D., Bern C., Herrero M., Desjeux P., Cano J., et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves E.B., Figueiredo F.B., Rocha M.F., Castro M.C., Werneck G.L. Effectiveness of insecticide-impregnated collars for the control of canine visceral leishmaniasis. Prev. Vet. Med. 2020;182 doi: 10.1016/j.prevetmed.2020.105104. [DOI] [PubMed] [Google Scholar]
- Amodu O.C., Richter M.S., Salami B.O. A scoping review of the health of conflict-iInduced internally displaced women in Africa. Int. J. Environ. Res. Publ. Health. 2020;17:1280. doi: 10.3390/ijerph17041280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amr Z.S., Kanani K., Shadfan B., Hani R.B. Cutaneous leishmaniasis among Syrian refugees in Jordan: A retrospective study. Bull. Soc. Pathol. Exot. 2018;111:295–300. doi: 10.3166/bspe-2019-0057. [DOI] [PubMed] [Google Scholar]
- Andersson N., Nava-Aguilera E., Arostegui J., Morales-Perez A., Suazo-Laguna H., Legorreta-Soberanis J., et al. Evidence-based community mobilization for dengue prevention in Nicaragua and Mexico (Camino Verde, the Green Way): Cluster randomized controlled trial. BMJ. 2015;351:h3267. doi: 10.1136/bmj.h3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade M.S., Brito M.E., Silva S.T., Lima B.S., Almeida E.L., Albuquerque E.L., et al. [American tegumentary leishmaniasis caused by Leishmania (Viannia) braziliensis in military training area of Zona da Mata in Pernambuco] Rev. Soc. Bras. Med. Trop. 2005;38:229–233. doi: 10.1590/s0037-86822005000300004. [DOI] [PubMed] [Google Scholar]
- Ant T.H., Mancini M.V., McNamara C.J., Rainey S.M., Sinkins S.P., et al. Wolbachia-virus interactions and arbovirus control through population replacement in mosquitoes. Pathog. Glob. Health. 2022;117:245–258. doi: 10.1080/20477724.2022.2117939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori S., Schifanella L., Corbellino M. Leishmaniasis: New insights from an old and neglected disease. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31:109–118. doi: 10.1007/s10096-011-1276-0. [DOI] [PubMed] [Google Scholar]
- Argaw D., Mulugeta A., Herrero M., Nombela N., Teklu T., Tefera T., et al. Risk factors for visceral leishmaniasis among residents and migrants in Kafta-Humera, Ethiopia. PLoS Negl. Trop. Dis. 2013;7:e2543. doi: 10.1371/journal.pntd.0002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arisco N.J., Peterka C., Castro M.C. Cross-border malaria in northern Brazil. Malar. J. 2021;20:135. doi: 10.1186/s12936-021-03668-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armed Forces Pest Management Board . Silver Spring; MD: 2015. Sand flies - significance, surveillance, and control in contingency operations. Diptera: Psychodidae: Phlebotominae; pp. 20910–21230.https://apps.dtic.mil/sti/citations/tr/ADA617143 [Google Scholar]
- Aschale Y., Ayehu A., Worku L., Tesfa H., Birhanie M., Lemma W. Malaria-visceral leishmaniasis co-infection and associated factors among migrant laborers in West Armachiho Ddistrict, North West Ethiopia: Community-based cross-sectional study. BMC Infect. Dis. 2019;19:239. doi: 10.1186/s12879-019-3865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asidi A., NʼGuessan R., Akogbeto M., Curtis C., Rowland M. Loss of household protection from use of insecticide-treated nets against pyrethroid-resistant mosquitoes, Benin. Emerg. Infect. Dis. 2012;18:1101–1106. doi: 10.3201/eid1807.120218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attractive Targeted Sugar Bait Phase III Trial Group Attractive targeted sugar bait phase III trials in Kenya, Mali, and Zambia. Trials. 2022 doi: 10.1186/s13063-022-06555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayhan N., Charrel R.N. Of phlebotomines (sand flies) and viruses: A comprehensive perspective on a complex situation. Curr. Opin. Insect Sci. 2017;22:117–124. doi: 10.1016/j.cois.2017.05.019. [DOI] [PubMed] [Google Scholar]
- Bacon K.M., Hotez P.J., Kruchten S.D., Kamhawi S., Bottazzi M.E., Valenzuela J.G., et al. The potential economic value of a cutaneous leishmaniasis vaccine in seven endemic countries in the Americas. Vaccine. 2013;31:480–486. doi: 10.1016/j.vaccine.2012.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaska S., Fotakis E.A., Chaskopoulou A., Vontas J. Chemical control and insecticide resistance status of sand fly vectors worldwide. PLoS Negl. Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0009586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft D., Power G.M., Jones R.T., Massad E., Iriat J.B., Preet R., et al. Vector control strategies in Brazil: A qualitative investigation into community knowledge, attitudes and perceptions following the 2015–2016 Zika virus epidemic. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2021-050991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks S.D., Murray N., Wilder-Smith A., Logan J.G. Insecticide-treated clothes for the control of vector-borne diseases: A review on effectiveness and safety. Med. Vet. Entomol. 2014;28(Suppl. 1):14–25. doi: 10.1111/mve.12068. [DOI] [PubMed] [Google Scholar]
- Bannister-Tyrrell M., Gryseels C., Sokha S., Dara L., Sereiboth N., James N., et al. Forest goers and multidrug-resistant malaria in Cambodia: An ethnographic study. Am. J. Trop. Med. Hyg. 2019;100:1170–1178. doi: 10.4269/ajtmh.18-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baomar A., Mohamed A. Malaria outbreak in a malaria-free region in Oman 1998: Unknown impact of civil war in Africa. Publ. Health. 2000;114:480–483. doi: 10.1038/sj.ph.1900699. [DOI] [PubMed] [Google Scholar]
- Battaglia V., Agostini V., Moroni E., Colombo G., Lombardo G., Rambaldi Migliore N., et al. The worldwide spread of Aedes albopictus: New insights from mitogenomes. Front. Genet. 2022;13 doi: 10.3389/fgene.2022.931163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer I.L. Knowledge and behavior of tourists to Manu National Park, Peru, in relation to leishmaniasis. J. Trav. Med. 2002;9:173–179. doi: 10.2310/7060.2002.24487. [DOI] [PubMed] [Google Scholar]
- Bayoh M.N., Akhwale W., Ombok M., Sang D., Engoki S.C., Koros D., et al. Malaria in Kakuma refugee camp, Turkana, Kenya: Facilitation of Anopheles arabiensis vector populations by installed water distribution and catchment systems. Malar. J. 2011;10:149. doi: 10.1186/1475-2875-10-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle C., Hoffman S.L. History of malaria in the United States naval forces at war: World War I through the Vietnam conflict. Clin. Infect. Dis. 1993;16:320–329. doi: 10.1093/clind/16.2.320. [DOI] [PubMed] [Google Scholar]
- Bedasso A.H., Gutto A.A., Waldetensai A., Eukubay A., Bokore G.E., Kinde S., et al. Malaria vector feeding, peak biting time and resting place preference behaviors in line with indoor-based intervention tools and its implication: Scenario from selected sentinel sites of Ethiopia. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens R.H., Carroll B., Hellgren U., Visser L.G., Siikamaki H., Vestergaard L.S., et al. The incidence of malaria in travellers to South-East Asia: Is local malaria transmission a useful risk indicator? Malar. J. 2010;9:266. doi: 10.1186/1475-2875-9-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellali H., Chemak F., Nouiri I., Ben Mansour D., Ghrab J., Chahed M.K., et al. Zoonotic cutaneous leishmaniasis prevalence among farmers in Central Tunisia, 2014. J. Agromed. 2017;22:244–250. doi: 10.1080/1059924x.2017.1318725. [DOI] [PubMed] [Google Scholar]
- Benedict M.Q., Levine R.S., Hawley W.A., Lounibos L.P. Spread of the tiger: Global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 2007;71:76–85. doi: 10.1089/vbz.2006.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benelli G., Wilke A.B.B., Beier J.C. Aedes albopictus (Asian tiger mosquito) Trends Parasitol. 2020;36:942–943. doi: 10.1016/j.pt.2020.01.001. [DOI] [PubMed] [Google Scholar]
- Berens-Riha N., Fleischmann E., Pratlong F., Bretzel G., von Sonnenburg F., Loscher T. Cutaneous leishmaniasis (Leishmania tropica) in a German tourist after travel to Greece. J. Trav. Med. 2009;16:220–222. doi: 10.1111/j.1708-8305.2008.00291.x. [DOI] [PubMed] [Google Scholar]
- Berger F., Romary P., Brachet D., Rapp C., Imbert P., Garrabe E., et al. [Outbreak of cutaneous leishmaniasis in military population coming back from French Guiana] Rev. Epidemiol. Sante Publique. 2006;54:213–221. doi: 10.1016/s0398-7620(06)76717-7. [DOI] [PubMed] [Google Scholar]
- Bern C., Courtenay O., Alvar J. Of cattle, sand flies and men: A systematic review of risk factor analyses for South Asian visceral leishmaniasis and implications for elimination. PLoS Negl. Trop. Dis. 2010;4:e599. doi: 10.1371/journal.pntd.0000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S., Weiss D.J., Cameron E., Bisanzio D., Mappin B., Dalrymple U., et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibbs C.S., Kaufman P.E. Volatile pyrethroids as a potential mosquito abatement tool: A review of pyrethroid-containing spatial repellents. J. Integrated Pest Managem. 2017;8:21. doi: 10.1093/jipm/pmx016. [DOI] [Google Scholar]
- Boggild A.K., Parise M.E., Lewis L.S., Kain K.C. Atovaquone-proguanil: Report from the CDC expert meeting on malaria chemoprophylaxis (II) Am. J. Trop. Med. Hyg. 2007;76:208–223. doi: 10.4269/ajtmh.2007.76.208. [DOI] [PubMed] [Google Scholar]
- Botros B.A., Watts D.M., Soliman A.K., Salib A.W., Moussa M.I., Mursal H., et al. Serological evidence of dengue fever among refugees, Hargeysa, Somalia. J. Med. Virol. 1989;29:79–81. doi: 10.1002/jmv.1890290202. [DOI] [PubMed] [Google Scholar]
- Boussery G., Boelaert M., van Peteghem J., Ejikon P., Henckaerts K. Visceral leishmaniasis (kala-azar) outbreak in Somali refugees and Kenyan shepherds, Kenya. Emerg. Infect. Dis. 2001;7(Suppl. 3):603–604. doi: 10.3201/eid0707.010746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady O.J., Hay S.I. The global expansion of dengue: how Aedes aegypti mosquitoes enabled the first pandemic arbovirus. Annu. Rev. Entomol. 2020;65:191–208. doi: 10.1146/annurev-ento-011019-024918. [DOI] [PubMed] [Google Scholar]
- Brady O.J., Kharisma D.D., Wilastonegoro N.N., OʼReilly K.M., Hendrickx E., Bastos L.S., et al. The cost-effectiveness of controlling dengue in Indonesia using wMel Wolbachia released at scale: A modelling study. BMC Med. 2020;18:186. doi: 10.1186/s12916-020-01638-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson M., Brisson P. Compliance with antimalaria chemoprophylaxis in a combat zone. Am. J. Trop. Med. Hyg. 2012;86:587–590. doi: 10.4269/ajtmh.2012.11-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.E., Webster H.K., Krinchai K., Chuenchitra C., Pipithkul J. Occupational malaria in Thai rangers: Epidemiological, clinical and immunological features. Mil. Med. 1990;155:406–410. doi: 10.1093/milmed/155.9.406. [DOI] [PubMed] [Google Scholar]
- Bruce-Chwatt L. Malaria: From eradication to control. New Scientist. 1984;102:1046. [Google Scholar]
- Buckner E.A., Alto B.W., Lounibos L.P. Vertical transmission of Key West dengue-1 virus by Aedes aegypti and Aedes albopictus (Diptera: Culicidae) mosquitoes from Florida. J. Med. Entomol. 2013;50:1291–1297. doi: 10.1603/me13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett D.A., Cope S.E., Strickman D.A., White G.B. The Deployed Warfighter Protection (DWFP) Research Program: Developing new public health pesticides, application technologies, and repellent systems. J. Integr. Pest Managem. 2013;4:A1–A7. doi: 10.1603/IPM11024. [DOI] [Google Scholar]
- Carreno-Almanzar F.R., Coronado-Galan A., Cala-Gomez S.A., Vega-Vera A. Malaria among migrants in a university hospital in Colombia during 2018: A case series. Trop. Doct. 2021;51:422–424. doi: 10.1177/0049475520981301. [DOI] [PubMed] [Google Scholar]
- Carvalho F.D., Moreira L.A. Why is Aedes aegypti Linnaeus so successful as a species? Neotrop. Entomol. 2017;46:243–255. doi: 10.1007/s13744-017-0520-4. [DOI] [PubMed] [Google Scholar]
- Casalle N., de Barros Pinto Grifoni L., Bosco Mendes A.C., Delort S., Massucato E.M.S. Mucocutaneous leishmaniasis with rare manifestation in the nasal mucosa and cartilage bone septal. Case Rep. Inf. Disp. 2020;2020 doi: 10.1155/2020/8876020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro M.C., Wilson M.E., Bloom D.E. Disease and economic burdens of dengue. Lancet Infect. Dis. 2017;17:e70–e78. doi: 10.1016/S1473-3099(16)30545-X. [DOI] [PubMed] [Google Scholar]
- CDC Dengue virus infections among travelers returning from Haiti,-- Georgia and Nebraska, October 2010. Morb. Mortal. Wkly. Rep. 2011;60:914–917. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6027a2.htm [PubMed] [Google Scholar]
- CDC . Centers for Disease Control and Prevention; Atlanta, GA, USA: 2020. Parasites - Leishmaniasis.https://www.cdc.gov/parasites/leishmaniasis/index.html [Google Scholar]
- CDC . Malaria. Centers for Disease Control and Prevention; Atlanta, GA, USA: 2020. Where malaria occurs.https://www.cdc.gov/malaria/data-research/index.html [Google Scholar]
- CDC . Centers for Disease Control and Prevention; Atlanta, GA, USA: 2020. About Leishmaniasis Parasites - Leishmaniasis.https://www.cdc.gov/leishmaniasis/about/index.html [Google Scholar]
- CDC . Centers for Disease Control and Prevention; Atlanta, GA, USA: 2021. Dengue Vaccine.https://www.cdc.gov/dengue/vaccine/index.html [Google Scholar]
- CDC . Centers for Disease Control and Prevention; Atlanta, GA, USA: 2021. Malaria's Impact Worldwide.https://www.cdc.gov/malaria/malaria_worldwide/impact.html [Google Scholar]
- CDC . Centers for Disease Control and Prevention; Atlanta, GA, USA: 2022. Dengue Vaccine Globally. Prevention.https://www.cdc.gov/dengue/healthcare-providers/hc-providers-prevention.html [Google Scholar]
- CDC . Centers for Disease Control and Prevention; Atlanta, GA, USA: 2022. Malaria. Frequently Asked Questions.https://www.cdc.gov/malaria/about/faqs.html [Google Scholar]
- Cecilio P., Cordeiro-da-Silva A., Oliveira F. Sand flies: Basic information on the vectors of leishmaniasis and their interactions with Leishmania parasites. Commun. Biol. 2022;5:305. doi: 10.1038/s42003-022-03240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlwood J.D., Nenhep S., Protopopoff N., Sovannaroth S., Morgan J.C., Hemingway J. Effects of the spatial repellent metofluthrin on landing rates of outdoor biting anophelines in Cambodia, Southeast Asia. Med. Vet. Entomol. 2016;30:229–234. doi: 10.1111/mve.12168. [DOI] [PubMed] [Google Scholar]
- Chen I., Doum D., Mannion K., Hustedt J., Sovannaroth S., McIver D., et al. Applying the COM-B behaviour change model to a pilot study delivering volatile pyrethroid spatial repellents and insecticide-treated clothing to forest-exposed populations in Mondulkiri Province, Cambodia. Malar. J. 2023;22:251. doi: 10.1186/s12936-023-04685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D.B., Grieco J.P., Apperson C.S., Schal C., Ponnusamy L., Wesson D.M., et al. Effect of spatial repellent exposure on dengue vector attraction to oviposition sites. PLoS Negl. Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury R., Chowdhury V., Faria S., Akter S., Dash A.P., Bhattacharya S.K., et al. Effect of insecticide-treated bed nets on visceral leishmaniasis incidence in Bangladesh. A retrospective cohort analysis. PLoS Negl. Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claborn D.M. The biology and control of leishmaniasis vectors. J. Global Infect. Dis. 2010;2:127–134. doi: 10.4103/0974-777X.62866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.M., Smith D.L., Cotter C., Ward A., Yamey G., Sabot O.J., et al. Malaria resurgence: A systematic review and assessment of its causes. Malar. J. 2012;11:122. doi: 10.1186/1475-2875-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J.P. Gene drives in our future: challenges of and opportunities for using a self-sustaining technology in pest and vector management. BMC Proc. 2018;12(Suppl. 8):9. doi: 10.1186/s12919-018-0110-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conteh L., Shuford K., Agboraw E., Kont M., Kolaczinski J., Patouillard E. Costs and cost-effectiveness of malaria control interventions: A systematic literature review. Value Health. 2021;24:1213–1222. doi: 10.1016/j.jval.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter C., Sturrock H.J., Hsiang M.S., Liu J., Phillips A.A., Hwang J., et al. The changing epidemiology of malaria elimination: New strategies for new challenges. Lancet. 2013;382:900–911. doi: 10.1016/S0140-6736(13)60310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawshaw A.F., Maung T.M., Shafique M., Sint N., Nicholas S., Li M.S., et al. Acceptability of insecticide-treated clothing for malaria prevention among migrant rubber tappers in Myanmar: A cluster-randomized non-inferiority crossover trial. Malar. J. 2017;16:92. doi: 10.1186/s12936-017-1737-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft A.M. Malaria: Prevention in travellers (non-drug interventions) Clin. Evid. 2014;2014:903. https://pubmed.ncbi.nlm.nih.gov/25399869/ [PMC free article] [PubMed] [Google Scholar]
- Dambach P., Schleicher M., Korir P., Ouedraogo S., Dambach J., Sié A., et al. Nightly biting cycles of Anopheles species in rural northwestern Burkina Faso. J. Med. Entomol. 2018;55:1027–1034. doi: 10.1093/jme/tjy043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbro J.M., Muzari M.O., Giblin A., Adamczyk R.M., Ritchie S.A., Devine G.J. Reducing biting rates of Aedes aegypti with metofluthrin: Investigations in time and space. Parasites Vectors. 2017;10:69. doi: 10.1186/s13071-017-2004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B., Mane E., Gurbuzer L.Y., Caivano G., Piedrahita N., Azhar N., et al. Food and Agriculture Organization of the Inited Nations; Rome: 2023. Estimating global and country-level employment in agrifood systems. FAO Statistics Working Paper Series, No. 23-34. [DOI] [Google Scholar]
- Day J.F. Mosquito oviposition behavior and vector control. Insects. 2016;7:65. doi: 10.3390/insects7040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beer P., el Harith A., Deng L.L., Semiao-Santos S.J., Chantal B., van Grootheest M. A killing disease epidemic among displaced Sudanese population identified as visceral leishmaniasis. Am. J. Trop. Med. Hyg. 1991;44:283–289. doi: 10.4269/ajtmh.1991.44.283. [DOI] [PubMed] [Google Scholar]
- de Laval F., Dia A., Plumet S., Decam C., Leparc Goffart I., Deparis X., et al. Dengue surveillance in the French armed forces: A dengue sentinel surveillance system in countries without efficient local epidemiological surveillance. J. Trav. Med. 2013;20:259–261. doi: 10.1111/j.1708-8305.2012.00674.x. [DOI] [PubMed] [Google Scholar]
- de Laval F., Simon F., Bogreau H., Rapp C., Wurtz N., Oliver M., et al. Emergence of Plasmodium ovale malaria among the French armed forces in the Republic of Ivory Coast: 20 years of clinical and biological experience. Clin. Infect. Dis. 2014;58:e122–e128. doi: 10.1093/cid/ciu021. [DOI] [PubMed] [Google Scholar]
- de Oliveira Guerra J.A., Talhari S., Paes M.G., Garrido M., Talhari J.M. [Clinical and diagnostic aspects of American tegumentary leishmaniosis in soldiers simultaneously exposed to the infection in the Amazon Region] Rev. Soc. Bras. Med. Trop. 2003;36:587–590. doi: 10.1590/S0037-86822003000500008. [DOI] [PubMed] [Google Scholar]
- Dedet J.P., Pradinaud R., Gay F. Epidemiological aspects of human cutaneous leishmaniasis in French Guiana. Trans. R. Soc. Trop. Med. Hyg. 1989;83:616–620. doi: 10.1016/0035-9203(89)90375-1. [DOI] [PubMed] [Google Scholar]
- Deller J.J., Jr., Russell P. An analysis of fevers of unknown origin in American soldiers in Vietnam. Ann. Intern. Med. 1967;66:1129–1143. doi: 10.7326/0003-4819-66-6-1129. [DOI] [PubMed] [Google Scholar]
- Denz A., Njoroge M.M., Tambwe M.M., Champagne C., Okumu F., van Loon J.J.A., et al. Predicting the impact of outdoor vector control interventions on malaria transmission intensity from semi-field studies. Parasites Vectors. 2021;14:64. doi: 10.1186/s13071-020-04560-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRaedt Banks S., Orsborne J., Gezan S.A., Kaur H., Wilder-Smith A., Lindsey S.W., et al. Permethrin-treated clothing as protection against the dengue vector, Aedes aegypti: Extent and duration of protection. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0004109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev V. In: Genetically modified and other innovative vector control technologies: Eco-bio-social considerations for safe application. Tyagi B.K., editor. Springer Singapore; Singapore: 2021. Long-lasting insecticidal nets: an evidence-based technology for malaria vector control and future perspectives; pp. 297–309. [Google Scholar]
- Devictor X., Do Q.-T. World Bank; Washington, D.C: 2016. How many years have refugees been in exile? Policy Research Working Paper, No. WPS 7810.https://documents1.worldbank.org/curated/en/549261472764700982/pdf/WPS7810.pdf [Google Scholar]
- Devine A., Pasaribu A.P., Teferi T., Pham H.T., Awab G.R., Contantia F., et al. Provider and household costs of Plasmodium vivax malaria episodes: A multicountry comparative analysis of primary trial data. Bull. World Health Organ. 2019;97:828–836. doi: 10.2471/BLT.18.226688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz J.H. Chemical and plant-based insect repellents: Efficacy, safety, and toxicity. Wilderness Environ. Med. 2016;27:153–163. doi: 10.1016/j.wem.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Doltario A.B., Menon L.J.B., Bollela V.R., Martinez R., de Almeida E.A.D.C., da Fonseca B.A.L., et al. Malaria and other febrile diseases among travellers: The experience of a reference centre located outside the Brazilian Amazon Region. Malar. J. 2016;15:294. doi: 10.1186/s12936-016-1347-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douine M., Mosnier E., Le Hingrat Q., Charpentier C., Corlin F., Hureau L., et al. Illegal gold miners in French Guiana: A neglected population with poor health. BMC Publ. Health. 2017;18:23. doi: 10.1186/s12889-017-4557-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douine M., Musset L., Corlin F., Pelleau S., Pasquier J., Mutricy L., et al. Prevalence of Plasmodium spp. in illegal gold miners in French Guiana in 2015: A hidden but critical malaria reservoir. Malar. J. 2016;15:315. doi: 10.1186/s12936-016-1367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douine M., Sanna A., Hiwat H., Briolant S., Nacher M., Belleoud D., et al. Investigation of a possible malaria epidemic in an illegal gold mine in French Guiana: An original approach in the remote Amazonian forest. Malar. J. 2019;18:91. doi: 10.1186/s12936-019-2721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du R., Hotez P.J., Al-Salem W.S., Acosta-Serrano A. Old World cutaneous leishmaniasis and refugee crises in the Middle East and North Africa. PLoS Negl. Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy P.E., Le Guillouzic H., Gass R.F., Innis B.L. Murine typhus identified as a major cause of febrile illness in a camp for displaced Khmers in Thailand. Am. J. Trop. Med. Hyg. 1990;43:520–526. doi: 10.4269/ajtmh.1990.43.520. [DOI] [PubMed] [Google Scholar]
- Durrani A.Z., Durrani H.Z., Kamal N. Prevalence of Leishmania in sand fly in Pakistan. Pakistan J. Zool. 2012;44:61–65. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [Google Scholar]
- Eisler R. Health risks of gold miners: A synoptic review. Environ. Geochem. Health. 2003;25:325–345. doi: 10.1023/a:1024573701073. [DOI] [PubMed] [Google Scholar]
- Ekawati L.L., Johnson K.C., Jacobson J.O., Cueto C.A., Zarlinda I., Elyazar I.R.F., et al. Defining malaria risks among forest workers in Aceh, Indonesia: A formative assessment. Malar. J. 2020;19:441. doi: 10.1186/s12936-020-03511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endy T.P., Anderson K.B., Nisalak A., Yoon I.K., Green S., Rothman A.L., et al. Determinants of inapparent and symptomatic dengue infection in a prospective study of primary school children in Kamphaeng Phet, Thailand. PLoS Negl. Trop. Dis. 2011;5:e975. doi: 10.1371/journal.pntd.0000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennibi K., Rabhi M., Boudlal M., Sbai Idrissi K., Chaari J., Toloune F. [Malaria in the United Nations troops in the Democratic Republic of the Congo during 2005] Med. Sante Trop. 2012;22:220–221. doi: 10.1684/mst.2012.0037. [DOI] [PubMed] [Google Scholar]
- EPA . U.S. Enviromental Protection Agency; Washington DC, USA: 2023. Repellent-treated clothing.https://www.epa.gov/insect-repellents/repellent-treated-clothing [Google Scholar]
- Erhart A., Ngo D.T., Phan V.K., Ta T.T., Van Overmeir C., Speybroeck N., et al. Epidemiology of forest malaria in central Vietnam: A large-scale cross-sectional survey. Malar. J. 2005;4:58. doi: 10.1186/1475-2875-4-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana G., Leidner A.J., Waterman S.H., Perkins T.A. Cost-effectiveness of dengue vaccination in Puerto Rico. PLoS Negl. Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0009606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks E.L., Saeung M., Pongsiri A., Vajda E., Wang Y., McIver D.J., et al. Inference for entomological semi-field experiments: Fitting a mathematical model assessing personal and community protection of vector-control interventions. Comput. Biol. Med. 2023;168 doi: 10.1016/j.compbiomed.2023.107716. [DOI] [PubMed] [Google Scholar]
- Ferede G., Tiruneh M., Abate E., Wondimeneh Y., Damtie D., Gadisa E., et al. A serologic study of dengue in northwest Ethiopia: Suggesting preventive and control measures. PLoS Negl. Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira M.U., Gamboa D., Torres K., Rodriguez-Ferrucci H., Soto-Calle V.E., Pardo K., et al. Evidence-based malaria control and elimination in the Amazon: Input from the International Center of Excellence in Malaria Research Network in Peru and Brazil. Am. J. Trop. Med. Hyg. 2022;107(Suppl. 4):160–167. doi: 10.4269/ajtmh.21-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira-de-Lima V.H., Lima-Camara T.N. Natural vertical transmission of dengue virus in Aedes aegypti and Aedes albopictus: A systematic review. Parasites Vectors. 2018;11:77. doi: 10.1186/s13071-018-2643-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorenzano J.M., Koehler P.G., Xue R.D. Attractive toxic sugar bait (ATSB) for control of mosquitoes and its impact on non-target organisms: A review. Int. J. Environ. Res. Publ. Health. 2017;14:398. doi: 10.3390/ijerph14040398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgearini J.C., Michalowski C.B., Assumpcao E., Pohlmann A.R., Guterres S.S. Development of an insect repellent spray for textile based on permethrin-loaded lipid-core nanocapsules. J. Nanosci. Nanotechnol. 2016;16:1301–1309. doi: 10.1166/jnn.2016.11665. [DOI] [PubMed] [Google Scholar]
- Frances S.P., Auliff A.M., Edstein M.D., Cooper R.D. Survey of personal protection measures against mosquitoes among Australian defense force personnel deployed to East Timor. Mil. Med. 2003;168:227–230. https://pubmed.ncbi.nlm.nih.gov/12685689/ [PubMed] [Google Scholar]
- Galactionova K., Tediosi F., Camponovo F., Smith T.A., Gething P.W., Penny M.A. Country-specific predictions of the cost-effectiveness of malaria vaccine RTS,S/AS01 in endemic Africa. Vaccine. 2017;35:53–60. doi: 10.1016/j.vaccine.2016.11.042. [DOI] [PubMed] [Google Scholar]
- Gawthrop M., Ford L. Malaria in children: Bite avoidance and prophylaxis. Prescriber. 2009;20:28–36. doi: 10.1002/psb.547. [DOI] [Google Scholar]
- GBD 2015 DALYs & HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1603–1658. doi: 10.1016/S0140-6736(16)31460-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genchi C., Rinaldi L., Mortarino M., Genchi M., Cringoli G. Climate and Dirofilaria infection in Europe. Vet. Parasitol. 2009;163:286–292. doi: 10.1016/j.vetpar.2009.03.026. [DOI] [PubMed] [Google Scholar]
- George S., Joy T.M., Kumar A., Panicker K.N., George L.S., Raj M., et al. Prevalence of neglected tropical diseases (leishmaniasis and lymphatic filariasis) and malaria among a migrant labour settlement in Kerala, India. J. Immigr. Minority Health. 2019;21:563–569. doi: 10.1007/s10903-018-0767-9. [DOI] [PubMed] [Google Scholar]
- Ghamari M., Khoobdel M. Permethrin-impregnated military uniform protection against different species of biting insects: Narrative review. J. Mil. Med. 2019;21:221–233. http://militarymedj.ir/article-1-2126-en.html [Google Scholar]
- Gibbons R.V., Streitz M., Babina T., Fried J.R. Dengue and US military operations from the Spanish-American War through today. Emerg. Infect. Dis. 2012;18:623–630. doi: 10.3201/eid1804.110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giladi M., Danon Y.L., Greenblatt C., Schinder E., Nili E. Keziot - a new endemic site of cutaneous leishmaniasis in Israel. An epidemiological and clinical study of a non-immune population entering an endemic area. Trop. Geogr. Med. 1985;37:298–303. https://www.ncbi.nlm.nih.gov/pubmed/4095766 [PubMed] [Google Scholar]
- Godói I.P., Lemos L.L., de Araújo V.E., Bonoto B.C., Godman B., Guerra Júnior A.A. CYD-TDV dengue vaccine: Systematic review and meta-analysis of efficacy, immunogenicity and safety. J. Comp. Eff. Res. 2017;6:165–180. doi: 10.2217/cer-2016-0045. [DOI] [PubMed] [Google Scholar]
- González U., Pinart M., Sinclair D., Firooz A., Enk C., Vélez I.D., et al. Vector and reservoir control for preventing leishmaniasis. Cochrane Database Syst. Rev. 2015:Cd008736. doi: 10.1002/14651858.CD008736.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossner C.M., Fournet N., Frank C., Fernandez-Martinez B., Del Manso M., Gomes D.J., et al. Dengue virus infections among European travellers, 2015 to 2019. Euro Surveill. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.2.2001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradoni L. In: The leishmaniases: Old neglected tropical diseases. Bruschi F., Gradoni L., editors. Springer; Cham: 2018. A brief introduction to leishmaniasis epidemiology; pp. 1–13. [DOI] [Google Scholar]
- Gratz N.G. Critical review of the vector status of Aedes albopictus. Med. Vet. Entomol. 2004;18:215–227. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- Gryseels C., Uk S., Sluydts V., Durnez L., Phoeuk P., Suon S., et al. Factors influencing the use of topical repellents: Implications for the effectiveness of malaria elimination strategies. Sci. Rep. 2015;5 doi: 10.1038/srep16847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler D.J. CRC Press Inc.; Boca Raton, USA: 1988. Epidemiology of arthropod-borne viral disease. [Google Scholar]
- Gubler D.J. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 1998;11:480–496. doi: 10.1128/CMR.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler D.J. Resurgent vector-borne diseases as a global health problem. Emerg. Infect. Dis. 1998;4:442–450. doi: 10.3201/eid0403.980326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler D.J. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10:100–103. doi: 10.1016/S0966-842X(01)02288-0. [DOI] [PubMed] [Google Scholar]
- Gunathilaka N., Semege S., Pathirana N., Manamperi N., Udayanga L., Wijesinghe H., et al. Prevalence of cutaneous leishmaniasis infection and clinico-epidemiological patterns among military personnel in Mullaitivu and Kilinochchi districts of the Northern Province, early war-torn areas in Sri Lanka. Parasites Vectors. 2020;13:263. doi: 10.1186/s13071-020-04137-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S.B., Wilder-Smith A. Severe dengue in travellers: Pathogenesis, risk and clinical management. J. Trav. Med. 2019;26 doi: 10.1093/jtm/taz062. [DOI] [PubMed] [Google Scholar]
- Halstead S.B. Dengue hemorrhagic fever: Two infections and antibody-dependent enhancement, a brief history and personal memoir. Rev. Cubana Med. Trop. 2002;54:171–179. https://www.ncbi.nlm.nih.gov/pubmed/15846943 [PubMed] [Google Scholar]
- Hayes C.G., OʼRourke T.F., Fogelman V., Leavengood D.D., Crow G., Albersmeyer M.M., et al. Dengue fever in American military personnel in the Philippines: Clinical observations on hospitalized patients during a 1984 epidemic. Southeast Asian J. Trop. Med. Publ. Health. 1989;20:1–8. https://www.ncbi.nlm.nih.gov/pubmed/2772694 [PubMed] [Google Scholar]
- Henderson A., Simon J., Melia W., Navein J., McCallum J. Polyresistant malaria in Gurkha soldiers returning from Papua New Guinea: Treatment and prevention. J. Roy. Army Med. Corps. 1986;132:37–41. doi: 10.1136/jramc-132-01-09. [DOI] [PubMed] [Google Scholar]
- Henry K., Mayet A., Hernandez M., Frechard G., Blanc P.A., Schmitt M., et al. Outbreak of cutaneous leishmaniasis among military personnel in French Guiana, 2020: Clinical, phylogenetic, individual and environmental aspects. PLoS Negl. Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0009938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepburn N.C., Tidman M.J., Hunter J.A. Cutaneous leishmaniasis in British troops from Belize. Br. J. Dermatol. 1993;128:63–68. doi: 10.1111/j.1365-2133.1993.tb00149.x. [DOI] [PubMed] [Google Scholar]
- Hesse E.M., Martinez L.J., Jarman R.G., Lyons A.G., Eckels K.H., De La Barrera R.A., et al. Dengue virus exposures among deployed U.S. military personnel. Am. J. Trop. Med. Hyg. 2017;96:1222–1226. doi: 10.4269/ajtmh.16-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill N., Lenglet A., Arnez A.M., Carneiro I. Plant-based insect repellent and insecticide-treated bed nets to protect against malaria in areas of early evening biting vectors: Double blind randomised placebo controlled clinical trial in the Bolivian Amazon. BMJ. 2007;335:1023. doi: 10.1136/bmj.39356.574641.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiwat H., Hardjopawiro L.S., Takken W., Villegas L. Novel strategies lead to pre-elimination of malaria in previously high-risk areas in Suriname, South America. Malar. J. 2012;11:10. doi: 10.1186/1475-2875-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoermann A., Tapanelli S., Capriotti P., Del Corsano G., Masters E.K.G., Habtewold T., et al. Converting endogenous genes of the malaria mosquito into simple non-autonomous gene drives for population replacement. eLife. 2021;10 doi: 10.7554/eLife.58791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honorio N.A., Codeco C.T., Alves F.C., Magalhaes M.A., Lourenco-De-Oliveira R. Temporal distribution of Aedes aegypti in different districts of Rio de Janeiro, Brazil, measured by two types of traps. J. Med. Entomol. 2009;46:1001–1014. doi: 10.1603/033.046.0505. [DOI] [PubMed] [Google Scholar]
- Hotez P.J. The rise of leishmaniasis in the twenty-first century. Trans. R. Soc. Trop. Med. Hyg. 2018;112:421–422. doi: 10.1093/trstmh/try075. [DOI] [PubMed] [Google Scholar]
- Hotez P.J., Savioli L., Fenwick A. Neglected tropical diseases of the Middle East and North Africa: Review of their prevalence, distribution, and opportunities for control. PLoS Negl. Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IDMC . Internal Displacement Monitoring Centre, Geneva, Switzerland. 2022. Global report on internal displacement.https://www.internal-displacement.org/publications/2022-global-report-on-internal-displacement-grid/ [Google Scholar]
- ILO . Vol. 3, 4th ed. International Labour Office; Geneva, Switzerland: 1998. Encyclopaedia of Occupational Health and Safety. [Google Scholar]
- Inci R., Ozturk P., Mulayim M.K., Ozyurt K., Alatas E.T., Inci M.F. Effect of the Syrian civil war on prevalence of cutaneous leishmaniasis in southeastern Anatolia, Turkey. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2015;21:2100–2104. doi: 10.12659/MSM.893977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis J.P., Lau J. Pooling research results: Benefits and limitations of meta-analysis. Jt. Comm. J. Qual. Improv. 1999;25:462–469. doi: 10.1016/s1070-3241(16)30460-6. [DOI] [PubMed] [Google Scholar]
- IVCC . IVCC, Liverpool School of Tropical Medicine; 2020. An expert review of spatial repellents for mosquito control.https://www.ivcc.com/resources/an-expert-review-of-spatial-repellents-for-mosquito-control/ [Google Scholar]
- Jacobson R.L. Leishmaniasis in an era of conflict in the Middle East. Vector Borne Zoonotic Dis. 2011;11:247–258. doi: 10.1089/vbz.2010.0068. [DOI] [PubMed] [Google Scholar]
- James A.A. Gene drive systems in mosquitoes: Rules of the road. Trends Parasitol. 2005;21:64–67. doi: 10.1016/j.pt.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Janaki M.D.S., Aryaprema V.S., Fernando N., Handunnetti S.M., Weerasena O.V.D.S.J., Pathirana P.P.S.L., et al. Prevalence and resting behaviour of dengue vectors, Aedes aegypti and Aedes albopictus in dengue high risk urban settings in Colombo, Sri Lanka. J. Asia Pac. Entomol. 2022;25 doi: 10.1016/j.aspen.2022.101961. [DOI] [Google Scholar]
- Jeffree S.M., Ahmed K., Safian N., Hassan R., Mihat O., Lukman K.A., et al. Falciparum malaria outbreak in Sabah linked to an immigrant rubber tapper. Am. J. Trop. Med. Hyg. 2018;98:45–50. doi: 10.4269/ajtmh.17-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek T. Dengue fever in international travelers. Clin. Infect. Dis. 2000;31:144–147. doi: 10.1086/313889. [DOI] [PubMed] [Google Scholar]
- Jones R.T., Tusting L.S., Smith H.M.P., Segbaya S., Macdonald M.B., Bangs M.J., et al. The impact of industrial activities on vector-borne disease transmission. Acta Trop. 2018;188:142–151. doi: 10.1016/j.actatropica.2018.08.033. [DOI] [PubMed] [Google Scholar]
- Joshi A.B., Das M.L., Akhter S., Chowdhury R., Mondal D., Kumar V., et al. Chemical and environmental vector control as a contribution to the elimination of visceral leishmaniasis on the Indian subcontinent: Cluster randomized controlled trials in Bangladesh, India and Nepal. BMC Med. 2009;7:54. doi: 10.1186/1741-7015-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kain K.C., Shanks G.D., Keystone J.S. Malaria chemoprophylaxis in the age of drug resistance. I. Currently recommended drug regimens. Clin. Infect. Dis. 2001;33:226–234. doi: 10.1086/321817. [DOI] [PubMed] [Google Scholar]
- Kakkar M. Dengue fever is massively under-reported in India, hampering our response. BMJ. 2012;345 doi: 10.1136/bmj.e8574. [DOI] [PubMed] [Google Scholar]
- Kanani K., Amr Z.S., Shadfan B., Khorma R., Ro G., Abid M., et al. Cutaneous leishmaniasis among Syrian refugees in Jordan. Acta Trop. 2019;194:169–171. doi: 10.1016/j.actatropica.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Karimkhani C., Wanga V., Coffeng L.E., Naghavi P., Dellavalle R.P., Naghavi M. Global burden of cutaneous leishmaniasis: A cross-sectional analysis from the Global Burden of Disease Study 2013. Lancet Infect. Dis. 2016;16:584–591. doi: 10.1016/S1473-3099(16)00003-7. [DOI] [PubMed] [Google Scholar]
- Kasap O.E., Votypka J., Alten B. The distribution of the Phlebotomus major complex (Diptera: Psychodidae) in Turkey. Acta Trop. 2013;127:204–211. doi: 10.1016/j.actatropica.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Kawar G.I., Maayah J.F., Rawashdeh B.T. Analysis of malaria cases among United Nations troops in Sierra Leone. Saudi Med. J. 2003;24:881–884. https://www.ncbi.nlm.nih.gov/pubmed/12939677 [PubMed] [Google Scholar]
- Kaya K., An I. Auricular cutaneous leishmaniasis: A retrospective evaluation of 12 patients. Electronic J. Otolaryngol. 2024;23:1. https://kbb-forum.net/journal/text.php?lang=en&id=639 [Google Scholar]
- Khallaayoune K., Qualls W.A., Revay E.E., Allan S.A., Arheart K.L., Kravchenko V.D., et al. Attractive toxic sugar baits: control of mosquitoes with the low-risk active ingredient dinotefuran and potential impacts on nontarget organisms in Morocco. Environ. Entomol. 2013;42:1040–1045. doi: 10.1603/EN13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheang S.T., Lin M.A., Lwin S., Naing Y.H., Yarzar P., Kak N., et al. Malaria case detection among mobile populations and migrant workers in Myanmar: Comparison of 3 service delivery approaches. Glob. Health Sci. Pract. 2018;6:384–389. doi: 10.9745/GHSP-D-17-00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimani E.W., Vulule J.M., Kuria I.W., Mugisha F. Use of insecticide-treated clothes for personal protection against malaria: A community trial. Malar. J. 2006;5:63. doi: 10.1186/1475-2875-5-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioko U.M. Economic burden of malaria on subsistence crop production in Kenya. Int. J. Educ. Res. 2013;1:1–20. https://www.ijern.com/images/February-2013/13.pdf [Google Scholar]
- Kitchen L.W., Lawrence K.L., Coleman R.E. The role of the United States military in the development of vector control products, including insect repellents, insecticides, and bed nets. J. Vector Ecol. 2009;34:50–61. doi: 10.1111/j.1948-7134.2009.00007.x. [DOI] [PubMed] [Google Scholar]
- Kitchener S., Leggat P.A., Brennan L., McCall B. Importation of dengue by soldiers returning from East Timor to north Queensland, Australia. J. Trav. Med. 2002;9:180–183. doi: 10.2310/7060.2002.24234. [DOI] [PubMed] [Google Scholar]
- Kitchener S., Nasveld P., Russell B., Elmes N. An outbreak of malaria in a forward battalion on active service in East Timor. Mil. Med. 2003;168:457–459. doi: 10.1093/milmed/168.6.457. [DOI] [PubMed] [Google Scholar]
- Kittayapong P., Olanratmanee P., Maskhao P., Byass P., Logan J., Tozan Y. Mitigating diseases transmitted by Aedes mosquitoes: A cluster-randomised trial of permethrin-impregnated school uniforms. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviat E. Hudsonia Ltd., Annandale; NY: 1993. Traditional protection against biting flies. [Google Scholar]
- Klein T.A., Pacha L.A., Lee H.C., Kim H.C., Lee W.J., Lee J.K., et al. Plasmodium vivax malaria among U.S. forces Korea in the Republic of Korea, 1993–2007. Mil. Med. 2009;174:412–418. doi: 10.7205/milmed-d-01-4608. [DOI] [PubMed] [Google Scholar]
- Klein T.A., Seyoum B., Forshey B.M., Ellis K.K., McCall H., Jackson, et al. Cluster of vivax malaria in U.S. soldiers training near the demilitarized zone, Republic of Korea during 2015. MSMR. 2018;25:4–9. https://www.ncbi.nlm.nih.gov/pubmed/30475636 [PubMed] [Google Scholar]
- Kolaczinski J., Brooker S., Reyburn H., Rowland M. Epidemiology of anthroponotic cutaneous leishmaniasis in Afghan refugee camps in northwest Pakistan. Trans. R. Soc. Trop. Med. Hyg. 2004;98:373–378. doi: 10.1016/j.trstmh.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Koltas I.S., Eroglu F., Alabaz D., Uzun S. The emergence of Leishmania major and Leishmania donovani in southern Turkey. Trans. R. Soc. Trop. Med. Hyg. 2014;108:154–158. doi: 10.1093/trstmh/trt119. [DOI] [PubMed] [Google Scholar]
- Kotwal R.S., Wenzel R.B., Sterling R.A., Porter W.D., Jordan N.N., Petruccelli B.P. An outbreak of malaria in US Army rangers returning from Afghanistan. JAMA. 2005;293:212–216. doi: 10.1001/jama.293.2.212. [DOI] [PubMed] [Google Scholar]
- Kraemer M.U.G., Reiner R.C., Jr., Brady O.J., Messina J.P., Gilbert M., Pigott D.M., et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019;4:854–863. doi: 10.1038/s41564-019-0376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Mandal R., Das S., Kesari S., Dinesh D.S., Pandey K., et al. Kala-azar elimination in a highly-endemic district of Bihar, India: A success story. PLoS Negl. Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0008254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwagonza L., Masiira B., Kyobe-Bosa H., Kadobera D., Atuheire E.B., Lubwama B., et al. Outbreak of yellow fever in central and southwestern Uganda, February-May 2016. BMC Infect. Dis. 2018;18:548. doi: 10.1186/s12879-018-3440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrou K., Hammond A.M., Galizi R., Kranjc N., Burt A., Beaghton A.K., et al. A CRISPR-Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nat. Biotechnol. 2018;36:1062–1066. doi: 10.1038/nbt.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainson R., Shaw J.J. A brief history of the genus Leishmania (Protozoa: Kinetoplastida) in the Americas with particular reference to Amazonian Brazil. Ciencia Cultura Sao Paulo. 1992;44:94–106. https://eurekamag.com/research/002/281/002281719.php [Google Scholar]
- Lalani T., Yun H., Tribble D., Ganesan A., Kunz A., Fairchok M., et al. A comparison of compliance rates with anti-vectorial protective measures during travel to regions with dengue or chikungunya activity, and regions endemic for Plasmodium falciparum malaria. J. Trav. Med. 2016;23 doi: 10.1093/jtm/taw043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn S.D., Whetham J., Chiodini P.L., Kanagalingam J., Watson J., Behrens R.H., et al. New World mucosal and cutaneous leishmaniasis: An emerging health problem among British travellers. QJM. 2004;97:781–788. doi: 10.1093/qjmed/hch127. [DOI] [PubMed] [Google Scholar]
- Lee J.S., Lee W.J., Cho S.H., Ree H.I. Outbreak of vivax malaria in areas adjacent to the demilitarized zone, South Korea, 1998. Am. J. Trop. Med. Hyg. 2002;66:13–17. doi: 10.4269/ajtmh.2002.66.13. [DOI] [PubMed] [Google Scholar]
- Leta S., Beyene T.J., De Clercq E.M., Amenu K., Kraemer M.U.G., Revie C.W. Global risk mapping for major diseases transmitted by Aedes aegypti and Aedes albopictus. Int. J. Infect. Dis. 2018;67:25–35. doi: 10.1016/j.ijid.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung S., Windbichler N., Wenger E.A., Bever C.A., Selvaraj P. Population replacement gene drive characteristics for malaria elimination in a range of seasonal transmission settings: A modelling study. Malar. J. 2022;21:226. doi: 10.1186/s12936-022-04242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Li Y., Lu S., Dong J., Xu H., Zhang Q., et al. Epidemiological survey and screening strategy for dengue virus in blood donors from Yunnan Province. BMC Infect. Dis. 2021;21:104. doi: 10.1186/s12879-021-05810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Yang Y., Xiao N., Zhou S., Lin K., Wang D., et al. Malaria imported from Ghana by returning gold miners, China, 2013. Emerg. Infect. Dis. 2015;21:864–867. doi: 10.3201/2105.141712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightburn E., Meynard J.B., Morand J.J., Garnotel E., Kraemer P., Hovette P., et al. [Epidemiologic surveillance of cutaneous leishmaniasis in Guiana. Summary of military data collected over 10 years] Med. Trop. 2002;62:545–553. https://pubmed.ncbi.nlm.nih.gov/12616949/ [PubMed] [Google Scholar]
- Lindback H., Lindback J., Tegnell A., Janzon R., Vene S., Ekdahl K. Dengue fever in travelers to the tropics, 1998 and 1999. Emerg. Infect. Dis. 2003;9:438–442. doi: 10.3201/eid0904.020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner A.K., Richter J., Gertler M., Nikolaus M., Equihua Martinez G., Muller K., et al. Cutaneous leishmaniasis in refugees from Syria: Complex cases in Berlin 2015–2020. J. Trav. Med. 2020;27 doi: 10.1093/jtm/taaa161. [DOI] [PubMed] [Google Scholar]
- Loiseau R., Nabet C., Simon S., Ginouves M., Brousse P., Blanchet D., et al. American cutaneous leishmaniasis in French Guiana: An epidemiological update and study of environmental risk factors. Int. J. Dermatol. 2019;58:1323–1328. doi: 10.1111/ijd.14625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londono-Renteria B., Patel J.C., Vaughn M., Funkhauser S., Ponnusamy L., Grippin C., et al. Long-lasting permethrin-impregnated clothing protects against mosquito bites in outdoor workers. Am. J. Trop. Med. Hyg. 2015;93:869–874. doi: 10.4269/ajtmh.15-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loney P.L., Chambers L.W., Bennett K.J., Roberts J.G., Stratford P.W. Critical appraisal of the health research literature: Prevalence or incidence of a health problem. Chron. Dis. Can. 1998;19:170–176. https://www.ncbi.nlm.nih.gov/pubmed/10029513 [PubMed] [Google Scholar]
- Lowe R. The impact of global environmental change on vector-borne disease risk: A modelling study. Lancet Planet. Health. 2018;2:S1. doi: 10.1016/S2542-5196(18)30086-X. [DOI] [Google Scholar]
- Lukwa A.T., Mawoyo R., Zablon K.N., Siya A., Alaba O. Effect of malaria on productivity in a workplace: The case of a banana plantation in Zimbabwe. Malar. J. 2019;18:390. doi: 10.1186/s12936-019-3021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupi E., Hatz C., Schlagenhauf P. The efficacy of repellents against Aedes, Anopheles, Culex and Ixodes spp. - a literature review. Trav. Med. Infect. Dis. 2013;11:374–411. doi: 10.1016/j.tmaid.2013.10.005. [DOI] [PubMed] [Google Scholar]
- MacDonald A.J., Mordecai E.A. Amazon deforestation drives malaria transmission, and malaria burden reduces forest clearing. Proc. Natl. Acad. Sci. USA. 2019;116:22212–22218. doi: 10.1073/pnas.1905315116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magris M., Rubio-Palis Y., Alexander N., Ruiz B., Galvan N., Frias D., et al. Community-randomized trial of lambdacyhalothrin-treated hammock nets for malaria control in Yanomami communities in the Amazon region of Venezuela. Trop. Med. Int. Health. 2007;12:392–403. doi: 10.1111/j.1365-3156.2006.01801.x. [DOI] [PubMed] [Google Scholar]
- Maia M.F., Kliner M., Richardson M., Lengeler C., Moore S.J. Mosquito repellents for malaria prevention. Cochrane Database Syst. Rev. 2018;2 doi: 10.1002/14651858.CD011595.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia M.F., Onyango S.P., Thele M., Simfukwe E.T., Turner E.L., Moore S.J. Do topical repellents divert mosquitoes within a community? Health equity implications of topical repellents as a mosquito bite prevention tool. PLoS One. 2013;8 doi: 10.1371/journal.pone.0084875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia M.F., Tenywa F.C., Nelson H., Kambagha A., Ashura A., Bakari I., et al. Attractive toxic sugar baits for controlling mosquitoes: a qualitative study in Bagamoyo, Tanzania. Malar. J. 2018;17:22. doi: 10.1186/s12936-018-2171-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaria Consortium Indoor residual spraying (Vector control) 2021. https://www.malariaconsortium.org/vector-control/indoor-residual-spraying-2017.htm
- Mansueto P., Seidita A., Vitale G., Cascio A. Leishmaniasis in travelers: A literature review. Trav. Med. Infect. Dis. 2014;12:563–581. doi: 10.1016/j.tmaid.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Marasinghe D.H., Cheaveau J., Meatherall B., Kuhn S., Vaughan S., Zimmer R., et al. Risk of malaria associated with travel to malaria-endemic areas to visit friends and relatives: A population-based case-control study. CMAJ Open. 2020;8:e60–e68. doi: 10.9778/cmajo.20190070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroli M., Feliciangeli M.D., Bichaud L., Charrel R.N., Gradoni L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med. Vet. Entomol. 2013;27:123–147. doi: 10.1111/j.1365-2915.2012.01034.x. [DOI] [PubMed] [Google Scholar]
- Marshall J.M., Akbari O.S., Friedman R.M. Gene drives: New and improved. Issues Sci. Technol. 2020;36:72–78. https://issues.org/gene-drives/ [Google Scholar]
- Martins J.F., Marques C., Nieto-Andrade B., Kelley J., Patel D., Nace D., et al. Malaria risk and prevention in Asian migrants to Angola. Am. J. Trop. Med. Hyg. 2020;103:1918–1926. doi: 10.4269/ajtmh.20-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins-Filho P.R., Damascena N.P., Araujo A.P.D., Silva M.C., Santiago B.M., Deitos A.R., et al. The devastating impact of illegal mining on indigenous health: A focus on malaria in the Brazilian Amazon. EXCLI J. 2023;22:400–402. doi: 10.17179/excli2023-6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masyeni S., Yohan B., Somia I.K.A., Myint K.S.A., Sasmono R.T. Dengue infection in international travellers visiting Bali, Indonesia. J.Travel Med. 2018;25 doi: 10.1093/jtm/tay061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly P. Genetical aspects of the Aedes aegypti problem: I. Taxonomy and bionomics. Ann. Trop. Med. Parasitol. 1957;51:392–408. [PubMed] [Google Scholar]
- Mazigo H.D., Rumisha S.F., Chiduo M.G., Bwana V.M., Mboera L.E.G. Malaria among rice farming communities in Kilangali village, Kilosa District, Central Tanzania: Prevalence, intensity and associated factors. Infect. Dis. Poverty. 2017;6:101. doi: 10.1186/s40249-017-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride W.J. Chemoprophylaxis of tropical infectious diseases. Pharmaceuticals. 2010;3:1561–1575. doi: 10.3390/ph3051561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medlock J.M., Hansford K.M., Van Bortel W., Zeller H., Alten B. A summary of the evidence for the change in European distribution of phlebotomine sand flies (Diptera: Psychodidae) of public health importance. J. Vector Ecol. 2014;39:72–77. doi: 10.1111/j.1948-7134.2014.12072.x. [DOI] [PubMed] [Google Scholar]
- Meek S.R. Epidemiology of malaria in displaced Khmers on the Thai-Kampuchean border. Southeast Asian J. Trop. Med. Publ. Health. 1988;19:243–252. https://www.ncbi.nlm.nih.gov/pubmed/3067373 [PubMed] [Google Scholar]
- Messenger L.A., Furnival-Adams J., Chan K., Pelloquin B., Paris L., Rowland M. Vector control for malaria prevention during humanitarian emergencies: A systematic review and meta-analysis. Lancet Global Health. 2023;11:e534–e545. doi: 10.1016/S2214-109X(23)00044-X. [DOI] [PubMed] [Google Scholar]
- Messina J.P., Brady O.J., Golding N., Kraemer M.U.G., Wint G.R.W., Ray S.E., et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 2019;4:1508–1515. doi: 10.1038/s41564-019-0476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mika F., De Sanctis S., Nicosia V., Vasile C., Dalida R. SPE International Conference on Health, Safety, and Environment in Oil and Gas Exploration and Production, Nice, France. 2008. The benefits of antimalarial chemoprophylaxis concerning offshore activities in high-risk malaria areas. [Google Scholar]
- Miller S.A., Bergman B.P., Croft A.M. Epidemiology of malaria in the British Army from 1982–1996. J. Roy. Army Med. Corps. 1999;145:20–22. doi: 10.1136/jramc-145-01-06. [DOI] [PubMed] [Google Scholar]
- Modell W. Malaria and victory in Vietnam. The first battle against drug-resistant malignant malaria is described. Science. 1968;162:1346–1352. doi: 10.1126/science.162.3860.1346. [DOI] [PubMed] [Google Scholar]
- Mody R.M., Lakhal-Naouar I., Sherwood J.E., Koles N.L., Shaw D., Bigley D.P., et al. Asymptomatic visceral Leishmania infantum infection in US soldiers deployed to Iraq. Clin. Infect. Dis. 2019;68:2036–2044. doi: 10.1093/cid/ciy811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Mondragon-Shem K. A story of two fevers: When malaria is the price of gold. BugBitten, BMC. 2022 https://blogs.biomedcentral.com/bugbitten/2022/01/14/when-malaria-is-the-price-of-gold/ [Google Scholar]
- Monroe A., Mihayo K., Okumu F., Finda M., Moore S., Koenker H., et al. Human behaviour and residual malaria transmission in Zanzibar: findings from in-depth interviews and direct observation of community events. Malar. J. 2019;18:220. doi: 10.1186/s12936-019-2855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe A., Moore S., Koenker H., Lynch M., Ricotta E. Measuring and characterizing night time human behaviour as it relates to residual malaria transmission in sub-Saharan Africa: a review of the published literature. Malar. J. 2019;18:6. doi: 10.1186/s12936-019-2638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S.J., Davies C.R., Cameron M.M. Are mosquitoes diverted from repellent-using individuals to non-users? Results of a field study in Bolivia. Trop. Med. Int. Health. 2007;12:532–539. doi: 10.1111/j.1365-3156.2006.01811.x. [DOI] [PubMed] [Google Scholar]
- Moore S.J., Mordue A.J., Logan J.G. Insect bite prevention. Infect. Dis. Clin. 2012;26:655–673. doi: 10.1016/j.idc.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Morrison A.C., Reiner R.C., Jr., Elson W.H., Astete H., Guevara C., Del Aguila C., et al. Efficacy of a spatial repellent for control of Aedes-borne virus transmission: A cluster-randomized trial in Iquitos, Peru. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2118283119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Msellemu D., Gavana T., Ngonyani H., Mlacha Y.P., Chaki P., Moore S.J. Knowledge, attitudes and bite prevention practices and estimation of productivity of vector breeding sites using a Habitat Suitability Score (HSS) among households with confirmed dengue in the 2014 outbreak in Dar es Salaam, Tanzania. PLoS Negl. Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0007278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muja-Bajraktari N., Kadriaj P., Zhushi-Etemi F., Sherifi K., Alten B., Petric D., et al. The asian tiger mosquito Aedes albopictus (Skuse) in Kosovo: First record. PLoS One. 2022;17 doi: 10.1371/journal.pone.0264300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller G.C., Beier J.C., Traore S.F., Toure M.B., Traore M.M., Bah S., et al. Successful field trial of attractive toxic sugar bait (ATSB) plant-spraying methods against malaria vectors in the Anopheles gambiae complex in Mali, West Africa. Malar. J. 2010;9:210. doi: 10.1186/1475-2875-9-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller G.C., Galili A. Westham Innovations; Geneva: 2016. Attractive toxic sugar baits (ATSB): From basic science to product, a new paradigm for vector control. [Google Scholar]
- Munn Z., Moola S., Lisy K., Riitano D., Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int. J. Evid. Base. Healthc. 2015;13:147–153. doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- Munn Z., Moola S., Lisy K., Riitano D., Tufanaru C. In: JBI Manual for Evidence Synthesis. Aromataris E., Munn Z., editors. JBI; 2020. Chapter 5: Systematic reviews of prevalence and incidence. [DOI] [Google Scholar]
- National Research Council . National Research Council; Washington (DC): 1994. Health effects of permethrin-impregnated army battle-dress uniforms.https://www.ncbi.nlm.nih.gov/books/NBK231566/ [PubMed] [Google Scholar]
- Nature Education Dengue transmission. 2014. https://www.nature.com/scitable/topicpage/dengue-transmission-22399758/
- Neel S.H. Department of the Army; Washington DC: 1991. Medical Support of the US Army in Vietnam, 1965–1970. [Google Scholar]
- Ng Lee Ching. Wolbachia mediated sterility suppresses Aedes aegypti populations in the urban tropics. medRxiv. 2021;2021.2006.2016 doi: 10.1101/2021.06.16.21257922. [DOI] [Google Scholar]
- NʼGuessan R., Corbel V., Akogbeto M., Rowland M. Reduced efficacy of insecticide-treated nets and indoor residual spraying for malaria control in pyrethroid resistance area, Benin. Emerg. Infect. Dis. 2007;13:199–206. doi: 10.3201/eid1302.060631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obwaller A.G., Köhsler M., Poeppl W., Herkner H., Mooseder G., Aspock H., et al. Leishmania infections in Austrian soldiers returning from military missions abroad: A cross-sectional study. Clin. Microbiol. Infect. 2018;24:E1101. doi: 10.1016/j.cmi.2018.01.006. [DOI] [PubMed] [Google Scholar]
- Odolini S., Parola P., Gkrania-Klotsas E., Caumes E., Schlagenhauf P., Lopez-Velez R., et al. Travel-related imported infections in Europe, EuroTravNet 2009. Clin. Microbiol. Infect. 2012;18:468–474. doi: 10.1111/j.1469-0691.2011.03596.x. [DOI] [PubMed] [Google Scholar]
- Ogoma S.B., Ngonyani H., Simfukwe E.T., Mseka A., Moore J., Killeen G.F. Spatial repellency of transfluthrin-treated hessian strips against laboratory-reared Anopheles arabiensis mosquitoes in a semi-field tunnel cage. Parasites Vectors. 2012;5:54. doi: 10.1186/1756-3305-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okwor I., Uzonna J. Social and economic burden of human leishmaniasis. Am. J. Trop. Med. Hyg. 2016;94:489–493. doi: 10.4269/ajtmh.15-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J., Larsen S., Stinear T.P., Hoffmann A., Crouch S., Gibney K.B. Reducing mosquito-borne disease transmission to humans: A systematic review of cluster randomised controlled studies that assess interventions other than non-targeted insecticide. PLoS Negl. Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0009601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S. Wolbachia goes to work in the war on mosquitoes. Nature. 2021;598:S32–S34. doi: 10.1038/d41586-021-02914-8. [DOI] [Google Scholar]
- Ore M., Saenz E., Cabrera R., Sanchez J.F., De Los Santos M.B., Lucas C.M., et al. Outbreak of cutaneous leishmaniasis in Peruvian military personnel undertaking training activities in the Amazon Basin, 2010. Am. J. Trop. Med. Hyg. 2015;93:340–346. doi: 10.4269/ajtmh.15-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orem J.N., Kirigia J.M., Azairwe R., Kasirye I., Walker O. Impact of malaria morbidity on gross domestic product in Uganda. Int. Arch. Med. 2012;5:12. doi: 10.1186/1755-7682-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsborne J., DeRaedt Banks S., Hendy A., Gezan S.A., Kaur H., Wilder-Smith A., et al. Personal protection of permethrin-treated clothing against Aedes aegypti, the vector of dengue and Zika virus, in the laboratory. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Östlund L., Öbom A., Löfdahl A., Rautio A.-M. Women in forestry in the early twentieth century - new opportunities for young women to work and gain their freedom in a traditional agrarian society. Scand. J. For. Res. 2020;35:403–416. doi: 10.1080/02827581.2020.1808054. [DOI] [Google Scholar]
- Oxman M. Informed Health Choices; 2018. Quality of news media reports about the effects and costs of health Interventions: Systematic review protocol.https://www.informedhealthchoices.org/publications/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbel Y., Toz S., Munoz C., Ortuno M., Jumakanova Z., Perez-Cutillas P., et al. The current epidemiology of leishmaniasis in Turkey, Azerbaijan and Georgia and implications for disease emergence in European countries. Zoonoses Public Health. 2022;69:395–407. doi: 10.1111/zph.12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pages F., Faulde M., Orlandi-Pradines E., Parola P. The past and present threat of vector-borne diseases in deployed troops. Clin. Microbiol. Inf. 2010;16:209–224. doi: 10.1111/j.1469-0691.2009.03132.x. [DOI] [PubMed] [Google Scholar]
- PAHO . Pan American Health Organization; Washington, DC: 2017. Leishmaniasis - fact sheet for health workers.https://www.paho.org/en/documents/leishmaniasis-americas-public-health-workers-2017 [Google Scholar]
- Paquet D., Jung L., Trawinski H., Wendt S., Lubbert C. Fever in the returning traveler. Dtsch. Arztebl. Int. 2022;119:400–407. doi: 10.3238/arztebl.m2022.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanasin S., Satitvipawee P., Wongklang W., Viwatwongkasem C., Bhumiratana A., Soontornpipit P., et al. Risk factors for malaria infection among rubber tappers living in a malaria control program area in southern Thailand. Southeast Asian J. Trop. Med. Publ. Health. 2012;43:1313–1325. https://www.ncbi.nlm.nih.gov/pubmed/23413693 [PubMed] [Google Scholar]
- Paupy C., Delatte H., Bagny L., Corbel V., Fontenille D. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microb. Infect. 2009;11:1177–1185. doi: 10.1016/j.micinf.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Pavli A., Maltezou H.C. Leishmaniasis, an emerging infection in travelers. Int. J. Infect. Dis. 2010;14:e1032–e1039. doi: 10.1016/j.ijid.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Pell C., Straus L., Andrew E.V., Menaca A., Pool R. Social and cultural factors affecting uptake of interventions for malaria in pregnancy in Africa: A systematic review of the qualitative research. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peragallo M.S., Nicoletti L., Lista F., DʼAmelio R., East Timor Dengue Study Group Probable dengue virus infection among Italian troops, East Timor, 1999–2000. Emerg. Infect. Dis. 2003;9:876–880. doi: 10.3201/eid0907.020496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera S., John D., Senanayaka B. Cost effectiveness of dengue vaccination following pre-vaccination serological screening in Sri Lanka. Int. J. Technol. Assess. Health Care. 2019;35:427–435. doi: 10.1017/S0266462319000680. [DOI] [PubMed] [Google Scholar]
- Perez-Ayala A., Norman F., Perez-Molina J.A., Herrero J.M., Monge B., Lopez-Velez R. Imported leishmaniasis: A heterogeneous group of diseases. J. Trav. Med. 2009;16:395–401. doi: 10.1111/j.1708-8305.2009.00341.x. [DOI] [PubMed] [Google Scholar]
- Perng G.C., Ho T.C., Shih H.I., Lee C.H., Huang P.W., Chung C.H., et al. Seroprevalence of Zika and dengue virus antibodies among migrant workers, Taiwan, 2017. Emerg. Infect. Dis. 2019;25:814–816. doi: 10.3201/eid2504.181449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuong H.L., De Vries P.J., Boonshuyar C., Binh T.Q., Nam N.V., Kager P.A., et al. Dengue risk factors and community participation in Binh Thuan Province, Vietnam, a household survey. Southeast Asian J. Trop. Med. Publ. Health. 2008;39:79–89. https://www.ncbi.nlm.nih.gov/pubmed/18567446 [PubMed] [Google Scholar]
- Pluess B., Mueller I., Levi D., King G., Smith T.A., Lengeler C. Malaria - a major health problem within an oil palm plantation around Popondetta, Papua New Guinea. Malar. J. 2009;8:56. doi: 10.1186/1475-2875-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluess B., Tanser F.C., Lengeler C., Sharp B.L. Indoor residual spraying for preventing malaria. Cochrane Database Syst. Rev. 2010;2010 doi: 10.1002/14651858.CD006657.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier de Santi V., Djossou F., Barthes N., Bogreau H., Hyvert G., Nguyen C., et al. Malaria hyperendemicity and risk for artemisinin resistance among illegal gold miners, French Guiana. Emerg. Infect. Dis. 2016;22:903–906. doi: 10.3201/eid2205.151957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier de Santi V., Girod R., Mura M., Dia A., Briolant S., Djossou F., et al. Epidemiological and entomological studies of a malaria outbreak among French armed forces deployed at illegal gold mining sites reveal new aspects of the diseaseʼs transmission in French Guiana. Malar. J. 2016;15:35. doi: 10.1186/s12936-016-1088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons M.J., Gomes C., del Valle-Mendoza J., Ruiz J. Carrion’s disease: More than a sand fly-vectored Illness. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovici J., Moreira L.A., Poinsignon A., Iturbe-Ormaetxe I., McNaughton D., OʼNeill S.L. Assessing key safety concerns of a Wolbachia-based strategy to control dengue transmission by Aedes mosquitoes. Mem. Inst. Oswaldo Cruz. 2010;105:957–964. doi: 10.1590/s0074-02762010000800002. [DOI] [PubMed] [Google Scholar]
- Powell J.R. Mosquito-borne human viral diseases: Why Aedes aegypti? Am. J. Trop. Med. Hyg. 2018;98:1563–1565. doi: 10.4269/ajtmh.17-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J.R., Tabachnick W.J. History of domestication and spread of Aedes aegypti -a review. Mem. Inst. Oswaldo Cruz. 2013;108(Suppl. 1):11–17. doi: 10.1590/0074-0276130395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce J., Richardson M., Lengeler C. Insecticide‐treated nets for preventing malaria. Cochrane Database Syst. Rev. 2018;11:CD000363. doi: 10.1002/14651858.CD000363.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaresima V., Agbenyega T., Oppong B., Awunyo J., Adu Adomah P., Enty E., et al. Are malaria risk factors based on gender? A mixed-methods survey in an urban setting in Ghana. Trav. Med. Infect. Dis. 2021;6 doi: 10.3390/tropicalmed6030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabaa M.A., Simmons C.P., Fox A., Le M.Q., Nguyen T.T., Le H.Y., et al. Dengue virus in sub-tropical northern and central Viet Nam: Population immunity and climate shape patterns of viral invasion and maintenance. PLoS Neglected Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahayu N., Suryatinah Y., Kusumaningtyas H. Discovery of malaria cases on forest workers in public health center, Teluk Kepayang, Tanah Bumbu, South Kalimantan. BIO Web Conf. 2020;20 doi: 10.1051/bioconf/20202001008. [DOI] [Google Scholar]
- Rahman H., Razzak M.A., Chanda B.C., Bhaskar K.R., Mondal D. Cutaneous leishmaniasis in an immigrant Saudi worker: A case report. J. Health Popul. Nutr. 2014;32:372–376. https://www.ncbi.nlm.nih.gov/pubmed/25076674 [PMC free article] [PubMed] [Google Scholar]
- Ramchurn S.K., Moheeput K., Goorah S.S. An analysis of a short-lived outbreak of dengue fever in Mauritius. Euro Surveill. 2009;14 doi: 10.2807/ese.14.34.19314-en. [DOI] [PubMed] [Google Scholar]
- Rebollo M.P., Bockarie M.J. Can lymphatic filariasis be eliminated by 2020? Trends Parasitol. 2017;33:83–92. doi: 10.1016/j.pt.2016.09.009. [DOI] [PubMed] [Google Scholar]
- Rehman K., Walochnik J., Mischlinger J., Alassil B., Allan R., Ramharter M. Leishmaniasis in northern Syria during civil war. Emerg. Infect. Dis. 2018;24:1973–1981. doi: 10.3201/eid2411.172146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyburn H., Ashford R., Mohsen M., Hewitt S., Rowland M. A randomized controlled trial of insecticide-treated bednets and chaddars or top sheets, and residual spraying of interior rooms for the prevention of cutaneous leishmaniasis in Kabul, Afghanistan. Trans. R. Soc. Trop. Med. Hyg. 2000;94:361–366. doi: 10.1016/s0035-9203(00)90104-4. [DOI] [PubMed] [Google Scholar]
- Rezza G. Aedes albopictus and the reemergence of dengue. BMC Publ. Health. 2012;12:72. doi: 10.1186/1471-2458-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijal S., Sundar S., Mondal D., Das P., Alvar J., Boelaert M. Eliminating visceral leishmaniasis in South Asia: The road ahead. BMJ. 2019;364 doi: 10.1136/bmj.k5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocklov J., Lohr W., Hjertqvist M., Wilder-Smith A. Attack rates of dengue fever in Swedish travellers. Scand. J. Infect. Dis. 2014;46:412–417. doi: 10.3109/00365548.2014.887222. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fernandez R., Ng N., Susilo D., Prawira J., Bangs M.J., Amiya R.M. The double burden of disease among mining workers in Papua, Indonesia: At the crossroads between old and new health paradigms. BMC Publ. Health. 2016;16:951. doi: 10.1186/s12889-016-3630-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossbach B., Appel K.E., Mross K.G., Letzel S. Uptake of permethrin from impregnated clothing. Toxicol. Lett. 2010;192:50–55. doi: 10.1016/j.toxlet.2009.06.863. [DOI] [PubMed] [Google Scholar]
- Rowland M., Munir A., Durrani N., Noyes H., Reyburn H. An outbreak of cutaneous leishmaniasis in an Afghan refugee settlement in north-west Pakistan. Trans. R. Soc. Trop. Med. Hyg. 1999;93:133–136. doi: 10.1016/s0035-9203(99)90285-7. [DOI] [PubMed] [Google Scholar]
- Rowland M., Rab M.A., Freeman T., Durrani N., Rehman N. Afghan refugees and the temporal and spatial distribution of malaria in Pakistan. Soc. Sci. Med. 2002;55:2061–2072. doi: 10.1016/s0277-9536(01)00341-0. [DOI] [PubMed] [Google Scholar]
- Russell B.M., Muir L.E., Weinstein P., Kay B.H. Surveillance of the mosquito Aedes aegypti and its biocontrol with the copepod Mesocyclops aspericornis in Australian wells and gold mines. Med. Vet. Entomol. 1996;10:155–160. doi: 10.1111/j.1365-2915.1996.tb00722.x. [DOI] [PubMed] [Google Scholar]
- Sachs J., Malaney P. The economic and social burden of malaria. Nature. 2002;415:680–685. doi: 10.1038/415680a. [DOI] [PubMed] [Google Scholar]
- Sadasivaiah S., Breman J.G., Tozan Y. Dichlorodiphenyltrichloroethane (DDT) for indoor residual spraying in Africa: How can it be used for malaria control? Am. J. Trop. Med. Hyg. 2007;77(Suppl. 6):249–263. doi: 10.4269/ajtmh.2007.77.249. [DOI] [PubMed] [Google Scholar]
- Saha S., Chant D., McGrath J. Meta-analyses of the incidence and prevalence of schizophrenia: Conceptual and methodological issues. Int. J. Methods Psychiatr. Res. 2008;17:55–61. doi: 10.1002/mpr.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salami B., Iwuagwu S., Amodu O., Tulli M., Ndikom C., Gommaa H., et al. The health of internally displaced children in sub-Saharan Africa: A scoping review. BMJ Glob. Health. 2020;5 doi: 10.1136/bmjgh-2020-002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salman I.S., Vural A., Unver A., Sacar S. [Cutaneous leishmaniasis cases in Nizip, Turkey after the Syrian civil war] Mikrobiyoloji Bulteni. 2014;48:106–113. https://www.ncbi.nlm.nih.gov/pubmed/24506720 [PubMed] [Google Scholar]
- Samuel M., Maoz D., Manrique P., Ward T., Runge-Ranzinger S., Toledo J., et al. Community effectiveness of indoor spraying as a dengue vector control method: A systematic review. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez J.F., Carnero A.M., Rivera E., Rosales L.A., Baldeviano G.C., Asencios J.L., et al. Unstable malaria transmission in the southern Peruvian Amazon and its association with gold mining, Madre de Dios, 2001–2012. Am. J. Trop. Med. Hyg. 2017;96:304–311. doi: 10.4269/ajtmh.16-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez J.L., Bendet I., Grogl M., Lima J.B., Pang L.W., Guimaraes M.F., et al. Malaria in Brazilian military personnel deployed to Angola. J. Trav. Med. 2000;7:275–282. doi: 10.2310/7060.2000.00077. [DOI] [PubMed] [Google Scholar]
- Sanders J.W., Putnam S.D., Frankart C., Frenck R.W., Monteville M.R., Riddle M.S., et al. Impact of illness and non-combat injury during operations Iraqi Freedom and Enduring Freedom (Afghanistan) Am. J. Trop. Med. Hyg. 2005;73:713–719. doi: 10.4269/ajtmh.2005.73.713. [DOI] [PubMed] [Google Scholar]
- Sandfort M., Vantaux A., Kim S., Obadia T., Pepey A., Gardais S., et al. Forest malaria in Cambodia: tThe occupational and spatial clustering of Plasmodium vivax and Plasmodium falciparum infection risk in a cross-sectional survey in Mondulkiri Province, Cambodia. Malar. J. 2020;19:413. doi: 10.1186/s12936-020-03482-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangkawibha N., Rojanasuphot S., Ahandrik S., Viriyapongse S., Jatanasen S., Salitul V., et al. Risk factors in dengue shock syndrome: A prospective epidemiologic study in Rayong, Thailand: I. The 1980 outbreak. Am. J. Epidemiol. 1984;120:653–669. doi: 10.1093/oxfordjournals.aje.a113932. [DOI] [PubMed] [Google Scholar]
- Sarma N., Patouillard E., Cibulskis R.E., Arcand J.L. The economic burden of malaria: Revisiting the evidence. Am. J. Trop. Med. Hyg. 2019;101:1405–1415. doi: 10.4269/ajtmh.19-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saroufim M., Charafeddine K., Issa G., Khalifeh H., Habib R.H., Berry A., et al. Ongoing epidemic of cutaneous leishmaniasis among Syrian refugees, Lebanon. Emerg. Infect. Dis. 2014;20:1712–1715. doi: 10.3201/eid2010.140288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schicker R.S., Hiruy N., Melak B., Gelaye W., Bezabih B., Stephenson R., et al. A venue-based survey of malaria, anemia and mobility patterns among migrant farm workers in Amhara Region, Ethiopia. PLoS One. 2015;10 doi: 10.1371/journal.pone.0143829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E., Weld L.H., Wilder-Smith A., von Sonnenburg F., Keystone J.S., Kain K.C., et al. Seasonality, annual trends, and characteristics of dengue among ill returned travelers, 1997–2006. Emerg. Infect. Dis. 2008;14:1081–1088. doi: 10.3201/eid1407.071412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seet R.C., Ooi E.E., Wong H.B., Paton N.I. An outbreak of primary dengue infection among migrant Chinese workers in Singapore characterized by prominent gastrointestinal symptoms and a high proportion of symptomatic cases. J. Clin. Virol. 2005;33:336–340. doi: 10.1016/j.jcv.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Shaha D.P., Pacha L.A., Eric C. Garges, Scoville S., Mancuso J.D. Confirmed malaria cases among active component U.S. Army personnel, January-September 2012. MSMR. 2013;20:6–7. [PubMed] [Google Scholar]
- Shanks G.D., Wongsrichanalai C. Mining-associated malaria epidemics. Am. J. Trop. Med. Hyg. 2021;106:33–37. doi: 10.4269/ajtmh.21-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S.K., Tyagi P.K., Upadhyay A.K., Haque M.A., Mohanty S.S., Raghavendra K., et al. Efficacy of permethrin-treated long-lasting insecticidal nets on malaria transmission and observations on the perceived side effects, collateral benefits and human safety in a hyperendemic tribal area of Orissa, India. Acta Trop. 2009;112:181–187. doi: 10.1016/j.actatropica.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Sharp T.M., Pillai P., Hunsperger E., Santiago G.A., Anderson T., Vap T., et al. A cluster of dengue cases in American missionaries returning from Haiti, 2010. Am. J. Trop. Med. Hyg. 2012;86:16–22. doi: 10.4269/ajtmh.2012.11-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp T.W., Wallace M.R., Hayes C.G., Sanchez J.L., DeFraites R.F., Arthur R.R., et al. Dengue fever in U.S. troops during operation Restore Hope, Somalia, 1992–1993. Am. J. Trop. Med. Hyg. 1995;53:89–94. https://www.ncbi.nlm.nih.gov/pubmed/7625541 [PubMed] [Google Scholar]
- Shea B.J., Reeves B.C., Wells G., Thuku M., Hamel C., Moran J., et al. Amstar 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrard-Smith E., Skarp J.E., Beale A.D., Fornadel C., Norris L.C., Moore S.J., et al. Mosquito feeding behavior and how it influences residual malaria transmission across Africa. Proc. Natl. Acad. Sci. USA. 2019;116:15086–15095. doi: 10.1073/pnas.1820646116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shropshire J.D., Leigh B., Bordenstein S.R. Symbiont-mediated cytoplasmic incompatibility: What have we learned in 50 years? Elife. 2020;9 doi: 10.7554/eLife.61989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbergeld E.K., Nash D., Trevant C., Strickland G.T., de Souza J.M., da Silva R.S. Mercury exposure and malaria prevalence among gold miners in Para, Brazil. Rev. Soc. Bras. Med. Trop. 2002;35:421–429. doi: 10.1590/s0037-86822002000500001. [DOI] [PubMed] [Google Scholar]
- Silva S., Gomes L.B., Carvalho P., Santos A., Borges L., Oliveira C.S.F., et al. Effectiveness of the mass use of deltamethrin-impregnated dog collars for preventing transmission of canine leishmaniasis by Lutzomyia spp.: A cluster randomized controlled trial. Prev. Vet. Med. 2019;171 doi: 10.1016/j.prevetmed.2019.104770. [DOI] [PubMed] [Google Scholar]
- Silveira F.T., Ishikawa E.A., De Souza A.A., Lainson R. An outbreak of cutaneous leishmaniasis among soldiers in Belem, Para State, Brazil, caused by Leishmania (Viannia) lindenbergi n. sp., a new leishmanial parasite of man in the Amazon region. Parasite. 2002;9:43–50. doi: 10.1051/parasite/200209143. [DOI] [PubMed] [Google Scholar]
- Singhasivanon P., Thimasarn K., Yimsamran S., Linthicum K., Nualchawee K., Dawreang D., et al. Malaria in tree crop plantations in south-eastern and western provinces of Thailand. Southeast Asian J. Trop. Med. Publ. Health. 1999;30:399–404. https://www.ncbi.nlm.nih.gov/pubmed/10774642 [PubMed] [Google Scholar]
- Sinka M.E., Bangs M.J., Manguin S., Chareonviriyaphap T., Patil A.P. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: Occurrence data, distribution maps and bionomic precis. Parasites Vectors. 2011;4:89. doi: 10.1186/1756-3305-4-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinka M.E., Bangs M.J., Manguin S., Coetzee M., Mbogo C.M., Hemingway J., et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic precis. Parasites Vectors. 2010;3:117. doi: 10.1186/1756-3305-3-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinka M.E., Bangs M.J., Manguin S., Rubio-Palis Y., Chareonviriyaphap T., Coetzee M., et al. A global map of dominant malaria vectors. Parasites Vectors. 2012;5:69. doi: 10.1186/1756-3305-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinka M.E., Rubio-Palis Y., Manguin S., Patil A.P., Temperley W.H., Gething P.W., et al. The dominant Anopheles vectors of human malaria in the Americas: occurrence data, distribution maps and bionomic precis. Parasites Vectors. 2010;3:72. doi: 10.1186/1756-3305-3-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobirk S.K., Inghammar M., Collin M., Davidsson L. Imported leishmaniasis in Sweden 1993–2016. Epidemiol. Infect. 2018;146:1267–1274. doi: 10.1017/S0950268818001309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sochantha T., Van Bortel W., Savonnaroth S., Marcotty T., Speybroeck N., Coosemans M. Personal protection by long-lasting insecticidal hammocks against the bites of forest malaria vectors. Trop. Med. Int. Health. 2010;15:336–341. doi: 10.1111/j.1365-3156.2009.02457.x. [DOI] [PubMed] [Google Scholar]
- Solomon M., Benenson S., Baum S., Schwartz E. Tropical skin infections among Israeli travelers. Am. J. Trop. Med. Hyg. 2011;85:868–872. doi: 10.4269/ajtmh.2011.10-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son D.H., Thuy-Nhien N., von Seidlein L., Le Phuc-Nhi T., Phu N.T., Tuyen N.T.K., et al. The prevalence, incidence and prevention of Plasmodium falciparum infections in forest rangers in bu gia map national park, binh phuoc province, Vietnam: A pilot study. Malar. J. 2017;16:444. doi: 10.1186/s12936-017-2091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soong L. Academic Press; Cambridge, MA: 2009. Vaccines for biodefense and emerging and neglected diseases. [Google Scholar]
- Soto J., Medina F., Dember N., Berman J. Efficacy of permethrin-impregnated uniforms in the prevention of malaria and leishmaniasis in Colombian soldiers. Clin. Infect. Dis. 1995;21:599–602. doi: 10.1093/clinids/21.3.599. [DOI] [PubMed] [Google Scholar]
- Sousa-Paula L.C., Otranto D., Dantas-Torres F. Lutzomyia longipalpis (Sand fly) Trends Parasitol. 2020;36:796–797. doi: 10.1016/j.pt.2020.05.007. [DOI] [PubMed] [Google Scholar]
- Stevenson J.C., Simubali L., Mudenda T., Cardol E., Bernier U.R., Vazquez A.A., et al. Controlled release spatial repellent devices (CRDs) as novel tools against malaria transmission: a semi-field study in Macha, Zambia. Malar. J. 2018;17:437. doi: 10.1186/s12936-018-2558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steverding D. The history of leishmaniasis. Parasites Vectors. 2017;10:82. doi: 10.1186/s13071-017-2028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienlauf S., Segal G., Sidi Y., Schwartz E. Epidemiology of travel-related hospitalization. J. Trav. Med. 2005;12:136–141. doi: 10.2310/7060.2005.12308. [DOI] [PubMed] [Google Scholar]
- Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Sturrock H.J., Hsiang M.S., Cohen J.M., Smith D.L., Greenhouse B., Bousema T., et al. Targeting asymptomatic malaria infections: active surveillance in control and elimination. PLoS Med. 2013;10 doi: 10.1371/journal.pmed.1001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung V., OʼBrien D.P., Matchett E., Brown G.V., Torresi J. Dengue fever in travelers returning from southeast Asia. J. Trav. Med. 2003;10:208–213. doi: 10.2310/7060.2003.40555. [DOI] [PubMed] [Google Scholar]
- Sunyoto T., Boelaert M., Meheus F. Understanding the economic impact of leishmaniasis on households in endemic countries: A systematic review. Expert Rev. Anti. Infect. Ther. 2019;17:57–69. doi: 10.1080/14787210.2019.1555471. [DOI] [PubMed] [Google Scholar]
- Sunyoto T., Verdonck K., El Safi S., Potet J., Picado A., Boelaert M. Uncharted territory of the epidemiological burden of cutaneous leishmaniasis in sub-Saharan Africa: A systematic review. PLoS Negl. Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susanna D., Eryando T., Pratiwi D., Nugraha F. The changed occupation and behavioral among imported malaria cases 2009–2011 in Sukabumi District, West Java, Indonesia. Malar. J. 2012;11(Suppl. 1):128. doi: 10.1186/1475-2875-11-S1-P128. Retrieved from. [DOI] [Google Scholar]
- Sutherst R.W. Global change and human vulnerability to vector-borne diseases. Clin. Microbiol. Rev. 2004;17:136–173. doi: 10.1128/cmr.17.1.136-173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwantika A.A., Supadmi W., Ali M., Abdulah R. Cost-effectiveness and budget impact analyses of dengue vaccination in Indonesia. PLoS Negl. Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0009664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swai J.K., Mmbando A.S., Ngowo H.S., Odufuwa O.G., Finda M.F., Mponzi W., et al. Protecting migratory farmers in rural Tanzania using eave ribbons treated with the spatial mosquito repellent, transfluthrin. Malar. J. 2019;18:414. doi: 10.1186/s12936-019-3048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swai J.K., Ntabaliba W., Ngonyani H., Mseka A., Makungwa N., Kibondo U., et al. PAMCA Ethiopia 2023, Addis Ababa. 2023. Efficacy of Guardian, a long-lasting indoor passive spatial repellent, against pyrethroid-resistant malaria vectors. [Google Scholar]
- Swai J.K., Soto A.C., Ntabaliba W.S., Kibondo U.A., Ngonyani H.A., Mseka A.P., et al. Efficacy of the spatial repellent product Mosquito Shield against wild pyrethroid-resistant Anopheles arabiensis in south-eastern Tanzania. Malar. J. 2023;22:249. doi: 10.1186/s12936-023-04674-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syafruddin D., Asih P.B.S., Rozi I.E., Permana D.H., Nur Hidayati A.P., Syahrani L., et al. Efficacy of a spatial repellent for control of malaria in Indonesia: A cluster-randomized controlled trial. Am. J. Trop. Med. Hyg. 2020;103:344–358. doi: 10.4269/ajtmh.19-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadesse Y., Irish S.R., Chibsa S., Dugassa S., Lorenz L.M., Gebreyohannes A., et al. Malaria prevention and treatment in migrant agricultural workers in Dangur District, Benishangul-Gumuz, Ethiopia: Social and behavioural aspects. Malar. J. 2021;20:224. doi: 10.1186/s12936-021-03766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takafuji E.T., Hendricks L.D., Daubek J.L., McNeil K.M., Scagliola H.M., Diggs C.L. Cutaneous leishmaniasis associated with jungle training. Am. J. Trop. Med. Hyg. 1980;29:516–520. doi: 10.4269/ajtmh.1980.29.516. [DOI] [PubMed] [Google Scholar]
- Tambwe M.M., Saddler A., Kibondo U.A., Mashauri R., Kreppel K.S., Govella N.J., et al. Semi-field evaluation of the exposure-free mosquito electrocuting trap and BG-Sentinel trap as an alternative to the human landing catch for measuring the efficacy of transfluthrin emanators against Aedes aegypti. Parasites Vectors. 2021;14:265. doi: 10.1186/s13071-021-04754-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambwe M.M., Swai J.K., Moore S.J. In: Advances in Arthropod Repellents. Coats J., et al., editors. Elsevier; 2021. Semi-field bioassays for the evaluation of spatial (and topical) arthropod repellents for indoor and outdoor use. [Google Scholar]
- Tangena J.A., Thammavong P., Chonephetsarath S., Logan J.G., Brey P.T., Lindsay S.W., et al. Field evaluation of personal protection methods against outdoor-biting mosquitoes in Lao PDR. Parasites Vectors. 2018;11:661. doi: 10.1186/s13071-018-3239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangena J.A., Thammavong P., Lindsay S.W., Brey P.T. Risk of exposure to potential vector mosquitoes for rural workers in Northern Lao PDR. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnas M.C., Desai A.N., Lassmann B., Abbara A. Increase in vector-borne disease reporting affecting humans and animals in Syria and neighboring countries after the onset of conflict: A ProMED analysis 2003–2018. Int. J. Infect. Dis. 2021;102:103–109. doi: 10.1016/j.ijid.2020.09.1453. [DOI] [PubMed] [Google Scholar]
- Tesh R.B. The epidemiology of Phlebotomus (sandfly) fever. Isr. J. Med. Sci. 1989;25:214–217. https://www.ncbi.nlm.nih.gov/pubmed/2496050 [PubMed] [Google Scholar]
- Thang N.D., Erhart A., Speybroeck N., Xa N.X., Thanh N.N., Ky P.V., et al. Long-lasting insecticidal hammocks for controlling forest malaria: A community-based trial in a rural area of central Vietnam. PLoS One. 2009;4 doi: 10.1371/journal.pone.0007369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The MENTOR Initiative Protecting conflict populations from cutaneous leishmaniasis and other insect-borne diseases. Creating Hope in Conflict: A Humanitarian Grand Challenge. 2020 https://humanitariangrandchallenge.org/innovator/mentor-initiative/ [Google Scholar]
- Thornton S.J., Wasan K.M., Piecuch A., Lynd L.L.D., Wasan E.K. Barriers to treatment for visceral leishmaniasis in hyperendemic areas: India, Bangladesh, Nepal, Brazil and Sudan. Drug Dev. Ind. Pharm. 2010;36:1312–1319. doi: 10.3109/03639041003796648. [DOI] [PubMed] [Google Scholar]
- Tickell-Painter M., Maayan N., Saunders R., Pace C., Sinclair D. Mefloquine for preventing malaria during travel to endemic areas. Cochrane Database Syst. Rev. 2017;10:CD006491. doi: 10.1002/14651858.CD006491.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres J.R., Falleiros-Arlant L.H., Gessner B.D., Delrieu I., Avila-Aguero M.L., Giambernardino H.I.G., et al. Updated recommendations of the international dengue initiative expert group for CYD-TDV vaccine implementation in Latin America. Vaccine. 2019;37:6291–6298. doi: 10.1016/j.vaccine.2019.09.010. [DOI] [PubMed] [Google Scholar]
- Torres-Guerrero E., Quintanilla-Cedillo M.R., Ruiz-Esmenjaud J., Arenas R. Leishmaniasis: A review. F1000Res. 2017;6:750. doi: 10.12688/f1000research.11120.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozan Y., Ratanawong P., Louis V.R., Kittayapong P., Wilder-Smith A. Use of insecticide-treated school uniforms for prevention of dengue in schoolchildren: A cost-effectiveness analysis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0108017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trofa A.F., DeFraites R.F., Smoak B.L., Kanesa-thasan N., King A.D., Burrous J.M., et al. Dengue fever in US military personnel in Haiti. JAMA. 1997;277:1546–1548. doi: 10.1001/jama.1997.03540430058033. [DOI] [PubMed] [Google Scholar]
- Trojanek M., Maixner J., Sojkova N., Kyncl J., Rohacova H., Maresova V., et al. Dengue fever in Czech travellers: A 10-year retrospective study in a tertiary care centre. Trav. Med. Infect. Dis. 2016;14:32–38. doi: 10.1016/j.tmaid.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Tuck J.J., Green A.D., Roberts K.I. A malaria outbreak following a British military deployment to Sierra Leone. J. Infect. 2003;47:225–230. doi: 10.1016/s0163-4453(03)00063-x. [DOI] [PubMed] [Google Scholar]
- Tungu P., Kabula B., Nkya T., Machafuko P., Sambu E., Batengana B., et al. Trends of insecticide resistance monitoring in mainland Tanzania, 2004–2020. Malar. J. 2023;22:100. doi: 10.1186/s12936-023-04508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNHCR . The UN Refugee Agency; Geneva, Switzerland: 2022. Global Trends: Forced Displacement.https://reliefweb.int/report/world/global-trends-forced-displacement-2022?gad_source=1&gclid=EAIaIQobChMIuKmK3O-IhgMVrgYGAB2oZAjYEAAYAiAAEgLwAPD_BwE [Google Scholar]
- UNHCR . The UN Refugee Agency; Geneva, Switzerland: 2022. Guidance Note on Malaria Programmes in Refugee Operations.https://www.unhcr.org/sites/default/files/legacy-pdf/53ba5cca9.pdf [Google Scholar]
- UNHCR . The UN Refugee Agency; Geneva, Switzerland: 2022. Refugee Statistics.https://www.unrefugees.org/refugee-facts/statistics/ [Google Scholar]
- UNHCR . The UN Refugee Agency; Geneva, Switzerland: 2023. Ethiopia humantarian crisis. Emergencies.https://www.unrefugees.org/emergencies/ethiopia/ [Google Scholar]
- United Nations Convention and protocol relating to the status of refugees. Annu. Rev. Popul. Law. 1989;16:175. https://www.ncbi.nlm.nih.gov/pubmed/12344222 [PubMed] [Google Scholar]
- Uranw S., Meheus F., Baltussen R., Rijal S., Boelaert M. The household costs of visceral leishmaniasis care in south-eastern Nepal. PLoS Negl. Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valbon W., Andreazza F., Oliveira E.E., Liu F., Feng B., Hall M., et al. Bioallethrin activates specific olfactory sensory neurons and elicits spatial repellency in Aedes aegypti. Pest Manag. Sci. 2022;78:438–445. doi: 10.1002/ps.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bogaart E., Berkhout M.M., Adams E.R., Mens P.F., Sentongo E., Mbulamberi D.B., et al. Prevalence, features and risk factors for malaria co-infections amongst visceral leishmaniasis patients from Amudat Hospital, Uganda. PLoS Negl. Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Thiel P.P., Zeegelaar J.E., van Gool T., Faber W.R., Kager P.A. Cutaneous leishmaniasis in three Dutch military cohorts following jungle training in Belize. Trav. Med. Infect. Dis. 2011;9:153–160. doi: 10.1016/j.tmaid.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Vavre F., Charlat S. Making (good) use of Wolbachia: What the models say. Curr. Opin. Microbiol. 2012;15:263–268. doi: 10.1016/j.mib.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Vazquez-Prokopec G.M., Kitron U., Montgomery B., Horne P., Ritchie S.A. Quantifying the spatial dimension of dengue virus epidemic spread within a tropical urban environment. PLoS Negl. Trop. Dis. 2010;4 doi: 10.1371/journal.pntd.0000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Prokopec G.M., Montgomery B.L., Horne P., Clennon J.A., Ritchie S.A. Combining contact tracing with targeted indoor residual spraying significantly reduces dengue transmission. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1602024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Lopez F., Ritchie S. In: Mansonʼs Tropical Infectious Diseases. 23rd ed. Farrar J., et al., editors. W.B. Saunders; London: 2014. 68. Dermatological problems; pp. 995–1026.e1021. [Google Scholar]
- Velez I.D., Tanamas S.K., Arbelaez M.P., Kutcher S.C., Duque S.L., Uribe A., et al. Reduced dengue incidence following city-wide wMel Wolbachia mosquito releases throughout three Colombian cities: iInterrupted time series analysis and a prospective case-control study. PLoS Negl. Trop. Dis. 2023;17 doi: 10.1371/journal.pntd.0011713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez R., Gallego M. Commercially approved vaccines for canine leishmaniosis: A review of available data on their safety and efficacy. Trop. Med. Int. Health. 2020;25:540–557. doi: 10.1111/tmi.13382. [DOI] [PubMed] [Google Scholar]
- Vilay P., Nonaka D., Senamonty P., Lao M., Iwagami M., Kobayashi J., et al. Malaria prevalence, knowledge, perception, preventive and treatment behavior among military in Champasak and Attapeu provinces, Lao PDR: A mixed methods study. Trop. Med. Health. 2019;47:11. doi: 10.1186/s41182-019-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villamizar-Pena R., Gutierrez-Ocampo E., Holguin-Rivera Y., Agudelo-Mejia K., Cortes-Gutierrez M., Sossa-Pinzon L., et al. Leishmaniasis among internally displaced people of Colombia, 2007–2018 - a comparative analysis with the general population. Trav. Med. Infect. Dis. 2021;41 doi: 10.1016/j.tmaid.2021.102043. [DOI] [PubMed] [Google Scholar]
- von Seidlein L., Peto T.J., Tripura R., Pell C., Yeung S., Kindermans J.M., et al. Novel approaches to control malaria in forested areas of Southeast Asia. Trends Parasitol. 2019;35:388–398. doi: 10.1016/j.pt.2019.03.011. [DOI] [PubMed] [Google Scholar]
- Wahid S., Stresman G.H., Kamal S.S., Sepulveda N., Kleinschmidt I., Bousema T., et al. Heterogeneous malaria transmission in long-term Afghan refugee populations: A cross-sectional study in five refugee camps in northern Pakistan. Malar. J. 2016;15:245. doi: 10.1186/s12936-016-1305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldetensai A., Sisay C., Asrat Y., Debebe Y., Kinde S., Alemnesh H., et al. Assessment of the resting behavior of Aedes aegypti during dengue fever outbreak of Dire Dawa, eastern Ethiopia. Int. J. Appl. Sci. Res. Rev. 2021;8:25. https://www.primescholars.com/articles/assessment-of-the-resting-behavior-of-aedes-aegypti-during-dengue-fever-outbreak-of-dire-dawa-eastern-ethiopia-95457.html [Google Scholar]
- Wall E.C., Watson J., Armstrong M., Chiodini P.L., Lockwood D.N. Epidemiology of imported cutaneous leishmaniasis at the Hospital for Tropical Diseases, London, United Kingdom: Use of polymerase chain reaction to identify the species. Am. J. Trop. Med. Hyg. 2012;86:115. doi: 10.4269/ajtmh.2012.10-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M.R., Sharp T.W., Smoak B., Iriye C., Rozmajzl P., Thornton S.A., et al. Malaria among United States troops in Somalia. Am. J. Med. 1996;100:49–55. doi: 10.1016/s0002-9343(96)90011-x. [DOI] [PubMed] [Google Scholar]
- Wangchuk S., Gyeltshen S., Dorji K., Wangdi T., Dukpa T., Namgay R., et al. Malaria elimination in Bhutan: Asymptomatic malaria cases in the Bhutanese population living in malaria-risk areas and in migrant workers from India. Rev. Inst. Med. Trop. Sao Paulo. 2019;61:e52. doi: 10.1590/S1678-9946201961052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangdahl A., Wyss K., Saduddin D., Bottai M., Ydring E., Vikerfors T., et al. Severity of Plasmodium falciparum and non-falciparum malaria in travelers and Migrants: A nationwide observational study over 2 decades in Sweden. J. Infect. Dis. 2019;220:1335–1345. doi: 10.1093/infdis/jiz292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangroongsar P., Hwang J., Thwing J., Karuchit S., Kumpetch S., Rand A., et al. Using respondent driven sampling to identify malaria risks and occupational networks among migrant workers in Ranong, Thailand. PLoS One. 2016;11 doi: 10.1371/journal.pone.0168371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver S.C., Reisen W.K. Present and future arboviral threats. Antivir. Res. 2010;85:328–345. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedell N., Price T.A.R., Lindholm A.K. Gene drive: Progress and prospects. Proc. Biol. Sci. 2019;286 doi: 10.1098/rspb.2019.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren J.H., Bartos J.D. Recombination in Wolbachia. Curr. Biol. 2001;11:431–435. doi: 10.1016/s0960-9822(01)00101-4. [DOI] [PubMed] [Google Scholar]
- Westaway E., Blok J. CAB International; London: 1997. Dengue and dengue hemorrhagic fever. [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2011. Comprehensive guideline for prevention and control of dengue and dengue haemorrhagic fever. Revised and expanded edition.https://iris.who.int/handle/10665/204894 [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2013. Leishmaniasis. Visceral Leishmaniasis.https://www.who.int/health-topics/leishmaniasis#tab=tab_1 [Google Scholar]
- WHO . second ed. World Health Organization; Geneva, Switzerland: 2013. Malaria Control in Humanitarian Emergencies: an Inter-agency Field Handbook.https://www.who.int/docs/default-source/documents/publications/gmp/malaria-control-in-humanitarian-emergencies-an-inter-agency-field-handbook.pdf [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2018. Vaccines and immunization: Dengue.https://www.who.int/news-room/questions-and-answers/item/dengue-vaccines [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2019. Dengue Increase Likely during Rainy Season: WHO Warns.https://www.who.int/westernpacific/news/item/11-06-2019-dengue-increase-likely-during-rainy-season-who-warns#:~:text=Several%20Asian%20countries%20are%20experiencing,illness%20and%20deaths%20from%20dengue [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2019. Guidelines for Malaria Vector Control.https://www.ncbi.nlm.nih.gov/books/NBK132015/ [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2021. World Malaria Report 2021.https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021#:~:text=The%202021%20edition%20of%20the,did%20not%20come%20to%20pass [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2021. WHO Guidelines for Malaria WHO/UCN/GMP/2021.01 Rev.1.https://iris.who.int/bitstream/handle/10665/343751/WHO-UCN-GMP-2021.01-Rev.1-eng.pdf?sequence=1 [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2021. WHO Recommends Groundbreaking Malaria Vaccine for Children at Risk.https://www.who.int/news/item/06-10-2021-who-recommends-groundbreaking-malaria-vaccine-for-children-at-risk [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2022. Disease Outbreak News: Dengue in Rohingya Refugee/forcibly Displaced Myanmar Nationals (FDMN) Camps in Cox's Bazar, Bangladesh.https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON401 [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2022. World Malaria Report 2022.https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022 [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2022. Leishmaniasis. Key Facts.https://www.who.int/news-room/fact-sheets/detail/leishmaniasis#:~:text=Leishmaniasis%20is%20caused%20by%20a,in%20over%2095%25%20of%20cases [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2022. Regional Strategic Framework for Accelerating and Sustaining Elimination of Kala-Azar in the South-East Asia Region.https://www.who.int/publications/i/item/9789290209812 [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2023. Malaria. Health Topics.https://www.who.int/health-topics/malaria#tab=tab_1 [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2023. WHO Recommends R21/Matrix-M Vaccine for Malaria Prevention in Updated Advice on Immunization (Press Release)https://www.who.int/news/item/02-10-2023-who-recommends-r21-matrix-m-vaccine-for-malaria-prevention-in-updated-advice-on-immunization [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2023. World Malaria Report 2023.https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023 [Google Scholar]
- WHO . Eighteenth Meeting of the WHO Vector Control Advisory Group. Meeting Report, 24–26 April 2023. World Health Organization; Geneva, Switzerland: 2023. https://www.who.int/publications/i/item/9789240077300 [Google Scholar]
- Wichmann O., Gascon J., Schunk M., Puente S., Siikamaki H., Gjorup I., et al. Severe dengue virus infection in travelers: Risk factors and laboratory indicators. J. Infect. Dis. 2007;195:1089–1096. doi: 10.1086/512680. [DOI] [PubMed] [Google Scholar]
- Wilder-Smith A. Dengue infections in travellers. Paediatr. Int. Child Health. 2012;32(Suppl. 1):28–32. doi: 10.1179/2046904712Z.00000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Smith A., Schwartz E. Dengue in travelers. N. Engl. J. Med. 2005;353:924–932. doi: 10.1056/NEJMra041927. [DOI] [PubMed] [Google Scholar]
- Wilken L., Rimmelzwaan G.F. Adaptive immunity to dengue virus: Slippery slope or solid ground for rational vaccine design? Pathogens. 2020;9:470. doi: 10.3390/pathogens9060470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard R.J., Jeffcoat A.M., Benson P.M., Walsh D.S. Cutaneous leishmaniasis in soldiers from Fort Campbell, Kentucky returning from Operation Iraqi Freedom highlights diagnostic and therapeutic options. J. Am. Acad. Dermatol. 2005;52:977–987. doi: 10.1016/j.jaad.2005.01.109. [DOI] [PubMed] [Google Scholar]
- Williams Y.A., Tusting L.S., Hocini S., Graves P.M., Killeen G.F., Kleinschmidt I., et al. Expanding the vector control toolbox for malaria elimination: A systematic review of the evidence. Adv. Parasitol. 2018 doi: 10.1016/bs.apar.2018.01.003. [DOI] [PubMed] [Google Scholar]
- World Bank International tourism, number of arrivals - sub-Saharan Africa. World Tourism Organization. Yearbook of Tourism Statistics. 2023 https://data.worldbank.org/indicator/ST.INT.ARVL?locations=ZG&most_recent_value_desc=true [Google Scholar]
- Wu H.M. Evaluation of the sick returned traveler. Semin. Diagn. Pathol. 2019;36:197–202. doi: 10.1053/j.semdp.2019.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young G.D., Evans S. Safety and efficacy of DEET and permethrin in the prevention of arthropod attack. Mil. Med. 1998;163:324–330. https://www.ncbi.nlm.nih.gov/pubmed/9597850 [PubMed] [Google Scholar]
- Yukich J., Digre P., Scates S., Boydens L., Obi E., Moran N., et al. Incremental cost and cost-effectiveness of the addition of indoor residual spraying with pirimiphos-methyl in sub-Saharan Africa versus standard malaria control: Results of data collection and analysis in the Next Generation Indoor Residual Sprays (NgenIRS) project, an economic-evaluation. Malar. J. 2022;21:185. doi: 10.1186/s12936-022-04160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapor M.J., Moran K.A. Infectious diseases during wartime. Curr. Opin. Infect. Dis. 2005;18:395–399. doi: 10.1097/01.qco.0000182102.50430.2c. [DOI] [PubMed] [Google Scholar]
- Zheng T.-T., Nie L.-F., Teng Z., Luo Y.-L. Modeling seasonal variation for mosquito-borne disease in the tropical monsoon environment. Adv. Differ. Equ. 2020;2020:469. doi: 10.1186/s13662-020-02807-6. [DOI] [Google Scholar]
- Zimmerman R.H., Lounibos L.P., Nishimura N., Galardo A.K., Galardo C.D., Arruda M.E. Nightly biting cycles of malaria vectors in a heterogeneous transmission area of eastern Amazonian Brazil. Malar. J. 2013;12:262. doi: 10.1186/1475-2875-12-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zutshi S., Kumar S., Chauhan P., Bansode Y., Nair A., Roy S., et al. Anti-leishmanial vaccines: Assumptions, approaches, and annulments. Vaccines. 2019;7:156. doi: 10.3390/vaccines7040156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the conclusions of this article are included within the article and its supplementary files.