Abstract
In autumn 2023, European vaccination campaigns predominantly administered XBB.1.5 vaccine. In a European multicentre study, we estimated 2023 COVID-19 vaccine effectiveness (VE) against laboratory-confirmed symptomatic infection at primary care level between September 2023 and January 2024. Using a test-negative case–control design, we estimated VE in the target group for COVID-19 vaccination overall and by time since vaccination. We included 1057 cases and 4397 controls. Vaccine effectiveness was 40 % (95 % CI: 26–53 %) overall, 48 % (95 % CI: 31–61 %) among those vaccinated < 6 weeks of onset and 29 % (95 % CI: 3–49 %) at 6–14 weeks. Our results suggest that COVID-19 vaccines administered to target groups during the autumn 2023 campaigns showed clinically significant effectiveness against laboratory-confirmed, medically attended symptomatic SARS-CoV-2 infection in the 3 months following vaccination. A longer study period will allow for further variant-specific COVID-19 VE estimates, better understanding decline in VE and informing booster administration policies.
Keywords: COVID-19, Vaccine effectiveness, Multicentre study, Test-negative design, Europe, SARS-CoV-2
1. Introduction
Between August and October 2023, the European Medicines Agency (EMA) authorised three adapted XBB.1.5 COVID-19 vaccines: Comirnaty Omicron XBB.1.5, Spikevax XBB.1.5 and Nuvaxovid XBB.1.5 [1]. In the European Union and European Economic Area (EU/EEA) the population groups targeted for COVID-19 vaccination included: older adults, persons with underlying medical conditions, immunocomprised individuals, healthcare workers and pregnant women [2]. The majority of vaccines administered (>98 %) were XBB.1.5 vaccines [3].
At time of the autumn 2023 COVID-19 vaccination campaigns in Europe, Omicron XBB.1.5-like, XBB.1.5-like + F456L and BA.2.86 viruses circulated, with BA.2.86 viruses comprising > 50 % of all variants from mid-December 2023 onwards [4].
Vaccine Effectiveness, Burden, and Impact Studies (VEBIS) of COVID-19 and influenza vaccines is a European Centre for Disease Prevention and Control (ECDC) project using post-marketing observational studies to understand effectiveness of vaccines. The primary care multicentre component of VEBIS is based on a well-established network, the I-MOVE network, estimating influenza VE since 2008 [5], [6].
Within VEBIS we aimed to estimate the 2023 autumn vaccination campaign vaccine effectiveness among patients in the COVID-19 vaccination target groups presenting to primary care physicians in the European Union/European Economic Area (EU/EEA), up to January 2024.
2. Methods
The VEBIS primary care study is a test-negative design case-control study conducted at primary care level in 11 European study sites: Croatia (HR); France (FR); Germany (DE); Hungary (HU); Ireland (IE); Portugal (PT); The Netherlands (NL); Romania (RO); Spain, national (ES); Spain, Navarre region (NA); Sweden (SE) (Supplementary Fig. 1).
Detailed methods are available elsewhere [7]. Briefly, in each site, primary care physicians or paediatricians recruit and swab all or a systematic sample of patients consulting with acute respiratory infection (ARI). Site-specific variations in case definition used for recruitment are available in Table 1. Demographic and clinical information is collected via interview or electronic medical records. COVID-19 vaccination information iss obtained from general practitioner records, interviews or national vaccination registries. Biological samples are tested using reverse transcription (RT)-PCR and all (or a random sample of) virus-positive specimens below a specified cycle threshold (Ct) value are sequenced. Patients meeting the EU-ARI case definition (sudden onset of symptoms with at least one of four respiratory symptoms: cough, sore throat, shortness of breath, coryza) testing RT-PCR positive are defined as cases and those testing negative as controls.
Table 1.
Autumn 2023 national COVID-19 vaccination campaign, vaccination and recruitment information, by study site, VEBIS primary care study, September 2023–January 2024.
| Study sites included in the analysis | Start of autumn 2023 national COVID-19 vaccination campaigns | Age-specific recommendation for COVID-19 vaccination for older adults | Data source for COVID-19 vaccination information | Case definition used for recruitment of patientsb |
|---|---|---|---|---|
| Croatia | 18 Sep 2023 | 65 | Medical records, vaccination registry, GP interview (self-report) | EU-ILI or EU-ARI |
| France | 2 Oct 2023 | 65 | GP interview (self-report) | Sentinelles ARI |
| Germany | 1 Sep 2023 | 60 | Medical records, patient’s certificate of vaccination, GP interview (self-report) | ARI |
| Hungary | 1 Oct 2023 | 60 | National Immunisation Registry (83.5 %), GP records, self-report | EU-ARI |
| Ireland | 2 Oct 2023 | 50 | Data linkage to vaccine registry | EU-ARI |
| The Netherlands | 2 Oct 2023 | 60 | GP interview (self-report) | EU-ILI or EU-ARI |
| Portugal | 29 Sep 2023 | 60 | Vaccine registry look-up by GPs | EU-ARI |
| Spain, national | 25 Sep 2023 | 60 | Data linkage to vaccine registry | EU-ARI |
| Spain, Navarre region | 9 Oct 2023 | 60 | Data linkage to vaccine registry | EU-ILI |
| Sweden | 7 Nov 2023 | 65 | Data linkage to vaccine registry | EU-ARI |
ARI: acute respiratory infection; EU: European Union; ILI: influenza-like illness; VEBIS: Vaccine Effectiveness, Burden and Impact Studies.
a Romania was excluded from the analysis due to too small a sample size (<10 cases).
EU-ARI: Sudden onset of symptoms and at least one of four respiratory symptoms (cough, sore throat, shortness of breath, coryza) and a clinician’s judgement that the illness is due to an infection; EU-ILI: Sudden onset of symptoms and at least one of four systemic symptoms (fever or feverishness, malaise, headache, or myalgia) and at least one of three respiratory symptoms (cough, sore throat, or shortness of breath); Sentinelles ARI: Sudden onset of fever (or feverishness), and respiratory signs; The ARI case definition in Germany includes patients with at least one of the following four symptoms: fever, cough, coryza or sore throat.
The study period for this analysis started 7 days after the start of the autumn 2023 COVID-19 vaccination campaigns in each country (Table 1), with dates ranging between 1 September and 7 November 2023.
We excluded patients who lived in a residential care facility, who were swabbed 10 days after symptom onset, who were vaccinated with a non-EMA approved vaccine for their autumn 2023 dose, who were reported by study sites where there were fewer than 10 cases, and who did not provide information for key covariates.
We included patients in the target groups for vaccination as recommended in each country. The age-specific recommendations varied by country, with the age above which older adults were recommended for vaccination ranging from 50 to 65 years (Table 1). All countries recommended patients with chronic conditions to be vaccinated, among those aged ≥ 6 months. As recommendations for COVID-19 vaccination for those aged under 5 years depended on previous COVID-19 vaccination history, we restricted the study population to those aged 5 years and over [2].
We considered a patient as vaccinated if they had received any dose of COVID-19 vaccine on or after the start date of the autumn 2023 vaccination campaign. Unvaccinated were those not receiving a COVID-19 vaccine as part of the autumn 2023 vaccination campaign, nor in the 6 months preceding the campaign. Those vaccinated 1–6 days before symptom onset were excluded.
We pooled individual patient data and used logistic regression to estimate the odds ratio (OR) of vaccination among cases and controls, adjusting for study site, age, sex, presence of four commonly collected chronic conditions (diabetes, heart disease, lung disease and immunodeficiency) and onset date. We selected the functional form of continuous variables among categories, linear terms, or restricted cubic splines using the Akaike Information Criterion. We estimated VE as (1-OR), expressed as a percentage.
We estimated VE among the overall target group and then stratified according to country-specific age limits for older adult vaccination (50 to 65 years, depending on the country). We refer to “the younger vaccination target group”, for the group including younger individuals who require medical risk conditions to be part of the target group, and to “the older adult vaccination target group”, for those vaccinated because they meet the age requirement. As recommendations vary by country, those aged 50–64 may be included in the “younger” or the “older” group, depending on the country-specific vaccination recommendations.
We estimated VE by time since vaccination, based on the median days between vaccination and onset, rounded to full weeks (two intervals: <6 weeks; 6–14 weeks). We linked epidemiological and sequencing data to estimate VE against Omicron XBB among all ages within the overall vaccination target group. Here, we restricted to sites providing at least five Omicron XBB cases and excluded any weeks of onset before and after the first and last sequenced case, respectively. Based on sequence data, viruses were classified as Omicron XBB if they belonged to the XBB variant and its descendants, e.g. XBB.1.16, XBB.1.5, XBB.1.9, XBB.2.3, as well as XBB lineages featuring the F456L mutation in Spike protein.
To handle sparse data, we carried out penalised logistic regression if the model violated the 10 events per parameter rule of thumb [8]. If the normal and penalised logistic regression VE point estimates differed by > 10 % we did not report the estimate. Additionally, we did not report any estimate where there were fewer than three patients in any cell of case and exposure status.
We carried out four sensitivity analyses: 1) varying the definition of vaccinated as someone who received vaccine at least 14 days before symptom onset, instead of 7 days as in the main analyses, meaning also that the study period started 14 days after COVID-19 vaccination campaigns; 2) varying the definition of the pre-campaign vaccination exclusion period for the reference group, using 3 months prior to the start of the vaccination campaign (instead of 6 months, as in the main analyses); 3) excluding influenza positive controls to account for potential correlation between influenza and COVID-19 vaccination behaviour [9]; 4) among those aged 65 years and over in order to have a more homogeneously aged older adult group.
3. Results
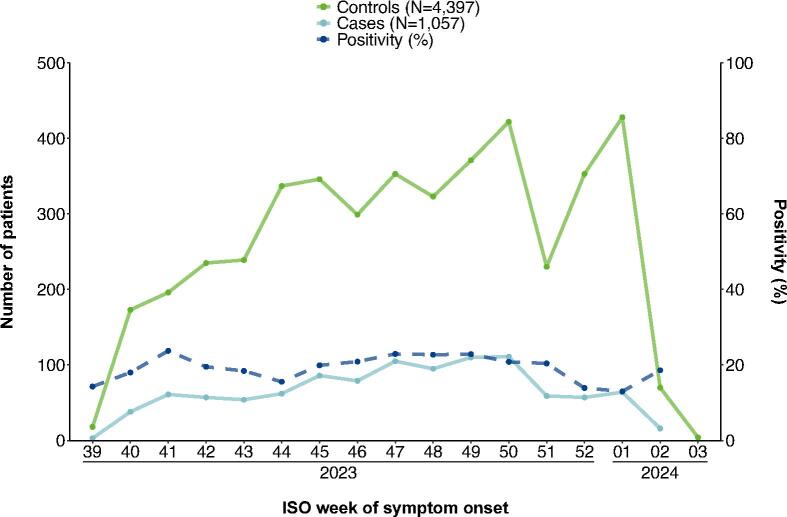
We included 5,454 patients; 1,057 cases and 4,397 controls aged ≥ 5 years (Fig. 1).
Fig. 1.
Patients included in the VEBIS primary care study by COVID-19 case status and ISO week of symptom onset, Europe, September 2023–January 2024 (n = 5,454). ISO: International Organisation for Standardisation; VEBIS: Vaccine Effectiveness, Burden and Impact Studies.
The median age was 64 years (interquartile range [IQR]: 53–73) among cases and 62 years (IQR: 47–72) among controls (Table 2). Sixty-two percent of cases and 60 % of controls were female. Sixty-nine percent of cases and 70 % of controls had one of four commonly collected chronic conditions (diabetes, heart disease, lung disease, immunodeficiency), with 74 % of both cases and controls in the younger vaccination target group with these chronic conditions, compared to 67 % and 68 % of cases and controls in the older vaccination target group. Among cases, 12 % had received COVID-19 vaccination as part of the autumn 2023 vaccination campaign, compared to 16 % of controls. Among vaccinated, 66 % (80/122) of cases and 68 % (482/707) of controls had received their fifth dose. COVID-19 vaccine brand was missing for 39 % (47/122) of vaccinated cases and for 26 % (186/707) of vaccinated controls. All patients but three were vaccinated with Comirnaty vaccine, where vaccine brand was known.
Table 2.
Baseline characteristics of patients included in the VEBIS primary care study by COVID-19 case status, Europe, September 2023–January 2024.
| Variables | Number of COVID-19 cases (%) 1057 |
Number of test-negative controls (%) 4397 |
|---|---|---|
| Median age (IQR) | 64 (53–73) | 62 (47–72) |
| Age groups (years) | ||
| 5–19 | 20/1057 (2 %) | 249/4397 (6 %) |
| 20–29 | 30/1057 (3 %) | 186/4397 (4 %) |
| 30–39 | 53/1057 (5 %) | 322/4397 (7 %) |
| 40–49 | 112/1057 (11 %) | 491/4397 (11 %) |
| 50–59 | 169/1057 (16 %) | 700/4397 (16 %) |
| 60–69 | 299/1057 (28 %) | 1096/4397 (25 %) |
| 70–79 | 243/1057 (23 %) | 901/4397 (20 %) |
| ≥80 | 131/1057 (12 %) | 452/4397 (10 %) |
| Female | 651/1057 (62 %) | 2631/4397 (60 %) |
| Presence of four commonly collected chronic conditionsa | 733/1057 (69 %) | 3093/4397 (70 %) |
| Pregnantb | 9/116 (8 %) | 60/616 (10 %) |
| Missing | 15 | 65 |
| COVID-19 vaccination in the autumn 2023 campaign | ||
| Received COVID-19 vaccination as part of this campaign | 122/1057 (12 %) | 713/4397 (16 %) |
| Detailed COVID-19 vaccination status in the autumn 2023 campaignc | ||
| First dose | 0/122 (0 %) | 4/707 (1 %) |
| Second dose | 2/122 (2 %) | 2/707 (0 %) |
| Third dose | 3/122 (2 %) | 17/707 (2 %) |
| Fourth dose | 19/122 (16 %) | 118/707 (17 %) |
| Fifth dose | 80/122 (66 %) | 482/707 (68 %) |
| Sixth dose | 12/122 (10 %) | 51/707 (7 %) |
| Seventh dose | 6/122 (5 %) | 32/707 (5 %) |
| Eighth dose | 0/122 (0 %) | 1/707 (0 %) |
| Missing | 0 | 6 |
| Brand of vaccine received in the autumn 2023 campaignc | ||
| Comirnaty | 74/75 (99 %) | 519/521 (100 %) |
| Spikevax | 0/75 (0 %) | 1/521 (0 %) |
| Jcovden | 0/75 (0 %) | 1/521 (0 %) |
| Bimervax | 1/75 (1 %) | 0/521 (0 %) |
| Missing | 47 | 186 |
| Median number of days since last COVID-19 vaccination received in the autumn 2023 campaign (IQR)c | 40 (22–54) | 37 (22–56) |
| COVID-19 vaccination target group | ||
| Younger target group | 374/1057 (35 %) | 1858/4397 (42 %) |
| Older adult target group | 683/1057 (65 %) | 2539/4397 (58 %) |
| Influenza virus RT-PCR positive | 35/1051 (3 %) | 706/4370 (16 %) |
| Missing | 6 | 27 |
| Study site | ||
| Croatia | 22/1057 (2 %) | 45/4397 (1 %) |
| France | 83/1057 (8 %) | 144/4397 (3 %) |
| Germany | 137/1057 (13 %) | 309/4397 (7 %) |
| Hungary | 121/1057 (11 %) | 150/4397 (3 %) |
| Ireland | 63/1057 (6 %) | 396/4397 (9 %) |
| Navarra, Spain | 15/1057 (1 %) | 111/4397 (3 %) |
| Portugal | 29/1057 (3 %) | 124/4397 (3 %) |
| Spain, national | 556/1057 (53 %) | 2981/4397 (68 %) |
| The Netherlands | 21/1057 (2 %) | 95/4397 (2 %) |
| Sequenced | 203/1057 (19 %) | NA |
| Among sequenced viruses | ||
| Omicron BA.2.75 | 3/203 (1 %) | NA |
| Omicron BA.2.86e | 73/203 (36 %) | NA |
| Non-JN.1 sublineages | 16 (22 %) | NA |
| JN.1 sublineages | 57 (78 %) | NA |
| Omicron XBB and sublineages | 123/203 (61 %) | NA |
| Otherd | 4/203 (2 %) | NA |
Abbreviations: IQR, Interquartile range; NA: Not applicable; VEBIS: Vaccine Effectiveness, Burden and Impact Studies.
The four commonly collected chronic conditions are: diabetes, heart disease, lung disease and immunodeficiency.
Among females aged 15–49.
Among those vaccinated as part of the 2023 autumn COVID-19 vaccination campaigns.
Recombinant strains detected in minimal proportions have been categorized as 'other'. Note that the members of this group (XCH.1, XDD, XDK, and XDL) lack topological congruence.
Among Omicron BA.2.86 viruses.
As of February 2024, 19 % (203/1057) of positive SARS-CoV-2 viruses were sequenced (Table 2). Of these sequenced viruses, 61 % (123/203) belonged to Omicron XBB and its sublineages and 36 % (73/203) belonged to Omicron BA.2.86 and its sublineages. Of the 73 BA.2.86 viruses, 78 % (57/73) belonged to JN.1 and its sublineages. During the study period, Omicron XBB viruses peaked in week 46 with 20 viruses and decreased to 6 viruses or fewer from week 48. In week 48, Omicron BA.2.86 viruses peaked with 15 sequenced viruses (Supplementary Figure 2).
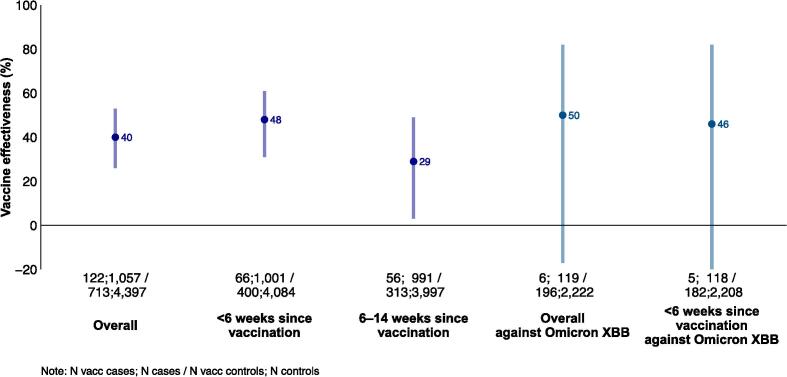
COVID-19 VE was 40 % (95 % CI: 26–53 %) overall (Fig. 2; Supplementary Table 1). The VE was 48 % (95 % CI: 31–61 %) among those vaccinated less than 6 weeks before onset and 29 % (95 % CI: 3–49 %) among those vaccinated 6–14 weeks before onset. The overall VE against Omicron XBB and its sublineages was 50 % (95 % CI: −17–82) and 46 % (95 % CI: –32–82) among those vaccinated less than 6 weeks before onset.
Fig. 2.
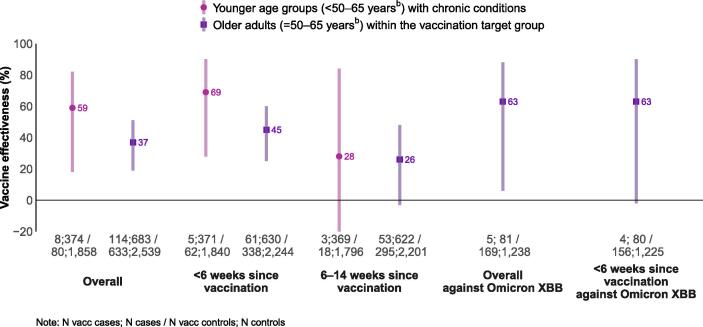
COVID-19 vaccine effectivenessa against medically-attended symptomatic SARS-CoV-2 infection among target groups for vaccination, (A) overall against any SARS-CoV-2 and against Omicron XBB and its sublineages and (B) against any SARS-CoV-2 stratified by age-specific vaccination target groups, Europe, VEBIS primary care study, September 2023–January 2024. Abbreviations: VEBIS: Vaccine Effectiveness, Burden and Impact Studies. The series of numbers below the x-axes provides the number of vaccinated cases; number of cases / number of vaccinated controls; number of controls. a All VE models included study site, sex and presence of chronic condition as adjustment variables. Using the Akaike information criterion to select the best functional form of onset date and age for each VE model, we included onset date as a restricted cubic spline (RCS) with 3 or 4 knots depending on the analysis, and age as a RCS with 3 or 5 knots, as a continuous variable or as a categorical variable with 5-year age bands depending on the analysis. b The target groups for older adults varied by country, see Table 1.
Among younger patients (aged less than 50–65 years, depending on country) VE was 59 % (95 % CI: 18–82 %) overall; 69 % (95 % CI: 28–90 %) if vaccinated less than 6 weeks before onset and 28 % (95 % CI: −128–84 %) if vaccinated 6–14 weeks before onset (Fig. 2; Supplementary Table 1).
Among older patients (aged over 50–65), the VE was 37 % (95 % CI:19–51 %) overall; 45 % (95 % CI: 26–60 %) if vaccinated less than 6 weeks before onset and 26 % (95 % CI: −3–48 %) if vaccinated 6–14 weeks before onset (Fig. 2; Supplementary Table 1). The overall VE against Omicron XBB and its sublineages was 63 % (95 % CI: 6–88) and 63 % (95 % CI: −2–90) among those vaccinated less than 6 weeks before onset.
When considering participants as vaccinated after 14 days instead of 7 days, the overall VE in the younger target group increased from 59 % (95 % CI: 18–82) to 70 % (95 % CI: 30–90); all other estimates differed by ≤ 7 % (Supplementary Table 2). When excluding unvaccinated patients vaccinated within 3 months of the autumn 2023 vaccination campaign instead of 6 months, VE point estimates differed by ≤ 2 %. When excluding controls positive to influenza virus, all VE point estimates were higher, differing by ≤ 8 %. VE point estimates among those aged 65 years and older were lower, differing by ≤ 6 %.
4. Discussion
Our results suggest that COVID-19 vaccines administered during the autumn 2023 campaigns, mainly Omicron XBB.1.5 vaccines, showed around 40 % effectiveness against laboratory-confirmed, medically attended symptomatic SARS-CoV-2 infection in the 3 months following vaccination among target groups. The COVID-19 VE point estimate was lower among older adults than among younger patients in the target group for vaccination (36 % vs. 59 %, respectively), although confidence intervals overlapped.
Within this approximately 3-month interval, point estimates declined with time since vaccination. However, due to the increase in circulation of BA.2.86 viruses and sublineages over time (Supplementary Figure 2), a variant-specific analysis by time since vaccination is needed to disentangle the variant and waning effects of vaccine protection. Omicron BA.2.86 viruses differ by more than 30 additional amino acid substitutions in their spike protein compared to XBB.1.5 and BA.2 viruses [10]. Additionally, JN.1, a descendant of BA.2.86 with the L455S substitution at the receptor binding site, is increasing in Europe and worldwide [11], [12]. Neutralisation studies indicate immune escape in JN.1 viruses compared to other recent variants/lineages, including XBB.1.5, although this immune escape is moderate [13]. Further studies in the BA.2.86/JN.1 era are needed to understand the impact on vaccine effectiveness. In our study, VE against Omicron XBB was 46 % and 63 % within 1–5 weeks since vaccination among all ages and among the older target group, respectively. These point estimates were similar or higher than those against any SARS-CoV-2 within the same time since vaccination period in our study, which may indicate that VE against BA.2.86 and its sublineages would be the same or lower, however precision was very low. Sample size was too small to estimate variant-specific VE by time since vaccination against non-XBB lineages in our study, and to estimate VE Omicron XBB by longer time since vaccination intervals.
Our VE results are similar to recently published COVID-19 VE estimates against symptomatic infection at outpatient level [14], [15]. In a similar study period, researchers from Canada reported a VE of 45 % among those aged 12 years and over, using similar methods to those in our study [15]. In a US study, younger adult VE was 57 % (compared to our 59 %) and older adult VE was 46 % (compared to our 40 %) [14]. While the US study observed less waning over a period of 4 months than our study did, variant-specific analyses by time since vaccination with more data will help better understand trends over time since vaccination.
Our 2023/24 COVID-19 VE point estimates among older adults within 90 days of vaccination are similar to those reported in the 2022/23 autumn–winter period [16]. In the 2022/23 season, our study suggested no protection against symptomatic infection conferred by the bivalent COVID-19 vaccine after 6 months (data not shown). This rapid waning was coherent with other studies at outpatient level in that season [17], [18]. We need a longer study period to understand if the waning of the XBB.1.5 vaccine shows a similar pattern, or if the protection conferred by the vaccine may have longer lasting effects, as indicated in a recent neutralisation study [19].
Our results are subject to limitations. These include unmeasured confounding and potential selection bias. However, the test-negative design aims to reduce potential confounding (mainly access to care) that is difficult to measure [20]. Our sensitivity analyses suggest that selection of influenza positive controls did not result in a selection bias that affected our VE by more than 8 %. We were unable to account for history of SARS-CoV-2 infection, which can potentially be both a confounder and an effect modifier of COVID-19 VE [21], [22]. Not all study sites collect information on previous SARS-CoV-2 infection, and this information is most often self-reported as a binary variable (yes/no). The risk of measurement error is then higher than with more objective measurements of previous infection (e.g., serological testing of infection-induced antibodies), and key information such as the date of infection, the variant period, and the number of past infections are not always available. In the context of reduced testing, it is difficult to obtain accurate information on previous mild infection. Sequencing information on SARS-CoV-2 viruses was only available for 19 % of cases. This resulted in low precision for the Omicron XBB-specific analysis, which hindered understanding if decline in VE was related to waning of vaccine protection and/or variant-specific effects. Vaccine coverage among the younger target group was low, limiting the precision of these estimates.
Strengths of the study include a multicentre study design, covering 10 European countries. The study is embedded on well-established platforms sharing a common protocol for robust results.
In conclusion, our study indicated that XBB.1.5 vaccination protected 40 % of vaccinated individuals from symptomatic infection in the short term. Estimating COVID-19 against Omicron BA.2.86 and its sublineages, by time since vaccination, will provide one ingredient to help public health decision-making around scheduling of future COVID-19 vaccination campaigns and vaccine composition.
Ethical statement
The planning, conduct and reporting of the studies was in line with the Declaration of Helsinki. Official ethical approval and patient consent was not required in Spain, as this study was classified as being part of routine care/surveillance. In the Netherlands, as the data are initially collected through surveillance, no formal ethical approval was necessary. Verbal informed consent from patients for participation in the national respiratory surveillance is required. In addition, patients have the option to opt out for participation in any further research (including influenza vaccine effectiveness studies). Other study sites received local ethical approval from a national or regional review board: Croatia: approved by the Ethics Committee of the Croatian Institute of Public Health (class 030–02/23–01/1); France: 471393; Germany: EA2/126/11; Hungary: IV/1885–5/2021/EKU; Ireland: ICGP2019.4.04; Portugal: approved 14 December 2022 by the Ethics Committee of Instituto Nacional de Saúde Doutor Ricardo Jorge, no registration number given; Romania: CE199/2022; Sweden: 2006/1040–31/2 revised Drn 2021–02791).
Funding
This study has received funding from the European Centre for Disease Prevention and Control under framework contract ECDC/2021/019.
GISAID
Participating laboratories submitted their sequences to GISAID (www.gisaid.org) for easy sharing with the central laboratory in Lisbon.
CRediT authorship contribution statement
Charlotte Laniece Delaunay: Writing – review & editing, Visualization, Validation, Methodology, Formal analysis, Data curation. Aryse Melo: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Marine Maurel: Writing – review & editing, Visualization, Validation, Methodology, Formal analysis, Data curation. Clara Mazagatos: Writing – review & editing, Resources, Methodology, Investigation, Data curation, Conceptualization. Luise Goerlitz: Writing – review & editing, Resources, Methodology, Investigation, Data curation, Conceptualization. Joan O’Donnell: Writing – review & editing, Resources, Investigation, Data curation, Conceptualization. Beatrix Oroszi: Writing – review & editing, Resources, Methodology, Investigation, Data curation, Conceptualization. Noémie Sève: Writing – review & editing, Resources, Methodology, Investigation, Data curation, Conceptualization. Ana Paula Rodrigues: Writing – review & editing, Resources, Methodology, Investigation, Data curation, Conceptualization. Iván Martínez-Baz: Writing – review & editing, Resources, Methodology, Investigation, Data curation, Conceptualization. Adam Meijer: Writing – review & editing, Resources, Methodology, Investigation, Data curation, Conceptualization. Ivan Mlinarić: Writing – review & editing, Resources, Methodology, Investigation, Data curation, Conceptualization. Neus Latorre-Margalef: Writing – review & editing, Resources, Methodology, Investigation, Data curation, Conceptualization. Mihaela Lazăr: Writing – review & editing, Resources, Methodology, Investigation, Data curation, Conceptualization. Gloria Pérez-Gimeno: Writing – review & editing, Resources, Methodology, Investigation, Data curation, Conceptualization. Ralf Dürrwald: Writing – review & editing, Resources, Methodology, Investigation, Data curation, Conceptualization. Charlene Bennett: Writing – review & editing, Resources, Investigation, Formal analysis, Conceptualization. Gergő Túri: Writing – review & editing, Resources, Methodology, Investigation, Data curation, Conceptualization. Marie-Anne Rameix-Welti: Writing – review & editing, Resources, Methodology, Investigation, Data curation, Conceptualization. Raquel Guiomar: Writing – review & editing, Resources, Methodology, Investigation, Data curation, Conceptualization. Jesús Castilla: Writing – review & editing, Resources, Methodology, Investigation, Data curation, Conceptualization. Mariëtte Hooiveld: Writing – review & editing, Resources, Methodology, Investigation, Data curation, Conceptualization. Sanja Kurečić Filipović: Writing – review & editing, Resources, Methodology, Investigation, Data curation, Conceptualization. Tove Samuelsson Hagey: Writing – review & editing, Resources, Methodology, Investigation, Data curation, Conceptualization. Frederika Dijkstra: Writing – review & editing, Resources, Methodology, Investigation, Data curation, Conceptualization. Vitor Borges: Writing – review & editing, Resources, Methodology, Investigation, Data curation, Conceptualization. Violeta Ramos Marín: Writing – review & editing, Resources, Methodology, Investigation, Data curation, Conceptualization. Sabrina Bacci: Writing – review & editing, Visualization, Project administration, Methodology, Funding acquisition, Conceptualization. Marlena Kaczmarek: Writing – review & editing, Resources, Methodology, Investigation, Data curation, Conceptualization. Esther Kissling: Writing – original draft, Visualization, Validation, Project administration, Methodology, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
All study teams are very grateful to all patients, general practitioners, paediatricians, laboratory teams, and regional epidemiologists who have contributed to the studies. We acknowledge the huge contribution by the European primary care group: Croatia - Ivana Ferenčak, Bernard Kaić, Maja Ilić, Vesna Višekruna Vučina, Croatian Institute of Public Health, Zagreb; Katica Čusek Adamić, Institute of Public Health, Varaždin County, Varaždin; Mirjana Lana Kosanović Ličina, ‘Dr Andrija Štampar’ Teaching Institute of Public Health, Zagreb; Danijela Lakošeljac, Teaching Institute of Public Health, Primorje-Gorski kotar County, Rijeka; Ivana Mihin Huskić, Teaching Institute of Public Health, Osijek-Baranja County, Osijek; Diana Nonković, Teaching Institute for Public Health, Split-Dalmatia County, Split. France – Thierry Blanchon, Caroline Guerrisi, Titouan Launay, Aubane Renard, Sorbonne Université, INSERM, Institut Pierre Louis d'épidémiologie et de Santé Publique (IPLESP UMRS 1136); Marie Chazelle, Alessandra Falchi, Shirley Masse, Laboratoire de Virologie, Université de Corse-Inserm; Vincent Enouf, Sylvie van der Werf, Centre National de Référence Virus des Infections Respiratoire (CNR VIR), Institut Pasteur. Epiconcept, Alain Moren, Anthony Nardone, Angela MC Rose, Epiconcept, Paris. Germany – Silke Buda, Annika Erdwiens, Ute Preuss, Kristin Tolksdorf, Department for Infectious Disease Epidemiology, Respiratory Infections Unit, Robert Koch Institute; Barbara Biere, Djin-Ye Oh, Janine Reiche, Marianne Wedde, National Reference Centre for Influenza, Robert Koch Institute. Hungary – Judit Krisztina Horváth, Krisztina Mucsányiné Juhász, Katalin Krisztalovics, Csaba Luca, National Laboratory for Health Security, Epidemiology and Surveillance Centre, Semmelweis University, Budapest; Katalin Kristóf, Institute of Laboratory Medicine, Semmelweis University, Budapest. The Hungarian study team works as part of the National Laboratory for Health Security Hungary (RRF-2.3.1-21-2022-00006) supported by the National Research, Development and Innovation Office (NKFIH). Ireland – Lisa Domegan, Adele McKenna, HSE Health Protection Surveillance Centre, Dublin; Jeff Connell, National Virus Reference Laboratory, Dublin; Michael Joyce, Olga Levis and the Irish sentinel GP network, Irish College of General Practitioners, Dublin; The Netherlands Lynn Aarts, Mariam Bagheri, Danytza Berry, Sanne Bos, Sharon van den Brink, Dirk Eggink, Rianne van Gageldonk-Lafeber, Gabriel Goderski, Liz Jenniskens, Femke Jongenotter, Marit de Lange, Tara Sprong, Anne Teirlinck, molecular pool technicians, National Institute for Public Health and the Environment (RIVM), Bilthoven; Nivel Primary Care Database – Sentinel Practices team, Ruud van den Broek, Safira Wortel, Ruben van der Burgh, Cathrien Kager, Mayra Klinkhamer, Bart Knottnerus, Marloes Riethof, Nienke Veldhuijzen, participating general practices and their patients, Nivel, Utrecht. Portugal – Nuno Verdasca, Licínia Gomes, Camila Henriques, Daniela Dias, Débora Pereira, Pais de Lacerda, Susana Maia Silva, Paula Pinto, Cristina Bárbara, Epidemiology Department, Instituto Nacional de Saúde Doutor Ricardo Jorge, Lisbon. Romania – Maria Elena Mihai, Alina Ivanciuc, Catalina Pascu, Iulia Bistriceanu, Sorin Dinu, Mihaela Oprea, Olivia Timnea, Adrian Jidovu, ‘Cantacuzino’ National Military-Medical Institute for Research and Development, Bucharest; Rodica Popescu, National Institute of Public Health, Bucharest. Spain – SiVIRA surveillance and vaccine effectiveness group. Spain: Navarre – Itziar Casado, Aitziber Echeverria, Camino Trobajo-Sanmartín, Manuel García Cenoz, Guillermo Ezpeleta, Instituto de Salud Pública de Navarra – IdiSNA, CIBERESP, Pamplona; Ana Navascués, Miguel Fernández-Huerta, Carmen Ezpeleta, Hospital Universitario de Navarra – IdiSNA, Pamplona. Sweden – Annasara Carnahan (epidemiology team), Emmi Andersson, Eva Hansson-Pihlainen, Elin Arvesen, Nora Nid, Anna-Lena Hansen and Lena Dillner (influenza virus surveillance team) and the NGS platform, Public Health Agency of Sweden, Stockholm, Sweden.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2024.05.067.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.European Medicines Agency. COVID-19 vaccines: authorised [Internet]. [cited 2024 Mar 21]. Available from: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-authorised#covid-19-vaccines:-strains,-use-and-age-ranges-(new)-section.
- 2.European Centre for Disease Prevention and Control. EMA and ECDC statement on updating COVID-19 vaccines to target new SARS-CoV-2 virus variants [Internet]. [cited 2024 Feb 12]. Available from: file:///C:/Users/esthe/Downloads/ecdc-ema_statement_on_updating_covid-19_vaccines_composition_for_new_sars-cov-2_virus_variants_en(1).pdf.
- 3.European Centre for Disease Prevention and Control. Interim COVID-19 vaccination coverage in the EU/EEA during the 2023–24 season campaigns [Internet]. 2024 Jan [cited 2024 Apr 19]. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/interim-vaccine-overage-eu-eea-2023-24.pdf.
- 4.European Centre for Disease Prevention and Control. European Respiratory Virus Surveillance Summary (ERVISS), 2024, Week 07. [Internet]. 202Available from: https://erviss.org/.
- 5.Valenciano M., Ciancio B. I-MOVE study team. I-MOVE: a European network to measure the effectiveness of influenza vaccines. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2012;17(39) doi: 10.2807/ese.17.39.20281-en. [DOI] [PubMed] [Google Scholar]
- 6.Kissling E., Pozo F., Martínez-Baz I., Buda S., Vilcu A., Domegan L., et al. Influenza vaccine effectiveness against influenza A subtypes in Europe: Results from the 2021–2022 I-MOVE primary care multicentre study. Influenza Other Respir Viruses [Internet] 2023 doi: 10.1111/irv.13069. https://onlinelibrary.wiley.com/doi/10.1111/irv.13069 Jan [cited 2023 Jan 17];17(1). Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Centre for Disease Prevention and Control. Core protocol for ECDC studies of vaccine effectiveness against symptomatic laboratory-confirmed influenza or SARS-CoV-2 infection at primary care level: version 1.0, September 2023. [Internet]. LU: Publications Office; 2023 [cited 2024 Jan 26]. Available from: https://data.europa.eu/doi/10.2900/25966.
- 8.Peduzzi P., Concato J., Kemper E., Holford T.R., Feinstein A.R. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 9.Doll M.K., Pettigrew S.M., Ma J., Verma A. Effects of confounding bias in COVID-19 and influenza vaccine effectiveness test-negative designs due to correlated influenza and COVID-19 vaccination behaviors. Clin Infect Dis Off Publ Infect Dis Soc Am. 2022 doi: 10.1093/cid/ciac234. Mar 24;ciac234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lasrado N., Collier A.ris.Y., Hachmann N.P., Miller J., Rowe M., Schonberg E.D., et al. Neutralization escape by SARS-CoV-2 Omicron subvariant BA.2.86. Vaccine. 2023;41(47):6904–6909. doi: 10.1016/j.vaccine.2023.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nextstrain. Genomic epidemiology of novel coronavirus - Europe-focused subsampling [Internet]. [cited 2021 Oct 30]. Available from: https://nextstrain.org/ncov/gisaid/europe.
- 12.Yang S., Yu Y., Xu Y., Jian F., Song W., Yisimayi A., et al. Fast evolution of SARS-CoV-2 BA.2.86 to JN.1 under heavy immune pressure. Lancet Infect Dis. 2024;24(2):e70–e72. doi: 10.1016/S1473-3099(23)00744-2. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Updated Risk Evaluation of JN.1, 09 January 2023 [Internet]. [cited 2024 May 16]. Available from: https://www.who.int/docs/default-source/coronaviruse/09022024_jn.1_ure.pdf?sfvrsn=a153518c_3.
- 14.Link-Gelles R, Ciesla AA, Mak J, Miller JD, Silk BJ, Lambrou AS, et al. Early Estimates of Updated 2023–2024 (Monovalent XBB.1.5) COVID-19 Vaccine Effectiveness Against Symptomatic SARS-CoV-2 Infection Attributable to Co-Circulating Omicron Variants Among Immunocompetent Adults — Increasing Community Access to Testing Program, United States, September 2023–January 2024. MMWR Morb Mortal Wkly Rep. 2024 Feb 1;73(4):77–83. [DOI] [PMC free article] [PubMed]
- 15.Skowronski D.M., Zhan Y., Kaweski S.E., Sabaiduc S., Khalid A., Olsha R., et al. 2023/24 mid-season influenza and Omicron XBB.1.5 vaccine effectiveness estimates from the Canadian Sentinel Practitioner Surveillance Network (SPSN) Eurosurveillance [Internet] 2024 doi: 10.2807/1560-7917.ES.2024.29.7.2400076. https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2024.29.7.2400076 Feb 15 [cited 2024 Feb 20];29(7). Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laniece Delaunay C, Mazagatos C, Martínez-Baz I, Túri G, Goerlitz L, Meijer A, et al. Effectiveness of COVID-19 vaccines administered in autumn and winter 2022/2023 in Europe. Submitt JAMA Netw Open. [DOI] [PMC free article] [PubMed]
- 17.Tartof S.Y., Slezak J.M., Puzniak L., Hong V., Frankland T.B., Ackerson B.K., et al. Effectiveness of BNT162b2 BA.4/5 bivalent mRNA vaccine against a range of COVID-19 outcomes in a large health system in the USA: a test-negative case–control study. Lancet Respir Med. 2023;11(12):1089–1100. doi: 10.1016/S2213-2600(23)00306-5. [DOI] [PubMed] [Google Scholar]
- 18.Plumb I.D., Briggs Hagen M., Wiegand R., Dumyati G., Myers C., Harland K.K., et al. Effectiveness of a bivalent mRNA vaccine dose against symptomatic SARS-CoV-2 infection among U.S. Healthcare personnel, September 2022–May 2023. Vaccine. 2023 doi: 10.1016/j.vaccine.2023.10.072. S0264410X23012872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Q., Guo Y., Bowen A., Mellis I.A., Valdez R., Gherasim C., et al. XBB.1.5 monovalent mRNA vaccine booster elicits robust neutralizing antibodies against emerging SARS-CoV-2 variants [Internet] Immunology. 2023 doi: 10.1016/j.chom.2024.01.014. http://biorxiv.org/lookup/doi/10.1101/2023.11.26.568730 Nov [cited 2024 Feb 25]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson M.L., Nelson J.C. The test-negative design for estimating influenza vaccine effectiveness. Vaccine. 2013 Apr;31(17):2165–2168. doi: 10.1016/j.vaccine.2013.02.053. [DOI] [PubMed] [Google Scholar]
- 21.Powell A.A., Kirsebom F., Stowe J., Ramsay M.E., Lopez-Bernal J., Andrews N., et al. Protection against symptomatic infection with delta (B.1.617.2) and omicron (B.1.1.529) BA.1 and BA.2 SARS-CoV-2 variants after previous infection and vaccination in adolescents in England, August, 2021–March, 2022: a national, observational, test-negative, case-control study. Lancet Infect Dis. 2023;(4):23435–23444. doi: 10.1016/S1473-3099(22)00729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ioannidis J.P.A. Factors influencing estimated effectiveness of COVID-19 vaccines in non-randomised studies. BMJ Evid-Based Med. 2022;27(6):324–329. doi: 10.1136/bmjebm-2021-111901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.