Abstract
The aim of the present study was to develop an in vitro system for presentation of bovine herpesvirus 1 (BHV-1) antigens to bovine T lymphocytes and to characterize the antigen-presenting cells (APC) which efficiently activate CD4+ T cells. Two approaches were used to monitor the infection of APC by BHV-1 as follows: (i) detection of viral glycoproteins at the cell surface by immunofluorescence staining and (ii) detection of UL26 transcripts by reverse transcription-PCR. The monocytes were infected, while dendritic cells (DC) did not demonstrate any detectable viral expression. These data suggest that monocytes are one site of replication, while DC are not. The capacities of monocytes and DC to present BHV-1 viral antigens in vitro were compared. T lymphocytes (CD2+ or CD4+) from BHV-1 immune cattle were stimulated in the presence of APC previously incubated with live or inactivated wild-type BHV-1. DC stimulated strong proliferation of Ag-specific T cells, while monocytes were poor stimulators of T-cell proliferation. When viral attachment to the surface of the APC was inhibited by virus pretreatment with soluble heparin, T-cell proliferation was dramatically decreased. Unexpectedly, incubation of DC and monocytes with the deletion mutant BHV-1 gD−/−, which displays impaired fusion capacity, resulted in strong activation of T lymphocytes by both APC types. Collectively, these results indicate that presentation of BHV-1 antigens to immune T cells is effective in the absence of productive infection and suggest that BHV-1 gD−/− mutant virus could be used to induce virus-specific immune responses in cattle.
Bovine herpesvirus 1 (BHV-1), a member of the Alphaherpesvirinae subfamily, is one of the major pathogens in cattle. BHV-1 is the causative agent of a variety of diseases, including infectious bovine rhinotracheitis (IBR), conjuntivitis, and pustular vulvovaginitis (reviewed in reference 13). The natural infection by BHV-1 occurs through mucous membranes of the upper respiratory tract, conjunctival epithelium, or genital tract (36).
The symptoms of the acute diseases are often associated with destruction of infected epithelial cells. The virus may spread in the infected host by viremia, gaining access to a broader range of tissues and organs. Furthermore, the virus is able to establish a latent infection and is eventually reactivated and reexcreted (1).
During BHV-1 infection, CD4+ T cells are considered to be essential for virus clearance in vivo. They are required for the generation of antibody-producing cells (2), class II-restricted CD4+ cytotoxic T lymphocytes (CTL) (12, 39), and NK-like cytotoxicity (9). Some effects appear to be mediated by cytokines such as interleukin-2 and gamma interferon (IFN-γ) (10–13).
Activation of CD4+ T helper lymphocytes requires specialized cells that process protein and present antigenic peptide fragments in the context of major histocompatibility complex class II (MHCII) molecules. The population of antigen-presenting cells (APC) is heterogenous and includes dendritic cells (DC), B cells, and macrophages. DC represent a discrete leukocyte population which has the unique capacity to sensitize naive T cells (reviewed in reference 23). Bovine alveolar macrophages (5, 6, 16, 17) and monocytes (31, 39) have been shown to be infected by BHV-1. However, consequences of infection of these cells on antigen-presenting function have not been evaluated.
We have recently described the purification and characterization of bovine DC from peripheral blood (33). In the present study, we develop an in vitro system, using bovine DC and monocytes as APC for BHV-1. Our data show that the susceptibility of APC to infection by BHV-1 and their capacity to activate BHV-1-specific T cells are unrelated.
MATERIALS AND METHODS
Animals and culture media.
Healthy cows were housed at the Veterinary and Agrochemical Research Centre (VAR). Six selected cows were vaccinated intranasally with attenuated gE-deleted virus (Bayovac IBR-marker vivum; Bayer) and boosted 4 months later by subcutaneous inoculation of inactivated gE-deleted vaccine (Bayovac IBR-marker inactivatum; Bayer). Blood samples were used as source of primed cells.
The culture medium used for the isolation and Ag pulsing of APC was RPMI 1640 (Gibco BRL; Merelbeke, Belgium) supplemented with 10% fetal calf serum (Byosis S.A., Compiègne, France), HEPES, 2-βmercaptoethanol, penicillin, streptomycin, l-glutamine, and sodium pyruvate (Flow ICN Biomedicals, Bucks, United Kingdom) as described elsewhere (33).
Isolation of bovine DC and monocytes.
DC (CD14−) and monocytes (CD14+) were purified as described previously (33), with some modifications. Peripheral blood mononuclear cells (PBMC) were obtained from whole blood by centrifugation on Ficoll-Hypaque (Pharmacia) gradients. PBMC were seeded onto gelatin plasma-coated flasks, and then DC and monocytes were allowed to attach for 2 h at 37°C. Nonadherent cells were removed by serial washes with phosphate-buffered saline (PBS). Cells attached to the gelatin were removed with Hank’s balanced salt solution containing 10 mM EDTA, washed, and cultured overnight. Nonadherent cells contained mainly DC and were further enriched by centrifugation in Nycodenz gradients (d = 1.068; Nycomed Pharma, Oslo, Norway).
FACS sorting of DC.
DC were further enriched by sorting of MHCII+, CD14−, and immunoglobulin M− (IgM−) cells. Cells were labelled with monoclonal antibodies (MAbs) specific for CD14 (CC-G33) and IgM (ILA30), revealed by fluorescein isothiocyanate (FITC)-coupled F(ab′)2 goat anti-mouse IgG1, and with mouse MAbs specific for MHCII (ILA21) followed by treatment with phycoerythrin-coupled F(ab′)2 goat anti-mouse IgG2a (Southern Biotechnology Associates, Birmingham, Ala.). MHCII+, CD14−, and IgM− cells were sorted by using fluorescence-activated cell sorter (FACS) Vantage (Becton Dickinson). Reanalysis of the sorted cell population confirmed a purity of >97%.
Isolation of T cells.
CD2+ cells were isolated by rosetting with sheep erythrocytes. CD4+ cells were obtained by treating CD2+ cells with MAbs to CD8+ (CC63) and complement. Purified CD2+ or CD4+ cells were used in the proliferation assays.
Viruses.
BHV-1 strain LAM was kindly provided by J. T. van Oirschot (Lelystad, The Netherlands). BHV-1 recombinant strain 8221 (genetic background BHV-1/Aus 12) carries the Escherichia coli β-galactosidase (β-Gal) gene inserted between gD and gI open reading frames and under control of the mouse cytomegalovirus immediate-early gene promoter. BHV-1 LAM and BHV-1 recombinant strain 8221 were produced on Madin-Darby bovine kidney (MDBK) cell monolayers. Virus supernatants were collected after the development of a complete cytopathic effect. Supernatants were cleared and kept at −80°C until use.
BHV1/80-221 (genetic background BHV-1/Aus 12) is a gD deletion mutant; the gD gene has been replaced by the E. coli β-Gal cassette (15). This virus was multiplied on MDBK cells that constitutively express the gD protein (MDBK-BUIV3-7 cells). The progeny virus was grown on MDBK cells, leading to the production of gD−/− virus (15).
Titration of BHV-1 8221 or LAM BHV-1 stocks was done by plaque assays on MDBK cells as described elsewhere (20).
In some experiments, 4 × 106 PFU of wild-type BHV-1 (strain LAM)/ml were exposed to shortwave UV radiation at 254 nm (UV General Electric 15 W germicidal, model no. G15T8) at a dose of 1,500 J/m2 (dosimeter Latarj model no. 162) for 5 min. Such exposure was shown to reduce virus infectivity in excess of 5 log, as assessed on MDBK cells (data not shown).
In the virus-cell attachment experiments, BHV-1 8221 or BHV-1/80-221 were incubated for 15 min at 37°C with soluble heparin at a final concentration of 500 U/ml. The treated viruses were added to the APC for 1 h. The cells were then extensively washed and immediately used in the proliferation test.
Virus purification.
BHV-1 recombinant strain 8221 was purified according to a protocol described previously (29). The virus preparations were clarified by centrifugation at 1,000 × g for 20 min and pelleted at 40,000 × g for 60 min at 4°C in a JA21 Beckman centrifuge. The viral pellets were suspended in PBS and centrifuged at 95,000 × g through 10 to 25% Ficoll 400 (Pharmacia) step gradient for 1 h at 4°C in a 60Ti rotor on an LS 5 Beckman ultracentrifuge. The virus band was removed by pipetting and resuspended in TNE buffer (1 M NaCl, 100 mM Trisma, 10 mM EDTA, [pH 7.5]), mixed well, and pelleted at 95,000 × g for 4°C in a 60Ti rotor on an LS 5 Beckman ultracentrifuge. The viruses were resuspended in 1 ml of PBS, aliquoted, titrated, and stored at −80°C until use.
T-cell proliferation assays.
DC and monocytes were incubated at a multiplicity of infection (MOI) of 1 with live or UV-inactivated BHV-1 LAM, BHV-1 8221, or BHV-1/80-221 (gD−/−) viruses for 60 min at 37°C. Serial dilutions of APC were added to 2 × 105 CD4+ or CD2+ T cells. Cells were cultured for 5 days and pulsed with 0.4 μCi of [3H]thymidine (specific activity, 2 Ci/mmol; Amersham Corp., Little Chalfont, United Kingdom) during the last 18 h of culture. Labeled DNA was collected onto filter paper, and thymidine incorporation was assessed by liquid scintillation. The results were expressed in counts per minute and corresponded to the means ± standard deviations of triplicate cultures.
Assays for viral penetration and replication. (i) Detection of viral glycoproteins by FACS.
DC or monocytes (106) were incubated for 1 h at 37°C with BHV-1 LAM at an MOI of 5. Cells were then washed three times and cultured for 18 h. Cells were washed twice with ice-cold PBS containing 1% bovine serum albumin and 0.1% sodium azide and labeled for 30 min with murine MAbs against BHV-1 glycoproteins gC (1507), gB (5106), and gD (3402). Cells were washed with PBS and incubated for 30 min on ice with FITC-polyclonal rabbit anti-mouse (Sigma Chemicals), washed again, and analyzed with a FACScan flow cytometer (Becton Dickinson). Infected MDBK cells were used as a positive control, while mock-infected DC, monocytes, and MDBK cells were used as negative controls.
(ii) Staining of cells for lacZ expression and determination of β-Gal activity.
DC, monocytes, and MDBK cells were incubated with BHV-1 8221 (LacZ+) at an MOI of 5 for 1 h and then washed. β-Gal expression was determined after 1, 3, 5, 8, 10, and 18 h. Cells were fixed for 30 min at 4°C with glutaraldehyde 0.5% in PBS, washed with PBS, and incubated overnight at 37°C with PBS containing 660 mM Na2HPO4 · 2H2O, 330 mM NaH2PO4, 30 mM K4[Fe(CN)6], 130 mM MgCl2, 30 mM K3[Fe(CN)6], and 20 mg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal)/ml in dimethyl sulfoxide. Cells were visualized under the microscope, and the number of blue cells was determined by counting at least 200 cells. Mock-infected cells and infected MDBK cells were used as negative and positive controls, respectively.
(iii) RNA isolation and reverse transcription (RT).
DC and monocytes (7 × 105) were incubated with the BHV-1 LAM strain at an MOI of 5 and collected 5 h postinfection. Similarly infected MDBK cells were used as a positive control. Messenger RNAs were extracted by using the Oligotex direct mRNA purification kit (Qiagen) according to the manufacturer’s instructions. Briefly, the cells were lysed, the cell lysate was homogenized, and poly(A)+ mRNA was captured on Oligotex oligo(dT)-coupled latex beads. After washing of the complex, mRNA was eluted in 20 μl of elution buffer.
The PCRs were done with two sets of primers, one specific for the cellular β-actin and one specific for the BHV-1 UL26 gene, respectively. The oligonucleotides β-actin 5′ (5′ GAG AAG CTG TGC TAC GTC GC 3′) and β-actin 3′ (5′ CCA GAC AGC ACT GTG TTG GC 3′) were located on two different exons. The size of the PCR product obtained after amplification of the mRNA sample—261 bp if the cDNA was amplified and 350 bp if the template was cellular DNA—was used to check the integrity of mRNA and the absence of contaminating DNA in the mRNA preparations. BHV-1 infection was monitored by the amplification of a 203-bp fragment of the γ UL26 gene by using the primers UL26-1 (5′ GAT CAA CAT TGA CCA CGC AAG C 3′) and UL26-2 (5′ TAG TTG CTG ACG AGG TAC AGG 3′).
The RT reaction was also performed in the absence of Moloney murine leukemia virus reverse transcriptase to exclude the possibility that the DNA products were amplified from contaminating DNA.
RT was done in a final volume of 20 μl containing 10 μl of mRNA, 10 mM dithiothreitol, 50 mM Tris-HCl, 3 mM MgCl2, 4 μg of random hexamers, 0.2 mM deoxynucleoside triphosphate, and 200 U of Moloney murine leukemia virus reverse transcriptase (Gibco BRL). The reaction mixture was incubated for 1 h at 37°C, and the reaction was terminated by incubation at 95°C for 5 min. The PCRs were carried out in a total volume of 50 μl containing commercial PCR buffer, Q solution, 1.5 mM MgCl2, 5 U of Taq DNA polymerase (Qiagen), 0.2 mM deoxynucleoside triphosphate, 150 pmol of each primer, and 2 μl of the RT reactions. Amplifications were carried out for 35 cycles by denaturating at 95°C for 1 min, annealing for 1 min at 51°C for the β-actin primers and at 49°C for the UL26 primers, and extending at 72°C for 1 min.
Measurement of cell viability.
Cell viability was determined at the single-cell level by using a double fluorescent approach (viability/cytotoxicity kit; Molecular Probes). Briefly, cells were washed and treated for 30 min with fluorescent dyes, 2 μM calcein AM, and 4 μM EthD-1, which stain viable and dead and dying cells, respectively. Stained cells were counted by using fluorescence microscopy.
RESULTS
DC are not susceptible to BHV-1 infection, whereas monocytes are sites of viral replication.
We tested whether bovine DC and monocytes could be productively infected by BHV-1. Enriched DC and monocytes were shown to contain 86% MHCII+ and CD14− cells and 86% MHCII+ and CD14+ cells, respectively.
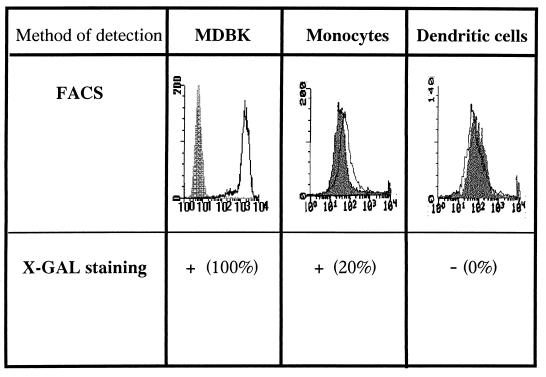
These cells were incubated with wild-type BHV-1 (strain LAM) at an MOI of 5. Cells were washed at 1 h postinfection and incubated in culture medium for 18 h. To detect BHV-1 infection, expression of viral glycoproteins was monitored by immunofluorescence staining with a cocktail of MAbs against gB, gC, and gD. Infected MDBK cells were used as a positive control, while mock-infected APC and MDBK cells were used as negative controls. All MDBK cells expressed high levels of BHV-1 glycoproteins (Fig. 1). Under the same experimental conditions, monocytes expressed viral glycoproteins at low levels, while the expression on DC was undetectable.
FIG. 1.
DC are not susceptible to BHV-1 infection, whereas monocytes are sites of viral replication. (Upper panels) The expression of viral glycoproteins was analyzed by FACS 18 h after infection with LAM wild-type BHV-1. Results are representative of three independent experiments. Dashed histogram, uninfected cells; open histograms, infected cells. (Lower panels) Percentages of cells expressing β-Gal activity. Monocytes, DC, or MDBK were incubated with BHV-1 8221 at an MOI of 5 during 1 h and washed, and β-Gal expression was determined 8 h postinfection.
In order to further assess the susceptibility of both APC populations, DC, monocytes, and MDBK were cultured with a β-Gal recombinant BHV-1 strain (BHV-1 8221 LacZ+) at an MOI of 5 and stained for lacZ expression.
More than 90% of MDBK cells and 20% of monocytes stained blue at 8 h postinfection (Fig. 1). The percentage of monocytes expressing β-Gal did not increase with time (data not shown). In contrast, no β-Gal activity was observed in the DC-enriched population under the same experimental conditions.
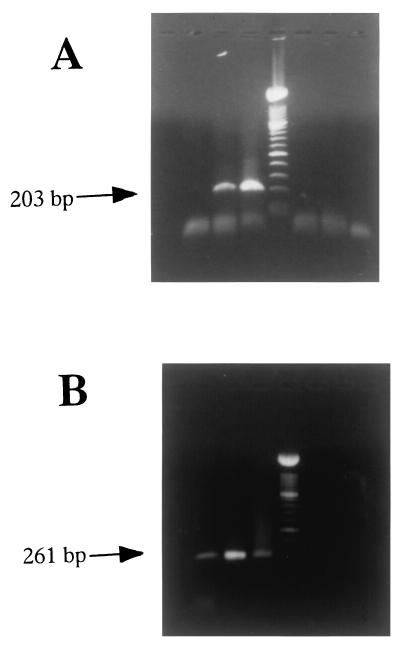
To further confirm that DC did not express viral messages, we monitored the presence of mRNA coding for the γ capsid protein UL26, using RT-PCR on RNA isolated from FACS-sorted DC (>97% MHCII+ and CD14−) incubated with BHV-1. As a positive control, we used enriched monocytes (86% MHCII+ CD14+) that express viral antigens. As UL26 mRNAs were detectable in monocytes at 5 h postinfection (data not shown), the presence of mRNA coding for the BHV-1 UL26 was tested on DC and monocytes at the same time point. The results shown in Fig. 2A indicate a positive signal for monocytes (lane 2) and MDBK (lane 3). In contrast, no fragment was amplified from mRNA extracted from DC (lane 1). Amplification in the absence of RT gave a negative result for the three cell types (lanes 5 to 7). To control for integrity of mRNA, we amplified a 261-bp fragment from the β-actin gene. The data obtained with the β-actin primers are illustrated in Fig. 2B. The expected 261-bp fragment was amplified from pulsed DC (lane 1), monocytes (lane 2), and MDBK cells (lane 3) incubated with BHV-1. No amplified fragments were detected in control reaction mixtures from which the reverse transcriptase was omitted (lanes 5 to 7).
FIG. 2.
UL26 transcript is detected in monocytes but not in DC. (A) Detection of UL26 mRNA expression by RT-PCR. A total of 7 × 105 FACS-sorted DC (>97% MHCII+, CD14−, and IgM−), enriched monocytes (86% MHCII+ and CD14+), or MDBK cells were infected with Lam BHV-1 at an MOI of 5 for 5 h. Lanes (from left): 1, DC; 2, monocytes; 3, MDBK, 4, molecular markers; 5 to 7, RT-PCR products of mRNA DC, monocytes, and MDBK, respectively, without reverse transcriptase. (B) Detection of β-actin mRNA expression by RT-PCR. Lanes are the same as for panel A.
To evaluate the PCR sensitivity, monocytes were infected with dilutions of BHV-1. The UL26 primers were capable of detecting viral mRNA 5 h after infection at an MOI of 0.001 (data not shown), suggesting that the difference observed between DC and the monocytes was not due to a lack of sensitivity of the RT-PCR.
DC, but not monocytes, induce strong proliferation of BHV-1-specific T lymphocytes in vitro.
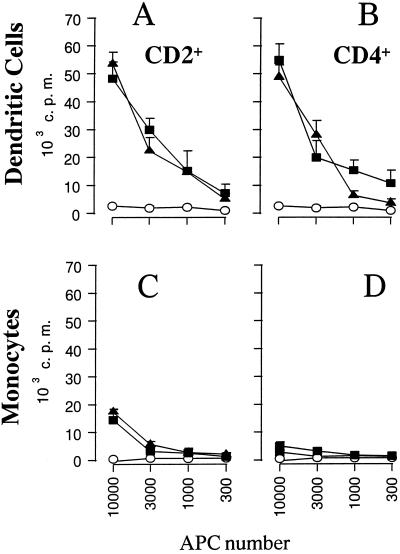
We next compared the ability of DC and monocytes to present BHV-1 antigens to T cells in vitro. Enriched DC (86% MHCII+ and CD14−) and monocytes (86% MHCII+ and CD14+) were incubated with live virus at an MOI of 1, washed extensively 1 h later, and cultured with autologous T cells from a BHV-1-immune animal. The data in Fig. 3 indicate that CD2+ T cells proliferated in the presence of DC incubated with live BHV-1 (Fig. 3A). Proliferation was equivalent for an enriched population of CD4+ T cells (Fig. 3B). Monocytes that were infected with the same viruses weakly activated CD2+ T-cell proliferation (Fig. 3C) and did not induce proliferation of purified CD4+ T lymphocytes (Fig. 3D). Notably, we detected IFN-γ in the culture supernatants of T cells activated by DC but not monocytes (data not shown), showing that T cells neither proliferated nor secreted IFN-γ.
FIG. 3.
DC sensitize BHV-1-specific T lymphocytes in vitro. Increasing numbers of DC or monocytes, incubated with BHV-1 LAM, were cultured with 2 × 105 autologous CD2+ (A and C) or CD4+ (B and D) lymphocytes. Proliferation was assessed by thymidine incorporation during the last 10 h of 5 days of culture. The data are expressed as counts per minute (c.p.m.), and each point represents the mean ± standard deviation of triplicate cultures. The results are representative of three independent experiments. Open circles, mock-infected APC; closed triangles, APC incubated with live BHV-1; closed squares, APC incubated with UV-inactivated BHV-1.
As presentation of BHV-1 by DC did not seem to depend on productive infection, DC were tested for their capacity to present UV-inactivated BHV-1. The same DC population was incubated with UV-inactivated BHV-1 and used in a proliferation test. The results shown in Fig. 3A and B indicate that live and inactivated viruses were presented with similar efficiencies, as measured per cell, to CD2+ and CD4+ T cells. Similar results were obtained with FACS-sorted DC (97% purity) and purified virus (data not shown), suggesting that DC process viral proteins and do not take up peptides that are released by contaminating monocytes or that are present in the viral inoculum. As expected, proliferation of T cells stimulated with similar numbers of monocytes incubated with UV-inactivated BHV-1 was undetectable (Fig. 3C and D).
Infected monocytes do not inhibit T-cell proliferation.
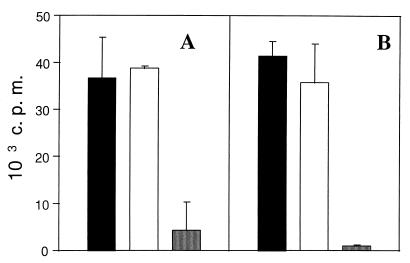
We next determined whether BHV-1-infected monocytes had a suppressive effect on T-cell proliferation. DC and monocytes were incubated with live virus, washed, and cocultured with CD2+ T cells. The data in Fig. 4 show that T-cell proliferation induced by DC was not decreased in the presence of infected monocytes. Indeed, comparable level of T-cell proliferation was observed when T lymphocytes were stimulated with DC alone or with a mixture of DC and monocytes (ratio of 1:1). These results indicate that the failure of BHV-1-infected monocytes to activate T-cell proliferation was not due to a mechanism of active suppression.
FIG. 4.
Infected monocytes do not inhibit T-cell proliferation induced by DC. DC were incubated with live BHV-1 LAM, washed, and cultured with 2 × 105 autologous CD2+ lymphocytes in the presence (black bars) or absence (open bars) of monocytes pretreated with the same virus. Controls include cocultures of T cells with mock-infected DC and monocytes (shaded bars). The total numbers of APC are 2 × 104 (A) and 6 × 103 (B). Proliferation was assessed by thymidine incorporation during the last 10 h of 5 days of culture. The data are expressed as counts per minute (c.p.m.), and each point represents the mean ± standard deviation of triplicate cultures. The results are representative of three independent experiments.
Effects of heparin and gD mutant in BHV-1 antigen presentation by DC and monocytes.
We next evaluated whether the difference between DC and monocytes in presentation of BHV-1 antigen to T cells was linked to virus penetration into the cell.
Attachment of BHV-1 to the cell membrane can be inhibited by the addition of exogenous heparin (32). The second step during infection involves the gD glycoprotein, which has been shown to be essential for penetration into cells (15, 18). We tested whether the addition of soluble heparin to purified gD+ virus (BHV-1 recombinant strain 8221) or gD−/− deletion mutant (BHV-1/80-221) would affect the presentation of BHV-1 antigens by DC and monocytes.
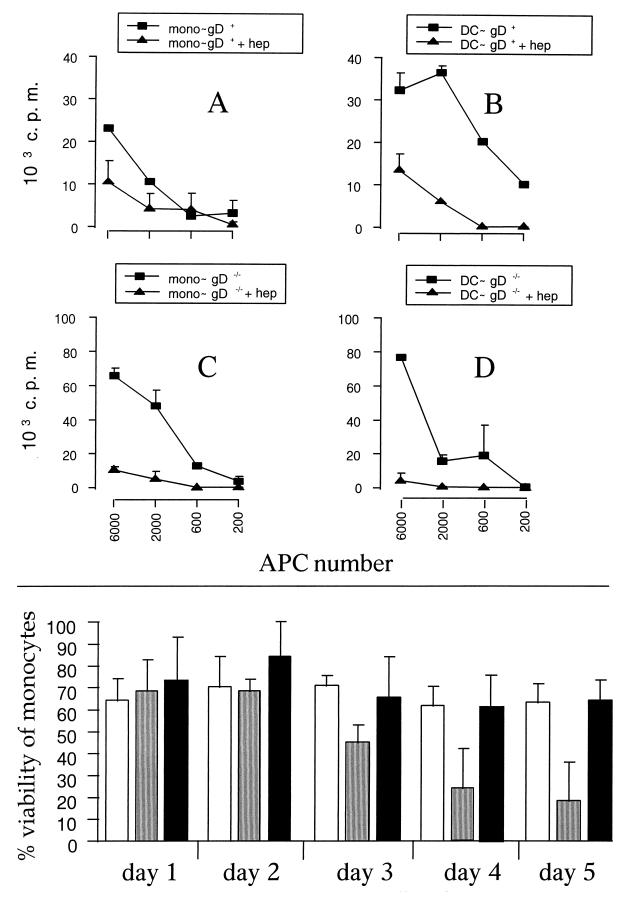
MDBK cells, infected either with gD+ or gD−/− virus, which were pretreated with soluble heparin, did not show evidence of infection, demonstrating that attachment and penetration were inhibited (data not shown). DC or monocyte populations isolated from the same animal were incubated with both types of viruses, pretreated with soluble heparin for 1 h or left untreated. Cells were washed extensively and cocultured with autologous T cells. The results are presented in Fig. 5. As expected, monocytes infected with purified virus in the presence or absence of soluble heparin induced a low proliferation of CD2+ T cells (Fig. 5A). The presence of soluble heparin diminished the presentation of BHV-1 antigen by DC to T cells (Fig. 5B). gD−/− deletion mutant virus was presented by DC, and this presentation was inhibited by pretreatment with heparin (Fig. 5D). Surprisingly, monocytes incubated with the gD−/− deletion mutant presented BHV-1 antigen to T cells with the same efficiency as DC (Fig. 5C).
FIG. 5.
(Upper panel) The interaction of BHV-1 with cell membrane through the gD glycoprotein is dispensable for efficient antigen presentation, while attachment is necessary. CD2+ T lymphocytes were cultured with increasing numbers of APC infected with gD+ (recombinant strain 8221) (A and B) or gD−/− (BHV-1/80-221) BHV-1 strains (C and D). The viruses were left untreated (closed squares) or pretreated (closed triangles) with 500 U of soluble heparin/ml for 15 min at 37°C and added to APC for 1 h. Proliferation was assessed by thymidine incorporation during the last 10 h of 5 days of culture. The data are expressed as counts per minute (c.p.m.), and each point represents the mean ± standard deviation of triplicate cultures. The results are representative of three independent experiments. (Lower panel) gD−/− does not reduce the viability of monocytes, in contrast to wild-type BHV-1. Monocytes were either mock infected (open bars) or incubated with live wild-type BHV-1 (shaded bars) or with gD−/− deletion mutant (black bars) at an MOI of 1. The monocytes were harvested at different days after treatment, and the percentage of viable cells was determined as described in Materials and Methods. The results represent the percentage of dead/dying cells (mean ± standard deviation of five randomly selected fields), as determined by the number of calcein AM-positive cells/number of calcein AM and EthD-1-positive cells.
Previous studies (21) have shown that monocytes infected with live BHV-1 undergo apoptosis, suggesting that the failure of infected monocytes to activate T cells could be due to decreased viability. We therefore compared the viability of monocytes incubated with live wild-type virus or gD−/− deletion mutant. The data in Fig. 5 (lower panel) show that wild-type BHV-1 did indeed reduce the viability of infected monocytes from day 3 postinfection. By contrast, under the same conditions, the gD−/− mutant virus did not affect monocyte viability at any time point tested.
DISCUSSION
The aim of this work was to evaluate the capacity of DC and monocytes to stimulate BHV-1-specific T lymphocytes in vitro. We first investigated the pathway of BHV-1 penetration in DC and monocytes. Viral glycoproteins gB, gC, and gD and the complex gH-gL represent a structural component of virions that is thought to be involved in attachment and penetration of the virion in susceptible cells (25, 38). The entry process begins with a low-affinity attachment on the cell surface mediated by an interaction between cell-surface heparan sulfate proteoglycan and virion glycoproteins gB and gC and, to a lesser extent, gD (25–27). Following this initial step, pH-independent fusion of the virus envelope with the cell surface requires at least four virion glycoproteins, including gB, gD, gH, and gL (25, 30, 38). The major role of gD is to ensure a penetration-competent conformation of the fusion complex (35) involving other cellular receptors (19, 37).
In order to evaluate the role of the entry process in the presentation of BHV-1, (i) the attachment of the virus to the cell surface was blocked by treatment of the virus with soluble heparin and (ii) the fusion step was impaired by the use of a gD−/− deletion mutant.
Our data show that DC efficiently present live, UV-inactivated, and gD−/− deletion mutant viruses. Pretreatment of virus with soluble heparin completely inhibited the presenting capacity of DC, showing that the virus attachment step through binding of gB and/or gC on heparan sulfate receptors was necessary for an efficient antigen presentation to immune T cells. Notably, the presentation of the gD−/− deletion mutant by DC was as efficient as that of the parental virus, suggesting that the fusion was not required. Our observations further indicate that BHV-1 did not productively infect DC, as assessed by the lack of transcription of a late capsid gene and the absence of viral glycoprotein expression.
Monocytes poorly presented live or UV-inactivated wild-type virus but strongly stimulated proliferation of virus-specific T cells when incubated with the gD−/− deletion mutant virus. In contrast to DC, monocytes were productively infected by wild-type BHV-1 as shown by immunofluorescence, RT-PCR, and β-Gal activity. Similarly, we found that monocytes incubated with gD−/− deletion mutant virus expressed mRNA coding for the γ capsid protein UL26 by RT-PCR (data not shown), suggesting that the virus could penetrate monocytes independently of gD, as previously reported (24, 34, 35).
The observation that monocytes incubated with UV-inactivated or live viruses were defective for BHV-1 presentation may result from inhibition of T-cell and/or monocyte function. It has indeed been reported that, in the presence of live BHV-1, activated CD4+ T cells lose their expression of CD4 and undergo apoptosis (14). However, the addition of infected monocytes to T cells and DC cocultures did not inhibit T cell proliferation (see Fig. 4), excluding an inhibitory effect of infected monocytes to T cells. Of note, several reports have shown that monocytes were susceptible to BHV-1 infection (31, 39) and that alveolar macrophages infected with BHV-1 displayed reduced Fc-mediated receptor activity, complement receptor activity, and phagocytosis (17). Live and inactivated BHV-1 were shown to induce apoptosis of bovine mitogen-stimulated PBMC (20). The data presented herein show a reduction of monocyte viability as a consequence of their susceptibility to BHV-1 infection (Fig. 5). By contrast, UV-inactivated BHV-1 had a limited effect on monocyte viability, compared to live virus (data not shown). Interestingly, gD−/− mutant did not affect cell viability (Fig. 5), as shown previously (22).
Our results are consistent with the model described for herpes simplex virus type 1, which proposed that this virus can penetrate cells by two mechanisms. The first mechanism is the fusion between the viral envelope and the plasma membrane, leading to the entry of the virus into cytosol, and the second corresponds to the endocytosis of viral particles. There is evidence that the virus particles contained in the endocytic vesicles are degraded (7, 8). Therefore, the entry of the virus by way of endocytosis would result in a decreased number of infectious viruses and therefore limit the cytopathic effect of the virus.
Collectively, these observations would be consistent with the hypothesis that APC have the capacity to present BHV-1 antigens that enter the cell by endocytosis but not by fusion of the envelope with the membrane. The efficient presentation of wild-type BHV-1 by DC could be due to a deficient fusion process and/or to a lack of cytopathic effect. It would be of interest to analyze whether DC and monocytes express gD receptor(s) (19).
The role of DC in the induction of virus-specific immune responses has been amply demonstrated both in vitro and in vivo. In particular, DC have been shown to present influenza virus and lymphocytic choriomeningitis virus (3, 28; reviewed in reference 4). Our data suggest that monocytes could be vehicles to disseminate virus in the host, while DC would initiate the immune response. Whether the difference in infectivity and presentation between both APC is of physiological relevance in vivo remains to be determined.
In conclusion, the interaction of BHV-1 with APC represents a complex event in which both routes of cellular penetration (infection and endocytosis) could occur. Our data suggest that BHV-1 infection could inhibit the presentation of monocytes by affecting their viability. By contrast, DC are not susceptible to infection but strongly activate T-cell function. We further show that a gD−/− deletion mutant virus has no cytopathic effect on APC and can be presented efficiently by both DC and monocytes. This mutant virus could be a promising tool for vaccine development.
ACKNOWLEDGMENTS
We are grateful to E. Hanon, B. Renjifo, O. Leo, and F. Rijsewijk for interesting discussions and helpful suggestions; to O. Leo for careful review of the manuscript; to C. Howard for providing MAbs specific for bovine cells; to G. Letchworth for providing the MAbs specific for BHV-1; and to G. Vandendaele and M. Verhoeven for excellent technical assistance. M. Moser and A. Vanderplasschen are research associates from the Belgian Fonds National de la Recherche Scientifique.
This work was supported by grants from the Ministère des classes moyennes et de l’agriculture and by the Biotech Programme of the European Commission (contract no. BIO2-CT93-0489).
REFERENCES
- 1.Ackermann M, Wyler R. The DNA of an IPV strain of bovid herpesvirus 1 in sacral ganglia during latency after intravaginal infection. Vet Microbiol. 1984;9:53–63. doi: 10.1016/0378-1135(84)90078-6. [DOI] [PubMed] [Google Scholar]
- 2.Babiuk L A, Van Drunen Littel-van den Hurk S, Tikoo S K. Immunology of bovine herpesvirus 1 infection. Vet Microbiol. 1996;53:31–42. doi: 10.1016/s0378-1135(96)01232-1. [DOI] [PubMed] [Google Scholar]
- 3.Bender A, Bui L K, Feldman M A V, Larson M, Bhardwaj N. Inactivated influenza virus, when presented on dendritic cells, elicits human CD8+ cytolytic T cell responses. J Exp Med. 1995;182:1663–1671. doi: 10.1084/jem.182.6.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhardwaj N. Interactions of viruses with dendritic cells: a double-edged sword. J Exp Med. 1997;186:795–799. doi: 10.1084/jem.186.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bielefeldt Ohmann H, Gilchrist J E, Babiuk L A. Effect of recombinant DNA-produced bovine interferon alpha (BoIFN-aplha 1) on the interaction between bovine alveolar macrophages and bovine herpesvirus type 1. J Gen Virol. 1984;65:1487–1495. doi: 10.1099/0022-1317-65-9-1487. [DOI] [PubMed] [Google Scholar]
- 6.Bielefeldt Ohmann H B, Babiuk L A. Alteration of alveolar macrophage functions after aerosol infection with bovine herpesvirus type 1. Infect Immun. 1986;51:344–347. doi: 10.1128/iai.51.1.344-347.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunetti C R, Dingwell K S, Wale C, Graham F L, Johnson D C. Herpes simplex virus gD and virions accumulate in endosomes mannose 6-phosphate-dependent and -independent mechanism. J Virol. 1998;72:3330–3339. doi: 10.1128/jvi.72.4.3330-3339.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campadelli-Fiume G, Arsenakis M, Farabegoli F, Roizman B. Entry of herpes simplex virus 1 in BJ cells that constitutively express viral glycoprotein D is by endocytosis and results in degradation of the virus. J Virol. 1988;62:159–167. doi: 10.1128/jvi.62.1.159-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campos M, Rossi C R. Cell-mediated cytotoxicity of bovine mononuclear cells to IBRV-infected cells: dependence on Sephadex G-10 adherent cells. Vet Immunol Immunopathol. 1985;8:363–375. doi: 10.1016/0165-2427(85)90006-6. [DOI] [PubMed] [Google Scholar]
- 10.Campos M, Bielefeldt-Ohmann H, Hutchings D, Rapin N, Babiuk L A, Lawman M J P. Role of interferon-γ in inducing cytotoxicity of peripheral blood mononuclear leukocytes to bovine herpesvirus type 1 (BHV-1)-infected cells. Cell Immunol. 1989;120:259–269. doi: 10.1016/0008-8749(89)90193-7. [DOI] [PubMed] [Google Scholar]
- 11.Campos M, Rossi C R, Bielefeldt-Ohmann H, Beskorwayne T, Rapin N, Babiuk L A. Characterization and activation requirements of bovine lymphocytes acquiring cytotoxic activity after interleukin-2 treatment. Vet Immunol Immunopathol. 1992;32:205–223. doi: 10.1016/0165-2427(92)90047-t. [DOI] [PubMed] [Google Scholar]
- 12.Choi S H, Splitter G A. Induction of MHC-unrestricted cytolytic CD4+ T cells against virally infected target cells by cross-linking CD4 molecules. J Immunol. 1994;153:3874–3881. [PubMed] [Google Scholar]
- 13.Denis M, Splitter G, Thiry E, Pastoret P-P, Babiuk L A. Infectious bovine rhinotracheitis (bovine herpesvirus 1): helper T cells, cytotoxicity T cells and NK cells. In: Goddeeris B, Morrison I, editors. Cell mediated immunity in ruminants. Boca Raton, Fla: CRC Press; 1994. pp. 157–172. [Google Scholar]
- 14.Eskra L, Splitter G. Bovine herpesvirus-1 infects activated CD4+ lymphocytes. J Gen Virol. 1997;78:2159–2166. doi: 10.1099/0022-1317-78-9-2159. [DOI] [PubMed] [Google Scholar]
- 15.Fehler F, Herrmann J M, Saalmüller A, Mettenleiter T C, Keil G M. Glycoprotein IV of bovine herpesvirus 1-expressing cell line complements and rescues a conditionally lethal viral mutant. J Virol. 1992;66:831–839. doi: 10.1128/jvi.66.2.831-839.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forman A J, Babiuk L A, Misra V, Baldwin F. Susceptibility of bovine macrophages to infectious bovine rhinotracheitis virus infection. Infect Immun. 1982;35:1048–1057. doi: 10.1128/iai.35.3.1048-1057.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forman A J, Babiuk L A. Effect of infectious bovine rhinotracheitis virus infection on bovine alveolar macrophage function. Infect Immun. 1982;35:1041–1047. doi: 10.1128/iai.35.3.1041-1047.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuller A O, Lee W C. Herpes simplex virus type 1 entry through a cascade of virus-cell interactions requires different roles of gD and gH in penetration. J Virol. 1992;66:5002–5012. doi: 10.1128/jvi.66.8.5002-5012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geraghty R J, Krummenacher C, Cohen G H, Eisenberg R J, Spear P G. Entry of alphaherpesvirus mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 20.Hanon E, Vanderplasschen A, Lyaku J R, Keil G M, Denis M, Pastoret P P. Inactivated bovine herpesvirus 1 induces apoptotic cell death of mitogen-stimulated bovine peripheral blood mononuclear cells. J Virol. 1996;70:4116–4120. doi: 10.1128/jvi.70.6.4116-4120.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanon E, Lambot M, Hoornaert S, Lyaku J, Pastoret P P. Bovine herpesvirus 1-induced apoptosis: phenotypic characterization of susceptible peripheral blood mononuclear cells. Arch Virol. 1998;143:441–452. doi: 10.1007/s007050050301. [DOI] [PubMed] [Google Scholar]
- 22.Hanon, E., G. Keil, S. Van Drunen Littel-van den Hurk, P. Griebel, A. Vanderplasschen, F. A. M. Rijsewijk, L. A. Babiuk, and P. P. Pastoret. Bovine herpesvirus induced apoptotic cell death: role of glycoprotein D. Virology, in press. [DOI] [PubMed]
- 23.Hart D N J. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–3287. [PubMed] [Google Scholar]
- 24.Karger A, Schmidt J, Mettenleiter T C. Infectivity of a pseudorabies virus mutant lacking attachment glycoproteins C and D. J Virol. 1998;72:7341–7348. doi: 10.1128/jvi.72.9.7341-7348.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Van Drunen Littel-van den Hurk S, Babiuk L A, Liang X. Characterization of cell-binding properties of bovine herpesvirus 1 glycoproteins B, C, and D: identification of a dual cell-binding function of gB. J Virol. 1995;69:4758–4768. doi: 10.1128/jvi.69.8.4758-4768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Liang X, van Drunen Littel-van den Hurk S, Attah-Poku S, Babiuk L A. Glycoprotein Bb, the N-terminal subunit of bovine herpesvirus 1 gB, can bind to heparan sulfate on the surfaces of Mardin-Darby bovine kidney cells. J Virol. 1996;70:2032–2037. doi: 10.1128/jvi.70.3.2032-2037.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang X, Babiuk L A, van Drunen Littel-van den Hurk S, Fitzpatrick D R, Zamb T J. Bovine herpesvirus 1 attachment to permissive cells is mediated by its major glycoproteins gI, gIII, and gIV. J Virol. 1991;65:1124–1132. doi: 10.1128/jvi.65.3.1124-1132.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ludewig B, Ehl S, Karrer U, Odermatt B, Hengartner H, Zinkernagel R. Dendritic cells efficiently induce protective antiviral immunity. J Virol. 1998;72:3812–3818. doi: 10.1128/jvi.72.5.3812-3818.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyaku J R S, Nettleton P F, Marsden H. A comparison of serological relationships among five ruminant alphaherpesviruses by ELISA. Arch Virol. 1992;124:333–341. doi: 10.1007/BF01309813. [DOI] [PubMed] [Google Scholar]
- 30.Meyer G, Hanon E, Georlette D, Pastoret P P, Thiry E. Glycoprotein gH of bovine herpesvirus type 1 (BHV-1) is essential for penetration and propagation in cell culture. J Gen Virol. 1998;79:1983–1987. doi: 10.1099/0022-1317-79-8-1983. [DOI] [PubMed] [Google Scholar]
- 31.Nyaga P N, McKercher D G. Pathogenesis of bovine herpesvirus 1 (BHV-1) infections: interactions of the virus with peripheral bovine blood cellular components. Comp Immunol Microbiol Infect Dis. 1980;2:587–602. doi: 10.1016/0147-9571(79)90100-0. [DOI] [PubMed] [Google Scholar]
- 32.Okazaki K, Matsuzaki T, Sugahara Y, Okada J, Hasebe M, Iwamura Y, Ohnishi M, Kanno T, Shimizu M, Honda E, Kono Y. BHV-1 adsorption is mediated by the interaction of glycoprotein gIII with heparinlike moiety on the cell surface. Virology. 1991;181:666–670. doi: 10.1016/0042-6822(91)90900-v. [DOI] [PubMed] [Google Scholar]
- 33.Renjifo X, Howard C, Kerkhofs P, Denis M, Urbain J, Moser M, Pastoret P P. Purification and characterization of bovine dendritic cells from peripheral blood. Vet Immunol Immunopathol. 1997;60:77–88. doi: 10.1016/s0165-2427(97)00092-5. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt J, Klupp B G, Karger A, Mettenleitter T C. Adaptability in herpesvirus: glycoprotein D-independent infectivity of pseudorabies virus. J Virol. 1997;71:17–24. doi: 10.1128/jvi.71.1.17-24.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schröder C, Linde G, Fehler F, Keil G M. From essential to beneficial: glycoprotein D loses importance for replication of bovine herpesvirus 1 in cell culture. J Virol. 1997;71:25–33. doi: 10.1128/jvi.71.1.25-33.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Straub O C. Infectious bovine rhinotracheitis virus. In: Dinter Z, Morein B, editors. Virus infections of ruminants. Amsterdam, The Netherlands: Elsevier Science Publishers B.V.; 1990. pp. 71–108. [Google Scholar]
- 37.Thaker S R, Stine D L, Zamb T J, Srikumuran S. Identification of a putative cellular receptor for bovine herpesvirus 1. J Gen Virol. 1994;75:2303–2309. doi: 10.1099/0022-1317-75-9-2303. [DOI] [PubMed] [Google Scholar]
- 38.Van Drunen Littel-van den Hurk S, Khattar S, Tikoo S K, Babiuk L A, Baranowski E, Plainchamp D, Thiry E. Glycoprotein H (gII/gp 108) and glycoprotein L form a functional complex which plays a role in penetration, but not in attachment, of bovine herpesvirus 1. J Gen Virol. 1996;77:1515–1520. doi: 10.1099/0022-1317-77-7-1515. [DOI] [PubMed] [Google Scholar]
- 39.Wang C, Splitter G A. CD4+ cytotoxic T-lymphocyte activity against macrophages pulsed with bovine herpesvirus 1 polypeptides. J Virol. 1998;72:7040–7047. doi: 10.1128/jvi.72.9.7040-7047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]