Abstract
Background
Asthma is a common condition characterised by airway inflammation and airway narrowing, which can result in intermittent symptoms of wheezing, coughing and chest tightness, possibly limiting activities of daily life. Water‐based exercise is believed to offer benefits for people with asthma through pollen‐free air, humidity and effects of exercise on physical function.
Objectives
To evaluate the effectiveness and safety of water‐based exercise for adults with asthma.
Search methods
We searched the Cochrane Airways Group Specialised Register of Trials (CAGR), the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Allied and Complementary Medicine Database (AMED), PsycINFO, the Latin American and Caribbean Health Science Information Database (LILACS), the Physiotherapy Evidence Database (PEDro), the System for Information on Grey Literature in Europe (SIGLE) and Google Scholar on 13 May 2014. We handsearched ongoing clinical trial registers and meeting abstracts of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the British Thoracic Society (BTS).
Selection criteria
We included all randomised controlled trials (RCTs) of adults with asthma comparing a water‐based exercise group versus one or more of the following groups: usual care, land‐based exercise, non‐exercise.
Data collection and analysis
Two review authors (AJG, VS) independently extracted data from the primary studies using a standard form developed for this purpose, which includes methods, participants, interventions and outcomes. We contacted trial authors to request additional data. Data were input by one review author and were double‐checked by a second review author.
Main results
In this systematic review, we provide a narrative synthesis of available evidence from three small studies including 136 adult participants. The studies were at high risk of bias. No meta‐analysis was possible because of methodological and interventional heterogeneity between included studies. The primary outcomes of quality of life and exacerbations leading to use of steroids were not reported by these studies. For exacerbations leading to health centre/hospital visits, uncertainty was wide because a very small number of events was reported (in a single study). Secondary outcomes symptoms, lung function, changes in medication and adverse effects, where available, described for each included study. The overall quality of the studies was very low, and no clear differences were noted between water‐based exercise and comparator treatments. Therefore, we remain very uncertain about the effects of water‐based exercise for adults with asthma.
Authors' conclusions
The small number of participants in the three included studies, the clinical and methodological heterogeneity observed and the high risk of bias assessed mean that we are unable to assess the place of water‐based exercise in asthma. Randomised controlled trials are needed to assess the efficacy and safety of water‐based exercise for adults with asthma. For future research, we suggest greater methodological rigour (participant selection, blinding of outcome assessors, reporting of all outcomes analysed and registering of the study protocol).
Keywords: Adult, Female, Humans, Male, Gymnastics, Swimming, Water, Anti‐Asthmatic Agents, Anti‐Asthmatic Agents/therapeutic use, Asthma, Asthma/therapy, Randomized Controlled Trials as Topic, Resistance Training, Resistance Training/methods
Plain language summary
Is water‐based exercise an effective and safe type of exercise for adults with asthma?
Asthma is a common condition in which inflammation and narrowing of the air conducting tubes may cause intermittent symptoms, possibly limiting activities of daily life. Some adults believe that exercise could trigger an asthma attack. However, research has shown the opposite—that adults who exercise may have less chance of having an asthma attack, and taking exercise in water may be more beneficial than taking exercise on land. In this review, we aimed to evaluate the effect and safety of water‐based exercise for adults with asthma.
We found a total of three studies involving 136 participants with an average age between 33 and 36 years with well‐controlled asthma. They underwent water‐based exercise from 40 to 60 minutes three to five times a week; the programme lasted 10 to 24 weeks in two studies, and one day only in one study.
We considered data reported on quality of life, asthma general symptoms or asthma exacerbations, measure of lung function (FEV1, forced expiratory volume of the lung in the first second of air expired) and adverse events. The quality of evidence is very low because of issues with selection of participants, small number of participants, differences in exercise duration and intensity and differences in levels of asthma. Often surrogate endpoints were measured instead of patient‐important outcomes.
To sum up, more studies are needed to find out the effect and safety of water‐based exercise for adults with asthma. The quality of evidence is very low because of issues with selection of participants, differences in exercise duration and intensity and differences in levels of asthma; surrogate endpoints were measured instead of patient‐important outcomes.
This plain language summary is current as of 13 May 2014.
Summary of findings
Summary of findings for the main comparison. Comparison of water‐based exercise versus control for adults with asthma.
| Comparison of water‐based exercise versus control for adults with asthma | ||||
| Patient or population: adults with asthma Settings: community Intervention: water‐based exercise Comparison: control or land‐based exercise | ||||
| Outcomes | Analysis in study report | No. of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Quality of life | See comment | See comment | See comment | None of the included studies analysed this outcome using validated scales |
| Exacerbations leading to health centre visits or exacerbations leading to hospitalisations Instrument: patient recorded Follow‐up: 10 weeks | No significant difference in the likelihood of an exacerbation leading to a health centre/hospital visit (RR 1.65, 95% CI 0.17 to 16.33, P value 0.67) | 31 (1 study) | ⊕⊝⊝⊝ very lowa,b,d | Parallel‐group study |
| Lung function (FEV1 (L)) Spirometer: scale from 0.5 to 8 Follow‐up: 1 to 180 days | One 24‐week study showed no difference in FEV1 between water‐based exercise + education versus medication (MD 0.32 L, 95% CI ‐0.13 to 0.77, P value 0.16). One study employing a single session of water‐based breathing exercise versus land‐based exercise showed no difference in FEV1 (MD ‐0.14 L, 95% CI ‐0.53 to 0.25, P value 0.48). The third study was not included for this outcome because of considerable baseline imbalance between groups | 105 (2 studies) | ⊕⊝⊝⊝ very lowa,b,c,d | Parallel‐group and cross‐over, respectively |
|
Medication usage Instrument: questionnaire Follow‐up: 10 weeks |
Same medication used throughout study | 31 (1 study) | ⊕⊝⊝⊝ very lowa,b,d | Parallel‐group |
| Adverse events Count of events Follow‐up: 10 weeks | 1 case of skin sensitivity in the water‐based exercise group | 31 (1 study) | ⊕⊝⊝⊝ very lowa,b,d | Parallel‐group |
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
a(‐2 risk of bias) Inadequate provision of random sequence generation and allocation concealment. Selective reporting likely. b(‐2 inconsistency) Clinical heterogeneity due to different frequency/intensity and length of the intervention, different levels of participant asthma on baseline. c(‐1 indirectness) Surrogate endpoints measured instead of patient‐important outcomes. d(‐1 imprecision) Small sample size compromising optimal information size (OIS)
Background
Description of the condition
Asthma is a disease characterised by inflammation of the airways, which causes airflow obstruction (CDC 2011; Lucas 2005; WHO 2011). Although frequency and intensity vary from person to person, the symptoms of asthma may include breathlessness, wheezing, coughing, chest tightness and pain (Banzett 1985; CDC 2011; WHO 2011). Epidemiological data from a survey applied in 70 countries estimate that 623 million individuals, or 8.6% (95% confidence interval (CI) 8.5 to 8.7) worldwide, are currently living with some level of asthma‐related symptoms (To 2012); asthma is ranked as one of the most prevalent conditions globally. It is also important to consider the economic impact of this disease; the USA spends around US $30 billion per year on emergency department visits, physician visits and medication (CDC 2011). The Centers for Disease Control and Prevention (CDC) estimated in 2006 that 1.6 million emergency department visits and 440,000 hospitalisations in the USA were related to asthma (CDC 2011).
Description of the intervention
It may be inferred that some people with asthma are limited in the physical activities they can undertake, and that this may result in a sedentary lifestyle. Indeed, people with asthma are more likely to suffer from cardiovascular and metabolic conditions (Corbin 2011; Goodman 2008; Hildenbrand 2010). Undertaking physical activity is important for improving quality of life in people with asthma (Lucas 2005; Pedersen 2006). Water‐based exercise is a good treatment option; these exercises increase muscular strength, cardiorespiratory fitness and flexibility, and they improve body composition (reducing body fat and increasing muscle mass) (Ambrosini 2010; Avelar 1999; Corbin 2011; Malkia 1998). Furthermore, muscle strength and cardiorespiratory fitness have a positive association with a reduction in the incidence of acute exacerbations (Gonçalves 2008). The most common water‐based exercises described in the literature are swimming, hydrogymnastics and hydrotherapy (Denning 2012; Hildenbrand 2010).
How the intervention might work
The aquatic environment has particular properties, such as temperature of the water and humid, pollen‐free air. These features have shown extra benefits in people with asthma, facilitating air exchange (Barbosa 2011; Hildenbrand 2010). Water‐based exercises have therefore been widely used, as they improve breathing and increase lung volume (Aranđelović 2007). Water‐based exercises also stimulate physiological pathways, which may raise forced expiratory volume in one second (FEV1) and forced vital capacity (FVC), both of which are objective measures for bronchospasm (Aranđelović 2007). Exercises increase cardiovascular fitness and, consequently, maximal consumption of oxygen (VO2max), reducing the minute ventilation required for exercise and decreasing the stimulus for bronchoconstriction. Regular and long‐term exercises may be helpful in asthmatic patients and may have a positive impact on quality of life (Carson 2013). In addition, during water‐based exercise, the air that is inhaled is humidified, attenuating asthma risks (O'Byrne 2012; Randolph 2009).
Why it is important to do this review
Cochrane systematic reviews for people with asthma have examined physical training (Carson 2013), breathing exercises (Freitas 2013), inspiratory muscle training (Silva 2013) and swimming training in children (Beggs 2013). However, the effectiveness and safety of water‐based exercise in adults with asthma remain unclear. No systematic reviews have focused on the relationship between water‐based exercise and asthma in adults. Therefore, in this systematic review, we sought to map the scientific literature and describe available evidence on this topic.
Objectives
To assess the effectiveness and safety of water‐based exercise for adults with asthma.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), irrespective of language, age of publication or publication status. We included cross‐over RCTs; however we analysed separately the data obtained from them. We planned to include cluster randomised trials.
Types of participants
We considered participants who met the following criteria.
Adults of either gender.
Adults with asthma diagnosed according to clinical criteria (American Thoracic Society (ATS), European Respiratory Society (ERS) or British Thoracic Society (BTS)) and other validated diagnostic criteria.
Types of interventions
We included studies that compared any type of water‐based exercise versus another type of water‐based exercise, land‐based exercise or usual care. We documented the duration and frequency of sessions and the overall length of the programme. At least one group in each comparison used water‐based exercise.
The following possible comparisons were made.
Water‐based exercise versus land‐based exercise.
Water‐based exercise versus usual care.
Types of outcome measures
Primary outcomes
Quality of life (measured using a validated questionnaire, e.g. Asthma Quality of Life Questionnaire (AQLQ)).
Exacerbations (leading to a course of oral steroids; health centre visits or exacerbations; or hospitalisations).
Secondary outcomes
Symptoms (measured on a validated scale (e.g. Lara Asthma Symptom Scale (LASS)).
Lung function by physiological measures (e.g. spirometry, FEV1, FVC).
Reduction in medication usage (e.g. inhaled corticosteroids or leukotriene inhibitors, short‐acting beta2‐agonists (SABAs) or symptom controllers (e.g. long‐acting beta2‐agonists (LABAs)).
Adverse events (reactions due to the water treatment process (e.g. chlorine), exercise induced‐bronchoconstriction (EIB)).
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group Specialised Register of Trials (CAGR), which is derived from systematic searches of the main health and medical bibliographic databases (see Appendix 1 for further details). The Cochrane Airways Group Trials Search Co‐ordinator searched the CAGR in May 2014 using the strategy provided in Appendix 2. We conducted additional searches on the Latin American and Caribbean Health Sciences Database (LILACS), the Physiotherapy Evidence Database (PEDro), the System for Information on Grey Literature (SIGLE) and Google Scholar. These searches were conducted in May 2014; the search strategies are reported in Appendix 3. We handsearched respiratory journals and meeting abstracts of the American Thoracic Society (ATS) from May 1994 to May 2014, the European Respiratory Society (ERS) from January 1993 to May 2014 and the British Thoracic Society (BTS) from December 2002 to May 2014.
We checked for ongoing studies in ClinicalTrials.gov (http://clinicaltrials.gov/); the metaRegister of Controlled Trials (mRCT) (http://www.controlled‐trials.com/); the International Clinical Trials Registry Platform (ICTRP) (http://www.who.int/ictrp/en/); Pan African Clinical Trials (http://www.pactr.org); and the EU Clinical Trials Register (https://www.clinicaltrialsregister.eu/). These registers were searched in May 2014.
We applied no language or date restrictions. We searched all databases from their inception to the individual reported dates.
We used Endnote X6 to manage the references and excluded duplicate articles found in the different searches.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We contacted the authors of identified trials and asked them to identify other published and unpublished studies. We also contacted exercise equipment manufacturers and experts in the field.
Data collection and analysis
We performed data collection and analysis according to recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Selection of studies
Two review authors (AJG, VS) independently screened titles and abstracts of all potential studies that we identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve.' We retrieved the full‐text study reports/publications, and two review authors (AJG, VS) independently screened the full text to identify studies for inclusion. We recorded the reasons for exclusion of ineligible studies. We resolved disagreements through discussion; if required, we consulted a third person (BNGA). We identified and excluded duplicates and collated multiple reports of the same study, so that each study, rather than each report, is the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow diagram and Characteristics of excluded studies table.
Data extraction and management
Two review authors (AJG, VS) independently extracted data from the primary studies using a standard form (Google Spreadsheet, e.g. http://goo.gl/pd0KZ) developed for this purpose, which includes methods, participants, interventions and outcomes. One review author transferred data into Review Manager (RevMan 2012). We double‐checked the data by comparing data presented in the systematic review versus the study reports. A second review author spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (AJG, VS) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). Disagreements were resolved by discussion or by involving a third review author (MSP). We assessed the risk of bias according to the following domains.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other bias (other sources of bias related to a particular trial design (cross‐over or cluster‐randomised) or to specific circumstances (interventions mixed)).
We classified the risk of bias as low, high or unclear (Higgins 2011b).
Measures of treatment effect
In light of the proposed objectives of this review, we used the following types of measurements of treatment effect for the primary studies.
Dichotomous data: outcome measures evaluated as binary responses. We summarised results as risk ratios (RRs). This type of data was found for the numbers of exacerbations and adverse events and for reduction in medication use.
Continuous data: outcome measures evaluated by numerical quantity. We combined results using the mean difference (MD) for measures using the same scale, or the standardised mean difference (SMD) when different scales were used to evaluate the same outcome. This type of data was found for symptoms and lung function.
Counts and rates: may mean that exacerbation episodes are counted twice or more frequently in the same person. We planned to use the risk ratio, as it represents the rate of events in the two groups attained by dividing one by the other; however these data were not found.
Time‐to‐event: use of the hazard ratio as planned to describe the risk ratio of exacerbation based on comparisons of event rates in both groups.
When we found more than one study that analysed the same outcome, we conducted a meta‐analysis. All statistical parameters used 95% confidence intervals (CIs) (Deeks 2011).
Unit of analysis issues
We considered the individual participant to be the unit of analysis. In the cross‐over design, we included only phase one data to avoid a carry‐over effect. If we had encountered cluster‐randomised trials, we planned to use the groups randomly assigned as the unit of analysis.
Dealing with missing data
We contacted authors of studies by email when reports did not describe the outcome measures of interest, the process of randomisation or intention‐to‐treat analysis, and when other necessary data were missing. We sent at least two emails to the corresponding author; if no answer was obtained, we discussed this in the results section of the review.
Assessment of heterogeneity
We would have assessed inconsistencies between studies by using the I2 statistic to describe the extent of statistical variation among study results, if we had found heterogeneity. We would have considered heterogeneity to be substantial when I2 > 50% (Deeks 2011). If we had found substantial heterogeneity among studies, we would have explored this subgroup analysis of study characteristics.
Assessment of reporting biases
Reporting biases can affect the likelihood of publication, and statistically significant results may be selectively reported. It is important that this is assessed because reporting biases may overestimate or underestimate the treatment effect. If mismatches had been identified, we would have contacted the study authors for further information. If necessary, we would have explored the impact of including such studies by conducting a sensitivity analysis. We did not perform a funnel plot asymmetry test because we found a limited number of included studies.
Data synthesis
We would have conducted a meta‐analyses if the combination of data had been possible. We would have used a fixed‐effect model unless heterogeneity had been substantial (I2 > 50%), in which case we would have chosen a random‐effects model. We presented data in forest plot graphics produced by RevMan 5.2. If data combination had not been possible, we would have described the individual studies.
We created Table 1 by adhering to the methods and recommendations described in Section 8.5 and Section 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b; Schünemann 2011) and by using GRADEpro software (GRADEpro 2008). We included the following outcomes: quality of life, number of acute exacerbations, level of severity of disease, lung function, medication usage and adverse events.
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analysis while considering participant, intervention and environmental characteristics.
Participant characteristics
Co‐morbidities.
Intervention characteristics
Type of exercise (swimming (horizontal), hydrogymnastics (vertical)).
Frequency of exercise (how many sessions/wk).
Intensity: light (1.6 to 2.9 metabolic equivalents (METs)), moderate (3 to 5.9 METs), vigorous (≥ 6 METs).
Environmental characteristics
Water temperature and air humidity.
Water treatment process (chlorinated vs non‐chlorinated).
Water depth.
Sensitivity analysis
If we had been able to pool data from included studies, we planned to test studies with limited information to check whether the robustness of the assumptions had influenced review findings. Moreover, we planned to conduct sensitivity analyses by removing studies at high or unclear risk of bias. We also planned to conduct sensitivity analyses to look at the effects of missing data.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
See Figure 1. A total of 1719 references were identified from the initial search combining all prespecified databases, handsearching of reference lists, grey literature and trial registers (Figure 1). A total of 1007 references remained after duplicates were removed, of which 953 were removed after titles were screened by two review authors. The remaining 54 references were screened by two review authors on the basis of their abstracts. From these, 48 studies were excluded. Only six studies were identified as potentially relevant, and full‐text articles for these were retrieved. Two study authors (Boulet 2005; Fitch 1971) were contacted for more information. The remaining three articles were excluded, and reasons for exclusion have been specified (see Excluded studies). The review authors agreed that three articles fulfilled the study inclusion criteria of this systematic review (see Included studies).
1.
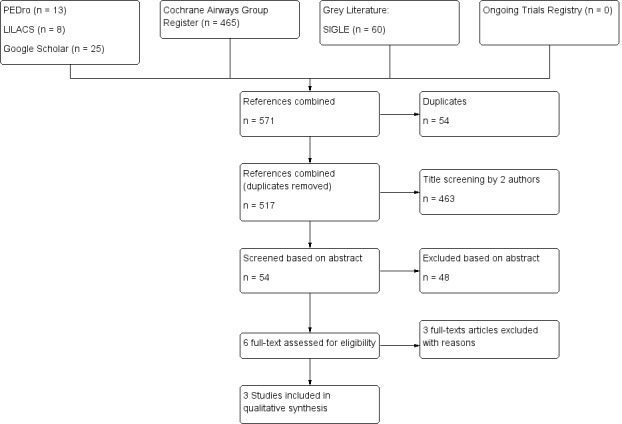
PRISMA flow diagram.
Included studies
We included three studies involving 136 adults, published between 1998 and 2007: two studies in the English language (Aranđelović 2007; Emtner 1998) and one in Portuguese (Pereira 2005). One study from Sweden (Emtner 1998), one from Serbia (Aranđelović 2007) and one from Brazil (Pereira 2005) met the inclusion criteria. Sample sizes ranged from 32 to 70 participants. Participants had a mean age of between 33 and 36 years. The proportion of female participants varied between 44% and 78%. Asthma was diagnosed on the basis of guidelines‐specified criteria in two studies (Aranđelović 2007; Emtner 1998). All studies were RCTs of adults with asthma; intervention groups were provided with water‐based exercise, swimming (Aranđelović 2007), gymnastics movements (Emtner 1998) and strength/resistance training (Pereira 2005). The intensity of the water‐based exercise programme varied, lasting between 40 and 60 minutes one to five times per week; the length of the programme ranged from one day (one session) to 24 weeks, and moderate‐ to high‐intensity exercises were delivered by a physiotherapist. Three pools were located indoors and were heated. One pool was non‐chlorinated (Aranđelović 2007), and in two studies, the water treatment process was not specified (Emtner 1998; Pereira 2005). The comparison group performed land‐based exercise in two studies (Emtner 1998; Pereira 2005), and in one study, the comparison group was given usual care medication (Aranđelović 2007). In one study, asthma was specified as persistent, with severity graded as mild (Aranđelović 2007). In another study, asthma was graded as mild/moderate (Emtner 1998), and another study did not specify the criteria for diagnosis; however, study authors specified asthma as persistent and graded it as moderate or severe (Pereira 2005). Participants' regular medication was continued during all three studies. All participants in Aranđelović 2007 were treated with low doses of inhaled corticosteroids and short‐acting beta2‐agonists (salbutamol); in Emtner 1998, 24 participants were treated with > 400 mg inhaled corticosteroids and long‐term beta2‐agonists, and eight participants with < 400 mg of inhaled corticosteroids. All participants in Pereira 2005 used exclusively short‐acting beta2‐agonists. No study specified its primary or secondary outcomes.
These studies met the inclusion criteria of the review; full information on each study is available in Characteristics of included studies. In addition, we provide a comparison of baseline characteristics in Table 2 and a comparison of intervention characteristics in Table 3.
1. Comparison of baseline characteristics of included studies.
| ID/Location/Full publication | n randomly assigned/Withdrawals | % female | Mean age (DP) | Asthma diagnosis | Asthma severity |
| Aranđelović 2007/Serbia/Yes | 70/5 | 75.4 | 33.31 (10.34) | GINA criteria | Mild persistent asthma |
| Emtner 1998/Sweden/Yes | 32/1 | 43.7 | 36 (10) | ATS criteria | Mild or moderate asthma |
| Pereira 2005/Brazil/Yes | 40/0 | 77.5 | 33.4 (8.77) | Not stated | Moderate and severe persistent asthma |
ATS = AmericanThoracic Society; GINA = Global Initiative for Asthma.
2. Comparision of intervention characteristics of included studies.
| ID/Location/Full publication | Asthma treatment | Water‐based time/frequency per week/n weeks/Pool type/ Supervision | Comparison group | Follow‐up point in weeks/outcomes in meta‐analysis |
| Aranđelović 2007/Serbia/Yes | Low doses of ICS and short‐acting beta2‐agonist salbutamol | 1 hour/twice a week/24 weeks/indoor heated non‐chlorinated/not informed. Included an educational component | Control group with medication (but no educational component) | 24 weeks/FEV1 (L), FVC (L), FEV1/FVC ratio, PEF (L/s), PD20 |
| Emtner 1998/Sweden/Yes | 24 participants > 400 mg ICS and long‐term beta2‐stimulants | 45 minutes/5 days in the first 2 weeks, then twice a week for 8 weeks/10 weeks/Indoor heated/Physiotherapist | Land‐based exercise | 10 weeks/FEV1 (L), FVC (L), FEV1/FVC ratio, PEF (L/s), PD20 |
| Pereira 2005/Brazil/Masters' dissertation | Short‐acting beta2‐agonists exclusively | 40 minutes/1 session only (1 day)/Indoor heated/Physiotherapist | Land‐based exercise | 1 day/ FEV1 (L), FVC (L), FEV1/FVC ratio, PEF (L/s) |
FEV1= forced expiratory volume in 1 second; FEV1/FVC ratio; FVC = forced vital capacity; ICS = inhaled corticosteroids; PD20 = provocative dose; PEF = peak expiratory flow.
Excluded studies
Three studies were excluded with reasons (see Characteristics of excluded studies): One study was not an RCT (Boulet 2005); another study analysed exercise‐induced asthma (Fitch 1971); and another other study did not include a control group (Hildenbrand 2010).
Risk of bias in included studies
All three included studies had at least three categories judged to be at high risk of bias (Figure 2; Figure 3).
2.
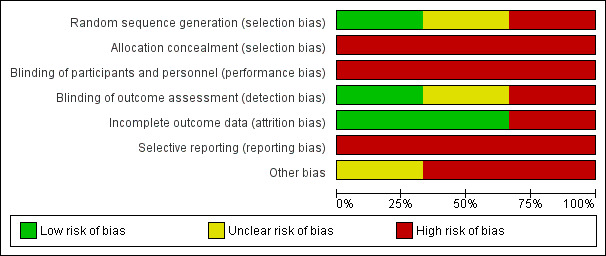
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
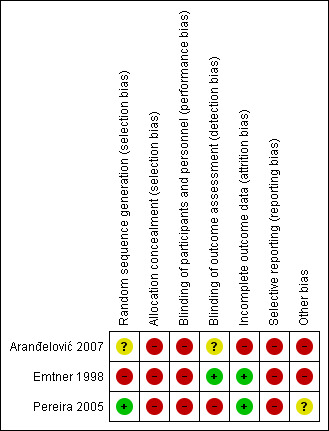
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The term randomisation was used in all studies. However, two studies did not explain how randomisation was conducted (Aranđelović 2007; Pereira 2005), so we contacted the study authors; only Pereira 2005 returned a satisfactory explanation of how this was done. Therefore Pereira 2005 was judged to be at low risk of bias, and Aranđelović 2007 at unclear risk of bias. One study had high risk of bias because participants were randomly assigned by odd and even birth dates (Emtner 1998). Allocation concealment was judged as high risk of bias for all three studies (Aranđelović 2007; Emtner 1998; Pereira 2005).
Blinding
Because of the nature of the study interventions, blinding of participants was not applicable; thus we classified all studies as high risk of performance bias. For detection bias, one study did not specify whether the outcome assessor was blinded (Aranđelović 2007), so we judged it as unclear risk of bias. Another study blinded the outcome assessor (Emtner 1998), so we classified it as low risk of bias. In another study, the researcher assessed participant outcomes, so we classified the study as high risk of bias (Pereira 2005).
Incomplete outcome data
One study reported loss of one participant (Emtner 1998), and another study no loss to follow‐up (Pereira 2005), so we judged both as being at low risk of bias. Another study reported loss of five participants in the control group, and investigators did not use intention‐to‐treat analysis, so we classified it as high risk of bias (Aranđelović 2007).
Selective reporting
All study protocols were not available, and some outcome results were not reported in all three studies, so we judged all studies as high risk of bias (Aranđelović 2007; Emtner 1998; Pereira 2005).
Other potential sources of bias
In Aranđelović 2007, no education was given to the control arm (high risk of bias). Emtner 1998 reported baseline imbalance, which may have been related to the quasi‐randomised design (high risk of bias). Pereira 2005 used a cross‐over design with a washout period of one week, so it is unclear whether washout time was sufficient for exercise; therefore we classified the study as unclear risk of bias.
Effects of interventions
See: Table 1
See Table 1.
Primary outcomes
First comparison: water‐based exercise versus land‐based exercise (10 weeks): parallel groups
Quality of life
None of the studies provided data on quality of life.
Exacerbations
Exacerbations leading to a course of oral steroids
No study explored this outcome.
Exacerbations leading to health centre/hospital visits
One study with 32 participants analysed exacerbations leading to health centre/hospital visits (Emtner 1998). In this study, water‐based exercise was compared with land‐based exercise. Two exacerbations were reported in the water‐based exercise group, and one in the land‐based group during 10 weeks of intervention. Thus, we are very uncertain of whether a difference in the likelihood of an exacerbation leading to a health centre/hospital visit existed between water‐based and land‐based exercise (RR 1.65, 95% CI 0.17 to 16.33, P value 0.67).
Second comparison: water‐based exercise versus land‐based exercise (one session): cross‐over design
Quality of life
None of the studies provided data on quality of life.
Exacerbations
Exacerbations leading to a course of oral steroids
No study explored this outcome.
Exacerbations leading to health centre/hospital visits
No study explored this outcome.
Third comparison: water‐based exercise + education versus medication (24 weeks): parallel groups
Quality of life
None of the studies provided data on quality of life.
Exacerbations
Exacerbations leading to a course of oral steroids
No study explored this outcome.
Exacerbations leading to health centre/hospital visits
No study explored this outcome.
Secondary outcomes
First comparison: water‐based exercise versus land‐based exercise (10 weeks): parallel groups
Symptoms
One study with 32 participants analysed general symptoms (Emtner 1998). Study authors used a non‐validated questionnaire called "Influence of asthma on everyday life," which is composed of 10 questions measured on a visual analogue scale (VAS) of 10 centimetres. This study did not report a significant difference in asthma symptoms between water‐based exercise and land‐based exercise (MD 0.20, 95% CI ‐1.18 to 1.58, P value 0.78).
Lung function
Forced expiratory volume in one second (FEV1)
One study with 32 participants analysed FEV1 (Emtner 1998). Investigators reported a statistically significant difference in FEV1 between water‐based exercise and land‐based exercise when data were analysed as final values (MD 0.7 L, 95% CI 0.17 to 1.23, P value 0.009). However, baseline imbalance between groups was evident, and the difference in FEV1 was no longer significant in comparisons of change from baseline (MD 0.30 L, 95% CI ‐0.23 to 0.83, P value 0.27), as shown in Analysis 1.3.
1.3. Analysis.
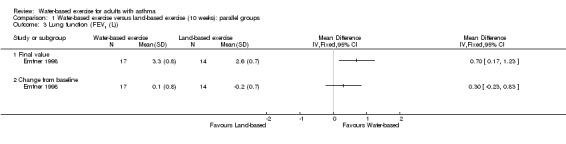
Comparison 1 Water‐based exercise versus land‐based exercise (10 weeks): parallel groups, Outcome 3 Lung function (FEV1 (L)).
Peak expiratory flow (PEF) (L/min)
One study with 32 participants analysed PEF. In Emtner 1998 (n = 32), researchers did not find a significant difference in PEF between the two groups (MD 26.00 L/min, 95% CI ‐44.16 to 96.16, P value 0.47).
Use of medication
Only one study mentioned use of medication (n = 32). In Emtner 1998, study authors mentioned that all participants continued with the same medication at the same dosage.
Adverse events
One study with 32 participants reported adverse events. In Emtner 1998, one person in the water group reported skin sensitivity, stopped water‐based exercise for three weeks and then was transferred to a land‐based group. Thus, considerable uncertainty remains about adverse events in the two groups (RR 2.65, 95% CI 0.12 to 60.21, P value 0.54).
Second comparison: water‐based exercise versus land‐based exercise (one session): cross‐over design
Symptoms
Only one cross‐over study with 40 participants reported anxiety symptoms immediately after a single treatment with land‐based or water‐based exercise (Pereira 2005). Study authors measured the symptom (anxiety) using the State‐Trait Anxiety Inventory (STAI), which is composed of 20 items scored on a four‐point scale; it was translated and adapted from Portuguese. A decrease in anxiety was noted after both types of exercise, which represented a statistically significant change from baseline for both arms when participants received water‐based exercise, and for one arm when land‐based exercise was provided. However, no appropriate comparison of the differences between land‐based and water‐based treatment was reported.
Lung function
Forced expiratory volume in one second (FEV1)
After a single session of exercise, we remain very uncertain about any difference in FEV1 in the first arm of the cross‐over study (MD ‐0.14 L, 95% CI ‐0.53 to 0.25, P value 0.48).
Peak expiratory flow (PEF) (L/s)
Similarly, after a single session of water‐based exercise versus land‐based exercise (n = 40), we remain very uncertain about any difference in PEF from the first arm of the cross‐over study (MD 23.70, 95% CI ‐37.18 to 84.58, P value 0.45).
Third comparison: water‐based exercise + education versus medication (24 weeks): parallel groups
Symptoms
No study explored this outcome.
Lung function
Forced expiratory volume in one second (FEV1)
In Aranđelović 2007 (N = 65), no significant difference in FEV1 was noted between water‐based exercise and usual care medication plus education (MD 0.32 L, 95% CI ‐0.13 to 0.77, P value 0.16).
Peak expiratory flow (PEF) (L/min)
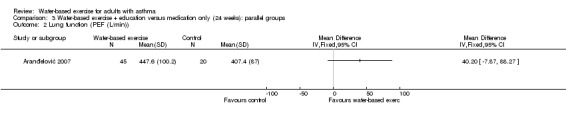
Aranđelović 2007 included 65 participants divided into water‐based exercise and usual care medication plus education groups; the duration of the intervention was 24 weeks, and no significant differences in PEF were reported (MD 40.20 L/min, 95% CI ‐7.87 to 88.27, P value 0.10).
Subgroup analysis
We prespecified subgroup analysis of participant, intervention and environmental characteristics. Because the included studies used different designs, different types and numbers of exercise sessions and different control groups, we were unable to pool the data and explore subgroup analysis.
Sensitivity analysis
We could not perform sensitivity analysis because of clinical and methodological differences among the included studies.
Discussion
Summary of main results
This review aimed to determine the effectiveness and safety of water‐based exercise as an intervention for adults with asthma. When the primary outcomes were analysed, quality of life was not explored before or after intervention in any of the studies, nor was the outcome of exacerbations leading to the use of steroids. Exacerbations leading to health centre visits or to hospitalisations were analysed by one study that compared water‐based exercise versus land‐based exercise over 10 weeks of exercise; however, very few events were reported, and we remain uncertain about whether differences existed between the groups (Emtner 1998).
In terms of the secondary outcome of symptoms, in Emtner 1998, no significant difference in general asthma symptoms was reported after 10 weeks of exercise.
To assess lung function, we explored FEV1 and PEF. For FEV1, Emtner 1998 found a statistically significant difference favouring water‐based exercise over land‐based exercise; however, we have to be cautious with these data because baseline imbalance was described among groups, and in analyses of change from baseline, the difference was no longer significant. No significant difference in FEV1 or PEF was seen in either of the other two studies.
The sole study reporting on the outcome of medication use (Emtner 1998) found that all study participants continued to take the same doses after training. One study reported adverse effects (skin sensitivity) in one person in the water‐based exercise group (Emtner 1998).
Overall completeness and applicability of evidence
The three included studies randomly assigned 142 participants who commenced the intervention, and overall six participants were lost to follow‐up. The number of included studies is small, and the data are too limited to support decision making. All studies evaluated some variable of lung function. An update of a Cochrane review on the effects of exercise on participants of all ages with asthma reported that exercise was well tolerated by study participants and found no evidence of adverse effects on asthma symptoms (Carson 2013). Our review sought to assess water‐based exercise specifically for adults with asthma and to summarise the best available evidence.
This review is unable to present strong evidence on the topic. Regarding the safety of water‐based exercise, it is important to mention that only one participant in the water‐based exercise group had an adverse event of a skin complaint (Emtner 1998). Additionally, some observational studies have suggested potential risks concerning respiratory or allergic disease (Bernard 2009; Font‐Ribera 2011; Kohlhammer 2006; Schoefer 2008). It is not possible to establish a cause‐effect relationship about exercise performed in an aquatic environment; further studies on this topic are needed.
It is important to mention that the nature of the water‐based exercise employed in studies varied, both in intensity (moderate and high) and in frequency (one day to six months), as did asthma severity (mild, moderate and severe). This makes it difficult to envisage a standardised intervention that will allow investigators to understand the effects of water‐based exercise. Noting such problems, Hildenbrand 2010 proposed a standard protocol of water‐based exercise for adults with asthma, but this intervention has not yet been tested by a randomised controlled clinical trial.
The age groups analysed by all three included studies were very similar; the mean age of participants was 34.23 (SD = 9.7) years. However, these studies examined the effects of exercise, a very complex intervention, on participants with different types of asthma and concluded that exercise is most effective when prescribed on the basis of asthma severity.
Quality of the evidence
The three included studies (Aranđelović 2007; Emtner 1998; Pereira 2005) were generally classified as high risk of bias. All studies analysed had high risk of bias in terms of allocation concealment and selective outcome reporting, which may have made it possible for the trialists to know which participants received which intervention, and this may present a risk of selection bias. Most studies reported only surrogate outcomes. In Aranđelović 2007, no category was classified as low risk of bias. We tried to contact the study author to obtain clarification but received no response.
Emtner 1998 had low risk of bias in blinding of the assessor and in attrition, because the evaluator was a professional who did not provide the intervention or perform the randomisation, and because loss of participants was minimal and was explained. The high risk of selection bias in Emtner 1998 could have impacted the baseline imbalances and could have influenced the intervention effects. Additionally, the high risk of reporting bias could have affected the outcomes presented by the study author (i.e. we are uncertain whether study authors published everything that they measured). In Pereira 2005, the study author provided information by email explaining how randomisation was conducted and that the study did not report sample loss; these were the only categories classified as low risk of bias. Also in Pereira 2005, high risk of bias in terms of allocation concealment could have influenced the selection of participants with asthma of different severity for inclusion in intervention and control groups. In Aranđelović 2007, many factors influenced the classification of high risk of bias, including the following: The asthma education intervention was a confounder in the exercise group, reporting of outcomes was selective and outcome data were incompletely reported.
On the basis of GRADE (Grades of Recommendation, Assessment, Development and Evaluation) criteria, the quality of evidence for primary outcomes such as exacerbations leading to health centre/hospital visits was classified as very low because of the mode of selection of individuals, the selective reporting and fact that very small numbers of participants were included. For secondary outcomes of lung function (FEV1, PEF), reduction in medication use and adverse effects, the quality of evidence was judged to be very low because of the high risk of bias, as the size and direction of the effect may have been influenced by the small numbers of participants and the high risk of bias in allocation concealment. Clinical variability, different severities of asthma and different exercise intensities among studies have also played an important role in lowering the quality of evidence.
Potential biases in the review process
We used a highly sensitive search strategy (i.e. avoided locating references that were not reports of randomised trials comparing water‐based or land‐based exercise vs standard therapy). Also, we did not limit the search on the basis of language, date of publication or publication status (grey literature) for the primary studies. All three studies did not register their trials, so it is important to consider this as a potential source of bias. We were unable to combine data in a meta‐analysis because of study heterogeneity and limitations. Additionally, we found no ongoing studies in the clinical trial registries.
Agreements and disagreements with other studies or reviews
The conclusions of seven systematic reviews on asthma and exercise—five Cochrane reviews (Beggs 2013; Carson 2013; Dennis 2012; Freitas 2013; Silva 2013) and two non‐Cochrane reviews (Eijkemans 2012; Posadzki 2011)—can be compared with the findings of our review
The systematic review of Beggs 2013 included eight trials on swimming for children and adolescents up to 18 years of age. The quality of evidence on outcomes, quality of life and asthma symptoms was judged as low. For the outcomes of emergency department visits, FEV1 and VO2max, the studies were judged as of moderate and high quality. This review concluded that swimming training is well tolerated in children and adolescents with stable asthma and can improve lung function and cardiopulmonary fitness.
Carson 2013 included 21 studies on exercise training for persons with asthma who were eight years of age or older. The quality of evidence was judged as very low or low for all outcomes examined in the 21 studies, and most studies were classified as unclear risk of bias. Despite the limitations, investigators found significant beneficial effects of exercise on oxygen consumption and concluded that exercise "was well tolerated as such, people with stable asthma should be encouraged to participate in regular exercise training, without fear of symptom exacerbation."
Silva 2013 included five studies on inspiratory exercises for adults with asthma. The quality of evidence was judged as very low or low for all outcomes analysed, and selection bias (randomisation and allocation concealment) for all five studies was judged as unclear. Benefits of exercise were found in maximal inspiratory pressure and maximal expiratory pressure. The review author concluded that study results cannot be generalised to children or people with severe asthma, and that the benefits vary according to the type of exercise performed.
Freitas 2013 investigated breathing exercises for adults with asthma; 13 studies were included, and the quality of evidence for all outcomes analysed was classified as very low. Only four of the 13 studies were considered at low risk of bias, and for most of the remaining studies, the risk of bias was judged as unclear. Review authors concluded that breathing exercises are safe and well tolerated for people with asthma. Additionally, the meta‐analysis showed positive effects of exercise on quality of life and asthma symptoms.
Dennis 2012 found no RCTs that examined techniques for Alexander chronic asthma.
Two non‐Cochrane systematic reviews were identified. Posadzki 2011 analysed yoga exercises for adults with asthma and included RCTs and non‐ RCTs. Seven studies were included in the review, and two were assessed as low risk of bias. Study authors were unable to summarise the results because of heterogeneity, and the review was inconclusive about the effects of yoga because of methodological limitations and the heterogeneity of primary studies. Eijkemans 2012 conducted a systematic review to assess the benefits of physical activity for patients with asthma; they included 39 studies: 34 cross‐sectional and five longitudinal. The review authors evaluated the articles using the Newcastle‐Ottawa Scale; 79% were deemed to be of moderate or good methodological quality. The review authors concluded that the level of physical activity is a protective factor in the development of asthma, and that some factors such as age groups, gender differences and sedentary lifestyle are major sources of heterogeneity that must be observed when future studies are planned.
The types of exercise involved in most of the systematic reviews included in this section differed from those examined in the current systematic review. Only one systematic review involved water exercise (swimming), and study participants were of a different age group (children and adolescents).
Authors' conclusions
Implications for practice.
The small numbers of participants in the three included studies, the clinical and methodological heterogeneity observed and the high risk of bias noted mean that we are unable to assess the place of water‐based exercise in the treatment of patients with asthma.
Implications for research.
Randomised controlled trials are needed to assess the efficacy and safety of water‐based exercise for adults with asthma. We suggest greater methodological rigour (participant selection, blinding of outcome assessors, reporting of all outcomes analysed and registering of the study protocol) for future research.
Acknowledgements
The review authors wish to acknowledge Emma Welsh, Chris Cates, Emma Jackson and Julia Walters for providing attention and assistance, and Liz Stovold for building the search strategy. We would like to thank the Cochrane Handbook Study Group from the Brazilian Cochrane Centre for providing the opportunity to discuss and explore Cochrane methodology.
Julia Walters was the Editor for this review and commented critically on the review.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| CENTRAL | Monthly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 2. Search strategy for Cochrane Airways Group Register
#1 AST:MISC1
#2 MeSH DESCRIPTOR Asthma Explode All
#3 asthma*:ti,ab
#4 #1 or #2 or #3
#5 MeSH DESCRIPTOR Water Explode All
#6 MeSH DESCRIPTOR Swimming Pools
#7 water*
#8 aquatic*
#9 immers*
#10 bath*
#11 whirlpool*
#12 pool*
#13 hydro*
#14 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13
#15 exercis*
#16 gym*
#17 aerobic*
#18 anaerobic*
#19 breathing*
#20 endurance*
#21 train*
#22 kinesiotherap*
#23 isokinetic*
#24 isometric*
#25 treadmill*
#26 muscle*
#27 muscular*
#28 pilates*
#29 plyometric*
#30 resistance*
#31 stretch*
#32 posture*
#33 movement*
#34 motion*
#35 physiotherap*
#36 physical*
#37 fitness*
#38 sport*
#39 walk*
#40 run*
#41 calisthenic*
#42 MeSH DESCRIPTOR Physical Therapy Modalities Explode All
#43 MeSH DESCRIPTOR Exercise Explode All
#44 MeSH DESCRIPTOR Sports Explode All
#45 MeSH DESCRIPTOR Gymnastics
#46 MeSH DESCRIPTOR Exercise Therapy
#47 #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46
#48 #14 and #47
#49 hydrotherap*
#50 swim*
#51 hydrogymnastic*
#52 aqua‐aerobic*
#53 "aqua aerobic*"
#54 aquafit*
#55 dive* or diving
#56 hydrokinetic*
#57 hydro‐kinetic*
#58 "Ai Chi"
#59 Halliwick
#60 balneotherap*
#61 MeSH DESCRIPTOR Hydrotherapy
#62 MeSH DESCRIPTOR Swimming
#63 MeSH DESCRIPTOR Balneology Explode All
#64 #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 or #63
#65 #48 or #64
#66 #4 and #65
(MISC1 = field in the Register where each record is coded for disease, e.g. AST = asthma)
Appendix 3. Additional database search stategies
LILACS via BVS (iaHx interface)
asthma AND Exercise AND (((PT:"Ensaio Clinico Controlado Aleatorio" OR PT:"Ensaio Clinico Controlado" OR PT:"Ensaio Clinico" OR MH:"Ensaios Clinicos Controlados Aleatorios como Assunto" OR MH:"Ensaios Clinicos Controlados como Assunto" OR MH:"Ensaios Clinicos como Assunto") OR ((PT:"Estudo Multicentrico" OR MH:"Estudos Multicentricos como Assunto" OR MH:"Distribuicao Aleatoria" OR MH:"Metodo Duplo‐Cego" OR MH:"Metodo Simples‐Cego" OR MH:"Grupos Controle" OR MH:"Estudos Cross‐Over") AND ((tw:ensaio$ or tw:ensayo$ or tw:trial$ or tw:estudo$ or tw:estudio$ or tw:study or tw:studies) AND (tw:azar or tw:acaso or tw:enmascarado or tw:placebo or tw:control$ or tw:aleat$ or tw:random$ or tw:dobleciego or tw:simpleciego or ((tw:simple$ or tw:single or tw:duplo$ or tw:doble$ or tw:double$) and (tw:cego$ or tw:ciego$ or tw:blind$ or tw:mask$))) AND tw:clinic$)) OR ((tw:ensaio$ or tw:ensayo$ or tw:trial$) AND (tw:azar or tw:acaso or tw:enmascarado or tw:placebo or tw:control$ or tw:aleat$ or tw:random$ or tw:dobleciego or tw:simpleciego or ((tw:simple$ or tw:single or tw:duplo$ or tw:doble$ or tw:double$) and (tw:cego$ or tw:ciego$ or tw:blind$ or tw:mask$))) AND tw:clinic$)) AND NOT (MH:animais OR MH:ratos$ OR MH:camundongo$ OR MH:gatos OR MH:primatas OR MH:caes OR MH:coelhos OR MH:suinos OR PT:"in vitro"))
PEDro
exercise AND Asthma; Swimming AND asthma; Water AND asthma
Google Scholar
asthma* "aquatic exercise" "Randomized Clinical Trial" ‐child*
SIGLE
asthma AND exercise OR swimming AND water
ICTRP WHO
exercise AND asthma
Data and analyses
Comparison 1. Water‐based exercise versus land‐based exercise (10 weeks): parallel groups.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbations leading to health centre visits or exacerbations leading to hospitalisations | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Symptoms (general symptoms) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Lung function (FEV1 (L)) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Final value | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Lung function (PEF (L/min)) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Reduction in medication use | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.
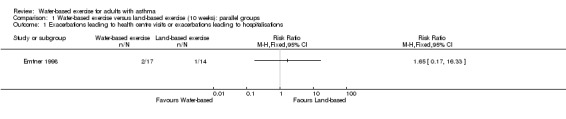
Comparison 1 Water‐based exercise versus land‐based exercise (10 weeks): parallel groups, Outcome 1 Exacerbations leading to health centre visits or exacerbations leading to hospitalisations.
1.2. Analysis.
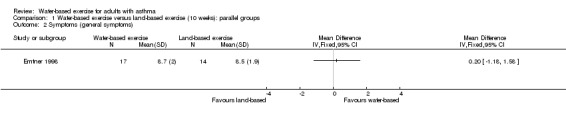
Comparison 1 Water‐based exercise versus land‐based exercise (10 weeks): parallel groups, Outcome 2 Symptoms (general symptoms).
1.4. Analysis.
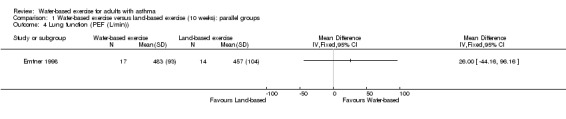
Comparison 1 Water‐based exercise versus land‐based exercise (10 weeks): parallel groups, Outcome 4 Lung function (PEF (L/min)).
1.5. Analysis.
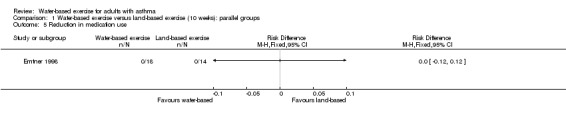
Comparison 1 Water‐based exercise versus land‐based exercise (10 weeks): parallel groups, Outcome 5 Reduction in medication use.
1.6. Analysis.
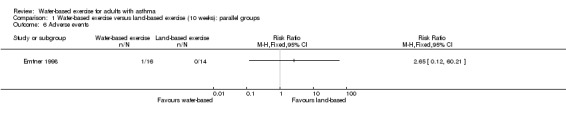
Comparison 1 Water‐based exercise versus land‐based exercise (10 weeks): parallel groups, Outcome 6 Adverse events.
Comparison 2. Water‐based exercise versus land‐based exercise (1 session): cross‐over.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lung function (FEV1 (L)) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Lung function (PEF (L/min)) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
2.1. Analysis.
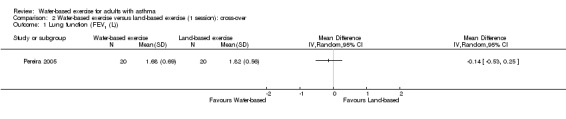
Comparison 2 Water‐based exercise versus land‐based exercise (1 session): cross‐over, Outcome 1 Lung function (FEV1 (L)).
2.2. Analysis.
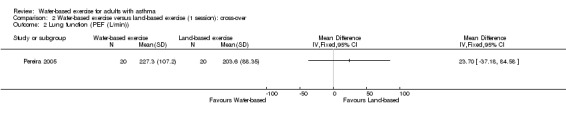
Comparison 2 Water‐based exercise versus land‐based exercise (1 session): cross‐over, Outcome 2 Lung function (PEF (L/min)).
Comparison 3. Water‐based exercise + education versus medication only (24 weeks): parallel groups.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lung function (FEV1 (L)) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Lung function (PEF (L/min)) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
3.1. Analysis.
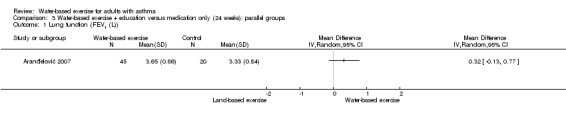
Comparison 3 Water‐based exercise + education versus medication only (24 weeks): parallel groups, Outcome 1 Lung function (FEV1 (L)).
3.2. Analysis.
Comparison 3 Water‐based exercise + education versus medication only (24 weeks): parallel groups, Outcome 2 Lung function (PEF (L/min)).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aranđelović 2007.
| Methods | STUDY DESIGN: parallel‐group, randomised, prospective study LOCATION, NUMBER OF CENTRES: asthmatic patients from departments of the Health Centre, University of Nis STUDY PERIOD: 6‐month programme; twice a week on a 1‐hour basis METHODS OF ANALYSIS: At the beginning and the end of the study, spirometry forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC) and FEV1/FVC × 100 were recorded using a flow‐volume spirometer (Jaeger) according to the recommendations of the European Respiratory Society; a broncho provocative test was performed with a histamine dose of 15 μg, which was then increased by doubling doses until FEV1, measured within 1 minute of histamine inhalation, decreased by 20% or more when compared with baseline FEV1; the dose of histamine causing a 20% decrease in FEV1 (provocative dose (PD20) histamine) was calculated by interpolation of the dose‐response curves. The skin prick test was performed to examine atopic status versus a panel of 5 common aero‐allergens and was defined as positive if values ≥ 3 mm to 1 or more allergens were reported STATISTICAL ANALYSIS: not specified |
|
| Participants | RECRUITMENT MEANS: patients from the Health Centre, University of Nis TARGET PARTICIPANTS: adults N SCREENED: 70 adult asthmatic patients N RANDOMLY ASSIGNED: 70 N COMPLETED: 65 : 45 in the experimental group and 20 in the control group GENDER M = 49; experimental 34, control 15 F = 16; experimental 11, control 5 AGE, years: experimental 33.07 ± 9.81, control 33.55 ± 10.88 ASTHMA DIAGNOSIS CRITERIA: patients with mild, persistent asthma selected according to GINA given documented treatment with low doses of ICS and short‐acting beta2‐agonists OTHER BASELINE DETAILS: age, sex, atopic, smoking status |
|
| Interventions | SETTING OF INTERVENTION: Experimental group used an indoor swimming pool with natural warm water without chlorination, located in a recreational spa centre in the vicinity of their home DESCRIPTION OF INTERVENTION: Experimental group practiced swimming twice a week on a 1‐hour basis for 6 months DELIVERED BY: not informed TREATMENT PERIOD: 6 months FOLLOW‐UP PERIOD: 6 months CO‐INTERVENTIONS: asthma education within an asthma school, which provided information on therapy, breathing, diet, mental hygiene, smoking, physical activities and quality of life through related courses for experimental groups. Both groups continued treatment with low doses of ICS and short‐acting beta2‐agonists |
|
| Outcomes | LUNG FUNCTION: FVC, FEV1, FEV1/FVC, PEF and BHR ATOPIC STATUS: atopic, non‐atopic FEV1 BASELINE = control group 3.99 (SD = 0.92) L; experimental group 4.27 (SD = 0.97) L; post‐intervention control group 4.01 (SD = 0.7) L; experimental group 4.37 (SD = 0.93) L PRESPECIFIED: at the beginning of the programme FOLLOW‐UP PERIOD: 6 months after the programme start |
|
| Notes | Study author did not supply information STUDY FUNDING: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Term randomised was used; however the randomisation process was not described |
| Allocation concealment (selection bias) | High risk | No mention was made in the text of how allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Because of the characteristics of the intervention, participants could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information regarding blinding of those who assessed participants was stated in the text |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss of 5 participants in the control group was described by the study author. Investigators did not use ITT analysis |
| Selective reporting (reporting bias) | High risk | Protocol was not available. Study was not registered on a clinical trials registry. Only subrogate outcomes were analysed |
| Other bias | High risk | Asthma education was restricted to the experimental group; this was a confounding factor |
Emtner 1998.
| Methods | STUDY DESIGN: parallel‐group, quasi‐randomised, prospective study LOCATION, NUMBER OF CENTRES: asthmatic patients from the Lung Clinic, Uppsala, Sweden STUDY PERIOD: 10‐week rehabilitation programme; Monday to Friday during the first 2 weeks, period 1; twice a week during the following 8 weeks, period 2 METHODS OF ANALYSIS: Participants underwent the tests described below at the start of the programme and after 2 and 10 weeks. A submaximal 6‐minute ergometry test was performed on a cycle ergometer with a mechanical friction load at a constant pedaling rate, attaining a heart rate of 140 to 180 beats/min after 6 minutes. A 12‐minute walking test was performed in a hospital corridor. Spirometry was carried out with a body plethysmograph (Jaeger Masterlab body 175,050) for measurement of lung volume and specific airway conductance, and a wedge spirometer (Ohio) was used for recording of flow–volume curves. PEFR was measured with a peak flow meter. The highest value of 3 attempts was used. A methacholine provocation test was carried out to measure airway reactivity, expressed as the amount of inhaled methacholine required to cause a fall of 20% in FEV1. Three different questionnaires were administered, with questions concerning physical exercise, effects of asthma on everyday life and participants' experiences. Answers were given on a 10‐cm visual analogue scale (0 to 10 cm), on which 0 indicates the worst value and 10 the best. The number of emergency department visits for acute asthmatic attacks during the 10 weeks before the start of the rehabilitation programme and the 10 weeks after the start of the programme was recorded. A training log was kept by each participant to record the frequency and intensity of training and perceived exertion after each exercise session. A diary was kept by each participant. Participants were asked to record their asthma symptoms on a 4‐graded scale every evening during the 10‐week programme STATISTICAL ANALYSIS: Data were analysed for statistically significant differences between groups using the unpaired t‐test (parametric data) and Mann‐Whitney’s U‐test (non‐parametric data). One‐factor analysis of variance for repeated measurements (Fischer PLSD) was used for parametric data, and Friedman’s test was used for non‐parametric data. A paired t‐test (parametric data) and the Wilcoxon signed‐rank test (non‐parametric data) were used for comparison between values obtained 10 weeks before and at the start, and between those obtained at the start and after 10 weeks |
|
| Participants | RECRUITMENT MEANS: All were outpatients from the Lung Clinic, Uppsala, Sweden TARGET PARTICIPANTS: adults N SCREENED: 32 adult asthmatic patients N RANDOMLY ASSIGNED: 32 N COMPLETED: 31: 17 water group and 14 land group GENDER M = 18; water 9, land 9 F = 14; water 9, land 5 AGE, mean, years: land 38 ± 12, water 34 ± 8 ASTHMA DIAGNOSIS CRITERIA: (1) chronic well‐controlled asthma (according to ATS) of mild or moderate severity, (2) FEV1% > 75 after inhalation of a beta2‐agonist and (3) no concomitant disease OTHER BASELINE DETAILS: age, gender, physical condition, baseline spirometry and reversibility |
|
| Interventions | SETTING OF INTERVENTION: for land group: gymnastic hall at room temperature of 22°C and relative humidity of 20% to 30%; for water group: indoor swimming pool with water temperature of 33°C at room temperature of 24°C and relative humidity of at least 60% DESCRIPTION OF INTERVENTION: period 1, 2 weeks, 5 days‐a‐week training session on land or in the pool (hydrogymnastics) for 45 minutes; daily theoretical lessons in anatomy, physiology, pathophysiology, medication or physical training; and daily practical sessions on techniques for inhalation, breathing, relaxation or incontinence. Period 2, 8 weeks, 2‐days‐a‐week training session on land or in the pool for 45 minutes. Each training session, whether on land or in water, started with a warming‐up period (15 minutes) followed by arm and leg exercises, walking and low‐intensity jogging. Exercise continued with interval training, comprising five 2‐minute periods of intensive exercise separated by eleven 2‐minute periods of low‐intensity exercise (total 16 minutes). These periods of interval training consisted of varied repetitive large muscle, dynamic exercises with a target pulse rate of 80% to 100% of maximal intensity. A cooling‐down period (7 minutes) and stretching exercises (7 minutes) completed the 45‐minute session DELIVERED BY: All training sessions and practical sessions were led by 1 of 2 physiotherapists; the theoretical sessions were led by a physiotherapist, a nurse or a physician TREATMENT PERIOD: 10 weeks FOLLOW‐UP PERIOD: 10 weeks CO‐INTERVENTIONS: Medication was kept constant during the study period |
|
| Outcomes | FEV1, PEF, heart rate, walking distance, breathing rate. Questionnaire measurements (made by study authors): emergency department visits, medication use, missing workdays, asthma symptoms, perceived exertion FEV1 BASELINE = land group 2.8 (SD = 0.8) L; water group 3.2 (SD = 0.8) L; post‐intervention land group 2.6 (SD = 0.7) L; water group 3.3 (SD = 0.8) L PRESPECIFIED: 10 weeks before and at the start of the programme FOLLOW‐UP PERIOD: 2 weeks and 8 weeks after the programme start |
|
| Notes | Study author supplied information regarding randomisation and allocation STUDY FUNDING: Swedish Heart‐Lung Foundation, Stockholm; Heart and Lung Patients National Association, Stockholm; Bror Hjerpstedt Foundation and Lilly and RagnarA° Kerhamn Foundation, Uppsala |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random sequence was generated by odd and even days of birth |
| Allocation concealment (selection bias) | High risk | Allocation was in accordance with date of birth |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Because of the characteristics of the interventions, participants could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researcher who assessed participants was blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of 1 participant in the water group as the result of skin sensitivity was reported; participant completed the exercise during remaining weeks on land |
| Selective reporting (reporting bias) | High risk | Protocol was not available. The study was not registered on a clinical trials registry |
| Other bias | High risk | Baseline imbalance may be related to sequence generation |
Pereira 2005.
| Methods | STUDY DESIGN: cross‐over group; randomised, prospective study LOCATION, NUMBER OF CENTRES: Physical Therapy Clinic, therapeutic swimming pool at City University of Sao Paulo (UNICID) STUDY PERIOD: October 2003 to December 2004 METHODS OF ANALYSIS: questionnaires: asthma symptoms and exacerbations; Beck Depression Inventory; quality of life questionnaire for asthmatic patients; State‐Trait Anxiety Inventory (STAI); breathing discomfort for adults with asthma. Cirtometry (axillary, xiphoid, umbilical) with measuring tape; strength of respiratory muscles (MV‐120 manometer); pulse oximeter (model 9500, Nonin Medical Inc; Spirometry Vitalograph 2120) (FVC, FEV1, FEV1/FVC, PEF and FEF 25% to 75%); peak flow meter STATISTICAL ANALYSIS: descriptive statistics for all variables plus Kolmogorov Sminoff for normality and Levene for homogeneity of variance. For comparisons, student t‐test was used |
|
| Participants | RECRUITMENT MEANS: interest list, advertisement in train station and newspaper, last 3 years, asthmatic patients from Sao Paulo Clinics Hospital TARGET PARTICIPANTS: adults 18 to 50 years of age N SCREENED: 40 adult asthmatic patients N RANDOMLY ASSIGNED: 40 N COMPLETED: 40: 20 water group and 20 land group GENDER M = 9; water 5, land 4 F = 31; water 15, land 16 AGE, years: water group 34.75 ± 8.32, land group 32.05 ± 9.23 ASTHMA DIAGNOSIS CRITERIA: inclusion criteria: mild and severe asthma for at least 5 years; use of beta2‐agonists of short action; age between 18 and 50 years. Exclusion criteria: smokers or ex‐smokers for less than 5 years; response lower than 12% and 200 mL FEV1 after use of bronchodilator; 40% < FEV1 > 80% estimated; pregnancy; airways infection in the past 30 days; associated diseases (pulmonary and extrapulmonary); participation in any other physical activity aimed at pulmonary rehabilitation OTHER BASELINE DETAILS: asthma classification, age, gender, weight, height, skin colour, rhinitis, sinusitis, dermatitis, smoking status, practice of physical activity |
|
| Interventions | SETTING OF INTERVENTION: for land group: exercises conducted on land; for water group: swimming pool with water temperature of 35°C DESCRIPTION OF INTERVENTION: Study was composed 1 session of water‐based exercise and 1 session of land‐based exercise, crossed‐over after 7 days of washout. Water‐based exercise lasted 40 minutes. Exercise protocol was divided into the following: exercise for thoracic mobility (10 minutes); muscular strength of expiratory muscles (10 minutes); resistance training for inspiratory muscles (10 minutes); and self‐stretching of inspiratory muscles (10 minutes); each exercise was performed 10 times. Land‐based exercise had the same protocol of exercises. After the intervention, both groups rested 10 minutes on land and proceeded to assessment DELIVERED BY: researcher TREATMENT PERIOD: 1 day. 1 session in the water and 1 session on land FOLLOW‐UP PERIOD: finished after last session CO‐INTERVENTIONS: short‐acting beta2‐agonists kept constant during study period |
|
| Outcomes | SIGNS AND SYMPTOMS: depression and anxiety (depression score; anxiety score); quality of life (physical limitation domain, frequency and severity domain; adherence domain, socioeconomic domain, psychosocial domain); degree of respiratory discomfort; forced expiratory volume in 1 second (FEV1); peak expiratory flow (PEF); maximal inspiratory pressure (MIP); maximal expiratory pressure (MEP); cirtometry (axillary, xiphoid, umbilical) FEV1 BASELINE = land group 2.12 (SD = 0.69) L; water group 1.83 (SD = 0.62) L; post‐intervention land group 1.82 (SD = 0.56) L; water group 1.68 (SD = 0.69) L PRESPECIFIED: tested before and after each session of the intervention FOLLOW‐UP PERIOD: every session |
|
| Notes | Study author supplied information regarding randomisation and allocation STUDY FUNDING: not declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study was randomised. We contacted the author who explained that randomisation was done by drawing numbered pieces of paper from a container. |
| Allocation concealment (selection bias) | High risk | This was not possible because the author conducted the assessments and the randomisation, so the concealment was not adequate. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the characteristics of the intervention participants could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The researcher who assessed the patients have also conducted the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow up. The flow of patients were clear from baseline through the end. |
| Selective reporting (reporting bias) | High risk | Protocol not available. The study was not registered on Clinical Trials Registry. The author did not present some outcomes such as: Quality of life global measure, she presented only the domains; FVC; FEV1; FEV1/FVC; PEF; FEF 25% to 75%; the author did not present the comparisons and she claimed not to be relevant. |
| Other bias | Unclear risk | The study design was a cross‐over with a washout period of 1 week. Since the aim of the study was assess the acute effects after intervention, it is unclear if it was enough time of washout for exercise. |
ATS: American Thoracic Society; BHR: bronchial hyperresponsiveness; FEF: forced expiratory flow; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; GINA: Global Initiative for Asthma; ICS: inhaled corticosteroids; ITT: intention‐to‐treat; MEP: maximal expiratory pressure; MIP: maximal inspiratory pressure; PD20: provocative dose 20; PEF: peak expiratory flow; PEFR: peak expiratory flow rate; PLSD: protected least significant difference; SD: standard deviation; STAI: State‐Trait Anxiety Inventory.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Boulet 2005 | Although the study was randomised, we contacted the study author, who explained that most participants were not given a diagnosis of asthma |
| Fitch 1971 | The study analysed exercise‐induced asthma |
| Hildenbrand 2010 | The study did not include a control group |
Differences between protocol and review
We deleted the head‐to‐head comparisons (i.e. swimming vs hydrogymnastics and swimming vs hydrotherapy).
In the protocol, we envisaged three different situations for exacerbations. We still believe they have to be analysed differently; however for this review, we believed it made sense to put all of them together and call them exacerbations.
In the protocol, we included the outcome of use of rescue medications (e.g. short‐acting beta2‐agonists (SABAs)) or symptom controllers (e.g. long‐acting beta2‐agonists (LABAs)); however in the review, we decided to include the need for SABAs under the outcome of reduction in medication usage.
We planned to used either OR or RR to analyse dichotomous data, however we decided that using RR was more appropriate. We did not derive number needed to treat to benefit (NNTB).
We deleted the outcome 'level of severity of disease' from the summary of findings table as it was included in the protocol through error.
Contributions of authors
Antonio Jose Grande (AJG) co‐ordinated retrieval of papers, identified papers for inclusion, extracted and checked data and wrote the background, methods, results, discussion and conclusion sections of the review.
Valter Silva (VS) participated in retrieval of papers, identified papers for inclusion, extracted and checked data and co‐wrote the background, methods, results, discussion and conclusion sections of the review.
Brenda NG Andriolo (BNGA) analysed data, wrote statistical methods, co‐wrote results and interpreted the data.
Rachel Riera (RR) co‐wrote the background and methods sections and provided a critical review of the existing review.
Sergio Alencar Parra (SAP) co‐wrote the background and methods sections and provided a critical review of the existing review.
Maria Stella Peccin (MSP) arbitrated on quality assessment, co‐wrote the background and methods and provided a critical review of the existing review.
All authors participated in preparing and approved the revised text for publication.
Sources of support
Internal sources
-
None, Other.
None
External sources
-
None, Other.
None
Declarations of interest
None known.
New
References
References to studies included in this review
Aranđelović 2007 {published data only}
- Aranđelović M, Stanković I, Nikolić M. Swimming and persons with mild persistent asthma. The Scientific World Journal 2007;7:1182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Emtner 1998 {published data only}
- Emtner M, Finne M, Stalenheim G. High‐intensity physical training in adults with asthma. A comparison between training on land and in water. Scandinavian Journal of Rehabilitation Medicine 1998;30:201‐9. [DOI] [PubMed] [Google Scholar]
Pereira 2005 {published data only}
- Pereira KS. A comparative study of breathing exercises in warm‐water pool for moderate and severe asthma patients: a singles session effects. Estudo comparativo de exercícios respiratórios em piscina aquecida para asmáticos graves e moderados: impacto de uma sessão [Dissertation]. Sao Paulo: University of Sao Paulo, 2005. [Google Scholar]
References to studies excluded from this review
Boulet 2005 {published data only}
- Boulet LP, Turcotte H, Langdeau JB, Bernier MC. Lower airway inflammatory responses to high‐intensity training in athletes. Clinical and Investigative Medicine 2005;28(1):15‐22. [PubMed] [Google Scholar]
Fitch 1971 {published data only}
- Fitch KD, Morton AR. Specificity of exercise in exercise‐induced asthma. British Medical Journal 1971;4(5787):577‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hildenbrand 2010 {published data only}
- Hildenbrand K, Nordio S, Freson TS, Becker B. Development of an aquatic exercise training protocol for the asthmatic population. International Journal of Aquatic Research and Education 2010;4(3):278‐99. [Google Scholar]
Additional references
Ambrosini 2010
- Ambrosini AB, Brentano MA, Coertjens M, Kruel LFM. The effects of strength training in hydrogymnastics for middle‐age women. International Journal of Aquatic Research and Education 2010;4:153‐62. [Google Scholar]
Avelar 1999
- Avelar NCP, Bastone AC, Alcantara MA, Gomes WF. Effectiveness of aquatic and non‐aquatic lower limb muscle endurance training in the static and dynamic balance of elderly people. Revista Brasileira de Fisioterapia 2010;14:229‐36. [PubMed] [Google Scholar]
Banzett 1985
- Banzett RB, Lansing RW, Reid MB. Reflex compensation of voluntary inspiration when immersion changes diaphragm length. Journal of Applied Physiology 1985;59(2):611–8. [DOI] [PubMed] [Google Scholar]
Barbosa 2011
- Barbosa TM, Marinho DA, Reis VM, Silva AJ, Bragada JA. Physiological assessment of head‐out aquatic exercises in healthy subjects: a qualitative review. Journal of Sports Science and Medicine 2009;8:179‐89. [PMC free article] [PubMed] [Google Scholar]
Beggs 2013
- Beggs S, Foong YC, Le HC, Noor D, Wood‐Baker R, Walters JA. Swimming training for asthma in children and adolescents aged 18 years and under. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD009607.pub2] [DOI] [PubMed] [Google Scholar]
Bernard 2009
- Bernard A, Nickmilder M, Voisin C, Sardella A. Impact of chlorinated swimming pool attendance on the respiratory health of adolescents. Pediatrics 2009;124(4):1110‐8. [DOI] [PubMed] [Google Scholar]
Carson 2013
- Carson KV, Chandratilleke MG, Picot J, Brinn MP, Esterman AJ, Smith BJ. Physical training for asthma. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD001116.pub4] [DOI] [PubMed] [Google Scholar]
CDC 2011
- Centers for Disease Control and Prevention. Asthma surveillance data. http://www.cdc.gov/asthma/asthmadata.htm (accessed 12 November 2012).
Corbin 2011
- Corbin CB. Concepts of Fitness and Wellness: A Comprehensive Lifestyle Approach. New York: McGraw Hill, 2011. [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Intervention. Chichester: John Wiley & Sons, 2011. [Google Scholar]
Denning 2012
- Denning WM, Bressel E, Dolny D, Bressel M, Seeley MK. A review of biophysical differences between aquatic and land‐based exercise. International Journal of Aquatic Research and Education 2012;6:46‐67. [Google Scholar]
Dennis 2012
- Dennis JA, Cates CJ. Alexander technique for chronic asthma. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD000995.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eijkemans 2012
- Eijkemans M, Mommers M, Draaisma JM, Thijs C, Prins MH. Physical activity and asthma: a systematic review and meta‐analysis. PLoS One 2013;7(12):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Font‐Ribera 2011
- Font‐Ribera L, Villanueva CM, Nieuwenhuijsen MJ, Zock J‐P, Kogevinas M, Henderson J. Swimming pool attendance, asthma, allergies, and lung function in the Avon Longitudinal Study of Parents and Children cohort. American Journal of Respiratory and Critical Care Medicine 2011;183(5):582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freitas 2013
- Freitas DA, Holloway EA, Bruno SS, Chaves GS, Fregonezi GA, Mendonca KM. Breathing exercises for adults with asthma. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD001277.pub3] [DOI] [PubMed] [Google Scholar]
Gonçalves 2008
- Gonçalves RC, Nunes MPT, Cukier A, Stelmach R, Martins MA, Carvalho CRF. Effects of an aerobic physical training program on psychosocial characteristics, quality‐of‐life, symptoms and exhaled nitric oxide in individuals with moderate or severe persistent asthma. Revista Brasileira de Fisioterapia 2008;12(2):127‐35. [Google Scholar]
Goodman 2008
- Goodman M, Hays S. Asthma and swimming: a meta‐analysis. Journal of Asthma 2008;45:639–47. [DOI: 10.1080/02770900802165980] [DOI] [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows. GRADE Group, 2008.
Higgins 2011a
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2011. [Google Scholar]
Higgins 2011b
- Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2011. [Google Scholar]
Kohlhammer 2006
- Kohlhammer Y, Döring A, Schäfer T, Wichmann HE, Heinrich J. Swimming pool attendance and hay fever rates later in life. Allergy 2006;61(11):1305‐9. [DOI] [PubMed] [Google Scholar]
Lucas 2005
- Lucas SR, Platts‐Mills TA. Physical activity and exercise in asthma: relevance to etiology and treatment. Journal of Allergy and Clinical Immunology 2005;115(5):928‐34. [DOI] [PubMed] [Google Scholar]
Malkia 1998
- Malkia E, Impivaara O. Intensity of physical activity and respiratory function in subjects with and without bronchial asthma. Scandinavian Journal of Medicine and Science in Sports 1998;8(1):27‐32. [DOI] [PubMed] [Google Scholar]
O'Byrne 2012
- O'Byrne PM. Exercise‐induced bronchoconstriction. http://www.uptodate.com/contents/exercise‐induced‐bronchoconstriction?source=search_result&search=Exercise‐induced+bronchoconstriction&selectedTitle=1˜36. Waltham, MA: UpToDate, (accessed 26 November 2013).
Pedersen 2006
- Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scandinavian Journal of Medicine and Science in Sports 2006;16(1):3‐63. [DOI] [PubMed] [Google Scholar]
Posadzki 2011
- Posadzki P, Ernst E. Yoga for asthma? A systematic review of randomized clinical trials. The Journal of Asthma 2011;48(6):632‐9. [DOI] [PubMed] [Google Scholar]
Randolph 2009
- Randolph C. An update on exercise‐induced bronchoconstriction with and without asthma. Current Allergy and Asthma Reports 2009;9(6):433‐8. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Schoefer 2008
- Schoefer Y, Zutavern A, Brockow I, Schäfer T, Krämer U, Schaaf B, et al. Health risks of early swimming pool attendance. International Journal of Hygiene and Environmental Health 2008;211(3):367‐73. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Chichester: John Wiley & Sons, 2011. [Google Scholar]
Silva 2013
- Silva IS, Fregonezi GA, Dias FA, Ribeiro CT, Guerra RO, Ferreira GM. Inspiratory muscle training for asthma. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD003792.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
To 2012
- To T, Stanojevic S, Moores G, Gershon AS, Bateman ED, Cruz AA, et al. Global asthma prevalence in adults: findings from the cross‐sectional world health survey. BMC Public Health 2012;12:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2011
- World Health Organization. Chronic respiratory disease. Asthma definition, 2011. http://www.who.int/respiratory/asthma/definition/en/ Vol. (accessed 12 November 2012).


