Abstract
Recurrent spontaneous abortion (RSA) is a prevalent pregnancy complication with a complex and poorly understood pathogenesis. Shoutai Wan (STW), a traditional Chinese medicine formula, is renowned for its kidney tonifying and fetus tranquilizing effects. It is used to treat miscarriages associated with kidney deficiency, hyperemesis gravidarum, and fetal restlessness. Recently, there has been an increase in experimental studies exploring the use of STW for RSA treatment, making progress in understanding its molecular mechanisms and signaling pathways. This review aims to systematically elucidate the mechanisms by which STW enhances cellular antioxidant capacity, attenuates inflammation, and improves the environment for embryo implantation. This involves regulating multiple signaling pathways, including Nuclear factor-erythroid 2-related factor 2/Heme oxygenase-1, JAK kinase 1/Signal transducer and activator of transcription 3, NOD-like receptor pyrin domain-containing protein/Caspase-1/Gasdermin D, Human Leukocyte Antigen G, Mitogen-activated protein kinase, and Serum and glucocorticoid-regulated kinase 1/Epithelial sodium channel. This review provides a theoretical reference for the clinical application and further experimental researches on the treatment of RSA with STW.
Keywords: Shoutai Wan, Recurrent spontaneous abortion, Signaling pathway, Molecular mechanisms, Experimental research
Abbreviations
- (RSA)
Recurrent spontaneous abortion
- (STW)
Shoutai Wan
- (LIF)
Leukemia Inhibitory Factor
- (EMT)
Epithelial-Mesenchymal Transition
- (VEGF)
Vascular Endothelial Growth Factor
- (HIF-1α)
Hypoxia-Inducible Transcription Factor 1-α
- (HK2)
Hexokinase 2
- (PKM2)
Pyruvate Kinase type M2
- (LDHA)
Lactate Dehydrogenase A
- (MCT4)
Monocarboxylic acid transporter 4
- (G6PD)
Glucose-6-phosphate dehydrogenase
- (GLUT1)
Glucose transporter protein 1
- (GPR81)
G-protein-coupled receptor 81
- (Nrf2)
Nuclear factor erythroid 2-related factor 2
- (HO-1)
Heme Oxygenase-1
- (JAK1)
JAK Kinase 1
- (STAT3)
Signal Transducer and Activator of Transcription 3
- (NLRP3)
NOD-like receptor thermal protein domain associated protein 3
- (GSDMD)
Gasdermin D
- (HLA-G)
Human Leukocyte Antigen
- (MAPK)
Mitogen-Activated Protein Kinase
- (SGK1)
Serum and Glucocorticoid Regulated Kinase 1
- (ENaC)
Epithelial Sodium Channel
1. Introduction
Recurrent spontaneous abortion (RSA) is a prevalent and challenging condition in the field of obstetrics and gynecology [1]. It occurs when a woman experiences two or more consecutive spontaneous abortions in the early stages of pregnancy [2]. This condition not only increases psychological pressure, decreases self-esteem, and leads to psychological problems such as anxiety and depression but also frequent abortions may trigger endometrial abnormalities. These abnormalities subsequently impair embryo implantation and heighten the risk of further abortions, creating a vicious cycle [3]. According to epidemiological survey data, about 1%–5% of women of reproductive age have a history of RSA [4]. The etiology of RSA is complex and may be influenced by genetic, environmental, age, and other related factors. It involves chromosomal abnormalities, uterine structural abnormalities, immunologic factor abnormalities, endocrine factor abnormalities, and coagulation system abnormalities, among other causes [5,6].
Shoutai Wan (STW), originating from Zhang Xichun's "Records of Tradition Chinese and Western Medicine in Combination" in the first volume during the Republic of China era, consists of four medicinal components: Cuscuta chinensis lam, Taxillus chinensis (DC.) Danser, Dipsacus asper Wall. ex DC., and Asini Corii Colla [7]. This formula is widely used in clinical practice for treating various pregnancy-related symptoms, such as slippage of the fetus due to kidney deficiency, hematemesis in pregnancy, restlessness of the fetus, and fetus withering without growth. Several clinical studies [[8], [9], [10]] have demonstrated that STW could effectively regulate immune homeostasis and coagulation function in patients with RSA, advanced preterm miscarriage, and RSA due to embolism. It optimizes hemodynamic parameters in spiral uterine arteries and Leukemia Inhibitory Factor (LIF) levels, reducing miscarriage risk and increasing the success rate of fetal preservation. A systematic evaluation and meta-analysis has also provided high-quality evidence supporting the efficacy and safety of STW combined with dextroprogesterone in treating preterm labor [11]. Recently, researches on STW for treating RSA, especially in delving into its molecular mechanisms, have significantly progressed. This review aims to systematically summarize the main pharmacological effects and signaling pathways of STW in the treatment of RSA, providing a scientific reference for its clinical application.
2. Methods
To ensure a thorough literature review, we systematically searched the PubMed Embase, Web of Science, China National Knowledge Infrastructure and China Wanfang Database for articles published up to March 1, 2024, to retrieve relevant publications. We use the keywords"Shoutai Wan", "Recurrent spontaneous abortion", "physiological mechanisms", and "signaling pathways", confined to article titles and abstracts. Two researchers independently conducted an initial screening based on these titles and abstracts, and articles deemed irrelevant were excluded. Full-text reviews were subsequently performed to further confirm the eligibility of the studies.
3. Principal pharmacological effects of STW in the regulation of RSA
3.1. Regulating hormone balance
In early pregnancy, the progesterone secreted by the placenta is essential for maintaining the endometrium and placental structures, which are crucial for safeguarding embryo attachment and development. Insufficient progesterone levels may lead to endometrial detachment or failure of embryo implantation, thus triggering miscarriage [12]. During pregnancy, the balanced relationship between estrogen and progesterone plays a mutual regulatory role: estrogen promotes endometrial proliferation, while progesterone maintains its stability and development. An imbalance between the two hormones may result in endometrial abnormalities and affect embryo implantation [13]. Additionally, hormones such as follicle-stimulating hormone, luteinizing hormone, and testosterone also play roles in RSA. Abnormal levels of these hormones may lead to ovarian dysfunction or ovulation problems, which in turn may trigger miscarriage [14]. A clinical study [15] has shown that combining STW with progesterone or dydrogesterone could significantly increase levels of chorionic gonadotropin, progesterone, and estradiol. This combination effectively alleviates symptoms of preeclampsia, improves clinical manifestations such as lumbar and knee soreness and weakness, fear of cold and cold limbs, and dizziness and tinnitus. Additionally, it enhances therapeutic effects, reduces the incidence of late-pregnancy complications, and improves the success rate of fetal preservation [16,17].
3.2. Promoting Epithelial-Mesenchymal Transition of embryonic trophoblast cells
Epithelial-Mesenchymal Transition (EMT) is a crucial process during embryonic development, particularly in embryonic trophoblasts. It involves the conversion of epithelial cells into more mobile and invasive mesenchymal cells [18]. This transformation is crucial for embryo implantation, formation, and subsequent tissue and organ development. During embryo implantation and early development, trophoblasts must undergo EMT, transforming from tightly connected, less invasive cells into highly invasive cells capable of deeply penetrating the endometrium [19]. Impairment of the EMT process could diminish the migration and invasive capabilities of trophoblast cells, potentially leading to RSA development [20]. An in vitro study [21] discovered that serum containing STW could reduce the apoptosis of trophoblast cells in RSA patients in a dose-dependent manner, while enhancing their proliferation and migration abilities, thereby aiding in pregnancy maintenance. Tang et al. [22] revealed that STW could promote embryonic development and reduce embryo loss in RSA mice by activating β-catenin signaling in trophoblast cells. This activation led to a decrease in E-cadherin expression, an epithelial cell marker, and an increase in Vimentin expression, a mesenchymal cell marker.
3.3. Promoting angiogenesis
The healthy development of the fetus and placenta largely depends on the effective differentiation of trophoblasts and the construction of the utero-placental vascular network [23]. During fertilization, trophoblasts penetrate the endometrium and spiral arteries, activating neovascularization and promoting the expansion of these arteries. This expansion increases blood flow to the implantation site, further promoting endometrial metaplasia and neovascularization [19]. Additionally, the hypoxic environment within the placenta creates optimal conditions for trophoblast invasion and placenta formation, while changes in oxygen partial pressure adversely affect the proliferation and invasive capacity of trophoblasts [24]. A study [25] has shown significant changes in the expression levels of Vascular Endothelial Growth Factor (VEGF) and Hypoxia-Inducible Transcription Factor 1-α (HIF-1α) in the chorionic tissues of patients with early RSA. STW, by decreasing the expression level of HIF-1α mRNA and protein and simultaneously promoting the expression of VEGF and its receptor mRNA and protein, enhances the proliferation and reinforces functions of placental trophoblast cells. This enhancement accelerates the process of vascular remodeling at the maternal-fetal interface, optimizing the developmental environment for the fetus and placenta and improving pregnancy maintenance [26,27].
3.4. Modulating immune-inflammatory
Inflammation plays a significant role in the development of RSA, which involving mechanisms such as aberrant activation of immune cells, cytokine release, immune regulation imbalance, and adverse effects on embryo implantation and pregnancy maintenance. Abnormal activation of immune cells such as T-cells, B-cells and natural killer cells in RSA patients exacerbates the inflammatory response. This exacerbation leads to the release of pro-inflammatory cytokines, including Tumor Necrosis factor-alpha (TNF-α), interleukin (IL)-1β, and IL-6, which could negatively affect embryo implantation [28]. Additionally, an altered balance between T helper type 1 cells (Th1) and T helper type 2 cells (Th2) may result in a pro-inflammatory immune response, increasing the risk of an immune attack on the embryo [29]. The inflammatory response also impacts the structure, function, nutritional status, and cell adhesion molecule expression in the endometrium, affecting the requisite environment for embryo attachment [30]. While immune tolerance is crucial for maintaining pregnancy, inflammation could disrupt this balance, enabling the immune system to attack the embryo and placenta, thus elevating the risk of miscarriage [31].
An experimental animal study [32] has found that STW stimulates the expression of IL-4, IL-6, IL-10, LIF, and LIF receptors, while levels of interferon-gamma (INF-γ) and IL-2 in ovulating rats. This could to help maintain the balance of Th1/Th2 cytokines, reduces immune responses and inflammation, lower the risk of immune system attacks on embryos, improves endometrial tolerance, as well as promote embryo implantation [33]. Lai et al. [34] further demonstrated that STW tend to bias Th1/Th2 cytokines towards Th2. Additionally, STW has been shown to improve the immune function of pregnant rats exposed to Di(2-ethylhexyl) by antagonizing its estrogen-like effects and modulating the levels of serum immune factors such as IL-2, IL-5, and TNF-ɑ [35].
3.5. Improve blood coagulation balance
Achieving a successful pregnancy necessitates a balance between coagulation and fibrinolysis. In the physiological hypercoagulable state of pregnancy, precise regulation of the coagulation-fibrinolysis system is critical for embryo implantation and trophoblast invasion. Inadequate fibrin formation could result in ischemia, which hinders the proper implantation of the fertilized egg in the uterus, potentially leading to miscarriage [36]. Patients with coagulation abnormalities such as hemorrhagic defects, fibrinogen deficiency, and antiphospholipid syndrome are particularly susceptible to RSA [37]. Han et al. [38] found that STW could effectively improve the prethrombotic state in patients with RSA, restoring balance in the anticoagulation-fibrinolysis system in uterine embryonic tissues. This improvement is achieved by enhancing the balanced expression of coagulation factors such as serum activated protein S, serum activated protein, antithrombin, tissue factor, fibrinogen, fibrinopeptide A, and tissue fibrinogen activator between endothelial tissue cells and trophoblast cells in the placenta. In addition, a proteomic study [39] has shown that STW could effectively improve the hypercoagulable state of fetal fecal tissues, promote the expression of membrane-bound protein A2, as well as increase the production of fibrinogen, thus preventing thrombosis and ensuring that the embryo has an adequate blood supply to support pregnancy.
3.6. Regulating glycolytic balance
Glycolytic homeostasis involves the switch of cellular metabolism from oxidative phosphorylation to glycolytic metabolism. Normally, cells produce large amounts of Adenosine Triphosphate (ATP) primarily through oxidative phosphorylation, the main energy source for cell survival and function. During embryo implantation, the endometrium initially undergoes metamorphosis, with early pregnancy relying on glycolysis and a high-lactate environment in endometrial stromal cells [40]. Several studies [41,42] have shown that STW upregulates mRNA expression of lactate, ATP, Hexokinase 2 (HK2), Pyruvate Kinase type M2 (PKM2), Lactate Dehydrogenase A (LDHA), Monocarboxylic acid transporter 4 (MCT4), Glucose-6-phosphate dehydrogenase (G6PD), Glucose transporter protein 1 (GLUT1), and G-protein-coupled receptor 81 (GPR81) in preeclamptic abortions and improves glycolysis homeostasis at the maternal-fetal interface to improve the embryo survival rate.
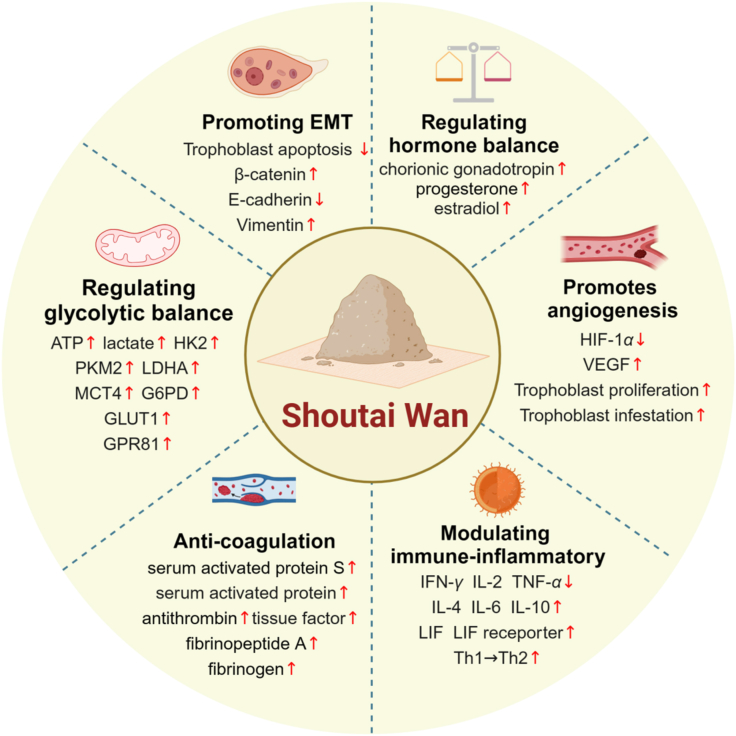
In conclusion, the mechanism of STW in treating RSA involves regulating hormonal balance, promoting EMT, angiogenesis, modulating immune-inflammatory responses, enhancing anti-coagulation, as well as regulating glycolytic balance. These actions contribute to maintaining the health of the uterine endometrium and placenta, improving the energy requirements of the placenta and embryo, enhancing the success rate of embryo implantation, and reducing the risk of miscarriage in patients with RSA (Fig. 1). However, the specific molecular mechanisms underpinning these effects warrant further in-depth study.
Fig. 1.
The main pharmacological effects of STW in regulating RSA.
4. The principal signaling pathways of STW in the treatment of RSA
4.1. Nrf2/HO-1 signaling pathway
The Nuclear factor erythroid 2-related factor 2(Nrf2)/Heme Oxygenase-1(HO-1) signaling pathway is a key intracellular antioxidant pathway that regulates cellular oxidative stress and inflammatory responses. Nrf2 serves as a transcription factor, migrating to the nucleus and binding to the Antioxidant Response Element (ARE) to activate the transcription of enzymes like HO-1 upon oxidative or inflammatory stress stimulation [43]. Activation of the Nrf2/HO-1 pathway enhances cellular antioxidant capabilities, reduces reactive oxygen species and free radical accumulation, and protects cells from oxidative stress damage [44]. Moreover, this pathway could mitigate inflammatory responses, modulate immune system function, decrease embryonic immune rejection, and improve the embryo's survival environment in the uterus, thereby increasing embryo implantation rates [45]. A study [46] found that serum in STW activates the Nrf2/HO-1 signaling pathway, promoting the expression of downstream genes such as quinone oxidoreductase (NQO1) and B-cell lymphoma-2 (BCL-2), while inhibiting Bcl-2-associated X protein (BAX) and cystatinase-3 expression. This results in reduced hydrogen peroxide-induced apoptosis in trophoblast cells and enhanced antioxidant capacity. A further research [47] revealed that STW activates the Nrf2/HO-1 pathway in metaphase tissues, increases the uterine organ index in rats, improves cytoplasmic edema and nucleus pulposus consolidation in metaphase cells, lowers embryo loss rates, and enhances the oxidative stress resistance in RSA rats.
In conclusion, by activating the Nrf2/HO-1 signaling pathway and regulating the interaction between downstream antioxidant enzymes and apoptotic proteins, STW effectively mitigates oxidative stress-induced apoptosis, safeguards the embryo and intrauterine environment, and diminishes the risk of RSA.
4.2. JAK1/STAT3 signaling pathway
The JAK Kinase 1 (JAK1)/Signal Transducer and Activator of Transcription 3 (STAT3) signaling pathway plays a pivotal role in regulating trophoblast cell growth, proliferation, migration, and invasion [48]. Disruptions in STAT3 activation during placental development could lead to abnormal embryonic development and placenta previa [17]. Notably, reduced levels of STAT3 phosphorylation in the nuclei of trophoblast cells, placental stromal cells, and meconium stromal cells have been observed in patients with early miscarriage and RSA, leading to decreased proliferative capacity of these cells [14]. Blockade or down-regulation of the JAK1/STAT3 signaling pathway may hinder vasculogenesis of low-resistance uterine spiral arterioles at the maternal-fetal interface, which is critical for a normal pregnancy. This may result in trophoblast cell ischemia and hypoxia, diminished invasiveness, and potential miscarriage. Serum of containing STW has been demonstrated to significantly boost the proliferation, invasion, and migration of trophoblast cells HTR-8/Svneo. This effect is likely related to STW activating the JAK1/STAT3 signaling pathway, promoting the high expression of proteins or genes such as VEGF receptor, matrix metalloproteinase 9 (MMP9), Minichromosome maintenance protein 2 (MCM2), and proliferative nuclear antigen (PCNA) [49]. Furthermore, a study by Jenny Mou et al. [50] revealed that STW activates the JAK1/STAT3 pathway, promotes the expression of autophagy-associated proteins 5 (ATG5), Beclin1, and microtubule-associated protein 1A/1B light chain 3B (LC3B), alleviates intracellular waste accumulation and cellular function abnormalities, and improves the morphology of metaphyses and placental tissues. This contributes to a protective effect in pregnancy for RSA mice.
However, it has also been shown that aberrant activation of the JAK1/STAT3 signaling pathway is critical in exacerbating the endometrial inflammatory response and embryo implantation failure [51]. Therefore, more in-depth and definitive studies are needed to investigate the exact mechanism of the role of STW in regulating the JAK1/STAT3 signaling pathway in RSA.
4.3. NLRP3/caspase-1/GSDMD signaling pathway
In regulating cellular immune response and apoptosis, two signaling pathways, NOD-like receptor thermal protein domain associated protein 3, (NLRP3)/caspase-1 and Caspase-1/Gasdermin D, (GSDMD), are crucial and jointly contribute to the pathogenesis of RSA. NLRP3, an essential host immune receptor, activates in response to infection and intracellular injury signals. This activation involves NLRP3 inflammatory vesicles first activating Caspase-1, transforming it from a precursor to an active enzyme, which then contributes to the cleavage of pro-inflammatory cytokine precursors like IL-1β and IL-18 into active cytokines, triggering inflammatory responses [52]. Additionally, activated Caspase-1 directly cleaves GSDMD proteins into GSDMD-N-terminal and GSDMD-C-terminal fragments. The GSDMD-N-terminal forms a pore structure in the cell membrane, facilitating the release of intracellular substances such as lactic acid and cytokines, thereby triggering apoptosis and pro-inflammatory responses [52]. Moreover, caspase-1 activation promotes the production of pro-inflammatory factors IL-1β and IL-18, exacerbating the inflammatory response, increasing placental tissue damage, and disrupting immune regulation, thus elevating the risk of RSA.
A study [53] has demonstrated that STW significantly reduces serum levels of Caspase-1, IL-1β, and IL-18 in RSA model mice, and inhibits the apoptosis of uterine metamorphic cells mediated by the Caspase-1/GSDMD signaling pathway. This reduction could decrease the miscarriage rate and provide a tranquilizing effect on the fetus. A further research [54] has shown that STW may maintain maternal-fetal immune balance and promote fetal preservation by dose-dependently inhibiting NLRP3/Caspase-1 pathway-mediated apoptosis in metamorphic cells and reducing serum levels of IL-1β and IL-18.
In conclusion, the NLRP3/Caspase-1 and Caspase-1/GSDMD pathways are potentially involved in the pathogenesis of RSA by regulating inflammatory responses and apoptosis, impacting the function and survival of placental tissues and uterine meconium cells.
4.4. HLA-G signaling pathway
Human Leukocyte Antigen (HLA-G) is an atypically expressed form of the human leukocyte antigen class I molecule, playing a crucial role in immune regulation. The HLA-G immune signaling pathway involves the interaction of HLA-G proteins with their receptors on immune cells, thereby regulating immune responses and promoting embryonic immune tolerance [55]. During RSA development, there is a marked increase in the expression of HLA-G proteins in placental trophoblast cells, which is critical for embryonic development and immune tolerance. HLA-G proteins protect the embryo from immune rejection by inhibiting maternal immune cells from attacking the embryo through their receptor binding. This inhibition also reduces the maternal immune system's inflammatory response and maintains a conducive immune environment for fetal implantation and development [56]. A clinical study [57] has indicated that patients with RSA exhibit significantly lower levels of HLA-G expression, potentially leading to embryos being susceptible to maternal immune attack and increasing the risk of miscarriage. Conversely, STW could upregulate the HLA-G immune signaling pathway, promote the expression of seven isoforms of the HLA-G gene and its receptors Immunoglobulin-like Transcript 2 (ILT2) and Killer Cell Immunoglobulin-like Receptor, Two Ig Domains and Long Cytoplasmic Tail 4 (KIR2DL4), activate inhibitory signaling in metaphase NK cells, and induce immune tolerance at the maternal-fetal interface, thus effectively treating unexplained RSA [58].
4.5. MAPK signaling pathway
The Mitogen-Activated Protein Kinase (MAPK) signaling pathway plays an essential role in cellular signaling, governing cell growth, proliferation, differentiation, apoptosis, and stress responses [59]. In response to external stimuli such as growth factors, cellular stress, and hormones, MAPK translocates to the nucleus or cytoplasm and regulates cellular functions and physiological activities by phosphorylating downstream target proteins [60]. A study [61] indicates that in the endometrium of RSA patients, the expression levels of MAPK signaling pathway components may be abnormal, leading to altered proliferation, apoptosis, and immunoregulatory dysfunction of endometrial cells. This is particularly evident in the case of Extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and aberrant activation of MAPK family members such as p38 MAPK, which may play a significant role in RSA development. Additionally, the MAPK signaling pathway may be linked to the activation of inflammatory cells and the production of inflammatory factors, exacerbating the endometrium's inflammatory response and potentially leading to embryo implantation failure [62].
It was shown that STW could bi-directionally regulate the phosphorylation levels of MAPK pathway-related proteins, such as up-regulating the phosphorylation levels of Raf1, Ask1, Mek1, Mek2, and JKK1 proteins, and down-regulating the phosphorylation levels of MEK6 proteins. This regulation occurs through the up-regulation of the MAPK signaling pathway in CD4+ T cells within the decidual membrane and placental tissues of RSA mice. This, in turn, modulates cellular function and physiological activity, affecting endometrial cell activity, promoting embryo implantation, fostering fetal immune tolerance, and reducing the incidence of RSA [63]. Furthermore, a subsequent study [64] has shown that STW also regulates the activation of STAT3 and STAT6 signaling pathways via ERK and JNK pathways. It up-regulates the expression of inhibitory cytokine signaling protein 3, influencing the differentiation of Th1 cells towards Th2. Additionally, the primary compound components in the herbal formula of STW, specifically total flavonoids of Cuscuta chinensis and quercetin, activate the Notch/AKT/MAPK pathway in a time- and dose-dependent manner. These components down-regulate the phosphorylation of p-ERK, p-p38, and JNK, and enhance the activity of extrachorionic trophoblastic cells. This leads to an up-regulation of estradiol, progesterone, and prolactin hormone receptor levels, reduces oxidative stress damage, and promotes embryonic implantation [65].
In conclusion, aberrant activation of MAPK signaling pathway components in the endometrium may lead to impaired embryo implantation and abnormal immune tolerance, potentially triggering RSA. Conversely, STW may reduce the occurrence of RSA by regulating MAPK signaling pathway components and pathways such as ERK, JNK, and STAT3. This regulation affects Th cell differentiation and endometrial cell activity, thereby promoting embryo implantation and fetal immunotolerance.
4.6. SGK1/ENaC signaling pathway
Serum and Glucocorticoid Regulated Kinase 1 (SGK1) and Epithelial Sodium Channel (ENaC) are signaling components that play crucial roles in cellular physiology, particularly in maintaining electrolyte homeostasis, regulating cell volume, and facilitating ion transport. SGK1, a phosphorylation-regulated protein kinase, is typically modulated by hormones such as glucocorticoids and androgens [66]. ENaC, an ion channel protein complex, is predominantly found in various cell types, especially epithelial cells, and is critical in sodium ion uptake and efflux [67]. The activation of SGK1 leads to the phosphorylation of ENaC, thereby increasing its opening probability and enhancing cellular sodium ion uptake [68].
The SGK1/ENaC signaling pathway is implicated in embryo implantation and the immune environment of the endometrium during the development of RSA. Modulation of SGK1 activity could influence ENaC channels in endometrial cells, thereby affecting intra- and extracellular ion homeostasis and fluid transport. This modulation may impact the endometrium's immune environment, leading to immune dysregulation and impaired embryo implantation, ultimately triggering RSA [69]. An experimental study [70] has indicated that the protein and mRNA expression levels of SGK1, ENaC-a, estrogen receptor β, progesterone receptor, E-cadherin, Vimentin, prolactin receptor, and insulin-like growth factor-binding protein 1 were significantly decreased in the endometrial tissues of RSA patients. Conversely, treatment with Jiawei STW in an RSA mouse model markedly reversed the trend of reduced SGK1/ENaC signaling pathway and related protein expression. It facilitated endometrial proliferation and the EMT process, alleviated endometrial detachment and damage, and consequently decreased the rate of embryo loss while increasing litter size in mice [71].
However, the detailed mechanisms underlying the SGK1/ENaC signaling pathway's role in recurrent miscarriage requires further elucidation through more in-depth studies. Specifically, the precise mechanism by which STW treats recurrent miscarriage via the SGK1/ENaC signaling pathway needs to be comprehensively understood, encompassing both its beneficial and potential adverse effects. This might include the modulation of ion channel activity in endometrial cells, regulation of cell volume, and the corresponding immune and inflammatory responses. The major signaling pathways implicated in RSA treatment with STW are detailed in (Table 1).
Table 1.
Primary signaling pathways in the treatment of RSA with STW.
| pathways | Mechanism of action | Model | Reference |
|---|---|---|---|
| Nrf2/HO-1 | Nrf2: ↑, HO-1: ↑, NQO1: ↑, BCL-2: ↑, BAX: ↓, cystatinase-3: ↓ | HTR-8/Svneo cell; RSA model rats | [42,43] |
| JAK1/STAT3 | p-JAK: ↑, p-STAT3: ↑, Ki67: ↑, VEGF receptors: ↑, MMP9: ↑ MCM2: ↑, PCNA: ↑, ATG5: ↑, Beclin1: ↑, LC3B: ↑ | HTR-8/Svneo cell | [44,45] |
| NLRP3/Caspase-1/GSDMD | NLRP3: ↓, Caspase-1: ↓, GSDMD: ↓, IL-1: ↓, IL-1β: ↓, IL-18: ↓ | HTR-8/Svneo cell; RSA model mice | [46,47] |
| HLA-G | 7 subtypes of the HLA-G: ↑, ILT2: ↑, KIR2DL4: ↑ | NK92 cell | [52,53] |
| MAPK | p-Raf1: ↑,p-A-Raf: ↑, p-Ask1: ↑, p-Mek1: ↑, p-Mek2: ↑, p-JKK1: ↑, p-ERK1: ↑, p-ERK2: ↑, p-c-fos: ↑, p-c-Jun: ↑, p-CREB: ↑, p-MEK6: ↓, SOCS3: ↑, Th1→Th2; p-ERK: ↑, p-p38: ↑, p-JNK: ↑. | CD4+T cell; HTR-8/Svneo cell |
[[57], [58], [59]] |
| SGK1/ENaC | SGK1: ↑, ENaC-a: ↑, ERβ: ↑, PR: ↑, E-cadherin: ↑, Vimentin: ↑, PRLR: ↑, IGFBP-1: ↑ | RSA model mice | [62] |
5. Conclusion and future remarks
The efficacy of STW as a treatment for RSA is established, and there has been some progress in understanding its therapeutic mechanisms. However, comprehensive understanding of its signaling pathways necessitates further scientific researches to elaborate on the molecular regulatory mechanisms of STW in RSA treatment. Additionally, the exploration of STW's influence on regulatory mechanisms involving microRNAs, long non-coding RNAs, competitive endogenous RNAs, and histone modifications in RSA treatment is emerging as a significant research area. These investigations not only broaden the theoretical understanding of STW's role in RSA treatment but also offer novel insights for clinical application. The intricate interactions among various signaling pathways present substantial challenges in clarifying STW role in treating RSA. Nevertheless, with the utilization of contemporary medical research methodologies and advanced technologies, notable advances in understanding the molecular mechanisms of RSA treatment by STW are anticipated. This paper comprehensively reviews the pharmacological effects and primary pathways of STW in RSA treatment, revealing that its primary regulatory mechanism is intricately linked to the Nrf2/HO-1, JAK1/STAT3, NLRP3/Caspase-1, HLA-G, MAPK, and SGK1/ENaC signaling pathways. These insights furnish a critical theoretical foundation and direction for further investigation into STW's mechanism and the refinement of clinical treatment protocols.
Funding
This work was supported by National Natural Science Foundation of China: (Grant no. 82104730, 81974564); Zhongyuan Talent Plan--leading Talent Project for Scientific and Technological Innovation (No. 224200510027).
Data availability statement
Data availability is not applicable to this article as no new data were created or analyzed in this study.
CRediT authorship contribution statement
Xue Dang: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Investigation. Yanchen Feng: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Investigation. Pan Zheng: Writing – review & editing, Visualization, Investigation. Diyan Liu: Writing – review & editing, Visualization, Investigation. Yusupu Nuerbiye: Writing – original draft, Investigation. Ziyun Liao: Writing – review & editing, Methodology. Feixiang Liu: Writing – review & editing, Writing – original draft, Visualization, Supervision, Project administration, Investigation. Zhiying Che: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to express our sincere gratitude to Biorende for providing the platform for generating figures used in this research work.
Contributor Information
Feixiang Liu, Email: spiritofwest@126.com.
Zhiying Che, Email: chezy365@126.com.
References
- 1.ESHRE Guideline Group on RPL. Bender Atik R., Christiansen O.B., Elson J., Kolte A.M., Lewis S., Middeldorp S., Mcheik S., Peramo B., Quenby S., Nielsen H.S., van der Hoorn M.-L., Vermeulen N., Goddijn M. ESHRE guideline: recurrent pregnancy loss: an update in 2022. Hum Reprod Open. 2023;2023 doi: 10.1093/hropen/hoad002. hoad002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deng T., Liao X., Zhu S. Recent advances in treatment of recurrent spontaneous abortion. Obstet. Gynecol. Surv. 2022;77:355–366. doi: 10.1097/OGX.0000000000001033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karavani G., Alexandroni H., Sheinin D., Dior U.P., Simon A., Ben-Meir A., Reubinoff B. Endometrial thickness following early miscarriage in IVF patients - is there a preferred management approach? Reprod. Biol. Endocrinol. 2021;19:93. doi: 10.1186/s12958-021-00780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rai R., Regan L. Recurrent miscarriage. Lancet. 2006;368:601–611. doi: 10.1016/S0140-6736(06)69204-0. [DOI] [PubMed] [Google Scholar]
- 5.Kwak-Kim J., Yang K.M., Gilman-Sachs A. Recurrent pregnancy loss: a disease of inflammation and coagulation. J. Obstet. Gynaecol. Res. 2009;35:609–622. doi: 10.1111/j.1447-0756.2009.01079.x. [DOI] [PubMed] [Google Scholar]
- 6.Ndjapa-Ndamkou C., Govender L., Chauke L. Role of genetic factors in recurrent miscarriages - a review. Afr. J. Reprod. Health. 2022;26:72–82. doi: 10.29063/ajrh2022/v26i10.9. [DOI] [PubMed] [Google Scholar]
- 7.Jia L., Zhou J., Yu Q., Zhao Y., Jia L., Yan H. CiteSpace-based visualization of traditional Chinese medicine for recurrent miscarriages. Modern Chinese Medicine. 2023;43:6–13. doi: 10.13424/j.cnki.mtcm.2023.04.002. [DOI] [Google Scholar]
- 8.Dong X. Clinical observation of Shoutai Pill combined with Danggui Powder combined with low molecular weight heparin in the treatment of recurrent abortion thrombolism. J. North Pharmacy. 2021;18:19–20. [Google Scholar]
- 9.Chen Y., Ning Y., Hu S., Liao J., Huang S., Tian Y. Clinical observation on modified Shoutaiwan with Si Junzitang combined with didroxyprogesterone tablets in treating threatened abortion in advanced age patients. Chin. J. Exp. Tradit. Med. Formulae. 2020;26:71–75. doi: 10.13422/j.cnki.syfjx.20201979. [DOI] [Google Scholar]
- 10.Hou M. Nanjing University of Chinese Medicine; 2022. To Observe the Clinical Effect of Pregnancy Outcome by Using Shoutai Pills Combined with Anticoagulant Drugs in Thetreatment for Recurrent Spontaneous Abortion Due to Thrombophilia, M.S.Thesis. [DOI] [Google Scholar]
- 11.Song C., Zhang S., Gao X., Zhang H., Zuo S., Qin Y., Bi X., Chen H. Shoutai pills for threatened abortion: a protocol for systematic review and meta-analysis. Medicine (Baltim.) 2023;102 doi: 10.1097/MD.0000000000033173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raghupathy R., Szekeres-Bartho J. Progesterone: a unique hormone with immunomodulatory roles in pregnancy. Int. J. Mol. Sci. 2022;23:1333. doi: 10.3390/ijms23031333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng S. Effect analysis of estradiol tablets/didrogesterone tablets packaged in combination for the treatment of perimenopausal syndrome. Modern Med. Health Res. Electron. J. 2023;7:58–60. [Google Scholar]
- 14.Pluchino N., Drakopoulos P., Wenger J.M., Petignat P., Streuli I., Genazzani A.R. Hormonal causes of recurrent pregnancy loss (RPL) Hormones (Basel) 2014;13:314–322. doi: 10.14310/horm.2002.1505. [DOI] [PubMed] [Google Scholar]
- 15.Guo W., Chen Y. Effect of shoutai pills combined with dydrogesterone tablets in the treatment of threatened abortion. Chin. J. Drug Abuse Prevent. Treatment. 2023;29:1062–1066. doi: 10.15900/j.cnki.zylf1995.2023.06.036. [DOI] [Google Scholar]
- 16.Ding C., Zuo J., Shi H. Clinical efficacy of jiao ai tang combined with modified shoutai pill in the treatment of threatened miscarriage with kidney deficiency and blood stasis. Chin. J. Fam. Plan. 2022;30:2744–2748. [Google Scholar]
- 17.Xia R., Li Y., Song Y. Clinical observation of modified shoutai pills combined with progesterone and low-molecular-weight heparin in the treatment of recurrent abortion. World J. Integrated Traditional and Western Med. 2023;18:796–800+805. doi: 10.13935/j.cnki.sjzx.230433. [DOI] [Google Scholar]
- 18.Kalluri R., Weinberg R.A. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gauster M., Moser G., Wernitznig S., Kupper N., Huppertz B. Early human trophoblast development: from morphology to function. Cell. Mol. Life Sci. 2022;79:345. doi: 10.1007/s00018-022-04377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.E Davies J., Pollheimer J., Yong H.E.J., Kokkinos M.I., Kalionis B., Knöfler M., Murthi P. Epithelial-mesenchymal transition during extravillous trophoblast differentiation. Cell Adhes. Migrat. 2016;10:310–321. doi: 10.1080/19336918.2016.1170258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y., Liu X., Wang J., Liu Y., Teng H. Effects of shoutai pil containing serum on bioactivity behavior of trophoblast cells of spontaneous abortion patients. Chin. J. Integrated Tradit. West Med. 2016;36:586–591. [PubMed] [Google Scholar]
- 22.Tang S., Liang X., Li R., Su Z., Liu X., Du H., Duan Y. Effects of Shoutai Pills on EMT of embryonic trophoblasts in recurrent spontaneous abortion mice. J. Beijing Univ. Traditional Chin. Med. 2023;46:1139–1149. [Google Scholar]
- 23.Ji L., Brkić J., Liu M., Fu G., Peng C., Wang Y.-L. Placental trophoblast cell differentiation: physiological regulation and pathological relevance to preeclampsia. Mol. Aspect. Med. 2013;34:981–1023. doi: 10.1016/j.mam.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Zhao H., Wong R.J., Stevenson D.K. The impact of hypoxia in early pregnancy on placental cells. Int. J. Mol. Sci. 2021;22:9675. doi: 10.3390/ijms22189675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu X., Ye X., Zhu M., Zhang Q., Li X., Yan J. FtMt reduces oxidative stress-induced trophoblast cell dysfunction via the HIF-1α/VEGF signaling pathway. BMC Pregnancy Childbirth. 2023;23:131. doi: 10.1186/s12884-023-05448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu J. Hubei University of Chinese Medicine; 2016. Study on the Intervention Mechanism of Bushen Antai Chongji on Vascular Remodeling at the Maternal-Fetal Interface in RSA Mice. Ph.D.dissertation. [Google Scholar]
- 27.Hou A., Wang X., Zhang Y., Liu M., Liang X., Chu J. Effects of bushen antai granules medicated serum on the function of placental trophoblast and the expression of VEGF and its receptor in recurrent spontaneous abortion mice. Chin. J. Inf. Tradit. Chin. Med. 2023;30:69–73. doi: 10.19879/j.cnki.1005-5304.202206111. [DOI] [Google Scholar]
- 28.Li D., Zheng L., Zhao D., Xu Y., Wang Y. The role of immune cells in recurrent spontaneous abortion. Reprod. Sci. 2021;28:3303–3315. doi: 10.1007/s43032-021-00599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia D., Zhang Y., Chen L., Feng X. Research progress on the correlation between Th1/Th2 and Th17/treg patterns and recurrent spontaneous abortion. Curr. Immunol. 2021;41:244–248. [Google Scholar]
- 30.Agostinis C., Balduit A., Mangogna A., Zito G., Romano F., Ricci G., Kishore U., Bulla R. Immunological basis of the endometriosis: the complement system as a potential therapeutic target. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.599117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang L., Mao B., Liu Q., Wang Y., Li J., Dai Z., Li Y. Interaction effect between IL-6 polymorphisms and environmental factors on risk of unexplained recurrent spontaneous abortion. Chin. J. Immunol. 2022;38:2640–2645. [Google Scholar]
- 32.Zhang J., Chen L., Zheng C.-H., Wang J., Xie D., Zhou Y.-X. Effect of shoutai pills on Th1/Th2 cytokines in serum and endometrium of rats with stimulated ovulation. Curr Med Sci. 2019;39:285–290. doi: 10.1007/s11596-019-2032-4. [DOI] [PubMed] [Google Scholar]
- 33.Piccinni M.-P., Raghupathy R., Saito S., Szekeres-Bartho J. Cytokines, hormones and cellular regulatory mechanisms favoring successful reproduction. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.717808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai M., You Z., Ma H., Lei L., Lu F., He D., Liu H., Yin S. Effects of shoutai pills on expression of Th1/Th2 cytokine in maternal-fetal interface and pregnancy outcome. Zhongguo Zhongyao Zazhi. 2010;35:3065–3068. [PubMed] [Google Scholar]
- 35.Jin M., Chuan J., Shen Y., Fu P. Effects of Shoutai pills on immune function and oxidative stress in pregnant rats with di(2-ethylhexyl) phthalate exposure. Nan Fang Yi Ke Da Xue Xue Bao. 2020;40:850–855. doi: 10.12122/j.issn.1673-4254.2020.06.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang P., Cai D., Du L., Shen F., Zhang C., Li M. Relationship between polymorphism of thrombin-activatable fibrinolysis inhibitor gene +1040C/T and a cohort of Chinese women with recurrent spontaneous abortion. Clin. Appl. Thromb. Hemost. 2021;27 doi: 10.1177/10760296211029720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sucker C., Geisen C., Schmitt U., Zawislak B. Hypofibrinogenemia and miscarriage: report of a first successful pregnancy under fibrinogen substitution and short review of the literature. Arch Clin Cases. 2022;9:100–103. doi: 10.22551/2022.36.0903.10211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han C., Sun Z., Hu X., Han J. Effect of modified shoutaiwan on recurrent abortion due to prethrombotic status and effect on coagulation factors. Chin. J. Exp. Tradit. Med. Formulae. 2020;26:51–56. doi: 10.13422/j.cnki.syfjx.20201023. [DOI] [Google Scholar]
- 39.Tan Z. Ph.D.dissertation; 2012. Preliminary Study on the Effect of Shoutai Pill on the Proteome of Decidua in Mice with Recurrent Spontaneous Abortion. [Google Scholar]
- 40.S. Wang, L. Zhang, Y. Hao, M. Jiang, J. Lv, Y. Zhang, Z. Yu, M. Liu, H. Du, Effect of Shoutai Pill on Aerobic Glycolysis Inhibition in Decidual Tissue of Recurrent Spontaneous Abortion Mice, Chin. J. Integrated Tradit. West Med. 44 (20240228) 56–62. 10.7661/j.cjim.20230726.281. [DOI]
- 41.Liang X., Tang S., Li D., Song Y., He M., Duan Y., Du H. Shoutai wan improves embryo survival by regulating aerobic glycolysis of trophoblast cells in a mouse model of recurrent spontaneous abortion. Evid Based Complement Alternat Med. 2022;2022 doi: 10.1155/2022/8251503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L., Wang S., Ma Y., Song Y., Li D., Liang X., Hao Y., Jiang M., Lv J., Du H. Shoutai Wan regulates glycolysis imbalance at the maternal-fetal interface in threatened abortion mice. J. Ethnopharmacol. 2023;312 doi: 10.1016/j.jep.2023.116502. [DOI] [PubMed] [Google Scholar]
- 43.Loboda A., Damulewicz M., Pyza E., Jozkowicz A., Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016;73:3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu A., Zhao Y., Yu R., Zhou J., Tuo Y. Untargeted metabolomics analysis reveals the metabolic disturbances and exacerbation of oxidative stress in recurrent spontaneous abortion. PLoS One. 2023;18 doi: 10.1371/journal.pone.0296122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vomund S., Schäfer A., Parnham M.J., Brüne B., von Knethen A. Nrf2, the master regulator of anti-oxidative responses. Int. J. Mol. Sci. 2017;18:2772. doi: 10.3390/ijms18122772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen S., Mou Z., Tang L., Qiao Z., Lei L. Effects of shoutai pills on recurrent spontaneous abortion mice based on Nrf2/HO-1 signaling pathway. Chin. J. Inf. Tradit. Chin. Med. 2023;30:63–68. doi: 10.19879/j.cnki.1005-5304.202203816. [DOI] [Google Scholar]
- 47.Shen S., Mou Z., Tang L., Lei L. Shoutaiwan ameliorates oxidative damage of human chorionic trophoblast cells by regulating Nrf2 signaling pathway to treat recurrent abortion. Chin. J. Exp. Tradit. Med. Formulae. 2023;29:44–51. doi: 10.13422/j.cnki.syfjx.20222240. [DOI] [Google Scholar]
- 48.Xin P., Xu X., Deng C., Liu S., Wang Y., Zhou X., Ma H., Wei D., Sun S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharm. 2020;80 doi: 10.1016/j.intimp.2020.106210. [DOI] [PubMed] [Google Scholar]
- 49.Mou Z., Shen S., Tang L., Liu Y., Zhou Z., Lei L. Effects of shoutai pills-containing serum on the proliferation, invasion and migration of human chorionic trophoblast cells based on JAK1/STAT3 signaling pathway. Chin. J. Inf. Tradit. Chin. Med. 2023;30:84–90. doi: 10.19879/j.cnki.1005-5304.202204037. [DOI] [Google Scholar]
- 50.Mou Z., Shen S., Tang L., Liu Y., Zhou Z., Lei L. Effects of shoutai pills on autophagy in mice with recurrent spontaneous abortion based on JAK1/STAT3 pathway. Chin. J. Inf. Tradit. Chin. Med. 2023;30:87–93. doi: 10.19879/j.cnki.1005-5304.202207237. [DOI] [Google Scholar]
- 51.Li H., Sun N., Zhu Y., Wang W., Cai M., Luo X., Xia W., Quan S. Growth hormone inhibits the JAK/STAT3 pathway by regulating SOCS1 in endometrial cells in vitro: a clue to enhance endometrial receptivity in recurrent implantation failure. Eur. J. Histochem. 2023;67:3580. doi: 10.4081/ejh.2023.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blevins H.M., Xu Y., Biby S., Zhang S. The NLRP3 inflammasome pathway: a review of mechanisms and inhibitors for the treatment of inflammatory diseases. Front. Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.879021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qiao Z., Shen S., Deng D., Zhang S., Lei L. Shoutaiwan regulate lipopolysaccharide-induced oxidative stress and pyroptosis in human extravillous trophoblast cells. Chin. J. Exp. Tradit. Med. Formulae. 2022;28:17–24. doi: 10.13422/j.cnki.syfjx.20221637. [DOI] [Google Scholar]
- 54.Zhang S. Hunan university of chinese medicine; 2023. Study on the Intervention Effect of Shoutai Pill on Cell Pyroptosis in RSA Rat Model Based on the NLRP3/Caspase-1 Pathway, M.S.Thesis. [DOI] [Google Scholar]
- 55.Bu X., Zhong J., Li W., Cai S., Gao Y., Ping B. Immunomodulating functions of human leukocyte antigen-G and its role in graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Ann. Hematol. 2021;100:1391–1400. doi: 10.1007/s00277-021-04486-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu X., Zhou Y., Wei H. Roles of HLA-G in the maternal-fetal immune microenvironment. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.592010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marik B., Nomani K., Agarwal N., Dadhwal V., Sharma A. Role of the HLA-G regulatory region polymorphisms in idiopathic recurrent spontaneous abortions (RSA) Am. J. Reprod. Immunol. 2023;90 doi: 10.1111/aji.13740. [DOI] [PubMed] [Google Scholar]
- 58.Yan Q. The preliminary study of the effect and mechanism of showtaiwan play on HLA-G immune signaling pathway. Guangzhou University of Chinese Medicine, Ph.D.dissertation. 2015 [Google Scholar]
- 59.Ma Y., Nicolet J. Specificity models in MAPK cascade signaling. FEBS Open Bio. 2023;13:1177–1192. doi: 10.1002/2211-5463.13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo Y.-J., Pan W.-W., Liu S.-B., Shen Z.-F., Xu Y., Hu L.-L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020;19:1997–2007. doi: 10.3892/etm.2020.8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang J., Liu X., Gao Y. Abnormal H3K27 histone methylation of RASA1 gene leads to unexplained recurrent spontaneous abortion by regulating Ras-MAPK pathway in trophoblast cells. Mol. Biol. Rep. 2021;48:5109–5119. doi: 10.1007/s11033-021-06507-6. [DOI] [PubMed] [Google Scholar]
- 62.Santulli P., Marcellin L., Tosti C., Chouzenoux S., Cerles O., Borghese B., Batteux F., Chapron C. MAP kinases and the inflammatory signaling cascade as targets for the treatment of endometriosis? Expert Opin. Ther. Targets. 2015;19:1465–1483. doi: 10.1517/14728222.2015.1090974. [DOI] [PubMed] [Google Scholar]
- 63.You Z., He D., Liu H., Lai M., Lv F., Lei L. Effects of ShouTaiWan on the MAPK signal transduction pathway of CD4’T cells in maternal-fetal interface of mice with recurrent spontaneous abortion. Chin. Archiv. Traditional Chin. Med. 2010;28:459–461. doi: 10.13193/j.archtcm.2010.03.12.youzhl.031. [DOI] [Google Scholar]
- 64.He D. Hunan University of Chinese Medicine; 2011. Molecular Mechanism of Shoutaiwan Affectting the Outcome in Mouse Models of Recurrent Spontaneous Abortion, Ph.D.Dissertation. [Google Scholar]
- 65.Gao F., Zhou C., Qiu W., Wu H., Li J., Peng J., Qiu M., Liang C., Gao J., Luo S. Total flavonoids from Semen Cuscutae target MMP9 and promote invasion of EVT cells via Notch/AKT/MAPK signaling pathways. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-35732-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Q., Tian Y., Fu Z., Wu S., Lan H., Zhou X., Shen W., Lou Y. The role of serum-glucocorticoid regulated kinase 1 in reproductive viability: implications from prenatal programming and senescence. Mol. Biol. Rep. 2024;51:376. doi: 10.1007/s11033-024-09341-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rossier B.C., Pradervand S., Schild L., Hummler E. Epithelial sodium channel and the control of sodium balance: interaction between genetic and environmental factors. Annu. Rev. Physiol. 2002;64:877–897. doi: 10.1146/annurev.physiol.64.082101.143243. [DOI] [PubMed] [Google Scholar]
- 68.Lang F., Pearce D. Regulation of the epithelial Na+ channel by the mTORC2/SGK1 pathway. Nephrol. Dial. Transplant. 2016;31:200–205. doi: 10.1093/ndt/gfv270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fisher S.J., Giudice L.C. SGK1: a fine balancing act for human pregnancy. Nat. Med. 2011;17:1348–1349. doi: 10.1038/nm.2549. [DOI] [PubMed] [Google Scholar]
- 70.Di X., Hao Y., Duan Z., Ma Y., Cao Y., Tan Z., Song C., Lin X. Activation of SGK1/ENaC signaling pathway improves the level of decidualization in unexplained recurrent spontaneous abortion. Reprod. Sci. 2023;30:3273–3284. doi: 10.1007/s43032-023-01273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Di X., Duan Z., Ma Y., Song X., Hao Y., Li G., Tan Z., Lou Y., Lin X. Jiawei Shoutai Pill promotes decidualization by regulating the SGK1/ENaC pathway in recurrent spontaneous abortion. J. Ethnopharmacol. 2024;318 doi: 10.1016/j.jep.2023.116939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availability is not applicable to this article as no new data were created or analyzed in this study.