Abstract
The human T-cell leukemia virus type 1 (HTLV-1) transcriptional trans-activator Tax has been demonstrated to have transforming activity in multiple cell culture and transgenic-mouse models. In addition to activating transcription from the viral long terminal repeat (LTR) through the cyclic AMP response element binding protein/activating transcription factor (CREB/ATF) family of transcription factors, Tax activates the expression of multiple cellular promoters through the NF-κB pathway of transcriptional activation. The Tax mutants M22 and M47 have previously been demonstrated to selectively abrogate the ability of Tax to activate transcription through the NF-κB or CREB/ATF pathway, respectively. These mutations were introduced in the tax gene of the ACH functional molecular clone of HTLV-1, and virus produced from the mutant ACH clones was examined for the ability to replicate and immortalize primary human lymphocytes. While virus derived from the clone containing the M47 mutation retained the ability to immortalize T lymphocytes, the M22 mutant lost the ability to immortalize infected cells. These results indicate that activation of the CREB/ATF pathway by Tax is dispensable for the immortalization of T cells by HTLV-1, whereas activation of the NF-κB pathway may be critical.
The human T-cell leukemia virus type 1 (HTLV-1) infects and immortalizes T lymphocytes in vitro and is the causative agent of adult T-cell leukemia/lymphoma and HTLV-1-associated myelopathy/tropical spastic paraparesis in vivo (16, 22, 53, 57). The HTLV-1 genome encodes a 40-kDa transcriptional trans-activator known as Tax, which has been demonstrated to have transforming activity (2, 17, 43, 63, 70, 78). In addition to activating transcription from the viral long terminal repeat (LTR) (10, 13, 27, 66), Tax activates the expression of a number of cellular genes, many of which either encode proteins involved in the regulation of cellular proliferation (i.e., interleukin 2 [IL-2] [68], IL-2 receptor α chain [3, 25, 40, 68], and proliferating cell nuclear antigen [PCNA] [58]) or are proto-oncogenes (c-fos [14] and c-sis [75]). Furthermore, Tax alters the activity of a number of cell cycle regulators, including cyclin D (49), the mitotic checkpoint regulator MAD1 (30), the cyclin-dependent kinases (Cdk) Cdk4 and Cdk6 (65), the Cdk inhibitor p16INK4a (41, 72), and the tumor suppressor p53 (46, 55, 56). Thus, it is likely that Tax disregulates the cell cycle through many different mechanisms, leading to the eventual immortalization and transformation of the infected cell.
Tax activates transcription through a number of different transcription factors, including cyclic AMP response element binding protein/activating transcription factor (CREB/ATF) (4, 7, 8, 74, 80), NF-κB (3, 15, 24, 32, 44), p67 serum response factor (SRF) (14, 71), Sp1 (75), and NGFI-A/Egr-1 (75). Tax activates transcription through the CREB/ATF pathway by at least two distinct mechanisms. First, Tax binds CREB1 and increases the affinity of CREB1 for the three 21-bp repeats in the HTLV-1 LTR, which contain nonconsensus CREB response elements and are involved in Tax-activated and basal expression of the LTR (1, 5, 39, 80, 81). Second, Tax interacts with the CREB transcriptional coactivators CREB-binding protein (CBP) and p300, thereby recruiting them to the CREB–Tax–21-bp repeat complex (6, 20, 37). Likewise, Tax has been shown to interact with various members of the NF-κB family and their inhibitors, including p50, p100, and p105 (23, 47, 64). Recent evidence suggests that Tax also increases NF-κB activity by increasing the activity of mitogen-activated protein/extracellular signal-related kinase kinase 1 (MEKK1) and NF-κB-inducing kinase (NIK), which phosphorylate and activate the IκB kinases IKKα and IKKβ (15, 79). The IKK kinases then phosphorylate the NF-κB inhibitors IκBα and IκBβ, leading to IκB degradation and nuclear translocation of the active NF-κB subunits (44, 45).
A number of mutations in Tax have been described which selectively abrogate the ability of Tax to upregulate transcription through the CREB/ATF and NF-κB transcriptional activation pathways (67, 69, 78). Two of these mutations, termed M22 and M47, have been extensively characterized and were chosen for examination in this study (69). The M22 mutation is a double-amino-acid substitution of an alanine and serine for the threonine and leucine amino acids at positions 130 and 131, respectively. The M22 mutant is defective for NF-κB activation, but the mutation has only a minimal effect on CREB/ATF activity (69). Conversely, the M47 mutation is a substitution of arginine and serine for the two leucine amino acid residues at positions 319 and 320, and the mutant is defective for CREB/ATF activation while retaining the ability to activate NF-κB (69). In addition to M22 and M47, other Tax point mutations have been described which also selectively abrogate CREB/ATF (C29S, H52Q, L296G, and L320G) or NF-κB (C23S, S258A, and G148V) activation (67, 78).
The effects of these mutations on the ability of Tax to immortalize or transform various cell types has been examined, including established rat fibroblast cell lines and primary human peripheral blood mononuclear cells (PBMC) (2, 62, 70, 78). However, the results of these studies have been conflicting, and no overall consensus has been reached as to whether Tax activation of the CREB/ATF or NF-κB pathway is critical for cellular immortalization or transformation. In this study, we examined the effects of these mutations on the ability of an infectious molecular clone of HTLV-1 to immortalize primary human PBMC. These results indicate that while CREB/ATF activation is dispensable for cellular immortalization, NF-κB activation appears to be important. Furthermore, activation of CREB/ATF by Tax may be important in the preferential immortalization of CD4+ T cells by HTLV-1.
MATERIALS AND METHODS
Construction of ACH Tax mutants.
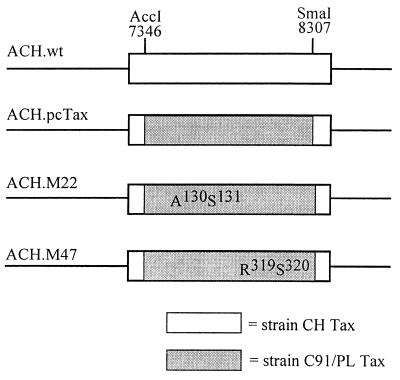
Construction of the ACH molecular clone of HTLV-1 has been previously described (34). Tax expression clones containing wild-type Tax (pcTax), the M22 mutation, and the M47 mutation were generous gifts from Warner C. Greene (61, 69). The Tax expression clones were digested with AccI and SmaI (New England Biolabs, Beverly, Mass.), and a 961-bp fragment encoding amino acids 18 to 336 of Tax was cloned into an AccI-SmaI-digested ACH subclone (ACHΔKpnI), which consists of nucleotides 6121 to 11813 of the ACH plasmid. A 2,344-bp NsiI-EcoRI fragment from ACHΔKpnI was then cloned into NsiI-EcoRI-digested ACH to generate ACH.pcTax, ACH.M22, and ACH.M47.
Viral particle production assay.
Human 293T kidney fibroblasts seeded to 30% confluence in 10-cm-diameter dishes were transfected with 10 μg of ACH.pcTax, ACH.M22, ACH.M47, or pBluescript KS (Stratagene, La Jolla, Calif.) (empty vector) with 30 μl of Lipofectamine (Life Technologies, Gaithersburg, Md.) in OPTI-MEM I (Life Technologies). After transfection, the cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum (FCS), 2 mM l-glutamine, 50 μg of penicillin/ml, and 50 U of streptomycin/ml. Cell culture supernatants were collected at various time points posttransfection, and virus particle production was monitored by p19 enzyme-linked immunosorbent assay (ELISA) (Cellular Products, Buffalo, N.Y.) according to the manufacturer’s instructions.
Transfection of PBMC.
Human PBMC were purified from healthy donors by Ficoll-Paque (Pharmacia, San Diego, Calif.) centrifugation and activated for 72 h with 10 μg of phytohemagglutinin-P (PHA) (Sigma, St. Louis, Mo.)/ml and 50 U of recombinant human IL-2/ml in RPMI 1640 medium supplemented with 10% FCS, 2 mM l-glutamine, 50 μg of penicillin/ml, and 50 U of streptomycin/ml (cRPMI). The cells were electroporated with 25 μg of ACH plasmid at 250 V, 1,800 μF capacitance, and 720 Ω resistance with an ECM 600 electroporation apparatus (BTX, San Diego, Calif.). The cells were then cultured in cRPMI supplemented with 5 μg of PHA/ml and 50 U of IL-2/ml for 6 weeks, after which they were cultured in the same medium without PHA. At various time points, cell culture supernatants were collected for p19 determination, and relative cellular viability was assayed by MTT conversion assays on 100-μl aliquots of cells as described by Hansen et al. (19).
PCR amplification of Tax.
Genomic DNA was purified from ACH.pcTax- and ACH.M47-immortalized cell lines by the Wizard Prep genomic DNA purification system (Promega, Madison, Wis.) according to the manufacturer’s instructions. PCR (95°C for 1 min; 60°C for 2 min; 72°C for 3 min; 35 cycles) was performed on 200 ng of genomic DNA with the following primers: 5′-CGGAATTCATGGCCCACTTCCCAGGGTTTGG-3′ and 5′-CGGGATCCCTAGTCACTTAGACTTCTGTTTCTCGGAAATG-3′, which amplifies the entire Tax open reading frame (ORF). The PCR product was then digested with BglII to assess the presence of the M47 mutation. Alternatively, the PCR product was digested with AccI and XmaI and cloned into AccI-XmaI-digested Tax expression plasmid pIEX (kindly provided by O. John Semmes). For activity determination, 5 μg of each Tax expression clone was cotransfected with 1 μg of HTLV LTR-luciferase in 293T cells with Lipofectamine. The cells were harvested 24 h posttransfection and lysed by repeated freeze-thaw (four times) in 0.25 M Tris (pH 7.8), and luciferase activity was measured with an OPTOCOMP I luminometer (MGM Instruments, Hamden, Conn.).
Western immunoblotting for Tax in immortalized cell extracts.
Ten million cells were lysed by incubation at 4°C for 30 min in 500 μl of RIPA buffer (50 mM Tris, 150 mM NaCl, 0.1% sodium dodecyl sulfate, 1.0% Nonidet P-40, 0.5% sodium deoxycholate, 0.5 mM phenylmethylsulfonyl fluoride, 1 μg of leupeptin/ml, 5 μg of pepstatin A/ml, and 8 μg of aprotinin [Sigma, St. Louis Mo.]/ml). Cellular debris was removed by centrifugation at 8,000 × g for 20 min, and 100 μl of cleared extract was electrophoresed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After transfer to a nitrocellulose membrane, the blot was sequentially probed with pooled anti-Tax monoclonal antibodies (no. 168A51-2, 168A51-42, and 168B17-46-34; AIDS Reagent Program, Rockville, Md.) (38) and anti-mouse immunoglobulin G-horseradish peroxidase-conjugated antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.), and Tax protein was detected by chemiluminescence.
Transfection of immortalized cell lines.
Twenty million ACH.pcTax- or ACH.M47-immortalized cells or PHA-IL-2-stimulated PBMC were transfected by electroporation (250V; 1,800 μF; 720 Ω) with 20 μg of HTLV LTR-luciferase and 5 μg of a cytomegalovirus (CMV) enhancer-chloramphenicol acetyltransferase (CAT) expression plasmid. The cells were harvested 24 h posttransfection, and luciferase activity was measured in 30 μg of whole-cell extract as described above. Transfection efficiency was normalized by measurement of CAT activity in 3 μg of cell extracts. CAT activity was determined by phase extraction of butyrated chloramphenicol with xylenes (35).
Infection of PBMC and microtiter infectivity assay.
For the viral replication assay, 5 × 105 PCTAX-2 and M47-3 cells were lethally gamma irradiated (6,000 rads) and cocultured with 5 × 106 PBMC activated with PHA and IL-2. The cells were cultured in cRPMI supplemented with 50 U of IL-2/ml, and supernatants were collected at various time points and assayed for virus particles by p19 ELISA. The microtiter infectivity assays were performed as previously described by Persaud et al. (54). In brief, 104 PHAIL-2-activated PBMC were cocultured with either 103, 102, or 10 lethally irradiated (6,000 rads) PCTAX-2, PCTAX-3, M47-2, or M47-3 cells in replicates of 20 at each dilution in 96-well microtiter plates. The cells were cultured in cRPMI with 50 U of IL-2/ml were split 1:2 approximately once per week. At 8 to 10 weeks postcoculture, individual wells were examined microscopically for the presence of viable cells.
FACS analysis of immortalized cell lines.
One million ACH.pcTax- or ACH.M47-immortalized cells were washed in fluorescence-activated cell sorter (FACS) staining buffer (1× phosphate-buffered saline, 10% FCS, 0.05% sodium azide) and incubated in the same buffer for 30 min on ice with mouse anti-human CD3-phycoerytherin (PE), anti-CD25-PE, anti-CD4-fluorescein isothiocyanate, or anti CD8-PE (Pharmingen). The stained cells were washed twice in the staining buffer, fixed in 1% paraformaldehyde–phosphate-buffered saline, and analyzed on a FACSCalibur flow cytometer (Becton-Dickinson, San Jose, Calif.).
RESULTS
Construction and activity of ACH Tax mutants.
A molecular clone of HTLV-1 known as ACH, which produces infectious viral particles capable of immortalizing primary human lymphocytes and establishing productive infections in rabbits, has been previously described (11, 34). The ACH Tax mutant clones were constructed as described in Materials and Methods and are diagrammed in Fig. 1. The resulting ACH Tax mutant clones contained the tax gene from HTLV-1 C91/PL between nucleotides 7346 and 8307, which differs from the CH strain in the ACH clone by 5 amino acid codons. As the first 17 and the last 7 amino acids are identical in the CH and C91/PL strains, the resulting tax gene in the ACH clones is identical to C91/PL tax. Since the C91/PL and CH strains differ by 5 amino acids, we also constructed a positive control clone which contained wild-type C91/PL tax (ACH.pcTax). The CREB/ATF- and NF-κB-activating activities of the M22 and M47 mutants have been previously examined in a number of cell lines and in a variety of expression systems (2, 69). However, we wanted to confirm that these mutants had the expected activities with respect to CREB and NF-κB activation when expressed from the ACH clone. The ACH.pcTax, ACH.M22, and ACH.M47 clones were cotransfected with either an HTLV LTR-luciferase reporter construct or human immunodeficiency virus (HIV) LTR-CAT reporter construct in human 293T kidney fibroblasts, and luciferase or CAT activity was measured (Table 1). As expected, the ACH.M47 clone expressed a Tax protein which failed to transactivate the HTLV LTR-luciferase construct, which is dependent on CREB, but had wild-type activity for the HIV LTR-CAT reporter plasmid, which is activated by NF-κB. Conversely, the ACH.M22 clone expressed a Tax protein which activated the HTLV LTR to near-wild-type levels but did not activate the HIV LTR. Therefore, when expressed from the ACH clone, the M22 and M47 Tax mutants have the originally described activity with respect to CREB and NF-κB activation.
FIG. 1.
Construction of ACH Tax mutants. The ACH.pcTax, ACH.M22, and ACH.M47 constructs used in this study contain the tax gene of the HTLV-1 strain C91/PL between the AccI site at position 7346 and the SmaI site at position 8307 in the ACH molecular clone. The M22 mutation results in a Thr130Leu131-to-Ala130Ser131 substitution, while the M47 mutation is a Leu319Leu320-to-Arg319Ser320 substitution.
TABLE 1.
Activity of Tax mutants and summary of immortalization assays
| Clone | Activity (%)a
|
No. of immortalized cultures/no. of transfections (no. of donor PBMC) | |
|---|---|---|---|
| HTLV LTR-luciferase | HIV LTR-CAT | ||
| ACH.wt | ND | ND | 4/5 (5) |
| ACH.pcTax | 100 | 100 | 5/6 (4) |
| ACH.M22 | 80 | 7 | 0/6 (4) |
| ACH.M47 | 2 | 138 | 5/5 (4) |
| Empty vector | 0 | 0 | 0/8 (7) |
Luciferase or CAT activity above empty vector cotransfection normalized to 100% for the wild-type ACH.pcTax clone. A 100% activity corresponds to an approximately 20-fold increase in luciferase activity for HTLV LTR-luciferase and a 2-fold increase in CAT activity for HIV LTR-CAT. ND, not done.
Viral particle production.
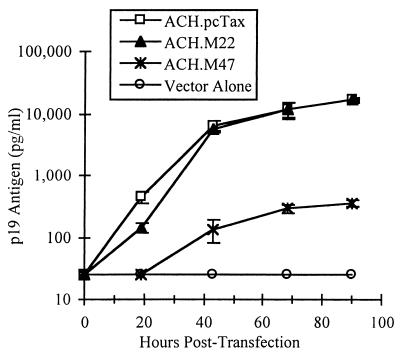
To assess the abilities of the ACH.pcTax, ACH.M22, and ACH.M47 clones to produce viral particles, 293T cells were transfected with 10 μg of each clone and viral particle production was assayed by p19 antigen ELISA approximately every 24 h posttransfection (Fig. 2). The ACH.pcTax and ACH.M22 clones were both capable of producing relatively high levels of viral particles, with up to 15,000 pg of p19 antigen/ml being detected in the culture supernatants at 90 h posttransfection. Therefore, the M22 mutation in the ACH clone does not appear to affect viral particle production, as would be expected from its ability to activate the HTLV LTR-luciferase construct efficiently. This result rules out any major defect in the Rex protein, a splicing regulator required for efficient gag expression (26, 27), whose ORF overlaps that of Tax and in which the M22 mutation also changes two amino acids (Pro149Cys150 to Leu149Ala150). However, the ACH.M47 clone produced much lower amounts of virus in this transient transfection assay, with approximately 400 pg of p19/ml being detected by 90 h posttransfection. This result was expected due to the defect in LTR activation by the M47 mutant.
FIG. 2.
Viral particle production by ACH Tax mutants. Human 293T cells were transfected with the ACH.pcTax, ACH.M22, and ACH.M47 clones or empty vector, and viral particle production was measured by p19 antigen ELISA. The error bars indicate the standard deviations of three replicate transfections. Whereas the ACH.M22 clone produces viral particles at amounts similar to wild-type levels, the ACH.M47 clone produces much lower levels of virus.
Immortalization of primary human lymphocytes.
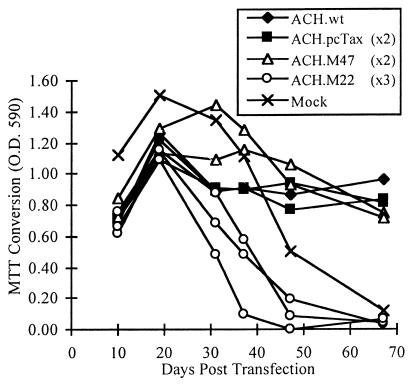
To test the ability of the ACH Tax mutant clones to induce immortalization, 20 million Ficoll-Paque-purified human PBMC were activated with 10 μg of PHA/ml and 50 U of IL-2/ml for 72 h and transfected by electroporation with the ACH.wt, ACH.pcTax, ACH.M47, and ACH.M22 plasmids. Cellular viability was monitored by MTT conversion assays. An example of one experiment, in which two separate transfections were done with ACH.pcTax and ACH.M47 and three transfections were done with ACH.M22, is shown in Fig. 3. As expected, the cells transfected with the ACH.wt and ACH.pcTax clones continued to proliferate indefinitely while the mock-transfected cells proliferated transiently and died by approximately 60 days posttransfection. Interestingly, like the mock-transfected cells, the ACH.M22-transfected cells also ceased to proliferate. Surprisingly, the ACH.M47-transfected cells also became immortalized, despite the fact that virus produced by this clone is not predicted to replicate as efficiently as ACH.wt- or ACH.pcTax-derived virus. PBMC from multiple donors were likewise transfected and monitored, and the results are summarized in Table 1. These results indicate that while CREB/ATF activation activity appears to be dispensable for immortalization of primary PBMC, NF-κB activation by Tax may be important for cellular immortalization.
FIG. 3.
Immortalization of PBMC by ACH Tax mutants. PHA-IL-2-activated human PBMC were transfected by electroporation with ACH.wt (one replicate), ACH.pcTax (two replicates), ACH.M47 (two replicates), and ACH.M22 (three replicates), and cellular viability was monitored by MTT conversion assays. Like the clones containing wild-type Tax, the ACH.M47 clone retains immortalizing activity. However, the M22 mutant fails to immortalize the infected cells.
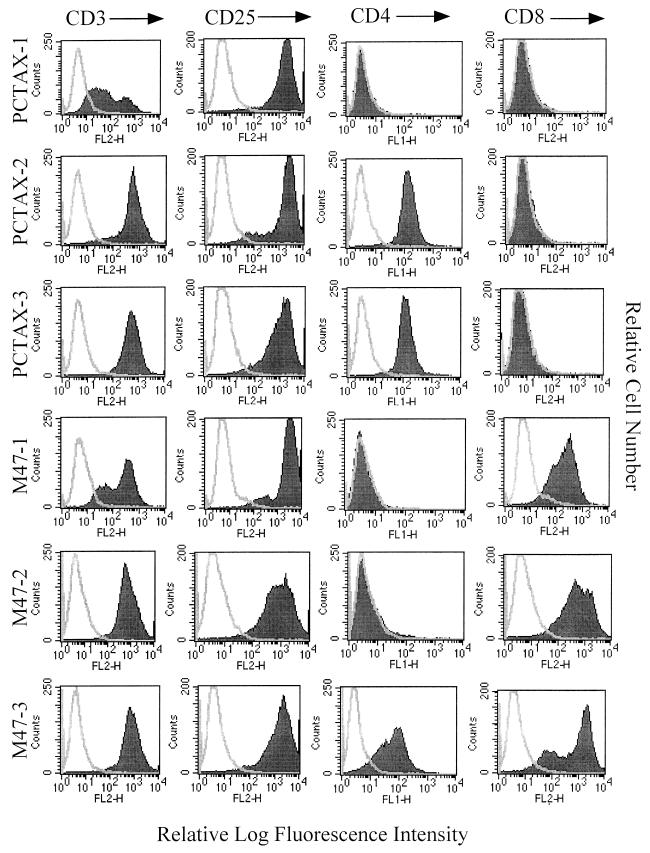
Three ACH.pcTax-immortalized cell lines and three ACH.M47-immortalized cell lines were chosen for further analysis and will be referred to as PCTAX-1, PCTAX-2, PCTAX-3, M47-1, M47-2, and M47-3. The PCTAX-1 and M47-1 cell lines were derived from PBMC from the same donor, whereas the PCTAX-2, PCTAX-3, M47-2, and M47-3 cell lines were derived from PBMC of a different donor. These cell lines were examined for cell surface marker expression by FACS analysis, and all were determined to be activated T cells based on the expression of CD3 and CD25 (Fig. 4). Expression of CD4 and CD8, however, was varied among the six cell lines. Two of the ACH.pcTax-immortalized cell lines were CD4+ CD8−, while the third was CD4− CD8−. In contrast, two of the ACH.M47 cell lines were CD4− CD8+, while one expressed both CD4 and CD8. To assess the pattern of proviral integration in the immortalized cells, Southern blot analysis was performed on BamHI-digested genomic DNA with a probe derived from an 8.5-kb SacI fragment of the HTLV-1 genome. This analysis revealed both clonal and oligoclonal patterns of proviral integration in the immortalized cells and a lack of pBluescript vector sequences from the ACH clone flanking the 3′ LTR (data not shown). Thus, integration of the HTLV-1 provirus occurred through the viral replication cycle and not through random integration of the transfected plasmid.
FIG. 4.
Cell surface phenotype of immortalized cell lines. ACH.pcTax- and ACH.M47-immortalized cells were stained with anti-human CD3, CD25, CD4, and CD8 and analyzed by flow cytometry. Although all cell lines express CD3 and CD25, expression of CD4 and CD8 is variable, with a tendency for CD4 expression by ACH.pcTax-transfected cells and CD8 expression by cells immortalized with the ACH.M47 clone. The open histogram on each plot corresponds to unstained cells, while the shaded histogram corresponds to cells stained with the indicated antibody.
Conservation of M47 mutation and lack of Tax activity in M47-immortalized cell lines.
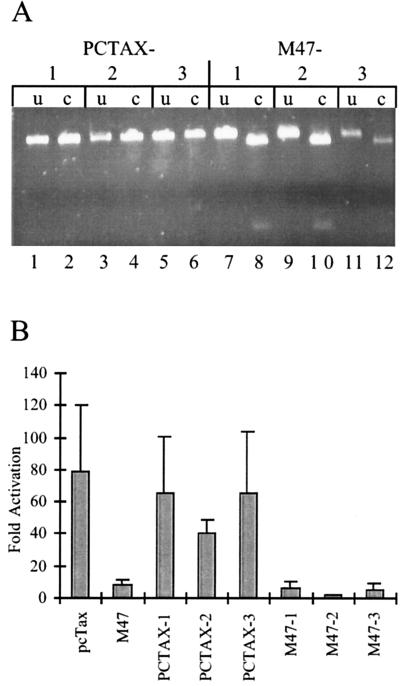
One potential explanation for the ability of the ACH.M47 mutant to immortalize primary PBMC despite being impaired for replication is that a reversion may have occurred in the tax gene which restored the ability of the M47 mutant Tax protein to activate the CREB/ATF pathway. To assess this possibility, the tax ORF was PCR amplified from proviral genomic DNA isolated from the three ACH.pcTax- and three ACH.M47-immortalized cultures. The PCR product was then digested with BglII, which is diagnostic for the presence of the M47 mutation. Whereas the three PCR products from the ACH.pcTax-immortalized cells were not cleaved with BglII, all three of the PCR products derived from the ACH.M47-immortalized cells were cut (Fig. 5A). This analysis rules out the possibility of a change at any of the 6 nucleotides which comprise the M47 mutation, as any change would have resulted in the loss of the BglII site. Additionally, the PCR products were cloned and sequenced and confirmed for the presence of the M47 mutation (data not shown).
FIG. 5.
Lack of reversion of M47 mutation. (A) The Tax ORF was amplified by PCR from genomic DNA and digested with the restriction endonuclease BglII. Whereas the PCR products from all three ACH.pcTax-immortalized cell lines lack the BglII site, the site is present in the Tax fragment amplified from the ACH.M47-immortalized cells. Lanes 1, 3, 5, 7, 9, and 11 are undigested (u), while lanes 2, 4, 6, 8, 10, and 12 are digested with BglII (c [cut]). (B) The PCR-amplified tax genes from ACH.pcTax- and ACH.M47-immortalized cells were cloned into a CMV expression vector and cotransfected with HTLV-LTR-luciferase reporter construct in 293T cells. As positive and negative controls, the wild-type and M47 mutant tax genes were amplified from the ACH.pcTax and ACH.M47 clones. The data are expressed as fold increase in luciferase activity relative to the HTLV LTR-luciferase construct transfected alone, and the error bars represent the standard deviations of four replicate transfections.
This analysis, however, does not rule out the possibility of the presence of a mutation elsewhere in the Tax protein which is somehow suppressing or compensating for the 2-amino-acid M47 mutation. To examine this possibility, the AccI-XmaI fragment containing the coding region for Tax amino acids 18 to 336 from the above-described PCR products were cloned into a CMV promoter-driven Tax expression clone (pIEX; provided by O. J. Semmes) and cotransfected with the HTLV LTR-luciferase reporter construct in 293T cells (Fig. 5B). Positive and negative control plasmids were likewise constructed by PCR amplifying and cloning the tax ORFs from the original ACH.pcTax and ACH.M47 clones. As expected, the control expression plasmids derived from the original ACH.pcTax and ACH.M47 clones had the expected activity with respect to HTLV LTR activation, with the pcTax clone activating the LTR approximately 30-fold and the M47 clone having <10% of the activity of the wild-type expression clone. Furthermore, the expression clones derived from the PCR-amplified proviral tax genes from the PCTAX-1, -2, and -3 and the M47-1, -2, and -3 cell lines also had the expected activity for activation of the HTLV LTR. Therefore, a mutation had not occurred elsewhere in the tax gene which had restored the ability of Tax to activate the CREB/ATF pathway.
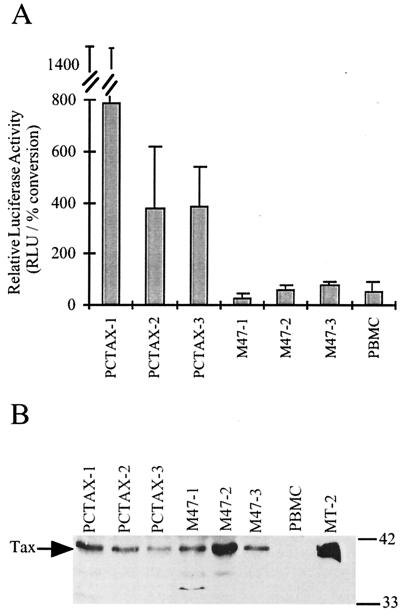
Although the above analysis rules out the possibility of a reversion in Tax, it does not address the possibility of a cellular mutation or alteration in cellular environment which results in an increase of CREB/ATF activity in the ACH.M47-immortalized cells. This could potentially be due to an increased level of CREB expression or CREB phosphorylation or an enhanced CREB-CBP–p300 interaction. Alternatively, the cells immortalized with the ACH.M47 mutant may be the result of an inadvertent selection for cellular clones which express abnormally high levels of Tax, thus compensating for the lack of CREB activation by the M47 mutant. To assess this possibility, the three ACH.pcTax- and ACH.M47-immortalized cell lines were directly transfected by electroporation with the HTLV LTR-luciferase reporter construct. The cells were additionally cotransfected with a CMV-CAT reporter to normalize for transfection efficiency. The cell lines immortalized with the ACH.pcTax clone all had higher HTLV-1 LTR activity than the ACH.M47-immortalized cell lines, which expressed levels of luciferase similar to those of PHA-IL-2-stimulated PBMC (Fig. 6A). Furthermore, Western immunoblot analysis indicated that while Tax expression varied among the cell lines, expression of Tax in the M47 cell lines was not consistently upregulated and was similar to the levels in the PCTAX cell lines (Fig. 6B). Finally, the PCTAX and M47 cell lines were examined for activated NF-κB activity by electrophoretic mobility shift assay analysis with a probe containing the NF-κB binding site from the IL-2Rα promoter. As expected, all PCTAX- and M47-immortalized cell lines had increased nuclear NF-κB compared to activated PBMC, indicating the expression of a functional Tax protein (data not shown). Therefore, these results indicate that the ACH.M47-immortalized cells do not have abnormally altered CREB/ATF activity or Tax expression which suppresses or otherwise compensates for the M47 mutation.
FIG. 6.
Activation of HTLV-1 LTR and Tax expression in immortalized cells. (A) ACH.pcTax- and ACH.M47-immortalized cells were transfected with 20 μg of HTLV LTR-luciferase and 5 μg of CMV-CAT. Luciferase activity was measured in 30 μg of whole-cell extract, and CAT activity was measured in 3 μg of extract. The data are expressed as relative light units per 1% conversion CAT activity. The error bars indicate the standard deviations of three replicate transfections. (B) Tax expression in whole-cell extracts prepared from PCTAX and M47 cell lines. For negative and positive controls. Tax expression was also examined in PHA-IL-2-stimulated PBMC and the HTLV-1-infected cell line MT-2.
Viral replication and microtiter infectivity assay.
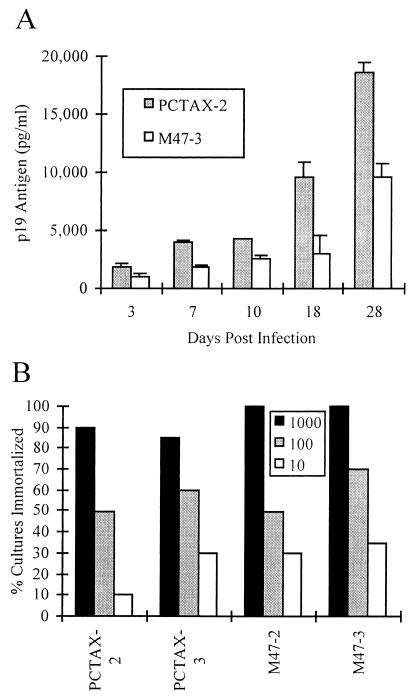
Despite being impaired for replication in the transient transfection assay (Fig. 2) and producing lower amounts of virus early in the transfection of PBMC (data not shown), the ACH.M47-immortalized PBMC produce virus at levels similar to ACH.pcTax-immortalized cells. We examined whether or not the virus being shed from the ACH.M47-immortalized cells was as infectious for activated PBMC as wild-type virus. One ACH.pcTax-immortalized cell line (PCTAX-2) and one ACH.M47-immortalized cell line (M47-3) were chosen that expressed very similar amounts of viral particles (approximately 500 pg/ml/106 cells/24 h). Furthermore, these two cell lines were both derived from the same donor, and both expressed CD4 on their surfaces (although M47-3 also expressed CD8). Therefore, PCTAX-2 and M47-3 are similar in many respects, except for the presence of wild-type or M47 mutant Tax in the integrated provirus. Five hundred thousand cells were lethally irradiated (6,000 rads) and cocultured with 5 × 106 PHA-IL-2-activated PBMC, and viral replication was monitored by p19 ELISA on cell culture supernatants. As expected, the virus from the ACH.M47-immortalized cells replicated less efficiently than the wild-type virus due to the inability of M47 Tax to efficiently transactivate the LTR (Fig. 7A).
FIG. 7.
Replication and immortalization by ACH.M47 virus. (A) Five hundred thousand PCTAX-2- or M47-3-immortalized cells were lethally gamma irradiated (6,000 rads) and cocultured with 5 × 106 PHA-activated uninfected PBMC. Viral replication was determined by p19 antigen ELISA. The standard deviation of two replicate infections is indicated by the error bars. (B) Results of microtiter infectivity-immortalization assay. Ten thousand PHA-IL-2-activated PBMC were cocultured with 103, 102, or 101 gamma-irradiated PCTAX-2-, PCTAX-3-, M47-2-, or M47-3-immortalized cells in replicates of 20 in 96-well plates. The cultures were examined at 8 weeks postcoculture for the presence of viable cells, and the number of cultures which were immortalized at each dilution was determined.
To further examine the replication and immortalizing activity of the ACH.M47 virus, a quantitative microtiter infectivity-immortalization assay was employed as described by Persaud et al. (54). In this assay, 104 PBMC were cocultured with 10-fold dilutions (103, 102, 10) of irradiated PCTAX-2, PCTAX-3, M47-2, or M47-3 cells in replicates of 20 in 96-well microtiter plates. The cultures were examined at 8 to 10 weeks postcoculture, and the percentage of cultures which became immortalized at each dilution of infected cells was quantitated (Fig. 7B). Despite being less able to replicate efficiently, the virus from the M47-2 and M47-3 cell lines infected and immortalized the uninfected PBMC at each dilution of infected cells at a rate similar to that of the PCTAX-2- and PCTAX-3-derived virus. Thus, there does not seem to be a quantitative difference in the immortalizing activity of the M47 mutant Tax.
Cell surface phenotype of immortalized cell lines.
Analysis of the cells immortalized by transfection of PBMC by the ACH.pcTax and ACH.M47 proviral clones revealed that while all cell lines were activated T cells, expression of CD4 and CD8 varied, with a slight bias for CD4 expression by ACH.pcTax-immortalized cells and for CD8 expression by ACH.M47-immortalized cells. To examine this phenomenon further, 12 ACH.pcTax and 12 ACH.M47 cell lines produced during the course of the microtiter infectivity assay were examined for cell surface marker expression (Table 2). As was observed for the ACH-transfected PBMC, the immortalized cell lines derived from infection with the M47-2- or M47-3-derived virus were more likely to be either CD8+ T cells or mixed cultures of CD4+ and CD8+ cells. Likewise, the cultures infected with the PCTAX-2- or PCTAX-3-derived virus were more likely to be CD4+ T lymphocytes. This suggests that activation of the CREB/ATF pathway by Tax may be important for the preferential immortalization of CD4+ cells compared to that of CD8+ cells by HTLV-1, despite the fact that the virus can infect both types of cells.
TABLE 2.
Cell surface phenotype of immortalized PBMC from transfections and infections
| Status and clone | No. of cultures with phenotype/total cultures
|
|||
|---|---|---|---|---|
| CD4− CD8− | CD4+ CD8−c | CD4− CD8+c | Mixed CD4+ CD8+ | |
| Transfecteda | ||||
| ACH.wt | 0/4 | 4/4 | 0/4 | 0/4 |
| ACH.pcTax | 1/4 | 3/4 | 0/4 | 0/4 |
| ACH.M47 | 0/5 | 1/5 | 2/5 | 2/5 |
| Infectedb | ||||
| PCTAX-2 | 0/6 | 6/6 | 0/6 | 0/6 |
| PCTAX-3 | 0/6 | 3/6 | 0/6 | 3/6 |
| M47-2 | 0/6 | 0/6 | 3/6 | 3/6 |
| M47-3 | 0/6 | 0/6 | 2/6 | 4/6 |
Immortalized cell cultures which were derived from electroporation of PBMC with ACH molecular clones. The tabulation includes the data shown in Fig. 4.
Immortalized cell cultures created from microtiter infectivity-immortalization assay.
Cultures were considered to be CD4+ CD8− or CD4− CD8+ if >90% of cells expressed CD4 or CD8, respectively.
DISCUSSION
The Tax protein of HTLV-1 has been demonstrated to have a number of activities which may all lead directly or indirectly to cellular immortalization and transformation of the infected cell. Tax interacts with the mitotic checkpoint protein MAD1, and this interaction leads to the loss of MAD1 function (30). Tax also interacts with Cdk4 and Cdk6 and increases their activities in primary human T cells (65). Tax has also been shown to interact with and inactivate the Cdk inhibitor p16INK4a (41, 72). Additionally, Tax functionally inactivates p53 through an increase in phosphorylation, although the mechanistic details are still unclear (46, 55, 56). Finally, expression of Tax leads to DNA damage, which may be associated with the transcriptional repression of DNA polymerase-β (29) and/or increased PCNA expression (58).
The ability of Tax to upregulate the expression of a variety of genes involved in cellular proliferation or cell cycle control has also been proposed as contributing to the ability of Tax to transform cells. Among the genes demonstrated to be upregulated by Tax are c-fos and the genes for PCNA, platelet-derived growth factor (c-sis), IL-2, and IL-2Rα (3, 14, 25, 40, 58, 68, 75). Additionally, Tax has been shown to repress the transcription of p53 (76) and bax (9), which may lead to an inhibition of apoptosis in the infected cell.
Tax has been demonstrated to have transforming activity in a number of cell culture systems (2, 17, 43, 62, 63, 70, 78). However, the relative importance of the activation of the CREB/ATF and NF-κB pathways for transforming activity is controversial. Studies examining the transformation of established rodent cell lines have produced conflicting results. Smith and Greene examined the ability of the M22 and M47 mutations to transform Rat2 fibroblasts and determined that the CREB pathway is important for transformation (i.e., M47 was defective for cellular transformation, while M22 retained transforming activity) (70). However, subsequently Yamaoka et al. and Matsumoto et al. have demonstrated that the NF-κB pathway appears to be important for the transformation of Rat1 fibroblasts (43, 78). Studies utilizing viral transduction systems to evaluate the ability of Tax mutants to immortalize primary human PBMC have also produced varied results. Rosin et al. demonstrated that a Tax point mutation defective for NF-κB induction (S258A) retains the capability to immortalize primary lymphocytes when transduced by a herpesvirus saimiri vector (62). On the contrary, Akagi et al. demonstrated that the M22 Tax mutation fails to immortalize PBMC when transduced by a retroviral expression vector (2). Finally, antisense oligonucleotides to NF-κB can inhibit the proliferation of Tax-transformed tumors from Tax-transgenic mice (36). The apparent discrepancies among these results are likely due to a number of factors, including differences in the experimental systems utilized (i.e., rodent fibroblasts versus human primary PBMC). Furthermore, subtle differences in the activities of the mutants used (NF-κB mutations M22, S258A, and G148V) and the levels of Tax expression may also account for differences in the observed phenotypes. None of these studies, however, examined the role of Tax in immortalization or transformation in the context of a replicating HTLV-1 virus in primary PBMC, which may be the closest model of what occurs in vivo.
Our results indicate that the activation of the NF-κB pathway by Tax is critical for the immortalization of PBMC by HTLV-1. Disregulated NF-κB activity has been demonstrated to be transforming and associated with other human cancers. The IκB family member bcl-3 is located at a site of chromosomal translocations in a specific type of B-cell leukemia (51). Likewise, the NF-κB family member p52 was identified in the cloning of a chromosomal translocation in a non-Hodgkin’s B-cell lymphoma (48). Additionally, the Epstein-Barr virus LMP-1 protein activates the NF-κB pathway by interaction with the tumor necrosis factor receptor (TNFR)-associated factors (TRAFs) and the TNFR-associated death domain protein (TRADD) through a pathway that involves NIK, IKKα, and IKKβ (18, 73). Furthermore, activation of NF-κB by LMP-1 through its interactions with the TRAFs and TRADD has been demonstrated to be important for its B-lymphocyte growth-transforming activity (12, 28).
As mentioned previously, the M22 Tax mutant has been demonstrated to lack NF-κB-activating activity in a variety of cell types, including Jurkat T cells (69) and primary human PBMC (2). Furthermore, it appears that M22 Tax fails to activate NF-κB by a defect in the activation of the IKK-activating kinases MEKK1 and NIK (15, 79). Although the effect of the M22 mutation on NF-κB activation is clear, other effects that the M22 mutation may have on other Tax functions is less well established. For example, Pise-Masison et al. have demonstrated that M22 mutant Tax is not able to functionally inactivate p53 as wild-type Tax is capable of doing (55). However, Mulloy et al. have shown that M22 retains the ability to inactivate p53 function (46). In addition, the M22 mutant Tax protein does not dimerize as efficiently as wild-type Tax (74), which may also influence various Tax activities, including HTLV LTR activation (1, 81). Therefore, we cannot rigorously exclude the possibility that the lack of immortalizing activity observed with the ACH.M22 clone may be due to a defect in Tax to interact with or activate or inactivate some additional factor. Examination of additional Tax mutants defective for NF-κB induction may help to conclusively define a role for NF-κB induction in HTLV-1-mediated cellular immortalization.
Our results also indicate that activation of the CREB/ATF pathway by Tax is not necessary for immortalization. This is somewhat surprising, as the ACH.M47-derived virus replicates to much lower levels than the wild-type ACH.pcTax-derived virus, due to the defect in CREB activation. However, the ACH.M47-immortalized cells produce viral particles to levels similar to those of ACH.pcTax-immortalized cells (data not shown). There are a number of possible explanations for this observation. The possibility exists that once the cells become immortalized, there are sufficient levels of CREB or other transcription factors to drive levels of viral gene expression that lead to relatively large amounts of viral particle production. Second, regulation of viral gene expression in the immortalized cell may be more complex than simple activation of the LTR by Tax, as a number of studies have identified suppressors of the LTR, possibly in the R and U5 regions (52, 77). In fact, expression of viral genes by the ACH clone appears to be restricted in lymphoid cell lines but not in primary PBMC (34). In addition, the fact that CREB activation is not necessary for immortalization may not be surprising, in that few cellular genes have been shown to be activated by Tax through the CREB/ATF pathway. However, though activation of the CREB/ATF pathway is dispensable for cellular immortalization (IL-2-dependent growth), our results do not rule out the possibility that Tax interaction with the CREB/ATF pathway is important for the emergence of fully transformed cells which proliferate independently of IL-2.
In vivo, CD4+ T cells represent the major infected cell type in asymptomatic individuals (59, 60), and the leukemic cells in patients with adult T-cell leukemia/lymphoma are CD4+ T cells in the majority of cases (21, 31, 33). In vitro, HTLV-1 can infect and immortalize CD4+ as well as CD8+ T cells, although immortalization and transformation of CD4+ cells is more common (42, 54). While the M47 mutant virus retains the ability to immortalize infected T cells, there appears to be a difference in the phenotype of the immortalized cells, as CD8+ cells become immortalized at a much higher frequency than that observed with the wild-type virus. Newbound et al. have demonstrated that the ability of Tax to activate the HTLV-1 LTR is greatly enhanced in CD4+ cells compared to CD8+ cells (50). Furthermore, it was proposed that this difference may account for the higher frequency of CD4+ HTLV-1-immortalized cells than CD8+ immortalized cells (50). This hypothesis is consistent with our findings. Since the virus with the M47 mutation does not efficiently transactivate the LTR, there would be no selective advantage for replication in CD4+ cells versus CD8+ cells. Therefore, one would expect an approximately equal frequency of CD4+ and CD8+ immortalized cells with the M47 mutant virus. Alternatively, activation of the CREB/ATF pathway may be important for increased proliferation and outgrowth of CD4+ cells, as was proposed by Akagi et al., who observed immortalization of CD4+ T cells by retrovirally transduced wild-type Tax and CD8+ cells by transduced M47 mutant Tax (2).
The results presented here support the possibility that NF-κB activation by Tax is important for the immortalization of primary T lymphocytes by HTLV-1. In addition, it appears that activation of the CREB/ATF pathway by Tax is dispensable for cellular immortalization. Examination of the immortalizing phenotypes of various Tax point mutants in a functional molecular clone of HTLV-1 likely mimics the infection of primary lymphocytes in vivo more closely than other cell culture models which have been used to study cellular transformation by HTLV-1. This system will be useful for the determination of immortalizing activity of additional Tax mutants defective for other activities of Tax which may contribute to the immortalization and transformation of infected cells, which will lead to a more complete understanding of Tax function and the pathogenesis of HTLV-1 infection.
ACKNOWLEDGMENTS
We thank Warner C. Greene for the gift of the M22 and M47 Tax mutant expression plasmids and Cetus for the gift of IL-2. We also thank Fen-Hwa Wong for construction of the ACH Tax mutant plasmids and Samuel R. Trejo, Toni Portis, and Nancy Vander-Heyden for helpful discussions and critical reviews of the manuscript.
This work was supported by PHS grant CA64317 and training grant GM07076 (M.D.R.).
REFERENCES
- 1.Adya N, Giam C-Z. Distinct regions in human T-cell lymphotropic virus type I Tax mediate interactions with activator protein CREB and basal transcription factors. J Virol. 1995;69:1834–1841. doi: 10.1128/jvi.69.3.1834-1841.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akagi T, Ono H, Nyunoya H, Shimotohno K. Characterization of peripheral blood T-lymphocytes transduced with HTLV-1 Tax mutants with different trans-activating phenotypes. Oncogene. 1997;14:2071–2080. doi: 10.1038/sj.onc.1201045. [DOI] [PubMed] [Google Scholar]
- 3.Ballard D W, Bohnlein E, Lowenthal J W, Wano Y, Franza B R, Greene W C. HTLV-1 tax induces cellular proteins that activate the κB element in the IL-2 receptor alpha gene. Science. 1988;241:1652–1655. doi: 10.1126/science.241.4873.1652. [DOI] [PubMed] [Google Scholar]
- 4.Bantignies F, Rousset R, Desbois C, Jalinot P. Genetic characterization of transactivation of the human T-cell leukemia virus type 1 promoter: binding of Tax to Tax-responsive element 1 is mediated by the cyclic AMP-responsive members of the CREB/ATF family of transcription factors. Mol Cell Biol. 1996;16:2174–2182. doi: 10.1128/mcb.16.5.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnhart M K, Connor L M, Marriott S J. Function of the human T-cell leukemia virus type 1 21-base-pair repeats in basal transcription. J Virol. 1997;71:337–344. doi: 10.1128/jvi.71.1.337-344.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bex F, Yin M J, Burney A, Gaynor R B. Differential transcriptional activation by human T-cell leukemia virus type 1 Tax mutants is mediated by distinct interactions with CREB binding protein and p300. Mol Cell Biol. 1998;18:2392–2405. doi: 10.1128/mcb.18.4.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodor J, Walker W, Flemington E, Spetz A-L, Habener J F. Modulation of Tax and PKA-mediated expression of HTLV-1 promoter via cAMP response element binding and modulator proteins CREB and CREM. FEBS Lett. 1995;377:413–418. doi: 10.1016/0014-5793(95)01299-0. [DOI] [PubMed] [Google Scholar]
- 8.Brauweiler A, Garl P, Franklin A A, Giebler H A, Nyborg J K. A molecular mechanism for human T-cell leukemia virus latency and Tax transactivation. J Biol Chem. 1995;270:12814–12822. doi: 10.1074/jbc.270.21.12814. [DOI] [PubMed] [Google Scholar]
- 9.Brauweiler A, Garrus J E, Reed J C, Nyborg J K. Repression of bax gene expression by the HTLV-1 Tax protein: implications for suppression of apoptosis in virally infected cells. Virology. 1997;231:135–140. doi: 10.1006/viro.1997.8509. [DOI] [PubMed] [Google Scholar]
- 10.Cann A J, Rosenblatt J D, Wachsman W, Shah N P, Chen I S Y. Identification of the gene responsible for human T-cell leukemia virus transcriptional regulation. Nature. 1985;318:571–574. doi: 10.1038/318571a0. [DOI] [PubMed] [Google Scholar]
- 11.Collins N D, Newbound G C, Ratner L, Lairmore M D. In vitro CD4+ lymphocyte transformation and infection in a rabbit model with a molecular clone of human T-cell leukemia virus type 1. J Virol. 1996;70:7241–7246. doi: 10.1128/jvi.70.10.7241-7246.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devergne O, Hatzivassiliou E, Izumi K M, Kaye K M, Kleijnen M F, Kieff E, Mosialos G. Association of TRAF1, TRAF2, and TRAF3 with an Epstein-Barr virus LMP-1 domain important for B-lymphocyte transformation: role in NF-κB activation. Mol Cell Biol. 1996;16:7098–7108. doi: 10.1128/mcb.16.12.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felber B K, Paskalis H, Kleinman-Ewing C, Wong-Staal F, Pavlakis G N. The pX protein of HTLV-I is a transcriptional transactivator of its long terminal repeats. Science. 1985;229:675–679. doi: 10.1126/science.2992082. [DOI] [PubMed] [Google Scholar]
- 14.Fujii M, Tsuchiya H, Chuhjo T, Akizawa T, Seiki M. Interaction of HTLV-1 Tax1 with p67SRF causes the aberrant induction of cellular immediate early genes through CArG boxes. Genes Dev. 1992;6:2066–2076. doi: 10.1101/gad.6.11.2066. [DOI] [PubMed] [Google Scholar]
- 15.Geleziunas R, Ferrell S, Lin X, Mu Y, Cunningham E T, Jr, Grant M, Connelly M A, Hambor J E, Marcu K B, Greene W C. Human T-cell leukemia virus type 1 Tax induction of NF-κB involves activation of the IκB kinase α (IKKα) and IKK β cellular kinases. Mol Cell Biol. 1998;18:5157–5165. doi: 10.1128/mcb.18.9.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gessain A, Barin F, Vernant J-C, Gout O, Maurs L, Calendar A, de The G. Antibodies to human T-lymphotropic virus type 1 in patients with tropical spastic paraparesis. Lancet. 1985;ii:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 17.Grassmann R, Dengler C, Muller-Fleckenstein I, Fleckenstein B, McGuire K, Dokhelar M-C, Sodroski J G, Haseltine W A. Transformation to continuous growth of primary human T lymphocytes by human T-cell leukemia virus type I X-region genes transduced by a herpesvirus saimiri vector. Proc Natl Acad Sci USA. 1989;86:3351–3355. doi: 10.1073/pnas.86.9.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammarskjold M L, Simurda M C. Epstein-Barr virus latent membrane protein transactivates the human immunodeficiency virus type 1 long terminal repeat through induction of NF-κB activity. J Virol. 1992;66:6496–6501. doi: 10.1128/jvi.66.11.6496-6501.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen M B, Nielsen S E, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 20.Harrod R, Tang Y, Nicot C, Lu H S, Vassilev A, Nakatani Y, Giam C Z. An exposed KID-like domain in human T-cell lymphotropic virus type 1 Tax is responsible for the recruitment of coactivators CBP/p300. Mol Cell Biol. 1998;18:5052–5061. doi: 10.1128/mcb.18.9.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hattori T, Uchiyama T, Toibana T, Takatsuki K, Uchino H. Surface phenotype of Japanese adult T-cell leukemia cells characterized by monoclonal antibodies. Blood. 1981;58:645–647. [PubMed] [Google Scholar]
- 22.Hinuma Y, Nagata K, Hanaoka M, Nakai M, Matsumoto T, Kinoshita K, Shirakawa S, Miyoshi I. Adult T-cell leukemia antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA. 1981;78:6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirai H, Fujisawa J, Suzuki T, Ueda K, Muramatsu M, Tsuboi A, Arai N, Yoshida M. Transcriptional activator Tax of HTLV-1 binds to the NF-κB precursor p105. Oncogene. 1992;7:1737–1742. [PubMed] [Google Scholar]
- 24.Hirai H, Suzuki T, Fujisawa J, Inoue J, Yoshida M. Tax protein of human T-cell leukemia virus type 1 binds to the ankyrin motifs of inhibitory factor κB and induces nuclear translocation of transcription factor NF-κB proteins for transcriptional activation. Proc Natl Acad Sci USA. 1994;91:3584–3588. doi: 10.1073/pnas.91.9.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoue J, Seiki M, Taniguchi T, Tsuru S, Yoshida M. Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type 1. EMBO J. 1986;5:2883–2888. doi: 10.1002/j.1460-2075.1986.tb04583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoue J, Seiki M, Yoshida M. The second pX product p27X-III of HTLV-1 is required for gag gene expression. FEBS Lett. 1986;209:187–190. doi: 10.1016/0014-5793(86)81108-5. [DOI] [PubMed] [Google Scholar]
- 27.Inoue J, Yoshida M, Seiki M. Transcriptional (p40X) and post transcriptional (p27X-III) regulators are required for the expression and replication of human T-cell leukemia virus type I. Proc Natl Acad Sci USA. 1987;84:3653–3657. doi: 10.1073/pnas.84.11.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izumi K M, Kieff E. The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-κB. Proc Natl Acad Sci USA. 1997;94:12592–12597. doi: 10.1073/pnas.94.23.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeang K T, Widen S G, Semmes O J, Wilson S H. HTLV-1 transactivator protein, Tax, is a trans-repressor of the human β-polymerase gene. Science. 1990;247:1082–1084. doi: 10.1126/science.2309119. [DOI] [PubMed] [Google Scholar]
- 30.Jin D-Y, Spencer F, Jeang K-T. Human T cell leukemia virus type 1 oncoprotein tax targets the human mitotic checkpoint protein MAD1. Cell. 1998;93:81–91. doi: 10.1016/s0092-8674(00)81148-4. [DOI] [PubMed] [Google Scholar]
- 31.Kamihira S, Sohda H, Atogami S, Toriya K, Yamada Y, Tsukazaki K, Momita S, Ikeda S, Kusano M, Amagasaki T, Kinoshita K-I, Tomonaga M. Phenotypic diversity and prognosis of adult T-cell leukemia. Leuk Res. 1992;16:435–441. doi: 10.1016/0145-2126(92)90168-7. [DOI] [PubMed] [Google Scholar]
- 32.Kanno T, Brown K, Siebenlist U. Evidence in support of a role for human T-cell leukemia virus type 1 Tax in activating NF-κB via stimulation of signaling pathways. J Biol Chem. 1995;270:11745–11748. doi: 10.1074/jbc.270.20.11745. [DOI] [PubMed] [Google Scholar]
- 33.Kikuchi M, Mitsui T, Takeshita M, Okamura H, Naitoh H, Eimoto T. Virus associated adult T-cell leukemia (ATL) in Japan: clinical, histological, and immunological studies. Hematol Oncol. 1984;4:67–81. doi: 10.1002/hon.2900040109. [DOI] [PubMed] [Google Scholar]
- 34.Kimata J T, Wong F H, Wang J J, Ratner L. Construction and characterization of infectious human T-cell leukemia virus type 1 molecular clones. Virology. 1994;204:656–664. doi: 10.1006/viro.1994.1581. [DOI] [PubMed] [Google Scholar]
- 35.Kingston R E, Sheen J. Phase extraction assay for CAT activity. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1995. pp. 9.7.5–9.7.11. [Google Scholar]
- 36.Kitajima I, Shinohara T, Bilakovics J, Brown D A, Xu X, Nerenberg M. Ablation of transplanted HTLV-1 Tax-transformed tumors in mice by antisense inhibition of NF-κB. Science. 1992;258:1792–1795. doi: 10.1126/science.1299224. [DOI] [PubMed] [Google Scholar]
- 37.Kwok R P S, Laurance M E, Lundblad J R, Goldman P S, Shih H-M, Connor L M, Marriott S J, Goodman R H. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the co-activator CBP. Nature. 1996;380:642–646. doi: 10.1038/380642a0. [DOI] [PubMed] [Google Scholar]
- 38.Langton B C, Sliwkowski M, Tran K V, Knapp S, Keitelman E, Smith C, Wallingford S, Liu H-L, Ralston J S, Brandis J, Coats S. Development and characterization of monoclonal antibodies to the HTLV-1 Tax (p40X) protein. Med Virol. 1988;8:295–302. [Google Scholar]
- 39.Lenzmeier B A, Giebler H A, Nyborg J K. Human T-cell leukemia virus type 1 Tax requires direct access to DNA for recruitment of CREB binding to the viral promoter. Mol Cell Biol. 1998;18:721–731. doi: 10.1128/mcb.18.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leung K, Nabel G J. HTLV-1 transactivator induces interleukin-2 receptor expression through an NF-κB-like factor. Nature. 1988;333:776–778. doi: 10.1038/333776a0. [DOI] [PubMed] [Google Scholar]
- 41.Low K G, Doener L F, Fernando D B, Grossman J, Jeang K-T, Comb M J. Human T-cell leukemia virus type 1 Tax releases cell cycle arrest induced by p16INK4a. J Virol. 1997;67:1956–1962. doi: 10.1128/jvi.71.3.1956-1962.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mann D L, Popovic M, Murray C, Neuland C, Strong D M, Sarin P, Gallo R C, Blattner W A. Cell surface antigen expression in newborn cord blood lymphocytes infected with HTLV-1. J Immunol. 1983;131:2021–2024. [PubMed] [Google Scholar]
- 43.Matsumoto K, Shibata H, Fujisawa J-I, Inoue H, Hakura A, Tsukahara T, Fujii M. Human T-cell leukemia virus type 1 Tax protein transforms rat fibroblasts via two distinct pathways. J Virol. 1997;71:4445–4451. doi: 10.1128/jvi.71.6.4445-4451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKinsey T A, Brockman J A, Scherer D C, Al-Murrani S W, Green P L, Ballard D W. Inactivation of IκBβ by the Tax protein of human T-cell leukemia virus type 1: a potential mechanism for constitutive induction of NF-κB. Mol Cell Biol. 1996;16:2083–2090. doi: 10.1128/mcb.16.5.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mercurio F, Zhu H, Murray B W, Shevchenko A, Bennett B L, Li J, Young D B, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 46.Mulloy J C, Kislyakova T, Cereseto A, Casareto L, LoMonico A, Fullen J, Lorenzi M V, Cara A, Nicot C, Giam C-Z, Franchini G. Human T-cell lymphotropic/leukemia virus type 1 Tax abrogates p53-induced cell cycle arrest and apoptosis through its CREB/ATF functional domain. J Virol. 1998;72:8852–8860. doi: 10.1128/jvi.72.11.8852-8860.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murakami T, Hirai H, Suzuki T, Fujisawa J-I, Yoshida M. HTLV-1 Tax enhances NF-κB2 expression and binds to the products p52 and p100, but does not suppress the inhibitory function of p100. Virology. 1995;206:1066–1074. doi: 10.1006/viro.1995.1029. [DOI] [PubMed] [Google Scholar]
- 48.Neri A, Chang C C, Lombardi L, Salina M, Corradini P, Maiolo A T, Chaganti R S, Dalla-Favera R. B cell lymphoma-associated chromosomal translocation involves candidate oncogene lyt-10, homologous to NF-κB, a rel-related polypeptide. Cell. 1991;67:1075–1087. doi: 10.1016/0092-8674(91)90285-7. [DOI] [PubMed] [Google Scholar]
- 49.Neuveut C, Low K G, Maldarelli F, Schmitt I, Majone F, Grassmann R, Jeang K T. Human T-cell leukemia virus type 1 Tax and cell cycle progression: role of cyclin D-cdk and p110Rb. Mol Cell Biol. 1998;18:3620–3632. doi: 10.1128/mcb.18.6.3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newbound G C, Andrews J M, O’Rourke J P, Brady J N, Lairmore M D. Human T-cell lymphotropic virus type 1 Tax mediates enhanced transcription in CD4+ T lymphocytes. J Virol. 1996;70:2101–2106. doi: 10.1128/jvi.70.4.2101-2106.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohno H, Takimoto G, McKeithan T W. The candidate proto-oncogene bcl-3 is related to genes implicated in cell lineage determination and cell cycle control. Cell. 1990;60:991–997. doi: 10.1016/0092-8674(90)90347-h. [DOI] [PubMed] [Google Scholar]
- 52.Okumura K, Tagaki S, Sakaguchi G, Naito K, Minoura-Tada N, Kobayashi H, Mimori T, Hinuma Y, Igarashi H. Autoantigen Ku protein is involved in DNA binding proteins which recognize the U5 repressive element of human T-cell leukemia virus type I long terminal repeat. FEBS Lett. 1994;356:94–100. doi: 10.1016/0014-5793(94)01243-1. [DOI] [PubMed] [Google Scholar]
- 53.Osame M, Usuku K, Izumo S, Ijichi N, Amitini H, Igata A. HTLV-I associated myelopathy: a new clinical entity. Lancet. 1986;i:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 54.Persaud D, Munoz J L, Tarsis S L, Parks E S, Parks W P. Time course and cytokine dependence of human T-cell lymphotropic virus type 1 T-lymphocyte transformation as revealed by a microtiter infectivity assay. J Virol. 1995;69:6297–6303. doi: 10.1128/jvi.69.10.6297-6303.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pise-Masison C A, Choi K-S, Radonovich M, Dittmer J, Kim S-J, Brady J N. Inhibition of p53 transactivation function by the human T-cell lymphotropic virus type 1 Tax protein. J Virol. 1998;72:1165–1170. doi: 10.1128/jvi.72.2.1165-1170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pise-Masison C A, Radonovich M, Sakaguchi K, Appella E, Brady J H. Phosphorylation of p53: a novel pathway for p53 inactivation in human T-cell lymphotropic virus type 1-transformed cells. J Virol. 1998;72:6348–6355. doi: 10.1128/jvi.72.8.6348-6355.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poiesz B J, Ruscetti F W, Gazdar A F, Bunn P A, Minna J D, Gallo R C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ressler S, Morris G F, Marriott S J. Human T-cell leukemia virus type 1 Tax transactivates the human proliferating cell nuclear antigen promoter. J Virol. 1997;71:1181–1190. doi: 10.1128/jvi.71.2.1181-1190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richardson J H, Edwards A J, Cruikshank J K, Rudge P, Dalgleish A G. In vivo cellular tropism of human T-cell leukemia virus type 1. J Virol. 1990;64:5682–5687. doi: 10.1128/jvi.64.11.5682-5687.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Richardson J H, Hollsberg P, Windhagen A, Child L A, Hafler D A, Lever A M L. Variable immortalizing potential and frequent virus latency in blood-derived T-cell clones infected with human T-cell leukemia virus type 1. Blood. 1997;89:3303–3314. [PubMed] [Google Scholar]
- 61.Rimsky L, Hauber J, Dukovich M, Malim M H, Langlois A, Cullen B R, Greene W C. Functional replacement of the HIV-1 rev protein by the HTLV-1 rex protein. Nature. 1988;335:738–740. doi: 10.1038/335738a0. [DOI] [PubMed] [Google Scholar]
- 62.Rosin R, Koch C, Schmitt I, Semmes O J, Jeang K-T, Grassmann R. A human T-cell leukemia virus Tax variant incapable of activating NF-κB retains its immortalizing potential for primary T-lymphocytes. J Biol Chem. 1998;273:6698–6703. doi: 10.1074/jbc.273.12.6698. [DOI] [PubMed] [Google Scholar]
- 63.Ross T M, Pettiford S M, Green P L. The tax gene of human T-cell leukemia virus type 2 is essential for transformation of human T lymphocytes. J Virol. 1996;70:5194–5202. doi: 10.1128/jvi.70.8.5194-5202.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rousset R, Desbois C, Bantignies F, Jalinot P. Effects on NF-κB1/p105 processing of the interaction between the HTLV-1 transactivator Tax and the proteosome. Nature. 1996;381:328–331. doi: 10.1038/381328a0. [DOI] [PubMed] [Google Scholar]
- 65.Schmitt I, Rosin O, Rohwer P, Gossen M, Grassmann R. Stimulation of cyclin-dependent kinase activity and G1- to S-phase transition in human lymphocytes by the human T-cell leukemia/lymphotropic virus type 1 Tax protein. J Virol. 1998;72:633–640. doi: 10.1128/jvi.72.1.633-640.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seiki M, Inoue J, Takeda T, Yoshida M. Direct evidence that the p40x of human T-cell leukemia virus type-1 is a trans-activating transcriptional transactivator. EMBO J. 1986;5:561–565. doi: 10.1002/j.1460-2075.1986.tb04247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Semmes O J, Jeang K-T. Mutational analysis of human T-cell leukemia virus type 1 Tax: regions necessary for function determined with 47 mutant proteins. J Virol. 1992;66:7183–7192. doi: 10.1128/jvi.66.12.7183-7192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siekevitz M, Feinberg M, Holbrook N, Wong-Staal F, Greene W C. Activation of interleukin 2 and interleukin 2-receptor (tac) promoter expression by the transactivator (tat) gene product of human T-cell leukemia virus type 1. Proc Natl Acad Sci USA. 1987;84:5389–5393. doi: 10.1073/pnas.84.15.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith M R, Greene W C. Identification of HTLV-1 tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1990;4:1875–1885. doi: 10.1101/gad.4.11.1875. [DOI] [PubMed] [Google Scholar]
- 70.Smith M R, Greene W C. Type 1 human T cell leukemia virus Tax protein transforms rat fibroblasts through the cyclic adenosine monophosphate response element binding protein/activating transcription factor pathway. J Clin Investig. 1991;88:1038–1042. doi: 10.1172/JCI115364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suzuki T, Hirai H, Fujisawa J, Fujita T, Yoshida M. A transactivator Tax of human T-cell leukemia virus type 1 binds to NF-κB p50 and serum response factor (SRF) and associates with enhancer DNAs of the NF-κB site and CArG box. Oncogene. 1993;8:2391–2397. [PubMed] [Google Scholar]
- 72.Suzuki T, Kitao S, Matsushime H, Yoshida M. HTLV-1 Tax protein interacts with cyclin-dependent kinase inhibitor p16INK4a and counteracts its inhibitory activity towards cdk4. EMBO J. 1996;15:1607–1614. [PMC free article] [PubMed] [Google Scholar]
- 73.Sylla B S, Hung S C, Davidson D M, Hatzivassiliou E, Malinin N L, Wallach D, Gilmore T D, Kieff E, Mosialos G. Epstein-Barr virus-transforming protein latent membrane protein 1 activates transcription factor NF-κB through a pathway that includes the NF-κB-inducing kinase and the IκB kinases IKKα and IKKβ. Proc Natl Acad Sci USA. 1998;95:10106–10111. doi: 10.1073/pnas.95.17.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tie F, Adya N, Greene W C, Giam C-Z. Interaction of the human T-lymphotropic virus type 1 Tax dimer with CREB and the viral 21-base-pair repeat. J Virol. 1996;70:8368–8374. doi: 10.1128/jvi.70.12.8368-8374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Trejo S R, Fahl W E, Ratner L. The Tax protein of human T-cell leukemia virus type 1 mediates the transactivation of the c-sis/platelet-derived growth factor-B promoter through interactions with the zinc finger transcription factors Sp1 and NGFI-A/Egr-1. J Biol Chem. 1997;272:27411–27421. doi: 10.1074/jbc.272.43.27411. [DOI] [PubMed] [Google Scholar]
- 76.Uittenbogaard M N, Giebler H A, Reisman D, Nyborg J K. Transcriptional repression of p53 by human T-cell leukemia virus type 1 Tax protein. J Biol Chem. 1995;270:28503–28506. doi: 10.1074/jbc.270.48.28503. [DOI] [PubMed] [Google Scholar]
- 77.Xiao X, Brown D A, Kitajima I, Bilakovics J, Fey L W, Nerenberg M I. Transcriptional suppression of the human T-cell leukemia virus type I long terminal repeat occurs by an unconventional interaction of a CREB factor with the R region. Mol Cell Biol. 1994;14:5371–5383. doi: 10.1128/mcb.14.8.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamaoka S, Inoue H, Sakurai M, Sugiyama T, Hazama M, Yamada T, Hatanaka M. Constitutive activation of NF-κB is essential for transformation of rat fibroblasts by the human T-cell leukemia virus type 1 Tax protein. EMBO J. 1996;15:873–887. [PMC free article] [PubMed] [Google Scholar]
- 79.Yin M-J, Christerson L B, Yamamoto Y, Kwak Y-T, Xu S, Mercurio F, Barbosa M, Cobb M H, Gaynor R B. HTLV-1 Tax protein binds to MEKK1 to stimulate IκB kinase activity and NF-κB activation. Cell. 1998;93:875–884. doi: 10.1016/s0092-8674(00)81447-6. [DOI] [PubMed] [Google Scholar]
- 80.Yin M-J, Gaynor R B. Complex formation between CREB and Tax enhances the binding affinity of CREB for the human T-cell leukemia virus type 1 21-base-pair repeats. Mol Cell Biol. 1996;16:3156–3168. doi: 10.1128/mcb.16.6.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yin M J, Paulssen E J, Seeler J-S, Gaynor R B. Protein domains involved in both in vivo and in vitro interactions between human T-cell leukemia virus type 1 Tax and CREB. J Virol. 1995;69:3420–3432. doi: 10.1128/jvi.69.6.3420-3432.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]