Significance
The molecular basis for evolutionary gain and loss remains elusive. Here, we report that a G protein-coupled receptor (GPCR) triggered mitogen-activated protein kinase (MAPK) signaling cascades trans -regulates a key P450 gene CYP6CM1 by activating a transcription factor (TF) cAMP-response element binding protein (CREB), contributing to insecticide resistance. However, this same signaling pathway also negatively modulates three key oogenesis genes Ex, Va, and Bg via recruiting another TF CncC, which leads to reproductive cost. We further find a positive feedback loop between the MAPK p38 and GPCR NPFF2 that continuously activates the MAPK pathways, thereby promoting resistance evolution but with a strong reproductive cost. Thus, our results resolve in fine detail the pleiotropic role of GPCR–MAPK signaling cascades in evolutionary trade-offs.
Keywords: adaptive evolution, fitness trade-offs, GPCR, MAPK, P450
Abstract
Trade-offs between evolutionary gain and loss are prevalent in nature, yet their genetic basis is not well resolved. The evolution of insect resistance to insecticide is often associated with strong fitness costs; however, how the fitness trade-offs operates remains poorly understood. Here, we show that the mitogen-activated protein kinase (MAPK) pathway and its upstream and downstream actors underlie the fitness trade-offs associated with insecticide resistance in the whitefly Bemisia tabaci. Specifically, we find a key cytochrome P450 gene CYP6CM1, that confers neonicotinoids resistance to in B. tabaci, is regulated by the MAPKs p38 and ERK through their activation of the transcription factor cAMP-response element binding protein. However, phosphorylation of p38 and ERK also leads to the activation of the transcription repressor Cap “n” collar isoform C (CncC) that negatively regulates exuperantia (Ex), vasa (Va), and benign gonial cell neoplasm (Bg), key genes involved in oogenesis, leading to abnormal ovary growth and a reduction in female fecundity. We further demonstrate that the transmembrane G protein-coupled receptor (GPCR) neuropeptide FF receptor 2 (NPFF2) triggers the p38 and ERK pathways via phosphorylation. Additionally, a positive feedback loop between p38 and NPFF2 leads to the continuous activation of the MAPK pathways, thereby constitutively promoting neonicotinoids resistance but with a significant reproductive cost. Collectively, these findings provide fundamental insights into the role of cis-trans regulatory networks incurred by GPCR–MAPK signaling pathways in evolutionary trade-offs and applied knowledge that can inform the development of strategies for the sustainable pest control.
Nature selection not only favors the evolution of ever greater benefit but also comes with costly side effects on fitness, resulting in rapid evolutionary trade-offs. The evolution of beneficial traits, such as resistance to harmful xenobiotics, pests, or pathogens, often incurs fitness costs in the form of negative impacts on other traits (1). For instance, the evolution of plant resistance to virus or pathogen attack can come at the costs of reduced growth rate (2). In the case of animals, the marine threespine stickleback can gain immune resistance to cestode parasitism, but this is associated with a substantial fitness cost on fecundity (3). Notably, the evolution of insect resistance to insecticides is frequently accompanied by fitness costs (4). Despite the widespread existence of these evolutionary gain and loss in many organisms, our understanding of their mechanistic basis remains poor.
Insects are an excellent system with which to address fundamental questions on evolutionary trade-offs (5). Over the last century, overuse of insecticides has resulted in the rapid evolution of resistance in more than 600 different insect species (6). For many highly damaging insect pests worldwide, the evolution of insecticide resistance now risks outpacing human innovation, threatening to reverse the gains made in crop yields and reduction in human mortality from vector-borne diseases (1, 7). This worrying situation provides a strong incentive to develop and implement strategies to slow the development of insecticide resistance. However, designing such strategies requires an understanding of the fitness of resistant and susceptible genotypes in the presence and absence of pesticide and any fitness costs associated with resistance. Indeed, many current resistant management strategies, such as those based on rotation of insecticides of different modes of action, explicitly rely on the assumption that resistance carries a cost in the absence of insecticide (4, 8). A body of empirical works have provided support for this hypothesis and revealed that the evolution of insecticide resistance in many insect species is frequently accompanied by fitness costs (4, 9). In 2000, Coustau et al. postulated that fitness costs associated with resistance “can only be fully interpreted in the light of the molecular mutations that might underlie them” (10). However, over two decades later, our understanding of the pleiotrophic effect of most resistance mutations on insect fitness, and the precise mechanisms by which trade-offs between resistance and their associated costs operate remain surprisingly poor.
The whitefly Bemisia tabaci is a notorious pest of a wide range of important field and protected crops worldwide (11). However, field-evolved resistance to neonicotinoids has developed in B. tabaci around the world (12). Several studies investigating the molecular basis of resistance to this insecticide class in B. tabaci have implicated the upregulation of one or more cytochrome P450 enzymes in metabolic resistance (13). Of these, the P450 CYP6CM1 has been most frequently shown to detoxify several neonicotinoids by metabolizing these compounds to less-toxic forms (14, 15). Regarding this, our recent work demonstrates that CYP6CM1 is trans-regulated by the transcription factor (TF) cAMP-response element binding protein (CREB) in the mitogen-activated protein kinase (MAPK)-dependent pathway (16). Resistance mediated by P450s is assumed to carry associated fitness costs as a result of energy reallocation to support the enhanced metabolic activity (4, 17). While many studies have identified specific fitness costs phenotype associated with P450-mediated neonicotinoid resistance (18, 19), the molecular mechanisms underlying such fitness trade-offs have never been elucidated.
Here, we addressed this knowledge gap by characterizing a significant reproductive cost associated with neonicotinoid resistance in the global pest B. tabaci. We unravel the role of components of the MAPK signaling pathway and its upstream and downstream actors in simultaneously mediating neonicotinoid resistance and its associated fitness cost in this pest. Our findings largely advance the understanding of the genetic basis for evolutionary trade-offs and have profound implications for currently proposed strategies aimed at sustainable pest control of humans health and agriculture crop.
Results
Field Strains of B. tabaci MED Exhibit Broad-Spectrum Resistance to Neonicotinoids.
We first examined the sensitivity of two field-collected strains (R#1 and R#2) of B. tabaci MED to seven commonly used neonicotinoids, compared to our laboratory reference susceptible strain (S#1) and a field-collected susceptible population (S#2) (SI Appendix, Tables S1 and S2). We also examined the sensitivity of these strains to another insecticide, the avermectin abamectin, belonging to a completely different mode of action. All strains showed similar survival rates when exposed to abamectin at all concentrations tested (SI Appendix, Fig. S1A). In contrast, a significantly higher survival rate was observed in the R#1 and R#2 strains compared to S#1 and S#2 following exposure to all of the different neonicotinoids with the exception of dinotefuran in the case of R#2 strain (SI Appendix, Fig. S1A). To more precisely quantify the level of resistance in the R#1 and R#2 strains, full dose–response insecticide bioassays were conducted to determine the median lethal concentration (LC50) for each compound and calculate resistance ratios (RR) based on comparisons with LC50 values obtained for a susceptible strain. In these assays the LC50 (SI Appendix, Fig. S1B) and RR (Fig. 1A) values obtained for the R#1 and R#2 strains for each neonicotinoid were higher than those obtained for both the S#1 and S#2 strains with the exception of dinotefuran in the case of R#2 strain. Compared to the reference strain S#2, the calculated RR for R#1 and R#2 ranged from 12 to 230 (SI Appendix, Table S3). In contrast, both strains exhibited no resistance to abamectin. Together, these results reveal a broad spectrum of neonicotinoid resistance in field-collected strains of B. tabaci MED (SI Appendix, Fig. S1C).
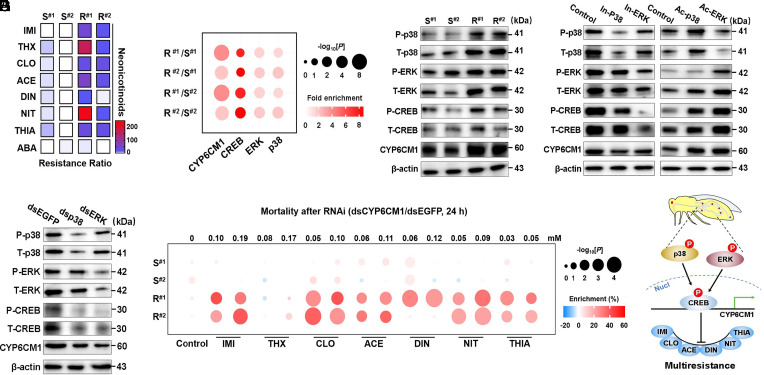
Fig. 1.
The MAPK–CREB signaling pathway underlies CYP6CM1-mediated broad-spectrum resistance to neonicotinoids. (A) Determination of RR values in comparison of neonicotinoid sensitive (S#1 and S#2) and resistant whitefly strains (R#1 and R#2) to seven commonly used neonicotinoids. Insecticides tested include imidacloprid (IMI), nitenpyram (NIT), acetamiprid (ACE), thiamethoxam (THX), thiacloprid (THIA), clothianidin (CLO), dinotefuran (DIN), and the avermectin abamectin (ABA). (B) Quantification of the expression of genes involved in the MAPK-CREB-CYP6CM1 pathway among the experimental strains. (C) Western blot analysis of protein level involved in the MAPK–CREB–CYP6CM1 pathway in the experimental strains. T-p38, total p38 protein; P-p38, phosphorylated form of p38 protein; T-ERK, total ERK protein; P-ERK, phosphorylated form of ERK protein; T-CREB, total CREB protein; P-CREB, phosphorylated form of CREB protein. (D) Effect of specific chemical inhibitors and activators of p38 and ERK on the expression of key proteins involved in the MAPK–CREB–CYP6CM1 pathway. In-p38, inhibitor of p38; In-ERK, inhibitor of p38; Ac-p38, activator of p38; Ac-ERK, activator of ERK. (E) Western blot analysis of the expression of key proteins involved in the MAPK–CREB–CYP6CM1 pathway after RNAi knockdown of p38 and ERK. (F) Sensitivity of the experimental strains to seven neonicotinoids after RNAi knockdown of CYP6CM1. (G) Schematic of the role of the MAPK–CREB–CYP6CM1 pathway in conferring the broad-spectrum resistance to neonicotinoids in B. tabaci MED. Data were presented as the mean ± SEM of at least three independent experiments. Note that the resistant strain R#1 was used in RNAi experiments and inhibitor tests, and the susceptible strain S#2 was used in activator tests. Whiteflies fed on dsEGFP were severed as a negative control in RNAi experiments. β-actin was used as a loading control in western blot.
The MAPK–CREB Pathways Confer Neonicotinoid Resistance.
Next, we investigate the role of target-site mutation in the broad-spectrum neonicotinoid resistance observed in the R#1 and R#2 strains by cloning and sequencing nine nicotinic acetylcholine receptor (nAChR, the neonicotinoid target site) from these strains and S#1 and S#2. No nonsynonymous mutations were observed in the sequences obtained that consistently distinguish the four experimental strains (Dataset S1). Thus, this result suggests that target-site mutation plays no role in neonicotinoid resistance in the R#1 and R#2 strains. To investigate the role of metabolic mechanisms in resistance, we examined the expression of CYP6CM1 in the experimental strains, as overexpression of this P450 gene has been frequently implicated in the resistance of B. tabaci to neonicotinoids (20). Quantitative PCR (qRT-PCR) revealed that CYP6CM1 is strongly overexpressed in the resistant strain R#1 and R#2 compared to the susceptible strain S#1 and S#2 (Fig. 1B). This result was further confirmed by western blot, which showed that CYP6CM1 protein was expressed at higher levels in R#1 and R#2 strains than that in S#1 and S#2 (Fig. 1C). Given that the functional capacity of CYP6CM1 to detoxify several neonicotinoids has been demonstrated previously (21), these findings suggest that overexpression of CYP6CM1 plays a role in the broad-spectrum resistance to neonicotinoids in the whitefly.
We have previously shown that the MAPK-activated protein kinases p38 and ERK can activate the TF CREB, thereby contributing to overexpression of CYP6CM1 that underlies imidacloprid resistance in B. tabaci MED (16). To investigate the role of this signaling pathway in the resistance of the R#1 and R#2 strains to neonicotinoids, qPCR and western blot analysis were first used to examine the expression level of p38, ERK, and CREB in the experimental strains. Both the mRNA level of p38, ERK, and CREB and their total and phosphorylated protein level were elevated in the R#1 and R#2 strains compared to the S#1 or S#2 strain (Fig. 1 B and C). To further confirm the role of the MAPK–CREB signaling pathway in CYP6CM1-mediated resistance, we conducted loss-and-gain of function experiments using specific chemical inhibitors (In-p38 and In-ERK) and activators (Ac-p38 and Ac-ERK) in vivo. Inhibition of p38 and ERK decreased the level of mRNA, total protein level and phosphorylated form of p38 and ERK, and CREB, and the mRNA and protein level of CYP6CM1. In contrast, activation of p38 and ERK showed the opposite effect (Fig. 1D). To functionally characterize the role of the MAPK–CREB pathway in regulation of CYP6CM1, RNAi was performed to knockdown p38 and ERK in the resistant strain R#1. Depletion of either p38 or ERK (SI Appendix, Fig. S1D) significantly decreased the mRNA expression level (SI Appendix, Fig. S1D) of CREB (p38, P = 7.41 × 10−9; ERK, P = 1.41 × 10−9) and CYP6CM1 (p38, P = 2.40 × 10−11; ERK, P = 6.29 × 10−11), as well as reducing the total protein level and phosphorylated form of p38, ERK, and CREB, and total protein level of CYP6CM1 (Fig. 1E). Taken together, these findings indicate that the MAPK p38 and ERK pathways play a pivotal role in modulating the key resistance gene CYP6CM1 via activation of CREB.
To further demonstrate the causal role of CYP6CM1 in the resistance to neonicotinoids, RNAi knockdown of this gene (SI Appendix, Fig. S1E) was performed and the effect of this on the survival rates of the experimental strains assessed using insecticide bioassays. With exception of THX, knockdown of CYP6CM1 significantly increased the mortality rate of R#1 and R#2 compared to the control dsEGFP at 24 h (Fig. 1F) and 48 h (SI Appendix, Fig. S1F) when adult whiteflies were exposed to both a high and low concentration of a range of neonicotinoids. Collectively, these results provide clear evidence that the MAPK–CREB pathway positively regulates the key resistance gene CYP6CM1 and thus explains the broad-spectrum resistance to neonicotinoid insecticides in the whitefly (Fig. 1G).
MAPK-Mediated CYP6CM1 Resistance Is Associated with Strong Reproductive Cost.
To identify whether neonicotinoid resistance is associated with a fitness cost, the “age stage-two sex” life table methodology developed by Chi (22) (Fig. 2A and SI Appendix, Fig. S2A) was used to compare life-history traits in the experimental strains. The R#1 and R#2 strains showed a prolonged developmental time (SI Appendix, Fig. S2B and Table S4), decreased survival rate of immature stages (SI Appendix, Fig. S3 A and B), and a shortened adult longevity (SI Appendix, Fig. S2D) and life expectancy (SI Appendix, Fig. S3C) compared to the S#1 and S#2 strains (SI Appendix, Table S5). Notably, the oviposition period (OP) (SI Appendix, Fig. S2C), adult longevity (SI Appendix, Fig. S2D), fecundity (Fig. 2B), and reproductive value (SI Appendix, Fig. S3D) were decreased in the R#1 and R#2 strains relative to the S#1 and S#2 strains. Furthermore, the relative fitness (Rf) of R#1 and R#2were calculated as 0.85 and 0.88 using the value of intrinsic rate of increase (r) and as 0.59 and 0.62 using the net reproduction rate (R0) when compared to S#1 (SI Appendix, Table S6), suggesting a strong reproductive fitness cost associated with resistance. To confirm whether this reproductive cost is caused by female fecundity, we subsequently measured the fecundity dynamics. As shown in Fig. 2C, R#1 and R#2 females showed a reduced rate of egg production over time compared to S#1 and S#2. Thus, our data provide clear evidence of a female-based reproductive cost of neonicotioid resistance in the whitefly.
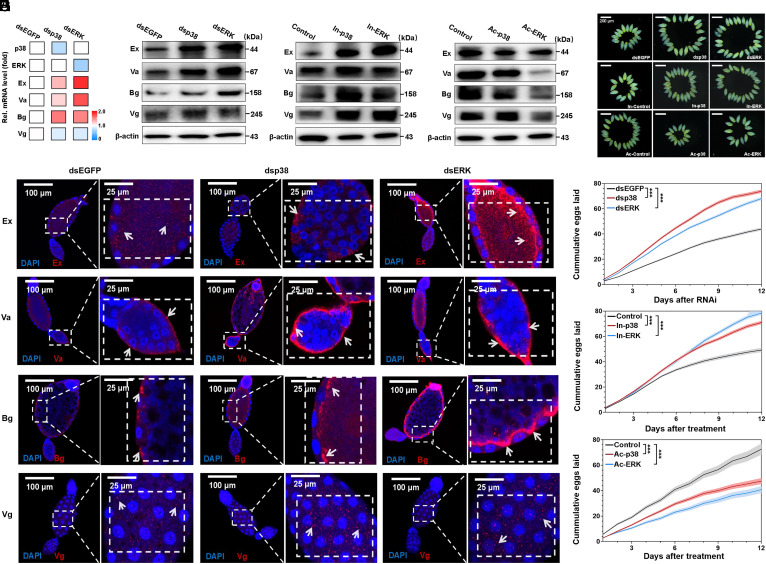
Fig. 2.
Downregulation of the key oogenesis genes Ex, Va, Bg, and Vg causes abnormal ovarian development, leading to a reproductive cost. (A) A schematic representation of life-table approach and observation of whiteflyovary growth. Green arrow, ovariole. White arrow, immature oocytes. Red arrow, mature oocytes. Yellow arrow, symbionts. (B) Comparison of total egg production in the neonicotinoid sensitive (S#1 and S#2) and resistant strains (R#1 and R#2). (C) Investigation of female fecundity of neonicotinoid sensitive (S#1 and S#2) and resistant strains (R#1 and R#2) after eclosion. (D) Investigation of the average number of ovarioles and oocytes per female of the experimental strains on days 2 to 16 after eclosion. (E) Representative images showing ovary morphology and ovarian development of the experimental strains on days 2, 6, and 10 after eclosion. (Scale bar, 200 μm.) (F) Expression of genes involved in oogenesis in female adults of the experimental strains sampled on days 6 and 14 after eclosion. (G) Relative expression level of Ex, Va, Bg, and Vg in female adults of the experimental strains sampled on days 2, 6, 10, 14, and 18 after eclosion. (H) Western blot analysis of the Ex, Va, Bg, and Vg protein level in the female adults of the experimental strains sampled 6 d after eclosion. (I) The effect of RNAi knockdown of Ex, Va, Bg, and Vg on female fecundity in the S#2 strain. (J) Representative images displaying ovary morphology of female adults sampled on days 3 and 8 after RNAi knockdown of Ex, Va, Bg, and Vg. (Scale bar, 200 μm.) (K) Schematic of downregulation of Ex, Va, Bg, and Vg leading to abnormal ovarian development that confers the reproductive cost associated with neonicotinoid resistance in B. tabaci MED. Data were presented as the mean ± SEM of at least three independent experiments. Note that newly emerged (1-d-old) female adults of susceptible strain S#2 were used in the RNAi experiments. Whiteflies fed on dsEGFP served as a negative controls in RNAi experiments. β-actin was used as a loading control in western blot. Data were analyzed with Student’s t test: *P < 0.05, **P < 0.01, ***P < 0.001.
The growth of the ovary, the female reproductive system, is indispensable for maintaining reproduction capacity (23); we therefore investigated whether the reproductive cost observed in the R#1 and R#2 strains is caused by a deficiency in ovarian development. We first dissected females of the S#2 strain to determine ovary morphology (SI Appendix, Fig. S4A) and development pattern (SI Appendix, Fig. S4D) of the whitefly. The ovary was formed by a mass of 10 to 28 ovarioles at various developmental stages (SI Appendix, Fig. S4 D and E) and each ovariole had two or three oocytes of different maturities (SI Appendix, Fig. S4 F and G). The mean number of ovarioles (SI Appendix, Fig. S4B) and oocytes (SI Appendix, Fig. S4C) per ovary increased during the 10 d after eclosion and then declined gradually with age. We then compared ovarian development in the four experimental strains. Both the number of ovarioles and oocytes in the R#1 and R#2 strains were found to be reduced at each female developmental age compared to the S#1 and S#2 strains (Fig. 2D), largely consistent with their morphology at different stages of ovary growth (Fig. 2E). Collectively, these findings provide direct evidence that abnormal ovary growth, primarily manifest as a reduction in ovariole and oocyte number, causes a reproductive cost associated with neonicotinoid resistance.
The Key Oogenesis Genes Ex, Va, Bg, and Vg Underlie the Reproductive Cost.
Oogenesis (SI Appendix, Fig. S5A) is the key event for ovary growth and involves the coordinated action of numerous genes (24, 25). We hypothesized that the expression of one or more of these oogenesis genes may be modified in the neonicotinoid-resistant strains and thus play a role in the observed deficiency in ovarian development. Based on research conducted on the model insect Drosophila melanogaster (26), we selected 31 genes that play key roles in oogenesis (SI Appendix, Table S7) and examined their expression in the experimental strains using qPCR. Nine of the 31 genes were found to be down-regulated in adults of the R#1 and R#2 strains compared to S#1 and S#2 strains (SI Appendix, Fig. S5B). To investigate the expression of these nine genes in more detail, we compared their expression in the females of experimental strains on day 6 and 14 after eclosion. Of the genes analyzed, we found that exuperantia (Ex), vasa (Va), benign gonialcell neoplasm (Bg), and vitellogein (Vg) were consistently expressed at a lower level in the R#1 and R#2 strains than in S#1 and S#2 (Fig. 2F and SI Appendix, Fig. S5C). Phylogenetic and protein domain analysis of Ex, Va, Bg, and Vg provided support for a functional role in insect reproduction (SI Appendix, Figs. S6 and S7). Additional investigation of the expression of these genes using qPCR in females from day 2 to 18 after eclosion showed a similar pattern of downregulation in the R#1 and R#2 strains compared to the S#1 and S#2 strains (Fig. 2G). Furthermore, western blot analysis revealed that the protein level of Ex, Va, Bg, and Vg increased with female age from day 2 to 10 while decreasing at day 16 after eclosion (SI Appendix, Fig. S5E), which was largely consistent with the ovarian development pattern (SI Appendix, Fig. S4 B and C). Likewise, the protein level of these four genes were reduced in females of the R#1 and R#2 strains compared to the S#1 and S#2 strains sampled both on day 6 (Fig. 2H) and 12 (SI Appendix, Fig. S5F) after eclosion. Overall, these results suggest that downregulation of Ex, Va, Bg, and Vg is implicated in reproductive cost in the whitefly.
To determine whether the four candidate genes are expressed in the whitefly ovary, visualization analysis using immunofluorescence (IF) staining assays was performed. Ex and Va were specifically detected in the immature oocyte at early stages 1 and 2 (SI Appendix, Fig. S8 A and B), while Bg and Vg were specifically detected in mature oocytes at late stages 3 and 4 (SI Appendix, Fig. S8 C and D). This finding supports an important role for Ex, Va, Bg, and Vg at specific stages of oogenesis to maintain ovary growth (SI Appendix, Fig. S5D). IF staining assay also revealed a lower protein signal for Ex, Va, Bg, and Vg in the oocytes in the R#1 and R#2 strains compared to S#1 and S#2 (SI Appendix, Fig. S9). These findings corroborate the link between the downregulation of these four genes at specific stages of oogenesis and the observed reproductive cost.
To investigate whether the expression of Va, Ex, Bg, and Vg is impacted by neonicotinoid exposure, we investigated the effect of a high and low dose of seven neonicotinoids on the expression of these genes at two time points. Exposure to neonicotinoids resulted in reduced expression of Ex, Va, Bg, and Vg (SI Appendix, Fig. S10 A and B), with a “time-dose” effect of a greater downregulation at the higher dose and later time-point observed (SI Appendix, Fig. S10 C and D). Taken together, the above results demonstrate a clear link between downregulation of Ex, Va, Bg, and Vg and a fitness cost of abnormal ovarian development in neonicotinoid resistant strains.
To validate the causal role of Ex, Va, Bg, and Vg in this reproductive cost, RNAi knockdown of the four genes in S#2 strain was performed and the effect of this on ovarian development and female fecundity was examined (SI Appendix, Fig. S11A). Silencing of the four candidate genes (SI Appendix, Fig. S11B) decreased their protein level in western blot analysis (SI Appendix, Fig. S11 C–F) and reduced their protein signal in oocytes through IF staining analysis (SI Appendix, Fig. S12), relative to the control dsEGFP. Knockdown of the four genes also reduced female egg production (Fig. 2I), ovariole (SI Appendix, Fig. S11G) and oocyte (SI Appendix, Fig. S11H) number compared to the control dsEGFP, and resulted in an abnormal ovarian development phenotype (Fig. 2J). These data collectively indicate that downregulation of Ex, Va, Bg, and Vg in the whitefly ovary causes an abnormal ovary growth and a reduction in female fecundity, thereby contributing to the observed reproductive cost (Fig. 2K).
The p38 and ERK Pathways Negatively Modulate Ex, Va, and Bg.
The MAPK signaling pathway has been shown to play a key role in the regulation of development, reproduction, and metabolism (27). Given the role of p38 and ERK in regulating neonicotinoid resistance in the whitefly (Fig. 1), we hypothesized that this same MAPK signaling may play a pleiotropic role in modulating reproductive cost identified in this study. To test this, RNAi was performed to knockdown p38 and ERK (SI Appendix, Fig. S13 A and B) in the resistant strain R#1 and the effect of this on the expression of Ex, Va, Bg, and Vg examined. Knockdown of either p38 or ERK dramatically increased the expression of Ex (dsp38, P = 4.30 × 10−3; dsERK, P = 6.89 × 10−5), Va (dsp38, P = 6.10 × 10−3; dsERK, P = 2.98 × 10−6), and Bg (dsp38, P = 2.00 × 10−4; dsERK, P = 1.70 × 10−3) but had little impact on Vg compared to the control dsEGFP (Fig. 3A), and this was reflected in changes in their protein level in both western blot (Fig. 3B) and IF staining assays (Fig. 3E). To further confirm the function of p38 and ERK in regulating the four candidate genes, a loss-and-gain of function experiment was conducted in vivo using specific chemical inhibitors and activators. Similarly, inhibition of p38 and ERK (SI Appendix, Fig. S13C) significantly enhanced the mRNA expression of Ex (In-p38, P = 6.40 × 10−6; In-ERK, P = 3.82 × 10−5), Va (In-p38, P = 3.05 × 10−6; In-ERK, P = 6.85 × 10−6), and Bg (In-p38, P = 7.38 × 10−8; In-ERK, P = 8.63 × 10−5), while the opposite effect was observed after activation of p38 and ERK (SI Appendix, Fig. S13D). These results were corroborated by western blot analysis which revealed an increased protein level of Ex, Va, and Bg after inhibition of p38 and ERK (Fig. 3C) and a decreased protein level after activation of p38 and ERK (Fig. 3D). Taken together, the above findings clearly demonstrate that the p38 and ERK negatively regulate the expression of Ex, Va, and Bg which in turn is associated with a reproductive cost.
Fig. 3.
The MAPK signaling pathway negatively regulate Ex, Va, and Bg that confers the reproductive cost. (A) The impact of RNAi knockdown of p38 and ERK on the expression of Ex, Va, Bg, and Vg. (B) Western blot analysis of Ex, Va, Bg, and Vg protein level after RNAi knockdown of p38 and ERK. (C and D) Western blot analysis of Ex, Va, Bg, and Vg protein level after treatment with specific chemical inhibitors (C) or activators (D) of p38 and ERK. In-p38, inhibitor of p38; In-ERK, inhibitor of ERK; Ac-p38, activator of p38; Ac-ERK, activator of ERK. (E) Immunofluorescence staining assays of Ex, Va, Bg, and Vg protein locating in oocytes of female ovaries after RNAi knockdown of p38 and ERK. Cell nuclei were stained with DAPI (blue). White arrows represents location of the enriched target proteins in the oocyte. Scale bars, 100 μm for original images and 25 μm for zoom-in images. (F) Representative images displaying ovary morphology of female adult B. tabaci MED sampled on day 6 after RNAi knockdown of p38 and ERK, and following treatment with chemical inhibitors and activators of p38 and ERK. (Scale bar, 200 μm.) (G–I) Investigation of female fecundity after RNAi knockdown of p38 and ERK (G), and after treatment with the specifically chemical inhibitor (H) and activator (I) p38 and ERK. Data were presented as the mean ± SEM of at least three independent experiments. Note that newly emerged (1-d-old) female adults of resistant strain R#1 were used in the RNAi and inhibitor experiments, while the susceptible strain S#2 was used in activator experiments. Whiteflies fed on dsEGFP were severed as a negative control in RNAi experiments. Whiteflies fed on diet solution served as a negative control in the chemical inhibitor and activator experiments. β-actin was used as a loading control in western blot. Data were analyzed with Student’s t test: *P < 0.05, **P < 0.01, ***P < 0.001.
To further investigate the role of MAPK pathway in conferring the reproductive cost associated with neonicotinoid resistance, ovarian development and female fecundity were examined in the resistant strain R#1 following RNAi knockdown of p38 and ERK. Silencing of either p38 or ERK resulted in an increase in the number of ovarioles (SI Appendix, Fig. S13 E–G), oocytes (SI Appendix, Fig. S13 H–J), and female fecundity (Fig. 3G), consistent with a phenotype of enhanced ovarian development (Fig. 3F). These results were further confirmed by p38 and ERK inhibition and activation experiments (Fig. 3 F, H, and I and SI Appendix, Fig. S13 E–J). Conclusively, these results suggest that the MAPK p38 and ERK pathways negatively regulate Ex, Va, and Bg that leads to abnormal ovarian development, thereby contributing to the reproductive cost. Together with the demonstration of the role of MAPK p38 and ERK pathway in resistance to neonicotinoids (Fig. 1), these findings demonstrate a pleiotropic effect of the MAPKs p38 and ERK in regulation of the trade-off between neonicotinoids resistance and its associated fitness costs in the whitefly.
The TF CncC Is a General Repressor of Ex, Va, and Bg.
The MAPK signaling pathway typically activates downstream TFs by phosphorylation (16). For this reason, we investigated potential TFs downstream of the MAPK pathway that might regulate Ex, Va, and Bg transcription (Fig. 4A). We first used dual luciferase reporter gene assays to identify the promoter of Ex, Va, and Bg by creating a series of constructs containing various lengths of the sequences upstream of the translation start site of these genes (SI Appendix, Table S9). The regions −1,742 to +1 for Ex, −1,215 to +1 for Va, and −2,336 to +1 for Bg region exhibited the highest activity when compared to the reference reporter plasmid pGL4.10 (Fig. 4A) and suggested that the core promoter region was located from −1,742 to −1,418 bp for Ex, −1,215 to −1,004 bp for Va, and −2,336 to −1,839 bp for Bg (SI Appendix, Fig. S15A). Reporter gene assays in the presence of the p38 and ERK inhibitors increased the transcriptional activity of the promoters of Ex, Va, and Bg when compared to the DMSO control (SI Appendix, Fig. S15B), confirming the negative regulation of Ex, Va, and Bg by p38 and ERK in vitro. To search for a general TF that regulates Ex, Va, and Bg, we next selected a total of 24 TFs with potential roles in insect development, reproduction, and xenobiotic stress response based on an extensive literature search (SI Appendix, Tables S8 and S9). In dual luciferase reporter assays (SI Appendix, Fig. S16 A–C), only S2 cells overexpressing the CncC protein demonstrated a greater than threefold decrease in the transcriptional activity of the three target genes (Fig. 4 B and C), suggesting that the Cap “n” collar isoforms C (CncC) could be a general TF for Ex, Va, and Bg (SI Appendix, Fig. S16 D and E).
Fig. 4.
The MAPK pathway activates the TF CncC that negatively modulates Ex, Va, and Bg expression, thus contributing to the reproductive cost. (A) Transcriptional activity of putative promoter sequences upstream of Ex, Va, and Bg in dual luciferase reporter gene assays. (B) Effects of 24 TFs on the activity of the Ex, Va, and Bg promoters in reporter gene assays. Empty pAC5.1b was used as a control. (C) Venn diagram illustrating the relationship of different TFs on the activity of the Ex, Va, and Bg promoters in dual luciferase reporter gene assays. (D) Determination of CncC gene expression and protein level in the experimental strains by qPCR and western blot analysis. (E) Effects of RNAi knockdown of CncC on the protein level of Ex, Va, and Bg in the resistant strain R#1. (F) Immunoprecipitation analysis of the direct interaction of p38 or ERK with CncC protein in the resistant strain R#1. Input and IgG were used as a positive and negative control, respectively. P-p38, the phosphorylated form of p38. P-ERK, the phosphorylated form of ERK. P-CncC, the phosphorylated form of CncC. (G) Y2H assays showing the protein interactions of ERK or p38 with CncC in vitro. SD, yeast drop-out culture medium. pGBKT7-53 and pGADT7-T were used as a positive control. pGBKT7-lam and pGADT7-T were used as a negative control. (H) Detection of the phosphorylated level of CncC after coincubation with purified p38 or ERK protein in vitro. Note that Phos-TagTM gel was used in western blot. P-CncC, the phosphorylated form of CncC; T-CncC, the total protein of CncC. (I) Eletrophoretic mobility shift assay of the binding of purified CncC protein to the putative promoter sequences of Ex, Va, and Bg in vitro. (J) ChIP-qPCR analysis of the binding of CncC protein to the putative promoter sequences of Ex, Va, and Bg in the experimental strains. (K–M) Investigation of ovariole number (K), oocyte number (L), and female fecundity (M) after RNAi knockdown of CncC in female adults of the resistant strain R#1. (N) Representative images displaying ovary morphology of female adults sampled on day 3 and day 7 after RNAi knockdown of CncC in the resistant strain R#1. (Scale bar, 200 μm.) Data were presented as the mean ± SEM of at least three independent experiments. Whiteflies fed on dsEGFP were severed as a negative control in RNAi experiments. β-actin was used as a loading control in western blot. Data were analyzed with Student’s t test: *P < 0.05, **P < 0.01, ***P < 0.001.
Belonging to one of the Cap “n” collar (Cnc) isoforms, CncC is a central modulator of the oxidative stress response and owning a very broad target gene spectrum (16). To address whether Cnc gene can produce different splice forms, we cloned this gene according to the Whitefly Genome Database. We found that CncC was one of three identified Cnc isoforms in the resistant R#1 and R#2 strains (SI Appendix, Fig. S14 A–C), implying a conserved role of CncC in the whitefly as reported by the functionally homologous gene to mammalian Nrf2 (SI Appendix, Fig. S14 D and E). Phylogenetic, protein domain, phosphorylation sites analysis of the whitefly CncC provided support for a conserved role as a TF (SI Appendix, Fig. S14 D and F–H). To investigate the importance of the three conserved domains of CncC (SI Appendix, Fig. S14D) in activating the promoter of Ex, Va, and Bg, we constructed a CncC-wild type (WT) construct and three mutant CncC constructs that lack either the bZIP_Maf basic region (M1), the CNC domain (M2), and the DNA-binding region (M3) (SI Appendix, Table S11). These constructs were then cotransfected into S2 cells with pGL4.10-Ex−1,742 to +1, -Va−1,215 to +1, and -Bg−2,336 to +1. Compared to the WT control, coexpression with all three mutant constructs was associated with an increase in the transcriptional activity of the promoters of the three target genes (SI Appendix, Fig. S15C), revealing the importance of the three domains of CncC in transcriptional activation of Ex, Va, and Bg. To investigate the role of CncC in regulating Ex, Va, and Bg in vivo, qPCR and western blot analysis were conducted to quantify CncC expression in the experimental strains. The mRNA levels, total protein levels and the phosphorylated form of CncC were found to be elevated in the R#1 and R#2 strains compared to the S#1 and S#2 strains (Fig. 4D). To validate the cause role of CncC in regulating Ex, Va, and Bg, RNAi was used to knockdown CncC in the R#1 strain and the effect of this on gene expression and protein level of the three target genes examined. Depletion of CncC significantly enhanced gene expression of Ex (P = 1.00 × 10−3), Va (P = 8.00 × 10−4), and Bg (P < 1.00 × 10−4) compared to the control dsEGFP (SI Appendix, Fig. S15D). This result was also corroborated by western blot (Fig. 4E) and IF staining (SI Appendix, Fig. S17) assay, which showed an increased protein level of Ex, Va, and Bg after knockdown of CncC. Collectively, these results confirm that CncC is a general transcription repressor of Ex, Va, and Bg.
p38 and ERK Activate CncC Which Then Binds to the Core Promoter of Ex, Va, and Bg.
To investigate whether CncC is also regulated by the MAPK pathway, RNAi knockdown of p38 and ERK in the R#1 strain was conducted and the impact of this on CncC mRNA expression and protein level was examined. Both the mRNA and total protein levels of CncC and its phosphorylated form were found to be reduced following RNAi knockdown of p38 and ERK (SI Appendix, Fig. S15 E and F). This result was corroborated by p38 and ERK inhibitor and activator experiments (SI Appendix, Fig. S15G), which provides additional evidence that the p38 and ERK pathways positively regulate CncC. To support the concept that MAPK activates CncC by phosphorylation in vivo, immunoprecipitation (IP) was then performed to investigate the interaction of CncC with p38 or ERK in the R#1 strain. A western blot showed that the phosphorylated forms of CncC and p38 or ERK were detected after the whitefly protein incubated with their antibodies in IP assay (Fig. 4F), suggesting the direct interactions between CncC and p38 or ERK by phosphorylation. Likewise, Y2H assays showed that yeast cells cotransformed with plasmid DNAs the GAL4 activation domain AD-fused CncC and BD-p38 or BD-ERK grew well on the quadruple dropout media (Fig. 4G), which further confirms that CncC could interact with p38 or ERK protein in vitro. To further investigate whether p38 and ERK activate CncC by phosphorylation, IP was used to check the level of phosphorylation of CncC after RNAi-mediated knockdown of p38 or ERK in the R#1 strain. As shown in SI Appendix, Fig. S15H, the phosphorylated form of CncC decreased after RNAi-IP assay compared to the dsEGFP control, further confirming that p38 or ERK can modulate CncC phosphorylation in vivo. To further investigate the regulatory mechanism of CncC by p38 and ERK via phosphorylation in vitro, the level of phosphorylated CncC was examined after coincubation of in vitro-purified CncC protein with p38 or ERK protein. The level of phosphorylated CncC was found to be increased after coincubation with in vitro-expressed p38 or ERK proteins (SI Appendix, Fig. S15I). This result was also confirmed by western blot with Phos-TagTM gel in vitro (Fig. 4H). Taken together, both in vivo and in vitro assays unequivocally demonstrate that either p38 or ERK can interact with CncC and regulate CncC phosphorylation.
To identify putative CncC binding sites in the core promoter of target genes, the candidate binding sequence for Ex, Va, and Bg promoter were identified through the JASPAR database (SI Appendix, Table S10). A series of reporter gene constructs were created containing the promoters with these binding sequences (WT) and a second set where these sites were mutated (E1-M, E2-M, V1-M, V2-M, B1-M, B2-M, and B3-M) (SI Appendix, Table S11). Following cotransfection of these plasmids into S2 cells with pAC5.1-CncC, they were then used in reporter gene assays. The constructs E2-M (SI Appendix, Fig. S18A), V1-M (SI Appendix, Fig. S18B), and B1-M (SI Appendix, Fig. S18C) mutants showed the highest transcriptional activity compared to their respective WT control, suggesting that the regions mutated could contain CncC binding sites (SI Appendix, Fig. S18D). Electrophoretic mobility shift assays (EMSA) were then conducted to confirm the direct binding of purified CncC protein to DNA probes of these identified sequences in vitro. Binding was confirmed in each case as a shifted band, which disappeared when an unlabeled competitor DNA or a mutated probe was used (Fig. 4I). ChIP-qPCR assays were then conducted to confirm direct binding of CncC to the core promoter region of Ex, Va, and Bg in the whitefly (SI Appendix, Fig. S24 A–D). Further ChIP-qPCR assays found that the relative expression of CncC-DNA fragments was higher in the R#1 and R#2 strains than in the S#1 and S#2 strains (Fig. 4J), revealing a greater level of binding of CncC to the promoter sequences in the resistant strains.
By alignment of the identified binding sequences, we identified a potential consensus CncC binding site 5′-TGCC(T)GAA-3′ (SI Appendix, Fig. S18D). To functionally pinpoint this binding site in the promoters of Ex, Va, and Bg, reporter gene plasmids were constructed where the putative binding sequence was disrupted with a series of five-nucleotide mutations (M1 to M5) in the identified binding sequence (SI Appendix, Table S11) and the response of this on gene expression in the presence of CncC examined using reporter gene assays. Compared to the WT control, the construct where the site (M3) containing the consensus sequence was mutated exhibited the highest transcriptional activity in the case of all three target genes (SI Appendix, Fig. S18 E–G). Thus, the functional binding sites of CncC in the core promoter of the target genes are identified as 5′-TGCC(T)GAA-3′.
To elucidate the causal role of CncC in mediating the reproductive fitness cost associated with neonicotinoid resistance, RNAi knockdown of this gene in the R#1 strain was performed. Silencing of CncC led to an increase in the number of ovarioles (7 d: Fig. 4K; 3 d: SI Appendix, Fig. S18H), oocytes (7 d: Fig. 4L; 3 d: SI Appendix, Fig. S18I), and female fecundity (Fig. 4M) compared to the dsEGFP control and led to enhanced ovary growth (Fig. 4N). Collectively, these findings suggest that p38 and ERK can activate the transcriptional repressor CncC by phosphorylation, which leads to the binding of this TF to the core promoter of Ex, Va, and Bg resulting in a reproductive cost (Fig. 4N).
The Transmembrane G Protein-Coupled Receptor NPFF2 Triggers the MAPK Pathway.
The activation of CncC phosphorylation by components of the MAPK signaling pathway raises a question in regard to the upstream activators of p38 and ERK, which ultimately initiates the signaling cascades. G protein-coupled receptors (GPCRs) (Fig. 5A) are the largest family of transmembrane receptors with a well-known role in activating intracellular signaling transduction (28), thereby regulating a variety of pivotal physiological processes (29)). To probe the potential role of GPCRs in regulating p38 and ERK, we first examined the effect of inhibition of this receptor family in B. tabaci MED. Inhibition of GPCRs caused a reduction in p38 and ERK gene expression in qPCR analysis (SI Appendix, Fig. S19A), and this was corroborated by western blot analysis of their total and phosphorylated protein level (SI Appendix, Fig. S19B). This result revealed an involvement of GPCRs in controlling components of the MAPK pathway in B. tabaci MED, which then motivated us to explore the possible role of GPCRs in neonicotinoid resistance and its associated reproductive fitness cost. Inhibition of GPCRs was found to decrease gene expression of CREB and CYP6CM1 and their protein level (SI Appendix, Fig. S19 A and B) and reduced the resistance of B. tabaci MED to neonicotinoids (SI Appendix, Fig. S19C). In contrast, GPCR inhibition enhanced Ex, Va, and Bg expression while decreasing CncC expression (SI Appendix, Fig. S19 D and E), thereby promoting female fecundity (SI Appendix, Fig. S19F) and ovarian development (SI Appendix, Fig. S19 G–I). Together, this inhibitor assay suggests that GPCRs may positively modulate the p38 and ERK pathways in the whitefly.
Fig. 5.
NPFF2 underpins the evolutionary trade-offs between neonicotinoid resistance and its reproductive cost by activating the MAPK pathway. (A) A schematic representation of the GPCR-triggered signaling pathway. (B) Quantification of NPFF2 gene expression and its protein level in neonicotinoid sensitive (S#1 and S#2) and resistant strains (R#1 and R#2). T-NPFF2, total NPFF2 protein; P-NPFF2, phosphorylated form of NPFF2 protein. (C) Effect of neonicotinoids exposure on the protein level of NPFF2 in a resistant (R#1) strain. (D) Immunoprecipitation analysis of the interaction of phosphorylated NPFF2 with p38 and ERK in the resistant strain R#1. Input and IgG were used as a positive and negative control, respectively. (E) Y2H assays showing the protein interactions of ERK or p38 with NPFF2 in vitro. SD, yeast drop-out culture medium. pGBKT7-53 and pGADT7-T were used as a positive control. pGBKT7-lam and pGADT7-T were used as a negative control. (F) Detection of the phosphorylation level of p38 and ERK in vitro after coincubation with purified NPFF2 protein in a reaction buffer containing ATP. Phos-TagTM gel was used in western blot. (G) Western blot analysis of protein level of key genes in the MAPK-CREB pathway and CYP6CM1 following RNAi knockdown of NPFF2 in the resistant strain R#1. T-p38, total p38 protein; P-p38, phosphorylated form of p38 protein; T-ERK, total ERK protein; P-ERK, phosphorylated form of ERK protein; T-CREB, total CREB protein; P-CREB, phosphorylated form of CREB protein. (H) Sensitivity of adults of the experimental strains to neonicotinoids after RNAi knockdown of NPFF2. Mortality was scored 24 and 48 h after first exposure to a high concentration of each of seven neonicotinoids. IMI, imidacloprid; THX, thiamethoxam; CLO, clothianidin; ACE, acetamiprid; DIN dinotefuran; NIT, nitenpyram; THIA, thiacloprid. (I) Western blot analysis of the level of CncC, Ex, Va, Bg, and Vg protein after RNAi knockdown of NPFF2. (J) Investigation of female fecundity after RNAi knockdown of NPFF2 in the resistant strain R#1. (K) Representative images displaying ovary morphology of female adults sampled on day 3 and day 7 after RNAi knockdown of NPFF2 in the resistant strain R#1. (Scale bar, 200 μm.) (L) Protein level of NPFF2 and its phosphorylated form following knockdown of p38 and ERK, and chemical inhibition and activation of p38 and ERK. In-p38, inhibitor of p38; In-ERK, inhibitor of ERK; Ac-p38, activator of p38; Ac-ERK, activator of ERK. (M) RNAi-IP analysis of the level of immunoprecipited NPFF2 after silencing of p38 in the resistant strain R#1. dsEGFP was used as a negative control. (N) Detection of the level of phosphorylated NPFF2 in vitro after coincubation with purified p38 protein in a reaction buffer containing ATP. Phos-TagTM gel was used in western blot. (O) Schematic showing a proposed model of a feedback loop mediated by p38 that triggers the phosphorylation of NPFF2, thereby promoting the resistance but with a reproductive cost. Data were presented as the mean ± SEM of at least three independent experiments. Whiteflies fed on dsEGFP served as a negative control in RNAi experiments. Whiteflies fed on diet solution served as a negative control in chemical inhibitor experiments. β-actin was used as a loading control in western blot. Data were analyzed by Student’s t test: *P < 0.05, **P < 0.01, ***P < 0.001.
Building on the results, we next investigated the precise mechanism underlying the activation of p38 and ERK by a specific GPCR. We have previously shown by transcriptome profiling that a key GPCR gene, named neuropeptide FF2 receptor (NPFF2), was highly expressed in a neonicotinoid resistant strain of B. tabaci (30). The deduced amino acid sequence of NPFF2 contains conserved domains common to the 7TM-GPCR rhodopsin family (SI Appendix, Fig. S20 A and B), which are involved in the recognition and transduction of extracellular ligand signals to the intracellular environment (31–33). Notably, similar to human and rat NPFF2 (SI Appendix, Fig. S20 C and D), putative phosphorylation sites were present in the C terminus of NPFF2 of B. tabaci MED (SI Appendix, Fig. S20 E and F), which may be important in NPFF2 activity as reported on human and rat (33, 34). qPCR and western blot analysis revealed that NPFF2 gene expression, its total protein, and the phosphorylated form were elevated in the R#1 and R#2 strains compared to S#1 and S#2 (Fig. 5B). Furthermore, exposure of the whitefly to a range of neonicotinoids increased the total protein and phosphorylated form of NPFF2 (Fig. 5C and SI Appendix, Fig. S21A). These results suggest that NPFF2 may act as a receptor of neonicotinoids or be activated indirectly by Ca entry through the activation of nicotinic receptors by the pesticides.
To investigate whether the MAPK pathway is activated by NPFF2, RNAi knockdown of NPFF2 in the R#1 strain was performed and the impact of this on p38 and ERK was examined. Western blot analysis revealed a reduction in the phosphorylated form of p38 and ERK (Fig. 5G) after silencing of NPFF2, suggesting NPFF2 can modulate the phosphorylation of p38 and ERK. Supporting this hypothesis, the phosphorylated NPFF2 and p38 or ERK proteins were detected after the whitefly protein coincubated with their antibodies in IP assays (Fig. 5D), which demonstrated a direct interaction of these proteins via phosphorylation in vivo. This result was further clarified by Y2H assay in vitro, showing that yeast cells cotransformed with plasmid DNAs the GAL4 activation domain AD-fused NPFF2 and BD-p38 and BD-ERK grew well on the quadruple dropout media (Fig. 5E). The regulation of CncC by p38 and ERK were also confirmed by RNAi-IP, which demonstrated a decreased level of immunoprecipitated phosphorylated p38 and ERK after knockdown of NPFF2 in the R#1 strain (SI Appendix, Fig. S21B). To further explore whether NPFF2 can activate p38 or ERK phosphorylation in vitro, we measured phosphorylated level of p38 and ERK after coincubation of their purified proteins with NPFF2 in a reaction buffer containing ATP. Coincubation of p38 or ERK with NPFF2 promoted the level of phosphorylated p38 and ERK by in vitro-phosphorylation assay (SI Appendix, Fig. S21C), which was further supported by an increased level of phosphorylated p38 and ERK in western blot analysis using Phos-TagTM gel (Fig. 5F). Taken together, these findings demonstrate the importance of NPFF2 in activating the phosphorylation of p38 and ERK, thereby initiating key MAPK signaling cascades.
NPFF2 Underpins Trade-Offs between Neonicotinoids Resistance and Reproductive Cost.
Considering the essential role of NPFF2 in activating p38 and ERK, we conducted RNAi knockdown of NPFF2 in the R#1 strain to examine the role of this gene in mediating neocniotinoid resistance. Silencing of NPFF2 led to a decrease in the mRNA expression of CREB and CYP6CM1 (SI Appendix, Fig. S21D), which was corroborated by western blot showing a reduced level of CREB and CYP6CM1 protein (Fig. 5G). Subsequent insecticide bioassays demonstrated an increased sensitivity of resistant B. tabaci MED to each of the seven neonicotinoids after RNAi knockdown of NPFF2 (Fig. 5H and SI Appendix, Fig. S21E). Thus, we conclude that NPFF2 plays a key role in the resistance of B. tabaci MED to neonicotinoids via its activation of the MAPK-CREB pathway.
To test whether NPFF2 also plays a role in the reproductive fitness cost associated with resistance, RNAi knockdown of the gene was performed and the effect of this on the expression of Ex, Va, and Bg was examined. Knockdown of NPFF2 enhanced the expression of Ex, Va, and Bg (SI Appendix, Fig. S21F) and the level of protein produced by these genes (Fig. 5I). This result was further confirmed by IF staining assays showing an increased intensity of protein signal of Ex, Va, and Bg in the B. tabaci ovary following silencing of NPFF2 (SI Appendix, Fig. S22). Further phenotypic assays showed that the mean number of ovarioles (SI Appendix, Fig. S21G), oocytes (SI Appendix, Fig. S21H) and fecundity (Fig. 5J) of NPFF2-depleted females was higher than that of females fed dsEGFP, which was supported by a phenotype of increased ovarian development after RNAi knockdown of NPFF2 (Fig. 5K). Thus, the above results reveal an essential role of NPFF2 in the trade-offs between neonicotinoid resistance and reproductive cost via its action on activating MAPK pathways.
A Feedback Loop Mediated by p38 Promotes the Phosphorylation of NPFF2.
Phosphorylation of intracellular residues is a key mechanism in regulating GPCR activity and thus the downstream signaling pathways activated by these receptors (32, 34). Considering the enhanced level of NPFF2 phosphorylation observed in the R#1 and R#2 strains (Fig. 5B), we asked how this phosphorylation is mediated. Given that phosphorylation of the protein kinases p38 and ERK often interact with other intracellular proteins by phosphorylation, we hypothesized that these kinases may play a role in the phosphorylation of NPFF2. To test this, RNAi knockdown of p38 and ERK in the R#1 strain was performed, and the effect of this on the total protein level of NPFF2 and its phosphorylated form was examined. Depletion of p38 but not ERK decreased the phosphorylated form of NPFF2 but with no influence on the total protein level (Fig. 5L), which suggests that p38 phosphorylation could be necessary to activate the phosphorylation of NPFF2. Likewise, chemical inhibition of p38 but not ERK resulted in a reduction in the phosphorylated form of NPFF2, while chemical activation of p38 resulted in an increased level of phosphorylated NPFF2 (Fig. 5L). This suggests that p38 activated NPFF2 by phosphorylation. RNAi-IP assays showed that the immunoprecipitated phosphorylated form of NPFF2 in the whitefly was reduced after the knockdown of p38 (Fig. 5M), further confirming the importance of p38 in regulating NPFF2 phosphorylation in vivo. To determine whether phosphorylation of p38 can activate NPFF2 in vitro, we detected the level of phosphorylated NPFF2 after coincubation of their purified proteins in a reaction buffer containing ATP. Coincubation of NPFF2 with p38 enhanced the level of phosphorylated NPFF2 detected by western blot using Mn2+-Phos-TagTM SDS-PAGE gel (Fig. 5N). Together these data indicate that p38 phosphorylation is pivotal in regulating NPFF2 activity, thereby continuously activating the p38 and ERK signaling cascades that promote the evolution of resistance but with a strong reproductive cost (Fig. 5O).
Discussion
Our work conclusively provides a precise mechanism by which GPCR–MAPK signaling pathways act as “master regulator” to govern the evolutionary gain and loss. Especially, our data represent a whole regulatory network involved in the evolutionary trade-offs associated with insecticide resistance has been mapped out. Such trade-offs are mediated by the opposing action of a GPCR–MAPK signaling cascades on alternate downstream trans-acting factors (Fig. 6). These findings provide fundamental insights into the pleiotrophic effects of key genes involved in resistance on other traits, the mechanisms underpinning fitness trade-offs, and knowledge that can inform the development of strategies for sustainable pest control.
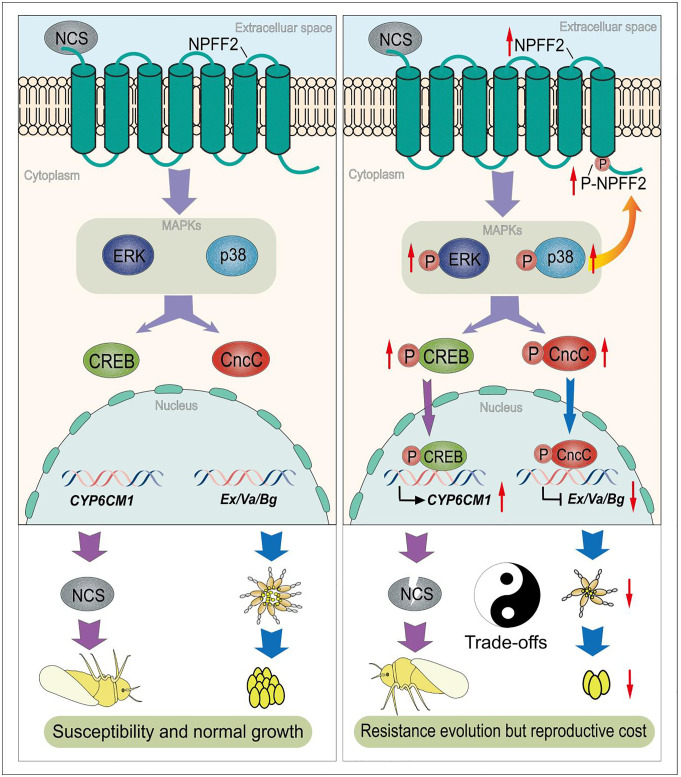
Fig. 6.
A proposed working model for GPCR–MAPK signaling cascades underlying trade-offs between insecticide resistance and reproductive costs. Exposure to neonicotinoids (NCS) is detected by the GPCR NPFF2 that then initiates the MAPK signaling pathway by phosphorylation. MAPK p38 and ERK not only activate the TF CREB contributing to overexpression of CYP6CM1 that confers resistance to neonicotinoids but also recruit another TF CncC that negatively regulates Ex, Va, and Bg leading to abnormal ovary growth and reduced fecundity. Notably, a positive feedback loop mediated by p38 triggers the phosphorylation of NPFF2, which in turn continuously activates the MAPK pathways, thereby promoting resistance evolution but with strong reproductive cost.
The MAPK signaling pathway plays an important role in development, metabolism, and the stress response (35). Here, we further demonstrate that the MAPK–CREB–CYP6CM1 signaling pathway plays a pivotal role in conferring broad-spectrum resistance to neonicotinoids in B. tabaci MED. Work by others has also implicated MAPK-dependent hormonal signaling in Bt resistance in Plutella xylostella (36). These studies demonstrate convergent recruitment of the MAPK pathway in insect resistance to xenobiotics. However, to date exactly what triggers the initiation of the MAPK signaling cascade that leads to resistance is unknown (16). Here, we identify, a key GPCR NPFF2, as an upstream activator of the MAPK pathway associated with xenobiotic resistance. This finding resolves the complete signaling pathway of GPCR–MAPK–CREB–CYP6CM1 involved in B. tabaci MED resistance to neonicotinoids. Understanding this pathway sets out a framework for future studies to elucidate the genetic changes at key points in this pathway that result in the constitutive overexpression of CYP6CM1 observed in neonicotinoid resistance.
In the current study, we show that field-evolved resistance to neonicotinoids in B. tabaci MED is accompanied by strong reproductive cost. This phenotype is consistent with previous studies on the whitefly, where thiamethoxam and acetamiprid-resistant strains exhibited fitness disadvantages in reproduction (18). We further show that the reproductive cost associated with neonicotinoid resistance in B. tabaci MED is caused by abnormal ovarian development, primarily manifesting as a significant reduction in ovariole and oocyte number. This finding has parallels with work on fitness costs associated with insecticide resistance in other species, such as Bt Cry-toxin resistant Helicoverpa armigera (37) and spinosad resistant Frankliniella occidentalis (38). Our further investigation of the molecular mechanisms underlying this cost provides compelling evidence that the reduced expression of key genes involved in oogenesis, Ex, Va, Bg, and Vg, specifically in the oocyte, have a previously unappreciated role in the observed defects in ovarian development, thereby leading to the reproductive cost. Beyond whiteflies, delayed ovarian development in Cry-toxin resistant H. armigera, has also been associated with the downregulation of Vg (37). Beyond Vg, our results provide insight into the role of Va, Ex, and Bg in ovarian development in a nonmodel insect pest. Further work is now merited to investigate the precise functional role of these genes in oogenesis and how this function is disrupted in insecticide-resistant insects. Intriguingly, we find the decreased expression of Va, Ex, and Bg are negatively regulated by p38 and ERK owing to the pleotropic effects of MAPK pathway. In addition to the role of MAPK pathway in regulating insecticide resistance, these findings conclusively demonstrate the dual function of components of the MAPK cascades in fitness trade-offs associated with it.
Genes involved in resistance to xenobiotics and important physiological processes are commonly regulated by core TFs, which belong to the nuclear receptor (NR), bHLH-PAS, and bZIP superfamilies (33). Among them, the bZIP members CREB (21) and CncC (39) have been shown to play a key role in regulating xenobiotic detoxification and insecticide resistance in insect pests. In the current study, we further demonstrate the role of CREB as an activator of CYP6CM1 in broad-spectrum resistance to neonicotinoids. We also show that CncC negatively regulates Ex, Va, and Bg. Importantly, we demonstrate that both CREB and CncC are activated by the same MAPK p38/ERK pathway. Collectively, these findings reveal that activation of this common signaling pathway in resistant B. tabaci MED leads to both the beneficial resistance trait and the negative impacts on reproductive fitness. More broadly, we illustrate the key role of the MAPK pathway as a master regulator underpinning an evolutionary trade-offs. In agreement with a recent study reporting the regulation of CncC by adenosine monophosphate-activated protein kinase (AMPK) (40), our study provides additional evidence that the protein kinases p38 and ERK can act as upstream activators of CncC. Previous research has shown that CncC heterodimerizes with another TF, small-muscle aponeurrosis fibromatosis (Maf), which then translocates to the nucleus to regulate gene expression (40). Thus, further work is required to investigate the interaction between CncC and Maf and the role, if any, of the latter in regulating insecticide resistance and the associated fitness cost in B. tabaci MED.
A key question emerging from our previous work on the regulation of resistance genes in B. tabaci MED (16) is related to the nature of the upstream actor that triggers the MAPK signaling cascade in insecticide-resistant strains. Here, we show that NPFF2, belonging to a member of the rhodopsin family of GPCRs, can activate components of the MAPK signaling pathway associated with neonicotinoid resistance and its reproductive cost. GPCRs are well known for their functions in the transduction of extracellular signals to the intracellular environment (41). In insects, GPCRs are involved in regulating development, reproduction, and behavior (42). In relation to our findings, a recent study has revealed juvenile hormone (JH)/GPCR-stimulated vitellogenin receptor (VgR) phosphorylation, Vg internalization, and VgR recycling (43), indicating the potential role of GPCRs in regulating reproduction by their action on VgR. Although several previous studies have implicated GPCRs in insecticide resistance (44), their precise role in this trait remains poorly understood. Thus, our findings provide insight into the role of GPCRs in insecticide resistance while also demonstrating their pleiotropic effect on reproduction. Phosphorylation of intracellular residues is the most extensively studied posttranslation modification (PTM) regulating GPCR activity (31). Here, we show that exposure to neonicotinoids increased the phosphorylated form of NPFF2, suggesting NPFF2 phosphorylation can be activated by xenobiotic exposure. Furthermore, we identify a positive feedback loop whereby phosphorylation of p38 can promote phosphorylation of NPFF2, which in turn activates the MAPK p38 and ERK pathways. This phosphorylation-mediated feedback loop between NPFF2 and p38 ensures that resistance mediated by a typically responsive signaling pathway (MAPK) is converted into a constitutive mechanism. Constitutive mechanisms of resistance are often more advantageous in the context of insecticide resistance as they provide immediate protection against fast-acting chemical insecticides. However, in this case, the evolution of constitutive resistance also results in a continuous cost on female fecundity.
In conclusion, we find a complete GPCR–MAPK-initiated signaling cascades underpinning fitness trade-offs-associated xenobiotic resistance in an insect (Fig. 6). More than twenty years ago, it was proposed that trans-acting regulatory loci which upregulate P450s involved in insecticide detoxification might also coregulate genes involved in other functions leading to a physiological cost via negative pleiotropy (45). Our findings thus provide a compelling empirical example that this can and does happen and illustrate how the evolution of resistance involving broad-spectrum master regulators, such as components of the GPCR–MAPK signaling pathways, can carry an Achilles’s Heel in the form of a significant fitness cost due to the pleiotropic roles of these genes. From an applied perspective, our findings provide promise that it should be possible to effectively manage resistance mediated by the GPCR–MAPK signaling pathway by employing alternation of insecticides with differing modes of action. Such management strategies rely on the fact that resistance to insecticides belonging to one mode of action will carry a fitness cost resulting in the frequency of resistance to this insecticide class declining when a compound of a different mode of action is used.
Materials and Methods
A detailed description of insects, insecticides, and bioassays, life-table study, evaluation of fitness costs, female fecundity analysis, ovarian development measurement, microscopy, RNA extraction, molecular cloning, phylogenetic and bioinformatic analysis, qRT-PCR, screening of oogenesis genes, western blot, antibody availability, immunofluorescence, RNAi, inhibitor and activator test, cell culture and transfection, plasmid construction, dual-lucifersae reporter assay, promoter analysis, TF analysis, binding site analysis, recombinant protein preparation, EMSA, IP, Y2H, RNAi-IP, ChIP-qPCR, protein phosphorylation detection, Phos-tag assays, statistical analyses, and data visualization are described in SI Appendix, Materials and Methods.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
This research was supported by the National Natural Science Foundation of China (32122073, 32221004, 32202360, and 32272598), Beijing Natural Science Foundation (6212031), China Agriculture Research System (CARS-24-C-02), the 2020 Research Program of Sanya Yazhou Bay Science and Technology City (SKJC-2020-02-012) and the European Research Council under the European Union’s Horizon 2020 research and innovation programme (646625).
Author contributions
C.B., X.Y., and Y.Z. designed research; B.F., J.L., J.H., T.D., Q.T., and M.H. performed research; C.H., X.W., J.Y., L.G., K.L., and R.N. contributed new reagents/analytic tools; B.F., J.H., P.G., S.L., L.G., K.L., and X.Y. analyzed data; and B.F., X.Z., C.B., and X.Y. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Chris Bass, Email: c.bass@exeter.ac.uk.
Xin Yang, Email: yangxin@caas.cn.
Youjun Zhang, Email: zhangyoujun@caas.cn.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Gould F., et al. , Wicked evolution: Can we address the sociobiological dilemma of pesticide resistance? Science 360, 728–732 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Gao M. J., et al. , Ca2+ sensor-mediated ROS scavenging suppresses rice immunity and is exploited by a fungal effector. Cell 184, 5391–5404 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Weber J. N., et al. , Evolutionary gain and loss of a pathological immune response to parasitism. Science 377, 1206–1211 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kliot A., Ghanim M., Fitness costs associated with insecticide resistance. Pest Manag. Sci. 68, 1431–1437 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Stork N. E., How many species of insects and other terrestrial arthropods are there on Earth? Annu. Rev. Entomol. 63, 31–45 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Arthropod Pesticide Resistance Database (APRD). https://www.pesticideresistance.org/. Accessed 30 December 2023.
- 7.Liu N. N., Insecticide resistance in mosquitoes: Impact, mechanisms, and research directions. Annu. Rev. Entomol. 60, 537–559 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Ffrench-Constant R. H., Bass C., Does resistance really carry a fitness cost? Curr. Opin. Insect Sci. 21, 39–46 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tchouakui M., et al. , Cytochrome P450 metabolic resistance (CYP6P9a) to pyrethroids imposes a fitness cost in the major African malaria vector Anopheles funestus. Heredity 124, 621–632 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coustau C., et al. , Resistance to xenobiotics and parasites: Can we count the cost? Trends Ecol. Evol. 15, 378–383 (2000). [DOI] [PubMed] [Google Scholar]
- 11.Xia J. X., et al. , Whitefly hijacks a plant detoxification gene that neutralizes plant toxins. Cell 184, 1693–1705 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Mastuda K., et al. , Neonicotinoid insecticides: Molecular target, resistance, and toxicity. Annu. Rev. Pharmacol. Toxicol. 60, 241–245 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Horowitz A. R., et al. , Insecticide resistance and its management in Bemisia tabaci species. J. Pest Sci. 93, 893–910 (2020). [Google Scholar]
- 14.Karunker I., et al. , Structural model and functional characterization of the Bemisia tabaci CYP6CM1vQ, a cytochrome P450 associated with high level of imidacloprid resistance. Insect Biochem. Mol. Biol. 39, 697–706 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Nauen R., et al. , Development of a lateral flow test to detect metabolic resistance in Bemisia tabaci mediated by CYP6CM1, a cytochrome P450 with broad spectrum catalytic efficiency. Pestic. Biochem. Physiol. 121, 3–11 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Yang X., et al. , MAPK-directed activation of the whitefly transcription factor CREB leads to P450-mediated imidacloprid resistance. Proc. Natl. Acad. Sci. U.S.A. 117, 10246–10253 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivero A., et al. , Energetic cost of insecticide resistance in Culex pipiens mosquitoes. J. Med. Entomol. 48, 694–700 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Hu J. Y., et al. , CYP4CS5-mediated thiamethoxam and clothianidin resistance is accompanied by fitness cost in the whitefly Bemisia tabaci. Pest Manag. Sci. 80, 910–921 (2023), 10.1002/ps.7826. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y. C., et al. , Dinotefuran resistance in Nilaparvata lugens: Resistance monitoring, inheritance, resistance mechanism and fitness costs. J. Pest Sci. 96, 1213–1227 (2023). [Google Scholar]
- 20.Nauen R., et al. , The role of cytochrome P450s in insect toxicology and resistance. Annu. Rev. Entomol. 67, 105–124 (2022). [DOI] [PubMed] [Google Scholar]
- 21.Pym A., et al. , A single point mutation in the Bemisia tabaci cytochrome-P450 CYP6CM1 causes enhanced resistance to neonicotinoids. Insect Biochem. Mol. Biol. 156, 103934 (2023). [DOI] [PubMed] [Google Scholar]
- 22.Chi H., Su H. Y., Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 35, 10–21 (2006). [Google Scholar]
- 23.Roy S., et al. , Regulatory pathways controlling female insect reproduction. Annu. Rev. Entomol. 63, 489–511 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Luo W., et al. , Juvenile hormone signaling promotes ovulation and maintains egg shape by inducing expression of extracellular matrix genes. Proc. Natl. Acad. Sci. U.S.A. 118, e2104461118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie T., Spradling A. C., A niche maintaining germ line stem cells in the Drosophila ovary. Science 290, 328–330 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Hayashi Y., et al. , The regulation of Drosophila ovarian stem cell niches by signaling crosstalk. Curr. Opin. Insect Sci. 37, 23–29 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Chang L., Karin M., Mammalian MAP kinase signaling cascades. Nature 410, 37–40 (2001). [DOI] [PubMed] [Google Scholar]
- 28.Goldsmith Z. G., Dhanasekaran D. N., G protein regulation of MAPK networks. Oncogene 26, 3122 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Kwon Y., et al. , Non-canonical β-adrenergic activation of ERK at endosomes. Nature 611, 173–179 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang N. N., et al. , Transcriptome profiling of the whitefly, Bemisia tabaci, reveals stage-specific gene expression signatures for thiamethoxam resistance. Insect Mol. Biol. 22, 485–496 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonini J. A., et al. , Identification and characterization of two G protein-coupled receptors for neuropeptide FF. J. Biol. Chem. 275, 39324–39331 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Mollereau C., et al. , Neuropeptide FF receptor modulates potassium currents in a dorsal root ganglion cell line. Pharmacol. Rep. 63, 1061–1065 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Bray L., et al. , Identification and functional characterization of the phosphorylation sites of the neuropeptide FF2 receptor. J. Biol. Chem. 289, 33754–33766 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amezian D., et al. , Transcriptional regulation of xenobiotic detoxification genes in insects—An overview. Pestic. Biochem. Physiol. 174, 104822 (2021). [DOI] [PubMed] [Google Scholar]
- 35.Ragab A., et al. , Drosophila Ras/MAPK signaling regulates innate immune responses in immune and intestinal stem cells. EMBO J. 30, 1123–1136 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo Z. J., et al. , A single transcription factor facilitates an insect host combating Bacillus thuringiensis infection while maintaining fitness. Nat. Commun. 13, 6024 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang W. N., et al. , Tradeoff between reproduction and resistance evolution to Bt-toxin in Helicoverpa armigera: Regulated by vitellogenin gene expression. Bull. Entomol. Res. 104, 444–452 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Hua D. K., et al. , Fitness cost of spinosad resistance related to vitellogenin in Frankliniella occidentalis (Pergande). Pest Manag. Sci. 79, 771–780 (2022). [DOI] [PubMed] [Google Scholar]
- 39.Kalsi M., et al. , Transcription factor cap n collar C regulates multiple cytochrome P450 genes conferring adaptation to potato plant allelochemicals and resistance to imidacloprid in Leptinotarsa decemlineata (Say). Insect Biochem. Mol. Biol. 83, 1–12 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Jiang H., et al. , The pleiotropic AMPK-CncC signaling pathway regulates the trade-off between detoxification and reproduction. Proc. Natl. Acad. Sci. U.S.A. 120, e2214038120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hill C. A., et al. , G protein-coupled receptors in Anopheles gambiae. Science 298, 176–178 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Audsley N., Down R. N., G protein coupled receptors as targets for next generation pesticides. Insect Biochem. Mol. Biol. 67, 27–37 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Jing Y. P., et al. , The vitellogenin receptor functionality of the migratory locust depends on its phosphorylation by juvenile hormone. Proc. Natl. Acad. Sci. U.S.A. 118, e2106908118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma Z., et al. , The role of G protein-coupled receptor-related genes in cytochrome P450-mediated resistance of the house fly, Musca domestica (Diptera: Muscidae), to imidacloprid. Insect Mol. Biol. 29, 92–103 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Feyereisen R., Insect P450 enzymes. Annu. Rev. Entomol. 44, 507–533 (1999). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.