Abstract
Poor air quality accounts for more than 9 million deaths a year globally according to recent estimates. A large portion of these deaths are attributable to cardiovascular causes, with evidence indicating that air pollution may also play an important role in the genesis of key cardiometabolic risk factors. Air pollution is not experienced in isolation but is part of a complex system, influenced by a host of other external environmental exposures, and interacting with intrinsic biologic factors and susceptibility to ultimately determine cardiovascular and metabolic outcomes. Given that the same fossil fuel emission sources that cause climate change also result in air pollution, there is a need for robust approaches that can not only limit climate change but also eliminate air pollution health effects, with an emphasis of protecting the most susceptible but also targeting interventions at the most vulnerable populations. In this review, we summarize the current state of epidemiologic and mechanistic evidence underpinning the association of air pollution with cardiometabolic disease and how complex interactions with other exposures and individual characteristics may modify these associations. We identify gaps in the current literature and suggest emerging approaches for policy makers to holistically approach cardiometabolic health risk and impact assessment.
Keywords: air pollution, cardiometabolic, cardiovascular diseases, disparities, exposome, particulate matter
Globally, poor air quality accounts for >6 million deaths per year according to the Global Burden of Disease in 2019 although recent estimates have suggested that this number may actually be above 9 million.1 Air pollution accounts for 2.9 fewer years of life on average worldwide compared with 2.2 years for tobacco smoking, highlighting the importance of ambient air pollution as a global risk factor.2 In 2019, the total economic cost of air pollution equated to over $8 trillion, which is equivalent to over 6.1% of the global annual GDP.2 In setting new aspirational global guidelines for air quality with an annual average for particulate matter <2.5 μm (PM2.5) of 5 μg/m3, the World Health Organization acknowledges that over 99% of the world’s population lives in an area with harmful levels of air pollution.2,3
There is a large body of epidemiological evidence indicating that air pollution–mediated effects on global mortality and morbidity are predominantly driven by its effects on atherosclerotic cardiovascular disease (CVD). Growing evidence also links air pollution to cardiometabolic risk factors including obesity, diabetes, and hypertension that may represent important pathways to clinical atherosclerotic CVD events.1,4 It has also become increasingly clear that air pollution is seldom experienced in isolation from other environmental factors but occurs and is influenced by exposures such as the built and natural environment and interacts with a variety of extrinsic, as well as intrinsic biological factors including intrinsic susceptibility, in a complex system to impact cardiovascular health (Figure 1).5 Persistent disparities in air pollution health impacts and new sources of air pollution attributable to climate change, have renewed the urgency to better understand sources, characterize health effects, and disseminate this information for personal protection and policy impact.6
Figure 1.
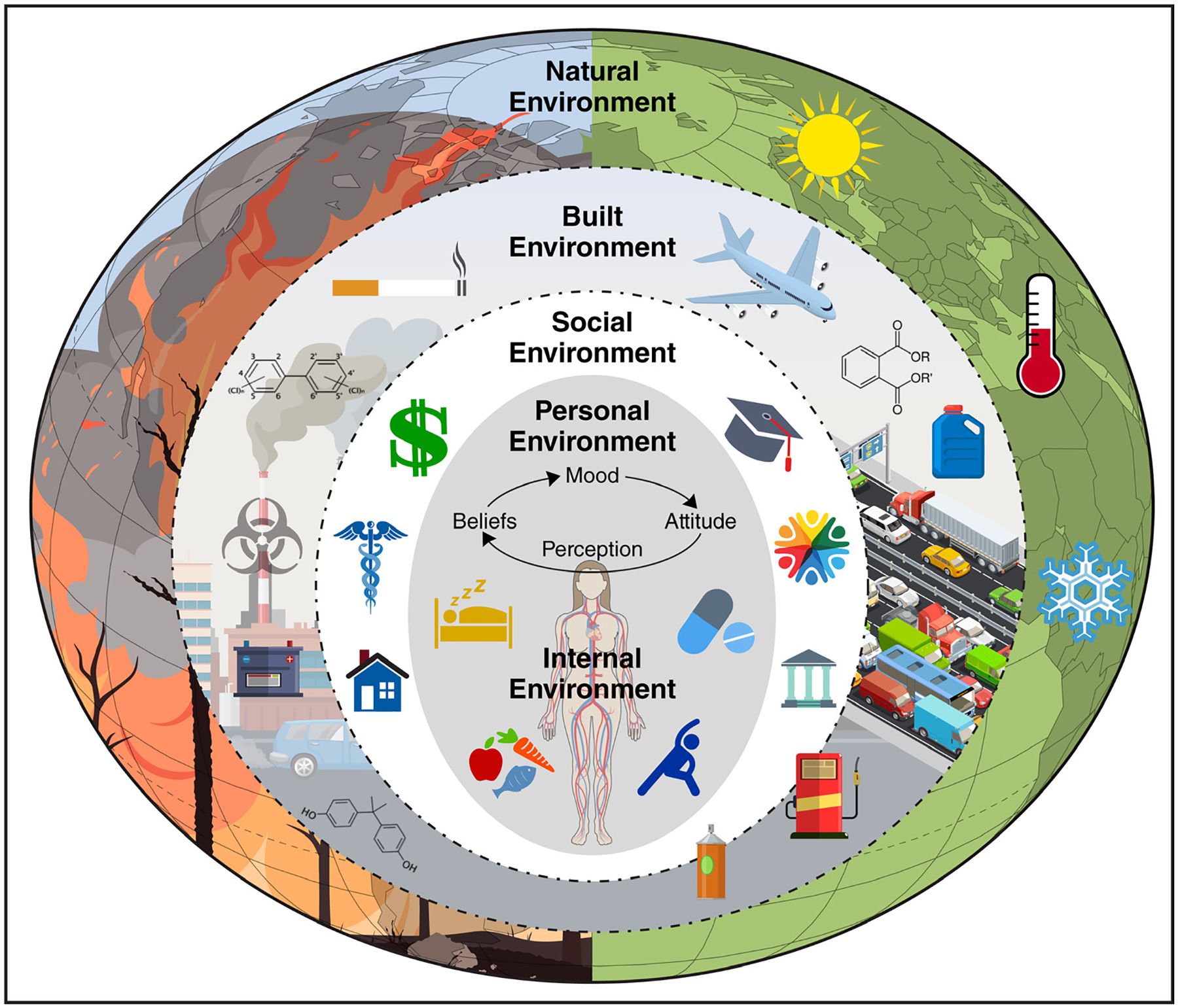
The relationship between intrinsic susceptibility and extrinsic factors in the environment, which determines vulnerable environments and populations.
An integrated understanding that requires a shift in a one-exposure-at-a-time approach to a systems framework is much needed. In 2005, Wild7 introduced the term exposome to encompass the totality of exposures from a variety of external and internal sources over a complete lifetime. Current epidemiological studies are focusing on operationalizing the concept of the exposome, at least with regard to a list of important exposures, to improve our understanding of the effects of multiple factors in the external environment, experienced concurrently.8 Additionally, research has focused on identifying vulnerable populations, defined by age, sex, race, and socioeconomic factors. This integrated understanding is important for policymakers, health care providers, and public health professionals to help facilitate integrated solutions that may mitigate the effects of air pollution, especially among the most vulnerable subgroups, and to develop health impact assessment tools. In this review, we summarize the current state of the epidemiological evidence of the association of air pollution with cardiovascular health, the mechanisms by which air pollution affects cardiovascular health often together with other exposures, and how these complex interactions and individual characteristics may modify these associations. Finally, we suggest current challenges and opportunities in the field. In this review, the use of the word exposome is limited to exposures in the external environment and their health impact on atherosclerotic cardiovascular outcomes.
AIR POLLUTION SOURCES AND CONSTITUENTS
The sources of air pollution and its complex chemistry have been reviewed extensively in prior reviews, but, briefly, both natural and anthropogenic sources emit primary air pollutants comprising of particulate and gaseous components, such as particulate matter (PM), sulfur dioxide, and nitrogen oxides (NOx).9,10 Primary pollutants are formed and emitted directly from sources, while secondary pollutants are those that are formed as part of atmospheric chemistry and include secondary NOx gases, ozone, secondary nitrates, and sulfate particles. The Environmental Protection Agency sets National Ambient Air Quality Standards for 6 principal criteria air pollutants—NOx, sulfur oxides, PM, carbon monoxide, O3, and lead—all of which have been shown to be harmful to public health and the environment. In February 2024, the EPA finalized revisions to the annual health–based National Ambient Air Quality Standard for fine PM (PM2.5) from a level of 12 to 9 μg/m3.11 The EPA estimates that this revision alone will prevent up to 4500 premature deaths, yielding up to $46 billion in net health benefits in 2032.
PM is a blend of solids and liquids that arise from natural (desert dust, sand, wildfires, and salt) and anthropogenic sources (burning fossil fuels). PM can be categorized by size into thoracic particles (PM10—aerodynamic diameter <10 μm), fine particles (PM2.5—aerodynamic diameter <2.5 μm), and ultrafine particles (UFPs or PM0.1—aerodynamic diameter <0.1 μm). The size of PM determines its final destination within the body; PM10 is filtered by the respiratory tract, while PM2.5 and PM0.1 can migrate from the lungs, eliciting systemic inflammatory and prothrombotic effects. The toxicity of air pollutant components is directly related to characteristics of its physicochemical characteristics including composition, size/morphology, oxidative stress potential, charge, solubility, surface area, particle count, and lung deposition. Meteorologic conditions such as wind speed, humidity, temperature, and diurnal changes can alter the composition and propagation of air pollution, affecting the size of the population exposed.9,12
Both anthropogenic and nonanthropogenic sources may contribute to PM2.5 in urban environments.13,14 Differences in air pollutant concentrations within cities or regions are greater for primary (ie, directly emitted) pollutants, such as nitrogen dioxide (NO2) and UFPs, compared with secondary pollutants, such as ozone and a portion of PM2.5.1,15 Anthropogenic PM2.5 is directly emitted from sources such as power plants, traffic, factories, transportation, and port activities but can be secondarily formed in the atmosphere from myriad sources and chemical interactions.13 Desert dust, sand, wildfires, and salt are common sources of nonanthropogenic PM2.5. Biomass burning due to forest fires, dust derived from construction, crustal material, tire wear and road dust, and secondary organic aerosols may dominate PM2.5 mass in some environments and its oxidative potential.13,16
The evidence to date suggests that the cardiovascular toxicity of nonfossil fuel sources may not be any less deleterious, and, in the case of wildfire PM2.5 toxicity, there is evidence that the cardiovascular and respiratory toxicity may even be higher.16 The sources of PM2.5 have a major impact on its composition and consequent health effects.17,18 Traffic-related air pollution is often composed of a large component of organic aerosols. Ultrafine components in the particulate phase are particularly prevalent in vehicular exhaust and include volatile organic compounds that change dramatically in size, composition, and oxidative potential, both spatially and temporally. Hence, with quantification and characterization of traffic-related air pollution and its health impact, an emerging and important area of research may mandate high spatial resolution and more sophisticated chemical speciation approaches.19,20
NOx is a group of highly reactive gases formed primarily from the combustion of fossil fuels and may serve as a surrogate for traffic-related air pollution and other reactive components such as volatile organic compounds.21,22 NO2 is created during combustion not only from coal, oil, methane gas, or diesel from cars and other vehicles, power plants, and off-road equipment but also from biomass burning. In urban areas, stationary power generation and traffic are the dominant sources. Sulfur oxides are generated in a similar manner as NOx, from fuels high in sulfur content such as coal. Sulfates and nitrate salts are generated via complex photochemical reactions initiated by intermediate free radicals catalyzed by metals and dependent on atmospheric humidity. Haze episodes in winter are dominated by drastically elevated concentrations of PM2.5 containing high levels of nitrate and sulfates. Ambient SO42– formation is also chemically linked to NOx and volatile organic compounds through the intermediate formation of reactive oxygen species and, thus, is dependent on NOx levels.
Multiple aspects of the physical and social environment can also modify the health effects of air pollution exposure, including poor housing, lack of green space, poor diet, and access to health care.23–26 Household air pollution encompasses a range of pollution from diverse sources, including solid fuels used for cooking and heat, and remains a considerable problem globally. In countries with high levels of ambient air pollution, penetration of outdoor particles is well known to determine indoor air pollution levels and health effects.27
AIR POLLUTION AND CARDIOMETABOLIC RISK EPIDEMIOLOGY
An extensive body of epidemiological literature exists on the adverse cardiovascular and metabolic effects of both short- and long-term exposures to air pollution.1,19,28–31 Specifically, there is substantial evidence of association with CVD mortality, ischemic heart disease incident acute myocardial infarction and stroke (ischemic and hemorrhagic), heart failure hospitalizations or death, mortality in heart transplant recipients, hypertension, and dyslipidemia. These studies have been compiled and interpreted in many recent reviews.32–35
In a recent umbrella analysis of systematic reviews and meta-analyses, a total of 56 reviews (10 systematic reviews and 46 meta-analyses) were identified.34 Nearly 21 studies (4 systematic reviews and 17 meta-analyses) were published between 2010 and 2020, therefore representing rather recent trends in air pollution levels across the globe. Both short- and long-term associations of PM2.5 were robustly associated with cardiovascular mortality.34 The meta meta-analytic estimates for cardiovascular mortality ranged from 0.64% to 1.00%/10-μg/m3 increase in PM2.5. The estimates for myocardial infarction with short-term increases in PM2.5 ranged from 1.2 to 2.4, while that for stroke ranged from 0.8 to 2.2 for every 10-μg/m3 increase in PM2.5. These estimates are broadly consistent with prior meta-analytical estimates where short-term exposure (over hours to days) increases the risk for cardiovascular mortality, myocardial infarction, stroke, heart failure, and sudden death, each by about 1% to 2% per 10 μg/m3.10 Long-term exposure over months to years amplifies these risks by nearly an order of magnitude for cardiovascular mortality.19 Results for cardiovascular mortality, stroke, and myocardial infarction with chronic exposures in the umbrella review were broadly consistent with prior estimates of 5% to 10% per 10 μg/m3.10,34 In contrast, the long-term effect of PM2.5 and NOx on heart failure demonstrated uninformative associations with large CIs.34 The equivalent estimates for NO2 with regard to cardiovascular mortality were 0.88 to 1.62 and 3 to 23 for each 10-μg/m3 increase in short- and long-term exposures, respectively.34 In contrast to the relatively robust associations between PM2.5 and stroke and myocardial infarction, few studies have noted stable associations between the long-term effects of NOx. This may partly relate to the fact that short-term changes in NOx, which are typically influenced by traffic, are substantial and could account for a large proportion of the short-term attributable risk. In contrast, long-term levels may not vary as much. Biological explanations, relating to the potent chemistry of NOx (highly reactive in the short term but may be less potent in the longer term), may be plausible. Studies on the impact of long-term exposure to PM2.5 and peripheral arterial disease are scarce.36 In a population-based study in Germany, an increase in PM2.5 exposure was associated with abnormal ankle-brachial index (high or low; odds ratio, 1.59 [95% CI, 1.01–2.51]).37 There is also growing evidence of associations between air pollutants and multiple surrogate markers for atherosclerosis including arterial stiffness, endothelial function, coronary artery calcium score, and carotid artery intima thickness.19 Although there are emerging data suggesting that UFPs may exert potent cardiovascular effects, the epidemiology of UFPs continues to evolve. One challenge is the accurate quantification of these particles. In the National Particle Component Toxicity research program, fossil fuel combustion source categories were most consistently associated with both short- and long-term adverse effects of PM2.5 exposure.38 From a source perspective, traffic sources and industrial and power categories result in residual oil combustion and are most closely associated with short-term effects and components, with coal combustion closely associated with long-term effects.39
There is substantial geographic variability across the globe in health effects attributable to air pollution due to differences in concentrations, exposure sources, and susceptible subpopulations.34,40 The Global Burden of Disease exposure-response function derived from many of the same studies included in the umbrella review helps understand how even low concentrations of air pollution have a cardiovascular impact. In the Global Exposure Mortality Model, involving only ambient air pollution cohorts (41 cohorts from 16 countries, including China, allowing a broad range of exposures), the global burden of deaths attributable to PM2.5 was 8.9 million deaths with >50% from ischemic heart disease and stroke.41 The shape of this dose-response curve for ischemic heart disease mortality is nearly linear, with little evidence of flattening across current global pollutant levels and no lower concentration threshold, below which exposures can be considered safe at the population level. Air pollution is well known to cross national boundaries and has substantial cross-boundary and even cross-continental impacts. A prior study demonstrated that about 12% of air pollution attributed to PM2.5 was emitted in a region of the world other than that in which the death occurred, and about 22% were associated with goods and services produced in 1 region for consumption in another.42
Air pollution has been extensively implicated in the pathogenesis of hypertension.30,31 A recent study estimated the extent of global blood pressure (BP) elevation attributable to air pollution above the World Health Organization recommended safe threshold of 5 μg/m3 for PM2.5 assuming a mean global annual population-weighted PM2.5 exposure of 42.6 μg/m3 (Figure 2).29 PM2.5 levels above the World Health Organization Air Quality Guidelines of 5 μg/m3 in 2019 was associated with 2.4 and 1.2 mmHg increase in systolic and diastolic BP, respectively (Figure 3). Countries with the highest excess BP levels (in millimeters of mercury) included India (4.9/2.4), Nepal (4.9/2.4), Niger (4.7/2.3), Qatar (4.5/2.2), and Nigeria (4.1/2.0). Given relatively low PM2.5 levels in the United States (7.7 μg/m3), the associated excess BP was small (0.2/0.1).29
Figure 2. Global relationship between excess blood pressure (BP) and particulate matter <2.5 μm (PM2.5) levels.
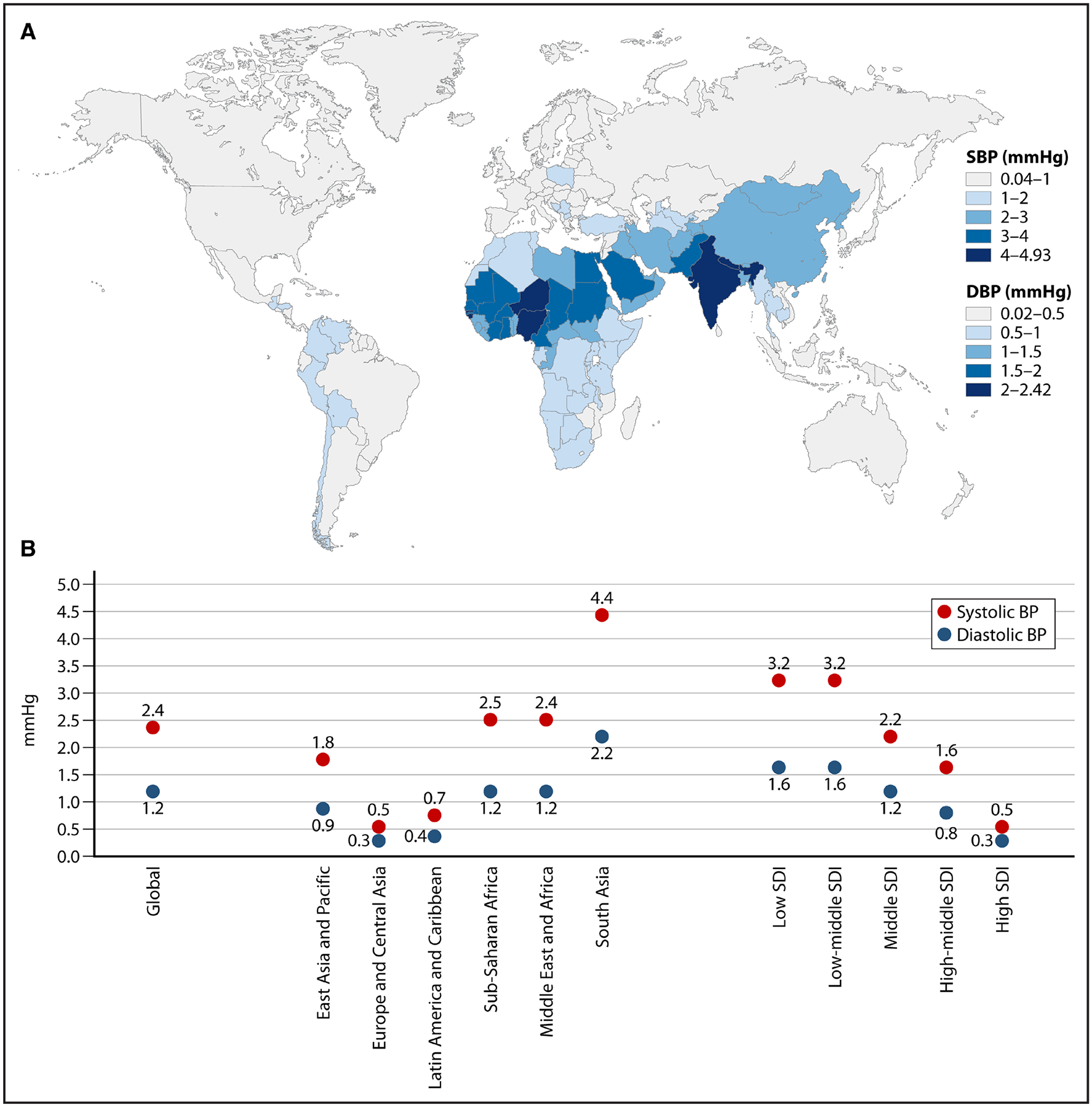
A, Geographic distribution of estimated excess BP associated with fine PM2.5 levels above the World Health Organization Air Quality Guidelines of 5 μg/m3 in 2019. B, World map showing excess systolic and diastolic BP (using the 2021 World Health Organization Air Quality Guidelines) in each country and by specific groupings (World Bank regions and sociodemographic index [SDI]). DBP indicates diastolic blood pressure; and SBP, systolic blood pressure.
Figure 3. Data science approaches to derive exposomic signatures.
CNN indicates convolutional neural network; DL, deep learning; and ML, machine learning.
A recent review has extensively detailed the impact of air pollution on insulin resistance and diabetes including underlying mechanisms.28 The Global Burden of Disease investigators found that 20% of all diabetes-related deaths and disability-adjusted life-years globally were attributed to PM2.5 (≈13.5% ambient and 6.5% household air pollution).43 Several nationwide cohort studies in adults in China have also demonstrated a link between air pollution, obesity, and other metabolic syndrome components particularly at higher levels of air pollutants.44 In a meta-analysis to estimate the effects of childhood exposure to air pollutants, obesity and body mass index (BMI) were both associated with PM10, PM2.5, and PM0.1.45 Longitudinal studies examining links between PM2.5 and obesity have been largely negative at lower annual PM2.5 concentrations (median ≤20 μg/m3).46–48
In summary, evidence supports a link between air pollution and clinical CVD, as well as multiple key cardiometabolic risk factors that may be important in driving CVD risk.
SUSCEPTIBLE SUBPOPULATIONS
Increased susceptibility defines individuals (eg, elderly adults, diabetics) who are at higher risk for adverse health effects than the general population when facing the same level of exposure.49 Vulnerability from an epidemiological and policy standpoint generally refers to a broader construct of populations who face disproportionately high exposures and, hence, a greater burden of health effects either because of their built, social, or natural environment (Figure 1). The Clean Air Act requires the US EPA to identify and protect not only the general public but also the most vulnerable populations to guide interventions and inform regulatory policy.50 The World Health Organization specifies vulnerable populations based on both innate and acquired environmental, social, or behavioral factors.51 This has been a growing area of emphasis in the air pollution and cardiovascular literature, driven by the increased emphasis on environmental justice.49,50,52 In the following, we highlight key vulnerable subgroups consistently examined in the literature—specifically defined by age, sex, race, and socioeconomic status (SES).
Age
Given the progressive nature of the atherosclerotic disease, cardiovascular risk due to exposure to air pollution has generally been found to be highest in older populations53–55 These populations have higher CVD risk even in the absence of air pollution, but air pollution has been shown to worsen cardiovascular health by accelerating atherosclerotic change.56 In the European Study of Cohorts for Air Pollution Effects (ESCAPE) project, a meta-analysis of 11 European cohorts consisting of 100 166 participants, the highest air pollution-related cardiovascular risk was among those over 60. The study found that for every 5-μg/m3 increase in long-term PM2.5 exposure, nonfatal acute coronary event risk increased by 13% among this population.57 In many large US cohorts, age continues to represent one of the most important determinants of air pollution health effects.54,55
Sex
There has been long-term interest in understanding sex differences in air pollution/CVD associations. In one recent review of over 100 studies, researchers found a stronger association between PM2.5 and CVD morbidity in women compared with men.58 In a separate review and meta-analyses of studies that included 3.6 million ischemic heart disease (IHD) and 1.3 million stroke cases among 63.7 million participants, researchers found that a higher level of PM2.5 exposure was associated with an increased risk of IHD in women than in men; similar differences were not observed for stroke.59 Consistent sex differences have not been identified in all studies, and the mechanism for these differences is not clear. Therefore, additional research is needed to understand whether air pollution is a sex-specific risk factor for CVD and the underlying causal processes in the association.
Race/Ethnicity/Nativity
It is well-documented that racial/ethnic minorities, particularly in the United States, are at a higher risk of CVD due to air pollution.55,60–62 In other parts of the world, where the social construct of race is not as applicable, these disparities play out more frequently by nativity.60,63,64 There are 2 hypotheses driving these disparities, either that the populations themselves are more susceptible due to chronic disadvantage or they have higher risks because of increased exposures.65,66 Also, often, it is difficult to disentangle the effects of minority status from differences in SES.
Environmental racism refers to the fact that environmental exposures disproportionately impact disadvantaged individuals and communities.67 These adverse exposures at least in the United States are often rooted in environmental policies and decisions throughout history that has disproportionately impacted marginalized groups and placed them at increased risk for health outcomes.67–70 A recent report showed that, on average, Black, Asian, and Hispanic or Latino populations have higher exposures to PM2.5 than their white counterparts in the United States,71 with similar patterns playing out around the world.72–75 Conversely, the pollution reduction benefits are also likely to be unevenly distributed within a population and require additional knowledge on which subgroups may derive preferential benefit.65
Socioeconomic Status
SES has been shown to be associated with both levels of exposure to air pollution and the magnitude of the association of air pollution with CVD risk. A review of 37 studies found that economically disadvantaged communities in North America, Asia, and Africa may experience higher levels of air pollution, but this relationship is less clear in Europe.23 A 2012 analysis of long-term exposures over 6 years in 215 US Census tracts found that individuals with less than a high school education had 6.2% higher PM2.5 exposure concentration than individuals with a college education.76 Similar results were found in studies on populations in New Zealand, Hong Kong, Ghana, and Europe.77–79 The association between low SES and air pollution was noted several decades ago and, indeed, had been shown to worsen the adverse effects of short- and long-term exposures to air pollution.54,80–82 More recently, researchers have begun to use indices capturing multiple domains of SES and deprivation as modifiers. In US population-level studies, these social deprivation indices have been found to modify the association between ambient air pollution and cardiovascular mortality.83–85 Social deprivation is a construct that may reflect an increased risk for adverse health outcomes that are dependent on many factors, including income, education, employment, access to transportation, and housing. Validated indices such as the social deprivation index or the social vulnerability index compute a score using a mix of factors that reflect living conditions, education, income, and access to services. Higher CVD mortality has been noted in counties with high social vulnerability, measured using the Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry social vulnerability index, with similar results among subgroups of CVD mortality.32 In a US study that linked county-level cardiovascular mortality with PM2.5 levels, both PM2.5 (β [SE], 7.584 [0.938]; P<0.001) and social deprivation index scores (β [SE], 0.591 [0.140]; P<0.001) were independently associated with age-adjusted cardiovascular mortality (R2=0.341). The association between PM2.5 and cardiovascular mortality was stronger among counties with the highest social deprivation index (Pinteraction=0.012). The Environmental Justice Index developed using data from the US Census Bureau, the US Environmental Protection Agency, the US Mine Safety and Health Administration, and the US Centers for Disease Control and Prevention provides additional information beyond the social vulnerability index by accounting for environmental factors.1
FROM MULTIPLE EXTERNAL EXPOSURES TO THE EXPOSOME
Increasingly, researchers and policymakers have begun to explore the interactions between multiple components of the exposome on the risk of CVD.15,86 This is vitally important because exposures are not experienced in isolation and, especially now due to climate change, these interactions between exposures may be changing in complex ways with unforeseen consequences. Additionally, as the world explores climate change mitigation and adaptation strategies, considering the potential impacts on other exposures and health is vital to maximize potential cobenefits.
A growing number of studies, especially from Europe, have conducted analyses exploring the independent and joint associations between transportation-related noise and air pollution on cardiovascular outcomes.87,88 The rationale behind exploring these exposures simultaneously is the common exposure source of transportation. In many studies, noise appears to modestly attenuate air pollution associations. For example, Héritier et al89 conducted an analysis in a nationwide study in Switzerland with the aim of understanding the concurrent risks of air pollution (PM2.5 and NO2) and transportation noise sources (road, railway, and aircraft) on myocardial infarction mortality in the Swiss National Cohort. The hazard ratios (HRs) for both PM2.5 (HR per 10 μg/m3, 1.02 [95% CI, 1.01–1.04]) and NO2 (HR per 10 μg/m3, 1.05 [95% CI, 1.01–1.09]) were attenuated by noise (PM2.5: HR, 0.99 [95% CI, 0.97–1.02]; NO2: HR, 1.02 [95% CI, 0.97–1.07]).89 In a study in Germany, noise slightly attenuated associations of PM2.5 with hypertension from an odds ratio of 1.15 (95% CI, 1.02–130) to 1.11 (95% CI, 0.98–1.27) for each 1-μg/m3 increase.90
A smaller number of studies have started to explore interactions between air pollution and the built and natural environment.5 The built environment refers to places built or designed by humans including parks, buildings, transportation infrastructure, and man-made green spaces.91 The natural environment and green spaces have been shown to modulate cardiovascular and metabolic risk factors and have been previously reviewed in a policy review from the American Heart Association.92 Similar to the rationale for exploring interactions with transportation noise, many aspects of urban design and land use characteristics may directly influence air pollution exposure, as well as directly impacting cardiometabolic health including mobility, housing, transport mode, and recreational and natural tree covers.93 For example, car-centric infrastructure reduces active mobility and physical activity, simultaneously leads to higher levels of environmental stressors, such as air pollution, noise, and heat islands, and increases cardiovascular morbidity and mortality. In contrast, green residential communities, active transportation, and community layouts that reduce air pollution exposure while promoting social interactions may help attenuate the cardiovascular health effects of air pollution.5 There is good evidence that individual components of urban design can reduce disparate environmental exposures through synergistic impacts. In a study in rural China, the negative associations of PM1 on cardiac conduction parameters were mitigated with increasing greenness surrounding the residence.94 Similar attenuations have been observed in studies of other cardiac outcomes around the world.95–97
Driven by an interest in how associations with air pollution may change under climate change and reflecting differences in air pollution sources and components in different seasons, many studies have explored associations between various air pollutants and climatic components (eg, temperature [especially extremes], humidity) with a variety of cardiovascular outcomes.98,99 In a time-series study in Hong Kong, the detrimental effects of air pollution on emergency IHD admissions were highest in the cool and dry season.100 In another study in Lanzhou city, China,101 the associations of air pollution with coronary heart disease hospitalizations were also greater during the cold season. However, the bulk of the literature to date focuses on the impacts of air pollution on the effects of temperature as opposed to the other way around.
Recent studies highlight the necessity and challenges of a multiexposure framework to understand the environmental CVD burden.32 Poulsen et al102 used data from a nationwide Danish cohort and considered road traffic noise and green space together with air pollution exposures. To quantify the cumulative burden of multiple exposures, they calculated a cumulative risk index per interquartile range increase. In multipollutant models adjusted for individual and neighborhood sociodemographic covariates, PM2.5, noise, and lack of green space within 150 m of the residence were all independently associated with the risk of myocardial infarction. The cumulative risk index was 1.09 [95% CI, 1.08–1.10], and all 3 factors were statistically significant contributors.102 A cohort study in Denmark investigated the health effects of 4 air pollutants (PM2.5, ultrafine, elemental carbon, and NO2), traffic noise, and lack of green space within 150 and 1000 m from the residence.103 The exposure assessments were sophisticated and obtained at high spatial and temporal resolutions. Combustion-related air pollution typified by UFPs and NO2, road traffic noise, and lack of green space were all independently associated with a higher risk of type 2 diabetes with the estimated cumulative risk for all exposures, higher than for any single exposure. These studies, while being a step in the right direction, do not address multiple other exposures including those attributable to chemical pollution.
To move from a limited framework of external exposures to a true exposomic framework will involve the incorporation of geospatial features of the natural and built environment, light pollution, and other chemical exposures in the environment in the air, water, and soil, constituting the pollutome and ultimately predictors in the internal environment such as genetic, metabolomic, radiomic, transcriptomic, epigenomic, and epitranscriptomic features.32
AIR POLLUTION PATHOPHYSIOLOGICAL MECHANISMS
The mechanistic pathways linking air pollution to cardiovascular outcomes have been well-described in prior studies.1,10,104 Mechanisms linking air pollution to CVD can be broadly summarized in 3 main pathways: (1) initial local response in the lung, (2) systemic transmission and dissemination, and (3) end-organ effector pathways.19 Many of these pathways intersect and may occur simultaneously and not necessarily in the same temporal sequence to produce complex effects on cardiovascular health. Upon exposure to PM, oxidative stress and local inflammatory response in the lungs and pulmonary vasculature trigger activation of both innate and adaptive immunity mechanisms.19 Inhaled PM2.5 may result in the release of inflammatory mediators that may result in the activation of pattern recognition receptors such as TLRs (toll-like receptors).105 Long-term exposure to PM2.5 leads to upregulation of prothrombotic and inflammatory pathways.106 The rapidity of onset of changes in vasomotor tone together with changes in BP and autonomic tone strongly implicates activation of neural pathways that may transmit signals systemically.1,10,19 Systemic effector pathways involved in atherosclerosis are also mediated by increased production of biological oxidative intermediates, all of which may lead to exaggerated inflammatory responses.107,108 Unlike larger PM, ultrafine PM can bypass pulmonary defenses and enter the systemic circulation more easily, where it can generate local inflammatory responses and cause direct cellular injury.106,109 These particles are also thought to cross the blood-brain barrier and upregulate central nervous system efferent inflammatory pathways, increasing sympathetic tone and arterial pressure.109 Ultrafine PM may disrupt the hypothalamic-pituitary-adrenal axis directly activating the sympathetic nervous system activation and contributing to insulin resistance.10 PM leads to pulmonary and peripheral platelet sensitization, increasing the potential for prothrombotic responses.10 PM2.5 exposure in animal models results in circadian rhythm disruptions that may link long-term PM exposure to chronic cardiometabolic dysfunction.110 There is evidence of epigenetic changes after PM exposure as well, but additional research is required, and the pathophysiologic mechanisms are not well understood.10 In a retrospective, mechanistic evaluation of the combined effect of air pollution and noise, 474 individuals without active cancer or known CVD in Boston were imaged with clinical (18)F-FDG-PET/CT (2-deoxy-2-[fluorine-18]fluoro- D-glucose integrated with computed tomography) for the evaluation of residual cancer. Arterial inflammation was measured as a secondary read out and linked to regional monitors reporting PM2.5 and transportation noise. Higher exposures were defined as noise >55 dBA (World Health Organization cutoff) and PM2.5 >median. Exposure to an increasing number of pollutants associated with higher arterial inflammation (standardized beta, 0.195 [95% CI, 0.052–0.339]; P=0.008) and >10-fold increased risk of major adverse cardiovascular events (MACE; relative to pollutant unexposed) in fully adjusted models (11.844 [95% CI, 3.154–44.475]; P<0.001). In mediation analysis, arterial inflammation partially mediated the relationship between pollutant exposures and MACE (P<0.05).111
ALLOSTATIC LOAD IN RESPONSE TO ENVIRONMENTAL STRESSORS
Allostatic load is a concept that was introduced in 1993 by McEwen and Stellar112 as a way to explain how cumulative, chronic exposure to stressors (including SES and environmental exposures) may result in noncommunicable diseases. Today, the allostatic load concept provides a unified pathway of how simultaneous, chronic exposure to the exposome would lead to physiological stress and disturb baseline homeostatic functions. Cortisol levels have been used as a primary marker of allostatic load.113 As mentioned above, stress hormones and sympathetic activation result in circadian rhythm disruption. In a metanalysis of 6 studies, the allostatic load was associated with cardiovascular mortality (HR, 1.31 [95% CI, 1.10–1.57]; I2>90%).113 There is growing evidence that allostatic load increases with increasing exposure to air pollution, offering a potential way to assess cumulative impacts of the overall burden of stressor experienced.114,115 Prior experimental studies have demonstrated that circadian genes ranked among the top differentially expressed genes in response to air pollution exposure.116 Mice exposed to concentrated ambient PM2.5 demonstrated insulin resistance, reduced energy expenditure, metabolism, thermogenesis, and dysregulated circadian genes, which reversed with cessation.116 In a study comparing exposure to PM2.5 exposure and light at night exposure, although PM2.5 and light at night induced an identical phenotype of insulin resistance and metabolic dysfunction, the transcriptional and epigenetic pathways, including differentially expressed circadian genes, seemed to differ between these 2 exposures. In humans, metabolic syndrome parameters, sleep deprivation, and depression (circadian syndrome) have been highly correlated with PM2.5 exposure.110 The cascade of circadian disruption is commonly associated with stress mediators such as catecholamines and cortisol. Cortisol levels indeed have been used as a primary marker of allostatic load. Increased secretion of corticosterone and catecholamines has been demonstrated in animal models and humans in response to both PM2.5 and ozone exposure.110,117–120 In a post hoc analysis of the MESA cohort, higher levels of annual NO2 (a traffic pollutant) and PM2.5 were associated with higher epinephrine and dopamine levels.121
FUTURE DIRECTIONS MEASURING AND QUANTIFYING AIR POLLUTION EXPOSURE LEVELS
While remote estimates of PM concentrations using satellites by aerosol optical depth, chemical transport models, and ensemble approaches have been transformative for research and policymaking purposes, accurate estimations of personal exposure based on such approaches may be challenging. Therefore, as technologies have become widely available, there has been a move towards deploying low-cost stationary and portable sensor systems to help provide data more accurate estimation of personal exposures and to extend the spatial and temporal resolutions of existing networks. To date, there has not been a study of sufficient size combining personal monitoring and CVD outcomes other than biological markers or BP/electrophysiology.122 Deploying personal monitoring is still not feasible for large cohorts or for the decades needed to examine associations with CVD mortality, IHD, or stroke. A growing literature has combined personal monitoring in small subsamples with model predictions in the full sample to adjust effect estimates for measurement error.123–128 The availability of low-cost monitors embedded in the urban environments in multiple locations may be able to provide the next best solution to personal monitoring, but standardization of measurements and the challenge using of high temporal resolution data (eg, minutes or even hourly data) remain challenges.129
INTEGRATED TOOLS FOR CUMULATIVE HEALTH IMPACT
Integrated approaches that incorporate multiple exposures and their aggregate effects defined as the exposome have been proposed by us and others.32,86,130–132 Addressing that the sum totality of exposures is a departure from the current one exposure at a time framework to the simultaneous examination of multiple exposures is necessary as the impact of many aggregate exposures may not be linear or additive.80 The integration of climate, environmental, social, and health data into common platforms and the use of machine learning and artificial intelligence to explore climate and human health effects provides an unprecedented opportunity for Health Impact Assessment (HIA) and policy.133–135
In the field of exposure science, new sensor technologies in wearable monitors can capture multiple human microenvironments and offer the promise of true exposomic assessment. High-resolution mass spectrometry allows the measurement and identification of vast numbers of exogenous and endogenous chemicals in a single analytical run. High-resolution mass spectrometry methods can detect small molecules such as pharmaceuticals, pesticides, microbial metabolites, and other exogenous chemicals that may be present in the soil, water, or even in the human plasma and can be mapped at least at the census tract level for a start. Identification of chemical species is currently complicated, as chemicals undergo transformation. While computational tools can predict many transformations, predicting first-order reactions alone can be challenging, let alone second-order reactions. Approaches to reduce complexity and dimension reduction such as grouping chemicals by their sources into groups and grouping exposures, analogous to genotype variants, and linking their relationship to a phenotype of interest are increasingly being used. Additional complexities in the statistical toolset and assumptions, which assumes that all chemicals are randomly distributed and measured with equal precision, can be fallacious. Thus, identifying exposomic relationships from high-dimensional data comprising the pollutome poses major statistical and computational challenges.
Currently, most health risk–assessment tools are at the census tract level, which is not helpful for individual-level decision-making but can be important in population health interventions and policy. A good example of this type of integrated online mapper is the California Communities Environmental Health Screening Tool (CalEnviroScreen), developed by the California Office of Environmental Health Hazard Assessment. CalEnviroScreen enables researchers, community groups, and the public to identify communities burdened by multiple sources of pollution and health and socioeconomic vulnerabilities136 The 2021 version of CalEnviroScreen is composed of 21 indicators, 13 of which estimate exposures to different environmental pollutants and 8 of which identify population characteristics that may increase vulnerability (eg, education, unemployment, poverty, comorbidities). These scores are combined to calculate an overall cumulative impact score.137 However, despite these limitations, the use of such tools may provide useful information for future policy recommendations. A recent study evaluated the impact of future climate emission mitigation scenarios, using the Climate and Economic Justice Screening Tool to identify disadvantaged communities and prioritize them for government programs and funding based on climate and environmental burdens and socioeconomic indicators.65 In the study, the Black population was the most exposed racial-ethnic group to PM2.5. Under the business-as-usual scenario for limiting fossil fuel emissions, exposure disparities to PM2.5 by race and ethnicity persisted and remained higher than average for Black, Hispanic, and Asian populations. The 2 scenarios with enhanced emission reductions eliminated absolute and relative disparities for disadvantaged communities and for low-income populations but not the disparities by race or ethnicity. This finding underscores that new regulatory strategies for emission reduction (deviating from business-as-usual) are needed to also help address PM2.5 exposure disparities. Additional geospatial tools and artificial intelligence approaches can markedly enhance the ability to integrate features in the built environment to predict heart disease. In a recent article, we demonstrated that built environment features extracted from Google Street Views from 7 cities across the United States using deep learning predicted 63% of the census tract variation in coronary heart disease prevalence. The study obtained 0.53 million Google street views (GSV) images covering 789 census tracts in seven US cities (Cleveland, OH; Fremont, CA; Kansas City, MO; Detroit, MI; Bellevue, WA; Brownsville, TX; and Denver, CO). The addition of Google Street View features improved a model that included census tract level age, sex, race, income, and education or composite indices of social determinants of health. Google Street View images of seven US cities were associated with 63% of the variance in prevalence of coronary heart disease (CHD; Figure 4). Compared with a model including age, sex, race, income, education, and composite indices for social determinants of health, the addition of GSV features enhanced the association of CHD. Activation maps from the features revealed a set of neighborhood features represented by buildings and roads that were associated with high coronary disease prevalence.138 Systems science approaches such as community-based systems can additionally help resolve complex issues related to climate change, pollution, and health equity in a contextually rich approach that includes spatial relationships and community-based systems dynamic modeling.139,140
Figure 4. Artificial intelligence–enabled approach to detect novel geospatial determinants of health.
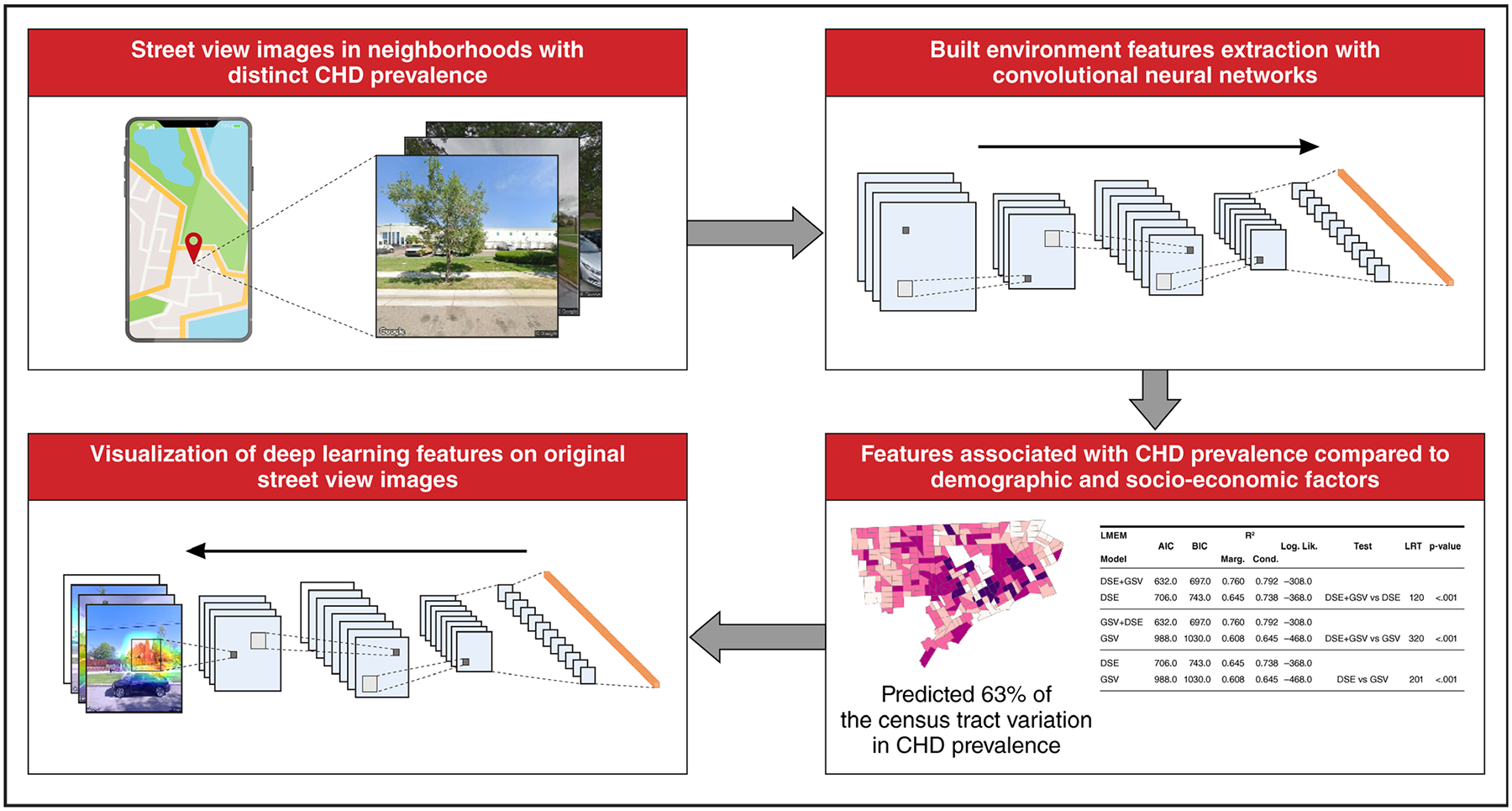
AIC indicates Akaike information criterion; BIC, Bayesian information criterion; CHD, coronary heart disease; DSE, demographic, social and environmental (city + sex + age + race + income + education); GSV, Google street views; LMEM, linear mixed effects model; and LRT, likelihood ratio test.
CONCLUSIONS
As we have summarized above, there is strong and consistent evidence that air pollution is a major driver of CVD and cardiometabolic risk factors based on robust mechanistic and epidemiological findings. However, there is an urgent need to incorporate additional domains of the external environment and, ultimately, the incorporation of measures of the pollutome and that of the internal environment. The successful implementation of a true exposomic framework remains a challenge and will need the satisfactory resolution of many issues. The toll of air pollution and environmental pollution at large disproportionately affects vulnerable populations defined by age, sex, race/ethnicity/nativity, and SES. It is also vital to identify other individual or area-level factors that increase resilience to pollutant exposure. The integration of climate, environmental, social, and health data into common platforms and the use of machine learning and artificial intelligence to explore climate and human health effects provide an unprecedented opportunity for HIA and policy.
Sources of Funding
S. Rajagopalan was supported by grants 1R35ES031702, R01ES017290, R01 ES033670-01, and P50 MD017351-01.
Nonstandard Abbreviations and Acronyms
- BP
blood pressure
- CVD
cardiovascular disease
- HR
hazard ratio
- NO2
nitrogen dioxide
- NOx
nitrogen oxides
- PM
particulate matter
- PM2.5
particulate matter <2.5 μm
- SES
socioeconomic status
- TLR
toll-like receptor
- UFP
ultrafine particle
REFERENCES
- 1.Rajagopalan S, Landrigan PJ. Pollution and the heart. N Engl J Med. 2021;385:1881–1892. doi: 10.1056/NEJMra2030281 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO global air quality guidelines: particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide: executive summary. World Health Organization. Accessed April 2, 2024. https://apps.who.int/iris/handle/10665/345334 [PubMed] [Google Scholar]
- 3.World Health Organization. WHO global air quality guidelines: executive summary. World Health Organization; 2021. Accessed on March 20, 2024. 2021. https://www.who.int/publications/i/item/9789240034228 [Google Scholar]
- 4.Rajagopalan S, Brook RD, Salerno P, Bourges-Sevenier B, Landrigan P, Nieuwenhuijsen MJ, Munzel T, Deo SV, Al-Kindi S. Air pollution exposure and cardiometabolic risk. Lancet Diabetes Endocrinol. 2024;12:196–208. doi: 10.1016/S2213-8587(23)00361-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajagopalan S, Ramaswami A, Bhatnagar A, Brook RD, Fenton M, Gardner C, Neff R, Russell AG, Seto KC, Whitsel LP; on behalf of the American Heart Association Council on Hypertension; Council on Lifestyle and Cardiometabolic Health; Council on Peripheral Vascular Disease; Council on Lifelong Congenital Heart Disease and Heart Health in the Young; Council on Cardiovascular Surgery and Anesthesia; and the American Heart Association Advocacy Coordinating Committee. Toward heart-healthy and sustainable cities: a policy statement from the American Heart Association. Circulation. 2024;149:e1067–e1089. doi: 10.1161/CIR.0000000000001217 [DOI] [PubMed] [Google Scholar]
- 6.Kaufman JD, Elkind MSV, Bhatnagar A, Koehler K, Balmes JR, Sidney S, Burroughs Pena MS, Dockery DW, Hou L, Brook RD, et al. ; American Heart Association Advocacy Coordinating Committee. Guidance to reduce the cardiovascular burden of ambient air pollutants: a policy statement from the American Heart Association. Circulation. 2020;142:e432–e447. doi: 10.1161/CIR.0000000000000930 [DOI] [PubMed] [Google Scholar]
- 7.Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14:1847–1850. doi: 10.1158/1055-9965.EPI-05-0456 [DOI] [PubMed] [Google Scholar]
- 8.Miller GW, Jones DP. The nature of nurture: refining the definition of the exposome. Toxicol Sci. 2014;137:1–2. doi: 10.1093/toxsci/kft251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, et al. ; American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1 [DOI] [PubMed] [Google Scholar]
- 10.Rajagopalan S, Al-Kindi SG, Brook RD. Air pollution and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72:2054–2070. doi: 10.1016/j.jacc.2018.07.099 [DOI] [PubMed] [Google Scholar]
- 11.EPA finalizes stronger standards for harmful soot pollution. Accessed on March 20, 2024. 2024. https://www.epa.gov/newsreleases/epa-finalizes-stronger-standards-harmful-soot-pollution-significantly-increasing
- 12.Kumar P, Morawska L, Birmili W, Paasonen P, Hu M, Kulmala M, Harrison RM, Norford L, Britter R. Ultrafine particles in cities. Environ Int. 2014;66:1–10. doi: 10.1016/j.envint.2014.01.013 [DOI] [PubMed] [Google Scholar]
- 13.Daellenbach KR, Uzu G, Jiang J, Cassagnes LE, Leni Z, Vlachou A, Stefenelli G, Canonaco F, Weber S, Segers A, et al. Sources of particulate-matter air pollution and its oxidative potential in Europe. Nature. 2020;587:414–419. doi: 10.1038/s41586-020-2902-8 [DOI] [PubMed] [Google Scholar]
- 14.Rajagopalan S, Vergara-Martel A, Zhong Z, Khraishah H, Kosiborod M, Neeland IJ, Munzel T, Brook RD, Nieuwenhuijsen M, Hovmand P, et al. The urban environment and cardiometabolic health. Circulation. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motairek I, Makhlouf MHE, Rajagopalan S, Al-Kindi S. The exposome and cardiovascular health. Can J Cardiol. 2023;39:1191–1203. doi: 10.1016/j.cjca.2023.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadley MB, Henderson SB, Brauer M, Vedanthan R. Protecting cardiovascular health from wildfire smoke. Circulation. 2022;146:788–801. doi: 10.1161/CIRCULATIONAHA.121.058058 [DOI] [PubMed] [Google Scholar]
- 17.Hopke PK, Ito K, Mar T, Christensen WF, Eatough DJ, Henry RC, Kim E, Laden F, Lall R, Larson TV, et al. PM source apportionment and health effects: 1. Intercomparison of source apportionment results. J Expo Sci Environ Epidemiol. 2006;16:275–286. doi: 10.1038/sj.jea.7500458 [DOI] [PubMed] [Google Scholar]
- 18.Laden F, Neas LM, Dockery DW, Schwartz J. Association of fine particulate matter from different sources with daily mortality in six U.S. cities. Environ Health Perspect. 2000;108:941–947. doi: 10.1289/ehp.00108941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bevan GH, Al-Kindi SG, Brook RD, Münzel T, Rajagopalan S. Ambient air pollution and atherosclerosis: insights into dose, time, and mechanisms. Arterioscler Thromb Vasc Biol. 2021;41:628–637. doi: 10.1161/atvbaha.120.315219 [DOI] [PubMed] [Google Scholar]
- 20.Karagulian F, Belis CA, Dora CFC, Prüss-Ustün AM, Bonjour S, Adair-Rohani H, Amann M. Contributions to cities’ ambient particulate matter (PM): a systematic review of local source contributions at global level. Atmos Environ. 2015;120:475–483. doi: 10.1016/j.atmosenv.2015.08.087 [DOI] [Google Scholar]
- 21.International Agency for Research on Cancer. Outdoor Air Pollution: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans (IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans, 109). World Health Organization; 2016. [Google Scholar]
- 22.U.S. Environmental Protection Agency (EPA). Basic Information about NO2. Accessed February 2. https://www.epa.gov/no2-pollution/basic-information-about-no2#:~:text=Nitrogen%20Dioxide%20(NO2)%20is,from%20the%20burning%20of%20fuel
- 23.Hajat A, Hsia C, O’Neill MS. Socioeconomic disparities and air pollution exposure: a global review. Curr Environ Health Rep. 2015;2:440–450. doi: 10.1007/s40572-015-0069-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Havard S, Deguen S, Zmirou-Navier D, Schillinger C, Bard D. Traffic-related air pollution and socioeconomic status: a spatial autocorrelation study to assess environmental equity on a small-area scale. Epidemiology. 2009;20:223–230. doi: 10.1097/EDE.0b013e31819464e1 [DOI] [PubMed] [Google Scholar]
- 25.Mitchell R, Popham F. Effect of exposure to natural environment on health inequalities: an observational population study. Lancet. 2008;372:1655–1660. doi: 10.1016/S0140-6736(08)61689-X [DOI] [PubMed] [Google Scholar]
- 26.Rajagopalan S, Brook RD. The indoor-outdoor air-pollution continuum and the burden of cardiovascular disease: an opportunity for improving global health. Glob Heart. 2012;7:207–213. doi: 10.1016/j.gheart.2012.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balmes JR. Household air pollution from domestic combustion of solid fuels and health. J Allergy Clin Immunol. 2019;143:1979–1987. doi: 10.1016/j.jaci.2019.04.016 [DOI] [PubMed] [Google Scholar]
- 28.Rajagopalan S, Brook RD, Salerno P, Bourges-Sevenier B, Landrigan P, Nieuwenhuijsen MJ, Munzel T, Deo SV, Al-Kindi S. Air pollution exposure and cardiometabolic risk. Lancet Diabetes Endocrinol. 2024;12:196–208. doi: 10.1016/S2213-8587(23)00361-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brook RD, Motairek I, Rajagopalan S, Al-Kindi S. Excess global blood pressure associated with fine particulate matter air pollution levels exceeding World Health Organization guidelines. J Am Heart Assoc. 2023;12:e029206. doi: 10.1161/JAHA.122.029206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hahad O, Rajagopalan S, Lelieveld J, Sorensen M, Frenis K, Daiber A, Basner M, Nieuwenhuijsen M, Brook RD, Munzel T. Noise and air pollution as risk factors for hypertension: part I-epidemiology. Hypertension. 2023;80:1375–1383. doi: 10.1161/HYPERTENSIONAHA.122.18732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hahad O, Rajagopalan S, Lelieveld J, Sorensen M, Kuntic M, Daiber A, Basner M, Nieuwenhuijsen M, Brook RD, Munzel T. Noise and air pollution as risk factors for hypertension: part II-pathophysiologic insight. Hypertension. 2023;80:1384–1392. doi: 10.1161/HYPERTENSIONAHA.123.20617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Münzel T, Sørensen M, Hahad O, Nieuwenhuijsen M, Daiber A. The contribution of the exposome to the burden of cardiovascular disease. Nat Rev Cardiol. 2023;20:651–669. doi: 10.1038/s41569-023-00873-3 [DOI] [PubMed] [Google Scholar]
- 33.Dominski FH, Lorenzetti Branco JH, Buonanno G, Stabile L, Gameiro da Silva M, Andrade A. Effects of air pollution on health: a mapping review of systematic reviews and meta-analyses. Environ Res. 2021;201:111487. doi: 10.1016/j.envres.2021.111487 [DOI] [PubMed] [Google Scholar]
- 34.de Bont J, Jaganathan S, Dahlquist M, Persson A, Stafoggia M, Ljungman P. Ambient air pollution and cardiovascular diseases: an umbrella review of systematic reviews and meta-analyses. J Intern Med. 2022;291:779–800. doi: 10.1111/joim.13467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhatnagar A Cardiovascular effects of particulate air pollution. Annu Rev Med. 2022;73:393–406. doi: 10.1146/annurev-med-042220-011549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kloog I Fine particulate matter (PM2.5) association with peripheral artery disease admissions in northeastern United States. Int J Environ Health Res. 2016;26:572–577. doi: 10.1080/09603123.2016.1217315 [DOI] [PubMed] [Google Scholar]
- 37.Zhang S, Wolf K, Breitner S, Kronenberg F, Stafoggia M, Peters A, Schneider A. Long-term effects of air pollution on ankle-brachial index. Environ Int. 2018;118:17–25. doi: 10.1016/j.envint.2018.05.025 [DOI] [PubMed] [Google Scholar]
- 38.Vedal S, Campen MJ, McDonald JD, Larson TV, Sampson PD, Sheppard L, Simpson CD, Szpiro AA. National Particle Component Toxicity (NPACT) initiative report on cardiovascular effects. Res Rep Health Eff Inst. 2013;5:8. [PubMed] [Google Scholar]
- 39.Lippmann M, Chen LC, Gordon T, Ito K, Thurston GD. National Particle Component Toxicity (NPACT) Initiative: integrated epidemiologic and toxicologic studies of the health effects of particulate matter components. Res Rep Health Eff Inst. 2013;5:13. [PubMed] [Google Scholar]
- 40.Vohra K, Vodonos A, Schwartz J, Marais EA, Sulprizio MP, Mickley LJ. Global mortality from outdoor fine particle pollution generated by fossil fuel combustion: results from GEOS-Chem. Environ Res. 2021;195:110754. doi: 10.1016/j.envres.2021.110754 [DOI] [PubMed] [Google Scholar]
- 41.Burnett R, Chen H, Szyszkowicz M, Fann N, Hubbell B, Pope CA, Apte JS, Brauer M, Cohen A, Weichenthal S, et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci USA. 2018;115:9592–9597. doi: 10.1073/pnas.1803222115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Q, Jiang X, Tong D, Davis SJ, Zhao H, Geng G, Feng T, Zheng B, Lu Z, Streets DG, et al. Transboundary health impacts of transported global air pollution and international trade. Nature. 2017;543:705–709. doi: 10.1038/nature21712 [DOI] [PubMed] [Google Scholar]
- 43.Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, Basu N, Baldé AB, Bertollini R, Bose-O’Reilly S, Boufford JI, et al. The lancet commission on pollution and health. Lancet. 2018;391:462–512. doi: 10.1016/S0140-6736(17)32345-0 [DOI] [PubMed] [Google Scholar]
- 44.Li X, Wang M, Song Y, Ma H, Zhou T, Liang Z, Qi L. Obesity and the relation between joint exposure to ambient air pollutants and incident type 2 diabetes: a cohort study in UK Biobank. PLoS Med. 2021;18:e1003767. doi: 10.1371/journal.pmed.1003767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang C, Li C, Zhao F, Zhu J, Wang S, Sun G. The association between childhood exposure to ambient air pollution and obesity: a systematic review and meta-analysis. Int J Environ Res Public Health. 2022;19:4491. doi: 10.3390/ijerph19084491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matthiessen C, Lucht S, Hennig F, Ohlwein S, Jakobs H, Jockel KH, Moebus S, Hoffmann B; Heinz Nixdorf Recall Study Investigative Group., Heinz Nixdorf Recall Study Investigative G. Long-term exposure to airborne particulate matter and NO2 and prevalent and incident metabolic syndrome - results from the Heinz Nixdorf Recall Study. Environ Int. 2018;116:74–82. doi: 10.1016/j.envint.2018.02.035 [DOI] [PubMed] [Google Scholar]
- 47.Kim JS, Chen Z, Alderete TL, Toledo-Corral C, Lurmann F, Berhane K, Gilliland FD. Associations of air pollution, obesity and cardiometabolic health in young adults: the Meta-AIR study. Environ Int. 2019;133:105180. doi: 10.1016/j.envint.2019.105180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voss S, Schneider A, Huth C, Wolf K, Markevych I, Schwettmann L, Rathmann W, Peters A, Breitner S. ENVINT-D-20–01309: long-term exposure to air pollution, road traffic noise, residential greenness, and prevalent and incident metabolic syndrome: results from the population-based KORA F4/FF4 cohort in Augsburg, Germany. Environ Int. 2021;147:106364. doi: 10.1016/j.envint.2020.106364 [DOI] [PubMed] [Google Scholar]
- 49.Casey JA, Daouda M, Babadi RS, Do V, Flores NM, Berzansky I, Gonzalez DJX, Van Horne YO, James-Todd T. Methods in public health environmental justice research: a scoping review from 2018 to 2021. Curr Environ Health Rep. 2023;10:312–336. doi: 10.1007/s40572-023-00406-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.National Institute of Environmental Health Sciences (NIEHS). Environmental health disparities and environmental justice. Accessed April 2, 2024. https://www.niehs.nih.gov/research/supported/translational/justice
- 51.Makri A, Stilianakis NI. Vulnerability to air pollution health effects. Int J Hyg Environ Health. 2008;211:326–336. doi: 10.1016/j.ijheh.2007.06.005 [DOI] [PubMed] [Google Scholar]
- 52.World Health Organization. U.S. EPA and WHO partner to protect public health. Accessed April 2, 2024. https://www.who.int/news/item/20-01-2022-u.s.-epa-and-world-health-organization-partner-to-protect-public-health
- 53.Newman JD, Bhatt DL, Rajagopalan S, Balmes JR, Brauer M, Breysse PN, Brown AGM, Carnethon MR, Cascio WE, Collman GW, et al. Cardiopulmonary impact of particulate air pollution in high-risk populations: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76:2878–2894. doi: 10.1016/j.jacc.2020.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di Q, Dai L, Wang Y, Zanobetti A, Choirat C, Schwartz JD, Dominici F. Association of short-term exposure to air pollution with mortality in older adults. JAMA. 2017;318:2446–2456. doi: 10.1001/jama.2017.17923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, Dominici F, Schwartz JD. Air pollution and mortality in the Medicare population. N Engl J Med. 2017;376:2513–2522. doi: 10.1056/NEJMoa1702747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brook RD, Newby DE, Rajagopalan S. The global threat of outdoor ambient air pollution to cardiovascular health: time for intervention. JAMA Cardiol. 2017;2:353–354. doi: 10.1001/jamacardio.2017.0032 [DOI] [PubMed] [Google Scholar]
- 57.Cesaroni G, Forastiere F, Stafoggia M, Andersen ZJ, Badaloni C, Beelen R, Caracciolo B, de Faire U, Erbel R, Eriksen KT, et al. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE project. BMJ. 2014;348:f7412. doi: 10.1136/bmj.f7412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liao M, Braunstein Z, Rao X. Sex differences in particulate air pollution-related cardiovascular diseases: a review of human and animal evidence. Sci Total Environ. 2023;884:163803. doi: 10.1016/j.scitotenv.2023.163803 [DOI] [PubMed] [Google Scholar]
- 59.Zhang J, Wang X, Yan M, Shan A, Wang C, Yang X, Tang N. Sex differences in cardiovascular risk associated with long-term PM2.5 exposure: a systematic review and meta-analysis of cohort studies. Front Public Health. 2022;10:802167. doi: 10.3389/fpubh.2022.802167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.So R, Chen J, Stafoggia M, de Hoogh K, Katsouyanni K, Vienneau D, Samoli E, Rodopoulou S, Loft S, Lim YH, et al. Long-term exposure to elemental components of fine particulate matter and all-natural and cause-specific mortality in a Danish nationwide administrative cohort study. Environ Res. 2023;224:115552. doi: 10.1016/j.envres.2023.115552 [DOI] [PubMed] [Google Scholar]
- 61.Kioumourtzoglou MA, Schwartz J, James P, Dominici F, Zanobetti A. PM2.5 and mortality in 207 US cities: modification by temperature and city characteristics. Epidemiology. 2016;27:221–227. doi: 10.1097/EDE.0000000000000422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bell ML, Zanobetti A, Dominici F. Evidence on vulnerability and susceptibility to health risks associated with short-term exposure to particulate matter: a systematic review and meta-analysis. Am J Epidemiol. 2013;178:865–876. doi: 10.1093/aje/kwt090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.So R, Andersen ZJ, Chen J, Stafoggia M, de Hoogh K, Katsouyanni K, Vienneau D, Rodopoulou S, Samoli E, Lim YH, et al. Long-term exposure to air pollution and mortality in a Danish nationwide administrative cohort study: beyond mortality from cardiopulmonary disease and lung cancer. Environ Int. 2022;164:107241. doi: 10.1016/j.envint.2022.107241 [DOI] [PubMed] [Google Scholar]
- 64.World Health Organization. Environmental health inequalities in Europe. Second assessment report. WHO Regional Office for Europe; 2019. [Google Scholar]
- 65.Wang Y, Apte JS, Hill JD, Ivey CE, Johnson D, Min E, Morello-Frosch R, Patterson R, Robinson AL, Tessum CW, et al. Air quality policy should quantify effects on disparities. Science. 2023;381:272–274. doi: 10.1126/science.adg9931 [DOI] [PubMed] [Google Scholar]
- 66.Nunez Y, Benavides J, Shearston JA, Krieger EM, Daouda M, Henneman LRF, McDuffie EE, Goldsmith J, Casey JA, Kioumourtzoglou MA. An environmental justice analysis of air pollution emissions in the United States from 1970 to 2010. Nat Commun. 2024;15:268. doi: 10.1038/s41467-023-43492-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bullard RD. Confronting Environmental Racism: Voices From the Grassroots. First Edition ed. South End Press; 1999. [Google Scholar]
- 68.Schinasi LH, Kanungo C, Christman Z, Barber S, Tabb L, Headen I. Associations between historical redlining and present-day heat vulnerability housing and land cover characteristics in Philadelphia, PA. J Urban Health. 2022;99:134–145. doi: 10.1007/s11524-021-00602-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Motairek I, Lee EK, Janus S, Farkouh M, Freedman D, Wright J, Nasir K, Rajagopalan S, Al-Kindi S. Historical neighborhood redlining and contemporary cardiometabolic risk. J Am Coll Cardiol. 2022;80:171–175. doi: 10.1016/j.jacc.2022.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Al-Kindi S, Motairek I, Kreatsoulas C, Wright JT, Dobre M, Rahman M, Rajagopalan S. Historical neighborhood redlining and cardiovascular risk in patients with chronic kidney disease. Circulation. 2023;148:280–282. doi: 10.1161/CIRCULATIONAHA.123.064215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jbaily A, Zhou X, Liu J, Lee TH, Kamareddine L, Verguet S, Dominici F. Air pollution exposure disparities across US population and income groups. Nature. 2022;601:228–233. doi: 10.1038/s41586-021-04190-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fong KC, Heo S, Lim CC, Kim H, Chan A, Lee W, Stewart R, Choi HM, Son JY, Bell ML. The intersection of immigrant and environmental health: a scoping review of observational population exposure and epidemiologic studies. Environ Health Perspect. 2022;130:96001. doi: 10.1289/EHP9855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van den Brekel L, Lenters V, Mackenbach JD, Hoek G, Wagtendonk A, Lakerveld J, Grobbee DE, Vaartjes I. Ethnic and socioeconomic inequalities in air pollution exposure: a cross-sectional analysis of nationwide individual-level data from the Netherlands. Lancet Planet Health. 2024;8:e18–e29. doi: 10.1016/S2542-5196(23)00258-9 [DOI] [PubMed] [Google Scholar]
- 74.Ehler I, Bader F, Rüttenauer T, Best H. The air pollution disadvantage of immigrants in Germany: partly a matter of urbanity [published online August 5, 2023]. Eur Sociol Rev. doi: 10.1093/esr/jcad046 [DOI] [Google Scholar]
- 75.Cooper N, Green D, Knibbs LD. Inequalities in exposure to the air pollutants PM2.5 and NO2 in Australia. Environ Res Lett. 2019;14:115005. doi: 10.1088/1748-9326/ab486a [DOI] [Google Scholar]
- 76.Bell ML, Ebisu K. Environmental inequality in exposures to airborne particulate matter components in the United States. Environ Health Perspect. 2012;120:1699–1704. doi: 10.1289/ehp.1205201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rooney MS, Arku RE, Dionisio KL, Paciorek C, Friedman AB, Carmichael H, Zhou Z, Hughes AF, Vallarino J, Agyei-Mensah S, et al. Spatial and temporal patterns of particulate matter sources and pollution in four communities in Accra, Ghana. Sci Total Environ. 2012;435–436:107–114. doi: 10.1016/j.scitotenv.2012.06.077 [DOI] [PubMed] [Google Scholar]
- 78.Pearce J, Kingham S. Environmental inequalities in New Zealand: a national study of air pollution and environmental justice. Geoforum. 2008;39:980–993. doi: 10.1016/j.geoforum.2007.10.007 [DOI] [Google Scholar]
- 79.Fan X, Lam KC, Yu Q. Differential exposure of the urban population to vehicular air pollution in Hong Kong. Sci Total Environ. 2012;426:211–219. doi: 10.1016/j.scitotenv.2012.03.057 [DOI] [PubMed] [Google Scholar]
- 80.Zeka A, Zanobetti A, Schwartz J. Individual-level modifiers of the effects of particulate matter on daily mortality. Am J Epidemiol. 2006;163:849–859. doi: 10.1093/aje/kwj116 [DOI] [PubMed] [Google Scholar]
- 81.O’Neill MS, Jerrett M, Kawachi I, Levy JI, Cohen AJ, Gouveia N, Wilkinson P, Fletcher T, Cifuentes L, Schwartz J; Workshop on Air Pollution and Socioeconomic Conditions. Health, wealth, and air pollution: advancing theory and methods. Environ Health Perspect. 2003;111:1861–1870. doi: 10.1289/ehp.6334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Finkelstein MM, Jerrett M, DeLuca P, Finkelstein N, Verma DK, Chapman K, Sears MR. Relation between income, air pollution and mortality: a cohort study. CMAJ. 2003;169:397–402. [PMC free article] [PubMed] [Google Scholar]
- 83.Motairek I, Sharara J, Makhlouf MHE, Dobre M, Rahman M, Rajagopalan S, Al-Kindi S. Association between particulate matter pollution and CKD mortality by social deprivation. Am J Kidney Dis. 2023;81:497–499. doi: 10.1053/j.ajkd.2022.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khan SU, Javed Z, Lone AN, Dani SS, Amin Z, Al-Kindi SG, Virani SS, Sharma G, Blankstein R, Blaha MJ, et al. Social vulnerability and premature cardiovascular mortality among US counties, 2014 to 2018. Circulation. 2021;144:1272–1279. doi: 10.1161/CIRCULATIONAHA.121.054516 [DOI] [PubMed] [Google Scholar]
- 85.Bevan G, Pandey A, Griggs S, Dalton JE, Zidar D, Patel S, Khan SU, Nasir K, Rajagopalan S, Al-Kindi S. Neighborhood-level social vulnerability and prevalence of cardiovascular risk factors and coronary heart disease. Curr Probl Cardiol. 2023;48:101182. doi: 10.1016/j.cpcardiol.2022.101182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Montone RA, Camilli M, Calvieri C, Magnani G, Bonanni A, Bhatt DL, Rajagopalan S, Crea F, Niccoli G. Exposome in ischaemic heart disease: beyond traditional risk factors. Eur Heart J. 2024;45:419–438. doi: 10.1093/eurheartj/ehae001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hahad O, Rajagopalan S, Lelieveld J, Sørensen M, Frenis K, Daiber A, Basner M, Nieuwenhuijsen M, Brook RD, Münzel T. Noise and air pollution as risk factors for hypertension: part I-epidemiology. Hypertension. 2023;80:1375–1383. doi: 10.1161/HYPERTENSIONAHA.122.18732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hahad O, Rajagopalan S, Lelieveld J, Sørensen M, Kuntic M, Daiber A, Basner M, Nieuwenhuijsen M, Brook RD, Münzel T. Noise and air pollution as risk factors for hypertension: part II-pathophysiologic insight. Hypertension. 2023;80:1384–1392. doi: 10.1161/HYPERTENSIONAHA.123.20617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Héritier H, Vienneau D, Foraster M, Eze IC, Schaffner E, de Hoogh K, Thiesse L, Rudzik F, Habermacher M, Köpfli M, et al. A systematic analysis of mutual effects of transportation noise and air pollution exposure on myocardial infarction mortality: a nationwide cohort study in Switzerland. Eur Heart J. 2019;40:598–603. doi: 10.1093/eurheartj/ehy650 [DOI] [PubMed] [Google Scholar]
- 90.Luo H, Huemer MT, Petrera A, Hauck SM, Rathmann W, Herder C, Koenig W, Hoyer A, Peters A, Thorand B. Association of plasma proteomics with incident coronary heart disease in individuals with and without type 2 diabetes: results from the population-based KORA study. Cardiovasc Diabetol. 2024;23:53. doi: 10.1186/s12933-024-02143-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.National Research Council. Committee on Physical Activity HT, Land U, National Research Council. Transportation Research B, Institute of M. Does the Built Environment Influence Physical Activity?: Examining the Evidence (Special Report (National Research Council (U S) Transportation Research Board)). Transportation Research Board; 2005. [Google Scholar]
- 92.Nieuwenhuijsen MJ. Green infrastructure and health. Annu Rev Public Health. 2021;42:317–328. doi: 10.1146/annurev-publhealth-090419-102511 [DOI] [PubMed] [Google Scholar]
- 93.Meyfroidt P, de Bremond A, Ryan CM, Archer E, Aspinall R, Chhabra A, Camara G, Corbera E, DeFries R, Díaz S, et al. Ten facts about land systems for sustainability. Proc Natl Acad Sci USA. 2022;119:e2109217118. doi: 10.1073/pnas.2109217118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baheti B, Chen G, Ding Z, Wu R, Zhang C, Zhou L, Liu X, Song X, Wang C. Residential greenness alleviated the adverse associations of long-term exposure to ambient PM1 with cardiac conduction abnormalities in rural adults. Environ Res. 2023;237:116862. doi: 10.1016/j.envres.2023.116862 [DOI] [PubMed] [Google Scholar]
- 95.Klompmaker JO, Hart JE, James P, Sabath MB, Wu X, Zanobetti A, Dominici F, Laden F. Air pollution and cardiovascular disease hospitalization - are associations modified by greenness, temperature and humidity? Environ Int. 2021;156:106715. doi: 10.1016/j.envint.2021.106715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li T, Yu Z, Xu L, Wu Y, Yu L, Yang Z, Shen P, Lin H, Shui L, Tang M, et al. Residential greenness, air pollution, and incident ischemic heart disease: a prospective cohort study in China. Sci Total Environ. 2022;838:155881. doi: 10.1016/j.scitotenv.2022.155881 [DOI] [PubMed] [Google Scholar]
- 97.Kasdagli MI, Katsouyanni K, de Hoogh K, Lagiou P, Samoli E. Associations of air pollution and greenness with mortality in Greece: an ecological study. Environ Res. 2021;196:110348. doi: 10.1016/j.envres.2020.110348 [DOI] [PubMed] [Google Scholar]
- 98.Borchert W, Grady ST, Chen J, DeVille NV, Roscoe C, Chen F, Mita C, Holland I, Wilt GE, Hu CR, et al. Air pollution and temperature: a systematic review of ubiquitous environmental exposures and sudden cardiac death. Curr Environ Health Rep. 2023;10:490–500. doi: 10.1007/s40572-023-00414-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rus AA, Mornoş C. The impact of meteorological factors and air pollutants on acute coronary syndrome. Curr Cardiol Rep. 2022;24:1337–1349. doi: 10.1007/s11886-022-01759-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qiu H, Yu ITS, Wang X, Tian L, Tse LA, Wong TW. Cool and dry weather enhances the effects of air pollution on emergency IHD hospital admissions. Int J Cardiol. 2013;168:500–505. doi: 10.1016/j.ijcard.2012.09.199 [DOI] [PubMed] [Google Scholar]
- 101.Liu M, Yu J, Zhu A, Ling J, Chen R, Zhang Y, Ruan Y. Association between air pollution and coronary heart disease hospitalizations in Lanzhou City, 2013–2020: a time series analysis. J Urban Health. 2023;100:1246–1257. doi: 10.1007/s11524-023-00797-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Poulsen AH, Sorensen M, Hvidtfeldt UA, Christensen JH, Brandt J, Frohn LM, Ketzel M, Andersen C, Jensen SS, Munzel T, et al. Concomitant exposure to air pollution, green space, and noise and risk of stroke: a cohort study from Denmark. Lancet Reg Health Eur. 2023;31:100655. doi: 10.1016/j.lanepe.2023.100655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sorensen M, Poulsen AH, Hvidtfeldt UA, Brandt J, Frohn LM, Ketzel M, Christensen JH, Im U, Khan J, Munzel T, et al. Air pollution, road traffic noise and lack of greenness and risk of type 2 diabetes: a multi-exposure prospective study covering Denmark. Environ Int. 2022;170:107570. doi: 10.1016/j.envint.2022.107570 [DOI] [PubMed] [Google Scholar]
- 104.Bevan GH, Al-Kindi SG, Brook R, Rajagopalan S. Ambient air pollution and atherosclerosis: recent updates. Curr Atheroscler Rep. 2021;23:63. doi: 10.1007/s11883-021-00958-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hiraiwa K, van Eeden SF. Contribution of lung macrophages to the inflammatory responses induced by exposure to air pollutants. Mediators Inflamm. 2013;2013:619523. doi: 10.1155/2013/619523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.O’Neill LAJI. How frustration leads to inflammation. Science. 2008;320:619–620. doi: 10.1126/science.1158398 [DOI] [PubMed] [Google Scholar]
- 107.Berliner JA, Leitinger N, Tsimikas S. The role of oxidized phospholipids in atherosclerosis. J Lipid Res. 2009;50:S207–S212. doi: 10.1194/jlr.R800074-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rao X, Zhong J, Maiseyeu A, Gopalakrishnan B, Villamena FA, Chen LC, Harkema JR, Sun Q, Rajagopalan S. CD36-dependent 7-ketocholesterol accumulation in macrophages mediates progression of atherosclerosis in response to chronic air pollution exposure. Circ Res. 2014;115:770–780. doi: 10.1161/CIRCRESAHA.115.304666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Miller MR, Raftis JB, Langrish JP, McLean SG, Samutrtai P, Connell SP, Wilson S, Vesey AT, Fokkens PHB, Boere AJF, et al. Inhaled nanoparticles accumulate at sites of vascular disease. ACS Nano. 2017;11:4542–4552. doi: 10.1021/acsnano.6b08551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Palanivel R, Vinayachandran V, Biswal S, Deiuliis JA, Padmanabhan R, Park B, Gangwar RS, Durieux JC, Ebreo Cara EA, Das L, et al. Exposure to air pollution disrupts circadian rhythm through alterations in chromatin dynamics. iScience. 2020;23:101728–101728. doi: 10.1016/j.isci.2020.101728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Osborne MT, Abohashem S, Naddaf N, Abbasi T, Zureigat H, Mezue K, Ghoneem A, Dar T, Cardeiro AJ, Mehta NN, et al. The combined effect of air and transportation noise pollution on atherosclerotic inflammation and risk of cardiovascular disease events. J Nucl Cardiol. 2023;30:665–679. doi: 10.1007/s12350-022-03003-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153:2093–2101. doi: 10.1001/archinte.153.18.2093 [DOI] [PubMed] [Google Scholar]
- 113.Parker HW, Abreu AM, Sullivan MC, Vadiveloo MK. Allostatic load and mortality: a systematic review and meta-analysis. Am J Prev Med. 2022;63:131–140. doi: 10.1016/j.amepre.2022.02.003 [DOI] [PubMed] [Google Scholar]
- 114.Xu H, Yang T, Guo B, Silang Y, Dai Y, Baima K, Gao Y, Tang S, Wei J, Jiang Y, et al. ; China Multi-Ethnic Cohort (CMEC) Collaborative Group. Increased allostatic load associated with ambient air pollution acting as a stressor: cross-sectional evidence from the China multi-ethnic cohort study. Sci Total Environ. 2022;831:155658. doi: 10.1016/j.scitotenv.2022.155658 [DOI] [PubMed] [Google Scholar]
- 115.Thomson EM. Air pollution, stress, and allostatic load: linking systemic and central nervous system impacts. J Alzheimers Dis. 2019;69:597–614. doi: 10.3233/JAD-190015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rajagopalan S, Park B, Palanivel R, Vinayachandran V, Deiuliis JA, Gangwar RS, Das LM, Yin J, Choi Y, Al-Kindi S, et al. Metabolic effects of air pollution exposure and reversibility. J Clin Invest. 2020;130:6034–6040. doi: 10.1172/jci137315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li H, Cai J, Chen R, Zhao Z, Ying Z, Wang L, Chen J, Hao K, Kinney PL, Chen H, et al. Particulate matter exposure and stress hormone levels: a randomized, double-blind, crossover trial of air purification. Circulation. 2017;136:618–627. doi: 10.1161/CIRCULATIONAHA.116.026796 [DOI] [PubMed] [Google Scholar]
- 118.Ying Z, Xu X, Bai Y, Zhong J, Chen M, Liang Y, Zhao J, Liu D, Morishita M, Sun Q. Long-term exposure to concentrated ambient PM2.5 increases mouse blood pressure through abnormal activation of the sympathetic nervous system: a role for hypothalamic inflammation. Environ Health Perspect. 2013;122:79–86. doi: 10.1289/ehp.1307151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Miller DB, Ghio AJ, Karoly ED, Bell LN, Snow SJ, Madden MC, Soukup J, Cascio WE, Gilmour MI, Kodavanti UP. Ozone exposure increases circulating stress hormones and lipid metabolites in humans. Am J Respir Crit Care Med. 2016;193:1382–1391. doi: 10.1164/rccm.201508-1599OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kodavanti UP. Stretching the stress boundary: linking air pollution health effects to a neurohormonal stress response. Biochim Biophys Acta. 2016;1860:2880–2890. doi: 10.1016/j.bbagen.2016.05.010 [DOI] [PubMed] [Google Scholar]
- 121.Hajat A, Diez Roux AV, Castro-Diehl C, Cosselman K, Golden SH, Hazlehurst MF, Szpiro A, Vedal S, Kaufman JD. The association between long-term air pollution and urinary catecholamines: evidence from the multi-ethnic study of atherosclerosis. Environ Health Perspect. 2019;127:57007. doi: 10.1289/EHP3286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bi J, Carmona N, Blanco MN, Gassett AJ, Seto E, Szpiro AA, Larson TV, Sampson PD, Kaufman JD, Sheppard L. Publicly available low-cost sensor measurements for PM2.5 exposure modeling: guidance for monitor deployment and data selection. Environ Int. 2022;158:106897. doi: 10.1016/j.envint.2021.106897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hart JE, Liao X, Hong B, Puett RC, Yanosky JD, Suh H, Kioumourtzoglou MA, Spiegelman D, Laden F. The association of long-term exposure to PM2.5 on all-cause mortality in the Nurses’ Health Study and the impact of measurement-error correction. Environ Health. 2015;14:38. doi: 10.1186/s12940-015-0027-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hart JE, Spiegelman D, Beelen R, Hoek G, Brunekreef B, Schouten LJ, van den Brandt P. Long-term ambient residential traffic-related exposures and measurement error-adjusted risk of incident lung cancer in the Netherlands cohort study on diet and cancer. Environ Health Perspect. 2015;123:860–866. doi: 10.1289/ehp.1408762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bergen S, Sheppard L, Kaufman JD, Szpiro AA. Multipollutant measurement error in air pollution epidemiology studies arising from predicting exposures with penalized regression splines. J R Stat Soc Ser C Appl Stat. 2016;65:731–753. doi: 10.1111/rssc.12144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Keller JP, Chang HH, Strickland MJ, Szpiro AA. Measurement error correction for predicted spatiotemporal air pollution exposures. Epidemiology. 2017;28:338–345. doi: 10.1097/EDE.0000000000000623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Szpiro AA, Paciorek CJ. Measurement error in two-stage analyses, with application to air pollution epidemiology. Environmetrics. 2013;24:501–517. doi: 10.1002/env.2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Katsouyanni K, Evangelopoulos D. Invited perspective: impact of exposure measurement error on effect estimates-an important and neglected problem in air pollution epidemiology. Environ Health Perspect. 2022;130:71302. doi: 10.1289/EHP11277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rajagopalan S, Brauer M, Bhatnagar A, Bhatt DL, Brook JR, Huang W, Münzel T, Newby D, Siegel J, Brook RD; On behalf of the American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; and Stroke Council. Personal-level protective actions against particulate matter air pollution exposure: a scientific statement from the American Heart Association. Circulation. 2020;142:411–431. doi: 10.1161/CIR.0000000000000931 [DOI] [PubMed] [Google Scholar]
- 130.Motairek I, Makhlouf MHE, Rajagopalan S, Al-Kindi S. The exposome and cardiovascular health. Can J Cardiol. 2023;39:1191–1203. doi: 10.1016/j.cjca.2023.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Munzel T, Sorensen M, Lelieveld J, Hahad O, Al-Kindi S, Nieuwenhuijsen M, Giles-Corti B, Daiber A, Rajagopalan S. Heart healthy cities: genetics loads the gun but the environment pulls the trigger. Eur Heart J. 2021;42:2422–2438. doi: 10.1093/eurheartj/ehab235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vermeulen R, Schymanski EL, Barabasi AL, Miller GW. The exposome and health: where chemistry meets biology. Science. 2020;367:392–396. doi: 10.1126/science.aay3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Khoury MJ, Iademarco MF, Riley WT. Precision public health for the era of precision medicine. Am J Prev Med. 2016;50:398–401. doi: 10.1016/j.amepre.2015.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pearson TA, Califf RM, Roper R, Engelgau MM, Khoury MJ, Alcantara C, Blakely C, Boyce CA, Brown M, Croxton TL, et al. Precision health analytics with predictive analytics and implementation research: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76:306–320. doi: 10.1016/j.jacc.2020.05.043 [DOI] [PubMed] [Google Scholar]
- 135.Al-Kindi S, Paneni F, Brook RD, Rajagopalan S. Residual environmental risk in patients with cardiovascular disease: an overlooked paradigm. Eur Heart J. 2023;44:4612–4614. doi: 10.1093/eurheartj/ehad412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.California Office of Environmental Health Hazard Assessment (COEHHA). CalEnviroScreen 4.0. Accessed April 2, 2024. https://oehha.ca.gov/calenviroscreen/report/calenviroscreen-40
- 137.California Office of Environmental Health Hazard Assessment (COEHHA). Draft CalEnviroScreen 4.0. Accessed April 2, 2024. https://oehha.ca.gov/calenviroscreen/report/draft-calenviroscreen-40.
- 138.Chen Z, Dazard JE, Khalifa Y, Motairek I, Al-Kindi S, Rajagopalan S. Built environment features obtained from Google Street View are associated with coronary artery disease prevalence: a deep-learning framework [published online March 28, 2024]. Eur Heart J. doi: 10.1101/2023.03.28.23287888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gullett HL, Brown GL, Collins D, Halko M, Gotler RS, Stange KC, Hovmand PS. Using community-based system dynamics to address structural racism in community health improvement. J Public Health Manag Pract. 2022;28:S130–S137. doi: 10.1097/PHH.0000000000001492 [DOI] [PubMed] [Google Scholar]
- 140.Rosenthal J, Arku RE, Baumgartner J, Brown J, Clasen T, Eisenberg JNS, Hovmand P, Jagger P, Luke DA, Quinn A, et al. Systems science approaches for global environmental health research: enhancing intervention design and implementation for Household Air Pollution (HAP) and Water, Sanitation, and Hygiene (WASH) programs. Environ Health Perspect. 2020;128:105001. doi: 10.1289/EHP7010 [DOI] [PMC free article] [PubMed] [Google Scholar]


