Abstract

Polysaccharides (PSAs) are carbohydrate-based macromolecules widely used in the biomedical field, either in their pure form or in blends/nanocomposites with other materials. The relationship between structure, properties, and functions has inspired scientists to design multifunctional PSAs for various biomedical applications by incorporating unique molecular structures and targeted bulk properties. Multiple strategies, such as conjugation, grafting, cross-linking, and functionalization, have been explored to control their mechanical properties, electrical conductivity, hydrophilicity, degradability, rheological features, and stimuli-responsiveness. For instance, custom-made PSAs are known for their worldwide biomedical applications in tissue engineering, drug/gene delivery, and regenerative medicine. Furthermore, the remarkable advancements in supramolecular engineering and chemistry have paved the way for mission-oriented biomaterial synthesis and the fabrication of customized biomaterials. These materials can synergistically combine the benefits of biology and chemistry to tackle important biomedical questions. Herein, we categorize and summarize PSAs based on their synthesis methods, and explore the main strategies used to customize their chemical structures. We then highlight various properties of PSAs using practical examples. Lastly, we thoroughly describe the biomedical applications of tailor-made PSAs, along with their current existing challenges and potential future directions.
Keywords: tailor-made, polysaccharides, biomedical applications, tissue engineering, drug delivery
1. Introduction
Polynucleotides, lipids, proteins, and polysaccharides (PSAs) represent distinct classes of covalently synthesized multifunctional biomacromolecules that are essential for life in all animals, including humans. Among these, PSAs stand out as the most abundant natural biopolymers. They are used by microorganisms, algae, plants, and animals for energy storage and/or structural support.1 PSAs are typically synthesized through a natural carbon-capture process in various photosynthetic organisms, such as plants. In this process, which harnesses the energy of sunlight, inorganic carbon present in the atmosphere as carbon dioxide is converted into organic carbons, including high-energy storage molecules such as lipids and PSAs.2 In contrast to proteins and nucleic acids, the cellular machinery does not use a template to make PSAs. Therefore, PSA synthesis naturally forms a variety of microstructures whose composition depends on the type of cells or microorganisms performing the synthesis.
PSAs are ubiquitous components of cells. A dense and complex coating of oligosaccharides (OSAs) or PSAs, also called glycans, has been observed to cover the plasma membrane of nearly all cell types in nature. The OSAs or PSAs may be conjugated with proteins or lipids, a process known as glycosylation, to aid protein folding and to make multifunctional biomacromolecules that can mediate or regulate various functions in diverse cellular interactions.3 In addition, PSAs play prominent roles in many biological processes, such as cell adhesion and recognition, differentiation, signaling, and microbial attachment.4 Multivalent protein–carbohydrate interactions are vital in such biological processes.5 Due to the bioactivity of PSAs, many PSA-containing plants, fungi, and algae have been traditionally used as medications, such as in traditional Chinese medicine.6 Since the discovery that they possess bioactivities, PSAs have also gained many clinical applications. One notable example occurred in 1988 when PSAs were observed to be immunomodulating agents with numerous activities and antiviral properties.7
PSAs and their derivatives have been utilized in many applications, such as food and therapeutics.8 For example, carboxymethyl cellulose (CMC), a cellulose ether, is a widely used water-soluble food additive, and alginate/alginate derivatives are commonly used in wound dressing applications and hemostasis agent production.9,10 On the other hand, PSA-based vaccines, which are based on capsular PSAs (CPSAs) and lipopolysaccharides, are an essential class of vaccines utilized to prevent various infections.11 Nevertheless, due to the structural diversity of PSAs (in terms of structural monomers, glycosidic linkages, and pendant functional groups), their chemical complexity, and hurdles associated with isolating them from various natural resources, understanding their structure–function relationships remains a significant challenge. Consequently, designing and fabricating tailor-made PSAs has also proven to be quite challenging.
Both chemical structure (e.g., molecular weight, glycosidic linkages, branching, and functional groups) and molecular conformation affect the physiochemical characteristics and bioactivity of PSAs.12,13 For example, low molecular weight and the presence of anionic and hydrophilic groups are factors that enhance the water solubility of PSAs. The water solubility of carboxymethyl chitosan (CMCS), which bears carboxymethyl functional groups, is significantly higher at nearly neutral pH than unmodified chitosan (CS). As a Lewis base, CS can only dissolve in a mildly acidic environment (pH < 6.5) because of the creation of soluble −NH3+ moieties on glycosamine units. Furthermore, acetylation that affects the conformation of a PSA may significantly increase its solubility by exposing its hydroxyl groups to water molecules. Chemical, physical, and biological modifications of PSAs can govern the physiochemical and bioactivity of those PSAs. Bioactivity refers to the capacity of a chemical to interact with biological systems, leading to a biological effect or response. This can manifest as antibacterial effects, immunogenicity, inflammation, or antioxidant properties. Grafting or conjugation with other (bio)(macro)molecules, oligomers, and polymers can endow PSAs with increased complexity, conformational versatility, and enhanced biological activities. Furthermore, the rheological and gelling properties of PSA aqueous solutions are determined by the chemical structure and conformation of the polymer chains. In addition, physical or chemical cross-linking features (e.g., cross-linker type and cross-linking density) of PSAs significantly affect the mechanical properties of PSA-based hydrogels.14−18 Utilization of PSA-based hydrogels for tissue engineering, drug delivery, and three-dimensional (3D) or four-dimensional (4D) bioprinting is highly dependent on their physicochemical, mechanical, rheological, and biological properties.19−21 Property alternation/reversal in PSAs originates from chemical and conformational features that correspond to unique electronic structures. Thus, tailoring PSAs is critical for adjusting their properties to meet specific applications in the biomedical arena.22−24
Tailor-made polymers have been at the center of attention over the past three decades. There has also been a continued push for tailor-made macromolecules, long-chain molecules with predefined chemical architectures, for the purpose of obtaining polymeric materials with anticipated properties.25−27 The concept of tailor-made macromolecules was inspired by the idea of using polymerization reactions or design systems to seek outstanding properties of macromolecules via an inverse design strategy, i.e., beginning with a desired property and then achieving this property through rounds of design and experimentation.28−31 Tailoring provides a vast playground for materials scientists to design and manufacture diverse polymers with unique features based on different (co)monomers, oligomers, side chains, and cross-linking states. On the other hand, this strategy requires the ability to pattern macromolecules with controlled molecular weights and architectures among the multitude of potential macromolecular configurations.32 Copolymerization of different types of monomers has traditionally been considered an essential strategy for making novel polymers with unique properties.33−35 For example, poly(ethylene glycol)-b-poly(propylene glycol)-b-poly(ethylene glycol), known as poloxamer or Pluronic, is a nonionic triblock (ABA) copolymer. Two hydrophilic terminal oligomer segments, i.e., poly(ethylene oxide), and one hydrophobic central oligomer segment, i.e., poly(propylene oxide), give this polymer its amphiphilic nature and thermosensitive properties.36,37 This enables poloxamer to be utilized in micelle-based intelligent drug delivery systems (DDSs). Additionally, it is possible to make injectable hydrogels based on poloxamer for tissue engineering applications.38,39 As another example, poly(lactic-co-glycolic acid) (PLGA) is a synthetic biodegradable random copolymer that has been approved by the Food and Drug Administration (FDA) and has a broad range of applications in drug delivery and cancer treatment.40−44 Hydrolysis of ester linkages results in PLGA degradation both in vitro and in vivo. The degradation rate of this copolymer can be tailored primarily by varying the ratio of lactide to glycolide (i.e., the comonomer ratio, L/G).45,46 Tailor-made hydrogels with desired properties can be created by selecting appropriate (co)monomers and adjusting their ratio, as well as the type of cross-linker(s), density, and feeding policy during polymerization.47−49
Several review articles have explored the application of PSA-based adhesives or their derivatives in various biomedical applications. However, many of these reviews focus on individual PSAs or limited applications, providing readers with a narrower perspective. For instance, Kim et al. concentrated on hyaluronic acid (HA) and its derivatives for specific biomedical applications but did not delve into the chemical tailoring procedures.50 Similarly, Liu et al. examined various strategies for the chemical modification of PSAs but did not explore their biomedical applications in depth.51
PSAs, as abundant natural biopolymers, have been widely utilized for biomedical applications such as cell therapy, gene or drug delivery, different facets of tissue engineering, wound healing, and bioprinting technology.52−54 However, their properties must be modified to expand their applications in future medicine. The pressing need for modification comes from existing weaknesses in specific mechanical or biological properties and a lack of unique functionalities. For instance, naturally thermoresponsive PSAs (like agarose and carrageenan) can be converted from solution to gel state upon cooling, i.e., a sol–gel transition that is undesirable for some biomedical applications.55 For instance, the sol phase is preferred for cell encapsulation, but this requires raising the temperature in thermoresponsive PSAs, which can damage living cells.56 Accordingly, making tailored thermoresponsive PSAs with lower critical solution temperatures can preserve cell viability and result in enhanced biomedical applications.57 In addition, the increased use of PSA-based materials in biomedical applications has encouraged scientists to look for state-of-the-art methods or facilities to improve the extraction processes. One practical improvement is an enzymatic modification that leads to the generation of smaller and better-defined molecules.58 Among the different modifications, the easiest and most common is a chemical modification that explicitly focuses on the oxidation of the PSA backbone.59 This introduction has presented a general overview of the modification strategies for PSAs and touched on the chemical processes, ranging from the synthesis of PSAs to the modification of their microstructure. The following section discusses the properties and applications of modified PSAs in more detail.
2. Chemical modifications of PSAs
As shown in Table 1, various PSAs can be found in abundance in natural resources. Most studies rely on the isolation of natural PSAs from multiple species, such as plants and microorganisms. However, some bacteria are purposely cultured to make exopolysaccharides (EPSAs). Glycosaminoglycans (GAGs), or mucopolysaccharides, are long, unbranched PSAs with high water absorption capabilities. These alternating copolymers are ubiquitous in animal tissues and possess vital features, including water retention, promotion of cell attachment and proliferation, superior ability to direct cell differentiation, and binding to cytokines and growth factors. GAGs such as heparin, heparan sulfate, keratan sulfate (KS), dermatan sulfate, chondroitin sulfate, and HA play essential roles in the biological functions of animal tissues.60,61 On the other hand, the total synthesis of OSAs/PSAs has been extensively investigated over the last three decades to prepare well-defined oligomers or polymers, particularly for fundamental studies such as structure–property-function relationships.62,63 While these studies have led to outstanding achievements such as automated glycan assembly (AGA), the corresponding methods may require further improvement and will not be covered here.
Table 1. Classification of Naturally Derived Polysaccharides (PSAs) Is Based on Their Origins, Chemical Structures, Functional Groups, Properties, and Biomedical Applications64−90.
Chemical, physical, and biological modification strategies are robust methods for improving the physicochemical properties and biological activities of PSAs. Chemical modification of naturally derived PSAs is a versatile and robust strategy for creating tailor-made PSAs. Partial substitution of functional groups of PSAs with other functionalities, small molecules, oligomers, or polymers provides a leading strategy to tailor PSAs for specific applications. In addition, changes in the PSA backbone (e.g., oxidation of some of the pyranose rings, backbone truncation) can result in new polymeric structures. However, designing new tailor-made PSAs with special features requires basic knowledge of how the various chemical modifications affect the physicochemical properties and bioactivities of PSAs. For example, a PSA-based, targeted DDS for delivery of hydrophobic chemotherapeutics requires the design or selection of an amphiphilic PSA with pH-responsiveness so that it releases its payload in the slightly acidic tumor microenvironment (TME). Furthermore, PSAs possessing inherent antitumor properties can be especially useful in designing DDSs for cancer treatment since they can act as dual-action anticancer nanomedicines.91 The designer can select a natural PSA with inherent antitumor activity and enhance its anticancer activity via chemical modification strategies such as sulfation. Bioactive PSAs can serve as therapeutics, and DDSs can improve their curative efficacy.92 Enhancing the solubility of PSAs, optimizing gelling/melting temperature, endowing responsiveness against various stimuli (e.g., pH, redox moieties, light, and temperature), and adjusting degradation rate would significantly enhance in vivo applications of PSAs.93 For example, xanthan gum (XG) is a microbial EPSA with various biomedical uses. However, it has several limitations, such as poor water solubility and inadequate mechanical stability. Accordingly, several strategies for chemical modification and cross-linking have been used to tailor the properties of XG.94
The molecular weight of PSAs can be reduced by chain scission via chemical, physical, or biological methods. On the other hand, the ring opening of sugar units via partial oxidation results in the formation of reactive functional groups (e.g., aldehyde), facilitating further modifications. Moreover, sugar units in PSAs are rich in various functionalities, such as primary alcohol, carboxylic acid, and amine. Based on the chemical functional groups, PSAs can be classified into three categories, as shown in Table 1. These functional groups can be involved in the PSAs’ modifications.95 These strategies apply to both molecular and surface modifications (usually in heterogeneous reactions).
2.1. Esterification
Esterification is one of the most important processes used in the chemical functionalization of PSAs. Esterification is a process that can improve the stability and water solubility of PSAs, while also allowing for customization of their physicochemical properties such as viscosity and gelation time.96,97 For example, the esterification of κ-carrageenan with various fatty acids has been shown to affect properties such as gelling behavior, swelling, viscosity, thermal stability, and mechanical properties of the resulting PSA.98 Consequently, harnessing esterification for tailoring the properties of PSAs can lead to the development of hydrogels with desirable gelling properties and enhanced mechanical strength, making them highly attractive for applications in tissue engineering.
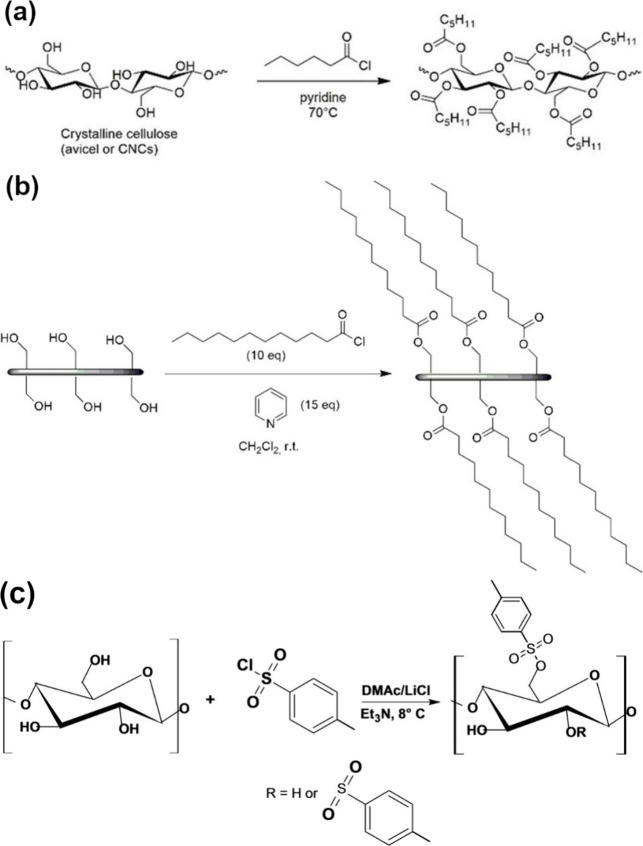
Cellulose esters (e.g., nitrocellulose and cellulose acetate) have many critical industrial applications.99 These applications include, but are not limited to, sensors, reinforcement agents, biomaterials, and interfacial materials. The specific application field depends on the type of ester moieties introduced.100 Esterification of cellulose nanomaterials results in the formation of functional nanomaterials that can be classified into inorganic (e.g., cellulose nitrate and cellulose phosphate) and organic (e.g., cellulose acetate) cellulose esters.101 Cellulose acetate is typically produced using Fischer esterification, which involves refluxing acetic acid and acetic anhydride with cellulose in the presence of a strong acid catalyst, such as sulfuric acid.102 Activated derivatives of carboxylic acids (e.g., acid halides or anhydrides) can react with alcohols in basic pH. In addition to these activated derivatives, vinyl carboxylates can also act as acyl donors.103 Note that selecting proper solvents is critical for the homogeneous functionalization of PSAs. Surface modification of cellulose nanocrystals (CNC) with lauroyl chloride also resulted in lauroyl ester groups on the hydrophobically modified nanopaper, as schematically presented in Figure 1.104 The esterification reaction was conducted in dichloromethane (DCM)/pyridine using lauroyl chloride as the acyl donor.105,106
Figure 1.
Enhanced hydrophobicity of cellulose. (a) Esterification of cellulose nanocrystals (CNC) using an acid halide (i.e., hexanoyl chloride) in the presence of pyridine, resulting in hexanoyl derivatives of CNC with suppressed hydrogen bonding, soluble in (non)polar solvents. (b) Making hydrophobic, freestanding cellulose nanopaper through surface treatment using lauroyl chloride as acyl donor, resulting in ester bond formation. Adapted with permission from ref (104). Copyright 2018, Royal Society of Chemistry. (c) Cellulose tosylation using p-tosyl chloride in a DMAc/LiCl system containing trimethylamine.107 Reproduced with permission from ref (107). Copyright 2020, Wiley-VCH.
Sulfonate esterification of PSAs can be performed using toluenesulfonates and methanesulfonates. For instance, cellulose was dissolved in N,N-dimethylacetamide (DMAc)/LiCl (8%), followed by a reaction with tosyl chloride and mesyl chloride under homogeneous conditions to yield tosylated/mesylated cellulose.108,109 As schematically illustrated in Figure 1, tosylation can occur in cellulose in a DMAc/LiCl system with trimethylamine (Et3N) as the basic catalyst.107
Ester functional groups can be created through the chemical reaction between carboxylic acid groups and hydroxyl groups or epoxides. PSAs like alginate, which contain carboxyl residues, can react with the oxirane functionalities of other molecules to create ester linkages. For example, the esterification of pristine hydrophilic sodium alginate with propylene oxide results in surface-active propylene glycol alginate (PGA), an ester of alginic acid.110 Moreover, the esterification of alginate using poly(vinyl alcohol) (PVA) results in the creation of ester linkages (i.e., cross-linking) and improved physicochemical and mechanical properties of the obtained polymer network.111 Hydroxyl groups on sugar units can react with monochloroacetic acid (MCA) to create carboxymethyl derivatives of PSAs, such as CMCS. Carboxymethyl groups can further react with alcohols to produce methyl ester derivatives that are more reactive compared to carboxylic acid groups. For example, amino-dealkoxylation of methyl ester–functionalized CS and β-glucan results in the amidation of these PSAs.112 Oxidized dextran (OD) bearing aldehyde groups was conjugated with the cyclic peptide cyclo-(Arg-Gly-Asp-d-Phe-Lys) (c(RGDfK)) and the anticancer drug doxorubicin (DOX) via Schiff base reaction.113 Additionally, bortezomib (BTZ), another anticancer drug, was conjugated to dextran (Dex) via boronic ester linkages, as shown in Figure 2.
Figure 2.
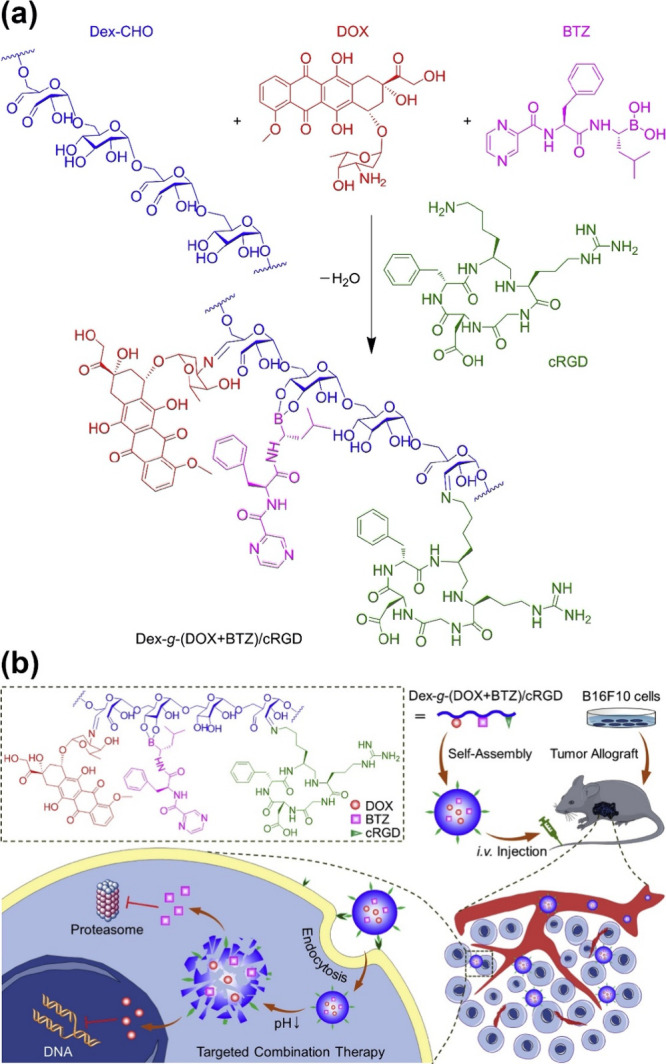
Targeting cancer cells using chemically modified Dex-g-(DOX+BTZ)/cRGD. (a) The Schiff base reaction between aldehyde functional groups on oxidized dextran (Dex-CHO) and primary amines on the cyclic arginine-glycine-aspartic acid peptide (cRGD) or doxorubicin (DOX). Boronic esterification of adjacent OH on pyranose ring and boronic acid on bortezomib (BTZ). (b) Self-assembly of modified dextran into micelles in an aqueous environment; intravenous injections of micelles followed by tumor-site accumulation due to EPR effect; micelle uptake by cancerous cells and disassembly of nanomedicine in the acidic endosome environment followed by drug release in the cytosol.113 Reproduced with permission from ref (113). Copyright 2020, Elsevier.
The obtained PSA, containing two anticancer drugs and a cyclic peptide, self-assembles into 80 nm micelles in aqueous media. This nanomedicine is a synergistic cancer therapy that accumulates in tumor tissue via the enhanced permeability and retention (EPR) effect. Integrin αvβ3, which plays an essential role in angiogenesis and tumor progression, can be targeted via the cRGD sequence of cRGDfK in the nanomedicine formulation. The nanomedicine can target cancer cells via αvβ3–cRGD interplay before entering cancer cells through endocytosis. The acidic microenvironment within the endosome triggers the release of the anticancer drugs into the cytosol, as depicted in Figure 2.
Another research team reported that the esterification of alginic acid can be considered a standard chemical route for improving the functionality of tableting excipients. They claimed that higher levels of methylation brought greater tensile strength and compressibility, in addition to the slower disintegration resulting from the introduced hydrophobicity.114 Interestingly, it was found that the immunomodulatory effect of pectin is a function of methyl-esterification and molecular mass. For instance, 56% methyl-esterification of pectin confers an immunomodulatory effect on this PSA. It can increase macrophage cell activity by 39%, phagocytic activation by 30% (after 2 days), and peritoneal macrophage activity by 490% after 1 day.115
2.2. Etherification
Etherification is an essential class of chemical functionalization of PSAs. Cellulose ethers (e.g., CMC, methylcellulose (MC), hydroxyethyl cellulose, hydroxypropyl cellulose (HPC), ethylcellulose (EC), and hydroxypropyl methylcellulose (HPMC)) are produced at industrial scale for various applications, such as food, pharmaceuticals, cosmetics and personal care, adhesives, paints, and coatings.116 Etherification, another chemical modification process, can enhance the solubility, stability, and chemical versatility of PSAs, while also improving their film-forming ability and reducing immunogenicity.117 Furthermore, etherification can introduce additional functionalities such as stimuli-responsiveness. For instance, by etherifying chitosan, it is possible to produce thermosensitive hydrogels based on hydroxybutyl chitosan, which can serve as an intelligent drug delivery platform.118
The reaction between the alcoholic hydroxide group on PSAs and an alkylating agent (e.g., alkyl halides) in the presence of a strong base results in ether linkage formation. In such reactions, the saccharide oxygen acts as a nucleophile. For example, etherification of pullulans using two alkyl halides (i.e., 1-bromopropane and 1-bromobutane) was carried out in the presence of sodium hydroxide (NaOH).119 The most crucial ether derivatives of PSAs can be classified as alkyl, benzyl, carboxymethyl, and hydroxyethyl ethers. MC and its salts serve as alkylating agents to create carboxymethyl ethers in the presence of NaOH.120,121 Ring-opening reactions of epoxides (e.g., in ethylene oxide and propylene oxide) are vital to creating PSA ethers such as hydroxypropyl starch and hydroxyethyl starch (HES), which is used as a blood plasma volume expander.122,123
2.3. Amidation
Amide linkage formation is possible between amine-bearing PSAs, such as CS or synthetic aminated PSAs, and carboxylic acid groups. Amine nucleophiles react with electrophiles to produce various functionalities such as amides, imines, and quaternary ammonium salts. The ammonium salts usually come from the acid-based reaction unless the temperature is elevated. The amine group of CS can be activated using carbodiimides (e.g., 1-ethyl-3-(3-(dimethylamino)propyl) carbodiimide (ECD)) to react with carboxylic acid-bearing molecules such as fatty acids to make amphiphilic PSAs. Carboxylic acids can serve as electrophiles for amine nucleophiles.
Amidation is a process that significantly increases the solubility and stability of PSAs, while also enhancing or inducing bioactivity within them.124 For example, incorporating amide bonds into chitosan has been shown to impart antibacterial and antioxidant properties.125 Additionally, since amide bonds can participate in hydrogen bonding, PSAs modified through amidation can form hydrogels with superior mechanical strength.
However, carboxylic acid functional groups should be activated before amidation. Carbodiimides are commonly employed as bifunctional coupling reagents to activate and link these functional groups. Among carbodiimide chemistry’s coupling agents, water-soluble EDC and organo-soluble N,N′-dicyclohexylcarbodiimide (DCC) are the most widely used.
The amidation reaction is of particular interest in pharmaceutical applications. It plays a crucial role in the synthesis of more than 25% of commercially available drugs and similarly bioactive products with anticancer, antifungal, and antibacterial properties.127 However, a large amount of waste is usually produced during the amidation process, resulting in environmental and economic concerns. Enzymatic amidation was proposed as an alternative to address this challenge.128 Furthermore, new approaches with interesting mechanistic pathways have also been presented. For instance, Srivastava et al. reported a state-of-the-art green approach for amidating carboxylic acids using a photoredox catalysis technique. It acts by direct amidation using visible light and eosin Y as a catalyst and represents a cheap and eco-sustainable system. This technique can be used at room temperature. Hence, this novel approach enables amide bond formation, even under physiological conditions (Figure 3).126
Figure 3.
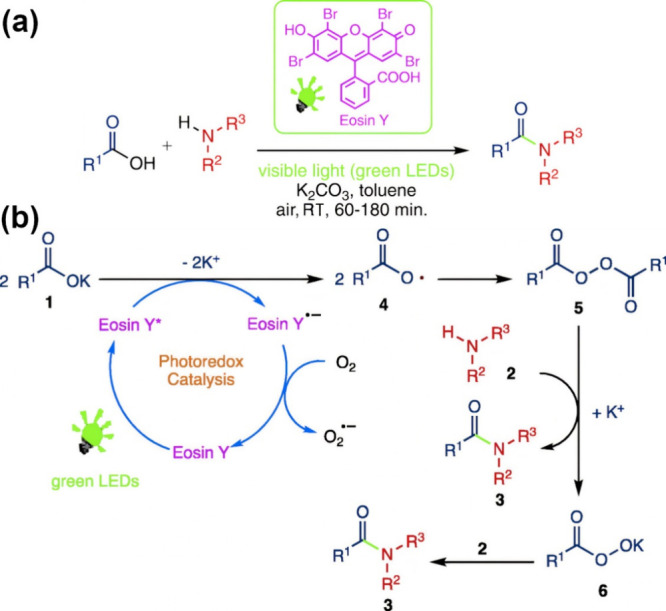
Sustainable amidation of carboxylic acids under mild conditions. (a) Schematic illustration of the amidation process using visible light (green LEDs). (b) The mechanism of amidation of carboxylic acids with amines in the presence of visible light and eosin Y.126 Adapted with permission from ref (126). Copyright 2019, Elsevier.
2.4. Oxidation
Oxidation of PSAs may occur on free primary alcohol functional groups or by oxidative cleavage of 1,2-diols. Using chemical or enzymatic reactions, primary alcohols in the C6 position can be oxidized to aldehyde or carboxylic acid (i.e., containing a uronic acid residue). The discovery of a stable aminoxyl radical (e.g., (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO)) allowed researchers to oxidize the primary alcohol on PSAs through a regioselective reaction to get polyuronic acids.129,130 TEMPO-mediated oxidation, in the presence of a primary oxidant such as sodium hypochlorite, selectively converts the primary alcohols of PSAs into carboxylic acid functional groups.131
Oxidation of PSAs can increase their reactivity, induce bioactivity, improve biostability, and modify their physical properties.132,133 Reactive functional groups, such as aldehydes generated from main chain oxidation, can be leveraged for further chemical modification of PSAs or for cross-linking purposes, particularly in the fabrication of injectable hydrogels.134 Furthermore, the degree of oxidation can be controlled to adjust the degradation behavior of PSAs.135
Reactive aldehyde functional groups can be used for further chemical modifications. For example, reductive amination of aldehydes can be performed using hydrogen as a reductant. Using a kind of one-pot, two-step technique, reductive amination can be done at 50–100 °C utilizing molecular dihydrogen and manganese pyridinyl-phosphine as reductant and precatalyst, respectively, with a high yield of 90%.137 Additionally, Mendoza et al. suggested a one-pot reaction for oxidizing cellulose that utilizes a combination of sodium periodate, TEMPO, NaClO, and NaBr. These oxidants synergistically form oxidized 2,3,6-tricarboxycellulose in water-soluble and water-insoluble fractions, depending on the periodate concentration. Their results showed that increasing the concentration of periodate enhances cellulose crystallinity, and the degree of substitution (DS) regulates the solubility of carboxylated cellulose (Figure 4).136
Figure 4.
Adjustable one-pot cellulose oxidation. (a) Illustration of (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO)-periodate oxidation of cellulose, which forms water-insoluble and water-soluble carboxylated fractions, which synergistically produces oxidized 2,3,6-tricarboxycellulose as a function of periodate concentration. (b) Presentation of selective oxidation (for cellulose). (A) TEMPO/sodium hypochlorite/sodium bromide oxidation at pH = 10.5. (B) Sodium periodate reaction. (C) combination of TEMPO/sodium hypochlorite/sodium bromide oxidation and sodium periodate reaction.136 Adapted with permission from ref (136). Copyright 2019, Elsevier.
2.5. Nucleophilic Displacement Reactions
Typically, organic synthesis requires the use of a protecting group strategy that enables selective functionalization. However, some limitations exist (e.g., multiple steps and cumbersome procedures) relevant to regio- or chemo-selective synthesis. This highlights the importance of atom economy in organic synthesis.136 As discussed earlier, the oxygen atoms in PSAs serve as nucleophiles and react with an alkylating agent to produce ethers/esters. However, alcohols can attack saccharides, which serve as electrophiles. For this purpose, nucleophilic displacement of groups containing nitrogen (e.g., amines and azides) or sulfur (e.g., thiols) is carried out, which is of interest for click chemistry.8 For example, synthesizing 6-amino-6-deoxycellulose without any protecting groups involves a nucleophilic substitution of the tosyl group with sodium azide. In one study, regioselective and complete iodination was carried out by nucleophilic substitution of the tosyl functional group at 60 °C.138 Interestingly, Hamdaoui et al. reported a novel method of synthesizing cellulose-l-methionine with strong antibacterial activity. They utilized an esterification reaction of microcrystalline cellulose with tosyl chloride, completed by a nucleophilic displacement reaction. In this reaction, displacement of the tosyl group was done by l-methionine, an amino acid, as illustrated in Figure 5.139
Figure 5.
Innovative approach for the synthesis of antibacterial cellulose derivatives. Schematic representation of the reaction pathway for l-methionine-modified cellulose using nucleophilic displacement.139 Reproduced from ref (139). The copyright is licensed under the Creative Commons Attribution License 2021, Hindawi.
2.6. Grafting Reactions
Grafting reactions are a class of chemical reactions that conjugate small molecules and oligomers/polymers onto PSAs’ main chains to introduce new properties into the original PSAs. Grafting is a process that significantly enhances the chemical versatility of PSAs. It involves attaching polymerizable and functional monomers onto PSA chains to create interpenetrating polymer networks (IPNs) or stimuli-responsive PSAs. For instance, grafting gallic acid to CMCS enhances antioxidation activities and protects against peroxide-induced oxidative damage.140 In addition, grafting peptides to dextran has provided a platform with a more accurate endosomal release profile.141 Furthermore, a starch–cellulose hydrogel grafted with N-maleoyl-β-alanine (MAA) is another example of such a reaction. A biocompatible platform was designed for the controlled release of 5-fluorouracil (5-Fu) using a combination of the Diels–Alder click reaction and photopolymerization, as shown in Figure 6.142 It is worth mentioning that grafting with catechol-containing molecules (e.g., dopamine and caffeic acid) endows PSAs with wet-adhesive properties, while grafting with alkyl chains (e.g., fatty acids) produces PSAs with hydrophobicity.143,144
Figure 6.
Schematic illustration of the mechanism of starch–cellulose interpenetration network formation. (a) Raw materials. (b) The mechanism for forming the first network. (c) The mechanism for forming the second network.142 Reproduced with permission from ref (142). Copyright 2022, Elsevier.
In addition to grafting PSAs with small molecules, grafting with oligomers has attracted much attention. For example, cationic PSAs with grafted oligomers are exciting materials for making nonviral vectors for gene delivery.145 These cationic polymers can enhance transfection efficiency through strong electrostatic interactions (or complexation) with DNA, which is negatively charged. Various oligoamines (e.g., spermin) can be grafted onto the PSA backbone. For example, dextran–spermine conjugates are efficient nonviral vectors for gene delivery.146 Reductive amination is the most critical strategy for making amines, such as in pharmaceuticals.147 For example, monoquaternary (MQ) ammonium oligoamines were grafted to OD and used for gene delivery.148 Furthermore, reductive amination can create PSA–protein conjugates.149
The grafting of oligomers and polymers onto the backbone of PSAs is typically categorized into two main strategies: graft-to and graft-from. This process results in hybrid polymers that inherit properties and functionalities from both the constructing polymers and the PSAs.150 In the graft-to strategy, a polymeric material is attached to the PSA backbone via reactive sites, such as clickable moieties.151 For instance, grafting-to has been employed to confer antibacterial properties to chitosan by grafting antimicrobial peptides (AMP) through a photoclick reaction.152 On the other hand, the grafting-from strategy involves polymerizing a monomer from reactive sites along the main chain of PSAs. For example, grafting poly(N-isopropylacrylamide) from PSA derivatives can impart thermosensitive properties to the resulting polymer.153 Controlled radical polymerization (CRP) techniques, including Nitroxide-mediated polymerization (NMP), atom transfer radical polymerization (ATRP), and reversible addition–fragmentation chain transfer (RAFT), are commonly used for these purposes.154−156 These CRP methods facilitate the growth of well-defined polymer chains with high density, allowing precise control over polymer length, graft density, chemical composition, and topology. Utilizing these techniques has opened up new avenues for customizing PSA-based materials.148,157,158
Although molecular-scale modification of PSAs is a critical step toward their real-world biomedical application, complementary modifications are also necessary. Many of their chemical, biological, or mechanical features can be changed using modification techniques, such as physical, biological, and chemical modification. The following section is a comprehensive presentation of different modification processes crucial to tailor-making PSAs with desirable properties for biomedical applications.
3. Strategies for PSA Modification
3.1. Physical Modification
Physical modification aims to induce changes in the molecular structure of PSAs, such as reducing molecular weight, via physical triggers. PSAs with a reduced molecular weight typically show increased water solubility and bioactivity. A physical modification strategy can enhance the solubility of CS, starch, and cellulose. For example, the water solubility of CS at neutral pH is essential for designing drug carriers and tissue scaffolds. Three main strategies exist for the physical modification of PSAs: ultrasonic disruption, microwave exposure, and radiation-induced physical modification. In ultrasonic disruption, low- or high-frequency sound waves create mechanical vibrations that induce chemical bond cleavage. In other words, the energy transferred via ultrasonic waves results in vibration in chemical bonds, which ultimately leads to the clipping of covalent bonds in the chemical structure of PSAs. Cleavage of chemical bonds in the PSA backbone results in PSAs with lower molecular weights and enhanced water solubility.159,160
Microwave exposure is another facile physical modification strategy that can save even more time and energy than the above approach. Microwave exposure, like ultrasonic disruption, also improves the water solubility and biological activity of PSAs by breaking chemical bonds and reducing molecular weight. Instead of using mechanical waves, microwave exposure uses electromagnetic waves to transfer energy to chemical bonds throughout the structure of the PSA. Specific chemical bonds absorb specific spectra of electromagnetic waves, producing vibrational and rotational motions. These motions can result in bond cleavage and can increase local temperature via a frictional phenomenon, which eventually reduces the molecular weight of PSAs.161
The third physical modification strategy is known as radiation-induced treatment. Instead of electromagnetic waves of longer wavelengths, this technique utilizes high-energy radiation such as γ rays to induce chemical bond cleavage in PSAs. Radiation-induced treatment produces low molecular weight fragments of PSAs, which possess enhanced water solubility and antioxidation activities.162 Cobalt-60 (60Co), a well-known radioactive isotope of cobalt, is the most frequently used gamma-ray source in this technique.
3.2. Chemical Modification
Chemical modification of PSAs refers to a process where small molecules or macromolecules induce bond cleavage and/or formation in the chemical structures of PSAs. Utilizing biological macromolecules for chemical modification represents a strategy within this class of modifications. The biological modification of PSAs is considered equivalent to enzymatic modification. Enzymatic modification of PSAs involves chemical bond cleavage or chemical transformation using various enzymes, such as hydrolases and transferases.163 Compared to chemical modification, an enzymatic modification strategy is more specific, produces less waste, and possesses higher efficacy.164,165 Moreover, it results in a more uniform degradation along the chemical structure of the PSA. Although enzymatic modification is a favorable process for PSA modification, it is limited to some specific types of PSAs and represents a limited number of modifications. However, enzymatic modification is an excellent alternative to chemical modification, which often involves the use of toxic materials.166,167
Advancements in modern organic chemistry have driven chemical modification of PSAs, leading to new possibilities for creating chemically modified PSAs with enhanced versatility. Section 2 delves into the fundamental chemistry behind the chemical modification of PSAs, detailing specific and commonly used methods for incorporating specialized functional groups. These chemical modifications introduce unique characteristics to PSAs, facilitating the creation of customized formulations, as summarized below.
3.2.1. Sulfation
Sulfation is one of the most utilized chemical modification strategies for PSAs. It includes the chemical attachment of a sulfate group to a hydroxyl group on the pyranose ring of PSAs. Sulfation can enhance the existing bioactivities of PSAs or can introduce new functionalities, such as antiviral, antioxidant, and antitumor activities. For example, marine-derived OSA/PSA (e.g., carrageenans, alginates, and fucans) and their sulfated derivatives usually exhibit antiviral activities.168In vivo studies have shown that sulfated alginate possesses antioxidant, anti-inflammatory, and anti-immunogenic properties.169 Recent studies have shown that heparin, a sulfated PSA known for its anticoagulant properties, exhibits remarkable binding affinity to the spike protein (S-protein) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).170 Furthermore, in vitro investigations have demonstrated that specific sulfated PSAs possess high binding affinity to S-proteins of SARS-CoV-2, thus displaying antiviral activity.171 These sulfated PSAs interfere with S-proteins, which bind to heparan sulfate coreceptors on host cells, thereby preventing viral infection.172 Moreover, sulfated PSAs demonstrate antitumor activity by influencing various pathways involved in tumor growth and progression.173 They interfere with the cell cycle progression of cancerous cells, leading to tumor cell cycle arrest and inhibiting their growth, division, and uncontrolled proliferation. Additionally, sulfated PSAs exhibit antiangiogenic effects, induce apoptosis, and modulate the immune response, all contributing to their ability to inhibit tumor growth.174−176 Sulfated PSAs typically exhibit immunomodulatory activity and affect various signaling pathways, as depicted in Figure 7.177 Several sulfated PSAs, such as heparin or fucoidan, have the capability to directly bind to Toll-like receptor 4 (TLR4) and myeloid differentiation factor-2 (MD-2).178 This binding of sulfated PSAs to the TLR4-MD-2 complex leads to the activation of receptors, subsequently initiating downstream signaling cascades, as depicted in Figure 7. Similarly, lipopolysaccharides (LPS), components of the cell wall in Gram-negative bacteria, can also trigger the TLR4 and downstream signaling pathways.179 Thus, sulfation of PSAs enhances their bioactivity by facilitating interaction with biomacromolecular receptors on the plasma membrane, ultimately resulting in the activation of various intracellular signaling pathways.
Figure 7.
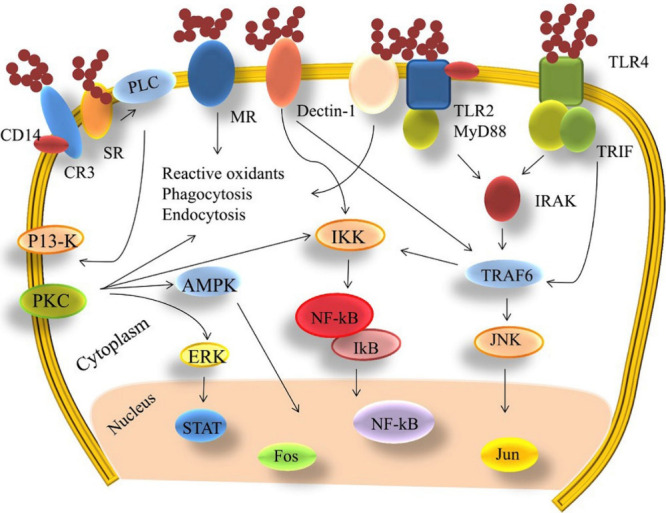
Immunomodulatory activity of sulfated PSAs. Schematic illustration of sulfated polysaccharides (PSAs)-induced regulation of functioning and metabolism of immune cells by activating signaling pathways involved in macrophage activation; CD14: a cluster of differentiation antigen 14; CR3: complement receptor 3; Dectin-1: dendritic cell-associated C-type lectin-1; ERK: extracellular signal-regulated kinase; IKK: inhibitor of nuclear factor kappa-B kinase; IRAK: interleukin-1 receptor-associated kinase; IκB: inhibitor of nuclear factor kappa-B; JNK: Jun N-terminal kinase; MR: mannose receptors; MyD88: myeloid differentiation factor 88; NF-κB: nuclear factor kappa-B; PI3-K: phosphatidylinositol 3 kinase; PKC: protein kinase C; PLC: phospholipases C; SR: scavenger receptors; STAT: signal transducers and activators of transcription; TLR2: Toll-like receptor 2; TLR4: Toll-like receptor 4; TRAF6: tumor necrosis factor receptor-associated factor 6; TRIF: Toll/IL-1 domain-containing adaptor inducing interferon β.177 Reproduced with permission from ref (177). Copyright 2019, Elsevier.
Naturally occurring sulfated PSAs (e.g., chondroitin sulfate, carrageenan, and fucadion) play essential roles in biological systems. In addition, the sulfation of GAGs strongly affects human health conditions.180 GAGs are anionic linear PSAs and have been observed in all animal tissues. They affect the physical properties of the extracellular matrix (ECM) and tissues and modulate cells’ biological functions. Intrinsic negative charges originate from sulfate functional groups in all GAGs except for HA. Extracellular signals are modulated by sulfation, while phosphorylation is involved in intracellular signal transduction.181 GAGs play critical roles in many biological processes, a consequence of their interactions with many different proteins. However, many PSAs fail to mimic GAGs in the ECM (e.g., heparin) in terms of interactions with cells and other biomolecules. Sulfation may help PSAs in better mimicking the properties of GAGs, which are essential for applications in tissue engineering and regenerative medicine. For example, sulfated alginates mimic the properties of heparin, making them comparable to heparin analogs.182 Sulfation of GAGs, a posttranslational modification process, modulates cell signaling pathways and mediates interactions with other cells or nearby ECM. It is worth mentioning that sulfation patterns of GAGs (known as sulfation code) affect various biological processes at different levels.183 Sulfated GAGs can be divided into four classes based on the primary monosaccharide unit and sulfation pattern: 1) HA, 2) chondroitin sulfate or dermatan sulfate (stereoisomers), 3) KS, and 4) heparin or heparan sulfate.184
Heparin has the highest degree of sulfation among GAGs, which endows it with a high number of interactions with various proteins. Sulfated GAGs (e.g., heparan sulfate and chondroitin sulfate) are anionic linear PSAs that play essential roles in animal tissues; they are ubiquitous in ECM and cell membrane, serve as structural components in ECM and act as receptors/coreceptors via electrostatic interactions with proteins. Chondroitin sulfate is a sulfated GAG and a critical structural component of cartilage with high compression resistance.185 Dermatan sulfate is another GAG found in the skin, lung blood vessels, and heart valves, playing roles in wound healing, cardiovascular disease, and infection.186 KS is a sulfated GAG found in cartilage, bone, cornea, and the central nervous system.187 These PSAs are highly hydrated and can absorb mechanical vibrations. Heparan sulfate (HS) octadecasaccharide was found to show hepatoprotective activity against acetaminophen-induced acute liver failure.188−190
3.2.2. Carboxymethylation
Carboxymethylation is a chemical modification strategy in which some of the hydroxyl groups on sugar units of PSAs are substituted with carboxymethyl groups (−CH2–COOH). It includes a facile chemical process that utilizes low-cost reagents to modify PSAs. Increased water solubility and enhanced bioactivity are the general features of carboxymethylated PSAs.191 Compared to sulfation, carboxymethylation benefits from process simplicity, low-cost raw materials, and minor toxic waste production.192,193 Kappa-carrageenan (KC) is a sulfated PSA that shows antitumor and immunomodulatory activities.194 This anionic linear PSA can create double-helix structures when interacting with divalent cations.195 The carboxymethyl functionality enhances the bioactivities of KC, which includes antiproliferative, antibacterial, and antioxidant activities, and alters its rheological properties in solutions.196
3.2.3. Acetylation
Acetylation is typically utilized to modify PSA side chains, resulting in a significant enhancement in solubility. In addition, acetylation is an essential posttranslational modification related to a wide range of biological processes, such as gene regulation.197 For example, CS is a linear heteropolysaccharide obtained by the deacetylation of chitin. The degree of acetylation of CS affects its physicochemical properties, biocompatibility, biodegradation, and cell interaction, highlighting the importance of the acetylation of PSAs.198 In addition, the anticoagulant activity of heparin pentasaccharide is drastically reduced upon acetylation.199 The acetylation pattern has also been found to affect the bioactivity of partially acetylated CS or chitosan oligosaccharide (COS), regardless of the degree of acetylation or the degree of polymerization.200−202 Cellulose acetylation results in water-soluble cellulose acetate, one of the most-used cellulose derivatives. The negligible solubility of cellulose in aqueous media or organic solvents is an obstacle to its chemical modification. Although cellulose is typically insoluble in most solvents, it can be dissolved in certain ionic liquids (ILs). These ILs can then be used to acetylate cellulose and produce high molecular weight cellulose acetate.203,204
3.2.4. Phosphorylation
Phosphate groups bearing electric charge can alter the solubility and chain conformation of PSAs. It should be noted that the strong acids used in the phosphorylation process may result in PSA degradation. It has been revealed that modification by phosphorylation enhances the antiaging effect of a PSA isolated from Trichosanthes peel.205 Phosphorylated GAGs are resistant to GAG-specific glycosidases.206 In addition, enhanced antioxidant activity was observed for phosphorylated PSAs isolated from different natural resources such as garlic, ginseng, and pumpkin.207−209 Various chemicals, such as phosphoric acid or anhydride phosphoric, phosphate, and phosphorus oxychloride, have been utilized in the phosphorylation of PSAs. For instance, Jiang et al. used a mixture of sodium trimetaphosphate and sodium tripolyphosphate to synthesize phosphorylated ulvan (a cell wall PSA).210In vivo studies on mouse models revealed that ulvan phosphorylation enhances its antioxidant and antihyperlipidemic properties.211 Although CS acts as an osteoconductive PSA, its osteoconductive properties can be improved through phosphorylation.212,213 Additionally, it is worth mentioning that, based on results from Cao et al., several PSAs derived from Amana edulis showed great antioxidant potential not only after phosphorylation but also after sulfation and carboxymethylation.214 Furthermore, it was recently revealed that influenza A viruses can bind to phosphorylated glycans from the human lung.215
3.2.5. Selenylation
Selenium (Se), as a micronutrient, supplies this essential trace element to the human body and other living organisms. Selenoproteins, containing selenocysteine residues, provide various pleiotropic effects, such as antioxidant and anti-inflammatory activities. Se contributes to the structure of Se-dependent antioxidant enzymes (e.g., glutathione peroxidase and thioredoxin reductase), which protect cells against oxidation induced by free radicals.216 Se deficiency can weaken the immune system and increase mortality rates. In contrast, maintaining an adequate level of Se in the body can have antiviral and antitumor effects, and it may also reduce the risk of autoimmune thyroid disease.217,218
Selenylation of PSAs significantly increases their antioxidant, anticancer, and antidiabetic activities, as well as their antimetal-poisoning, immunomodulatory, and hepatoprotective properties.219,220 However, natural resources for Se-containing PSAs are minimal (e.g., Cucurbita pepo L. and Ganoderma lucidum) and contain very trace and insufficient Se content in their structure.220 Despite some successful results, adding inorganic Se-containing compounds (e.g., Na2SeO3) to the culture media has many complications.221 The most well-accepted selenylation process uses a combination of diluted nitric acid and Na2SeO3 to produce H2SeO3in situ, followed by selective esterification of primary OH groups on sugar units of PSAs.222,223 Excellent antioxidant, antitumor, and antidiabetic properties were observed for selenylated PSA isolated from sweet potato tuber.224 Additionally, it was revealed that selenylation of PSA extracted from Ulmus pumila L. (PPU) significantly enhanced the antioxidant activity of PPU.225 It is worth stating that PSAs themselves, as bioactive macromolecules, can be used as stabilizers for the fabrication and delivery of organic Se to the human body.226
3.2.6. Alkylation
Alkylation is a chemical modification that improves solubility, gel strength, and antibacterial activity. Among different PSAs, alkylation is most frequently used with pectin and can be classified as either methoxylation or long-chain alkylation. Methoxylation brings the minimum number of carbons within the chains, while long-chain alkylation usually connects more carbons to the carboxyl groups of pectin. A literature overview indicates that the physicochemical properties and functional features of the obtained PSAs are different for these alkylation reactions.227 Liu et al. reported that the molecular weight of alkylated pectin is affected by DS and degradation.228 They also showed that the random coil chain conformation in pectin is transformed into a spherical conformation for alkylated pectin with a higher DS. Moreover, they found that the gel strength of pectin was increased after the alkylation reaction.228 Yataka et al. studied the effect of alkyl chain length on the self-assembly of alkylated cellulose oligomers.229 They revealed that the self-assembly and allomorphs of cellulose oligomers critically depend on the alkyl chain length. Alkyl chains significantly affect the intermolecular interactions between different cellulose chains, leading to modified assemblies and diverse multidimensional structures. This is of interest for controlling the morphology and crystal structures of these cellulose derivatives. Alternatively, alkylation using alkyl halides, in the presence of sodium hydroxide and a catalyst, proceeds under mild reaction conditions, utilizes low-cost reagents and enables the creation of a variety of alkylated monosaccharides that can be polymerized to alkylated OSAs/PSAs.230Table 2 summarizes different types of chemical modification used for PSAs and the properties of the obtained products.
Table 2. Approaches for the Chemical Modification of Different Types of Polysaccharides (PSAs) and the Impact of Their Structure and Performance Are Essential for Biomedical Applications.
| Modification Strategy | PSA Type | Results | Ref |
|---|---|---|---|
| Carboxylation | Kappa-carrageenan | • Increased viscosity in water and decreased viscosity in synthetic human sweat | (196) |
| • Higher viability and lower cytotoxicity for human adipose-derived stem cells (hADSCs) | |||
| • No hemolytic activity in human red blood cells (RBC) | |||
| • Increased antioxidant activity | |||
| • Antibacterial activity | |||
| Chitosan | • Enhanced water solubility at various pH | (85) | |
| • Support cell growth and tissue regeneration | |||
| • Improved bioactivity, including antimicrobial, anticancer, antitumor, antioxidant, and antifungal | |||
| Mutan | • Higher thermal stability | (231) | |
| • Enhanced solubility | |||
| • Antioxidative, radical-scavenging activity | |||
| Sulfation | Alginate | • Antioxidant, anti-inflammatory, and anti-immunogenic properties | (169) |
| • Free radical–scavenging properties | |||
| Chitosan | • Antimicrobial activity | (190) | |
| • Higher solubility | |||
| • Less chain depolymerization | |||
| • Higher yield | |||
| • Greater thermal stability | |||
| Polysaccharide isolated from Gracilaria caudate (a marine alga) | • Reduced hypernociception and edema | (232) | |
| • Reduced inflammatory response | |||
| • Great option for treating arthritis | |||
| Polysaccharide isolated from Myriophyllum spicatum L. | • Immunostimulatory effect | (233) | |
| • Heterogeneous molecular weight | |||
| • Activated macrophages | |||
| Acetylation | Cellulose | • Enhanced crystallinity, up to 70% | (190, 234) |
| • Enhanced hydrophobicity | |||
| • Dispersible in water and other organic solvents | |||
| Chitosan | • Higher solubility | (235) | |
| • Lower molecular weight | |||
| • Random distribution of functional groups | |||
| Phosphorylation | Polysaccharide isolated from Ulva pertusa | • Enhanced antioxidant and antihyperlipidemic properties | (210) |
| Chitosan | • Improved osteoinduction | (213) | |
| Polysaccharides extracted from Pleurotus ostreatus | • Enhanced hepatoprotective effect | (236) | |
| Chitosan | • Improved osteoinduction | (213) | |
| • Large pore sizes (850–1097 μm), microroughness and thickness | |||
| • Low level of thrombogenicity | |||
| • Long degradation time | |||
| • Low cytotoxicity | |||
| Polysaccharide isolated from native ginseng | • Enhanced radical-scavenging ability (antioxidant activity) | (209) | |
| Polysaccharide isolated from pumpkin | • Enhanced antioxidant activity, scavenging ability to hydroxyl radicals | (208) | |
| Polysaccharide isolated from garlic | • Enhanced antioxidant activity | (207) | |
| • Scavenging hydroxyl radicals and superoxide anions | |||
| Radix Cyathula officinalis Polysaccharide | • Improved immune-enhancing activity | (237) | |
| • Increased secretion of cytokines | |||
| • Immune-adjuvant activity | |||
| Selenylation | Polysaccharide isolated from Momordica charantia L. | • Antidiabetic properties: reduced fasting blood glucose levels and enhanced insulin levels | (238) |
| • Improved antioxidant enzyme activities | |||
| • Preventing liver, kidney, and pancreatic islet damage in diabetic mice | |||
| Chinese angelica polysaccharide | • Enhanced immune-enhancing activity | (222, 223) | |
| Chuanminshen violaceum Polysaccharide | • Enhanced immune-enhancing activity | (239) | |
| Artemisia sphaerocephala Polysaccharide | • Immunomodulatory effect | (240) | |
| Polysaccharide isolated from Ulmus pumila L. | • Improved anti-inflammatory activity | (241) | |
| Polysaccharide isolated from Ulmus pumila L. | • Significantly enhanced antioxidant activity | (225) | |
| • Hydroxyl radical–scavenging activity | |||
| Mycelial PSA from Catathelasma ventricosum | • Enhanced antidiabetic activity by increasing selenium content | (242) | |
| • Damaged triple-helical structure | |||
| Cellulose | • Controllable morphology and crystal structure | (229) | |
| Pectin | • High stability | (227) | |
| • Long release time | |||
| • Good mechanical characteristics | |||
| Pectin | • Controllable degradation profile | (228) | |
| • Random coil conformation |
4. Tailor-Made PSAs for Biomedical Applications
Modifying the chemical structure of materials at the molecular scale can introduce specific features, such as stimulus responsiveness or bioactivity, and import new functionalities into conventional materials. Strategies such as introducing cleavable covalent bonds, functionalizing with molecular recognition motifs, inserting hydrophilic and hydrophobic segments in one (macro)molecule, grafting specific lipids or molecules onto polymer chains, and performing partial oxidation can be employed to create multifunctional materials with specific functionalities and bioactivities.243,244 These chemical modifications can be used to design new macromolecules from small molecules or to modify OSAs and PSAs. At higher length scales, the resulting multifunctional materials may exhibit new properties, including different self-assembly behavior, which can be leveraged to create advanced biomaterials.
Unprecedented progress in chemistry and materials science, including dynamic chemistry, clip chemistry, click chemistry, supramolecular chemistry, and CRP strategies, has made it possible to create novel biomaterials with customized properties and functionalities. The strength of these strategies can be utilized in the field of OSA/PSA to create multifunctional PSAs with dynamic behavior and customized properties. This approach has garnered attention from biologists and biomedical engineers, who can synergistically leverage the power of biology and chemistry to tackle some of the outstanding challenges in the biomedical field.
This section addresses the modification of PSAs to introduce tailor-made features. However, it is worth mentioning that biomaterials’ bulk properties are affected not only by chemical structure and composition but also by ordering and hierarchy at higher length scales, which can be dictated by thermodynamic equilibrium, kinetic trapping, or out-of-equilibrium self-organization.245,246 Even at higher length scales, we can observe properties or functionalities induced by naturally occurring (e.g., gecko feet) or artificial (e.g., metamaterials) microstructures. However, this section focuses on the chemical modification of the molecular structure of PSAs, rather than examining phenomena on a larger length scale.
4.1. Tailored Body/Immune Responses
The human body, including its organs, tissues, and individual cells, constitutes a highly dynamic microenvironment with a myriad of biomolecules engaged in continuous interactions.247 These interactions play a crucial role in coordinating various intracellular processes, facilitating cell–cell interactions, mediating receptor–ligand interactions, and triggering cellular movements. Cell interactions with other cells and biomaterials, such as the surfaces of DDSs or implanted devices, are mediated by a dense array of biomacromolecules, such as glycoconjugates, decorating the plasma membranes of cells.248 The interactions between cells or native biomolecules and biomaterials are primarily determined by the chemical structure of the biomaterials, ultimately influencing the in vivo fate of DDSs or tissue engineering scaffolds. For instance, the cytotoxicity, biocompatibility, and degradation behavior of biomaterials used in tissue scaffolds are predominantly influenced by interactions between immune cells or enzymes and the biomaterials.249
Drug delivery is one of the most critical research areas of biomedical engineering, especially cancer drug delivery.250,251 The efficient delivery of therapeutics primarily depends on delivery vehicles’ structural features and chemical composition. Smart DDSs should be able to target particular types of cells (e.g., cancer cells) and release therapeutics in a controlled and sustained manner. Additionally, they should pass through biological barriers (e.g., blood–brain barrier (BBB)) and evade the immune system. All of these features depend primarily on the interactions of cells or biomacromolecules with the constructing materials of delivery platforms. For example, decorating DDSs with targeting ligands is a popular strategy for making targeted DDSs that can target cancer cells (Section 4.7). DDSs can be coated with a polyethylene glycol (PEG) layer to evade immune cells before reaching the target site, such as tumor tissue. Similarly, it is possible to graft PEG onto PSAs to make a DDS that can evade the immune cells. These surface treatment strategies are also applicable to tissue engineering constructs. Similar to drug delivery systems, the surfaces of tissue scaffolds are exposed to the biological environment and may undergo events such as protein adsorption and detection by the immune system as potentially harmful. Therefore, the design of the composition and surface modification of tissue engineering constructs is critical for preventing immune responses.252,253 PSAs play a valuable role in the formulation and surface modification of tissue scaffolds and wound dressings, as they have the ability to modulate immune responses.254,255
A protein corona is a tightly packed layer of protein that is adsorbed onto nanoparticles to form a coating around them, as shown in Figure 8.256 A variety of proteins with molecular masses in the range of 10–100 kDa can contribute to the protein corona, including albumin, fibronectin, and globulin in the blood. The protein corona phenomenon can significantly affect nanoparticles’ identity and final fate. Various ligands, stealth polymer coatings, and protein shields have been used to overcome this challenge and minimize protein corona formation.257 Formation of protein coronas around PSA-based nanoparticles is possible via protein–PSA interactions. In particular, CS-based nanomedicine, which is surrounded by a cationic surface, adsorbs different cells and proteins through electrostatic interactions (due to the negative charge on proteins’ surfaces). This phenomenon can limit the bioavailability and clinical applications of CS-based DDSs. Hence, overcoming the challenges associated with protein adsorption onto nanoparticulate delivery platforms is of utmost importance in biomedical engineering.258 The nonspecific interactions related to biofouling can alter a DDS’s pharmacokinetic properties function. Given the above data, a severe demand exists for a deep study of tailoring PSA-based nanoparticles’ interactions with their drug delivery media.
Figure 8.
Schematic illustration of adsorption of abundant, negatively charged proteins (e.g., albumin) onto nanoparticles, ultimately leading to protein corona formation. Based on the Vroman effect, higher molecular weight proteins replace the soft layer, which leads to the formation of a hard corona.258 Reproduced with permission from ref (258). Copyright 2021, Elsevier.
Preventing biofouling is a critical challenge in implanted devices, such as tissue engineering scaffolds, where the accumulation of biological materials on the surface can interfere with their inherent function. When the body detects potentially harmful pathogens or materials, a series of physiological processes is triggered to protect against these foreign substances, collectively known as immune responses. The immune system comprises two main components: the innate and adaptive immune systems.259 The innate system acts as the first line of defense, providing a nonspecific immune response that is immediately activated upon threat detection. It includes physical barriers such as the skin and mucous membranes, as well as specific cells like phagocytes. Additionally, it initiates inflammatory responses and recruits immune cells to the site of infection. Implantation of a biomaterial activates the innate immune response, leading to the activation of immune cells that secrete factors to call additional immune cells to the infection site. These secreted factors and subsequent processes constitute the initial stages of the biofouling process, which is further complicated by the expression of specific molecules by microbes.260 On the other hand, the adaptive immune system elicits a specific immune response that develops over time following antigen detection. This system involves specialized immune cells such as B cells and T cells, as well as antibodies that target specific antigens like viruses and bacteria.261 Processes such as macrophage engulfment through phagocytosis and renal clearance are crucial for clearing foreign bodies from the body.
The biofilm formation process begins when a device (e.g., pacemaker) or tissue scaffolds (Ti mesh) is implanted. PSAs can be chemically modified to make antibiofouling materials to protect implants and scaffolds from fouling. For example, dopamine–HA–HA conjugates were used as an antibiofouling coating for various substrates such as metals and polymers.262 Dopamine–HA coatings prevent protein adsorption and cell attachment on substrates using mussel-inspired chemistry.263 Alternatively, zwitterionic copolymers can be used to tailor the antibiofouling of PSAs. For example, methacrylated HA was in situ-gelled with a zwitterionic copolymer based on carboxybetaine methacrylate using a thiol–ene click reaction.264 The obtained PSA-based hydrogel with zwitterionic cross-linkers showed diminished protein adsorption. Recently, there have been several comprehensive reviews of ionically gelled PSAs intended for drug delivery applications.48,251
Antimicrobial materials are a class of materials that inhibit biofilm formation by killing bacteria. For example, grafting antimicrobial peptides (AMPs) onto PSAs can be a robust strategy for making antibiofouling PSA-based scaffolds. AMPs are small molecules that exhibit a broad range of antimicrobial properties. They are part of the innate immune system and are produced by cells in response to microbial infection. These positively charged peptides, which are typically amphiphiles, interact with and disrupt microbial membranes, resulting in antimicrobial effects.265 An example of an AMP is histatin, found in human saliva, which protects the oral cavity from pathogens.266
Methacrylated AMPs were grafted to dextran to make cationic peptidopolysaccharides with antimicrobial properties.267 In similar research, an AMP was grafted onto amino and hydroxyl functionalities on sugar units of CS via click reaction to make antibacterial coatings.268 The CS-g-AMP showed lower cytotoxicity against mammalian cells than did free AMP molecules.
4.2. Tailored Adhesiveness
It is essential to consider the adhesion of biomaterials to biological tissues, such as skin, mucus, bone, and other internal parts of the human body when designing tissue engineered scaffolds, medical devices, and surgical adhesives.269−271 The adhesion process involves multiple factors and can be mediated by various mechanisms, including covalent bonding, physical interactions (such as electrostatic interactions and hydrogen bonding), and mechanical interlocking.272,273 Additionally, protein adsorption acts as an interlayer or bridge between tissue and biomaterials, further facilitating adhesion. Understanding and tailoring these adhering mechanisms is essential for designing superior tissue engineering constructs.274 The ability to tailor tissue adhesiveness relies not only on the chemical composition of the biomaterials used but also on the surface topology of tissue engineering scaffolds.
Most internal organs and tissues are hydrated or covered by a highly hydrated mucus layer. Accordingly, wet adhesion is a prerequisite for tissue attachment, and designing wet-adhesive biomaterials is vital for these applications, as represented in Table 3. Mussel-inspired chemistry has been at the center of attention in developing wet-adhesive biomaterials.275 Some chemical functionalities, such as catechol, significantly increase the wet adhesion of tissue scaffolds, wound dressings, and surgical adhesives.276 Various physical and chemical interactions play vital roles in tissue-adhesive properties. Catechol moieties can chemically interact with amine and thiol functional groups on the outer surface of tissues. Furthermore, surface patterns also impact wet/dry adhesion.277,278 Surface chemistry, topology, and mechanics significantly affect the adhesion of hydrogels.279
Table 3. Tailor-Made Polysaccharides (PSAs), Applied Modifiers, Resulting Functionalities, Resulting Tissue-/Wet-Adhesive Properties, and Corresponding Biomedical Applications.
| PSA Type | Other Constituents/Functionalities | Properties (Processing, Physicochemical, Mechanical, Biomedical) | Application | Ref |
|---|---|---|---|---|
| Chitosan | Catechol | • Thermosensitive | • Wound dressing | (143) |
| • Antibacterial | ||||
| • Promote angiogenesis | ||||
| • Adjustable mechanical properties via catechol degree of substitution | • Surgical adhesives | (284) | ||
| • High adhesive shear strength (64.8 kPa to porcine skin) | ||||
| Lauric acid | • Good physicochemical features | • Mucosal drug delivery | (285) | |
| • No cytotoxicity | ||||
| Chitosan | Gelatin | • Good adhesiveness | • Tissue adhesive for infected wound closure | (286) |
| • Antimicrobial activities | ||||
| • Good wound-healing properties | ||||
| Benzaldehyde-terminated Pluronic F127 and carbon nanotubes | • Desirable gelation time | • Treatment option for infected wounds | (287) | |
| • High water absorption | ||||
| • Stable mechanical features | ||||
| • Antimicrobial activities | ||||
| • Hemostatic properties | ||||
| • Good biodegradability | ||||
| Polyethylene glycol propionaldehyde | • Facile preparation | • Wound closure | (288) | |
| • Injectable | ||||
| • Fast gelation time | ||||
| • High adhesive strength | ||||
| • Low cytotoxicity | ||||
| • Great wound healing responses | ||||
| Oxidized konjac glucomannan | • Great tissue adhesiveness | • Wound healing | (289) | |
| • Great antibacterial activities | ||||
| Pyrogallol | • Good electrospinning capability | • Hemostasis applications | (290) | |
| • Good adhesiveness to wet media | ||||
| Methylenebis(acrylamide) | • Good swelling ratio | • Different biomedical applications | (291) | |
| • Good mechanical properties | ||||
| • Antibacterial | ||||
| Polyethylene glycol | • Rapid gelation | • Tissue adhesive devices | (292) | |
| • Great tissue adhesiveness | ||||
| • Hemostatic ability | ||||
| Glycol chitosan | 3-(4-Hydroxyphenyl) propionic acid | • Tissue adhesiveness | • Wound dressing | (293) |
| • Antibacterial activities | ||||
| • Hemostasis ability | ||||
| • Controlled drug release | ||||
| Gelatin or chitosan | Poly(acrylic acid), N-hydrosuccinimide ester | • Perfect adhesiveness to wet tissue | • Sealant | (294) |
| Carboxymethyl Chitosan | Aldehyde hydroxyethyl starch | • Controllable gelation time | • Hemostasis applications | (292, 295) |
| • Controllable mechanical properties | ||||
| • Biocompatibility | ||||
| • Controllable degradation | ||||
| Oxidized sodium alginate | Polyacrylamide | • Mechanical stability | • Wound dressing | (296) |
| • High tensile strength | ||||
| • High stretchability | ||||
| • Great self-healing ability | ||||
| Agarose | Polyethylene glycol | • Fast gelation | • Wound dressing | (297) |
| • Good mechanical properties | ||||
| • High deformability | ||||
| • Short hemostasis time | ||||
| Dextran | Aldehyde group | • Strong blood absorption | • Hemostasis applications | (298) |
| • Strong tissue adhesiveness | ||||
| Levan | Chitosan and alginate | • Surface homogeneity | • Free-standing layer-by-layer film | (299) |
| • Great mechanical performance | ||||
| • Great biological features | ||||
| Chitin | Fibrin, gelatin | • Bioadhesive | • Surgical adhesives | (300) |
| • Hemostatic | ||||
| • Antibacterial |
Grafting catechol-containing molecules and short peptides onto PSAs has been a widely used strategy to tailor wet- and tissue-adhesive properties of PSAs. For example, Hasani-Sadrabadi et al. fabricated a cell-laden bioinspired hydrogel based on dopamine-modified methacrylated alginate for craniofacial bone tissue regeneration.280 As shown in Figure 9, they grafted dopamine onto methacrylated alginate using carbodiimide chemistry. Methacrylate functional groups enabled photo-cross-linking, while catechol moieties of dopamine endowed the hydrogel with a wet-adhesion feature. It was further modified with a short peptide mimicking collagen to improve cell adhesion. The hydrogel can be cross-linked physically by adding Ca2+ cations or chemically via oxidizing dopamine moieties on alginate chains. In addition, methacrylate functionality on alginate chains enables cross-linking via radical polymerization.279,281 Hydroxyapatite microparticles (HAP MPs), which can induce osteogenic differentiation, were incorporated into gingival mesenchymal stem cells (G-MSCs). The obtained injectable mesenchymal stem cell (MSC)-laden hydrogel showed osteoconductive, wet-adhesive, and biodegradation properties, as well as adjustable mechanical properties, making it appealing for bone tissue engineering in the oral cavity. In vivo investigations in a rat model showed that MSC-laden adhesive hydrogels can completely regenerate bone tissue around dental implants.280
Figure 9.
Schematic illustration of a synthetic pathway for cell-laden and injectable alginate-based hydrogel with tailored wet and tissue adhesion properties.280 Reproduced with permission from ref (280). Copyright 2020, The American Association for the Advancement of Science.
A hydrocaffeic acid-grafted CS with tailored wet-adhesive properties (Gel1) was designed and used as a hemostatic, adhesive wound dressing because of excellent adhesiveness and inherent hemostatic capability.282 In addition, CS was modified with lactic acid, resulting in hydrophobically modified CS lactate (hmCS lactate) with tailored hydrophobic properties. Integration of hydrocaffeic acid-grafted CS with hmCS lactate in ratios of 4:1 (Gel2) and 2:1 (Gel3) introduced amphiphilic features into the obtained PSA, thereby enhancing the hemostatic capability of CS. Catechol functionalities on hydrocaffeic acid induce wet adhesion to CS and enhance its antimicrobial properties. As shown in Figure 10, modified CS with tailored properties is an adhesive hydrogel with excellent anti-infective and antibleeding features.
Figure 10.

Schematic illustration of in vitro and in situ characteristics of chitosan (CS)-based adhesive gel as a hemostasis agent. (a) Blood clotting index (BCI) values at 7 min, (b) images of in vitro blood-clotting process at 7 min, (c) red blood cells (RBC)attachment percentages, (d) percentages of platelet adhesion, (e) photographs of in situ antibleeding effects, (f) total blood loss mass, and (g) hemostasis time in response to CS-based adhesive gels (Gel1, Gel2, and Gel3).282 Reproduced from ref (282). Copyright 2020, American Chemical Society.
In a similar vein, phosphate groups play a crucial role in adhesion to inorganic materials. Accordingly, adhesive materials for bone/tooth tissue engineering could be designed based on phosphate-bearing PSAs to tailor their adhesiveness to hard tissues. For example, phosphocreatine-modified CS was used to fabricate porous scaffolds for bone tissue engineering with excellent adhesiveness behavior.280,283
4.3. Tailoring Mechanical Properties
Improving and tuning the mechanical properties (e.g., stiffness, tensile strength, flexibility, and elasticity) of PSA-based biomaterials is of utmost importance in many biomedical applications, such as fabricating tissue engineering constructs.301 For instance, tuning the mechanical properties of a hydrogel (e.g., modulus) so that it conforms to surrounding tissue is vital for injectable hydrogels. In addition, the time-dependent mechanical properties of injected or implanted scaffolds are essential for maintaining their integrity over the defined time needed to effectively deliver their payload (e.g., cells, therapeutics).
The tailoring of mechanical properties in PSAs for biomedical applications can be categorized into physical and chemical methods. These approaches leverage physical and chemical interactions to enhance the resulting mechanical properties of biomaterials. Chemical interactions, such as using cross-linkers, effectively increase the mechanical integrity and stiffness of the resulting materials.302,303 Moreover, the cross-linking strategy typically influences the porosity and drug delivery features of PSA-based scaffolds/DDSs.304 Hence, increasing active sites on PSA, enabling cross-linking (e.g., clickable functional groups), emerges as a critical factor for customizing the mechanical properties of the resulting biomaterials.
Cross-linkers may include cleavable linkages that respond to certain stimuli, enabling degradable scaffolds. Accordingly, stimuli-cleavable linkages (e.g., o-nitrobenzene) can be used to tailor the degradation and time-dependent mechanical behavior of PSA-based biomaterials.305 Furthermore, incorporating or grafting onto nanofibers, such as cellulose, is another strategy to improve the mechanical properties of PSAs.306−308
On the other hand, physical interactions (e.g., hydrogen bonding and electrostatic interactions) can not only improve the mechanical integrity but also induce further functionalities such as self-healing and dynamic mechanical behavior. If the potential of physical interactions is high, as seen in agarose, hydrogel formation is possible without the need for additional cross-linkers. Supramolecular interactions, such as host–guest interactions and hydrogen bonding, can be harnessed to create self-healing scaffolds with dynamic properties.309 Recently, the fabrication of dynamic biomaterials with time-dependent mechanical properties has gained significant attention in the biomedical field.310−312 Although self-healing is not directly correlated with the mechanical properties of biomaterials, it can contribute to maintaining mechanical integrity over time. For instance, self-healable biomaterials can better withstand mechanical stresses in vivo, resulting in durable mechanical properties. Additionally, adjusting the dynamic mechanical properties of PSA-based hydrogels can be achieved by functionalizing PSAs with hydrogen-bond forming functionalities (e.g., amide, carboxyl, urethane). Another strategy involves employing hydrogen bond forming cross-linkers (e.g., N-acryloyl glycinamide and N,N′-methylenebis(acrylamide)) represents another strategy.313,314
Other applications require robust biomaterials with enhanced mechanical properties that cannot be obtained with cross-linked PSAs. Double-network hydrogels could be designed to improve their mechanical strength and toughness based on an IPN.315−317
Among the different mechanical properties, self-healing features depend considerably on interactions at the molecular scale because supramolecular interactions can affect mechanical behavior.269 For example, strong electrostatic interactions between negatively charged alginate chains and divalent cations result in spontaneous gelation. Electrostatic interactions between polyelectrolytes can result in polyplexes. Moreover, strong hydrogen bonding is responsible for gelation in agarose hydrogel. Metal complexation in catechol-bearing polymers increases the strength and stiffness of a hydrogel to endow it with self-healing capability.318,319 Self-healing hydrogels can be made using physical or dynamic covalent cross-linking methods.320 For example, acrylic acid (AA) and acrylamide (AM) copolymer grafted onto CS (CS-g-P(AM-r-AA)) is a self-healing copolymer with multiple noncovalent interactions.321 These interactions include electrostatic attraction forces (negatively charged AA and positively charged amino groups on CS) and hydrogen bonding (AM and CS backbone). Grafting catechol moieties onto the polymer backbone provides another robust structure for self-healing hydrogels.322−324
4.4. Tailoring Gelation Time and Rheological Behavior
Gelation time is a key parameter in biomaterials design, especially when injectable hydrogels serve as delivery platforms for cells or therapeutics. A low gelation time is desirable to maintain hydrogel integrity and prevent displacement post subcutaneous injections. However, with a low gelation time, a double-syringe injection system should be used to avoid solidification before injection. Similar challenges are encountered when using hydrogel bioinks for 3D bioprinting. Accordingly, precise adjustment of gel time is essential for biomedical applications of PSA-based biomaterials, as presented in Table 4.
Table 4. Customizing the Gelation Time of PSA-Based Platforms and Exploring Their Potential Applicationsa.
| Main Constituents | Other Components | Gelation Mechanism | Gelation Time at 37 °C | Results | Applications | Ref |
|---|---|---|---|---|---|---|
| N,O-carboxymethyl chitosan (N,O-CMCS) and oxidized chondroitin sulfate | None | Chemical: Schiff base reaction | 133 s | • Excellent hemostatic ability | • Wound dressing | (330) |
| • Great biological properties | ||||||
| • Antibacterial properties | ||||||
| Oxidized gellan gum and carboxymethyl chitosan | Drug-loaded gellan gum microspheres | Chemical: Schiff base reaction | 139 s | • Excellent antibacterial activity | • Wound regeneration | (331) |
| Physical: ionic | • Sustained drug release | • Drug delivery | ||||
| Chitosan | β-Glycerol phosphate disodium salt (β-GP), genipin | Physical/chemical | 3–5 min | • Enzymatic degradation | • Drug delivery | (332) |
| • Great mechanical properties | ||||||
| • Great rheological properties | ||||||
| Pluronic-chitosan | Aniline-pentamer | Physical | 4–7 min | • Enhanced gelation time by aniline tetramer incorporation | • Protein delivery | (333) |
| • Increase gelation time by decreasing Pluronic content | ||||||
| Chitosan and dialdehyde debranched starch | None | Physical/chemical | <30 s | • Rapid gelling | • Food applications | (334) |
| • Self-healing | • Medicine applications | |||||
| • Fluorescence-responsive | ||||||
| • Good mechanical properties | ||||||
| N-Carboxyethyl chitosan and benzaldehyde-terminated Pluronic F127 | Carbon nanotubes | Physical/chemical | <40–120 s | • Antimicrobial activities | • Treatment of infected wounds | (287) |
| • Desirable gelation time | ||||||
| • Stable mechanical features | ||||||
| • Hemostatic properties | ||||||
| • High water absorption | ||||||
| • Good biodegradability | ||||||
| N-Carboxyethyl chitosan and dibenzaldehyde-terminated poly (ethylene glycol) | Doxorubicin | Physical/chemical | 35–90 s | • Controlled release | • Drug delivery (cancer) | (335) |
| • Self-healing behavior without external stimulus | ||||||
| •pH responsiveness | ||||||
| Oxidized alginate and carboxymethyl chitosan | Tetracycline hydrochloride-loaded gelatin microspheres | Physical/chemical | 70–150 s | • Antibacterial properties | • Wound healing | (336) |
| • Controlled drug release | ||||||
| • Biodegradability | ||||||
| • Great mechanical properties | ||||||
| N-Carboxyethyl chitosan and oxidized HA-g-aniline tetramer | Amoxicillin | Physical/chemical | 15–67 s | • Free radical–scavenging ability | • Wound dressing | (337) |
| • Antibacterial properties | ||||||
| • Wound healing capacity | ||||||
| • High swelling ratio | ||||||
| • Great biodegradability | ||||||
| Carboxyethyl cellulose-g-dithiodipropionate dihydrazide and dibenzaldehyde-terminated poly (ethylene glycol) | 4-Amino-dl-phenylalanine | Physical/chemical | 60 s | • Sustained drug release | • Drug delivery | (338) |
| • Good mechanical properties | • 3D cell culture | |||||
| • Self-healing performance | ||||||
| • Cell encapsulation ability | ||||||
| • pH/redox dual responsiveness | ||||||
| Chitosan-g-dihydrocaffeic acid and oxidized pullulan | doxorubicin, amoxicillin | Physical/chemical | 4–20 s | •pH-responsiveness | • Drug delivery (cancer) | (339) |
| • Injectability | ||||||
| • Tissue adhesiveness | ||||||
| •pH-dependent swelling ratio | ||||||
| • Antibacterial activity | ||||||
| Chitosan-g-polyaniline and oxidized dextran | Amoxicillin or ibuprofen | Physical/chemical | 105–444 s | • Injectability | • Drug delivery | (340) |
| • Antibacterial properties | ||||||
| • Conductivity | ||||||
| • Dual response to electric field and pH | ||||||
| Agarose–ethylenediamine conjugate and dialdehyde-functionalized polyethylene glycol | None | Physical/chemical | 10–60 s | • Short hemostasis time | • Wound dressing | (297) |
| • Good mechanical properties | ||||||
| • Fast gelation | ||||||
| • High deformability |
While most of these biomaterials are primarily designed for bioadhesive applications, a comprehensive review on this subject has already been published.329.
However, gelation time is not the only important factor when designing a hydrogel bioink or injectable hydrogel for cell delivery. The dynamic rheological properties of the hydrogel are also essential to preserve cell viability. Because the pregel solution exhibits viscoelastic behavior, encapsulated cells may experience shear stress as they pass through the nozzle/needle. Therefore, tailoring the rheological properties of these biomaterials is essential to maintain cell viability and ensure the appropriate spatial resolution of printed constructs.
The gelation phenomenon originates from physical (e.g., electrostatic) or chemical interactions between various constituents of the hydrogel, i.e., from cross-linking. Hydrogels formed via chemical cross-linking are usually stronger. However, physically cross-linked hydrogels can be created faster and are generally reversible. The kinetics of physical/chemical interactions significantly affect the gelation kinetics or gel time.
The kinetics of water absorption, swelling/deswelling, and water retention of hydrogel systems emanate from the chemical and porous structures of hydrogels. Water swelling/absorption depends on hydrogen bonding interactions between water molecules and functional groups on the hydrogel constituents. Additionally, thermodynamic parameters, such as temperature and pressure, also affect the equilibrium water content of hydrogels. Water molecules affect hydrogels’ mechanical properties, such as softness and flexibility. Accordingly, installing highly hydrophilic species on the PSA backbone also affects the mechanical properties of the obtained PSA-based hydrogel.
Similarly, the conformation of polymer chains also plays a significant role in the hydrogel’s water solubility and absorption capability. The base polymer should not only possess suitable functional groups (such as hydroxyl and carboxyl groups) but also be capable of interacting with water molecules. For example, cellulose has many hydroxyl groups in its structure that result in strong intermolecular interactions and the formation of a supramolecular structure. This structure, in which OH groups are mainly shielded, prevents cellulose from dissolving in water or organic solvents. However, chemical modifications (e.g., carboxymethylation or acetylation) disturb the supramolecular structure and expose hydroxyl groups to water molecules. These chemical modifications of cellulose will result in a hydrogel that is softer than the original cellulose.
Adjustable gelation time is crucial in biomedical applications, such as injectable hydrogels and bioprinting. The gelation behavior of thermosensitive hydrogels depends on temperature changes. For example, they are liquid at room temperature and then solidify at body temperature (∼37 °C). In these thermosensitive hydrogels, the gelation kinetics depend on water diffusion across polymer chains (a mass transfer-controlled process) and the creation of hydrogen bonds (physical interactions) between water molecules and PSA functional groups. Since creating physical interactions is fast, gelation is mainly governed by diffusion mass transfer.
On the other hand, chemical reaction kinetics play a significant role in the gelation behavior of chemically cross-linked hydrogels. For example, an aldehyde is a fast-reacting group, while the ketone group reacts slowly with hydrazide. Accordingly, an injectable mixing-induced hydrogel with adjustable gelation time can be designed using a hydrazide-functionalized polymer as the first component, with the second polymer having aldehyde and ketone functional groups.325 Clickable PSAs can make a mixing-induced hydrogel with in situ gel-formation features.8 Because of their orthogonality, click reactions enable the design of injectable hydrogels for in vivo gelling.326 Fast-gelling and other superior gel properties were observed for a hydrogel based on sodium alginate, which benefited from dual cross-linking mechanisms, i.e., hydrogen bonding and thiol–ene click chemistry.327 An interesting self-healing and shear-sensitive PSA hydrogel was devised by Shitrit et al. CS nanogels embedded within pectin created a two-component whole PSA hydrogel with self-healing and shear-thinning properties.328 The nanogels act as macroscale cross-linkers, which physically interact with the pectin chains. The reversible physical bonds dissociate under shear stresses and recreate upon shear removal, corresponding to a shear-thinning behavior.
4.5. Tailoring Responsiveness
Stimuli-responsive polymers, known as smart polymers, are a group of polymers whose properties change in response to an external stimulus such as pressure alternation, change in temperature or ionic strength, exposure to radiation or electric/magnetic fields, and the presence of redox species (Table 5). Fabrication of stimuli-responsive polymers is at the center of attention for many materials scientists for their usefulness in tissue engineering and drug delivery applications (Figure 11).341 For example, thermoresponsive polymers, which undergo a phase change in response to temperature change, have been used in tissue engineering and wound healing because physiological temperature is precisely controlled in a narrow range above room temperature.342
Table 5. Classification of Stimuli-Responsive Polysaccharides (PSAs) Based on Type, Responsive Groups, Responsible Stimuli, and Recommended Biomedical Applications.
| Stimuli | Responsive groups | PSA | Applications | Ref |
|---|---|---|---|---|
| pH | Phenylboronic ester | Dextran | • Drug delivery | (357) |
| Thiol | Chitosan | • Wound healing | (359) | |
| Reactive oxygen species (ROS) | Boronic ester | Dextran | • Drug delivery | (360) |
| H2O2 (redox)/light | MnO2 | Chitosan | • Wound healing | (361) |
| GSH (redox)/temperature | Disulfide linkage | Cellulose | • Drug delivery | (362) |
| Thermosensitive/pH responsive/cell penetrating | H6R6-chitosan-g-poly(N-vinylcaprolactam) | Chitosan | • Promoting tumor apoptosis | (363) |
| Glucose (chemicals) | Glucose oxidase | Dextran | • Insulin delivery | (364) |
| Near-infrared | Mussel-inspired polydopamine | Glycol chitosan | • Combating bacterial infection | (365) |
| Polydopamine | Chitosan | • Coagulopathy | (366) | |
| • Tissue regeneration | ||||
| Gold nanoparticles | Chitosan | • Cancer treatment | (367) | |
| Gold nanorod | Chitosan | • Drug delivery vehicle | (368) | |
| Polyaniline | Glycol chitosan | • Photothermal therapy | (369) | |
| Quantum dots | Cellulose | • Printing industry | (370) | |
| • Packaging industry | ||||
| Photo- and redox-responsive | Dithioerythritol | Carboxymethyl cellulose | • Shape memory hydrogel | (371) |
| Electric/pH responsive | Polyaniline | Chitosan | •“Smart” drug release | (340) |
Figure 11.
Thermosensitive polymers in medicine. (a) Schematic illustration of different applications of stimulus-responsive polysaccharide (PSA) in tissue and biomedical engineering.341 Reproduced with permission from ref (341). Copyright 2019, Elsevier. (b) Drug release mechanism of temperature-responsive hydrogels depends on the heating intensity.345 Reproduced with permission from ref (345). Copyright 2009, Elsevier. (c) Fabrication and in vivo fate of nanocarrier based on chitosan, with oleic acid tail (CMO), hyaluronidase (HAase), and oxidized hyaluronic acid (OHA) deposited onto the 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP)/survivin-shRNA lipoplex (LR).346 Reproduced from ref (346). Copyright 2020, American Chemical Society. (d) Scheme of the UV-induced chemical cross-linking of alginate with PEG via exposure to CaCl2. (e) Mechanism of the cross-linking induced by UV and calcium ions during bioprinting of hollow tubes. (f) Diameter of the multilayer coaxial nozzles of 4D bioprinter and the pattern applied for the printing of responsive scaffold.347 Reproduced with permission from ref (347). Copyright 2016, Elsevier.
Thermosensitive polymers based on grafted PSAs are an essential subclass of tailor-made smart PSAs. Grafting a thermosensitive polymer, usually poly(N-isopropylacrylamide) (PNIPAM), onto the PSA main chain is the most widely used procedure for making thermoresponsive PSAs.343 For instance, CS-g-PNIPAM was synthesized by Bao et al. using a combination of ATRP and click chemistry.344 Because of the inherent pH sensitivity of CS and the thermosensitivity of PNIPAM, we can expect that CS-g-PNIPAM would possess double sensitivity. It was observed that at acidic pH (pH < 4) and temperatures above the lower critical solution temperature (LCST), polymeric micelles were formed, in which PNIPAM and CS constituted the hydrophobic core and the hydrophilic shell, respectively. Such structures can be utilized to make injectable hydrogels or drug delivery platforms.
This procedure is also applicable for other PSAs, with some modifications. For example, PNIPAM-g-alginate was synthesized by grafting amine-terminated PNIPAM (PNIPAM-NH2) onto the main alginate chain.348 Thiolated chemicals, such as cysteamine or 2-aminoethanethiol hydrochloride (AET), can make PNIPAM that contains a reactive amine functional group (i.e., PNIPAM-NH2) before grafting it to alginate. The obtained PNIPAM-g-alginate can be used as an injectable thermosensitive hydrogel for drug delivery applications.349 In another study, a graft copolymer based on PNIPAM-g-alginate was synthesized with unique gelling properties.350 The so-called schizophrenic gelling properties of this graft copolymer resulted in the gelling of the polymer both when cooling and heating.
However, making thermosensitive PSA is not limited to PNIPAM grafting. Poly(N-vinylcaprolactam)-grafted-aminated alginate (PNVCL-g-Alg-NH2) also shows thermoresponsive features.351 Moreover, making IPNs based on a PSA and a thermosensitive polymer is another robust method to make PSA-based thermosensitive polymers.352 In addition, thermosensitive cross-linkers (e.g., based on PNIPAM) can be used to create smart PSA-based hydrogels with tailored responsiveness.
On the other hand, magnetic-responsive PSA-based systems can be manufactured by grafting PSAs onto magnetic nanoparticles (e.g., superparamagnetic iron oxide nanoparticles, SPIONs), which can be used in imaging or drug delivery platforms.353,354
In addition, PSAs may be tailored to respond to other stimuli, such as light and redox moieties, some of which are discussed in the following sections.355,356 It is worth mentioning that the pH sensitivity of boronate esters (an acid-responsive linkage that hydrolyzes under mildly acidic conditions) can be utilized to make pH-sensitive PSA-based DDSs.357,358 Clip chemistry provides an invaluable toolbox to make PSA-based smart biomaterials that respond to various stimuli.244 Some stimuli-responsive PSA-based biomaterials are summarized in Table 5.
4.6. Tailoring Conductivity
Scaffolds that are conductive, containing inherently conducting polymers (CPs) or polymer composites with conductive nanomaterials, have found numerous applications in the biomedical field, particularly in cardiac and neural tissue engineering.372,373 Electroconductive hydrogels based on PSAs have been extensively used in tissue engineering, wound healing, drug delivery, and bioelectronic applications.13 Incorporating conductive (nano)materials or grafting conjugated polymers/oligomers provides a robust strategy to make conductive PSA-based materials for tissue engineering or wearable electronics.374 As depicted in Table 6, metallic nanomaterials (such as gold nanostructures) and carbon-based nanomaterials (such as graphene and carbon nanotubes) have been the most utilized conducting materials in biomedical applications. However, they suffer from biodegradation and low biocompatibility, which have limited their in vivo applications.
Table 6. Classification of Tailor-Made Polysaccharides (PSAs) Reported in the Literature Works Based on the Grafted Compounds and Their Applications.
| Material | Graft | PSA | Other Components | Applications | Ref |
|---|---|---|---|---|---|
| Conducting polymers | Aniline trimer | Dextran | Hexamethylene diisocyanate | Localized drug delivery | (381) |
| Aniline pentamer | Chitosan | Gelatin/agarose | Treating motor neuron–related diseases | (375) | |
| Polyaniline | Hydroxyethyl cellulose | Soy protein isolate | Electrical stimulation for enhanced recovery of peripheral nerve injury | (382) | |
| Polyaniline | Chitosan | N-Acetylneuraminic acid aldolase and pyruvate oxidase | Biosensors | (383) | |
| Poly(3,4-ethylenedioxythiophene) | Alginate | Poly-β-cyclodextrin | Cell culture | (384) | |
| Tissue engineering | |||||
| Carbon nanomaterials | carbon nanotube | Quaternized Chitosan | Gelatin | Injectable hydrogel for hemorrhage | (385) |
| Wound healing |
On the other hand, oligomers of semiconducting polymers, especially oligoanilines, enable the construction of conductive hydrogels for in vivo applications, as they benefit from electrical conductivity and can be removed from the human body through renal clearance.375 Accordingly, grafting aniline oligomers onto the PSA backbone is a robust strategy for making conductive hydrogels for tissue engineering constructs. However, oligomers have lower electrical conductivity compared to conjugated polymers. For applications that require higher electrical conductivity values, semiconducting polymers such as PANI and poly(3,4-ethylenedioxythiophene) (PEDOT) are usually preferred over their oligomers.376−378 The deterioration of electrical conductivity over time, known as aging, is also a challenge when using small-molecule dopants (such as protonic acids).379,380 In this regard, macromolecular dopants are preferred over small-molecule dopants.
4.7. Tailoring Targeting Capabilities
Targeted drug delivery systems (TDDSs) have been widely studied for various diseases, especially cancers.386,387 Because of the systemic toxicity of chemotherapeutics, tremendous efforts have been directed toward developing targeted nanocarriers to treat cancers. Distinctive characteristics between diseased and normal tissue (e.g., slightly acidic TME) have directed researchers to design targeted delivery platforms that can release therapeutics in appropriate locations, such as tumor tissue (Table 7).
Table 7. Insights into the Development of Tailor-Made Polysaccharides (PSAs) with Engineered Modes of Action for Targeted Drug Delivery along with Their Mechanism of Action and Dedicated Therapy.
| Target | PSA | Other Constituents/Ligands | Indications/Tumor Cells | Cancer Type/Therapy | Ref |
|---|---|---|---|---|---|
| Aptamer | Dextran | Graphene oxide | 4T1 and MCF-7 nucleolin | Mammary carcinoma | (402) |
| Enzyme-sensitive polysaccharide | Guar gum | Mesoporous silica nanoparticles | Colon carcinoma cells | Colon | (403) |
| Magnetic navigation | Dextran | Fucoidan | Anti-PD-L1 and T-cell activators | Immunotherapy | (404) |
| Mitochondria-targeted | Chitosan | Fluorescent carbon quantum dots | Mitochondria | Mitochondria-targeted photodynamic cancer therapy | (405) |
| Hyaluronic acid | Chitosan | 5-Fluorouracil | Expressed CD44 | Targeted cancer drug delivery | (406) |
| pH-responsive | Collagen-peptide-functionalized chitosan nanoparticles | Doxorubicin | HeLa cells | Cancer drug encapsulation | (407) |
| pH-responsive | Xylan–curcumin (xyl–cur) conjugate | none | Human colon cancer cells | Prodrug | (408) |
| Reactive oxygen species -responsive | Chondroitin sulfate | Protoporphyrin, Doxorubicin | MCF-7/ARD cells | Chemo-photodynamic therapy | (409) |
| Up-regulation of cytokine secretion and expression | Functionalized alginate | Ovalbumin/mannose | Dendritic cell | Cancer immunotherapy | (410) |
| Receptor-mediated endocytic uptake | Dextran sulfate | Alginate, doxorubicin, cisplatin | Resistant/sensitive breast cancer cells | Breast | (411) |
| Thermoresponsive | Chitosan | Maleic anhydride, sodium dodecyl sulfate, erlotinib | A549 and OVCAR-3 cells | Injectable tumor-targeting platform | (412) |
| Glutathione-sensitive | Carboxymethyl chitosan | Cisplatin-doxorubicin | HeLa cells | Combination tumor therapy | (413) |
| Glutathione-sensitive | Chondroitin sulfate | Deoxycholic acid | HGC7 cells | Gastric cancer | (414) |
| Selective delivery of Akt2 siRNA | glycol chitosan | Taurocholic acid | CT26 cells | Colorectal and liver Metastases | (415) |
Some TDDSs rely solely on passive targeting strategies, especially the EPR effect, while others also benefit from active targeting strategies. Abnormalities in the TME have been widely used to develop TDDSs. These abnormalities include a mildly acidic pH, unique vascular architecture, and distinctive features of cancer cells (e.g., overexpression of specific receptors or enzymes and abnormal metabolic activities). Various nanocarriers, such as nanoparticles, polymer micelles, liposomes, dendrimers, and polymer-drug conjugates, have been developed to fabricate TDDSs.388 Stimuli-responsive polymers that respond to changes in the internal stimuli (e.g., pH or temperature) and external stimuli (magnetic field, ultrasound, electromagnetic waves) have played a critical role in making smart and targeted delivery systems.
Most therapeutics, such as chemotherapeutics, are hydrophobic molecules and insoluble under physiological conditions. For this reason, much attention has been given to the fabrication of hydrophobically modified PSAs (which, unmodified, are usually hydrophilic).389 Chemical modification of PSAs with fatty acids, which contain alkyl chains in their chemical structure, is a popular method for making amphiphilic PSAs, i.e., tailoring their amphiphilicity. Amphiphilic polymers can form micelles in solutions (when concentrations exceed their critical micelle concentration), with the core and shell capable of storing hydrophobic and hydrophilic therapeutics.
PSAs tailored to target the TME are not limited to pH-sensitive PSAs. It is possible to target particular types of receptors that are overexpressed on cancer cell membranes. For example, folate receptors are overexpressed on many cancer cells and can be used to design TDDSs based on PSAs.390,391 In addition, HA, which binds to multiple receptors on tumor cells, can be used to make TDDSs.392
Molecular recognition, which consists of specific noncovalent interactions that are ubiquitous in biological systems (e.g., between receptor and ligand), can be exploited for TDDSs.393,394 Recognition-capable soft materials can be used in various fields, such as drug delivery and biosensing applications.395 Some monoclonal antibodies (mAB), capable of molecular recognition, have been designed to target tumor antigens (usually growth factor receptors).396,397 Synthetic molecular recognition, especially molecularly imprinted polymers (MIPs), can also target cancer cells.398 Molecularly imprinted polymer nanoparticles, known as nanoMIPs, are another subclass of MIPs that can be used in designing TDDSs.399
Another appealing strategy for targeting cancer cells is to make artificial receptors on tumor cells, which can then be targeted through complementary species. A hot field of research is installing clickable groups into the plasma membrane in the form of artificial clickable receptors that can be targeted via bioorthogonal click reactions.400 This technique includes installing clickable glycoconjugates on a cell membrane using metabolic glycoengineering (MGE), followed by targeting these clickable, artificial receptors via complementary clickable groups on therapeutics-loaded nanocarriers (Figure 12).401 Glycol chitosan nanoparticles (CNPs) were loaded with unnatural sugars to enable the installation of clickable groups via MGE. Afterward, a complementary clickable group targeted clickable glycoconjugates on the cell membrane. Orthogonal click reactions allow for improved targeting compared to targeting biological receptors on cancer cells.
Figure 12.
Schematic illustration of TDDS based on artificial receptors. In the first step, glycol chitosan nanoparticles (CNPs) containing tetraacetylated N-azidoacetyl-d-mannosamine (Ac4ManNAz) are injected, and site-specific generation of azide groups on tumor tissue occurs via metabolic glycoengineering. The second injection contains bicyclo[6.1.0]nonyne (BCN)-modified CNPs, which yields click reactions between BCN groups and azide groups, enhancing tumor targeting and photodynamic therapy in vivo.401 Reproduced from ref (401). Copyright 2014, American Chemical Society.
5. Conclusions
The properties and functionalities of materials must be adjusted to meet the requirements of specific applications, especially for novel materials and technology-based systems.416 Examples include mechanically robust ultralightweight materials for making aircraft/spacecraft, high-temperature superconductors, electrically conductive thermally insulative materials, high absorbance materials with low emissivity, and semiconductive polymers with metal-like conductivity. Furthermore, some novel applications may necessitate multiple material properties that seem mutually exclusive. For example, some applications require the fabrication of tissue engineering scaffolds with high mechanical strength and dynamic behavior (e.g., self-healing features).389,417 This combination is challenging to achieve with conventional PSAs.
Based on the intended applications, various PSAs possessing distinct properties may be needed, which result in an interdependence of materials for a target application. Regardless of the materials selected, further modifications may be required to create new and suitable properties. For instance, customizing the chemical structure of materials to obtain desired bulk properties has been sought through various strategies, including chemical modifications.95,418,419 An exploration of altering the physical and biological characteristics of PSAs has also been conducted to fine-tune their properties.
Although PSAs are biocompatible with an appeal in the biomedical field, their poor mechanical properties and limited bioactivities can hinder their widespread application. In this regard, tailoring their mechanical properties can increase their suitability for biomedical applications, such as fabricating tissue engineering scaffolds. Chemical modification strategies, such as cross-linking and grafting, provide invaluable tools for enhancing the appealing features of PSAs or for introducing new properties. Designing PSA-based smart materials that respond to various stimulants, preparing PSA-based hydrogels with improved mechanical properties and tissue-adhesive features, and endowing PSA-based hydrogels with electrical conductivity, are some strategies that enable the creation of tailor-made PSAs for special biomedical applications. Although this review has focused on the biomedical applications of tailor-made PSAs, there are also significant opportunities to utilize these customized carbohydrate polymers for applications in other fields.
Chemical modification strategies play pivotal roles in developing novel tailor-made PSAs. However, modern biomedical applications increasingly require the fabrication of novel tailor-made PSAs with finely defined properties and functionalities, which cannot be achieved through simple chemical modification methods. An important application in cancer therapy is the development of nanocarriers with superior biocompatibility and controllable biodegradation. These carriers are designed to evade the immune system and selectively target cancer cells prior to cellular uptake.420−422 However, individual PSAs, or a combination of conventional PSAs, fail to make such systems. The chemical modification of PSAs—using special functional groups, grafting polymers and biomolecules, utilizing stimuli-responsive cross-linkers, and conjugating with targeting biomacromolecules—can be used as strategies to fulfill these requirements.
This review article discusses the creation of tailor-made PSAs with specific properties from a chemistry and materials science perspective. Throughout the article, general knowledge from these fields was utilized to design PSAs that meet the requirements of various biomedical applications. However, this simplified perspective has its limitations. For example, similar chemical modifications on different PSAs may result in other properties. Emergent computer-aided molecular design and inverse materials design strategies, combined with high-throughput synthesis, are enabling faster and more efficient development of new molecules, such as new drugs.423 These molecules can be used to modify PSAs and introduce new properties. Furthermore, simulation of molecular dynamics can be used as a robust strategy to predict the properties of bulk materials based on molecular structure.424,425 However, the computational cost of these methods is relatively high, especially for macromolecules and polymers, which limits their applicability in designing tailor-made PSAs.426 Alternatively, machine learning strategies, such as deep generative modeling, can be used to create novel materials based on a collection of material properties and chemical structures. This robust technique has the potential to revolutionize the design of tailor-made materials in the near future.427−445 With the advancement of computational strategies, quantum computers, and artificial intelligence (using adaptable machine learning-based algorithms), there is potential to design PSAs without relying on cumbersome experimental work involving trial and error. However, we must remain patient as these technologies are still in the development stage.
Acknowledgments
M.K.Y. acknowledges financial support from Nobelium Joining Gdańsk Tech Research Community (DEC 35/2022/IDUB/l.1). A.H. acknowledges financial support from the Ministry of Science and Higher Education in Poland (0613/SBAD/4820). J.K.-L. acknowledges financial support received under the IDUB GUT RADIUM LEARNING THROUGH RESEARCH PROGRAMS number 13/Radium/2021. S.A.B. acknowledges financial support provided by the National Institutes of Health (1R01EB027705) and the National Science Foundation (DMR1847843).
Glossary
Abbreviations
- 5-Fu
5-fluorouracil
- AA
acrylic acid
- Ac4ManNAz
tetraacetylated N-azidoacetyl-d-mannosamine
- AET
2-aminoethanethiol hydrochloride
- AGA
automated glycan assembly
- AM
acrylamide
- AMPs
antimicrobial peptides
- ATRP
atom transfer radical polymerization
- β-GP
β-glycerol phosphate disodium salt
- BBB
blood–brain barrier
- BCI
blood clotting index
- BCN
bicyclo[6.1.0]nonyne
- BTZ
bortezomib
- c(RGDfK)
cyclo-(Arg-Gly-Asp-d-Phe-Lys)
- CMC
carboxymethyl cellulose
- CMCS
carboxymethyl chitosan
- CMO
chitosan with oleic acid tail
- CNC
cellulose nanocrystals
- CNPs
glycol chitosan nanoparticles
- CNTs
carbon nanotubes
- COS
chitosan oligosaccharide
- CPs
conducting polymers
- CPSAs
capsular polysaccharides
- cRGD
cyclic arginine-glycine-aspartic acid peptide
- CRP
controlled radical polymerization
- CS
chitosan
- DCC
N,N′-dicyclohexylcarbodiimide
- DCM
dichloromethane
- DDSs
drug delivery systems
- Dex
dextran
- Dex-CHO
oxidized dextran
- DMAc,N
N-dimethylacetamide
- DOTAP
1,2-dioleoyl-3-trimethylammonium-propane
- DOX
doxorubicin
- DS
degree of substitution
- DTE
dithioerythritol
- EC
ethylcellulose
- ECD
1-ethyl-3-(3-(dimethylamino)propyl) carbodiimide
- ECM
extracellular matrix
- EPR
enhanced permeability and retention
- EPSAs
exopolysaccharides
- ES
electrical stimulation
- FDA
Food and Drug Administration
- GAGs
glycosaminoglycans
- GCS
glycol chitosan
- G-MSCs
gingival mesenchymal stem cells
- GO
graphene oxide
- HA
hyaluronic acid
- HAase
hyaluronidase
- hADSCs
human adipose-derived stem cells
- HAP MPs
hydroxyapatite microparticles
- HES
hydroxyethyl starch
- HPC
hydroxypropyl cellulose
- HPMC
hydroxypropyl methylcellulose
- HS
heparan sulfate
- ILs
ionic liquids
- IPN
interpenetrating polymer network
- KC
kappa-carrageenan
- KS
keratan sulfate
- LCST
lower critical solution temperature
- LR
survivin-shRNA lipoplex
- MAA
N-maleoyl-β-alanine
- mAB
monoclonal antibodies
- MC
methylcellulose
- MCA
monochloroacetic acid
- MGE
metabolic glycoengineering
- MIPs
molecularly imprinted polymers
- MQ
monoquaternary
- MSC
mesenchymal stem cell
- MSN
mesoporous silica nanoparticle
- NAL
N-acetylneuraminic acid aldolase
- NMP
nitroxide-mediated polymerization
- N
O-CMCS,N,O-carboxymethyl chitosan
- OD
oxidized dextran
- OHA
oxidized hyaluronic acid
- OSAs
oligosaccharides
- PANI
polyaniline
- PDA
polydopamine
- PEDOT
poly(3,4-ethylenedioxythiophene)
- PEG
polyethylene glycol
- PGA
propylene glycol alginate
- PLGA
poly(lactic-co-glycolic acid)
- PNIPAM
poly(N-isopropylacrylamide)
- PNIPAM-NH2
amine-terminated poly(N-isopropylacrylamide)
- PNVCL-
g-Alg-NH2, poly(N-vinylcaprolactam)-grafted-aminated alginate
- POP
Pleurotus ostreatus
- PPU
Ulmus pumila L. hyaluronic acid (HA)
- PSAs
polysaccharides
- PVA
poly(vinyl alcohol)
- QD
quantum dot
- RAFT
reversible addition–fragmentation chain transfer
- RBC
red blood cell
- RCPS
Radix Cyathula officinalis polysaccharide
- ROS
reactive oxygen species
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- S-protein
spike protein
- SPIONs
superparamagnetic iron oxide nanoparticles
- TDDSs
targeted drug delivery systems
- TEMPO
(2,2,6,6-tetramethylpiperidin-1-yl)oxyl
- TH
tetracycline hydrochloride
- TME
tumor microenvironment
- XG
xanthan gum
Data Availability Statement
Data will be made available on request.
Author Contributions
Mohsen Khodadadi Yazdi: Writing - Original draft preparation, Methodology, Visualization. Farzad Seidi: Supervision. Aleksander Hejna: Writing - Original draft preparation, Writing - Review and Editing, Methodology. Payam Zarrintaj: Investigation. Navid Rabiee: Writing - Editing, Investigation. Justyna Kucinska-Lipka: Investigation, Formal analysis. Mohammad Reza Saeb: Conceptualization, Supervision, Writing - Review and Editing. Sidi A. Bencherif: Conceptualization, Supervision, Writing - Review and Editing.
The authors declare no competing financial interest.
References
- Shokrani H.; Shokrani A.; Sajadi S. M.; Seidi F.; Jouyandeh M.; Zarrintaj P.; Kar S.; Kim S.; Kuang T.; Rabiee N. Polysaccharide-based nanocomposites for biomedical applications: a critical review. Nanoscale Horizons 2022, 7, 1136. 10.1039/D2NH00214K. [DOI] [PubMed] [Google Scholar]
- Nogia P.; Sidhu G. K.; Mehrotra R.; Mehrotra S. Capturing atmospheric carbon: biological and nonbiological methods. International Journal of Low-Carbon Technologies 2016, 11 (2), 266–274. 10.1093/ijlct/ctt077. [DOI] [Google Scholar]
- Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature 2007, 446 (7139), 1023–1029. 10.1038/nature05816. [DOI] [PubMed] [Google Scholar]
- Varki A.; Cummings R. D.; Esko J. D.; Stanley P.; Hart G. W.; Aebi M.; Darvill A. G.; Kinoshita T.; Packer N. H.; Prestegard J. H. . Essentials of Glycobiology [Internet] 2015. [PubMed] [Google Scholar]
- Brewer C. F.; Miceli M. C.; Baum L. G. Clusters, bundles, arrays and lattices: novel mechanisms for lectin-saccharide-mediated cellular interactions. Curr. Opin. Struct. Biol. 2002, 12 (5), 616–623. 10.1016/S0959-440X(02)00364-0. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Huang G. Extraction and derivatisation of active polysaccharides. Journal of Enzyme Inhibition and Medicinal Chemistry 2019, 34 (1), 1690–1696. 10.1080/14756366.2019.1660654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto K.; Sugawara I.; Ito W.; Kodama T.; Hayami M.; Mori S. Sulfated homopolysaccharides with immunomodulating activities are more potent anti-HTLV-III agents than sulfated heteropolysaccharides. Japanese journal of experimental medicine 1988, 58 (3), 145–151. [PubMed] [Google Scholar]
- Khodadadi Yazdi M.; Sajadi S. M.; Seidi F.; Rabiee N.; Fatahi Y.; Rabiee M.; Dominic C.D. M.; Zarrintaj P.; Formela K.; Saeb M. R.; Bencherif S. A. Clickable Polysaccharides for Biomedical Applications: A Comprehensive Review. Prog. Polym. Sci. 2022, 133, 101590. 10.1016/j.progpolymsci.2022.101590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aderibigbe B. A.; Buyana B. Alginate in wound dressings. Pharmaceutics 2018, 10 (2), 42. 10.3390/pharmaceutics10020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal M. I. H.; Yeasmin M. S.; Rahman M. S. Preparation of food grade carboxymethyl cellulose from corn husk agrowaste. Int. J. Biol. Macromol. 2015, 79, 144–150. 10.1016/j.ijbiomac.2015.04.061. [DOI] [PubMed] [Google Scholar]
- Mettu R.; Chen C.-Y.; Wu C.-Y. Synthetic carbohydrate-based vaccines: challenges and opportunities. Journal of Biomedical Science 2020, 27 (1), 1–22. 10.1186/s12929-019-0591-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidi F.; Yazdi M. K.; Jouyandeh M.; Habibzadeh S.; Munir M. T.; Vahabi H.; Bagheri B.; Rabiee N.; Zarrintaj P.; Saeb M. R. Crystalline polysaccharides: a review. Carbohydr. Polym. 2022, 275, 118624. 10.1016/j.carbpol.2021.118624. [DOI] [PubMed] [Google Scholar]
- Khodadadi Yazdi M.; Zarrintaj P.; Khodadadi A.; Arefi A.; Seidi F.; Shokrani H.; Saeb M. R.; Mozafari M. Polysaccharide-based electroconductive hydrogels: Structure, properties and applications in biomedical engineering. Carbohydr. Polym. 2022, 278, 118998. 10.1016/j.carbpol.2021.118998. [DOI] [PubMed] [Google Scholar]
- Taghizadeh M.; Taghizadeh A.; Yazdi M. K.; Zarrintaj P.; Stadler F. J.; Ramsey J. D.; Habibzadeh S.; Rad S. H.; Naderi G.; Saeb M. R.; et al. Chitosan-based inks for 3D printing and bioprinting. Green Chem. 2022, 24 (1), 62–101. 10.1039/D1GC01799C. [DOI] [Google Scholar]
- Rezaeeyazdi M.; Colombani T.; Memic A.; Bencherif S. A. Injectable hyaluronic acid-co-gelatin cryogels for tissue-engineering applications. Materials 2018, 11 (8), 1374. 10.3390/ma11081374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gsib O.; Duval J.-L.; Goczkowski M.; Deneufchatel M.; Fichet O.; Larreta-Garde V.; Bencherif S. A.; Egles C. Evaluation of fibrin-based interpenetrating polymer networks as potential biomaterials for tissue engineering. Nanomaterials 2017, 7 (12), 436. 10.3390/nano7120436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prata J. E.; Barth T. A.; Bencherif S. A.; Washburn N. R. Complex fluids based on methacrylated hyaluronic acid. Biomacromolecules 2010, 11 (3), 769–775. 10.1021/bm901373x. [DOI] [PubMed] [Google Scholar]
- Kim J.; Bencherif S. A.; Li W. A.; Mooney D. J. Cell-Friendly Inverse Opal-Like Hydrogels for a Spatially Separated Co-Culture System. Macromol. Rapid Commun. 2014, 35 (18), 1578–1586. 10.1002/marc.201400278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdi M. K.; Seidi F.; Jin Y.; Zarrintaj P.; Xiao H.; Esmaeili A.; Habibzadeh S.; Saeb M. R. Crystallization of polysaccharides. Polysaccharides: Properties and Applications 2021, 283–300. 10.1002/9781119711414.ch13. [DOI] [Google Scholar]
- Yazdi M. K.; Khodadadi A.; Zarrintaj P.; Ganjali M. R.; Salehnia F.; Rezapour M.; Habibzadeh S.; Saeb M. R.. Cross-linked polysaccharides in drug delivery. In Tailor-Made Polysaccharides in Drug Delivery; Elsevier, 2023; pp 107–127. [Google Scholar]
- Khodadadi Yazdi M.; Taghizadeh A.; Taghizadeh M.; Stadler F. J.; Farokhi M.; Mottaghitalab F.; Zarrintaj P.; Ramsey J. D.; Seidi F.; Saeb M. R.; Mozafari M. Agarose-based biomaterials for advanced drug delivery. J. Controlled Release 2020, 326, 523–543. 10.1016/j.jconrel.2020.07.028. [DOI] [PubMed] [Google Scholar]
- Al-Hazmi H. E.; Łuczak J.; Habibzadeh S.; Hasanin M.; Mohammadi A.; Esmaeili A.; Kim S.-J.; Yazdi M. K.; Rabiee N.; Badawi M.; et al. Polysaccharide nanocomposites in water treatment: A review. Chemosphere 2024, 347, 140578. 10.1016/j.chemosphere.2023.140578. [DOI] [PubMed] [Google Scholar]
- Khodadadi Yazdi M.; Jabbour K.; Sajadi S. M.; Esmaeili A. Drug delivery systems based on renewable polymers: a conceptual short review. Polymers from Renewable Resources 2022, 13 (1–2), 44–54. 10.1177/20412479221107469. [DOI] [Google Scholar]
- Shokrani H.; Shokrani A.; Sajadi S. M.; Yazdi M. K.; Seidi F.; Jouyandeh M.; Zarrintaj P.; Kar S.; Kim S.-J.; Kuang T.; et al. Polysaccharide-based nanocomposites for biomedical applications: a critical review. Nanoscale Horizons 2022, 7 (10), 1136–1160. 10.1039/D2NH00214K. [DOI] [PubMed] [Google Scholar]
- Khodadadi Yazdi M.; Zarrintaj P.; Saeb M. R.; Mozafari M.; Bencherif S. A. Progress in ATRP-derived Materials for Biomedical Applications. Prog. Mater. Sci. 2024, 143, 101248. 10.1016/j.pmatsci.2024.101248. [DOI] [Google Scholar]
- Mohammadi Y.; Saeb M. R.; Penlidis A.; Jabbari E.; Stadler F. J.; Zinck P.; Matyjaszewski K. Intelligent machine learning: tailor-making macromolecules. Polymers 2019, 11 (4), 579. 10.3390/polym11040579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek P.; Vudayagiri S.; Skov A. L. How to tailor flexible silicone elastomers with mechanical integrity: A tutorial review. Chem. Soc. Rev. 2019, 48 (6), 1448–1464. 10.1039/C8CS00963E. [DOI] [PubMed] [Google Scholar]
- Saeb M. R.; Mohammadi Y.; Rastin H.; Kermaniyan T. S.; Penlidis A. Visualization of bivariate sequence length-chain length distribution in free radical copolymerization. Macromol. Theory Simul. 2017, 26 (5), 1700041. 10.1002/mats.201700041. [DOI] [Google Scholar]
- Mohammadi Y.; Saeb M. R.; Penlidis A.; Jabbari E.; Zinck P.; Stadler F. J.; Matyjaszewski K. Intelligent Monte Carlo: a new paradigm for inverse polymerization engineering. Macromol. Theory Simul. 2018, 27 (3), 1700106. 10.1002/mats.201700106. [DOI] [Google Scholar]
- Mohammadi Y.; Saeb M. R.; Penlidis A. Heuristic search strategy for transforming microstructural patterns to optimal copolymerization recipes. Macromol. Theory Simul. 2018, 27 (2), 1700088. 10.1002/mats.201700088. [DOI] [Google Scholar]
- Zunger A. Inverse design in search of materials with target functionalities. Nature Reviews Chemistry 2018, 2 (4), 0121. 10.1038/s41570-018-0121. [DOI] [Google Scholar]
- Saeb M. R.; Mohammadi Y.; Kermaniyan T. S.; Zinck P.; Stadler F. J. Unspoken aspects of chain shuttling reactions: Patterning the molecular landscape of olefin multi-block copolymers. Polymer 2017, 116, 55–75. 10.1016/j.polymer.2017.03.033. [DOI] [Google Scholar]
- Khodadadi Yazdi M.; Hashemi Motlagh G.. Synthesis, characterization, and thermal aging behavior of HCl-doped polyaniline/TRGO nanocomposites. J. Appl. Polym. Sci. 2017, 134 ( (17), ). 10.1002/app.44635 [DOI] [Google Scholar]
- Mohammadi Y.; Ahmadi M.; Saeb M. R.; Khorasani M. M.; Yang P.; Stadler F. J. A detailed model on kinetics and microstructure evolution during copolymerization of ethylene and 1-octene: from coordinative chain transfer to chain shuttling polymerization. Macromolecules 2014, 47 (14), 4778–4789. 10.1021/ma500874h. [DOI] [Google Scholar]
- Saeb M. R.; Mohammadi Y.; Ahmadi M.; Khorasani M. M.; Stadler F. J. A Monte Carlo-based feeding policy for tailoring microstructure of copolymer chains: Reconsidering the conventional metallocene catalyzed polymerization of α-olefins. Chemical Engineering Journal 2015, 274, 169–180. 10.1016/j.cej.2015.02.095. [DOI] [Google Scholar]
- Nilforoushzadeh M. A.; Khodadadi Yazdi M.; Baradaran Ghavami S.; Farokhimanesh S.; Mohammadi Amirabad L.; Zarrintaj P.; Saeb M. R.; Hamblin M. R.; Zare M.; Mozafari M. Mesenchymal stem cell spheroids embedded in an injectable thermosensitive hydrogel: an in situ drug formation platform for accelerated wound healing. ACS Biomaterials Science & Engineering 2020, 6 (9), 5096–5109. 10.1021/acsbiomaterials.0c00988. [DOI] [PubMed] [Google Scholar]
- Zarrintaj P.; Ramsey J. D.; Samadi A.; Atoufi Z.; Yazdi M. K.; Ganjali M. R.; Amirabad L. M.; Zangene E.; Farokhi M.; Formela K.; et al. Poloxamer: A versatile tri-block copolymer for biomedical applications. Acta biomaterialia 2020, 110, 37–67. 10.1016/j.actbio.2020.04.028. [DOI] [PubMed] [Google Scholar]
- Yazdi M. K.; Vatanpour V.; Taghizadeh A.; Taghizadeh M.; Ganjali M. R.; Munir M. T.; Habibzadeh S.; Saeb M. R.; Ghaedi M. Hydrogel membranes: A review. Materials Science and Engineering: C 2020, 114, 111023. 10.1016/j.msec.2020.111023. [DOI] [PubMed] [Google Scholar]
- Zarrintaj P.; Ahmadi Z.; Saeb M. R.; Mozafari M. Poloxamer-based stimuli-responsive biomaterials. Materials Today: Proceedings 2018, 5 (7), 15516–15523. 10.1016/j.matpr.2018.04.158. [DOI] [Google Scholar]
- Zarrintaj P.; Ramsey J. D.; Samadi A.; Atoufi Z.; Yazdi M. K.; Ganjali M. R.; Amirabad L. M.; Zangene E.; Farokhi M.; Formela K.; Saeb M. R.; Mozafari M.; Thomas S. Poloxamer: A versatile tri-block copolymer for biomedical applications. Acta Biomater 2020, 110, 37–67. 10.1016/j.actbio.2020.04.028. [DOI] [PubMed] [Google Scholar]
- Russo E.; Villa C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11 (12), 671. 10.3390/pharmaceutics11120671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdi M. K.; Vatanpour V.; Taghizadeh A.; Taghizadeh M.; Ganjali M. R.; Munir M. T.; Habibzadeh S.; Saeb M. R.; Ghaedi M. Hydrogel membranes: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 111023. 10.1016/j.msec.2020.111023. [DOI] [PubMed] [Google Scholar]
- Rezvantalab S.; Drude N. I.; Moraveji M. K.; Guvener N.; Koons E. K.; Shi Y.; Lammers T.; Kiessling F. PLGA-Based Nanoparticles in Cancer Treatment. Front Pharmacol 2018, 9, 1260. 10.3389/fphar.2018.01260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghaei B.; Jafari S. H.; Khonakdar H. A.; Saeb M. R.; Wagenknecht U.; Heinrich G. A multioptimization approach to assessment of drug delivery of PLGA nanoparticles: simultaneous control of particle size and release behavior. Int. J. Polym. Mater. 2015, 64 (12), 641–652. 10.1080/00914037.2014.996714. [DOI] [Google Scholar]
- Chung T. W.; Tsai Y. L.; Hsieh J. H.; Tsai W. J. Different ratios of lactide and glycolide in PLGA affect the surface property and protein delivery characteristics of the PLGA microspheres with hydrophobic additives. J. Microencapsulation 2006, 23 (1), 15–27. 10.1080/02652040500286110. [DOI] [PubMed] [Google Scholar]
- Keles H.; Naylor A.; Clegg F.; Sammon C. Investigation of factors influencing the hydrolytic degradation of single PLGA microparticles. Polymer degradation and stability 2015, 119, 228–241. 10.1016/j.polymdegradstab.2015.04.025. [DOI] [Google Scholar]
- Khodadadi Yazdi M.; Hashemi Motlagh G.; Saeedi Garakani S.; Boroomand A. Effects of multiwall carbon nanotubes on the polymerization model of aniline. Journal of Polymer Research 2018, 25, 1–15. 10.1007/s10965-018-1655-7. [DOI] [Google Scholar]
- Yazdi M. K.; Ganjali M. R.; Rezapour M.; Zarrintaj P.; Habibzadeh S.; Saeb M. R.. Ionically gelled polysaccharide-based interpenetrating polymer network systems for drug delivery; Springer, 2021. [Google Scholar]
- Bagheri B.; Zarrintaj P.; Surwase S. S.; Baheiraei N.; Saeb M. R.; Mozafari M.; Kim Y. C.; Park O. O. Self-gelling electroactive hydrogels based on chitosan-aniline oligomers/agarose for neural tissue engineering with on-demand drug release. Colloids Surf., B 2019, 184, 110549. 10.1016/j.colsurfb.2019.110549. [DOI] [PubMed] [Google Scholar]
- Kim H.; Jeong H.; Han S.; Beack S.; Hwang B. W.; Shin M.; Oh S. S.; Hahn S. K. Hyaluronate and its derivatives for customized biomedical applications. Biomaterials 2017, 123, 155–171. 10.1016/j.biomaterials.2017.01.029. [DOI] [PubMed] [Google Scholar]
- Liu T.; Ren Q.; Wang S.; Gao J.; Shen C.; Zhang S.; Wang Y.; Guan F. Chemical Modification of Polysaccharides: A Review of Synthetic Approaches, Biological Activity and the Structure-Activity Relationship. Molecules 2023, 28 (16), 6073. 10.3390/molecules28166073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manouchehri S.; Bagheri B.; Rad S. H.; Nezhad M. N.; Kim Y. C.; Park O. O.; Farokhi M.; Jouyandeh M.; Ganjali M. R.; Yazdi M. K.; et al. Electroactive bio-epoxy incorporated chitosan-oligoaniline as an advanced hydrogel coating for neural interfaces. Prog. Org. Coat. 2019, 131, 389–396. 10.1016/j.porgcoat.2019.03.022. [DOI] [Google Scholar]
- Mohebbi S.; Nezhad M. N.; Zarrintaj P.; Jafari S. H.; Gholizadeh S. S.; Saeb M. R.; Mozafari M. Chitosan in biomedical engineering: a critical review. Current stem cell research & therapy 2019, 14 (2), 93–116. 10.2174/1574888X13666180912142028. [DOI] [PubMed] [Google Scholar]
- Yazdi M. K.; Zarrintaj P.; Bagheri B.; Kim Y. C.; Ganjali M. R.; Saeb M. R.. Nanotechnology-based biosensors in drug delivery. In Nanoengineered biomaterials for advanced drug delivery; Elsevier, 2020; pp 767–779. [Google Scholar]
- Seidi F.; Yazdi M. K.; Jouyandeh M.; Dominic M.; Naeim H.; Nezhad M. N.; Bagheri B.; Habibzadeh S.; Zarrintaj P.; Saeb M. R.; et al. Chitosan-based blends for biomedical applications. Int. J. Biol. Macromol. 2021, 183, 1818–1850. 10.1016/j.ijbiomac.2021.05.003. [DOI] [PubMed] [Google Scholar]
- Mohebbi S.; Nezhad M. N.; Zarrintaj P.; Jafari S. H.; Gholizadeh S. S.; Saeb M. R.; Mozafari M. Chitosan in Biomedical Engineering: A Critical Review. Curr. Stem Cell Res. Ther 2019, 14 (2), 93–116. 10.2174/1574888X13666180912142028. [DOI] [PubMed] [Google Scholar]
- Graham S.; Marina P. F.; Blencowe A. Thermoresponsive polysaccharides and their thermoreversible physical hydrogel networks. Carbohydr. Polym. 2019, 207, 143–159. 10.1016/j.carbpol.2018.11.053. [DOI] [PubMed] [Google Scholar]
- Jönsson M.; Allahgholi L.; Sardari R. R.; Hreggviđsson G. O.; Nordberg Karlsson E. Extraction and modification of macroalgal polysaccharides for current and next-generation applications. Molecules 2020, 25 (4), 930. 10.3390/molecules25040930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K.; Rajan R. Oxidized polysaccharides as green and sustainable biomaterials. Curr. Org. Chem. 2021, 25 (13), 1483–1496. 10.2174/1385272825666210428140052. [DOI] [Google Scholar]
- Esko J. D.; Kimata K.; Lindahl U.. Proteoglycans and sulfated glycosaminoglycans. Essentials of Glycobiology, 2nd ed. 2009. [PubMed] [Google Scholar]
- Pomin V. H.; Mulloy B.. Glycosaminoglycans and proteoglycans; MDPI, 2018; Vol. 11, p 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph A. A.; Pardo-Vargas A.; Seeberger P. H. Total synthesis of polysaccharides by automated glycan assembly. J. Am. Chem. Soc. 2020, 142 (19), 8561–8564. 10.1021/jacs.0c00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.; Xiong D. C.; Chen S. C.; Wang Y. S.; Ye X. S. Total synthesis of mycobacterial arabinogalactan containing 92 monosaccharide units. Nat. Commun. 2017, 8 (1), 14851. 10.1038/ncomms14851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W.; Zhang Z.; Zheng Y.; Qiao S.; Xie Y.; Sun Y.; Qiao K.; Feng Z.; Wang X.; Wang J. Preparation of aminoalkyl-grafted bacterial cellulose membranes with improved antimicrobial properties for biomedical applications. J. Biomed. Mater. Res., Part A 2020, 108 (5), 1086–1098. 10.1002/jbm.a.36884. [DOI] [PubMed] [Google Scholar]
- Bhat A.; Khan I.; Usmani M. A.; Umapathi R.; Al-Kindy S. M. Cellulose an ageless renewable green nanomaterial for medical applications: An overview of ionic liquids in extraction, separation and dissolution of cellulose. Int. J. Biol. Macromol. 2019, 129, 750–777. 10.1016/j.ijbiomac.2018.12.190. [DOI] [PubMed] [Google Scholar]
- Wu W.-C.; Hsiao P.-Y.; Huang Y.-C. Effects of amylose content on starch-chitosan composite film and its application as a wound dressing. Journal of Polymer Research 2019, 26, 1–13. 10.1007/s10965-019-1770-0. [DOI] [Google Scholar]
- Zhang X.-D.; et al. The effect of amylose on kernel phenotypic characteristics, starch-related gene expression and amylose inheritance in naturally mutated high-amylose maize. JOURNAL OF INTEGRATIVE AGRICULTURE 2020, 19 (6), 1554–1564. 10.1016/S2095-3119(19)62779-6. [DOI] [Google Scholar]
- Wang L.; Li X.; Sun T.; Tsou Y. H.; Chen H.; Xu X. Dual-functional dextran-PEG hydrogel as an antimicrobial biomedical material. Macromol. Biosci. 2018, 18 (2), 1700325. 10.1002/mabi.201700325. [DOI] [PubMed] [Google Scholar]
- Bechtner J.; Hassler V.; Wefers D.; Vogel R. F.; Jakob F. Insights into extracellular dextran formation by Liquorilactobacillus nagelii TMW 1.1827 using secretomes obtained in the presence or absence of sucrose. Enzyme Microb. Technol. 2021, 143, 109724. 10.1016/j.enzmictec.2020.109724. [DOI] [PubMed] [Google Scholar]
- Singh R. S.; Kaur N.; Hassan M.; Kennedy J. F. Pullulan in biomedical research and development-A review. Int. J. Biol. Macromol. 2021, 166, 694–706. 10.1016/j.ijbiomac.2020.10.227. [DOI] [PubMed] [Google Scholar]
- Wang S.; Gu B.-J.; Ganjyal G. M. Impacts of the inclusion of various fruit pomace types on the expansion of corn starch extrudates. Lwt 2019, 110, 223–230. 10.1016/j.lwt.2019.03.094. [DOI] [Google Scholar]
- Cai Z.; Wu J.; Wu M.; Li R.; Wang P.; Zhang H. Rheological characterization of novel carboxymethylated Curdlan-silica hybrid hydrogels with tunable mechanical properties. Carbohydr. Polym. 2020, 230, 115578. 10.1016/j.carbpol.2019.115578. [DOI] [PubMed] [Google Scholar]
- Chaudhari V.; Buttar H. S.; Bagwe-Parab S.; Tuli H. S.; Vora A.; Kaur G. Therapeutic and industrial applications of Curdlan with overview on its recent patents. Frontiers in Nutrition 2021, 8, 646988. 10.3389/fnut.2021.646988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N.; Jangid A. K.; Pooja D.; Kulhari H. Inulin: A novel and stretchy polysaccharide tool for biomedical and nutritional applications. Int. J. Biol. Macromol. 2019, 132, 852–863. 10.1016/j.ijbiomac.2019.03.188. [DOI] [PubMed] [Google Scholar]
- Morillo-Santander G.; Curtin L.; Williams S.; Edwards C.; Garcia A. Food sources and inulin consumption in school-aged children. Proceedings of the Nutrition Society 2021, 80 (OCE2), E61 10.1017/S0029665121000732. [DOI] [Google Scholar]
- Nechita P.; Mirela R.; Ciolacu F. Xylan Hemicellulose: A renewable material with potential properties for food packaging applications. Sustainability 2021, 13 (24), 13504. 10.3390/su132413504. [DOI] [Google Scholar]
- Wijaya C. J.; Ismadji S.; Gunawan S. A review of lignocellulosic-derived nanoparticles for drug delivery applications: lignin nanoparticles, xylan nanoparticles, and cellulose nanocrystals. Molecules 2021, 26 (3), 676. 10.3390/molecules26030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talodthaisong C.; Boonta W.; Thammawithan S.; Patramanon R.; Kamonsutthipaijit N.; Hutchison J. A.; Kulchat S. Composite guar gum-silver nanoparticle hydrogels as self-healing, injectable, and antibacterial biomaterials. Materials Today Communications 2020, 24, 100992. 10.1016/j.mtcomm.2020.100992. [DOI] [Google Scholar]
- Gunasekaran A.; Sorrentino A.; Asiri A. M.; Anandan S. Guar gum-based polymer gel electrolyte for dye-sensitized solar cell applications. Sol. Energy 2020, 208, 160–165. 10.1016/j.solener.2020.07.084. [DOI] [Google Scholar]
- Ding Q.; Zhuang T.; Fu P.; Zhou Q.; Luo L.; Dong Z.; Li H.; Tang S. Alpha-terpineol grafted acetylated lentinan as an anti-bacterial adhesion agent. Carbohydr. Polym. 2022, 277, 118825. 10.1016/j.carbpol.2021.118825. [DOI] [PubMed] [Google Scholar]
- Murphy E. J.; Masterson C.; Rezoagli E.; O’Toole D.; Major I.; Stack G. D.; Lynch M.; Laffey J. G.; Rowan N. J. β-Glucan extracts from the same edible shiitake mushroom Lentinus edodes produce differential in-vitro immunomodulatory and pulmonary cytoprotective effects—Implications for coronavirus disease (COVID-19) immunotherapies. Sci. Total Environ. 2020, 732, 139330. 10.1016/j.scitotenv.2020.139330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taemeh M. A.; Shiravandi A.; Korayem M. A.; Daemi H. Fabrication challenges and trends in biomedical applications of alginate electrospun nanofibers. Carbohydr. Polym. 2020, 228, 115419. 10.1016/j.carbpol.2019.115419. [DOI] [PubMed] [Google Scholar]
- Aitouguinane M.; Bouissil S.; Mouhoub A.; Rchid H.; Fendri I.; Abdelkafi S.; Ould El-Hadj M. D.; Boual Z.; Dubessay P.; Gardarin C.; et al. Induction of natural defenses in tomato seedlings by using alginate and oligoalginates derivatives extracted from Moroccan brown algae. Marine drugs 2020, 18 (10), 521. 10.3390/md18100521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.; Waterhouse G. I.; Xu F.; He Z.; Du Y.; Lian Y.; Wu P.; Sun-Waterhouse D. Recent advances in utilization of pectins in biomedical applications: A review focusing on molecular structure-directing health-promoting properties. Critical Reviews in Food Science and Nutrition 2023, 63 (19), 3386–3419. 10.1080/10408398.2021.1988897. [DOI] [PubMed] [Google Scholar]
- Shariatinia Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. 10.1016/j.ijbiomac.2018.09.131. [DOI] [PubMed] [Google Scholar]
- Manigandan V.; Karthik R.; Ramachandran S.; Rajagopal S.. Chitosan applications in food industry. In Biopolymers for food design; Elsevier, 2018; pp 469–491. [Google Scholar]
- He C.; Ji H.; Qian Y.; Wang Q.; Liu X.; Zhao W.; Zhao C. Heparin-based and heparin-inspired hydrogels: size-effect, gelation and biomedical applications. J. Mater. Chem. B 2019, 7 (8), 1186–1208. 10.1039/C8TB02671H. [DOI] [PubMed] [Google Scholar]
- Rnjak-Kovacina J.; Tang F.; Whitelock J. M.; Lord M. S. Glycosaminoglycan and proteoglycan-based biomaterials: current trends and future perspectives. Adv. Healthcare Mater. 2018, 7 (6), 1701042. 10.1002/adhm.201701042. [DOI] [PubMed] [Google Scholar]
- Stellavato A.; Restaino O. F.; Vassallo V.; Finamore R.; Ruosi C.; Cassese E.; De Rosa M.; Schiraldi C. Comparative analyses of pharmaceuticals or food supplements containing chondroitin sulfate: are their bioactivities equivalent?. Advances in Therapy 2019, 36, 3221–3237. 10.1007/s12325-019-01064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews S.; Cheng A.; Stevens H.; Logun M. T.; Webb R.; Jordan E.; Xia B.; Karumbaiah L.; Guldberg R. E.; Stice S. Chondroitin sulfate glycosaminoglycan scaffolds for cell and recombinant protein-based bone regeneration. Stem cells translational medicine 2019, 8 (6), 575–585. 10.1002/sctm.18-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitenbach B. B.; Schmid I.; Wich P. R. Amphiphilic polysaccharide block copolymers for pH-responsive micellar nanoparticles. Biomacromolecules 2017, 18 (9), 2839–2848. 10.1021/acs.biomac.7b00771. [DOI] [PubMed] [Google Scholar]
- Li Z.; Wang L.; Lin X.; Shen L.; Feng Y. Drug delivery for bioactive polysaccharides to improve their drug-like properties and curative efficacy. Drug delivery 2017, 24 (2), 70–80. 10.1080/10717544.2017.1396383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokri Z.; Seidi F.; Karami S.; Li C.; Saeb M. R.; Xiao H. Laccase immobilization onto natural polysaccharides for biosensing and biodegradation. Carbohydr. Polym. 2021, 262, 117963. 10.1016/j.carbpol.2021.117963. [DOI] [PubMed] [Google Scholar]
- Patel J.; Maji B.; Moorthy N. H. N.; Maiti S. Xanthan gum derivatives: Review of synthesis, properties and diverse applications. RSC Adv. 2020, 10 (45), 27103–27136. 10.1039/D0RA04366D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumpstey I. Chemical modification of polysaccharides. International scholarly research notices 2013, 2013, 1. 10.1155/2013/417672. [DOI] [Google Scholar]
- Zhou L.; Ke K.; Yang M.-B.; Yang W. Recent progress on chemical modification of cellulose for high mechanical-performance Poly (lactic acid)/Cellulose composite: A review. Composites Communications 2021, 23, 100548. 10.1016/j.coco.2020.100548. [DOI] [Google Scholar]
- Lease J.; Kawano T.; Andou Y. Esterification of cellulose with long fatty acid chain through mechanochemical method. Polymers 2021, 13 (24), 4397. 10.3390/polym13244397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardhana Y. W.; Aanisah N.; Sopyan I.; Hendriani R.; Chaerunisaa A. Y. Gelling Power Alteration on Kappa-Carrageenan Dispersion through Esterification Method with Different Fatty Acid Saturation. Gels 2022, 8 (11), 752. 10.3390/gels8110752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar K. J.; Buchanan C. M.; Debenham J. S.; Rundquist P. A.; Seiler B. D.; Shelton M. C.; Tindall D. Advances in cellulose ester performance and application. Prog. Polym. Sci. 2001, 26 (9), 1605–1688. 10.1016/S0079-6700(01)00027-2. [DOI] [Google Scholar]
- Mulyadi A.; Deng Y. Surface modification of cellulose nanofibrils by maleated styrene block copolymer and their composite reinforcement application. Cellulose 2016, 23, 519–528. 10.1007/s10570-015-0787-8. [DOI] [Google Scholar]
- Heinze T.; Liebert T.; Koschella A.. Esterification of polysaccharides; Springer Science & Business Media, 2006. [Google Scholar]
- Klemn D.; Philipp B.; Heinze T.; Heinze U.; Wagenknecht W.. Comprehensive cellulose chemistry: Functionalization of cellulose; Wiley-VCH: Weinhein. Germany, 1998. [Google Scholar]
- Heinze T.; Liebert T. F.; Pfeiffer K. S.; Hussain M. A. Unconventional cellulose esters: synthesis, characterization and structure-property relations. Cellulose 2003, 10 (3), 283–296. 10.1023/A:1025117327970. [DOI] [Google Scholar]
- Operamolla A.; Casalini S.; Console D.; Capodieci L.; Di Benedetto F.; Bianco G. V.; Babudri F. Tailoring water stability of cellulose nanopaper by surface functionalization. Soft Matter 2018, 14 (36), 7390–7400. 10.1039/C8SM00433A. [DOI] [PubMed] [Google Scholar]
- Sealey J. E.; Samaranayake G.; Todd J. G.; Glasser W. G. Novel cellulose derivatives. IV. Preparation and thermal analysis of waxy esters of cellulose. J. Polym. Sci., Part B: Polym. Phys. 1996, 34 (9), 1613–1620. . [DOI] [Google Scholar]
- Grote C.; Heinze T. Starch derivatives of high degree of functionalization 11: Studies on alternative acylation of starch with long-chain fatty acids homogeneously in N, N-dimethyl acetamide/LiCl. Cellulose 2005, 12 (4), 435–444. 10.1007/s10570-005-2178-z. [DOI] [Google Scholar]
- El Hamdaoui L.; Es-said A.; El Marouani M.; El Bouchti M.; Bchitou R.; Kifani-Sahban F.; El Moussaouiti M. Tosylation Optimization, Characterization and Pyrolysis Kinetics of Cellulose Tosylate. ChemistrySelect 2020, 5 (26), 7695–7703. 10.1002/slct.202001906. [DOI] [Google Scholar]
- Fox S. C.; Li B.; Xu D.; Edgar K. J. Regioselective esterification and etherification of cellulose: a review. Biomacromolecules 2011, 12 (6), 1956–1972. 10.1021/bm200260d. [DOI] [PubMed] [Google Scholar]
- Rahn K.; Diamantoglou M.; Klemm D.; Berghmans H.; Heinze T. Homogeneous synthesis of cellulose p-toluenesulfonates in N, N-dimethylacetamide/LiCl solvent system. Die Angewandte Makromolekulare Chemie: Applied Macromolecular Chemistry and Physics 1996, 238 (1), 143–163. 10.1002/apmc.1996.052380113. [DOI] [Google Scholar]
- Nilsen-Nygaard J.; Hattrem M. N.; Draget K. I. Propylene glycol alginate (PGA) gelled foams: A systematic study of surface activity and gelling properties as a function of degree of esterification. Food Hydrocolloids 2016, 57, 80–91. 10.1016/j.foodhyd.2016.01.011. [DOI] [Google Scholar]
- Amri C.; Mudasir M.; Siswanta D.; Roto R. In vitro hemocompatibility of PVA-alginate ester as a candidate for hemodialysis membrane. Int. J. Biol. Macromol. 2016, 82, 48–53. 10.1016/j.ijbiomac.2015.10.021. [DOI] [PubMed] [Google Scholar]
- Taubner T.; Marounek M.; Synytsya A. Preparation and characterization of hydrophobic and hydrophilic amidated derivatives of carboxymethyl chitosan and carboxymethyl β-glucan. Int. J. Biol. Macromol. 2020, 163, 1433–1443. 10.1016/j.ijbiomac.2020.07.257. [DOI] [PubMed] [Google Scholar]
- Li D.; Su T.; Ma L.; Yin F.; Xu W.; Ding J.; Li Z. Dual-acidity-labile polysaccharide-di-drugs conjugate for targeted cancer chemotherapy. Eur. J. Med. Chem. 2020, 199, 112367. 10.1016/j.ejmech.2020.112367. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ballester N. M.; Bataille B.; Benabbas R.; Alonso B.; Soulairol I. Development of alginate esters as novel multifunctional excipients for direct compression. Carbohydr. Polym. 2020, 240, 116280. 10.1016/j.carbpol.2020.116280. [DOI] [PubMed] [Google Scholar]
- Busato B.; de Almeida Abreu E. C.; de Oliveira Petkowicz C. L.; Martinez G. R.; Noleto G. R. Pectin from Brassica oleracea var. italica triggers immunomodulating effects in vivo. Int. J. Biol. Macromol. 2020, 161, 431–440. 10.1016/j.ijbiomac.2020.06.051. [DOI] [PubMed] [Google Scholar]
- Heinze T.; El Seoud O. A.; Koschella A.. Etherification of cellulose. In Cellulose derivatives; Springer, 2018; pp 429–477. [Google Scholar]
- Panda P. K.; Verma A.; Jain S. K.. Etherified polysaccharides in biomedical applications. In Tailor-Made Polysaccharides in Biomedical Applications; Elsevier, 2020; pp 35–50. [Google Scholar]
- Wang Q. Q.; Kong M.; An Y.; Liu Y.; Li J. J.; Zhou X.; Feng C.; Li J.; Jiang S. Y.; Cheng X. J.; et al. Hydroxybutyl chitosan thermo-sensitive hydrogel: a potential drug delivery system. J. Mater. Sci. 2013, 48, 5614–5623. 10.1007/s10853-013-7356-z. [DOI] [Google Scholar]
- Shibata M.; Nozawa R.; Teramoto N.; Yosomiya R. Synthesis and properties of etherified pullulans. European polymer journal 2002, 38 (3), 497–501. 10.1016/S0014-3057(01)00198-7. [DOI] [Google Scholar]
- Petzold K.; Schwikal K.; Heinze T. Carboxymethyl xylan—synthesis and detailed structure characterization. Carbohydr. Polym. 2006, 64 (2), 292–298. 10.1016/j.carbpol.2005.11.037. [DOI] [Google Scholar]
- Bukzem A. L.; Signini R.; dos Santos D. M.; Lião L. M.; Ascheri D. P. R. Optimization of carboxymethyl chitosan synthesis using response surface methodology and desirability function. Int. J. Biol. Macromol. 2016, 85, 615–624. 10.1016/j.ijbiomac.2016.01.017. [DOI] [PubMed] [Google Scholar]
- El-Hinnawy S.; Fahmy A.; El-Saied H.; El-Shirbeeny A.; El-Sahy K. Preparation and evaluation of hydroxyethyl starch. Starch-Stärke 1982, 34 (2), 65–68. 10.1002/star.19820340208. [DOI] [Google Scholar]
- Fu Z.; Zhang L.; Ren M. H.; BeMiller J. N. Developments in Hydroxypropylation of Starch: a review. Starch-Stärke 2019, 71 (1–2), 1800167. 10.1002/star.201800167. [DOI] [Google Scholar]
- Gilarska A.; Lewandowska-Łańcucka J.; Guzdek-Zajac K.; Karewicz A.; Horak W.; Lach R.; Wójcik K.; Nowakowska M. Bioactive yet antimicrobial structurally stable collagen/chitosan/lysine functionalized hyaluronic acid-based injectable hydrogels for potential bone tissue engineering applications. Int. J. Biol. Macromol. 2020, 155, 938–950. 10.1016/j.ijbiomac.2019.11.052. [DOI] [PubMed] [Google Scholar]
- Hu F.; Sun T.; Xie J.; Xue B.; Li X.; Gan J.; Li L.; Shao Z. Functional properties and preservative effect on Penaeus vannamei of chitosan films with conjugated or incorporated chlorogenic acid. Int. J. Biol. Macromol. 2020, 159, 333–340. 10.1016/j.ijbiomac.2020.05.089. [DOI] [PubMed] [Google Scholar]
- Srivastava V.; Singh P. K.; Singh P. P. Visible light photoredox catalysed amidation of carboxylic acids with amines. Tetrahedron letters 2019, 60 (1), 40–43. 10.1016/j.tetlet.2018.11.050. [DOI] [Google Scholar]
- Pedrood K.; Bahadorikhalili S.; Lotfi V.; Larijani B.; Mahdavi M. Catalytic and non-catalytic amidation of carboxylic acid substrates. Molecular Diversity 2022, 26, 1–34. 10.1007/s11030-021-10252-0. [DOI] [PubMed] [Google Scholar]
- Manova D.; Gallier F.; Tak-Tak L.; Yotava L.; Lubin-Germain N. Lipase-catalyzed amidation of carboxylic acid and amines. Tetrahedron letters 2018, 59 (21), 2086–2090. 10.1016/j.tetlet.2018.04.049. [DOI] [Google Scholar]
- Fraschini C.; Chauve G.; Bouchard J. TEMPO-mediated surface oxidation of cellulose nanocrystals (CNCs). Cellulose 2017, 24 (7), 2775–2790. 10.1007/s10570-017-1319-5. [DOI] [Google Scholar]
- De Nooy A.; Besemer A.; Van Bekkum H. Highly selective TEMPO mediated oxidation of primary alcohol groups in polysaccharides. Recueil des Travaux Chimiques des Pays-Bas 1994, 113 (3), 165–166. 10.1002/recl.19941130307. [DOI] [Google Scholar]
- Kato Y.; Matsuo R.; Isogai A. Oxidation process of water-soluble starch in TEMPO-mediated system. Carbohydr. Polym. 2003, 51 (1), 69–75. 10.1016/S0144-8617(02)00159-5. [DOI] [Google Scholar]
- Abou-Okeil A.; Fahmy H.; Fouda M. M.; Aly A.; Ibrahim H. Hyaluronic acid/oxidized ϰ-carrageenan electrospun nanofibers synthesis and antibacterial properties. BioNanoScience 2021, 11, 687–695. 10.1007/s12668-021-00884-9. [DOI] [Google Scholar]
- Bao Z.; Yu A.; Shi H.; Hu Y.; Jin B.; Lin D.; Dai M.; Lei L.; Li X.; Wang Y. Glycol chitosan/oxidized hyaluronic acid hydrogel film for topical ocular delivery of dexamethasone and levofloxacin. Int. J. Biol. Macromol. 2021, 167, 659–666. 10.1016/j.ijbiomac.2020.11.214. [DOI] [PubMed] [Google Scholar]
- Li H.; Cheng F.; Wei X.; Yi X.; Tang S.; Wang Z.; Zhang Y. S.; He J.; Huang Y. Injectable, self-healing, antibacterial, and hemostatic N, O-carboxymethyl chitosan/oxidized chondroitin sulfate composite hydrogel for wound dressing. Materials Science and Engineering: C 2021, 118, 111324. 10.1016/j.msec.2020.111324. [DOI] [PubMed] [Google Scholar]
- Wang H.; Chen X.; Wen Y.; Li D.; Sun X.; Liu Z.; Yan H.; Lin Q. A study on the correlation between the oxidation degree of oxidized sodium alginate on its degradability and gelation. Polymers 2022, 14 (9), 1679. 10.3390/polym14091679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza D. J.; Browne C.; Raghuwanshi V. S.; Simon G. P.; Garnier G. One-shot TEMPO-periodate oxidation of native cellulose. Carbohydr. Polym. 2019, 226, 115292. 10.1016/j.carbpol.2019.115292. [DOI] [PubMed] [Google Scholar]
- Wei D.; Bruneau-Voisine A.; Valyaev D. A.; Lugan N.; Sortais J.-B. Manganese catalyzed reductive amination of aldehydes using hydrogen as a reductant. Chem. Commun. 2018, 54 (34), 4302–4305. 10.1039/C8CC01787E. [DOI] [PubMed] [Google Scholar]
- Liu C.; Baumann H. Exclusive and complete introduction of amino groups and their N-sulfo and N-carboxymethyl groups into the 6-position of cellulose without the use of protecting groups. Carbohydrate research 2002, 337 (14), 1297–1307. 10.1016/S0008-6215(02)00132-5. [DOI] [PubMed] [Google Scholar]
- El Hamdaoui L.; Talbaoui A.; El Moussaouiti M. Nucleophilic displacement reaction on tosyl cellulose by L-methionine to the synthesis of novel water-soluble cellulose derivative and its antibacterial activity. International Journal of Polymer Science 2021, 2021, 1. 10.1155/2021/6613684. [DOI] [Google Scholar]
- Bai R.; Yong H.; Zhang X.; Liu J.; Liu J. Structural characterization and protective effect of gallic acid grafted O-carboxymethyl chitosan against hydrogen peroxide-induced oxidative damage. Int. J. Biol. Macromol. 2020, 143, 49–59. 10.1016/j.ijbiomac.2019.12.037. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Wang H.; Song H.; Young M.; Fan Y.; Xu F.-J.; Qu X.; Lei X.; Liu Y.; Cheng G. Peptide-grafted dextran vectors for efficient and high-loading gene delivery. Biomaterials science 2019, 7 (4), 1543–1553. 10.1039/C8BM01341A. [DOI] [PubMed] [Google Scholar]
- Wei H.; Li S.; Liu Z.; Chen H.; Liu Y.; Li W.; Wang G. Preparation and characterization of starch-cellulose interpenetrating network hydrogels based on sequential Diels-Alder click reaction and photopolymerization. Int. J. Biol. Macromol. 2022, 194, 962–973. 10.1016/j.ijbiomac.2021.11.154. [DOI] [PubMed] [Google Scholar]
- Zheng Z.; Bian S.; Li Z.; Zhang Z.; Liu Y.; Zhai X.; Pan H.; Zhao X. Catechol modified quaternized chitosan enhanced wet adhesive and antibacterial properties of injectable thermo-sensitive hydrogel for wound healing. Carbohydr. Polym. 2020, 249, 116826. 10.1016/j.carbpol.2020.116826. [DOI] [PubMed] [Google Scholar]
- Qiao D.; Li S.; Yu L.; Zhang B.; Simon G.; Jiang F. Effect of alkanol surface grafting on the hydrophobicity of starch-based films. Int. J. Biol. Macromol. 2018, 112, 761–766. 10.1016/j.ijbiomac.2018.01.205. [DOI] [PubMed] [Google Scholar]
- Azzam T.; Raskin A.; Makovitzki A.; Brem H.; Vierling P.; Lineal M.; Domb A. J. Cationic polysaccharides for gene delivery. Macromolecules 2002, 35 (27), 9947–9953. 10.1021/ma0209592. [DOI] [Google Scholar]
- Hosseinkhani H.; Azzam T.; Tabata Y.; Domb A. Dextran-spermine polycation: an efficient nonviral vector for in vitro and in vivo gene transfection. Gene therapy 2004, 11 (2), 194–203. 10.1038/sj.gt.3302159. [DOI] [PubMed] [Google Scholar]
- Afanasyev O. I.; Kuchuk E.; Usanov D. L.; Chusov D. Reductive amination in the synthesis of pharmaceuticals. Chem. Rev. 2019, 119 (23), 11857–11911. 10.1021/acs.chemrev.9b00383. [DOI] [PubMed] [Google Scholar]
- Yudovin-Farber I.; Domb A. Cationic polysaccharides for gene delivery. Materials Science and Engineering: C 2007, 27 (3), 595–598. 10.1016/j.msec.2006.05.044. [DOI] [Google Scholar]
- Gildersleeve J. C.; Oyelaran O.; Simpson J. T.; Allred B. Improved procedure for direct coupling of carbohydrates to proteins via reductive amination. Bioconjugate Chem. 2008, 19 (7), 1485–1490. 10.1021/bc800153t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim M. S.; Abd El-Mageed H.; Azmy A. F.; El-Deeb M.; Kamal E. H.; Abd El-Salam H. Synthesis, characterization, and molecular docking analysis of Chitosan-gr-Polysulphanilic acid as antimicrobial water-soluble polymers. International Journal of Polymeric Materials and Polymeric Biomaterials 2023, 72 (4), 271–284. 10.1080/00914037.2021.2006656. [DOI] [Google Scholar]
- Seidi F.; Salimi H.; Shamsabadi A. A.; Shabanian M. Synthesis of hybrid materials using graft copolymerization on non-cellulosic polysaccharides via homogenous ATRP. Prog. Polym. Sci. 2018, 76, 1–39. 10.1016/j.progpolymsci.2017.07.006. [DOI] [Google Scholar]
- Alves P. M.; Pereira R. F.; Costa B.; Tassi N.; Teixeira C.; Leiro V.; Monteiro C.; Gomes P.; Costa F.; Martins M. C. L. Thiol-norbornene photoclick chemistry for grafting antimicrobial peptides onto chitosan to create antibacterial biomaterials. ACS Applied Polymer Materials 2022, 4 (7), 5012–5026. 10.1021/acsapm.2c00563. [DOI] [Google Scholar]
- Pilipenko I.; Korzhikov-Vlakh V.; Zakharova N.; Urtti A.; Tennikova T. Thermo-and pH-sensitive glycosaminoglycans derivatives obtained by controlled grafting of poly (N-isopropylacrylamide). Carbohydr. Polym. 2020, 248, 116764. 10.1016/j.carbpol.2020.116764. [DOI] [PubMed] [Google Scholar]
- Moreira G.; Fedeli E.; Ziarelli F.; Capitani D.; Mannina L.; Charles L.; Viel S.; Gigmes D.; Lefay C. Synthesis of polystyrene-grafted cellulose acetate copolymers via nitroxide-mediated polymerization. Polym. Chem. 2015, 6 (29), 5244–5253. 10.1039/C5PY00752F. [DOI] [Google Scholar]
- Tizzotti M.; Charlot A.; Fleury E.; Stenzel M.; Bernard J. Modification of polysaccharides through controlled/living radical polymerization grafting—Towards the generation of high performance hybrids. Macromol. Rapid Commun. 2010, 31 (20), 1751–1772. 10.1002/marc.201000072. [DOI] [PubMed] [Google Scholar]
- Bontempo D.; Masci G.; De Leonardis P.; Mannina L.; Capitani D.; Crescenzi V. Versatile grafting of polysaccharides in homogeneous mild conditions by using atom transfer radical polymerization. Biomacromolecules 2006, 7 (7), 2154–2161. 10.1021/bm0601373. [DOI] [PubMed] [Google Scholar]
- Afanasyev O. I.; Kuchuk E.; Usanov D. L.; Chusov D. Reductive Amination in the Synthesis of Pharmaceuticals. Chem. Rev. 2019, 119 (23), 11857–11911. 10.1021/acs.chemrev.9b00383. [DOI] [PubMed] [Google Scholar]
- Gildersleeve J. C.; Oyelaran O.; Simpson J. T.; Allred B. Improved procedure for direct coupling of carbohydrates to proteins via reductive amination. Bioconjug Chem. 2008, 19 (7), 1485–1490. 10.1021/bc800153t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W.; Lin L.; Xie J.; Wang Z.; Wang H.; Dong Y.; Shen M.; Xie M. Effect of ultrasonic treatment on the physicochemical properties and antioxidant activities of polysaccharide from Cyclocarya paliurus. Carbohydr. Polym. 2016, 151, 305–312. 10.1016/j.carbpol.2016.05.078. [DOI] [PubMed] [Google Scholar]
- Zhong K.; Zhang Q.; Tong L.; Liu L.; Zhou X.; Zhou S. Molecular weight degradation and rheological properties of schizophyllan under ultrasonic treatment. Ultrasonics Sonochemistry 2015, 23, 75–80. 10.1016/j.ultsonch.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Li S.; Xiong Q.; Lai X.; Li X.; Wan M.; Zhang J.; Yan Y.; Cao M.; Lu L.; Guan J.; et al. Molecular modification of polysaccharides and resulting bioactivities. Comprehensive Reviews in Food Science and Food Safety 2016, 15 (2), 237–250. 10.1111/1541-4337.12161. [DOI] [PubMed] [Google Scholar]
- Pang G.; Chen C.; Liu Y.; Jiang T.; Yu H.; Wu Y.; Wang Y.; Wang F.-J.; Liu Z.; Zhang L. W. Bioactive polysaccharide nanoparticles improve radiation-induced abscopal effect through manipulation of dendritic cells. ACS Appl. Mater. Interfaces 2019, 11 (45), 42661–42670. 10.1021/acsami.9b16814. [DOI] [PubMed] [Google Scholar]
- Kadokawa J.-i. Precision polysaccharide synthesis catalyzed by enzymes. Chem. Rev. 2011, 111 (7), 4308–4345. 10.1021/cr100285v. [DOI] [PubMed] [Google Scholar]
- Wu M.; Qu J.; Tian X.; Zhao X.; Shen Y.; Shi Z.; Chen P.; Li G.; Ma T. Tailor-made polysaccharides containing uniformly distributed repeating units based on the xanthan gum skeleton. Int. J. Biol. Macromol. 2019, 131, 646–653. 10.1016/j.ijbiomac.2019.03.130. [DOI] [PubMed] [Google Scholar]
- McCarty N. S.; Ledesma-Amaro R. Synthetic biology tools to engineer microbial communities for biotechnology. Trends Biotechnol. 2019, 37 (2), 181–197. 10.1016/j.tibtech.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.; Chen F.; Cheng H.; Huang G. Modification and application of polysaccharide from traditional Chinese medicine such as Dendrobium officinale. Int. J. Biol. Macromol. 2020, 157, 385–393. 10.1016/j.ijbiomac.2020.04.141. [DOI] [PubMed] [Google Scholar]
- Karaki N.; Aljawish A.; Humeau C.; Muniglia L.; Jasniewski J. Enzymatic modification of polysaccharides: Mechanisms, properties, and potential applications: A review. Enzyme Microb. Technol. 2016, 90, 1–18. 10.1016/j.enzmictec.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Wang W.; Wang S.-X.; Guan H.-S. The antiviral activities and mechanisms of marine polysaccharides: an overview. Marine drugs 2012, 10 (12), 2795–2816. 10.3390/md10122795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschenmeyer A.; Arlov Ø.; Malheiro V.; Steinwachs M.; Rottmar M.; Maniura-Weber K.; Palazzolo G.; Zenobi-Wong M. Anti-oxidant and immune-modulatory properties of sulfated alginate derivatives on human chondrocytes and macrophages. Biomaterials science 2017, 5 (9), 1756–1765. 10.1039/C7BM00341B. [DOI] [PubMed] [Google Scholar]
- Kim S. Y.; Jin W.; Sood A.; Montgomery D. W.; Grant O. C.; Fuster M. M.; Fu L.; Dordick J. S.; Woods R. J.; Zhang F.; et al. Characterization of heparin and severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) spike glycoprotein binding interactions. Antiviral research 2020, 181, 104873. 10.1016/j.antiviral.2020.104873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon P. S.; Oh H.; Kwon S.-J.; Jin W.; Zhang F.; Fraser K.; Hong J. J.; Linhardt R. J.; Dordick J. S. Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro. Cell Discovery 2020, 6 (1), 1–4. 10.1038/s41421-020-00192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mycroft-West C.; Su D.; Pagani I.; Rudd T.; Elli S.; Guimond S.; Miller G.; Meneghetti M.; Nader H.; Li Y. Heparin inhibits cellular invasion by SARS-CoV-2: structural dependence of the interaction of the surface protein (spike) S1 receptor binding domain with heparin. bioRxiv 2020, 120, 1700. 10.1055/s-0040-1721319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan A. R.; Lokeshwar S. D.; Lopez L. E.; Hennig M.; Chipollini J.; Yates T.; Hupe M. C.; Merseburger A. S.; Shiedlin A.; Cerwinka W. H.; et al. Antitumor activity of sulfated hyaluronic acid fragments in pre-clinical models of bladder cancer. Oncotarget 2017, 8 (15), 24262. 10.18632/oncotarget.10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartel N. J.; Post M. Abrogation of apoptosis through PDGF-BB-induced sulfated glycosaminoglycan synthesis and secretion. American Journal of Physiology-Lung Cellular and Molecular Physiology 2005, 288 (2), L285–L293. 10.1152/ajplung.00275.2004. [DOI] [PubMed] [Google Scholar]
- Zamboni F.; Vieira S.; Reis R. L.; Oliveira J. M.; Collins M. N. The potential of hyaluronic acid in immunoprotection and immunomodulation: chemistry, processing and function. Prog. Mater. Sci. 2018, 97, 97–122. 10.1016/j.pmatsci.2018.04.003. [DOI] [Google Scholar]
- Mao Y.; Hu Y.; Feng W.; Yu L.; Li P.; Cai B.; Li C.; Guan H. Effects and mechanisms of PSS-loaded nanoparticles on coronary microcirculation dysfunction in streptozotocin-induced diabetic cardiomyopathy rats. Biomedicine & Pharmacotherapy 2020, 121, 109280. 10.1016/j.biopha.2019.109280. [DOI] [PubMed] [Google Scholar]
- Huang L.; Shen M.; Morris G. A.; Xie J. Sulfated polysaccharides: Immunomodulation and signaling mechanisms. Trends in Food Science & Technology 2019, 92, 1–11. 10.1016/j.tifs.2019.08.008. [DOI] [Google Scholar]
- Mulloy B. The specificity of interactions between proteins and sulfated polysaccharides. Anais da Academia Brasileira de Ciencias 2005, 77, 651–664. 10.1590/S0001-37652005000400007. [DOI] [PubMed] [Google Scholar]
- Shimazu R.; Akashi S.; Ogata H.; Nagai Y.; Fukudome K.; Miyake K.; Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. Journal of experimental medicine 1999, 189 (11), 1777–1782. 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares da Costa D.; Reis R. L.; Pashkuleva I. Sulfation of glycosaminoglycans and its implications in human health and disorders. Annu. Rev. Biomed. Eng. 2017, 19, 1–26. 10.1146/annurev-bioeng-071516-044610. [DOI] [PubMed] [Google Scholar]
- Gama C. I.; Tully S. E.; Sotogaku N.; Clark P. M.; Rawat M.; Vaidehi N.; Goddard W. A.; Nishi A.; Hsieh-Wilson L. C. Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat. Chem. Biol. 2006, 2 (9), 467–473. 10.1038/nchembio810. [DOI] [PubMed] [Google Scholar]
- Arlov Ø.; Skjåk-Bræk G. Sulfated alginates as heparin analogues: a review of chemical and functional properties. Molecules 2017, 22 (5), 778. 10.3390/molecules22050778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares da Costa D.; Reis R. L.; Pashkuleva I. Sulfation of Glycosaminoglycans and Its Implications in Human Health and Disorders. Annu. Rev. Biomed Eng. 2017, 19, 1–26. 10.1146/annurev-bioeng-071516-044610. [DOI] [PubMed] [Google Scholar]
- Kang Z.; Zhou Z.; Wang Y.; Huang H.; Du G.; Chen J. Bio-based strategies for producing glycosaminoglycans and their oligosaccharides. Trends Biotechnol. 2018, 36 (8), 806–818. 10.1016/j.tibtech.2018.03.010. [DOI] [PubMed] [Google Scholar]
- Baeurle S.; Kiselev M.; Makarova E.; Nogovitsin E. Effect of the counterion behavior on the frictional-compressive properties of chondroitin sulfate solutions. Polymer 2009, 50 (7), 1805–1813. 10.1016/j.polymer.2009.01.066. [DOI] [Google Scholar]
- Trowbridge J. M.; Gallo R. L. Dermatan sulfate: new functions from an old glycosaminoglycan. Glycobiology 2002, 12 (9), 117R–125R. 10.1093/glycob/cwf066. [DOI] [PubMed] [Google Scholar]
- Miller B.; Sheppard A. M.; Pearlman A. L. Developmental expression of keratan sulfate-like immunoreactivity distinguishes thalamic nuclei and cortical domains. Journal of Comparative Neurology 1997, 380 (4), 533–552. . [DOI] [PubMed] [Google Scholar]
- Arnold K.; Xu Y.; Sparkenbaugh E. M.; Li M.; Han X.; Zhang X.; Xia K.; Piegore M.; Zhang F.; Zhang X. Design of anti-inflammatory heparan sulfate to protect against acetaminophen-induced acute liver failure. Science Translational Medicine 2020, 12 (535), eaav8075. 10.1126/scitranslmed.aav8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo H. E.; Straub J. E.; Grinstaff M. W. Design, synthesis, and biomedical applications of synthetic sulphated polysaccharides. Chem. Soc. Rev. 2019, 48 (8), 2338–2365. 10.1039/C7CS00593H. [DOI] [PubMed] [Google Scholar]
- Pires N. R.; Cunha P. L.; Maciel J. S.; Angelim A. L.; Melo V. M.; de Paula R. C.; Feitosa J. P. Sulfated chitosan as tear substitute with no antimicrobial activity. Carbohydr. Polym. 2013, 91 (1), 92–99. 10.1016/j.carbpol.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Chen T.; Liu H.; Liu J.; Li J.; An Y.; Zhu M.; Chen B.; Liu F.; Liu R.; Si C.; et al. Carboxymethylation of polysaccharide isolated from Alkaline Peroxide Mechanical Pulping (APMP) waste liquor and its bioactivity. Int. J. Biol. Macromol. 2021, 181, 211–220. 10.1016/j.ijbiomac.2021.03.125. [DOI] [PubMed] [Google Scholar]
- Leong K. H.; Chung L. Y.; Noordin M. I.; Mohamad K.; Nishikawa M.; Onuki Y.; Morishita M.; Takayama K. Carboxymethylation of kappa-carrageenan for intestinal-targeted delivery of bioactive macromolecules. Carbohydr. Polym. 2011, 83 (4), 1507–1515. 10.1016/j.carbpol.2010.09.062. [DOI] [Google Scholar]
- Tranquilan-Aranilla C.; Barba B. J. D.; Vista J. R. M.; Abad L. V. Hemostatic efficacy evaluation of radiation crosslinked carboxymethyl kappa-carrageenan and chitosan with varying degrees of substitution. Radiat. Phys. Chem. 2016, 124, 124–129. 10.1016/j.radphyschem.2016.02.003. [DOI] [Google Scholar]
- Yuan H.; Song J.; Li X.; Li N.; Dai J. Immunomodulation and antitumor activity of κ-carrageenan oligosaccharides. Cancer letters 2006, 243 (2), 228–234. 10.1016/j.canlet.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Schefer L.; Usov I.; Mezzenga R. Anomalous stiffening and ion-induced coil-helix transition of carrageenans under monovalent salt conditions. Biomacromolecules 2015, 16 (3), 985–991. 10.1021/bm501874k. [DOI] [PubMed] [Google Scholar]
- Madruga L. Y.; Sabino R. M.; Santos E. C.; Popat K. C.; Balaban R. d. C.; Kipper M. J. Carboxymethyl-kappa-carrageenan: A study of biocompatibility, antioxidant and antibacterial activities. Int. J. Biol. Macromol. 2020, 152, 483–491. 10.1016/j.ijbiomac.2020.02.274. [DOI] [PubMed] [Google Scholar]
- Basu A.; Rose K. L.; Zhang J.; Beavis R. C.; Ueberheide B.; Garcia B. A.; Chait B.; Zhao Y.; Hunt D. F.; Segal E.; et al. Proteome-wide prediction of acetylation substrates. Proc. Natl. Acad. Sci. U. S. A. 2009, 106 (33), 13785–13790. 10.1073/pnas.0906801106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S.; Song D.; Cho K.; Oh S.; Lee-Yoon D.; Bae E.; Lee J. H.. Cell adhesion and degradation behaviors of acetylated chitosan films. In 3rd Kuala Lumpur International Conference on Biomedical Engineering 2006; Springer, 2007; pp 94–97. [Google Scholar]
- Peter Wessel H.; Labler L.; Tschopp T. B. Synthesis of an N-Acetylated Heparin Pentasaccharide and its anticoagulant activity in comparison with the heparin pentasaccharide with high anti-factor-Xa activity. Helv. Chim. Acta 1989, 72 (6), 1268–1277. 10.1002/hlca.19890720613. [DOI] [Google Scholar]
- Cord-Landwehr S.; Richter C.; Wattjes J.; Sreekumar S.; Singh R.; Basa S.; El Gueddari N. E.; Moerschbacher B. M. Patterns matter part 2: Chitosan oligomers with defined patterns of acetylation. React. Funct. Polym. 2020, 151, 104577. 10.1016/j.reactfunctpolym.2020.104577. [DOI] [Google Scholar]
- Wattjes J.; Sreekumar S.; Richter C.; Cord-Landwehr S.; Singh R.; El Gueddari N. E.; Moerschbacher B. M. Patterns matter part 1: Chitosan polymers with non-random patterns of acetylation. React. Funct. Polym. 2020, 151, 104583. 10.1016/j.reactfunctpolym.2020.104583. [DOI] [Google Scholar]
- Basa S.; Nampally M.; Honorato T.; Das S. N.; Podile A. R.; El Gueddari N. E.; Moerschbacher B. M. The pattern of acetylation defines the priming activity of chitosan tetramers. J. Am. Chem. Soc. 2020, 142 (4), 1975–1986. 10.1021/jacs.9b11466. [DOI] [PubMed] [Google Scholar]
- Chen M.; Li R.-M.; Runge T.; Feng J.; Feng J.; Hu S.; Shi Q.-S. Solvent-Free Acetylation of Cellulose by 1-Ethyl-3-methylimidazolium Acetate-Catalyzed Transesterification. ACS Sustainable Chem. Eng. 2019, 7 (20), 16971–16978. 10.1021/acssuschemeng.8b06333. [DOI] [Google Scholar]
- Wu J.; Zhang J.; Zhang H.; He J.; Ren Q.; Guo M. Homogeneous acetylation of cellulose in a new ionic liquid. Biomacromolecules 2004, 5 (2), 266–268. 10.1021/bm034398d. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Su N.; Huang Q.; Zhang Q.; Wang Y.; Li J.; Ye M. Phosphorylation and antiaging activity of polysaccharide from Trichosanthes peel. Journal of food and drug analysis 2017, 25 (4), 976–983. 10.1016/j.jfda.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojarski K. K.; Becher J.; Riemer T.; Lemmnitzer K.; Möller S.; Schiller J.; Schnabelrauch M.; Samsonov S. A. Synthesis and in silico characterization of artificially phosphorylated glycosaminoglycans. J. Mol. Struct. 2019, 1197, 401–416. 10.1016/j.molstruc.2019.07.064. [DOI] [Google Scholar]
- Chen J.; Huang G. Antioxidant activities of garlic polysaccharide and its phosphorylated derivative. Int. J. Biol. Macromol. 2019, 125, 432–435. 10.1016/j.ijbiomac.2018.12.073. [DOI] [PubMed] [Google Scholar]
- Chen L.; Huang G. Antioxidant activities of phosphorylated pumpkin polysaccharide. Int. J. Biol. Macromol. 2019, 125, 256–261. 10.1016/j.ijbiomac.2018.12.069. [DOI] [PubMed] [Google Scholar]
- Xiong X.; Huang G.; Huang H. The antioxidant activities of phosphorylated polysaccharide from native ginseng. Int. J. Biol. Macromol. 2019, 126, 842–845. 10.1016/j.ijbiomac.2018.12.266. [DOI] [PubMed] [Google Scholar]
- Jiang N.; Li B.; Wang X.; Xu X.; Liu X.; Li W.; Chang X.; Li H.; Qi H. The antioxidant and antihyperlipidemic activities of phosphorylated polysaccharide from Ulva pertusa. Int. J. Biol. Macromol. 2020, 145, 1059–1065. 10.1016/j.ijbiomac.2019.09.198. [DOI] [PubMed] [Google Scholar]
- Chen L.; Huang G. Antioxidant activities of phosphorylated pumpkin polysaccharide. Int. J. Biol. Macromol. 2019, 125, 256–261. 10.1016/j.ijbiomac.2018.12.069. [DOI] [PubMed] [Google Scholar]
- Liu H.; Li F.; Luo P. Effect of Carboxymethylation and Phosphorylation on the Properties of Polysaccharides from Sepia esculenta Ink: Antioxidation and Anticoagulation in Vitro. Marine Drugs 2019, 17 (11), 626. 10.3390/md17110626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombaldi de Souza R. F.; Bombaldi de Souza F. C.; Thorpe A.; Mantovani D.; Popat K. C.; Moraes A. M. Phosphorylation of chitosan to improve osteoinduction of chitosan/xanthan-based scaffolds for periosteal tissue engineering. Int. J. Biol. Macromol. 2020, 143, 619–632. 10.1016/j.ijbiomac.2019.12.004. [DOI] [PubMed] [Google Scholar]
- Cao Y.-Y.; Ji Y.-H.; Liao A.-M.; Huang J.-H.; Thakur K.; Li X.-L.; Hu F.; Zhang J.-G.; Wei Z.-J. Effects of sulfated, phosphorylated and carboxymethylated modifications on the antioxidant activities in-vitro of polysaccharides sequentially extracted from Amana edulis. Int. J. Biol. Macromol. 2020, 146, 887–896. 10.1016/j.ijbiomac.2019.09.211. [DOI] [PubMed] [Google Scholar]
- Byrd-Leotis L.; Jia N.; Dutta S.; Trost J. F.; Gao C.; Cummings S. F.; Braulke T.; Müller-Loennies S.; Heimburg-Molinaro J.; Steinhauer D. A.; et al. Influenza binds phosphorylated glycans from human lung. Science advances 2019, 5 (2), eaav2554 10.1126/sciadv.aav2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmartín C.; Plano D.; Sharma A. K.; Palop J. A. Selenium compounds, apoptosis and other types of cell death: an overview for cancer therapy. International journal of molecular sciences 2012, 13 (8), 9649–9672. 10.3390/ijms13089649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozmanová J.; Mániková D.; Vlčková V.; Chovanec M. Selenium: a double-edged sword for defense and offence in cancer. Archives of toxicology 2010, 84 (12), 919–938. 10.1007/s00204-010-0595-8. [DOI] [PubMed] [Google Scholar]
- Rayman M. P. Selenium and human health. Lancet 2012, 379 (9822), 1256–1268. 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- Santi C.; Bagnoli L.. Celebrating two centuries of research in selenium chemistry: state of the art and new prospective; Multidisciplinary Digital Publishing Institute, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorito S.; Epifano F.; Preziuso F.; Taddeo V. A.; Genovese S. Selenylated plant polysaccharides: A survey of their chemical and pharmacological properties. Phytochemistry 2018, 153, 1–10. 10.1016/j.phytochem.2018.05.008. [DOI] [PubMed] [Google Scholar]
- Li X.; Liu F.; Li Z.; Ye N.; Huang C.; Yuan X. Atractylodes macrocephala polysaccharides induces mitochondrial-mediated apoptosis in glioma C6 cells. Int. J. Biol. Macromol. 2014, 66, 108–112. 10.1016/j.ijbiomac.2014.02.019. [DOI] [PubMed] [Google Scholar]
- Qin T.; Chen J.; Wang D.; Hu Y.; Zhang J.; Wang M.; Qiu S.; Gao Z.; Yu Y.; Huang Y.; et al. Selenylation modification can enhance immune-enhancing activity of Chinese angelica polysaccharide. Carbohydr. Polym. 2013, 95 (1), 183–187. 10.1016/j.carbpol.2013.02.072. [DOI] [PubMed] [Google Scholar]
- Qin T.; Chen J.; Wang D.; Hu Y.; Wang M.; Zhang J.; Nguyen T. L.; Liu C.; Liu X. Optimization of selenylation conditions for Chinese angelica polysaccharide based on immune-enhancing activity. Carbohydr. Polym. 2013, 92 (1), 645–650. 10.1016/j.carbpol.2012.08.097. [DOI] [PubMed] [Google Scholar]
- Yuan B.; Yang X.-q.; Kou M.; Lu C.-y.; Wang Y.-y.; Peng J.; Chen P.; Jiang J.-h. Selenylation of polysaccharide from the sweet potato and evaluation of antioxidant, antitumor, and antidiabetic activities. Journal of agricultural and food chemistry 2017, 65 (3), 605–617. 10.1021/acs.jafc.6b04788. [DOI] [PubMed] [Google Scholar]
- Lee J.-H.; Lee Y.-K.; Chang Y. H. Effects of selenylation modification on structural and antioxidant properties of pectic polysaccharides extracted from Ulmus pumila L. Int. J. Biol. Macromol. 2017, 104, 1124–1132. 10.1016/j.ijbiomac.2017.06.121. [DOI] [PubMed] [Google Scholar]
- Huang S.; Yang W.; Huang G. Preparation and activities of selenium polysaccharide from plant such as Grifola frondosa. Carbohydr. Polym. 2020, 242, 116409. 10.1016/j.carbpol.2020.116409. [DOI] [PubMed] [Google Scholar]
- Zheng X. F.; Lian Q.; Yang H.; Zhu H. Alkyl pectin: Hydrophobic matrices for controlled drug release. J. Appl. Polym. Sci. 2015, 132 (3), 41302. 10.1002/app.41302. [DOI] [Google Scholar]
- Liu C.-m.; Guo X.-j.; Liang R.-h.; Liu W.; Chen J. Alkylated pectin: Molecular characterization, conformational change and gel property. Food Hydrocolloids 2017, 69, 341–349. 10.1016/j.foodhyd.2017.03.008. [DOI] [Google Scholar]
- Yataka Y.; Sawada T.; Serizawa T. Multidimensional self-assembled structures of alkylated cellulose oligomers synthesized via in vitro enzymatic reactions. Langmuir 2016, 32 (39), 10120–10125. 10.1021/acs.langmuir.6b02679. [DOI] [PubMed] [Google Scholar]
- Madhusudan S. K.; Agnihotri G.; Negi D. S.; Misra A. K. Direct one-pot conversion of acylated carbohydrates into their alkylated derivatives under heterogeneous reaction conditions using solid NaOH and a phase transfer catalyst. Carbohydrate research 2005, 340 (7), 1373–1377. 10.1016/j.carres.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Boddapati S.; Rai R.; Gummadi S. N. Structural analysis and antioxidative properties of mutan (water-insoluble glucan) and carboxymethyl mutan from Streptococcus mutans. Process Biochemistry 2020, 97, 130–139. 10.1016/j.procbio.2020.07.006. [DOI] [Google Scholar]
- de Oliveira F. F. B.; Bingana R. D.; Morais P. A. F.; Oliveira S. R. B. D.; dos Reis Barbosa A. L.; de Sousa Chaves L.; Alencar P. D. O. C.; Soares P. M. G.; Souza M. H. L. P.; Freitas A. L. P.; Barros F.C. N.; Medeiros J. R.; Damasceno R. O. S. Sulfated polysaccharide from Gracilaria caudata reduces hypernociception and inflammatory response during arthritis in rodents. Int. J. Biol. Macromol. 2020, 161, 1061–1069. 10.1016/j.ijbiomac.2020.06.060. [DOI] [PubMed] [Google Scholar]
- Alavi M.; Tabarsa M.; You S.; Gavlighi H. A. Structural characteristics, molecular properties and immunostimulatory effects of sulfated polysaccharide from freshwater Myriophyllum spicatum L. Int. J. Biol. Macromol. 2020, 153, 951–961. 10.1016/j.ijbiomac.2019.11.109. [DOI] [PubMed] [Google Scholar]
- Xu J.; Wu Z.; Wu Q.; Kuang Y. Acetylated cellulose nanocrystals with high-crystallinity obtained by one-step reaction from the traditional acetylation of cellulose. Carbohydr. Polym. 2020, 229, 115553. 10.1016/j.carbpol.2019.115553. [DOI] [PubMed] [Google Scholar]
- Kubota N.; Tatsumoto N.; Sano T.; Toya K. A simple preparation of half N-acetylated chitosan highly soluble in water and aqueous organic solvents. Carbohydrate research 2000, 324 (4), 268–274. 10.1016/S0008-6215(99)00263-3. [DOI] [PubMed] [Google Scholar]
- Duan Z.; Zhang Y.; Zhu C.; Wu Y.; Du B.; Ji H. Structural characterization of phosphorylated Pleurotus ostreatus polysaccharide and its hepatoprotective effect on carbon tetrachloride-induced liver injury in mice. Int. J. Biol. Macromol. 2020, 162, 533. 10.1016/j.ijbiomac.2020.06.107. [DOI] [PubMed] [Google Scholar]
- Lin L.; Yang J.; Yang Y.; Zhi H.; Hu X.; Chai D.; Liu Y.; Shen X.; Wang J.; Song Y.; et al. Phosphorylation of Radix Cyathula officinalis polysaccharide improves its immune-enhancing activity. Journal of Carbohydrate Chemistry 2020, 39 (1), 50–62. 10.1080/07328303.2019.1700996. [DOI] [Google Scholar]
- Ru Y.; Liu K.; Kong X.; Li X.; Shi X.; Chen H. Synthesis of selenylated polysaccharides from Momordica charantia L. and its hypoglycemic activity in streptozotocin-induced diabetic mice. International Journal of Biological Macromolecules 2020, 152, 295–304. [DOI] [PubMed] [Google Scholar]
- Haibo F.; Fan J.; Bo H.; Tian X.; Bao H.; Wang X. Selenylation modification can enhance immune-enhancing activity of Chuanminshen violaceum polysaccharide. Carbohydr. Polym. 2016, 153, 302–311. 10.1016/j.carbpol.2016.07.055. [DOI] [PubMed] [Google Scholar]
- Li R.; Qin X.; Liu S.; Zhang X.; Zeng X.; Guo H.; Wang T.; Zhang Y.; Zhang J.; Zhang J.; et al. HSO4 catalyzed synthesis of selenized polysaccharide and its immunomodulatory effect on RAW264. 7 cells via MAPKs pathway. Int. J. Biol. Macromol. 2020, 160, 1066–1077. 10.1016/j.ijbiomac.2020.05.261. [DOI] [PubMed] [Google Scholar]
- Lee J.-H.; Lee Y.-K.; Choi Y.-R.; Park J.; Jung S. K.; Chang Y. H. The characterization, selenylation and anti-inflammatory activity of pectic polysaccharides extracted from Ulmus pumila L. Int. J. Biol. Macromol. 2018, 111, 311–318. 10.1016/j.ijbiomac.2018.01.005. [DOI] [PubMed] [Google Scholar]
- Liu Y.; You Y.; Li Y.; Zhang L.; Yin L.; Shen Y.; Li C.; Chen H.; Chen S.; Hu B.; et al. The characterization, selenylation and antidiabetic activity of mycelial polysaccharides from Catathelasma ventricosum. Carbohydr. Polym. 2017, 174, 72–81. 10.1016/j.carbpol.2017.06.050. [DOI] [PubMed] [Google Scholar]
- Shahi S.; Roghani-Mamaqani H.; Talebi S.; Mardani H. Chemical stimuli-induced reversible bond cleavage in covalently crosslinked hydrogels. Coord. Chem. Rev. 2022, 455, 214368. 10.1016/j.ccr.2021.214368. [DOI] [Google Scholar]
- Shieh P.; Hill M. R.; Zhang W.; Kristufek S. L.; Johnson J. A. Clip chemistry: diverse (bio)(macro) molecular and material function through breaking covalent bonds. Chem. Rev. 2021, 121 (12), 7059–7121. 10.1021/acs.chemrev.0c01282. [DOI] [PubMed] [Google Scholar]
- Das K.; Gabrielli L.; Prins L. J. Chemically fueled self-assembly in biology and chemistry. Angew. Chem., Int. Ed. 2021, 60 (37), 20120–20143. 10.1002/anie.202100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesides G. M.; Grzybowski B. Self-assembly at all scales. Science 2002, 295 (5564), 2418–2421. 10.1126/science.1070821. [DOI] [PubMed] [Google Scholar]
- Tibbitt M. W.; Anseth K. S. Dynamic microenvironments: the fourth dimension. Science translational medicine 2012, 4 (160), 160ps124–160ps124. 10.1126/scitranslmed.3004804. [DOI] [PubMed] [Google Scholar]
- Disney M. D. A glimpse at the glycoRNA world. Cell 2021, 184 (12), 3080–3081. 10.1016/j.cell.2021.05.025. [DOI] [PubMed] [Google Scholar]
- Anselme K.; Ploux L.; Ponche A. Cell/material interfaces: influence of surface chemistry and surface topography on cell adhesion. J. Adhes. Sci. Technol. 2010, 24 (5), 831–852. 10.1163/016942409X12598231568186. [DOI] [Google Scholar]
- Yazdi M. K.; Zarrintaj P.; Ganjali M. R.; Salehnia F.; Rezapour M.; Seidi F.; Saeb M. R.. Grafted polysaccharides in drug delivery. In Tailor-Made Polysaccharides in Drug Delivery; Elsevier, 2023; pp 157–175. [Google Scholar]
- Yazdi M. K.; Ganjali M. R.; Zarrintaj P.; Bagheri B.; Kim Y. C.; Saeb M. R. Ionically gelled carboxymethyl polysaccharides for drug delivery. ionically gelled biopolysaccharide based systems in drug delivery 2021, 93–103. 10.1007/978-981-16-2271-7_5. [DOI] [Google Scholar]
- Xue K.; Wang F.; Suwardi A.; Han M.-Y.; Teo P.; Wang P.; Wang S.; Ye E.; Li Z.; Loh X. J. Biomaterials by design: Harnessing data for future development. Materials Today Bio 2021, 12, 100165. 10.1016/j.mtbio.2021.100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.-l.; Li B.-c.; Li Z.-j.; Ren K.-f.; Jin L.-j.; Zhang S.-m.; Chang H.; Sun Y.-x.; Ji J. Electropolymerization of dopamine for surface modification of complex-shaped cardiovascular stents. Biomaterials 2014, 35 (27), 7679–7689. 10.1016/j.biomaterials.2014.05.047. [DOI] [PubMed] [Google Scholar]
- Murphy E. J.; Fehrenbach G. W.; Abidin I. Z.; Buckley C.; Montgomery T.; Pogue R.; Murray P.; Major I.; Rezoagli E. Polysaccharides—Naturally Occurring Immune Modulators. Polymers 2023, 15 (10), 2373. 10.3390/polym15102373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. N.; Birkinshaw C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92 (2), 1262–1279. 10.1016/j.carbpol.2012.10.028. [DOI] [PubMed] [Google Scholar]
- Zhdanov V. P. Formation of a protein corona around nanoparticles. Current opinion in colloid & interface science 2019, 41, 95–103. 10.1016/j.cocis.2018.12.002. [DOI] [Google Scholar]
- Barui A. K.; Oh J. Y.; Jana B.; Kim C.; Ryu J. H. Cancer-targeted nanomedicine: overcoming the barrier of the protein corona. Advanced therapeutics 2020, 3 (1), 1900124. 10.1002/adtp.201900124. [DOI] [Google Scholar]
- Caprifico A. E.; Foot P. J.; Polycarpou E.; Calabrese G. Overcoming the protein corona in chitosan-based nanoparticles. Drug Discovery Today 2021, 26 (8), 1825–1840. 10.1016/j.drudis.2021.04.014. [DOI] [PubMed] [Google Scholar]
- Hillion S.; Arleevskaya M. I.; Blanco P.; Bordron A.; Brooks W. H.; Cesbron J. Y.; Kaveri S.; Vivier E.; Renaudineau Y. The innate part of the adaptive immune system. Clinical reviews in allergy & immunology 2020, 58, 151–154. 10.1007/s12016-019-08740-1. [DOI] [PubMed] [Google Scholar]
- Flemming H.-C.; van Hullebusch E. D.; Neu T. R.; Nielsen P. H.; Seviour T.; Stoodley P.; Wingender J.; Wuertz S. The biofilm matrix: Multitasking in a shared space. Nature Reviews Microbiology 2023, 21 (2), 70–86. 10.1038/s41579-022-00791-0. [DOI] [PubMed] [Google Scholar]
- Hosseini A.; Hashemi V.; Shomali N.; Asghari F.; Gharibi T.; Akbari M.; Gholizadeh S.; Jafari A. Innate and adaptive immune responses against coronavirus. Biomedicine & Pharmacotherapy 2020, 132, 110859. 10.1016/j.biopha.2020.110859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.; Kim S.; Park J.; Lee J. Y. Universal surface modification using dopamine-hyaluronic acid conjugates for anti-biofouling. Int. J. Biol. Macromol. 2020, 151, 1314–1321. 10.1016/j.ijbiomac.2019.10.177. [DOI] [PubMed] [Google Scholar]
- Taghizadeh A.; Taghizadeh M.; Yazdi M. K.; Zarrintaj P.; Ramsey J. D.; Seidi F.; Stadler F. J.; Lee H.; Saeb M. R.; Mozafari M. Mussel-inspired biomaterials: From chemistry to clinic. Bioengineering & Translational Medicine 2022, 7, e10385 10.1002/btm2.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Liu S.; Li T.; Zhang L.; Azhar U.; Ma J.; Zhai C.; Zong C.; Zhang S. Cytocompatible and non-fouling zwitterionic hyaluronic acid-based hydrogels using thiol-ene “click” chemistry for cell encapsulation. Carbohydr. Polym. 2020, 236, 116021. 10.1016/j.carbpol.2020.116021. [DOI] [PubMed] [Google Scholar]
- Gong H.; Hu X.; Zhang L.; Fa K.; Liao M.; Liu H.; Fragneto G.; Campana M.; Lu J. R. How do antimicrobial peptides disrupt the lipopolysaccharide membrane leaflet of Gram-negative bacteria?. J. Colloid Interface Sci. 2023, 637, 182–192. 10.1016/j.jcis.2023.01.051. [DOI] [PubMed] [Google Scholar]
- Kavanagh K.; Dowd S. Histatins: antimicrobial peptides with therapeutic potential. J. Pharm. Pharmacol. 2010, 56 (3), 285–289. 10.1211/0022357022971. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Yu L.; Zhang B.; Feng W.; Xu M.; Gao L.; Liu N.; Wang Q.; Huang X.; Li P.; et al. Design and synthesis of biocompatible, hemocompatible, and highly selective antimicrobial cationic peptidopolysaccharides via click chemistry. Biomacromolecules 2019, 20 (6), 2230–2240. 10.1021/acs.biomac.9b00179. [DOI] [PubMed] [Google Scholar]
- Pranantyo D.; Xu L. Q.; Kang E.-T.; Chan-Park M. B. Chitosan-based peptidopolysaccharides as cationic antimicrobial agents and antibacterial coatings. Biomacromolecules 2018, 19 (6), 2156–2165. 10.1021/acs.biomac.8b00270. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Song S.; Ren X.; Zhang J.; Lin Q.; Zhao Y. Supramolecular Adhesive Hydrogels for Tissue Engineering Applications. Chem. Rev. 2022, 122 (6), 5604–5640. 10.1021/acs.chemrev.1c00815. [DOI] [PubMed] [Google Scholar]
- Ghobril C.; Grinstaff M. The chemistry and engineering of polymeric hydrogel adhesives for wound closure: a tutorial. Chem. Soc. Rev. 2015, 44 (7), 1820–1835. 10.1039/C4CS00332B. [DOI] [PubMed] [Google Scholar]
- Guo Y.; Wang Y.; Zhao X.; Li X.; Wang Q.; Zhong W.; Mequanint K.; Zhan R.; Xing M.; Luo G. Snake extract-laden hemostatic bioadhesive gel cross-linked by visible light. Science Advances 2021, 7 (29), eabf9635 10.1126/sciadv.abf9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place E. S.; Evans N. D.; Stevens M. M. Complexity in biomaterials for tissue engineering. Nature materials 2009, 8 (6), 457–470. 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- Chen S.; Gil C. J.; Ning L.; Jin L.; Perez L.; Kabboul G.; Tomov M. L.; Serpooshan V. Adhesive tissue engineered scaffolds: mechanisms and applications. Frontiers in Bioengineering and Biotechnology 2021, 9, 683079. 10.3389/fbioe.2021.683079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmany M. B.; Van Dyke M. Biomimetic approaches to modulate cellular adhesion in biomaterials: A review. Acta biomaterialia 2013, 9 (3), 5431–5437. 10.1016/j.actbio.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Wu B.; Zhou Y.; Zhou F.; Liu W.; Wang Z. Mussel-inspired hydrogels: from design principles to promising applications. Chem. Soc. Rev. 2020, 49, 3605. 10.1039/C9CS00849G. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Wang R.; Sun Z.; Zhu X.; Zhao Q.; Zhang T.; Cholewinski A.; Yang F. K.; Zhao B.; Pinnaratip R.; et al. Catechol-functionalized hydrogels: biomimetic design, adhesion mechanism, and biomedical applications. Chem. Soc. Rev. 2020, 49 (2), 433–464. 10.1039/C9CS00285E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geim A. K.; Dubonos S.; Grigorieva I.; Novoselov K.; Zhukov A.; Shapoval S. Y. Microfabricated adhesive mimicking gecko foot-hair. Nature materials 2003, 2 (7), 461–463. 10.1038/nmat917. [DOI] [PubMed] [Google Scholar]
- Lee H.; Lee B. P.; Messersmith P. B. A reversible wet/dry adhesive inspired by mussels and geckos. Nature 2007, 448 (7151), 338–341. 10.1038/nature05968. [DOI] [PubMed] [Google Scholar]
- Yang J.; Bai R.; Chen B.; Suo Z. Hydrogel adhesion: A supramolecular synergy of chemistry, topology, and mechanics. Adv. Funct. Mater. 2020, 30 (2), 1901693. 10.1002/adfm.201901693. [DOI] [Google Scholar]
- Hasani-Sadrabadi M. M.; Sarrion P.; Pouraghaei S.; Chau Y.; Ansari S.; Li S.; Aghaloo T.; Moshaverinia A. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Science translational medicine 2020, 12 (534), eaay6853 10.1126/scitranslmed.aay6853. [DOI] [PubMed] [Google Scholar]
- Memic A.; Rezaeeyazdi M.; Villard P.; Rogers Z. J.; Abdullah T.; Colombani T.; Bencherif S. A. Effect of polymer concentration on autoclaved cryogel properties. Macromol. Mater. Eng. 2020, 305 (5), 1900824. 10.1002/mame.201900824. [DOI] [Google Scholar]
- Du X.; Liu Y.; Yan H.; Rafique M.; Li S.; Shan X.; Wu L.; Qiao M.; Kong D.; Wang L. Anti-infective and pro-coagulant chitosan-based hydrogel tissue adhesive for sutureless wound closure. Biomacromolecules 2020, 21 (3), 1243–1253. 10.1021/acs.biomac.9b01707. [DOI] [PubMed] [Google Scholar]
- Liu L.; He Y.; Shi X.; Gao H.; Wang Y.; Lin Z. Phosphocreatine-modified chitosan porous scaffolds promote mineralization and osteogenesis in vitro and in vivo. Applied Materials Today 2018, 12, 21–33. 10.1016/j.apmt.2018.03.010. [DOI] [Google Scholar]
- Park M. K.; Li M.-X.; Yeo I.; Jung J.; Yoon B.-I.; Joung Y. K. Balanced Adhesion and Cohesion of Chitosan Matrices by Conjugation and Oxidation of Catechol for High-Performance Surgical Adhesives. Carbohydr. Polym. 2020, 248, 116760. 10.1016/j.carbpol.2020.116760. [DOI] [PubMed] [Google Scholar]
- Kazemi M. S.; Mohammadi Z.; Amini M.; Yousefi M.; Tarighi P.; Eftekhari S.; Tehrani M. R. Thiolated chitosan-lauric acid as a new chitosan derivative: Synthesis, characterization and cytotoxicity. Int. J. Biol. Macromol. 2019, 136, 823–830. 10.1016/j.ijbiomac.2019.06.132. [DOI] [PubMed] [Google Scholar]
- He X. Y.; Sun A.; Li T.; Qian Y. J.; Qian H.; Ling Y. F.; Zhang L. H.; Liu Q. Y.; Peng T.; Qian Z. Mussel-inspired antimicrobial gelatin/chitosan tissue adhesive rapidly activated in situ by H2O2/ascorbic acid for infected wound closure. Carbohydr. Polym. 2020, 247, 116692. 10.1016/j.carbpol.2020.116692. [DOI] [PubMed] [Google Scholar]
- He J.; Shi M.; Liang Y.; Guo B. Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds. Chemical Engineering Journal 2020, 394, 124888. 10.1016/j.cej.2020.124888. [DOI] [Google Scholar]
- Li S.; Zhou J.; Huang Y.; Roy J.; Zhou N.; Yum K.; Sun X.; Tang L. Injectable click chemistry-based bioadhesives for accelerated wound closure. Acta Biomaterialia 2020, 110, 95–104. 10.1016/j.actbio.2020.04.004. [DOI] [PubMed] [Google Scholar]
- Chen H.; Cheng J.; Ran L.; Yu K.; Lu B.; Lan G.; Dai F.; Lu F. An injectable self-healing hydrogel with adhesive and antibacterial properties effectively promotes wound healing. Carbohydr. Polym. 2018, 201, 522–531. 10.1016/j.carbpol.2018.08.090. [DOI] [PubMed] [Google Scholar]
- Sanandiya N. D.; Lee S.; Rho S.; Lee H.; Kim I. S.; Hwang D. S. Tunichrome-inspired pyrogallol functionalized chitosan for tissue adhesion and hemostasis. Carbohydr. Polym. 2019, 208, 77–85. 10.1016/j.carbpol.2018.12.017. [DOI] [PubMed] [Google Scholar]
- Sharma S.; Kumar R.; Kumari P.; Kharwar R. N.; Yadav A. K.; Saripella S. Mechanically magnified chitosan-based hydrogel as tissue adhesive and antimicrobial candidate. Int. J. Biol. Macromol. 2019, 125, 109–115. 10.1016/j.ijbiomac.2018.12.018. [DOI] [PubMed] [Google Scholar]
- Lih E.; Lee J. S.; Park K. M.; Park K. D. Rapidly curable chitosan-PEG hydrogels as tissue adhesives for hemostasis and wound healing. Acta biomaterialia 2012, 8 (9), 3261–3269. 10.1016/j.actbio.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Lu M.; Liu Y.; Huang Y.-C.; Huang C.-J.; Tsai W.-B. Fabrication of photo-crosslinkable glycol chitosan hydrogel as a tissue adhesive. Carbohydr. Polym. 2018, 181, 668–674. 10.1016/j.carbpol.2017.11.097. [DOI] [PubMed] [Google Scholar]
- Yuk H.; Varela C. E.; Nabzdyk C. S.; Mao X.; Padera R. F.; Roche E. T.; Zhao X. Dry double-sided tape for adhesion of wet tissues and devices. Nature 2019, 575 (7781), 169–174. 10.1038/s41586-019-1710-5. [DOI] [PubMed] [Google Scholar]
- Liu J.; Li J.; Yu F.; Zhao Y. X.; Mo X. M.; Pan J. F. In situ forming hydrogel of natural polysaccharides through Schiff base reaction for soft tissue adhesive and hemostasis. Int. J. Biol. Macromol. 2020, 147, 653–666. 10.1016/j.ijbiomac.2020.01.005. [DOI] [PubMed] [Google Scholar]
- Chen T.; Chen Y.; Rehman H. U.; Chen Z.; Yang Z.; Wang M.; Li H.; Liu H. Ultratough, self-healing, and tissue-adhesive hydrogel for wound dressing. ACS Appl. Mater. Interfaces 2018, 10 (39), 33523–33531. 10.1021/acsami.8b10064. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Wang X.; Wang Y.; Hao J. Rapid-forming and self-healing agarose-based hydrogels for tissue adhesives and potential wound dressings. Biomacromolecules 2018, 19 (3), 980–988. 10.1021/acs.biomac.7b01764. [DOI] [PubMed] [Google Scholar]
- Liu C.; Liu X.; Liu C.; Wang N.; Chen H.; Yao W.; Sun G.; Song Q.; Qiao W. A highly efficient, in situ wet-adhesive dextran derivative sponge for rapid hemostasis. Biomaterials 2019, 205, 23–37. 10.1016/j.biomaterials.2019.03.016. [DOI] [PubMed] [Google Scholar]
- Gomes T. D.; Caridade S. G.; Sousa M. P.; Azevedo S.; Kandur M. Y.; Öner E. T.; Alves N. M.; Mano J. F. Adhesive free-standing multilayer films containing sulfated levan for biomedical applications. Acta biomaterialia 2018, 69, 183–195. 10.1016/j.actbio.2018.01.027. [DOI] [PubMed] [Google Scholar]
- Sundaram M. N.; Krishnamoorthi Kaliannagounder V.; Selvaprithiviraj V.; Suresh M. K.; Biswas R.; Vasudevan A. K.; Varma P. K.; Jayakumar R. Bioadhesive, hemostatic, and antibacterial in situ chitin-fibrin Nanocomposite gel for controlling bleeding and preventing infections at mediastinum. ACS Sustainable Chem. Eng. 2018, 6 (6), 7826–7840. 10.1021/acssuschemeng.8b00915. [DOI] [Google Scholar]
- Mohammadinejad R.; Maleki H.; Larrañeta E.; Fajardo A. R.; Nik A. B.; Shavandi A.; Sheikhi A.; Ghorbanpour M.; Farokhi M.; Govindh P.; et al. Status and future scope of plant-based green hydrogels in biomedical engineering. Applied Materials Today 2019, 16, 213–246. 10.1016/j.apmt.2019.04.010. [DOI] [Google Scholar]
- Dimida S.; Barca A.; Cancelli N.; De Benedictis V.; Raucci M. G.; Demitri C. Effects of genipin concentration on cross-linked chitosan scaffolds for bone tissue engineering: Structural characterization and evidence of biocompatibility features. International Journal of Polymer Science 2017, 2017, 1. 10.1155/2017/8410750. [DOI] [Google Scholar]
- Jang J.; Seol Y.-J.; Kim H. J.; Kundu J.; Kim S. W.; Cho D.-W. Effects of alginate hydrogel cross-linking density on mechanical and biological behaviors for tissue engineering. journal of the mechanical behavior of biomedical materials 2014, 37, 69–77. 10.1016/j.jmbbm.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Shokrani H.; Shokrani A.; Jouyandeh M.; Seidi F.; Gholami F.; Kar S.; Munir M. T.; Kowalkowska-Zedler D.; Zarrintaj P.; Rabiee N.; et al. Green polymer nanocomposites for skin tissue engineering. ACS Applied Bio Materials 2022, 5 (5), 2107–2121. 10.1021/acsabm.2c00313. [DOI] [PubMed] [Google Scholar]
- Lunzer M.; Shi L.; Andriotis O. G.; Gruber P.; Markovic M.; Thurner P. J.; Ossipov D.; Liska R.; Ovsianikov A. A modular approach to sensitized two-photon patterning of photodegradable hydrogels. Angew. Chem. 2018, 130 (46), 15342–15347. 10.1002/ange.201808908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsad N. J.; Ngadi N.; Razak M.; Fatimah S.. Preparation of Chitosan-Grafted Nanocellulose via Microwave-Initiate Method. In Applied Mechanics and Materials; Trans Tech Publ, 2016; Vol. 818, pp 281–284. [Google Scholar]
- Yataka Y.; Suzuki A.; Iijima K.; Hashizume M. Enhancement of the mechanical properties of polysaccharide composite films utilizing cellulose nanofibers. Polym. J. 2020, 52, 1–9. 10.1038/s41428-020-0311-3. [DOI] [Google Scholar]
- Arianita A.; Amalia B.; Pudjiastuti W.; Melanie S.; Fauzia V.; Imawan C.. Effect of glutaraldehyde to the mechanical properties of chitosan/nanocellulose. In Journal of Physics: Conference Series; IOP Publishing, 2019; Vol. 1317, p 012045. [Google Scholar]
- Singh P.; Chen Y.; Tyagi D.; Wu L.; Ren X.; Feng J.; Carrier A.; Luan T.; Tang Y.; Zhang J.; et al. β-Cyclodextrin-grafted hyaluronic acid as a supramolecular polysaccharide carrier for cell-targeted drug delivery. Int. J. Pharm. 2021, 602, 120602. 10.1016/j.ijpharm.2021.120602. [DOI] [PubMed] [Google Scholar]
- Panja S.; Adams D. J. Stimuli responsive dynamic transformations in supramolecular gels. Chem. Soc. Rev. 2021, 50 (8), 5165–5200. 10.1039/D0CS01166E. [DOI] [PubMed] [Google Scholar]
- Webber M. J.; Appel E. A.; Meijer E.; Langer R. Supramolecular biomaterials. Nature materials 2016, 15 (1), 13–26. 10.1038/nmat4474. [DOI] [PubMed] [Google Scholar]
- Zhou J.; Rao L.; Yu G.; Cook T. R.; Chen X.; Huang F. Supramolecular cancer nanotheranostics. Chem. Soc. Rev. 2021, 50 (4), 2839–2891. 10.1039/D0CS00011F. [DOI] [PubMed] [Google Scholar]
- Boustta M.; Vert M. Hyaluronic Acid-Poly (N-acryloyl glycinamide) Copolymers as Sources of Degradable Thermoresponsive Hydrogels for Therapy. Gels 2020, 6 (4), 42. 10.3390/gels6040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Rivera G. J.; Post A.; John M.; Buchan S.; Bernard D.; Razavi M.; Cosgriff-Hernandez E. Injectable hydrogel electrodes as conduction highways to restore native pacing. Nat. Commun. 2024, 15 (1), 64. 10.1038/s41467-023-44419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdougall L. J.; Pérez-Madrigal M. M.; Shaw J. E.; Inam M.; Hoyland J. A.; O’Reilly R.; Richardson S. M.; Dove A. P. Self-healing, stretchable and robust interpenetrating network hydrogels. Biomaterials science 2018, 6 (11), 2932–2937. 10.1039/C8BM00872H. [DOI] [PubMed] [Google Scholar]
- Grijalvo S.; Eritja R.; Díaz Díaz D. On the Race for More Stretchable and Tough Hydrogels. Gels 2019, 5 (2), 24. 10.3390/gels5020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A.; Maity S.; Das R. K. A Highly Stretchable, Tough, Self-Healing, and Thermoprocessable Polyacrylamide-Chitosan Supramolecular Hydrogel. Macromol. Mater. Eng. 2018, 303 (12), 1800322. 10.1002/mame.201800322. [DOI] [Google Scholar]
- Pan J.; Jin Y.; Lai S.; Shi L.; Fan W.; Shen Y. An antibacterial hydrogel with desirable mechanical, self-healing and recyclable properties based on triple-physical crosslinking. Chemical Engineering Journal 2019, 370, 1228–1238. 10.1016/j.cej.2019.04.001. [DOI] [Google Scholar]
- Liu H.; Xiong C.; Tao Z.; Fan Y.; Tang X.; Yang H. Zwitterionic copolymer-based and hydrogen bonding-strengthened self-healing hydrogel. RSC Adv. 2015, 5 (42), 33083–33088. 10.1039/C4RA15003A. [DOI] [Google Scholar]
- Ding F.; Li H.; Du Y.; Shi X. Recent advances in chitosan-based self-healing materials. Res. Chem. Intermed. 2018, 44 (8), 4827–4840. 10.1007/s11164-018-3339-7. [DOI] [Google Scholar]
- Zhang Z.-X.; Liow S. S.; Xue K.; Zhang X.; Li Z.; Loh X. J. Autonomous Chitosan-Based Self-Healing Hydrogel Formed through Noncovalent Interactions. ACS Applied Polymer Materials 2019, 1 (7), 1769–1777. 10.1021/acsapm.9b00317. [DOI] [Google Scholar]
- Wan H.; Shen J.; Gao N.; Liu J.; Gao Y.; Zhang L. Tailoring the mechanical properties by molecular integration of flexible and stiff polymer networks. Soft Matter 2018, 14 (12), 2379–2390. 10.1039/C7SM02282D. [DOI] [PubMed] [Google Scholar]
- Gao N.; Hou G.; Liu J.; Shen J.; Gao Y.; Lyulin A. V.; Zhang L. Tailoring the mechanical properties of polymer nanocomposites via interfacial engineering. Phys. Chem. Chem. Phys. 2019, 21 (34), 18714–18726. 10.1039/C9CP02948F. [DOI] [PubMed] [Google Scholar]
- Yavvari P. S.; Srivastava A. Robust, self-healing hydrogels synthesised from catechol rich polymers. J. Mater. Chem. B 2015, 3 (5), 899–910. 10.1039/C4TB01307G. [DOI] [PubMed] [Google Scholar]
- Patenaude M.; Campbell S.; Kinio D.; Hoare T. Tuning gelation time and morphology of injectable hydrogels using ketone-hydrazide cross-linking. Biomacromolecules 2014, 15 (3), 781–790. 10.1021/bm401615d. [DOI] [PubMed] [Google Scholar]
- Devaraj N. K. The future of bioorthogonal chemistry. ACS Central Science 2018, 4 (8), 952–959. 10.1021/acscentsci.8b00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S.; Liang W.; Xu G.; Huang C.; Zhang J.; Lang M. A fast and dual crosslinking hydrogel based on vinyl ether sodium alginate. Appl. Surf. Sci. 2020, 515, 145811. 10.1016/j.apsusc.2020.145811. [DOI] [Google Scholar]
- Shitrit Y.; Davidovich-Pinhas M.; Bianco-Peled H. Shear thinning pectin hydrogels physically cross-linked with chitosan nanogels. Carbohydr. Polym. 2019, 225, 115249. 10.1016/j.carbpol.2019.115249. [DOI] [PubMed] [Google Scholar]
- Devaraj N. K. The Future of Bioorthogonal Chemistry. ACS Cent Sci. 2018, 4 (8), 952–959. 10.1021/acscentsci.8b00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; Cheng F.; Wei X.; Yi X.; Tang S.; Wang Z.; Zhang Y. S.; He J.; Huang Y. Injectable, self-healing, antibacterial and hemostatic N, O-Carboxymethyl chitosan/oxidized chondroitin sulfate composite hydrogel for wound dressing. Materials Science and Engineering: C 2021, 118, 111324. 10.1016/j.msec.2020.111324. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Pan Y.; Li S.; Xing L.; Du S.; Yuan G.; Li J.; Zhou T.; Xiong D.; Tan H. Doubly crosslinked biodegradable hydrogels based on gellan gum and chitosan for drug delivery and wound dressing. Int. J. Biol. Macromol. 2020, 164, 2204. 10.1016/j.ijbiomac.2020.08.093. [DOI] [PubMed] [Google Scholar]
- Maiz-Fernández S.; Guaresti O.; Pérez-Álvarez L.; Ruiz-Rubio L.; Gabilondo N.; Vilas-Vilela J. L.; Lanceros-Mendez S. β-Glycerol Phosphate/Genipin Chitosan Hydrogels: A Comparative Study of their Properties and Diclofenac Delivery. Carbohydr. Polym. 2020, 248, 116811. 10.1016/j.carbpol.2020.116811. [DOI] [PubMed] [Google Scholar]
- Nourbakhsh M.; Zarrintaj P.; Jafari S. H.; Hosseini S. M.; Aliakbari S.; Pourbadie H. G.; Naderi N.; Zibaii M. I.; Gholizadeh S. S.; Ramsey J. D.; et al. Fabricating an Electroactive Injectable Hydrogel Based on Pluronic-Chitosan/Aniline-Pentamer Containing Angiogenic Factor for Functional Repair of the Hippocampus Ischemia Rat Model. Materials Science and Engineering: C 2020, 117, 111328. 10.1016/j.msec.2020.111328. [DOI] [PubMed] [Google Scholar]
- Liu Q.; Ji N.; Xiong L.; Sun Q. Rapid gelling, self-healing, and fluorescence-responsive chitosan hydrogels formed by dynamic covalent crosslinking. Carbohydr. Polym. 2020, 246, 116586. 10.1016/j.carbpol.2020.116586. [DOI] [PubMed] [Google Scholar]
- Qu J.; Zhao X.; Ma P. X.; Guo B. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomaterialia 2017, 58, 168–180. 10.1016/j.actbio.2017.06.001. [DOI] [PubMed] [Google Scholar]
- Chen H.; Xing X.; Tan H.; Jia Y.; Zhou T.; Chen Y.; Ling Z.; Hu X. Covalently antibacterial alginate-chitosan hydrogel dressing integrated gelatin microspheres containing tetracycline hydrochloride for wound healing. Materials Science and Engineering: C 2017, 70, 287–295. 10.1016/j.msec.2016.08.086. [DOI] [PubMed] [Google Scholar]
- Qu J.; Zhao X.; Liang Y.; Xu Y.; Ma P. X.; Guo B. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chemical Engineering Journal 2019, 362, 548–560. 10.1016/j.cej.2019.01.028. [DOI] [Google Scholar]
- Yang X.; Liu G.; Peng L.; Guo J.; Tao L.; Yuan J.; Chang C.; Wei Y.; Zhang L. Highly efficient self-healable and dual responsive cellulose-based hydrogels for controlled release and 3D cell culture. Adv. Funct. Mater. 2017, 27 (40), 1703174. 10.1002/adfm.201703174. [DOI] [Google Scholar]
- Liang Y.; Zhao X.; Ma P. X.; Guo B.; Du Y.; Han X. pH-responsive injectable hydrogels with mucosal adhesiveness based on chitosan-grafted-dihydrocaffeic acid and oxidized pullulan for localized drug delivery. J. Colloid Interface Sci. 2019, 536, 224–234. 10.1016/j.jcis.2018.10.056. [DOI] [PubMed] [Google Scholar]
- Qu J.; Zhao X.; Ma P. X.; Guo B. Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized “smart” drug release. Acta biomaterialia 2018, 72, 55–69. 10.1016/j.actbio.2018.03.018. [DOI] [PubMed] [Google Scholar]
- Zarrintaj P.; Jouyandeh M.; Ganjali M. R.; Hadavand B. S.; Mozafari M.; Sheiko S. S.; Russell A. J.; Vatankhah-Varnoosfaderani M.; Gutiérrez T. J.; Saeb M. R. Thermo-sensitive polymers in medicine: A review. Eur. Polym. J. 2019, 117, 402. 10.1016/j.eurpolymj.2019.05.024. [DOI] [Google Scholar]
- Doberenz F.; Zeng K.; Willems C.; Zhang K.; Groth T. Thermoresponsive polymers and their biomedical application in tissue engineering-a review. J. Mater. Chem. B 2020, 8 (4), 607–628. 10.1039/C9TB02052G. [DOI] [PubMed] [Google Scholar]
- Sabino M. A.; Carrero M. G.; Rodriguez C. J. Intelligent copolymers based on poly (N-isopropilacrylamide). Part ii: Grafts polysaccharide to obtain new biomaterials for biomedical and pharmacological applications. International Journal of Advances in Medical Biotechnology-IJAMB 2019, 2 (1), 17–26. 10.25061/2595-3931/IJAMB/2019.v2i1.31. [DOI] [Google Scholar]
- Bao H.; Li L.; Leong W. C.; Gan L. H. Thermo-responsive association of chitosan-graft-poly (N-isopropylacrylamide) in aqueous solutions. J. Phys. Chem. B 2010, 114 (32), 10666–10673. 10.1021/jp105041z. [DOI] [PubMed] [Google Scholar]
- Liu T.-Y.; Hu S.-H.; Liu D.-M.; Chen S.-Y.; Chen I.-W. Biomedical nanoparticle carriers with combined thermal and magnetic responses. Nano Today 2009, 4 (1), 52–65. 10.1016/j.nantod.2008.10.011. [DOI] [Google Scholar]
- Chen H.; Fan X.; Zhao Y.; Zhi D.; Cui S.; Zhang E.; Lan H.; Du J.; Zhang Z.; Zhang S.; et al. Stimuli-responsive polysaccharide enveloped liposome for targeting and penetrating delivery of survivin-shRNA into breast tumor. ACS Appl. Mater. Interfaces 2020, 12 (19), 22074–22087. 10.1021/acsami.9b22440. [DOI] [PubMed] [Google Scholar]
- Jia W.; Gungor-Ozkerim P. S.; Zhang Y. S.; Yue K.; Zhu K.; Liu W.; Pi Q.; Byambaa B.; Dokmeci M. R.; Shin S. R.; et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 2016, 106, 58–68. 10.1016/j.biomaterials.2016.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocoiu O.-N.; Staikos G.; Vasile C. Thermoresponsive behavior of sodium alginate grafted with poly (N-isopropylacrylamide) in aqueous media. Carbohydr. Polym. 2018, 184, 118–126. 10.1016/j.carbpol.2017.12.059. [DOI] [PubMed] [Google Scholar]
- Liu M.; Song X.; Wen Y.; Zhu J.-L.; Li J. Injectable thermoresponsive hydrogel formed by alginate-g-poly (N-isopropylacrylamide) that releases doxorubicin-encapsulated micelles as a smart drug delivery system. ACS Appl. Mater. Interfaces 2017, 9 (41), 35673–35682. 10.1021/acsami.7b12849. [DOI] [PubMed] [Google Scholar]
- Guo H.; de Magalhaes Goncalves M.; Ducouret G.; Hourdet D. Cold and Hot Gelling of Alginate-graft-PNIPAM: a Schizophrenic Behavior Induced by Potassium Salts. Biomacromolecules 2018, 19 (2), 576–587. 10.1021/acs.biomac.7b01667. [DOI] [PubMed] [Google Scholar]
- Durkut S.; Elçin Y. M. Synthesis and Characterization of Thermosensitive Poly (N-Vinyl Caprolactam)-Grafted-Aminated Alginate Hydrogels. Macromol. Chem. Phys. 2020, 221 (2), 1900412. 10.1002/macp.201900412. [DOI] [Google Scholar]
- Chang C.; Han K.; Zhang L. Structure and properties of cellulose/poly (N-isopropylacrylamide) hydrogels prepared by IPN strategy. Polym. Adv. Technol. 2011, 22 (9), 1329–1334. 10.1002/pat.1616. [DOI] [Google Scholar]
- Song W.; Su X.; Gregory D. A.; Li W.; Cai Z.; Zhao X. Magnetic alginate/chitosan nanoparticles for targeted delivery of curcumin into human breast cancer cells. Nanomaterials 2018, 8 (11), 907. 10.3390/nano8110907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B.-L.; Zhang J.-Z.; Lu L.-J.; Mao J.-J.; Cao M.-H.; Mao X.-H.; Zhang F.; Duan X.-H.; Zheng C.-S.; Zhang L.-M.; et al. Superparamagnetic iron oxide nanoparticles-complexed cationic amylose for in vivo magnetic resonance imaging tracking of transplanted stem cells in stroke. Nanomaterials 2017, 7 (5), 107. 10.3390/nano7050107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duceppe N.; Tabrizian M.. Design and development of light-sensitive chitosan-based nanocarriers for gene delivery. In Advances in Science and Technology; Trans Tech Publ, 2013; Vol. 86, pp 75–80. [Google Scholar]
- Ghassami E.; Varshosaz J.; Taymouri S. Redox sensitive polysaccharide based nanoparticles for improved cancer treatment: a comprehensive review. Current pharmaceutical design 2018, 24 (28), 3303–3319. 10.2174/1381612824666180813114841. [DOI] [PubMed] [Google Scholar]
- Li L.; Bai Z.; Levkin P. A. Boronate-dextran: an acid-responsive biodegradable polymer for drug delivery. Biomaterials 2013, 34 (33), 8504–8510. 10.1016/j.biomaterials.2013.07.053. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Lin Y.; Wang J.; Yao W.; Wu W.; Jiang X. A facile strategy for constructing boron-rich polymer nanoparticles via a boronic acid-related reaction. Macromol. Rapid Commun. 2011, 32 (6), 534–539. 10.1002/marc.201000757. [DOI] [PubMed] [Google Scholar]
- Esmaeilzadeh P.; Köwitsch A.; Liedmann A.; Menzel M.; Fuhrmann B.; Schmidt G.; Klehm J.; Groth T. Stimuli-responsive multilayers based on thiolated polysaccharides that affect fibroblast cell adhesion. ACS Appl. Mater. Interfaces 2018, 10 (10), 8507–8518. 10.1021/acsami.7b19022. [DOI] [PubMed] [Google Scholar]
- Lv W.; Xu J.; Wang X.; Li X.; Xu Q.; Xin H. Bioengineered boronic ester modified dextran polymer nanoparticles as reactive oxygen species responsive nanocarrier for ischemic stroke treatment. ACS Nano 2018, 12 (6), 5417–5426. 10.1021/acsnano.8b00477. [DOI] [PubMed] [Google Scholar]
- Wang S.; Zheng H.; Zhou L.; Cheng F.; Liu Z.; Zhang H.; Zhang Q. Injectable redox and light responsive MnO2 hybrid hydrogel for simultaneous melanoma therapy and multidrug-resistant bacteria-infected wound healing. Biomaterials 2020, 260, 120314. 10.1016/j.biomaterials.2020.120314. [DOI] [PubMed] [Google Scholar]
- Pan Y.; Liu J.; Yang K.; Cai P.; Xiao H. Novel multi-responsive and sugarcane bagasse cellulose-based nanogels for controllable release of doxorubicin hydrochloride. Materials Science and Engineering: C 2021, 118, 111357. 10.1016/j.msec.2020.111357. [DOI] [PubMed] [Google Scholar]
- Chen X.; Niu S.; Bremner D. H.; Zhang X.; Zhang H.; Zhang Y.; Li S.; Zhu L.-M. Co-delivery of doxorubicin and oleanolic acid by triple-sensitive nanocomposite based on chitosan for effective promoting tumor apoptosis. Carbohydr. Polym. 2020, 247, 116672. 10.1016/j.carbpol.2020.116672. [DOI] [PubMed] [Google Scholar]
- Jamwal S.; Ram B.; Ranote S.; Dharela R.; Chauhan G. S. New glucose oxidase-immobilized stimuli-responsive dextran nanoparticles for insulin delivery. Int. J. Biol. Macromol. 2019, 123, 968–978. 10.1016/j.ijbiomac.2018.11.147. [DOI] [PubMed] [Google Scholar]
- Gao G.; Jiang Y.-W.; Jia H.-R.; Wu F.-G. Near-infrared light-controllable on-demand antibiotics release using thermo-sensitive hydrogel-based drug reservoir for combating bacterial infection. Biomaterials 2019, 188, 83–95. 10.1016/j.biomaterials.2018.09.045. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Liang Y.; Guo B.; Yin Z.; Zhu D.; Han Y. Injectable dry cryogels with excellent blood-sucking expansion and blood clotting to cease hemorrhage for lethal deep-wounds, coagulopathy and tissue regeneration. Chemical Engineering Journal 2021, 403, 126329. 10.1016/j.cej.2020.126329. [DOI] [Google Scholar]
- Reddy B. P. K.; Mishra S. K.; Ravichandran G.; Chauhan D. S.; Srivastava R.; De A. Preclinical evaluation of multi stimuli responsive core-plasmonic nanoshell for photo-triggered tumor ablation: A disintegrable nanohybrid. Applied Materials Today 2020, 20, 100684. 10.1016/j.apmt.2020.100684. [DOI] [Google Scholar]
- Pourjavadi A.; Bagherifard M.; Doroudian M. Synthesis of micelles based on chitosan functionalized with gold nanorods as a light sensitive drug delivery vehicle. Int. J. Biol. Macromol. 2020, 149, 809–818. 10.1016/j.ijbiomac.2020.01.162. [DOI] [PubMed] [Google Scholar]
- Yan L. X.; Chen L. J.; Zhao X.; Yan X. P. pH switchable nanoplatform for in vivo persistent luminescence imaging and precise photothermal therapy of bacterial infection. Adv. Funct. Mater. 2020, 30 (14), 1909042. 10.1002/adfm.201909042. [DOI] [Google Scholar]
- Li P.; Zeng J.; Wang B.; Cheng Z.; Xu J.; Gao W.; Chen K. Waterborne fluorescent dual anti-counterfeiting ink based on Yb/Er-carbon quantum dots grafted with dialdehyde nano-fibrillated cellulose. Carbohydr. Polym. 2020, 247, 116721. 10.1016/j.carbpol.2020.116721. [DOI] [PubMed] [Google Scholar]
- Li Z.; Davidson-Rozenfeld G.; Vázquez-González M.; Fadeev M.; Zhang J.; Tian H.; Willner I. Multi-triggered supramolecular DNA/bipyridinium dithienylethene hydrogels driven by light, redox, and chemical stimuli for shape-memory and self-healing applications. J. Am. Chem. Soc. 2018, 140 (50), 17691–17701. 10.1021/jacs.8b10481. [DOI] [PubMed] [Google Scholar]
- Nezakati T.; Seifalian A.; Tan A.; Seifalian A. M. Conductive polymers: opportunities and challenges in biomedical applications. Chem. Rev. 2018, 118 (14), 6766–6843. 10.1021/acs.chemrev.6b00275. [DOI] [PubMed] [Google Scholar]
- Zarrintaj P.; Zangene E.; Manouchehri S.; Amirabad L. M.; Baheiraei N.; Hadjighasem M. R.; Farokhi M.; Ganjali M. R.; Walker B. W.; Saeb M. R.; et al. Conductive biomaterials as nerve conduits: Recent advances and future challenges. Applied Materials Today 2020, 20, 100784. 10.1016/j.apmt.2020.100784. [DOI] [Google Scholar]
- Lim C.; Shin Y.; Jung J.; Kim J. H.; Lee S.; Kim D.-H. Stretchable conductive nanocomposite based on alginate hydrogel and silver nanowires for wearable electronics. APL Materials 2019, 7 (3), 031502. 10.1063/1.5063657. [DOI] [Google Scholar]
- Bagher Z.; Atoufi Z.; Alizadeh R.; Farhadi M.; Zarrintaj P.; Moroni L.; Setayeshmehr M.; Komeili A.; Kamrava S. K. Conductive hydrogel based on chitosan-aniline pentamer/gelatin/agarose significantly promoted motor neuron-like cells differentiation of human olfactory ecto-mesenchymal stem cells. Materials Science and Engineering: C 2019, 101, 243–253. 10.1016/j.msec.2019.03.068. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Li P.; Guo B.; Ma P. X. Antibacterial and conductive injectable hydrogels based on quaternized chitosan-graft-polyaniline/oxidized dextran for tissue engineering. Acta biomaterialia 2015, 26, 236–248. 10.1016/j.actbio.2015.08.006. [DOI] [PubMed] [Google Scholar]
- Ferlauto L.; D’Angelo A. N.; Vagni P.; Leccardi A.; Ildelfonsa M. J.; Mor F. M.; Cuttaz E. A.; Heuschkel M. O.; Stoppini L.; Ghezzi D. Development and characterization of PEDOT: PSS/alginate soft microelectrodes for application in neuroprosthetics. Frontiers in neuroscience 2018, 12, 648. 10.3389/fnins.2018.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abedi A.; Hasanzadeh M.; Tayebi L. Conductive nanofibrous Chitosan/PEDOT: PSS tissue engineering scaffolds. Mater. Chem. Phys. 2019, 237, 121882. 10.1016/j.matchemphys.2019.121882. [DOI] [Google Scholar]
- Khodadadi Yazdi M.; Hashemi Motlagh G. Improved electrical and thermal aging properties of DBSA-doped PANI using MWCNT and GO. J. Electron. Mater. 2020, 49, 5326–5334. 10.1007/s11664-020-08256-x. [DOI] [Google Scholar]
- Khodadadi Yazdi M.; Noorbakhsh B.; Nazari B.; Ranjbar Z. Preparation and EMI shielding performance of epoxy/non-metallic conductive fillers nano-composites. Prog. Org. Coat. 2020, 145, 105674. 10.1016/j.porgcoat.2020.105674. [DOI] [Google Scholar]
- Qu J.; Liang Y.; Shi M.; Guo B.; Gao Y.; Yin Z. Biocompatible conductive hydrogels based on dextran and aniline trimer as electro-responsive drug delivery system for localized drug release. Int. J. Biol. Macromol. 2019, 140, 255–264. 10.1016/j.ijbiomac.2019.08.120. [DOI] [PubMed] [Google Scholar]
- Wu P.; Zhao Y.; Chen F.; Xiao A.; Du Q.; Dong Q.; Ke M.; Liang X.; Zhou Q.; Chen Y. Conductive hydroxyethyl cellulose/soy protein isolate/polyaniline conduits for enhancing peripheral nerve regeneration via electrical stimulation. Frontiers in bioengineering and biotechnology 2020, 8, 709. 10.3389/fbioe.2020.00709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatoni A.; Numnuam A.; Kanatharana P.; Limbut W.; Thavarungkul P. A conductive porous structured chitosan-grafted polyaniline cryogel for use as a sialic acid biosensor. Electrochim. Acta 2014, 130, 296–304. 10.1016/j.electacta.2014.03.036. [DOI] [Google Scholar]
- Xu Y.; Cui M.; Patsis P. A.; Günther M.; Yang X.; Eckert K.; Zhang Y. Reversibly Assembled Electroconductive Hydrogel via a Host-Guest Interaction for 3D Cell Culture. ACS Appl. Mater. Interfaces 2019, 11 (8), 7715–7724. 10.1021/acsami.8b19482. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Guo B.; Wu H.; Liang Y.; Ma P. X. Injectable antibacterial conductive nanocomposite cryogels with rapid shape recovery for noncompressible hemorrhage and wound healing. Nat. Commun. 2018, 9 (1), 1–17. 10.1038/s41467-018-04998-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X.; Wei X.; Chen Z.; Zhang X.; Yang G.; Zhou S. Multifunctional nanoplatforms for subcellular delivery of drugs in cancer therapy. Prog. Mater. Sci. 2020, 107, 100599. 10.1016/j.pmatsci.2019.100599. [DOI] [Google Scholar]
- Mi P.; Cabral H.; Kataoka K. Ligand-installed Nanocarriers toward precision therapy. Adv. Mater. 2020, 32 (13), 1902604. 10.1002/adma.202070101. [DOI] [PubMed] [Google Scholar]
- Danhier F.; Feron O.; Préat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. Journal of controlled release 2010, 148 (2), 135–146. 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- Niu S.; Williams G. R.; Wu J.; Wu J.; Zhang X.; Chen X.; Li S.; Jiao J.; Zhu L.-M. A chitosan-based cascade-responsive drug delivery system for triple-negative breast cancer therapy. J. Nanobiotechnol. 2019, 17, 1–18. 10.1186/s12951-019-0529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q.; Zhong S.; He S.; Gao Y.; Cui X. Constructing of pH and reduction dual-responsive folic acid-modified hyaluronic acid-based microcapsules for dual-targeted drug delivery via sonochemical method. Colloid and Interface Science Communications 2021, 44, 100503. 10.1016/j.colcom.2021.100503. [DOI] [Google Scholar]
- Wei X.; Liao J.; Davoudi Z.; Zheng H.; Chen J.; Li D.; Xiong X.; Yin Y.; Yu X.; Xiong J.; et al. Folate receptor-targeted and GSH-responsive carboxymethyl chitosan nanoparticles containing covalently entrapped 6-mercaptopurine for enhanced intracellular drug delivery in leukemia. Marine drugs 2018, 16 (11), 439. 10.3390/md16110439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G.; Huang H. Hyaluronic acid-based biopharmaceutical delivery and tumor-targeted drug delivery system. Journal of controlled release 2018, 278, 122–126. 10.1016/j.jconrel.2018.04.015. [DOI] [PubMed] [Google Scholar]
- Saxena S.; Kandasubramanian B. Glycopolymers in molecular recognition, biomimicking and glycotechnology: a review. International Journal of Polymeric Materials and Polymeric Biomaterials 2022, 71 (10), 756–776. 10.1080/00914037.2021.1900181. [DOI] [Google Scholar]
- Liu R.; Zuo R.; Hudalla G. A. Harnessing molecular recognition for localized drug delivery. Adv. Drug Delivery Rev. 2021, 170, 238–260. 10.1016/j.addr.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J. R.; Peppas N. A. Molecular recognition with soft biomaterials. Soft Matter 2020, 16 (4), 856–869. 10.1039/C9SM01981B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner L. M.; Surana R.; Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nature Reviews Immunology 2010, 10 (5), 317–327. 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A. M.; Allison J. P.; Wolchok J. D. Monoclonal antibodies in cancer therapy. Cancer immunity 2012, 12 (1), 14. [PMC free article] [PubMed] [Google Scholar]
- Liu R.; Poma A. Advances in molecularly imprinted polymers as drug delivery systems. Molecules 2021, 26 (12), 3589. 10.3390/molecules26123589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S.; Wang L.; Liu Z. Molecularly imprinted polymer nanoparticles: an emerging versatile platform for cancer therapy. Angew. Chem., Int. Ed. 2021, 60 (8), 3858–3869. 10.1002/anie.202005309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S.; Yee N. A.; Wu K.; Zakharian M.; Mahmoodi A.; Royzen M.; Mejía Oneto J. M. SQ3370 Activates Cytotoxic Drug via Click Chemistry at Tumor and Elicits Sustained Responses in Injected and Non-Injected Lesions. Advanced therapeutics 2021, 4 (3), 2000243. 10.1002/adtp.202000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.; Koo H.; Na J. H.; Han S. J.; Min H. S.; Lee S. J.; Kim S. H.; Yun S. H.; Jeong S. Y.; Kwon I. C.; et al. Chemical tumor-targeting of nanoparticles based on metabolic glycoengineering and click chemistry. ACS Nano 2014, 8 (3), 2048–2063. 10.1021/nn406584y. [DOI] [PubMed] [Google Scholar]
- Alibolandi M.; Mohammadi M.; Taghdisi S. M.; Ramezani M.; Abnous K. Fabrication of aptamer decorated dextran coated nano-graphene oxide for targeted drug delivery. Carbohydr. Polym. 2017, 155, 218–229. 10.1016/j.carbpol.2016.08.046. [DOI] [PubMed] [Google Scholar]
- Kumar B.; Kulanthaivel S.; Mondal A.; Mishra S.; Banerjee B.; Bhaumik A.; Banerjee I.; Giri S. Mesoporous silica nanoparticle based enzyme responsive system for colon specific drug delivery through guar gum capping. Colloids Surf., B 2017, 150, 352–361. 10.1016/j.colsurfb.2016.10.049. [DOI] [PubMed] [Google Scholar]
- Chiang C.-S.; Lin Y.-J.; Lee R.; Lai Y.-H.; Cheng H.-W.; Hsieh C.-H.; Shyu W.-C.; Chen S.-Y. Combination of fucoidan-based magnetic nanoparticles and immunomodulators enhances tumour-localized immunotherapy. Nature Nanotechnol. 2018, 13 (8), 746–754. 10.1038/s41565-018-0146-7. [DOI] [PubMed] [Google Scholar]
- Hua X.-W.; Bao Y.-W.; Chen Z.; Wu F.-G. Carbon quantum dots with intrinsic mitochondrial targeting ability for mitochondria-based theranostics. Nanoscale 2017, 9 (30), 10948–10960. 10.1039/C7NR03658B. [DOI] [PubMed] [Google Scholar]
- Wang T.; Hou J.; Su C.; Zhao L.; Shi Y. Hyaluronic acid-coated chitosan nanoparticles induce ROS-mediated tumor cell apoptosis and enhance antitumor efficiency by targeted drug delivery via CD44. J. Nanobiotechnol. 2017, 15 (1), 7. 10.1186/s12951-016-0245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anandhakumar S.; Krishnamoorthy G.; Ramkumar K.; Raichur A. Preparation of collagen peptide functionalized chitosan nanoparticles by ionic gelation method: An effective carrier system for encapsulation and release of doxorubicin for cancer drug delivery. Materials Science and Engineering: C 2017, 70, 378–385. 10.1016/j.msec.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Sauraj; Kumar S. U.; Kumar V.; Priyadarshi R.; Gopinath P.; Negi Y. S. pH-responsive prodrug nanoparticles based on xylan-curcumin conjugate for the efficient delivery of curcumin in cancer therapy. Carbohydr. Polym. 2018, 188, 252–259. 10.1016/j.carbpol.2018.02.006. [DOI] [PubMed] [Google Scholar]
- Wei X.; Liu L.; Guo X.; Wang Y.; Zhao J.; Zhou S. Light-activated ROS-responsive nanoplatform codelivering apatinib and doxorubicin for enhanced chemo-photodynamic therapy of multidrug-resistant tumors. ACS Appl. Mater. Interfaces 2018, 10 (21), 17672–17684. 10.1021/acsami.8b04163. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Shi G.; Zhang J.; Song H.; Niu J.; Shi S.; Huang P.; Wang Y.; Wang W.; Li C.; et al. Targeted antigen delivery to dendritic cell via functionalized alginate nanoparticles for cancer immunotherapy. J. Controlled Release 2017, 256, 170–181. 10.1016/j.jconrel.2017.04.020. [DOI] [PubMed] [Google Scholar]
- Ruttala H. B.; Ramasamy T.; Gupta B.; Choi H.-G.; Yong C. S.; Kim J. O. Multiple polysaccharide-drug complex-loaded liposomes: A unique strategy in drug loading and cancer targeting. Carbohydr. Polym. 2017, 173, 57–66. 10.1016/j.carbpol.2017.05.062. [DOI] [PubMed] [Google Scholar]
- Fathi M.; Zangabad P. S.; Aghanejad A.; Barar J.; Erfan-Niya H.; Omidi Y. Folate-conjugated thermosensitive O-maleoyl modified chitosan micellar nanoparticles for targeted delivery of erlotinib. Carbohydr. Polym. 2017, 172, 130–141. 10.1016/j.carbpol.2017.05.007. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Li L.; Li C.; Zheng H.; Song H.; Xiong F.; Qiu T.; Yang J. Cisplatin-crosslinked glutathione-sensitive micelles loaded with doxorubicin for combination and targeted therapy of tumors. Carbohydr. Polym. 2017, 155, 407–415. 10.1016/j.carbpol.2016.08.072. [DOI] [PubMed] [Google Scholar]
- Liu H.; Wu S.; Yu J.; Fan D.; Ren J.; Zhang L.; Zhao J. Reduction-sensitive micelles self-assembled from amphiphilic chondroitin sulfate A-deoxycholic acid conjugate for triggered release of doxorubicin. Materials Science and Engineering: C 2017, 75, 55–63. 10.1016/j.msec.2017.02.030. [DOI] [PubMed] [Google Scholar]
- Kang S. H.; Revuri V.; Lee S.-J.; Cho S.; Park I.-K.; Cho K. J.; Bae W. K.; Lee Y.-k. Oral siRNA delivery to treat colorectal liver metastases. ACS Nano 2017, 11 (10), 10417–10429. 10.1021/acsnano.7b05547. [DOI] [PubMed] [Google Scholar]
- Lutz J.-F.; Lehn J.-M.; Meijer E.; Matyjaszewski K. From precision polymers to complex materials and systems. Nature Reviews Materials 2016, 1 (5), 1–14. 10.1038/natrevmats.2016.24. [DOI] [Google Scholar]
- Webber M. J.; Tibbitt M. W. Dynamic and reconfigurable materials from reversible network interactions. Nature Reviews Materials 2022, 7 (7), 541–556. 10.1038/s41578-021-00412-x. [DOI] [Google Scholar]
- do Nascimento Marques N.; dos Santos Alves K.; Vidal R. R. L.; da Silva Maia A. M.; Madruga L. Y. C.; Curti P. S.; de Carvalho Balaban R.. Chemical Modification of Polysaccharides and Applications in Strategic Areas. In Emerging Research in Science and Engineering Based on Advanced Experimental and Computational Strategies; Springer, 2020; pp 433–472. [Google Scholar]
- Xie L.; Shen M.; Hong Y.; Ye H.; Huang L.; Xie J. Chemical modifications of polysaccharides and their anti-tumor activities. Carbohydr. Polym. 2020, 229, 115436. 10.1016/j.carbpol.2019.115436. [DOI] [PubMed] [Google Scholar]
- Fadeel B.Hide and seek: nanomaterial interactions with the immune system. Frontiers in immunology 2019, 10. 10.3389/fimmu.2019.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Chan H. F.; Leong K. W. Advanced materials and processing for drug delivery: the past and the future. Advanced drug delivery reviews 2013, 65 (1), 104–120. 10.1016/j.addr.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder J.; Taratula O.; Minko T. Nanocarrier-based systems for targeted and site specific therapeutic delivery. Advanced drug delivery reviews 2019, 144, 57–77. 10.1016/j.addr.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin N. D.; Sahinidis N. V.; Trahan D. W. Computer-aided molecular design: An introduction and review of tools, applications, and solution techniques. Chem. Eng. Res. Des. 2016, 116, 2–26. 10.1016/j.cherd.2016.10.014. [DOI] [Google Scholar]
- Saeb M. R.; Nonahal M.; Rastin H.; Shabanian M.; Ghaffari M.; Bahlakeh G.; Ghiyasi S.; Khonakdar H. A.; Goodarzi V.; Puglia D.; et al. Calorimetric analysis and molecular dynamics simulation of cure kinetics of epoxy/chitosan-modified Fe3O4 nanocomposites. Prog. Org. Coat. 2017, 112, 176–186. 10.1016/j.porgcoat.2017.07.015. [DOI] [Google Scholar]
- Zhao H.; Caflisch A. Molecular dynamics in drug design. European journal of medicinal chemistry 2015, 91, 4–14. 10.1016/j.ejmech.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Gartner III T. E.; Jayaraman A. Modeling and simulations of polymers: A Roadmap. Macromolecules 2019, 52 (3), 755–786. 10.1021/acs.macromol.8b01836. [DOI] [Google Scholar]
- Colombani T.; Rogers Z. J.; Bhatt K.; Sinoimeri J.; Gerbereux L.; Hamrangsekachaee M.; Bencherif S. A. Hypoxia-inducing cryogels uncover key cancer-immune cell interactions in an oxygen-deficient tumor microenvironment. Bioactive Materials 2023, 29, 279–295. 10.1016/j.bioactmat.2023.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt K.; Nukovic A.; Colombani T.; Bencherif S. A. Biomaterial-assisted local oxygenation safeguards the prostimulatory phenotype and functions of human dendritic cells in hypoxia. Frontiers in Immunology 2023, 14, 1278397. 10.3389/fimmu.2023.1278397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.; Kondo Y.; Kakimoto M. A.; Yang B.; Yamada H.; Kuwajima I.; Lambard G.; Hongo K.; Xu Y.; Shiomi J.; et al. Machine-learning-assisted discovery of polymers with high thermal conductivity using a molecular design algorithm. npj Computational Materials 2019, 5 (1), 1–11. 10.1038/s41524-019-0203-2. [DOI] [Google Scholar]
- Rana D.; Colombani T.; Saleh B.; Mohammed H. S.; Annabi N.; Bencherif S. A. Engineering injectable, biocompatible, and highly elastic bioadhesive cryogels. Materials Today Bio 2023, 19, 100572. 10.1016/j.mtbio.2023.100572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombani T.; Eggermont L. J.; Hatfield S. M.; Rogers Z. J.; Rezaeeyazdi M.; Memic A.; Sitkovsky M. V.; Bencherif S. A. Oxygen-generating cryogels restore T-cell mediated cytotoxicity in hypoxic tumors. Adv. Funct. Mater. 2021, 31, 2102234. 10.1002/adfm.202102234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaeeyazdi M.; Colombani T.; Eggermont L. J.; Bencherif S. A. Engineering hyaluronic acid-based cryogels for CD44-mediated breast tumor reconstruction. Materials Today Bio 2022, 13, 100207. 10.1016/j.mtbio.2022.100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Zhang K.; Lee Y. Machine learning enabled tailor-made design of application-specific metal-organic frameworks. ACS Appl. Mater. Interfaces 2020, 12 (1), 734–743. 10.1021/acsami.9b17867. [DOI] [PubMed] [Google Scholar]
- Rogers Z. J.; Zeevi M. P.; Koppes R.; Bencherif S. A. Electroconductive hydrogels for tissue engineering: current status and future perspectives. Bioelectricity 2020, 2 (3), 279–292. 10.1089/bioe.2020.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencherif S. A.; Sheehan J. A.; Hollinger J. O.; Walker L. M.; Matyjaszewski K.; Washburn N. R. Influence of cross-linker chemistry on release kinetics of PEG-co-PGA hydrogels. J. Biomed. Mater. Res., Part A 2009, 90 (1), 142–153. 10.1002/jbm.a.32069. [DOI] [PubMed] [Google Scholar]
- Kennedy S.; Bencherif S.; Norton D.; Weinstock L.; Mehta M.; Mooney D. Rapid and extensive collapse from electrically responsive macroporous hydrogels. Adv. Healthcare Mater. 2014, 3 (4), 500–573. 10.1002/adhm.201300260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J. A.; Bencherif S. A.; Aksak B.; Kim E. K.; Kowalewski T.; Oh J. K.; Matyjaszewski K. Thermoresponsive hydrogel scaffolds with tailored hydrophilic pores. Chemistry An Asian Journal 2011, 6 (1), 128–136. 10.1002/asia.201000514. [DOI] [PubMed] [Google Scholar]
- Joshi Navare K.; Colombani T.; Rezaeeyazdi M.; Bassous N.; Rana D.; Webster T.; Memic A.; Bencherif S. A. Needle-injectable microcomposite cryogel scaffolds with antimicrobial properties. Sci. Rep. 2020, 10, 18370. 10.1038/s41598-020-75196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulais L.; Jellali R.; Pereira U.; Leclerc E.; Bencherif S. A.; Legallais C. Cryogel-integrated biochip for liver tissue engineering. ACS Applied Bio Materials 2021, 4 (7), 5617–5626. 10.1021/acsabm.1c00425. [DOI] [PubMed] [Google Scholar]
- Colombani T.; Eggermont L.; Rogers Z. J.; McKay L.; Avena L.; Johnson R.; Storm N.; Griffiths A.; Bencherif S. A. Biomaterials and oxygen join forces to shape the immune response and boost COVID-19 vaccines. Advanced Science 2021, 8 (18), 2170111. 10.1002/advs.202100316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gsib O.; Eggermont L.; Egles C.; Bencherif S. A. Engineering a macroporous fibrin-based sequential interpenetrating polymer network for dermal tissue engineering. Biomaterials Sciencee 2020, 8, 7106–7116. 10.1039/D0BM01161D. [DOI] [PubMed] [Google Scholar]
- Wisniewska P.; Saeb M. R.; Bencherif S. A. Biomaterials recycling: a promising pathway to sustainability. Frontiers in Biomaterials Science 2023, 2, 1260402. 10.3389/fbiom.2023.1260402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers Z. J.; Bencherif S. A. Cryogelation and cryogels. Gels 2019, 5 (4), 46. 10.3390/gels5040046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdavi S.; Amirsadeghi A.; Jafari A.; Niknezhad S. V.; Bencherif S. A. Avian egg: a multifaceted biomaterial for tissue engineering. Ind. Eng. Chem. Res. 2021, 60 (48), 17348–17364. 10.1021/acs.iecr.1c03085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Lengeling B.; Aspuru-Guzik A. Inverse molecular design using machine learning: Generative models for matter engineering. Science 2018, 361 (6400), 360–365. 10.1126/science.aat2663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.