Abstract
Comorbidities in patients with multiple sclerosis (MS) and antibody-mediated diseases of the central nervous system (CNS) including neuromyelitis optica spectrum disorder (NMOSD), and myelin oligodendrocyte glycoprotein (MOG)-antibody-associated disease (MOGAD) are common and may influence the course of their neurological disease. Comorbidity may contribute to neuronal injury and therefore limit recovery from attacks, accelerate disease progression, and increase disability. This study aims to explore the impact of comorbidity, particularly vascular comorbidity, and related risk factors on clinical and paraclinical parameters of MS, NMOSD and MOGAD. We propose COMMIT, a prospective multicenter study with longitudinal follow-up of patients with MS, NMOSD, and MOGAD, with or without comorbidities, as well as healthy subjects as controls. Subjects will be stratified by age, sex and ethnicity. In consecutive samples we will analyze levels of inflammation and neurodegeneration markers in both fluid and cellular compartments of the peripheral blood and cerebrospinal fluid (CSF) using multiple state-of-the-art technologies, including untargeted proteomics and targeted ultrasensitive ELISA assays and quantitative reverse transcription polymerase chain reaction (RT-qPCR) as well as high-dimensional single-cell technologies i.e., mass cytometry and single-cell RNA sequencing. Algorithm-based data analyses will be used to unravel the relationship between these markers, optical coherence tomography (OCT) and magnetic resonance imaging (MRI), and clinical outcomes including frequency and severity of relapses, long-term disability, and quality of life. The goal is to evaluate the impact of comorbidities on MS, NMOSD, and MOGAD which may lead to development of treatment approaches to improve outcomes of inflammatory demyelinating diseases of the CNS.
Keywords: multiple sclerosis, neuromyelitis optica spectrum disorder, myelin oligodendrocyte glycoprotein antibody-associated disease, antibody-mediated diseases of the central nervous system, comorbidity
1. Introduction
Inflammatory demyelinating diseases (IDDs) of the central nervous system (CNS) are immune-mediated disorders that lead to the destruction of myelin, either primarily [multiple sclerosis (MS) and myelin oligodendrocyte glycoprotein-antibody-associated disease (MOGAD)] or secondarily [neuromyelitis optica spectrum disorder (NMOSD)]. There is a variable degree of axonal injury and loss, which is the ultimate substrate of disability (1, 2). The development and pathogenesis of IDDs are complicated immune mechanisms, presumably involving both innate and adaptive immunity, reflecting defects in autoimmune tolerance mechanisms. Likewise, the immunobiology of injury and repair is multifactorial and influenced by genetic and environmental factors. Remyelination may protect against axonal degeneration by oligodendrocyte precursor cell (OPC) proliferation and maturation to oligodendrocytes (OLs) in the case of actual OL loss to a variable degree (3). OLs have high metabolic requirements to maintain myelin synthesis and metabolic support of axons (3). The most well-defined IDDs are multiple sclerosis (MS), widely believed to be T-cell-mediated, and NMOSD and MOGAD, both of which are believed to be antibody-mediated.
Clusters of chronic conditions and comorbidities may occur in IDDs and are an area of current interest (4–12). Comorbidity refers to the burden caused by the presence and severity of any distinct additional entity other than the specific index disease and may result from similar predisposing genetic or environmental factors (13) or from treatment of IDDs (14, 15). For instance, corticosteroids can precipitate the onset of diabetes and hyperglycemia, while other certain immunosuppressive drugs are linked to an increased risk of cardiovascular disease (4). The co-occurrence of two or more chronic conditions, i.e., so-called multimorbidity, may be present even at the time of diagnosis (4).
In IDDs, comorbidity may contribute to remyelination failure and neuronal injury and adversely affect a broad range of outcomes, including the risk of relapse, diminished quality of life (QoL), and long-term disability.
Multimorbidity is a growing concern, with an increase in the prevalence of chronic somatic conditions as well as mental disorders, in part reflecting an aging population (10–12, 16, 17). Many factors such as lifestyle and socioeconomic status may lead to the onset of multimorbidity at a younger age (13). Emerging evidence underscores a high prevalence of comorbidity, i.e., vascular comorbidities and related risk factors in patients with multiple sclerosis (18), exemplified by type 2 diabetes mellitus (T2DM), which is a highly prevalent disorder worldwide. Patients with T2DM are at high risk for microvascular complications, owing to hyperglycemia and insulin resistance (metabolic syndrome) (19). Oral antidiabetic drugs such as metformin and pioglitazone may beneficially impact MS disease progression by reducing inflammatory mediators such as interferon-γ, interleukin (IL)-6, and TNF-alpha (20). T2DM may influence brain function and may cause brain insulin resistance, neuroinflammation including microglia activation, blood–brain barrier (BBB) impairment, and mitochondrial dysfunction, resulting in neuronal metabolic defects and neuronal cell death (21). The most common comorbidities in patients with NMOSD are hypertension and cardiovascular disease (4, 22), although uniquely in NMOSD, other autoimmune conditions, such as systemic lupus erythematosus, rheumatoid arthritis, Sjögren’s syndrome, and autoimmune encephalitis, may occur as concurrent comorbid diseases because of defective B-cell development checkpoints (5, 6). MOGAD has only recently been recognized and may be regarded as a relatively rare disorder (23), so the pattern of comorbidity has not yet been elucidated; however, anti-N-methyl-D-aspartate receptor encephalitis (anti-NMDAR encephalitis) and autoimmune thyroid disorders have been reported to occur (7).
Investigations of the impact of comorbidities on clinical outcomes of IDD have only been conducted to a limited extent. Comorbid conditions can be associated with a prolonged interval between the disease onset and its subsequent diagnosis. An increase in the frequency of relapses has been observed in NMOSD patients with comorbidities compared to NMOSD patients without comorbidities, with severe motor episodes and severe optic neuritis (ON) (15). Multifocal CNS lesions as a presenting symptom are more prevalent in patients with comorbidities compared to those without. Moreover, comorbidity can be indicative of reduced treatment responsiveness, heightened disability, and an elevated mortality rate (15, 24).
However, the current knowledge is mainly based on retrospective and cross-sectional studies with a small number of patients. A prospective and longitudinal study with an evaluation of the interaction between clinical and paraclinical parameters of comorbidity of subjects with MS, NMOSD, and MOGAD will be a timely effort.
1.1. Advancing data collection and protocols for comorbidity analysis and management
The high occurrence of comorbidities suggests screening for concomitant medical conditions as a routine part of patient care, as comorbidities may influence the clinical features, prognosis, and treatment outcomes. So far unexplored, the comorbidity and certain clusters of chronic conditions may have a negative impact on the levels of proinflammatory and neurodegenerative mediators, and they may negatively influence disease progression. Such knowledge will be important for optimizing treatment strategies and thereby hopefully improve QoL. Comorbidities may vary depending on age, sex, ethnicity, geographic region, socioeconomic composition, and healthcare system, highlighting the importance of a multicenter design. Furthermore, analytical pipelines for the determination of molecular and cellular markers as well as downstream algorithm-based (integrative) data analysis have been established and are in the process of validation. This multicentric study will analyze data from different sets of patients without or with one or more comorbidities, with follow-up visits. We aim to determine the levels of inflammatory and neurodegenerative mediators in patients with MS, NMOSD, and MOGAD, both with and without comorbidities, and evaluate whether changes may affect disease outcomes. Furthermore, we aim to assess whether comorbidity influences optical coherence tomography (OCT) and magnetic resonance imaging (MRI) parameters and its association with clinical outcomes.
2. Methods and analysis
2.1. Study design
The study comprises a prospective, multicenter investigation of patients with MS, AQP4-IgG-positive NMOSD, and MOGAD, with or without comorbidities with 3 years of follow-up in collaboration with multiple centers from Denmark (2), Germany (2), the USA (4), UK (1), Iran (2), Italy (1), Israel (1), and South Korea (1). All patients will be offered follow-up after 6, 12, 18, 24, and 36 months after baseline. The baseline is defined as the time of entry into the study, i.e., any time point during the disease course.
The COMMIT study will adhere to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines for reporting (25). Approval will be sought from local ethical institutional review boards in all centers, and informed consent will be obtained from the patients. The study will be conducted in accordance with the provisions of the Declaration of Helsinki and the guidelines for good clinical practice set forth by the International Conference on Harmonization (26).
2.1.1. Study population and diagnosis
We will recruit MS (27), NMOSD (seropositive for AQP4-IgG) (28), and MOGAD (23) patients, age-matched with or without comorbidities. Participants will undergo an assessment to determine the presence of comorbidity using a predefined list tailored for each organ system. All comorbidities will be listed, including common comorbidities such as cardiovascular diseases and their associated risk factors, as well as autoimmune disorders. For comorbidities with predefined quantitative criteria, screening will be conducted to confirm that patients have advanced beyond the pre-diagnostic stage (e.g., pre-diabetes) to actual diagnosis (e.g., T2DM). In addition to identifying diseases (e.g., ischemic heart disease), we will screen for risk factors such as obesity, hypertension, and hyperlipidemia (29). We will recruit a healthy control group from each region without known chronic conditions or comorbidities matched for age and sex.
2.1.2. Inclusion and exclusion criteria
Eligible participants must possess competence for written informed consent, be ≥18 years of age, and have a diagnosis of MS, NMOSD, or MOGAD with an Expanded Disability Status Scale (EDSS) assessment score from 0 to 6.5 inclusive at the time of recruitment (30, 31).
Patients will be excluded if they are unable to cooperate, have active drug or alcohol abuse, are pregnant, or have an active systemic infection, ON within the preceding 3 months, and steroid treatment in the past 1 month. Dropout criteria will be evaluated during follow-up visits ( Table 1 ).
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria | Dropout criteria |
|---|---|---|
| – Competent to give written informed consent – Signed consent—age ≥18 years – Diagnosis of MS (27), NMOSD (28), and MOGAD (23) – Expanded Disability Status Scale (EDSS) assessment score ranging from 0 to 6.5 (inclusive) at baseline |
– History of drug or alcohol abuse – Evidence of active systemic infection – Optic neuritis within 3 months prior to the baseline assessment – Any steroid treatment within the preceding month – Pregnancy – Inability to cooperate |
– Patient’s withdrawal of consent – Lost to follow-up – Non−compliance with protocol (decision by study board) – Condition hindering study continuation (decision by the study board) |
Exclusion criteria are only considered at baseline, while dropout criteria are checked at each follow−up visit.
2.1.3. Medical history and clinical examination
The patients will undergo a clinical examination at baseline visit and on follow-up after 6, 12, 24, and 36 months. Blood sampling, cerebrospinal fluid (CSF) testing, MRI, and OCT will be performed at baseline and annually during the follow-up period. The process is outlined in Table 2 . Clinical information including age, sex, ethnicity, height, weight, and lifestyle factors such as tobacco and alcohol use, as well as drug abuse history, is recorded. After a combination of interviews and review of past medical records, we will determine disease onset; assess current symptoms; and record histories of relapses or attacks, comorbidities, and comprehensive treatment details, encompassing both pharmacological and non-pharmacological therapies. The neurological evaluation employs the EDSS in accordance with neurostatus definitions (30, 31). The visual system score is determined through best-corrected high-contrast visual acuity testing (15) ( Table 1 ).
Table 2.
Timeline of key assessments and measurements throughout the study period.
| Assessment | Detail | Baseline | 6 months | 12 months | 24 months | 36 months |
|---|---|---|---|---|---|---|
| Demographics: | ✓ | |||||
| Age, sex, weight | ✓ | |||||
| Body mass index (BMI), waist circumference | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Height | ✓ | |||||
| Race/ethnicity | Caucasian/White; Black/African descent; Hispanic/Latino; Asian (including East, South, Southeast, and Central Asian); indigenous/native peoples; Middle Eastern/Arab; mixed/multiple races; other | ✓ | ||||
| Education level | No education; low education (primary/elementary); mid-level education (secondary/high school); vocational/technical training; higher education (undergraduate); advanced education (postgraduate) | ✓ | ||||
| Geographic location | The classification of geographic location is based on the center that prepared the data. | ✓ | ||||
| Medical history: | Diagnosis; disease onset; attack/relapse history [number of relapse(s)**]; current symptoms and complaints; vaccination history; previous infections; fertility history | ✓ | ✓ | ✓ | ✓ | ✓ |
| Comorbid conditions: | The list of comorbid conditions, along with the corresponding questionnaires and clinical examinations, can be found in Table 3 . This also includes the date of diagnosis, the severity (when available), the number of comorbid conditions grouped by main categories, and the actual raw numbers. | ✓ | ✓ | ✓ | ✓ | ✓ |
| Risk factors: | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Smoking | Status: never smoked; former smoker; current smoker Duration: number of years smoking Intensity: average number of cigarettes/day (e.g., 1 pack/day = 20 cigarettes) |
✓ | ✓ | ✓ | ✓ | ✓ |
| Alcohol consumption | Frequency: daily; weekly; monthly; rarely; never Quantity: average number of drinks/sessions |
✓ | ✓ | ✓ | ✓ | ✓ |
| Family medical history Dietary and lifestyle factors |
Specific illnesses in first-degree relatives (e.g., cardiovascular disease and diabetes) Level of physical activity |
✓ | ✓ | ✓ | ✓ | |
| Previous injuries/surgeries | Type of injury/surgery (e.g., fractures, surgeries, and traumatic events) | ✓ | ✓ | ✓ | ✓ | |
| Therapies: | Treatment status; relapse therapy; full drug list | ✓ | ✓ | ✓ | ✓ | ✓ |
| Neurological examinations | EDSS*** | ✓ | ✓ | ✓ | ✓ | ✓ |
| Quantitative assessment of motor functions | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Hand grip force | Handheld dynamometer, 3× each side | |||||
| Timed tests | T25−FW, 9−HPT | |||||
| Visuo−perceptive motion analysis PASS−MS |
Short walks, static balance, stand−up and sit, stepping in place, finger tapping, finger−nose test | |||||
| Patient-reported outcomes (PRO) | Neuro-QOL Fatigue Scale, European Quality of Life–5 Dimensions (EQ-5D) index, EQ-5D visual analog scale (VAS) | ✓ | ✓ | ✓ | ✓ | ✓ |
| Cognitive assessment | ✓ | ✓ | ✓ | |||
| Interview | Handedness, education | ✓ | ✓ | ✓ | ||
| Cognitive function: includes the Montreal Cognitive Assessment (MoCA) | Various cognitive domains such as memory, attention, language, abstraction, delayed recall, orientation, and visuospatial skills | ✓ | ✓ | ✓ | ||
| Biospecimens* | Blood samples, cerebrospinal fluid | ✓ | ✓ | ✓ | ✓ | |
| Vision and the visual system* | Refraction, high− and low−contrast visual acuity, perimetry, VEP, OCT of the macula and optic nerve head, OCT/A (optional: multifocal VEP), NEI-VFQ-25 | ✓ | ✓ | ✓ | ||
| CNS magnetic resonance imaging* | Cerebral: MPRAGE, T2−SPACE, FLAIR, MPM, DWI, rsfMRI Spinal: STIR (whole spine), PSIR (C2 and C3 and C7/T1) |
✓ | ✓ | ✓ |
*Further details can be found in the Method section.
**Number of relapse(s) (a new or worsening acute neurologic symptom lasting ≥24 h and not explained by fever or infection).
***Disability assessment with alteration in EDSS score of at least 0.5 from baseline at the clinical visits.
– All are relevant for healthy controls and are only assessed at the baseline visit.
2.1.4. Comorbid conditions
At the start of the study, comorbidity data, including the date of diagnosis, the characteristics, and the associated treatments of the identified comorbidities, will be abstracted from the ‘patients’ medical records ( Table 4 ). In cases where medical records are either ambiguous or lack specific details, patients will be interviewed to obtain information on their distinct comorbid conditions. Each comorbidity will be categorized into main categories utilizing global standard codes for primary and secondary diagnoses using the International Statistical Classification of Diseases and Related Health Problem (ICD) system (version 10 or 11) ( Table 3 ). For enhanced analytical clarity, patients will be grouped based on whether they have a single comorbid condition or multiple conditions. Special emphasis will be placed on cardiovascular comorbidities for subanalysis. Regular medical assessments at predetermined intervals will enable detection through ICD diagnosis, documentation, and treatment of any new comorbidities.
Table 4.
Comorbidity: key terminology and definitions.
| Comorbidity (coincidence and concurrent diseases or comorbid conditions) | This general term refers to the presence of one or more additional diseases or disorders occurring concurrently with a primary disease or disorder. Based on Table 3 categories. |
| Multimorbidity (polypathology or polymorbidity) | This is the coexistence of two or more chronic diseases or conditions in a patient. The term is often used interchangeably with comorbidity, but while comorbidity refers to the effect of all other diseases an individual patient might have other than the primary disease, multimorbidity refers to the presence of two or more chronic diseases without establishing a primary disease. |
| Health determinants and indicators | These broader terms encompass a variety of conditions and factors that affect health. For example, in the context of vascular health, this could include both comorbid conditions and risk factors related to vascular diseases. |
| Comorbid conditions (concurrent disorders and co-existing diseases) | These refer to actual health conditions or diseases an individual has in addition to their primary disease. For instance, in the realm of vascular health, examples could include conditions like heart disease, stroke, and hypertension. These comorbid conditions co-occur with the primary disease and can interact with it in complex ways, potentially impacting treatment and outcomes. |
| Risk factors (predictors) | These are characteristics or behaviors that increase the likelihood of developing certain diseases. For example, in the case of vascular health, risk factors might include behaviors like smoking, lifestyle factors like lack of exercise or obesity, and genetic predispositions such as family history of vascular disease. |
Table 3.
Overview of common comorbidities associated with MS, NMOSD, and MOGAD.
| Comorbidity categories | Detailed conditions | Severity metrics |
|---|---|---|
| Cardiovascular disease | Hypertension, coronary artery disease, heart failure, cardiac arrhythmia, cardiac valvular disease, peripheral vascular disease, smoking, alcohol consumption | ASCVD Risk Calculator: a tool assessing the 10-year and lifetime risks of atherosclerotic cardiovascular disease based on factors like age, cholesterol levels, and blood pressure. Current/former/never smoker |
| Endocrine, nutritional, and metabolic diseases | Diabetes mellitus type II, thyroid disorders, malnutrition, metabolic disorders, obesity, hyperlipidemia | TSH, BMI Fasting plasma glucose and insulin will be measured to derive the Homeostasis Model Assessment (HOMA) score, indicating glucose tolerance and insulin resistance. For non-diabetic patients, an oral glucose tolerance test (OGTT) will be conducted with samples taken at 0, 30, and 120 min. These measurements will help calculate indices for insulin sensitivity, secretion, and gauge glucose tolerance, including potential impaired glucose tolerance. Additionally, HbA1c will be assessed. Lipid profile (LDL, HDL, TG, cholesterol) |
| Mental, behavioral, and neurodevelopmental disorders | Insomnia, hypersomnia, sleepwalking [somnambulism], sleep terrors [night terrors], nightmare disorder, circadian rhythm sleep disorders, sleep apnea, narcolepsy and cataplexy, parasomnia, sleep-related movement disorders (RLS), depression, anxiety, bipolar disorder, personality disorders, psychosis, drug abuse, alcohol abuse, fatigue, pain | Beck’s Depression Inventory-II (depression severity), Hamilton Anxiety Rating Scale (anxiety severity), Fatigue Severity Scale (chronic fatigue assessment), FSMC - Fatigue Scale for Motor and Cognitive Functions, Short Form of Brief Pain Inventory (pain evaluation), Pittsburgh Sleep Quality Index and Epworth Sleepiness Scale (sleep quality and propensity), STOP-Bang (sleep apnea risk), Restless Legs Syndrome Rating Scale (RLS severity) |
| Diseases of the nervous system | Extrapyramidal and movement disorders, Alzheimer’s disease, epilepsy and recurrent seizures, migraine, other headache syndromes, cerebrovascular disease, CNS structural disorders, traumatic brain injury | Hoehn and Yahr staging (Parkinson’s disease progression), seizure frequency (number of epileptic events), Global Assessment of Migraine Severity and frequency of headache per month (migraine and headache assessment) |
| Diseases of the eye and adnexa | Cataract, glaucoma, visual problems | Visual Functioning Questionnaire-25 and Vision Performance Scale assess visual impairment and its impact on daily activities |
| Diseases of the respiratory system | Bronchitis, chronic obstructive pulmonary disease, asthma, bronchiectasis, interstitial pulmonary diseases, allergic disease, vascular pulmonary diseases, pleural cavity disease, inflammatory pulmonary disease, non-specific pulmonary diseases | Evaluates lung function, with the GOLD criteria categorizing chronic obstructive pulmonary disease (COPD) severity |
| Diseases of the digestive system | Irritable bowel syndrome, liver disease, peptic ulcer disease, inflammatory bowel disease, GERD, biliary tract disorders, hemorrhoids and other anal disorders | Patient Assessment of Upper Gastrointestinal Disorder Symptoms (PAGI-SYM) evaluates upper GI symptoms. Alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), and serum bilirubin are liver function tests |
| Neoplasms (cancer) | Brain and nervous system cancer, lung cancer, breast cancer, bones and joints cancer, digestive system cancer, endocrine system cancer, eye and orbit cancer, blood cancer, skin cancer, genitourinary system cancer, blood cancer, respiratory system cancer | Stage of cancer: categorizes cancer based on its size, spread, and key characteristics |
| Diseases of the immune system | Rheumatoid arthritis, other inflammatory arthritis, lupus, celiac disease, Sjogren’s syndrome, sarcoidosis, anti-phospholipid antibody syndrome, ankylosing spondylitis, systemic sclerosis, autoimmune encephalitis, myasthenia gravis, peripheral neuropathies, autoimmune thyroid disease, other non-neurological organ-specific autoimmune diseases | |
| Diseases of the musculoskeletal system and connective tissue | Gout, arthropathy, arthritis, osteoarthritis, spondylopathy, myositis, osteoporosis, osteopathy, chronic low back pain, fibromyalgia, joint replacement, fracture, spinal stenosis, disc herniation | Nordic Musculoskeletal Questionnaire (NMQ): evaluates musculoskeletal pain |
| Diseases of the blood and blood-forming organs | Anemias, coagulation defects, blood clots (thrombus) disorder, purpura and other hemorrhagic conditions, hemophilia, sickle cell disease, thalassemia | Hb (hemoglobin level), aPTT, PT, TT (clotting time tests), INR (blood clotting measure), vitamin K, platelets, bleeding time (BT) (platelet function test), clotting factor tests (specific clotting factors), WBC (white blood cell count) |
| Diseases of the skin | Eczema, psoriasis, pemphigus, alopecia areata, atopic dermatitis, epidermolysis bullosa, Raynaud’s phenomenon, rosacea, hidradenitis suppurativa, scleroderma, ichthyosis, vitiligo, pachyonychia | Dermatological Life Quality Index (impact of skin diseases on life quality), Psoriasis Area Severity Index (psoriasis severity), SCORAD (eczema assessment) |
| Diseases of the genitourinary system | Glomerular diseases, chronic kidney disease, renal failure, renal tubulo-interstitial diseases, urolithiasis, benign prostatic hyperplasia, disorders of the breast, disorders of the female/male genital tract | Stages of CKD (chronic kidney disease progression), The International Prostate Symptom Score (IPSS) (prostate-related symptoms) |
| Infections and parasitic diseases | Tuberculosis, malaria, HIV/AIDS, Epstein–Barr virus (EBV), herpesvirus 6, varicella-zoster virus, Helicobacter pylori, Clostridium perfringens | Antibody level: a measure of specific antibodies in the blood, indicating immune system activity or response to vaccination |
2.1.5. Cognitive assessment
The ‘participant’s level of education and hand preference will be determined through an interview (32). A professional will administer the Montreal Cognitive Assessment (MoCA) in alignment with its official guidelines. MoCA serves as a comprehensive clinical tool designed to evaluate a broad spectrum of cognitive functions related to MS and other conditions (33). This assessment includes cognitive domains such as memory, attention, language, abstraction, delayed recall, orientation, and visuospatial skills. The entire cognitive assessment with MoCA typically takes about 15 min. Additionally, the European Quality of Life–5 Dimensions (EQ-5D) index and the EQ-5D visual analog scale (VAS) (34) will be performed.
2.1.6. Biospecimens
Venous blood and CSF from patients will be collected. CSF will be sampled and stored according to international research standards (35). We will analyze the levels of markers of inflammation and BBB disruption, including matrix metalloproteases (MMPs) (MMP-2, MMP-3, MMP-7, MMP-9, MMP-12, MMP-13), vascular endothelial growth factor A (VEGF-A), and microfibril-associated protein 4 (MFAP4) using enzyme-linked immunosorbent assay (ELISA). Additionally, cytokines and chemokines such as interleukin-1 beta (IL-1beta), IL-6, IL-7, IL-10, IL-17A, tumor necrosis factor alpha (TNF-alpha), C-C chemokine receptor type 2 (CCR2), C-X-C motif chemokine ligand 10 (CXCL10), C-X-C motif chemokine ligand 12 (CXCL12), and C-X-C motif chemokine ligand 13 (CXCL13); type I interferons; neurofilament light chain (NfL); and glial fibrillary acidic protein (GFAP) will be measured as markers of neuronal and astrocyte injury. These biomarkers will be measured on a Quanterix™ Simoa HD-1 Analyzer using the appropriate Simoa assay kits (36).
At baseline and at the 24-month follow-up, peripheral blood mononuclear cells (PBMCs) will be isolated and undergo flow cytometry for quantitative analysis to determine the numbers of CD4, CD8 T lymphocytes, B lymphocytes, dendritic cells, monocytes, monocyte-derived macrophages, FOXP3+ regulatory T cells, and FoxA1+ Treg and natural killer T cells, as well as mass cytometry for in-depth immune characterization. Additionally, hydrophilic interaction chromatography/mass spectrometry-based proteomics for unbiased screening of plasma/serum will be performed, backed up by targeted highly sensitive assays (Simoa), and RT-qPCR performed centrally. All biospecimens will be collected and analyzed at the University of Southern Denmark.
2.1.7. Patient-reported outcomes
For assessing fatigue, the short-form Neuro-QOL Fatigue Scale (37) will be utilized to measure current levels of fatigue. This scale provides a valuable insight into the impact of fatigue on the quality of life of participants.
2.1.8. Vision and the visual system
Participants will undergo ophthalmological examinations at baseline and at the final visit for the study, which include refraction, high- and low-contrast visual acuity tests, visual fields, and visual evoked potentials (VEPs). Additionally, the National Eye Institute 25-item Visual Function Questionnaire (NEI-VFQ-25) will be utilized to evaluate patients’ visual health-related quality of life (38, 39).
High-resolution OCT images of the retina will be created for all patients and controls (majority of the data for this study will be derived from the Heidelberg Spectralis SD-OCT device as per our retrospective dataset. However, data may be obtained from other OCT devices, including Cirrus HD-OCT, Carl Zeiss; Topcon, Optovue, Canon). The imaging scans for this study will be conducted following the guidelines set forth by the Advised Protocol for OCT Study Terminology and Elements (APOSTEL) 2.0 9-point recommendations (40) and the OSCAR-IB quality criteria (41, 42). We will not consider patients whose documentation is incomplete or who underwent OCT imaging using time-domain devices.
The OCT protocol encompasses various scans such as a peripapillary ring scan, a volume scan of the macula, and a volume scan of the optic nerve head. The measurements taken by the OCT include the peripapillary retinal nerve fiber layer (pRNFL) thickness, ganglion cell-inner plexiform layer (GCIP) thickness, inner nuclear layer (INL) thickness, outer nuclear layer (ONL) thickness, and macular thickness.
Additionally, OCT angiography (OCTA) will be utilized, providing a detailed view of the retinal and choroidal vasculature. Equipment from both Heidelberg and Zeiss will be used to obtain images. Through these images, vascular density, the foveal avascular zone, flow regions, and qualitative vascular irregularities will be assessed. A high standard of image quality will be maintained. Scans meeting an acceptable signal strength index will be selected for analysis centrally, and those compromised by artifacts or other quality issues will be carefully excluded (1). All OCT data will be collected at Charite University, Berlin, Germany.
2.1.9. CNS MRI
In the comparative analysis of MRI findings for the index disease, both with and without comorbidity, our initial primary focus centers on vascular comorbid conditions. MRI scans will be conducted using a 3T system compatible with Siemens, Philips, and GE scanners. The core structural protocol includes the brain, orbit, and spinal cord.
2.1.9.1. Brain MRI
The core structural protocol will comprise 3D fluid-attenuated inversion recovery (FLAIR), T2 sampling enhanced with specialized contrasts (SPACE), susceptibility-weighted imaging (SWI), and T1 magnetization prepared-rapid gradient echo (MPRAGE) sequences (optimized for 1-mm isotropic pixels). Post-contrast T1 imaging will be performed in cases of acute relapse (~45 min) (43, 44).
Additionally, the research protocol for brain MRI for selective patients includes a specialized subgroup measurement that involves multiparametric mapping (MPM) for detailed tissue characterization, extending the scan duration by approximately 20 min (45, 46). Procedures such as diffusion-weighted imaging (DWI) (47), resting-state functional MRI (RS-fMRI), and advanced sequences like susceptibility-weighted imaging (SWI) and T2*-weighted imaging will be performed, with durations estimated at 3–5 min each (a total of ~30 min) (48).
2.1.9.2. Spinal cord
The protocol includes a 3D T2 SPACE sequence, a 3D T1 MPRAGE sequence for the cervical cord, and a 3D T2 sequence for the thoracic cord (~12 min).
2.1.9.3. Optic nerve
The MRI protocol will feature a high-resolution, 3D T2-weighted orbit sequence focused on the optic nerves with a slice thickness of 2 mm or less. Fat suppression techniques will be employed to enhance contrast and reduce artifacts, and a post-contrast T1-weighted sequence may also be used to assess inflammatory or demyelinating changes in the optic nerve (~10–15 min) (49–52). All MRI data will be evaluated at the University of Oxford, UK.
2.1.10. Outcome measures and assessment timeline
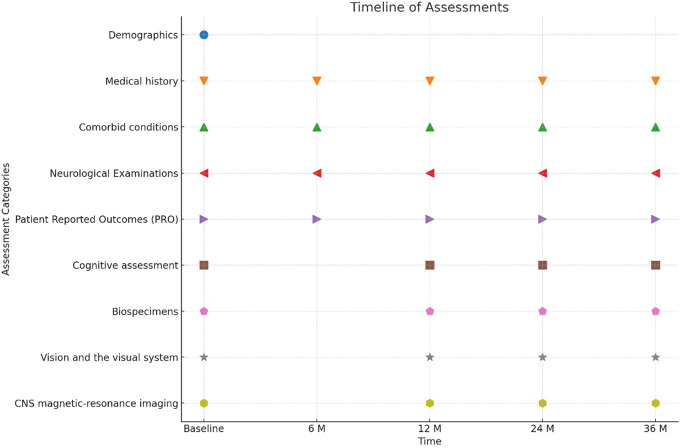
In this study, a comprehensive set of outcome parameters, as detailed above, will be monitored and analyzed at each clinical visit to the participating centers ( Table 2 ). Assessments are scheduled to be conducted at the initiation of the study (baseline) and subsequently at 6, 12, 24, and 36 months. This scheduling allows for the tracking of changes over time ( Table 2 , Figure 1 ). As an interim analysis specifically oriented at regional differences, we will conduct subgroup analyses to explore cross-sectional region-specific effects.
Figure 1.
Timeline of patient assessments and interventions in a clinical study spanning 36 months.
2.1.11. Assessments and endpoints
In MS, the evaluation of disease progression is refined through advanced measures, with a primary focus on no evidence of disease activity (NEDA-4) (40, 53, 54). NEDA-4 assessment in our study includes four criteria: no clinical relapses; no new/enlarged T2-weighted MRI lesions; no disability progression over 6 months, assessed by the EDSS score; and a mean annual brain volume loss rate under 0.4%.
For NMOSD and MOGAD, the primary endpoint is relapse frequency [annualized relapse rate (ARR)] and time to relapse. Secondary endpoints are clinically important change from baseline in Hauser Ambulation Index (HAI) score (55), changes from baseline in EQ-5D index and EQ-5D visual analog scale (VAS), disability worsening from baseline in EDSS score (30) and change from baseline in low-contrast visual acuity binocular score (by low-contrast Landolt C broken ring chart), and cumulative total number of active MRI lesions (new gadolinium-enhancing lesions, or new or enlarging T2 lesions, measured across the optic nerve, brain, brainstem, and spinal cord).
The number of IDD-related hospital admissions will be recorded. Patient-reported outcomes also provide further insights into the patient’s experience of the disease in this study (56). Exploratory outcomes include OCT parameters and clinically relevant biomarkers like NfL levels and GFAP for NMOSD (57, 58).
2.1.12. Data management and quality control
Data associated with the study will be stored within the REDCap database system (59). Monthly data monitoring will be conducted using a REDCap query function for completeness and accuracy by the research team to ensure that any errors or inconsistencies are identified and addressed in a timely manner.
2.2. Streamlined dataset protocol for multicenter coordination
To achieve consistent data collection across various centers, it is crucial to establish a standardized minimal dataset. This standardized approach is key to facilitating data sharing and analysis, ensuring uniformity and reliability in study outcomes across diverse facilities. It is planned that the specifics of the data subsets for each center will be thoroughly discussed with all participating centers. A primary milestone for the multicentric part of this project is the initiation of cross-sectional data collection.
2.3. Statistical analysis
The data will be analyzed in two stages: first cross-sectionally and then longitudinally. The cross-sectional analysis will focus on examining the relationships among the variables at a single point in time, while the longitudinal analysis will explore changes in these relationships over time.
In the initial stage of analysis, the data will be evaluated cross-sectionally. This will encompass descriptive statistics to summarize central aspects like means, medians for continuous variables, and frequencies for categorical ones. Additionally, correlation analyses will gauge the linear relationships between pairs of variables, while regression analysis will investigate the predictive influence of independent variables on a designated dependent variable. In the subsequent phase, the focus will be on the evolution and patterns in relationships over time. Repeated measures ANOVA will be employed to compare means across different times and identify any significant changes. Growth curve modeling will be done to understand the trajectory of variables over time and to determine if there are individual differences in the change trajectories. Furthermore, to accommodate instances of missing data or irregular time intervals, mixed-effects models will be utilized, considering both fixed and random influences.
To determine the sample size, the study’s primary objectives and expected effect sizes will be considered by using R statistical software. A-priori power analyses will be utilized to establish the needed sample size with an optimal power, usually set at 0.80, for a typical significance level of 0.05. Practical significance of the observed relationships or differences will be assessed.
Association analyses of outcomes with comorbidity and age (including comorbidity–age interactions) as well as relations between the different outcomes will be a major focus. Predictive modeling of disease progression will be the content of a follow-up analysis beyond this project’s period, where we intend to use both disease biomarkers and the related risk factor markers for vascular comorbidities as predictors together with techniques from machine learning and algorithm-based data analyses.
The study has a confounder bias, as subjects with comorbidity will receive treatment as the standard of care at different time points. Comorbidity treatment initiated between the baseline and follow-up will be analyzed as potential confounders. The influence of confounders will be minimized by advanced biostatistical techniques, including confounder adjustment.
2.4. Discussion
Comorbidity refers to the total burden of any additional disease entity other than the specific index disease and may result from predisposing genetic or environmental factors or from treatment for IDDs. Comorbidity is prevalent among people with IDDs of the CNS, consistent with the rising prevalence of multimorbidity, estimated to affect approximately 40% of the European population. The prevalence of comorbidity not only signifies elevated mortality rates but also correlates with heightened healthcare resource utilization (17). Comorbidities may have decisive importance in terms of diagnosis, prognosis, and treatment effectiveness and safety. The scarcity of established modifiable risk factors for the burden of multimorbidity indicates that new knowledge is required to develop effective prevention and treatment strategies.
The COMMIT research program focuses on the three best-defined IDDs: MS, NMOSD, and MOGAD. NMOSD and MOGAD are antibody-associated diseases of the CNS. In NMOSD, an autoantibody targets the astrocyte water channel AQP4 and defines NMOSD as a primary astrocytopathy in AQP4-IgG-positive cases (60, 61). In MOGAD, an autoantibody targets myelin antigens, i.e., MOG, which is produced by oligodendrocytes and thus is involved in demyelination (62). MS is thought to be an autoimmune disease and to be a mainly myelin-directed disease although causative specificities have not been identified, and considerable overlap may occur between MS and MOGAD. The goal of the present study is to assess whether comorbidity has an impact on the levels of proinflammatory and neurodegenerative mediators and in practical terms whether they influence OCT and MRI parameters and is associated with clinical outcome.
In the COMMIT study, the patients with IDDs and comorbidity are the primary target group. This project advances the management of IDD patients with chronic comorbidities and may contribute to the pathogenesis of diseases. Identification of reliable biomarkers will allow evaluation of the impact of comorbidity on IDDs and enable early diagnosis and new therapeutic strategies. Our prospective multicenter study, which will be conducted over a 3-year follow-up period, aims to compile a detailed and systematic database for patients who exhibit various comorbid conditions.
A strength of the study is the multicenter design, with longitudinal, consecutive follow-up with sample collection and a uniform protocol. Given the exhaustive nature of the study protocol, it can be particularly challenging for individuals with disabilities. We will introduce a streamlined, standardized assessment to ease follow-ups. With an adequate sample size, our objective is to curtail the effects of confounding.
3. Conclusion
In conclusion, the intricate relationship between IDD and other chronic conditions represents an area for further research. The COMMIT study will address if the clinical and biochemical status of patients with MS, NMOSD, and MOGAD is affected by comorbid conditions. In addition, the study is cross-disciplinary, contributing to the characterization of disease mechanisms. The study will identify clinically relevant biomarkers, as well as address survival and quality of life. Recognizing the frequency and patterns of comorbidities and risk factors can be instrumental in developing preventive strategies. The results of the study are anticipated to have a strong and immediate impact on the perception and treatment of IDDs due to improvement in diagnostic and monitoring biomarkers.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The Research Ethical Committees for the Region of Southern Denmark approved the study protocol. Approval by the local ethical institutional review boards from all centers for this multicenter study was obtained following informed consent from the patients. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SS: Visualization, Formal analysis, Methodology, Investigation, Writing – review & editing, Writing – original draft. RA: Writing – review & editing, Writing – original draft. PB: Writing – review & editing, Writing – original draft. MB: Investigation, Writing – review & editing, Writing – original draft. BD: Formal analysis, Methodology, Writing – review & editing, Writing – original draft. SR: Methodology, Writing – review & editing, Writing – original draft. ML: Supervision, Resources, Investigation, Writing – review & editing, Writing – original draft. AP: Supervision, Methodology, Investigation, Writing – review & editing, Writing – original draft. JP: Validation, Supervision, Methodology, Investigation, Writing – review & editing, Writing – original draft. EF: Validation, Supervision, Methodology, Investigation, Writing – review & editing, Writing – original draft. SMa: Supervision, Methodology, Investigation, Writing – review & editing, Writing – original draft. STS: Writing – review & editing, Writing – original draft. AF: Validation, Supervision, Methodology, Investigation, Writing – review & editing, Writing – original draft. IL: Methodology, Investigation, Writing – review & editing, Writing – original draft. SMe: Methodology, Writing – review & editing, Writing – original draft. RG: Methodology, Investigation, Writing – review & editing, Writing – original draft. SA: Methodology, Investigation, Writing – review & editing, Writing – original draft. HS-K: Methodology, Investigation, Writing – review & editing, Writing – original draft. FO: Supervision, Methodology, Investigation, Writing – review & editing, Writing – original draft. VS: Supervision, Investigation, Writing – review & editing, Writing – original draft. MS: Supervision, Investigation, Writing – review & editing, Writing – original draft. HK: Validation, Supervision, Methodology, Investigation, Writing – review & editing, Writing – original draft. JB: Validation, Supervision, Methodology, Writing – review & editing, Writing – original draft. CB: Methodology, Investigation, Writing – review & editing, Writing – original draft. HZ: Supervision, Methodology, Investigation, Writing – review & editing, Writing – original draft. BW: Validation, Supervision, Investigation, Writing – review & editing, Writing – original draft. FP: Validation, Supervision, Methodology, Investigation, Writing – review & editing, Writing – original draft. NA: Validation, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization, Writing – review & editing, Writing – original draft.
Glossary
- AT2R
angiotensin II type 2 receptor
- BBB
blood–brain barrier
- CCR2
C-C motif chemokine receptor 2
- CD
cluster of differentiation
- CNS
central nervous system
- CSF
cerebrospinal fluid
- CXCL
C-X-C motif chemokine ligand
- DMT
disease-modifying therapy
- EDSS
Expanded Disability Status Scale
- ELISA
enzyme-linked immunosorbent assay
- FOXP3
forkhead box P3
- FoxA1+ Treg
forkhead box A1-positive regulatory T cells
- GCIP
ganglion cell-inner plexiform layer
- GFAP
glial fibrillary acidic protein
- IDDs
inflammatory demyelinating diseases
- IL
interleukin
- INL
inner nuclear layer
- MFAP4
microfibril-associated protein 4
- MOG
myelin oligodendrocyte glycoprotein
- MOGAD
MOG-associated disease
- MRI
magnetic resonance imaging
- MMPs
matrix metalloproteases
- MS
multiple sclerosis
- NEI-VFQ25
the National Eye Institute 25-item Visual Function Questionnaire
- NfL
neurofilament light
- NMDAR EN
N-methyl-D-aspartate receptor encephalitis
- NMOSD
neuromyelitis optica spectrum disorder
- OCT
optical coherence tomography
- ON
optic neuritis
- ONL
outer nuclear layer
- OPC
oligodendrocyte precursor cell
- OL(s)
oligodendrocytes
- PBMCs
peripheral blood mononuclear cells
- pRNFL
peripapillary retinal nerve fiber layer
- PPMS
primary progressive MS
- QoL
quality of life
- REDCap
Research Electronic Data Capture
- RRMS
relapsing–remitting MS
- RT-qPCR
real-time quantitative polymerase chain reaction
- SD-OCT
spectral domain optical coherence tomography
- SPMS
secondary progressive MS
- T2DM
type 2 diabetes mellitus
- TNF-alpha
tumor necrosis factor alpha
- TREM2
triggering receptor expressed on myeloid cells 2
- pTau-181
phosphorylated tau at position 181
- VEGF-A
vascular endothelial growth factor-A.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The research was supported by the University of Southern Denmark, The Slagelse Hospital Research Fund, and The Region of Zealand Research Fund.
Conflict of interest
STS as received personal fees from Munksgaard, TrustMe-Ed, and Nestlé Health Science outside the submitted work; is a co-founder of GLA:D®, a not-for-profit initiative hosted at the University of Southern Denmark aimed at implementing clinical guidelines for osteoarthritis in clinical practice; and has received a program grant from Region Zealand Exercise First and two grants from the European Union’s Horizon 2020 research and innovation program, one from the European Research Council MOBILIZE, grant agreement No. 801790 and the other under grant agreement No. 945377 ESCAPE. ML has received consulting fees and research grants from Alexion AstraZeneca, Horizon Amgen, and Genentech Roche. AP has received grant support for remyelination trials in multiple sclerosis from the Amsterdam University Medicam Centre, Department of Neurology, MS Centre RESTORE trial and UCL, London RECOVER trial and from Fight for Sight nimodipine in optic neuritis trial; has received royalties or licenses from UptoDate Wolters Kluwer on a book chapter and speaker fees from the Heidelberg Academy; has participated in the Advisory Board SC Zeiss OCTA Angi-Network; is the chairman of ERN-EYE Neuro-ophthalmology until OCT-2020 and a board member of the National Dutch Neuro-ophthalmology Association; and has received equipment OCTA from Zeiss Plex Elite. JP has received support for scientific meetings and honorariums for advisory work from Merck Serono, Novartis, Chugai, Alexion, Roche, Medimmune, Argenx, UCB, Mitsubishi, Amplo, Janssen, and Sanofi; grants from Alexion, Roche, Medimmune, UCB, and Amplo Biotechnology; and patent ref P37347WO and license agreement Numares multimarker MS diagnostics shares in AstraZeneca. She also acknowledges partial funding from the highly specialized services of NHS England. SMe has received travel grants from Merck, Roche, and Sanofi and speaking honoraria from UCB. RG has received support for scientific meetings and courses and honoraria for advisory work from Bayer, Biogen, Merck, Novartis, and Jasen. EF has served on advisory boards for Alexion, Genentech, Horizon Therapeutics, and UCB. He has received speaker honoraria from Pharmacy Times and royalties from UpToDate. He was a site primary investigator in a randomized clinical trial on inebilizumab in neuromyelitis optica spectrum disorder run by Medimmune/Viela-Bio/Horizon Therapeutics. He has received funding from the NIH R01NS113828 and is a member of the medical advisory board of the MOG project and an editorial board member of the Journal of the Neurological Sciences and Neuroimmunology Reports. SMa has received speaker honoraria from Biogen, Novartis, Horizon, and Sanofi. SA has received speaker’s honoraria from Alexion, Bayer, and Roche. HS-K has received support for scientific meetings from Roche. FO has received grants from the National Multiple Sclerosis Society US, American Academy of Neurology, Hertie Foundation for Excellence in Clinical Neuroscience, and Novartis—all independent of this project. HK has received a grant from the National Research Foundation of Korea and research support from AprilBio, Eisai, and UCB; has received consultancy/speaker fees from Alexion, Altos Biologics, AstraZeneca, Biogen, Daewoong Pharmaceutical, Eisai, GC Pharma, Handok Pharmaceutical, Kaigene, Kolon Life Science, MDimune, Merck Serono, Mitsubishi Tanabe Pharma, Roche, and Sanofi Genzyme; and is a co-editor for the Multiple Sclerosis Journal and an associated editor for the Journal of Clinical Neurology. MS and VS has received educational research grants, lecture honorarium, and travel support to attend scientific meetings from Biogen-Idec, Merck, Bayer, Novartis, Cinnagen, Osveh, Zistdaru, Zahravi, Abidi, and NanoAvland. JB reported personal fees from Roche, Genentech, Horizon, Chugai Pharma, Clene Nanoscience, Reistone-Bio, Beigene, and Imcyse; grants and personal fees from Alexion; and grants from the National Institutes of Health. JB has a patent aquaporumab issued. CB has received a grant from Roche, unrelated to this study. HZ has received grants from Novartis, unrelated to this study. BW has received royalties from RSR Ltd, Oxford University, Hospices Civil de Lyon, and MVZ Labor PD Dr. Volkmann und Kollegen GbR for a patent of NMO-IgG as a diagnostic test for neuromyelitis optica spectrum disorders; served on the adjudication committee for clinical trials conducted by MedImmune/VielaBio, Alexion, and UCB Biosciences; and consulted for Chugai/Roche/Genentech, Horizon Therapeutics, Mitsubishi-Tanabe, and CANbridge Pharmaceuticals regarding neuromyelitis optica spectrum disorders. He has received honoraria for speaking at internal meetings of Genentech, Novartis, and Horizon and at external meetings for Roche. FP served on the scientific advisory boards of Novartis and MedImmune; received travel funding and/or speaker honoraria from Bayer, Novartis, Biogen, Teva, Sanofi-Aventis/Genzyme, Merck Serono, Alexion, Chugai, MedImmune, and Shire; is an associate editor of Neurology: Neuroimmunology & Neuroinflammation; is an academic editor of PLoS ONE; consulted for Sanofi Genzyme, Biogen, MedImmune, Shire, and Alexion; received research support from Bayer, Novartis, Biogen, Teva, Sanofi-Aventis/Genzyme, Alexion, and Merck Serono; and received research support from the German Research Council, Werth Stiftung of the City of Cologne, German Ministry of Education and Research, Arthur Arnstein Stiftung Berlin, EU FP7 Framework Program, Arthur Arnstein Foundation Berlin, Guthy-Jackson Charitable Foundation, and NMSS.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Wicklein R, Yam C, Noll C, Aly L, Banze N, Romahn EF, et al. The OSCAR-MP consensus criteria for quality assessment of retinal optical coherence tomography angiography. Neurol Neuroimmunol Neuroinflamm. (2023) 10(6). doi: 10.1212/NXI.0000000000200169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuhlmann T, Moccia M, Coetzee T, Cohen JA, Correale J, Graves J, et al. Multiple sclerosis progression: time for a new mechanism-driven framework. Lancet Neurol. (2023) 22(1):78–88. doi: 10.1016/S1474-4422(22)00289-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. (2007) 17(2):210–8. doi: 10.1111/j.1750-3639.2007.00064.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barzegar M, Mirmosayyeb O, Nehzat N, Vaheb S, Shaygannejad V, Asgari N. Frequency of comorbidities in Neuromyelitis Optica spectrum disorder. Mult Scler Relat Disord. (2021) 48:102685. doi: 10.1016/j.msard.2020.102685 [DOI] [PubMed] [Google Scholar]
- 5. Exuzides A, Sheinson D, Sidiropoulos P, Magrini F, Gholizadeh S, Surinach A, et al. Burden and cost of comorbidities in patients with neuromyelitis optica spectrum disorder. J Neurol Sci. (2021) 427:117530. doi: 10.1016/j.jns.2021.117530 [DOI] [PubMed] [Google Scholar]
- 6. Gkaniatsou T, Papadopoulou A, Paul F, Brandt AU, Oertel FC. Frequency of autoimmune disorders and autoantibodies in European patients with neuromyelitis optica spectrum disorders. Acta Neurol Belg. (2020) 120(1):223–5. doi: 10.1007/s13760-019-01176-6 [DOI] [PubMed] [Google Scholar]
- 7. Molazadeh N, Bose G, Lotan I, Levy M. Autoimmune diseases and cancers overlapping with myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD): A systematic review. Mult Scler J Exp Transl Clin. (2022) 8(4):20552173221128170. doi: 10.1177/20552173221128170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maric GD, Pekmezovic TD, Mesaros ST, Tamas OS, Ivanovic JB, Martinovic VN, et al. The prevalence of comorbidities in patients with multiple sclerosis: population-based registry data. Neurol Sci. (2021) 42(5):1887–93. doi: 10.1007/s10072-020-04727-5 [DOI] [PubMed] [Google Scholar]
- 9. Sparaco M, Lavorgna L, Bonavita S. Psychiatric disorders in multiple sclerosis. J Neurol. (2021) 268(1):45–60. doi: 10.1007/s00415-019-09426-6 [DOI] [PubMed] [Google Scholar]
- 10. Chertcoff AS, Yusuf FLA, Zhu F, Evans C, Fisk JD, Zhao Y, et al. Psychiatric comorbidity during the prodromal period in patients with multiple sclerosis. Neurology. (2023) 101(20):e2026–34. doi: 10.1212/WNL.0000000000207843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marrie RA, Fisk JD, Fitzgerald K, Kowalec K, Maxwell C, Rotstein D, et al. Etiology, effects and management of comorbidities in multiple sclerosis: recent advances. Front Immunol. (2023) 14:1197195. doi: 10.3389/fimmu.2023.1197195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marrie RA. The Barancik lecture: Comorbidity in multiple sclerosis-Looking backward, looking forward. Mult Scler. (2023) 29(9):1049–56. doi: 10.1177/13524585231167740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iris SSH, Amaya A-L, Ashley A, Jim D, Kamlesh K, Umesh TK, et al. Measuring multimorbidity in research: Delphi consensus study. BMJ Med. (2022) 1(1):e000247. doi: 10.1136/bmjmed-2022-000247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gholizadeh S, Exuzides A, Lewis KE, Palmer C, Waltz M, Rose JW, et al. Clinical and epidemiological correlates of treatment change in patients with NMOSD: insights from the CIRCLES cohort. J Neurol. (2023) 270(4):2048–58. doi: 10.1007/s00415-022-11529-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cai L, Chen H, Shi Z, Wang X, Du Q, Zhang Y, et al. Non-immune system comorbidity in neuromyelitis optica spectrum disorders. J Clin Neurosci. (2023) 107:16–22. doi: 10.1016/j.jocn.2022.11.008 [DOI] [PubMed] [Google Scholar]
- 16. Chowdhury SR, Chandra Das D, Sunna TC, Beyene J, Hossain A. Global and regional prevalence of multimorbidity in the adult population in community settings: a systematic review and meta-analysis. EClinicalMedicine. (2023) 57:101860. doi: 10.1016/j.eclinm.2023.101860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Skou ST, Mair FS, Fortin M, Guthrie B, Nunes BP, Miranda JJ, et al. Multimorbidity. Nat Rev Dis Primers. (2022) 8(1):48. doi: 10.1038/s41572-022-00376-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thormann A, Sørensen PS, Koch-Henriksen N, Laursen B, Magyari M. Comorbidity in multiple sclerosis is associated with diagnostic delays and increased mortality. Neurology. (2017) 89(16):1668–75. doi: 10.1212/WNL.0000000000004508 [DOI] [PubMed] [Google Scholar]
- 19. DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. (2015) 1:15019. doi: 10.1038/nrdp.2015.19 [DOI] [PubMed] [Google Scholar]
- 20. Negrotto L, Farez MF, Correale J. Immunologic effects of metformin and pioglitazone treatment on metabolic syndrome and multiple sclerosis. JAMA Neurol. (2016) 73(5):520–8. doi: 10.1001/jamaneurol.2015.4807 [DOI] [PubMed] [Google Scholar]
- 21. Blázquez E, Velázquez E, Hurtado-Carneiro V, Ruiz-Albusac JM. Insulin in the brain: its pathophysiological implications for States related with central insulin resistance, type 2 diabetes and Alzheimer's disease. Front Endocrinol (Lausanne). (2014) 5:161. doi: 10.3389/fendo.2014.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu J, Zhang X, Zhong Y, Liu X. The prevalence of depression, anxiety, and sleep disturbances in patients with neuromyelitis optica spectrum disorders (NMOSD): A systematic review and meta-analysis. Multiple Sclerosis Related Disord. (2023) 79:105007. doi: 10.1016/j.msard.2023.105007 [DOI] [PubMed] [Google Scholar]
- 23. Banwell B, Bennett JL, Marignier R, Kim HJ, Brilot F, Flanagan EP, et al. Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: International MOGAD Panel proposed criteria. Lancet Neurol. (2023) 22(3):268–82. doi: 10.1016/S1474-4422(22)00431-8 [DOI] [PubMed] [Google Scholar]
- 24. Barzegar M, Badihian S, Mirmosayyeb O, Ashtari F, Jamadi M, Emami S, et al. Comparative study of quality of life, anxiety, depression, and fatigue among patients with neuromyelitis optica spectrum disorder and multiple sclerosis: The first report from Iran. Mult Scler Relat Disord. (2018) 22:161–5. doi: 10.1016/j.msard.2018.04.009 [DOI] [PubMed] [Google Scholar]
- 25. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. (2008) 61:344–9. doi: 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 26. World Medical Association . Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama. (2013) 310(20):2191–4. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 27. Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. (2018) 17(2):162–73. doi: 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 28. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. (2015) 85(2):177–89. doi: 10.1212/WNL.0000000000001729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abboud H, Steingo B, Vargas D, Patel J, Nealon N, Alissa Willis M, et al. Satralizumab treatment in adults with AQP4-IgGseropositive neuromyelitis optica spectrum disorder: a retrospective case series. Multiple Sclerosis J. (2023) 29:672–3. [Google Scholar]
- 30. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. (1983) 33(11):1444–52. doi: 10.1212/WNL.33.11.1444 [DOI] [PubMed] [Google Scholar]
- 31. Kappos L, D'Souza M, Lechner-Scott J, Lienert C. On the origin of neurostatus. Mult Scler Relat Disord. (2015) 4(3):182–5. doi: 10.1016/j.msard.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 32. Jensen VM, Rasmussen AW. Danish education registers. Scand J Public Health. (2011) 39(7 Suppl):91–4. doi: 10.1177/1403494810394715 [DOI] [PubMed] [Google Scholar]
- 33. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53(4):695–9. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 34. EuroQol . EQ-5D-5L user guide(2019). Available online at: https://euroqol.org/publications/user-guides/.
- 35. Petzold A, Wattjes MP, Costello F, Flores-Rivera J, Fraser CL, Fujihara K, et al. The investigation of acute optic neuritis: a review and proposed protocol. Nat Rev Neurol. (2014) 10(8):447–58. doi: 10.1038/nrneurol.2014.108 [DOI] [PubMed] [Google Scholar]
- 36. Olesen MN, Soelberg K, Debrabant B, Nilsson AC, Lillevang ST, Grauslund J, et al. Cerebrospinal fluid biomarkers for predicting development of multiple sclerosis in acute optic neuritis: a population-based prospective cohort study. J Neuroinflamm. (2019) 16(1):59. doi: 10.1186/s12974-019-1440-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Victorson D, Cavazos JE, Holmes GL, Reder AT, Wojna V, Nowinski C, et al. Validity of the Neurology Quality-of-Life (Neuro-QoL) measurement system in adult epilepsy. Epilepsy Behav. (2014) 31:77–84. doi: 10.1016/j.yebeh.2013.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Graves JS, Oertel FC, Van der Walt A, Collorone S, Sotirchos ES, Pihl-Jensen G, et al. Leveraging visual outcome measures to advance therapy development in neuroimmunologic disorders. Neurol Neuroimmunol Neuroinflamm. (2022) 9(2). doi: 10.1212/NXI.0000000000001126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schmidt F, Zimmermann H, Mikolajczak J, Oertel FC, Pache F, Weinhold M, et al. Severe structural and functional visual system damage leads to profound loss of vision-related quality of life in patients with neuromyelitis optica spectrum disorders. Multiple Sclerosis Related Disord. (2017) 11:45–50. doi: 10.1016/j.msard.2016.11.008 [DOI] [PubMed] [Google Scholar]
- 40. Aytulun A, Cruz-Herranz A, Aktas O, Balcer LJ, Balk L, Barboni P, et al. APOSTEL 2.0 recommendations for reporting quantitative optical coherence tomography studies. Neurology. (2021) 97(2):68–79. doi: 10.1212/WNL.0000000000012125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tewarie P, Balk L, Costello F, Green A, Martin R, Schippling S, et al. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PloS One. (2012) 7(4):e34823. doi: 10.1371/journal.pone.0034823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schippling S, Balk LJ, Costello F, Albrecht P, Balcer L, Calabresi PA, et al. Quality control for retinal OCT in multiple sclerosis: validation of the OSCAR-IB criteria. Mult Scler. (2015) 21(2):163–70. doi: 10.1177/1352458514538110 [DOI] [PubMed] [Google Scholar]
- 43. Wattjes MP, Ciccarelli O, Reich DS, Banwell B, de Stefano N, Enzinger C, et al. 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. (2021) 20(8):653–70. doi: 10.1016/S1474-4422(21)00095-8 [DOI] [PubMed] [Google Scholar]
- 44. Carnero Contentti E, Okuda DT, Rojas JI, Chien C, Paul F, Alonso R. MRI to differentiate multiple sclerosis, neuromyelitis optica, and myelin oligodendrocyte glycoprotein antibody disease. J Neuroimaging. (2023) 33(5):688–702. doi: 10.1111/jon.13137 [DOI] [PubMed] [Google Scholar]
- 45. Vandeleene N, Guillemin C, Dauby S, Requier F, Charonitis M, Chylinski D, et al. Using quantitative magnetic resonance imaging to track cerebral alterations in multiple sclerosis brain: A longitudinal study. Brain Behav. (2023) 13(5):e2923. doi: 10.1002/brb3.2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cooper G, Hirsch S, Scheel M, Brandt AU, Paul F, Finke C, et al. Quantitative multi-parameter mapping optimized for the clinical routine. Front Neurosci. (2020) 14:611194. doi: 10.3389/fnins.2020.611194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schwartz DL, Tagge I, Powers K, Ahn S, Bakshi R, Calabresi PA, et al. Multisite reliability and repeatability of an advanced brain MRI protocol. J Magn Reson Imaging. (2019) 50:878–88. doi: 10.1002/jmri.26652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Enzinger C, Barkhof F, Ciccarelli O, Filippi M, Kappos L, Rocca MA, et al. Nonconventional MRI and microstructural cerebral changes in multiple sclerosis. Nat Rev Neurol. (2015) 11:676–86. doi: 10.1038/nrneurol.2015.194 [DOI] [PubMed] [Google Scholar]
- 49. Trip SA, Schlottmann PG, Jones SJ, Li WY, Garway-Heath DF, Thompson AJ, et al. Optic nerve atrophy and retinal nerve fibre layer thinning following optic neuritis: evidence that axonal loss is a substrate of MRI-detected atrophy. Neuroimage. (2006) 31(1):286–93. doi: 10.1016/j.neuroimage.2005.11.051 [DOI] [PubMed] [Google Scholar]
- 50. Trip SA, Schlottmann PG, Jones SJ, Li WY, Garway-Heath DF, Thompson AJ, et al. Optic nerve magnetization transfer imaging and measures of axonal loss and demyelination in optic neuritis. Mult Scler. (2007) 13(7):875–9. doi: 10.1177/1352458507076952 [DOI] [PubMed] [Google Scholar]
- 51. Trip SA, Wheeler-Kingshott C, Jones SJ, Li WY, Barker GJ, Thompson AJ, et al. Optic nerve diffusion tensor imaging in optic neuritis. Neuroimage. (2006) 30(2):498–505. doi: 10.1016/j.neuroimage.2005.09.024 [DOI] [PubMed] [Google Scholar]
- 52. Vidal-Jordana A, Rovira A, Calderon W, Arrambide G, Castilló J, Moncho D, et al. Adding the optic nerve in multiple sclerosis diagnostic criteria. Neurology. (2024) 102(1):e200805. doi: 10.1212/WNL.0000000000207805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kappos L, De Stefano N, Freedman MS, Cree BA, Radue EW, Sprenger T, et al. Inclusion of brain volume loss in a revised measure of 'no evidence of disease activity' (NEDA-4) in relapsing-remitting multiple sclerosis. Mult Scler. (2016) 22(10):1297–305. doi: 10.1177/1352458515616701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pandit L. No evidence of disease activity (NEDA) in multiple sclerosis - shifting the goal posts. Ann Indian Acad Neurol. (2019) 22(3):261–3. doi: 10.4103/aian.AIAN_159_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hauser SL, Dawson DM, Lehrich JR, Beal MF, Kevy SV, Propper RD, et al. Intensive immunosuppression in progressive multiple sclerosis. A randomized, three-arm study of high-dose intravenous cyclophosphamide, plasma exchange, and ACTH. N Engl J Med. (1983) 308(4):173–80. doi: 10.1056/NEJM198301273080401 [DOI] [PubMed] [Google Scholar]
- 56. Meca-Lallana V, Berenguer-Ruiz L, Carreres-Polo J, Eichau-Madueño S, Ferrer-Lozano J, Forero L, et al. Deciphering multiple sclerosis progression. Front Neurol. (2021) 12:608491. doi: 10.3389/fneur.2021.608491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schindler P, Aktas O, Ringelstein M, Wildemann B, Jarius S, Paul F, et al. Glial fibrillary acidic protein as a biomarker in neuromyelitis optica spectrum disorder: a current review. Expert Rev Clin Immunol. (2023) 19:71–91. doi: 10.1080/1744666X.2023.2148657 [DOI] [PubMed] [Google Scholar]
- 58. Ning L, Wang B. Neurofilament light chain in blood as a diagnostic and predictive biomarker for multiple sclerosis: A systematic review and meta-analysis. PloS One. (2022) 17(9):e0274565. doi: 10.1371/journal.pone.0274565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42(2):377–81. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Melamed E, Levy M, Waters PJ, Sato DK, Bennett JL, John GR, et al. Update on biomarkers in neuromyelitis optica. Neurol Neuroimmunol Neuroinflamm. (2015) 2(4):e134. doi: 10.1212/NXI.0000000000000134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jarius S, Paul F, Weinshenker BG, Levy M, Kim HJ, Wildemann B. Neuromyelitis optica. Nat Rev Dis Primers. (2020) 6(1):85. doi: 10.1038/s41572-020-0214-9 [DOI] [PubMed] [Google Scholar]
- 62. Jarius S, Paul F, Aktas O, Asgari N, Dale RC, de Seze J, et al. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflamm. (2018) 15(1):134. doi: 10.1186/s12974-018-1144-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.