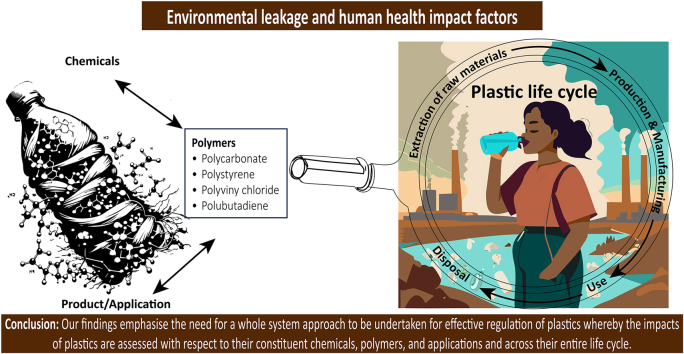
Abstract
Polymers are the main building blocks of plastic, with the annual global production volume of fossil carbon-based polymers reaching over 457 million metric tons in 2019 and this figure is anticipated to triple by 2060. There is potential for environmental harm and adverse human health impacts associated with plastic, its constituent polymers and the chemicals therein, at all stages of the plastic life cycle, from extraction of raw materials, production and manufacturing, consumption, through to ultimate disposal and waste management. While there have been considerable research and policy efforts in identifying and mitigating the impacts associated with problematic plastic products such as single-use plastics and hazardous chemicals in plastics, with national and/or international regulations to phase out their use, plastic polymers are often overlooked. In this review, the polymer dimension of the current knowledge on environmental release, human exposure and health impacts of plastic is discussed across the plastic life cycle, including chemicals used in production and additives commonly used to achieve the properties needed for applications for which the polymers are generally used. This review focuses on polycarbonate, polystyrene, polyvinyl chloride, and polybutadiene, four common plastic polymers made from the hazardous monomers, bisphenol, styrene, vinyl chloride and 1,3-butadiene, respectively. Potential alternative polymers, chemicals, and products are considered. Our findings emphasise the need for a whole system approach to be undertaken for effective regulation of plastics whereby the impacts of plastics are assessed with respect to their constituent polymers, chemicals, and applications and across their entire life cycle.
Keywords: Environmental leakage, Exposure, Health, PVC, Plastic-associated chemicals
Graphical abstract
Highlights
-
•
Impact of plastic depends on plastic polymers, chemicals and products/applications.
-
•
This review focuses on polycarbonate, polystyrene, PVC, and polybutadiene.
-
•
Constituent chemicals, products/applications and entire life cycle are considered.
-
•
Environmental leakage, human exposure and human health impacts are highlighted.
-
•
Insights and recommendations are provided for effective regulation of plastic.
1. Introduction
Plastic is ubiquitous in everyday life and in the environment. The annual global production volume of fossil carbon-based plastic reached over 457 million metric tons (Mt) in 2019 and this figure is anticipated to triple by 2060 [1]. In 2022, the United Nation Environment Assembly endorsed a resolution to end plastic pollution to safeguard the well-being of current and future generations. The resolution incorporates a holistic approach, considering the complete life cycle of plastic, from the extraction of raw materials to production and manufacturing, use and disposal, with the goal of either restricting or improving the systems as necessary within the plastic supply chain. This will be achieved through establishing an international legally binding agreement known as the ‘Global Plastics Treaty’ [2]. However, comprehensive life cycle assessment (LCA) of plastic, particularly its impact on the environment as well as human health, is complicated by the complex composition of plastics and the diverse array of chemicals involved from cradle to grave. Importantly, despite being the main constituent of plastic, polymers are often overlooked in regulations [3].
1.1. What are plastics?
Plastics are complex chemical mixtures which demand a nuanced understanding to highlight their environmental and health impacts. They are a group of predominantly fossil carbon-based, complex, highly heterogeneous, synthetic chemical materials. Plastics are characterised by their ability to be formed into various shapes, typically during manufacturing processes, and can function as a structural component of a final product. They are comprised of at least one type of synthetic or chemically modified natural polymer – made from individual functional chemical units called monomers – and a range of chemicals [4]. Depending on the polymer matrix, plastics can be grouped into thermoplastics (e.g., polycarbonate, polystyrene) or thermosets (e.g., synthetic rubber, epoxy resins). More than 10,000 different chemicals are used in plastic production, majority of which are known to be hazardous to the environmental and human health or are lacking hazard data to evaluate safety [4]. The resulting plastic materials have widely varied chemical compositions, including the intended polymer matrix and additives, as well as unreacted monomers, processing aids (e.g., persisting solvents or catalysts), and non-intentionally added substances (NIAS) such as byproducts (e.g., oligomers), impurities/contaminants, as well as degradation and transformation products [5]. Importantly, chemicals in plastic materials are usually not covalently bound to the polymer matrix and can migrate out of the plastic material [6]. Environmental and human health are negatively impacted throughout the plastic life cycle due to industrial emissions, occupational risks and local pollution during extraction of raw materials, production and manufacturing of plastic and disposal/waste management as well as due to migrating chemicals and particulates across the plastic life cycle [7], including during use by consumers [8,9].
1.2. The plastic life cycle and impacts of polymers, chemicals, and products at each stage
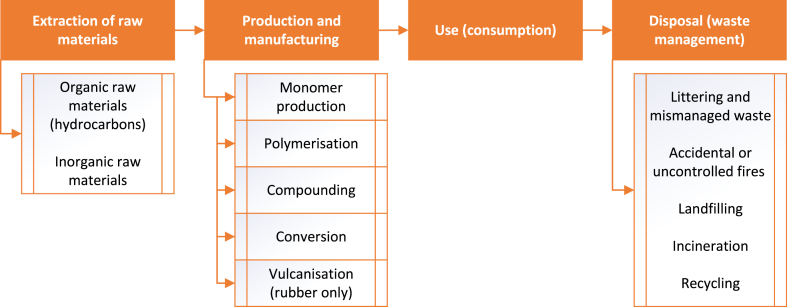
Fig. 1 shows the different stages and sub-stages of the plastic life cycle. Briefly, extraction of raw materials is the first stage of the plastic life cycle. Most (99 %) plastic polymers produced globally are derived from fossil carbon [10]. Oil and gas extraction and coal mining lead to significant environmental pollution and health hazards [7]. While biopolymer alternatives derived from renewable resources are beginning to emerge for some polymer types [10], similar considerations as fossil carbon-based plastics may apply throughout the remainder of the life cycle. Fossil carbon-based derivatives from the petrochemical industry are also the basis for most chemicals used in plastic production. Other plastic chemicals, such as many catalysts in polymerisation reactions and certain functional additives rely on the extraction of various (heavy) metals. Heavy metal mining contaminates surrounding soil, farmland and water bodies, including ground water, which negatively impacts both the ecosystems and human health [[11], [12], [13]].
Fig. 1.
The life cycle of plastic. The plastic life cycle involves four main stages: the extraction of raw materials, production and manufacturing, use/consumption, and disposal/waste management. Several steps are involved in production and manufacturing: 1) Monomer units are produced from the carbon feedstock; 2) Monomer units undergo polymerisation to produce polymers; 3) Polymers are compounded with additives to produce the desired material properties; and 4) this mixture is converted into the final plastic products. 5) Rubbers also undergo vulcanisation, which enhances their mechanical properties by causing the polymer chains to cross-link, forming a three-dimensional network structure.
The second stage of the plastic life cycle, production and manufacturing, involves the transformation of carbon feedstocks into a range of plastic products. It relies heavily on fossil carbon derived from coal (67 %), petroleum (23 %) and natural gas (10 %), both as feedstock for monomer production and as fuel [14]. The scale of plastic production (460 Mt in 2019 [1]) and anticipated increases present serious issues regarding sustainability, as the use of fossil resources results in a myriad of climate, environmental and health externalities [15] such as greenhouse gas emissions and particulate matter (PM) air pollution [16]. Monomer units produced from the carbon feedstock then undergo polymerisation, facilitated by initiators, catalysts, and solvents, to produce polymers in granular or pellet form (also called nurdles) [7]. Next, additives are added to the polymers during the compounding process to produce the desired material properties such as colour, transparency, flexibility, and flame resistance [7]. During conversion, this mixture is converted into the final plastic products using various mechanical processes, often involving heat. An additional step involved in rubber production is vulcanisation which enhances the mechanical properties of the rubber by causing the polymer chains to cross-link, forming a three-dimensional network structure. At any step of the plastic production and manufacturing stage, additional chemicals such as processing aids are added to enable or ease production or processing of plastics [7]. Hazardous plastic-associated chemicals may be released during transportation and manufacture [17], posing significant risks to the environment and human health (e.g., vinyl chloride leakage and explosion [18]). Releases of hazardous chemicals are often concentrated in areas where other petrochemical production is occurring, thereby presenting disproportionate risks to nearby communities, known as fenceline communities, which are typically vulnerable populations [7]. Furthermore, plastic pellets – primary microplastics used for manufacturing plastic products – are released into the environment via factory run-off into drains, loss during transport/handling and via accidental spills during shipping. They can also leach hazardous chemicals such as polychlorinated biphenyls (PCBs) and polycyclic aromatic hydrocarbons (PAHs) into seawater [19], can act as vectors of environmental pollutants by adsorbing persistent organic pollutants (POPs) from the environment [20,21], and can act as a vector for pathogenic microorganisms such as E. coli and Vibrio species, including V. cholerae [22,23].
During use (or consumption [24]), the third stage of the plastic life cycle, plastic-associated chemicals and micro- and nanoplastics (MNPs) are sources of environmental contamination and human exposure [25]. There is leakage of monomers, oligomers, additives, and other chemicals present in plastic products with known or likely hazard, either individually or as hazardous chemical mixtures, into the environment. For example, phthalates, which are plasticisers added to plastics to provide flexibility, have been detected in water, air, and soil [26]. Humans are inevitably exposed to these chemicals from their surrounding environment, for example, via ingestion of contaminated drinking water or inhalation of contaminated air [27]. Similarly, degradation of plastic products during use (e.g., tyre abrasion, washing of textiles, and paint wear) lead to the release of secondary MNPs into the environment [1]. These secondary microplastics add to primary microplastic waste entering the environment such as from plastic microbead exfoliants in personal care products, with humans then being exposed to via ingestion or inhalation [7,28]. Importantly, MNPs have unknown safety [7,29,30]. Humans can also be exposed to plastic-associated chemicals and MNPs directly from product use, for example, via ingestion due to chemicals and MNPs migrating into food/drinks from food contact materials [8,31,32].
Disposal of plastic, the fourth and final stage of the plastic life cycle, also poses major environmental and human health challenges, which heavily depend on waste management processes [33]. In 2019, 353 out of the 460 Mt of plastic produced became waste. Of this waste, 50 % was landfilled, 19 % incinerated, and 9 % recycled [1]. The remaining 22 % was mismanaged, for example, through littering, uncontrolled dumpsites, accidental or uncontrolled fires including open burning, or leakage to the environment [34]. During disposal, there are several potential risks to environmental and human health due to leakage/release of hazardous monomers, polymers and plastic-associated chemicals, formation of MNPs from environmental degradation of plastic products, and greenhouse gas emissions from incineration/open burning, landfilling or dumping. Importantly, MNP pollution is likely to be transboundary [35]. Additionally, persistence and bioaccumulation (in the environment and in humans) of chemicals with potential to migrate from plastic materials are also of concern. Furthermore, plastic-associated chemicals may adversely impact the recyclability of plastic materials/products, and the safety of resulting materials [33].
Table 1 summarises environmental and human health impact factors of plastic-associated chemicals, polymers, and products throughout the life cycle of fossil carbon-based plastic [7,25] – extraction of raw materials, production and manufacturing, use (consumption) and disposal (waste management) [24].
Table 1.
Examples of environmental and human health impact factors of plastic chemicals, polymers, and products/applications throughout the life cycle of fossil carbon-based plastic.
| Impact factors | Chemicals | Polymer | Application/product | |
|---|---|---|---|---|
| Extraction of raw materials | ||||
| Environmental impact factors | Environmental release during extraction | Environmental release of metals (e.g., for use as catalysts or stabilisers) from mining, leading to soil contamination and water pollution | The estimated share of fossil carbon used in plastic production was 6 % in 2014 and is predicted to increase to 20 % by 2050 [36] Oil and gas extraction and coal mining lead to significant air pollution (e.g., particulate matter (PM), oxides of nitrogen (NOx), oxides of sulphur (SOx)) and water pollution (e.g., benzene, toluene, ethylbenzene, and xylene (BTEX), metals, radioactive isotopes) Release of volatile hydrocarbons, leading to formation of ground-level ozone Greenhouse gas emissions during coal, gas and oil extraction (methane) Accidental oil spills |
Relevant to all applications/products |
| Human health (safety) impact factors | Occupational risks during extraction | Heavy metal mining is a physically hazardous occupation with elevated risk of acute and chronic injury/disease and death | Oil and gas extraction and coal mining are physically hazardous occupations with elevated risk of acute and chronic injury/disease and death | Relevant to all applications/products |
| Occupational and fenceline community exposure during extraction | Occupational exposure during heavy metal mining and exposure in fenceline communities due to environmental release and contamination of farmland | Exposure in workers and fenceline communities to hazardous chemicals (e.g., ozone and volatile organic compounds (VOCs) such as BTEX, formaldehyde) due to air pollution and contamination of drinking water | Relevant to all applications/products | |
| Production & Manufacturing | ||||
| Environmental impact factors | Greenhouse gases and climate impact | Refinement of fossil feedstock to base chemicals including separation, conversion, and treatment [37] Production of additives and other chemicals used for plastic production |
Refinement of fossil feedstock to base chemicals including separation, conversion, and treatment Due to polymer feedstock (e.g., fossil carbon-based plastics) and energy use for plastic production |
Relevant to all applications/products |
| Environmental release during production & manufacturing | Environmental release of chemicals used in polymer production (e.g., solvents, catalysts) and plastic manufacture (e.g., additives) | Environmental release of plastic pellets/nurdles (primary micro- and/or nanoplastics (MNPs)), hazardous monomers and by-products (e.g., bisphenol A (BPA) and oligomers during injection moulding of polycarbonate (PC)) | Environmental release of hazardous chemicals which are application-specific during plastic manufacture (e.g., blowing agents for expanded polystyrene (EPS)) | |
| Human health (safety) impact factors | Occupational exposure during production & manufacturing | Occupational exposure to chemicals used in polymer production (e.g., solvents, catalysts) and plastic manufacture (e.g., additives) | Occupational exposure to hazardous monomers, VOCs, and by-products (e.g., vinyl chloride and hydrogen chloride respectively during manufacture of polyvinyl chloride (PVC)) | Occupational exposure to hazardous chemicals which are application specific (e.g., plasticisers for plasticised/soft PVC) during plastic manufacture |
| Fenceline community exposure during production & manufacturing | Exposure to chemicals used in polymer production and plastic manufacture due to their emissions/releases into waste, air, water, and soil (e.g., additives and catalysts) | Exposure to hazardous monomers, VOCs, and other by-products due to their emissions into waste, air, water, and soil | Exposure to hazardous chemicals which are application specific (e.g., plasticisers for plasticised/soft PVC) due to their emissions/releases into waste, air, water, and soil | |
| Use (consumption) | ||||
| Environmental impact factors | Known environmental release during use | Most chemical additives and non-intentionally added substances (NIAS) in plastics are not covalently bound to the polymer matrix, and may migrate from plastic products (e.g., plasticisers and flame retardants) | MNPs released during use (e.g., tyre abrasion) | MNPs released during use of personal care products containing plastic microbeads |
| Additional unintentional environmental release during use | Potential for chemicals used in production to remain in final product (e.g., processing aids and catalysts) and migrate into the environment | Potential for residual monomers and by-products, including polymer breakdown products, to remain in final product and migrate into the environment (e.g., BPA from PC) | Potential for application-specific chemicals in the final product to migrate into the environment during use Potential for release of chemicals, NIAS and residual monomers during use due to application (e.g., heat or mechanical stress) Application-based degradation during use (e.g., PC contact with heat), leading to environmental release of breakdown products & MNPs |
|
| Human health (safety) impact factors | Human exposure during use | Most chemical additives (e.g., plasticisers and flame retardants) and NIAS in plastics are not covalently bound to the polymer matrix, and therefore migrate from plastic products, leading to human exposure during use Potential for chemicals that were used in manufacture (e.g., processing aids and catalysts) to also remain in final product and migrate from plastic products |
Residual monomers and polymer breakdown products may be present in and migrate from plastic products (e.g., BPA from PC) Exposure to MNPs during use (e.g., PVC pipes) |
Applications whereby direct exposure to chemicals and polymer breakdown products occur, such as food contact materials and medical products (e.g., intravenous tubing) Exposure to hazardous chemicals which are application specific (e.g., plasticisers emitted into indoor environments from plasticised/soft PVC flooring) Potential for release of chemicals, NIAS, residual monomers and MNPs during use due to application, leading to exposure (e.g., release of BPA from PC container due to polymer degradation when exposed to heat [38,39] or acidic/alkaline solutions or use of detergents [40]) |
| Disposal (waste management) | ||||
| Environmental impact factors | Greenhouse gases and climate impact | Emission of greenhouse gases, occurring with degradation in the environment, but especially due to incineration, and accidental or uncontrolled fires, such as landfill fires, house fires, backyard burning and burning for recycling other materials (e.g., PVC-insulated cables for copper retrieval) Energy use for all disposal and recycling methods |
Release of ozone depleting substances with high global warming potential (e.g., historical use of chlorofluorocarbons (CFCs) as blowing agents for EPS) End-of-life management such as transport of EPS |
|
| Persistence | Persistent and bio-accumulative potential of the chemicals in the environment (e.g., persistent organic pollutants (POPs)) | Biodegradability of the polymer. Most fossil carbon-based polymers do not easily degrade in the environment/landfills | Availability of end-of-life management options (e.g., tyres – issues with recycling, downcycling, landfilling & incineration) | |
| Landfilling | Chemicals migrating from plastic products in landfills contaminate the air, soil, and groundwater, even long after the landfills are decommissioned | Monomers/oligomers and MNPs formed due to degradation in landfills and potentially released into the environment via reuse of mineralised refuse | Tyres can cause landfill instability, which can allow pollutants from other municipal waste to migrate into the environment Tyres are also a fire hazard (act as a fire propellant) and can be hazardous to human health by acting as a breeding ground for vector-borne illnesses |
|
| Littering and mismanaged waste | Chemicals, including additives and NIAS, migrate from plastic products, leading to environmental impacts | Leakage of monomers/oligomers or as MNPs due to degradation in the environment | Primary MNPs and other applications whereby there is a high likelihood for mismanaged waste/littering (e.g., single-use plastics such as cutlery and straws) | |
| Incineration | Potential for release of hazardous pollutants (e.g., polybrominated dibenzo-p-dioxins and dibenzofurans (PBDD/Fs) produced as by-products of brominated flame retardants) into the environment from malfunctioning incinerator and/or depending on the scavenging methods used and whether optimal operation conditions are met Chemicals (e.g., heavy metals used as stabilisers) migrate from incineration residues which are landfilled or processed, resulting in both air and soil contamination |
Potential for release of hazardous pollutants (e.g., chlorine gas, polychlorinated dibenzodioxins and/or dibenzofurans (PCDD/Fs), hydrogen chloride) into the environment depending on the scavenging methods used and whether optimal operation conditions are met | Relevant to all applications/products | |
|
Recyclability Not recyclable and/or hinder the recycling of other items |
Additives in plastic products are rarely recoverable for reuse (except via feedstock recycling, which is not currently scalable) Use of chemicals of concern (e.g., legacy POPs) in plastics hinders circularity of the products Additives added during recycling can also degrade the quality of recycled plastic due to incompatibility with additives already present in the product |
Halogenated polymers are less recyclable due to their intrinsic susceptibility to heat and they contaminate recycling streams, leading to release of hazardous chemicals (e.g., PVC) Use of polymer mixtures in production of plastic products affects recyclability of the product (e.g., cling films) |
Some plastic products (e.g., plastic films, soft plastics) are hard to recycle irrespective of the specific chemicals or polymers used to produce them Products made with a variety of materials (e.g., carton lined with plastic) are also harder to recycle Some polymers are not easy to recycle due to their application (e.g., EPS) |
|
| By-products of recycling | Dispersion of hazardous additives (e.g., flame retardants) and their transformation products (e.g., PBDD/Fs) into the broader environment during recycling | When polymers are melted in an extruder during mechanical recycling, VOCs are formed as degradation products MNPs released into the environment during mechanical recycling |
Downcycling (e.g., tyres as crumbed rubber) increases the leakage of hazardous chemicals into the environment | |
| Human health (safety) impact factors | Occupational exposure during collection and sorting | Occupational exposure to hazardous additives (e.g., flame retardants) during collection and sorting | Occupational exposure to monomers during collection and sorting (e.g., styrene when cutting foam boards) | Application-specific occupational exposure to hazardous chemicals (e.g., waste pickers) |
| Exposure due to landfilling | Hazardous chemicals (e.g., additives) migrate from plastic products in landfills, contaminate the air, soil, and groundwater, even long after the landfills are decommissioned, leading to occupational exposure and exposure in fenceline communities | Exposure to hazardous chemicals (e.g., hydrochloric acid from PVC degradation in landfills) migrating from plastic products in landfills and contaminating the air, soil, and groundwater, even long after the landfills are decommissioned | Tyres can cause landfill instability, which can allow pollutants from other municipal waste to migrate into the environment, leading to human exposure | |
| Occupational and fenceline community exposure due to incineration | Exposure in workers and fenceline communities to hazardous pollutants (e.g., PBDD/Fs produced as by-products of brominated flame retardants) due to their release from malfunctioning incinerator and/or depending on the scavenging methods used and whether optimal operation conditions are met | Occupational exposure to hazardous monomers (e.g., BPA and bisphenol analogues from PC) during incineration Potential exposure in fenceline communities to hazardous pollutants such as PCDD/Fs due to their release from malfunctioning incinerator and/or depending on the scavenging methods used and whether optimal operation conditions are met |
Relevant to all applications/products | |
| Occupational exposure during recycling | Occupational exposure in recycling workers to hazardous additives (e.g., flame retardants) and their transformation products (e.g., PBDD/Fs) | Occupational exposure to hazardous by-products such as VOCs formed during the melting process of recycling (e.g., hydrochloric acid from PVC) and MNPs during recycling | Application-specific occupational exposure to hazardous chemicals during recycling (e.g., informal e-waste recycling workers) | |
| Fenceline community exposure near recycling facilities | Exposure to hazardous additives (e.g., flame retardants) and their transformation products (e.g., PBDD/Fs) due to their emissions/releases into waste, air, water, and soil | Exposure to hazardous by-products (e.g., hydrochloric acid from PVC) and MNPs due to their emissions/releases into waste, air, water, and soil | Application-specific exposure to hazardous chemicals due to their emissions/releases into waste, air, water, and soil (e.g., living close to e-waste recycling facilities) | |
| Recyclability Cannot safely be recycled with regard to hazardous content | Use of chemicals of concern (e.g., legacy POPs) in plastics hinders safe circularity of the products | Halogenated polymers contaminate recycling streams, leading to release of and exposure to hazardous chemicals (e.g., PVC) | Potential for food safety issues with open-loop plastics | |
| Exposure via recycled products | Recycled products contain higher concentrations of additives, leading to greater exposure levels Introduction of additional NIAS to recycled products due to contamination during disposal (e.g., metals and POPs adsorbed from the environment) |
Introduction of monomers/oligomers formed during melting process of recycling as NIAS to recycled plastic products | Introduction of additional NIAS into recycled plastic due to application-specific contamination during use (e.g., chemicals from previously packaged food) | |
1.3. Motivation for this review
The impacts of plastic across its life cycle are dependent on the associated polymers, chemicals, and applications/products. As a result, it is imperative that policies and regulations aimed at mitigating the impacts of plastic on environmental and human health consider all three dimensions of plastics by looking at the plastic problem from each of the three lenses. While there are national and/or international regulations to phase out the use of hazardous chemicals and problematic plastic products, no global framework for identifying and regulating polymers of concern in plastics throughout their life cycle exists [3,5,41,42]. Although there have been several reviews on the impacts of plastic on environmental and human health, these mainly focus on the impacts associated with plastic-associated chemicals [7,43,44], MNPs [7,45], or specific applications of plastic [7,46]. Similarly, LCA studies are largely focused on human toxicity impacts from non-plastic specific exposure to chemical substances [47] or environmental sustainability issues of plastic products/specific plastic applications [48]. Importantly, LCA studies on plastic polymers typically focus on one or more focal areas of concern with the primary focus being on overall global warming potential, and the impacts associated with disposal scenarios [16,[49], [50], [51], [52]]; with far less consideration of environmental/human exposure to the chemicals associated with the plastic polymers (e.g., processing aids, additives, NIAS) during production, use as well as disposal/recycling [53,54]. Furthermore, studies on the human health impacts of exposure to the polymers themselves have almost exclusively been conducted using skin patch tests [44]. This review provides a synthesis of scientific evidence on the impacts of plastic across its life cycle, viewed through a polymer lens and accounting for environmental release of and human exposure to plastic-associated chemicals and MNPs, including in product/application-specific situations, which will provide critical information for consideration in the negotiations for the Global Plastics Treaty, which aims to end plastic pollution by addressing all stages of its life cycle [[55], [56], [57], [58]].
1.4. Prioritised plastic polymers
Plastic materials can be made of various polymers depending on the properties required such as strength, flexibility, resistance to corrosion, heat/electrical conductivity, transparency and cost. To examine the impacts of plastic from a polymer lens, four examples of problematic polymers were selected for this review. The prioritisation process considered the hazardous properties of monomers and their degree of migration from food contact plastics, the amount and hazardous properties of additives and particulates associated with the polymers, as well as the polymer type and uniquely pressing challenges associated with the processing of, or end-of-life management of, particular polymers.
Specifically, the substances of potential concern list by Wiesinger and colleagues (2021) [4] was used to identify monomers which are known organic carcinogens, mutagens or reproductive toxicants with very high production volumes (>10 Mt per year) and are not regulated by any international convention. A total of 13 chemicals were identified from the list, namely BPA (CASRN: 80-05-7; monomer for polycarbonate (PC) and epoxy resins), styrene (CASRN: 100-42-5; monomer for polystyrene (PS), styrene acrylonitrile copolymer (SAN), acrylonitrile butadiene styrene (ABS), and styrene-butadiene rubber), vinyl chloride (CASRN: 75-01-4; monomer for polyvinyl chloride (PVC) and polyvinylidene chloride), 1,3-butadiene (CASRN: 106-99-0; monomer for polybutadiene, styrene-butadiene rubber, and ABS), ethanol (CASRN: 64-17-5; not a monomer), phenol (CASRN: 108-95-2; phenol-formaldehyde resin monomer), acrylonitrile (CASRN: 107-13-1; monomer for ABS, polyacrylonitrile (PAN), SAN), formaldehyde (CASRN: 50-00-0; monomer for phenol-formaldehyde resin, urea-formaldehyde resins, melamine-formaldehyde resins, and polyformaldehyde), methanol (CASRN: 67-56-1; not a monomer), propylene oxide (CASRN: 75-56-9; monomer for polypropylene glycol), ethylene glycol (CASRN: 107-21-1; monomer for polyethylene terephthalate (PET) and polyethylene glycol), vinyl acetate (CASRN: 108-05-4; monomer for polyvinyl acetate and polyvinyl alcohol), isobutylene (CASRN: 115-11-7; polyisobutylene monomer) [59].
The FCCmigex database [9] was then used to link the monomers identified above to polymers of concern with respect to their degree of migration from food contact plastics. Monomers BPA and styrene had the highest evidence of leaching/migration from plastic food contact materials with 191 and 109 database entries respectively compared to ≤31 for the other 11 chemicals. When grouped by material, PC and PS materials had the highest evidence of leaching their respective monomers (77 and 76 database entries respectively compared to ≤49 for the other nine known polymers included in the database).
In addition to PC and PS, PVC was chosen for this review because the plasticisers diethylhexyl phthalate (DEHP, CASRN 117-81-7) and di-n-butyl phthalate (DnBP, CASRN 84-74-2) were the two most common food contact chemicals in plastics in the FCCmigex database. About 80 % of all plasticisers produced are used to make plasticised PVC (PPVC), with the typical amount being 10 % – 70 % weight/weight of PVC material [6]. Additionally, PVC is used in a range of consumer products such that human exposure to PVC-associated chemicals occurs via all exposure pathways [60]: inhalation (e.g., vinyl flooring [60]), ingestion (e.g., food contact materials [61]), dermal absorption (e.g., personal care products packaging [62]), and direct exposure (e.g., medical devices [63]). Moreover, PVC MNPs have shown the greatest particle toxicity (greatest increase in pro-inflammatory cytokine release) in primary human monocytes in vitro [64]. Importantly, this effect of polymer has been found to be independent of leached chemicals and particle size/shape, with an independent effect of leached chemicals and particle size/shape also demonstrated. Furthermore, chlorinated polymers like PVC pose unique challenges, including in end-of-life management, compared to other plastics [65].
Lastly, polybutadiene rubber was chosen due to impacts associated with the vulcanisation process and thermosetting resins (PC, PS and PVC are thermoplastics). Also, end-of-life management of tyres pose unique challenges, and road transport, in particular the wear of tyres and brake pads, is one of the main sources of microplastic pollution, with tyre abrasion accounting for 3 % of total mismanaged plastic waste and 25 % of total microplastic waste [34].
It is important to note that there are several other important polymers which may require additional considerations, not covered here by the four prioritised polymers. There is no established priority list of “problematic polymers” or “polymers of concern” and criteria and/or framework for identifying polymers of concern with respect to environmental and human health impacts are currently being proposed for the Treaty and other regulatory bodies [3,66,67]. Production volume and microplastic toxicity have been consistently proposed to be included in the criteria. It has been reported that 24 % of all plastics used in 2019 were made of polyethylene (PE), 16 % were made of polypropylene (PP), 5 % were made of PET, and 4 % were made of polyurethane (PUR) [34]. A recent study ranking polymers based on hazards of microplastics in the environment reported that PUR, PVC, PAN, ABS, poly(methyl methacrylate), SAN, thermoplastic polyurethanes, unsaturated polyester, PET, PS, and high-density PE were the top-ranking polymers of concern in descending order [68]. Another study evaluated the risk of harm from microplastics across the entire material life cycle and found that PVC, PP and PS had the highest cumulative risk ranking [67]. Polyamine (e.g., nylon) is also considered an important polymer of concern due to evidence of hazardous impacts of inhaled microfibers [7]. Despite the common occurrence of these polymers in the environment and their relevance for consideration, they were beyond the scope of this review due to our prioritisation process, which places more emphasis on unavoidable issues inherent to the polymer such as hazardous properties of monomers and additives, their migration from food packaging, and end-of-life processing.
1.5. Objectives
This review focuses on four examples of common polymers made from hazardous monomers: PCs made from bisphenols, PS made from styrene, PVC made from vinyl chloride, and polybutadiene made from 1,3-butadiene. The main objective of this review is to identify and compile evidence of environmental leakage, human exposure, and health impacts associated with PC, PS, PVC, and polybutadiene across their life cycle, with regards to the chemicals and processes used to produce them and the additives commonly incorporated to achieve the properties needed for applications for which the polymers are generally used. For each polymer, we have provided the type of polymer (thermoplastic vs thermoset), the properties of the polymer, its common uses/applications, production volume and percentage of total plastic production, the different types of polymers within the category and their differences (where applicable), information on alternatives (where applicable), and a table compiling extensive evidence of environmental leakage, human exposure, and health impacts associated with the polymer.
The table contains information on the life cycle stages and sub-stages (see Fig. 1) to which the plastic pollutants and their impacts are associated with, and the plastic pollutants relevant for each stage/sub-stage, including monomers, oligomers, additives, processing aids, NIAS, PM, and MNPs. Chemical classes and/or specific chemicals are listed and the functions of all known intentionally added substances are provided, along with hazard information on specific plastic-associated chemicals obtained from Wiesinger and colleagues (2021) [4], the International Agency for Research on Cancer (IARC) Classified Agents List [69], the Substance of Very High Concern (SVHC) list by the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) [70], the ChemSec Substitute it Now (SIN) list [71], and the list of persistent, bioaccumulative and toxic (PBT) substances in the Stockholm Convention [72]. Evidence of environmental leakage, human exposure, and health impacts for each pollutant/group of pollutants/chemical class at each stage/sub-stage are summarised. Lastly, information on alternatives for the polymer, chemical, or product is provided, where relevant, and we have noted how their environmental and human health impacts differ.
The CAS registration number and hazard classification of the specific chemicals identified within this review are provided in the Supplementary Material (Excel Tables S1–S5). Hazard classifications were obtained from Wiesinger and colleagues (2021) [4], in which chemicals with “high level of concern” were defined as substances that have a production volume of ≥1000 metric tons per year and fulfil one or more of the following hazard criteria under EU REACH: PBT/very persistent and very bioaccumulative, carcinogenicity, mutagenicity, reproductive toxicity, specific target organ toxicity upon repeated exposure, chronic aquatic toxicity, respiratory sensitisation, and endocrine disruption.
Overall, this review demonstrates important considerations, in addition to the hazardous properties of constituent monomers, when evaluating/prioritising ‘problematic’ polymers. This review is therefore broadly relevant to scientists, policy makers and other stakeholders involved in the development of criteria for polymer assessment and management across the globe.
2. Polycarbonate
PC is a thermoplastic polymer with good thermal resistance, mechanical properties, durability, and high optical transparency, making it suitable for a range of commodities and for engineering applications in construction, automotive, aircraft, data storage, electrical, and telecommunication hardware [73]. The annual global demand for PC was 4.6 Mt in 2019 [74], accounting for 1 % of total plastic production [1].
PC resins can be divided into two structural classes based on their carbon chain backbone: aliphatic and aromatic PCs. The main differences between the chemical structures of the aliphatic and aromatic PCs are due to the different types of monomers used in their synthesis. Aliphatic PCs have backbones of repeating carbonate (-O–C(O)–O-) linkages, while aromatic PCs are formed when the carbonate group is directly connected to an aromatic carbon [75]. There are no aromatic groups between the carbonate linkages for the aliphatic PCs [75]. These structural differences mainly influence the physicochemical properties and degradability of PCs [75].
-
•
Aliphatic PCs consist of non-aromatic (aliphatic) repeating units and are generally biodegradable. Due to their aliphatic chemical structure, they are generally more flexible with lower glass transition temperature and more biocompatibility than aromatic PCs [76]. This makes aliphatic PCs more suitable for biomedical and pharmaceutical applications [[76], [77], [78]].
-
•
Aromatic PCs consist of aromatic repeating units. Their structure results in higher glass transition temperatures, more heat resistance and higher rigidity in comparison with the aliphatic PCs [75]. Higher mechanical and thermal stabilities of aromatic PCs make them more suitable for electrical and automotive industry [75]. Aromatic PCs are used to make thermoplastics. The most important and widely used aromatic PC is poly (bisphenol A) carbonate [79,80]. Regulatory bans and restrictions placed on BPA use have led to its substitution with BPA analogues, such as bisphenol S (BPS) and bisphenol F (BPF) [81]. These substitute chemicals are likely to present similar health risks [[82], [83], [84]], being structurally similar, but human biomonitoring data are currently sparse [84,85].
While aliphatic and aromatic PCs have distinct properties, there are some applications where both types of PCs can be used interchangeably or where their properties overlap, e.g., electronics, automotive parts. Nevertheless, aliphatic PCs cannot be used as an alternative to aromatic PCs for most applications. Table 2 outlines the life cycle of PCs, providing insights into the pollutants associated with each stage, along with their environmental leakage and human exposure or health impacts.
Table 2.
Environmental leakage and human health impact factors of polycarbonate (PC) throughout its life cycle.
| Life cycle stage | Plastic pollutants | Environmental leakage and/or human exposure/health impacts |
|---|---|---|
| Extraction of raw materials | ||
| Extraction of fossil resources – for fossil carbon | Greenhouse gases, particulate matter, metal(loid)s, volatile organic compounds such as benzene, toluene, ethylbenzene, and xylene [7] | Emitted during plastic production, transport and/or end-of-life processes [7], leading to climate, environmental and health impacts [14,15]. Alternatives: Bio-based PCs are derived from carbon dioxide and renewable feedstocks such as plant oils, industrial by-products (crude glycerol) and pure fatty acids [86]. Bio-based PCs require similar considerations as fossil carbon-based PCs across the rest of their life cycle as detailed below. Some bio-based PCs may be aromatic bio-PCs [87] and others may be aliphatic bio-PCs [88], which have added end-of-life options such as enhanced biodegradability [89]. Nevertheless, similar to fossil carbon-based PCs, aliphatic and aromatic bio-PCs have distinct properties and while there can be overlaps in application, aliphatic bio-PCs cannot be used as an alternative for aromatic bio-PCs for most applications. |
| Production & Manufacturing | ||
| Monomer production, Polymerisation & Conversion | Phosgene - used in polymerisation of bisphenols to aromatic PCs | Highly toxic and corrosive [90] reproductive toxicant [4] – leaks in chemical facilities [91,92]. |
| Methylene chloride (CH2Cl2) – polymerisation solvent | A carcinogenic, mutagenic, reproductive toxicant [4] – occupational exposure linked to increased risk of cancer [93]. | |
| Bisphenol A (BPA) and bisphenol analogues - monomer | BPA is an endocrine disruptor [4] with several known health impacts, including increased risk of cardiometabolic disorders [94,95], polycystic ovary syndrome [96], type 2 diabetes [97,98], and obesity [[99], [100], [101]]. Potential emissions from BPA manufacturing facilities, during transport or during processing of BPA [80]. BPA is released in the indoor atmosphere [102], leading to occupational exposure [85,103]. Alternatives: BPA-free aromatic PCs can be made with bisphenol analogues such as BPS and BPF, which may have similar health effects [[82], [83], [84]]. Some bio-based PCs may be BPA-free [87], in particular, aliphatic bio-PCs do not contain BPA or bisphenol analogues [88]. |
|
| Use (consumption) | ||
| BPA and bisphenol analogues | Migrates from the PC products leading to dietary exposure [80,104,105], transdermal exposure [106] or inhalation exposure via BPA in air and dust [107]. See health impacts above. | |
| Non-intentionally added substances (NIAS) | Several NIAS such as oligomers and other PC-degradation products migrate from PC products [8,9,108] – potential for human exposure and unknown safety. | |
| Disposal (waste management) | ||
| Landfilling | BPA and bisphenol analogues | Release and/or migrate from PC at environmental temperatures and normal pH in landfills [80]. |
| Micro- and/or nanoplastics (MNPs) | Formed due to PC degradation, potential release into the environment via reuse of mineralised refuse [109]. | |
| Littering and mismanaged waste | BPA and bisphenol analogues | Released into the environment due to PC degradation [110,111] – detected in fresh fish tissues and seafood, which affects humans via the food chain [112]. |
| MNPs | Released into the environment due to PC degradation [80]. MNPs have unknown safety [7]. | |
| Incineration | BPA and bisphenol analogues | Occupational exposure with subsequent health risks [113,114]. |
| Recycling | PC products are usually not accepted in curbside collection, but drop-off and special collection services exist. Collected PC products can be mechanically recycled or depolymerized [115,116]. | |
3. Polystyrene
PS is a thermoplastic polymer made from the styrene monomer. It is a lightweight material and can exhibit high thermal insulation and transparency. Different forms of PS are used in both short- and long-lived applications, such as packaging materials, food containers, insulation, and electronics casings. It is one of the most widely used plastics. The annual production volume of PS was 21 Mt in 2019, accounting for 5 % of all polymers in plastic [1]. PS can be either solid or foamed and there are four common forms.
-
•
Rigid PS refers to solid, non-foamed PS, often used in applications where a solid, durable material is required, such as disposable cutlery, food containers, and packaging trays.
-
•
EPS is a lightweight, foamed PS material produced by expanding PS beads with steam, then moulding them into shape. EPS is known for its excellent thermal insulation properties, making it ideal for use in construction as insulation boards, packaging materials, and protective packaging for fragile items. It is also rigid and provides cushioning against impact.
-
•
Extruded PS (XPS) is another type of foamed PS, but it is produced through an extrusion process rather than bead expansion, providing it with greater strength, moisture resistance, and thermal insulation properties. It is commonly used in applications where high compressive strength and moisture resistance are required, such as insulation for roofs, walls, and floors in construction.
-
•
PS microbeads are tiny spherical particles of PS and are classified as primary microplastics. PS microbeads can provide texture, exfoliation, or abrasiveness in cosmetic products like facial scrubs and toothpaste.
There are minimal opportunities to substitute or vary the materials used in PS production [117]. Table 3 outlines the life cycle of PS, providing insights into the pollutants associated with each stage, along with their environmental leakage and human exposure or health impacts, distinguishing between the different forms of PS where applicable.
Table 3.
Environmental leakage and human health impact factors of polystyrene (PS) throughout its life cycle, distinguishing between rigid PS, expanded PS (EPS), extruded PS (XPS), and PS microbeads where applicable.
| Life cycle stage | Plastic pollutants | Environmental leakage and/or human exposure/health impacts |
|---|---|---|
| Extraction of raw materials | ||
| Extraction of fossil resources – for fossil carbon | Greenhouse gases, particulate matter (PM), metal(loid)s, volatile organic compounds (VOCs) such as benzene, toluene, ethylbenzene, and xylene [7] | Emitted during plastic production, transport and/or end-of-life processes [7], leading to climate, environmental and health impacts [14,15]. Alternatives: Bio-based PS are made from renewable resources [118]. However, they have the same issues for consideration for the rest of their life cycle as the fossil carbon-based PS as detailed below. Bio-sourced alternatives like polyhydroxyalkanoates (PHAs) have added end-of-life benefit of being biodegradable/compostable and have the potential to replace PS in certain applications, without the production, use and end-of-life issues related to styrene and PS. |
| Production & Manufacturing | ||
| Monomer production | Benzene - primary chemical needed for production of styrene monomer | A carcinogen [69,119] on the ChemSec Substitute it Now (SIN) list [71] – potential for occupational exposure [120,121]. |
| Monomer production & Polymerisation | Styrene - monomer | A probable carcinogen [122] on the SIN list [71] with known toxicity to animals and possibly hazardous to humans [[122], [123], [124]] – potential emissions at styrene and PS manufacturing facilities [117]. Risk of runaway reactions [125], which have caused numerous injuries and fatalities in multiple events (see Table 1 [126]). |
| Polymerisation | Azobisisobutyronitrile (AIBN) – polymerisation initiator | Hazardous chemical on the SIN list [71] – potential for occupational exposure [117]. |
| Compounding & Conversion | Flame retardants including historical use of brominated flame retardants such as hexabromocyclododecane (HBCD) and polybrominated diphenyl ethers (PBDEs) | Flame retardants can be hazardous by nature [67] – potential for environmental release and occupational exposure. HBCD is bio-accumulative [4], carcinogenic [4], and a persistent organic pollutant (POP) [117] listed in Annex A of the Stockholm Convention for phasing out and eventual elimination [72]. Environmental emissions [127,128] and occupational exposure (HBCD production and processing plants [127], PS manufacturing plants [129] and from thermal cutting of PS [130]) can lead to environmental and human health risks [127,131], including type 2 diabetes [132]. PBDEs are POPs with known health impacts [7], including impaired neurodevelopment [133,134], reduced birth weight [135] and increased risk of gestational diabetes [136]. Several PBDEs are on Annex A of the Stockholm Convention for phasing out and eventual elimination [72]. There is potential for occupational exposure during extrusion and moulding due to high thermal stress [137]. Alternatives: Substitute flame retardants such as organophosphate esters (OPEs) also have known health impacts [7,138], including neurotoxicity [139] and altered pregnancy and birth outcomes [140]. |
| Blowing agents for EPS including historical use of different types of chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) | CFCs and HCFCs are ozone depleting substances with high global warming potential [117]. May be retained in the final EPS product – potential for environmental release during use and disposal [117]. |
|
| Conversion | Styrene and other VOCs | Emitted into the workplace air (see Tables X and XI [141]), posing risks to workers. See health impacts above. |
|
Use (consumption) Alternatives: Conventional plastics with less concern for human health than PS such as high-density polyethylene (HDPE), polypropylene (PP), and polyethylene terephthalate (PET) are widely used to replace PS in a range of applications. Bioplastics, including bio-derived and biodegradable/compostable bioplastics, are being used as alternatives in some applications such as coffee lids. | ||
| Styrene | Migrates into food [142] and hot liquids [143] and is released into air (e.g., when cutting of insulation foam boards) [117], leading to human exposure and potential health risks. See health impacts above. | |
| AIBN | Hazardous chemical on the SIN list [71] – can still be found in the final product [144], and therefore there is potential for impacts on environmental and human health [145]. | |
| Tetramethylsuccinonitrile (TMSN) | Release/migration of this decomposition product of AIBN during use [9,146] – potential for human exposure and health impacts. | |
| Styrene dimers/oligomers & other non-intentionally added substances (NIAS) [147] | Release/migration during use [108] – potential for human exposure and health impacts. | |
| Flame retardants including historical use of HBCD | Present in indoor environments [148], leading to inhalation and ingestion exposure [117,149]. See health impacts above. | |
| PS microbeads - Primary micro- and/or nanoplastics (MNPs) | Leakage and direct human exposure via ingestion, inhalation or potentially absorption via the skin due to use as exfoliants in personal care products [150,151] – potential impacts on the environment [152] and human health [153]. | |
| Secondary MNPs | Migrates (leaches) into food from food contact materials such as plastic food containers [154,155] and food trays [156], which can lead to human exposure and potential health impacts [7]. | |
|
Disposal (waste management) Alternatives: Substitute biodegradable materials for common PS applications are currently emerging since the high volume-to-weight ratio of foamed PS makes it impractical to transport or landfill. These can range from packaging solutions with traditional insulating materials (such as wool [157]) or new technologies like chitin-based [158] or starch-based [159,160] (biodegradable) foams. | ||
| Landfilling | PBDE, HBCD, CFC and HCFC | Potential for legacy chemicals to migrate from PS in landfills [117,161]. See impacts above. |
| Secondary MNPs | Found in landfill leachates [162] and act as a reservoir of antibiotic resistance genes [163] – potential for environmental and human health impacts. | |
| Littering and mismanaged waste | Secondary MNPs | Released into the environment (one of the most common MNPs in the marine environment [164]) – potential impacts on human health [153] and the environment [152,165]. |
| Flame retardants and other additives | Migrating from PS into the environment [166,167] – potential impacts on the environment [168] and on human health due to indirect exposure from contaminated food products [169,170]. | |
| Styrene oligomers & other NIAS | NIAS produced from PS degradation and migrate into the environment [171]. | |
| Accidental or uncontrolled fires | Styrene monomers, halogenated flame retardants (including HBCD and PBDE), polybrominated dibenzo-p-dioxins and dibenzofurans (PBDD/Fs), sulphur dioxide, nitrogen dioxide, PM, carbon monoxide | Released into the environment [117,137,172] – potential environmental impacts and exposure can lead to several health impacts [7,173]. See specific health impacts above. PBDD/Fs show reproductive effects and immunotoxicity in animal studies [174,175]. |
| Incineration | PBDD/Fs | Produced as by-products of brominated flame retardants and released into the environment from malfunctioning incinerators [117], leading to potential exposure and health impacts in workers and fenceline communities. |
|
Recycling Alternatives: Low recycling rate relative to other plastic types [176], for example, due to the high volume-to-weight ratio of EPS. PS requires a modified process for mechanical recycling compared to other plastics. Consequently, most recycling facilities do not offer PS recycling. Conventional plastics with less concern for human health and better recyclability than PS (e.g., HDPE, PP, PET) can be used to replace rigid PS in a range of applications. | ||
| Processing | Flame retardants including historical use of HBCD and PBDEs and their transformation products under thermal stress (e.g., PBDD/Fs) | Potential exposure for recycling workers and dispersion into the broader environment when the material is processed [117,137]. See health impacts above. |
| Recycled products | Legacy POPs (e.g., HBCD) | Risk of re-introduction into recycled PS product [177]. See health impacts above. |
4. Polyvinyl chloride
PVC is a thermoplastic polymer with good durability, flexibility and low cost, making it suitable for various applications such as in the building and construction sector, in food packaging, children's toys, furniture, medical devices, and adhesives [[178], [179], [180], [181]]. The annual production volume of PVC was 51 Mt in 2019, accounting for 11 % of all polymers in plastic [1].
There are two main types of PVC, namely rigid or unplasticised PVC (uPVC) and flexible or plasticised PVC (PPVC), with the main difference being that in contrast to PPVC, uPVC does not incorporate plasticisers (e.g., phthalates) at the compounding step and therefore, no plasticiser leakage/exposure occurs throughout the production, use, or disposal/recycling stages. Both uPVC and PPVC contain other additives such as stabilisers [182], which are added to PVC resin powder used to form PVC pellets. These additives may migrate/leak throughout the production, use, and disposal/recycling stages of the life cycle of both PPVC and uPVC. Alternatives for PVC are dependent on short-life vs long-life applications and rigid uPVC vs flexible PPVC. Table 4 outlines the life cycle of PVC, providing insights into the pollutants associated with each stage, along with their environmental leakage and human exposure or health impacts.
Table 4.
Environmental leakage and human health impact factors of polyvinyl chloride (PVC) throughout its life cycle, distinguishing between unplasticised PVC (uPVC) and plasticised PVC (PPVC) where applicable.
| Life cycle stage | Plastic pollutants | Environmental leakage and/or human exposure/health impacts |
|---|---|---|
| Extraction of raw materials | ||
| Extraction of fossil resources – for fossil carbon | Greenhouse gases, particulate matter (PM), metal(loid)s, volatile organic compounds (VOCs) such as benzene, toluene, ethylbenzene, and xylene [7] | Emitted during plastic production, transport and/or end-of-life processes [7], leading to climate, environmental and health impacts [14,15]. Alternatives: Bio-PVC is derived from ethanol obtained from renewable resources such as starch crops, sugar crops and lignocellulosic biomass [118]. Bio-PVC otherwise still require similar considerations as fossil carbon-based PVC materials for the rest of their life cycle as detailed below. |
| Production & Manufacturing | ||
| Monomer production | Mercury – About 50 metric tons utilised in 2020 [183] by older technologies that are still operational for chlorine production [184]; also used as a catalyst for production of vinyl chloride monomer [185] | Highly hazardous chemical, classified as a reproductive toxicant [4,186] – released into the air, water, and landfills from mercury cell plants [187], leading to occupational exposures [188], potential exposures in fenceline communities [189], and potential health impacts, even at low levels [190]. |
| Asbestos – About 100–800 metric tons [191] is used by older technologies for chlorine production [184] per year | A carcinogen [[192], [193], [194]] – released into the air and landfills from asbestos diaphragm cell plants [187] and there is potential for occupational exposure [191]. | |
| Per- and polyfluoroalkyl substance (PFAS) – PFAS diaphragms or PFAS-coated membranes such as Nafion used in chlorine production [185] | PFAS are known persistent organic pollutants (POPs) with several human health impacts [195,196], including reduced birth weight [197], increased allergic outcomes [198], altered thyroid and immune function [199,200], and impaired neurodevelopment [201] – unregulated and undocumented releases from PFAS diaphragms or PFAS-coated membranes during chlorine gas production [187]. Nafion by-products are novel PFAS, with evidence of potential human health impacts [202] – released into the environment [203], leading to exposure in fenceline communities [204]. |
|
| Chlorine gas – used for production of vinyl chloride monomer | Inherently hazardous (aquatic toxicity and specific target organ toxicity upon repeated exposure) [4] and can contribute to the formation of polychlorinated dibenzodioxins and/or dibenzofurans (PCDD/Fs) – can be released into the air [187]. See health impacts of PCDD/Fs below. | |
| Ethylene dichloride – produced as an intermediate during production of vinyl chloride monomer | A possible carcinogen [4,205] on the Substance of Very High Concern (SVHC) list by the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) [70] as well as the ChemSec Substitute it Now (SIN) list [71] – released into the air [185], leading to exposure and health impacts in workers [206,207] and fenceline communities [208]. | |
| Other chlorinated compounds, including polychlorinated biphenyls (PCBs), hexachlorobutadiene (HCBD), PCDD/Fs, carbon tetrachloride, and chloroform | Released into the environment [187,209,210] – potential for occupational exposure [185]; exposure in fenceline communities reported [211]. PCBs and HCBD are POPs listed in Annex A of the Stockholm Convention for phasing out and eventual elimination and in Annex C for reducing unintentional releases [72]. PCBs have several known health impacts, including lower birth weight [212] and increased risk of endometriosis [213], type 2 diabetes [214], and breast cancer [215,216]. HBCD has known human and animal toxicity [217], and is a possible human carcinogen [72]. PCDD/Fs are POPs listed in Annex C of the Stockholm Convention for reducing unintentional releases with the goal of eventual elimination [72]. Have several health impacts, including change in body composition [218] and increased risk of endometriosis [213] and incidence of type 2 diabetes [219]. Carbon tetrachloride is a probable carcinogen [205] on the SIN list [71] and is an ozone depleting substance with high global warming potential [185] and hazardous properties [220], including liver toxicity and cancer [221]. Chloroform exhibits specific target organ toxicity upon repeated exposure [4] and is on the SIN list [185]. It has known human and animal toxicity, including reproductive and developmental toxicity and carcinogenicity [222]. |
|
| Polymerisation | Azobisisobutyronitrile (AIBN) [223] – polymerisation initiator | Hazardous chemical on the SIN list [71] – potential for occupational exposure. |
| Monomer production, Polymerisation & Compounding | Vinyl chloride – monomer | Primary health concern during PVC production [224]. Known human carcinogen [205] on the SVHC list [225] – released into the environment via waste, in air and in water [226], leading to potential exposure in workers and fenceline communities [208]. Occupational exposure via inhalation or skin contact can lead to respiratory problems [[227], [228], [229]], liver damage [207], and an increased risk of developing cancer [224], particularly angiosarcoma of the liver [225,230]. |
| Compounding & Conversion of PPVC | Phthalates, e.g., diethylhexyl phthalate (DEHP) and di-n-butyl phthalate (DnBP) – plasticisers (PPVC can contain up to 60 % plasticisers [226]) | DEHP and DnBP are a known carcinogenic and reproductive toxicants [4] on the SIN list [71]. Released into external environment via air emissions and in water [226]. Occupational exposures are well documented [231], as are the health impacts of phthalates, including impaired neurodevelopment [232], increased risk of type 2 diabetes and insulin resistance [98,233] and reproductive outcomes [234,235]. |
| Bisphenol A (BPA) – antioxidant | Endocrine disruptor [4] – Potential for environmental release and occupational exposure [103]. | |
| Conversion of PPVC and uPVC | Hydrogen chloride | Emitted into the workplace air (see table IX [141]), posing risks to workers. |
| Tetramethylsuccinonitrile (TMSN) | Shows specific target organ toxicity upon repeated exposure [4]. Occupational exposure to this decomposition product of AIBN via inhalation can lead to convulsions and hypoglycemia [236]. | |
| Use (consumption) | ||
| Use of PPVC products | Plasticisers such as phthalates (e.g., DEHP and DnBP) and adipates, e.g., di-2-Ethylhexyl adipate (DEHA) | Migrates into patients [237,238], food [6,239,240], water [226], and indoor environments [185], leading to human exposure [238,241]. See health impacts of phthalates above. DEHA is an aquatic toxicant [4] and a potential endocrine-disrupting chemical [242,243]. Alternatives: Conventional plastics with less concern for human health than PVC (e.g., low-density polyethylene (LDPE), high-density polyethylene (HDPE), polypropylene (PP), polyethylene terephthalate (PET)) are widely used to replace PVC in a range of applications such as food packaging or medical tubing. This can avoid exposure to hazardous PVC additives such as the widely used phthalates. |
| Use of both PPVC and uPVC | Stabilisers, e.g., heavy metal stabilisers including organotins and historical use of lead and cadmium | Lead and cadmium are carcinogens [4] on the SVHC [70] and SIN lists [71] – migrate from PVC products (higher migration out of the polymer matrix from PPVC than uPVC although migration occurs from both) [244], posing potential environmental and health hazards [7,185], including increased risk for cardiovascular and renal disease [[245], [246], [247]]. Organotin exposure is associated with increased risk of congenital cryptorchidism [248] and can lead to endocrine and metabolic disturbances [7] – can migrate from PVC products [249]. |
| BPA | Endocrine disruptor [4] – migrates into water [250,251] and food, especially when the plastic product is exposed to heat or acidic/alkaline solutions due to polymer degradation during use [252], which can lead to human exposure and impact health. See health impacts above. | |
| TMSN | Found in and migrates from the final product [9,146], leading to potential for environmental and human exposure. | |
| Non-intentionally added substances (NIAS) [147] | Several NIAS such as vinyl chloride tetramer and antioxidant derivatives migrate from PVC products [8,9,108] – potential for human exposure and unknown safety. Nonylphenol, which is formed during the degradation of tris(nonylphenol) phosphite, an antioxidant used in PVC [244], is a known endocrine disrupting chemical associated with increased risk of ovarian, uterine, pituitary, and testicular cancers [253] – migrate into food and water [250,251,254]. |
|
| Residual vinyl chloride monomer | Can be present in and migrate from PVC products [[255], [256], [257]], leading to human exposure and potential health impacts. See health impacts above. | |
| Secondary micro- and/or nanoplastics (MNPs) | Migrates (leaches) from PVC products and PPVC MNPs act as a vector for contaminants such as phthalates [258] – potential for human exposure and health impacts. | |
|
Disposal (waste management) Alternatives: Bio-based alternatives such as cellulose acetate bioplastic wood, pulp/cotton fibres and polylactic acid (PLA) [259] have added end-of-life options such as enhanced biodegradability and compostability [260]. | ||
| Landfilling | Hazardous chemicals, including hydrochloric acid and above-mentioned additives | Known to contaminate the air, soil, and groundwater, even long after the landfills are decommissioned [244]. |
| Littering and mismanaged waste | Secondary MNPs | Released into the environment [261] and can leach hazardous chemicals including phthalates from PPVC MNPs [262,263] and heavy metals from both PPVC and uPVC MNPs [264]. |
| Accidental or uncontrolled fires (e.g., landfill fires, house fires) and burning PVC-insulated cables for copper retrieval | Hazardous pollutants such as PM, polycyclic aromatic hydrocarbons (PAHs), heavy metal stabilisers such as lead and cadmium, benzene, chlorine gas, carbon monoxide, hydrogen chloride, SOx, PCBs, and PCDD/Fs. | Released into the environment, posing risks to environmental (e.g., acid rain) and human health [7,244,[265], [266], [267]]. See health impacts above. |
| Incineration | Hazardous pollutants such as PM, PAHs, heavy metal stabilisers such as lead and cadmium, benzene, chlorine gas, carbon monoxide, hydrogen chloride, SOx, PCBs, and PCDD/Fs. | Potential for release into the environment depending on the scavenging methods used and whether optimal operation conditions are met [244]. See health impacts above. |
| Soluble salts, heavy metals, PCBs, and PCDD/Fs. | Migrating (leaching) from incineration residues which are landfilled or processed, resulting in both air and soil contamination [244]. See health impacts above. Alternatives: Conventional plastics (e.g., LDPE, HDPE, PP, PET) do not include chloride ions and do not produce PCBs and PCDD/Fs when incinerated, although other pollutants may be released similar to the above. |
|
| Mechanical/chemical recycling | Monoaromatics, oxygenated VOCs, chlorinated VOCs, hydrochloric acid and additives, such as plasticisers and stabilisers | Released into indoor environment [268] – potential exposure for recycling workers and dispersion into the broader environment due to its inherent instability to thermal and photo-induced stress [269]. Alternatives: Conventional plastics with less concern for human health and better recyclability than PVC (e.g., LDPE, HDPE, PP, PET) are widely used to replace PVC in a range of applications such as food packaging or medical tubing. This can avoid exposure to hazardous PVC additives such as the widely used phthalates and increase material circularity. |
| Recycled products | DEHP and legacy substances (e.g., POP flame retardants, lead, and cadmium) | Can be re-introduced into recycled PVC products during recycling [185,226] – potential for exposure. See health impacts above. |
| Landfilling or recycling e-waste (PPVC) | Flame retardants, plasticisers, metals and MNPs | Hazardous chemicals migrate from e-waste [7], with waste pickers and informal e-waste recycling workers, including women and children, being particularly exposed [270], leading to health impacts [271,272]. See health impacts of flame retardants, plasticisers, and heavy metals above. MNPs released into the environment during mechanical recycling of e-waste [273]. MNPs have unknown safety. Alternatives: Greener alternatives (phthalate-free, plasticiser-free, flame retardant-free, and/or halogen/PVC-free) to PVC cables are emerging [274]. Health and environmental concerns regarding these alternatives will need to be considered throughout their life cycle. |
5. Polybutadiene
Polybutadiene is a thermosetting plastic known for its high flexibility, tensile strength, and resistance to abrasion. It is predominantly used in the manufacture of tyres, specifically the treads and sidewalls [275]. Polybutadiene (estimated to constitute around 30 % of the synthetic polymers used in tyres) [276], along with other synthetic polymers, comprise approximately 24 % of a passenger or light truck tyre and 11 % of a truck tyre [277]. Other applications of polybutadiene include footwear, sporting equipment (e.g., golf ball cores), conveyor belts, and cable insulation [278]. Polybutadiene may also be added to other polymers, such as PS and ABS, where it is used as a toughening agent [275]. The annual global market volume for polybutadiene was 3.99 Mt in 2019 [279], accounting for 0.87 % of total plastic production [1].
As a thermoset material, and because of the chemical composition and hazardous properties of constituent materials, vulcanised polybutadiene tyres cannot be recycled via simple mechanical recycling [280]. Used tyres can instead be retreaded [281] or downcycled for use as crumbed/ground rubber in playgrounds and sports facilities [282] or used in other applications [[283], [284], [285]]. There are a range of potential alternatives to polybutadiene across its numerous applications. Biodegradable and photodegradable alternatives include utilisation of polybutadiene composites with biodegradable polymers such as co-polymers of polybutadiene and polylactic acid (PLA) [286]. Polybutadiene alternatives for use in tyres include natural rubber, though scalability may be a challenge, and degradable PLA co-polymers of elastomers [287], which also present environmental and health concerns.
Table 5 outlines the environmental leakage and human exposure, or health impacts associated with polybutadiene, particularly in the context of a synthetic tyre rubber, throughout its life cycle.
Table 5.
Environmental leakage and human health impact factors of polybutadiene throughout its life cycle, particularly in the context of synthetic tyre rubber.
| Life cycle stage | Plastic pollutants | Environmental leakage and/or human exposure/health impacts |
|---|---|---|
| Extraction of raw materials | ||
| Extraction of fossil resources – for fossil carbon | Greenhouse gases, particulate matter (PM), metal(loid)s, volatile organic compounds (VOCs) such as benzene, toluene, ethylbenzene, and xylene [7] | Emitted during plastic production, transport and/or end-of-life processes [7], leading to climate, environmental and health impacts [14,15]. Alternatives: Bio-polybutadiene derived from renewable resources such as sugarcane or corn is being investigated as an alternative [288]. Bio-polybutadiene otherwise still requires similar considerations as fossil carbon-based polybutadiene materials for the rest of their life cycle as detailed below. |
| Production & Manufacturing | ||
| Monomer production & Polymerisation | Butadiene – monomer | A known human carcinogen [289] and one of the top air pollution-related health risk drivers in the United States [290] – documented release of butadiene into the atmosphere during production [291] and polymerisation [292], leading to occupational exposure [293] and detectable level of butadiene in drinking and ground water [294]. Products of degradation and atmospheric reactions of butadiene (e.g., 1,3-butadiene monoxide and 1,3-butadiene diepoxide) are considered more carcinogenic than butadiene itself [295]. |
| Polymerisation | Titanium tetrachloride – catalyst | Shows specific target organ toxicity upon repeated exposure [4]. Chronic occupational exposure associated with lung diseases [296]. Low environmental exposure risk due to their rapid breakdown in the environment [296]. |
| Compounding | Carbon black – UV stabiliser and reinforcing filler in tyres | Shows specific target organ toxicity upon repeated exposure [4] (known lung irritant) – occupational exposure increases the risk of chronic inflammatory and fibrotic lung diseases, such as pneumoconiosis [297]. |
| Benzophenone-type UV stabilisers | Potential for occupational exposure, which can lead to adverse human health effects, including altered kidney function [298,299] and increased risk of osteoarthritis [300]. | |
| Vulcanisation | VOCs and semi-VOCs (e.g., benzene, hydrazine, and n-Nitrosodi-n-propylamine) | Benzene, hydrazine, and n-Nitrosodi-n-propylamine are carcinogens [4] on the ChemSec Substitute it Now (SIN) list [71]. Occupational exposure associated with higher risk of cancer [301,302]. |
| Polycyclic aromatic hydrocarbons (PAHs) | Carcinogenic [303] – occupational exposure documented [302]. | |
| Use (consumption) | ||
| Tyre wear particles – secondary micro- and/or nanoplastics (MNPs) | Released into the environment [304,305] and a major source of MNP in air and water [1] – leachates are hazardous [[306], [307], [308], [309]] and can contain PAHs and metals such as zinc, lead, cadmium [309,310]. | |
| N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine (6PPD) stabiliser | Migrates from tyre wear particles and transforms into 6PPD-quinone, causing aquatic toxicity [311,312] and potentially affect human health based on animal studies showing multi-organ toxicity [313]. | |
| Disposal (waste management) | ||
| Landfilling | Whole tyres | Tyres can cause landfill instability, which can allow pollutants from other municipal waste to migrate into the environment [314,315]. Tyre leachate can contaminate soil, groundwater and surface water [314]. The synthetic components of tyres do not biodegrade and therefore, accumulate in landfills which have limits for the amount of tyres they can accept [314,315]. Alternatives: A biodegradable alternative include utilisation of polylactic acid (PLA) co-polymers of elastomers [287]. |
| Accidental or uncontrolled fires, e.g., landfill fires and burning of scrap tyres | PM, carbon monoxide, SOx, NOx, VOCs, benzene, PAHs, polychlorinated dibenzodioxins and/or dibenzofurans (PCDD/Fs), and polychlorinated biphenyls (PCBs), and some trace elements such as arsenic, cadmium, nickel, zinc, mercury, chromium, vanadium | Hazardous pollutants [4] released into the air and aquatic environment [314,316,317], leading to serious threat to the environment [315], human exposure and potential health risks [318,319]. |
| Tyre-derived fuel | PM, carbon monoxide, SOx, NOx, PAHs, PCDD/Fs, metals such as zinc | Potentially released into the environment [[320], [321], [322]], leading to environmental and human health impacts. |
| Recycling/downcycling for use as crumbed rubber | PAHs and metals such as zinc, lead, and cobalt | Migrate into the environment [323,324] and may impact marine life [[324], [325], [326]] and human health [327]. |
6. Discussion and conclusions
6.1. Discussion
In this review, we explore the known impacts and potential risks to the environment and human health posed by plastics across their life cycle, focusing on four key problematic polymers. By analysing their entire life cycle, we examine how the specific impacts and risks depend on chemical, polymer and product/application aspects of plastic materials. Specifically, we synthesized published evidence on environmental and human health impacts of resource extraction, production and manufacturing, use/consumption, and disposal/waste management of PC, PS, PVC, and polybutadiene rubber. We show that for every plastic polymer examined, there is evidence of environmental leakage, human exposure, and human health impacts at every stage of the life cycle, and we demonstrate that each aspect of plastic – the polymer itself, the chemicals therein, and the product/application – is associated with unique impacts. Considering these findings, fulfilling the Global Plastics Treaty objective of ending plastic pollution [328] cannot be achieved via effective waste management practices or increased circularity alone. Our findings emphasise the urgent need for a more refined and comprehensive approach to assessing and mitigating the risks associated with the plastics economy.
While extensive research and policy focus has been dedicated to understanding and mitigating the global environmental and human health impacts of plastics, these efforts have largely been limited to plastic chemicals (e.g., POPs [72]), problematic plastic products such as single-use plastic [42,329,330] and problematic applications such as primary MNPs in personal care products [329,330]. Less attention has been given to environmental toxicity assessments, human health risk assessments and management of polymers [3]. Applying a polymer lens is important because several environmental and human health harms are polymer-specific.
-
1.Monomers can be hazardous.
-
a.Polymerisation is never 100 % and some unreacted/residual monomers remain in and leach from the final plastic product.
-
b.Monomers and oligomers can also be produced from degradation of the polymer during product use.
-
c.Monomers and oligomers can also be produced and released into the environment due to degradation of managed and mismanaged waste.
-
a.
-
2.
Micro and/or nanoplastic toxicity is dependent on polymer type [7,68,331,332]. For example, polyvinyl chloride MNPs have shown the greatest particle toxicity in primary human monocytes in vitro (greatest increase in pro-inflammatory cytokine release) [64]. Importantly, this effect of polymer has been found to be independent of leached chemicals and particle size/shape, with an independent effect of leached chemicals and particle size/shape also demonstrated.
-
3.
Polymers can present barriers to circularity [333]. The choice of recycling methods, recyclability of the plastics, and quality of the recyclate are affected by the identity of polymers and their combinations (co-polymers, multi-layer). For example, halogenated polymers are less recyclable due to their intrinsic susceptibility to heat. They contaminate recycling streams, leading to the release of toxic chemicals (e.g., volatile organic chemicals released as degradation products). Additionally, use of complex polymer mixtures affects recyclability of the product (e.g., cling films).
Therefore, a whole system approach is needed whereby plastics are assessed by viewing through all lenses – polymer, chemical, and product/application. One example of such an approach being taken is the recommendation for a complete phase out of PVC in healthcare by academics [334] and non-governmental organisations such as Health Care Without Harm [238]. PVC products, including disposable and short-lived medical materials like respiratory tubing, blood/plasma bags, and IV tubing, frequently contain phthalates like DEHP, which can leach into patients, particularly newborns, resulting in direct exposure [237,[334], [335], [336]]. Exposure to phthalates at vulnerable moments of human development is a serious concern as it can alter development and have lifelong consequences [336]. To ensure patient safety, Health Care Without Harm recommends substitution of hazardous chemicals (chemical lens). Since continued use of PVC produced using safer alternative plasticisers will not address the environmental and human health risks associated with PVC production and disposal, additional recommendations include the use of PVC-free alternatives (polymer lens) [334], and prioritisation of the phasing out of PVC medical devices that can result in direct patient exposure to migrating hazardous chemicals (product/application lens) [238].
6.2. Conclusions and recommendations
To date, while a small portion of the many thousands of plastic-associated chemicals have been regulated [333], polymers remain largely unregulated [3]. At the third session of the Intergovernmental Negotiating Committee meeting to develop an international legally binding instrument on plastic pollution, several delegations questioned the need for the Treaty to include controls on polymers of concern, in addition to provisions targeting harmful chemicals and/or products [337]. Ongoing assessments of what constitutes problematic plastics will be improved by viewing plastics through polymer, chemical, and product/application lenses, offering opportunities to minimise unintended impacts on the environmental and human health across the plastic life cycle. Based on our findings, polymers need separate consideration within the Treaty for future proofing the agreement and in order to adequately fulfill its goal of ending plastic pollution by:
-
1.
Introducing globally binding requirements on transparency of plastic composition. Material transparency and traceability measures proposed for chemicals should also apply to polymers [3] as they are the main constituents of plastic. Currently, there is a shortage of information that directly associates plastic chemicals with specific types of polymers or polymer types [333].
-
2.
Establishing of a Comprehensive Global Inventory of polymers, with classification and registration utilising a grouping approach based on structural features. This will facilitate the identification of polymers requiring regulatory oversight. Various criteria (see Table 1 in Groh et al., 2023 [3]) are used to classify polymers, but these vary between jurisdictions and many are debated. No global framework exists yet for the identification of plastic polymers that are problematic at some point or throughout their lifecycle. The European Commission is currently developing proposals to identify polymers requiring registration based on specific criteria (molecular weight, ionicity criteria, degradation criteria and hazard criteria) [3].
-
3.
Establishing a negative list of polymers of concern for which there is a body of evidence on their toxic effects, in order to minimise their use and enable the phase-out of their use in non-essential and problematic applications. Despite their ubiquitous use, high production volumes and associated exposure risks, plastic polymers have historically been overlooked by regulators worldwide. Several jurisdictions have defined “polymers of low concern” based on intrinsic polymer properties such as molecular weight, which exempt from regulatory/registration requirements [3,338].
-
4.
Integrating an effective science-policy interface which can keep step with emerging knowledge, respond to industry innovation and avoid regrettable substitution. This will help identify priorities for further research with respect to comprehensive life cycle assessment of polymers, including in terms of environmental and human health impacts of their degradation products (monomers, oligomers, and micro- and nanoplastics), in order to fill critical knowledge gaps. This body can also advise on the creating and updating of criteria, standard testing protocols and listing of existing and new polymers for which environmental and human health risks, including those of degradation products, become known over time. Currently, countries that do regulate plastic polymers often rely on assessment schemes that were developed in the early 1990s [3].
We recommend consideration of the following insights into LCA evaluation practices and development of the Global Plastics Treaty criteria for assessing problematic plastics:
-
•
Circularity is usually assessed at a product level, rather than at a polymer level [333]. The size of the problem should be considered, including high volumes of production, limited re-use and ineffective recycling resulting in high waste production.
-
•
A recent report identified over 4,200 known hazardous plastic-associated chemicals, of which about 3,600 are not currently regulated globally [333]. Hazardous properties of the chemicals used in plastics should be considered (only some are listed in this review).
-
•
More than 9,000 chemicals found in plastic lack publicly available information regarding their origins or uses in plastics [333]. Which plastic-associated chemicals remain in, and can migrate from, the final plastic material should be considered as this can expose individuals to several hazardous chemicals (e.g., heavy metals), that they would not otherwise encounter in typical daily activities.
-
•
A range of plastic products have been banned or restricted at regional and national levels due to their high environmental contamination potential (e.g., single-use plastic [42,329,330] and primary MNPs in personal care products [329,330]). Given that no single nation possesses the capability to tackle the transboundary issue of plastic pollution, a unified global effort is essential. Evidence for environmental contamination via emissions and disposal in wastewater, land, and air throughout the plastic life cycle should be considered for all plastic products/applications, including through a chemical and polymer lens, as should evidence of the effects from leakage on environmental health.
-
•
More than 6300 plastic-associated chemicals have been reported to have a high exposure potential [333]. While we recommend a hazard-based approach over a risk-based approach due to the scarcity of information of exposure, evidence for human exposure in the general population and evidence of exposure in workers in manufacturing plants, fenceline communities, consumers, and those working in formal and informal waste disposal and recycling facilities should also be considered, as should existence of systematic post-market human biomonitoring to detect human exposure and evidence of associated health impacts.
-
•
Lastly, it is important to consider mitigation measures that effectively prevent environmental leakage and human exposure to chemicals.
Overall, there is a need for:
-
•
Whole system consideration of environmental and human health impacts of plastic polymers, chemicals, and products/applications throughout the plastic life cycle in the Global Plastics Treaty.
-
•
Implementation of stronger regulatory measures to phase out hazardous polymers and chemicals in plastics and promote safer alternatives.
-
•
Integration of cost externalities from human health and environmental damage into fossil carbon-based plastics (e.g., via a global plastic levy).
-
•
Increasing funding for research and development of sustainable and less hazardous plastic alternatives, particularly for the replacement of the most problematic plastics, with respect to polymers, chemicals, and products/applications.
-
•
Fostering collaborative efforts among industry, government, and academia to innovate in sustainable plastic production, recycling technologies, and waste management.
-
•
Enhancing public awareness and education on the impacts of plastics to drive informed consumer choices and support sustainable practices.
Funding
This review was funded by Minderoo Foundation, an independent not-for-profit philanthropic organization. Neither the foundation, nor its benefactors, had any influence on the conduct or the findings of this review.
Data availability statement
No data was used for the research described in the article.
CRediT authorship contribution statement
Bhedita J. Seewoo: Writing – review & editing, Writing – original draft, Visualization, Validation, Project administration, Methodology, Investigation, Data curation, Conceptualization. Enoch V.S. Wong: Writing – review & editing, Investigation. Yannick R. Mulders: Writing – review & editing, Investigation. Louise M. Goodes: Writing – review & editing, Investigation. Ela Eroglu: Writing – review & editing, Investigation. Manuel Brunner: Writing – review & editing, Visualization. Aleksandra Gozt: Investigation. Priyanka Toshniwal: Investigation. Christos Symeonides: Writing – review & editing, Supervision, Methodology, Conceptualization. Sarah A. Dunlop: Writing – review & editing, Supervision, Methodology, Conceptualization.
Declaration of AI and AI-assisted technologies in the writing process
The graphical abstract was designed by BJS and MB with the assistance of the Text to Vector Graphic tool in Adobe Illustrator and Dall•E 2. After using these tools, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to gratefully acknowledge valuable contributions from Lizzie Fuller (editing) and Sarah McEvoy (tyres). The authors also acknowledge the University of Western Australia's Library for providing access to journal articles.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e32912.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.OECD . 2022. Global Plastics Outlook: Policy Scenarios to 2060. [DOI] [Google Scholar]
- 2.End Plastic Pollution - towards an International Legally Binding Instrument. 2022. pp. 1–4. UNEP/EA.5/Res.14. https://wedocs.unep.org/xmlui/bitstream/handle/20.500.11822/39764/END%20PLASTIC%20POLLUTION%20-%20TOWARDS%20AN%20INTERNATIONAL%20LEGALLY%20BINDING%20INSTRUMENT%20-%20English.pdf?sequence=1&isAllowed=y. [Google Scholar]
- 3.Groh K.J., Arp H.P.H., MacLeod M., Wang Z. Assessing and managing environmental hazards of polymers: historical development, science advances and policy options. Environ Sci Process Impacts. 2023;25(1):10–25. doi: 10.1039/D2EM00386D. [DOI] [PubMed] [Google Scholar]
- 4.Wiesinger H., Wang Z., Hellweg S. Deep dive into plastic monomers, additives, and processing aids. Environ. Sci. Technol. 2021;55(13):9339–9351. doi: 10.1021/acs.est.1c00976. [DOI] [PubMed] [Google Scholar]
- 5.UNEP . United Nations Environment Programme (UNEP) and Secretariat of the Basel, Rotterdam and Stockholm Conventions; 2023. Chemicals in Plastics - A Technical Report.http://www.unep.org/resources/report/chemicals-plastics-technical-report [Google Scholar]
- 6.Hahladakis J.N., Velis C.A., Weber R., Iacovidou E., Purnell P. An overview of chemical additives present in plastics: migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard Mater. 2018;344:179–199. doi: 10.1016/j.jhazmat.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 7.Landrigan P.J., Raps H., Cropper M., et al. The Minderoo-Monaco Commission on plastics and human health. Ann Glob Health. 2023;89(1):23. doi: 10.5334/aogh.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geueke B., Groh K.J., Maffini M.V., et al. Systematic evidence on migrating and extractable food contact chemicals: most chemicals detected in food contact materials are not listed for use. Crit. Rev. Food Sci. Nutr. 2022;0(0):1–11. doi: 10.1080/10408398.2022.2067828. [DOI] [PubMed] [Google Scholar]
- 9.Food Packaging Forum Foundation . 2022. FCCmigex Database.https://www.foodpackagingforum.org/fccmigex [Google Scholar]
- 10.Skoczinski P., Carus M., Tweddle G., et al. Production and Trends 2022-2027. nova-Institut GmbH; 2023. Bio-based building blocks and polymers – global Capacities. [DOI] [Google Scholar]
- 11.Li Z., Ma Z., van der Kuijp T.J., Yuan Z., Huang L. A review of soil heavy metal pollution from mines in China: pollution and health risk assessment. Sci. Total Environ. 2014;468–469:843–853. doi: 10.1016/j.scitotenv.2013.08.090. [DOI] [PubMed] [Google Scholar]
- 12.Sun Z., Xie X., Wang P., Hu Y., Cheng H. Heavy metal pollution caused by small-scale metal ore mining activities: a case study from a polymetallic mine in South China. Sci. Total Environ. 2018;639:217–227. doi: 10.1016/j.scitotenv.2018.05.176. [DOI] [PubMed] [Google Scholar]
- 13.Kim J.Y., Kim K.W., Ahn J.S., Ko I., Lee C.H. Investigation and risk assessment modeling of as and other heavy metals contamination around five abandoned metal mines in Korea. Environ. Geochem. Health. 2005;27(2):193–203. doi: 10.1007/s10653-005-0127-2. [DOI] [PubMed] [Google Scholar]
- 14.Cabernard L., Pfister S., Oberschelp C., Hellweg S. Growing environmental footprint of plastics driven by coal combustion. Nat. Sustain. 2022;5(2):139–148. doi: 10.1038/s41893-021-00807-2. [DOI] [Google Scholar]
- 15.Fact Sheet | Climate, environmental, and health impacts of fossil fuels . 2021. White Papers | EESI.https://www.eesi.org/papers/view/fact-sheet-climate-environmental-and-health-impacts-of-fossil-fuels-2021 [Google Scholar]
- 16.Zhou X., Zhai Y., Ren K., et al. Life cycle assessment of polycarbonate production: proposed optimization toward sustainability. Resour. Conserv. Recycl. 2023;189 doi: 10.1016/j.resconrec.2022.106765. [DOI] [Google Scholar]
- 17.Chu J., Hu X., Kong L., et al. Dynamic flow and pollution of antimony from polyethylene terephthalate (PET) fibers in China. Sci. Total Environ. 2021;771 doi: 10.1016/j.scitotenv.2020.144643. [DOI] [PubMed] [Google Scholar]
- 18.Long L.A., Lay J., Leskin K., McClure R., Smith A. Vinyl chloride monomer explosion. Process Saf. Prog. 2008;27(1):72–79. doi: 10.1002/prs.10233. [DOI] [Google Scholar]
- 19.Rendell-Bhatti F., Paganos P., Pouch A., et al. Developmental toxicity of plastic leachates on the sea urchin Paracentrotus lividus. Environ Pollut. 2021;269 doi: 10.1016/j.envpol.2020.115744. [DOI] [PubMed] [Google Scholar]
- 20.Ogata Y., Takada H., Mizukawa K., et al. International Pellet Watch: global monitoring of persistent organic pollutants (POPs) in coastal waters. 1. Initial phase data on PCBs, DDTs, and HCHs. Mar. Pollut. Bull. 2009;58(10):1437–1446. doi: 10.1016/j.marpolbul.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Wang L.C., Lin J.C.T., Dong C.D., Chen C.W., Liu T.K. The sorption of persistent organic pollutants in microplastics from the coastal environment. J. Hazard Mater. 2021;420 doi: 10.1016/j.jhazmat.2021.126658. [DOI] [PubMed] [Google Scholar]
- 22.Rodrigues A., Oliver D.M., McCarron A., Quilliam R.S. Colonisation of plastic pellets (nurdles) by E. coli at public bathing beaches. Mar. Pollut. Bull. 2019;139:376–380. doi: 10.1016/j.marpolbul.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Ormsby M.J., Woodford L., White H.L., Fellows R., Oliver D.M., Quilliam R.S. Toxigenic Vibrio cholerae can cycle between environmental plastic waste and floodwater: Implications for environmental management of cholera. J. Hazard Mater. 2024;461 doi: 10.1016/j.jhazmat.2023.132492. [DOI] [PubMed] [Google Scholar]
- 24.Brs, Raubenheimer K., Urho N. United Nations Environment Programme; Geneva: 2023. Global Governance of Plastics and Associated Chemicals. Secretariat of the Basel, Rotterdam and Stockholm Conventions. [Google Scholar]
- 25.Bolan N.S., Kirkham M., Bolan S.S., Tsang D.C.W., Tsang Y.F., Wang H. Particulate Plastics in Terrestrial and Aquatic Environments. first ed. CRC Press; 2020. Particulate plastics and human health. [DOI] [Google Scholar]
- 26.Net S., Sempéré R., Delmont A., Paluselli A., Ouddane B. Occurrence, fate, behavior and ecotoxicological state of phthalates in different environmental matrices. Environ. Sci. Technol. 2015;49(7):4019–4035. doi: 10.1021/es505233b. [DOI] [PubMed] [Google Scholar]
- 27.Ji Y., Wang F., Zhang L., et al. A comprehensive assessment of human exposure to phthalates from environmental media and food in Tianjin, China. J. Hazard Mater. 2014;279:133–140. doi: 10.1016/j.jhazmat.2014.06.055. [DOI] [PubMed] [Google Scholar]
- 28.Okoye C.O., Addey C.I., Oderinde O., et al. Toxic chemicals and persistent organic pollutants associated with micro-and nanoplastics pollution. Chem Eng J Adv. 2022;11 doi: 10.1016/j.ceja.2022.100310. [DOI] [Google Scholar]
- 29.Mehmood T., Peng L. Polyethylene scaffold net and synthetic grass fragmentation: a source of microplastics in the atmosphere? J. Hazard Mater. 2022;429 doi: 10.1016/j.jhazmat.2022.128391. [DOI] [PubMed] [Google Scholar]
- 30.Mehmood T., Hassan M.A., Faheem M., Shakoor A. Why is inhalation the most discriminative route of microplastics exposure? Environ. Sci. Pollut. Res. 2022;29(33):49479–49482. doi: 10.1007/s11356-022-20653-9. [DOI] [PubMed] [Google Scholar]
- 31.Groh K.J., Backhaus T., Carney-Almroth B., et al. Overview of known plastic packaging-associated chemicals and their hazards. Sci. Total Environ. 2019;651:3253–3268. doi: 10.1016/j.scitotenv.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 32.Zangmeister C.D., Radney J.G., Benkstein K.D., Kalanyan B. Common single-use consumer plastic products release trillions of sub-100 nm nanoparticles per liter into water during normal use. Environ. Sci. Technol. 2022;56(9):5448–5455. doi: 10.1021/acs.est.1c06768. [DOI] [PubMed] [Google Scholar]
- 33.Rustagi N., Pradhan S.K., Singh R. Public health impact of plastics: an overview. Indian J. Occup. Environ. Med. 2011;15(3):100. doi: 10.4103/0019-5278.93198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.OECD . Organisation for Economic Co-operation and Development; 2022. Global Plastics Outlook: Economic Drivers, Environmental Impacts and Policy Options. [DOI] [Google Scholar]
- 35.Mehmood T., Peng L., Salam A., Prakash J., Haider M. Neglected atmospheric microplastic pollution in South Asia reflects a wider failure. Ecol. Inf. 2023;73 doi: 10.1016/j.ecoinf.2022.101949. [DOI] [Google Scholar]
- 36.World Economic Forum, Ellen MacArthur Foundation, McKinsey & Company The New Plastics Economy — Rethinking the Future of Plastics. 2016:120. https://emf.thirdlight.com/link/faarmdpz93ds-5vmvdf/@/preview/1?o [Google Scholar]
- 37.US EPA . Greenhouse Gas Reporting Program. GHGRP) of US Environment Protection Agency (EPA); 2013. 2013 GHGRP industrial profiles of petroleum Refineries sector.https://www.epa.gov/sites/default/files/2016-11/documents/refineries_2013_112516.pdf [Google Scholar]
- 38.Brede C., Fjeldal P., Skjevrak I., Herikstad H. Increased migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food Addit. Contam. 2003;20(7):684–689. doi: 10.1080/0265203031000119061. [DOI] [PubMed] [Google Scholar]
- 39.Agarwal A., Gandhi S., Tripathi A.D., Iammarino M., Homroy S. Analysis of Bisphenol A migration from microwaveable polycarbonate cups into coffee during microwave heating. Int. J. Food Sci. Technol. 2022;57(12):7477–7485. doi: 10.1111/ijfs.16103. [DOI] [Google Scholar]
- 40.Maia J., Cruz J.M., Sendón R., Bustos J., Sanchez J.J., Paseiro P. Effect of detergents in the release of bisphenol A from polycarbonate baby bottles. Food Res. Int. 2009;42(10):1410–1414. doi: 10.1016/j.foodres.2009.07.003. [DOI] [Google Scholar]
- 41.UNEP . United Nations Environment Programme (UNEP); 2022. Addendum Document on Priorities, Needs, Challenges and Barriers to End Plastic Pollution at National Level (UNEP/PP/INC.1/11) [Google Scholar]
- 42.Nordic Council of Ministers . Secretary of the Nordic Council of Ministers, Nordic Council of Ministers; 2024. Global Criteria to Address Problematic, Unnecessary and Avoidable Plastic Products.https://pub.norden.org/temanord2024-508/ [Google Scholar]
- 43.Eales J., Bethel A., Galloway T., et al. Human health impacts of exposure to phthalate plasticizers: an overview of reviews. Environ. Int. 2022;158 doi: 10.1016/j.envint.2021.106903. [DOI] [PubMed] [Google Scholar]
- 44.Seewoo B.J., Goodes L.M., Mofflin L., et al. The plastic health map: a systematic evidence map of human health studies on plastic-associated chemicals. Environ. Int. 2023;181 doi: 10.1016/j.envint.2023.108225. [DOI] [PubMed] [Google Scholar]
- 45.Koelmans A.A., Redondo-Hasselerharm P.E., Nor N.H.M., de Ruijter V.N., Mintenig S.M., Kooi M. Risk assessment of microplastic particles. Nat. Rev. Mater. 2022;7(2):138–152. doi: 10.1038/s41578-021-00411-y. [DOI] [Google Scholar]
- 46.Yates J., Deeney M., Rolker H.B., White H., Kalamatianou S., Kadiyala S. A systematic scoping review of environmental, food security and health impacts of food system plastics. Nat Food. 2021;2(2):80–87. doi: 10.1038/s43016-021-00221-z. [DOI] [PubMed] [Google Scholar]
- 47.Fantke P., Chiu W.A., Aylward L., et al. Exposure and toxicity characterization of chemical emissions and chemicals in products: global recommendations and implementation in USEtox. Int. J. Life Cycle Assess. 2021;26(5):899–915. doi: 10.1007/s11367-021-01889-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marson A., Piron M., Zuliani F., Fedele A., Manzardo A. Comparative life cycle assessment in the plastic sector: a systematic literature review. Clean Environ Syst. 2023;9 doi: 10.1016/j.cesys.2023.100119. [DOI] [Google Scholar]
- 49.Lindstrom T., Hicks A.L. Life cycle assessment of expanded polystyrene shipping boxes at a public research institution: insights for infrastructure at the end of life. Environ Res Infrastruct Sustain. 2022;2(3) doi: 10.1088/2634-4505/ac80d1. [DOI] [Google Scholar]
- 50.Kamalakkannan S., Abeynayaka A., Kulatunga A.K., Singh R.K., Tatsuno M., Gamaralalage P.J.D. Life cycle assessment of selected single-use plastic products towards evidence-based policy recommendations in Sri Lanka. Sustainability. 2022;14(21) doi: 10.3390/su142114170. [DOI] [Google Scholar]
- 51.Maga D., Aryan V., Blömer J. A comparative life cycle assessment of tyre recycling using pyrolysis compared to conventional end-of-life pathways. Resour. Conserv. Recycl. 2023;199 doi: 10.1016/j.resconrec.2023.107255. [DOI] [Google Scholar]
- 52.Marten B., Hicks A. Expanded polystyrene life cycle analysis literature review: an analysis for different disposal scenarios. Sustainability. 2018;11(1):29–35. doi: 10.1089/sus.2017.0015. [DOI] [Google Scholar]
- 53.Bishop G., Styles D., Lens P.N.L. Environmental performance comparison of bioplastics and petrochemical plastics: a review of life cycle assessment (LCA) methodological decisions. Resour. Conserv. Recycl. 2021;168 doi: 10.1016/j.resconrec.2021.105451. [DOI] [Google Scholar]
- 54.Ernstoff A., Niero M., Muncke J., et al. Challenges of including human exposure to chemicals in food packaging as a new exposure pathway in life cycle impact assessment. Int. J. Life Cycle Assess. 2019;24(3):543–552. doi: 10.1007/s11367-018-1569-y. [DOI] [Google Scholar]
- 55.Wang Z., Praetorius A. Integrating a chemicals perspective into the global plastic treaty. Environ. Sci. Technol. Lett. 2022;9(12):1000–1006. doi: 10.1021/acs.estlett.2c00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simon N., Raubenheimer K., Urho N., et al. A binding global agreement to address the life cycle of plastics. Science. 2021;373(6550):43–47. doi: 10.1126/science.abi9010. [DOI] [PubMed] [Google Scholar]
- 57.Dey T., Trasande L., Altman R., et al. Global plastic treaty should address chemicals. Science. 2022;378(6622):841–842. doi: 10.1126/science.adf5410. [DOI] [PubMed] [Google Scholar]
- 58.Bergmann M., Almroth B.C., Brander S.M., et al. A global plastic treaty must cap production. Science. 2022;376(6592):469–470. doi: 10.1126/science.abq0082. [DOI] [PubMed] [Google Scholar]
- 59.PubChem. PubChem. Accessed March 7, 2024. https://pubchem.ncbi.nlm.nih.gov/.
- 60.Hauser R., Calafat A.M. Phthalates and human health. Occup. Environ. Med. 2005;62(11):806–818. doi: 10.1136/OEM.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carlos K.S., de Jager L.S., Begley T.H. Investigation of the primary plasticisers present in polyvinyl chloride (PVC) products currently authorised as food contact materials. Food Addit. Contam. 2018;35(6):1214–1222. doi: 10.1080/19440049.2018.1447695. [DOI] [PubMed] [Google Scholar]
- 62.Amberg-Müller J.P., Hauri U., Schlegel U., Hohl C., Brüschweiler B.J. Migration of phthalates from soft PVC packaging into shower and bath gels and assessment of consumer risk. J Für Verbraucherschutz Leb. 2010;5(3):429–442. doi: 10.1007/s00003-010-0620-0. [DOI] [Google Scholar]
- 63.Gosetti F., Bolfi B., Robotti E., et al. Study of endocrine disrupting compound release from different medical devices through an on-line SPE UHPLC-MS/MS method. Anal. Chim. Acta. 2018;1042:141–154. doi: 10.1016/j.aca.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 64.Weber A., Schwiebs A., Solhaug H., et al. Nanoplastics affect the inflammatory cytokine release by primary human monocytes and dendritic cells. Environ. Int. 2022;163 doi: 10.1016/j.envint.2022.107173. [DOI] [PubMed] [Google Scholar]
- 65.Al Alshaikh A., Bara J.E. Chlorinated plastics offer unique opportunities and challenges in upcycling. Polym. Int. 2024;(n/a) doi: 10.1002/pi.6621. n/a. [DOI] [Google Scholar]
- 66.Suh S., Boulay A.M., Fantke P., et al. Conceptual framework for identifying polymers of concern. Front Sustain. 2024;5 doi: 10.3389/frsus.2024.1399431. [DOI] [Google Scholar]
- 67.Senathirajah K., Kemp A., Saaristo M., Ishizuka S., Palanisami T. Polymer prioritization framework: a novel multi-criteria framework for source mapping and characterizing the environmental risk of plastic polymers. J. Hazard Mater. 2022;429 doi: 10.1016/j.jhazmat.2022.128330. [DOI] [PubMed] [Google Scholar]
- 68.Yuan Z., Nag R., Cummins E. Ranking of potential hazards from microplastics polymers in the marine environment. J. Hazard Mater. 2022;429 doi: 10.1016/j.jhazmat.2022.128399. [DOI] [PubMed] [Google Scholar]
- 69.International Agency on Cancer Research . 2022. List of Classifications – IARC Monographs on the Identification of Carcinogenic Hazards to Humans.https://monographs.iarc.who.int/list-of-classifications/ Published July 9, [Google Scholar]
- 70.ECHA Candidate list of substances of very high concern for authorisation. European Chemicals Agency (ECHA) 2021 https://echa.europa.eu/candidate-list-table [Google Scholar]
- 71.ChemSec. SIN list. 2023. https://sinlist.chemsec.org/
- 72.Stockholm Convention Listing of POPs in the Stockholm convention. 2019. http://chm.pops.int/TheConvention/ThePOPs/ListingofPOPs/tabid/2509/Default.aspx
- 73.Saad N.A., Jwad E.R. Investigation of addition Titanium dioxide on general properties of polycarbonate. OALib. 2018;5(1):1–11. doi: 10.4236/oalib.1104229. [DOI] [Google Scholar]
- 74.Polycarbonates global demand . Statista; 2022. https://www.statista.com/statistics/750965/polycarbonates-demand-worldwide/ [Google Scholar]
- 75.Takeuchi K. In: Polymer Science: A Comprehensive Reference. Matyjaszewski K., Möller M., editors. Elsevier; 2012. 5.16 - polycarbonates; pp. 363–376. [DOI] [Google Scholar]
- 76.Xu J., Feng E., Song J. Renaissance of aliphatic polycarbonates: new techniques and biomedical applications. J. Appl. Polym. Sci. 2014;131(5) doi: 10.1002/app.39822. doi:10.1002/app.39822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu W., Maynard E., Chiaradia V., Arno M.C., Dove A.P. Aliphatic polycarbonates from cyclic carbonate monomers and their application as biomaterials. Chem Rev. 2021;121(18):10865–10907. doi: 10.1021/acs.chemrev.0c00883. [DOI] [PubMed] [Google Scholar]
- 78.Wei P., Bhat G.A., Darensbourg D.J. Enabling new approaches: recent advances in processing aliphatic polycarbonate-based materials. Angew. Chem. Int. Ed. 2023;62(48) doi: 10.1002/anie.202307507. [DOI] [PubMed] [Google Scholar]
- 79.Cimmino I., Fiory F., Perruolo G., et al. Potential mechanisms of bisphenol A (BPA) contributing to human disease. Int. J. Mol. Sci. 2020;21(16):5761. doi: 10.3390/ijms21165761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hahladakis J.N., Iacovidou E., Gerassimidou S. An overview of the occurrence, fate, and human risks of the bisphenol-A present in plastic materials, components, and products. Integrated Environ. Assess. Manag. 2023;19(1):45–62. doi: 10.1002/ieam.4611. [DOI] [PubMed] [Google Scholar]
- 81.Chen D., Kannan K., Tan H., et al. Bisphenol analogues other than BPA: environmental occurrence, human exposure, and toxicity – a review. Environ. Sci. Technol. 2016;50(11):5438–5453. doi: 10.1021/acs.est.5b05387. [DOI] [PubMed] [Google Scholar]
- 82.Pelch K., Wignall J.A., Goldstone A.E., et al. A scoping review of the health and toxicological activity of bisphenol A (BPA) structural analogues and functional alternatives. Toxicology. 2019;424 doi: 10.1016/j.tox.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 83.Rochester J.R., Bolden A.L. Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ. Health Perspect. 2015;123(7):643–650. doi: 10.1289/ehp.1408989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moon M.K. Concern about the safety of bisphenol A substitutes. Diabetes Metab. J. 2019;43(1):46–48. doi: 10.4093/dmj.2019.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bousoumah R., Leso V., Iavicoli I., et al. Biomonitoring of occupational exposure to bisphenol A, bisphenol S and bisphenol F: a systematic review. Sci. Total Environ. 2021;783 doi: 10.1016/j.scitotenv.2021.146905. [DOI] [PubMed] [Google Scholar]
- 86.Cui S., Borgemenke J., Liu Z., Li Y. Recent advances of “soft” bio-polycarbonate plastics from carbon dioxide and renewable bio-feedstocks via straightforward and innovative routes. J. CO2 Util. 2019;34:40–52. doi: 10.1016/j.jcou.2019.05.027. [DOI] [Google Scholar]
- 87.Park S.A., Eom Y., Jeon H., et al. Preparation of synergistically reinforced transparent bio-polycarbonate nanocomposites with highly dispersed cellulose nanocrystals. Green Chem. 2019;21(19):5212–5221. doi: 10.1039/C9GC02253H. [DOI] [Google Scholar]
- 88.López Rocha CJ., Álvarez-Castillo E., Estrada Yáñez M.R., Bengoechea C., Guerrero A., Orta Ledesma M.T. Development of bioplastics from a microalgae consortium from wastewater. J. Environ. Manag. 2020;263 doi: 10.1016/j.jenvman.2020.110353. [DOI] [PubMed] [Google Scholar]
- 89.Li X., Meng L., Zhang Y., et al. Research and application of polypropylene carbonate composite materials: a review. Polymers. 2022;14(11):2159. doi: 10.3390/polym14112159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim W.B., Joshi U.A., Lee J.S. Making polycarbonates without employing phosgene: an overview on catalytic chemistry of intermediate and precursor syntheses for polycarbonate. Ind. Eng. Chem. Res. 2004;43(9):1897–1914. doi: 10.1021/ie034004z. [DOI] [Google Scholar]
- 91.Fukuoka S., Fukawa I., Adachi T., Fujita H., Sugiyama N., Sawa T. Industrialization and expansion of green sustainable chemical process: a review of non-phosgene polycarbonate from CO 2. Org. Process Res. Dev. 2019;23(2):145–169. doi: 10.1021/acs.oprd.8b00391. [DOI] [Google Scholar]
- 92.CSB. DuPont Belle Toxic Chemical Releases |. Accessed May 26, 2023. https://www.csb.gov/dupont-belle-toxic-chemical-releases-/.
- 93.Liu T., Xu Q er, Zhang C hui, Zhang P. Occupational exposure to methylene chloride and risk of cancer: a meta-analysis. Cancer Causes Control. 2013;24(12):2037–2049. doi: 10.1007/s10552-013-0283-0. [DOI] [PubMed] [Google Scholar]
- 94.Fu X., Xu J., Zhang R., Yu J. The association between environmental endocrine disruptors and cardiovascular diseases: a systematic review and meta-analysis. Environ. Res. 2020;187:109464. doi: 10.1016/j.envres.2020.109464. 109464. [DOI] [PubMed] [Google Scholar]
- 95.Rancière F., Lyons J.G., Loh V.H.Y., et al. Bisphenol A and the risk of cardiometabolic disorders: a systematic review with meta-analysis of the epidemiological evidence. Environ. Health. 2015;14:46. doi: 10.1186/s12940-015-0036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu Y., Wen S., Yuan D., et al. The association between the environmental endocrine disruptor bisphenol A and polycystic ovary syndrome: a systematic review and meta-analysis. Gynecol. Endocrinol. 2018;34(5):370–377. doi: 10.1080/09513590.2017.1405931. [DOI] [PubMed] [Google Scholar]
- 97.Hwang S., Lim J eun, Choi Y., Jee S.H. Bisphenol A exposure and type 2 diabetes mellitus risk: a meta-analysis. BMC Endocr. Disord. 2018;18(1):1–10. doi: 10.1186/S12902-018-0310-Y. 181. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Song Y., Chou E.L., Baecker A., et al. Endocrine-disrupting chemicals, risk of type 2 diabetes, and diabetes-related metabolic traits: a systematic review and meta-analysis. J. Diabetes. 2016;8(4):516–532. doi: 10.1111/1753-0407.12325. [DOI] [PubMed] [Google Scholar]
- 99.Wu W., Li M., Liu A., et al. Bisphenol A and the risk of obesity: a systematic review with meta-analysis of the epidemiological evidence. Dose Response. 2020;18(2) doi: 10.1177/1559325820916949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim K.Y., Lee E., Kim Y. The association between bisphenol A exposure and obesity in children-A systematic review with meta-analysis. Int. J. Environ. Res. Publ. Health. 2019;16(14) doi: 10.3390/ijerph16142521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ribeiro C.M., Beserra B.T.S., Silva N.G., et al. Exposure to endocrine-disrupting chemicals and anthropometric measures of obesity: a systematic review and meta-analysis. BMJ Open. 2020;10(6) doi: 10.1136/bmjopen-2019-033509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chao Y., Chen J., Yang W., Ho T., Yen F. Exposure hazard to bisphenol A for labor and particle size distribution at polycarbonate molding plants. Iran. J. Public Health. 2015;44(6):783–790. [PMC free article] [PubMed] [Google Scholar]
- 103.Ribeiro E., Ladeira C., Viegas S. Occupational exposure to bisphenol a (BPA): a reality that still needs to be unveiled. Toxics. 2017;5(3):22. doi: 10.3390/toxics5030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nam S.H., Seo Y.M., Kim M.G. Bisphenol A migration from polycarbonate baby bottle with repeated use. Chemosphere. 2010;79(9):949–952. doi: 10.1016/j.chemosphere.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 105.Srivastava R.K., Godara S. Use of polycarbonate plastic products and human health. Int. J. Basic Clin. Pharmacol. 2013;2(1):12–17. [Google Scholar]
- 106.Zalko D., Jacques C., Duplan H., Bruel S., Perdu E. Viable skin efficiently absorbs and metabolizes bisphenol A. Chemosphere. 2011;82(3):424–430. doi: 10.1016/j.chemosphere.2010.09.058. [DOI] [PubMed] [Google Scholar]
- 107.Vasiljevic T., Harner T. Bisphenol A and its analogues in outdoor and indoor air: properties, sources and global levels. Sci. Total Environ. 2021;789 doi: 10.1016/j.scitotenv.2021.148013. [DOI] [PubMed] [Google Scholar]
- 108.Kato L.S., Conte-Junior C.A. Safety of plastic food packaging: the challenges about non-intentionally added substances (NIAS) discovery, identification and risk assessment. Polymers. 2021;13(13):2077. doi: 10.3390/polym13132077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang Y., Peng Y., Peng C., et al. Comparison of detection methods of microplastics in landfill mineralized refuse and selection of degradation degree indexes. Environ. Sci. Technol. 2021;55(20):13802–13811. doi: 10.1021/acs.est.1c02772. [DOI] [PubMed] [Google Scholar]
- 110.Artham T., Doble M. Fouling and degradation of polycarbonate in seawater: field and lab studies. J. Polym. Environ. 2009;17(3):170–180. doi: 10.1007/s10924-009-0135-x. [DOI] [Google Scholar]
- 111.Hermabessiere L., Dehaut A., Paul-Pont I., et al. Occurrence and effects of plastic additives on marine environments and organisms: a review. Chemosphere. 2017;182:781–793. doi: 10.1016/j.chemosphere.2017.05.096. [DOI] [PubMed] [Google Scholar]
- 112.Russo G., Barbato F., Mita D.G., Grumetto L. Occurrence of Bisphenol A and its analogues in some foodstuff marketed in Europe. Food Chem. Toxicol. 2019;131 doi: 10.1016/j.fct.2019.110575. [DOI] [PubMed] [Google Scholar]
- 113.Lu S., Yang D., Ge X., et al. The internal exposure of phthalate metabolites and bisphenols in waste incineration plant workers and the associated health risks. Environ. Int. 2020;145 doi: 10.1016/j.envint.2020.106101. [DOI] [PubMed] [Google Scholar]
- 114.Muthukrishnan S., Kua H.W. 2016. https://papers.ssrn.com/abstract=3466780 (Computational study of potential impact of end-of-life stage of polycarbonate waste due to dispersion of bisphenol-A from incinerator). Published online January 14. [Google Scholar]
- 115.Covestro Press. Covestro launches mechanical recycling polycarbonate compounding line in China. Pioneering the circular journey. Published October 18, 2023. Accessed December 15, 2023. https://www.covestro.com/press/covestro-launches-mechanical-recycling-polycarbonate-compounding-line-in-china/.
- 116.Covestro Press, Chemical recycling of polycarbonates reaches a major milestone, Circular Economy (2023) Published August 16 https://www.covestro.com/press/chemical-recycling-of-polycarbonates-reaches-a-major-milestone/. (Accessed 15 December 2023).
- 117.OECD . Environment, Health and Safety, Environment Directorate. OECD; 2021. Case study on insulation: an example of chemical considerations for sustainable plastics design. [Google Scholar]
- 118.Mendieta C.M., Vallejos M.E., Felissia F.E., Chinga-Carrasco G., Area M.C. Review: bio-polyethylene from wood wastes. J. Polym. Environ. 2020;28(1):1–16. doi: 10.1007/s10924-019-01582-0. [DOI] [Google Scholar]
- 119.Loomis D., Guyton K.Z., Grosse Y., et al. Carcinogenicity of benzene. Lancet Oncol. 2017;18(12):P1574–P1575. doi: 10.1016/S1470-2045(17)30832-X. [DOI] [PubMed] [Google Scholar]
- 120.Blanc-Lapierre A., Sauvé J.F., Parent M.E. Occupational exposure to benzene, toluene, xylene and styrene and risk of prostate cancer in a population-based study. Occup. Environ. Med. 2018;75(8):562–572. doi: 10.1136/oemed-2018-105058. [DOI] [PubMed] [Google Scholar]
- 121.Peters C.E., Ge C.B., Hall A.L., Davies H.W., Demers P.A. CAREX Canada: an enhanced model for assessing occupational carcinogen exposure. Occup. Environ. Med. 2015;72(1):64–71. doi: 10.1136/oemed-2014-102286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.IARC . International Agency for Research on Cancer (IARC) Press; 2019. Styrene, Styrene-7,8-Oxide, and Quinoline.http://www.ncbi.nlm.nih.gov/books/NBK551039/ [PubMed] [Google Scholar]
- 123.Gibbs B.F., Mulligan C.N. Styrene toxicity: an ecotoxicological assessment. Ecotoxicol. Environ. Saf. 1997;38(3):181–194. doi: 10.1006/eesa.1997.1526. [DOI] [PubMed] [Google Scholar]
- 124.Bond J.A., Bolt H.M. Review of the toxicology of styrene. CRC Crit. Rev. Toxicol. 1989;19(3):227–249. doi: 10.3109/10408448909037472. [DOI] [PubMed] [Google Scholar]
- 125.Zhao L., Shen Y., Sun Y., Mannan M.S., Akbulut M. Thermal hazards analysis of styrene in contact with impurities. J. Loss Prev. Process. Ind. 2020;68 doi: 10.1016/j.jlp.2020.104315. [DOI] [Google Scholar]
- 126.Zhao L., Zhu W., Papadaki M.I., Mannan M.S., Akbulut M. Probing into styrene polymerization runaway hazards: effects of the monomer mass fraction. ACS Omega. 2019;4(5):8136–8145. doi: 10.1021/acsomega.9b00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yi S., Liu J.G., Jin J., Zhu J. Assessment of the occupational and environmental risks of hexabromocyclododecane (HBCD) in China. Chemosphere. 2016;150:431–437. doi: 10.1016/j.chemosphere.2016.01.047. [DOI] [PubMed] [Google Scholar]
- 128.Covaci A., Gerecke A.C., Law R.J., et al. Hexabromocyclododecanes (HBCDs) in the environment and humans: a review. Environ. Sci. Technol. 2006;40(12):3679–3688. doi: 10.1021/es0602492. [DOI] [PubMed] [Google Scholar]
- 129.Thomsen C., Molander P., Daae H.L., et al. Occupational exposure to hexabromocyclododecane at an industrial plant. Environ. Sci. Technol. 2007;41(15):5210–5216. doi: 10.1021/es0702622. [DOI] [PubMed] [Google Scholar]
- 130.Zhang H., Kuo Y.Y., Gerecke A.C., Wang J. Co-release of hexabromocyclododecane (HBCD) and nano- and microparticles from thermal cutting of polystyrene foams. Environ. Sci. Technol. 2012;46(20):10990–10996. doi: 10.1021/es302559v. [DOI] [PubMed] [Google Scholar]
- 131.Marques M.L., Cairrao E. Occurrence and health effects of hexabromocyclododecane: an updated review. Toxics. 2023;11(5):409. doi: 10.3390/toxics11050409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ongono J.S., Dow C., Gambaretti J., et al. Dietary exposure to brominated flame retardants and risk of type 2 diabetes in the French E3N cohort. Environ. Int. 2019;123:54–60. doi: 10.1016/j.envint.2018.11.040. [DOI] [PubMed] [Google Scholar]
- 133.Lam J., Lanphear B.P., Bellinger D., et al. Developmental PBDE exposure and IQ/ADHD in childhood: a systematic review and meta-analysis. Environ. Health Perspect. 2017;125(8) doi: 10.1289/EHP1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Herbstman J.B., Sj ödin A., Kurzon M., et al. Prenatal exposure to PBDEs and neurodevelopment. Environ. Health Perspect. 2010;118(5):712–719. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhao X., Peng S., Xiang Y., et al. Correlation between prenatal exposure to polybrominated diphenyl ethers (PBDEs) and infant birth outcomes: a meta-analysis and an experimental study. Int. J. Environ. Res. Publ. Health. 2017;14(3):268. doi: 10.3390/ijerph14030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yan D., Jiao Y., Yan H., Liu T., Yan H., Yuan J. Endocrine-disrupting chemicals and the risk of gestational diabetes mellitus: a systematic review and meta-analysis. Environ. Health. 2022;21(1):53. doi: 10.1186/s12940-022-00858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ortuño N., Lundstedt S., Lundin L. Emissions of PBDD/Fs, PCDD/Fs and PBDEs from flame-retarded high-impact polystyrene under thermal stress. Chemosphere. 2015;123:64–70. doi: 10.1016/j.chemosphere.2014.10.085. [DOI] [PubMed] [Google Scholar]
- 138.Blum A., Behl M., Birnbaum L., et al. Organophosphate ester flame retardants: are they a regrettable substitution for polybrominated diphenyl ethers? Environ. Sci. Technol. Lett. 2019;6(11):638–649. doi: 10.1021/acs.estlett.9b00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhao J yi, xiang Zhan Z., Lu M juan, Tao F biao, Wu D., Gao H. A systematic scoping review of epidemiological studies on the association between organophosphate flame retardants and neurotoxicity. Ecotoxicol. Environ. Saf. 2022;243 doi: 10.1016/j.ecoenv.2022.113973. [DOI] [PubMed] [Google Scholar]
- 140.Gan H., Zhang Y., Wang Y fei, biao Tao F., Gao H. Relationships of prenatal organophosphate ester exposure with pregnancy and birth outcomes: a systematic scoping review of epidemiological studies. Ecotoxicol. Environ. Saf. 2023;252 doi: 10.1016/j.ecoenv.2023.114642. [DOI] [PubMed] [Google Scholar]
- 141.Patel S.H., Xanthos M. Volatile emissions during thermoplastics processing—a review. Adv. Polym. Technol. 1995;14(1):67–77. doi: 10.1002/adv.1995.060140107. [DOI] [Google Scholar]
- 142.Pilevar Z., Bahrami A., Beikzadeh S., Hosseini H., Jafari S.M. Migration of styrene monomer from polystyrene packaging materials into foods: characterization and safety evaluation. Trends Food Sci. Technol. 2019;91:248–261. doi: 10.1016/j.tifs.2019.07.020. [DOI] [Google Scholar]
- 143.Khaksar M.R., Ghazi-Khansari M. Determination of migration monomer styrene from GPPS (general purpose polystyrene) and HIPS (high impact polystyrene) cups to hot drinks. Toxicol. Mech. Methods. 2009;19(3):257–261. doi: 10.1080/15376510802510299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Moad G., Solomon D.H., Johns S.R., Willing R.I. Fate of the initiator in the azobisisobutyronitrile-initiated polymerization of styrene. Macromolecules. 1984;17(5):1094–1099. doi: 10.1021/ma00135a021. [DOI] [Google Scholar]
- 145.OECD . United Nations Environment Programme (UNEP); 1999. 2,2’-Azobis(2-Methylpropionitrile) Screening Information Dataset (SIDS) Initial Assessment Report for 9th SIAM.https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=a82bd6a2df707308aa52defb488609185e0e49e2 [Google Scholar]
- 146.Ishiwata H, Inoue T, Yamamoto M, Yoshihira K. Determination of Tetramethylsuccinonitrile in Food Containers and Packaging Made of Plastics. ACS Publications. doi:10.1021/jf00084a046.
- 147.Fordham P.J., Gramshaw J.W., Castle L. Analysis for organic residues from aids to polymerization used to make plastics intended for food contact. Food Addit. Contam. 2001;18(5):461–471. doi: 10.1080/02652030119958. [DOI] [PubMed] [Google Scholar]
- 148.Dedeo M., Drake S. Healthy environments: strategies for avoiding flame retardants in the built environment. Perkins & Will Research; 2017. pp. 1–59.https://research.perkinswill.com/articles/healthy-environments-strategies-for-avoiding-flame-retardants-in-the-built-environment/ [Google Scholar]
- 149.Abdallah M.A.E., Harrad S. Personal exposure to HBCDs and its degradation products via ingestion of indoor dust. Environ. Int. 2009;35(6):870–876. doi: 10.1016/j.envint.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 150.Duis K., Coors A. Microplastics in the aquatic and terrestrial environment: sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016;28(1):2. doi: 10.1186/s12302-015-0069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhou Y., Ashokkumar V., Amobonye A., et al. Current research trends on cosmetic microplastic pollution and its impacts on the ecosystem: a review. Environ Pollut. 2023;320 doi: 10.1016/j.envpol.2023.121106. [DOI] [PubMed] [Google Scholar]
- 152.Kik K., Bukowska B., Sicińska P. Polystyrene nanoparticles: sources, occurrence in the environment, distribution in tissues, accumulation and toxicity to various organisms. Environ Pollut. 2020;262 doi: 10.1016/j.envpol.2020.114297. [DOI] [PubMed] [Google Scholar]
- 153.Hwang J., Choi D., Han S., Jung S.Y., Choi J., Hong J. Potential toxicity of polystyrene microplastic particles. Sci. Rep. 2020;10(1):7391. doi: 10.1038/s41598-020-64464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang J., Lee J., Kwon E.E., Jeong S. Quantitative analysis of polystyrene microplastic and styrene monomer released from plastic food containers. Heliyon. 2023;9(5) doi: 10.1016/j.heliyon.2023.e15787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hee Y.Y., Weston K., Suratman S. The effect of storage conditions and washing on microplastic release from food and drink containers. Food Packag. Shelf Life. 2022;32 doi: 10.1016/j.fpsl.2022.100826. [DOI] [Google Scholar]
- 156.Kedzierski M., Lechat B., Sire O., Le Maguer G., Le Tilly V., Bruzaud S. Microplastic contamination of packaged meat: occurrence and associated risks. Food Packag. Shelf Life. 2020;24 doi: 10.1016/j.fpsl.2020.100489. [DOI] [Google Scholar]
- 157.Planet Protector Planet protector packaging | packaging for the planet. https://planetprotectorpackaging.com/
- 158.Cruz foam. Cruz foam. https://www.cruzfoam.com
- 159.Soykeabkaew N., Thanomsilp C., Suwantong O. A review: starch-based composite foams. Compos Part Appl Sci Manuf. 2015;78:246–263. doi: 10.1016/j.compositesa.2015.08.014. [DOI] [Google Scholar]
- 160.Tapia-Blácido D.R., Aguilar G.J., de Andrade M.T., Rodrigues-Júnior M.F., Guareschi-Martins F.C. Trends and challenges of starch-based foams for use as food packaging and food container. Trends Food Sci. Technol. 2022;119:257–271. doi: 10.1016/j.tifs.2021.12.005. [DOI] [Google Scholar]
- 161.Zhang Y., Yuan Y., Tan W. Influences of humic acid on the release of polybrominated diphenyl ethers from plastic waste in landfills under different environmental conditions. Ecotoxicol. Environ. Saf. 2022;230 doi: 10.1016/j.ecoenv.2021.113122. [DOI] [PubMed] [Google Scholar]
- 162.He P., Chen L., Shao L., Zhang H., Lü F. Municipal solid waste (MSW) landfill: a source of microplastics? Evidence of microplastics in landfill leachate. Water Res. 2019;159:38–45. doi: 10.1016/j.watres.2019.04.060. [DOI] [PubMed] [Google Scholar]
- 163.Shi J., Wu D., Su Y., Xie B. Selective enrichment of antibiotic resistance genes and pathogens on polystyrene microplastics in landfill leachate. Sci. Total Environ. 2021;765 doi: 10.1016/j.scitotenv.2020.142775. [DOI] [PubMed] [Google Scholar]
- 164.Shim W.J., Hong S.H., Eo S. In: Microplastic Contamination in Aquatic Environments. Zeng E.Y., editor. Elsevier; 2018. Chapter 1 - marine microplastics: abundance, distribution, and composition; pp. 1–26. [DOI] [Google Scholar]
- 165.Turner A. Foamed polystyrene in the marine environment: sources, additives, transport, behavior, and impacts. Environ. Sci. Technol. 2020;54(17):10411–10420. doi: 10.1021/acs.est.0c03221. [DOI] [PubMed] [Google Scholar]
- 166.De-la-Torre G.E., Dioses-Salinas D.C., Pizarro-Ortega C.I., Saldaña-Serrano M. Global distribution of two polystyrene-derived contaminants in the marine environment: a review. Mar. Pollut. Bull. 2020;161 doi: 10.1016/j.marpolbul.2020.111729. [DOI] [PubMed] [Google Scholar]
- 167.Barhoumi B., Metian M., Oberhaensli F., et al. Extruded polystyrene microplastics as a source of brominated flame retardant additives in the marine environment: long-term field and laboratory experiments. Environ. Int. 2023;172 doi: 10.1016/j.envint.2023.107797. [DOI] [PubMed] [Google Scholar]
- 168.Aminot Y., Lanctôt C., Bednarz V., et al. Leaching of flame-retardants from polystyrene debris: bioaccumulation and potential effects on coral. Mar. Pollut. Bull. 2020;151 doi: 10.1016/j.marpolbul.2019.110862. [DOI] [PubMed] [Google Scholar]
- 169.Thomsen C., Knutsen H.K., Liane V.H., et al. Consumption of fish from a contaminated lake strongly affects the concentrations of polybrominated diphenyl ethers and hexabromocyclododecane in serum. Mol. Nutr. Food Res. 2008;52(2):228–237. doi: 10.1002/mnfr.200700123. [DOI] [PubMed] [Google Scholar]
- 170.Schecter A., Haffner D., Colacino J., et al. Polybrominated diphenyl ethers (PBDEs) and hexabromocyclodecane (HBCD) in composite U.S. food samples. Environ. Health Perspect. 2010;118(3):357–362. doi: 10.1289/ehp.0901345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Amamiya K., Saido K., Chung S.Y., Hiaki T., Lee D.S., Kwon B.G. Evidence of transport of styrene oligomers originated from polystyrene plastic to oceans by runoff. Sci. Total Environ. 2019;667:57–63. doi: 10.1016/j.scitotenv.2019.02.383. [DOI] [PubMed] [Google Scholar]
- 172.Jose J., Uvais K.N., Sreenadh T.S., Deepak A.V., Rejeesh C.R. Investigations into the development of a mycelium biocomposite to substitute polystyrene in packaging applications. Arabian J. Sci. Eng. 2021;46(3):2975–2984. doi: 10.1007/s13369-020-05247-2. [DOI] [Google Scholar]
- 173.Doroudiani S., Omidian H. Environmental, health and safety concerns of decorative mouldings made of expanded polystyrene in buildings. Build. Environ. 2010;45(3):647–654. doi: 10.1016/j.buildenv.2009.08.004. [DOI] [Google Scholar]
- 174.Fernandes A.R., Falandysz J. Polybrominated dibenzo-p-dioxins and furans (PBDD/Fs): contamination in food, humans and dietary exposure. Sci. Total Environ. 2021;761 doi: 10.1016/j.scitotenv.2020.143191. [DOI] [PubMed] [Google Scholar]
- 175.Birnbaum L.S., Staskal D.F., Diliberto J.J. Health effects of polybrominated dibenzo-p-dioxins (PBDDs) and dibenzofurans (PBDFs) Environ. Int. 2003;29(6):855–860. doi: 10.1016/S0160-4120(03)00106-5. [DOI] [PubMed] [Google Scholar]
- 176.Thakur S., Verma A., Sharma B., Chaudhary J., Tamulevicius S., Thakur V.K. Recent developments in recycling of polystyrene based plastics. Curr. Opin. Green Sustainable Chem. 2018;13:32–38. doi: 10.1016/j.cogsc.2018.03.011. [DOI] [Google Scholar]
- 177.Abdallah M.A.E., Sharkey M., Berresheim H., Harrad S. Hexabromocyclododecane in polystyrene packaging: a downside of recycling? Chemosphere. 2018;199:612–616. doi: 10.1016/j.chemosphere.2018.02.084. [DOI] [PubMed] [Google Scholar]
- 178.Murphy J. second ed. Elsevier; 2001. The Additives for Plastics Handbook. [DOI] [Google Scholar]
- 179.Wilkes C.E., Summers J.W., Daniels C.A., Berard M.T. Hanser Publications; 2005. PVC Handbook. [Google Scholar]
- 180.Sampson J., de Korte D. DEHP-plasticised PVC: relevance to blood services. Transfus. Med. 2011;21(2):73–83. doi: 10.1111/j.1365-3148.2010.01056.x. [DOI] [PubMed] [Google Scholar]
- 181.Qadeer A., Kirsten K.L., Ajmal Z., Jiang X., Zhao X. Alternative plasticizers as emerging global environmental and health threat: another regrettable substitution? Environ. Sci. Technol. 2022;56(3):1482–1488. doi: 10.1021/acs.est.1c08365. [DOI] [PubMed] [Google Scholar]
- 182.Petrović E.K., Hamer L.K. Improving the healthiness of sustainable construction: example of polyvinyl chloride (PVC) Buildings. 2018;8(2):28. doi: 10.3390/buildings8020028. [DOI] [Google Scholar]
- 183.World Chlorine Council . United Nations Environment Programme (UNEP); 2021. World Chlorine Council Report to UNEP on Chlor-Alkali Partnership-Data 2020.https://wedocs.unep.org/20.500.11822/36451 [Google Scholar]
- 184.Crook J., Mousavi A. The chlor-alkali process: a review of history and pollution. Environ. Forensics. 2016;17(3):211–217. doi: 10.1080/15275922.2016.1177755. [DOI] [Google Scholar]
- 185.OECD . Environment, Health and Safety, Environment Directorate. OECD; 2021. Case study on flooring: an example of chemical considerations for sustainable plastics design.https://www.oecd.org/chemicalsafety/risk-management/sustainable-plastic-products-flooring.pdf [Google Scholar]
- 186.Kumar S., Sharma A., Sedha S. Occupational and environmental mercury exposure and human reproductive health - a review. J. Turk. Ger. Gynecol. Assoc. 2022;23(3):199–210. doi: 10.4274/jtgga.galenos.2022.2022-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Vallette J. Healthy Building Network; 2018. Chlorine and Building Materials: A Global Inventory of Production Technologies, Markets, and Pollution. [Google Scholar]
- 188.Shirkhanloo H., Golbabaei F., Hassani H., Eftekhar F., Kian M.J. Occupational exposure to mercury: air exposure assessment and biological monitoring based on dispersive ionic liquid-liquid microextraction. Iran. J. Public Health. 2014;43(6):793–799. [PMC free article] [PubMed] [Google Scholar]
- 189.Navarro A., Quirós L., Casado M., et al. Physiological responses to mercury in feral carp populations inhabiting the low Ebro River (NE Spain), a historically contaminated site. Aquat. Toxicol. 2009;93(2):150–157. doi: 10.1016/j.aquatox.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 190.Neghab M., Amin Norouzi M., Choobineh A., Reza Kardaniyan M., Hassan Zadeh J. Health effects associated with long-term occupational exposure of employees of a chlor-alkali plant to mercury. Int. J. Occup. Saf. Ergon. 2012;18(1):97–106. doi: 10.1080/10803548.2012.11076920. [DOI] [PubMed] [Google Scholar]
- 191.Office of Chemical Safety and Pollution Prevention . U.S. Environmental Protection Agency (EPA); 2020. Risk Evaluation for Asbestos Part 1: Chrysotile Asbestos.https://www.epa.gov/sites/default/files/2020-12/documents/1_risk_evaluation_for_asbestos_part_1_chrysotile_asbestos.pdf [Google Scholar]
- 192.McCormack V., Peto J., Byrnes G., Straif K., Boffetta P. Estimating the asbestos-related lung cancer burden from mesothelioma mortality. Br. J. Cancer. 2012;106(3):575–584. doi: 10.1038/bjc.2011.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.GBD 2016 Occupational Carcinogens Collaborators Global and regional burden of cancer in 2016 arising from occupational exposure to selected carcinogens: a systematic analysis for the Global Burden of Disease Study 2016. Occup. Environ. Med. 2020;77(3):151–159. doi: 10.1136/oemed-2019-106012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.IARC . Vol. 14. International Agency for Research on Cancer (IARC) Press; 1977. https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Asbestos-1977 (Asbestos). [Google Scholar]
- 195.Carlson L.M., Angrish M., Shirke A.V., et al. Systematic evidence map for over one hundred and fifty per- and polyfluoroalkyl substances (PFAS) Environ. Health Perspect. 2022;130(5) doi: 10.1289/EHP10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pelch K.E., Reade A., Kwiatkowski C.F., et al. The PFAS-Tox Database: a systematic evidence map of health studies on 29 per- and polyfluoroalkyl substances. Environ. Int. 2022;167 doi: 10.1016/j.envint.2022.107408. [DOI] [PubMed] [Google Scholar]
- 197.Negri E., Metruccio F., Guercio V., et al. Exposure to PFOA and PFOS and fetal growth: a critical merging of toxicological and epidemiological data. Crit. Rev. Toxicol. 2017;47(6):489–515. doi: 10.1080/10408444.2016.1271972. [DOI] [PubMed] [Google Scholar]
- 198.Luo Y., Deji Z., Huang Z. Exposure to perfluoroalkyl substances and allergic outcomes in children: a systematic review and meta-analysis. Environ. Res. 2020;191 doi: 10.1016/j.envres.2020.110145. [DOI] [PubMed] [Google Scholar]
- 199.Kim M.J., Moon S., Oh B.C., et al. Association between perfluoroalkyl substances exposure and thyroid function in adults: a meta-analysis. PLoS One. 2018;13(5) doi: 10.1371/journal.pone.0197244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang L., Louie A., Rigutto G., et al. A systematic evidence map of chronic inflammation and immunosuppression related to per- and polyfluoroalkyl substance (PFAS) exposure. Environ. Res. 2023;220 doi: 10.1016/j.envres.2022.115188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Forns J., Verner M.A., Iszatt N., et al. Early life exposure to perfluoroalkyl substances (PFAS) and ADHD: a meta-analysis of nine European population-based studies. Environ. Health Perspect. 2020;128(5) doi: 10.1289/EHP5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yao J., Pan Y., Sheng N., et al. Novel perfluoroalkyl ether carboxylic acids (PFECAs) and sulfonic acids (PFESAs): occurrence and association with serum biochemical parameters in residents living near a fluorochemical plant in China. Environ. Sci. Technol. 2020;54(21):13389–13398. doi: 10.1021/acs.est.0c02888. [DOI] [PubMed] [Google Scholar]
- 203.Guillette T.C., McCord J., Guillette M., et al. Elevated levels of per- and polyfluoroalkyl substances in Cape Fear River Striped Bass (Morone saxatilis) are associated with biomarkers of altered immune and liver function. Environ. Int. 2020;136 doi: 10.1016/j.envint.2019.105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kotlarz N., McCord J., Collier D., et al. Measurement of novel, drinking water-associated PFAS in blood from adults and children in wilmington, North Carolina. Environ. Health Perspect. 2020;128(7) doi: 10.1289/EHP6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.International Agency for Research on Cancer (IARC) IARC Monographs on the Identification of Carcinogenic Hazards to Humans. 2022. Agents classified by the IARC monographs, volumes 1–132.https://monographs.iarc.who.int/list-of-classifications Published September 7. [Google Scholar]
- 206.Cheng T.J., Chou P.Y., Huang M.L., Du C.L., Wong R.H., Chen P.C. Increased lymphocyte sister chromatid exchange frequency in workers with exposure to low level of ethylene dichloride. Mutat Res Toxicol Environ Mutagen. 2000;470(2):109–114. doi: 10.1016/S1383-5742(00)00045-4. [DOI] [PubMed] [Google Scholar]
- 207.Cheng T.J., Huang M.L., You N.C., Du C.L., Chau T.T. Abnormal liver function in workers exposed to low levels of ethylene dichloride and vinyl chloride monomer. J. Occup. Environ. Med. 1999;41(12):1128–1133. doi: 10.1097/00043764-199912000-00018. [DOI] [PubMed] [Google Scholar]
- 208.Yuan T.H., Chen J.L., Shie R.H., Yeh Y.P., Chen Y.H., Chan C.C. Liver fibrosis associated with potential vinyl chloride and ethylene dichloride exposure from the petrochemical industry. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139920. [DOI] [PubMed] [Google Scholar]
- 209.Koistinen J., Herve S., Ruokojärvi P., Koponen J., Vartiainen T. Persistent organic pollutants in two Finnish watercourses: levels, congener profiles and source estimation by mussel incubation. Chemosphere. 2010;80(6):625–633. doi: 10.1016/j.chemosphere.2010.04.058. [DOI] [PubMed] [Google Scholar]
- 210.Olivares A., Quirós L., Pelayo S., et al. Integrated biological and chemical analysis of organochlorine compound pollution and of its biological effects in a riverine system downstream the discharge point. Sci. Total Environ. 2010;408(22):5592–5599. doi: 10.1016/j.scitotenv.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 211.Garí M., Bosch C., Grimalt J.O., Sunyer J. Impacts of atmospheric chlor-alkali factory emissions in surrounding populations. Environ. Int. 2014;65:1–8. doi: 10.1016/j.envint.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 212.Govarts E., Nieuwenhuijsen M., Schoeters G., et al. Birth weight and prenatal exposure to polychlorinated biphenyls (PCBs) and dichlorodiphenyldichloroethylene (DDE): a meta-analysis within 12 European birth cohorts. Environ. Health Perspect. 2012;120(2):162–170. doi: 10.1289/ehp.1103767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Cano-Sancho G., Ploteau S., Matta K., et al. Human epidemiological evidence about the associations between exposure to organochlorine chemicals and endometriosis: systematic review and meta-analysis. Environ. Int. 2019;123:209–223. doi: 10.1016/j.envint.2018.11.065. [DOI] [PubMed] [Google Scholar]
- 214.Wu H., Bertrand K.A., Choi A.L., et al. Persistent organic pollutants and type 2 diabetes: a prospective analysis in the Nurses' Health Study and meta-analysis. Environ. Health Perspect. 2013;121(2):153–161. doi: 10.1289/ehp.1205248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Leng L., Li J., mei Luo X., et al. Polychlorinated biphenyls and breast cancer: a congener-specific meta-analysis. Environ. Int. 2016;88:133–141. doi: 10.1016/j.envint.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 216.Zhang J., Huang Y., Wang X., Lin K., Wu K. Environmental polychlorinated biphenyl exposure and breast cancer risk: a meta-analysis of observational studies. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0142513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.US EPA, Health effects support Document for hexachlorobutadiene. Office of water (4304T), Health and Ecological Criteria Division (2003). Report number: EPA 822-R-03-002. pp. 1-1–10-17. https://www.epa.gov/sites/default/files/2014-09/documents/support_cc1_hexachlorobutadiene_healtheffects.pdf. (Accessed 30 June 2023).
- 218.Duque-Cartagena T., Mundstock E., Dala Bernardina Dalla M., Vontobel Padoin A., Cañon-Montañez W., Mattiello R. The role of environmental pollutants in body composition: systematic review and meta-analysis. Environ. Res. 2023;228 doi: 10.1016/j.envres.2023.115840. [DOI] [PubMed] [Google Scholar]
- 219.Gang N., Van Allen K., Villeneuve P.J., MacDonald H., Bruin J.E. Sex-specific associations between type 2 diabetes incidence and exposure to dioxin and dioxin-like pollutants: a meta-analysis. Front Toxicol. 2022;3 doi: 10.3389/ftox.2021.685840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.US EPA . IRIS); 2010. Toxicological Review of Carbon Tetrachloride (CAS No. 56-23-5) in Support of Summary Information on the Integrated Risk Information System.http://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0020tr.pdf [Google Scholar]
- 221.US EPA . U.S. Environmental Protection Agency (EPA); 2022. Final Revised Unreasonable Risk Determination for Carbon Tetrachloride. [Google Scholar]
- 222.US EPA Toxicological review of chloroform (CAS No. 67-66-3) in support of summary information on the integrated risk information system (IRIS) 2001. https://iris.epa.gov/static/pdfs/0025tr.pdf
- 223.Höfer R., Hinrichs K. In: Polymers - Opportunities And Risks II: Sustainability, Product Design And Processing. The Handbook of Environmental Chemistry. Eyerer P., Weller M., Hübner C., editors. Springer; 2010. Additives for the manufacture and processing of polymers; pp. 97–145. [DOI] [Google Scholar]
- 224.Brandt-Rauf P.W., Li Y., Long C., Monaco R., Kovvali G., Marion M.J. Plastics and carcinogenesis: the example of vinyl chloride. J. Carcinog. 2012;11:5. doi: 10.4103/1477-3163.93700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.US EPA . U.S. Environmental Protection Agency; 2000. Toxicological Review of Vinyl Chloride CAS No. 75-01-4.https://nepis.epa.gov/Exe/ZyPDF.cgi/P1006B7P.PDF?Dockey=P1006B7P.PDF [Google Scholar]
- 226.Baitz D.M., Kreißig M.J., Byrne E., et al. European Commission; 2004. Final Report: Life Cycle Assessment of PVC and of Principal Competing Materials.https://ec.europa.eu/docsroom/documents/13049/attachments/1/translations/en/renditions/pdf [Google Scholar]
- 227.Ng T.P., Lee H.S., Low Y.M., Phoon W.H., Ng Y.L. Pulmonary effects of polyvinyl chloride dust exposure on compounding workers. Scand. J. Work. Environ. Health. 1991;17(1):53–59. doi: 10.5271/sjweh.1734. [DOI] [PubMed] [Google Scholar]
- 228.Mastrangelo G., Fedeli U., Fadda E., Milan G., Turato A., Pavanello S. Lung cancer risk in workers exposed to poly(vinyl chloride) dust: a nested case-referent study. Occup. Environ. Med. 2003;60(6):423–428. doi: 10.1136/oem.60.6.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Girardi P., Barbiero F., Baccini M., et al. Mortality for lung cancer among PVC baggers employed in the vinyl chloride industry. Int. J. Environ. Res. Publ. Health. 2022;19(10):6246. doi: 10.3390/ijerph19106246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Scarselli A., Corfiati M., Di Marzio D., Massari S., Marinaccio A., Iavicoli S. The impact of vinyl chloride exposure on the health of Italian workers: an evaluation from SIREP compliance data. Arch. Environ. Occup. Health. 2022;77(5):372–381. doi: 10.1080/19338244.2021.1900045. [DOI] [PubMed] [Google Scholar]
- 231.Fréry N., Santonen T., Porras S.P., et al. Biomonitoring of occupational exposure to phthalates: a systematic review. Int. J. Hyg Environ. Health. 2020;229 doi: 10.1016/j.ijheh.2020.113548. [DOI] [PubMed] [Google Scholar]
- 232.Lee D.W., Kim M.S., Lim Y.H., Lee N., Hong Y.C. Prenatal and postnatal exposure to di-(2-ethylhexyl) phthalate and neurodevelopmental outcomes: a systematic review and meta-analysis. Environ. Res. 2018;167:558–566. doi: 10.1016/j.envres.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 233.Shoshtari-Yeganeh B., Zarean M., Mansourian M., et al. Systematic review and meta-analysis on the association between phthalates exposure and insulin resistance. Environ. Sci. Pollut. Res. 2019;26(10):9435–9442. doi: 10.1007/s11356-019-04373-1. [DOI] [PubMed] [Google Scholar]
- 234.Zhang H., Gao F., Ben Y., Su Y. Association between phthalate exposure and risk of spontaneous pregnancy loss: a systematic review and meta-analysis. Environ Pollut. 2020;267 doi: 10.1016/j.envpol.2020.115446. [DOI] [PubMed] [Google Scholar]
- 235.Cai H., Zheng W., Zheng P., et al. Human urinary/seminal phthalates or their metabolite levels and semen quality: a meta-analysis. Environ. Res. 2015;142:486–494. doi: 10.1016/J.ENVRES.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 236.Ensslin A.S., Koller M.F. Convulsions and hypoglycemia due to tetramethyl succinonitrile intoxication in the polyvinyl chloride (PVC) industry: a 4-year follow-up. Int. J. Occup. Med. Environ. Health. 2014;27(2):188–195. doi: 10.2478/s13382-014-0244-1. [DOI] [PubMed] [Google Scholar]
- 237.Ruzickova K., Cobbing M., Rossi M., Belazzi T. Preventing harm from phthalates, avoiding PVC in hospitals. Health Care Without Harm. 2024:1–28. https://noharm.org/sites/default/files/lib/downloads/pvc/Preventing_Harm_From_Phthalates.pdf [Google Scholar]
- 238.Health Care Without Harm, Towards PVC-free healthcare, Health Care Without Harm (2023) 1–31. https://noharm-global.org/sites/default/files/documents-files/7382/2023-05-Towards-PVC-free-healthcare.pdf. (Accessed 18 May 2023).
- 239.Fankhauser-Noti A., Grob K. Migration of plasticizers from PVC gaskets of lids for glass jars into oily foods: amount of gasket material in food contact, proportion of plasticizer migrating into food and compliance testing by simulation. Trends Food Sci. Technol. 2006;17(3):105–112. doi: 10.1016/j.tifs.2005.10.013. [DOI] [Google Scholar]
- 240.Goulas A.E., Zygoura P., Karatapanis A., Georgantelis D., Kontominas M.G. Migration of di(2-ethylhexyl) adipate and acetyltributyl citrate plasticizers from food-grade PVC film into sweetened sesame paste (halawa tehineh): Kinetic and penetration study. Food Chem. Toxicol. 2007;45(4):585–591. doi: 10.1016/j.fct.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 241.Shu H., Jönsson B.A.G., Gennings C., Lindh C.H., Nånberg E., Bornehag C.G. PVC flooring at home and uptake of phthalates in pregnant women. Indoor Air. 2019;29(1):43–54. doi: 10.1111/ina.12508. [DOI] [PubMed] [Google Scholar]
- 242.Sheikh I.A., Beg M.A. Structural characterization of potential endocrine disrupting activity of alternate plasticizers di-(2-ethylhexyl) adipate (DEHA), acetyl tributyl citrate (ATBC) and 2,2,4-trimethyl 1,3-pentanediol diisobutyrate (TPIB) with human sex hormone-binding globulin. Reprod. Toxicol. 2019;83:46–53. doi: 10.1016/j.reprotox.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 243.Rajkumar A., Luu T., Beal M.A., Barton-Maclaren T.S., Hales B.F., Robaire B. Phthalates and alternative plasticizers differentially affect phenotypic parameters in gonadal somatic and germ cell lines. Biol. Reprod. 2022;106(3):613–627. doi: 10.1093/biolre/ioab216. [DOI] [PubMed] [Google Scholar]
- 244.European Commission, Directorate-General for Environment . Publications Office of the European Union; 2022. The Use of PVC (Poly Vinyl Chloride) in the Context of a Non-toxic Environment: Final Report. [DOI] [Google Scholar]
- 245.Sanders A.P., Mazzella M.J., Malin A.J., et al. Combined exposure to lead, cadmium, mercury, and arsenic and kidney health in adolescents age 12–19 in NHANES 2009–2014. Environ. Int. 2019;131 doi: 10.1016/j.envint.2019.104993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Tellez-Plaza M., Jones M.R., Dominguez-Lucas A., Guallar E., Navas-Acien A. Cadmium exposure and clinical cardiovascular disease: a systematic review. Curr. Atherosclerosis Rep. 2013;15(10):356. doi: 10.1007/s11883-013-0356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lu X., Xu X., Zhang Y., Zhang Y., Wang C., Huo X. Elevated inflammatory Lp-PLA2 and IL-6 link e-waste Pb toxicity to cardiovascular risk factors in preschool children. Environ Pollut. 2018;234:601–609. doi: 10.1016/j.envpol.2017.11.094. [DOI] [PubMed] [Google Scholar]
- 248.Rantakokko P., Main K.M., Wohlfart-Veje C., et al. Association of placenta organotin concentrations with congenital cryptorchidism and reproductive hormone levels in 280 newborn boys from Denmark and Finland. Hum. Reprod. 2013;28(6):1647–1660. doi: 10.1093/humrep/det040. [DOI] [PubMed] [Google Scholar]
- 249.Boettner E., Ball G., Hollingsworth Z., Aquino R. U.S. Environmental Protection Agency (EPA); 2004. Organic and Organotin Compounds Leached from PVC and CPVC Pipe.https://cfpub.epa.gov/si/si_public_record_Report.cfm?Lab=ORD&dirEntryID=30597 [Google Scholar]
- 250.Lamprea K., Bressy A., Mirande-Bret C., Caupos E., Gromaire M.C. Alkylphenol and bisphenol A contamination of urban runoff: an evaluation of the emission potentials of various construction materials and automotive supplies. Environ. Sci. Pollut. Res. 2018;25(22):21887–21900. doi: 10.1007/s11356-018-2272-z. [DOI] [PubMed] [Google Scholar]
- 251.Cheng Y.C., Chen H.W., Chen W.L., Chen C.Y., Wang G.S. Occurrence of nonylphenol and bisphenol A in household water pipes made of different materials. Environ. Monit. Assess. 2016;188(10):562. doi: 10.1007/s10661-016-5556-0. [DOI] [PubMed] [Google Scholar]
- 252.Wang H., Jiang L., Gu S., Wang X. Migration of bisphenol A from polyvinyl chloride plastics to solvents of different polarities and packaged food in China. Packag. Technol. Sci. 2021;34(2):127–137. doi: 10.1002/pts.2545. [DOI] [Google Scholar]
- 253.Noorimotlagh Z., Mirzaee S.A., Martinez S.S., Rachoń D., Hoseinzadeh M., Jaafarzadeh N. Environmental exposure to nonylphenol and cancer progression risk–A systematic review. Environ. Res. 2020;184 doi: 10.1016/j.envres.2020.109263. [DOI] [PubMed] [Google Scholar]
- 254.Loyo-Rosales J.E., Rosales-Rivera G.C., Lynch A.M., Rice C.P., Torrents A. Migration of nonylphenol from plastic containers to water and a milk surrogate. J. Agric. Food Chem. 2004;52(7):2016–2020. doi: 10.1021/jf0345696. [DOI] [PubMed] [Google Scholar]
- 255.Akovali G. In: Toxicity of Building Materials. Pacheco-Torgal F., Jalali S., Fucic A., editors. Woodhead Publishing Series in Civil and Structural Engineering. Woodhead Publishing; 2012. 2 - plastic materials: polyvinyl chloride (PVC) pp. 23–53. [DOI] [Google Scholar]
- 256.Walter R.K., Lin P.H., Edwards M., Richardson R.E. Investigation of factors affecting the accumulation of vinyl chloride in polyvinyl chloride piping used in drinking water distribution systems. Water Res. 2011;45(8):2607–2615. doi: 10.1016/j.watres.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 257.Al-Malack M.H., Sheikheldin S.Y., Fayad N.M., Khaja N. Effect of water quality parameters on the migration of vinyl chloride monomer from unplasticized PVC pipes. Water Air Soil Pollut. 2000;120(1):195–208. doi: 10.1023/A:1005249808374. [DOI] [Google Scholar]
- 258.Ye X., Wang P., Wu Y., Zhou Y., Sheng Y., Lao K. Microplastic acts as a vector for contaminants: the release behavior of dibutyl phthalate from polyvinyl chloride pipe fragments in water phase. Environ. Sci. Pollut. Res. 2020;27(33):42082–42091. doi: 10.1007/s11356-020-10136-0. [DOI] [PubMed] [Google Scholar]
- 259.7 PVC Alternatives That Are Eco Friendly . 2023. https://ourecofriendlylife.com/7-pvc-alternatives-that-are-eco-friendly/ (Our eco friendly life). Published March 6, 2023. [Google Scholar]
- 260.Naser A.Z., Deiab I., Darras B.M. Poly(lactic acid) (PLA) and polyhydroxyalkanoates (PHAs), green alternatives to petroleum-based plastics: a review. RSC Adv. 2021;11(28):17151–17196. doi: 10.1039/D1RA02390J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Fernández-González V., Andrade-Garda J.M., López-Mahía P., Muniategui-Lorenzo S. Misidentification of PVC microplastics in marine environmental samples. TrAC Trends Anal Chem. 2022;153 doi: 10.1016/j.trac.2022.116649. [DOI] [Google Scholar]
- 262.Henkel C., Hüffer T., Hofmann T. Polyvinyl chloride microplastics leach phthalates into the aquatic environment over decades. Environ. Sci. Technol. 2022;56(20):14507–14516. doi: 10.1021/acs.est.2c05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Cao Y., Lin H., Zhang K., et al. Microplastics: a major source of phthalate esters in aquatic environments. J. Hazard Mater. 2022;432 doi: 10.1016/j.jhazmat.2022.128731. [DOI] [PubMed] [Google Scholar]
- 264.Turner A., Filella M. Polyvinyl chloride in consumer and environmental plastics, with a particular focus on metal-based additives. Environ Sci Process Impacts. 2021;23(9):1376–1384. doi: 10.1039/D1EM00213A. [DOI] [PubMed] [Google Scholar]
- 265.Yu J., Sun L., Wang B., et al. Study on the behavior of heavy metals during thermal treatment of municipal solid waste (MSW) components. Environ. Sci. Pollut. Res. 2016;23(1):253–265. doi: 10.1007/s11356-015-5644-7. [DOI] [PubMed] [Google Scholar]
- 266.Zhang M., Buekens A., Jiang X., Li X. Dioxins and polyvinylchloride in combustion and fires. Waste Manag. Res. 2015;33(7):630–643. doi: 10.1177/0734242X15590651. [DOI] [PubMed] [Google Scholar]
- 267.Velis C.A., Cook E. Mismanagement of plastic waste through open burning with emphasis on the global south: a systematic review of risks to occupational and public health. Environ. Sci. Technol. 2021;55(11):7186–7207. doi: 10.1021/acs.est.0c08536. [DOI] [PubMed] [Google Scholar]
- 268.He Z., Li G., Chen J., Huang Y., An T., Zhang C. Pollution characteristics and health risk assessment of volatile organic compounds emitted from different plastic solid waste recycling workshops. Environ. Int. 2015;77:85–94. doi: 10.1016/j.envint.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 269.Schyns Z.O.G., Shaver M.P. Mechanical recycling of packaging plastics: a review. Macromol. Rapid Commun. 2021;42(3) doi: 10.1002/marc.202000415. [DOI] [PubMed] [Google Scholar]
- 270.World Health Organization . World Health Organization; 2021. Children and Digital Dumpsites: E-Waste Exposure and Child Health.https://apps.who.int/iris/handle/10665/341718 [Google Scholar]
- 271.Grant K., Goldizen F.C., Sly P.D., et al. Health consequences of exposure to e-waste: a systematic review. Lancet Global Health. 2013;1(6):e350–e361. doi: 10.1016/S2214-109X(13)70101-3. [DOI] [PubMed] [Google Scholar]
- 272.Parvez S.M., Jahan F., Brune M.N., et al. Health consequences of exposure to e-waste: an updated systematic review. Lancet Planet. Health. 2021;5(12):e905–e920. doi: 10.1016/S2542-5196(21)00263-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Zhan L., Zhang Q., Bulati A., Wang R., Xu Z. Characteristics of microplastics and the role for complex pollution in e-waste recycling base of Shanghai, China. Environ. Int. 2022;169 doi: 10.1016/j.envint.2022.107515. [DOI] [PubMed] [Google Scholar]
- 274.Murray C. Suppliers challenge PVC cables with green alternatives. designnews.com. 2015 https://www.designnews.com/suppliers-challenge-pvc-cables-green-alternatives [Google Scholar]
- 275.Valentini L., Lopez-Manchado M.A. In: High-Performance Elastomeric Materials Reinforced by Nano-Carbons. Valentini L., Lopez Manchado M.A., editors. Elsevier; 2020. Chapter 1 - classification of rubbers and components for harsh environmental systems; pp. 1–14. [DOI] [Google Scholar]
- 276.Francik W.P., Alwardt C.L., Rachita M.J., Rodewald S. 2013. https://patents.google.com/patent/EP2607103A2/en (Rubber composition and tire containing functionalized polybutadiene and functionalized styrene/butadiene elastomers). Published online June 26. [Google Scholar]
- 277.U.S. Tire Manufacturers Association What's in a tire. 2020. https://www.ustires.org/whats-tire-0
- 278.KEMAT Polybutenes. Natural and synthetic rubber. KEMAT Polybutenes. Accessed May 17, 2023. https://www.kematbelgium.com/portfolio/kemarub/.
- 279.Polybutadiene global market volume 2015-2029. Statista. Accessed June 28, 2023. https://www.statista.com/statistics/1245213/polybutadiene-market-volume-worldwide/.
- 280.Mohajerani A., Burnett L., Smith J.V., et al. Recycling waste rubber tyres in construction materials and associated environmental considerations: a review. Resour. Conserv. Recycl. 2020;155 doi: 10.1016/j.resconrec.2020.104679. [DOI] [Google Scholar]
- 281.Qiang W., Li J., Yunlong W., Xiaojie Q., Guotian W. Discussion on tire retreading and reuse technology. IOP Conf. Ser. Earth Environ. Sci. 2020;512(1) doi: 10.1088/1755-1315/512/1/012146. [DOI] [Google Scholar]
- 282.Valentini F., Pegoretti A. End-of-life options of tyres. A review. Adv Ind Eng Polym Res. 2022;5(4):203–213. doi: 10.1016/j.aiepr.2022.08.006. [DOI] [Google Scholar]
- 283.Shulman V.L. In: Waste. second ed. Letcher T.M., Vallero D.A., editors. Academic Press; 2019. Chapter 26 - tire recycling; pp. 489–515. [DOI] [Google Scholar]
- 284.2013 U.S. Scrap Tire Management Summary. Rubber Manufacturers Association; 2014. https://www.ustires.org/sites/default/files/MAR_027_USTMA.pdf [Google Scholar]
- 285.Fazli A., Rodrigue D. Recycling waste tires into ground tire rubber (GTR)/rubber compounds: a review. J Compos Sci. 2020;4(3):103. doi: 10.3390/jcs4030103. [DOI] [Google Scholar]
- 286.Arrington K.J., Waugh J.B., Radzinski S.C., Matson J.B. Photo- and biodegradable thermoplastic elastomers: combining ketone-containing polybutadiene with polylactide using ring-opening polymerization and ring-opening metathesis polymerization. Macromolecules. 2017;50(11):4180–4187. doi: 10.1021/acs.macromol.7b00479. [DOI] [Google Scholar]
- 287.Naddeo M., Viscusi G., Gorrasi G., Pappalardo D. Degradable elastomers: is there a future in tyre compound formulation? Molecules. 2021;26(15):4454. doi: 10.3390/molecules26154454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Clarke L.J. Synthetic biology – pathways to commercialisation. Eng Biol. 2019;3(1):2–5. doi: 10.1049/enb.2018.5009. [DOI] [Google Scholar]
- 289.IARC . Vol. 97. International Agency for Research on Cancer (IARC) Press; 2008. 1,3-Butadiene.https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/1-3-Butadiene-Ethylene-Oxide-And-Vinyl-Halides-Vinyl-Fluoride-Vinyl-Chloride-And-Vinyl-Bromide--2008 (Ethylene Oxide and Vinyl Halides (Vinyl Fluoride, Vinyl Chloride and Vinyl Bromide)). [Google Scholar]
- 290.Hendler A.H., Goodmanson Bunch A.T., Crow W.L. Long-term trends in ambient air 1,3-butadiene levels in Houston, Texas. Environ. Sci. Technol. 2010;44(19):7383–7390. doi: 10.1021/es101511u. [DOI] [PubMed] [Google Scholar]
- 291.US EPA Locating and estimating air emissions from sources of 1,3-butadiene. https://www3.epa.gov/ttnchie1/le/butadien.pdf
- 292.Sadeghi-Yarandi M., Karimi A., Ahmadi V., Sajedian A.A., Soltanzadeh A., Golbabaei F. Cancer and non-cancer health risk assessment of occupational exposure to 1,3-butadiene in a petrochemical plant in Iran. Toxicol. Ind. Health. 2020;36(12):960–970. doi: 10.1177/0748233720962238. [DOI] [PubMed] [Google Scholar]
- 293.Albertini R.J., Sram R.J., Vacek P.M., et al. Biomarkers for assessing occupational exposures to 1,3-butadiene. Chem. Biol. Interact. 2001;135–136:429–453. doi: 10.1016/S0009-2797(01)00181-8. [DOI] [PubMed] [Google Scholar]
- 294.US EPA Final scope of the risk evaluation for 1,3-butadiene. https://www.epa.gov/sites/default/files/2020-09/documents/casrn_106-99-0_13-butadiene_finalscope.pdf
- 295.Vallecillos L., Sanmartin J., Marcé R.M., Borrull F. Determination of 1,3-butadiene degradation products in air samples by thermal desorption-gas chromatography-mass spectrometry. Atmos. Environ. 2019;196:95–102. doi: 10.1016/j.atmosenv.2018.10.010. [DOI] [Google Scholar]
- 296.US EPA Titanium tetrachloride. https://www.epa.gov/sites/default/files/2016-09/documents/titanium-tetrachloride.pdf
- 297.Szozda R. Pneumoconiosis in carbon black workers. J. UOEH. 1996;18(3):223–228. doi: 10.7888/juoeh.18.223. [DOI] [PubMed] [Google Scholar]
- 298.Mao J.F., Li W., Ong C.N., He Y., Jong M.C., Gin K.Y.H. Assessment of human exposure to benzophenone-type UV filters: a review. Environ. Int. 2022;167 doi: 10.1016/j.envint.2022.107405. [DOI] [PubMed] [Google Scholar]
- 299.Kang H., Lee J.P., Choi K. Exposure to phthalates and environmental phenols in association with chronic kidney disease (CKD) among the general US population participating in multi-cycle NHANES (2005-2016) Sci. Total Environ. 2021;791 doi: 10.1016/j.scitotenv.2021.148343. [DOI] [PubMed] [Google Scholar]
- 300.Li Y., Zhu J., Fan J., et al. Associations of urinary levels of phenols and parabens with osteoarthritis among US adults in NHANES 2005–2014. Ecotoxicol. Environ. Saf. 2020;192 doi: 10.1016/j.ecoenv.2020.110293. [DOI] [PubMed] [Google Scholar]
- 301.Durmusoglu E., Aslan S., Can E., Bulut Z. Health risk assessment of workers' exposure to organic compounds in a tire factory. Hum. Ecol. Risk Assess. 2007;13(1):209–222. doi: 10.1080/10807030601105134. [DOI] [Google Scholar]
- 302.Powers C., Lampel H.P. The rubber manufacturing industry: a case report and review of cutaneous exposure and sequelae. J Occup Med Toxicol Lond Engl. 2015;10:33. doi: 10.1186/s12995-015-0075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.IARC . Vol. 92. International Agency for Research on Cancer (IARC) Press; 2010. https://publications.iarc.fr/110 (Some Non-heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures). [PMC free article] [PubMed] [Google Scholar]
- 304.Müller A., Kocher B., Altmann K., Braun U. Determination of tire wear markers in soil samples and their distribution in a roadside soil. Chemosphere. 2022;294 doi: 10.1016/j.chemosphere.2022.133653. [DOI] [PubMed] [Google Scholar]
- 305.Monira S., Bhuiyan M.A., Haque N., Pramanik B.K. In: Plastic Waste for Sustainable Asphalt Roads. Giustozzi F., Nizamuddin S., editors. Woodhead Publishing Series in Civil and Structural Engineering. Woodhead Publishing; 2022. 13 - road dust-associated microplastics from vehicle traffics and weathering; pp. 257–271. [DOI] [Google Scholar]
- 306.Kolomijeca A., Parrott J., Khan H., et al. Increased temperature and turbulence alter the effects of leachates from tire particles on fathead minnow (Pimephales promelas) Environ. Sci. Technol. 2020;54(3):1750–1759. doi: 10.1021/acs.est.9b05994. [DOI] [PubMed] [Google Scholar]
- 307.Gualtieri M., Andrioletti M., Vismara C., Milani M., Camatini M. Toxicity of tire debris leachates. Environ. Int. 2005;31(5):723–730. doi: 10.1016/j.envint.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 308.McCarty K., Mian H.R., Chhipi-Shrestha G., Hewage K., Sadiq R. Ecological risk assessment of tire and road wear particles: a preliminary screening for freshwater sources in Canada. Environ Pollut. 2023;325 doi: 10.1016/j.envpol.2023.121354. [DOI] [PubMed] [Google Scholar]
- 309.Wik A., Dave G. Occurrence and effects of tire wear particles in the environment – a critical review and an initial risk assessment. Environ Pollut. 2009;157(1):1–11. doi: 10.1016/j.envpol.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 310.Davis A.P., Shokouhian M., Ni S. Loading estimates of lead, copper, cadmium, and zinc in urban runoff from specific sources. Chemosphere. 2001;44(5):997–1009. doi: 10.1016/S0045-6535(00)00561-0. [DOI] [PubMed] [Google Scholar]
- 311.Brinkmann M., Montgomery D., Selinger S., et al. Acute toxicity of the tire rubber-derived chemical 6PPD-quinone to four fishes of commercial, cultural, and ecological importance. Environ. Sci. Technol. Lett. 2022;9(4):333–338. doi: 10.1021/acs.estlett.2c00050. [DOI] [Google Scholar]
- 312.Hiki K., Yamamoto H. The tire-derived chemical 6PPD-quinone is lethally toxic to the white-spotted char salvelinus leucomaenis pluvius but not to two other salmonid species. Environ. Sci. Technol. Lett. 2022;9(12):1050–1055. doi: 10.1021/acs.estlett.2c00683. [DOI] [Google Scholar]
- 313.Hua X., Wang D. Tire-rubber related pollutant 6-PPD quinone: a review of its transformation, environmental distribution, bioavailability, and toxicity. J. Hazard Mater. 2023;459 doi: 10.1016/j.jhazmat.2023.132265. [DOI] [PubMed] [Google Scholar]
- 314.Matthews M. S3 - Sustainable Strategic Solutions; 2006. Review of Management of Used Tyres at Landfill Sites.https://www.wasteauthority.wa.gov.au/images/resources/files/2019/11/Review_of_Management_of_Used_Tyres_at_Landfill_Sites.pdf [Google Scholar]
- 315.Zainol N.A., Gunny A.A.N., Aziz H.A., Hung Y.T. In: Solid Waste Engineering And Management: Volume 3. Handbook of Environmental Engineering. Wang L.K., Wang M.H.S., Hung Y.T., editors. Springer International Publishing; 2022. Rubber tire recycling and disposal; pp. 55–114. [DOI] [Google Scholar]
- 316.Reisman J.I. U.S. Environmental Protection Agency; 1997. Air Emissions from Scrap Tire Combustion; pp. 1–117.https://www3.epa.gov/ttncatc1/dir1/tire_eng.pdf [Google Scholar]
- 317.Dabrowska D., Rykala W., Nourani V. Causes, types and consequences of municipal waste landfill fires—literature review. Sustainability. 2023;15(7):5713. doi: 10.3390/su15075713. [DOI] [Google Scholar]
- 318.Nadal M., Rovira J., Díaz-Ferrero J., Schuhmacher M., Domingo J.L. Human exposure to environmental pollutants after a tire landfill fire in Spain: health risks. Environ. Int. 2016;97:37–44. doi: 10.1016/j.envint.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 319.Piotrowska K., Kruszelnicka W., Bałdowska-Witos P., et al. Assessment of the environmental impact of a car tire throughout its lifecycle using the LCA method. Materials. 2019;12(24):4177. doi: 10.3390/ma12244177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Levendis Y.A. American Society of Mechanical Engineers Digital Collection; 2009. Emissions from Direct or Indirect Combustion of Tire-Derived-Fuel; pp. 481–489. [DOI] [Google Scholar]
- 321.Pegg M.J., Amyotte P.R., Fels M., Cumming C.R.R., Poushay J.C. Dalhousie University; 2007. An Assessment Of the Use Of Tires As an Alternative Fuel. Faculty of Engineering.https://www.novascotia.ca/nse/waste/docs/tireusealternativefuelassessment.pdf [Google Scholar]
- 322.Kazemi M., Parikhah Zarmehr S., Yazdani H., Fini E. Review and perspectives of end-of-life tires applications for fuel and products. Energy Fuels. 2023;37(15):10758–10774. doi: 10.1021/acs.energyfuels.3c00459. [DOI] [Google Scholar]
- 323.Rhodes E.P., Ren Z., Mays D.C. Zinc leaching from tire crumb rubber. Environ. Sci. Technol. 2012;46(23):12856–12863. doi: 10.1021/es3024379. [DOI] [PubMed] [Google Scholar]
- 324.Halsband C., Sørensen L., Booth A.M., Herzke D. Car tire crumb rubber: does leaching produce a toxic chemical cocktail in coastal marine systems? Front. Environ. Sci. 2020;8 https://www.frontiersin.org/articles/10.3389/fenvs.2020.00125 [Google Scholar]
- 325.Sarker I., Moore L.R., Tetu S.G. Investigating zinc toxicity responses in marine Prochlorococcus and Synechococcus. Microbiology. 2021;167(6) doi: 10.1099/mic.0.001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Birkholz D.A., Belton K.L., Guidotti T.L. Toxicological evaluation for the hazard assessment of tire crumb for use in public playgrounds. J. Air Waste Manag. Assoc. 2003;53(7):903–907. doi: 10.1080/10473289.2003.10466221. [DOI] [PubMed] [Google Scholar]
- 327.Kim S., Yang J.Y., Kim H.H., Yeo I.Y., Shin D.C., Lim Y.W. Health risk assessment of lead ingestion exposure by particle sizes in crumb rubber on artificial turf considering bioavailability. Environ Health Toxicol. 2012;27 doi: 10.5620/eht.2012.27.e2012005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.UNEP . UNEP - UN Environment Programme; 2022. Historic Day in the Campaign to Beat Plastic Pollution: Nations Commit to Develop a Legally Binding Agreement.http://www.unep.org/news-and-stories/press-release/historic-day-campaign-beat-plastic-pollution-nations-commit-develop [Google Scholar]
- 329.Karasik R., Virdin J., Vegh T. Nicholas Institute for Environmental Policy Solutions; 2023. Plastics Policy Inventory.https://nicholasinstitute.duke.edu/plastics-policy-inventory [Google Scholar]
- 330.Plastic pollution coalition. Global plastic laws. 2023. https://www.globalplasticlaws.org/map
- 331.Brehm J., Wilde M.V., Reiche L., et al. In-depth characterization revealed polymer type and chemical content specific effects of microplastic on Dreissena bugensis. J. Hazard Mater. 2022;437 doi: 10.1016/j.jhazmat.2022.129351. [DOI] [PubMed] [Google Scholar]
- 332.Frère L., Maignien L., Chalopin M., et al. Microplastic bacterial communities in the Bay of Brest: influence of polymer type and size. Environ Pollut. 2018;242:614–625. doi: 10.1016/j.envpol.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 333.Wagner M., Monclús L., Arp H.P.H., et al. 2024. State of the Science on Plastic Chemicals - Identifying and Addressing Chemicals and Polymers of Concern. [DOI] [Google Scholar]
- 334.Tickner J.A., Schettler T., Guidotti T., McCally M., Rossi M. Health risks posed by use of Di-2-ethylhexyl phthalate (DEHP) in PVC medical devices: a critical review. Am. J. Ind. Med. 2001;39(1):100–111. doi: 10.1002/1097-0274(200101)39:1<100::AID-AJIM10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 335.Stroustrup A., Bragg J.B., Busgang S., et al. Sources of clinically significant neonatal intensive care unit phthalate exposure. J. Expo. Sci. Environ. Epidemiol. 2020;30(1):137–148. doi: 10.1038/s41370-018-0069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Fischer C.J., Bickle Graz M., Muehlethaler V., Palmero D., Tolsa J.F. Phthalates in the NICU: is it safe? J. Paediatr. Child Health. 2013;49(9):E413–E419. doi: 10.1111/jpc.12244. [DOI] [PubMed] [Google Scholar]
- 337.UNEP Revised draft Text of the international legally binding instrument on plastic pollution, including in the marine environment. 2023. https://wedocs.unep.org/bitstream/handle/20.500.11822/44526/RevisedZeroDraftText.pdf
- 338.OECD Polymers of low concern. https://www.oecd.org/env/ehs/oecddefinitionofpolymer.htm
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.