Abstract
Background
A semistructured patient-reported outcome measure (PROM) wherein patients rate the importance of structured items and the magnitude of the psychometric properties to be investigated (e.g., disability and satisfaction) facilitates patient engagement in their treatment and patient-centered clinical practice. The Satisfaction and Recovery Index (SRI) is one such semistructured PROM that was originally developed to measure recovery from a whiplash injury. Exploratory factor analysis demonstrated a one-factor structure among ambulatory community-dwelling people with traumatic musculoskeletal injuries. However, a confirmatory factor analysis has not been conducted among patients with various musculoskeletal disorders, and the internal structure of the SRI has not been established yet. Thus, this study aimed to investigate the internal structure of the SRI among patients with diverse musculoskeletal disorders.
Methodology
An anonymous survey was performed for patients who were referred for physical therapy for musculoskeletal disorders at a local orthopedic clinic. A confirmatory factor analysis was conducted. The goodness-of-fit criteria were as follows: chi-square/degree of freedom < 3, goodness-of-fit index > 0.90, adjusted goodness-of-fit index > 0.95, and root mean square error of approximation < 0.08.
Results
Data from 217 participants were analyzed. All goodness-of-fit criteria were satisfied.
Conclusion
This study confirmed the acceptable internal structure of the SRI among patients with diverse musculoskeletal disorders.
Keywords: survey, reliability and validity, patient reported outcome measures, musculoskeletal disorders, factor analysis
Introduction
In patient-centered clinical practice, the subjective status of the patient must be understood, and a patient-reported outcome measure (PROM) is needed. The integration of PROM facilitates patient engagement in their treatment [1]. A semistructured PROM wherein patients rate the importance of structured items and the magnitude of the psychometric properties to be investigated (e.g., disability and satisfaction) is considered a feasible and promising patient-centered PROM [2], and some have been developed recently [3-5]. Among these semistructured PROMs, the Satisfaction and Recovery Index (SRI) is a potentially applicable region-agnostic PROM [4,6].
Originally, the SRI was developed to measure recovery from a whiplash injury via a focus group of patients with whiplash injuries, and cognitive interviews with patients with upper extremity disorders were conducted [4]. In the SRI, patients rate the importance of nine items and their satisfaction on two 11-point scales. Among ambulatory community-dwelling people with traumatic musculoskeletal injuries, the SRI demonstrated single-factor loading in the exploratory factor analysis, convergent validity with the SF-12v2® Health Survey [7], and better responsiveness than the SF-12v2® Health Survey [4]. However, the unidimensionality of the SRI has not been established as a confirmatory factor analysis has not been performed yet. Furthermore, during the development of a Japanese version, patients with various musculoskeletal disorders were found to be able to understand the SRI [6].
Therefore, this study aimed to investigate the internal structure of the SRI among patients with diverse musculoskeletal disorders. The unidimensionality of the SRI was hypothesized to be acceptable in those patients.
Materials and methods
Design
Because an anonymous survey was performed, the need for informed consent was waived. This study was approved by the Ethics Committee of the Saitama Prefectural University (No. 22040).
Participants
Convenience sampling was used to recruit participants from June 2021 to May 2024. Inclusion criteria were (1) patients who were referred for physical therapy for musculoskeletal disorders at a local orthopedic clinic (Tokyo, Japan), (2) ≥ 18 years old, and (3) whose first language is Japanese. Patients with respiratory, neurological, or cognitive comorbidities (e.g., dementia) who were diagnosed by a medical doctor were not eligible. Participants were recruited until 200 analyzable SRI data were collected, considering the adequate quality criterion for the examination of construct validity according to the COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) guidelines [8,9].
Variables
Before the initial physical therapy session, participants were asked to respond to the survey. This study collected data including age, sex, duration of symptoms that resulted in a referral for physical therapy (≤ 7 days, eight days to three months, and ≥ three months), location of the symptoms, and pain intensity using the four-item pain intensity measure (P4) and the SRI. The duration of symptoms was defined as the period since the last time the patient had no such symptoms at all for > 1 month [10-12]. The symptom location was assessed using a body chart with 23 areas [13-15]. The P4 is a valid and reliable PROM for subjective pain intensity on a four-item 11-point numerical rating scale to indicate the average pain intensity over the previous two days in the morning, afternoon, and evening and during activities [16,17]; the higher the total P4 score, the greater the perceived pain intensity (0-40).
The SRI is composed of 10 items, where one item (item 6) is a dummy to check whether the respondents fill a number mindlessly. The SRI score is calculated based on the following two steps from nine items: (1) calculating a weighted score for each item and (2) calculating the total score.
Analysis
A confirmatory factor analysis was conducted with Analysis of Moment Structures Statistical Product and Service Solutions (AMOS SPSS) (version 20; IBM SPSS Statistics for Windows, Armonk, NY) for the weighted scores of the nine items. The goodness-of-fit criteria were as follows: chi-square/degree of freedom < 2 (good fit) and < 3 (acceptable fit), goodness-of-fit index > 0.95 (good fit) and > 0.90 (acceptable fit), adjusted goodness-of-fit index > 0.97 (good fit) and > 0.95 (acceptable fit), and root mean square error of approximation < 0.05 (good fit) and < 0.08 (acceptable fit) [18,19]. Data with missing SRI values or dummy items with inappropriate responses were excluded from the analysis. A post hoc model modification was conducted according to the suggested modification indices.
Cronbach’s alpha was calculated for internal consistency. The values were interpreted as acceptable (≥ 0.7) and not acceptable (< 0.7) [9]. In the SRI, the total score is calculated by weighting item importance. Therefore, the item-total correlation (ITC) was assessed using Pearson’s correlation coefficient (r). The correlation between the weighted score of each item and the total SRI score excluding the item was calculated. Values of ≤ 0.2 were interpreted as acceptable and > 0.2 as not acceptable [20,21].
Results
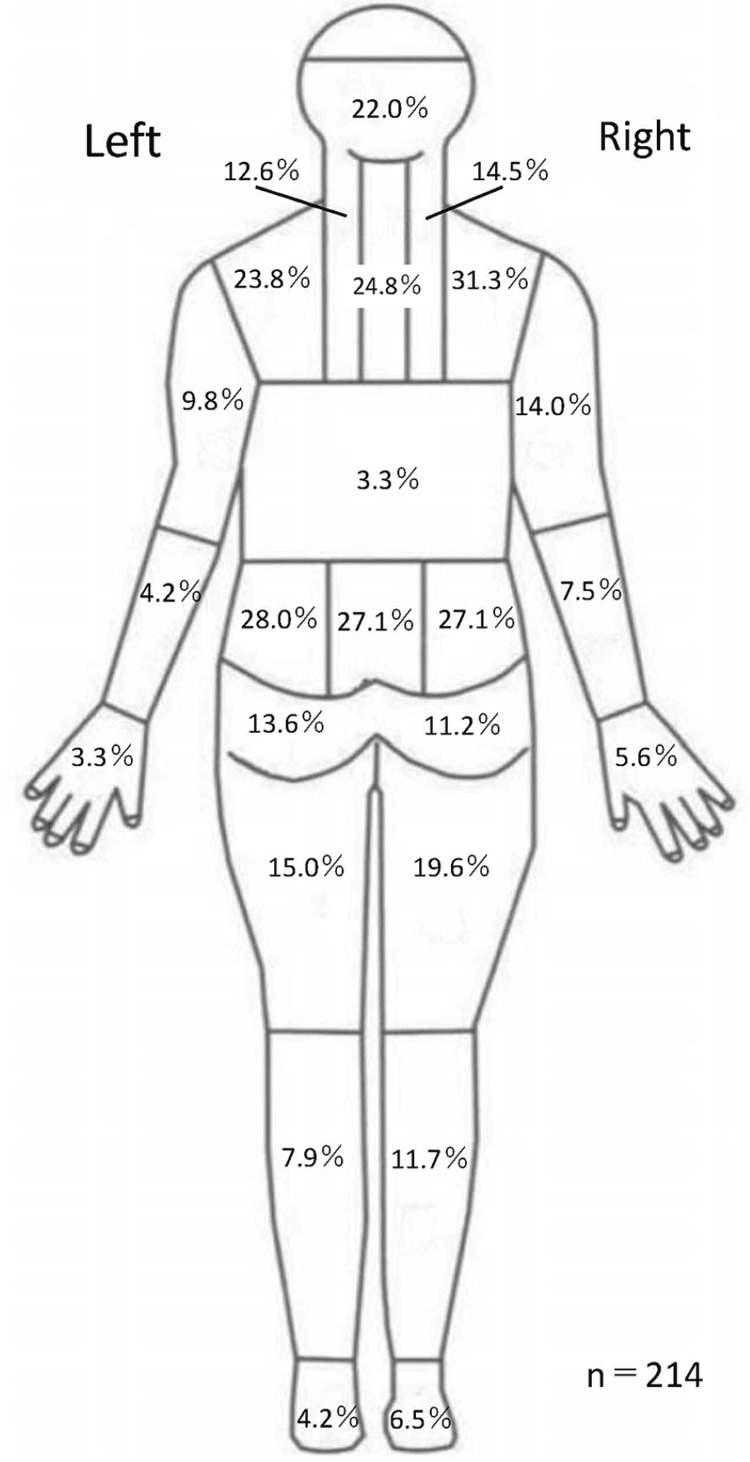
Approximately 233 patients participated in the study. There were 16 participants who had missing data on SRI and were excluded, and data from 217 participants were analyzed (Table 1). The proportion of symptom locations is shown in Figure 1.
Table 1. Summary of the 217 participants.
Abbreviation; SD, standard deviation; n, number. *n = 215; †n = 213; ‡n = 216
| Variables | N = 217 |
| Age* (years), mean (SD) | 48.5 (17.8) |
| Sex† | |
| n of males (%) | 98 (46%) |
| n of females (%) | 115 (54%) |
| Symptom duration‡ | |
| n of those with < 7 days (%) | 16 (7%) |
| n of those with 8 days–3 months (%) | 116 (54%) |
| n of those with > 3 months (%) | 84 (39%) |
| 4-item Pain Intensity Measure‡ (0–40), mean (SD) | 14.9 (8.7) |
| Satisfaction and Recovery Index score (0–100), mean (SD) | 73.7 (15.2) |
Figure 1. Symptom distributions of the 217 participants.
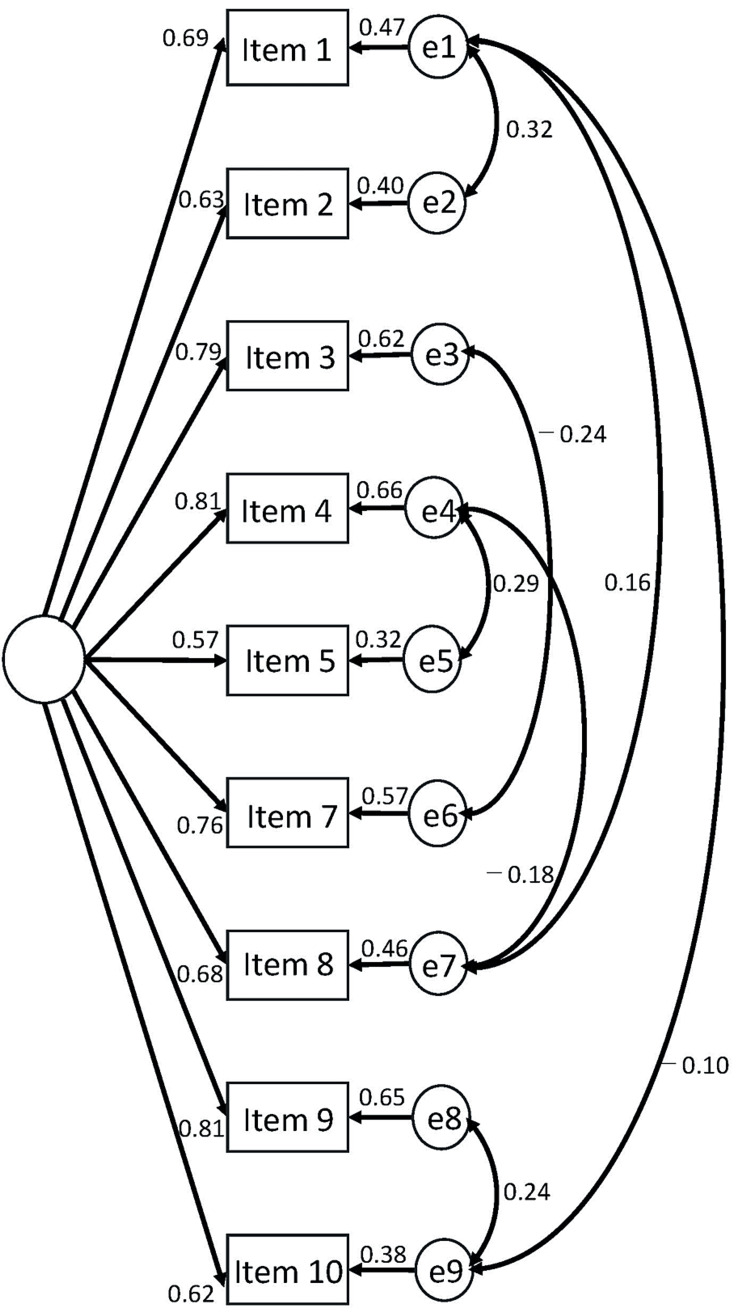
Figure 2 presents the structure of the models. Table 2 shows the goodness-of-fit criteria, satisfying all criteria of good or acceptable fit.
Table 2. Goodness-of-fit results.
Chi-square/degree of freedom < 2 (good fit) and < 3 (acceptable fit); goodness-of-fit index > 0.95 (good fit) and > 0.90 (acceptable fit); adjusted goodness-of-fit index > 0.97 (good fit) and > 0.95 (acceptable fit); root mean square error of approximation < 0.05 (good fit) and < 0.08 (acceptable fit).
| Goodness-of-fit statistics | Value |
| Chi-square/degree of freedom | 1.051 |
| Goodness-of-fit index | 0.980 |
| Adjusted goodness-of-fit index | 0.954 |
| Root mean square error of approximation | 0.015 |
Figure 2. The structure model with standardized coefficients.
Acceptable internal consistency was confirmed with a Cronbach’s alpha of 0.902 (95% confidence intervals: 0.881-0.920). ITCs are presented in Table 3, where all items satisfied the predetermined criteria.
Table 3. Item-total correlation with Pearson’s correlation coefficient.
| Item No. | Pearson’s R | P value | 95% confidence intervals |
| 1 | 0.68 | < 0.001 | 0.60–0.75 |
| 2 | 0.60 | < 0.001 | 0.51–0.68 |
| 3 | 0.66 | < 0.001 | 0.58–0.73 |
| 4 | 0.70 | < 0.001 | 0.62–0.76 |
| 5 | 0.51 | < 0.001 | 0.40–0.60 |
| 7 | 0.60 | < 0.001 | 0.50–0.68 |
| 8 | 0.62 | < 0.001 | 0.53–0.70 |
| 9 | 0.73 | < 0.001 | 0.66–0.79 |
| 10 | 0.57 | < 0.001 | 0.47–0.65 |
Discussion
In this study, a confirmatory factor analysis demonstrated that all items met the goodness-of-fit criteria of being either good or acceptable. Therefore, this result suggests that the SRI exhibits a single-factor structure among patients with diverse musculoskeletal diseases. The internal consistency was also acceptable. These findings indicate that the second step of the reliability and validity testing process indicated in the COSMIN (i.e., the confirmation of the internal structure, has been completed). In the future, the third step of the reliability and validity testing process as indicated in COSMIN (i.e., test-retest reliability and responsiveness) must be verified. Particularly, clinical trials are recommended to use the method of determining whether the 95% confidence interval of the difference exceeds the minimum clinically important difference (MCID), rather than a statistically significant difference [22]. Such a new clinical trial has begun to be reported [12]. Therefore, the verification of the MCID of the SRI is considered a priority research agenda.
The total SRI score is divided by the total number of importance scores, which is an ingenious way to enhance the individuality of each patient. Herein, the ITC was evaluated to confirm the structural validity of the total score, even with this unique calculation method. Consequently, all items met the ITC criteria. Therefore, the internal structure of the total SRI score is deemed reliable.
Previous studies have commonly used pain intensity, degree of functional impairment, and cost-effectiveness as primary outcomes. However, patients’ demands for an intervention are diverse, and developing a new outcome that reflects patient individuality was one of the research priorities in musculoskeletal research [23]. However, the above concern, namely, that the intervention effects cannot be truly reflected, would arise because the value of outcomes such as pain intensity, functional limitations, and cost-effectiveness vary among patients. Therefore, evaluating the effectiveness of a patient-centered approach when the outcome is well-being, a psychological characteristic that is the goal for any patient [24], may be possible. Such a conceptually higher level of outcomes for well-being would include life satisfaction, which is measured by the SRI. To the best of the author's knowledge, SRI is the only free, semistructured PROM for life satisfaction. The SRI may be a promising primary outcome for future clinical trials of musculoskeletal disorders as this study confirmed that the SRI has sufficient internal structure in patients with various musculoskeletal disorders.
One limitation of this study is that data were collected in a single center. Second, because this was an anonymized survey and considering patients might not remember their exact diagnosis, we asked only for the symptomatic area rather than the name of the diagnosis. However, we do not believe that these limitations overshadow the results of this study. Nevertheless, there is a limitation that the present findings are based on the Japanese population, raising uncertainty regarding whether the SRI is unidimensional in other cultures. Verification of cross-cultural validity will be required in the future.
Conclusions
This study confirmed the acceptable internal structure of the SRI among patients with diverse musculoskeletal disorders. Therefore, it is now possible to verify the third step of the reliability and validity testing process as indicated in COSMIN to use the SRI in clinical practice. The SRI is a semistructured and region-agnostic PROM that is free of charge and thus may be widely used to facilitate patient-centered interventions for patients with musculoskeletal disorders.
Disclosures
Human subjects: Consent was obtained or waived by all participants in this study. Ethics Committee of the Saitama Prefectural University issued approval 22040.
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Hiroshi Takasaki
Acquisition, analysis, or interpretation of data: Hiroshi Takasaki, Soma Ishida
Drafting of the manuscript: Hiroshi Takasaki
Supervision: Hiroshi Takasaki
Critical review of the manuscript for important intellectual content: Soma Ishida
References
- 1.Are patient reported outcome measures (PROMs) useful in low back pain? Experiences of physiotherapists in primary health care in Sweden. Rasmussen-Barr E, Lindqvist C, Östhols S, Boström C. https://pubmed.ncbi.nlm.nih.gov/34153691/ Musculoskelet Sci Pract. 2021;55 doi: 10.1016/j.msksp.2021.102414. [DOI] [PubMed] [Google Scholar]
- 2.Initial development of a patient-reported outcome measure of disability due to Katakori via evaluating patient comprehensibility and comprehensiveness. Takasaki H, Handa Y. J Phys Ther Sci. 2022;34:13–17. doi: 10.1589/jpts.34.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Developing a final format of a patient-reported outcome measure for disability in daily living due to stiff neck/shoulders, Katakori disability index, through internal structure assessments. Takasaki H. Musculoskeletal Care. 2024;22 [Google Scholar]
- 4.Development and initial validation of the Satisfaction and Recovery Index (SRI) for measurement of recovery from musculoskeletal trauma. Walton DM, MacDermid JC, Pulickal M, Rollack A, Veitch J. Open Orthop J. 2014;8:316–325. doi: 10.2174/1874325001408010316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Standardized measurement of recovery from nonspecific back pain. Hush JM, Kamper SJ, Stanton TR, Ostelo R, Refshauge KM. Arch Phys Med Rehabil. 2012;93:849–855. doi: 10.1016/j.apmr.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 6.Cross-cultural adaptation of the Satisfaction and Recovery Index among Japanese people with musculoskeletal disorders. Takasaki H. J Phys Ther Sci. 2022;34:374–378. doi: 10.1589/jpts.34.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Ware J Jr, Kosinski M, Keller SD. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 8.COSMIN methodology for evaluating the content validity of patient-reported outcome measures: a Delphi study. Terwee CB, Prinsen CA, Chiarotto A, et al. Qual Life Res. 2018;27:1159–1170. doi: 10.1007/s11136-018-1829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.COSMIN risk of bias checklist for systematic reviews of patient-reported outcome measures. Mokkink LB, de Vet HC, Prinsen CA, Patrick DL, Alonso J, Bouter LM, Terwee CB. Qual Life Res. 2018;27:1171–1179. doi: 10.1007/s11136-017-1765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Immediate neck hypoalgesic effects of craniocervical flexion exercises and cervical retraction exercises among individuals with non-acute neck pain and a directional preference for retraction or extension: preliminary pretest-posttest randomized experimental design. Takasaki H, Yamasaki C. J Man Manip Ther. 2023;31:368–375. doi: 10.1080/10669817.2023.2201918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Definitions of recurrence of an episode of low back pain: a systematic review. Stanton TR, Latimer J, Maher CG, Hancock M. Spine (Phila Pa 1976) 2009;34:0–22. doi: 10.1097/BRS.0b013e318198d073. [DOI] [PubMed] [Google Scholar]
- 12.Mechanical diagnosis and therapy has a clinically meaningful effect on neck derangement syndrome with a directional preference for cervical retraction or extension in comparison to a wait-and-see approach: An assessor-blinded randomized controlled trial for 6 weeks. Takasaki H, Handa Y, Kikkawa K. JOSPT Open. 2024;1:26. [Google Scholar]
- 13.Predictors of 1-year perceived recovery, absenteeism, and expenses due to low back pain in workers receiving mechanical diagnosis and therapy: a prospective cohort study. Takasaki H. Healthcare (Basel) 2023;11 doi: 10.3390/healthcare11091293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.No increase in 6-week treatment effect of mechanical diagnosis and therapy with the use of the LUMOback in people with non-acute non-specific low back pain and a directional preference of extension: a pilot randomized controlled trial. Takasaki H, Aoki S, May S. Physiotherapy. 2018;104:347–353. doi: 10.1016/j.physio.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Investigation on the effectiveness of abdominal hollowing home-exercises using a portable ultrasound: randomized controlled trial. Takasaki H, Kawazoe S. J Electromyogr Kinesiol. 2021;58 doi: 10.1016/j.jelekin.2021.102532. [DOI] [PubMed] [Google Scholar]
- 16.The evaluation of change in pain intensity: a comparison of the P4 and single-item numeric pain rating scales. Spadoni GF, Stratford PW, Solomon PE, Wishart LR. J Orthop Sports Phys Ther. 2004;34:187–193. doi: 10.2519/jospt.2004.34.4.187. [DOI] [PubMed] [Google Scholar]
- 17.The development and cross-validation of the P4: a self-report pain intensity measure. Spadoni GF, Stratford PW, Solomon PE, Wishart LR. Physiother Canada. 2003;55:32–40. [Google Scholar]
- 18.Evaluating the fit of structural equation models: tests of significance and descriptive goodness-of-fit measures. Schermelleh-Engel K, Moosbrugger H, Müller H. Methods Psych Res Online. 2003;8:23–74. [Google Scholar]
- 19.Confirmatory factor analysis of the Japanese health locus of control scales among people with musculoskeletal disorders. Nemoto S, Miki T, Kondo Y, Takasaki H. J Phys Ther Sci. 2023;35:7–11. doi: 10.1589/jpts.35.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kline P. New York: Methuen & Co; 1986. A handbook of test construction (psychology revivals): introduction to psychometric design. [Google Scholar]
- 21.Internal consistency and item-total correlation of patient-reported outcome instruments and hemophilia joint health score v2.1 in US adult people with hemophilia: results from the pain, functional impairment, and quality of life (P-FiQ) study. Wang M, Batt K, Kessler C, Neff A, Iyer NN, Cooper DL, Kempton CL. Patient Prefer Adherence. 2017;11:1831–1839. doi: 10.2147/PPA.S141391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Statistical inference through estimation: recommendations from the International Society of Physiotherapy journal editors. Elkins MR, Pinto RZ, Verhagen A, et al. Braz J Phys Ther. 2022;26 doi: 10.1016/j.bjpt.2021.100387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Research priorities for non-pharmacological therapies for common musculoskeletal problems: nationally and internationally agreed recommendations. Foster NE, Dziedzic KS, van der Windt DA, Fritz JM, Hay EM. BMC Musculoskelet Disord. 2009;10:3. doi: 10.1186/1471-2474-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Physiotherapist-led neck-specific exercise improves pain, disability and self-efficacy in chronic whiplash-associated disorders [commentary] Takasaki H. J Physiother. 2015;61:161. doi: 10.1016/j.jphys.2015.05.006. [DOI] [PubMed] [Google Scholar]