Abstract
Adeno-associated virus (AAV) vectors are promising gene therapy candidates, but pre-existing anti-AAV neutralizing antibodies (NAbs) pose a significant challenge to successful gene delivery. Knowledge of NAb seroprevalence remains limited and inconsistent. We measured activity of NAbs against six clinically relevant AAV serotypes across 10 countries in adults (n = 502) and children (n = 50) using a highly sensitive transduction inhibition assay. NAb prevalence was generally highest for AAV1 and lowest for AAV5. There was considerable variability across countries and geographical regions. NAb prevalence increased with age and was higher in females, participants of Asian ethnicity, and participants in cancer trials. Co-prevalence was most frequently observed between AAV1 and AAV6 and less frequently between AAV5 and other AAVs. Machine learning analyses revealed a unique clustering of AAVs that differed from previous phylogenetic classifications. These results offer insights into the biological relationships between the immunogenicity of AAVs in humans beyond that observed previously using standard clades, which are based on linear capsid sequences. Our findings may inform improved vector design and facilitate the development of AAV vector-mediated clinical gene therapies.
Keywords: AAV1, AAV5, AAV6, AAV8, AAV9, AAVRh74var, rare disease, genetic diseases, biotechnology, clinical trials
Graphical abstract
Rasko and colleagues use a standardized transduction inhibition assay to determine the prevalence of NAbs against 6 AAV serotypes used for gene therapies in a large global population of adult and pediatric subjects from 10 countries.
Introduction
Adeno-associated virus (AAV) is a non-enveloped parvovirus that is non-pathogenic in humans. AAV vectors provide compelling advantages for the delivery of therapeutic genes.1,2,3,4 AAV-based gene therapies have been approved for the treatment of spinal muscular atrophy,5 familial lipoprotein lipase deficiency,6 RPE65 mutation-associated retinal degeneration,7 and hemophilia.8 Over a thousand human participants have received AAV-based gene therapies in clinical trials.9,10,11,12,13,14,15,16,17,18,19,20,21,22 Despite relatively few long-term safety assessments,23 trials have been mostly free of vector-related adverse events.24,25,26
AAVs can provide durable therapeutic gene expression and are thus attractive candidates for gene therapeutics,27 notwithstanding their restricted pro-inflammatory profile.28 Immune responses to most systemically delivered viral vectors are influenced by prior exposure of the human immune system to both the wild-type virus capsid from which the vector was engineered29 and cross-reacting serotypes. Since human populations are first exposed to AAVs during childhood and adolescence,30,31 there is a high prevalence of anti-AAV immunity, with detectable antibody titers in adults reported to exceed 60% for some serotypes.32 This adaptive immunity (particularly humoral immunity in the form of neutralizing antibodies [NAbs]) to wild-type AAVs represents a significant challenge to successful systemic gene delivery via AAV vectors.33,34
Prior infection with AAVs may limit viral gene transfer efficiency and the durability of transgene expression35,36; however, successful gene transfer has been documented in some patients with pre-existing anti-AAV NAbs.37,38,39 In re-administration studies using preclinical animal models, little to no transduction was detected after the second administration, and poor transduction was associated with the presence of NAb activity against AAV capsid proteins and/or transgenes elicited by the first vector administration.29,40,41,42,43 Indeed, sustained high-titer antibody levels following systemic AAV-mediated gene therapy may prevent the possibility of re-administration of cross-reacting AAV therapies in humans.23
In response to these findings, the field of AAV gene therapy has used serological assays to exclude seropositive patients from receiving systemic AAV vector infusions. Despite the challenges posed by anti-AAV NAbs, knowledge of NAb seroprevalence is limited and inconsistent, with wide geographical variation observed in published and ongoing gene therapy and epidemiological studies.35,44,45 The immunology of human responses to AAV exposure is also poorly understood, highlighted by unexpected and unexplained differences in therapeutic success following administration of AAVs when pre-existing NAbs are present to AAV537,38,39 versus other serotypes.21,29,46,47 Finally, assays to determine NAb levels are poorly standardized across studies and vary in methodology, cutoffs used to determine sample positivity, and cutoffs used to determine patient eligibility.34 Consequently, the same patient may be deemed eligible for administration of AAV gene therapy in one trial but ineligible in another.48,49
This global multi-country observational study assessed the seroprevalence of NAbs against six AAV serotypes across 10 countries using a robust, highly sensitive, cell-based transduction inhibition assay.50,51,52 We also characterized the relationships between neutralizing titers of the six different AAV serotypes, along with demographic variables, using innovative machine learning algorithms. The results will inform vector design, planning of future gene therapy studies, and assessment of potential eligible populations in anticipation of the wider adoption of AAV vector-mediated gene therapies.
Results
Blood samples from 552 participants who previously participated in selected Pfizer clinical studies were analyzed. Most participants were adults (n = 502, 90.9%) with a mean (standard deviation [SD]) age of 48.6 (9.8) years (Table 1). Most adult participants were male (n = 276, 55%) and of White (n = 334, 66.5%) or Asian (n = 129, 25.7%) ethnicity. Fifty participants (9.1%) were pediatric patients with a mean (SD) age of 7.9 (1.5) years. Pediatric participants were exclusively male (n = 50, 100%) and predominantly of White ethnicity (n = 42, 84.0%).
Table 1.
Participant demographic and clinical characteristics
| Variables | Overall (N = 552) n (%) | Adultsa (N = 502) n (%) | Childrenb (N = 50) n (%) |
|---|---|---|---|
| Age group (years) | |||
| 6–10 | 47 (8.5) | 0 | 47 (94.0) |
| 11–15 | 3 (0.5) | 0 | 3 (6.0) |
| 16–40 | 95 (17.2) | 95 (18.9) | 0 |
| 41–60 | 407 (73.7) | 407 (81.1) | 0 |
| Age (years) | |||
| n | 552 | 502 | 50 |
| Mean (SD) | 44.89 (14.98) | 48.57 (9.83) | 7.92 (1.50) |
| Min, max | 6, 60 | 19, 60 | 6, 12 |
| Biological sex | |||
| Male | 326 (59.1) | 276 (55.0) | 50 (100.0) |
| Female | 226 (40.9) | 226 (45.0) | 0 |
| Ethnicity | |||
| African | 7 (1.3) | 7 (1.4) | 0 |
| Asian | 136 (24.6) | 129 (25.7) | 7 (14.0) |
| White | 376 (68.1) | 334 (66.5) | 42 (84.0) |
| Other | 9 (1.6) | 8 (1.6) | 1 (2.0) |
| Missing | 24 (4.3) | 24 (4.8) | 0 |
| Geographical region | |||
| North America | 137 (24.8) | 100 (19.9) | 37 (74.0) |
| Europe | 261 (47.3) | 252 (50.2) | 9 (18.0) |
| Asia-Pacific | 154 (27.9) | 150 (29.9) | 4 (8.0) |
| Country | |||
| US | 81 (14.7) | 50 (10.0) | 31 (62.0) |
| Canada | 56 (10.1) | 50 (10.0) | 6 (12.0) |
| Germany | 50 (9.1) | 50 (10.0) | 0 |
| Spain | 42 (7.6) | 42 (8.4) | 0 |
| France | 61 (11.1) | 61 (12.2) | 0 |
| UK | 58 (10.5) | 52 (10.4) | 6 (12.0) |
| Italy | 50 (9.1) | 47 (9.4) | 3 (6.0) |
| Australia | 50 (9.1) | 50 (10.0) | 0 |
| Japan | 54 (9.8) | 50 (10.0) | 4 (8.0) |
| South Korea | 50 (9.1) | 50 (10.0) | 0 |
| Site location within four US regions | |||
| Midwest | 27 (33.3) | 8 (16.0) | 19 (61.3) |
| Northeast | 18 (22.2) | 14 (28.0) | 4 (12.9) |
| South | 24 (29.6) | 16 (32.0) | 8 (25.8) |
| West | 12 (14.8) | 12 (24.0) | 0 |
| Site location within two US regions | |||
| North | 45 (55.6) | 22 (44.0) | 23 (74.2) |
| South | 36 (44.4) | 28 (56.0) | 8 (25.8) |
| Immunomodulation | |||
| Exposed | 461 (83.5) | 461 (91.8) | 0 |
| Non-exposed | 91 (16.5) | 41 (8.2) | 50 (100.0) |
| Disease area | |||
| Cancers | 340 (61.6) | 340 (67.7) | 0 |
| Others | 212 (38.4) | 162 (32.3) | 50 (100.0) |
| Indications | |||
| Alopecia areata | 28 (5.1) | 28 (5.6) | 0 |
| Dermatitis atopic | 50 (9.1) | 50 (10.0) | 0 |
| Diabetic retinal edema | 4 (0.7) | 4 (0.8) | 0 |
| Duchenne muscular dystrophy | 50 (9.1) | 0 | 50 (100.0) |
| Fallopian tube cancer, malignant peritoneal neoplasm, ovarian cancer | 115 (20.8) | 115 (22.9) | 0 |
| Metastatic renal cell carcinoma | 103 (18.7) | 103 (20.5) | 0 |
| Non-small cell lung cancer | 12 (2.2) | 12 (2.4) | 0 |
| Parkinson’s disease | 8 (1.4) | 8 (1.6) | 0 |
| Psoriasis | 14 (2.5) | 14 (2.8) | 0 |
| Psoriatic arthropathy | 37 (6.7) | 37 (7.4) | 0 |
| Squamous cell carcinoma of head and neck | 59 (10.7) | 59 (11.8) | 0 |
| Transitional cell carcinoma | 51 (9.2) | 51 (10.2) | 0 |
| Type 2 diabetes mellitus | 21 (3.8) | 21 (4.2) | 0 |
SD, standard deviation.
16–60 years.
<16 years.
By design, participants were located across 10 countries with approximately 50 participants per country. Among adults, there were slightly higher proportions of participants from France (n = 61, 12.2%) and the UK (n = 52, 10.4%), and slightly lower proportions from Spain (n = 42, 8.4%) and Italy (n = 47, 9.4%); the remaining participants were equally distributed across the US (n = 50, 10%), Canada (n = 50), Germany (n = 50), Australia (n = 50), Japan (n = 50), and South Korea (n = 50). Pediatric participants were mostly from the US (n = 31, 62.0%), followed by Canada (n = 6, 12%), the UK (n = 6, 12%), Japan (n = 4, 8.0%) and Italy (n = 3, 6.0%). A total of 50.2% (n = 252) of the adult participants was from Europe, 19.9% (n = 100) were from North America, and 29.9% (n = 150) were from the Asia-Pacific region. Among adult participants from the US (n = 50), most were from the South (n = 28, 56% [US divided into two regions]; or n = 16, 32.0% [US divided into four regions]). A total of 74% (n = 37) of the pediatric participants was from North America, 18% (n = 9) were from Europe, and 8.0% (n = 4) were from the Asia-Pacific region. Among the pediatric participants from the US (n = 31), the majority were from the North (n = 23, 74.2%) and the Midwest (n = 19, 61.3%).
Most participants (n = 461, 83.5%) were exposed to immunomodulatory drugs (study drugs and/or concomitant medications), predominantly avelumab (n = 328, 59.4%). Exposure to immunomodulatory drugs was documented in 31 participants with baseline serum samples collected prior to the date of first study drug exposure, and in 430 participants with post-baseline samples collected after or on the date of first study drug exposure. Most adult participants (n = 340, 67.7%) were enrolled in clinical studies for cancers. All the pediatric participants were enrolled in clinical studies for Duchenne muscular dystrophy.
The present report includes the results for the analysis comprising AAV1, AAV5, AAV6, AAV8, AAV9, and AAVRh74var vectors. Further assay development is required for AAV2, AAV3b, and AAV-DJ.
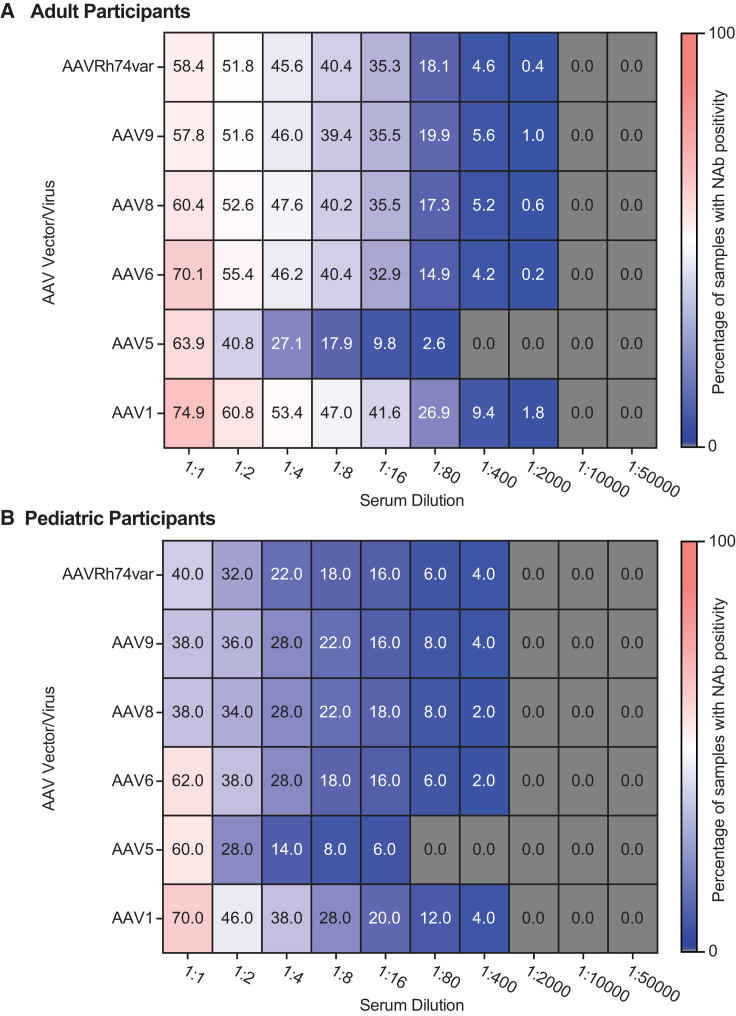
NAb seroprevalence was highest for AAV1 and lowest for AAV5
With the exception of the 1:1 serum dilution, NAb activity directed against AAV1 was the most prevalent, while NAb activity directed against AAV5 was the least prevalent, across dilutions in all countries and in both the adult and pediatric populations (Figures 1 and 2). In adult participants at the 1:1 serum dilution, the most prevalent AAV NAb activities were AAV1 (74.9% of evaluable participants had positive serum samples), AAV6 (70.1%), and AAV5 (63.9%), followed by AAV8 (60.4%), AAVRh74var (58.4%), and AAV9 (57.8%; Figure 1A). At the 1:2 serum dilution and above, a consistent pattern emerged with NAb prevalence highest for AAV1 and lowest for AAV5. Specifically, prevalence at the 1:2 dilution was 60.8% for AAV1 and 40.8% for AAV5. At the 1:4 serum dilution, NAb activity against AAV1 was again the most prevalent (53.4%), and NAb activity against AAV5 was the least prevalent (27.1%). NAb activity against AAV6, AAV8, AAV9, and AAVRh74var was intermediate, ranging from 51.6% to 55.4% at the 1:2 dilution and from 45.6% to 47.6% at the 1:4 dilution. Similar trends were observed at the 1:8, 1:16, and 1:80 dilutions. At the 1:400 and 1:2,000 dilutions, NAb activity against AAV5 was no longer detectable in the samples. NAb activity against all AAVs was undetectable at dilutions >1:10,000.
Figure 1.
Prevalence of NAb positivity in global adult and pediatric participants by AAV serotype and serum dilution
(A) Heatmap of the prevalence of NAb activity for adult participants (n = 502). (B) Heatmap of the prevalence of NAb activity for pediatric participants (n = 50). Heatmaps show the prevalence of NAb activity against six AAV serotypes across all serum dilutions. Numbers inside squares indicate the percentage of serum samples positive for NAb activity against the designated serotype at the designated serum dilution (out of all samples tested for that AAV at that serum dilution). Gray squares indicate that all tested serum samples were negative for NAb activity against the serotype at the specified dilution. AAV, adeno-associated virus; Nab, neutralizing antibody.
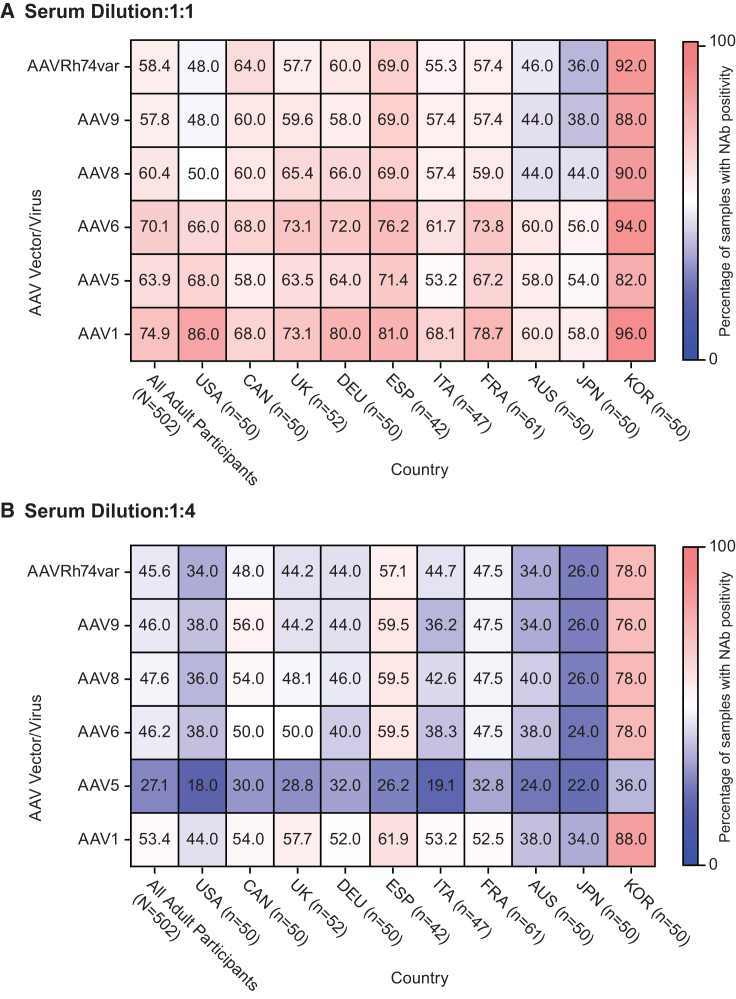
Figure 2.
Prevalence of NAb positivity in adult participants by AAV serotype and country at 1:1 and 1:4 serum dilutions
Heatmaps show the prevalence of NAb activity against six AAV serotypes for all adult participants (left column; n = 502) and the subset of participants from each country for the (A) 1:1 and (B) 1:4 serum dilutions. Numbers inside squares indicate the percentage of serum samples positive for NAb activity against the designated serotype in the designated country (out of all samples tested for that AAV in that country). AAV, adeno-associated virus; Nab, neutralizing antibody; USA, United States; CAN, Canada; UK, United Kingdom; DEU, Germany; ESP, Spain; ITA, Italy; FRA, France; AUS, Australia; JPN, Japan; KOR, South Korea.
Pediatric samples (Figure 1B) exhibited lower prevalence of anti-AAV NAbs compared with adult samples (Figure 1A), although the sample size was considerably smaller for the pediatric population (n = 50 compared with n = 502 for adults). At the 1:1 serum dilution in all pediatric participants (Figure 1B), the three most prevalent NAb activities were directed against AAV1 (70.0%), AAV6 (62.0%), and AAV5 (60.0%), similar to adults. At the 1:2 serum dilution, the lowest prevalence of NAb activity was observed for AAV5 (28.0%), followed by AAVRh74var (32.0%); the highest positivity was observed for AAV1 (46.0%). At the 1:4 serum dilution, the lowest prevalence of NAb activity was directed against AAV5 (14.0%) and AAVRh74var (22.0%), and the highest was directed against AAV1 (38.0%). Similar trends were observed at 1:8 and 1:16 serum dilutions. At 1:80 and 1:400 serum dilutions, NAb activity against AAV5 was not detected. At the 1:2,000, 1:10,000, and 1:50,000 serum dilutions, all pediatric samples were negative for anti-AAV NAb activity. NAb prevalence in pediatric participants was similar for the global (Figure 1B) and US-only (Figure S1A) populations, although it is noted that the US pediatric participants represented the majority (n = 31, 62%) of all pediatric participants.
In adults at the 1:1 serum dilution, South Korea exhibited the highest prevalence of NAb positivity against all six AAVs. The highest prevalence in South Korea was observed for AAV1 (96.0%) and AAV6 (94%), and the lowest prevalence was observed for AAV5 (82%; Figure 2A). The lowest overall prevalence of NAb activity at the 1:1 serum dilution was observed against AAVRh74var in Japan (36.0%). In Australia, seropositivity ranged from 44% for AAV9 and AAV8 to 60% for AAV1 and AAV6. In the US, the prevalence of NAb positivity ranged considerably by AAV vector, from 48% for AAVRh74var and AAV9 to 86% for AAV1. The remaining countries (Spain, Germany, UK, France, and Canada) presented intermediate levels of NAb positivity. At the 1:2 serum dilution, similar variation in NAb positivity was observed, with Japan, Australia, and the US presenting the lowest prevalence and South Korea the highest (Figure S1B). At 1:4 and 1:8 serum dilutions, NAb positivity was the lowest against AAV5 across all 10 countries (Figures 2B and S1C).
NAb seroprevalence varied across geographical regions, was higher in females versus males, was higher in those of Asian ethnicity, and increased with age
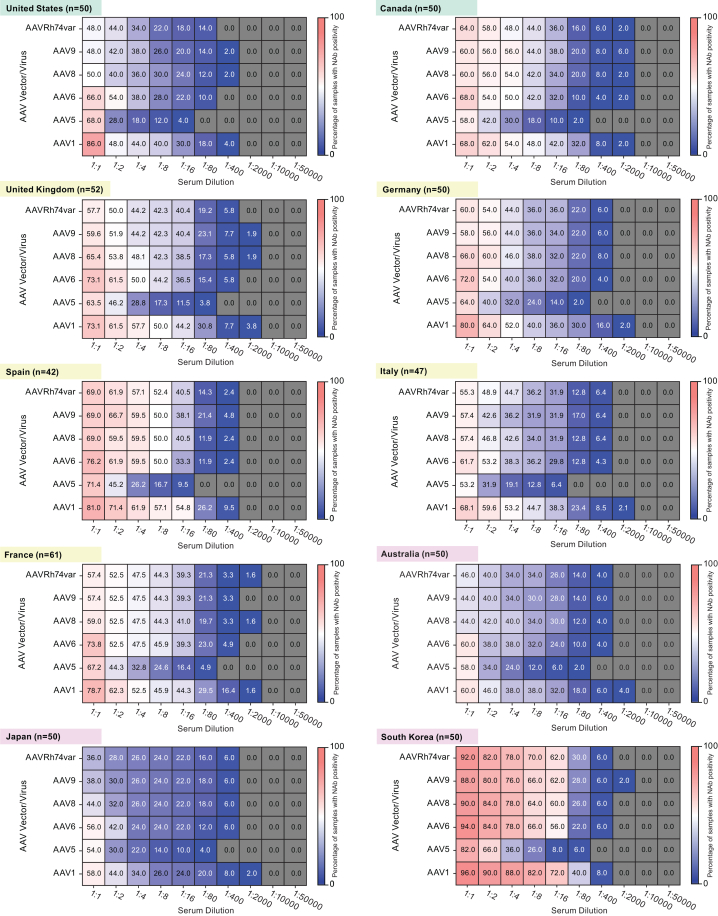
Further analysis of pre-defined exploratory aims noted no consistent trends in the prevalence of NAb positivity in adults by AAV vector and serum dilution across geographical regions, although some patterns were observed (Figure 3). In the Asia-Pacific region, Australia and Japan had similarly low levels of NAb positivity across AAV vectors and serum dilutions, which contrasted with the high prevalence observed in South Korea. In Europe, France, Germany, Italy, and the UK presented similar patterns while Spain exhibited a higher NAb prevalence. In North America, Canada and the US exhibited different patterns of seropositivity, with more variation observed across AAV vectors and serum dilutions in the US than in Canada.
Figure 3.
Prevalence of NAb positivity in adult participants by AAV serotype and serum dilution across geographical regions
Heatmaps show the prevalence of NAb activity against six AAV serotypes for adult participants (n = 502) across all serum dilutions on a per country basis, sorted by geographical region (green, North America; yellow, Europe; pink, Asia-Pacific). Numbers inside squares indicate the percentage of serum samples positive for NAb activity against the designated serotype at the designated serum dilution (out of all samples tested for that AAV at that serum dilution). Gray squares indicate that all tested serum samples were negative for NAb activity against the serotype at the specified dilution. AAV, adeno-associated virus; Nab, neutralizing antibody.
There was a numerically higher prevalence of NAb positivity in females (n = 226) compared with males (n = 276; Table S1). Prevalence differed for AAV9 at the 1:400 dilution, from 3.6% (95% confidence interval [CI], 1.8%, 6.6%; n = 10) in adult males to 8.0% (95% CI 4.8%, 12.3%; n = 18) in adult females. In adults, differences between ethnicities were observed at the 1:2 serum dilution for AAV1 and AAV5, with higher prevalence among those with Asian or missing ethnicity information. Specifically, the prevalence of NAb activity against AAV1 at the 1:2 serum dilution was 75.0% (95% CI 53.3%, 90.2%; n = 18) among those with missing ethnicity information, 68.2% (95% CI 59.4%, 76.1%; n = 88) in Asian participants, 57.8% (95% CI 52.3%, 63.1%; n = 193) in White participants, 28.6% (95% CI 3.7%, 71.0%; n = 2) in African participants, and 50.0% (95% CI 15.7%, 84.3%; n = 4) in other ethnic groups. Prevalence of NAb activity against AAV5 at the 1:2 serum dilution was 50.0% (95% CI 29.1%, 70.9%; n = 12) among those with missing ethnicity information, 51.2% (95% CI 42.2%, 60.1%; n = 66) in Asian participants, 36.8% (95% CI 31.6%, 42.2%; n = 123) in White participants, 28.6% (95% CI 3.7%, 71.0%; n = 2) in participants of African ethnicity, and 25.0% (95% CI 3.2%, 65.1%; n = 2) in other ethnic groups. Similar trends were observed for other serotypes and serum dilutions (data not shown).
The prevalence and titers of anti-AAV NAb activity increased gradually with age from childhood to late adulthood (Figure 1). The overall prevalence of pre-existing NAb activity against each AAV serotype was generally higher in adults aged 41 to 60 years (n = 407) than in those aged 16 to 40 years (n = 95) across serotypes and serum dilutions (Table S2).
NAb seroprevalence varied across US subregions and was higher in participants with cancer
The prevalence of NAb positivity in adults varied across AAV serotypes and serum dilutions in the four US regions (Midwest, Northeast, South, and West). A lower prevalence of NAb activity was observed in the Midwest, and a higher prevalence was observed in the West. At the 1:1 serum dilution, the prevalence of NAb activity directed against AAV1 was 100% (n = 12) in the West, 93.8% (n = 15) in the South, 78.6% (n = 11) in the Northeast, and 62.5% (n = 5) in the Midwest. The prevalence of NAb activity directed against AAV5 was 83.3% (n = 10) in the West, 75.0% (n = 12) in the South, 64.3% (n = 9) in the Northeast, and 37.5% (n = 3) in the Midwest. When dividing the US into two regions, there was a trend toward higher prevalence of NAb positivity in the South compared with the North across AAV serotypes and serum dilutions. At the 1:1 serum dilution, the prevalence of NAb activity directed against AAV1 was 96.4% (n = 27) in the South and 72.7% (n = 16) in the North; against AAV5, it was 78.6% (n = 22) in the South and 54.5% (n = 12) in the North.
NAb prevalence was numerically higher in adult participants with cancer (n = 340) compared with adult participants with other types of disease under investigation (n = 162; Table S3). At the 1:1 serum dilution, among participants in cancer clinical studies, the prevalence of NAb activity directed against AAV1, AAV5, and AAVRh74var was 77.4% (95% CI 72.5%, 81.7%; n = 263), 68.2% (95% CI 63.0%, 73.2%; n = 232), and 63.8% (95% CI 58.5%, 68.9%; n = 217). Among participants in other clinical studies, the prevalence was 69.8% (95% CI 62.1%, 76.7%; n = 113), 54.9% (95% CI 46.9%, 62.8%; n = 89), and 46.9% (95% CI 39.0%, 54.9%; n = 76), respectively. Furthermore, the prevalence of NAb positivity varied greatly by study disease indication, although some trends were observed. At the 1:1 serum dilution, participants with a diabetes-related condition (diabetic retinal edema and type 2 diabetes mellitus) presented the highest prevalence of NAb activity against AAV1 (100%, n = 4 and 95.2%, n = 20, respectively) compared with other disease indications. A similar prevalence of NAb activity against AAV1 was observed in participants with cancer (non-small cell lung cancer [83.3%, n = 10], squamous cell carcinoma of the head and neck [83.1%, n = 49], and transitional cell carcinoma [88.2%, n = 45]) and in participants with skin-related disorders (alopecia areata [60.7%, n = 17], atopic dermatitis [58.0%, n = 29], and psoriasis [50.0%, n = 7]) at the 1:1 serum dilution. Participants in non-small cell lung cancer trials presented the highest prevalence of NAb positivity, and participants in skin-related disorders trials presented with the lowest prevalence. Ninety-two percent of adult study subjects were exposed to immunomodulatory drugs; however, similar trends were observed between those exposed and non-exposed to immunomodulatory drugs (data not shown).
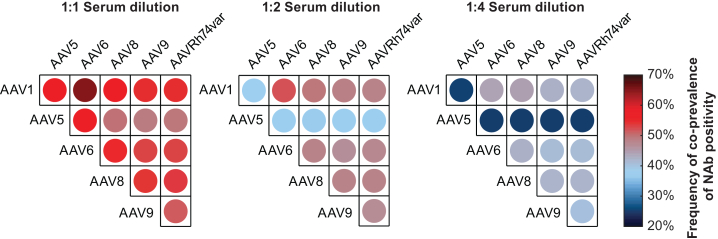
Co-prevalence of NAb positivity, the percentage of participants positive for NAb activity against both AAVs in a pair, varied across serotypes. At the 1:1 serum dilution in all participants (adults and pediatrics), 46.4% (n = 256) of participants were positive for all AAVs and 15.0% (n = 83) were negative for all AAVs (data not shown). Co-prevalence was most frequently observed between AAV1 and AAV6 across serum dilution levels (Figure 4). Co-prevalence between AAV5 and the five other AAVs was less frequent as the serum dilution level increased. Discordance of NAb prevalence, the percentage of participants positive for NAb activity against one AAV while negative for NAb activity against another AAV, also varied across pairs of AAVs. Up to the 1:4 serum dilution, all possible discordant pairs of AAVs were observed. Across all dilutions, discordance of NAb prevalence was observed most frequently between AAV1 (positive) and AAV5 (negative).
Figure 4.
Pairwise co-prevalence of NAb positivity in all participants at the 1:1, 1:2, and 1:4 serum dilutions
Circles show the co-prevalence of NAb activity against pairs of the six AAV serotypes across serum dilutions for all participants (n = 552). Circle colors indicate the percentage of serum samples positive for NAb activity against both AAV serotypes in pair. AAV, adeno-associated virus; Nab, neutralizing antibody.
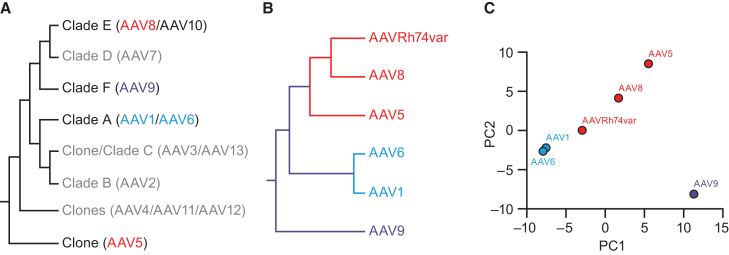
Machine learning analysis of NAb seroprevalence suggests a different clade classification for AAV5 and AAV9 compared with previous phylogenetic analyses
Ward’s hierarchical clustering of anti-AAV NAb ID50 titers across patients was compared with clustering of AAVs into clades based on phylogenetic analysis (Figures 5A and 5B).53,54 Ward’s method iteratively clusters the AAVs based on the similarity across patient ID50 values. With this method, AAV1 and AAV6 clustered together indicating that they were closely related. AAVRh74var formed a cluster with AAV8. These two clusters were consistent with the literature53,54; however, AAV5 was found to be closer to clade E (AAVRh74var:AAV8) than prior phylogenetic analyses had revealed. AAV9 was an outlier and had the lowest similarity relative to the other five AAVs. These hierarchical clustering results were further supported by a two-dimensional projection using principal-component analysis (PCA) that characterized similarities across patient ID50 values by distance in the projected space (Figure 5C).
Figure 5.
Machine learning analysis of relationship between different AAV serotypes
(A) Cladogram showing phylogenetic classifications of AAV serotypes, based on Gao et al.53 and Mietzsch et al.53,54 (B) Results of Ward’s hierarchical clustering of AAV NAb titers showing the relationship between the six analyzed AAV serotypes. AAV1 and AAV6 formed a cluster, indicating that they are closely related. AAV8 and AAVRh74var also formed a cluster, which was found to be related to AAV5. AAV9 was least similar to the other AAVs. (C) Principal-component analysis (PCA) of normalized NAb titers in all participants for the six AAV serotypes. AAVs that are nearer one another in the two-dimensional projection are more closely related. As observed with the clustering analysis (B), AAV1 and AAV6 were closest to one another, followed by AAVRh74var, AAV8, and AAV5, while AAV9 was most distant from all the other AAVs. AAV, adeno-associated virus; Nab, neutralizing antibody; PC, principal component.
Beyond the dimensionality reduction and clustering, the relationships between anti-AAV NAb titers and demographic data were assessed using regression models. For each AAV, a regression model was created from the other AAVs and demographic data as predictors. The variable importance or p values assessed the strength of the predictors. A random forest prediction model (Table 2) showed that the strongest predictors of each anti-AAV NAb titer were other AAVs, and the most predictive AAVs were those identified as most similar in the clustering analysis (i.e., AAV1 and AAV6, AAV8 and AAVRh74var). For example, the strongest predictor of anti-AAV6 NAb titer was AAV1, and the strongest predictor of AAV1 was AAV6 (variable importance: 0.6864 and 0.6791, respectively). Similarly, the strongest predictor of AAV8 was AAVRh74var, and the strongest predictor of AAVRh74var was AAV8 (variable importance: 0.6887 and 0.5435, respectively). Demographic and clinical characteristics of study participants had far less predictive power. The highest estimate of variable importance for any demographic characteristic was 0.0497 (for age and AAV9). Similar results were also obtained with linear and XGBoost regression analyses.
Table 2.
Variable importance in predicting AAV NAb titer from six AAVs and additional features using a random forest prediction model
| Features | AAV1 | AAV5 | AAV6 | AAV8 | AAV9 | AAV Rh74var |
|---|---|---|---|---|---|---|
| AAVRh74var | 0.1903 | 0.2219 | 0.1547 | 0.6887 | 0.1853 | – |
| AAV8 | 0.0595 | 0.2143 | 0.0694 | – | 0.4256 | 0.5435 |
| AAV6 | 0.6791 | 0.2728 | – | 0.0683 | 0.0455 | 0.1015 |
| AAV1 | – | 0.1397 | 0.6864 | 0.0649 | 0.0584 | 0.1621 |
| AAV9 | 0.0268 | 0.0866 | 0.0321 | 0.0895 | – | 0.1373 |
| AAV5 | 0.0189 | – | 0.0358 | 0.0593 | 0.1622 | 0.0212 |
| Age | 0.0024 | 0.0108 | 0.0017 | 0.0045 | 0.0497 | 0.0033 |
| Fallopian tube cancer, malignant peritoneal neoplasm, ovarian cancer | 0.0004 | 0.0063 | 0.0004 | 0.0039 | 0.0161 | 0.0004 |
| North America region | 0.0062 | 0.0021 | 0.0015 | 0.0008 | 0.0095 | 0.0062 |
| Canada | 0.0037 | 0.0017 | 0.0029 | 0.0010 | 0.0101 | 0.0069 |
| Metastatic renal cell carcinoma | 0.0009 | 0.0042 | 0.0021 | 0.0006 | 0.0016 | 0.0048 |
| France | 0.0029 | 0.0052 | 0.0008 | 0.0008 | 0.0009 | 0.0010 |
| White ethnicity | 0.0015 | 0.0014 | 0.0041 | 0.0010 | 0.0024 | 0.0008 |
| UK | 0.0002 | 0.0015 | 0.0002 | 0.0050 | 0.0036 | 0.0007 |
| Ethnicity not reported | 0.0016 | 0.0045 | 0.0009 | 0.0007 | 0.0013 | 0.0007 |
| Asian ethnicity | 0.0011 | 0.0018 | 0.0008 | 0.0008 | 0.0034 | 0.0016 |
| Europe region | 0.0005 | 0.0031 | 0.0005 | 0.0012 | 0.0026 | 0.0007 |
| Male | 0.0009 | 0.0010 | 0.0011 | 0.0006 | 0.0038 | 0.0005 |
| South Korea | 0.0000 | 0.0018 | 0.0001 | 0.0003 | 0.0044 | 0.0001 |
| Ethnicity not Hispanic or Latino | 0.0010 | 0.0018 | 0.0004 | 0.0004 | 0.0022 | 0.0009 |
| Female | 0.0002 | 0.0009 | 0.0004 | 0.0006 | 0.0034 | 0.0005 |
AAV, adeno-associated virus; Nab, neutralizing antibody.
Discussion
This retrospective, multi-country, observational study employed a sensitive, robust, and standardized cell-based transduction inhibition assay to measure anti-AAV NAb activity directed against several serotypes that are commonly used in AAV-mediated human gene therapy.50,51,52 The assays were performed by a central laboratory that specializes in NAb testing. The study determined the prevalence of NAb activity directed against six different AAV vectors in a large global population of adult and pediatric subjects.
We found that the prevalence of NAb activity directed against AAVs varied by serotype, serum dilution (also known as titer), and study participant demographics, including age, sex, ethnicity, and the 10 countries examined. NAb activity directed against AAV1 was most prevalent and NAb activity directed against AAV5 was the least prevalent across all countries and in both adult and pediatric populations. Similarities in prevalence were most frequently observed between AAV1 and AAV6 across a range of serum dilution levels, whereas similarities in prevalence between AAV5 and other AAVs were less frequently observed. The results reported here also suggest that increased age may be associated with increased NAb positivity. There were also trends favoring higher prevalence of NAb positivity in samples obtained from females, those identified with Asian ethnicity, and participants enrolled in cancer treatment studies across all AAVs.
The finding that NAb activity directed against AAV1 was most prevalent is consistent with prior studies.32,35,36,55 A study with samples from 10 countries and four continents found a higher prevalence of anti-AAV1 NAbs compared with anti-AAV7 or AAV8 NAbs.35 A higher prevalence of anti-AAV1 NAbs versus anti-AAV5, AAV6, AAV8, or AAV9 NAbs was also reported based on an adult population from France.32 The lower prevalence of NAb activity directed against AAV5 is also consistent with prior reports56,57 and relevant in light of recent clinical trial findings showing that treatment of hemophilia B with AAV5 vector-based gene therapy was largely successful even in study subjects with pre-existing anti-AAV5 NAb activity.37,38,39 AAV5 is one of the most divergent serotypes in sequence,53 antigenic properties,58 and structure,59 and its distinct structural features may contribute to the lower NAb activity observed in this and other studies. The relatively high co-prevalence of NAbs across multiple specific AAV serotypes is also consistent with prior studies.18,33,36,56,57 High co-prevalence could be the result of multiple natural infections with various AAVs; however, it is more likely that the immune response to AAV infection generates cross-reactive NAbs based on regions of antigenic homology within different AAV capsids.60 It is also possible that different NAb specificities co-exist in the same samples, rather than the same NAb exhibiting cross-reactivity.
The observed increase in the prevalence of anti-AAV NAbs with increasing age is consistent with prior observations.30,45,57,61,62,63 First exposure to AAV likely occurs in early childhood, most commonly with AAV2.64 While NAbs have been detected in newborns, this is likely a result of maternal transmission.30,64 In addition, biological sex has been reported previously as a factor in the prevalence of anti-AAV NAbs, with females more likely to exhibit anti-AAV NAbs than males.45 These findings are consistent with studies demonstrating an influence of biological sex on immune responses, with higher immunity in females than in males.65,66 However, duration of exposure, proximity to children, and other factors may also contribute to this observation.
Geographical35,44,45 and ethnic56 differences in anti-AAV NAb prevalence have been observed previously. This study revealed a higher prevalence of anti-AAV NAbs among Asians (or those with missing ethnicity information) compared with African, White, or other ethnic/racial groups; however, a previous study reported a higher NAb prevalence among Black and Hispanic versus White populations.56 The later study included only participants from the US,56 which may account for the difference in findings compared with the present study, which included a global population. Together, these findings suggest that genetic and environmental factors (population density, living conditions, socioeconomic status) may play a role in human exposure to and infection with AAVs, and, potentially, differences in immune recognition and NAb responses. It will be important to consider these factors when designing gene therapy vectors, particularly when seeking to harmonize regulatory approvals across geographical regions.
Participants from cancer studies exhibited higher prevalence of anti-AAV NAbs compared with participants from studies with other diseases. While previous reports have found differences in anti-AAV NAb seroprevalence based on participant health,31,49,67,68 none found a difference related specifically to cancer. Because a majority of adult samples (67.7%) were collected from participants in cancer studies, the findings of this study may not represent the prevalence of anti-AAV NAbs in the general population. While cancer affects immune system functioning,69 its impact on the development of NAbs is not fully understood. Several recent studies suggest that patients with cancer may exhibit lower prevalence of NAbs in response to vaccination or virus exposure.70,71,72 Therefore, it is possible that the results of this study may underestimate the prevalence of NAbs in the general population. While some cancer treatments are immunomodulatory, we did not observe a difference in the prevalence of NAb positivity between those exposed to immunomodulatory drugs and those not exposed. However, as disease under investigation and exposure to immunomodulatory drugs were exploratory aims of this study, we cannot draw strong conclusions. It is possible that there are demographic differences between those in the cancer versus non-cancer trials, and a high percentage of adult participants were exposed to immunomodulatory drugs. Future studies should provide a better understanding of the role of cancer or immunomodulatory drugs in modulating anti-AAV NAb levels.
The clustering results of this study mostly support the phylogenetic clade classification reported previously.53,54 However, our results also suggest a need for further investigation into AAV5 and AAV9, which showed a surprisingly different relationship than reported previously. Specifically, AAV5 was clustered more closely with AAV8 and AAVRh74var than shown previously, while AAV9 was more distant from the other AAVs. These differences might be related to the differing biological elements used to generate the clade classifications. In previous studies,53,54 clade classifications were performed based on the genetic similarity among serotypes that share a common ancestor, while in this study NAb titers were used. It should be noted that while the previous clade organizations were based on two-dimensional analysis of linear amino acid sequences, NAb titer clustering reflects the three-dimensional in vivo immune relationship across AAV serotypes. Previous work has found discrepancies between sequence-based and structure-based phylogenies.73 These results are an important point of distinction from earlier published reports of NAb seroprevalence30,35,44,45,49,56,57,61,67,68 and may be particularly useful for the rational design of gene therapy vectors.
Due to the observational retrospective design of this study, it may be subject to selection bias (in participant demographics and clinical characteristics). Specifically, most participants were enrolled in clinical trials for cancer and most had been exposed to immunomodulatory drugs. All pediatric participants were males with Duchenne muscular dystrophy, and most were White and from North America. Some pediatric and adult serum samples had insufficient volume to be tested in the assay, so the initial random sampling plan could not be applied fully. Changes included no random sampling for pediatric participants or adults from France or Italy. A subset of US adult participants were analyzed by sequentially selecting from the original random sampling list. Finally, cellular immunity (i.e., T cell responses to either wild-type AAVs or transgene vectors) was not assessed in this study but may provide evidence of prior exposure to AAVs and influence immune responses to AAV vectors even when patients are seronegative.74
Conclusions
This study brings insights into the global distribution of NAb activity directed against AAV vectors. It provides critical knowledge for the rational design of AAV-mediated gene therapy studies and for the assessment of eligible populations for AAV-mediated gene therapies.
Materials and methods
Experimental model and subject details
Human subjects
This global, multi-country, observational, retrospective, cross-sectional, epidemiologic study included participants who were enrolled in previous Pfizer clinical studies between 2015 and 2019 in the following 10 countries: Australia, Canada, France, Germany, Italy, Japan, South Korea, Spain, UK, and US. The study assayed residual serum samples stored in the Pfizer Biobank as part of the prior unrelated clinical studies.
The algorithm for trial and sample selection is detailed in Figure S2. Clinical studies were selected if they met the following criteria: (1) residual serum samples were stored in the Biobank, (2) final approved protocol date was between January 1, 2015, and December 31, 2019, (3) specimens were approved by the respective study team for use in this study, and (4) trial was conducted in at least one of the following countries: Australia, Canada, France, Germany, Italy, Japan, Korea, Spain, UK, and US.
Male and female participants could be included if they were enrolled in one of the selected clinical studies (described above) and met all of the following criteria: previously consented to post hoc/future exploratory assays using their blood/serum samples, aged up to 60 years, and had available a baseline serum sample if in a treatment arm or any serum sample if in a placebo arm or long-term follow-up study. One serum sample per participant was analyzed and, if available, the baseline sample collected in the clinical trial was used for this study. Participants were excluded if their serum sample was of insufficient quality (e.g., hemolyzed sample) or volume (<0.6 mL).
The target study size was 550 participants comprising 500 adults (∼50 serum samples per country) and 50 children. For adult participants, a random sampling scheme was used across countries, although for certain countries (i.e., France) more samples were selected to compensate for smaller numbers of samples in other neighboring countries (i.e., Italy, Spain). All samples from France were selected for analysis to make up for those not available from Italy and Spain. For pediatric participants, it was not possible to employ a random sampling scheme across non-US countries due to the smaller sample size.
Ethics statement
Informed consent was obtained at the time of clinical trial participation regarding the storage of blood samples and their use for future research. The study was conducted in accordance with legal and regulatory requirements and followed accepted research practices described in the International Ethical Guidelines for Biomedical Research Involving Human Subjects (Council for International Organizations of Medical Sciences 2002), International Council for Harmonisation Guideline for Good Clinical Practice, Good Epidemiology Practices, Good Pharmacoepidemiology Practices, and the Declaration of Helsinki.
Method details
Research question and objectives
The primary objective of this study was to estimate the global seroprevalence of NAbs against clinically relevant AAV serotypes (AAV1, AAV5, AAV6, AAV8, AAV9, AAVRh74var [also known as AAV-Spark100], AAV2, AAV3b, and AAVDJ) in adult (aged 16–60 years; overall and by country) and pediatric (aged younger than 16 years; overall and US only) clinical study participants. Secondary objectives included estimation of the seroprevalence of anti-AAV NAbs in subgroups of adults stratified by geographical region (North America, Europe, Asia-Pacific), biological sex (male, female), and ethnicity (African, Asian, White, other). Although not specified as a secondary objective in the study protocol, seroprevalence by age group (16–40 or 41–60 years of age) was also explored. Exploratory objectives included estimation of the seroprevalence of NAbs in subgroups stratified by site location within four US regions (Midwest, Northeast, South, West), clinical study disease area (cancer, other) and indication, and immunomodulation (exposed versus non-exposed to immunomodulatory drugs). Although not specified as an exploratory objective in the study protocol, seroprevalence by site location within two US regions (North, South) was also explored. The co-prevalence of NAb positivity in the combined pediatric and adult populations was also assessed. Finally, machine learning analyses were performed to examine clustering of AAV serotypes as well as assess the relationship between NAbs and demographic data.
Clinical trial data management
Trained Biobank staff obtained previously collected clinical trial data for the selected participants using unique de-identified participant identifiers. Variables from the clinical trial database included participant baseline characteristics (age, biological sex, ethnicity, and country), study drug and study drug start and stop dates, concomitant medications and date of medication start and stop dates, date of serum sample collection, clinical study indication (derived from the clinical trial number), disease area of previous clinical trial (derived from the study indication), geographical region (derived from country; if the sample was from the US, the site location within two [North, South] or four US regions [Northeast, Midwest, South, West] was derived), and immunomodulation status (derived from the list of concomitant medication and study drugs). No safety data were collected.
Participants’ immunomodulation status was derived using an in-house algorithm. Specifically, participants were first categorized as having a baseline sample (if their serum sample collection date was before the date of their first study treatment exposure) or post-baseline sample (if their serum sample collection date was on or after the date of their first study treatment exposure). For immunomodulatory study drugs, participants with a post-baseline sample were considered exposed if the study drug was recorded as ongoing, if the stop date of the study drug was within 7 days (≤7 days) of the sample collection date, or if the start date of the study drug was within 7 days (≤7 days) of the sample collection date (regardless of study drug stop date). For immunomodulatory concomitant medications, participants with a baseline or post-baseline sample were considered exposed if the concomitant medication was recorded as ongoing, if the stop date of the concomitant medication was within 7 days (≤7 days) of the sample collection date, or if the start date of the concomitant medication was within 7 days (≤7 days) of the sample collection date (regardless of concomitant medication stop date). Participants who did not have study treatment start and end dates (n = 39) were assumed to have a baseline serum sample and were considered non-exposed to immunomodulatory drugs.
Neutralizing antibody assay
Serum samples (0.6 mL) were shipped to and analyzed by a central laboratory (Labcorp-Monogram Biosciences, South San Francisco, CA). Samples were analyzed for NAb activity directed against AAV1, AAV5, AAV6, AAV8, AAV9, and AAVRh74var using a standardized anti-AAV NAb assay (Figure S3). A cell-based transduction inhibition assay was used to determine NAb titers in serum specimens by detecting reporter gene expression. In brief, a recombinant AAV vector comprising an AAV capsid containing a recombinant genome encoding a firefly luciferase reporter gene was incubated with serial dilutions (1:1, 1:2, 1:4, 1:8, 1:16, 1:80, 1:400, 1:2,000, 1:10,000, and 1:50,000) of human serum followed by addition to target cells (human embryonic kidney 293 [HEK293] cell line) maintained in tissue culture. Transduction of target cells is inhibited in the presence of anti-AAV NAb, measured as a proportional reduction in luciferase activity (taken 22–26 h after addition of target cells). Percent inhibition of luciferase activity was plotted against serum dilution, and inhibition curves were fit to the data using a four-parameter function. NAb titers were reported as ID50, the interpolated serum dilution that produces 50% inhibition of the luciferase activity achieved in the absence of human serum. Seroprevalence was assessed based on qualitative NAb determinations (positive, negative) at each of the following serum dilutions: 1:1, 1:2, 1:4, 1:8, 1:16, 1:80, 1:400, 1:2,000, 1:10,000, and 1:50,000.
Positive and negative controls were included on every assay plate. The assay positive control was a unit of polyclonal plasma with positive anti-AAV NAb activity against all AAV vectors in the study. The assay negative control was a unit of human polyclonal plasma negative for anti-AAV NAb activity against all AAV vectors in the study. Acceptance criteria for each assay included the titer of the positive control falling within a predefined range of ID50 results for each virus serotype. Under the standard test conditions, the test results for AAV2, AAV3b, and AAV-DJ were positive for all samples tested. Follow-up studies confirmed that further assay development would be necessary for each of these three AAV serotypes.
Quantification and statistical analysis
Seroprevalence
NAb prevalence was estimated for overall populations and designated subgroups. Outcomes estimating prevalence were evaluated by dividing the number of participants who were classified as NAb positive against a specific AAV at a specific serum dilution by the total number of participants tested for that specific AAV at a particular serum dilution. Exact 95% CIs for the prevalence estimates were calculated using the Clopper and Pearson method. Continuous variables including NAb titer were described using mean, SD, and minimum and maximum values. Titers reported as <1 were imputed as 0.5 to allow summarization and analyses. Categorical variables, including country, biological sex, ethnicity, clinical study indication, disease area, geographical region, site location within two or four US regions, and immunomodulatory drug exposure status, were summarized descriptively using frequency tables. Percentages were calculated using the specified denominator in the frequency table. A chi-square test was used to explore risk factors for NAb positivity. Fisher’s exact test was used if any one of the cells had ≤5 participants.
Machine learning analysis
Two sets of approaches were used to gain insight from AAV values where the data were converted to have a single ID50 per AAV per participant. From this table of results, the initial focus was to determine the relationship between the AAVs using the data across participants. First, the unsupervised techniques were used, which included Ward’s hierarchical clustering and the PCA dimensionality reduction techniques. For each of these techniques, the similarity between the AAVs could be established using clusters or similarity of the projections between the AAVs based on their ID50 values across participants. These results could then be compared with the patterns from an established cladogram or hierarchical clustering of the AAV sequences.
To further understand the relationships between the AAVs and the other factors, three machine learning models were explored to model and predict the ID50 values for each AAV. To predict a single AAV, the participant data from the other AAVs as well as demographic variables including age, gender, geographical region, and ethnicity were used. Three machine learning models were used for these predictions: random forests, linear regression, and XGBoost. A key use of these supervised models is not to predict the AAV but identify the critical variables in each of the models.
Data and code availability
Select data reported in this paper will be shared by the corresponding author upon request.
Any additional information required to reanalyze the data reported in this paper is available from the corresponding author upon request.
Acknowledgments
This study was sponsored by Pfizer. Editorial support was provided by Courtney M. Cameron, PhD, of Engage Scientific Solutions (Fairfield, CT) and was funded by Pfizer. The authors thank Nicole Williams, Michael Swietek, Bhupinder Singh, Michelle Casey, and Sandra Okala for their support in conducting the study and Samuel Jauregui, Benjamin Huang, and Jennifer Fischer for running the neutralizing antibody assay and processing some data.
Author contributions
Conceptualization, I.W. and J.E.J.R. (equal); resources, I.W. (lead); project administration, A.C. and G.B. (equal); investigation, C.J.P. and T.W. (equal); methodology, C.J.P. and T.W. (equal); writing – review & editing, A.C., G.B., C.J.P., T.W., Y.P., P.V.H., S.S., C.d.F.P., I.W., and J.E.J.R.; formal analysis, A.C., G.B., C.J.P., T.W., Y.P., P.V.H., S.S., C.d.F.P., I.W., and J.E.J.R.
Declaration of interests
J.E.J.R. has received supply of material (Material Transfer Agreement), consultancy, or honoraria from bluebird bio, Cynata, Novartis, Pfizer, RareCyte, Roche, and SPARK Therapeutics and has shareholdings with RareCyte and Woke. Y.P., C.d.F.P., and I.W. are employees of, and own stock/options in, Pfizer. G.B., P.V.H., S.S., and A.C. were employed at Pfizer at the time of this study. C.J.P. and T.W. are employees of, and hold equity in, Labcorp.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtm.2024.101273.
Supplemental information
References
- 1.Wang D., Tai P.W.L., Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019;18:358–378. doi: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coura R.d.S., Nardi N.B. The state of the art of adeno-associated virus-based vectors in gene therapy. Virol. J. 2007;4:99. doi: 10.1186/1743-422X-4-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naso M.F., Tomkowicz B., Perry W.L., 3rd, Strohl W.R. Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs. 2017;31:317–334. doi: 10.1007/s40259-017-0234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziccardi L., Cordeddu V., Gaddini L., Matteucci A., Parravano M., Malchiodi-Albedi F., Varano M. Gene therapy in retinal dystrophies. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20225722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Food and Drug Administration FDA approves innovative gene therapy to treat pediatric patients with spinal muscular atrophy, a rare disease and leading genetic cause of infant mortality. 2024. https://www.fda.gov/news-events/press-announcements/fda-approves-innovative-gene-therapy-treat-pediatric-patients-spinal-muscular-atrophy-rare-disease
- 6.Kastelein J.J.P., Ross C.J.D., Hayden M.R. From mutation identification to therapy: discovery and origins of the first approved gene therapy in the Western world. Hum. Gene Ther. 2013;24:472–478. doi: 10.1089/hum.2013.063. [DOI] [PubMed] [Google Scholar]
- 7.2024. U.S. Food and Drug Administration FDA approves novel gene therapy to treat patients with a rare form of inherited vision loss.https://www.fda.gov/news-events/press-announcements/fda-approves-novel-gene-therapy-treat-patients-rare-form-inherited-vision-loss [Google Scholar]
- 8.U.S. Food and Drug Administration FDA approves first gene therapy to treat adults with hemophilia B. 2024. https://public4.pagefreezer.com/browse/FDA/31-12-2022T07:59/https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapy-treat-adults-hemophilia-b
- 9.Chamberlain J.R., Chamberlain J.S. Progress toward gene therapy for Duchenne muscular dystrophy. Mol. Ther. 2017;25:1125–1131. doi: 10.1016/j.ymthe.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abreu N.J., Waldrop M.A. Overview of gene therapy in spinal muscular atrophy and Duchenne muscular dystrophy. Pediatr. Pulmonol. 2021;56:710–720. doi: 10.1002/ppul.25055. [DOI] [PubMed] [Google Scholar]
- 11.Wagner J.A., Reynolds T., Moran M.L., Moss R.B., Wine J.J., Flotte T.R., Gardner P. Efficient and persistent gene transfer of AAV-CFTR in maxillary sinus. Lancet. 1998;351:1702–1703. doi: 10.1016/s0140-6736(05)77740-0. [DOI] [PubMed] [Google Scholar]
- 12.Mendell J.R., Al-Zaidy S., Shell R., Arnold W.D., Rodino-Klapac L.R., Prior T.W., Lowes L., Alfano L., Berry K., Church K., et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 2017;377:1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 13.Pan R.Y., Chen S.L., Xiao X., Liu D.W., Peng H.J., Tsao Y.P. Therapy and prevention of arthritis by recombinant adeno-associated virus vector with delivery of interleukin-1 receptor antagonist. Arthritis Rheum. 2000;43:289–297. doi: 10.1002/1529-0131(200002)43:2<289::Aid-anr8>3.0.Co;2-h. [DOI] [PubMed] [Google Scholar]
- 14.Goater J., Müller R., Kollias G., Firestein G.S., Sanz I., O'Keefe R.J., Schwarz E.M. Empirical advantages of adeno associated viral vectors in vivo gene therapy for arthritis. J. Rheumatol. 2000;27:983–989. [PubMed] [Google Scholar]
- 15.Kay J.D., Gouze E., Oligino T.J., Gouze J.N., Watson R.S., Levings P.P., Bush M.L., Dacanay A., Nickerson D.M., Robbins P.D., et al. Intra-articular gene delivery and expression of interleukin-1Ra mediated by self-complementary adeno-associated virus. J. Gene Med. 2009;11:605–614. doi: 10.1002/jgm.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuller-Carter P.I., Basiri H., Harvey A.R., Carvalho L.S. Focused update on AAV-based gene therapy clinical trials for inherited retinal degeneration. BioDrugs. 2020;34:763–781. doi: 10.1007/s40259-020-00453-8. [DOI] [PubMed] [Google Scholar]
- 17.Brantly M.L., Chulay J.D., Wang L., Mueller C., Humphries M., Spencer L.T., Rouhani F., Conlon T.J., Calcedo R., Betts M.R., et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc. Natl. Acad. Sci. USA. 2009;106:16363–16368. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flotte T.R., Trapnell B.C., Humphries M., Carey B., Calcedo R., Rouhani F., Campbell-Thompson M., Yachnis A.T., Sandhaus R.A., McElvaney N.G., et al. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing α1-antitrypsin: interim results. Hum. Gene Ther. 2011;22:1239–1247. doi: 10.1089/hum.2011.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mueller C., Gernoux G., Gruntman A.M., Borel F., Reeves E.P., Calcedo R., Rouhani F.N., Yachnis A., Humphries M., Campbell-Thompson M., et al. 5-year expression and neutrophil defect repair after gene therapy in alpha-1 antitrypsin deficiency. Mol. Ther. 2017;25:1387–1394. doi: 10.1016/j.ymthe.2017.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nathwani A.C., Tuddenham E.G.D., Rangarajan S., Rosales C., McIntosh J., Linch D.C., Chowdary P., Riddell A., Pie A.J., Harrington C., et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.George L.A., Sullivan S.K., Giermasz A., Rasko J.E.J., Samelson-Jones B.J., Ducore J., Cuker A., Sullivan L.M., Majumdar S., Teitel J., et al. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N. Engl. J. Med. 2017;377:2215–2227. doi: 10.1056/NEJMoa1708538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doshi B.S., Arruda V.R. Gene therapy for hemophilia: what does the future hold? Ther. Adv. Hematol. 2018;9:273–293. doi: 10.1177/2040620718791933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.George L.A., Ragni M.V., Rasko J.E.J., Raffini L.J., Samelson-Jones B.J., Ozelo M., Hazbon M., Runowski A.R., Wellman J.A., Wachtel K., et al. Long-term follow-up of the first in human intravascular delivery of AAV for gene transfer: AAV2-hFIX16 for severe hemophilia B. Mol. Ther. 2020;28:2073–2082. doi: 10.1016/j.ymthe.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mingozzi F., High K.A. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat. Rev. Genet. 2011;12:341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 25.Flotte T.R., Cataltepe O., Puri A., Batista A.R., Moser R., McKenna-Yasek D., Douthwright C., Gernoux G., Blackwood M., Mueller C., et al. AAV gene therapy for Tay-Sachs disease. Nat. Med. 2022;28:251–259. doi: 10.1038/s41591-021-01664-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lek A., Wong B., Keeler A., Blackwood M., Ma K., Huang S., Sylvia K., Batista A.R., Artinian R., Kokoski D., et al. Unexpected Death of a Duchenne Muscular Dystrophy Patient in an N-of-1 Trial of rAAV9-delivered CRISPR-transactivator. medRxiv. 2023 doi: 10.1101/2023.05.16.23289881. Preprint at. [DOI] [Google Scholar]
- 27.Muhuri M., Levy D.I., Schulz M., McCarty D., Gao G. Durability of transgene expression after rAAV gene therapy. Mol. Ther. 2022;30:1364–1380. doi: 10.1016/j.ymthe.2022.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vandendriessche T., Thorrez L., Acosta-Sanchez A., Petrus I., Wang L., Ma L., DE Waele L., Iwasaki Y., Gillijns V., Wilson J.M., et al. Efficacy and safety of adeno-associated viral vectors based on serotype 8 and 9 vs. lentiviral vectors for hemophilia B gene therapy. J. Thromb. Haemost. 2007;5:16–24. doi: 10.1111/j.1538-7836.2006.02220.x. [DOI] [PubMed] [Google Scholar]
- 29.Mingozzi F., High K.A. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122:23–36. doi: 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calcedo R., Morizono H., Wang L., McCarter R., He J., Jones D., Batshaw M.L., Wilson J.M. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin. Vaccine Immunol. 2011;18:1586–1588. doi: 10.1128/cvi.05107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu H., Meadows A.S., Pineda R.J., Kunkler K.L., Truxal K.V., McBride K.L., Flanigan K.M., McCarty D.M. Differential prevalence of antibodies against adeno-associated virus in healthy children and patients with mucopolysaccharidosis iii: perspective for AAV-mediated gene therapy. Hum. Gene Ther. Clin. Dev. 2017;28:187–196. doi: 10.1089/humc.2017.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boutin S., Monteilhet V., Veron P., Leborgne C., Benveniste O., Montus M.F., Masurier C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum. Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 33.Calcedo R., Wilson J.M. Humoral immune response to AAV. Front. Immunol. 2013;4:341. doi: 10.3389/fimmu.2013.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulz M., Levy D.I., Petropoulos C.J., Bashirians G., Winburn I., Mahn M., Somanathan S., Cheng S.H., Byrne B.J. Binding and neutralizing anti-AAV antibodies: Detection and implications for rAAV-mediated gene therapy. Mol. Ther. 2023;31:616–630. doi: 10.1016/j.ymthe.2023.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calcedo R., Vandenberghe L.H., Gao G., Lin J., Wilson J.M. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kruzik A., Fetahagic D., Hartlieb B., Dorn S., Koppensteiner H., Horling F.M., Scheiflinger F., Reipert B.M., de la Rosa M. Prevalence of anti-adeno-associated virus immune responses in international cohorts of healthy donors. Mol. Ther. Methods Clin. Dev. 2019;14:126–133. doi: 10.1016/j.omtm.2019.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Majowicz A., Nijmeijer B., Lampen M.H., Spronck L., de Haan M., Petry H., van Deventer S.J., Meyer C., Tangelder M., Ferreira V. Therapeutic hFIX activity achieved after single AAV5-hFIX treatment in hemophilia B patients and NHPs with pre-existing anti-AAV5 NAbs. Mol. Ther. Methods Clin. Dev. 2019;14:27–36. doi: 10.1016/j.omtm.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leebeek F.W., Miesbach W., Recht M., S Key N., Lattimore S., Castaman G., K Sawyer E., Cooper D., Ferriera V., W Pipe S., HOPE-B Investigators Clinical outcomes in adults with hemophilia B with and without pre-existing neutralizing antibodies to AAV5: 6 month data from the phase 3 etranacogene dezaparvovec HOPE-B gene therapy trial [abstract] Res. Pract. Thromb. Haemost. 2021;5:92–93. https://abstracts.isth.org/abstract/clinical-outcomes-in-adults-with-hemophilia-b-with-and-without-pre-existing-neutralizing-antibodies-to-aav5-6-month-data-from-the-phase-3-etranacogene-dezaparvovec-hope-b-gene-therapy-trial/ [Google Scholar]
- 39.Miesbach W., Leebeek F.W.G., Recht M., Key N.S., Lattimore S., Castaman G., Coppens M., Cooper D., Slawka S., Verweij S., et al. Final analysis from the pivotal phase 3 HOPE-B gene therapy trial: stable steady-state efficacy and safety of etranacogene dezaparvovec in adults with severe or moderately severe hemophilia B. Haemophilia. 2022;28:25–126. [Google Scholar]
- 40.Fisher K.J., Jooss K., Alston J., Yang Y., Haecker S.E., High K., Pathak R., Raper S.E., Wilson J.M. Recombinant adeno-associated virus for muscle directed gene therapy. Nat. Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 41.Halbert C.L., Standaert T.A., Aitken M.L., Alexander I.E., Russell D.W., Miller A.D. Transduction by adeno-associated virus vectors in the rabbit airway: efficiency, persistence, and readministration. J. Virol. 1997;71:5932–5941. doi: 10.1128/jvi.71.8.5932-5941.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halbert C.L., Standaert T.A., Wilson C.B., Miller A.D. Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J. Virol. 1998;72:9795–9805. doi: 10.1128/jvi.72.12.9795-9805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manning W.C., Zhou S., Bland M.P., Escobedo J.A., Dwarki V. Transient immunosuppression allows transgene expression following readministration of adeno-associated viral vectors. Hum. Gene Ther. 1998;9:477–485. doi: 10.1089/hum.1998.9.4-477. [DOI] [PubMed] [Google Scholar]
- 44.Greenberg B., Butler J., Felker G.M., Ponikowski P., Voors A.A., Pogoda J.M., Provost R., Guerrero J., Hajjar R.J., Zsebo K.M. Prevalence of AAV1 neutralizing antibodies and consequences for a clinical trial of gene transfer for advanced heart failure. Gene Ther. 2016;23:313–319. doi: 10.1038/gt.2015.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Q., Huang W., Zhao C., Zhang L., Meng S., Gao D., Wang Y. The prevalence of neutralizing antibodies against AAV serotype 1 in healthy subjects in China: implications for gene therapy and vaccines using AAV1 vector. J. Med. Virol. 2013;85:1550–1556. doi: 10.1002/jmv.23647. [DOI] [PubMed] [Google Scholar]
- 46.Manno C.S., Pierce G.F., Arruda V.R., Glader B., Ragni M., Rasko J.J., Ozelo M.C., Hoots K., Blatt P., Konkle B., et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 47.Mingozzi F., High K.A. Immune responses to AAV in clinical trials. Curr. Gene Ther. 2007;7:316–324. doi: 10.2174/156652307782151425. [DOI] [PubMed] [Google Scholar]
- 48.Falese L., Sandza K., Yates B., Triffault S., Gangar S., Long B., Tsuruda L., Carter B., Vettermann C., Zoog S.J., Fong S. Strategy to detect pre-existing immunity to AAV gene therapy. Gene Ther. 2017;24:768–778. doi: 10.1038/gt.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stanford S., Pink R., Creagh D., Clark A., Lowe G., Curry N., Pasi J., Perry D., Fong S., Hayes G., et al. Adenovirus-associated antibodies in UK cohort of hemophilia patients: A seroprevalence study of the presence of adenovirus-associated virus vector-serotypes AAV5 and AAV8 neutralizing activity and antibodies in patients with hemophilia A. Res. Pract. Thromb. Haemostasis. 2019;3:261–267. doi: 10.1002/rth2.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gorovits B., Fiscella M., Havert M., Koren E., Long B., Milton M., Purushothama S. Recommendations for the development of cell-based anti-viral vector neutralizing antibody assays. AAPS J. 2020;22:24. doi: 10.1208/s12248-019-0403-1. [DOI] [PubMed] [Google Scholar]
- 51.Gorovits B., Azadeh M., Buchlis G., Harrison T., Havert M., Jawa V., Long B., McNally J., Milton M., Nelson R., et al. Evaluation of the humoral response to adeno-associated virus-based gene therapy modalities using total antibody assays. AAPS J. 2021;23:108. doi: 10.1208/s12248-021-00628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meliani A., Leborgne C., Triffault S., Jeanson-Leh L., Veron P., Mingozzi F. Determination of anti-adeno-associated virus vector neutralizing antibody titer with an in vitro reporter system. Hum. Gene Ther. Methods. 2015;26:45–53. doi: 10.1089/hgtb.2015.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao G., Vandenberghe L.H., Alvira M.R., Lu Y., Calcedo R., Zhou X., Wilson J.M. Clades of adeno-associated viruses are widely disseminated in human tissues. J. Virol. 2004;78:6381–6388. doi: 10.1128/jvi.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mietzsch M., Jose A., Chipman P., Bhattacharya N., Daneshparvar N., McKenna R., Agbandje-McKenna M. Completion of the AAV structural atlas: serotype capsid structures reveals clade-specific features. Viruses. 2021;13 doi: 10.3390/v13010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Louis Jeune V., Joergensen J.A., Hajjar R.J., Weber T. Pre-existing anti-adeno-associated virus antibodies as a challenge in AAV gene therapy. Hum. Gene Ther. Methods. 2013;24:59–67. doi: 10.1089/hgtb.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khatri A., Shelke R., Guan S., Somanathan S. Higher seroprevalence of anti-adeno-associated viral vector neutralizing antibodies among racial minorities in the United States. Hum. Gene Ther. 2022;33:442–450. doi: 10.1089/hum.2021.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klamroth R., Hayes G., Andreeva T., Gregg K., Suzuki T., Mitha I.H., Hardesty B., Shima M., Pollock T., Slev P., et al. Global seroprevalence of pre-existing immunity against AAV5 and other AAV serotypes in people with hemophilia A. Hum. Gene Ther. 2022;33:432–441. doi: 10.1089/hum.2021.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tseng Y.S., Gurda B.L., Chipman P., McKenna R., Afione S., Chiorini J.A., Muzyczka N., Olson N.H., Baker T.S., Kleinschmidt J., Agbandje-McKenna M. Adeno-associated virus serotype 1 (AAV1)- and AAV5-antibody complex structures reveal evolutionary commonalities in parvovirus antigenic reactivity. J. Virol. 2015;89:1794–1808. doi: 10.1128/jvi.02710-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Govindasamy L., DiMattia M.A., Gurda B.L., Halder S., McKenna R., Chiorini J.A., Muzyczka N., Zolotukhin S., Agbandje-McKenna M. Structural insights into adeno-associated virus serotype 5. J. Virol. 2013;87:11187–11199. doi: 10.1128/jvi.00867-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao G., Alvira M.R., Somanathan S., Lu Y., Vandenberghe L.H., Rux J.J., Calcedo R., Sanmiguel J., Abbas Z., Wilson J.M. Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc. Natl. Acad. Sci. USA. 2003;100:6081–6086. doi: 10.1073/pnas.0937739100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Daniel H.D.J., Kumar S., Kannangai R., Farzana J., Joel J.N., Abraham A., Lakshmi K.M., Agbandje-McKenna M., Coleman K.E., Srivastava A., et al. Age-stratified adeno-associated virus serotype 3 neutralizing and total antibody prevalence in hemophilia A patients from India. J. Med. Virol. 2022;94:4542–4547. doi: 10.1002/jmv.27859. [DOI] [PubMed] [Google Scholar]
- 62.Ertl H.C.J. T cell-mediated immune responses to AAV and AAV vectors. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.666666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perocheau D.P., Cunningham S., Lee J., Antinao Diaz J., Waddington S.N., Gilmour K., Eaglestone S., Lisowski L., Thrasher A.J., Alexander I.E., et al. Age-related seroprevalence of antibodies against AAV-LK03 in a UK population cohort. Hum. Gene Ther. 2019;30:79–87. doi: 10.1089/hum.2018.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen C.L., Jensen R.L., Schnepp B.C., Connell M.J., Shell R., Sferra T.J., Bartlett J.S., Clark K.R., Johnson P.R. Molecular characterization of adeno-associated viruses infecting children. J. Virol. 2005;79:14781–14792. doi: 10.1128/jvi.79.23.14781-14792.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jacobsen H., Klein S.L. Sex differences in immunity to viral infections. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.720952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 67.Halbert C.L., Miller A.D., McNamara S., Emerson J., Gibson R.L., Ramsey B., Aitken M.L. Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: Implications for gene therapy using AAV vectors. Hum. Gene Ther. 2006;17:440–447. doi: 10.1089/hum.2006.17.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boyce S., James I., Rangarajan S., Curry N., Bagot C., Austin S., Laffan M., Mangles S., Chandrakumaran K., Mundy C. Seroprevalence to adeno-associated virus type 6 in people with hemophilia B from a UK adult cohort. Res. Pract. Thromb. Haemostasis. 2022;6 doi: 10.1002/rth2.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chang R.B., Beatty G.L. The interplay between innate and adaptive immunity in cancer shapes the productivity of cancer immunosurveillance. J. Leukoc. Biol. 2020;108:363–376. doi: 10.1002/JLB.3MIR0320-475R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Felip E., Pradenas E., Romeo M., Marfil S., Trinité B., Urrea V., Hernández A., Ballana E., Cucurull M., Mateu L., et al. Impact of chemotherapy and/or immunotherapy on neutralizing antibody response to SARS-CoV-2 mRNA-1237 vaccine in patients with solid tumors. Mol. Oncol. 2023;17:686–694. doi: 10.1002/1878-0261.13359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fendler A., Shepherd S.T.C., Au L., Wilkinson K.A., Wu M., Byrne F., Cerrone M., Schmitt A.M., Joharatnam-Hogan N., Shum B., et al. Adaptive immunity and neutralizing antibodies against SARS-CoV-2 variants of concern following vaccination in patients with cancer: the CAPTURE study. Nat. Cancer. 2021;2:1305–1320. doi: 10.1038/s43018-021-00274-w. [DOI] [PubMed] [Google Scholar]
- 72.Zagouri F., Papatheodoridi A., Liontos M., Briasoulis A., Sklirou A.D., Skafida E., Fiste O., Markellos C., Andrikopoulou A., Koutsoukos K., et al. Assessment of Postvaccination Neutralizing Antibodies Response against SARS-CoV-2 in Cancer Patients under Treatment with Targeted Agents. Vaccines (Basel) 2022;10 doi: 10.3390/vaccines10091474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Balaji S., Srinivasan N. Comparison of sequence-based and structure-based phylogenetic trees of homologous proteins: Inferences on protein evolution. J. Biosci. 2007;32:83–96. doi: 10.1007/s12038-007-0008-1. [DOI] [PubMed] [Google Scholar]
- 74.Mingozzi F., Maus M.V., Hui D.J., Sabatino D.E., Murphy S.L., Rasko J.E.J., Ragni M.V., Manno C.S., Sommer J., Jiang H., et al. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Select data reported in this paper will be shared by the corresponding author upon request.
Any additional information required to reanalyze the data reported in this paper is available from the corresponding author upon request.